-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
Nicotine metabolism rate significantly varies between individuals and affects smoking behavior. Individuals with fast nicotine metabolism typically smoke more and thus have a greater risk for smoking-induced diseases. Further, the efficacy of smoking cessation pharmacotherapy is dependent on nicotine metabolism rate. Twin and family studies have shown that genes influence nicotine metabolism; however, only a minor fraction of variance in inter-individual differences in nicotine metabolism is accounted for by known reduced activity variants in CYP2A6, the main metabolic enzyme for nicotine. Here we utilized a biomarker of nicotine metabolism (nicotine metabolite ratio, NMR) in a genome-wide association study of three Finnish cohorts to identify novel genetic variants influencing nicotine metabolism rate. Our results enclose three independent novel signals in CYP2A6. The detected variants explain a strikingly large fraction of variance (up to 31%) in NMR in the study samples. A genetic risk score constructed using the independent signals predicts smoking quantity in two independent Finnish samples. Further, we enclose evidence for plausible epigenetic mechanisms influencing NMR. With the advent of other nicotine delivery devices than tobacco, such as e-cigarettes, the need to understand the long-term consequences and action mechanisms of nicotine and its metabolism are of high public health relevance.
Published in the journal: . PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005498
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005498Summary
Nicotine metabolism rate significantly varies between individuals and affects smoking behavior. Individuals with fast nicotine metabolism typically smoke more and thus have a greater risk for smoking-induced diseases. Further, the efficacy of smoking cessation pharmacotherapy is dependent on nicotine metabolism rate. Twin and family studies have shown that genes influence nicotine metabolism; however, only a minor fraction of variance in inter-individual differences in nicotine metabolism is accounted for by known reduced activity variants in CYP2A6, the main metabolic enzyme for nicotine. Here we utilized a biomarker of nicotine metabolism (nicotine metabolite ratio, NMR) in a genome-wide association study of three Finnish cohorts to identify novel genetic variants influencing nicotine metabolism rate. Our results enclose three independent novel signals in CYP2A6. The detected variants explain a strikingly large fraction of variance (up to 31%) in NMR in the study samples. A genetic risk score constructed using the independent signals predicts smoking quantity in two independent Finnish samples. Further, we enclose evidence for plausible epigenetic mechanisms influencing NMR. With the advent of other nicotine delivery devices than tobacco, such as e-cigarettes, the need to understand the long-term consequences and action mechanisms of nicotine and its metabolism are of high public health relevance.
Introduction
Nicotine is a neuro-stimulant with high addiction potential [1]. Similar to other drugs causing dependence, nicotine increases dopamine levels in the nucleus accumbens and activates the mesolimbic brain reward pathway [2]. Nicotine metabolism rate varies significantly between individuals and is strongly correlated to total nicotine clearance thus altering nicotine levels from a given intake [3]. Smokers are known to titrate their nicotine levels via cigarette consumption, number and volume of puffs, and depth of inhalation, to achieve and maintain desired levels; thus, nicotine clearance rate influences smoking behavior [4]. Individuals with fast metabolism typically smoke more, are less likely to succeed in quitting, and are more prone to nicotine dependence (ND) [5]. Consequently, those who smoke more have a greater risk for smoking-induced diseases [6]. Beyond the evident toxic effects of tobacco smoke, there is increasing evidence that, in addition to the known acute effects of nicotine [7], chronic nicotine exposure as such may also increase the risk of cancer by multiple mechanisms [8]. With the advent of other nicotine delivery devices than tobacco, such as e-cigarettes, the need to understand the long-term consequences and action mechanisms of nicotine and its metabolism are of high public health relevance.
A member of the cytochrome P450 family, CYP2A6, is the main metabolic enzyme for nicotine accounting for up to 80% of nicotine clearance [9]. A large number of distinct CYP2A6 (ENSG00000255974) alleles have been identified, including SNPs, duplications, deletions, and conversions (www.cypalleles.ki.se/cyp2a6.htm). CYP2A6 variations have been phenotypically grouped as slow (<50% of activity), intermediate (80% of activity), and normal (100% of activity) metabolizers. Another member of the cytochrome P450 family, CYP2B6, has an approximately 10% catalytic efficiency of the CYP2A6 enzyme in vitro in nicotine c-oxidation, and may play a minor role in nicotine clearance at higher nicotine levels [10] or in the absence of functional CYP2A6. While CYP2A6 is expressed primarily in the liver, CYP2B6 (ENSG00000197408) is expressed at higher levels in the brain, where it may influence localized metabolism of nicotine [11, 12]. Cytochrome P450 drug metabolizing enzymes are rarely highlighted in genome-wide association studies (GWAS) as the allele frequencies of the functional variants are low in most populations [13]. However, in 2010 a very large GWAS meta-analysis of smoking quantity revealed associations of CYP2A6 and CYP2B6 with SNPs that are in strong linkage disequilibrium (LD) with known functional variants [14].
Nicotine metabolism involves multiple steps and several enzymatic pathways. Up to 75% of nicotine is converted to cotinine mainly by CYP2A6, 15% of nicotine is metabolized through other metabolic pathways, and a minor fraction (10–15%) is excreted to urine unchanged. The majority of cotinine is further converted to 3-hydroxycotinine exclusively by CYP2A6; up to 40% is excreted to urine as 3-hydroxycotinine while 10% is further metabolized into 3-hydroxycotinine-glucuronide by UGT enzymes prior to excretion. Approximately 15% of cotinine is converted to cotinine-glucuronide by UGT enzymes, and the remaining is metabolized through other pathways. [9]
Cotinine is a relatively stable compound with a half-life of 15–20h, and is superior as a biomarker of nicotine intake compared to self-reported smoking quantity (cigarettes per day, CPD) [15]. The ratio of 3-hydroxycotinine/cotinine (i.e. nicotine metabolite ratio, NMR) is an established biomarker of CYP2A6 activity, as well as nicotine metabolism rate, and it correlates strongly with total nicotine clearance [3]. Twin studies suggest an important genetic contribution to nicotine metabolism, measured using the nicotine metabolite ratio. Swan and colleagues reported that the estimated additive genetic effects on plasma NMR were 0.67 (95% CI 0.56–0.76), dropped to 0.61 (95% CI 0.48–0.71) after adjusting for non-genetic covariates, and further reduced to 0.49 (95% CI 0.33–0.63) when CYP2A6 was adjusted for [16], suggesting that known CYP2A6 variants identified at the time accounted for approximately 15–20% of NMR heritability.
In addition to the strong effect of CYP2A6 and other genetic influences, NMR is also influenced by various demographic and hormonal factors. According to a recent study [17], factors affecting NMR include ethnicity, likely due to the varying frequency of reduced activity CYP2A6 variants among different ethnicities [13]. Other affecting factors include sex, hormone replacement therapy, and use of estrogen containing contraceptive pills, all related to CYP2A6 being induced by estrogen [18], as well as body mass index (BMI), alcohol consumption, and cigarette consumption [17]. Altogether these above mentioned factors were estimated to account for approximately 8% of inter-individual variance in NMR [17].
In addition to significantly affecting smoking behavior, nicotine clearance rate has been shown to contribute to the efficacy of cessation pharmacotherapy in various retrospective studies [19–21]. In a recent randomized prospectively NMR-stratified placebo-controlled clinical trial, varenicline (a prescription medication for smoking cessation) was more efficacious for normal metabolizers compared to nicotine patch [22]. Nicotine patch was equally effective in slow metabolizers with less side-effects than in varenicline treatment, suggesting that tailored pharmacotherapy, i.e. stratifying on NMR level, can be one approach for improving smoking cessation rates [22]. This first clinical trial to take nicotine metabolism rate into account is a landmark paper highlighting the importance of the metabolic component cigarette smoking and use of other nicotine products.
GWAS using metabolites measured from serum have been highly successful in identifying underlying genes [23, 24], highlighting the power of informative phenotypes. Our objective was to utilize NMR, a genetically informed biomarker of nicotine metabolism, in a GWAS meta-analysis of cotinine-verified (≥10ng/ml) current smokers from three Finnish cohorts to identify novel genetic variants influencing nicotine metabolism rate. In Caucasians, with up to 90% of individuals being normal metabolizers, a minor fraction of variance in inter-individual differences in nicotine metabolism is accounted for by known reduced activity CYP2A6 variants. Thus, we expected that other contributing factors, such as other genes, but also novel regulators of CYP2A6 action or expression, including epigenetic mechanisms, are bound to exist. Our data highlighted novel genetic and epigenetic influences on NMR, deepening our understanding on factors influencing nicotine metabolism and providing valuable guidelines for further focused studies.
Material and Methods
Study samples
FinnTwin12
FinnTwin12 is a population-based longitudinal study of five consecutive birth cohorts (1983–1987) of Finnish twins, designed to examine genetic and environmental determinants of health-related behaviors [25, 26]. The study has a two-stage sampling design. The first-stage is an epidemiological investigation, with four waves of data collection (at ages 12, 14, 17, and at early adulthood) providing data on approximately 2700 families with twins. The second-stage is an intensive assessment of a sub-sample nested within the epidemiological study. Most of the sub-sample is selected at random, but this random sample is then enriched with twins from families with a history of elevated familial risk for alcoholism. The 1295 subjects of the intensive sample have DNA available while serum was available on 780 subjects. Among this sample, cotinine and 3-hydroxycotinine were measured from all self-identified current smokers. A total of 211 subjects (55 monozygotic (MZ) individuals (one co-twin from each MZ pair), 80 dizygotic (DZ) individuals from 40 full DZ pairs and additional 76 DZ individuals (one co-twin from each DZ pair)) with cotinine above 10ng/ml and 1000Genomes imputed genome-wide genotype data available were included in the NMR GWAS analyses. Genome-wide Infinium 450k Methylation BeadChip data (from whole blood DNA) were available on 157 of the NMR GWAS subjects.
FinnTwin16
FinnTwin16 is a population–based longitudinal study of five consecutive birth cohorts (1975–1979) of Finnish twins and their families [25, 26]. Initially, 3065 families (6130 twins) were contacted, with a 91% response rate. Questionnaire assessments including detailed alcohol and smoking data were conducted on twins as they reached the ages of 16, 17, 18½, and young adulthood (ages 22–25). For an intensive study on predictors of alcoholism, both twin pairs with very similar alcohol use and twin pairs with dissimilar alcohol use were identified [27, 28]. Additionally, some randomly picked twin pairs functioning as a control group were selected. The 602 subjects of the intensive sample have DNA and serum available. Among this sample, cotinine and 3-hydroxycotinine were measured from all self-identified current smokers, and 174 subjects (40 MZ individuals (one co-twin from each MZ pair), 76 DZ individuals from 38 full DZ pairs, and additional 58 DZ individuals (one co-twin from each DZ pair)) with cotinine above 10ng/ml and 1000Genomes imputed genome-wide genotype data available were included in the NMR GWAS analyses. Genome-wide Infinium 450k Methylation BeadChip data (from whole blood DNA) were available on 14 of the NMR GWAS subjects.
The Young Finns Study (YFS)
The Young Finns Study (YFS) is a large follow-up study of cardiovascular risk factors from childhood to adulthood [29]. In total, 3596 children and adolescents aged 3–18 from all around Finland participated in the baseline study in 1980; they were followed up after, 3, 6, 9, 12, 21, 27, and 30 years, with comprehensive risk factor assessments, including smoking status and alcohol use. Cotinine and 3-hydroxycotinine were measured from all self-identified current smokers with available serum samples, and 714 subjects with cotinine above 10ng/ml and 1000Genomes imputed genome-wide genotype data available were included in the NMR GWAS analyses. For 166 subjects with repeated serum samples as current smoker, i.e. longitudinal metabolite data the NMR was assessed at the time of heaviest regular smoking (i.e. when cotinine was highest), although NMR can be used in light regular smokers [30].
The FINRISK
The FINRISK studies are population surveys of the Finnish adult population conducted every five years since 1972 [31], examining risk factors of chronic diseases. The FINRISK2007 study consists of 6258 men and women aged 25–74 years drawn from the national population register in five large geographical areas in Finland at the end of 2006. The sample was stratified by sex, 10-year age category, and area. FINRISK2007 includes a smoking-specific questionnaire given to a subsample of ever-smokers who attended an in-person clinical examination and blood draw. Cotinine and 3-hydroxycotinine were measured among self-identified current smokers and recent quitters as part of an analysis of the validity of self-reported smoking status. A total of 419 subjects with cotinine above 10ng/ml and 1000Genomes imputed genome-wide genotype data available were included in the NMR GWAS analyses. For scrutiny of linkage disequilibrium (LD) patterns and allele frequencies, as well as for Genetic Risk Score analyses, additional non-overlapping samples from FINRISK cohorts 1992, 1997, 2002, and 2007 (N = 19857 after individuals with pi-hat>0.4 were excluded; cotinine and 3-hydroxycotinine not available in these) with 1000Genomes imputed genome-wide genotype data available were used.
The NAG-FIN
The NAG-FIN sample was ascertained from the Older Finnish Twin Cohort consisting of adult twins born in 1938–1957. Based on earlier questionnaires, twin pairs concordant for ever-smoking were recruited along with their family members (mainly siblings) for the Nicotine Addiction Genetics (NAG) consortium [32]. Data collection took place in 2001–2005. A total of 747 families including 2193 subjects were assessed by DNA sample collection, structured psychiatric interview, and additional questionnaires, yielding detailed phenotypes of lifetime smoking behavior (including initiation, quantity, and cessation). For the Genetic Risk Score analyses 2054 subjects from 745 families (including 141 MZ twins (one co-twin from each MZ pair), 856 DZ individuals from 428 full DZ pairs, additional 147 DZ individuals (one co-twin from each DZ pair), 50 individuals of unknown zygosity, and 860 other family members (mainly siblings)) with 1000Genomes imputed genome-wide genotype data were used. Serum samples for cotinine and NMR analyses were not collected.
Ethics permissions
Written informed consent, according to the current edition of the Declaration of Helsinki, was obtained from all subjects who were interviewed and/or gave DNA samples before the beginning of the studies. The collection of blood samples followed the recommendations given in the Declaration of Helsinki and its amendments. For the FinnTwin12, FinnTwin16, and NAG-FIN studies, data collection has been approved by the hospital district of Helsinki and Uusimaa, the ethics committee for epidemiology and public health (HUS-113-E3-01, HUS-346-E0-05, HUS 136/E3/01). FinnTwin12 and FinnTwin16 have also been approved by the IRB of Indiana University at Bloomington, Indiana, while NAG-FIN was also approved by the IRB of Washington University (St. Louis). Young Finns Study data collection has been approved by the hospital district of Southwest Finland ethics committee (ETMK: no. 88/180/2010). The ethics approvals for FINRISK have been obtained from the Coordinating Ethics Committee of Helsinki and Uusimaa Hospital District.
Phenotypes and covariates
Cotinine and 3-hydroxycotinine phenotypes were measured from frozen serum samples using liquid chromatography/tandem mass spectrometry (University of Toronto, prof. Rachel Tyndale’s laboratory) for the FinnTwin12, FinnTwin16, and YFS samples, as previously described [33], and using gas chromatograph-mass spectrometer (at the National Institute for Health and Welfare, Helsinki, Finland) for the FINRISK2007 sample, as previously described [34]; these assays for NMR were demonstrated to be fully concordant (R2 = 0.93, P<0.0001) [35]. Cotinine threshold of ≥10ng/ml was applied to restrict the analyses to regular smokers.
Several potential factors influencing on NMR (sex, age, BMI, smoking quantity, alcohol consumption, genotyping batch) [17] were considered as covariates. Sex and BMI were associated with NMR (p<0.05) in the initial linear univariate regression models, and were selected as covariates for the study specific GWAS. Further, age was selected as a covariate due to the sampling design of the population-based samples. Neither smoking quantity, alcohol consumption, nor genotyping batch, were significant confounders (p>0.05) and thus were not included as covariates. Details of phenotype and covariate distributions in the three samples are presented in Table 1.
Tab. 1. Phenotype and covariate proportions (%) and means (min-max, SD) in the FinnTwin, Young Finns Study (YFS), and FINRISK2007 samples. 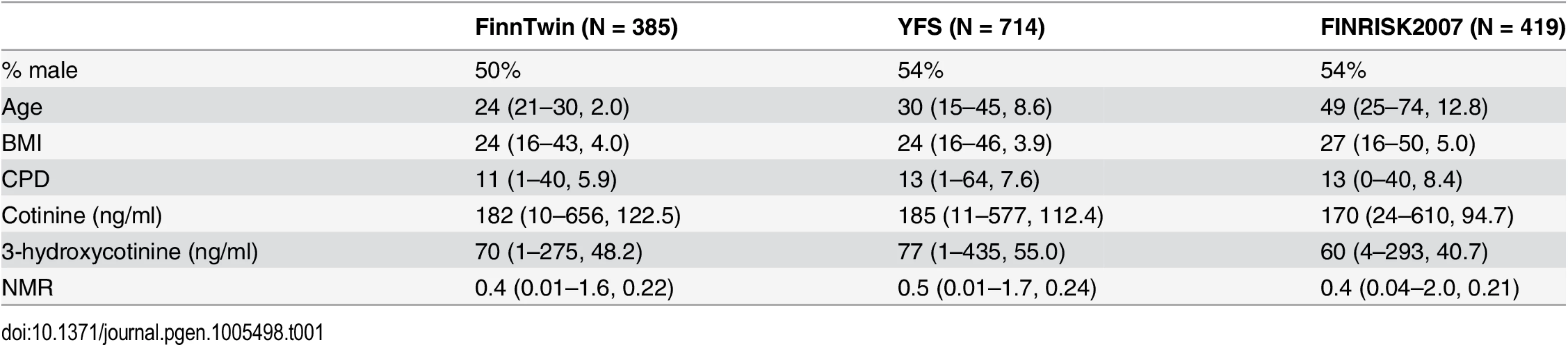
Genotyping and imputation
Genotyping was performed with the Human670-QuadCustom Illumina BeadChip at the Welcome Trust Sanger Institute, and with the Illumina Human Core Exome BeadChip at the Welcome Trust Sanger Institute and at the Broad Institute of MIT and Harvard. Standard post-genotyping quality control thresholds were applied for SNPs (minor allele frequency (MAF) <0.01, SNP call rate <0.95, and Hardy Weinberg Equilibrium (HWE) p<1E-06). Further, subjects with a call rate <0.95 were excluded, and a sample heterozygosity test, as well as sex and Multidimensional Scaling (MDS) outlier checks were done. Pre-phasing of the data was done with SHAPEIT2 [36] and imputation with IMPUTE2 [37] using the 1000 Genomes Phase I integrated haplotypes (produced using SHAPEIT2) reference panel [38]. For data generated with the Human670-QuadCustom Illumina BeadChip the following post-imputation exclusion criteria were applied for SNPs: MAF<0.01, SNP call rate <0.95 (<0.99 for SNPs with MAF<0.05), HWE p<1E-06, and imputation info <0.4. For data generated with the Illumina Human Core Exome BeadChip the SNP exclusion criteria were otherwise identical, except that a threshold of minor allele count <2 was applied instead of a MAF cut-off. Further, the same sample quality thresholds as in post-genotyping quality control were applied. Quality controls and imputation for all Finnish GWAS data were done centrally at the Institute for Molecular Medicine, University of Helsinki, Helsinki, Finland.
Statistical analyses
Twin modelling
Altogether 81 DZ twin pairs (32 same-sex and 49 opposite-sex) and 54 MZ twin pairs with cotinine (>10 ng/ml in both co-twins) and 3-hydroxycotine measures available in the FinnTwin12 and FinnTwin16 samples were used to estimate the heritability of NMR. Intraclass correlations were calculated and quantitative genetic modelling was used to estimate alternative variance components models in Mx [39]. We evaluated the presence of additive genetic (A), genetic effects due to dominance (D), shared environmental effects (C), and environmental effects not shared by twins (E).
Power analyses
We estimated the power of our GWAS meta-analysis sample to detect signals at the p<5E-08 significance threshold assuming an LD (r2) of 0.8 between the causal allele and the marker allele, as previously suggested [40]. To account for intraclass correlation within 78 DZ pairs included in the FinnTwin sample, we estimated the effective sample size (Neff) for these 156 DZ twins using the formula Neff = N/(1 + (m ‐ 1)ICC), where N is the number of DZ twins (156 twins in our case), m is the number of observations in a group (2 in our case), and ICC is the intraclass correlation (0.26 estimated from FinnTwin data), resulting in an estimated effective sample size of 124 (a reduction of 32). Thus, the effective sample size in our meta-analysis was reduced from 1518 to 1486. We performed power analyses using the Genetic Power Calculator [41].
GWAS analyses
Prior to the analyses NMR was transformed using rank transformation to obtain a normal distribution. The transformation was employed with the rntransform() function available in the R statistical software package GenABEL [42], which replaces the median NMR value with the value zero and transforms the rest of the values so that they are N(0,1)-normally distributed around this value. NMR distributions before and after the transformation are shown in S1 Fig.
GWAS analyses were done using GEMMA (Genome-wide efficient mixed-model association) [43], separately for FinnTwin (FinnTwin12 and FinnTwin16 samples were pooled together), Young Finns Study, and FINRISK2007 cohorts. Allelic dosage data were used to account for genotype uncertainties. The genetic associations were acquired with a linear mixed model in which the rank transformed NMR was the dependent variable and the coded allele dose (represented by the posterior mean genotypes) was the independent variable. The model included age, sex, and BMI as covariates (fixed effects). In addition, population stratification and relatedness within the sample were accounted for by the covariance matrix of the random effect in the model. The covariance matrix was determined by a relatedness matrix, calculated from genome-wide genotype data and representing genetic similarity across individuals. An estimate of the genomic control inflation factor (λ) was calculated for all three cohorts with the estlambda() function of the R library GenABEL [42]. P-values below 5E-08 were considered as genome-wide significant.
GWAS meta-analysis
Meta-analysis was performed with the software META [44]. The fixed effects model, using the inverse variance method, was chosen as all cohorts had the same phenotype and measurement scale. Briefly, the cohort-specific beta estimates were summed together by weighting the cohort-specific beta estimates by the inverse of their variance in order to take into account the contribution of each cohort. In addition, the variances of the cohort-specific beta estimates were first multiplied by the cohort-specific genomic control λ estimates. A total of 8970401 SNPs were included in the meta-analysis (the number of SNPs in the cohort-specific GWAS analyses was 9087865 in FinnTwin, 8829569 in YFS, and 9050503 in FINRISK2007). The genomic control inflation factor (λ) in the meta-analysis was 1.027, while in cohort-specific GWAS they were 1.038 in FinnTwin, 1.016 in YFS, and 1.000 in FINRISK2007.
Conditional analyses
Genomic loci reaching genome-wide significance in the meta-analysis were further targeted with conditional analyses to estimate the number of independent signals. We ran association analyses separately in each of the three cohorts conditioning on our SNP with the lowest p-value, followed by a meta-analysis of the three cohorts. The next signal was identified from the conditional meta-analysis results, and included in the second round of conditional analyses. This process was repeated in an iterative fashion until no residual genome-wide significant signal (p<5E-08) remained.
After conditioning on the top-SNP (rs56113850), rs113288603 emerged as the second independent SNP. As this SNP showed no association in either the cohort-specific GWAS or in meta-analysis, we further scrutinized the plausible interplay between rs56113850 and rs113288603 within the largest of our cohorts (YFS), by testing how adding the other SNP or an interaction term (rs56113850*rs113288603) to the model affects the effect sizes.
Percentage of variance explained by the SNPs
Percentage of variance in rank transformed NMR explained by the independent SNPs, as well as the known CYP2A6 alleles [CYP2A6*2 (= rs1801272), CYP2A6*9 (= rs28399433), CYP2A6*14 (= rs28399435), CYP2A6*18 (= rs1809810), CYP2A6*21 (= rs6413474), allele naming according to the CYP2A6 Allele Nomenclature (www.cypalleles.ki.se/cyp2a6.htm)) included in our 1000Genomes imputed data set, was estimated by running linear regression models separately in each of the three cohorts, with NMR as the dependent variable and age, sex, BMI, and SNPs as explanatory variables. The variance explained by a specific SNP or SNPs was acquired by subtracting the R2 from the model including only the covariates (age, sex, BMI) from the R2 of the model including also the SNP/SNPs of interest as explanatory variables.
Genetic risk score analyses
We constructed 3-SNP weighted genetic risk scores (wGRS) using the independent 19q13.2 SNPs from the meta-analysis (rs56113850, rs113288603, esv2663194) by summing the number of major alleles (0/1/2) for each of the three SNPs weighted by their estimated effect sizes obtained from the GWAS meta-analysis. In addition, a 4-SNP wGRS comprising of the four independent SNPs in FINRISK2007 (rs56113850, rs113288603, esv2663194, rs12461964) was constructed. We then tested whether the wGRSs predict smoking behavior using two independent Finnish samples. In the FINRISK sample (no overlap with the FINRISK2007 sample used in NMR GWAS), logistic regression was used to model current daily (N = 3138) vs. former (abstinence of ≥6 months; N = 2710) smoking, and hurdle regression was used to model smoking quantity among current smokers (quantitative CPD; N = 3138). In the family-based NAG-FIN sample, mixed effects logistic regression was used to model current daily (N = 816) vs. former (abstinence of ≥6 months; N = 833) smoking, and a mixed effects poisson regression was used to model smoking quantity among current smokers (quantitative CPD; N = 816). Odds ratios (ORs) obtained for current vs. former smoking depict whether it is more (OR>1) or less (OR<1) likely to fall into category of ‘former smoker’ as wGRS increases. Regression coefficients (betas) obtained for smoking quantity indicate the increase (positive beta) or decrease (negative beta) in CPD as wGRS increases. All analyses were adjusted for age, sex, and BMI, and families were assumed to form clusters in the NAG-FIN sample. Finally, a meta-analysis of the two samples was performed. Additionally, we utilized available questionnaire data of potential confounders of cessation. In a sensitivity analysis we excluded (i) 62 (7.4%) NAG-FIN former smokers who report quitting due to adverse health effects, and (ii) 188 (23%) current and 176 (21%) former smokers who reported having a DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition) major depressive disorder diagnosis. For FINRISK we excluded 820 (26%) current smokers and 775 (29%) former smokers who either (i) reported having a diagnosis of cancer of cardiovascular disease (angina pectoris, heart failure, asthma, pulmonary emphysema, or bronchitis), (ii) reported being on a disability pension, or (iii) reported self-rated health as ‘poor’ or ‘very poor’. Further, we adjusted the analyses for alcohol use, a known confounder, i.e. factor associated with both NMR and smoking behavior (grams of ethanol consumed during the last week in FINRISK, and grams of ethanol consumed on average per week in NAG-FIN). We also ran additional wGRS analyses with an rs56113850*rs113288603 interaction term included as a covariate, to account for the detected interplay between these two SNPs. As four independent SNPs emerged in the FINRISK2007 GWAS sample, and the main wGRS sample was derived from FINRISK cohorts, we report results for the 4-SNP wGRS; however, the 3-SNP wGRS analyses yielded highly similar results.
Annotation
For annotation of the plausible role of the highlighted SNPs, we considered three alternative hypotheses: (A) SNPs directly affect the function of CYP2A6 enzyme, or are in LD with functional variants, resulting in changes in NMR, (B) DNA methylation mediates the effect of SNPs on NMR, and (C) NMR mediates the effect of SNPs on methylation. To test hypothesis A, the potential functional consequences of the associating SNPs were predicted using the Ensembl Variant Effect Predictor database (http://www.ensembl.org/index.html) (includes predictions PolyPhen and SIFT). LD patterns were estimated by using the ‘solid spine of LD’ method of the program Haploview [45] from GWAS data generated in the FINRISK 1992, 1997, 2002, and 2007 cohorts (N = 19857). Similarly, minor allele frequencies representative of the Finnish population were obtained from the FINRISK sample (N = 19857). To test hypotheses B and C we proceeded with methylation quantitative loci analyses and Causal Inference Test (CIT).
Methylation quantitative trait loci (meQTL) analyses
In order to test whether the 719 genome-wide significant SNPs affect methylation we performed meQTL analyses. Methylation probes targeting CpG sites within the 4.2 Mb target region (i.e. the region with genome-wide significant association signal) with 500 kb flanking regions were identified from the Infinium HumanMethylation450 BeadChip (Illumina) data available in 171 FinnTwin12 and FinnTwin16 subjects included in the NMR GWAS. Array preprocessing and normalization was performed using the Bioconductor package ‘minfi’ [46], with Stratified Quantile Normalization using the function PreprocessQuantile. Altogether 2268 methylation probes map to the 4.2 Mb target region; 844 of these were excluded as they have been reported as unreliable based on various criteria (mapping to multiple genomic locations and known repeat regions, containing SNPs, etc.) [47]. Variance in methylation levels was estimated in the remaining 1424 CpG sites; as we had a limited sample size (N = 171) and thus limited power to detect association, we restricted the meQTL analyses to 158 CpG sites with reasonable variance (interquartile range ≥0.05; S2 Fig). We constructed linear regression models of DNA methylation levels at each CpG site as the dependent variable and dosage of coded allele at each SNP as the explanatory variable while adjusting for age and sex, as previously suggested [48, 49]. To account for multiple testing we used the Benjamini and Hochberg method [50] and considered false discovery rate (FDR) corrected p-values below 0.05 as statistically significant. Five CpG sites mapped to CYP2A6 in the genome-wide methylation data; however, we were unable to scrutinize these as four probes were filtered out based on the quality control criteria and the fifth probe had a low (0.02) interquartile range.
Causal Inference Test (CIT)
We proceeded with a CIT with the 16 CpG sites highlighted in the meQTL analyses to establish the direction of relationship between a causal factor (G, genotype), a potential mediator (M, methylation), and an outcome (Y, NMR). Briefly, the conditions for CIT are (1) G and Y are associated, (2) G is associated with M after adjusting for Y, (3) M is associated with Y after adjusting for G, and (4) G is independent of Y after adjusting for M [51]. We performed CIT using the R script provided by Millstein and colleagues [51]. This CIT R script gives a formal p-value for causal model (‘DNA methylation mediates the effect of SNPs on NMR’), reactive model (‘NMR mediates the effect of SNPs on methylation’), and a causal call based on the two p-values obtained. To account for multiple testing we considered FDR corrected p-values below 0.05 as statistically significant.
Results
MZ and DZ twins of the FinnTwin12 and FinnTwin16 cohorts were used to provide heritability estimates for NMR. The intraclass correlation for NMR was 0.80 for MZ and 0.26 for DZ pairs. The pattern of correlations suggested that in addition to additive genetic effects, dominance effects may be present but shared environmental were unlikely to be present. The data were consistent with both AE and ADE models, but not the ACE model as the MZ correlation was much larger than twice the DZ correlation (in such a situation the C component will be zero). In the AE model, A effects accounted for 0.81 (95% CI 0.70–0.88) of variance in NMR. The ADE model fit difference was not statistically significant from the more parsimonious AE model (p = 0.087, Δχ2 = 2.94, Δdf = 1); in the ADE model A effects accounted for 0.20 (95%CI 0.00–0.85) and D effects for 0.62 (95%CI 0.00–0.88) of the variance in NMR.
According to the power analyses we had inadequate power to detect signals with rare SNPs (MAF <5%) unless they have very large effect sizes, but high power to detect signals with common SNPs (MAF >5%) that have medium to high effect sizes (beta>±0.6) (S1 Table).
Each of the three Finnish cohorts independently showed genome-wide significant association on 19q13.2. In our GWAS meta-analysis 719 SNPs exceeded the genome-wide significance threshold within a 4.2 Mb region on 19q13.2 (chr19 : 39546965–43710562; according to GRCh37/hg19) (S2 Table). Manhattan and QQ plots are presented in Figs 1 and 2. The strongest evidence for association emerged for CYP2A6 (minimum p = 5.77E-86 for rs56113850, in intron 4) (S3 Table). Other genes of relevance with genome-wide significant signals included CYP2B6 (minimum p = 1.95E-24 for rs7260329, in intron 8), CYP2A7 (ENSG00000198077) (minimum p = 1.43E-38 for rs28602288, beta = -0.48, -83C>T, within predicted promoter region), EGLN2 (ENSG00000269858) (minimum p = 7.87E-16 for rs76443752, in intron 3), and NUMBL (ENSG00000105245) (minimum p = 1.40E-20 for rs4802082, in intron 5) (S2 Table).
Fig. 1. QQ plot of the GWAS meta-analysis results of the three Finnish cohorts (λ = 1.027). 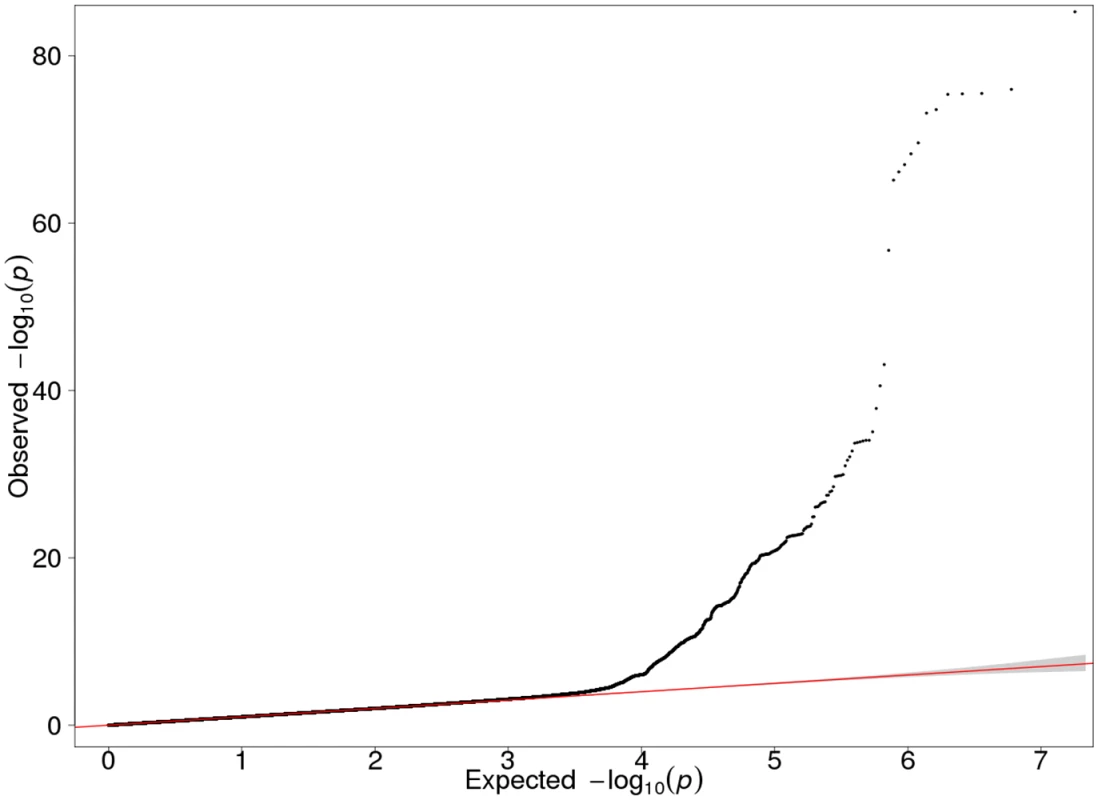
Fig. 2. Manhattan plot of the GWAS meta-analysis results of the three Finnish cohorts. The horizontal line represents the genome-wide significance threshold (p<5E-08). 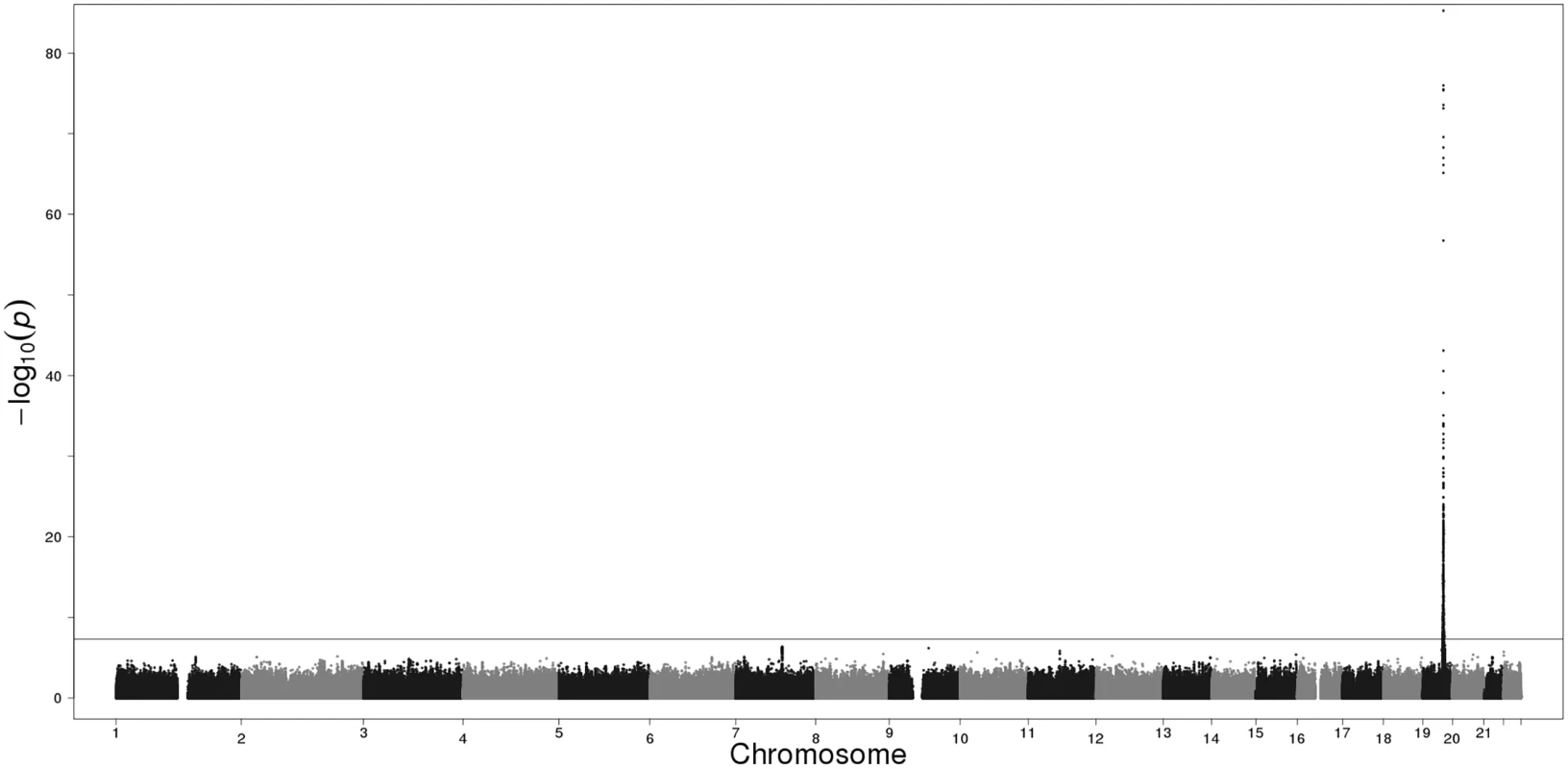
Conditional analyses of the 19q13.2 locus revealed three independent genome-wide significant signals tagged by rs56113850, rs113288603, and esv2663194 (Fig 3 and Table 2). The same independent SNPs emerged in all the three cohorts. A fourth independent signal (rs12461964) emerged in the conditional analysis of the FINRISK2007 sample; however, this was not seen in the meta-analysis of the three samples. All the independent SNPs are imputed, and are located either within CYP2A6 or at most at a distance of 8 kb from the gene. For all the independent SNPs the minor allele decreases NMR, i.e. decreases nicotine clearance rate (Table 2). None of the independent SNPs have predicted functional effects.
Fig. 3. Regional plot of 19q13.2. 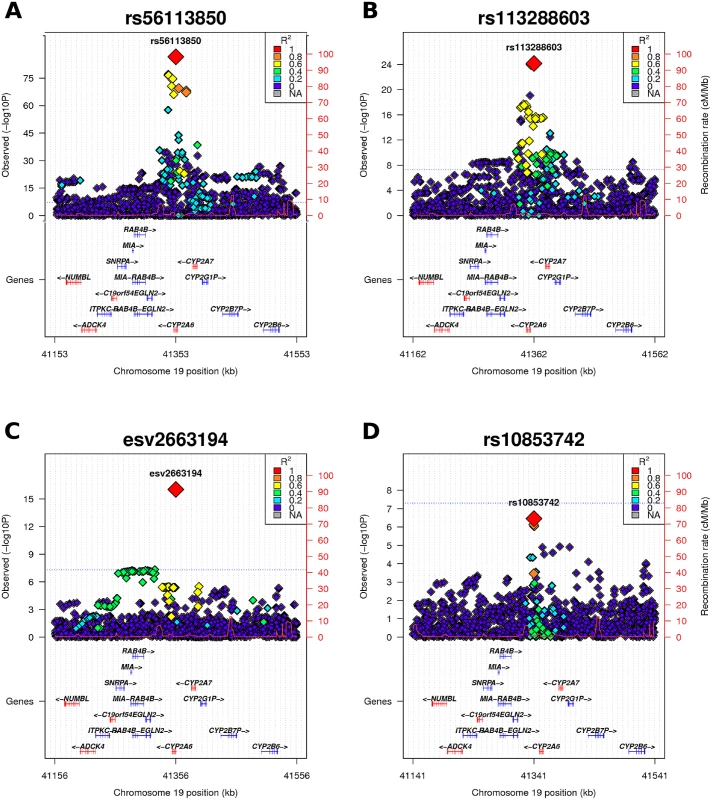
(A) In the GWAS meta-analysis a minimum p-value of 5.77E-86 was obtained for rs56113850, in intron 4 of CYP2A6. (B) Analyses conditioned on the top-SNP (rs56113850) revealed rs113288603 as a second independent genome-wide significant signal (pcond = 7.03E-25). (C) Analyses conditioned on the two independent SNPs (rs56113850, rs113288603) revealed esv2663194 as a third independent genome-wide significant signal (pcond = 9.3E-17). (D) Analyses conditioned on the three independent SNPs yielded no additional signal exceeding the genome-wide significance threshold. All plots are generated with LocusTrack [52]. LD (R2) values were obtained from the FINRISK sample (N = 19857). Tab. 2. Detailed results for the most interesting signals on 19q13.2. 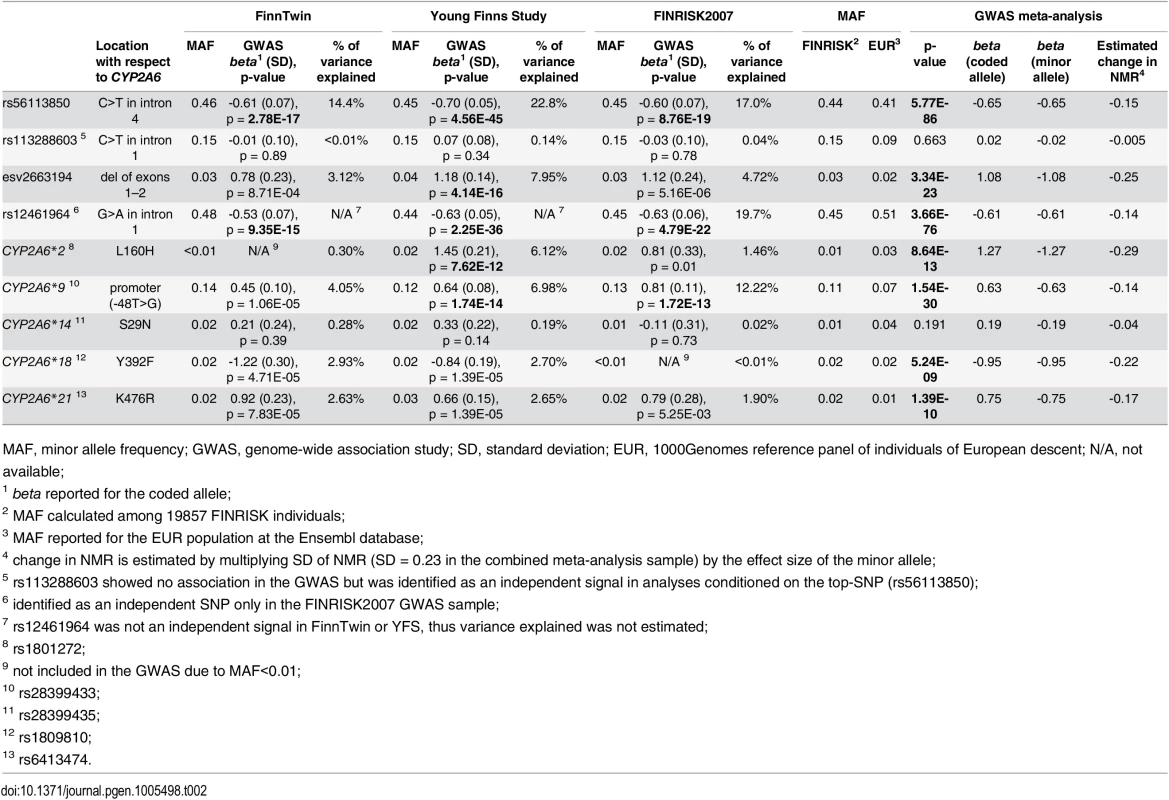
First four rows represent the independent signals in the ranking order from the meta-analysis of conditional analyses. Last five rows represent known CYP2A6 alleles present in our data. Distribution of NMR and rank transformed NMR among the different genotype groups of these nine SNPs in the YFS sample are presented in S6 Fig. A plausible interplay between the top-SNP rs56113850 and rs113288603 was detected. When analysed within the largest of our cohorts (YFS) the minor allelic effect size of rs113288603 increased from non-significant (beta = 0.05, p = 0.522) to highly significant (beta = -0.47, p = 1.32E-09) when rs56113850 was added to the model; when an interaction term (rs56113850*rs113288603) was also added to the model the effect size further increased (beta = -0.62, p = 1.32E-03). Similarly, the effect size of rs56113850 increased from -0.67 (p<2E-16) to -0.83 (p<2E-16) when rs113288603 was added to the model; addition of an interaction term (rs56113850*rs113288603) to the model did not further affect the results (beta = -0.82, p<2E-16).
According to LD estimation in the large FINRISK sample (N = 19857), rs56113850 and rs12461964 share an LD block with the known reduced activity allele CYP2A6*2, and esv2663194 shares a block with the known reduced activity allele CYP2A6*9, while rs113288603 is located outside these LD blocks (S5 Fig). Based on pairwise LD values both CYP2A6*2 and CYP2A6*9 exhibit strong LD with all four independent SNPs (S4 Table), and after conditioning on the top-SNP (rs56113850) neither were genome-wide significant (S5 Table).
Age, sex, and BMI explained altogether 8.9%, 6.1%, and 0.53% of the variance in NMR in FinnTwin, YFS, and FINRISK2007 samples, respectively. The top-SNP rs56113850 alone explains 14–23% of the variance in NMR in the three cohorts (Table 2). Further, the percentage of variance explained by the three independent SNPs (rs56113850, rs113288603, and esv2663194) when jointly included in the model was 20.8% in FinnTwin, 31.4% in YFS, and 26.3% in FINRISK (increasing to 27.7% when rs12461964 was included).
In the wGRS analyses highly similar results were obtained for the 3-SNP and 4-SNP wGRSs; only results for the 4-SNP wGRS are presented. For ease of interpretation, the wGRS was constructed as a weighted sum of major alleles (all of which increase nicotine clearance rate). In the wGRS meta-analysis of 3954 current smokers statistically significant association was detected for quantitative CPD (beta = 0.10, 95% CI 0.04–0.16, p = 0.0019), suggesting that individuals with faster metabolism smoke more. The wGRS meta-analysis for current (N = 3954) vs. former (N = 3543) smoking showed association with increased likelihood of being a former smoker (OR = 1.39, 95% CI 1.09–1.76, p = 0.007). Similarly, the major allele of CYP2A6*2 showed a trend of association with increased likelihood of being a former smoker (OR = 1.03, 95% CI 0.72–1.47, p = 0.89), although the results were not statistically significant. To scrutinize potential confounders for cessation, we utilized available questionnaire data and excluded NAG-FIN individuals who either have a DSM-IV major depression disorder diagnosis or report quitting due to adverse health consequences and FINRISK individuals who have a relevant somatic diagnosis, are on disability pension, or rate their health as ‘poor’. Further, we adjusted the analyses for alcohol consumption. After these exclusions, 2946 current and 2591 former smokers remained, and the wGRS result no longer was statistically significant (OR = 1.30, 95% CI 0.95–1.78, p = 0.10). We also tested these models with the rs56113850*rs113288603 interaction term included as a covariate; in all the models the interaction term was non-significant (p>0.05) and so a more complex model was not justified.
In the meQTL analyses of the 719 genome-wide significant SNPs and 158 CpG sites within the target region, methylation values of 16 CpG sites showed statistically significant association with 173 of the SNPs (FDR corrected p<0.05) (S6 Table). Among the highlighted genes were EGLN2, CYP2A7, CYP2F1 (Cytochrome P450, Family 2, Subfamily F, Polypeptide 1) (ENSG00000197446), and DLL3 (Delta-like 3 (Drosophila)) (ENSG00000090932) (S7 Table). To distinguish between our hypotheses B (‘SNPs affect NMR via methylation’) and C (‘SNPs affect methylation via NMR’) we performed CIT, and confirmed that methylation at the CpG site tagged by cg08551532 (in DLL3) mediates the effect of SNPs on NMR (S8 Table). We detected no evidence supporting hypothesis C.
Discussion
Nicotine metabolism rate is one of the key factors affecting smoking behavior, and has also been shown to contribute to the efficacy of cessation pharmacotherapy [22]. Many smokers find it exceedingly difficult to succeed in quitting, even when they have a major cardiovascular illness and smoking cessation would greatly improve their prognosis [53]. Current pharmacotherapies and behavioral counselling enhance smoking cessation rate on average by only approximately 2-fold [54], highlighting the need for more effective cessation support. Unraveling the genetic architecture of nicotine metabolism may enhance development of tailored smoking cessation pharmacotherapies.
MZ and DZ twins of FinnTwin12 and FinnTwin16 cohorts yielded a heritability estimate of 0.81 for NMR, confirming that genetic effects are major determinants of inter-individual variance in NMR. This estimate is higher than previous estimates obtained in an experimental setting [16], perhaps due to less heterogeneity in the Finnish sample. Our aim was to identify novel genetic variants affecting nicotine metabolism. We utilized NMR, a biomarker of nicotine metabolism, in a GWAS meta-analysis of three Finnish cohorts, and identified association on 19q13.2. This locus harbours a number of genes, including several members of the cytochrome P450 gene family. Three independent genome-wide significant signals were detected, all located either within or in the immediate vicinity of CYP2A6, the gene encoding the main metabolic enzyme for nicotine. A fourth independent signal in CYP2A6 emerged in one of the samples (FINRISK2007); however, this was not seen in the meta-analysis. The minor alleles of all of the independent variants associated with decreased NMR values, i.e. decreased nicotine clearance rate. All the independent variants are novel signals and have not been previously been highlighted in any smoking-related GWAS.
Our top-SNP (rs56113850) is located in intron 4 of CYP2A6, has a high minor allele frequency (MAFFINRISK = 0.44) and a prominent effect size (beta = -0.65), and alone accounts for a substantial percentage of variance (14–23%) in NMR in the three Finnish cohorts. Our second independent SNP (rs113288603) is located 5.9 kb upstream of CYP2A6, seems to be enriched in the Finnish population (MAFFINRISK = 0.15 vs. MAFEUR = 0.09), has a small effect size (beta = -0.02), and accounts for less than 1% of variance in NMR in the three cohorts. Neither of these variants has predicted functional consequences. Interestingly, our data support a plausible interplay between rs113288603 and rs56113850, as the effect sizes significantly increase when the other SNP is added to the model. These findings are in line with our GWAS data, as rs113288603 shows no association in any of the cohort-specific GWAS or in the meta-analysis, but in an analysis conditioned on rs56113850 it emerges as the second independent genome-wide significant SNP. The plausible mechanism underlying the interplay remains to be determined.
Our third independent variant (esv2663194) tags a 32 kb deletion that affects both CYP2A6 and CYP2A7. Esv2663194 has a low minor allele frequency (MAFFINRISK = 0.03) and a prominent effect size (beta = -1.08), and accounts for 3–8% of variance in NMR in the three cohorts. The 32 kb (chr19 : 41355715–41387669, according to GRCh37/hg19) deletion abolishes exons 1–2 in CYP2A6 and exons 2–9 in CYP2A7 when compared to the reference sequence; both genes have multiple isoforms and the consequence of the deletion varies between the isoforms. The 32 kb deletion may produce a similar construct as CYP2A6*12, which is a hybrid allele formed by an unequal crossover between CYP2A6 and CYP2A7. CYP2A6*12 is composed of the 5′-regulatory region and exons 1–2 of CYP2A7 and exons 3–9 and the 3′-regulatory region of CYP2A6, and harbors 10 amino acid differences when compared to the wild-type CYP2A6 allele, with an allele frequency of 2.2% reported among Spaniards [51]. CYP2A6*12 is shown to have reduced enzyme activity in vivo [55, 56]; in line with this, the minor allele of esv2663194 associates with decreased clearance rate. Further studies are needed to confirm whether the 32 kb deletion tagged by esv2663194 indeed creates a construct with properties similar to CYP2A6*12.
Our fourth independent SNP (rs12461964; detected in the FINRISK2007 sample) is located 8.2 kb downstream of CYP2A6, has a high minor allele frequency (MAFFINRISK = 0.45) and a prominent effect size (beta = -0.61). Rs12461964 is in high LD with the top-SNP rs56113850 (D’ = 0.85); although it was identified as an independent SNP in conditional analyses in the FINRISK2007 sample, the detected association likely reflects LD with rs56113850.
To date, the most widely studied CYP2A6 variants include CYP2A6*2 (rs1801272, L160H) which encodes a catalytically inactive enzyme, a whole gene deletion allele CYP2A6*4, CYP2A6*9 (rs28399433, -48T>G), which has an alteration in the TATA box resulting in lower expression of a structurally normal protein, and CYP2A6*12 discussed above [13]. Of these characterized reduced-activity variants, our data included CYP2A6*2 and CYP2A6*9, both of which showed genome-wide significant association but were not identified as independent signals in conditional analyses. A previous very large (N = 85997) meta-analysis of self-reported CPD showed genome-wide significant association on 19q13.2, with strongest evidence obtained for rs4105144, which is in LD with CYP2A6*2 (D′ = 1.0 in CEU) [14]. Similarly, LD between our novel independent signals and both CYP2A6*2 and CYP2A6*9 was high (D’ = 0.89–1.00). The percentage of variance in NMR explained by CYP2A6*2 and CYP2A6*9 in our study cohorts was up to 6% and 12%, respectively. A previous study using an ethnically diverse sample suggested that 15–20% of variance in NMR is accounted for by the four characterized reduced-activity variants (CYP2A6*2, CYP2A6*4, CYP2A6*9, and CYP2A6*12) [16]; much of the genetic variation would not have been tested for in this study (i.e. CYP2A6*10 –*35). Interestingly, in our study CYP2A6*9 alone explained a large fraction of variance in NMR, possibly due to the higher minor allele frequency in the Finnish cohorts (MAF 0.12–0.14) compared to that reported in the Caucasian population (MAFEUR = 0.07). In addition to CYP2A6*2 and CYP2A6*9, our data set included three additional known alleles, CYP2A6*14 (rs28399435; S29N), CYP2A6*18 (rs1809810; Y392F), and CYP2A6*21 (rs6413474; K476R). Although they are non-synonymous variants, their effect on nicotine clearance likely is minimal. CYP2A6*14 does not appear to affect enzyme activity [57, 58], and although CYP2A6*18 has a decreased activity towards another substrate, coumarin, activity towards nicotine is unaffected [59]. Based on in vivo studies, CYP2A6*21 showed normal activity in a Caucasian population [60]. After conditioning on our top-SNP (rs56113850), none of the five known alleles were genome-wide significant, suggesting that rs56113850 captures information on all of these alleles.
The percentage of variance explained by our novel independent SNPs was very high (up to 31%), exceeding the estimates for the four functional variants frequently genotyped in Caucasians [16]. Our novel variants likely tag multiple functional variants, both known and unidentified ones, and thus capture information on relevant haplotypes. The population-attributable effect of the novel independent variants thus is far greater than that of the known functional variants, and for the purpose of estimating the rate of nicotine metabolism our novel SNPs are more informative than the known previously characterized reduced-activity variants.
Based on our twin modelling, the heritability of NMR is 0.81. Although our novel independent SNPs capture a strikingly large fraction of this, major fraction still remains unaccounted for. This may be due to limitations of GWAS with lack of coverage of CYP2A6 duplications, translocations, and rare variants. Further, the high sequence homology between CYP2A6, CYP2A7, and CYP2A13 may prohibit detection of variants within homologous regions. SNP genotyping technologies using short probes will not be able to detect these variants with high specificity; most likely such variants will fail the HWE threshold, and thus will be excluded from analyses. In addition, limitations of statistical power result in inability to detect all relevant signals. In our GWAS meta-analysis we had high power to detect signals with common SNPs (MAF >5%) that have medium to high effect sizes. This is reflected in our top-SNPs, three of which are not only common but also have large effect sizes, and one that is rare (MAF = 0.03) but has a large effect size (beta = -1.08). We had very low power to detect signals with rare SNPs that have low effect sizes, and it is likely that we missed those signals; larger studies are needed to capture these signals.
Ethnicity has been reported as a prominent factor affecting NMR; however, all the subjects in the current study were Caucasian (Finns). Age, sex, and BMI accounted for 8.9% and 6.1% of variance in NMR in the FinnTwin and YFS samples, respectively. This is in line with a previous study showing that various non-genetic factors account for altogether 8% of inter-individual variance in NMR [17]. However, in the FINRISK2007 sample age, sex, and BMI only accounted for 0.5% of variance in NMR, which may be due to older age and longer overall duration of smoking with more cessation than among the young adult FinnTwin and YFS samples.
Although only variants in CYP2A6 were highlighted as independent signals, other interesting genes, such as CYP2B6, CYP2A7, EGLN2, and NUMBL, reside within the 19q13 locus, and showed genome-wide significant association in our GWAS meta-analysis. Our top-SNP in CYP2B6 (rs7260329) has been highlighted in the large GWAS meta-analysis of CPD [14]. CYP2B6 can also catalyze nicotine metabolism to cotinine and to nornicotine [9]; the CYP2B6 gene sits adjacent to CYP2A6 and they share some common regulation. CYP2B6 also metabolizes several other drugs of abuse, as well as bupropion, an atypical antidepressant also used as a smoking cessation aid [5]. Several functional CYP2B6 variants have been identified. The most prevalent and clinically important variant is CYP2B6*6, characterized as a haplotype consisting of two linked non-synonymous variants CYP2B6*4 (rs2279343 (K262R)) and CYP2B6*9 (rs3745274 (Q172H)), resulting in a splice site variant with reduced function [61]. In the current study the non-synonymous SNPs defining CYP2B6*4, CYP2B6*9, as well as CYP2B6*5 (rs3211371 (R487C)) did not show genome-wide significant results, but were in high LD with the top-SNP in CYP2B6 (rs7260329) (S9 Fig). Further, rs7260329 shows modest LD with our independent SNPs rs56113850 (D’ = 0.40), esv2663194 (D’ = 0.65), and rs12461964 (D’ = 0.43), but not with rs113288603 (D’ = 0.04); thus, it is unclear whether the detected association simply reflects LD with the independent variants residing in CYP2A6.
The interpretation of the detected CYP2A7 association is challenging, as the possible role of CYP2A7 in nicotine metabolism is unknown. Our top SNP in CYP2A7 (rs28602288) is located within a predicted promoter region. It is plausible that the association between rs28602288 and NMR reflects the regulation of CYP2A6*12 (a hybrid allele of CYP2A6 and CYP2A7) or similar constructs, such as the one generated by the 32 kb deletion tagged by esv2663194, rather than implies a role for CYP2A7 in nicotine clearance. Rs28602288 is in LD with all of the independent variants (D’ = 0.67–1.00).
Two additional interesting genes were highlighted. Egl-9 Family Hypoxia-Inducible Factor 2 (EGLN2) is a key component of the oxygen-sensing pathway that regulates the expression of various downstream genes, and responses e.g. to carbon monoxide (CO) and cigarette smoke exposure. In a recent study, EGLN2 was associated with CPD and breath CO independent of CYP2A6, although it was not associated with nicotine metabolism [62]. The top-SNP in EGLN2 (rs76443752) is in LD with all of the independent CYP2A6 variants (D’ = 0.67–1.00). Another potentially interesting candidate is Numb Homolog (Drosophila)-Like (NUMBL) which encodes a protein that maintains progenitor cells during cortical neurogenesis [63] and has previously been implicated in lung cancer [64, 65]. The top-SNP in NUMBL (rs4802082) is in LD with all of the independent variants (D’ = 0.48–0.96).
We constructed wGRS using the independent 19q13.2 SNPs and tested whether the wGRS predicts smoking behavior in two independent Finnish samples. The wGRS constructed of major alleles (increasing the metabolism rate) was associated with increased smoking quantity. This is in line with previous evidence of faster metabolizers smoking more [66]. Adding the rs56113850*rs113288603 interaction term to the wGRS analyses did not improve model fit, suggesting that the simpler model with main effects was sufficient. Our unadjusted wGRS results for current vs. former smoking suggested that major alleles that associate with faster metabolism associate with increased odds of being a former smoker; after adjustment and exclusion for potential confounders the wGRS result no longer was significant. Although the sample size decreased, the confidence intervals did not significantly increase, suggesting that the change in results is due to reduced confounding rather than reduced power. Many clinical trials and epidemiological studies indicate that slow metabolizers quit more often than normal metabolizers [20, 67, 68]. Possibly our wGRS does not capture all the relevant aspects of measured NMR or the cross-sectional nature of the FINRISK smoking status data did not permit us to fully replicate earlier findings.
We followed up the 719 genome-wide significant SNPs by meQTL analyses in order to annotate their potential functional consequence. As smoking is known to induce significant changes in methylation patterns [69], only cotinine-verified current smokers (cotinine≥10ng/ml) were included in the analyses. Several SNPs showed significant association with methylation values of 16 CpG sites located within the target region. The 16 CpG sites overlap with relevant genes, such as members of the cytochrome P450 gene family (CYP2F1 and CYP2A7) and EGLN2. According to CIT, methylation in one CpG site mediate the effect of SNPs on the variance observed in NMR. This CpG site (tagged by cg08551532) is located in DLL3, which is involved in neurogenesis via its role in the Notch signaling pathway [70]. Further, DLL3 has been shown to be silenced by methylation in human hepatocellular carcinoma (HCC), leading to restricted growth of cancer cells [71]. Expression of NOTCH3, encoding for a receptor for DLL3, has been shown to be influenced by cigarette smoke [72]. The potential mechanism how DLL3 may affect nicotine metabolism remains to be determined.
To our knowledge this is the first GWAS of NMR reported to date. Despite a relatively small sample size (N = 1518), we detected a multitude of genome-wide significant signals on 19q13.2, highlighting the power of informative biomarkers in GWAS and demonstrating the power of Finnish population samples to identify novel genes even with a modest sample size. Our genetic and epigenetic analyses enclosed several genes as potential players in regulating NMR. Four members of the cytochrome P450 gene family (CYP2A6, CYP2B6, CYP2A7, and CYP2F1) were highlighted, although only CYP2A6 encompassed independent signals. Three additional highlighted genes (DLL3, NUMBL, and EGLN2) are functionally linked according to the GeneMania database (http://www.genemania.org/), suggesting that an interplay between these genes may influence NMR. Future studies are needed to elucidate the potential role of these genes in NMR. The detected novel CYP2A6 variants explain a strikingly large fraction of variance (up to 31%) in NMR in the current sample, suggesting that they tag both known and unidentified functional variants. The population-attributable effect of the detected independent variants is thus far greater than that of the four functional variants frequently genotyped in Caucasians (CYP2A6*2, *4, *9, and *12). Further, we enclose evidence for plausible epigenetic mechanisms on 19q13.2 influencing NMR.
Supporting Information
Zdroje
1. Moss HB, Chen CM, Yi HY. Measures of substance consumption among substance users, DSM-IV abusers, and those with DSM-IV dependence disorders in a nationally representative sample. J Stud Alcohol Drugs. 2012;73(5): 820–28. 22846246
2. Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009;32(5): 269–76. 19834992
3. Dempsey D, Tutka P, Jacob P 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1): 64–72. 15229465
4. Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2): 234–38. doi: 10.1158/1055-9965.EPI-10-0674 21212060
5. Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23(3): 252–61. doi: 10.1080/01677060802572887 19169923
6. Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007;7(2): 81–98. 17224913
7. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49 : 57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742 18834313
8. Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14(6): 419–29. doi: 10.1038/nrc3725 24827506
9. Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1): 79–115. 15734728
10. Yamazaki H, Inoue K, Hashimoto M, Shimada T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch Toxicol. 1999;73(2): 65–70. 10350185
11. Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45(1): 122–32. 12814665
12. Garcia KL, Coen K, Miksys S, Lê AD, Tyndale RF. Effect of Brain CYP2B Inhibition on Brain Nicotine Levels and Nicotine Self-Administration. Neuropsychopharmacology. 2015;40(8):1910–8. doi: 10.1038/npp.2015.40 25652250
13. Mwenifumbo JC, Tyndale RF. Molecular genetics of nicotine metabolism. Handb Exp Pharmacol. 2009;192 : 235–59. doi: 10.1007/978-3-540-69248-5_9 19184652
14. Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5): 448–53. doi: 10.1038/ng.573 20418888
15. Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192 : 29–60. doi: 10.1007/978-3-540-69248-5_2 19184645
16. Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3'hydroxycotinine to cotinine in plasma and urine. Pharmacogenet Genomics. 2009;19(5): 388–98. doi: 10.1097/FPC.0b013e32832a404f 19300303
17. Chenoweth MJ, Novalen M, Hawk LW Jr, Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(9): 1773–82. doi: 10.1158/1055-9965.EPI-14-0427 25012994
18. Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35(10): 1935–41. 17646279
19. Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–8. 16765148
20. Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(3): 320–25. doi: 10.1038/clpt.2008.57 18388868
21. Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1): 6–11. doi: 10.1016/j.pbb.2008.10.016 19000709
22. Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2): 131–38. doi: 10.1016/S2213-2600(14)70294-2 25588294
23. Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikäinen LP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44(3): 269–76. doi: 10.1038/ng.1073 22286219
24. Demirkan A, Henneman P, Verhoeven A, Dharuri H, Amin N, van Klinken JB, et al. Insight in genome-wide association of metabolite quantitative traits by exome sequence analyses. PLoS Genet. 2015;11(1): e1004835. doi: 10.1371/journal.pgen.1004835 25569235
25. Kaprio J. Twin studies in Finland 2006. Twin Res Hum Genet. 2006;9(6): 772–77. 17254406
26. Kaprio J. The Finnish Twin Cohort Study: an update. Twin Res Hum Genet. 2013;16(1): 157–62. doi: 10.1017/thg.2012.142 23298696
27. Latvala A, Tuulio-Henriksson A, Dick DM, Vuoksimaa E, Viken RJ, Suvisaari J, et al. Genetic origins of the association between verbal ability and alcohol dependence symptoms in young adulthood. Psychol Med. 2011;41(3): 641–51. doi: 10.1017/S0033291710001194 20529418
28. Dick DM, Aliev F, Viken R, Kaprio J, Rose RJ. Rutgers alcohol problem index scores at age 18 predict alcohol dependence diagnoses 7 years later. Alcohol Clin Exp Res. 2011;35(5): 1011–4. doi: 10.1111/j.1530-0277.2010.01432.x 21323682
29. Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37(6): 1220–6. doi: 10.1093/ije/dym225 18263651
30. Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30(6): 386–89. 16872570
31. Borodulin K, Vartiainen E, Peltonen M, Jousilahti P, Juolevi A, Laatikainen T, et al. Forty-year trends in cardiovascular risk factors in Finland. Eur J Public Health. 2015;25(3):539–46. doi: 10.1093/eurpub/cku174 25422363
32. Loukola A, Wedenoja J, Keskitalo-Vuokko K, Broms U, Korhonen T, Ripatti S, et al. Genome-wide association study on detailed profiles of smoking behavior and nicotine dependence in a twin sample. Mol Psychiatry. 2014;19(5): 615–24. doi: 10.1038/mp.2013.72 23752247
33. St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P 3rd, Aziziyeh A, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21(7): 1105–14. doi: 10.1158/1055-9965.EPI-12-0236 22552800
34. Broms U, Pennanen M, Patja K, Ollila H, Korhonen T, Kankaanpää A, et al. Diurnal Evening Type is Associated with Current Smoking, Nicotine Dependence and Nicotine Intake in the Population Based National FINRISK 2007 Study. J Addict Res Ther. 2012 Jan 25;S2. pii: 002. 22905332
35. Tanner J-A, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J, et al. Agreement and association between measures of the nicotine metabolite ratio by different analytical approaches in plasma and urine: Implications for clinical implementation. Cancer Epidemiology, Biomarkers & Prevention, in press
36. Delaneau O, Howie B, Cox AJ, Zagury JF, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93(4): 687–96. doi: 10.1016/j.ajhg.2013.09.002 24094745
37. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6): e1000529. doi: 10.1371/journal.pgen.1000529 19543373
38. Genomes Project Consortium, Abecasis GR, Auton A Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422): 56–65. doi: 10.1038/nature11632 23128226
39. Neale MC, Boker SM, Xie G, Maes HH (2006). Mx: Statistical Modeling. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry. 7th Edition.
40. Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, et al. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41(2): 216–20. doi: 10.1038/ng.275 19122664
41. Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003;19(1): 149–50. 12499305
42. Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23(10): 1294–96. 17384015
43. Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44(7): 821–24. doi: 10.1038/ng.2310 22706312
44. Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5): 436–40. doi: 10.1038/ng.572 20418889
45. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2): 263–65. 15297300
46. Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10): 1363–69. doi: 10.1093/bioinformatics/btu049 24478339
47. Naeem H, Wong NC, Chatterton Z, Hong MK, Pedersen JS, Corcoran NM, et al. Reducing the risk of false discovery enabling identification of biologically significant genome-wide methylation status using the HumanMethylation450 array. BMC Genomics. 2014;15 : 51. doi: 10.1186/1471-2164-15-51 24447442
48. Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 2011;12(1): R10. doi: 10.1186/gb-2011-12-1-r10 21251332
49. Almli LM, Stevens JS, Smith AK, Kilaru V, Meng Q, Flory J, et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5): 327–36. doi: 10.1002/ajmg.b.32315 25988933
50. Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Metallurgy) 1995;57(1): 289–300.
51. Millstein J, Zhang B, Zhu J, Schadt EE. Disentangling molecular relationships with a causal inference test. BMC Genet. 2009;10 : 23. doi: 10.1186/1471-2156-10-23 19473544
52. Cuellar-Partida G, Renteria ME, MacGregor S. LocusTrack: Integrated visualization of GWAS results and genomic annotation. Source Code Biol Med. 2015;10 : 1. doi: 10.1186/s13029-015-0032-8 25750659
53. Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S, et al. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168(18): 1961–67. doi: 10.1001/archinte.168.18.1961 18852396
54. Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction. 2013;108(10): 1711–21. doi: 10.1111/add.12291 23834141
55. Oscarson M, McLellan RA, Asp V, Ledesma M, Bernal Ruiz ML, Sinues B, et al. Characterization of a novel CYP2A7/CYP2A6 hybrid allele (CYP2A6*12) that causes reduced CYP2A6 activity. Hum Mutat. 2002;20(4): 275–83. 12325023
56. Benowitz NL, Swan GE, Jacob P 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80(5): 457–67. 17112802
57. Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80(3): 282–97. 16952495
58. Dempsey DA, St Helen G, Jacob P 3rd, Tyndale RF, Benowitz NL. Genetic and pharmacokinetic determinants of response to transdermal nicotine in white, black, and Asian nonsmokers. Clin Pharmacol Ther. 2013;94(6): 687–94. doi: 10.1038/clpt.2013.159 23933970
59. Fukami T, Nakajima M, Higashi E, Yamanaka H, Sakai H, McLeod HL, et al. Characterization of novel CYP2A6 polymorphic alleles (CYP2A6*18 and CYP2A6*19) that affect enzymatic activity. Drug Metab Dispos. 2005;33(8): 1202–10. 15900015
60. Al Koudsi N, Mwenifumbo JC, Sellers EM, Benowitz NL, Swan GE, Tyndale RF. Characterization of the novel CYP2A6*21 allele using in vivo nicotine kinetics. Eur J Clin Pharmacol. 2006;62(6): 481–84. 16758265
61. Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, et al. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2008;325(1): 284–92. doi: 10.1124/jpet.107.133306 18171905
62. Bloom AJ, Baker TB, Chen LS, Breslau N, Hatsukami D, Bierut LJ, et al. Variants in two adjacent genes, EGLN2 and CYP2A6, influence smoking behavior related to disease risk via different mechanisms. Hum Mol Genet. 2014;23(2): 555–61. doi: 10.1093/hmg/ddt432 24045616
63. Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997;124(10): 1887–97. 9169836
64. Yingjie L, Jian T, Changhai Y, Jingbo L. Numblike regulates proliferation, apoptosis, and invasion of lung cancer cell. Tumour Biol. 2013;34(5): 2773–80. doi: 10.1007/s13277-013-0835-7 23681800
65. Vaira V, Faversani A, Martin NM, Garlick DS, Ferrero S, Nosotti M, et al. Regulation of lung cancer metastasis by Klf4-Numb-like signaling. Cancer Res. 2013;73(8): 2695–2705. doi: 10.1158/0008-5472.CAN-12-4232 23440423
66. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24): 2295–2303. doi: 10.1056/NEJMra0809890 20554984
67. Chenoweth MJ, O'Loughlin J, Sylvestre MP, Tyndale RF. CYP2A6 slow nicotine metabolism is associated with increased quitting by adolescent smokers. Pharmacogenet Genomics. 2013;23(4): 232–35. doi: 10.1097/FPC.0b013e32835f834d 23462429
68. Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction. 2014;109(1): 128–37. doi: 10.1111/add.12353 24033696
69. Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4 : 132. doi: 10.3389/fgene.2013.00132 23882278
70. Nasarre P, Potiron V, Drabkin H, Roche J. Guidance molecules in lung cancer. Cell Adh Migr. 2010;4(1): 130–45. 20139699
71. Maemura K, Yoshikawa H, Yokoyama K, Ueno T, Kurose H, Uchiyama K, et al. Delta-like 3 is silenced by methylation and induces apoptosis in human hepatocellular carcinoma. Int J Oncol. 2013;42(3): 817–22. doi: 10.3892/ijo.2013.1778 23337976
72. Fragkiadaki P, Soulitzis N, Sifakis S, Koutroulakis D, Gourvas V, Vrachnis N, et al. Downregulation of notch signaling pathway in late preterm and term placentas from pregnancies complicated by preeclampsia. PLoS One. 2015;10(5): e0126163. doi: 10.1371/journal.pone.0126163 25962154
Štítky
Genetika Reprodukční medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 9
-
Všechny články tohoto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



