-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study
In a community-based cohort study, Serhiy Dekhtyar and colleagues examine the association between engagement in various cognitive reserve-enhancing factors at early, mid, and late life and risk of dementia concurrence after 75.
Published in the journal: . PLoS Med 14(3): e32767. doi:10.1371/journal.pmed.1002251
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002251Summary
In a community-based cohort study, Serhiy Dekhtyar and colleagues examine the association between engagement in various cognitive reserve-enhancing factors at early, mid, and late life and risk of dementia concurrence after 75.
Introduction
The relevance of cognitive reserve-enhancing factors as contributors to dementia risk has emerged from several longitudinal population-based studies [1–3] and has been confirmed by pathological and clinical data [4]. Thus, numerous studies have reported an increased risk of dementia in less educated persons [5,6]. Higher education could help build cognitive reserve—a set of skills or repertoires that increase an individual’s ability to cope with dementia pathology later in life [3,7]. Some studies have suggested that childhood socioeconomic status (SES) may be of importance for the development of dementia in late life [8,9]. A poor-quality environment during childhood or adolescence may prevent the brain from reaching full levels of maturation, leading to low cognitive reserve, which in turn could put people at higher risk for dementia. On the other hand, few years of schooling could be a marker of cognitive abilities that may have both gestational and genetic origin [10]. In any case, the first decade of life appears to be a critical period for developing dementia later in life [11,12].
In addition to early life contributors, the functional efficiency of cognitive networks may be promoted by adulthood factors, such as occupational attainment or leisure activities, leading to higher cognitive reserve. There is growing support for the hypothesis that mental stimulation in middle age (e.g., mental occupational demands [13] and work complexity [14–16]), as well as in old age (e.g., leisure activities [1,17]), may reduce the risk of dementia. Biologically, mental stimulation could selectively increase synaptogenesis in adulthood, whereas physical exercise might enhance non-neuronal components of the brain, such as vasculature [18]. The ability of the adult brain to respond to environmental stimuli by activating compensatory processes, thus, could be sustained in late life [19]. Therefore, it appears that factors associated with dementia risk originate in different periods throughout the entire life course, including early, adult, and late life.
Few studies have been able to examine the effects of cumulative exposure to cognitive reserve-enhancing factors across the life span. Exposure to these factors during different life periods may act alone or combine in clusters affecting health in later life [20,21]. It has been suggested that dementia is not determined at a single time period but rather results from a complex interplay between genetic and environmental exposures throughout the life course [22,23]. Although life-course models for late-onset diseases have received increased attention in the epidemiological field [21], this approach has not yet been widely applied to dementia [22], with the exception of a few studies [12,24,25]. Using a life-course approach, this study aims to test the hypotheses that (1) cognitive reserve-related factors operating at various life periods each are potentially associated with decreased risk of the occurrence of dementia, (2) cumulative exposure to reserve-related factors is associated with a progressively reduced risk of dementia, and (3) the risk of dementia later in life is influenced by the interaction between lifelong exposure to cognitive reserve-related factors and genetic factors (e.g., apolipoprotein E [APOE] ε4).
Methods
Ethical approval
All phases of the project received approval from the Ethics Committee at Karolinska Institutet (Dnrs: 87 : 234; 90 : 251; 94 : 122; 97 : 413; 99 : 308; 99 : 025; 01 : 020). All individuals participating in the study completed a written informed consent form as stipulated in the ethical approval. For those participants who became cognitively impaired over time, consent was obtained from the next of kin.
Study population
The study population is derived from the Kungsholmen Project, a longitudinal community-based study that included all inhabitants of the Kungsholmen district in Stockholm, Sweden, aged 75 y and older on 1 October 1987 (n = 2,368) [26,27]. Of the 1,810 baseline participants, 1,473 were diagnosed without dementia by the two-phased design. These participants were approached again every 3 y for first, second, and third follow-up examinations. Because impaired cognition or institutionalization may limit participants’ current activity [28], we excluded 98 individuals with a baseline Mini-Mental State Examination (MMSE) score of less than 23, as well as those residing in an institution. During the first follow-up examination 3 y after the baseline, 441 of the baseline participants could not be clinically examined (269 died, and 172 refused participation), whereas 158 individuals were diagnosed with dementia and were excluded from the study to avoid the possibility that they may already have been in the preclinical phase of dementia when interviewed at baseline [29]. Another 44 participants who refused the second follow-up examination were also excluded. The resulting population eligible for analysis consisted of 732 individuals without dementia. Of these, we removed individuals with missing information on education (n = 3) and occupation (n = 109), as well as 18 women who never entered the labour market, resulting in 602 dementia-free participants at both baseline and the first 3-y follow-up who were followed for up to 9 y to detect incident dementia cases.
Dementia diagnosis
The clinical diagnosis of dementia was obtained in accordance with the third revised Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), with some modification [30]. A two-phased diagnostic procedure [27] was employed, whereby two physicians working independently made a preliminary diagnosis, and a third opinion was sought in the event of discordant assessments by the original examiners. Modifications to the DSM-III-R criteria included adding a diagnosis of questionable dementia for individuals with evident impairment in all requisite functions except one and defining cognitive impairment on the basis of objective neuropsychological assessment [31]. In the current study, only clinically definite cases of dementia were included. The time of dementia onset was assumed to be the midpoint between two examinations or the midpoint between examination and the date of death due to dementia.
Assessment of life-course cognitive reserve factors
We assessed cognitive reserve-related indicators from three distinct periods of the life course: early life, adult life, and late life.
Early life cognitive reserve-related factors (before 20 y) included the following:
Educational attainment collected from participants at the study baseline (1987–1989) as a result of the structured questionnaire administered by one of the two trained nurses (S2 Text). The highest degree achieved was recorded and subsequently categorized as elementary, professional, intermediate school, high school, or university.
Early-life SES measured by the number of siblings grown up with and substantive work complexity at 20 y. A trained nurse recorded the number of siblings during the same baseline interview in which educational attainment was assessed. We expected that a larger number of siblings would be a proxy for lower SES, since research has shown that around the time of this study population’s birth (1885–1912), elite socioeconomic groups had started limiting their fertility, whereas the less privileged strata did not begin this transition until several decades later [32]. Substantive work complexity at age 20 was collected at an informant interview during the first follow-up examination (1990–1991; S2 Text) that aimed to retrospectively explore lifespan work activities, by inquiring about the employer, job title, time period, and tasks at all jobs lasting at least 6 mo [33]. Substantive work complexity at 20 y was recorded in accordance with the work complexity matrix [34] as reported below.
Adult life cognitive reserve-related factors (around 35–55 y) included the following:
Complexity of work with data and people for the longest-held occupation in adult life. Work complexity scores were recorded in accordance with a work complexity matrix [34] that was based on the estimation of more than 12,000 occupations rated during on-site occupational assessments in the United States. Occupational categories of the 1980 Swedish census were matched to the best-fitting category in the 1970 US census [14]. The measures for each occupation were created to reflect the levels of complexity at which a worker in a particular occupation functions according to four dimensions: substantive complexity of work (score range 0–10) and complexity of work with data (0–6), with people (0–8), and with things (0–7), with higher scores indicating greater complexity. Work complexity with things was excluded since it was not found to affect dementia in a previous study using the same material [16] and because of its weak contribution to the latent variable. A large sample study assessing inter-rater agreement of the complexity ratings of different occupations yielded reliability estimates: 0.85 for complexity of work with data and 0.87 for complexity of work with people [35].
Job demands and decision latitude for the longest-held occupation in adult life. Job demands designate the use of skills to perform job tasks, whereas decision latitude indicates the extent of decision authority at a workplace [36]. Both measures were derived in accordance with a psychosocial job exposure matrix [37] for the longest occupational period. If participants reported spending the longest portion of their life working inside the household, their second-longest occupation was used. A high score indicates a higher level of psychosocial job demands and decision latitude. The scores ranged from 2.5 to 9.0 for job demands and 2.2 to 8.6 for control at work. Job demand and job controls derived from the job exposure matrix have been shown to correlate with self-reported levels among the same individuals (average r = 0.6) in a validation study [38]. Information on occupational attainment was collected from informants (relatives or other knowledgeable person) at the first follow-up examination (1990–1991) during the interview on lifetime work history. Interviews were conducted by one of the two nurses specially trained in occupational coding. Informants were used for individuals with dementia as well as dementia-free participants (S2 Text). The interview questionnaire was developed by an expert in occupational medicine and aimed to explore the lifespan work activities of all jobs lasting at least 6 mo. Substantive complexity ratings were added to occupations at 20 y, whereas measures of demands, decision latitude, and complexity of work with data and with people were linked with the longest-held job.
Late-life cognitive reserve-related factors (75 y and older) included the following:
Late-life leisure activities. Information on leisure activities was obtained by means of a personal interview conducted by trained nurses at baseline (1987–1989) (S2 Text). Participants were asked whether they regularly engaged in activities and what those specific activities were, resulting in 29 being identified. A mental, social, and physical component score was assigned to each of these activities, with grading of the three components defined as follows: 0 = none, 1 = low, 2 = moderate, and 3 = high. The sum of the component scores, which had a range of 0–18, was calculated based on the grading for each of the three activities [39]. To validate the scoring, 13 cognitively intact raters, aged 75 y or older, were asked to individually complete a questionnaire containing a list of the 29 activities together with scoring instructions. Reliability analyses revealed a satisfactory result: values for Cronbach’s α were 0.89 for the mental component, 0.95 for the physical component, and 0.82 for the social component.
Covariates
All covariates were assessed at the study baseline (1987–1989). In addition to age and gender (both extracted from the National Population Register), baseline cognitive functioning was evaluated using the MMSE, with a score of 30 indicating unimpaired performance. Depression was assessed through self-reported symptoms such as feeling lonely and constantly being in a bad mood. Comorbidities were ascertained by reviewing the hospital discharge diagnoses through the Stockholm computerized inpatient register system with coverage since 1969. Based on the International Classification of Disease, 8th edition (ICD-8) [40], we identified coronary heart disease (ICD-8 : 410–414), cerebrovascular disease (ICD-8 : 430–438), diabetes mellitus (ICD-8 : 250), malignancy (ICD-8 : 140–208 and 230–239), and hip fracture (ICD-8 : 820). Comorbidity was defined as the presence of any of these conditions. Genomic DNA was prepared from peripheral blood samples at baseline, and APOE genotyping using a standard polymerase chain reaction procedure [41] was performed by two technicians who were blind to all other data.
Statistical analysis
Logistic regression was used to examine differences between participants and nonparticipants. Structural equation models (SEMs) were computed to derive the best-fitting measurement model for ten individual lifelong reserve-enhancing indicators. Using various fit criteria [42,43], three separate latent reserve measures were generated, comprised of early-, adult-, and late-life cognitive reserve-enhancing composite indicators. Cox regression models (age - and sex-adjusted) were then used to estimate the hazard ratio (HR) and 95% confidence intervals of dementia occurrence for each of the three latent composite reserve-enhancing factors. In an additional set of analyses, we further adjusted for late-life cognitive functioning as well as the presence of comorbidity and depressive symptoms. The three latent composite factors were analysed as continuous variables, quartile categories, and dichotomized variables contrasting the top three quartiles versus the lowest one. First, each composite indicator for a specific life period was analysed separately, and then all three were entered into the same model to verify their independent effects.
We decomposed the total effect of early-, adult-, and late-life composite factors on dementia risk. First, a full model including all three life-course factors and covariates was fit, with estimated parameters producing the direct effects of early-, adult-, and late-life factors. Next, a series of reduced models that included only early-, adult-, or late-life indicators was estimated, with parameters from these models producing the total effect of each life-course indicator. The difference between the total and the direct effect for each of the latent life-course factors yielded an estimate of its indirect effect through all mediating factors. The significance of the indirect effect was tested through the model likelihood ratio [44].
As the risk of dementia may be affected by an interaction between genetic and environmental factors [45], formal tests of statistical interactions between the life-course cognitive reserve-enhancing composite factors and APOE ε4 status were performed by introducing an interaction term. To further assess the possible interaction between genetic predisposition and cognitive reserve, we estimated the effects of reserve-enhancing composite factors among both APOE ε4 allele carriers and noncarriers.
Results
Logistic regression showed that the population analysed in the study (n = 602) and the eligible individuals who were not included because of missing data on covariates (n = 130) did not differ with respect to age (odds ratio [OR] 0.98, 95% CI 0.94–1.03), sex (OR 0.92, 95% CI 0.58–1.40), presence of depression (OR 1.16, 95% CI 0.76–1.77), or comorbidity (OR 1.27, 95% CI 0.89–1.81). Table 1 shows the characteristics of study participants 4–9 y before dementia diagnosis. As expected, individuals who would develop dementia later were relatively older, more likely to be female, had more depressive symptoms, reported poorer cognitive functioning, and were more likely to be APOE ε4 allele carriers.
Tab. 1. Baseline characteristics of the study population including 454 persons who remained free of dementia during the follow-up and the 148 individuals who developed dementia over an average of 6 y of the follow-up. 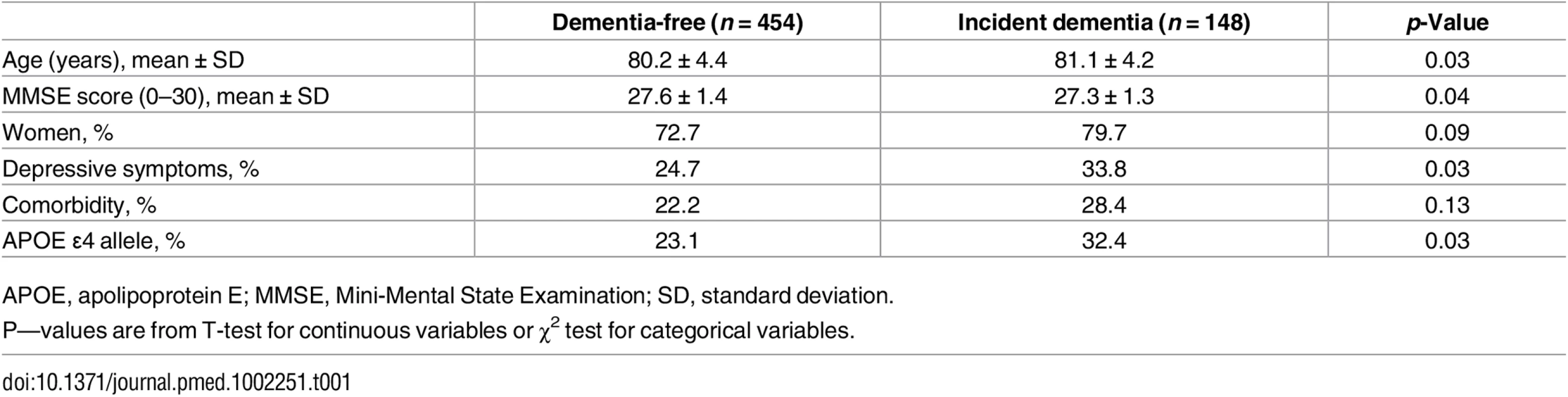
APOE, apolipoprotein E; MMSE, Mini-Mental State Examination; SD, standard deviation. The best-fitting latent measurement model for the three life-course cognitive reserve-enhancing composite factors (early, adult, and later life) is presented in Fig 1. For each latent factor, an individual’s factor score was estimated by (1) standardizing measurements on the raw indicators, (2) multiplying the standardized score for each indicator by its corresponding SEM factor-score weight, and (3) summing the products to yield three separate latent factor-score estimates for the early-, adult-, and late-life periods (for more details, see S1 Text). The distribution of the latent variables was as follows: early life (range from −1 to 1, mean = 0.02, and standard deviation [SD] = 0.491), adult life (range from −1 to 1, mean = 0.02, and SD = 0.372), and late life (range from −1 to 1, mean = 0.10, and SD = 0.655). Relative risks from models with latent variables should be interpreted as per a 1 unit SD increase in the underlying latent factor. The correlation between the early - and the adult-life latent factors was strong (γ = 0.9).
Fig. 1. Standardized estimates from the structural equation model (SEM) for the three composite factors and corresponding cognitive reserve indicators from three life periods (early life, adulthood, and late life). 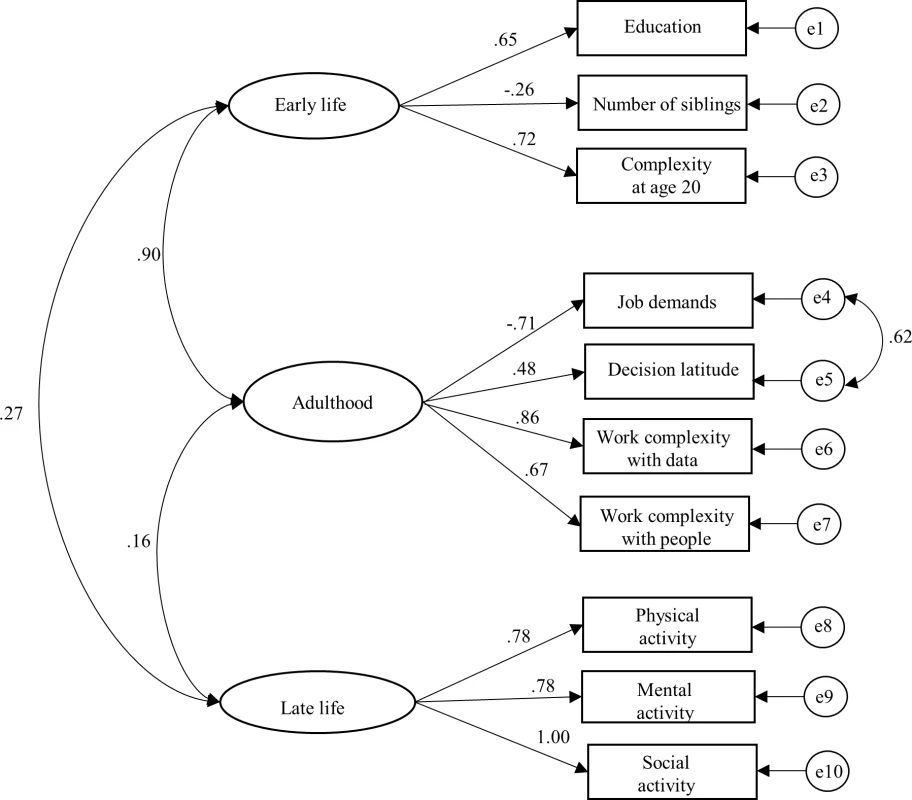
SEM fit statistics: χ2 = 99.81, df = 31, p < 0.001; χ2/df ratio = 3.22; goodness-of-fit index (GFI) = 0.967; comparative fit index (CFI) = 0.901; and root mean square error of approximation (RMSEA) = 0.061. We began by analysing the association between the risk of dementia occurrence and continuous cognitive reserve-enhancing composite factors derived from the SEM model on the risk of dementia (S1 Table). In separate minimally adjusted (age and sex) Cox models, a reduced risk of dementia was associated with early - (relative risk [RR] 0.61; 95% CI 0.41–0.90), adult - (RR 0.53; 95% CI 0.31–0.91), and late-life (RR 0.70; 95% CI 0.53–0.92) continuous composite factors. Estimating the association between dementia occurrence and all three life-course reserve factors in a simultaneous-entry model yielded nonsignificant risk estimates of early - (RR 1.19; 95% CI 0.22–6.39), adult - (RR 0.57; 95% CI 0.06–5.26), and late-life factors (RR 0.80; 95% CI 0.58–1.09).
We then converted the continuous reserve-enhancing factors into four categories based on the quartile distribution and assessed their associations with the risk of dementia occurrence, first in minimally adjusted separate Cox models and then in a fully adjusted model with all three factors estimated simultaneously (Table 2). In separate models, all quartile categories of the early-life reserve composite factor were associated with a reduced risk, relative to the bottom category, although only quartile four was statistically significant (relative risk [RR] 0.45; 95% CI 0.27–0.77). Similarly, all quartile categories of the adult-life reserve composite factor were associated with a reduced risk of dementia, relative to the bottom quartile (quartiles two and four were statistically significant; RR 0.57; 95% CI 0.37–0.89, and RR 0.46; 95% CI 0.27–0.77, respectively). All three quartiles of the late-life reserve composite factor were associated with a statistically reduced risk of dementia relative to the bottom quartile category. When all three composite factors were simultaneously entered into a single fully adjusted model, only the late-life composite factor (except quartile 2) preserved its statistical association with a reduced risk of dementia, whereas early - and adult-life reserve composite factors were no longer associated with a reduced risk of dementia.
Tab. 2. Number of participants, incident dementia cases, and relative risk (RR) (95% confidence interval [CI]) of dementia in relation to the cognitive reserve latent factors acting at different time periods in the life course. ![Number of participants, incident dementia cases, and relative risk (RR) (95% confidence interval [CI]) of dementia in relation to the cognitive reserve latent factors acting at different time periods in the life course.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/b7aa824048308df8999e6235dab5d71b.png)
Full adjustment: age, sex, depressive symptoms, comorbidity, and baseline cognitive function. Next, we converted all reserve factors into a dichotomous variable derived from a quartile distribution by combining the top three quartiles of each composite factor and contrasting them against the bottom quartile. The association between the risk of dementia occurrence and the dichotomized cognitive reserve latent factors was estimated in three separate minimally adjusted models, as well as in a single fully adjusted Cox regression (Fig 2). Whereas all three dichotomized life-course cognitive reserve-enhancing composite factors were associated with a reduced risk of dementia (early-life RR 0.57; 95% CI 0.36–0.90; adult-life RR 0.60; 95% CI 0.42–0.87; late-life RR 0.52; 95% CI 0.37–0.73) when analysed separately in minimally adjusted models, only the late-life factor remained statistically associated with a reduced risk of dementia (RR 0.65; 95% CI 0.45–0.94) in the simultaneous fully adjusted model. The association between dementia risk and the adult-life dichotomized composite factor was attenuated and remained marginally statistically significant (RR 0.73; 95% CI 0.50–1.06), whereas the early-life reserve-enhancing composite factor was the most attenuated (RR 0.76; 95% CI 0.47–1.23). In additional analyses (S2 Table), we found that 39.3% of the total association between the early-life composite factor and the hazard rate of dementia was attributed to its indirect association with adult - and late-life factors.
Fig. 2. RRs and 95% CIs of dementia in relation to early-life, adulthood, and late-life cognitive reserve-enhancing composite factors. 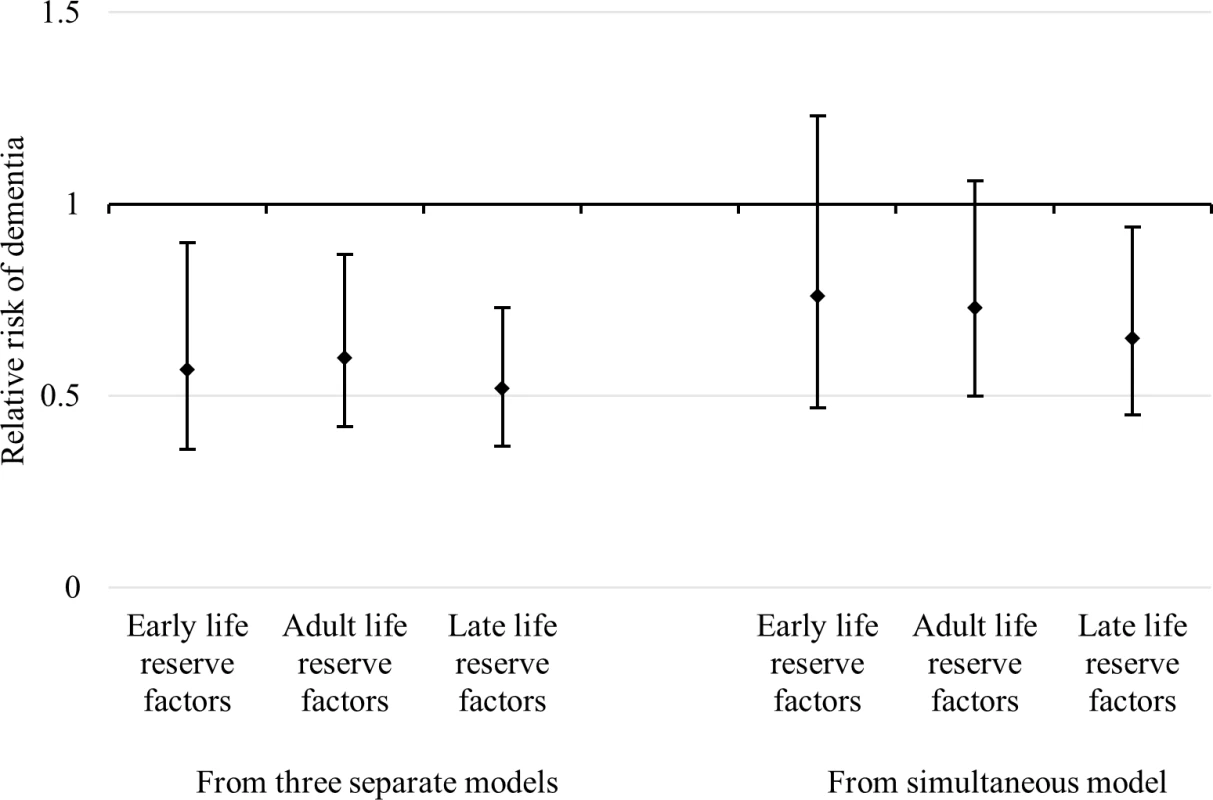
The RRs and 95% CIs on the left-hand side were estimated from three separate Cox models adjusted for age and sex. The RRs and 95% CIs on the right are from a single Cox model that simultaneously estimated the effects of composite indicators with additional adjustment for age, sex, depressive symptoms, comorbidity, and baseline cognitive function. Early-life, adulthood, and late-life cognitive reserve-enhancing composite indicators are dichotomized (top three quartiles versus the bottom quartile) based on the quartile transformation of continuous variables (factor scores) extracted from an SEM model. Early-life and adulthood latent factors were highly correlated (γ = 0.9). This implies that total effects of either factor on dementia from individually estimated models might not be independent and that the mutually adjusted effect of these variables might not be precisely estimated because of collinearity. To better understand the structure of exposure status without explicitly modelling the effect of each period, we divided participants into different groups according to their exposure status (high versus low) at the three time periods (early, adult, and late life). Because of study size limitations, we generated an index distinguishing between (1) a high-reserve level for just a single life period, (2) a high level for two out of the three life periods, and (3) a high reserve for all three life periods. We then estimated the risk of dementia for each of these three groups relative to those with a low level of reserve in all life periods (Fig 3).
Fig. 3. RRs and 95% CIs of dementia in relation to cumulative exposure to reserve-enhancing composite factors over the life course. 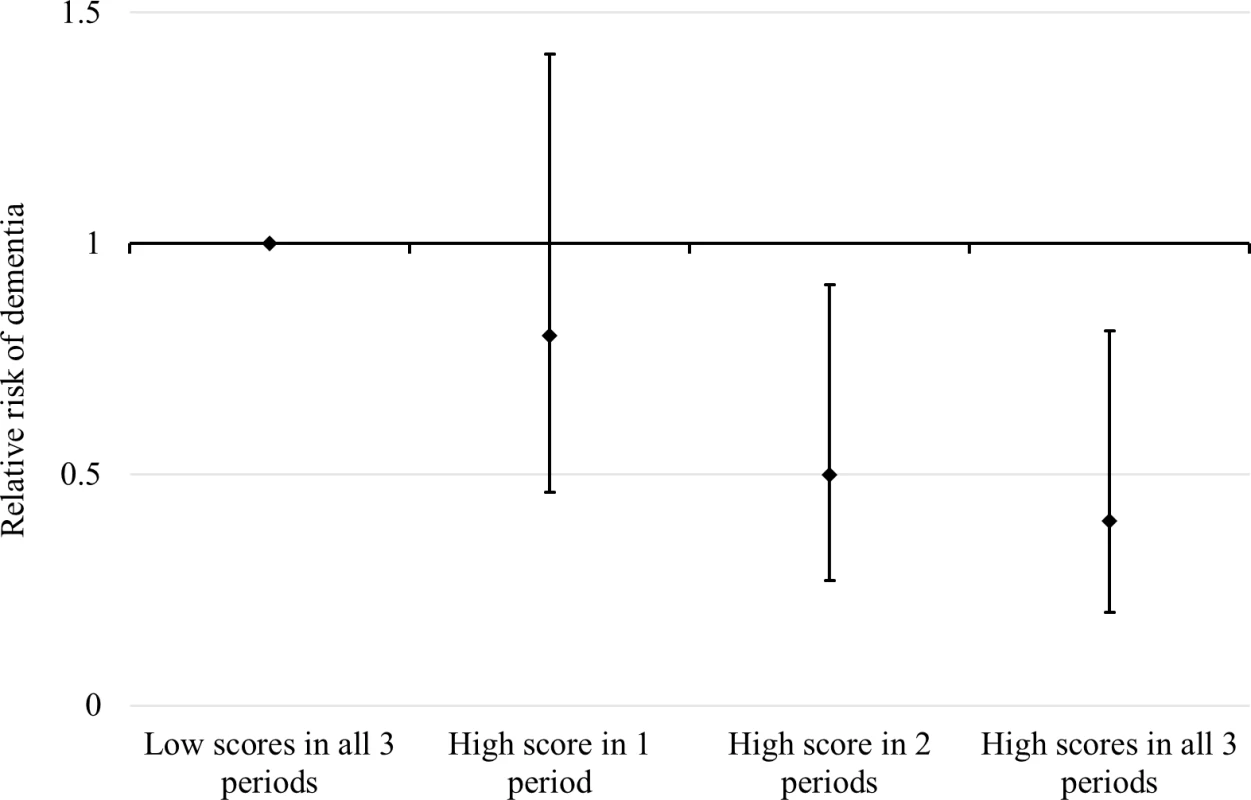
Estimated from a simultaneous-entry Cox regression model adjusted for age, sex, depressive symptoms, comorbidity, and baseline cognitive function. Relative to individuals with low scores on all three life-course cognitive reserve-enhancing composite factors over the life course, there was no significant reduction in the risk of dementia for individuals with high scores on the composite indicator in one life period (RR 0.80; 95% CI 0.46–1.41). Having high scores on the composite indicators in two life periods was associated with a significantly reduced risk of dementia (RR 0.50; 95% CI 0.27–0.91), with high scores in all three life periods associated with an even greater dementia risk reduction (RR 0.40; 95% CI 0.20–0.81) in the model adjusted for age, sex, depressive symptoms, comorbidity, and baseline cognitive function (Fig 3).
Finally, we found no evidence of a synergistic interaction between each of the life-course cognitive reserve-enhancing composite factors and genetic predisposition (APOE ɛ4 status) on risk of dementia (formal statistical test for interaction). Stratified analysis according to APOE ɛ4 status furthermore revealed that having high cognitive reserve in two or three periods over the life course was associated with a lower risk of dementia in both ɛ4 allele carriers (RR 0.5; 95% CI 0.3–0.9) and ɛ4 allele noncarriers (RR 0.7; 95% CI 0.5–0.97).
Discussion
Using a life-course approach, we found in our cohort that cognitive reserve-stimulating activities during early, adult, and late life were associated with a lower risk of dementia occurrence, although the early-life association was partially (39.3%) mediated by mid - and late-life activities. We observed a reverse dose-response relationship between dementia risk and increased duration of exposure to reserve-enhancing factors over the life course. However, the hypothesized interaction between cumulative exposure to cognitive reserve factors and genetic predisposition on the risk of dementia was not confirmed. The risk of dementia due to cumulative exposure to cognitive reserve factors over the life course was reduced irrespective of individuals’ genetic predisposition to dementia.
This study has several strengths. First, this prospective study collected information on exposures at least 4 y before incident dementia cases were diagnosed. Second, to avoid ascertainment bias [46], only cognitively intact community dwellers with no dementia were included, and people who developed dementia during the first 3 y were excluded from the study. Third, the three latent reserve-enhancing composite factors were estimated using structural equation modelling with a good overall fit. The use of latent factors has several advantages including (1) the incorporation of the interrelated observed measures, (2) the pooling of common variance across multiple index measures for each latent measure (increased convergent validity), and (3) the correction for unreliability in observed measures by attenuating measurement error.
Limitations of this study include possible measurement error in measuring adult-life work complexity, job demands, and decision latitude, as well as late-life leisure activities. However, since information was obtained from the same source for all participants, the misclassification is likely to be nondifferential and therefore should only lead to an underestimation of the true population effects. In addition, nonresponse bias may have occurred, but it should not have much bearing on the results because the distribution of participants and nonparticipants was largely comparable with respect to demographic and health indicators. The correlation between the early-life and the adulthood latent factors was strong (γ = 0.9). A respecified SEM model was fit, excluding occupational complexity at 20 y as an indicator of the early-life factor, in an attempt to reduce this association. While the correlation between the early and adult latent factors declined from 0.9 to 0.64, model fit deteriorated too. As one approach to circumventing collinearity concerns, we examined the general exposure structure by evaluating the effects of having high scores on latent factors over consecutive periods in life.
The results of the current study should first be considered in light of studies on cognitive reserve measures collected at single periods during the life course [5,12,23]. Thus, consistent with previous research, we have demonstrated that high scores on early-life measures of cognitive reserve [5,12,24,47,48], engagement in adult reserve-enhancing activities, such as complex occupational roles [6,12,16,24], and late-life engagement in leisure activities are all associated with a lowered risk of dementia [49]. Although reserve-enhancing activities from distinct periods of life have been individually linked with the risk of dementia previously, studies on reserve contributors spanning several periods over the life course have been lacking, with the exception of a few studies [12,24]. Notably, we extend this limited literature in an important way by adding previously unmeasured markers of late-life reserve factors, as well as by simultaneously examining the effects of early-, adult-, and, crucially, late-life cognitive reserve factors on dementia risk among the same individuals. Our findings underscore the contribution of factors acting at different periods of the life course.
A marked attenuation in the direct effect of the early-life composite factor on dementia risk could be due to its correlation with the adult-life cognitive reserve-enhancing composite factor. It is noteworthy that the late-life factor was not strongly correlated with either the early - or the adult-life composite factors. One possibility could be that disengagement from late-life leisure activities is a sign of impending dementia, rather than a reflection of earlier-life reserve contributors such as education or occupation. This could also account for the fact that associations between dementia risk and late-life factors were both significant and consistently estimated, whereas the associations between dementia and early-life, as well as adult-life, factors were less precise. Further studies using less correlated indicators are needed to explore the independent effects of early-life influences on dementia risk. Our finding of a dose-response relationship between the cumulative exposure to life-course cognitive reserve-enhancing composite factors and dementia risk underscores the importance of exposures occurring at multiple life periods.
Several biologically plausible hypotheses may explain the association between dementia risk and cognitive reserve factors acting at different periods over the life course. The environment plays a key role in influencing brain plasticity, which is the key element of the brain reserve hypothesis, as well as influencing memory formations and learning processes that provide the brain with a lifelong ability to change and to adjust [50]. Mental stimulation selectively increases synaptogenesis, whereas physical exercise may enhance non-neuronal components of the brain [18]. The ability of the brain to respond to environmental stimuli by adding new neurons or by activating compensatory processes can be sustained in late life [51]. Previous neuroimaging studies have shown that people with higher education, high occupational attainment, or higher levels of intellectual, social, or physical activity may cope with brain damage for a longer period of time [52–55].
We found no evidence of an interaction between APOE ɛ4 status and cognitive reserve factors over the life course on risk of dementia, suggesting that protective effects of cognitive reserve on dementia operate independently of genetic predisposition to the disease, which is consistent with findings from a smaller study using a younger study population [56]. It is known that the structural components of the nervous system are influenced not only by environmental exposures but also as a function of genetic endowment. Although APOE ɛ2 has been consistently shown to have neuroprotective effects and to increase synaptic plasticity [57], APOE ɛ4 has been associated with negative effects on neurites and synaptic functions [58,59]. Reserve-enhancing factors have been shown to enhance the functional organization of the brain through greater resilience in neural circuits involved in cognition and to modify the relationship between senile plaques and cognitive function [4]. Our findings suggest that enhanced neuroplasticity by reserve-enhancing factors may compensate for the deterioration of the brain function to the same extent in both APOE ε4 allele carriers and noncarriers.
Our findings point to the importance of adopting a life-course perspective in designing interventions aimed at enhancing cognitive reserve in order to prevent or postpone dementia incidence. It is never too late to initiate interventions because even late-life activities were associated with lower risk of dementia in our study. Nevertheless, interventions aimed at earlier life periods might be more beneficial, not only because greater exposure frequency has been linked with a reduced risk but also because of the correlated nature of reserve contributors over the life course. Importantly, these interventions should be equally effective among individuals with and without genetic susceptibility.
Supporting Information
Zdroje
1. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–53. doi: 10.1016/S1474-4422(04)00767-7 15157849
2. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychological medicine. 2006;36(4):441–54. doi: 10.1017/S0033291705006264 16207391
3. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004 19467352
4. Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–15. 12821732
5. Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22. 17851191
6. Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS ONE. 2012;7(6):e38268. doi: 10.1371/journal.pone.0038268 22675535
7. Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–33. doi: 10.1076/jcen.25.5.625.14576 12815500
8. Moceri VM, Kukull WA, Emanual I, van Belle G, Starr JR, Schellenberg GD, et al. Using census data and birth certificates to reconstruct the early-life socioeconomic environment and the relation to the development of Alzheimer's disease. Epidemiology. 2001;12(4):383–9. 11416775
9. Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer's disease. Neurology. 2000;54(2):415–20. 10668705
10. Gatz M, Svedberg P, Pedersen NL, Mortimer JA, Berg S, Johansson B. Education and the risk of Alzheimer's disease: findings from the study of dementia in Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P292–300. 11522804
11. De Ronchi D, Fratiglioni L, Rucci P, Paternico A, Graziani S, Dalmonte E. The effect of education on dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50(5):1231–8. 9595968
12. Dekhtyar S, Wang H-X, Scott K, Goodman A, Koupil I, Herlitz A. A Life-Course Study of Cognitive Reserve in Dementia—From Childhood to Old Age. The American Journal of Geriatric Psychiatry. 2015;23(9):885–96. doi: 10.1016/j.jagp.2015.02.002 25746486
13. Smyth KA, Fritsch T, Cook TB, McClendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63(3):498–503. 15304581
14. Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, et al. Complexity of work and risk of Alzheimer's disease: a population-based study of Swedish twins. J Gerontol B: Psychol Sci Soc Sci. 2005;60(5):P251–8. 16131619
15. Gatz M, Prescott CA, Pedersen NL. Lifestyle risk and delaying factors. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S84–8. 16917202
16. Karp A, Andel R, Parker MG, Wang H-X, Winblad B, Fratiglioni L. Mentally Stimulating Activities at Work During Midlife and Dementia Risk After Age 75: Follow-Up Study From the Kungsholmen Project. The American Journal of Geriatric Psychiatry. 2009;17(3):227–36. doi: 10.1097/JGP.0b013e318190b691 19454849
17. Akbaraly T, Portet F, Fustinoni S, Dartigues J-F, Artero S, Rouaud O, et al. Leisure activities and the risk of dementia in the elderly results from the Three-City Study. Neurology. 2009;73(11):854–61. doi: 10.1212/WNL.0b013e3181b7849b 19752452
18. Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–55. 12392797
19. Pham TM, Winblad B, Granholm AC, Mohammed AH. Environmental influences on brain neurotrophins in rats. Pharmacol Biochem Behav. 2002;73(1):167–75. 12076736
20. Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318(7196):1460–7. 10346775
21. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31(2):285–93. 11980781
22. Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):63–72. doi: 10.1097/01.wad.0000201854.62116.d7 16493239
23. Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. The Lancet Neurology. 2006;5(1):87–96. doi: 10.1016/S1474-4422(05)70286-6 16361026
24. Dekhtyar S, Wang HX, Fratiglioni L, Herlitz A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int J Epidemiol. 2016; 45(4):1207–1215. doi: 10.1093/ije/dyw008 26968481
25. Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE. Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. American journal of epidemiology. 2011;173(10):1148–58. doi: 10.1093/aje/kwq483 21430188
26. Fratiglioni L, Viitanen M, Backman L, Sandman PO, Winblad B. Occurrence of dementia in advanced age: the study design of the Kungsholmen Project. Neuroepidemiology. 1992;11 Suppl 1 : 29–36. 1603245
27. Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48(1):132–8. 9008508
28. Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: relationships to self-reported health and activity life style. J Gerontol. 1993;48(1):P1–11. 8418144
29. Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–7. 12048221
30. Diagnostic and statistical manual of mental disorders (DSM-III-R). American Psychiatric Association. 3rd, revised. ed. Washington, DC.1987. p. 97–163.
31. Fratiglioni L, Grut M, Forsell Y, Viitanen M, Winblad B. Clinical diagnosis of alzheimer's disease and other dementias in a population survey: Agreement and causes of disagreement in applying diagnostic and statistical manual of mental disorders, revised third edition, criteria. Archives of Neurology. 1992;49(9):927–32. 1520083
32. Molitoris J, Dribe M. Ready to stop: socioeconomic status and the fertility transition in Stockholm, 1878–1926. The Economic History Review. 2016; 69(2):679–704. doi: 10.1111/ehr.12275
33. Qiu C, Karp A, von Strauss E, Winblad B, Fratiglioni L, Bellander T. Lifetime principal occupation and risk of Alzheimer's disease in the Kungsholmen project. Am J Ind Med. 2003;43(2):204–11. doi: 10.1002/ajim.10159 12541276
34. Roos PA, Treiman DJ. DOT scales for the 1970 Census classification. In: Miller AR, Treiman DJ, Cain PS, Roos PA, editors. Work, jobs, and occupations: A critical review of occupational titles. Washington, DC: National Academy Press; 1980.
35. Cain P, Treiman D. The Dictionary of Occupational Titles as a Source of Occupational Data. Am Sociol Review. 1981;46 : 253–78.
36. Schnall PL, Landsbergis PA, Baker D. Job strain and cardiovascular disease. Ann Rev Public Health. 1994;15 : 381–411. doi: 10.1146/annurev.pu.15.050194.002121
37. Fredlund P, Hallqvist J, Diderichsen F. Psychosocial yrkesexponeringsmatris. En uppdatering av ett klassifikationssystem för yrkesrelaterade psykosociala exponeringar. Arbete och hälsa 2001(11).
38. Johnson JV, Stewart WF. Measuring work organization exposure over the life course with a job-exposure matrix. Scandinavian journal of work, environment & health. 1993;19(1):21–8.
39. Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21(2):65–73. doi: 10.1159/000089919 16319455
40. International classification of diseases: manual of the international statistical classification of diseases, injuries, and causes of death (ICD-8). Eighth revision. ed. Geneva, Switzerland. 1967.
41. Basun H, Corder EH, Guo Z, Lannfelt L, Corder LS, Manton KG, et al. Apolipoprotein E polymorphism and stroke in a population sample aged 75 years or more. Stroke. 1996;27(8):1310–5. 8711793
42. Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural equation modeling. 2002;9(2):233–55.
43. Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of psychological research online. 2003;8(2):23–74.
44. Liu X, Hermalin AI, Chuang YL. The effect of education on mortality among older Taiwanese and its pathways. J Gerontol B Psychol Sci Soc Sci. 1998;53(2):S71–82. 9520932
45. Fratiglioni L, Rocca W. Epidemiology of dementia. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 6. 2 ed. Oxford: Elsevier Science BV; 2001. p. 193–215.
46. Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635–41. doi: 10.1136/jech.2003.008466 15252064
47. Whalley L, Starr J, Athawes R, Hunter D, Pattie A, Deary I. Childhood mental ability and dementia. Neurology. 2000;55(10):1455–9. 11094097
48. Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: findings from the Nun Study. Jama. 1996;275(7):528–32. 8606473
49. Wang HX, Wahlberg M, Karp A, Winblad B, Fratiglioni L. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2012;8(2):114–20. doi: 10.1016/j.jalz.2011.03.001 22404853
50. Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1(4):351–63.
51. Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103(1):63–70. 10475165
52. Kidron D, Black SE, Stanchev P, Buck B, Szalai JP, Parker J, et al. Quantitative MR volumetry in Alzheimer's disease. Topographic markers and the effects of sex and education. Neurology. 1997;49(6):1504–12. 9409337
53. Perneczky R, Drzezga A, Diehl-Schmid J, Schmid G, Wohlschlager A, Kars S, et al. Schooling mediates brain reserve in Alzheimer's disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry. 2006;77(9):1060–3. doi: 10.1136/jnnp.2006.094714 16709580
54. Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45(1):55–60. 7824135
55. Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60(3):359–65. 12633147
56. Pettigrew C, Soldan A, Li S, Lu Y, Wang M-C, Selnes OA, et al. Relationship of Cognitive Reserve and APOE Status to the Emergence of Clinical Symptoms in Preclinical Alzheimer’s Disease. Cognitive neuroscience. 2013;4(3-4):136–142. doi: 10.1080/17588928.2013.831820 24168200
57. Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nature reviews Neurology. 2013;9(2):106–18. doi: 10.1038/nrneurol.2012.263 23296339
58. Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of aged human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97(4):685–92. 10842013
59. Masliah E, Samuel W, Veinbergs I, Mallory M, Mante M, Saitoh T. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 1997;751(2):307–14. 9099820
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- 2016 Reviewer and Editorial Board Thank You
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study
- Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery
- -related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
- Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
- Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study
- Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study
- Fine-mapping of the human leukocyte antigen locus as a risk factor for Alzheimer disease: A case–control study
- What’s the “Take Home” from Research on Dementia Trends?
- Cultural representations of dementia
- Dementia and aging populations—A global priority for contextualized research and health policy
- Dementia in the oldest old: Beyond Alzheimer disease
- Rehabilitation for people living with dementia: A practical framework of positive support
- Dementia in low-income and middle-income countries: Different realities mandate tailored solutions
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data
- Association between delirium superimposed on dementia and mortality in hospitalized older adults: A prospective cohort study
- Development of an adaptive, personalized, and scalable dementia care program: Early findings from the Care Ecosystem
- Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score
- Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study
- The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial
- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study
- Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study
- Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



