-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
LRRK2 is linked to autosomal dominant late-onset Parkinson’s disease, suggesting that LRRK2 gain-of-function mutations lead to age-dependent degeneration of the midbrain dopaminergic neurons. In this study, we describe two novel LRRK2-associated proteins HERC2 and NEURL4, which are a ubiquitin ligase and an adaptor-like protein, respectively. HERC2 and NEURL4 direct LRRK2 to Notch signaling pathway, in which the LRRK2-NEURL4-HERC2 complex promotes the recycling of the Notch ligand Delta-like 1 (Dll1)/Delta (Dl) through the modulation of endosomal trafficking. As a result, the amounts of Dll1/D1 on the plasma membrane are increased, which affects negatively Notch signaling through cis-inhibition. The effect is enhanced by a Parkinson’s-disease associated mutation of LRRK2. Inhibition of Notch signaling in adult dopaminergic neurons impairs its functions and survival. These findings indicate a possible link between Notch pathway and Parkinson’s disease.
Published in the journal: . PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005503
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005503Summary
LRRK2 is linked to autosomal dominant late-onset Parkinson’s disease, suggesting that LRRK2 gain-of-function mutations lead to age-dependent degeneration of the midbrain dopaminergic neurons. In this study, we describe two novel LRRK2-associated proteins HERC2 and NEURL4, which are a ubiquitin ligase and an adaptor-like protein, respectively. HERC2 and NEURL4 direct LRRK2 to Notch signaling pathway, in which the LRRK2-NEURL4-HERC2 complex promotes the recycling of the Notch ligand Delta-like 1 (Dll1)/Delta (Dl) through the modulation of endosomal trafficking. As a result, the amounts of Dll1/D1 on the plasma membrane are increased, which affects negatively Notch signaling through cis-inhibition. The effect is enhanced by a Parkinson’s-disease associated mutation of LRRK2. Inhibition of Notch signaling in adult dopaminergic neurons impairs its functions and survival. These findings indicate a possible link between Notch pathway and Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is thought to result from the combined effects of environmental and genetic factors. LRRK2, which encodes a ROCO protein with a Ras of complex proteins (ROC) domain, a conserved C-terminal of ROC (COR) domain, a kinase domain, leucine-rich repeats (LRRs), and WD40 repeats, has been identified as a causative gene for autosomal-dominant familial PD [1]. Although various amino acid substitutions have been identified throughout the multiple domains of LRRK2, including R1441G (RG) in the ROC domain and G2019S (GS) and I2020T (IT) in the kinase domain, the pathogenic role of LRRK2 mutations and the biochemical pathways involved remain largely unknown. LRRK2-associated familial PD is almost indistinguishable from the common sporadic form of PD in clinical and pathological aspects. LRRK2 mutations identified in familial PD patients have also been isolated in 1–5% of PD patients without apparent familial PD histories. Moreover, two recent genome-wide-association studies (GWAS) detected LRRK2 and SNCA/a-synuclein as two strong risk loci for sporadic PD [2]. The single-nucleotide polymorphisms (SNPs) in LRRK2 were situated in the non-coding region, upstream of the LRRK2 coding sequence, which suggests that even certain changes in the expression level of wild-type (WT) LRRK2, presumably incremental, could increase the risk of PD. Consistent with this possibility, experimental data from α-synuclein and LRRK2 double-transgenic mice suggest that the difference in LRRK2 protein expression levels is more important than LRRK2 kinase activity [3].
LRRK2 is highly expressed in the regions of active cell differentiation, migration and cell death during the development of the mouse embryo [4]. Loss of the LRRK2 gene in mice has been demonstrated to impair the autophagy-lysosome pathway, which leads to marked accumulations of α-Synuclein and ubiquitinated proteins [5] or enlarged and increased numbers of secondary lysosomes in the kidney with age [6]. A single LRRK gene (referred to hereafter as dLRRK) was identified in the Drosophila genome. Critical residues disrupted in familial PD are conserved between LRRK2 and dLRRK, although dLRRK structurally resembles LRRK1 [7, 8]. dLRRK is localized in the endosomes and has also been reported to regulate the function of Rab7 in the endosomal-lysosomal pathway [9, 10].
Notch is a large transmembrane receptor that is activated by ligand binding. The signaling pathway of Notch is an evolutionarily well-conserved signaling system and is known for its important roles in development, such as determining the timing and direction of cellular differentiation and the development and maintenance of borders in developing tissues. Numerous studies have revealed the importance of Notch in the nervous system, including in the maintenance of immature neurons, the control of neurite outgrowth of differentiated neurons, and the regulation of synaptic plasticity and olfactory functions in the adult brain [11–14].
Binding between Notch and Notch ligands on contacting cells results in the proteolytic cleavage of Notch at the transmembrane region and the subsequent release of the Notch intracellular domain (NICD). NICD then translocates into the nucleus and functions as a transcriptional activator for its target genes, such as Hes1 and Hes5, along with additional transcriptional factors. Notch signaling is regulated in multiple ways, such as glycosyl modification of Notch, cis-trans regulation by ligands, and endocytic trafficking and degradation of Notch signal components [15]. On a given cell, Notch can be trans-activated by Notch ligands presented by neighboring cells, whereas Notch ligands in the same cell have a cell-autonomous dominant-negative effect on the Notch receptor. This regulatory mechanism is called the ‘cis-inhibition’ of Notch signaling [16] and is thought to ensure polarized signaling in cell-cell communications.
Here, we report two novel LRRK2-binding proteins, NEURL4 and HERC2, both of which genetically and physically interact with the Notch ligand Dll1/Dl. Our data reveal that, in cooperation with NEURL4 and HERC2, LRRK2 modulates the Notch signaling through the regulation of the trafficking of Dll1/Dl, by which the function and survival of adult dopaminergic neurons are altered. Therefore, abnormal Notch signaling could be a component of the mechanism of neurodegeneration in PD.
Results
HERC2 and NEURL4 as novel LRRK2-binding proteins
To investigate the functions of LRRK2, we searched for novel LRRK2-binding proteins. N-terminally FLAG-tagged LRRK2 was expressed in HEK293 cells, and the LRRK2-associated protein complexes were affinity-purified from the cell lysate using anti-FLAG-conjugated beads. Of the binding proteins detected by liquid chromatography/tandem mass spectrometry (LC-MS/MS), we chose two novel proteins, HERC2 and NEURL4, for further investigation (Fig 1A).
Fig. 1. Identification of HERC2 and NEURL4 as LRRK2-binding proteins. 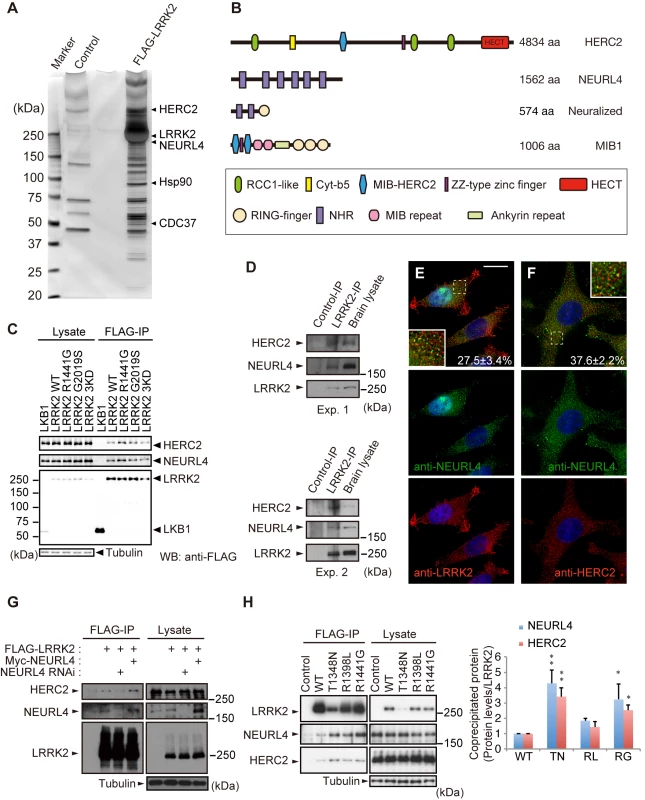
(A) Silver-stained gel image for LRRK2-binding proteins. LRRK2 and representative co-purified proteins are indicated. (B) Domain structures of HERC2, NEURL4 and their related proteins Neuralized and Mindbomb homolog 1 (MIB1). RCC1-like, Regulator of chromosome condensation 1-like domain; Cyt-b5, cytochrome b5-like motif; MIB-HERC2, MIB/HERC2 domain; NHR, Neuralized homology repeat domain. (C) HERC2 and NEURL4 specifically bind to LRRK2 in HEK293T cells. Lysates expressing FLAG-tagged LRRK2 or FLAG-tagged LKB1 were subjected to immunoprecipitation with anti-FLAG antibody (FLAG-IP) and were detected by Western blotting with anti-FLAG, anti-HERC2 and anti-NEURL4 antibodies. (D) Endogenous associations between LRRK2, NEURL4 and HERC2. Mouse brain lysates were subjected to immunoprecipitation with anti-LRRK2 (LRRK2-IP) or anti-dFoxO (Control-IP) antibodies as a control and analyzed by Western blotting with the indicated antibodies. The results of two independent experiments are shown. (E) LRRK2, NEURL4 and HERC2 are partially colocalized as vesicular signals in the cytosol. HeLa cells transfected with LRRK2 were visualized with anti-NEURL4 (green) and anti-LRRK2 (red) antibodies, and the nuclei were counterstained with DAPI (blue). Colocalization of the proteins appears as yellow. Insets show higher-magnification images of the boxed regions and the values ± SE in (E, F) indicates colocalized green signals with red (n = 4). Scale bar, 10 μm. (F) HeLa cells transfected with HERC2 were visualized with anti-NEURL4 (green) and anti-HERC2 (red) antibodies as in (E). (G) LRRK2 binds to HERC2 via NEURL4. HEK293T cell lysates transfected with FLAG-LRRK2, Myc-NEURL4, an siRNA duplex against NEURL4 and/or an empty plasmid were subjected to immunoprecipitation with an anti-FLAG antibody and detected by Western blotting with antibodies against the indicated proteins. (H) LRRK2 GTPase mutations (TN, T1348N; RL, R1398L; RG, R1441G) modulate the affinity to NEURL4 and HERC2. Note that LRRK2TN consistently exhibited lower expression levels, suggesting instability. The graph indicates the relative levels of endogenous proteins co-precipitated with FLAG-LRRK2. The data are shown as the mean ± SE from five repeated experiments (**, p < 0.01; *, p < 0.05 by one-way ANOVA). HERC2 is a large protein with a predicted molecular mass of more than 500 kDa. HERC2 has three RCC1-like domains (RLDs), a mind-bomb (MIB)/HERC2 domain, and a C-terminal HECT domain (Fig 1B). NEURL4 includes six Neuralized homology repeat (NHR) domains, which have been reported to associate with HERC2 and to regulate centrosome duplication (Fig 1B) [17–19].
Co-immunoprecipitation using the FLAG-tagged proteins confirmed that endogenous HERC2 and NEURL4 bind to FLAG-LRRK2, including pathogenic and kinase-dead (KD) mutants, but not to the unrelated LKB1-FLAG in HEK293 cells (Fig 1C). Physiological binding of these proteins was also detected by the co-immunoprecipitation of endogenous proteins from mouse brain lysates (Fig 1D), and these proteins partially colocalized with each other in cultured cells (Figs 1E, 1F and S1A–S1E). NEURL4 knockdown abolished the binding between HERC2 and LRRK2, and Myc-NEURL4 enhanced Flag-LRRK2 co-immunoprecipitation with HERC2 (Fig 1G), suggesting that LRRK2 binds to HERC2 through NEURL4. The binding of LRRK2 to NEURL4 was exclusively dependent on the ROC domain and partly on the WD40 domain (S1F, S1G and S1I Fig). The LRRK2 mutant LRRK2RG, a ROC domain pathogenic mutant, and LRRK2TN, an artificial mutant with impaired GTP-binding activity, stabilized the binding to HERC2 and NEURL4 (Fig 1H), whereas LRRK2GS, a kinase domain pathogenic mutant, and LRRK2RL, an artificial mutant with increased GTP hydrolysis activity, did not affect that binding (Fig 1C and 1H) [20, 21]. NEURL4 bound to LRRK2 through a region containing the third and fourth NHR domains of NEURL4 (S1H and S1J Fig). HERC2 bound to NEURL4 through a region containing the fifth and sixth NHR domains of NEURL4 (S1H and S1J Fig).
LRRK2 binds to Dll1 through NEURL4 and HERC2
Domain analysis of HERC2 and NEURL4 (Fig 1B) suggested that both proteins might be involved in the Notch signaling pathway. HERC2 has an MIB/HERC2 domain, and NEURL4 has four NHR domains. Neur, which is a ubiquitin-ligase for Dl, has been shown in Drosophila to recognize Dl through the NHR1 domain [22], and the MIB/HERC2 domain is also included in the Dl-binding region of MIB, another ubiquitin-ligase for Dl [23]. As expected, NEURL4 binds to the mammalian Dl homologue Dll1 (Fig 2A). In addition, NEURL4 also binds to Neur (S2A Fig). However, NEURL4 did not compete with Neur in the binding to Dll1; instead, NEURL4 enhanced the binding of Neur to Dll1 (S2B Fig). LRRK2 exhibited little binding with Dll1, whereas both NEURL4 and HERC2 enhanced the binding of LRRK2 to Dll1 (Fig 2B). Moreover, an immunoprecipitation assay indicated that LRRK2, NEURL4 and HERC2 specifically bound to several types of Rabs (S2C–S2E Fig). These results suggested that NEURL4 and HERC2 assist LRRK2 in dynamically regulating Dll1 in the endosomes.
Fig. 2. LRRK2, NEURL4 and HERC2 bind to the Notch ligand Dll1. 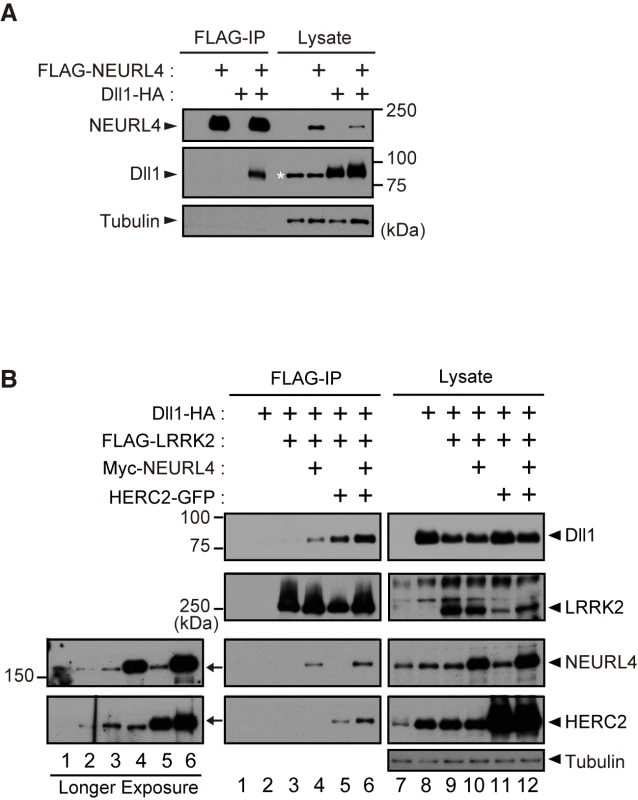
(A) NEURL4 binds to Dll1. HEK293T cell lysate transfected with the indicated plasmids was subjected to immunoprecipitation with an anti-FLAG antibody and analyzed by Western blotting with anti-FLAG and anti-HA antibodies. The asterisk indicates non-specific bands that appeared with anti-HA (clone 12CA5). (B) LRRK2 associates with Dll1 via NEURL4 and HERC2. A co-immunoprecipitation assay was performed as in (A). Western blotting was performed with anti-Dll1, anti-LRRK2, anti-NEURL4 and anti-HERC2 antibodies. Longer exposures of X-ray films revealed endogenous proteins that co-immunoprecipitate with FLAG-LRRK2 (Left). LRRK2, NEURL4 and HERC2 genetically interact with Dl in the fly
We next examined the influence of LRRK2, NEURL4 and HERC2 on Notch signaling in Drosophila. During wing development in Drosophila larvae, the future dorso-ventral border in the wing imaginal disc is maintained by the activity of Notch signaling along the border. Attenuation of Notch signaling in the border results in the diminution of the border, and the phenotype of the “notched” wing will be present in the adult fly. We utilized the Dpp-Gal4>UAS system, in which the Dpp-GAL4 driver drives transgenes with upstream activating sequences (UASs) in a temperature-dependent manner. The Dpp promoter is active along the long axis of the wing disc, orthogonally to the dorso-ventral border. Dpp-GAL4 expression had no effects on wing development (Fig 3A, Dpp-Gal4). Drosophila has single copies of homologous genes for LRRK2, NEURL4 and HERC2, which are dLRRK, Bluestreak (Blue) and dHERC2, respectively. Ectopic expression or knockdown of dLRRK, Blue or dHERC2 alone had no effects on wing formation. Ectopic expression of Dl along the long axis of the wing disc using Dpp-GAL4 resulted in a shrunken and completely deformed wing at 25°C. Reducing the expression of Dl by raising fly crosses at 18°C masked the wing phenotype (Fig 3A, Dl). However, the co-expression of human LRRK2 (Fig 3A, Dl; hLRRK2), Blue (Fig 3A, Dl; BlueEP, and S3A and S3B Fig) or dHERC2 (Figs 3A, Dl; dHERC2EP, and S3C) with Dl at 18°C caused “notching” of the wing margin. Expression of the pathogenic mutant LRRK2RG produced a similar but stronger phenotype (Fig 3A, Dl; hLRRK2RG) compared with the LRRK2 wild-type (WT) crosses (Fig 3A, Dl; hLRRK2). These results suggested that ectopic expression of LRRK2, NEURL4 and HERC2 compromises Notch signaling through Dl modulation.
Fig. 3. LRRK2, HERC2 and NEURL4 genetically interact with Dl signaling components in Drosophila. 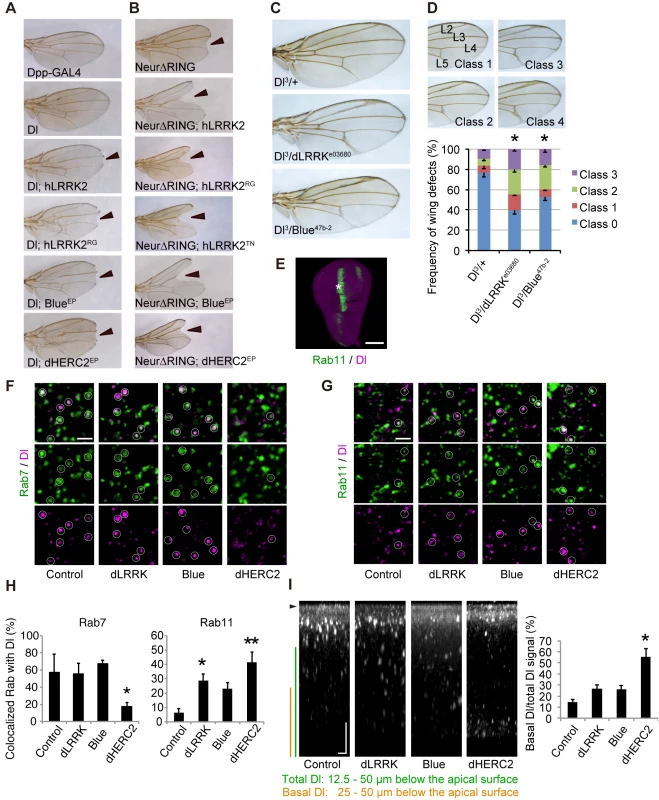
(A) The Dpp-GAL4 driver by itself produced normal wing morphology. A low level Dl expression causes a mild defect in the vein formation. hLRRK2, hLRRK2RG, Blue or dHERC2 co-expression together with Dl produces notched wing margins (arrowheads). EP lines were used for Blue and dHERC2 overexpression. (B) hLRRK2, Blue or dHERC2 exacerbates the phenotype of the dominant-negative Neur. Overexpression of Neur lacking the RING-finger domain (Dl-∆RING) influences the wing margin formation (arrowheads), and this effect is enhanced by hLRRK2, Blue or dHERC2 co-expression. (C, D) The Dl phenotype is enhanced by the reduced activity of dLRRK or Blue. Dl, dLRRK and Blue loss-of-function alleles were combined, and the wing phenotypes of (C) were quantified using a scoring system (D). Classification used to quantify frequencies of Dl phenotypes: Class 0, normal; Class 1, mild thickening of the longitudinal vein L2; Class 2, moderate thickening of the L2 and L5 veins and the distal tip of L3 and L4 veins; and Class 3, severe thickening of the L2-L5 veins. The data are shown as the mean ± SE from three experiments. * p < 0.05 vs. Dl3/+ by Steel-Dwass test. n = 108–200. Dpp-GAL4 drove all transgenes at 18°C (A) or 25°C (B-D). (E-H) Both dLRRK and dHERC2 promote Dl localization at the recycling endosomes. Colocalized Dl signals with Rab7 (circles in F) or Rab11 (circles in G) in the developing L3 vein region of the wing disc (asterisk in E) were imaged and graphed (H). Scale bars, 100 μm in E and 2 μm in F, G. (I) The LRRK2 complex does not inhibit Dl endocytosis. Images show the distribution of internalized anti-Dl in the confocal z-sections, and dHERC2 promotes Dl transcytosis to the basal direction. The graph indicates the basal distribution of the Dl signal. Arrowhead, apical surface. Scale bars on X/Z-axes, 2/10 μm. Graphs in (H, I) are shown as the mean ± SE. **, p < 0.01; *, p < 0.05 by Dunnett’s test. n = 3–6. Neur lacking its RING-finger domain (Neur∆RING) acts as a dominant-negative, and its expression results in Dl accumulation on the cell surface and the appearance of the wing phenotype by cis-inhibition (Fig 3B). LRRK2, Blue or dHERC2 co-expression further enhanced the notching phenotype (Fig 3B), and LRRK2RG, LRRK2TN, LRRK2GS, and LRRK2KD caused a size reduction in Neur∆RING (Figs 3B and S3D). However, the notching phenotype by LRRK2KD was milder (S3D Fig). The loss of dLRRK partly suppressed the notching phenotype by Neur∆RING (S3D Fig). Full-length Neur expression had little effect on wing development, whereas co-expression of dHERC2 led to wing size reduction (S3E Fig). Based on the genetic results of dHERC2 in Figs 3B and S3E, Neur and HERC2 appear to act differently rather than in synergy. We also performed a genetic test using loss-of-function alleles of Dl, dLRRK and Blue to substantiate our findings (Fig 3C and 3D). Approximately twenty-five per cent of Dl3 heterozygous flies exhibited the vein thickening phenotype to varying degrees, which was potentiated by a combination of dLRRK (approximately 60%) and Blue (approximately 47%) mutant alleles (Fig 3D). Our genetic tests revealed that the LRRK2 complex does not modulate phenotypes caused by the overexpression of Serrate, another ligand for Notch in Drosophila (S3F Fig), or by a mutation of Notch (S3G Fig), implying that the effects of the LRRK2 complex are specific to Dl/Dll1. These genetic results suggest that LRRK2, Blue and dHERC2 potentiate ‘cis-inhibition’, presumably by promoting the accumulation of endogenous Dl on the cell surface of Notch expressing (signal-receiving) cells as well as of signal-sending cells (Fig 3A and 3B) whereas the loss of dLRRK or Blue results in the reduction of Dl on the cell surface of signal-sending cells, leading to diminished Notch activation (Fig 3C).
Dl is subjected to endosomal trafficking after endocytosis mediated by ubiquitin ligases, such as Neur and MIB [24]. The small GTPases Rab5, Rab7 and Rab11 regulate early, late and recycling endosomes, respectively, and are known to influence the status of Notch signaling [25, 26]. Because LRRK2/dLRRK, NEURL4 and HERC2 binds to Rab proteins, the endosomal trafficking of Dl is likely regulated by the LRRK2 complex. To monitor the endosomal trafficking of Dl, an anti-Dl antibody uptake assay was performed in living wing imaginal discs expressing Rab7-GFP or Rab11-GFP (Fig 3E–3G) [27]. dHERC2 overexpression suppressed the transport of Dl to Rab7-positive late endosomes, whereas dLRRK and dHERC2 promoted the colocalization of Dl with Rab11-positive recycling endosomes. Blue overexpression also tended to increase the colocalization of Dl with Rab11. The same assay revealed that dLRRK and Blue did not inhibit the endocytosis of Dl but rather tended to promote it (Fig 3I). Interestingly, dHERC2 stimulated the transport of Dl in the basal direction (Fig 3I). In contrast, the LRRK2 complex did not change the distribution of Rab7-GFP and Rab11-GFP. These observations strongly suggest that the LRRK2 complex regulates the endosomal recycling of Dl to maintain the levels of Dl at the cell surface but does not inhibit Dl endocytosis.
LRRK2, NEURL4 and HERC2 suppress Notch signaling
We next examined the effects of LRRK2, NEURL4 or HERC2 on non-cell-autonomous Notch signaling using a luciferase-based reporter assay, in which the Hes1 promoter activity was monitored based on Notch signal intensity. We used SH-SY5Y cells as Notch signal-receiving cells and CHO cells stably expressing rat Dll1 or parental CHO cells co-cultured as Notch signal-sending cells (Fig 4A). SH-SY5Y cells express a certain level of endogenous Notch [28]. However, the Hes1 promoter activity was weak under a co-culture condition with CHO cells expressing Dll1, which suggests that the influence of endogenous Notch was minimal compared with the intensity generated by the addition of exogenous Notch1 (S4A Fig). In this setting, the co-expression of Notch1 and Dll1 lacking an intracellular domain (Dll1-∆ICD) effectively exerted a cis-inhibition effect, as reported in Drosophila (Fig 4B) [29]; thus, the Hes1 activity was suppressed to a level equivalent to that produced by cells co-cultured with parental CHO cells (Fig 4B).
Fig. 4. LRRK2, NEURL4 and HERC2 modulate Notch signal intensity in cultured cells. 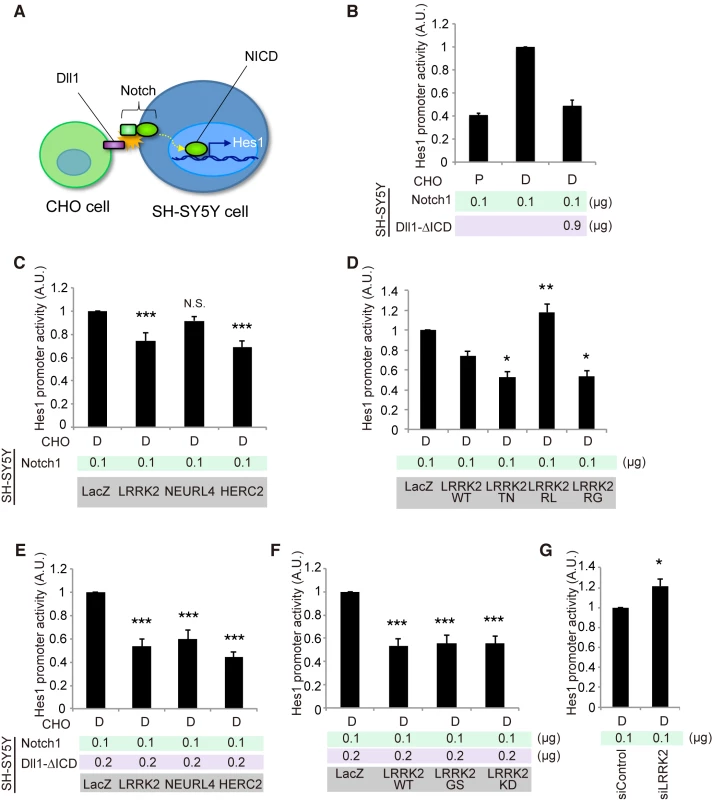
(A) Schematic of non-cell-autonomous Notch signaling and ‘cis-inhibition’ reconstituted in cultured cells. (B) Notch signal intensity assessed by the Hes1 promoter activity. SH-SY5Y cells were transfected with Hes1 reporter and Notch1 plasmids and control LacZ or Dll1-∆ICD plasmids. CHO cells stably expressing Dll1 (D) and parental CHO (P) cells were co-cultured as signal-sending and mock cells, respectively. (C) LRRK2 and HERC2 suppress Notch signal intensity. (D) LRRK2 ROC mutations affect the suppressive potency of Notch signaling. (E, F) ‘Weak cis-inhibition’ condition accentuates the apparent suppressive effects of LRRK2, NEURL4 and HERC2 on Notch signal intensity. (G) LRRK2 knockdown activates the Notch signal. (C, E, F) ***, p < 0.001; **, p < 0.01; N.S., not significant vs. LacZ and (D) **, p < 0.01; *, p < 0.05 vs. LRRK2 WT by one-way ANOVA. (G) *, p < 0.05 by Student’s t-test. The data represent the mean ± SE at least from three experiments performed in triplicate. KD, kinase-dead. The co-expression of LRRK2 or HERC2, instead of Dll1-∆ICD, moderately mimicked the cis-inhibition, whereas NEURL4 co-expression did not (Fig 4C). The cis-inhibitory activity of LRRK2 was enhanced by TN and RG mutations but not by the RL mutation (Fig 4D); the suppressive effect of LRRK2 was independent of its kinase activity although the effect of LRRK2KD was slightly milder (S4B Fig). Cis-inhibition by a minimal amount of Dll1-∆ICD (0.2 μg; 1/6 of total DNA transfected) was enhanced by combination with LRRK2, NEURL4, or HERC2 (Fig 4E). The extent of inhibition remained similar among the WT, GS and KD forms (Fig 4F), whereas the knockdown of endogenous LRRK2 increased it, suggesting that LRRK2 negatively regulates Notch activity through cis-inhibition (Fig 4G).
The LRRK2 complex regulates the endosomal trafficking of Dll1
The cis-inhibition by the LRRK2 complex observed in Drosophila and a reporter assay using mammalian co-cultured cells was likely caused by alterations in Dll1/Dl turnover or trafficking. The expression of each component or a combination of LRRK2 and NEURL4 did not change the turnover of newly synthesized Dll1 (S5A and S5B Fig), whereas the co-expression of LRRK2 with NEURL4 and HERC2 significantly slowed Dll1 turnover but not that of Notch1 (Figs 5A, 5B and S5C). Next, we explored the specific cellular compartment in which Dll1 was accumulated using a combination of live-cell imaging and SNAP-tag technology. HeLa cells stably expressing Dll1 with a SNAP-tag in the extracellular domain (SNAP-Dll1/HeLa cells) were covalently labeled with a cell-impermeable fluorescent substrate; thus, the dynamics of Dll1 on the cell surface at a specific point in time could be visualized. Internalized Dll1 was incorporated into cellular vesicles, and the cell-surface Dll1 decreased over time (Fig 5C). In this context, Dll1 was expressed more on the cell surface in the LRRK2 complex-expressing cells at 6 h post-labeling (Fig 5D). In contrast, the inactivation of the LRRK2 complex accelerated the disappearance of Dll1 on the plasma membrane (S5D–S5F Fig). To determine the physiological functions of LRRK2 in the endosomal pathway in detail, we used LRRK2-deficient cells expressing exogenous LacZ or LRRK2 for Dll1 trafficking. The compensatory expression of LRRK2 increased the colocalization of Dll1 with the Rab5 - and Rab11-positive endosomes, whereas it decreased the colocalization of Dll1 with Rab7-positive endosomes (Figs 5E and S6). These findings again suggested that the LRRK2 complex stimulates Dll1 recycling through the endosomal pathway to maintain the cell surface expression of Dll1, which likely explains the enhancement of cis-inhibition by the LRRK2 complex.
Fig. 5. LRRK2 complex modulates Dll1 turnover in the endosomal pathway. 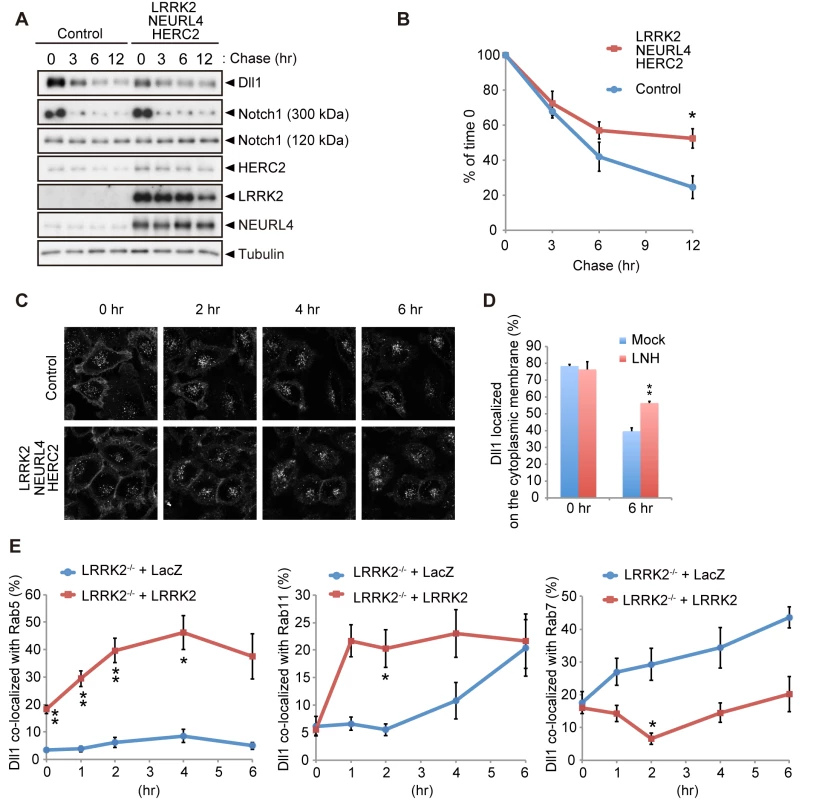
(A) Co-expression of LRRK2, NEURL4 and HERC2 stabilizes Dll1. HeLa cells stably expressing Dll1-HA were transfected with a mixture of plasmids for LRRK2/NEURL4/HERC2 or with LacZ as a control. At 24 h after transfection, the cells were then treated with cycloheximide (CHX, 100 μg ml-1) for the indicated times, and Western blotting assays were performed. (B) The level of Dll1 remaining at different time points was plotted as the percentage of the initial Dll1 level (0 h of CHX treatment). The data are shown as the mean ± SE from four repeated experiments (*, p < 0.05 by Student’s t-test). (C) Dynamics of cell surface Dll1. HeLa cells stably expressing Dll1-SNAP were transfected as in (A). Cell surface Dll1 was labeled with SNAP-Surface Alexa Fluor 647 for 20 min at 37°C. Representative grayscale images of Dll1 labeled with Alexa Fluor 647 0–6 h after washout of the tracking dye (t = 0) are shown. (D) LRRK2 complex increases the amount of cell-surface Dll1. The data are presented as the mean ± SE for six independent experiments, with 13–26 cells counted per sample. **, p < 0.01 vs. Mock at 6 h determined by Student’s t-test. (E) LRRK2 stimulates the recycling of Dll1 via the endosomes. LRRK2-deficient mouse embryonic fibroblasts stably expressing Dll1-SNAP along with EGFP-Rab5, EGFP-Rab7 or EGFP-Rab11 were transfected with LacZ or LRRK2 and were labeled with SNAP-Surface Alexa Fluor 647 as in (C). Graph showing that Dll1 colocalized with the indicated Rab proteins (mean ± SE for 3–7 independent experiments, with 5–7 cells counted per sample). ** p < 0.01, * p < 0.05 by Student’s t-test. The LRRK2 complex accelerates neuronal differentiation in the embryo
Given that the LRRK2 complex modulates Notch signaling by enhanced cis-inhibition, we next examined whether the expression of the LRRK2 complex affects neuronal differentiation in the development of the mouse brain, where the physiological Notch signal functions. The dorsolateral telencephalons of mouse embryos at embryonic day 13.5 (E13.5) were transfected with expression plasmids for LRRK2, NEURL4, HERC2, or a mixture of these plasmids via in utero electroporation and were then examined immunohistochemically 1 to 3 days post-transfection. TUJ1-positive differentiated neuronal cells emerged in the ventricular zones of the embryos transfected with LRRK2, HERC2 or the LRRK2/NEURL4/HERC2 mixture at 1 day post-transfection, and the immunoreactivity of NICD or Hes1 was decreased in areas of high transgene expression (Fig 6A). The appearance of TUJ1-positive cells was induced by ectopic HERC2 expression in WT embryos but not LRRK2-deficient embryos, suggesting that this phenomenon is LRRK2-dependent (S7A–S7C Fig). This observation suggests that Notch signaling is also suppressed by the LRRK2 complex in the mouse brain, leading to the accelerated neuronal differentiation of transfected cells. In contrast, the expression of NEURL4 alone failed to accelerate the neuronal differentiation of neural stem cells (Fig 6A), which is consistent with a result obtained in the reporter assay (Fig 4C).
Fig. 6. LRRK2 complex promotes neuronal differentiation in vivo. 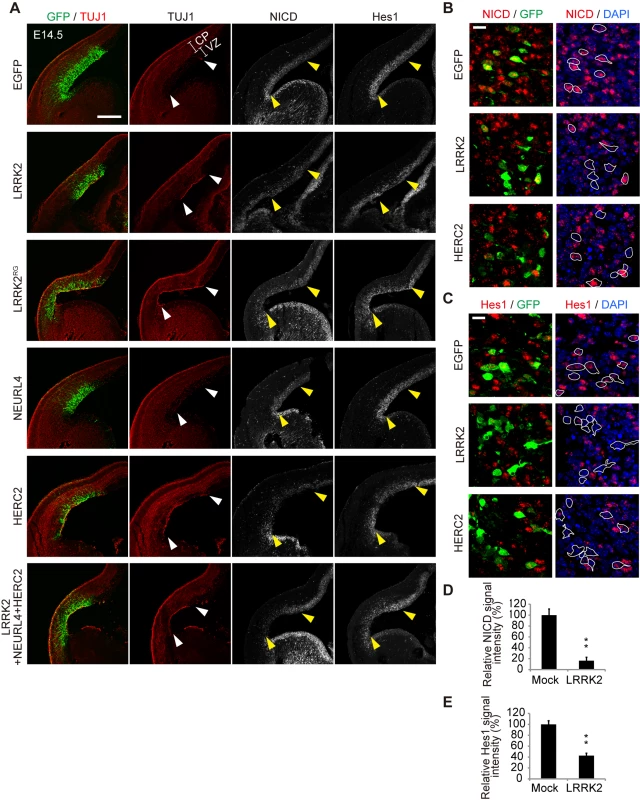
(A) Coronal sections of mouse dorsolateral telencephalon were immunostained for NICD, Hes1, neuron-specific class III β-tubulin (TUJ1) and GFP to stabilize the fates of cells transfected with the indicated genes 1 day after in utero electroporation at embryonic day 13.5 (E13.5). Empty vector pEF was used as a mock plasmid. EGFP was used as a reporter to visualize transfected cells. The regions of transgene expression are indicated by arrowheads. CP, the cortical plate and the intermediate zone; VZ, the ventricular and subventricular zones. Scale bar, 300 μm. (B, C) High-magnification images of samples from (A). Expression levels of NICD and Hes1 are suppressed in LRRK2- or HERC2-transfected cells. The boundaries of GFP-positive cells with intact nuclei are shown by white lines and are overlaid on the images stained for NICD or Hes1. Scale bars, 10 μm. (D, E) Immunoreactivity for NICD or Hes1 in EGFP-positive cells. Signal intensity was calculated from more than 200 cells from two animals in each group. **, p < 0.01 by Student’s t-test. Destruction of the normal ventricular zone structure or cell death by electroporation is unlikely to lead to the appearance of TUJ1-positive cells because NICD and Hes1 immunoreactivity were also decreased at E14.5 (i.e., 24 h post-transfection), when the overall structure of the ventricular zone was relatively preserved (Fig 6B–6E) and because the extent of cell death among LRRK2, HERC2 and NEURL4 transfectants was similar (S7D Fig).
At a later stage of development post-transfection with LRRK2, HERC2 or the mixture of the LRRK2 complex, the layer of cells positive for Pax6 (a marker for neuronal stem cells) in the ventricular zone was lost in the region of high transgene expression (S7E Fig). Conversely, ectopic TUJ1-positive cells were observed in the transfected regions (S7E Fig). Again, the transfection of NEURL4 alone did not have apparent effects (S7E Fig).
Notch signaling regulates dopaminergic neuron survival
We next examined whether the modification of Notch signaling by the LRRK2 complex is associated with the dopaminergic neurodegeneration observed in PD. Canonical Notch signaling is activated and inhibited by ligands expressed in nearby cells and by ligands expressed autonomously within cells, respectively. We manipulated Notch and Dl expression in dopaminergic and non-dopaminergic neurons in adult flies. Dopaminergic-specific inactivation of Dl improved the survival and motor activity of adult flies, whereas ectopic expression of Dl in the dopaminergic neurons shortened the fly lifespan (Fig 7A, 7B and 7E). Dl inactivation or overexpression outside of the dopaminergic neurons had little effect on lifespan (Fig 7C and 7D). These results suggested that the changes to Notch signaling activity through cis-inhibition by Dl are important for the function of adult dopaminergic neurons. In contrast, the alterations of Notch levels in both dopaminergic and non-dopaminergic neurons had toxic effects (Fig 7A–7D). The suppression of Dl in dopaminergic neurons also increased the brain dopamine levels and improved dopaminergic neuron survival (Figs 7F, 8A and S8A). In contrast, both Dl and Notch overexpression in dopaminergic neurons decreased its survivability (Figs 8A and S8A), and the neuronal loss by Dl overexpression was suppressed by dLRRK, Blue or dHERC2 inhibition and Neur overexpression (Figs 8B, S3H, S3I and S8B).
Fig. 7. Notch signaling modulates the function of dopaminergic neurons. 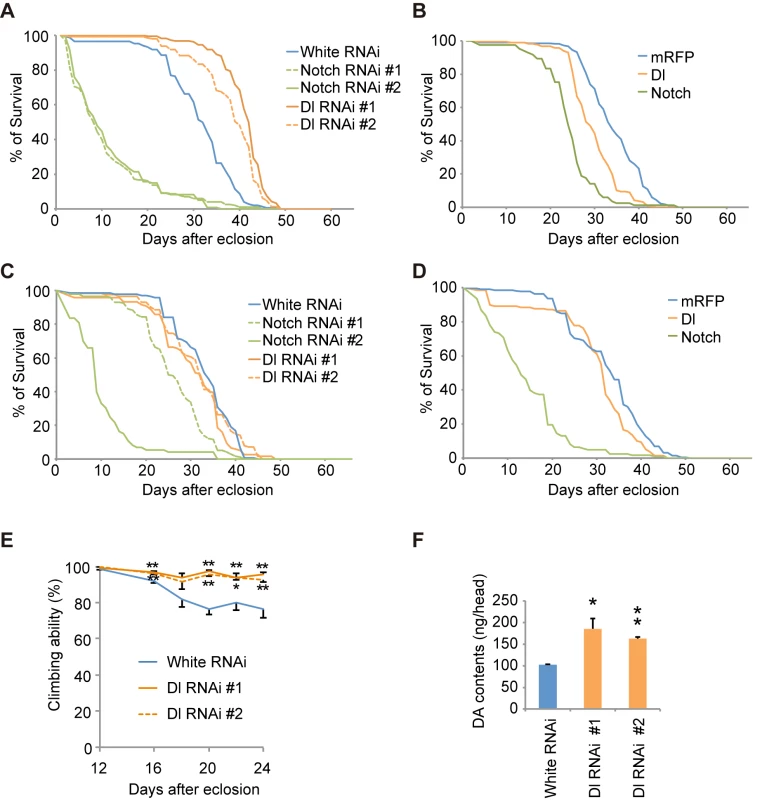
(A) Inhibition of Dl and Notch in the dopaminergic neurons during adulthood shortens and extends the lifespan, respectively, in Drosophila. p < 0.001 vs. control White RNAi, as determined by the log-rank test (n = 121–203). Flies harboring the indicated UAS-shRNAs were crossed with flies carrying tub-GAL80ts, TH-GAL4. Male offspring were raised at 18°C until eclosion, and the transgenes were expressed at 29°C during adulthood. (B) Ectopic expression of Dl and Notch in dopaminergic neurons during adulthood shortens the lifespan. p < 0.001 vs. mRFP, as determined by the log-rank test (n = 85–222). UAS-transgenes were expressed as in (A). (C) Inhibition of Dl in neurons other than dopaminergic neurons does not affect the lifespan, whereas Notch inhibition shortens the lifespan (p < 0.001, Notch RNAi #1 or #2 vs. White RNAi by the log-rank test. n = 78–178). Flies harboring the indicated UAS-shRNAs were crossed with flies carrying tub-GAL80ts, TH-GAL80, and elav-GAL4. Male offspring were raised at 18°C until eclosion, and the transgenes were expressed at 29°C during adulthood. (D) Ectopic expression of Dl in neurons other than dopaminergic neurons does not affect the lifespan, whereas ectopic Notch expression shortens the lifespan (p < 0.001 vs. mRFP by the log-rank test. n = 141–250). UAS-transgenes were expressed as in (C). (E) Age-dependent reduction of motor activity is improved by Dl inhibition. Flies were raised as in (A). (** p < 0.01, * p < 0.05 vs. White RNAi by one-way ANOVA). The data represent the mean ± SE from 2–6 trials. (F) Brain dopamine (DA) contents were increased by Dl inhibition (** p < 0.01, * p < 0.05 vs. White RNAi by one-way ANOVA. n = 3). Flies were raised as in (A), and adult male flies at 24 days of age were served. Fig. 8. Notch signaling modulates the survival of dopaminergic neurons. 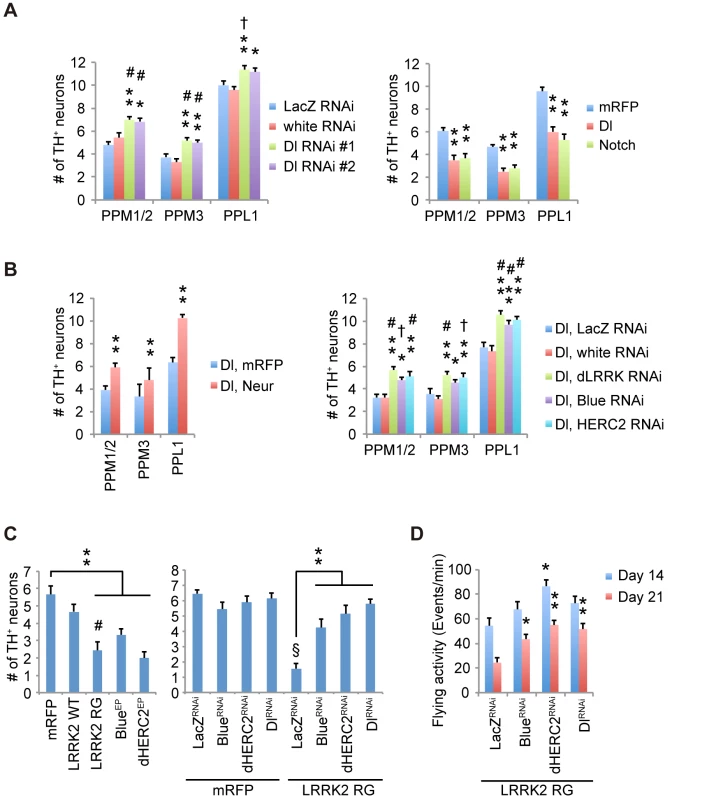
(A) Survivability of dopaminergic neurons is improved by Dl inhibition (** p < 0.01, * p < 0.05 vs. White RNAi; # p < 0.01, † p < 0.05 vs. LacZ RNAi by one-way ANOVA. n = 10–11), whereas ectopic Dl and Notch expression led to neuronal loss (** p < 0.01 vs. mRFP by one-way ANOVA. n = 10). Flies were raised as in Fig 7A, and the dopaminergic neurons in the PPM1/2, PPM3 and PPL1 clusters of adult male flies at 21 days old were counted. (B) Enhanced Dl endocytosis by Neur overexpression protects the dopaminergic neurons from Dl overexpression-mediated neuronal loss (** p < 0.01 by Student’s t-test. n = 9). Suppression of dLRRK, Blue and dHERC2 also protects the neurons (** p < 0.01, * p < 0.05 vs. White RNAi; # p < 0.01, † p < 0.05 vs. LacZ RNAi by one-way ANOVA. n = 9). Flies were raised as in Fig 7A, and the dopaminergic neurons in the PPM1/2, PPM3 and PPL1 clusters of adult male flies at 21 days of age were counted. (C) LRRK2RG, Blue or dHERC2 overexpression leads to neuronal loss, whereas the suppression of Blue, dHERC2 and Dl rescues the loss of dopaminergic neurons caused by LRRK2RG. Flies were raised as in Fig 7A, and the dopaminergic neurons in the PPM1/2 cluster of adult male flies at 40 days of age were counted (Left graph, ** p < 0.01 vs. mRFP; # p < 0.01 vs. LRRK2 WT by one-way ANOVA. n = 9. Right, ** p < 0.01 vs. LacZRNAi with LRRK2 RG; § p < 0.01 vs. LacZRNAi with mRFP by one-way ANOVA. n = 9). (D) The flying activity of 14- and 21-day-old males expressing LRRK2RG and the indicated shRNA constructs using the TH driver. Data are shown as the mean ± SE. ** p < 0.01, * p < 0.05 vs. LacZ RNAi. n = 10. LRRK2RG exhibited a strong cis-inhibition activity (Fig 4D), and dopaminergic expression of LRRK2RG led to dopaminergic neuronal loss in the PPM1/2 clusters, where neuronal excitation-dependent Notch activation could be detected (Figs 8C, S8C and S9). This neuronal loss and the reduction of flying behavior, which is associated with dopaminergic activity, were suppressed by Dl, Blue or dHERC2 inactivation (Fig 8C and 8D) [30]. In contrast, the overexpression of Blue or dHERC2 in dopaminergic neurons also caused neuronal loss (Fig 8C). Altogether, these results implied that the excess inhibition of Notch signaling affects the function and survival of adult dopaminergic neurons, and such inhibition could be mediated by the LRRK2 complex harboring ROC pathogenic mutations.
Discussion
We identified two novel LRRK2-associated proteins, NEURL4 and HERC2, which link LRRK2 to the regulation of Dll1/Dl turnover through the endosomal pathway. The LRRK2/NEURL4/HERC2 complex slows the turnover of Dll1, which negatively regulates Notch signaling. This complex promotes the differentiation of neural stem cells during mouse development and modulates the function and survival of mature dopaminergic neurons in adult Drosophila.
Roles of the LRRK2 complex in Dll1/Dl regulation
HERC2 belongs to the HERC family, which is characterized by the RCC1-like domain or RLD and HECT-type ubiquitin ligase domains. Another HERC family protein, HERC1, has been implicated in vesicle transport [31]. The RLD domains of HERC1 have a guanine-exchanging factor (GEF) activity for ARF1 and certain Rab proteins and can bind to the clathrin heavy chain, implying that HERC1 is involved in endocytosis and vesicular transport [31, 32]. NEURL4 belongs to the Neuralized family, a member of which is a RING-type ubiquitin ligase for Notch ligands [33]. Recently, it has been reported that HERC2 and NEURL4 regulate centrosome architecture [17]. dLRRK inactivation causes an abnormal centrosome phenotype in Drosophila cells, suggesting a functional link among LRRK2/dLRRK, HERC2 and NEURL4 [34].
Although the individual functions of LRRK2, HERC2 and NEURL4 remain to be fully elucidated, our results strongly suggest that the LRRK2 complex regulates the activity of Rab small GTPases. Based on the current results from fly genetic analysis and live-cell imaging, the LRRK2 complex can promote the endocytosis and recycling of Dll1 via early and recycling endosomes, thereby preventing Dll1 from trafficking to the late endosome and thus from lysosomal degradation (S10A Fig). The LRRK2 complex preferentially bound to Rab5 rather than Rab11 or Rab7, raising the possibility that the LRRK2 complex acts at the early endosomes to determine the fate of Dll1 (S2C–S2E Fig). The recycled Dll1 would presumably bind to Notch on the cell surface or at the endosomal compartments, thereby blocking Notch competency. HERC2 has the capacity to bind to Dll1, and NEURL4 appears to be a scaffold or adaptor protein that can form a four-part complex with LRRK2, HERC2 and Dll1. LRRK2 and NEURL4 appear to promote the ubiquitination of Dll1 by HERC2 (S10B and S10C Fig), which is likely to stimulate Dll1 endocytosis rather than degradation (Fig 5). Unlike Blue in Drosophila, overexpression of NEURL4 alone did not fully inhibit Notch signaling. This result might arise from a difference in molecular regulation between mammals and Drosophila. There is a good example of this situation in PINK1 and Parkin, two PD gene products. Overexpression of Parkin fully rescues PINK1 loss-of-function phenotypes in Drosophila, whereas Parkin cannot function in the absence of PINK1 in human cultured cells due to a more strict regulation of Parkin by PINK1 [35, 36]. Further studies will be required to understand the exact roles and regulatory mechanisms of LRRK2, NEURL4, HERC2 and other binding partners in the trafficking and regulation of Dll1.
Roles of LRRK2 GTPase and kinase activities in Notch cis-inhibition
LRRK2 associates with NEURL4 through the ROC domain, and the GTPase activity appears to regulate its binding to NEURL4. The pathogenic LRRK2RG mutant, which exhibits normal kinase activity but a modest impairment of GTP hydrolysis, increased the complex formation with NEURL4 and HERC2; LRRK2TN, which almost entirely lacks GTP hydrolysis and protein kinase activities, further enhanced the association compared with WT or LRRK2RL with increased GTP hydrolysis activity [37]. The stable formation of the LRRK2 complex showed a good correlation with the intensity of Notch cis-inhibition. In contrast, a limited effect of LRRK2 kinase activity on cis-inhibition was observed under our experimental conditions, and we have no evidence that LRRK2 directly phosphorylates NEURL4 and HERC2 (S10D and S10E Fig). However, considering that the Neur∆RING wing phenotype with LRRK2KD coexpression was milder than that with LRRK2GS (S3D Fig), and that the Hes1 suppression effect by LRRK2KD is slightly smaller than LRRK2WT or LRRK2GS (S4B Fig), we cannot exclude the possibility that there is a step that requires LRRK2 kinase activity in Dll1/Dl regulation.
LRRK1, the closest paralogue of LRRK2 in mammals, has both kinase activity-dependent and-independent functions to regulate the vesicular trafficking of the EGFR [38]. LRRK1 forms a complex with the EGFR in a kinase activity-independent manner, and its kinase activity regulates the transport of the EGFR from early to late endosomes. Thus, LRRK2 kinase activity may regulate the vesicular trafficking of Dll1 rather than the formation of the complex with Dll1. In this model, the kinase-dead LRRK2 will not transport Dll1 to either the cell surface or the lysosomes. The escaped Dll1 may bind to recycling Notch in the endosomal compartments, thereby suggesting that the total amount of Dll1, rather than the subcellular localization of Dll1, affects Notch cis-inhibition. The idea that LRRK2 has kinase-dependent and-independent functions might be supported by a comparative study of LRRK2 knockout mice and LRRK2 kinase-dead knock-in mice, where LRRK2 kinase-dead mice exhibit milder phenotypes than LRRK2 knockout mice or different phenotypes from LRRK2 knockout mice [6]. Another possibility is that endogenous LRRK2 may be recruited to the kinase-inactive LRRK2 complex, contributing to kinase-dependent regulation because LRRK2 can dimerize [39, 40]. Further molecular dissection will be required to address these possibilities.
Potential contribution of Notch signaling to PD etiology
LRRK2 is highly expressed in the regions of high proliferative and migratory activity and cell differentiation or cell death, including the ventricular and subventricular zones of the embryonic telencephalon during neurogenesis [4]. However, LRRK2-knockout mice exhibit no developmental abnormalities [41], suggesting that many regulators of Notch and other signaling molecules achieve robust regulation during development. It has been reported that Notch activity is impaired in the adult brain of α-Synuclein transgenic mice and in mouse embryonic stem cells overexpressing α-Synuclein, raising the possibility that the pathogenic mechanisms of α-Synuclein and LRRK2 could converge with the dysfunction of the endosomal trafficking that compromises Notch signaling because α-Synuclein inhibits Rab-dependent endosomal trafficking [42, 43]. The retromer component Vps35 is another gene product for autosomal-dominant PD [44, 45]. The retromer regulates the vesicular sorting from the endosome to the trans-Golgi network or the plasma membrane, and a PD-associated mutation of Vps35 impairs these functions [46]. Because LRRK2 has been reported to genetically associate with Vps35, the PD-associated Vps35 mutation may also modulate Notch signaling [47].
The findings that Notch and Dll1 are substrates of the γ-secretase complex and that Notch signaling participates in learning and memory and microtubule dynamics imply a potential link between Notch signaling and Alzheimer’s disease (AD) pathogenesis. However, the involvement of Notch signaling in the neurodegeneration process remains largely uncharacterized. Our study has revealed that the conditional suppression of Dl in adult dopaminergic neurons unexpectedly improved the function and survival of dopaminergic neurons in Drosophila. However, manipulation of the Notch expression level (both increased and decreased) exhibited neurotoxicity pan-neuronally. A recent study has demonstrated that Notch activation in olfactory receptor neurons is dependent on both the neuronal response and Dl [14]. In this study, Notch activation dependent on dopaminergic excitation was also detected in a few dopaminergic neurons of the PPM2 and PPM3 clusters and in unidentified cells, which supports the idea that Notch signaling contributes to dopaminergic activity in mature neurons (S9 Fig).
Notch signaling also contributes to the regulation of synaptic plasticity, spine density and neuronal morphology in the adult brain. Conditional deletion of Notch1 in the postnatal mouse hippocampus has revealed that Notch signaling is essential for both long-term potentiation and long-term depression, along with learning and short-term memory in response to synaptic activity of the hippocampal neurons [48]. Synaptic plasticity is important for adaptive motor control and procedural memory in the striatum, and its impairment could account for the onset and progression of the motor symptoms of PD.
Neurogenesis is impaired in many transgenic mouse models for AD, although its relevance to AD pathogenesis remains controversial [49]. Impaired neurogenesis originates from Notch signal suppression in mice that express the AD-associated mutant Presenilin 1 [50]. Because the suppression of Notch signal intensity will decrease the population of neural stem cells in the brain, an attractive explanation for age-dependent neurodegeneration in LRRK2 - or α-synuclein-linked PD is that impaired Notch signaling diminishes the maintenance of neuronal progenitor cells in the adult brain, making the brain more vulnerable to neuronal damage. The finding of impaired neurogenesis in LRRK2GS transgenic mice appears to support this idea [51]; however, the existence of neurogenesis in the mature substantia nigra continues to be debated [52, 53].
In conclusion, this study demonstrated that LRRK2 and newly identified binding proteins regulate Notch activity by regulating the vesicular trafficking of Dll1/Dl. The alteration of Notch signaling in adult dopaminergic neurons in Drosophila modulates the function and survival of these cells, which may be associated with the neurodegeneration caused by LRRK2 mutations. Future studies addressing the involvement of LRRK2-Notch signaling pathways in the mammalian striatal dopaminergic system will contribute to an understanding of PD development.
Materials and Methods
Ethics statement
This study was approved by the Ethics Committee of Kyoto University, Japan (approval number: MedKyo14306). Carbon dioxide inhalation was used for a euthanasia method of mice. All surgical procedures were performed according to the rules set forth by the Ethics Committee of Kyoto University.
Antibodies, plasmids and reagents
Western blot analysis using cultured cells, mouse brain tissue and Drosophila tissue samples was performed as described, using ECL prime solution (GE Healthcare) [30, 54]. The blot images were obtained with X-ray film or Image Quant LAS 4000 mini (GE Healthcare). All densitometry was performed using ImageJ software. Antibodies used in this study are described in S1 Text.
Reporter plasmid for Notch signaling activity using a Hes1 promoter (pHes1-luc) was generated in Kageyama’s lab. pCMV-Tag4-mouse Notch1-FLAG was a kind gift from Drs. K. Katsube and K. Sakamoto and subcloned into a pcDNA5/FRT vector. pMXs-rat Dll1-IG was a kind gift from Dr. Y. Goto, and the insert was sub-cloned into a pcDNA5/FRT vector. Rat Dll1 was PCR-amplified from pMXs-rat Dll-IG with new restriction enzyme cutting sites at both ends and with its stop codon deleted, and it was subcloned into a pcDNA3 vector with a C-terminal FLAG-6x His tag (pcDNA3-FLAG-His). For SNAP-Dll1, the SNAP-tag was PCR amplified from a pSNAPf vector (New England Biolabs) and inserted into the extracellular domain of rat Dll1 using In-Fusion cloning kit (Clontech). Plasmids for human LRRK2 have been reported elsewhere [9, 55] and were further sub-cloned into a pEF vector. Full-length cDNA of NEURL4 was obtained from Kazusa DNA Research Institute and sub-cloned into pcDNA3-FLAG-His or pEF. To clone full-length HERC2, total RNA was extracted from HEK293T cells, and cDNA was synthesized using a PrimeScript 1st strand cDNA synthesis kit (Takara Bio). Partial clones were created by RT-PCR using PfuUltraII Fusion HS DNA polymerase (Agilent Technologies) and sequenced. Potential PCR errors were fixed by PCR mutagenesis to code the same amino acid sequences as the reference sequence (NP_004658.3). Full-length HERC2 cDNA was made by the ligation of the partial clones and cloned into pEGFP-C, pcDNA3 and pEF vectors. pEGFP-Rab7 and pEGFP-Rab11 plasmids were kindly provided by Dr. M. Fukuda. Other Rab members were cloned in pEGFP-C in our lab.
Purification and identification of LRRK2-binding proteins
Flp-In 293 cells (Life Technologies) stably expressing FLAG-tagged human LRRK2 or parent cells were grown in suspension culture (Joklik-modified Eagle’s minimum essential medium with 5% (vol/vol) fetal bovine serum [FBS]). The cell pellet (2.4 x 108 cells) was homogenized in lysis buffer (50 mM Tris pH 7.4, 120 mM NaCl, 5 mM EDTA, 10% (vol/vol) glycerol, 1% (vol/vol) Trion-X100) supplemented with Complete protease inhibitor cocktail (Roche Applied Science). The soluble fraction of the suspension was immunoprecipitated with anti-FLAG M2 affinity gel (Sigma-Aldrich) and washed five times in lysis buffer. The fractions eluted with 200 μg ml-1 3x FLAG peptide (Sigma-Aldrich) were resolved using SDS-PAGE. Specific bands detected by silver staining were excised for in-gel digestion. The digest extracted from the gel was subjected to online HPLC-MS/MS, followed by informatics-based identification of the proteins (Nippon Proteomics).
Cell lines
SH-SY5Y and HEK293T cells have been described previously [56, 57]. Mouse embryonic fibroblasts (MEFs) were isolated from LRRK2-deficient mice. HeLa cell line was purchased from Clontech. SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (Sigma-Aldrich) supplemented with non-essential amino acid (NEAA) and 10% (vol/vol) FBS. HEK293T, HeLa cells and MEFs were cultured in DMEM supplemented with NEAA and 10% FBS. CHO cells were cultured in nutrient mixture F-12 (Sigma-Aldrich) supplemented with NEAA and 10% FBS. CHO and HeLa cell lines stably expressing transgenes were generated with the Flp-In system following the manufacturer’s instructions (Life Technologies). Briefly, pFRT/lacZeo plasmid was transfected into CHO or HeLa cells using Lipofectamine LTX (Life Technologies), and Zeocin (Life Technologies) was added to culture medium to select stably transfected cells. Zeocin-resistant cells were picked up and checked for single-site integrations of pFRT/lacZeo into the cell genomes by Southern blot. Among the cells with pFRT/lacZeo single-site integration, the cells with the highest β-galactosidase activity were chosen for further use. To produce individual stable cell lines, the genes of interest were subcloned into pcDNA5/FRT. pcDNA5/FRT plasmids containing the genes of interest were co-transfected with pOG44, and the cells stably expressing the genes of interest were selected with hygromycin. To select and maintain the stability of transfected cells, 300 μg ml-1 hygromycin or 250 μg ml-1 Zeocin for CHO cells and 300 μg ml-1 hygromycin or 150 μg ml-1 Zeocin for HeLa cells were used. MEFs stably expressing SNAP-Dll1 with EGFP-Rab5, EGFP-Rab7 or EGFP-Rab11 were produced by retroviral infection with pMX-puro harboring the transgenes and subsequent puromycin selection. For transient transfection, plasmids and siRNA duplexes (Silencer and Stealth, Life Technologies) were transfected using Lipofectamine 2000 (Life Technologies) and Lipofectamine RNAiMAX (Life Technologies), respectively, according to the manufacturer’s instructions.
Co-immunoprecipitation assays
The indicated plasmids were transfected into HEK293T cells using Lipofectamine 2000 (Life Technologies). Forty-eight hours after transfection, the cells were lysed with RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% (vol/vol) NP-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS), which was supplemented with Complete cocktail. For immunoprecipitation of the FLAG-tagged proteins, cell lysate was added to anti-FLAG gel. For immunoprecipitation of other proteins, specific antibodies were added to the cell or tissue lysates and precipitated with Protein G affinity gel (GE Healthcare). Following incubation at 4°C for 3 h, the gels were washed five times with RIPA buffer. The proteins immunoprecipitated with anti-FLAG gel were eluted with 200 μg ml-1 3x FLAG peptide, and the proteins immunoprecipitated with antibodies other than the anti-FLAG antibody were eluted in SDS-PAGE sample buffer. The eluted proteins were analyzed by SDS-PAGE followed by Western blotting.
Notch reporter assay
SH-SY5Y cells were used as ‘Notch cells’ and co-cultured with CHO cells stably transfected with rat Dll1-HA (Dll1-CHO cells), which were used as ‘Ligand cells’. SH-SY5Y cells were plated in 24-well plates the day prior to transfection. A 20%-confluent monolayer of cells was co-transfected with a reporter plasmid that encoded firefly luciferase in conjunction with the Hes1 promoter (pHes1-Luc) and a plasmid for Renilla luciferase (pGL4-TK-Rluc, Promega) to monitor the transfection efficiency, together with an empty plasmid or plasmids for the LRRK2 complex. Dll1-CHO cells (5 x 104) were added to each well of SH-SY5Y cells 12 h after transfection and co-cultured for an additional 36 h. The co-cultured cells were lysed and subjected to the reporter assay using a dual-luciferase reporter assay system (Promega) and Fluoroskan Ascent FL (ThermoFisher) following the manufacturers’ instructions.
Live cell imaging and image analyses
HeLa cells stably expressing Dll1 with a SNAP-tag in the extracellular region on an 8-well Lab-Tek chamber slide were labeled with 5 μM SNAP-Surface Alexa Fluor 647 for 20 min at 37°C; the cells were subsequently washed three times with normal medium without phenol red. The cell chamber was placed in a CO2 - and temperature-controlled microscope incubator and immediately imaged using a laser-scanning microscope system (LSM780/LSM7 Live, Carl Zeiss) with a 63x/1.2 water-immersion objective. Image stacks of multiple areas were taken every 15 min at 1.8-μm intervals. The Dll1 fluorescence intensity at the cytoplasmic membrane was measured by subtracting the Dll1 intensity at cellular vesicles from the total Dll1 intensity using the Surface tool in Imaris software (Bitplane). The quantifications of colocalized Dll1 with EGFP-Rabs and the quantifications of NICD and Hes1 in EGFP-positive cells were analyzed using the Coloc tool in Imaris software. LRRK2-deficient MEFs stably expressing Dll1-SNAP and Rab-EGFP were transiently transfected with LRRK2 or LacZ twice sequentially using Lipofectamine LTX, which achieved ~95% transfection efficiency estimated by EGFP transfection in parallel. Imaging analysis was performed as in HeLa cells.
Drosophila genetics
Fly culture and crosses were performed on standard fly food containing yeast, cornmeal and molasses, and flies were raised at 25°C unless otherwise indicated. To generate UAS-hLRRK2 mutant transgenic lines, the cDNA for hLRRK2RG, hLRRK2GS and hLRRK2KD from a pcDNA3.1 vector was subcloned into the pUAST vector. The introduction of transgenes into the Drosophila germline and the establishment of transgenic lines into a w−background were performed by BestGene Inc. (Chino Hills, CA). UAS-dHERC2 RNAi, UAS-Blue RNAi, UAS-dLRRK RNAi, UAS-Dl RNAi (#1, v37288; #2, v37287 [58]) and UAS-Notch RNAi (#1, v27229; #2, v1112 [59]) were obtained from the Vienna Drosophila RNAi Center. Dl3 was obtained from the Kyoto Stock Center. Notch-LexAVP16 and LexOP-dGFP were kind gifts of Drs. Lieber and Struhl [14]. UAS-Shits1 was a kind gift of Dr. Kitamoto. All other fly stocks and GAL4 lines used in this study were obtained from the Bloomington Drosophila Stock Center and have been previously described: UAS-dLRRK [9]; e03680 as a dLRRK null allele [9]; UAS-hLRRK2 [60]; and hs-Dl [61]. Full details of Drosophila genotypes used in this study are described in S2 Text.
Generation of a Blue mutant allele
The p{EPgy2}EY12221 insertion line (blueEP line) from the Bloomington Drosophila Stock Center was mobilized using ∆2–3 transposase. We screened for imprecise excision of the p{EPgy2}EY12221 by genomic PCR and identified a new insertion of an additional 11 bp of a genomic fragment containing 7 bp of the 5’ part of the Blue gene and 78 bp of a sequence derived from p{EPgy2} (named blue47b-2).
Dl endocytosis assay
The internalization assay of anti-Dl was performed basically following the reported procedures using hs-Dl crosses at 26°C [27, 33]. Briefly, third instar larvae wing imaginal discs were dissected in Drosophila Schneider’s medium containing 10% FBS and were incubated in the same medium at RT for 15 min with mouse anti-Delta antibody (C594.9B, Developmental Studies Hybridoma Bank, 1 : 100), which recognises the extracellular domain of Dl. The wing discs were washed three times for 15 min in Schneider’s medium with 10% FBS, fixed in 4% paraformaldehyde/PBS, washed three times for 15 min in 0.1% Triton X-100/PBS and visualized with a second antibody (anti-mouse Alexa 647, Life Technologies, 1 : 200). Dpp areas of the wing pouch were imaged using a laser-scanning microscope system (SP5, Leica Microsystems) with a 63x/1.40NA oil-immersion objective. Z-Stack images consisting of 80–100 slices were taken at a step size of 0.5-μm. Reconstituted confocal slices 5 to 8 μm below the apical surface were used for colocalization analysis of Dl with Rab7-GFP or Rab11-GFP, in which GFP and anti-Dl signals were outlined using Photoshop. Overlapped and non-overlapped signals over 0.06 μm2 were extracted using the analyze particles tool in ImageJ.
Drosophila lifespan assay, and climbing and flying assays
Approximately 20 adult flies per vial were maintained at 29°C, transferred to fresh fly food and scored for survival every 2 days. To control for isogeny, the fly lines were generated in the w- wild-type genetic background or were backcrossed to the w- background for six generations to match the genetic background. Climbing and flying assays were performed as previously described [9, 62].
Dopamine measurement
Dopamine measurement of the fly brain tissues was performed as previously described [9].
in utero electroporation and immunohistochemistry
in utero electroporation and immunohistochemistry were performed as previously described [63]. The embryos were sacrificed at 1 or 3 days after electroporation. The brains were excised, fixed in 4% (wt/vol) paraformaldehyde, embedded in OCT compound and sectioned at 16 μm. To detect NICD and Hes1, a TSA Plus Cyanine 3 System (Perkin-Elmer) was used for signal amplification.
Generation of LRRK2-deficient mice
A floxed Neo selection cassette was inserted at the end of exon 15 of murine LRRK2 gene by homologous recombination in TT2 ES cells, which were derived from F1 hybrid of C57BL/6 and CBA mice [64]. The homologous recombination events in the ES cells were confirmed by Southern blot with the 5′ and 3′ external probes and the neo probe. The ES cell clones were then injected into ICR 8-cell stage embryos. The chimeric offspring were crossed to C57BL/6J mice to obtain heterozygous mutant mice. To remove the neo gene and generate a frame-shift (LRRK2 KO) allele, mutant mice were crossed with CAG-Cre mice that express Cre recombinase in mature oocytes [65]. The mutant mice were subsequently backcrossed onto C57BL/6J background for seven generations, which were then intercrossed to obtain homozygous LRRK2 KO mice. Although the expression of an N-terminal fragment of LRRK2 (1–558 aa) with an additional 97 amino acids from the frame-shift allele was envisaged, we confirmed that no signals were detected at the expected migration position in Western blot analysis with an anti-LRRK2 antibody that recognized the N-terminal site of LRRK2. Genotyping of LRRK2 KO mice (Accession No. CDB0609K: http://www.cdb.riken.jp/arg/mutant%20mice%20list.html) was performed by PCR using following primer set (forward, 5’-TGCATACCCAGACATTGAATTATGTTTTACC-3’; reverse, 5’-GGTCCTGATGTTTTGTGCAGG-3’) and WT and mutant alleles were amplified as a 335-bp and a 275-bp DNA fragments, respectively. The LRRK2-deficeint MEF used here was obtained from this mouse line. Detailed information will be provided upon request.
Statistical analysis
Data are expressed in mean values ± SEM. To compare quantitative data of multiple groups, repeated measures one-way ANOVA was performed. p < 0.05 was considered as significant and if a statistically significant difference was detected by one-way ANOVA, the Bonferroni multiple comparison test was done as a post hoc test. Statistical significance of difference between two groups was determined by the two-tailed unpaired Student’s t-test.
Supporting Information
Zdroje
1. Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–7. 15541309.
2. Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41(12):1303–7. Epub 2009/11/17. doi: ng.485 [pii] doi: 10.1038/ng.485 19915576.
3. Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–27. Epub 2010/01/13. doi: S0896-6273(09)00889-7 [pii] doi: 10.1016/j.neuron.2009.11.006 20064389; PubMed Central PMCID: PMC2807409.
4. Zechel S, Meinhardt A, Unsicker K, von Bohlen Und Halbach O. Expression of leucine-rich-repeat-kinase 2 (LRRK2) during embryonic development. Int J Dev Neurosci. 2010;28(5):391–9. Epub 2010/04/21. doi: S0736-5748(10)00062-6 [pii] doi: 10.1016/j.ijdevneu.2010.04.002 20403420.
5. Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ 3rd, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9879–84. Epub 2010/05/12. doi: 1004676107 [pii] doi: 10.1073/pnas.1004676107 20457918; PubMed Central PMCID: PMC2906862.
6. Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Human molecular genetics. 2011;20(21):4209–23. Epub 2011/08/11. doi: ddr348 [pii] doi: 10.1093/hmg/ddr348 21828077; PubMed Central PMCID: PMC3188995.
7. Marin I, van Egmond WN, van Haastert PJ. The Roco protein family: a functional perspective. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(9):3103–10. 18523161.
8. Marin I. The Parkinson disease gene LRRK2: evolutionary and structural insights. Molecular biology and evolution. 2006;23(12):2423–33. doi: 10.1093/molbev/msl114 16966681.
9. Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. The EMBO journal. 2008;27(18):2432–43. 18701920. doi: 10.1038/emboj.2008.163
10. Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Human molecular genetics. 2012;21(6):1350–63. Epub 2011/12/16. doi: ddr573 [pii]doi: 10.1093/hmg/ddr573 22171073; PubMed Central PMCID: PMC3284123.
11. Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nature neuroscience. 2000;3(1):30–40. Epub 1999/12/22. doi: 10.1038/71104 10607392.
12. Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286(5440):741–6. Epub 1999/10/26. doi: 7935 [pii]. 10531053.
13. Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nature reviews Neuroscience. 2011;12(5):269–83. Epub 2011/04/21. doi: nrn3024 [pii]doi: 10.1038/nrn3024 21505516; PubMed Central PMCID: PMC3159580.
14. Lieber T, Kidd S, Struhl G. DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron. 2011;69(3):468–81. doi: 10.1016/j.neuron.2010.12.015 21315258; PubMed Central PMCID: PMC3216490.
15. Pratt EB, Wentzell JS, Maxson JE, Courter L, Hazelett D, Christian JL. The cell giveth and the cell taketh away: an overview of Notch pathway activation by endocytic trafficking of ligands and receptors. Acta Histochem. 2011;113(3):248–55. Epub 2010/02/04. doi: S0065-1281(10)00007-3 [pii]doi: 10.1016/j.acthis.2010.01.006 20122714; PubMed Central PMCID: PMC2939183.
16. del Alamo D, Rouault H, Schweisguth F. Mechanism and significance of cis-inhibition in Notch signalling. Current biology: CB. 2011;21(1):R40–7. Epub 2011/01/11. doi: S0960-9822(10)01298-4 [pii]doi: 10.1016/j.cub.2010.10.034 21215938.
17. Al-Hakim AK, Bashkurov M, Gingras AC, Durocher D, Pelletier L. Interaction proteomics identify NEURL4 and the HECT E3 ligase HERC2 as novel modulators of centrosome architecture. Mol Cell Proteomics. 2012;11(6):M111 014233. doi: 10.1074/mcp.M111.014233 22261722; PubMed Central PMCID: PMC3433907.
18. Li J, Kim S, Kobayashi T, Liang FX, Korzeniewski N, Duensing S, et al. Neurl4, a novel daughter centriole protein, prevents formation of ectopic microtubule organizing centres. EMBO Rep. 2012;13(6):547–53. doi: 10.1038/embor.2012.40 22441691; PubMed Central PMCID: PMC3367236.
19. Martinez-Noel G, Galligan JT, Sowa ME, Arndt V, Overton TM, Harper JW, et al. Identification and proteomic analysis of distinct UBE3A/E6AP protein complexes. Mol Cell Biol. 2012;32(15):3095–106. doi: 10.1128/MCB.00201-12 22645313; PubMed Central PMCID: PMC3434508.
20. Biosa A, Trancikova A, Civiero L, Glauser L, Bubacco L, Greggio E, et al. GTPase activity regulates kinase activity and cellular phenotypes of Parkinson's disease-associated LRRK2. Human molecular genetics. 2013;22(6):1140–56. doi: 10.1093/hmg/dds522 23241358.
21. Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, et al. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS genetics. 2010;6(4):e1000902. doi: 10.1371/journal.pgen.1000902 20386743; PubMed Central PMCID: PMC2851569.
22. Commisso C, Boulianne GL. The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling. Mol Biol Cell. 2007;18(1):1–13. Epub 2006/10/27. doi: E06-08-0753 [pii]doi: 10.1091/mbc.E06-08-0753 17065551; PubMed Central PMCID: PMC1751308.
23. Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132(10):2319–32. Epub 2005/04/15. doi: dev.01825 [pii]doi: 10.1242/dev.01825 15829515.
24. Daskalaki A, Shalaby NA, Kux K, Tsoumpekos G, Tsibidis GD, Muskavitch MA, et al. Distinct intracellular motifs of Delta mediate its ubiquitylation and activation by Mindbomb1 and Neuralized. The Journal of cell biology. 2011;195(6):1017–31. doi: 10.1083/jcb.201105166 22162135; PubMed Central PMCID: PMC3241720.
25. Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell. 2008;15(5):762–72. doi: 10.1016/j.devcel.2008.09.002 19000840.
26. Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122(5):763–73. doi: 10.1016/j.cell.2005.08.017 16137758.
27. Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS biology. 2005;3(4):e96. doi: 10.1371/journal.pbio.0030096 15760269; PubMed Central PMCID: PMC1064853.
28. Stockhausen MT, Sjolund J, Manetopoulos C, Axelson H. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92(4):751–9. Epub 2005/02/03. doi: 6602309 [pii]doi: 10.1038/sj.bjc.6602309 15685243; PubMed Central PMCID: PMC2361888.
29. Huppert SS, Jacobsen TL, Muskavitch MA. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development. 1997;124(17):3283–91. 9310323.
30. Shiba-Fukushima K, Inoshita T, Hattori N, Imai Y. PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila. PLoS genetics. 2014;10(6):e1004391. doi: 10.1371/journal.pgen.1004391 24901221; PubMed Central PMCID: PMC4046931.
31. Rosa JL, Casaroli-Marano RP, Buckler AJ, Vilaro S, Barbacid M. p619, a giant protein related to the chromosome condensation regulator RCC1, stimulates guanine nucleotide exchange on ARF1 and Rab proteins. The EMBO journal. 1996;15(16):4262–73. Epub 1996/08/15. 8861955; PubMed Central PMCID: PMC452152.
32. Rosa JL, Barbacid M. A giant protein that stimulates guanine nucleotide exchange on ARF1 and Rab proteins forms a cytosolic ternary complex with clathrin and Hsp70. Oncogene. 1997;15(1):1–6. doi: 10.1038/sj.onc.1201170 9233772.
33. Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1(6):783–94. 11740940.
34. Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, Balloux F, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432(7020):980–7. doi: 10.1038/nature03160 15616552.
35. Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(28):10793–8. Epub 2006/07/05. doi: 0602493103 [pii]doi: 10.1073/pnas.0602493103 16818890; PubMed Central PMCID: PMC1502310.
36. Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):378–83. Epub 2009/12/08. doi: 0911187107 [pii]doi: 10.1073/pnas.0911187107 19966284; PubMed Central PMCID: PMC2806779.
37. Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007;313(16):3658–70. 17706965.
38. Hanafusa H, Ishikawa K, Kedashiro S, Saigo T, Iemura S, Natsume T, et al. Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nature communications. 2011;2 : 158. Epub 2011/01/20. doi: ncomms1161 [pii]doi: 10.1038/ncomms1161 21245839; PubMed Central PMCID: PMC3105304.
39. Sen S, Webber PJ, West AB. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. The Journal of biological chemistry. 2009;284(52):36346–56. Epub 2009/10/15. doi: M109.025437 [pii]doi: 10.1074/jbc.M109.025437 19826009; PubMed Central PMCID: PMC2794750.
40. Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. The Journal of biological chemistry. 2008;283(24):16906–14. Epub 2008/04/10. doi: M708718200 [pii]doi: 10.1074/jbc.M708718200 18397888; PubMed Central PMCID: PMC2423262.
41. Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). J Neurosci. 2009;29(50):15846–50. Epub 2009/12/18. doi: 29/50/15846 [pii]doi: 10.1523/JNEUROSCI.4357-09.2009 20016100; PubMed Central PMCID: PMC2846613.
42. Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, et al. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28(16):4250–60. Epub 2008/04/18. doi: 28/16/4250 [pii]doi: 10.1523/JNEUROSCI.0066-08.2008 18417705; PubMed Central PMCID: PMC2666311.
43. Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):145–50. doi: 10.1073/pnas.0710685105 18162536; PubMed Central PMCID: PMC2224176.
44. Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–7. doi: 10.1016/j.ajhg.2011.06.001 21763482; PubMed Central PMCID: PMC3135796.
45. Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–75. doi: 10.1016/j.ajhg.2011.06.008 21763483; PubMed Central PMCID: PMC3135812.
46. Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, et al. Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy. Nature communications. 2014;5 : 3828. doi: 10.1038/ncomms4828 24819384; PubMed Central PMCID: PMC4024763.
47. MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron. 2013;77(3):425–39. doi: 10.1016/j.neuron.2012.11.033 23395371; PubMed Central PMCID: PMC3646583.
48. Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69(3):437–44. Epub 2011/02/15. doi: S0896-6273(11)00029-8 [pii]doi: 10.1016/j.neuron.2011.01.004 21315255; PubMed Central PMCID: PMC3056341.
49. Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: at the crossroads. Exp Neurol. 2010;223(2):267–81. Epub 2009/08/25. doi: S0014-4886(09)00327-6 [pii]doi: 10.1016/j.expneurol.2009.08.009 19699201; PubMed Central PMCID: PMC2864344.
50. Veeraraghavalu K, Choi SH, Zhang X, Sisodia SS. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J Neurosci. 2010;30(20):6903–15. Epub 2010/05/21. doi: 30/20/6903 [pii]doi: 10.1523/JNEUROSCI.0527-10.2010 20484632; PubMed Central PMCID: PMC2879010.
51. Winner B, Melrose HL, Zhao C, Hinkle KM, Yue M, Kent C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiology of disease. 2011;41(3):706–16. Epub 2010/12/21. doi: S0969-9961(10)00401-8 [pii]doi: 10.1016/j.nbd.2010.12.008 21168496; PubMed Central PMCID: PMC3059106.
52. Geraerts M, Krylyshkina O, Debyser Z, Baekelandt V. Concise review: therapeutic strategies for Parkinson disease based on the modulation of adult neurogenesis. Stem Cells. 2007;25(2):263–70. doi: 2006–0364 [pii] doi: 10.1634/stemcells.2006-0364 17082225.
53. Yoshimi K, Ren YR, Seki T, Yamada M, Ooizumi H, Onodera M, et al. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 2005;58(1):31–40. Epub 2005/05/25. doi: 10.1002/ana.20506 15912513.
54. Shiba-Fukushima K, Imai Y, Yoshida S, Ishihama Y, Kanao T, Sato S, et al. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Scientific reports. 2012;2 : 1002. doi: 10.1038/srep01002 23256036; PubMed Central PMCID: PMC3525937.
55. Kanao T, Venderova K, Park DS, Unterman T, Lu B, Imai Y. Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Human molecular genetics. 2010;19(19):3747–58. Epub 2010/07/14. doi: ddq289 [pii]doi: 10.1093/hmg/ddq289 20624856.
56. Kanao T, Sawada T, Davies SA, Ichinose H, Hasegawa K, Takahashi R, et al. The Nitric Oxide-Cyclic GMP Pathway Regulates FoxO and Alters Dopaminergic Neuron Survival in Drosophila. PloS one. 2012;7(2):e30958. doi: PONE-D-11-18262 [pii] doi: 10.1371/journal.pone.0030958 22393355; PubMed Central PMCID: PMCPMC3290610.
57. Wang HQ, Imai Y, Kataoka A, Takahashi R. Cell type-specific upregulation of Parkin in response to ER stress. Antioxid Redox Signal. 2007;9(5):533–42. Epub 2007/05/01. doi: 10.1089/ars.2006.1522 17465879.
58. Graves HK, Woodfield SE, Yang CC, Halder G, Bergmann A. Notch signaling activates Yorkie non-cell autonomously in Drosophila. PloS one. 2012;7(6):e37615. doi: 10.1371/journal.pone.0037615 22679484; PubMed Central PMCID: PMC3367968.
59. Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, et al. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458(7241):987–92. doi: 10.1038/nature07936 19363474; PubMed Central PMCID: PMC2988197.
60. Venderova K, Kabbach G, Abdel-Messih E, Zhang Y, Parks RJ, Imai Y, et al. Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson's disease. Human molecular genetics. 2009;18(22):4390–404. Epub 2009/08/21. doi: ddp394 [pii]doi: 10.1093/hmg/ddp394 19692353.
61. Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93(4):649–60. 9604939.
62. Shiba-Fukushima K, Arano T, Matsumoto G, Inoshita T, Yoshida S, Ishihama Y, et al. Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering. PLoS genetics. 2014;10(12):e1004861. doi: 10.1371/journal.pgen.1004861 25474007; PubMed Central PMCID: PMC4256268.
63. Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. The Journal of biological chemistry. 2001;276(32):30467–74. Epub 2001/06/16. doi: 10.1074/jbc.M102420200M102420200 [pii]. 11399758.
64. Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, et al. A novel ES cell line, TT2, with high germline-differentiating potency. Analytical biochemistry. 1993;214(1):70–6. doi: 10.1006/abio.1993.1458 8250257.
65. Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochemical and biophysical research communications. 1997;237(2):318–24. 9268708.
66. Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in neural circuits. 2009;3 : 5. doi: 10.3389/neuro.04.005.2009 19597562; PubMed Central PMCID: PMC2708966.
67. Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72(2):202–30. doi: 10.1016/j.neuron.2011.09.021 22017985; PubMed Central PMCID: PMC3232021.
68. Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. Journal of neurobiology. 2001;47(2):81–92. 11291099.
Štítky
Genetika Reprodukční medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 9
-
Všechny články tohoto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



