-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
article has not abstract
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001269
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001269Summary
article has not abstract
Summary Points
-
The evidence for a link between tuberculosis (TB) and diabetes is strong. Patients with diabetes are at increased risk of developing active TB, and have higher rates of treatment failure and death, even when placed on appropriate TB treatment.
-
This link may become even more meaningful in coming years, as the prevalence of diabetes is expected to rise dramatically in the resource-poor areas where TB thrives.
-
We discuss some of the financial challenges inherent in treating diabetes in patients with TB, as well as some opportunities for cost-saving and collaboration in the co-management of these two diseases.
-
We also propose strategies for funding and implementing efforts to control TB and diabetes concurrently in the developing world, and highlight areas of much-needed research in this field.
Introduction
Tuberculosis (TB) kills more than 3,500 people each day worldwide, leading to approximately 1.4 million deaths every year [1]. One-third of the world's population is currently infected with the causative agent of TB, and 8.8 million new cases of active TB are estimated to occur around the world each year [1]. TB is fueled by several social and economic factors, such as poverty or malnutrition [2], as well as other infectious diseases, such as HIV. People living with HIV/AIDS are 21–34 times more likely to develop active TB [1], and this harmful synergy has led public health systems to attempt to tackle the two diseases concurrently.
In recent years, strong evidence has been gathered to confirm a link between TB and yet another disease: diabetes mellitus. That link had been suspected for centuries [3]. Many studies now show that diabetes may be associated with an increased risk of developing active TB [4]–[6], and that TB patients who also have diabetes may have higher rates of treatment failure and death [7],[8]. These findings have led some to wonder if the diagnosis and treatment of diabetes in TB patients may improve TB outcomes. More research into this question is clearly needed [9],[10]. However, if further studies show that treating diabetes can improve TB outcomes, then should existing TB funding and treatment mechanisms in place in the developing world be expanded to include diabetes care? How could the existing funding and control efforts aimed at a mostly curable infectious disease be adapted to manage a chronic, non-communicable condition?
TB and Diabetes
Several recent publications have described the association between diabetes and TB, specifically the increased prevalence of active TB among patients with diabetes and the poorer treatment outcomes in these patients when compared to those without diabetes [11],[12]. For example, one recent systematic review of 13 studies reported that diabetic patients had about a 3-fold increased risk of developing TB when compared to those without diabetes [5].
Others have reported the poorer treatment outcomes in patients with diabetes and TB, including a higher risk of death among these patients. One prospective study examined sputum cultures at the completion of 6 months of TB treatment, finding positive cultures in 22.2% of patients with diabetes and 6.9% of those without diabetes [8]. Another systematic review of treatment outcomes described a relative risk of death of 1.89 among TB patients with diabetes when compared to non-diabetic patients [12]. After controlling for potential confounders, the pooled risk of death among TB patients with diabetes was nearly five times greater than those without diabetes [12].
The link between these two diseases may become even more meaningful in coming years, as the prevalence of obesity and diabetes are expected to rise dramatically in the resource-poor areas where TB thrives [13]. In sub-Saharan Africa, for example, the number of patients with diabetes is projected to increase by 161% between 2000 and 2030 [14].
Some groups have suggested that the burden of diabetes in these countries may hinder progress towards attaining the United Nations' (UN) Millennium Development Goal (MDG) and specific targets related to TB control [4],[15]. One study showed that in a southern Mexican population the impact of diabetes on the incidence of TB was greater than that of HIV [16]. This close relationship between TB and diabetes raises the question of whether diabetes treatment and TB care should become more integrated, similar to recent efforts to link the management of TB and HIV. For example, the Stop TB Partnership's Global Plan to Stop TB has called for greater collaboration between TB and HIV programs at multiple levels, and has estimated that US$2.8 billion would be required to fund TB-HIV co-infection programs between 2011 and 2015 [17].
In 2011, the World Health Organization (WHO) and The International Union Against Tuberculosis and Lung Disease released a report acknowledging the association between TB and diabetes and similarly calling for increased collaboration between TB and diabetes control efforts [9]. In addition to providing provisional suggestions for implementing these changes, this report highlights the lack of evidence to guide such work and advocates for more research into the efficacy and cost-effectiveness of any collaborative efforts.
Although numerous etiological risk factors affect TB incidence and treatment outcomes, some, such as poverty, malnutrition, or indoor air pollution, would be exceedingly difficult for existing health systems or TB control programs to address for logistical reasons. Diabetes and HIV are similar in that treating these illnesses presents an opportunity for feasible collaboration with current TB control efforts, if sufficient funding can be found.
Funding Challenges
A major impediment to expanding diabetes care in the developing world is the high cost. One study in Tanzania showed that the monthly cost of insulin treatment represents 25% of the minimum wage [18], while another showed that the yearly cost of diabetes treatment with insulin amounted to 75% of the per capita income in Mozambique and 61% in Mali [19]. In addition to the expenses linked to insulin and oral hypoglycemic medications, the direct costs of diabetes care include outpatient visits, inpatient admissions, and the management of the micro - and macrovascular complications of this disease. While these direct costs vary according to country, they were estimated at US$138 per patient per year in Tanzania [20], and US$149 per patient per year in India [21], demonstrating a stark contrast with the mean health expenditures per person with diabetes in 2010, estimated at US$112 in sub-Saharan Africa (US$30 in Tanzania) and US$53 in South East Asia (US$55 in India) [22]. Access to diabetes services also varies greatly among developing countries, where limited health care systems are often designed to address acute, not chronic, illnesses [23]–[25]. Although the UN and other international organizations are demonstrating increasing interest in addressing non-communicable diseases (NCDs) such as diabetes, clearly more funding will be needed [26].
One option might be for the organizations that fund global TB control efforts to expand their missions to include diabetes care. These organizations already have a robust fundraising and operational infrastructure in place that could help to rapidly expand diabetes treatment in developing countries.
To calculate the amount of funding that these organizations would need to allocate to treat diabetes in TB patients in developing countries, we used the estimated direct costs of diabetes treatment in Tanzania and India and figures from a study assessing the yield of finding additional diabetes cases through systematic screening of patients with active TB [27]. We estimated the cost of treating all additional diabetes cases detected in TB patients for 6 months, which is the duration of Directly Observed Therapy – Short Course (DOTS). This cost was calculated based on the estimated TB incidence (Table 1) and the number of actual TB cases detected (Table 2) in the two WHO-defined regions with the highest burden of TB, South East Asia and Africa.
Tab. 1. Additional funding required for diabetes care in TB patients using estimated TB incidence in Africa and South East Asia. 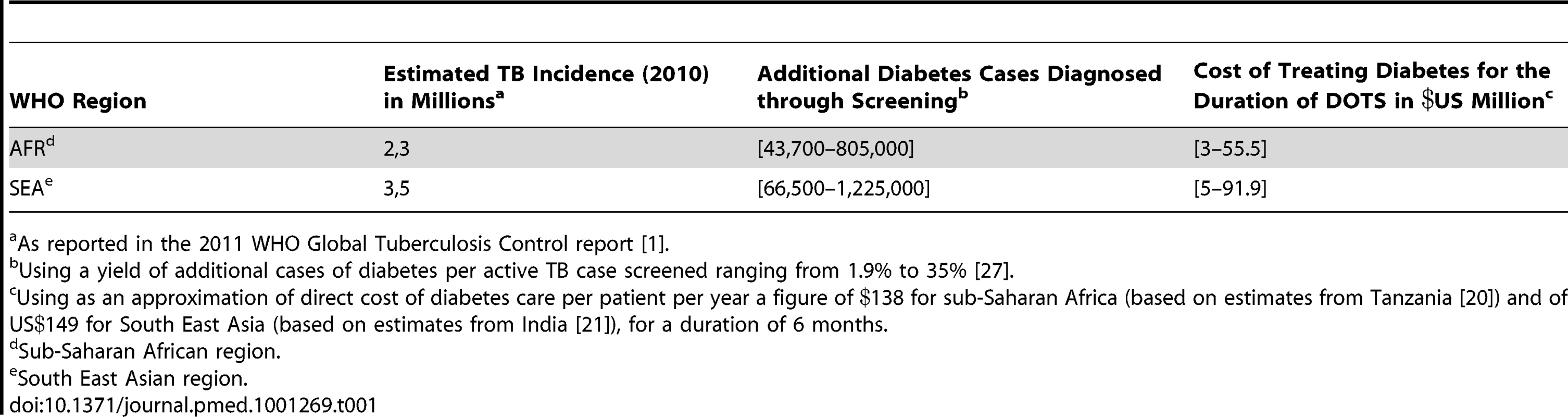
As reported in the 2011 WHO Global Tuberculosis Control report [1]. Tab. 2. Additional funding required for diabetes care in TB patients using actual number of TB cases diagnosed in Africa and South East Asia. 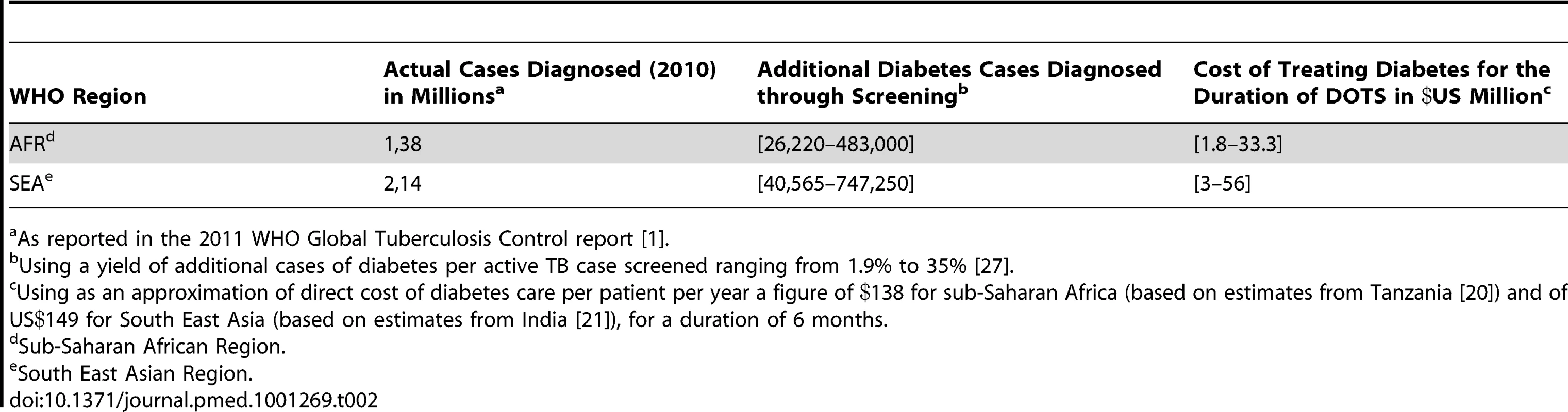
As reported in the 2011 WHO Global Tuberculosis Control report [1]. Using the estimated TB incidence (Table 1), the additional funding required for diabetes care ranged from US$3 million to US$55 million per year in Africa and from US$5 million to US$92 million per year in South East Asia. Based on actual numbers of TB cases detected (Table 2), we have estimated the additional funding for diabetes care to be US$2–33 million per year in Africa and US$3–56 million per year in South East Asia. The range in funding directly reflects the range in additional yield of diabetes cases, which was shown to vary from 1.9% to 35% [27].
As a point of comparison, the WHO reported the funding from domestic and donor sources for the 97 countries with 92% of the world's TB cases to amount to US$4.4 billion in 2012 (the funding gap reported by countries amounted to close to US$1 billion in 2012) [1], of which the contribution of the Global Fund to Fight AIDS, TB and Malaria accounts for 12% (or 82% of all international funding). The Global Fund has already provided US$3.2 billion in 112 countries to help fight TB. New grants allocated by the Global Fund might temporarily be in jeopardy due to its current financial situation [28]. If that predicament is solved, future TB-specific grants could include a diabetes co-management component to increase the score of TB proposals received. Similarly, the Global Plan to Stop TB has called for US$67 billion over 10 years to reduce the global burden of the disease, part of which could be specifically allocated to the co-management of the two diseases [17]. Whether these organizations will eventually help finance diabetes care in the future will ultimately be determined not only by the missions of these funds and their financial state, but also the expected benefit and cost-effectiveness of any new intervention. More research is surely needed to help inform these decisions.
Expanding TB treatment to include diabetes care would also create some foreseeable questions and challenges. Importantly, should the TB community pursue diabetes primary prevention in patients without active TB, since diabetes is clearly a risk factor for active TB, or should the focus be on treating diabetes in those who develop TB? And, if diabetes treatment is incorporated with TB care, who would fund the long-term management of diabetes in these patients after the TB is treated? Considering the current funding challenges, the co-management of the two diseases could initially be limited to patients with active TB while they are being treated for their TB, which served as reference for our funding estimates.
Opportunity for Collaboration
Although expanding TB treatment to include diabetes care would be expensive, perhaps existing health systems used for TB could be adapted to diabetes management, which may help control costs. For example, some have argued that DOTS, the framework already in place for TB control in many developing countries, could be modified to help with the management of NCDs like diabetes [29]. Although DOTS was originally designed to deliver a short course of TB treatment, a recent review has assessed that directly observed therapy can also be effective in the chronic management of HIV, particularly when targeting non-adherent patients [30]. Given that diabetes was shown to be effectively managed by non-physician clinicians in developing countries [31],[32], community health workers already in place to deliver TB care could be further trained to help manage diabetes as well.
In light of recent calls for increasing the presence of community health workers throughout the developing world [33], the training and implementation of this workforce could be another opportunity for those engaged in TB, HIV, and NCD efforts to work together. Future generations of community health workers could therefore be trained to manage both acute and chronic illnesses concurrently.
Another pillar of the DOTS framework is the creation of systems to insure a steady supply of TB medications. Diabetes patients would certainly benefit from the same logistics and commitment, as the supply of insulin has been shown to be unreliable in poor countries [34].
Yet another avenue for potential collaboration between TB and diabetes efforts is in the promotion of effective and affordable diagnostic testing. The DOTS framework includes a commitment to improving laboratory support, and the strengthening of laboratory systems has been identified as a major priority within the Global Plan to Stop TB [17]. Similarly, diagnostic testing for diabetes, including simple point-of-care blood glucose testing, is still lacking in many developing nations. A 2005 study reported that only 6% of health centers surveyed in Mozambique and 25% in Zambia were capable of testing blood glucose levels [35]. Although TB and diabetes diagnostics rely on very different technology, improving basic lab services in developing countries would undoubtedly benefit the diagnosis and management of both illnesses.
Our Proposed Strategy for Co-Management of TB and Diabetes
If the link between effective control of diabetes at the onset of TB treatment and improved outcomes among TB patients is clearly demonstrated by further studies, we propose that all confirmed TB patients be systematically screened for diabetes, and that all diabetic patients be screened for TB when symptomatic.
Co-afflicted patients without existing diabetes care should subsequently be managed by the relevant local or national TB program under DOTS for at least the duration of TB treatment to ensure proper and continuous management of both diseases. Although it would also be feasible to refer these patients to existing diabetes programs, these programs lack sufficient funding and infrastructure in many developing countries [25].
Expanding local TB programs to include diabetes care would necessitate the training of community health workers in basic diabetes care, such as blood glucose testing and medication management, as well as the consultation of nurses and physicians trained in diabetes management.
Following confirmed cure or completion of TB treatment, two options for ongoing diabetes care would be offered depending on the level of funding available to the TB control program:
-
Diabetic patients could be transferred to the diabetes control program, in countries where such programs exist, for continued management of their condition. As former TB patients, with a higher risk of relapse compared to non-diabetic patients, they would be required to return for regular TB screenings;
-
Diabetic patients would remain part of the DOTS structure after completion of TB treatment to ensure optimal follow-up and rapid detection of possible relapses.
The TB control programs should also develop educational materials to be distributed at diabetes treatment centers, to inform diabetic patients of their risk of developing active TB. More specifically, symptoms of TB (cough, fever, night sweats, weight loss, and chest pain) should be clearly highlighted to advise diabetic patients when to seek TB screening.
Looking Forward
Before TB funding or treatment resources are allocated for diabetes care, more research is needed to demonstrate unequivocally that controlling diabetes can reliably improve TB outcomes. Studies examining the effect of diabetes primary prevention and treatment on TB incidence and outcomes would be particularly helpful in informing how diabetes care could best be coordinated with existing TB programs, and in deciding if TB funding should be used for this purpose. In addition, cost-effectiveness studies will be necessary to determine if the scarce funding available for global TB efforts should be used for costly diabetes care. Perhaps further highlighting the link between these two diseases will help boost international fundraising efforts for both.
Diabetes and TB represent a critical intersection between communicable and non-communicable diseases in some of the world's poorest countries. As the prevalence of NCDs continues to rise, the effects of NCDs on the prevention and treatment of infectious diseases will likely become more evident. The proven detrimental effect of diabetes on TB incidence and outcomes raises some important questions about whether TB funding and infrastructure should be used for diabetes treatment in developing countries, while also revealing numerous opportunities for collaboration and progress in both patient care and research. The recent report on diabetes and TB from the WHO and The International Union Against Tuberculosis and Lung Disease indicates that international interest in this topic is rising. Even if the management of these complex illnesses cannot be readily integrated, it is clear that the growing burden of diabetes and its effect on TB in developing countries should not be ignored.
Zdroje
1. World Health Organization 2011 Global tuberculosis control 2011 Geneva WHO
2. LonnrothKJaramilloEWilliamsBGDyeCRaviglioneM 2009 Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med 68 2240 2246
3. RootHF 1934 The association of diabetes and tuberculosis. N Eng J Med 210 1 13
4. StevensonCRForouhiNGRoglicGWilliamsBGLauerJA 2007 Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health 7 234
5. JeonCYMurrayMB 2008 Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5 e152 doi:10.1371/journal.pmed.0050152
6. RestrepoBICamerlinAJRahbarMHWangWRestrepoMA 2011 Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull World Health Organ 89 352 359
7. DooleyKETangTGolubJEDormanSECroninW 2009 Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 80 634 639
8. AlisjahbanaBSahiratmadjaENelwanEJPurwaAMAhmadY 2007 The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 45 428 435
9. World Health Organization and The International Union Against Tuberculosis and Lung Disease 2011 Collaborative framework for care and control of tuberculosis and diabetes Geneva WHO
10. HarriesADMurrayMBJeonCYOttmaniSELonnrothK 2010 Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health 15 659 663
11. DooleyKEChaissonRE 2009 Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9 737 746
12. BakerMAHarriesADJeonCYHartJEKapurA 2011 The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 9 81
13. HossainPKawarBEl NahasM 2007 Obesity and diabetes in the developing world–a growing challenge. N Engl J Med 356 213 215
14. WildSRoglicGGreenASicreeRKingH 2004 Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27 1047 1053
15. StucklerDBasuSMcKeeM 2010 Drivers of inequality in Millennium Development Goal progress: a statistical analysis. PLoS Med 7 e1000241 doi:10.1371/journal.pmed.1000241
16. Ponce-De-LeonAGarcia-Garcia Md MdeLGarcia-SanchoMCGomez-PerezFJValdespino-GomezJL 2004 Tuberculosis and diabetes in southern Mexico. Diabetes Care 27 1584 1590
17. World Health Organization The global plan to stop TB 2011–2015: transforming the fight towards elimination of tuberculosis Geneva WHO Press
18. NeuhannHFWarter-NeuhannCLyaruuIMsuyaL 2002 Diabetes care in Kilimanjaro region: clinical presentation and problems of patients of the diabetes clinic at the regional referral hospital-an inventory before structured intervention. Diabet Med 19 509 513
19. BeranDYudkinJS 2010 Looking beyond the issue of access to insulin: what is needed for proper diabetes care in resource poor settings. Diabetes Res Clin Pract 88 217 221
20. ChaleSSSwaiABMujinjaPGMcLartyDG 1992 Must diabetes be a fatal disease in Africa? Study of costs of treatment. BMJ 304 1215 1218
21. KapurA 2007 Economic analysis of diabetes care. Indian J Med Res 125 473 482
22. ZhangPZhangXBrownJVistisenDSicreeR 2010 Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 87 293 301
23. BeranDYudkinJS 2006 Diabetes care in sub-Saharan Africa. Lancet 368 1689 1695
24. LevittNS 2008 Diabetes in Africa: epidemiology, management and healthcare challenges. Heart 94 1376 1382
25. WhitingDRHayesLUnwinNC 2003 Diabetes in Africa. Challenges to health care for diabetes in Africa. J Cardiovasc Risk 10 103 110
26. SachsJ 2010 The MDG decade: looking back and conditional optimism for 2015. Lancet 376 950 951
27. JeonCYHarriesADBakerMAHartJEKapurA 2010 Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health 15 1300 1314
28. [No authors listed] 2011 The Global Fund and a new modus operandi. Lancet 378 1896
29. HarriesADJahnAZachariahREnarsonD 2008 Adapting the DOTS framework for tuberculosis control to the management of non-communicable diseases in sub-Saharan Africa. PLoS Med 5 e124 doi:10.1371/journal.pmed.0050124
30. HartJEJeonCYIversLCBehforouzHLCaldasA 2010 Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr 54 167 179
31. ColemanRGillGWilkinsonD 1998 Noncommunicable disease management in resource-poor settings: a primary care model from rural South Africa. Bull World Health Organ 76 633 640
32. LabhardtNDBaloJRNdamMGrimmJJMangaE 2010 Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res 10 339
33. The Earth Institute 2011 One million community health workers: technical task force report New York The Earth Institute, Columbia University
34. YudkinJS 2000 Insulin for the world's poorest countries. Lancet 355 919 921
35. BeranDYudkinJSde CourtenM 2005 Access to care for patients with insulin-requiring diabetes in developing countries: case studies of Mozambique and Zambia. Diabetes Care 28 2136 2140
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



