-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
Background:
The lack of association found in several cohort studies between dietary saturated fat and coronary heart disease (CHD) risk has renewed debate over the link between dietary fats and CHD.Methods and Findings:
We assessed the relationship between plasma phospholipid fatty acid (PFA) concentration and incident CHD using a nested case control design within a prospective study (EPIC-Norfolk) of 25,639 individuals aged 40–79 years examined in 1993–1997 and followed up to 2009. Plasma PFA concentrations were measured by gas chromatography in baseline samples retrieved from frozen storage. In 2,424 men and women with incident CHD compared with 4,930 controls alive and free of cardiovascular disease, mean follow-up 13 years, saturated PFA (14 : 0, 16 : 0,18 : 0) plasma concentrations were significantly associated with increased CHD risk (odds ratio [OR] 1.75, 95% CI 1.27–2.41, p<0.0001), in top compared to bottom quartiles (Q), and omega-6 polyunsaturated PFA concentrations were inversely related (OR 0.77, 0.60–0.99, p<0.05) after adjusting for age, sex, body mass index, blood pressure, smoking, alcohol intake, plasma vitamin C, social class, education, and other PFAs. Monounsaturated PFA, omega-3 PFA, and trans PFA concentrations were not significantly associated with CHD. Odd chain PFA (15 : 0, 17 : 0) concentrations were significantly inversely associated with CHD (OR 0.73, 0.59–0.91, p<0.001, Q4 versus Q1). Within families of saturated PFA or polyunsaturated PFA, significantly heterogeneous relationships with CHD were observed for individual fatty acids.Conclusions:
In this study, plasma concentrations of even chain saturated PFA were found to be positively and omega-6 polyunsaturated PFA inversely related to subsequent coronary heart disease risk. These findings are consistent with accumulating evidence suggesting a protective role of omega-6 fats substituting for saturated fats for CHD prevention.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001255
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001255Summary
Background:
The lack of association found in several cohort studies between dietary saturated fat and coronary heart disease (CHD) risk has renewed debate over the link between dietary fats and CHD.Methods and Findings:
We assessed the relationship between plasma phospholipid fatty acid (PFA) concentration and incident CHD using a nested case control design within a prospective study (EPIC-Norfolk) of 25,639 individuals aged 40–79 years examined in 1993–1997 and followed up to 2009. Plasma PFA concentrations were measured by gas chromatography in baseline samples retrieved from frozen storage. In 2,424 men and women with incident CHD compared with 4,930 controls alive and free of cardiovascular disease, mean follow-up 13 years, saturated PFA (14 : 0, 16 : 0,18 : 0) plasma concentrations were significantly associated with increased CHD risk (odds ratio [OR] 1.75, 95% CI 1.27–2.41, p<0.0001), in top compared to bottom quartiles (Q), and omega-6 polyunsaturated PFA concentrations were inversely related (OR 0.77, 0.60–0.99, p<0.05) after adjusting for age, sex, body mass index, blood pressure, smoking, alcohol intake, plasma vitamin C, social class, education, and other PFAs. Monounsaturated PFA, omega-3 PFA, and trans PFA concentrations were not significantly associated with CHD. Odd chain PFA (15 : 0, 17 : 0) concentrations were significantly inversely associated with CHD (OR 0.73, 0.59–0.91, p<0.001, Q4 versus Q1). Within families of saturated PFA or polyunsaturated PFA, significantly heterogeneous relationships with CHD were observed for individual fatty acids.Conclusions:
In this study, plasma concentrations of even chain saturated PFA were found to be positively and omega-6 polyunsaturated PFA inversely related to subsequent coronary heart disease risk. These findings are consistent with accumulating evidence suggesting a protective role of omega-6 fats substituting for saturated fats for CHD prevention.
: Please see later in the article for the Editors' SummaryIntroduction
Dietary fat intake has long been implicated in the aetiology of coronary heart disease (CHD). The adverse relationship of transfats with CHD is widely accepted [1] such that recommendations and legislation controlling use of transfats are widespread.
A meta-analysis of prospective cohort studies concluding that there is no significant evidence that dietary saturated fat is associated with increased risk of cardiovascular disease has renewed debate over fat and CHD [2],[3]. Randomized trials are not always conclusive. The Women's Health Initiative (WHI) found no difference in cardiovascular outcomes in women randomized to a low fat diet compared to controls [4]. However, though the intervention group lowered total fat intake, the polyunsaturated fat/saturated fat ratio in the WHI trial was unchanged. It is notable that trials reporting significant differences in cardiovascular outcomes altered dietary fatty acid (FA) composition such as unsaturated/saturated fat ratios rather than simply lowering total fat intakes [5]–[7].
Observational studies using self-reported dietary instruments have limitations accurately assessing intake of different fats. Blood FA profiling may provide a more objective and accurate biomarker of FA intake [8]–[13]. Studies to date examining the prospective relationship between blood FA concentrations and CHD have had limited numbers of events, and few reported the whole range of FAs [14]–[23].
We tested the hypothesis that high saturated FA and low polyunsaturated plasma phospholipid fatty acid (PFA) status increase CHD risk in a prospective population study and explored the relationship of individual FAs with CHD.
Methods
The European Prospective Investigation into Cancer (EPIC)-Norfolk is a prospective study of 25,639 men and women aged 40–79 years in Norfolk, UK similar in characteristics to UK general population samples, who participated in a baseline survey in 1993–1997 [24]. Participants completed a health and lifestyle questionnaire including data on medical history, smoking, alcohol intake, physical activity, social class, and education [25] and attended a clinic for a health examination. Body mass index (BMI) was calculated as weight (kilograms) divided by height (metres) squared. Blood pressure was measured using an Accutorr non-invasive blood pressure monitor. Blood samples were spun, separated into 0.5 ml fractions of serum and citrated plasma, placed in straws, sealed, and stored in liquid nitrogen. Fresh samples were assayed for vitamin C and lipids [26].
Measurement of Plasma Phospholipid FA Concentrations
Funding was obtained for blood FA analyses in 2003–2008. Selection of participants for analyses was based on a series of nested case control studies with incident cases of cancers and cardiovascular disease and up to four disease-free controls for each case. This selection totalled about 10,000 individuals representing two-fifths of the cohort, enriched for incident diseases. Analyses on 8,000 samples were carried out in the WHO International Agency for Cancer Research laboratories, Lyon, France [27]. Because of laboratory constraints in Lyon, an additional 2,000 samples were analysed in Quotient Laboratories, UK, using the same methods and quality control standards.
Citrated plasma straws retrieved from storage were thawed, di-palmitoyl-D31-phosphatidylcholine Sigma) internal standard was added to each plasma sample, total lipids extracted, and purified by adsorption chromatography (LC-Si SPE, Supelco/Sigma). Plasma phospholipids were analysed by gas chromatography. Concentrations were measured by comparison of peak areas of individual FAs with the peak area of the internal standard using individual calibration curves. The method allowed for the analysis of individual FAs belonging to six classes of FAs. We analysed data for 22 FAs: saturated even chain fatty acid (SFA) (14 : 0, 16 : 0, 18 : 0); odd chain FA (15 : 0, 17 : 0); omega-6 polyunsaturated, n-6 polyunsaturated fatty acid (PUFA) (18 : 2n-6, 18 : 3n-6, 20 : 2n-6, 20 : 3n-6, 20 : 4n-6, 22 : 4n-6, and 22 : 5n-6); omega-3 polyunsaturated, n-3 PUFA (18 : 3n-3, 20 : 5n-3, 22 : 5n-3, 22 : 6n-3); mono-unsaturated fatty acid (MUFA) (16 : 1n-7, 18 : 1n-7cis, 18 : 1n-9cis, 20 : 1n-9); and trans-fatty acid (16 : 1n9trans, 18 : 1n9trans). The methods are detailed in Text S1.
Ascertainment of Coronary Heart Disease Events
All participants are flagged for death certification with the National Health Service (NHS) Central Register, UK with death certificates coded by nosologists according to the International Classification of Disease (ICD). CHD death was defined using ICD9 410–414 or ICD10 I22–I25 as underlying cause of death. Data linkage with the East Norfolk Health Authority database identifies all hospital contacts for participants using their NHS number. We used the ICD diagnostic codes listed to ascertain hospital episodes for CHD. Participants were identified as having a CHD event during follow-up if they had a hospital admission and/or died with CHD as cause of death, with clinical validation through medical record inspection of a sample [28]. We ascertained fatal and non-fatal CHD events to December 2009.
Selection of Cases and Controls
From all the individuals with available measurement of plasma phospholipid FAs, we identified 2,424 individuals who had incident CHD and 4,930 control individuals alive and free of known cardiovascular disease during follow-up to 2009.
Data Analysis
FAs were grouped into six families a priori: even chain SFA, odd chain FA, n-6 PUFA, n-3 PUFA, MUFA, and trans-fatty acid. Analyses used SPSS Version 17.0. We compared baseline characteristics in individuals with incident CHD and controls. We examined the odds ratios for CHD by quartile of concentration of FA families adjusting first for age and sex, then in multivariate models adjusting for all the other FA families, then additionally adjusting for covariates: BMI, physical activity, cigarette smoking habit, alcohol intake, social class, education, plasma vitamin C (used as a biomarker of fruit and vegetable intake [26]), personal history of diabetes, and systolic blood pressure then finally additionally adjusting for blood cholesterol. We repeated the analyses using FAs categorised using mol%
We then examined the odds ratios for CHD with individual FAs. In these models we used each individual FA as a continuous variable, adjusting for all the other individual acids as continuous variables. To enable comparisons, the interval used to estimate the odds ratio was approximately one standard deviation (SD) for each FA. All models were age and sex adjusted.
Trans-fatty acids were at very low concentrations near the lower limit of determination. Quality controlled results were only available from the laboratory for 1,979 cases and 3,995 controls. We analysed trans-fatty acid data two ways: coding missing data as missing or as zero on the basis that these were extremely low values. Since findings were similar using either approach, we present results for the more conservative approach of analysing the data as missing.
Results
Table 1 shows baseline characteristics of the 2,434 individuals with incident CHD (633 fatal) and 4,930 controls. Those who developed CHD compared to controls were older, had higher blood cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and blood pressure levels and lower high-density lipoprotein (HDL) cholesterol. They were more likely to have diabetes, be smokers and physically inactive, and have lower plasma vitamin C concentrations and lower mean alcohol intake. Quantitatively, the most abundant phospholipid FAs were palmitic (16 : 0), linoleic (18 : 2n-6), stearic (18 : 0), and arachidonic acid (AA) (20 : 4n-6). Plasma concentrations of phospholipid trans-fatty acid contributed less than 0.2% of the total.
Tab. 1. Baseline characteristics of men and women with incident CHD and controls EPIC-Norfolk 1993–2009. 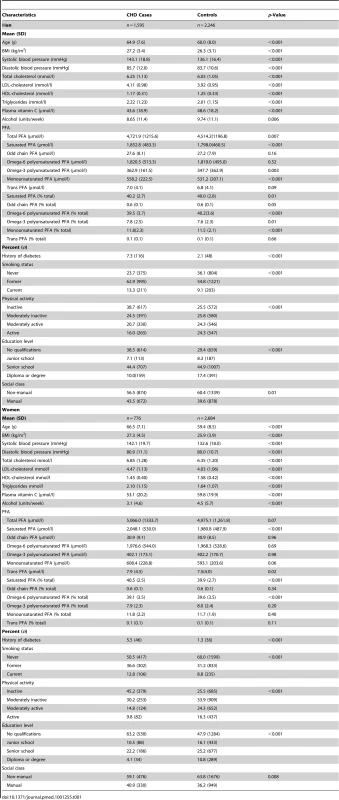
Table 2 shows characteristics of the participants by quartile (Q) of plasma phospholipid SFA. Plasma phospholipid SFA concentrations correlated with the other FA concentrations as well as age, blood pressure, and lipids. A positive relationship was seen with alcohol intake, but no consistent relationships with smoking habit, physical activity, diabetes history, and social class.
Tab. 2. Baseline characteristics of participants by quartile of plasma saturated PFA concentration. 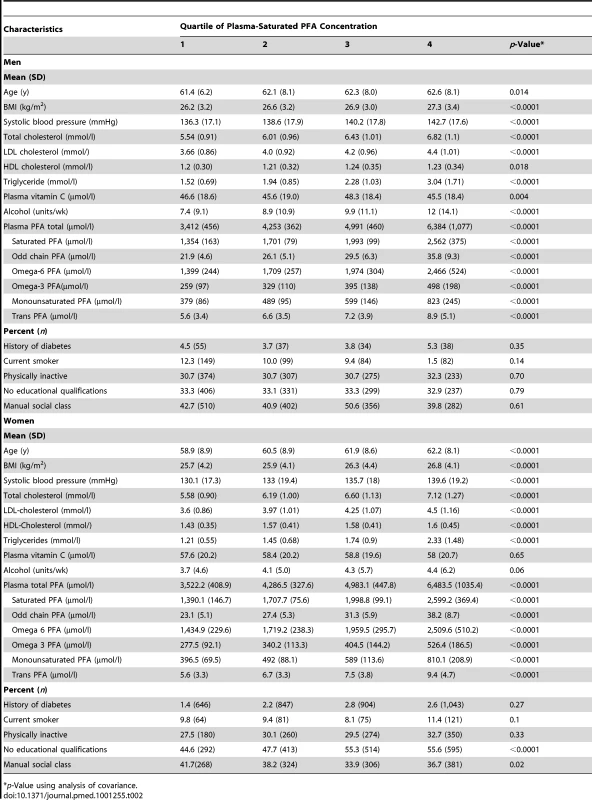
p-Value using analysis of covariance. Table 3 shows odds ratios for CHD by quartile of plasma phospholipid FA concentration using different multivariate models. The final column shows the odds ratio for CHD using FA concentrations as continuous variables, per approximate SD increase. Table 4 shows similar analyses using FA mol% quartiles.
Tab. 3. Odds ratios for CHD in men and women, EPIC-Norfolk 1993–2009 by quartile of plasma PFA concentration, age and sex adjusted, and multivariate adjusted and as a continuous variable, per approximate SD increase. 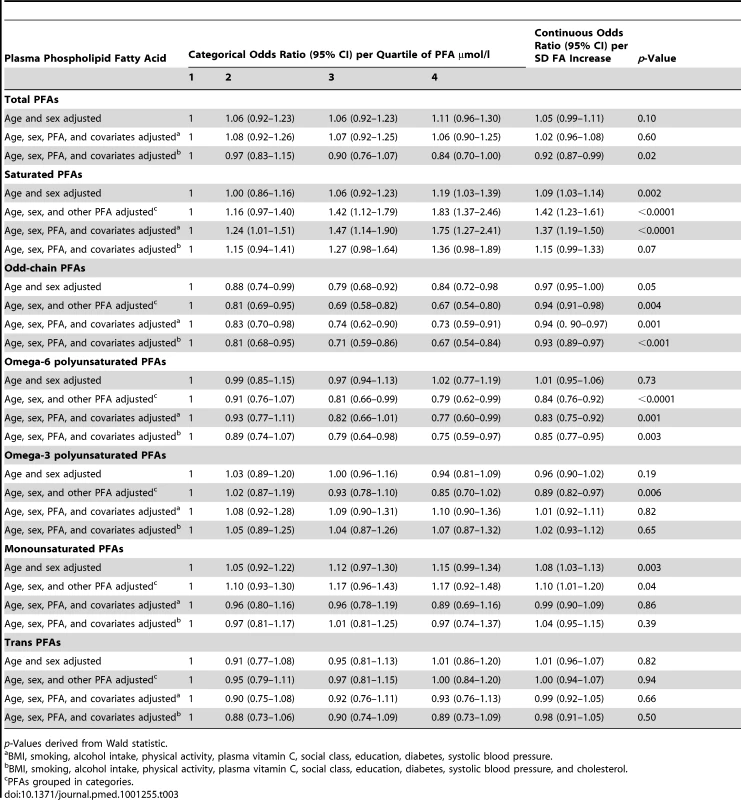
p-Values derived from Wald statistic. Tab. 4. Odds ratios for CHD in men and women, EPIC-Norfolk 1993–2009 by quartile of plasma PFA mol%, age and sex adjusted, and multivariate adjusted and as a continuous variable, per approximate standard deviation increase. 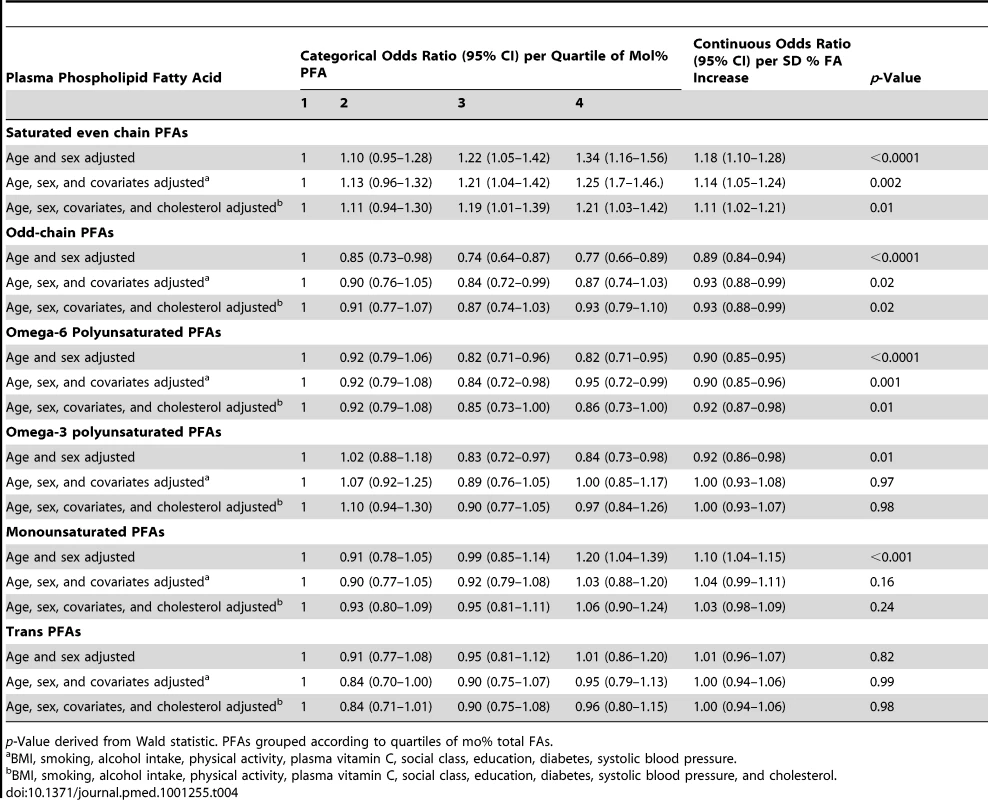
p-Value derived from Wald statistic. PFAs grouped according to quartiles of mo% total FAs. There was no overall significant relationship between total plasma phospholipid FA concentration and CHD. The weak but significantly positive association of CHD with plasma phospholipid SFA concentration increased in strength and significance after adjusting for other FA concentrations, with an odds ratio of 1.83 (95% CI 1.37–2.46, p<0.0001) in Q4 versus Q1 and a gradient of increasing risk throughout the distribution. This association hardly changed after multivariate adjustment apart from substantial attenuation after adjustment for cholesterol indicating much of the association between SFA and CHD was likely to be mediated through blood cholesterol levels.
In contrast, plasma phospholipid odd chain FA concentrations were inversely associated with lower CHD risk in all models. The odds ratio for Q4 versus Q1 was 0.67 (95% CI 0.54–0.80).
Plasma phospholipid n-6 PUFA concentrations were not significantly related to risk of CHD in the simple age - and sex-adjusted models, but after adjusting for other FAs, the relationship was significantly inverse. The odds ratio for CHD for Q4 versus Q1 was 0.84 (95% CI 0.76–0.92, p<0.0001).
Plasma phospholipid n-3 PUFA concentrations inversely related to CHD after adjusting for other FAs, but this was no longer significant after adjusting for other covariates. Plasma phospholipid MUFA concentrations showed a positive association with CHD before and after adjusting for other FAs though this relationship did not remain significant after adjusting for other covariates. Plasma phospholipid trans-fatty acid concentrations were not significantly associated with CHD.
Analyses using FA mol% rather than concentrations (Table 4) showed similar results with plasma phospholipid SFA positively and n-6 PUFA inversely significantly related to CHD.
Relationships were consistent in men and women in sex-stratified analyses (unpublished data) and also after further adjustment for dietary total energy, protein, carbohydrate, and fibre assessed using food frequency questionnaires (Text S2).
Table 5 shows mean plasma concentrations for individual phospholipid FAs.
Tab. 5. Mean plasma concentrations of individual PFAs in men and women, EPIC-Norfolk and age- and sex-adjusted odds ratios for CHD. 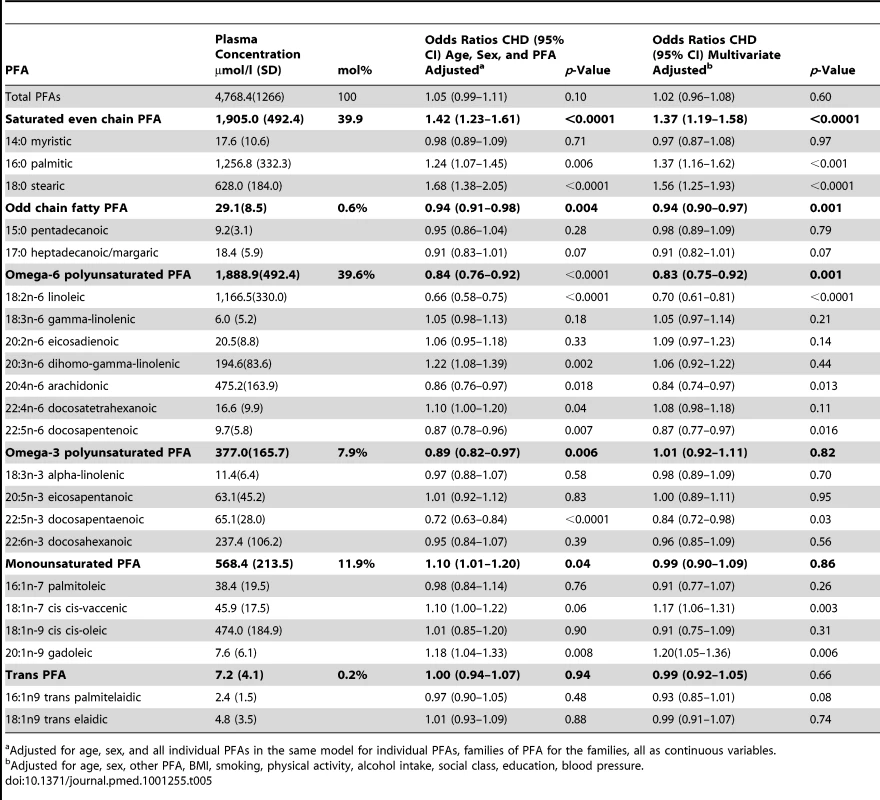
Adjusted for age, sex, and all individual PFAs in the same model for individual PFAs, families of PFA for the families, all as continuous variables. Table 5 also shows odds ratios for CHD for each individual FA, adjusting for age and sex and the other individual FAs listed, as continuous variables and then with multivariate adjustment excluding cholesterol. Odds ratios are shown per approximate SD increase in plasma concentration of the relevant phospholipid FA.
Stearic acid (18 : 0) concentration was most strongly positively related to CHD (OR 1.68, 1.38–2.05, p<0.0001) followed by palmitic acid (16 : 0) concentration (OR 1.24, 1.07–1.45, p = 0.006). There was no observed association with myristic acid (14 : 0) concentration (OR 0.98, 0.89–1.09).
Individual plasma phospholipid n-6 PUFA concentrations showed marked heterogeneity in the relationship with CHD. Linoleic acid (18 : 2n-6) was most strongly inversely related, OR 0.66 (0.58–0.75, p<0.001). Inverse associations were also observed for AA (20 : 4n-6), OR 0.86 (0.76–0.97, p = 0.02), and docosapentenoic acid (22 : 5n-6) OR 0.87 (0.78–0.96, p = 0.007). However, di-homo-gamma-linolenic acid (DGLA) (20 : 3n-6) positively related to CHD OR 1.22 (1.08–1.32, p = 0.002).
Of the n-3 PUFAs, only plasma phospholipid docosapentaenoic acid (22 : 5n-3) concentration was significantly inversely associated with CHD OR 0.72 (0.63 01500.84, p<0.00001). The main monounsaturated FA, cis-oleic was not related to CHD (OR 1.01, 0.85–1.20). Plasma phospholipid concentration of gadoleic acid (20 : 1n-9) was significantly positively related to CHD (OR 1.18,1.04–1.33, p = 0.008).
Plasma phospholipid concentrations of the two most common trans-fatty acids measured: palmitelaidic (16 : 1n9 trans) and elaidic (18 : 1n9 trans) were not related to CHD at these very low concentrations.
Discussion
There was no overall association between total plasma phospholipid FA concentration and CHD and only weak associations were observed for each family of FAs considered in isolation. However, when other FAs were taken into account, plasma phospholipid SFA concentrations strongly positively and n-6 PUFA concentrations inversely related to subsequent risk of incident CHD. For plasma phospholipid SFA concentrations, those in Q4 versus Q1 had approximately 75% higher CHD risk, and for n-6 PUFA concentrations, 25% lower risk in Q4 versus Q1. In contrast, plasma odd chain phospholipid FA concentrations were inversely related to CHD with a lower risk of about 30% in Q4 versus Q1. These relationships were independent of potential confounders including age, BMI, smoking, alcohol intake, physical activity, plasma vitamin C, history of diabetes, social class, education, as well as systolic blood pressure. The relationship between plasma phospholipid SFA concentration and CHD was attenuated after adjusting for blood cholesterol concentrations, indicating that at least some of the relationship could be mediated through lipid concentrations. Plasma phospholipid N-3 PUFA concentrations inversely related to CHD after adjusting for other FAs, but this relationship was no longer apparent after multivariate adjustment. There were no overall relationships with plasma phospholipid MUFA and trans-fatty acid concentrations. Results were consistent using FA mol%.
Observational and intervention studies indicate that blood FA profiles may be biomarkers for dietary FA intake though dietary associations are stronger for the FAs such as linoleic acid and alpha linolenic acid intake, which humans cannot produce [8],[10],[13].
In this study, a deuterium-labelled phospholipid internal standard allowed accurate determination of individual phospholipid FA plasma concentrations. Previous prospective studies examining individual FAs and CHD used percent composition of FAs in plasma rather than plasma concentration and not all measured the full range of FAs necessary to determine correctly the denominator [14],[15],[21].
Nevertheless, several such studies reported positive relationships between plasma phospholipid SFA composition and CHD, inverse relationships between plasma phospholipid PUFA composition, and no overall relationship with MUFA composition, consistent with our findings [14],[19],[21]. The consistent positive associations of SFA in cohorts using biomarker FA profiling is in marked contrast to reports using dietary assessments of saturated fat intake and CHD [2].
The lack of association between dietary saturated fat intake and CHD in population studies is unsurprising given the large measurement errors in dietary assessment of fat intake through recall errors, ubiquity of fats leading to quantification difficulties, and critically, huge variability in FA composition of foods such that discrimination between different fats is problematic.
Different types of fat using the conventional groupings of SFA, n-6 and n-3 PUFA, MUFA and trans-fatty acid, have varying biological and health effects. Though it is generally believed that saturated and transfats are adversely and unsaturated fats beneficially related to CHD risk [1],[7], the balance between the different fats may be more important than any single group alone. Keys noted the saturated/unsaturated fat ratio was critical for predicting cholesterol levels and CHD [29]. In the current study, we found no overall relationship between total FAs and CHD. Trials substituting unsaturated fat for saturated fat, altering the ratios have reported more consistently reduction in cardiovascular disease [6],[7], indicating that the balance between different fats is crucial.
Additionally even within these families, increasing evidence indicates individual FAs have different metabolic and health effects. Such heterogeneity in the relationship between individual SFAs or n-6 PUFAs and CHD noted in several prospective studies may explain variable associations of total SFAs and n-6 PUFA with CHD in different populations depending on the distribution of individual FAs.
In this study, stearic acid (18 : 0) plasma phospholipid concentration was the SFA most strongly positively related to CHD, followed by palmitic acid (16 : 0) concentration. Myristic acid (14 : 0) was not related to CHD risk. Of the n-3 PUFAs, only docosapentaenoic acid, (22 : 5n-3) plasma phospholipid concentration inversely related to CHD. This is not surprising as n-3PUFAs have primarily been associated with arrhythmic cardiac death [30].
Of the n-6PUFAs, the plasma phospholipid concentration of linoleic acid (18 : 2n-6) and AA (20 : 4n-6) inversely related to CHD, but DGLA (20 : 3n-6) positively related to CHD.
The n-6 PUFA AA (22 : 4n6) is the substrate for series 2 thromboxanes and prostanoids metabolites believed to be proinflammatory and thrombotic, in contrast to DGLA (22 : 3n6) for which the eicosanoid metabolites, series 1 thromboxanes and prostanoids are thought to be anti-inflammatory and antithrombotic [31]. DGLA is therefore believed to be beneficial for CHD in contrast to AA. However, we observed the converse, a positive relationship between DGLA and negative relationship between AA and CHD, unexpected findings also noted by other prospective studies [14],[18],[19],[21], including the ARIC study (282 CHD events) [21], a nested case control study within the Multiple Risk Factor Intervention Trial (94 CHD cases, 94 controls) [19], a Swedish cohort (153 CHD events) [18], and the Whitehall study (116 events) [14]. The Swedish investigators hypothesised their observed inverse relationship between AA/DGLA ratio and CHD reflected activity of delta5-desaturase enzyme [18].
Results for odd chain FAs (15 : 0; 17 : 0) require comment. Though often included with SFAs, they are metabolised differently from even chain SFAs. The 15 : 0 and 17 : 0 FAs are ruminant specific and suggested to be biomarkers of milk or dairy intake [12]. Studies reporting on odd chain FAs have inconsistent results. The Nurses Health Study (166 cases, 327 controls) reported an odds ratio of 2.36 for CHD between top and bottom tertiles of odd chain plasma FA composition [20]. In contrast, a Swedish cohort (446 cases, 558 controls) reported a standardized OR for CHD of 0.76 in women and 0.91 in men [23]. We found an odds ratio of 0.67 in Q4 versus Q1 for odd chain FAs. Despite the contribution of dairy produce to saturated FA composition of the diet, a recent reappraisal concluded there is no consistent evidence that dairy food consumption is associated with a higher risk of cardiovascular disease [32]. Milk or dairy consumption has been reported to be associated with lower risk of metabolic syndrome [33], heart disease [34], and hypertension [35], and the DASH trial reported lower blood pressure with a dietary intervention including low fat milk [36]. These associations need further exploration and confirmation.
The study has limitations. Although we conducted a large number of statistical tests, the analyses for the main FA families were designed using a priori hypotheses. Despite not being in line with the putative biological effects of their metabolites, the AA and DGLA results are consistent with previous studies in different settings in the US and Europe. The results for odd chain FAs have some previous support, but will need confirmation in future studies.
We only measured the two most common trans-fatty acids (16 : 1n9trans, 18 : 1n9trans). Though we were unable to assess the 18 : 2 transisomers associated with CHD in other studies [37], at the very low concentrations in a range more typical of European populations [11], there was little power to assess the trans-fatty acid–CHD relationship. We controlled for the major lifestyle and demographic factors related to CHD risk but cannot exclude residual confounding with other unknown factors.
We used only plasma FAs measured one point in time to characterise individuals. There is likely to be large intraindividual variation in FAs but such random measurement error is likely to attenuate any relationships, rather than produce spurious associations.
Future studies examining the relationship between FAs and CHD need to consider heterogeneity in the biological and health effects of individual FAs as well as the overall FA profile and balance between FAs. The ability to assess these associations in human populations has been constrained by the limitations of assessing dietary fat intake. Biochemical measures offer more objective and specific biomarkers, which may provide greater insight into exogenous dietary factors as well as endogenous metabolic processes which influence risk of chronic diseases.
These results indicate that high plasma phospholipid SFA and low PUFA (predominantly n-6 FAs) are associated with increased CHD risk but neither can be considered in isolation. It is beyond the remit of the current study to quantify in absolute amounts the relationship between blood FA concentrations and dietary intake of foods and nutrients, It is not clear how far the associations reflect complex interactions between dietary fat intake per se and FA metabolism, which may have both genetic and other exogenous influences. Nevertheless, the major sources of dietary n-6 PUFA, i.e., linoleic acid in most western populations, are vegetable oils such as sunflower oil, corn oil, soybean oil, and canola oil, and nuts and seeds.
While dietary recommendations should focus on general patterns of food intake rather than individual nutrients [38], recommendations still need to be based on knowledge of the biological roles of different nutrients and balance between different nutrients in foods. Just as recommendations now acknowledge differences in health effects of different families of fats, so they should evolve to reflect increasingly discriminating understanding of individual FA metabolism, and their interactions. Early guidelines to prevent CHD recommended reductions in saturated fat but little consistency as to what might be substituted: other fats, protein, or carbohydrate. Our results add to the accumulating evidence that substitution of saturated fat by n-6 polyunsaturated fat may have more CHD benefits [33],[39],[40].
Supporting Information
Zdroje
1. MozaffarianDAroAWillettWC 2009 Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 63 S5 21
2. Siri-TarinoPWSunQHuFBKraussRM 2010 Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 91 535 546
3. StamlerJ 2010 Diet-heart: a problematic revisit. Am J Clin Nutr 91 497 499
4. HowardBVVan HornLHsiaJMansonJEStefanickML 2006 Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 295 655 666
5. de LorgerilMRenaudSMamelleNSalenPMartinJL 1994 Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343 1454 1459
6. MozaffarianDMichaRWallaceS 2010 Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7 e1000252 doi:10.1371/journal.pmed.1000252
7. SkeaffCMMillerJ 2009 Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab 55 173 201
8. CantwellMM 2000 Assessment of individual fatty acid intake. Proc Nutr Soc 59 187 191
9. HodsonLSkeaffCMFieldingBA 2008 Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47 348 380
10. PoppittSDKilmartinPButlerPKeoghGF 2005 Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis 4 30
11. Saadatian-ElahiMSlimaniNChajesVJenabMGoudableJ 2009 Plasma phospholipid fatty acid profiles and their association with food intakes: results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 89 331 346
12. WolkAFuruheimMVessbyB 2001 Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr 131 828 833
13. ZockPLMensinkRPHarryvanJde VriesJHKatanMB 1997 Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am J Epidemiol 145 1114 1122
14. ClarkeRShipleyMArmitageJCollinsRHarrisW 2009 Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br J Nutr 102 279 284
15. GuallarEHennekensCHSacksFMWillettWCStampferMJ 1995 A prospective study of plasma fish oil levels and incidence of myocardial infarction in U.S. male physicians. J Am Coll Cardiol 25 387 394
16. LaaksonenDENyyssonenKNiskanenLRissanenTHSalonenJT 2005 Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 165 193 199
17. LemaitreRNKingIBMozaffarianDKullerLHTracyRP 2003 n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr 77 319 325
18. OhrvallMBerglundLSalminenILithellHAroA 1996 The serum cholesterol ester fatty acid composition but not the serum concentration of alpha tocopherol predicts the development of myocardial infarction in 50-year-old men: 19 years follow-up. Atherosclerosis 127 65 71
19. SimonJAHodgkinsMLBrownerWSNeuhausJMBernertJTJr 1995 Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol 142 469 476
20. SunQMaJCamposHHuFB 2007 Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 86 929 937
21. WangIFolsomAREckfeldtJH 2003 Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nut Metab Cardiovasc Dis 13 256 266
22. WarensjoESundstromJVessbyBCederholmTRiserusU 2008 Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 88 203 209
23. WarensjoEJanssonJHCederholmTBomanKEliassonM 2010 Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 92 194 202
24. DayNOakesSLubenRKhawKTBinghamS 1999 EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 80 95 103
25. KhawKTJakesRBinghamSWelchALubenR 2006 Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: The European Prospective Investigation into Cancer in Norfolk prospective population study. Int J Epidemiol 35 1034 1043
26. KhawKTBinghamSWelchALubenRWarehamN 2001 Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 357 657 663
27. Saadatian-ElahiMNoratTBueno-de-MesquitaHBClavelFGonzalezCA 2002 Plasma concentrations of fatty acids in nine European countries: cross-sectional study within the European Prospective Investigation into Cancer and Nutrition (EPIC). IARC Sci Publ 156 215 218
28. BoekholdtSMKuivenhovenJAWarehamNJPetersRJJukemaJW 2004 Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation 110 1418 1423
29. KeysAMenottiAKarvonenMJAravanisCBlackburnH 1986 The diet and 15-year death rate in the seven countries study. Am J Epidemiol 124 903 915
30. SiscovickDSLemaitreRNMozaffarianD 2003 The fish story: a diet-heart hypothesis with clinical implications: n-3 polyunsaturated fatty acids, myocardial vulnerability, and sudden death. Circulation 107 2632 2634
31. HarrisWSMozaffarianDRimmEKris-EthertonPRudelLL 2009 Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119 902 907
32. GermanJBGibsonRAKraussRMNestelPLamarcheB 2009 A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur J Nutr 48 191 203
33. PereiraMAJacobsDRJrVan HornLSlatteryMLKartashovAI 2002 Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA 287 2081 2089
34. ElwoodPCPickeringJEHughesJFehilyAMNessAR 2004 Milk drinking, ischaemic heart disease and ischaemic stroke II. Evidence from cohort studies. Eur J Clin Nutr 58 718 724
35. WangLMansonJEBuringJELeeIMSessoHD 2008 Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 51 1073 1079
36. AppelLJMooreTJObarzanekEVollmerWMSvetkeyLP 1997 A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336 1117 1124
37. LemaitreRNKingIBMozaffarianDSotoodehniaNReaTD 2006 Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation 114 209 215
38. MozaffarianDLudwigDS 2010 Dietary guidelines in the 21st century–a time for food. JAMA 304 681 682
39. Siri-TarinoPWSunQHuFBKraussRM 2010 Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 91 502 509
40. SkeaffCM 2009 Feasibility of recommending certain replacement or alternative fats. Eur J Clin Nutr 63 S34 S49
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



