-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Case for Junk DNA
article has not abstract
Published in the journal: . PLoS Genet 10(5): e32767. doi:10.1371/journal.pgen.1004351
Category: Viewpoints
doi: https://doi.org/10.1371/journal.pgen.1004351Summary
article has not abstract
Overview
With the advent of deep sequencing technologies and the ability to analyze whole genome sequences and transcriptomes, there has been a growing interest in exploring putative functions of the very large fraction of the genome that is commonly referred to as “junk DNA.” Whereas this is an issue of considerable importance in genome biology, there is an unfortunate tendency for researchers and science writers to proclaim the demise of junk DNA on a regular basis without properly addressing some of the fundamental issues that first led to the rise of the concept. In this review, we provide an overview of the major arguments that have been presented in support of the notion that a large portion of most eukaryotic genomes lacks an organism-level function. Some of these are based on observations or basic genetic principles that are decades old, whereas others stem from new knowledge regarding molecular processes such as transcription and gene regulation.
Introduction
The search for function in the genome
It has been known for several decades that only a small fraction of the human genome is made up of protein-coding sequences and that at least some noncoding DNA has important biological functions. In addition to coding exons, the genome contains sequences that are transcribed into functional RNA molecules (e.g., tRNA, rRNA, and snRNA), regulatory regions that control gene expression (e.g., promoters, silencers, and enhancers), origins of replication, and repeats that play structural roles at the chromosomal level (e.g., telomeres and centromeres).
New discoveries regarding potentially important sequences amongst the nonprotein-coding majority of the genome are becoming more prevalent. By far the best-known effort to identify functional regions in the human genome is the recently completed Encyclopaedia of DNA Elements (ENCODE) project [1], whose authors made the remarkable claim that a “biochemical function” could be assigned to 80% of the human genome [2]. Reports that ENCODE had refuted the existence of large amounts of junk DNA in the human genome received considerable media attention [3], [4]. Criticisms that these claims were based on an extremely loose definition of “function” soon followed [5]–[8] (for a discussion of the relevant function concepts, see [9]), and debate continues regarding the most appropriate interpretation of the ENCODE results. Nevertheless, the excitement and subsequent backlash served to illustrate the widespread interest among scientists and nonspecialists in determining how much of the human genome is functionally significant at the organism level.
The origin of “junk DNA”
Although the term “junk DNA” was already in use as early as the 1960s [10]–[12], the term's origin is usually attributed to Susumu Ohno [13]. As Ohno pointed out, gene duplication can alleviate the constraint imposed by natural selection on changes to important gene regions by allowing one copy to maintain the original function as the other undergoes mutation. Rarely, these mutations will turn out to be beneficial, and a new gene may arise (“neofunctionalization”) [14]. Most of the time, however, one copy sustains a mutation that eliminates its ability to encode a functional protein, turning it into a pseudogene. These sequences are what Ohno initially referred to as “junk” [13], although the term was quickly extended to include many types of noncoding DNA [15]. Today, “junk DNA” is often used in the broad sense of referring to any DNA sequence that does not play a functional role in development, physiology, or some other organism-level capacity. This broader sense of the term is at the centre of most current debate about the quantity—or even the existence—of “junk DNA” in the genomes of humans and other organisms.
It has now become something of a cliché to begin both media stories and journal articles with the simplistic claim that most or all noncoding DNA was “long dismissed as useless junk.” The implication, of course, is that current research is revealing function in much of the supposed junk that was unwisely ignored as biologically uninteresting by past investigators. Yet, it is simply not true that potential functions for noncoding DNA were ignored until recently. In fact, various early commenters considered the notion that large swaths of the genome were nonfunctional to be “repugnant” [10], [16], and possible functions were discussed each time a new type of nonprotein-coding sequence was identified (including pseudogenes, transposable elements, satellite DNA, and introns; for a compilation of relevant literature, see [17]).
Importantly, the concept of junk DNA was not based on ignorance about genomes. On the contrary, the term reflected known details about genome size variability, the mechanism of gene duplication and mutational degradation, and population genetics theory. Moreover, each of these observations and theoretical considerations remains valid. In this review, we examine several lines of evidence—both empirical and conceptual—that support the notion that a substantial percentage of the DNA in many eukaryotic genomes lacks an organism-level function and that the junk DNA concept remains viable post-ENCODE.
Genome Size and “The Onion Test”
There are several key points to be understood regarding genome size diversity among eukaryotes and its relationship to the concept of junk DNA. First, genome size varies enormously among species [18], [19]: at least 7,000-fold among animals and 350-fold even within vertebrates. Second, genome size varies independently of intuitive notions of organism complexity or presumed number of protein-coding genes (Figure 1). For example, a human genome contains eight times more DNA than that of a pufferfish but is 40 times smaller than that of a lungfish. Third, organisms that have very large genomes are not few in number or outliers—for example, of the >200 salamander genomes analyzed thus far, all are between four and 35 times larger than the human genome [18]. Fourth, even closely related species with very similar biological properties and the same ploidy level can differ significantly in genome size.
Fig. 1. Summary of haploid nuclear DNA contents (“genome sizes”) for various groups of eukaryotes. 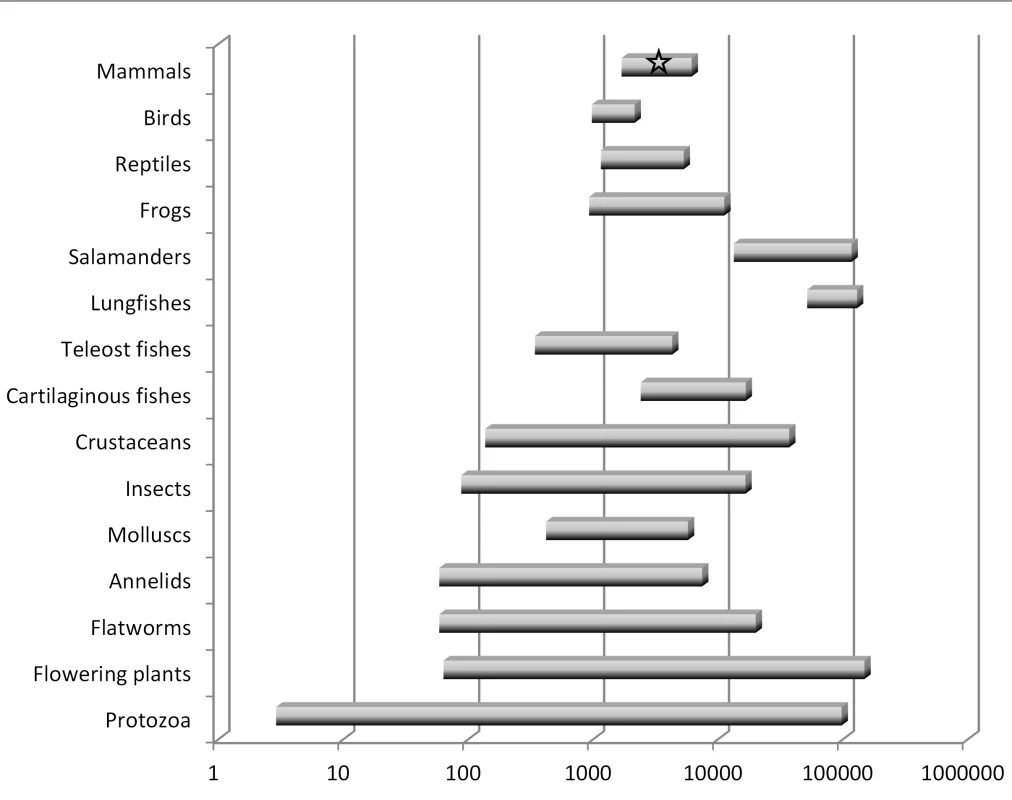
This graph is based on data for about 10,000 species [18], [19]. There is a wide range in genome sizes even among developmentally similar species, and there is no correspondence between genome size and general organism complexity. Humans, which have an average-sized genome for a mammal, are indicated by a star. Note the logarithmic scale. These observations pose an important challenge to any claim that most eukaryotic DNA is functional at the organism level. This logic is perhaps best illustrated by invoking “the onion test” [20]. The domestic onion, Allium cepa, is a diploid plant (2n = 16) with a haploid genome size of roughly 16 billion base pairs (16 Gbp), or about five times larger than humans. Although any number of species with large genomes could be chosen for such a comparison, the onion test simply asks: if most eukaryotic DNA is functional at the organism level, be it for gene regulation, protection against mutations, maintenance of chromosome structure, or any other such role, then why does an onion require five times more of it than a human? Importantly, the comparison is not restricted to onions versus humans. It could as easily be between pufferfish and lungfish, which differ by ∼350-fold, or members of the genus Allium, which have more than a 4-fold range in genome size that is not the result of polyploidy [21].
In summary, the notion that the majority of eukaryotic noncoding DNA is functional is very difficult to reconcile with the massive diversity in genome size observed among species, including among some closely related taxa. The onion test is merely a restatement of this issue, which has been well known to genome biologists for many decades [18].
Genome Composition
Another important consideration is the composition of eukaryotic genomes. Far from being composed of mysterious “dark matter,” the characteristics of the sequences constituting 98% or so of the human genome that is nonprotein-coding are generally well understood.
Transposable elements
By far the dominant type of nongenic DNA are transposable elements (TEs), including various well-described retroelements such as Short and Long Interspersed Nuclear Elements (SINEs and LINEs), endogenous retroviruses, and cut-and-paste DNA transposons. Because of their capacity to increase in copy number, transposable elements have long been described as “parasitic” or “selfish” [22], [23]. However, the vast majority of these elements are inactive in humans, due to a very large fraction being highly degraded by mutation. Due to this degeneracy, estimates of the proportion of the human genome occupied by TEs has varied widely, between one-half and two-thirds [24], [25]. Larger genomes, such as those of salamanders and lungfishes, almost certainly contain an even more enormous quantity of transposable element DNA [26], [27].
Many examples have been found in which TEs have taken on regulatory or other functional roles in the genome [28]. In recognition of the more complex interactions between transposable elements and their hosts, Kidwell and Lisch proposed an expansion of the “parasitism” framework where each TE can be classified along a spectrum from parasitism to mutualism [29]. Nevertheless, there is evidence of organism-level function for only a tiny minority of TE sequences. It is therefore not obvious that functional explanations can be extrapolated from a small number of specific examples to all TEs within the genome.
Highly repetitive DNA
Another large fraction of the genome consists of highly repetitive DNA. These regions are extremely variable even amongst individuals of the same population (hence their use as “DNA fingerprints”) and can expand or contract through processes such as unequal crossing over or replication slippage. Many repeats are thought to be derived from truncated TEs, but others consist of tandem arrays of di - and trinucleotides [30]. As with TEs, some highly repetitive sequences play a role in gene regulation (for example, [31]). Others, such as telomeric - and centromeric-associated repeats [32], [33], play critical roles in chromosomal maintenance. Despite this, there is currently no evidence that the majority of highly repetitive elements are functional.
Introns
According to Gencode v17, about 40% of the human genome is comprised of intronic regions; however, this figure is likely an overestimate as it includes all annotated events. It is also important to note that a large fraction of TEs and repetitive elements are found in introns. Although introns can increase the diversity of protein products by modulating alternative splicing, it is also clear that the vast majority of intronic sequence evolves in an unconstrained way, accumulating mutations at about the same rate as neutral regions. Although the median intron size in humans is ∼1.5 kb [30], data suggest that most of the constrained sequence is confined to the first and last 150 nucleotides [34].
Pseudogenes
The human genome is also home to a large number of pseudogenes. Estimates of the total number range from 12,600 to 19,700 [35]. These include both “classical” pseudogenes (direct duplicates, of the sort imagined by Ohno [13]) and “processed” pseudogenes, which are reverse transcribed from mRNA [36]. Once again, although some pseudogenes have been co-opted for organism-level function (for example see [37]), most are simply evolving without selective constraints on their sequences and likely have no function [38].
Conserved sequences
Several analyses of sequence conservation between humans and other mammals have found that about 5% of the genome is conserved [1], [39]–[42]. It is possible that an additional 4% of the human genome is under lineage-specific selection pressure [39]; however, this estimate appears to be somewhat questionable [43], [44] (also see [45]). Ignoring these problems, the idea that 9% of the human genome shows signs of functionality is actually consistent with the results of ENCODE and other large-scale genome analyses.
Besides protein-coding sequences (including associated untranslated regions), which make up 1.5%–2.5% of the human genome [24], data from ENCODE suggest that conserved long noncoding RNAs (lncRNAs) are generated from about 9,000 loci that add up to less than an additional 0.4% [46], [47]. Thus, even if a vast new untapped world of functional noncoding RNA is discovered, this will probably be transcribed from a small fraction of the human genome.
At first blush, sequences that are bound by transcription factors (TFs) appear to be very abundant, making up about 8.5% of the genome according to ENCODE [2]. This number, however, is an estimate of regions that are hypersensitive to DNase I treatment due to the displacement of nucleosomes by TFs. As pointed out by others [6], these regions are annotated as being several hundreds of nucleotides long and are thus much larger than the actual size of individual TF-binding motifs, which are typically 10 bp in length [48]. By ENCODE's own estimates, less than half of the nucleotide bases in these DNase I hypersensitivity regions contain actual TF recognition motifs [2], and only 60% of these are under purifying selection [49]. Others have found that weak and transient TF-binding events are routinely identified by chromatin IP experiments despite the fact that they do not significantly contribute to gene expression [50]–[53] and are poorly conserved [53]. Given that experiments performed in a diverse number of eukaryotic systems have found only a small correlation between TF-binding events and mRNA expression [54], [51], it appears that in most cases only a fraction of TF-binding sites significantly impacts local gene expression.
In summary, most of the major constituents of the genome have been well characterized. The majority of human DNA consists of repetitive, mutationally degraded sequences. There are unambiguous examples of nonprotein-coding sequences of various types having been co-opted for organism-level functions in gene regulation, chromosome structure, and other roles, but at present evidence from the published literature suggests that these represent a small minority of the human genome.
Evolutionary Forces
To understand the current state of the human genome, we need to examine how it evolved, and as Michael Lynch once wrote, “Nothing in evolution makes sense except in the light of population genetics” [55]. Unfortunately, concepts that have been generated by this field have not been widely recognized in other domains of the life sciences. In particular, what is underappreciated by many nonevolution specialists is that much of molecular evolution in eukaryotes is primarily the result of genetic drift, or the fixation of neutral mutations. This view has been widely appreciated by molecular evolutionary biologists for the past 35 years.
The nearly neutral theory of molecular evolution
An important development in the understanding of how various evolutionary forces shape eukaryotic genes and genomes came with the theories developed by Kimura, Ohta, King, and Jukes. They demonstrated that alleles that were slightly beneficial or deleterious behaved like neutral alleles, provided that the absolute value of their selection coefficient was smaller than the inverse of the “effective” population size [56]–[59]. In other words, it is important to keep in mind population size when thinking about whether deleterious mutations are subjected to purifying selection.
It is also important to realize that the “effective” population size is dependent on many factors and is typically much lower than the total number of individuals in a species [55]. For humans it has been estimated that the historical effective population size is approximately 10,000, and this is on the low side in comparison to most metazoans [60]. Given the overall low figures for multicellular organisms in general, we would expect that natural selection would be powerless to stop the accumulation of certain genomic alterations over the entirety of metazoan evolution. One type of mutation that fits this description is intergenic insertions, be they transposable elements, pseudogenes, or random sequence [55]. The creation and loss of TF-binding motifs or cryptic transcriptional start sites in these same intergenic regions will equally be invisible to natural selection, provided that these do not drastically alter the expression of any nearby genes or cause the production of stable toxic transcripts. Thus, a central tenet of the nearly neutral theory of molecular evolution is that extraneous DNA sequences can be present within genomes, provided that they do not significantly impact the fitness of the organism.
Genetic load
It has long been appreciated that there is a limit to the number of deleterious mutations that an organism can sustain per generation [61], [62]. The presence of these mutations is usually not harmful, because diploid organisms generally require only one functional copy of any given gene. However, if the rate at which these mutations are generated is higher than the rate at which natural selection can weed them out, then the collective genomes of the organisms in the species will suffer a meltdown as the total number of deleterious alleles increases with each generation [63]. This rate is approximately one deleterious mutation per generation. In this context it becomes clear that the overall mutation rate would place an upper limit to the amount of functional DNA. Currently, the rate of mutation in humans is estimated to be anywhere from 70–150 mutations per generation [64], [65]. By this line of reasoning, we would estimate that, at most, only 1% of the nucleotides in the genome are essential for viability in a strict sequence-specific way. However, more recent computational models have demonstrated that genomes could sustain multiple slightly deleterious mutations per generation [66]. Using statistical methods, it has been estimated that humans sustain 2.1–10 deleterious mutations per generation [66]–[68]. These data would suggest that at most 10% of the human genome exhibits detectable organism-level function and conversely that at least 90% of the genome consists of junk DNA. These figures agree with measurements of genome conservation (∼9%, see above) and are incompatible with the view that 80% of the genome is functional in the sense implied by ENCODE. It remains possible that large amounts of noncoding DNA play structural or other roles independent of nucleotide sequence, but it far from obvious how this would be reconciled with “the onion test.”
The evolution of the nucleus
When dealing with the evolution of any lineage, one must also keep in mind unique events, also known as historical contingencies, which constrain and shape subsequent evolutionary trajectories [69]. One of these key events in our own ancestry was the evolution of the eukaryotic nucleus. A further examination of why the nucleus evolved and how this altered cellular function may generate significant insights into the current shape of the eukaryotic genome.
One important event in early eukaryotic evolution was the development of a symbiotic relationship between the α-proteobacteria progenitor of mitochondria and an archaebacteria-like host [70], [71]. As with most endosymbiotically derived organelles [72], DNA was transferred from mitochondria to the host. In this way, Group II introns, which are still found in both mitochondria and α-proteobacteria [73], invaded the host genome. Group II introns are parasitic DNA fragments that replicate when they are transcribed, typically as part of a larger transcript. The intron then folds into a catalytic ribozyme that splices itself out of the precursor transcript and then reinserts itself at a new genomic locus by reversing the splicing reaction. Importantly, functional fragments of Group II introns can splice out inactive versions in a trans-splicing reaction [74], [75]. As described elsewhere, it is likely that Group II introns proliferated and evolved into two populations: inactivated copies that could be nonetheless spliced out in trans, and active fragments that promoted splicing of the former group. This latter group eventually evolved into the spliceosomal snRNAs [75]–[77]. This idea is supported by not only structural, catalytic, and functional similarities between Group II introns and snRNAs [78], [79] but also by the fact that expression of the U5 snRNA rescues the splicing of Group II introns that lack the corresponding U5-like region [80].
It is likely that the proliferation of trans-splicing triggered the spatial segregation of RNA processing (the nucleoplasm) from the translation machinery (the cytoplasm) [77]. This subdivision ensured that mRNAs were properly spliced before they encountered the translation machinery. Not only would this segregation prevent translating ribosomes from interfering with the splicing reaction (and vice versa) but would also prevent the translation of incompletely processed mRNAs, which often encode toxic proteins [81], [82]. Importantly, the segregation of translation from both transcription and RNA processing provided an opportunity for nuclear quality-control processes to eliminate misprocessed and spurious transcripts that did not meet the minimal requirements of “mRNA identity” (see below) before these RNAs ever encountered a ribosome. This in turn permitted intergenic DNA and cryptic transcriptional start sites to proliferate with minimal cost to the fitness of the organism. It should also be noted that the increase in ATP regeneration due to mitochondrial-derived metabolic pathways provided the surplus energy that is required to support an expansion not only in genome size and membranes [83], [84] but also wasteful transcription. Thus, by several independent mechanisms, the acquisition of mitochondria likely allowed the expansion of nonfunctional intergenic DNA and the evolution of a noisy transcriptional system.
Gene Expression in Eukaryotes
Eukaryotic transcription is inherently noisy
One of the most widely discussed discoveries of the past decade of transcriptome analysis is that much of the metazoan genome is transcribed at some level (although this, too, was already recognized in rough outline in the 1970s [15]). When nascent transcripts from mouse have been analyzed by deep sequencing, the total number of reads that map to intergenic loci is almost equivalent to the number mapping to exonic regions (Figure 2A, reproduced from reference [85]). This is consistent with the observation that a large fraction of the cellular pool of RNA Polymerase II is associated with intergenic regions [86] and that transcription can be initiated at random sequences (see Figure S4 in [87]) and nucleosome-free regions [88], [89]. Strikingly, when one examines the steady state level of polyadenylated RNA, very little maps to intergenic regions (Figure 2A, 2B, the latter reproduced from reference [46]; also see [85], [90]–[92]). In fact, when one eliminates the ∼9,000 transcript species that are thought to be derived from conserved lncRNA, then most of the annotated noncoding polyadenylated RNAs are present at levels below one copy per cell and are found exclusively in the nucleus (Figure 2B). The situation is no better in the unpolyadenylated pool, in which the amount of lncRNA and intergenic RNA is practically insignificant, especially in the cytoplasmic pool (Figure 2B). In aggregate, these data indicate that the majority of intergenic RNAs are degraded almost immediately after transcription. Consistent with this idea, the level of intergenic transcripts increase when RNA degradation machinery is inhibited [93]–[101]. Although pervasive transcription has been used as an argument against junk DNA [3], [4], it is in fact entirely in line with the idea that intergenic regions are evolving under little-to-no constraint, especially when one considers that this intergenic transcription is unstable.
Fig. 2. Levels of protein-coding and intergenic RNAs in mammalian cells. 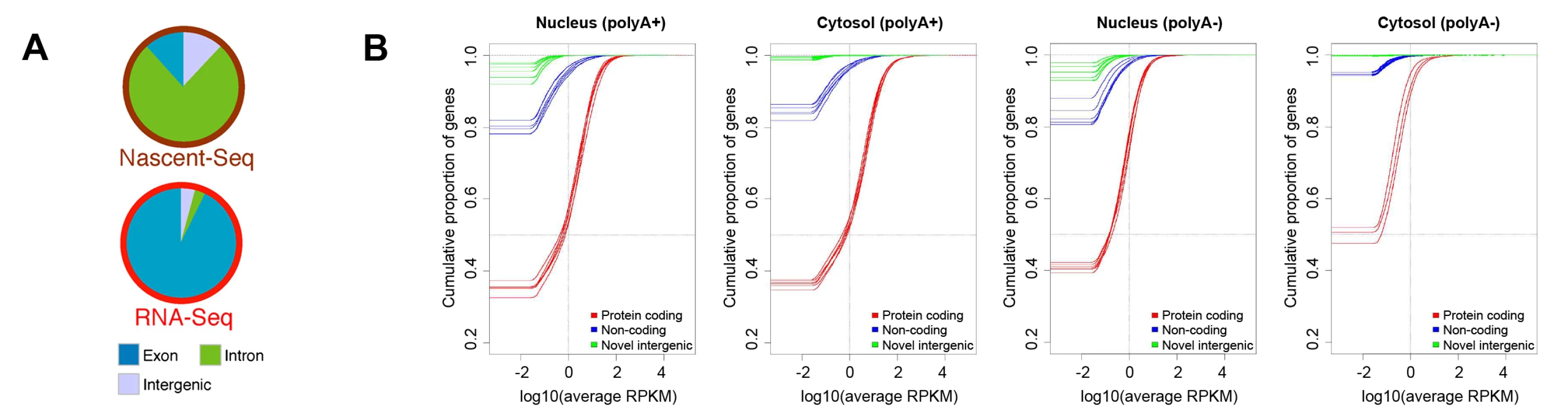
(A) Analysis of nascent and total poly(A)+ RNA levels from mouse liver nuclei. Nascent (i.e., polymerase-associated) RNA and poly(A)+ RNA were isolated from mouse liver nuclei and analyzed by high-throughput sequencing. Individual reads were categorized by their source. Exonic and intronic are from known referenced genes (i.e., “RefSeq” genes), while intergenic originate from nonreferenced loci (i.e., “non-RefSeq”) in the mouse genome. Reproduced from [85]. (B) Empirical Cumulative Distribution Function (ECDF) of transcript expression in each cell compartment as determined by the ENCODE consortia. Results for RNA that either contain (“polyA+”) or lack (“polyA−”) a poly(A)-tail in the nucleus and cytosolic fractions are shown. Each human cell line that was analyzed is represented by three lines, one for each pool of RNA (red for protein-coding RNAs, blue for lncRNAs [“noncoding”], and green for intergenic transcripts [“novel intergenic”]). The lines indicate the cumulative fraction of RNAs in a given pool (y-axis) that are expressed at levels that are equal or less than the reads per kilobase per million mapped reads (RPKM) on the x-axis. Total numbers in each pool are as follows: reference protein coding genes: 20,679, loci producing lncRNAs: 9,277, and regions producing intergenic transcripts: 41,204. Transcripts with expression levels of 0 RPKM were adjusted to an artificial value of 10−6 RPKM so that the onset of each graph represents the fraction of nonexpressed genes or loci. Note that 1–4 RPKM is approximately equivalent to one copy per tissue culture cell [46], [129]. Using this figure, one can easily deduce that the vast majority of intergenic transcripts are present at levels less than one copy per cell. Reproduced with permission from [46]. Identifying mRNA from intergenic transcription
A common theme that has emerged from the study of mRNA synthesis is that various steps in RNA synthesis and processing are biochemically coupled. In other words, cellular machineries that participate in one biochemical activity also promote subsequent steps. For example, during the splicing of the 5′most intron, the spliceosome collaborates with the 5′cap binding complex to deposit nuclear export factors onto the 5′end of the processed transcript [102], [103], and this helps to explain why splicing enhances the nuclear export of mRNA [104]–[106]. Countless other examples of coupling exist (for reviews, see [107]–[111]).
The ultimate goal of these coupling reactions is to sort protein-coding RNAs (i.e. mRNA) from intergenic transcripts [111], [112]. Given that, on average, protein-coding genes have eight introns [30], while the majority of annotated ENCODE intergenic transcripts tend not to be spliced [46], introns help distinguish these two populations and thus serve as “mRNA identity” markers. These mRNA identity features activate coupling reactions, which in turn promote the further processing, nuclear export, and translation of a particular transcript. Likewise, other classes of functional RNAs (e.g., tRNAs and snRNAs) have their own identity elements [113]. In contrast, transcripts that lack identity elements are targeted for degradation. In agreement with this model, intronless RNA molecules that have a random sequence are poorly exported from the nucleus and have a very short half-life [114], [115]. In contrast, intronless mRNAs have specialized motifs that promote their nuclear export [105], [116]–[119].
In light of the fact that many functional lncRNAs serve a role in regulating chromatin structure or transcription, it is not surprising that most localise to the nucleoplasm [46]. One would predict that lncRNAs contain a differential set of identity elements that not only serve to prevent their decay but also retain them in the nucleus. This would especially be critical for lncRNAs that are spliced. Despite this, the elements that regulate the localization and stability of these RNAs have received little attention, but can be informed by the view that they may have their own identity markers.
It is also important to point out that eukaryotes have other mechanisms that either degrade aberrant mRNAs (e.g., nonsense-mediated decay) or limit the amount of intergenic transcription (e.g., heterochromatin). Nevertheless, eukaryotes appear to have evolved an intricate network of coupling reactions that are required to cope with a large burden of junk RNA. These findings are consistent with the idea that eukaryotic genomes are filled with junk DNA that is transcribed at a low level.
An alternative view of transcription and conservation?
In an attempt to counter the argument that sequence conservation is a prerequisite for functionality, it has been recently proposed that certain transcriptional events may serve some role in regulating cellular function, despite the fact that the sequence of the transcriptional product is unconstrained [120]. Indeed, this view is in line with the findings that the transcription of certain yeast genes is inhibited as a consequence of the production of cryptic unstable transcripts originating from upstream and/or downstream promoters (for a review see [121]). Other examples have linked the generation of cryptic unstable transcripts to chromatin modifications [101], [122], DNA methylation [123], and DNA stability [124]. However, it remains unclear whether the majority of unstable noncoding RNAs have any effect on DNA or chromatin, let alone contribute to the fitness of the organism. In the cases where cryptic unstable transcriptional events impact gene expression, they usually consist of short transcripts that are synthesized from regions around the transcriptional start sites or within the gene itself [121]. Indeed most of the available data are consistent with the fact that transcriptional start sites are promiscuous, often generating bidirectional transcription [100], [101], and that subsequent coupling processes, such as the interaction between promoter-associated complexes and 3′end processing factors, are required to enforce proper transcriptional directionality [125]. Other unstable transcripts function to promote or maintain heterochromatin formation in the vicinity of the transcriptional site, likely because these regions produce toxic transcripts [122]. Although this form of transcription has a function (viz., to maintain a repressive state), it is not clear that the elimination of these regions would have any effect on the organism [8]. The transcription of other short unstable transcripts, mostly produced from enhancer regions, has been shown to promote gene expression [126]; however, again these “enhancer RNAs” are transcribed from a small fraction of the total genome [127]. As stated by others [128], it is imperative that those who claim that the vast majority of intergenic transcription is functional test their hypotheses. In the absence of this evidence, the declaration that we are in the midst of a paradigm shift with regards to eukaryotic genomes and gene expression [120] seems premature.
Concluding Remarks
For decades, there has been considerable interest in determining what role, if any, the majority of the DNA in eukaryotic genomes plays in organismal development and physiology. The ENCODE data are only the most recent contribution to a long-standing research program that has sought to address this issue. However, evidence casting doubt that most of the human genome possesses a functional role has existed for some time. This is not to say that none of the nonprotein-coding majority of the genome is functional—examples of functional noncoding sequences have been known for more than half a century, and even the earliest proponents of “junk DNA” and “selfish DNA” predicted that further examples would be found. Nevertheless, they also pointed out that evolutionary considerations, information regarding genome size diversity, and knowledge about the origins and features of genomic components do not support the notion that all of the DNA must have a function by virtue of its mere existence. Nothing in the recent research or commentary on the subject has challenged these observations.
Zdroje
1. BirneyE, StamatoyannopoulosJA, DuttaA, GuigóR, GingerasTR, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 : 799–816 doi:10.1038/nature05874
2. ENCODE Project Consortium (2012) BernsteinBE, BirneyE, DunhamI, GreenED, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74 doi:10.1038/nature11247
3. EckerJR, BickmoreWA, BarrosoI, PritchardJK, GiladY, et al. (2012) Genomics: ENCODE explained. Nature 489 : 52–55 doi:10.1038/489052a
4. PennisiE (2012) Genomics. ENCODE project writes eulogy for junk DNA. Science 337 : 1159, doi:10.1126/science.337.6099.1159
5. EddySR (2012) The C-value paradox, junk DNA and ENCODE. Curr Biol CB 22: R898–899 doi:10.1016/j.cub.2012.10.002
6. GraurD, ZhengY, PriceN, AzevedoRBR, ZufallRA, et al. (2013) On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biol Evol 5 : 578–590 doi:10.1093/gbe/evt028
7. DoolittleWF (2013) Is junk DNA bunk? A critique of ENCODE. Proc Natl Acad Sci U S A 110 : 5294–5300 doi:10.1073/pnas.1221376110
8. NiuD-K, JiangL (2013) Can ENCODE tell us how much junk DNA we carry in our genome? Biochem Biophys Res Commun 430 : 1340–1343 doi:10.1016/j.bbrc.2012.12.074
9. ElliottTA, LinquistS, GregoryTR (2014) Conceptual and empirical challenges of ascribing functions to transposable elements. Am Nat In press.
10. Aronson AI, Bolton ET, Britten RJ, Cowie DB, Duerksen JD, et al.. (1960) Biophysics. Year book - Carnegie Institution of Washington (1960). Volume 59. Baltimore, MD: Lord Baltimore Press. pp. 229–289.
11. EhertCF, de HallerG (1963) Origin, development, and maturation of organelles and organelle systems of the cell surface in Paramecium. J Ultrastruct Res 23: SUPPL6 : 1–42.
12. Graur D (2013) The Origin of Junk DNA: A Historical Whodunnit. Judge Starling. Available: http://judgestarling.tumblr.com/post/64504735261/the-origin-of-junk-dna-a-historical-whodunnit. Accessed 23 December 2013.
13. Ohno S (1972) So much “junk” DNA in our genome. In: Smith HH, editor. Evolution of Genetic Systems. New York: Gordon and Breach. pp. 366–370.
14. Ohno S (1970) Evolution by gene duplication. London, New York: Allen & Unwin; Springer-Verlag. 160 p.
15. ComingsDE (1972) The structure and function of chromatin. Adv Hum Genet 3 : 237–431.
16. BrittenRJ, KohneDE (1968) Repeated sequences in DNA. Science 161 : 529–540.
17. Gregory TR (2008) Junk DNA – the quotes of interest series. Available: http://www.genomicron.evolverzone.com/2008/02/junk-dna-quotes-of-interest-series/. Accessed 10 April 2014.
18. Gregory TR (2013) Animal Genome Size Database. Available: http://www.genomesize.com. Accessed 10 April 2014.
19. Bennett MD, Leitch IJ (2012) Plant DNA C-values Database (Release 6.0, Dec. 2012). Available: http://data.kew.org/cvalues/. Accessed 10 April 2014.
20. Gregory TR (2007) The onion test. Available: http://www.genomicron.evolverzone.com/2007/04/onion-test/. Accessed 10 April 2014.
21. RicrochA, YocktengR, BrownSC, NadotS (2005) Evolution of genome size across some cultivated Allium species. Genome Natl Res Counc Can Génome Cons Natl Rech Can 48 : 511–520 doi:10.1139/g05-017
22. OrgelLE, CrickFH (1980) Selfish DNA: the ultimate parasite. Nature 284 : 604–607.
23. DoolittleWF, SapienzaC (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284 : 601–603.
24. GregoryTR (2005) Synergy between sequence and size in large-scale genomics. Nat Rev Genet 6 : 699–708 doi:10.1038/nrg1674
25. De KoningAPJ, GuW, CastoeTA, BatzerMA, PollockDD (2011) Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet 7: e1002384 doi:10.1371/journal.pgen.1002384
26. SunC, ShepardDB, ChongRA, López ArriazaJ, HallK, et al. (2012) LTR retrotransposons contribute to genomic gigantism in plethodontid salamanders. Genome Biol Evol 4 : 168–183 doi:10.1093/gbe/evr139
27. MetcalfeCJ, FiléeJ, GermonI, JossJ, CasaneD (2012) Evolution of the Australian lungfish (Neoceratodus forsteri) genome: a major role for CR1 and L2 LINE elements. Mol Biol Evol 29 : 3529–3539 doi:10.1093/molbev/mss159
28. CowleyM, OakeyRJ (2013) Transposable elements re-wire and fine-tune the transcriptome. PLoS Genet 9: e1003234 doi:10.1371/journal.pgen.1003234
29. KidwellMG, LischDR (2001) Perspective: transposable elements, parasitic DNA, and genome evolution. Evol Int J Org Evol 55 : 1–24.
30. Scherer S (2008) A short guide to the human genome. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press. 173 p.
31. KunarsoG, ChiaN-Y, JeyakaniJ, HwangC, LuX, et al. (2010) Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet 42 : 631–634 doi:10.1038/ng.600
32. HemannMT, StrongMA, HaoLY, GreiderCW (2001) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107 : 67–77.
33. Torras-LlortM, Moreno-MorenoO, AzorínF (2009) Focus on the centre: the role of chromatin on the regulation of centromere identity and function. EMBO J 28 : 2337–2348 doi:10.1038/emboj.2009.174
34. GazaveE, Marqués-BonetT, FernandoO, CharlesworthB, NavarroA (2007) Patterns and rates of intron divergence between humans and chimpanzees. Genome Biol 8: R21 doi:10.1186/gb-2007-8-2-r21
35. PeiB, SisuC, FrankishA, HowaldC, HabeggerL, et al. (2012) The GENCODE pseudogene resource. Genome Biol 13: R51 doi:10.1186/gb-2012-13-9-r51
36. ZhangZ, GersteinM (2004) Large-scale analysis of pseudogenes in the human genome. Curr Opin Genet Dev 14 : 328–335 doi:10.1016/j.gde.2004.06.003
37. SalmenaL, PolisenoL, TayY, KatsL, PandolfiPP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146 : 353–358 doi:10.1016/j.cell.2011.07.014
38. ZhengD, GersteinMB (2007) The ambiguous boundary between genes and pseudogenes: the dead rise up, or do they? Trends Genet 23 : 219–224 doi:10.1016/j.tig.2007.03.003
39. WardLD, KellisM (2012) Evidence of abundant purifying selection in humans for recently acquired regulatory functions. Science 337 : 1675–1678 doi:10.1126/science.1225057
40. PontingCP, HardisonRC (2011) What fraction of the human genome is functional? Genome Res 21 : 1769–1776 doi:10.1101/gr.116814.110
41. Lindblad-TohK, GarberM, ZukO, LinMF, ParkerBJ, et al. (2011) A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478 : 476–482 doi:10.1038/nature10530
42. CooperGM, StoneEA, AsimenosG (2005) NISC Comparative Sequencing Program (2005) GreenED, et al. (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15 : 901–913 doi:10.1101/gr.3577405
43. BrayN, PachterL (2012) Comment on “Evidence of Abundant and Purifying Selection in Humans for Recently Acquired Regulatory Functions”. Cornell University Library arXiv:1212.3076 [q-bio.GN]. Available: http://arxiv.org/abs/1212.3076. Accessed 10 April 2014.
44. GreenP, EwingB (2013) Comment on “Evidence of abundant purifying selection in humans for recently acquired regulatory functions.”. Science 340 : 682 doi:10.1126/science.1233195
45. WardLD, KellisM (2013) Response to comment on “Evidence of abundant purifying selection in humans for recently acquired regulatory functions.”. Science 340 : 682 doi:10.1126/science.1233366
46. DjebaliS, DavisCA, MerkelA, DobinA, LassmannT, et al. (2012) Landscape of transcription in human cells. Nature 489 : 101–108 doi:10.1038/nature11233
47. DerrienT, JohnsonR, BussottiG, TanzerA, DjebaliS, et al. (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22 : 1775–1789 doi:10.1101/gr.132159.111
48. StewartAJ, HannenhalliS, PlotkinJB (2012) Why transcription factor binding sites are ten nucleotides long. Genetics 192 : 973–985 doi:10.1534/genetics.112.143370
49. VernotB, StergachisAB, MauranoMT, VierstraJ, NephS, et al. (2012) Personal and population genomics of human regulatory variation. Genome Res 22 : 1689–1697 doi:10.1101/gr.134890.111
50. LickwarCR, MuellerF, HanlonSE, McNallyJG, LiebJD (2012) Genome-wide protein-DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature 484 : 251–255 doi:10.1038/nature10985
51. BigginMD (2011) Animal transcription networks as highly connected, quantitative continua. Dev Cell 21 : 611–626 doi:10.1016/j.devcel.2011.09.008
52. LiX, MacArthurS, BourgonR, NixD, PollardDA, et al. (2008) Transcription factors bind thousands of active and inactive regions in the Drosophila blastoderm. PLoS Biol 6: e27 doi:10.1371/journal.pbio.0060027
53. ParisM, KaplanT, LiXY, VillaltaJE, LottSE, et al. (2013) Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet 9: e1003748 doi:10.1371/journal.pgen.1003748
54. SpitzF, FurlongEEM (2012) Transcription factors: from enhancer binding to developmental control. Nat Rev Genet 13 : 613–626 doi:10.1038/nrg3207
55. Lynch M (2007) The origins of genome architecture. Sunderland Mass.: Sinauer Associates. 494 p.
56. KimuraM (1968) Evolutionary rate at the molecular level. Nature 217 : 624–626.
57. KingJL, JukesTH (1969) Non-Darwinian evolution. Science 164 : 788–798.
58. OhtaT (1973) Slightly deleterious mutant substitutions in evolution. Nature 246 : 96–98.
59. Kimura M (1984) The Neutral theory of molecular evolution. Cambridge [Cambridgeshire]; New York: Cambridge University Press. 367 p.
60. CharlesworthB (2009) Fundamental concepts in genetics: effective population size and patterns of molecular evolution and variation. Nat Rev Genet 10 : 195–205 doi:10.1038/nrg2526
61. MullerHJ (1950) Our load of mutations. Am J Hum Genet 2 : 111–176.
62. KnudsonAGJr (1979) Presidential address. Our load of mutations and its burden of disease. Am J Hum Genet 31 : 401–413.
63. LynchM, ConeryJ, BurgerR (1995) Mutational meltdowns in sexual populations. Evolution 49 : 1067–1080.
64. KeightleyPD (2012) Rates and fitness consequences of new mutations in humans. Genetics 190 : 295–304 doi:10.1534/genetics.111.134668
65. ScallyA, DurbinR (2012) Revising the human mutation rate: implications for understanding human evolution. Nat Rev Genet 13 : 745–753 doi:10.1038/nrg3295
66. LesecqueY, KeightleyPD, Eyre-WalkerA (2012) A resolution of the mutation load paradox in humans. Genetics 191 : 1321–1330 doi:10.1534/genetics.112.140343
67. EoryL, HalliganDL, KeightleyPD (2010) Distributions of selectively constrained sites and deleterious mutation rates in the hominid and murid genomes. Mol Biol Evol 27 : 177–192 doi:10.1093/molbev/msp219
68. ReedFA, AkeyJM, AquadroCF (2005) Fitting background-selection predictions to levels of nucleotide variation and divergence along the human autosomes. Genome Res 15 : 1211–1221 doi:10.1101/gr.3413205
69. GouldSJ (1994) The evolution of life on the earth. Sci Am 271 : 84–91.
70. SaganL (1967) On the origin of mitosing cells. J Theor Biol 14 : 255–274.
71. WoeseCR (1977) Endosymbionts and mitochondrial origins. J Mol Evol 10 : 93–96.
72. MartinW (2003) Gene transfer from organelles to the nucleus: frequent and in big chunks. Proc Natl Acad Sci U S A 100 : 8612–8614 doi:10.1073/pnas.1633606100
73. FeratJL, MichelF (1993) Group II self-splicing introns in bacteria. Nature 364 : 358–361 doi:10.1038/364358a0
74. JarrellKA, DietrichRC, PerlmanPS (1988) Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol 8 : 2361–2366.
75. StoltzfusA (1999) On the possibility of constructive neutral evolution. J Mol Evol 49 : 169–181.
76. HickeyDA, BenkelBF, AbukashawaSM (1989) A general model for the evolution of nuclear pre-mRNA introns. J Theor Biol 137 : 41–53.
77. MartinW, KooninEV (2006) Introns and the origin of nucleus-cytosol compartmentalization. Nature 440 : 41–45 doi:10.1038/nature04531
78. ToorN, KeatingKS, TaylorSD, PyleAM (2008) Crystal structure of a self-spliced group II intron. Science 320 : 77–82 doi:10.1126/science.1153803
79. KeatingKS, ToorN, PerlmanPS, PyleAM (2010) A structural analysis of the group II intron active site and implications for the spliceosome. RNA 16 : 1–9 doi:10.1261/rna.1791310
80. HetzerM, WurzerG, SchweyenRJ, MuellerMW (1997) Trans-activation of group II intron splicing by nuclear U5 snRNA. Nature 386 : 417–420 doi:10.1038/386417a0
81. CaliBM, AndersonP (1998) mRNA surveillance mitigates genetic dominance in Caenorhabditis elegans. Mol Gen Genet 260 : 176–184.
82. KhajaviM, InoueK, LupskiJR (2006) Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet 14 : 1074–1081 doi:10.1038/sj.ejhg.5201649
83. LaneN, MartinW (2010) The energetics of genome complexity. Nature 467 : 929–934 doi:10.1038/nature09486
84. LaneN (2011) Energetics and genetics across the prokaryote-eukaryote divide. Biol Direct 6 : 35 doi:10.1186/1745-6150-6-35
85. MenetJS, RodriguezJ, AbruzziKC, RosbashM (2012) Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife 1: e00011 doi:10.7554/eLife.00011
86. StruhlK (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol 14 : 103–105 doi:10.1038/nsmb0207-103
87. WhiteMA, MyersCA, CorboJC, CohenBA (2013) Massively parallel in vivo enhancer assay reveals that highly local features determine the cis-regulatory function of ChIP-seq peaks. Proc Natl Acad Sci U S A 110 : 11952–11957 doi:10.1073/pnas.1307449110
88. CheungV, ChuaG, BatadaNN, LandryCR, MichnickSW, et al. (2008) Chromatin - and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 6: e277 doi:10.1371/journal.pbio.0060277
89. BuratowskiS (2008) Transcription. Gene expression–where to start? Science 322 : 1804–1805 doi:10.1126/science.1168805
90. BabakT, BlencoweBJ, HughesTR (2005) A systematic search for new mammalian noncoding RNAs indicates little conserved intergenic transcription. BMC Genomics 6 : 104 doi:10.1186/1471-2164-6-104
91. RamsköldD, WangET, BurgeCB, SandbergR (2009) An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol 5: e1000598 doi:10.1371/journal.pcbi.1000598
92. Van BakelH, NislowC, BlencoweBJ, HughesTR (2010) Most “dark matter” transcripts are associated with known genes. PLoS Biol 8: e1000371 doi:10.1371/journal.pbio.1000371
93. WyersF, RougemailleM, BadisG, RousselleJ-C, DufourM-E, et al. (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121 : 725–737 doi:10.1016/j.cell.2005.04.030
94. DavisCA, AresMJr (2006) Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 103 : 3262–3267 doi:10.1073/pnas.0507783103
95. ThiebautM, Kisseleva-RomanovaE, RougemailleM, BoulayJ, LibriD (2006) Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell 23 : 853–864 doi:10.1016/j.molcel.2006.07.029
96. ChekanovaJA, GregoryBD, ReverdattoSV, ChenH, KumarR, et al. (2007) Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell 131 : 1340–1353 doi:10.1016/j.cell.2007.10.056
97. VasiljevaL, KimM, TerziN, SoaresLM, BuratowskiS (2008) Transcription termination and RNA degradation contribute to silencing of RNA polymerase II transcription within heterochromatin. Mol Cell 29 : 313–323 doi:10.1016/j.molcel.2008.01.011
98. PrekerP, NielsenJ, KammlerS, Lykke-AndersenS, ChristensenMS, et al. (2008) RNA exosome depletion reveals transcription upstream of active human promoters. Science 322 : 1851–1854 doi:10.1126/science.1164096
99. MilliganL, DecourtyL, SaveanuC, RappsilberJ, CeulemansH, et al. (2008) A yeast exosome cofactor, Mpp6, functions in RNA surveillance and in the degradation of noncoding RNA transcripts. Mol Cell Biol 28 : 5446–5457 doi:10.1128/MCB.00463-08
100. NeilH, MalabatC, d' Aubenton-CarafaY, XuZ, SteinmetzLM, et al. (2009) Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 457 : 1038–1042 doi:10.1038/nature07747
101. XuZ, WeiW, GagneurJ, PerocchiF, Clauder-MünsterS, et al. (2009) Bidirectional promoters generate pervasive transcription in yeast. Nature 457 : 1033–1037 doi:10.1038/nature07728
102. MasudaS, DasR, ChengH, HurtE, DormanN, et al. (2005) Recruitment of the human TREX complex to mRNA during splicing. Genes Dev 19 : 1512–1517 doi:10.1101/gad.1302205
103. ChengH, DufuK, LeeC-S, HsuJL, DiasA, et al. (2006) Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 127 : 1389–1400 doi:10.1016/j.cell.2006.10.044
104. LuoMJ, ReedR (1999) Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci U S A 96 : 14937–14942.
105. PalazzoAF, SpringerM, ShibataY, LeeC-S, DiasAP, et al. (2007) The signal sequence coding region promotes nuclear export of mRNA. PLoS Biol 5: e322 doi:10.1371/journal.pbio.0050322
106. ValenciaP, DiasAP, ReedR (2008) Splicing promotes rapid and efficient mRNA export in mammalian cells. Proc Natl Acad Sci U S A 105 : 3386–3391 doi:10.1073/pnas.0800250105
107. ManiatisT, ReedR (2002) An extensive network of coupling among gene expression machines. Nature 416 : 499–506 doi:10.1038/416499a
108. BuratowskiS (2009) Progression through the RNA polymerase II CTD cycle. Mol Cell 36 : 541–546 doi:10.1016/j.molcel.2009.10.019
109. PeralesR, BentleyD (2009) “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol Cell 36 : 178–191 doi:10.1016/j.molcel.2009.09.018
110. MooreMJ, ProudfootNJ (2009) Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 136 : 688–700 doi:10.1016/j.cell.2009.02.001
111. PalazzoAF, AkefA (2012) Nuclear export as a key arbiter of “mRNA identity” in eukaryotes. Biochim Biophys Acta 1819 : 566–577 doi:10.1016/j.bbagrm.2011.12.012
112. PalazzoA, MahadevanK, TarnawskyS (2013) ALREX-elements and introns: two identity elements that promote mRNA nuclear export. WIREs RNA 4 : 523–533 doi:10.1002/wrna.1176
113. OhnoM, SegrefA, KuerstenS, MattajIW (2002) Identity elements used in export of mRNAs. Mol Cell 9 : 659–671.
114. DiasAP, DufuK, LeiH, ReedR (2010) A role for TREX components in the release of spliced mRNA from nuclear speckle domains. Nat Commun 1 : 97 doi:10.1038/ncomms1103
115. LeiH, DiasAP, ReedR (2011) Export and stability of naturally intronless mRNAs require specific coding region sequences and the TREX mRNA export complex. Proc Natl Acad Sci U S A 108 : 17985–17990 doi:10.1073/pnas.1113076108
116. HuangY, SteitzJA (2001) Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell 7 : 899–905.
117. CuljkovicB, TopisirovicI, SkrabanekL, Ruiz-GutierrezM, BordenKLB (2006) eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol 175 : 415–426 doi:10.1083/jcb.200607020
118. LeiH, ZhaiB, YinS, GygiS, ReedR (2012) Evidence that a consensus element found in naturally intronless mRNAs promotes mRNA export. Nucleic Acids Res doi:10.1093/nar/gks1314
119. KimuraT, HashimotoI, NishizawaM, ItoS, YamadaH (2010) Novel cis-active structures in the coding region mediate CRM1-dependent nuclear export of IFN-α 1 mRNA. Med Mol Morphol 43 : 145–157 doi:10.1007/s00795-010-0492-5
120. MattickJS, DingerME (2013) The extent of functionality in the human genome. HUGO J 7 : 2 doi:10.1186/1877-6566-7-2
121. TisseurM, KwapiszM, MorillonA (2011) Pervasive transcription - Lessons from yeast. Biochimie 93 : 1889–1896 doi:10.1016/j.biochi.2011.07.001
122. MoazedD (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457 : 413–420 doi:10.1038/nature07756
123. BartolomeiMS, ZemelS, TilghmanSM (1991) Parental imprinting of the mouse H19 gene. Nature 351 : 153–155 doi:10.1038/351153a0
124. KobayashiT, GanleyARD (2005) Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309 : 1581–1584 doi:10.1126/science.1116102
125. Tan-WongSM, ZauggJB, CamblongJ, XuZ, ZhangDW, et al. (2012) Gene loops enhance transcriptional directionality. Science 338 : 671–675 doi:10.1126/science.1224350
126. ØromUA, DerrienT, BeringerM, GumireddyK, GardiniA, et al. (2010) Long Noncoding RNAs with Enhancer-like Function in Human Cells. Cell 143 : 46–58 doi:10.1016/j.cell.2010.09.001
127. AnderssonR, GebhardC, Miguel-EscaladaI, HoofI, BornholdtJ, et al. (2014) An atlas of active enhancers across human cell types and tissues. Nature 507 : 455–461 doi:10.1038/nature12787
128. BirdA (2013) Genome biology: not drowning but waving. Cell 154 : 951–952 doi:10.1016/j.cell.2013.08.010
129. MortazaviA, WilliamsBA, McCueK, SchaefferL, WoldB (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5 : 621–628 doi:10.1038/nmeth.1226
Štítky
Genetika Reprodukční medicína
Článek Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin PathwayČlánek Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human DiseasesČlánek G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify LongevityČlánek PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial MatrixČlánek Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus ConflictsČlánek The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 5
-
Všechny články tohoto čísla
- Genetic Interactions Involving Five or More Genes Contribute to a Complex Trait in Yeast
- A Mutation in the Gene in Dogs with Hereditary Footpad Hyperkeratosis (HFH)
- Loss of Function Mutation in the Palmitoyl-Transferase HHAT Leads to Syndromic 46,XY Disorder of Sex Development by Impeding Hedgehog Protein Palmitoylation and Signaling
- Heterogeneity in the Frequency and Characteristics of Homologous Recombination in Pneumococcal Evolution
- Genome-Wide Nucleosome Positioning Is Orchestrated by Genomic Regions Associated with DNase I Hypersensitivity in Rice
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Single Nucleotide Variants in Transcription Factors Associate More Tightly with Phenotype than with Gene Expression
- Ribosomal Protein Mutations Induce Autophagy through S6 Kinase Inhibition of the Insulin Pathway
- Recent Mitochondrial DNA Mutations Increase the Risk of Developing Common Late-Onset Human Diseases
- Epistatically Interacting Substitutions Are Enriched during Adaptive Protein Evolution
- Meiotic Drive Impacts Expression and Evolution of X-Linked Genes in Stalk-Eyed Flies
- G×G×E for Lifespan in : Mitochondrial, Nuclear, and Dietary Interactions that Modify Longevity
- Population Genomic Analysis of Ancient and Modern Genomes Yields New Insights into the Genetic Ancestry of the Tyrolean Iceman and the Genetic Structure of Europe
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
- Whole Exome Re-Sequencing Implicates and Cilia Structure and Function in Resistance to Smoking Related Airflow Obstruction
- Allelic Expression of Deleterious Protein-Coding Variants across Human Tissues
- R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome
- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- The Impairment of MAGMAS Function in Human Is Responsible for a Severe Skeletal Dysplasia
- Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Males
- Mlh2 Is an Accessory Factor for DNA Mismatch Repair in
- Activating Transcription Factor 6 Is Necessary and Sufficient for Alcoholic Fatty Liver Disease in Zebrafish
- The Spatiotemporal Program of DNA Replication Is Associated with Specific Combinations of Chromatin Marks in Human Cells
- Rapid Evolution of PARP Genes Suggests a Broad Role for ADP-Ribosylation in Host-Virus Conflicts
- Genome-Wide Inference of Ancestral Recombination Graphs
- Mutations in Four Glycosyl Hydrolases Reveal a Highly Coordinated Pathway for Rhodopsin Biosynthesis and N-Glycan Trimming in
- SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates
- The Impact of Population Demography and Selection on the Genetic Architecture of Complex Traits
- Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation
- Genetic Dissection of the Female Head Transcriptome Reveals Widespread Allelic Heterogeneity
- Genome Sequencing and Comparative Genomics of the Broad Host-Range Pathogen AG8
- Copy Number Variation Is a Fundamental Aspect of the Placental Genome
- GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in
- Hox Transcription Factors Access the RNA Polymerase II Machinery through Direct Homeodomain Binding to a Conserved Motif of Mediator Subunit Med19
- Drosha Promotes Splicing of a Pre-microRNA-like Alternative Exon
- Predicting the Minimal Translation Apparatus: Lessons from the Reductive Evolution of
- PAX6 Regulates Melanogenesis in the Retinal Pigmented Epithelium through Feed-Forward Regulatory Interactions with MITF
- Enhanced Interaction between Pseudokinase and Kinase Domains in Gcn2 stimulates eIF2α Phosphorylation in Starved Cells
- A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in
- dGTP Starvation in Provides New Insights into the Thymineless-Death Phenomenon
- Phosphorylation Modulates Clearance of Alpha-Synuclein Inclusions in a Yeast Model of Parkinson's Disease
- RPM-1 Uses Both Ubiquitin Ligase and Phosphatase-Based Mechanisms to Regulate DLK-1 during Neuronal Development
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- More of a Good Thing or Less of a Bad Thing: Gene Copy Number Variation in Polyploid Cells of the Placenta
- Heritable Transmission of Stress Resistance by High Dietary Glucose in
- Revertant Mutation Releases Confined Lethal Mutation, Opening Pandora's Box: A Novel Genetic Pathogenesis
- Lifespan Extension by Methionine Restriction Requires Autophagy-Dependent Vacuolar Acidification
- A Genome-Wide Assessment of the Role of Untagged Copy Number Variants in Type 1 Diabetes
- Selectivity in Genetic Association with Sub-classified Migraine in Women
- A Lack of Parasitic Reduction in the Obligate Parasitic Green Alga
- The Proper Splicing of RNAi Factors Is Critical for Pericentric Heterochromatin Assembly in Fission Yeast
- Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma
- Six Homeoproteins and a linc-RNA at the Fast MYH Locus Lock Fast Myofiber Terminal Phenotype
- EDR1 Physically Interacts with MKK4/MKK5 and Negatively Regulates a MAP Kinase Cascade to Modulate Plant Innate Immunity
- Genes That Bias Mendelian Segregation
- The Case for Junk DNA
- An In Vivo EGF Receptor Localization Screen in Identifies the Ezrin Homolog ERM-1 as a Temporal Regulator of Signaling
- Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder
- Hyperactivated Wnt Signaling Induces Synthetic Lethal Interaction with Rb Inactivation by Elevating TORC1 Activities
- Mutations in the Cholesterol Transporter Gene Are Associated with Excessive Hair Overgrowth
- Scribble Modulates the MAPK/Fra1 Pathway to Disrupt Luminal and Ductal Integrity and Suppress Tumour Formation in the Mammary Gland
- A Novel CH Transcription Factor that Regulates Expression Interdependently with GliZ in
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics
- Spermatid Cyst Polarization in Depends upon and the CPEB Family Translational Regulator
- Insights into the Genetic Structure and Diversity of 38 South Asian Indians from Deep Whole-Genome Sequencing
- Intron Retention in the 5′UTR of the Novel ZIF2 Transporter Enhances Translation to Promote Zinc Tolerance in
- A Dominant-Negative Mutation of Mouse Causes Glaucoma and Is Semi-lethal via LBD1-Mediated Dimerisation
- Biased, Non-equivalent Gene-Proximal and -Distal Binding Motifs of Orphan Nuclear Receptor TR4 in Primary Human Erythroid Cells
- Ras-Mediated Deregulation of the Circadian Clock in Cancer
- Retinoic Acid-Related Orphan Receptor γ (RORγ): A Novel Participant in the Diurnal Regulation of Hepatic Gluconeogenesis and Insulin Sensitivity
- Extensive Diversity of Prion Strains Is Defined by Differential Chaperone Interactions and Distinct Amyloidogenic Regions
- Fine Tuning of the UPR by the Ubiquitin Ligases Siah1/2
- Paternal Poly (ADP-ribose) Metabolism Modulates Retention of Inheritable Sperm Histones and Early Embryonic Gene Expression
- Allele-Specific Genome-wide Profiling in Human Primary Erythroblasts Reveal Replication Program Organization
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PINK1-Parkin Pathway Activity Is Regulated by Degradation of PINK1 in the Mitochondrial Matrix
- Null Mutation in PGAP1 Impairing Gpi-Anchor Maturation in Patients with Intellectual Disability and Encephalopathy
- Phosphorylation of a WRKY Transcription Factor by MAPKs Is Required for Pollen Development and Function in
- p53 Requires the Stress Sensor USF1 to Direct Appropriate Cell Fate Decision
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



