-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
Background:
Many mathematical models have investigated the impact of expanding access to antiretroviral therapy (ART) on new HIV infections. Comparing results and conclusions across models is challenging because models have addressed slightly different questions and have reported different outcome metrics. This study compares the predictions of several mathematical models simulating the same ART intervention programmes to determine the extent to which models agree about the epidemiological impact of expanded ART.Methods and Findings:
Twelve independent mathematical models evaluated a set of standardised ART intervention scenarios in South Africa and reported a common set of outputs. Intervention scenarios systematically varied the CD4 count threshold for treatment eligibility, access to treatment, and programme retention. For a scenario in which 80% of HIV-infected individuals start treatment on average 1 y after their CD4 count drops below 350 cells/µl and 85% remain on treatment after 3 y, the models projected that HIV incidence would be 35% to 54% lower 8 y after the introduction of ART, compared to a counterfactual scenario in which there is no ART. More variation existed in the estimated long-term (38 y) reductions in incidence. The impact of optimistic interventions including immediate ART initiation varied widely across models, maintaining substantial uncertainty about the theoretical prospect for elimination of HIV from the population using ART alone over the next four decades. The number of person-years of ART per infection averted over 8 y ranged between 5.8 and 18.7. Considering the actual scale-up of ART in South Africa, seven models estimated that current HIV incidence is 17% to 32% lower than it would have been in the absence of ART. Differences between model assumptions about CD4 decline and HIV transmissibility over the course of infection explained only a modest amount of the variation in model results.Conclusions:
Mathematical models evaluating the impact of ART vary substantially in structure, complexity, and parameter choices, but all suggest that ART, at high levels of access and with high adherence, has the potential to substantially reduce new HIV infections. There was broad agreement regarding the short-term epidemiologic impact of ambitious treatment scale-up, but more variation in longer term projections and in the efficiency with which treatment can reduce new infections. Differences between model predictions could not be explained by differences in model structure or parameterization that were hypothesized to affect intervention impact.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001245
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001245Summary
Background:
Many mathematical models have investigated the impact of expanding access to antiretroviral therapy (ART) on new HIV infections. Comparing results and conclusions across models is challenging because models have addressed slightly different questions and have reported different outcome metrics. This study compares the predictions of several mathematical models simulating the same ART intervention programmes to determine the extent to which models agree about the epidemiological impact of expanded ART.Methods and Findings:
Twelve independent mathematical models evaluated a set of standardised ART intervention scenarios in South Africa and reported a common set of outputs. Intervention scenarios systematically varied the CD4 count threshold for treatment eligibility, access to treatment, and programme retention. For a scenario in which 80% of HIV-infected individuals start treatment on average 1 y after their CD4 count drops below 350 cells/µl and 85% remain on treatment after 3 y, the models projected that HIV incidence would be 35% to 54% lower 8 y after the introduction of ART, compared to a counterfactual scenario in which there is no ART. More variation existed in the estimated long-term (38 y) reductions in incidence. The impact of optimistic interventions including immediate ART initiation varied widely across models, maintaining substantial uncertainty about the theoretical prospect for elimination of HIV from the population using ART alone over the next four decades. The number of person-years of ART per infection averted over 8 y ranged between 5.8 and 18.7. Considering the actual scale-up of ART in South Africa, seven models estimated that current HIV incidence is 17% to 32% lower than it would have been in the absence of ART. Differences between model assumptions about CD4 decline and HIV transmissibility over the course of infection explained only a modest amount of the variation in model results.Conclusions:
Mathematical models evaluating the impact of ART vary substantially in structure, complexity, and parameter choices, but all suggest that ART, at high levels of access and with high adherence, has the potential to substantially reduce new HIV infections. There was broad agreement regarding the short-term epidemiologic impact of ambitious treatment scale-up, but more variation in longer term projections and in the efficiency with which treatment can reduce new infections. Differences between model predictions could not be explained by differences in model structure or parameterization that were hypothesized to affect intervention impact.
: Please see later in the article for the Editors' SummaryIntroduction
There has recently been increasing interest in expanding provision of antiretroviral therapy (ART) as a tool for reducing the spread of HIV in generalised epidemics in sub-Saharan Africa [1]–[5]. As momentum gathers for “HIV treatment as prevention", there is an urgent need to understand how ART might contribute to averting HIV transmissions, in addition to its direct benefits in reducing morbidity and mortality amongst treated patients. Mathematical modelling has supplied critical insights to discussions about treatment as prevention by providing a framework for combining information about the relationship between an infected individual's viral load and HIV transmissibility [6],[7], the reduction in a host's HIV viral load when on ART [8],[9], and the population-level contact structure over which HIV is transmitted [10],[11].
The idea of using medicines that suppress viral concentrations to reduce transmission of infection was posed almost as soon as the first HIV drugs were developed [12],[13]. Early models of the impact of ART focused on the opposing effects of reduced transmissibility and extended survival on new HIV infections, and whether associated increases in sexual risk behaviour would negate the prevention benefits of ART [10],[12],[14]–[23]. Since then, longitudinal observational data and one randomized controlled trial have demonstrated substantial reductions in the risk of heterosexual HIV transmission when the infective partner is virally suppressed [24]–[28], and continued follow-up of individuals receiving ART has confirmed the durability of viral suppression [29], including in sub-Saharan Africa [30],[31]. At the same time, there have been tremendous improvements in access to treatment in sub-Saharan Africa [32]. More recent modelling has shown more optimism about the potential for treatment to reduce new HIV infections in this region, with much work focused on the setting of South Africa, home to one in six people living with HIV globally [33].
Perhaps the most provocative of these modelling efforts has been the study by Granich and colleagues suggesting that a strategy involving annual testing and immediate treatment for all HIV-infected individuals, combined with other interventions, could eliminate HIV by the year 2050 [34]. Wagner and Blower implemented the same model but used different assumptions about treatment uptake amongst asymptomatic infected individuals that they characterised as being more realistic, and concluded that elimination would not be possible [35]. Kretzschmar et al. highlighted how choices in model structure affect the epidemic dynamics and intervention impact [36]. Dodd et al. showed that the potential for treatment to eliminate HIV depends on the patterns of sexual mixing in the population [11]. An age-structured model by Bacaër et al. found that elimination might be possible with less frequent testing than proposed by Granich et al., given recent epidemic trends and increases in condom usage [37]. Bendavid et al. used a microsimulation model to highlight that, in addition to increasing HIV testing, improving linkage to and retention in care are essential to achieving maximal benefits of test-and-treat interventions [38].
Other models have focused on the potential prevention benefits of providing treatment in line with current therapeutic guidelines. Eaton et al. estimated that 60 to 90 new infections could be averted for every 1,000 additional persons treated with CD4 cell count below 350 cells/µl (the current World Health Organization recommendation for when to start treatment [39]), depending on how well patients on treatment are retained in care [40]. The Goals model, used in the evaluation of the new UNAIDS Investment Framework, found that a US$46.5 billion incremental investment over the years 2011 to 2020, incorporating expanding access to ART, could avert 12.2 million new infections and 7.4 million deaths globally over that period [41]. Using a microsimulation model of the HIV epidemic in KwaZulu-Natal Province, Hontelez et al. found that expanding access to ART from those with CD4 cell count ≤200 cells/µl to those with ≤350 cells/µl required 28% more patients to receive treatment, but amounted to only a 7% increase in annual investment [42]. Cumulative net costs broke even after 16 y.
Models have also sought to understand the impact of past and current treatment policies; Johnson et al. used the ASSA2003 and STI-HIV Interaction models to assess the relative contributions of increased condom usage and ART scale-up to the declines in HIV incidence in South Africa up to 2008 [43]. Finally, other mathematical models have been used for short-term projections as a basis for power calculations for community-randomized trials of treatment as prevention [44].
Each of these models has predicted dramatic epidemiologic benefits of expanding access to ART, but models appear to diverge in their estimates of the possibility of eventually eliminating HIV using ART, the cost-effectiveness of increasing the CD4 threshold for treatment eligibility, and the benefits of immediate treatment compared to treatment based on the current World Health Organization eligibility guidelines. Directly comparing the models' predictions is challenging because each model has been applied to a slightly different setting, has used different assumptions regarding other interventions, has been used to answer different questions, and has reported different outcome metrics.
In this study we seek to understand the extent to which diverse mathematical models agree on the epidemiological impact of expanded access to ART by simulating the same set of intervention scenarios across the models and focusing on standardised outputs. The intervention scenarios are designed to be simple enough to be consistently implemented across different types of models in order to control several aspects of the treatment programme and isolate the effects of model structure, parameters, and assumptions about the underlying epidemic on estimates of intervention impact. The purpose of this study is not to make predictions about the impact of any particular intervention in any specific setting, but rather to better characterise the array of mathematical models being used to inform policy about treatment as prevention in hyperendemic settings such as South Africa.
Methods
Study Design
Literature and reports of meetings on related topics were reviewed in August 2011, and researchers who had previously developed mathematical models of the potential epidemiological impact of expanded access to ART, calibrated to the South African epidemic setting, were invited to participate in the model comparison exercise by simulating a standardised set of ART scale-up scenarios. Three aspects of the treatment programme were systematically varied: the CD4 threshold for treatment eligibility, access to treatment for those eligible, and the retention of patients on treatment. The timing of ART introduction and the rate at which individuals start treatment after becoming eligible were also standardised. The impact of an intervention was measured by comparing the number of new infections in the intervention scenario with that in a counterfactual epidemic simulation in which no ART is provided within the same model population. The counterfactual of no ART was chosen so that comparison between models would be independent of assumptions about the historic growth in ART uptake. As such, the results should not be interpreted as estimates of the future impact of treatment compared to current patterns of ART coverage, but can be generally taken as estimates of the overall net impact of treatment in a hypothetical scenario that assumes rapid ART scale-up and a homogenous rate of ART initiation across all ART-eligible adults. Although different models may incidentally have been calibrated using the same data, no standardisation was imposed on the specific epidemiologic data used for model calibration or on the calibration procedure itself in this exercise.
Mathematical Models
Twelve groups accepted the invitation to participate in the model comparison exercise. The collection of models encompasses a wide range of model structures, mechanisms for representing HIV transmission and disease progression, overall levels of complexity, and detail in the characterisation of treatment programmes. Table 1 summarises the names, authors, and key references for each model, and compares aspects of model structure. Four of the models are agent-based microsimulation models (i.e., models that track the behaviour and infection status of individual people) and use random-number generators to simulate particular events such as a new partnership formation or transmission events. The remaining eight models are deterministic compartmental models that stratify the population into groups according to each individual's characteristics and HIV infection status and use differential or difference equations to track the rate of movement of individuals between these groups. One of the models, the BBH model, solves the differential equations analytically, while the others numerically evaluate the differential equations. Ten of the models explicitly simulate both sexes and heterosexual HIV transmission, and six of the models include some form of age structure, although the extent to which age affects the natural history of HIV, the risk of HIV acquisition, and the risk of HIV transmission varies amongst these. All of the models simulate the national HIV epidemic in South Africa except for the STDSIM model, which simulates the higher prevalence Hlabisa subdistrict of KwaZulu-Natal Province, South Africa. Box 1 gives further comparative description of the structures and parameterization of the mathematical models.
Tab. 1. Description of mathematical models. 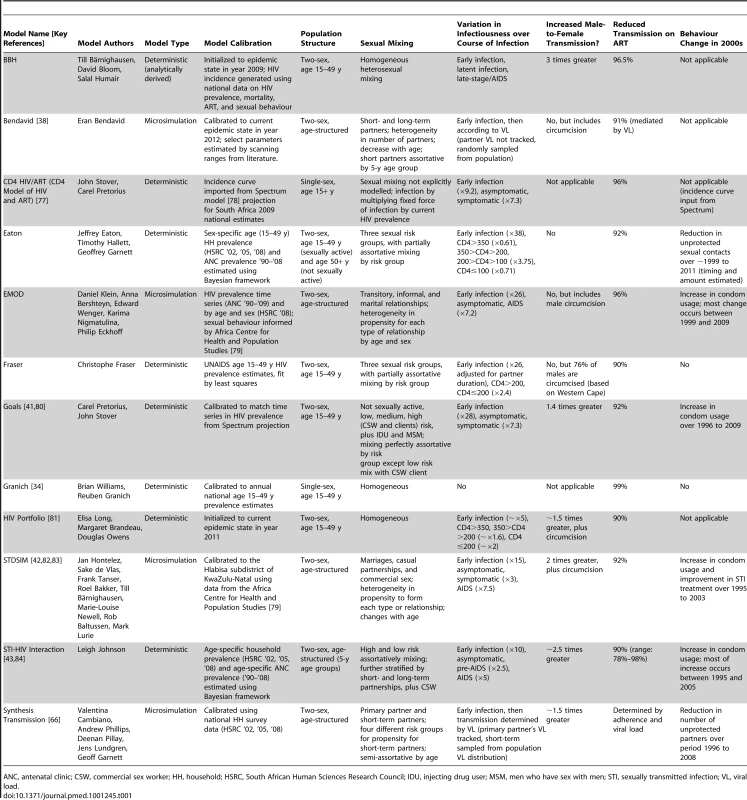
ANC, antenatal clinic; CSW, commercial sex worker; HH, household; HSRC, South African Human Sciences Research Council; IDU, injecting drug user; MSM, men who have sex with men; STI, sexually transmitted infection; VL, viral load. Box 1. Comparative Description of Mathematical Models
This box elaborates on the comparison of aspects of the models' structure, assumptions, and parameterization presented in Table 1. Specific details about the structure and implementation of each of the models are available in the references included in Table 1 or from the HIV Modelling Consortium (http://www.hivmodelling.org/plos-medicine-special-collection).
Many of the models allow individuals to have different propensities for sexual risk behaviour. Each of the microsimulation models allows individuals to have both long-term (or marital) partnerships and short-term (or informal or casual) partnerships that are different in duration, and individuals have heterogeneous propensities to form short-term partnerships. In the STDSIM model a proportion of the population engages in commercial sex work partnerships; in the EMOD model a proportion can have transitory partnerships, a third partnership type that is shorter than a casual partnership. Among the microsimulation models, EMOD and STDSIM explicitly simulate the sexual partnership network, while the Bendavid and Synthesis Transmission models calculate the risk of acquiring HIV for an individual in a partnership by sampling the distribution of viral load across, respectively, the entire population and potential partners.
The deterministic models assume that sexual contacts occur instantaneously. The BBH, Granich, and HIV Portfolio models assume that all individuals form new contacts at the same rate and mix homogeneously. The other deterministic models stratify the population into risk groups that form new contacts at different rates (Eaton, Fraser, and Goals: three groups; STI-HIV Interaction: two groups). The STI-HIV Interaction and Goals models additionally include commercial sex workers, and the Goals model includes transmission among men who have sex with men and injecting drug users. The STI-HIV Interaction model separates both the low - and high-risk groups into those with short-term or long-term partnerships or both. The Eaton, Fraser, and STI-HIV Interaction models all include a degree of “assortative" mixing (preferential partnership formation with those in the same risk group), and all partnerships are formed in the same risk group in the Goals model, except for low-risk men and women who are married to high-risk partners. The CD4 HIV/ART model does not explicitly model sexual mixing but rather calculates the number of new HIV infections by multiplying the current number of HIV-infected adults by a fixed force of infection calculated from the Spectrum model projection for South Africa.
All of the models except for the Granich model simulate different stages of HIV infection that affect the transmissibility of an individual, including a period of elevated infectiousness during the first few weeks of infection and increased transmission during later stage infection. Parameters governing the relative transmissibility during early infection are based principally on two sources: a meta-analysis of HIV transmission per coital act by Boily et al. [68], which estimated a 10-fold increase in transmission relative to asymptomatic infection (BBH, CD4 HIV/ART, Goals, and STI-HIV Interaction), or a reanalysis of data from Rakai, Uganda [70], by Hollingsworth et al. [69], which estimated a 26-fold increase (Eaton, EMOD, Fraser, and Synthesis Transmission). Relative transmissibility after the early stage is according to clinical stage (asymptomatic and AIDS: BBH, CD4 HIV/ART, EMOD, Goals; asymptomatic, pre-AIDS, and AIDS: STDSIM, STI-HIV Interaction) or CD4 count (Eaton, Fraser, and HIV Portfolio). The Bendavid and Synthesis Transmission models simulate the change in viral load for infected individuals and associate HIV transmission with this according to an empirically described relationship [6]. Many models assume an increased risk of male-to-female transmission compared to female-to-male transmission, and attenuation in female-to-male transmission due to male circumcision. The Goals, STDSIM, and Synthesis Transmission models include an increased risk of HIV transmission in the presence of other sexually transmitted infections.
The models that simulate each individual's viral load (Bendavid and Synthesis Transmission) mechanistically relate reduction in transmission on treatment to the effect of ART on viral load, while the other models all assume a reduction in transmission of greater than 90% for individuals on ART. The Bendavid, Eaton, and Synthesis Transmission models simulate a period of a few months of incomplete viral suppression after ART initiation before the full reduction in infectiousness is achieved. These three models and EMOD include a return to higher infectiousness during treatment failure. The remaining models assume a fixed reduction in transmissibility as soon as treatment is started, until either death on ART or dropout from treatment. The Bendavid and Synthesis Transmission models simulate switching to second-line ART upon an immunologic (Bendavid) or virologic (Synthesis Transmission) failure event. The Synthesis Transmission model is the only model to explicitly simulate heterogeneous adherence to treatment between patients and the emergence and impact of resistance. The models vary in their assumptions about what happens to an individual after dropping out from treatment. The CD4 HIV/ART, Fraser, Goals, Granich, and HIV Portfolio models return individuals who drop out to an untreated state, allowing them to restart treatment in exactly the same manner as those that have never been treated, while the Bendavid, STDSIM, STI-HIV Interaction, and Synthesis Transmission models do not allow individuals to start treatment again in the implementation for this exercise. Eaton allows individuals to restart treatment, but only once, and EMOD allows half of individuals to restart treatment after they once again satisfy the eligibility criterion.
Eleven of the models simulate the South African national HIV epidemic, while the STDSIM model has been calibrated specifically to the higher prevalence Hlabisa subdistrict of KwaZulu-Natal Province, South Africa. Nine models were calibrated to reproduce the historical time series of HIV prevalence in South Africa, while the BBH, HIV Portfolio, and Bendavid models were initialized using the current epidemic state in the years 2009, 2011, and 2012, respectively, and simulated forward from that point.
Most of the models were calibrated to yield a single set of model parameters and outputs. Two of the models (Eaton and STI-HIV Interaction) were calibrated using a Bayesian framework allowing for uncertainty in model parameters, which produces a joint posterior distribution of parameter combinations consistent with the observed HIV epidemic [43],[84]. The STI-HIV Interaction model allows for uncertainty in sexual behaviour, the natural history of HIV infection, and the effect of ART, while the Eaton model only allows for uncertainty in sexual behaviour and sexual mixing parameters.
Many of the models include facilities to simulate HIV testing and diagnosis, retention in care prior to treatment eligibility, and other processes related to achieving successful treatment, but these were not implemented for this exercise in order to conform to the simple intervention scenarios.
Intervention Scenarios
Three different CD4 cell count thresholds for treatment eligibility were considered: CD4 count ≤200 cells/µl, CD4 count ≤350 cells/µl, and all HIV-infected individuals. In each eligibility scenario, treatment initiation was simulated under the assumption that all eligible individuals had equal access, without prioritisation for any subpopulations. It was further assumed that eligible individuals with access to the intervention would initiate ART at a constant rate after reaching eligibility, such that average time from eligibility to treatment initiation would be 1 y.
Treatment access was defined as the proportion of eligible individuals who eventually initiate treatment. For example, 60% access and eligibility at CD4≤350 cells/µl implies that 60% of individuals will initiate treatment, on average 1 y after their CD4 count drops below 350 cells/µl, while 40% will never access treatment. Seven levels of treatment access were evaluated: 50%, 60%, 70%, 80%, 90%, 95%, and 100%.
Retention was defined as the percentage of individuals remaining on treatment after 3 y, excluding from both the numerator and the denominator those who had died while on treatment. The levels of retention were 75%, 85%, 95%, and 100% (no dropout), with individuals dropping out from treatment at a constant rate such that the desired level of retention was achieved at the 3-y time point. The prognosis and future treatment options for individuals who dropped out from treatment were not standardised.
Intervention Scale-Up
ART was assumed to be introduced into the population from the beginning of year 2012, with no treatment provision prior to this (in contrast to the rapid scale-up of treatment that has actually occurred prior to 2012 in South Africa). Intervention scale-up was immediate—a fraction (corresponding to the specified level of ART access) of individuals already eligible for treatment at the start of the intervention period were assumed to initiate treatment at a constant rate from that point, along with individuals who became eligible for treatment after the start of the intervention period.
Output Metrics
The measures of intervention impact were the percentage reduction in HIV incidence rate among adults (aged 15–49 y) in the ART scenario versus the no-ART counterfactual, the cumulative number of person-years of ART provided since the introduction of ART, and the cumulative number of person-years of ART provided per infection averted as a measure of the “efficiency" with which ART prevents infections. The percentage reduction in incidence was defined by calculating the difference in the adult HIV incidence rate between the intervention and no-ART counterfactual in the same year and dividing this by the incidence rate in the counterfactual scenario. The number of person-years of ART provided per infection averted was calculated by dividing the cumulative number of person-years of ART by the difference between the number of new adult infections since year 2012 in the intervention and the counterfactual scenario. Each of these metrics was reported at the midpoints of the years 2020 and 2050. Most of the models included in this study were not designed with the intention of making realistic projections to year 2050, but these results were included to gain some insight into the long-term dynamics of the models.
In addition to these measures of intervention impact, each model reported the HIV prevalence and HIV incidence rate amongst males and females aged 15–49 y for the no-treatment counterfactual simulation and the total size of the adult population (age 15 y and older). Each model also produced the proportion of the HIV-infected population in each CD4 count category (≤200, 200–350, and >350 cells/µl) and in early HIV infection in year 2012, and the proportion of HIV transmissions from individuals in each category.
The Eaton and STI-HIV Interaction models report posterior means and 95% credible intervals for model outcomes of interest (see Box 1). The Bendavid model completed simulations only for 50%, 80%, and 100% access, and 75%, 85%, and 100% retention scenarios, and only simulated to year 2040, so results for this model are reported for the year 2040 where other model results are reported for year 2050. The BBH model completed simulations only for the 85% and 100% retention scenarios. The Granich model did not simulate ART for the CD4≤200 cells/µl eligibility threshold, while the STI-HIV Interaction model did not simulate ART eligibility for all HIV-infected individuals. As a result of these models not completing all intervention scenarios and outputs, some analyses include only a subset of the models. To maximise comparability, the 40% reduction in transmission due to combination with other preventive interventions assumed by Granich and colleagues in [34] was not incorporated here.
Scenarios Representing the Existing ART Programme in South Africa
In a separate analysis, seven of the models (CD4 HIV/ART, Eaton, Fraser, Goals, Granich, STDSIM, and STI-HIV Interaction) were used to estimate the impact that the existing scale-up of ART in South Africa has had on HIV incidence and prevalence by comparing model simulations that include the ART scale-up over the past decade with the no-ART counterfactual. Models either used an existing calibration to the number of people on ART in South Africa (Fraser and STDSIM) or were calibrated using estimates of the number of adults starting and on ART in each year from 2001 to 2011 [45] (CD4 HIV/ART, Eaton, Goals, Granich, and STI-HIV Interaction).
Five models (Bendavid, CD4 HIV/ART, Eaton, Goals, and Granich) constructed short-term projections of HIV incidence in South Africa assuming different trajectories for continued ART scale-up from 2011 to 2016, the period covered by South Africa's national strategic plan [46]. Starting from the number of patients on ART in mid-2011, the numbers of adults starting ART in each of the years from mid-2011 through mid-2016 was specified. A “baseline" scenario was considered in which 400,000 adults would start ART in each of the next 5 y (approximately the number who started ART in 2009), for a total of 2 million new adults initiating ART. Three other scenarios were considered for the total numbers starting ART over the same period: (i) “low"—1.2 million start ART; (ii) “medium"—3 million start ART; and (iii) “high"—3.9 million start ART. (The exact number starting in each year is listed in Table 2.) The HIV incidence rate at the midpoint of 2016 and the cumulative number of new adult HIV infections over the period 2011 to 2016 were reported for each of these scenarios. For these projections, assumptions regarding CD4 distributions at ART initiation and rates of retention were based on actual treatment guidelines and programme experiences, but were not standardised across models.
Tab. 2. Number of adults starting ART each year in the short term. 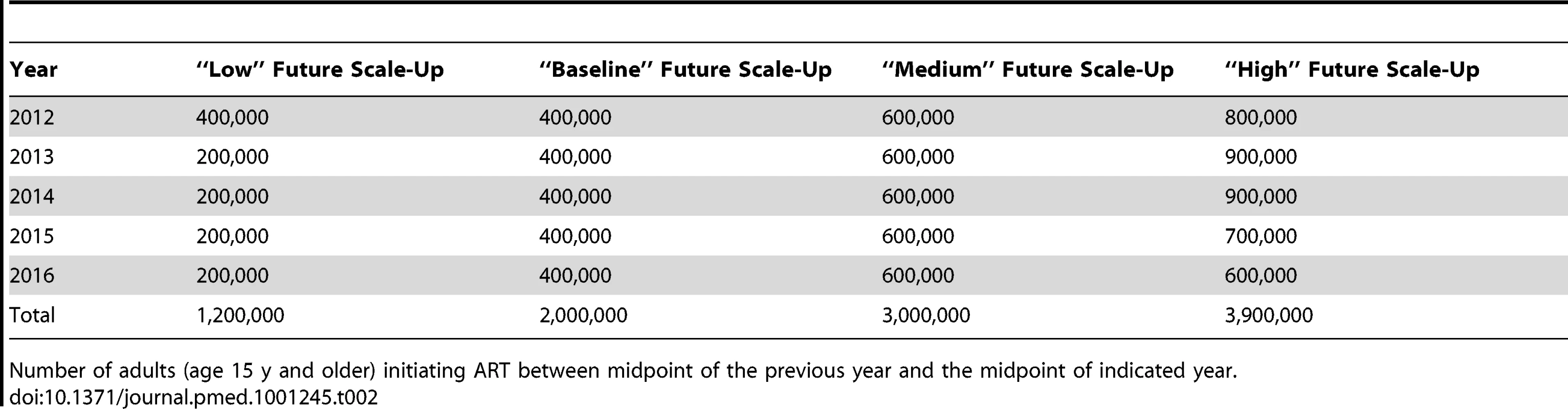
Number of adults (age 15 y and older) initiating ART between midpoint of the previous year and the midpoint of indicated year. Results
Figure 1 shows HIV prevalence and HIV incidence in 15 - to 49-y-old males and females simulated by each of the models under the counterfactual assumption of no ART provision.
Fig. 1. No-treatment counterfactual epidemic trends. 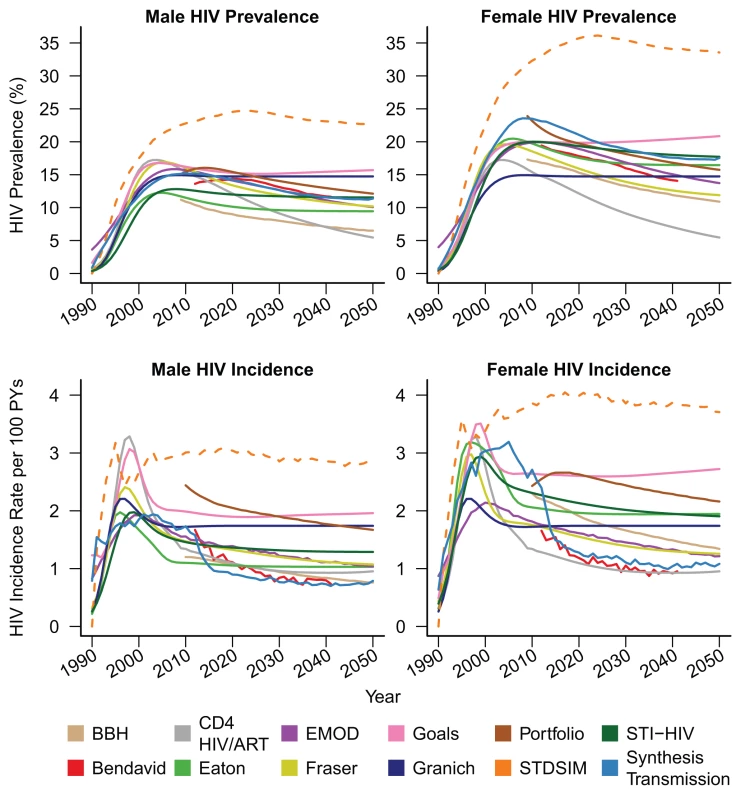
Male (left) and female (right) HIV prevalence (top) and incidence (bottom) amongst 15- to 49-y-olds for counterfactual HIV epidemics with no ART. The STDSIM model is calibrated to a more severe epidemic in the Hlabisa subdistrict of KwaZulu-Natal Province, South Africa. The CD4 HIV/ART and Granich models do not stratify by sex, and the same prevalence and incidence curves are plotted for both sexes for these models. PYs, person-years. Other epidemiologic statistics are presented in Table 3. The estimates of adult male HIV prevalence in year 2012 ranged between 10% and 16%, and estimates of female prevalence between 17% and 23%. Male HIV incidence in year 2012 ranged between 1.1 and 2.0 per 100 person-years, and female incidence ranged between 1.7 and 2.6. The STDSIM model calibrated to KwaZulu-Natal Province simulated a considerably larger burden of HIV, consistent with observation [47], with prevalences in year 2012 of 23% and 33% in males and females, respectively, and incidence rates of 3.0 and 3.9 per 100 person-years, respectively. All of the sex-stratified models simulated higher HIV prevalence in adult women than in men, with sex ratios in HIV prevalence in year 2012 between 1.2 and 1.7, and all of the models except for Bendavid simulated higher incidence in year 2012 in females than in males.
Tab. 3. Selected model outputs for counterfactual simulation with no ART. 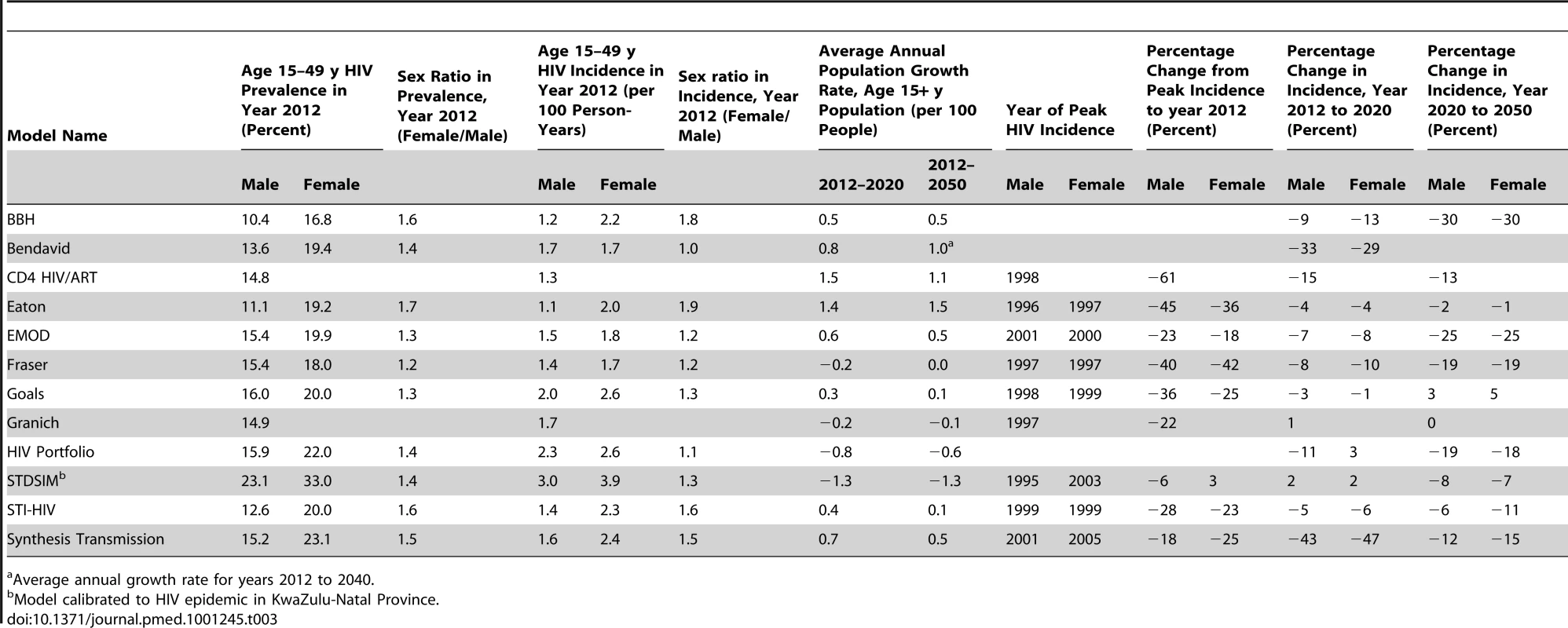
Average annual growth rate for years 2012 to 2040. Nearly all of the models projected declines in HIV incidence after 2012 in the absence of ART, but the magnitude of the projected natural changes between 2012 and 2050 varied widely from almost no change (Goals and Granich) to greater than 45% reduction (Bendavid and Synthesis Transmission).
Model projections of future national population growth in the absence of ART varied widely, ranging from a 6% reduction to a 13% increase in the population aged 15 y and older between the years 2012 and 2020. For comparison, the low and high variants for the projected total population growth from the United Nations Population Division over the same period (which incorporates some assumptions about ART provision) are 1.5% and 6.1% [48].
Impact of ART on HIV Incidence
Figure 2 presents the outcomes of an intervention starting in year 2012 with ART eligibility at CD4 count ≤350 cells/µl, reaching 80% of those requiring treatment, and retaining 85% of patients on ART after 3 y. This scenario reflects an optimised implementation of the current World Health Organization treatment guidelines [39] and the Joint United Nations Programme on HIV/AIDS definition for “universal access" of reaching 80% of those in need [32]. Compared to the no-treatment counterfactual scenario, ART provision reduced incidence in year 2020 by 35% to 54% across all models (Figure 2A). There was much greater variation, however, in the estimated long-term impact of the intervention. In year 2050, the range of the predicted reduction in incidence was from 32% to 74%. The relative impact of the ART intervention on HIV incidence decreased between 2020 and 2050 in four models and increased in seven.
Fig. 2. Impact of treatment for a scenario with eligibility at CD4≤350 cells/µl, 80% access, and 85% retention. 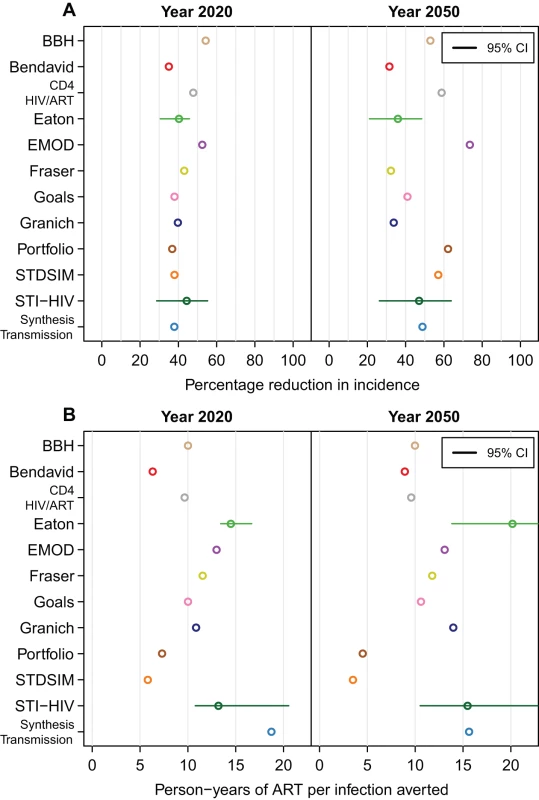
(A) The percentage reduction in HIV incidence in the years 2020 and 2050 when eligibility for treatment is at CD4 count ≤350 cells/µl, 80% of individuals are treated, and 85% are retained on treatment after 3 y. (B) The cumulative number of person-years of ART provided per infection averted for the same scenario. Horizontal lines indicate 95% credible intervals (CI). For the Bendavid model, results in year 2040 are reported in the right panels. Number of Person-Years of ART per Infection Averted
There was considerable variation between models in estimates of the number of person-years of treatment per infection averted. For the scenario described above, the range of estimates for the number of person-years of ART per infection averted between 2012 and 2020 was between 6.3 and 18.7, and over the period 2012 to 2050, the range was 4.5 to 20.2 (Figure 2B). The four models with the greatest estimates of the number of person-years of ART provided per infection averted (Eaton, EMOD, STI-HIV Interaction, and Synthesis Transmission) all explicitly included variation in transmission by age (e.g., allowing for reduced impact through ART provision to older adults who are less sexually active and hence less likely to expose susceptible individuals), whereas the other models did not assume reduced transmission by older people. (STDSIM allows for decreased sexual activity for those older than 50 and has the lowest estimate of person-years of ART per infection averted, but simulates a much higher HIV incidence.)
Determinants of Programme Impact
The impact on incidence of increasing access from 50% to 100%, improving 3-y programme retention from 85% to 100%, and changing the CD4 threshold for treatment eligibility, is shown for each model in Figure 3. The reduction in incidence increases approximately linearly with access in all models. In most models, improvements in retention in care led to greater impact of treatment on HIV incidence. The benefit of improving retention was minimal for the Fraser, Granich, and HIV Portfolio models. Each of these models regards individuals who have dropped out of treatment identically to untreated eligible individuals, allowing them to start treatment again on average within 1 y. In several models, improved retention means that the impact improves more rapidly with increasing access (i.e., the slope in reduction in incidence as access increases is steeper for higher retention).
Fig. 3. Proportion reduction in HIV incidence in year 2020. 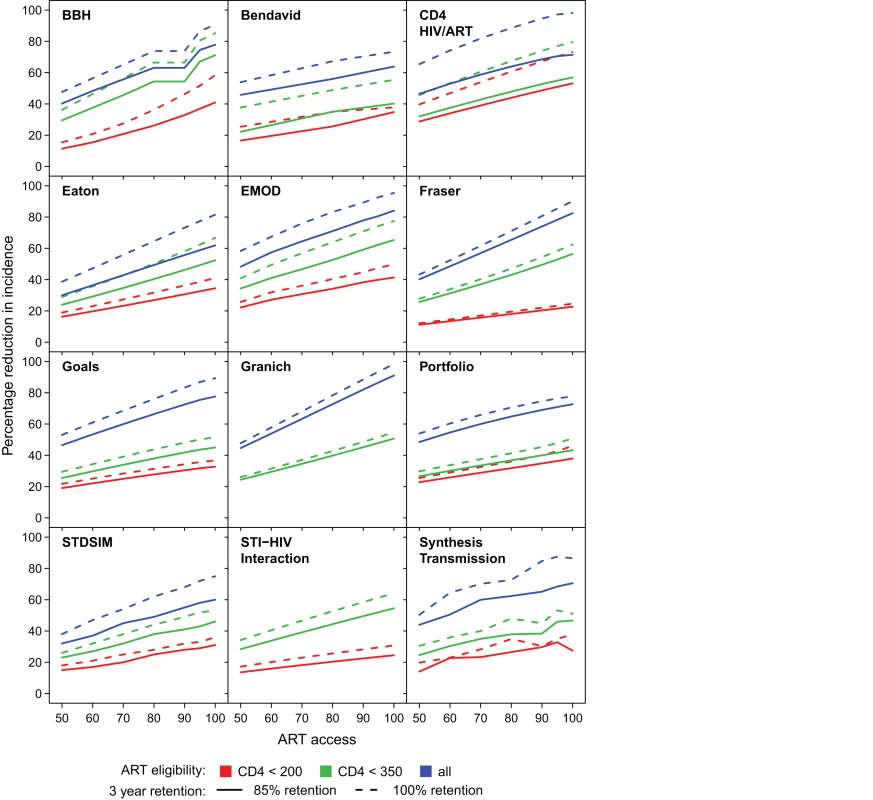
For each model, the proportion reduction in HIV incidence in year 2020 for increasing access levels from 50% to 100% (horizontal axis). ART eligibility thresholds are indicated by line colour; 85% retention is indicated by solid lines, and perfect 100% retention is indicated by dashed lines. Figure 4 shows how the number of person-years of ART provided per infection averted up to year 2020 varied in relation to the intervention programme. There were no consistent trends across all models. In some models, with earlier initiation of treatment, fewer years of ART were required per infection averted (efficiency increases), whereas the opposite was predicted in others. For all of the models except the Granich model, which does not include increased transmissibility during late-stage infection, it might be expected that treating at lower CD4 count would be more efficient, as it targets treatment towards individuals with the highest current infectiousness (as in the BBH, Bendavid, CD4 HIV/ART, Eaton, and Goals models). However this could be counteracted if stage of infection interacts with other processes such as decreased propensity to form new partnerships with ageing. For half of the models (BBH, Eaton, EMOD, Fraser, Goals, Granich, and HIV Portfolio), increasing the percentage of the population with access to treatment reduced the amount of treatment per infection averted, at least at earlier CD4 initiation thresholds. This increased efficiency is indicative of increasing returns due to “herd immunity" at high intervention coverage levels.
Fig. 4. Cumulative number of person-years of ART provided per infection averted through year 2020. 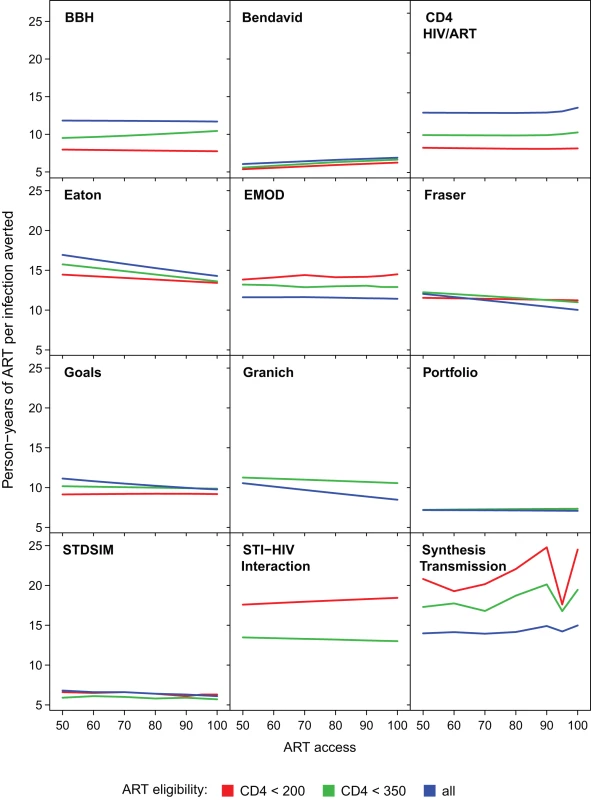
The cumulative person-years of ART provided per infection averted through the year 2020 for increasing access levels from 50% to 100% (horizontal axis), assuming 85% retention after 3 y. ART eligibility thresholds of are indicated by line colour. Varying retention did not affect trends between access and efficiency for any models. Treatment Eligibility and the Theoretical Impact of “Test and Treat"
The models varied in their predictions as to the relative benefit of increasing treatment eligibility from a CD4 threshold of ≤200 cells/µl (national guidelines in some settings and close to actual experience in many) to ≤350 cells/µl (international guidelines) compared to further increasing eligibility to all infected individuals (Figure 3). The Bendavid, CD4 HIV/ART, Goals, HIV Portfolio, and Synthesis Transmission models all predicted that there would be only a relatively modest benefit in moving from initiation at ≤200 cells/µl to ≤350 cells/µl, and a much greater benefit in moving from initiation at ≤350 cells/µl to immediately upon diagnosis of HIV infection. In contrast, the BBH model simulated very little benefit in moving from the ≤350 cells/µl threshold to immediate eligibility. The Eaton, Fraser, and EMOD models showed similar benefits associated with each of the increments at moderate levels of access.
One important argument that has been made for immediate ART is that commitment of a large amount of ART now could reduce the cumulative amount of ART required in the future as a result of averted HIV infections [2],[49]. Whether such savings could occur was evaluated by investigating whether the cumulative person-years of treatment through year 2050 to implement immediate treatment is less than the amount of ART required when treating after the CD4 count falls below 350 cells/µl for the same levels of access and retention. In six (BBH, CD4 HIV/ART, Fraser, Goals, HIV Portfolio, and STDSIM) out of eleven models (excluding STI-HIV Interaction) this was not the case: increasing eligibility from CD4≤350 cells/µl to immediate initiation always required more person-years of treatment, even with “perfect" ART programmes (100% access and 100% retention). However, for the EMOD model, expanding eligibility from CD4≤350 cells/µl to all HIV-infected adults required fewer cumulative person-years of treatment in all intervention scenarios (including access as low as 60% and retention in care as low as 75%). The Synthesis Transmission model found expanding access to be ART-saving with 70% access and retention above 95%, or with 80% access and retention above 85%. The other three models that found that expanding access could be ART-saving required more demanding assumptions about programmes: according to the Granich model, immediate initiation would be ART-saving if access were above 90% and retention above 95%; according to the Eaton model, access and retention would need to exceed 95%; and according to the Bendavid model, access and retention would both need to be 100%.
In an intervention treating all HIV-infected adults with 95% access and 95% retention, three (CD4 HIV/ART, EMOD, and HIV Portfolio) out of nine models (excluding BBH, Bendavid, and STI-HIV Interaction) predicted that HIV incidence would fall below 0.1% per year by 2050. The Granich model, which was used to argue the case for HIV elimination using treatment, projected that incidence in South Africa would be 0.13% under this scenario, a 92% reduction (in the original published projections, there was an assumption that risk of infection would fall by an additional 40% due to other interventions [34]).
Understanding Differences between Model Predictions
One factor expected to influence how much ART reduces HIV is the fraction of all transmission that occurs after individuals reach treatment eligibility thresholds, in the absence of any treatment [50]. Figure 5A shows the proportion of transmissions that occur from individuals in each CD4 count range in the counterfactual simulation in year 2012. Of the models that include a period of early infection, the percentage of new infections that occurs during this stage is between 4% and 28%, while between 20% and 51% of transmission results from individuals with CD4 cell count ≤200 cells/µl.
Fig. 5. Impact of treatment by transmission in each CD4 category. 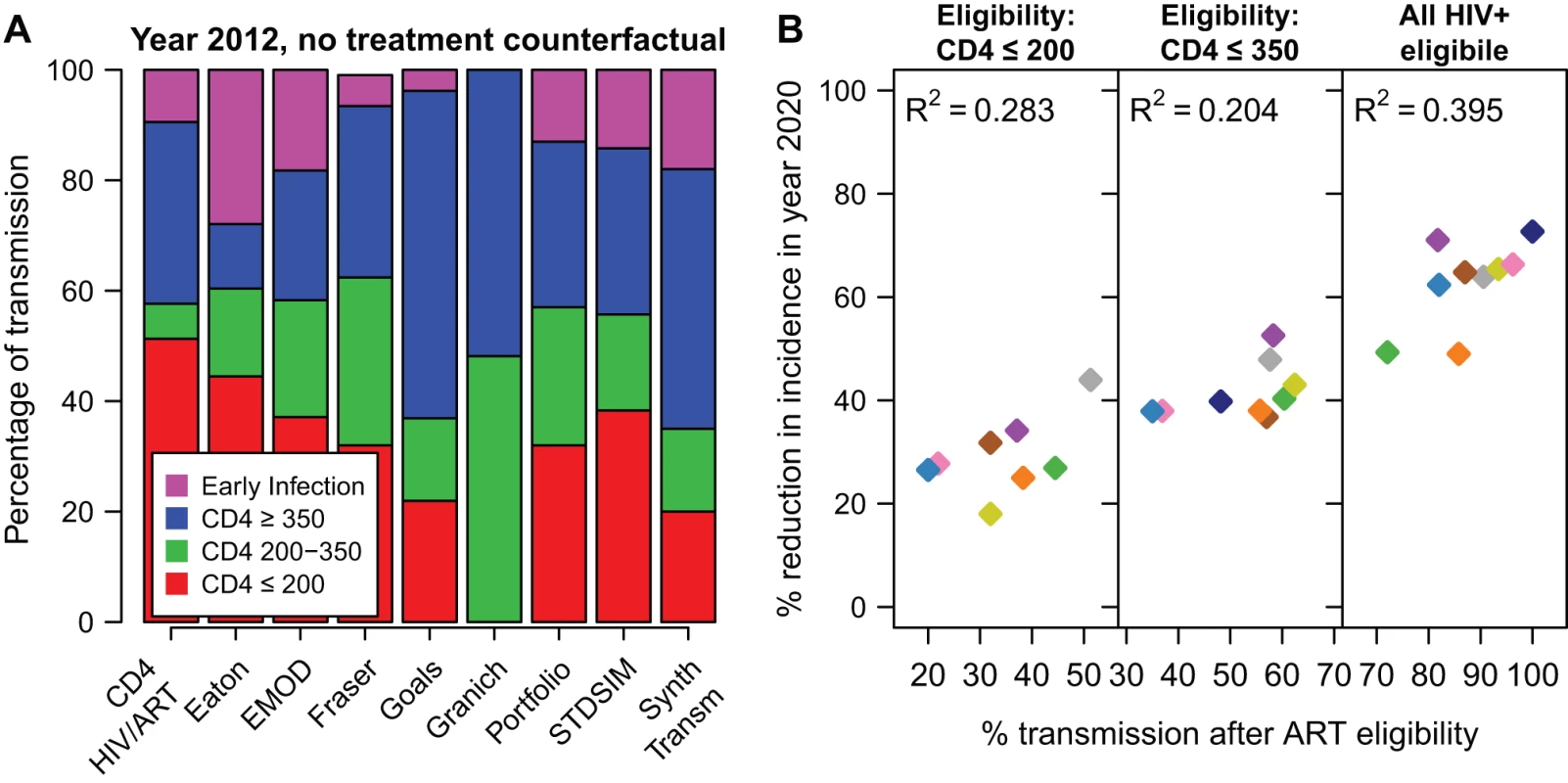
(A) The percentage of all HIV transmissions from individuals in each CD4 cell count category in year 2012, in the no-ART counterfactual simulation. (B) The reduction in incidence in year 2020, for the 80% access and 85% retention scenario, according to the cumulative proportion of transmission that occurs after eligibility (A). For the scenario where all HIV-positive adults are eligible (“all HIV+ eligible"), the percentage of transmission after ART eligibility is the percentage of transmission that occurs after the end of primary HIV infection. Colours for models are the same as in Figures 1 and 2. The BBH, Bendavid, and STI-HIV Interaction models do not estimate the proportion of transmission in each CD4 category and are not included in this figure. These percentages of transmission after ART eligibility can be compared with the percentage reduction in incidence in year 2020 (Figure 5B). Here, it is assumed that access is 80% and 3-y retention in care is 85%. Although this comparison explains why, within one model, earlier treatment initiation reduces HIV incidence more, the amount of between-model variation in projected impact explained by the distribution of transmission by CD4 count is modest. R2 values for this relationship were 0.28, 0.20, and 0.40 for eligibility at CD4≤200, eligibility at CD4≤350, and immediate eligibility, respectively. The correlation did not improve when considering higher access or higher retention scenarios.
Two other factors hypothesized to explain the differences between the model projections are different assumptions about the efficacy of ART in reducing transmission—between 90% and 99%—and different assumptions about the outcomes of individuals who drop out from treatment programmes. To test the importance of these factors, selected intervention scenarios were repeated under the artificial assumption that an individual never transmits after initiating treatment (treatment is 100% efficacious at preventing transmission, and retention on treatment is 100%). This assumption increased the intervention impact in every model, but, surprisingly, did not reduce the variation in the results between models or improve the ability of factors such as different model assumptions about CD4 progression, HIV transmission, or the future trajectory of HIV incidence to explain the variation.
Estimates of the Current Impact of ART in South Africa
Figure 6 shows the estimated impact of the current ART programme in South Africa on HIV prevalence and incidence. The CD4 HIV/ART, Eaton, Goals, Granich, and STI-HIV Interaction models used estimates of the number of adults starting treatment in South Africa in each year between 2001 and 2011 from [45], and the Fraser and STDSIM models used existing calibrations to ART coverage levels in the Western Cape and KwaZulu-Natal Provinces, respectively. All of the models predicted that ART should already have had a substantial impact on the HIV epidemic, estimating that HIV incidence in year 2011 was between 17% and 32% lower than it would have been in the absence of ART. The increasing impact on HIV incidence over time mirrors the steep increase every year in the number of people starting treatment during this period.
Fig. 6. The impact of the existing ART programme in South Africa on HIV prevalence and incidence. 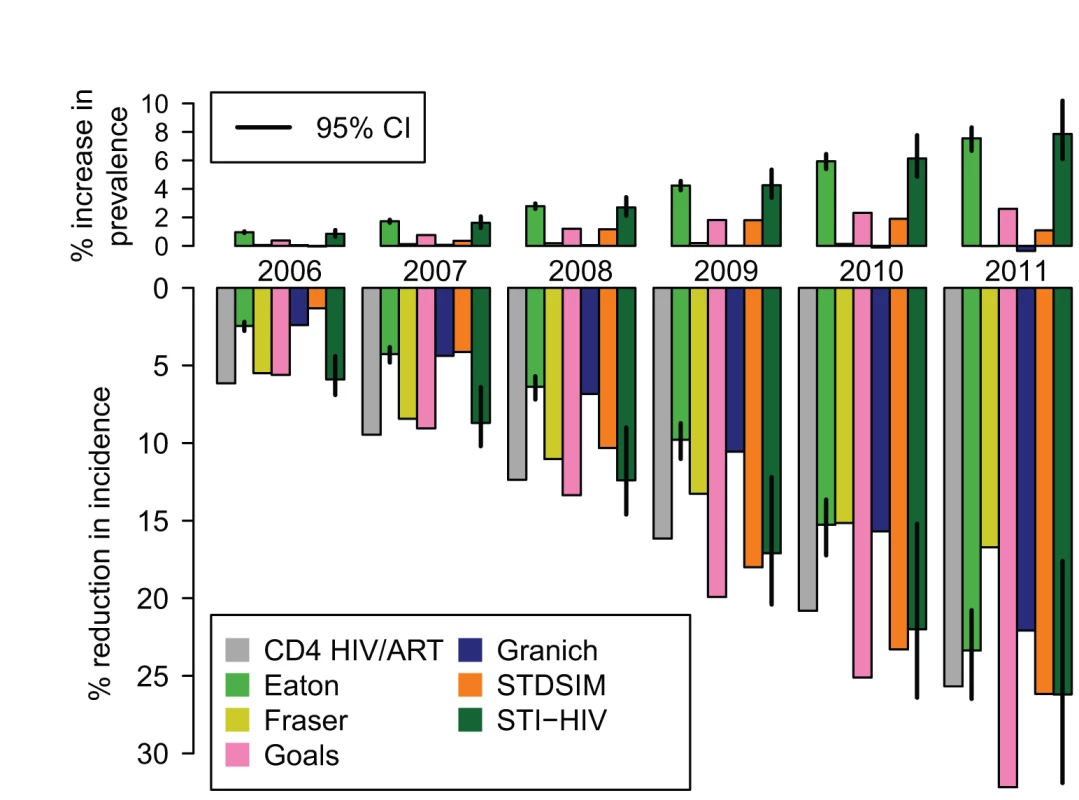
The percentage increase in HIV prevalence (top) and the percentage reduction in HIV incidence rate (bottom) compared to what would have occurred in the absence of any ART for years the 2006 to 2011. These are estimated by comparing HIV prevalence and incidence in a model calibrated to the existing scale-up of ART in South Africa from 2001 to 2011 with a model simulation with no ART provision. The CD4 HIV/ART, Eaton, Goals, Granich, and STI-HIV Interaction models use the same estimates of the number starting ART each year (from [45]). Fraser uses an existing calibration to the ART scale-up in the Western Cape Province. STDSIM is calibrated to the number of people on ART in the Hlabisa subdistrict. Vertical lines on the Eaton and STI-HIV Interaction models indicate 95% credible intervals (CI). The impact on prevalence was more modest and less consistent across models. The Eaton and STI-HIV Interaction models estimated that prevalence is around 8% higher than it would have been without treatment (an absolute increase in prevalence of one percentage point) due to the increased survival for those infected with HIV. The Fraser and Granich models suggest that this effect is offset by the reductions in incidence, so that there is no net change in prevalence. It is unlikely that standard surveillance methods based on monitoring trends in prevalence would have detected this impact, despite the significant underlying reductions in incidence.
The estimated potential impact of further ART scale-up is summarised in Table 4. In the baseline scenario, where 400,000 people are started on ART each year, the models estimated that incidence would be reduced in 2016 by between 13% and 26% compared to the incidence rate in 2011. If 800,000 fewer people are put on ART, then between 39,000 and 186,000 more new adult HIV infections would occur over the period 2012 to 2016 than under the baseline scenario. If more people are put on ART—3.0 or 3.9 million over the next 5 y—then the models estimated that the number of new infections over the 5-y period would be reduced by 64,000 to 327,000 and 270,000 to 521,000, respectively, compared to the baseline. The table underscores that there are still substantial potential preventive benefits from expanding ART coverage in South Africa. The models that tended to estimate the greatest reduction in incidence in hypothetical programmes over the medium term (CD4 HIV/ART, Goals, and Granich) also tended to project greater reductions in incidence over the short term in these more realistic scenarios.
Tab. 4. HIV incidence rate per 100 person-years in year 2016 for different potential scenarios of future ART scale-up. 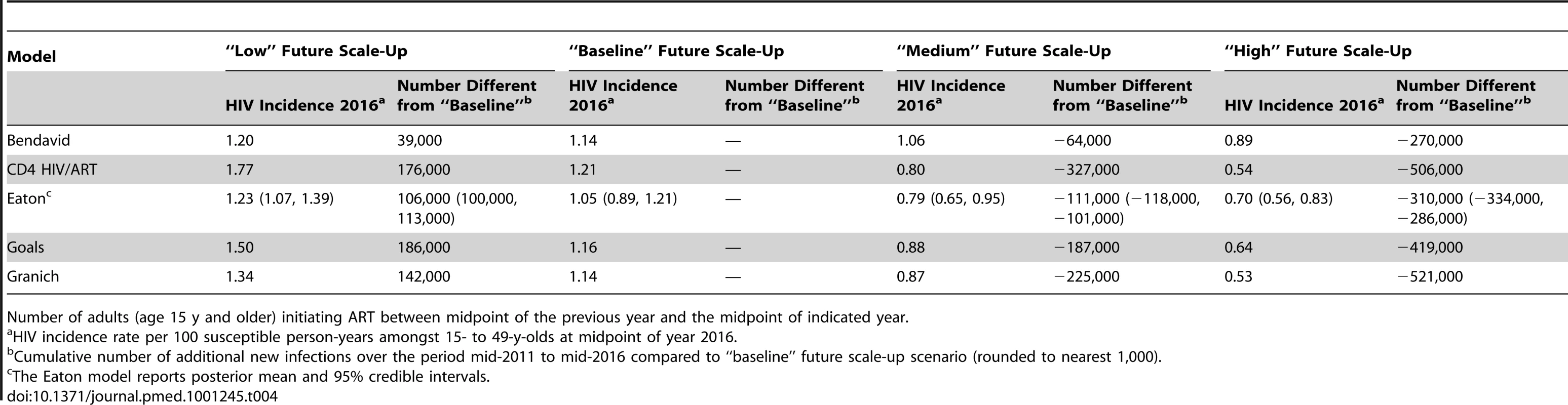
Number of adults (age 15 y and older) initiating ART between midpoint of the previous year and the midpoint of indicated year. Discussion
The mathematical models used to simulate the impact of treatment on HIV incidence in South Africa are diverse in their structure, level of complexity, representation of the HIV transmission process and the ART intervention, and parameter choices. All twelve of the models compared in this analysis predicted that treatment could substantially reduce HIV incidence—even using past or existing treatment guideline eligibility criteria, provided that coverage is high. Only three (CD4 HIV/ART, EMOD, and HIV Portfolio) out of nine models (excluding BBH, Bendavid, and STI-HIV Interaction), however, predicted that treatment could reduce HIV incidence below 0.1% by year 2050 (the definition of “elimination" established by [34]), even with very high access and retention. When simulating the historical scale-up of ART in South Africa, the models indicated that ART may already have reduced HIV incidence by between 17% and 32% in 2011, compared to what would have been expected in the absence of ART.
Although there have been ad hoc informal model comparison exercises [51], collections of work using standardised assumptions for interventions [52], and thorough model comparisons involving a few research groups [53],[54], to our knowledge, this exercise is the first to bring together such a large number of independent modelling groups to examine the same set of interventions. We hope that this will provide a foundation for much more collaborative work.
In this study we set out to test whether different models of the potential impact of treatment on new HIV infections in South Africa would make similar predictions when implementing the same intervention scenarios. We found substantial consistency between the model projections of the impact of ART interventions on HIV incidence in the short term (8 y). However, there was more variation in the predicted longer term (38 y) reductions in incidence, and models also produced divergent estimates of the number of person-years of ART provided per infection averted. While establishing where models agree and disagree about the epidemiological impact of ART represents an important scientific finding in itself, the substantial variation in the long-term impact and efficiency of interventions demands further investigation and explanation.
Based on epidemiological theory and previous modelling studies, we hypothesized a number of model attributes that might explain differences in model predictions about the impact of ART, including the amount of transmission in different stages of HIV infection, the assumed efficacy of ART for preventing transmission, opportunities for treatment reinitiation following dropout from a treatment programme, the age and sex structure of the population, future population growth rates, the degree of heterogeneity and assortativity in sexual mixing, the future trajectory of HIV incidence in the absence of intervention, and the inclusion of changes in sexual behaviour over the past decade. There was indeed substantial variation between the models in their characterisation of each of these aspects of the system, largely reflecting the true uncertainties that persist even after decades of tremendous research into the epidemiology of HIV in South Africa. We were able to show that crude differences in the proportion of transmission at each stage of infection explained a modest amount of the variation in the short-term impact of ART, but less of the long-term impact. However, beyond this, findings from the models did not appear to clearly support any of these hypotheses in univariate analyses, likely because of the large number of processes that interact nonlinearly to create HIV epidemics and interventions. For example, projecting a seemingly simple quantity such as the number of person-years of ART that will be provided in an intervention depends on future population growth, the natural trend in the epidemic, the proportion of HIV-infected individuals qualifying for treatment, retention and survival on ART, and the impact that ART provision has on future HIV incidence. This situation contrasts with that of an earlier exercise that compared predictions of the impact of male circumcision interventions [51], where the relationship between the established efficacy of the intervention and population-level impact was less complicated.
Having investigated the extent to which differences in ART programmes determine differences in results, the natural focus for future model comparison studies should be to explore the contribution of other hypotheses through incrementally standardising biological, behavioural, and demographic model parameters, and calibrating models to the same levels of HIV prevalence and incidence. A systematic approach to standardising model parameters would identify which parameters most significantly influence the results and guide priorities for future data collection. The HIV Modelling Consortium (http://www.hivmodelling.org) will coordinate such research efforts in coming months to investigate the extent to which variation in model predictions is driven by differences in underlying models of sexual mixing, or different models of the natural history of infection and epidemic trajectory.
Although our experiment and analysis has focused on how factors included in models can affect model predictions, it is important to note that if all models exclude an important aspect of the system, they could all be wrong. Early models of the impact of ART on HIV incidence were very focused on the concern that increased sexual risk behaviour might offset the reduction in transmission for those on treatment, but for this exercise all of the models assumed that population risk behaviour would not change in response to the introduction of ART. This may be a reasonable assumption given consistent evidence that patients report safer sexual behaviour after starting ART [55]–[59] and given the relative lack of information from sub-Saharan Africa about how the untreated and HIV-negative population responds to the availability of treatment [60]. But in other epidemic settings the availability of ART has been associated with receding gains in protective behaviour [61]–[63], and monitoring this in sub-Saharan African settings will be a priority for surveillance over coming years. The models also all assumed high efficacy of ART to reduce transmission. True effectiveness will depend on adherence and the level of viral suppression, which is mainly determined by adherence levels. While there are some data from South Africa on viral suppression rates outside carefully controlled trial settings [64], further information on this and on patterns of acquired and transmitted resistance will help in the calibration of models. Only one of the models in this exercise (Synthesis Transmission) explicitly incorporated the effect of antiretroviral drug resistance on the impact of ART interventions. Models have predicted that antiretroviral drug resistance could be widespread in sub-Saharan Africa in coming decades [20], which could eventually lead to the spread of transmitted drug resistance [65],[66]. This could affect the long-term costs and efficacy of treatment-as-prevention strategies [67].
Another finding from systematically comparing models is that often seemingly independent modelling studies rely on the same limited data. Nearly all of the models relied on two sources to derive parameters for elevated infectiousness during the first few weeks of infection [68],[69], but both of these sources are based principally on data from a few retrospective couples in Rakai, Uganda [70] (see [71]). This highlights both how invaluable these data are and also the importance of recognising the dependencies between seemingly independent modelling studies. However, even using the same data, models may reach different conclusions. The Eaton, EMOD, and Fraser models all in some way used the estimates of early HIV infectivity from [69] but estimate very different contributions of this stage to overall HIV transmission (Figure 4A), and the three models all reached different conclusions from those in another recent modelling study relying on these same estimates [72].
The purpose of this exercise was not to draw conclusions or recommendations about specific ART intervention strategies, but rather to test the hypothesis that a range of different models would come to similar conclusions about the impact of ART on HIV incidence when the same interventions were modelled. The simulated interventions were artificially simple and stylized to enable comparison between models. These did not explicitly simulate the steps of HIV testing, diagnosis, linkage to care, and adherence to ART required to achieve the access levels specified in the intervention scenarios (although several of the models include facility for this and have investigated this in independent analyses). Interpretation of models simulating high levels of treatment coverage should be cautioned by data suggesting that at present fewer than one-third of patients in sub-Saharan Africa are continuously retained in care from HIV diagnosis to ART initiation [73], and that barriers remain to access to and uptake of HIV testing [32]. The models assumed that all individuals eligible for treatment were equally likely to access treatment, which might not be true in practice (for example, women are more likely to start treatment than men [74]). The comparison scenario (counterfactual) against which interventions were evaluated assumed no treatment at all, which made it easier to compare models, but is clearly not the relevant benchmark for policy-makers. This study has also considered treatment in isolation from other interventions, even as there is broad consensus that “combination prevention" strategies are presently the best strategy for attacking the epidemic [41],[75].
We hope that this study will help to characterise the models that are being used to investigate questions related to the impact of HIV treatment and enable those who rely on models for decision-making to think critically about how the assumptions underlying models affect the results. The relative consistency between models' estimates of the short-term epidemiological impact of ART, including the impact of the existing ART programme, provides some reassurance that model projections on this time scale may be relatively robust to the substantial uncertainties in parameters and systems. This is a significant result considering that such short-term projections are often the most relevant for policy and resource allocation questions. On the other hand, the substantial variation in long-term epidemiological impacts and efficiency of ART, upon which arguments of substantial epidemic reduction and cost savings hinge, suggests that results in these areas from any single model should be extrapolated with caution. Care should be taken to ensure that models evaluating the long-term costs, benefits, and cost-effectiveness of treatment programmes adequately communicate the degree and myriad sources of uncertainty that influence these outputs.
A common question when faced with a diversity of model results is whether some models are “better" or “worse". Without data against which to test the predictions of models, it is not possible to answer this question in a study such as this, nor is this the correct question to be asking. Rather, users of model outputs should ask whether models include the necessary components to capably answer the specific questions at hand, and whether the models make credible assumptions in light of the information available, and choose models accordingly. Evaluated along these guidelines, the most appropriate models will vary between applications, so there is no single “best" model. However, in this exercise, the models that tended to project more “pessimistic" outcomes for the interventions seemed to do so for important reasons. For example, models that estimated poorer efficiency of ART for averting infections tended to be those that simulated ART provision for those at older ages, who might be at lower risk of transmitting, or included the elevated risk of transmission for those failing treatment, whereas models with more optimistic predictions assumed that risk behaviour did not vary by age or that transmission was fully suppressed immediately upon beginning treatment until death on ART or dropout. Artificial convergence of models should be avoided when true uncertainties persist about the system. It is incumbent upon modellers to incorporate and communicate uncertainty in projections, and identify which components of the system account for the uncertainty. For this exercise, only one model (STI-HIV Interaction) included a comprehensive analysis accounting for uncertainty about basic epidemiology and intervention efficacy. While the focus of the study was on variation between models, it is interesting to observe that the 95% credible interval representing parameter uncertainty for this model encompassed the point estimates of the other eleven models.
Fortunately there will be important new opportunities in the near future to test, validate, and improve epidemiological models of HIV treatment. These include comparing projections to the experience of expanded ART in industrialised countries [61],[63], the observed impact of ART in well-characterised communities [76], and results of a number of community-randomized trials of treatment as prevention that will soon be underway [44]. As new data are reported, the accuracy of models projecting the impact of treatment as prevention should improve, and we expect that validated and scientifically based model projections will continue to be central in understanding how ART can have the greatest impact in mitigating the global HIV epidemic.
Zdroje
1. MontanerJSGHoggRWoodEKerrTTyndallM 2006 The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368 531 536 doi:10.1016/S0140-6736(06)69162-9
2. De CockKMGilksCFLoY-RGuermaT 2009 Can antiretroviral therapy eliminate HIV transmission? Lancet 373 7 9 doi:10.1016/S0140-6736(08)61732-8
3. GarnettGPBaggaleyRF 2009 Treating our way out of the HIV pandemic: could we, would we, should we? Lancet 373 9 11 doi:10.1016/S0140-6736(08)61698-0
4. CohenJ 2011 Halting HIV/AIDS epidemics. Science 334 1338 1340
5. GranichRGuptaSSutharASmythCHoosD 2011 Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res 9 446 469 doi:10.2174/157016211798038597
6. QuinnTCWawerMJSewankamboNSerwaddaDLiC 2000 Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342 921 929 doi:10.1056/NEJM200003303421303
7. FraserCHollingsworthTDChapmanRde WolfFHanageWP 2007 Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A 104 17441 17446 doi:10.1073/pnas.0708559104
8. VernazzaPLTroianiLFleppMJConeRWSchockJ 2000 Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS 14 117 121
9. Cu-UvinSCaliendoAMReinertSChangAJuliano-RemollinoC 2000 Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS 14 415 421
10. BaggaleyRFFergusonNMGarnettGP 2005 The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol 2 9 doi:10.1186/1742-7622-2-9
11. DoddPJGarnettGPHallettTB 2010 Examining the promise of HIV elimination by “test and treat" in hyperendemic settings. AIDS 24 729 735 doi:10.1097/QAD.0b013e32833433fe
12. AndersonRMGuptaSMayRM 1991 Potential of community-wide chemotherapy or immunotherapy to control the spread of HIV-1. Nature 350 356 359 doi:10.1038/350356a0
13. GilliamBLDyerJRFiscusSAMarcusCZhouS 1997 Effects of reverse transcriptase inhibitor therapy on the HIV-1 viral burden in semen. J Acquir Immune Defic Syndr Hum Retrovirol 15 54 60
14. BlowerSMGershengornHBGrantRM 2000 A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 287 650 654
15. DangerfieldBCFangYRobertsCA 2001 Model-based scenarios for the epidemiology of HIV/AIDS: the consequences of highly active antiretroviral therapy. Syst Dyn Rev 17 119 150 doi:10.1002/sdr.211
16. LawMGPrestageGGrulichAVan de VenPKippaxS 2001 Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS 15 1287 1294
17. Velasco-HernandezJXGershengornHBBlowerSM 2002 Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis 2 487 493 doi:10.1016/S1473-3099(02)00346-8
18. XiridouMGeskusRDe WitJCoutinhoRKretzschmarM 2003 The contribution of steady and casual partnerships to the incidence of HIV infection among homosexual men in Amsterdam. AIDS 17 1029 1038 doi:10.1097/01.aids.0000050879.72891.1d
19. GrayRHLiXWawerMJGangeSJSerwaddaD 2003 Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS 17 1941 1951 doi:10.1097/01.aids.0000076313.76477.fc
20. BlowerSBodineEKahnJMcFarlandW 2005 The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS 19 1 14
21. BaggaleyRFGarnettGPFergusonNM 2006 Modelling the impact of antiretroviral use in resource-poor settings. PLoS Med 3 e124 doi:10.1371/journal.pmed.0030124
22. McCormickAWWalenskyRPLipsitchMLosinaEHsuH 2007 The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis 44 1115 1122 doi:10.1086/512816
23. SalomonJAHoganDR 2008 Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS 22 Suppl 1 S149 S159 doi:10.1097/01.aids.0000327636.82542.87
24. AttiaSEggerMMüllerMZwahlenMLowN 2009 Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 23 1397 1404 doi:10.1097/QAD.0b013e32832b7dca
25. ReynoldsSJMakumbiFNakigoziGKagaayiJGrayRH 2011 HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS 25 473 477 doi:10.1097/QAD.0b013e3283437c2b
26. DonnellDBaetenJMKiarieJThomasKKStevensW 2010 Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 375 2092 2098 doi:10.1016/S0140-6736(10)60705-2
27. GlynnJRPriceAFloydSMolesworthAKayuniN 2011 Anitretroviral therapy reduces HIV transmission in discordant couples in northern Malawi. Sex Transm Infect 87 A218 doi:10.1136/sextrans-2011-050108.277
28. CohenMSChenYQMcCauleyMGambleTHosseinipourMC 2011 Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 493 505 doi:10.1056/NEJMoa1105243
29. LodwickRCostagliolaDReissPTortiCTeiraR 2010 Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med 170 410 419 doi:10.1001/archinternmed.2009.472
30. BoulleAVan CutsemGHilderbrandKCraggCAbrahamsM 2010 Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 24 563 572 doi:10.1097/QAD.0b013e328333bfb7
31. NashDKatyalMBrinkhofMWGKeiserOMayM 2008 Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS 22 2291 2302 doi:10.1097/QAD.0b013e3283121ca9
32. Joint United Nations Programme on HIV/AIDS 2010 Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf. Accessed 19 December 2011
33. Joint United Nations Programme on HIV/AIDS 2011 World AIDS Day Report 2011. How to get to zero: faster, smarter, better. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf. Accessed 23 December 2011
34. GranichRMGilksCFDyeCDe CockKMWilliamsBG 2009 Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 48 57 doi:10.1016/S0140-6736(08)61697-9
35. WagnerBGBlowerS 2009 Voluntary universal testing and treatment is unlikely to lead to HIV elimination: a modeling analysis. doi:10.1038/npre.2009.3917.1
36. KretzschmarMEvan der LoeffMFCoutinhoRA 2012 Elimination of HIV by test and treat. AIDS 26 247 248 doi:10.1097/QAD.0b013e32834e1592
37. BacaërNPretoriusCAuvertB 2010 An age-structured model for the potential impact of generalized access to antiretrovirals on the South African HIV epidemic. Bull Math Biol 72 2180 2198 doi:10.1007/s11538-010-9535-2
38. BendavidEBrandeauMLWoodROwensDK 2010 Comparative effectiveness of HIV testing and treatment in highly endemic regions. Arch Intern Med 170 1347 1354 doi:10.1001/archinternmed.2010.249
39. World Health Organization 2010 Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2010 revision. Available: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 22 December 2011
40. PEPFAR Scientific Advisory Board 2011 PEPFAR Scientific Advisory Board recommendations for the Office of the US Global AIDS Coordinator: implications of HPTN 052 for PEPFAR's treatment programs. Available: http://www.pepfar.gov/documents/organization/177126.pdf. Accessed 19 December 2011
41. SchwartländerBStoverJHallettTAtunRAvilaC 2011 Towards an improved investment approach for an effective response to HIV/AIDS. Lancet 377 2031 2041 doi:10.1016/S0140-6736(11)60702-2
42. HontelezJAde VlasSJTanserFBakkerRBärnighausenT 2011 The impact of the new WHO antiretroviral treatment guidelines on HIV epidemic dynamics and cost in South Africa. PLoS ONE 6 e21919 doi:10.1371/journal.pone.0021919
43. JohnsonLFHallettTBRehleTMDorringtonRE 2012 The effect of changes in condom usage and antiretroviral treatment coverage on human immunodeficiency virus incidence in South Africa: a model-based analysis. J R Soc Interface 9 1544 1554 doi:10.1098/rsif.2011.0826
44. BoilyMCMâsseBAlsallaqRPadianNSEatonJW 2012 HIV treatment as prevention: considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. PLoS Med 9 e1001250 doi:10.1371/journal.pmed.1001250
45. JohnsonLF 2012 Access to antiretroviral treatment in South Africa, 2004–2011. South Afr J HIV Med 13 22 27
46. South Africa National AIDS Council 2011 National strategic plan on HIV, STIs and TB: 2012–2016 summary. Available: http://www.doh.gov.za/docs/stratdocs/2012/NSPsum.pdf. Accessed 25 May 2012
47. ShisanaORehleTSimbayiLCZumaKJoosteS 2009 South African national HIV prevalence, incidence, behaviour and communication survey 2008: a turning tide amongst teenagers? Cape Town HSRC Press
48. Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat 2011 World population prospects: the 2010 revision. Available: http://esa.un.org/unpd/wpp/index.htm. Accessed 19 December 2011
49. The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group 2012 HIV treatment as prevention: models, data, and questions—Towards evidence-based decision-making. PLoS Med 9 e1001259 doi:10.1371/journal.pmed.1001259
50. AndersonRMMayRM 1992 Infectious diseases of humans: dynamics and control Oxford Oxford University Press
51. UNAIDS/WHO/SACEMA Expert Group on Modelling the Impact and Cost of Male Circumcision for HIV Prevention 2009 Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS Med 6 e1000109 doi:10.1371/journal.pmed.1000109
52. HankinsCAGlasserJWChenRT 2011 Modeling the impact of RV144-like vaccines on HIV transmission. Vaccine 29 6069 6071 doi:10.1016/j.vaccine.2011.07.001
53. HalloranMEFergusonNMEubankSLonginiIMCummingsDA 2008 Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A 105 4639 4644 doi:10.1073/pnas.0706849105
54. AlthausCLTurnerKMSchmidBVHeijneJCKretzschmarM 2011 Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data. J R Soc Interface 9 136 146 doi:10.1098/rsif.2011.0131
55. BateganyaMColfaxGShaferLAKityoCMugyenyiP 2005 Antiretroviral therapy and sexual behavior: a comparative study between antiretroviral-naive and -experienced patients at an urban HIV/AIDS care and research center in Kampala, Uganda. AIDS Patient Care STDS 19 760 768
56. BunnellREkwaruJPSolbergPWamaiNBikaako-KajuraW 2006 Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS 20 85 92
57. EiseleTPMathewsCChopraMLurieMNBrownL 2009 Changes in risk behavior among HIV-positive patients during their first year of antiretroviral therapy in Cape Town South Africa. AIDS Behav 13 1097 1105 doi:10.1007/s10461-008-9473-2
58. McClellandRSGrahamSMRichardsonBAPeshuNMaseseLN 2010 Treatment with antiretroviral therapy is not associated with increased sexual risk behavior in Kenyan female sex workers. AIDS 24 891 897 doi:10.1097/QAD.0b013e32833616c7
59. VenkateshKKde BruynGLurieMNMohapiLPronykP 2010 Decreased sexual risk behavior in the era of HAART among HIV-infected urban and rural South Africans attending primary care clinics. AIDS 24 2687 2696 doi:10.1097/QAD.0b013e32833e78d4
60. VenkateshKKFlaniganTPMayerKH 2011 Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS 25 1939 1949 doi:10.1097/QAD.0b013e32834b4ced
61. BezemerDde WolfFBoerlijstMCvan SighemAHollingsworthTD 2008 A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS 22 1071 1077 doi:10.1097/QAD.0b013e3282fd167c
62. SmithMKPowersKAMuessigKEMillerWCCohenMS 2012 HIV treatment as prevention: the utility and limitations of ecological observation. PLoS Med 9 e1001260 doi:10.1371/journal.pmed.1001260
63. WilsonDP 2012 HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med 9 e1001231 doi:10.1371/journal.pmed.1001231
64. BarthREvan der LoeffMFSchuurmanRHoepelmanAIWensingAM 2010 Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis 10 155 166 doi:10.1016/S1473-3099(09)70328-7
65. VardavasRBlowerS 2007 The emergence of HIV transmitted resistance in Botswana: “when will the WHO detection threshold be exceeded?" PLoS ONE 2 e152 doi:10.1371/journal.pone.0000152
66. PhillipsANPillayDGarnettGBennettDVitoriaM 2011 Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS 25 843 850 doi:10.1097/QAD.0b013e328344037a
67. WagnerBGKahnJSBlowerS 2010 Should we try to eliminate HIV epidemics by using a “Test and Treat" strategy? AIDS 24 775 776 doi:10.1097/QAD.0b013e3283366782
68. BoilyM-CBaggaleyRFWangLMasseBWhiteRG 2009 Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis 9 118 129 doi:10.1016/S1473-3099(09)70021-0
69. HollingsworthTDAndersonRMFraserC 2008 HIV-1 transmission, by stage of infection. J Infect Dis 198 687 693 doi:10.1086/590501
70. WawerMJGrayRHSewankamboNKSerwaddaDLiX 2005 Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191 1403 1409 doi:10.1086/429411
71. CohenMSDyeCFraserCMillerWCPowersKA 2012 HIV treatment as prevention: debate and commentary—will early infection compromise treatment-as-prevention strategies? PLoS Med 9 e1001232 doi:10.1371/journal.pmed.1001232
72. PowersKAGhaniACMillerWCHoffmanIFPettiforAE 2011 The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 78 256 268 doi:10.1016/S0140-6736(11)60842-8
73. RosenSFoxMP 2011 Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 8 e1001056 doi:10.1371/journal.pmed.1001056
74. CornellMGrimsrudAFairallLFoxMPvan CutsemG 2010 Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS 24 2263 2270 doi:10.1097/QAD.0b013e32833d45c5
75. The US President's Emergency Plan for AIDS Relief 2011 August Guidance for the prevention of sexually transmitted HIV infections. Available: http://www.pepfar.gov/documents/organization/171303.pdf. Accessed 19 March 2012
76. MaherDBiraroSHosegoodVIsingoRLutaloT 2010 Translating global health research aims into action: the example of the ALPHA network. Trop Med Int Health 15 321 328 doi:10.1111/j.1365-3156.2009.02456.x
77. StoverJBollingerLAvilaC 2011 Estimating the impact and cost of the WHO 2010 recommendations for antiretroviral therapy. AIDS Res Treat 2011 738271 doi:10.1155/2011/738271
78. StoverJJohnsonPHallettTMarstonMBecquetR 2010 The Spectrum projection package: improvements in estimating incidence by age and sex, mother-to-child transmission, HIV progression in children and double orphans. Sex Transm Infect 86 Suppl 2 ii16 ii21 doi:10.1136/sti.2010.044222
79. TanserFHosegoodVBärnighausenTHerbstKNyirendaM 2008 Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol 37 956 962 doi:10.1093/ije/dym211
80. AIDS 2031 Commission 2010 AIDS in an uncertain world: the report of the AIDS 2031 Commission Upper Saddle River (New Jersey) Prentice Hall
81. LongEBrandeauM 2010 The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 158 778 789
82. HontelezJALurieMNNewellMLBakkerRTanserF 2011 Ageing with HIV in south africa. AIDS 25 1665 1667 doi:10.1097/QAD.0b013e32834982ea
83. HontelezJANagelkerkeNBärnighausenTBakkerRTanserF 2011 The potential impact of RV144-like vaccines in rural South Africa: a study using the STDSIM microsimulation model. Vaccine 29 6100 6106 doi:10.1016/j.vaccine.2011.06.059
84. JohnsonLDorringtonRBradshawDPillay-Van WykVRehleT 2009 Sexual behaviour patterns in South Africa and their association with the spread of HIV: insights from a mathematical model. Demogr Res 21 289 340 doi:10.4054/DemRes.2009.21.11
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



