-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies
Ambika Satija and colleagues study associations between plant-based diet indices and type 2 diabetes incidence in large prospective cohorts.
Published in the journal: . PLoS Med 13(6): e32767. doi:10.1371/journal.pmed.1002039
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002039Summary
Ambika Satija and colleagues study associations between plant-based diet indices and type 2 diabetes incidence in large prospective cohorts.
Introduction
Type 2 diabetes (T2D) is associated with increased morbidity, mortality, and healthcare costs in the US [1]. Several plant foods, such as whole grains, fruits, and vegetables, are associated with a lower risk of T2D [2–4], while certain animal foods, such as red and processed meats, are positively associated with T2D risk [5]. Additionally, the recently released 2015 Dietary Guidelines Advisory Committee report recommends shifting away from intake of certain animal foods and moving towards a plant-rich diet [6]. Thus, we evaluated the hypothesis that a plant-based diet is protective against T2D.
Prior studies on plant-based diets and T2D [7–9] have defined plant-based diets as “vegetarian” diets, categorizing study populations dichotomously into participants who do or do not consume some or all animal foods. An important question from clinical and public health standpoints, however, is whether gradually moving towards a plant-rich diet by progressively decreasing animal food intake lowers T2D risk. If so, public health recommendations could suggest incremental dietary changes. Existing studies of vegetarian diets and T2D are also limited by a lack of differentiation among plant foods with divergent effects on T2D, because less nutrient-dense plant foods, such as refined grains, potatoes, and sugar-sweetened beverages, are associated with higher T2D risk [10–12].
We thus conceptualized a graded dietary pattern that positively weighs plant foods and negatively weighs animal foods, similar to the approach used by Martínez-González et al. [13]. We examined the association of this overall plant-based diet and, a priori, healthful and unhealthful versions of a plant-based diet with T2D incidence in three large prospective cohort studies in the US. We hypothesized that these plant-based diets would be inversely associated with T2D risk.
Methods
Study protocols for all cohorts were approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health; completion of the self-administered questionnaire was considered to imply informed consent.
Study Population
The Nurses’ Health Study (NHS) started in 1976 with 121,701 female nurses (aged 30–55 y) [14], the Nurses’ Health Study 2 (NHS2) started in 1989 with 116,430 female nurses (aged 25–42 y) [15], and the Health Professionals Follow-Up Study (HPFS) started in 1986 with 51,529 male health professionals (aged 40–75 y) [16]; all three studies recruited participants from across the US. In all three studies, follow-up questionnaires collect information on lifestyle and medical history biennially, with a response rate of ~90% per cycle. In the current analysis, the 1984, 1991, and 1986 cycles were the baselines for NHS, NHS2, and HPFS, respectively, because these are the cycles in which data on most covariates of interest were first comprehensively measured. Participants with diabetes, cancer (except nonmelanoma skin cancer), cardiovascular disease (CVD), reported energy intake levels outside predefined limits (<600 or >3,500 kcal/d for women and <800 or >4,200 kcal/d for men), or incomplete dietary data at baseline were excluded. The final analysis included 69,949 women in NHS, 90,239 women in NHS2, and 40,539 men in HPFS at baseline.
Dietary Assessment
Dietary data were collected every 2–4 y using a semi-quantitative food frequency questionnaire. Participants were asked how often they consumed a defined portion of ~130 food items over the previous year. Response categories ranged from “never or less than once/month” to “≥6 times/day.” The reliability and validity of the questionnaires have been described previously [17–20].
Plant-Based Diet Indices
We created an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI). The procedure we used to create these indices is similar to the one used by Martínez-González et al. [13]; their “provegetarian food pattern” is similar in composition to our PDI. Frequencies of consumption of each food were converted into servings consumed per day. Then the number of servings of foods that belonged to each of 18 food groups were added up. The 18 food groups were created on the basis of nutrient and culinary similarities, within larger categories of animal foods and healthy and less healthy plant foods. We distinguished between healthy and less healthy plant foods using existing knowledge of associations of the foods with T2D, other outcomes (CVD, certain cancers), and intermediate conditions (obesity, hypertension, lipids, inflammation). Plant foods not clearly associated in one direction with several health outcomes, specifically alcoholic beverages, were not included in the indices. We also excluded margarine from the indices, as its fatty acid composition has changed over time from high trans fat to high unsaturated fat. We controlled for alcoholic beverages and margarine consumption in the analysis.
Healthy plant food groups included whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea/coffee, whereas less healthy plant food groups included fruit juices, sugar-sweetened beverages, refined grains, potatoes, and sweets/desserts. Animal food groups included animal fats, dairy, eggs, fish/seafood, meat (poultry and red meat), and miscellaneous animal-based foods.
S1 Table details examples of foods constituting the food groups. The 18 food groups were divided into quintiles of consumption, and each quintile was assigned a score between 1 and 5. For PDI, participants received a score of 5 for each plant food group for which they were above the highest quintile of consumption, a score of 4 for each plant food group for which they were above the second highest quintile but below the highest quintile, and so on, with a score of 1 for consumption below the lowest quintile (positive scores). On the other hand, participants received a score of 1 for each animal food group for which they were above the highest quintile of consumption, a score of 2 for each animal food group for which they were between the highest and second highest quintiles, and so on, with a score of 5 for consumption below the lowest quintile (reverse scores). For hPDI, positive scores were given to healthy plant food groups, and reverse scores to less healthy plant food groups and animal food groups. Finally, for uPDI, positive scores were given to less healthy plant food groups, and reverse scores to healthy plant food groups and animal food groups. The 18 food group scores for an individual were summed to obtain the indices, with a theoretical range of 18 (lowest possible score) to 90 (highest possible score). The observed ranges at baseline were 24–85 (PDI), 28–86 (hPDI), and 27–90 (uPDI) across the cohorts. The indices were analyzed as deciles, with energy intake adjusted at the analysis stage.
Ascertainment of Type 2 Diabetes
Participants who self-reported physician-diagnosed diabetes were sent a supplementary questionnaire with established validity to confirm diagnosis [21,22]. Only confirmed cases that met ≥1 of the following criteria were included (as per the National Diabetes Data Group) [23]: (a) ≥1 classic symptoms plus fasting blood glucose ≥ 140 mg/dl (>=7.8 mmol/l) or random blood glucose ≥ 200 mg/dl (≥11.1 mmol/l); (b) no symptoms, but raised blood glucose levels (i.e., fasting blood glucose ≥ 140 mg/dl or random blood glucose ≥ 200 mg/dl or 2-h blood glucose after oral glucose tolerance testing ≥ 200 mg/dl) on two different occasions; (c) treatment with hypoglycemic drugs. The threshold for fasting plasma glucose was changed to ≥126 mg/dl (7.0 mmol/l) starting in 1998 [24]. HbA1c ≥ 6.5% was further added to the diagnosis criteria starting in 2010 [25].
Assessment of Covariates
We collected height at baseline and updated information on weight, physical activity, smoking status, multivitamin use, ethnicity, family history of T2D, hypertension, and hypercholesterolemia through biennial questionnaires. In NHS and NHS2, we also assessed information on menopausal status, postmenopausal hormone use, and oral contraceptive use.
Statistical Analysis
We calculated person-time for each participant from questionnaire return date until T2D diagnosis, death, censoring, or end of follow-up (30 June 2012 in NHS, 30 June 2011 in NHS2, and 1 January 2010 in HPFS). For the primary analysis, we categorized the indices into deciles, so as to not make assumptions about linearity and to limit the influence of outlying observations. We used Cox proportional-hazards regression to evaluate the associations between deciles of each index and T2D incidence. Age (years) was used as the timescale, with stratification by calendar time (2-y intervals). We adjusted for smoking status, alcohol intake, physical activity, family history of diabetes, multivitamin use, margarine intake, energy intake, baseline hypertension and hypercholesterolemia, body mass index (BMI) categories, menopausal status and postmenopausal hormone use (women), and oral contraceptive use (NHS2). Continuous covariates were included in the model as categories for the reasons cited above for categorizing the indices.
All dietary variables were cumulatively updated, i.e., were averaged, over the entire follow-up duration to better capture long-term diet. Updating was stopped when major outcomes (CVD or cancer) developed, as diagnosis with these conditions could change an individual’s diet. Values of non-dietary covariates were updated every 2 y to account for changes in these variables over time. In order to examine potential nonlinear associations, we created continuous variables of the indices by assigning the median value to each decile and conducting tests for linear trend, examined associations per 10-unit increase in the indices, and used restricted cubic splines. We tested for effect modification by age, physical activity, family history of diabetes, and BMI, by including cross-product terms. The analysis was carried out separately for each cohort, and the cohort-specific HRs were combined using a fixed-effects model; the Cochrane Q statistic [26], the I2 statistic [27], and the between-study coefficient of variation [28,29] were used to assess heterogeneity among the cohorts. All statistical tests were two-sided (α = 0.05). All analyses were performed using SAS version 9.4 for UNIX (SAS Institute).
Results
Baseline Characteristics
The distribution of age-adjusted baseline characteristics according to the PDI and hPDI are shown in Tables S2 and 1, respectively. Participants with higher scores on PDI or hPDI were older, more active, leaner, and less likely to smoke than participants with lower scores. They also consumed a lower percentage of calories from saturated and monounsaturated fats, a higher percentage of calories from polyunsaturated fats and carbohydrates, and higher levels of fiber and folate.
Tab. 1. Age-standardized baseline characteristics by deciles of the healthful plant-based diet index. 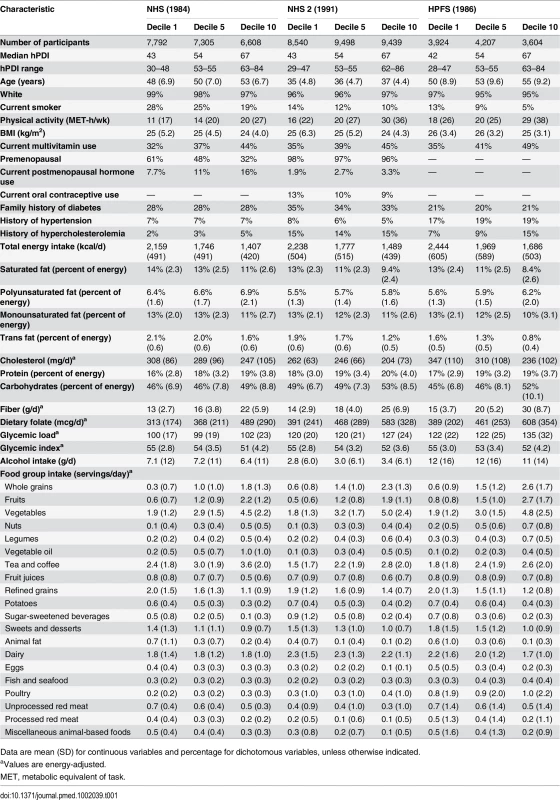
Data are mean (SD) for continuous variables and percentage for dichotomous variables, unless otherwise indicated. Plant-Based Diet Indices and Type 2 Diabetes Incidence
During 4,102,369 person-years of follow-up, we documented 16,162 T2D cases. PDI was inversely associated with T2D incidence in all three cohorts after adjusting for potential confounders (Table 2). Adjustment for BMI attenuated the relationship, but associations remained significant (pooled hazard ratio [HR] for extreme deciles 0.80, 95% CI 0.74–0.87; HR per 10-unit increase 0.88, 95% CI 0.86–0.91, p trend < 0.001).
Tab. 2. Hazard ratios (95% CIs) for type 2 diabetes according to deciles of the overall plant-based diet Index. 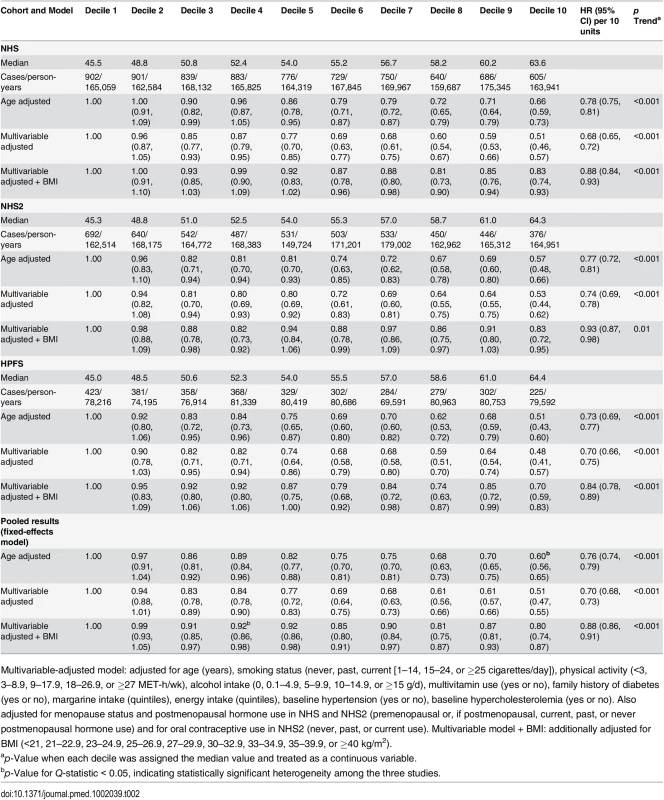
Multivariable-adjusted model: adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET-h/wk), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), multivitamin use (yes or no), family history of diabetes (yes or no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension (yes or no), baseline hypercholesterolemia (yes or no). Also adjusted for menopause status and postmenopausal hormone use in NHS and NHS2 (premenopausal or, if postmenopausal, current, past, or never postmenopausal hormone use) and for oral contraceptive use in NHS2 (never, past, or current use). Multivariable model + BMI: additionally adjusted for BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). After multivariable adjustment, a strong inverse association was observed between hPDI and T2D (Table 3), which was only modestly attenuated after BMI adjustment (pooled HR for extreme deciles 0.66, 95% CI 0.61–0.72; HR per 10-unit increase 0.83, 95% CI 0.80–0.85, p trend < 0.001). There was significant heterogeneity in the pooled estimates controlled for BMI due to greater attenuation in NHS2. In contrast, uPDI was positively associated with T2D (pooled HR for extreme deciles 1.16, 95% CI 1.08–1.25, p trend < 0.001) (Fig 1).
Fig. 1. Pooled hazard ratios (95% CIs) for type 2 diabetes according to deciles of the overall, healthful, and unhealthful plant-based diet indices. 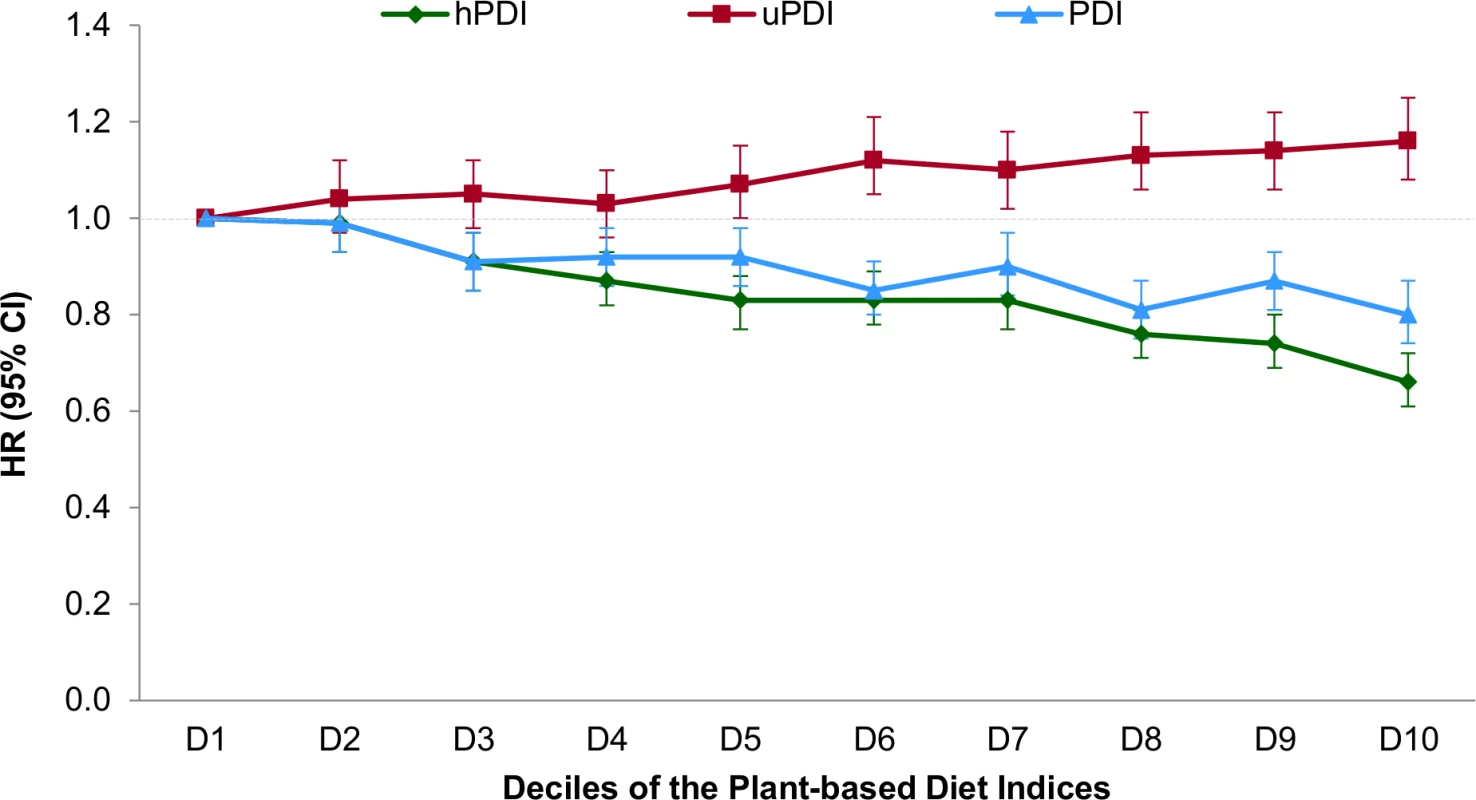
Results were pooled across the three cohorts using a fixed-effects model. Adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET-h/wk), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), multivitamin use (yes or no), family history of diabetes (yes or no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension (yes or no), baseline hypercholesterolemia (yes or no), and BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). Also adjusted for menopausal status and postmenopausal hormone use in NHS and NHS2 (premenopausal or, if postmenopausal, current, past, or never postmenopausal hormone use) and for oral contraceptive use in NHS2 (never, past, or current use). p trend < 0.001 for all indices. p-Value obtained by assigning the median value to each decile and entering this as a continuous variable in the model. Tab. 3. Hazard ratios (95% CI) for type 2 diabetes according to deciles of the healthful plant-based diet index. 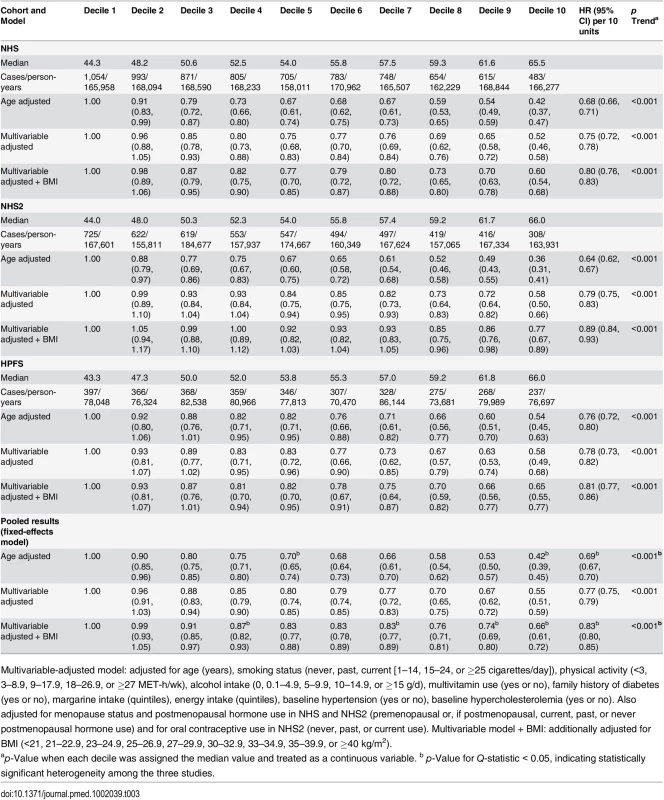
Multivariable-adjusted model: adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET-h/wk), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), multivitamin use (yes or no), family history of diabetes (yes or no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension (yes or no), baseline hypercholesterolemia (yes or no). Also adjusted for menopause status and postmenopausal hormone use in NHS and NHS2 (premenopausal or, if postmenopausal, current, past, or never postmenopausal hormone use) and for oral contraceptive use in NHS2 (never, past, or current use). Multivariable model + BMI: additionally adjusted for BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). Sensitivity Analyses
Our findings remained robust in several sensitivity analyses. In restricted cubic spline analysis, we did not find evidence for a nonlinear association of either PDI or hPDI with T2D incidence. Thus, both indices had significant linear associations with T2D incidence, with a stronger dose-response relationship for hPDI (S1 Fig). Similar inverse associations were observed in strata defined by physical activity and family history of diabetes (Fig 2). The inverse association of PDI with T2D incidence was stronger in non-obese than in obese participants (p interaction < 0.001), and the inverse associations of both PDI and hPDI were stronger in older participants (p interaction = 0.02) (S3 Table). The associations of both PDI and hPDI with T2D were virtually unchanged upon further adjustment for ethnicity, marital status, recent physical exam, diet beverage intake, and indicators of socioeconomic status (S4 Table). Results were also similar when the analysis was restricted to participants with fasting plasma glucose screening in the previous 2 y (PDI: HR for extreme deciles 0.78, 95% CI 0.71–0.85, p trend < 0.001; hPDI: HR for extreme deciles 0.65, 95% CI 0.59–0.71, p trend < 0.001). Continuously updating PDI and hPDI throughout follow-up did not change results (S5 Table). When we used baseline intakes of PDI and hPDI, associations were modestly attenuated but remained significant (PDI: HR for extreme deciles 0.86, 95% CI 0.80–0.93, p trend < 0.001; hPDI: HR for extreme deciles 0.70, 95% CI 0.64–0.75, p trend < 0.001). Associations were also modestly attenuated when we used the most recent scores prior to diagnosis of T2D (PDI: HR for extreme deciles 0.84, 95% CI 0.78–0.91, p trend < 0.001; hPDI: HR for extreme deciles 0.74, 95% CI 0.69–0.80, p trend < 0.001). Stratified analysis showed no significant effect modification by ethnicity for the diet indices (p interaction was 0.92 for PDI, 0.14 for hPDI, and 0.94 for uPDI; S2 Fig).
Fig. 2. Pooled hazard ratios (95% CI) for type 2 diabetes comparing extreme deciles of the plant-based diet indices, stratified by selected characteristics. 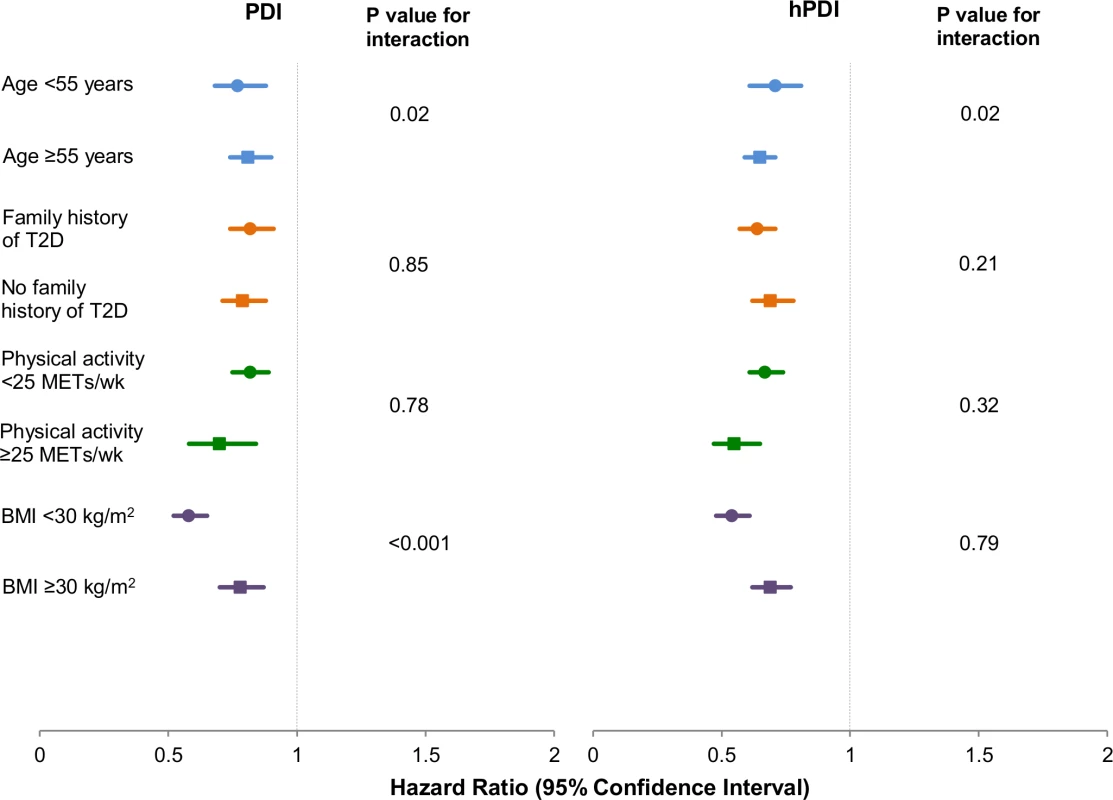
Results were pooled across the three cohorts using a fixed-effects model. Adjusted for age (years), smoking status (never, past, current [1–14, 15–24, or ≥25 cigarettes/day]), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET-h/wk), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), multivitamin use (yes or no), family history of diabetes (yes or no), margarine intake (quintiles), energy intake (quintiles), baseline hypertension (yes or no), baseline hypercholesterolemia (yes or no), and BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40 kg/m2). Also adjusted for menopause status and postmenopausal hormone use in NHS and NHS2 (premenopausal or, if postmenopausal, current, past, or never postmenopausal hormone use) and for oral contraceptive use in NHS2 (never, past, or current use). p trend < 0.001 for both indices across all strata. p-Value obtained by assigning the median value to each decile and entering this as a continuous variable in the model. To examine the individual contributions of healthy plant foods, less healthy plant foods, and animal foods to T2D risk, we included variables for all three food types simultaneously in the fully adjusted model; this allowed for mutual adjustment of the food types for one another, and hence an evaluation of their independent associations with T2D incidence. Healthy plant foods were inversely associated with T2D, while animal foods were positively associated, and less healthy plant foods were not associated, with risk (S3 Fig).
To examine the effect of consuming a healthful plant-based diet that is also high in intake of some animal foods known to be associated with reduced risk of several health outcomes (e.g., fish and yogurt [30–33]), we created two variations of hPDI. When we modified hPDI to score fish/seafood intake positively, the pooled HRs were slightly attenuated (HR for extreme deciles 0.73, 95% CI 0.68–0.79; HR per 10-unit increase 0.87, 95% CI 0.85–0.89, p trend < 0.001). Results for a modified hPDI with yogurt scored positively were not substantially different (HR for extreme deciles 0.65, 95% CI 0.60–0.71; HR per 10-unit increase 0.83, 95% CI 0.81–0.85, p trend < 0.001).
Previous analyses in these cohorts have found other dietary patterns such as the Mediterranean diet, the Alternate Healthy Eating Index (aHEI), and Dietary Approaches to Stop Hypertension (DASH) to be inversely associated with T2D [34–36]. Thus, in order to examine the independent associations of PDI and hPDI with T2D incidence, we individually controlled for these patterns (S6 and S7 Tables). Pooled HRs for both PDI and hPDI remained largely unchanged when the Mediterranean diet was controlled for, and were only slightly attenuated with aHEI or DASH in the same model.
Discussion
We found significant linear inverse associations of plant-based diets, especially a healthier version (captured by hPDI), with T2D incidence in three prospective cohorts in the US. In contrast, a less healthy version of a plant-based diet (captured by uPDI) was associated with increased T2D risk. These associations were independent of BMI and other diabetes risk factors.
There are several mechanisms through which a healthful plant-based diet could lower the risk of T2D [37,38]. Such a diet would be rich in dietary fiber, antioxidants, unsaturated fatty acids, and micronutrients such as magnesium, and low in saturated fat. Randomized clinical trials have shown beneficial effects of diets high in viscous and soluble fiber on improving postprandial glucose as well as long-term glucose metabolism [39]. In addition, several prospective studies have shown dietary fiber to be associated with reduced levels of inflammatory markers [40,41]. Animal studies and epidemiologic studies among humans have shown antioxidants such as polyphenols to have beneficial effects on glucose metabolism, probably through reduced oxidative stress and improved endothelial function [42]. High unsaturated fatty acid and low saturated fat contents in diets have also been shown to have anti-inflammatory properties [43], while specific micronutrients such as magnesium are known to play a key role in glucose metabolism [44]. Thus, a healthful plant-based diet could enhance glycemic control, improve insulin sensitivity, and decrease chronic inflammation, thereby reducing T2D risk. In addition, the high fiber and low calorie contents of many plant foods could further reduce T2D risk by promoting weight loss/maintenance [37,38]. Another less well understood mechanism could be through the gut microbiome. A healthful plant-based diet could promote a gut microbial environment that facilitates the metabolism of fiber and polyphenols and discourages the metabolism of bile acids, choline and L-carnitine, and amino acids, further reducing T2D risk [45]. An unhealthful plant-based diet, on the other hand, would have high glycemic index and load, reduced fiber, lower micronutrient content, and higher calorie content, which could adversely affect the above-mentioned pathways, resulting in increased T2D risk [2,10,12]. Such a diet would also have a high level of added sugar, which has been shown to be strongly associated with increased weight gain and T2D risk [12,46]. Given that BMI represents a pathway through which plant-based diets may affect T2D risk, controlling for it would have resulted in an underestimation of these diets’ true effects. Results from the final model controlling for BMI characterize plant-based diet associations that are independent of their potential beneficial effects on body weight. The association of PDI with decreased T2D incidence was also significantly stronger for non-obese individuals than for obese individuals, which could represent a true biological interaction of PDI with BMI (e.g., due to differential mediation by BMI in obese and non-obese individuals) or could be a methodological artifact (e.g., as a result of differential confounding or measurement error in the two strata).
Only a few prospective studies have examined the association of plant-based diets with T2D. The Adventist Health Studies found significantly higher T2D mortality (odds ratio 1.9, 95% CI 1.2–3.1) and incidence (odds ratio 1.38, 95% CI 1.06–1.80) among non-vegetarians than vegetarians [7,8]. They also found consumption of vegan, lacto-ovo vegetarian, and semi-vegetarian diets to be associated with lower T2D risk relative to non-vegetarian diets [9]. All of these studies were carried out among Seventh-day Adventists, a religious group that encourages a lacto-ovo vegetarian diet. Because the prevalence of vegetarianism is low in the US (~3% [47]), it is difficult to study the relationship between vegetarianism and health outcomes in the general US population. Defining a plant-based diet in terms of a continuous gradation of adherence to a diet high in plant and low in animal foods has allowed us to study the association of plant-based diets with T2D in more than 200,000 participants, utilizing detailed dietary data collected at multiple time points over more than two decades.
Our study highlights the varying risk profiles associated with different versions of plant-based diets, emphasizing the importance of considering the quality of plant foods consumed. Participants in the highest decile of uPDI consumed half the amount of healthy plant foods and almost double the amount of less healthy plant foods consumed by participants in the highest decile of hPDI. The healthier version of a plant-based diet proposed in this study may inform future public health recommendations regarding plant-based diets. We also found that even a modest lowering in animal food consumption was associated with substantially lower T2D incidence. For instance, in the highest decile of hPDI, participants consumed ~4 servings/day of animal foods, relative to 5–6 servings/day in the lowest decile. This has important public health implications, as plant-based diets need not completely exclude animal foods. Numerous studies have previously documented null or inverse associations of several animal foods (e.g., low-fat dairy, lean poultry, and fish and seafood) with T2D and other diseases, and consistent positive associations of certain animal foods (e.g., red and processed meats) with such diseases. Additionally, in our analysis the association of hPDI with T2D changed only slightly upon positively scoring fish and yogurt intake. Thus, the gradual reduction in animal food intake suggested here can be achieved largely through reducing intake of low-quality animal foods.
Our findings provide support for the 2015 Dietary Guidelines Advisory Committee conclusion that diets rich in healthy plant foods and lower in certain animal foods such as red and processed meats are beneficial for the prevention of chronic diseases [6]. Another rationale for shifting towards a plant-based diet is to improve food sustainability because food systems that rely heavily on animal foods require more natural resources than those more reliant on plant foods [48]. Thus, dietary guidelines that recommend a healthful plant-based diet would be compatible with the health of humans as well as our ecosystem. The hPDI was only moderately correlated with other commonly considered dietary patterns such as the Mediterranean diet, aHEI, and DASH, reflecting that this is a novel diet index that captures unique aspects of a healthful plant-based diet. This, coupled with the strong inverse association of the hPDI with T2D independent of these other dietary patterns, highlights the importance of focusing on a healthful plant-based diet for a potentially environmentally sustainable approach to T2D prevention.
Our study has several limitations. Because diet was self-reported, measurement errors are inevitable. However, the use of cumulative measures of diet over time not only reduces these errors but also represents long-term dietary habits [18]. We also made assumptions about the healthfulness of different plant foods, which, although based on prior evidence, has an element of subjectivity, and hence our findings need to be replicated in future studies. While we controlled for several potential confounders, given the observational nature of these studies, residual or unmeasured confounding cannot be ruled out. However, several randomized controlled trials have found vegetarian diets to positively impact intermediate endpoints, such as body weight, blood pressure, lipid profile, and insulin sensitivity, in those who were free of T2D [49–51] and in patients with the disease [52–56]. The socioeconomic homogeneity of the study population also enhances internal validity due to implicit control of confounders. Given that we found similar associations between the plant-based diet indices and T2D among different ethnic groups, it is likely that these findings are generalizable to diverse racial/ethnic groups. Nevertheless, these studies were carried out among health professionals in the US, and hence it would be important to replicate these findings in other populations representing diverse countries and occupational groups before translating these findings to other populations.
Conclusions
We found an inverse association between an overall plant-based diet and T2D incidence in three prospective cohorts. This inverse association was stronger for an index that captured a healthier version of the plant-based diet, but the association with T2D was positive for an index that captured an unhealthful version of a plant-based diet. Our study supports current recommendations to shift to diets rich in healthy plant foods, with lower intake of less healthy plant and animal foods.
Supporting Information
Zdroje
1. Centers for Disease Control and Prevention. Crude and age-adjusted incidence of diagnosed diabetes per 1,000 population aged 18–79 years, United States, 1980–2011. 2015 Dec 1 [cited 11 Apr 2016]. Available: http://www.cdc.gov/diabetes/statistics/incidence/fig2.htm.
2. Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28 : 845–858. doi: 10.1007/s10654-013-9852-5 24158434
3. Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012;66 : 1082–1092. doi: 10.1038/ejcn.2012.85 22854878
4. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001 23990623
5. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes: an updated review of the evidence. Curr Atheroscler Rep. 2012;14 : 515–524. doi: 10.1007/s11883-012-0282-8 23001745
6. US Department of Agriculture, US Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (District of Columbia): US Department of Health and Human Services; 2015.
7. Snowdon DA, Phillips RL. Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health. 1985;75 : 507–512. 3985239
8. Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52 : 96–104. doi: 10.1159/000121365 18349528
9. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23 : 292–299. doi: 10.1016/j.numecd.2011.07.004 21983060
10. Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454 22422870
11. Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83 : 284–290. 16469985
12. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33 : 2477–2483. doi: 10.2337/dc10-1079 20693348
13. Martínez-González MA, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Ros E, Arós F, et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(Suppl 1):320S–328S. doi: 10.3945/ajcn.113.071431 24871477
14. Liu S, Manson JE, Stampfer MJ, Hu FB, Giovannucci E, Colditz GA, et al. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. 2000;90 : 1409–1415. 10983198
15. van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29 : 398–403. 16443894
16. Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81 : 555–563. 15755822
17. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135 : 1114–1126. 1632423
18. Willett W. Nutritional epidemiology. 3rd ed. Oxford: Oxford University Press; 2013.
19. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122 : 51–65. 4014201
20. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69 : 243–249. 9989687
21. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161 : 1542–1548. 11427103
22. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338 : 774–778. 1681160
23. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28 : 1039–1057. 510803
24. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20 : 1183–1197. 9203460
25. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011 20042772
26. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10 : 101–129.
27. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327 : 557–560. 12958120
28. Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150 : 206–215. 10412966
29. Takkouche B, Khudyakov P, Costa-Bouzas J, Spiegelman D. Confidence intervals for heterogeneity measures in meta-analysis. Am J Epidemiol. 2013;178 : 993–1004. doi: 10.1093/aje/kwt060 23921232
30. Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012;15 : 725–737. doi: 10.1017/S1368980011002254 21914258
31. Xun P, Qin B, Song Y, Nakamura Y, Kurth T, Yaemsiri S, et al. Fish consumption and risk of stroke and its subtypes: accumulative evidence from a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2012;66 : 1199–1207. doi: 10.1038/ejcn.2012.133 23031847
32. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, et al. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12 : 215. doi: 10.1186/s12916-014-0215-1 25420418
33. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364 : 2392–2404. doi: 10.1056/NEJMoa1014296 21696306
34. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142 : 1009–1018. doi: 10.3945/jn.111.157222 22513989
35. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes risk among women with a history of gestational diabetes. Arch Intern Med. 2012;172 : 1566–1572. 22987062
36. de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34 : 1150–1156. doi: 10.2337/dc10-2352 21464460
37. Jenkins DJ, Kendall CW, Marchie A, Jenkins AL, Augustin LS, Ludwig DS, et al. Type 2 diabetes and the vegetarian diet. Am J Clin Nutr. 2003;78 : 610S–616S. 12936955
38. McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 2012;15 : 2287–2294. doi: 10.1017/S1368980012000936 22717188
39. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2 : 1266–1289. doi: 10.3390/nu2121266 22254008
40. North CJ, Venter CS, Jerling JC. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur J Clin Nutr. 2009;63 : 921–933. doi: 10.1038/ejcn.2009.8 19223918
41. Butcher JL, Beckstrand RL. Fiber’s impact on high-sensitivity C-reactive protein levels in cardiovascular disease. J Am Acad Nurse Pract. 2010;22 : 566–572. doi: 10.1111/j.1745-7599.2010.00555.x 21054629
42. Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients. 2016;8 : 17.
43. Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2 : 304–316. doi: 10.3945/an.111.000505 22332072
44. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4 : 378S–383S. doi: 10.3945/an.112.003483 23674807
45. Glick-Bauer M, Yeh MC. The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients. 2014;6 : 4822–4838. doi: 10.3390/nu6114822 25365383
46. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292 : 927–934. 15328324
47. Ruby MB. Vegetarianism. A blossoming field of study. Appetite. 2012;58 : 141–150. doi: 10.1016/j.appet.2011.09.019 22001025
48. Sabate J, Soret S. Sustainability of plant-based diets: back to the future. Am J Clin Nutr. 2014;100 : 476S–482S. doi: 10.3945/ajcn.113.071522 24898222
49. Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104 : 947–956. doi: 10.1016/j.amjcard.2009.05.032 19766762
50. Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015;115 : 954–969. doi: 10.1016/j.jand.2014.11.016 25620754
51. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174 : 577–587. doi: 10.1001/jamainternmed.2013.14547 24566947
52. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89 : 1588S–1596S. doi: 10.3945/ajcn.2009.26736H 19339401
53. Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28 : 549–559. doi: 10.1111/j.1464-5491.2010.03209.x 21480966
54. Kim MS, Hwang SS, Park EJ, Bae JW. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013;5 : 765–775. doi: 10.1111/1758-2229.12079 24115628
55. Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr. 2013;67 : 718–724. doi: 10.1038/ejcn.2013.92 23695207
56. Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med. 1999;29 : 87–91. 10446033
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Direct-to-consumer Marketing to People with Hemophilia
- Geographical Inequalities and Social and Environmental Risk Factors for Under-Five Mortality in Ghana in 2000 and 2010: Bayesian Spatial Analysis of Census Data
- Phosphodiesterase Type 5 Inhibitors and Risk of Malignant Melanoma: Matched Cohort Study Using Primary Care Data from the UK Clinical Practice Research Datalink
- Age, Spatial, and Temporal Variations in Hospital Admissions with Malaria in Kilifi County, Kenya: A 25-Year Longitudinal Observational Study
- Obesity and Multiple Sclerosis: A Mendelian Randomization Study
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Impact Evaluation of a System-Wide Chronic Disease Management Program on Health Service Utilisation: A Propensity-Matched Cohort Study
- Early Childhood Developmental Status in Low- and Middle-Income Countries: National, Regional, and Global Prevalence Estimates Using Predictive Modeling
- Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies
- Exclusive Breastfeeding and Cognition, Executive Function, and Behavioural Disorders in Primary School-Aged Children in Rural South Africa: A Cohort Analysis
- Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Weighing Evidence from Mendelian Randomization—Early-Life Obesity as a Causal Factor in Multiple Sclerosis?
- A Global Champion for Health—WHO’s Next?
- Malaria Epidemiology in Kilifi, Kenya during the 21st Century: What Next?
- Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
- Why Most Clinical Research Is Not Useful
- Delinking Investment in Antibiotic Research and Development from Sales Revenues: The Challenges of Transforming a Promising Idea into Reality
- Novel Three-Day, Community-Based, Nonpharmacological Group Intervention for Chronic Musculoskeletal Pain (COPERS): A Randomised Clinical Trial
- Prediction of Bladder Outcomes after Traumatic Spinal Cord Injury: A Longitudinal Cohort Study
- The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Why Most Clinical Research Is Not Useful
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



