-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNetwork Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
Autism Spectrum Disorders (ASD) are highly heritable and characterised by impairments in social interaction and communication, and restricted and repetitive behaviours. Considering four sets of de novo copy number variants (CNVs) identified in 181 individuals with autism and exploiting mouse functional genomics and known protein-protein interactions, we identified a large and significantly interconnected interaction network. This network contains 187 genes affected by CNVs drawn from 45% of the patients we considered and 22 genes previously implicated in ASD, of which 192 form a single interconnected cluster. On average, those patients with copy number changed genes from this network possess changes in 3 network genes, suggesting that epistasis mediated through the network is extensive. Correspondingly, genes that are highly connected within the network, and thus whose copy number change is predicted by the network to be more phenotypically consequential, are significantly enriched among patients that possess only a single ASD-associated network copy number changed gene (p = 0.002). Strikingly, deleted or disrupted genes from the network are significantly enriched in GO-annotated positive regulators (2.3-fold enrichment, corrected p = 2×10−5), whereas duplicated genes are significantly enriched in GO-annotated negative regulators (2.2-fold enrichment, corrected p = 0.005). The direction of copy change is highly informative in the context of the network, providing the means through which perturbations arising from distinct deletions or duplications can yield a common outcome. These findings reveal an extensive ASD-associated molecular network, whose topology indicates ASD-relevant mutational deleteriousness and that mechanistically details how convergent aetiologies can result extensively from CNVs affecting pathways causally implicated in ASD.
Published in the journal: . PLoS Genet 9(6): e32767. doi:10.1371/journal.pgen.1003523
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003523Summary
Autism Spectrum Disorders (ASD) are highly heritable and characterised by impairments in social interaction and communication, and restricted and repetitive behaviours. Considering four sets of de novo copy number variants (CNVs) identified in 181 individuals with autism and exploiting mouse functional genomics and known protein-protein interactions, we identified a large and significantly interconnected interaction network. This network contains 187 genes affected by CNVs drawn from 45% of the patients we considered and 22 genes previously implicated in ASD, of which 192 form a single interconnected cluster. On average, those patients with copy number changed genes from this network possess changes in 3 network genes, suggesting that epistasis mediated through the network is extensive. Correspondingly, genes that are highly connected within the network, and thus whose copy number change is predicted by the network to be more phenotypically consequential, are significantly enriched among patients that possess only a single ASD-associated network copy number changed gene (p = 0.002). Strikingly, deleted or disrupted genes from the network are significantly enriched in GO-annotated positive regulators (2.3-fold enrichment, corrected p = 2×10−5), whereas duplicated genes are significantly enriched in GO-annotated negative regulators (2.2-fold enrichment, corrected p = 0.005). The direction of copy change is highly informative in the context of the network, providing the means through which perturbations arising from distinct deletions or duplications can yield a common outcome. These findings reveal an extensive ASD-associated molecular network, whose topology indicates ASD-relevant mutational deleteriousness and that mechanistically details how convergent aetiologies can result extensively from CNVs affecting pathways causally implicated in ASD.
Introduction
Autism Spectrum Disorders (ASD) form a group of complex disorders affecting ∼1% of individuals [1]. ASD are characterised by impairments in social interaction, communication, and restricted and repetitive interests and behaviours [2], although other symptoms such as intellectual disability, seizures or auditory abnormalities frequently co-occur [3]. Despite the high estimates of heritability for ASD found from monozygotic twin studies (∼90%) [4], the genetic cause is recognized in only ∼20% of cases suggesting that there are many causal variants yet to be identified [5], [6]. ASD-causative alleles are likely to be rare as (i) they are under strong purifying selection from the population due to the low fertility (∼5%) of individuals with ASD [7], and (ii) there is a strong positive correlation between paternal age and ASD risk which suggests that ASD-contributing mutations frequently may be arising de novo in the continuously-replicating paternal germ line [8]. Thus, in this study we examine de novo variants, specifically de novo copy number variants (CNVs), found in individuals with ASD as a set of variants likely enriched in causal mutations [6].
By contrast to methods that require either recurrent or common genetic variation to identify disease-associated loci, functional enrichment analysis (FEA) approaches gain considerable power by simultaneously examining the contributions of many disparate variants across many individuals' genomes and thus may be particularly appropriate for investigating the many rare and distributed variants underlying autism [9], [10]. FEA approaches hypothesise that dispersed variants observed in patients with shared symptoms may be affecting genes that participate in a common biological process and it is the disruption of the same process within each of these patients that underlies their common symptoms [11], [12]. Thus, FEA considers whether there is a functional category that is exceptionally common for genes overlapped by dispersed CNVs identified in the genomes of patients that present the same disorder. It thus associates function with the disorder and nominates those copy number variable genes that participate in that function as candidate disease genes [9].
The functional category types used in FEA approaches are key to the biological insights that they can provide. Different approaches have been applied to investigate the genetics underlying autism, including literature annotations [6], [13], protein-protein interactions [14], [15], mouse model phenotypes [13], gene co-expression [16] and functional linkage networks [6], [17]. As the application of these different approaches in autism studies often accompanies the publication of a novel genetic dataset, each method has highlighted many, usually novel, candidate genes that add to a rapidly growing list [18] and replication of significant functional enrichments has only rarely been attempted, let alone achieved [13], [19]. Synaptic functioning has been recurrently associated with ASD by many of the recent studies [13], [17] but the small proportions of genes that form these associations along with the functional diversity broadly exhibited by genes implicated in ASD has led authors to question specific associations [20]. However, given that it appears likely that the variants of several hundred genes contribute to autism [6], identifying those biological process(es) that are commonly disrupted may provide a more explanatory approach than to collate individual causative genes. In particular, FEA, when applied to ASD CNVs, should not just aim to identify unifying functional themes but should also provide a framework for interpreting how these variants exert their proposed phenotypic effects.
In this study, we examined the genes affected by four previously-published sets of rare, de novo CNVs identified in autistic patients. Given that ASD is a behavioural disorder, we initially considered the phenotype-level gene associations provided by mouse gene models before moving on to consider more molecular gene descriptions. We identified a significant enrichment of genes whose orthologues' disruption in mouse yields an abnormal synaptic transmission phenotype in 3 of 4 sets. We show that the protein products of the genes contributing to these enrichments form an extensive physical interaction network with genes previously implicated in autism and that extends to many other genes located in CNVs (herein termed CNV genes). We show that many of the autistic individuals considered here possess multiple CNV genes that reside within the network, suggesting extensive epistasis, and provide evidence that the number of interactions a gene has within the network is related to the propensity of its copy change to cause autism. Finally, within this network we find that whereas genes deleted in ASD are significantly enriched in those that positively regulate biological processes, the converse is also true: genes that are duplicated are enriched in negative regulators of biological processes. We provide several examples of how the direction of copy number change reinforces the biological interpretation of the ASD-associated physical interaction network.
Results
Initially, we sought to objectively identify autism-related mouse behaviours among phenotypes that are over-represented among de novo CNV genes for individuals with ASD (herein termed ASD dn CNVs). For this, we obtained 4 sets of ASD dn CNVs (Table S1 and Materials and Methods). The set collated by the AGP is likely to be most powerful due both to its higher number of CNVs and their generally smaller sizes, although all methodologies employed in this study account for variations in the numbers of genes affected by each CNV set (Table S2). We considered Gain or Loss CNVs separately, in addition to the set formed from their union (All).
Many mouse model phenotypes are associated with ASD dn CNVs
The Mammalian Phenotype Ontology, the set of terms by which the MGI annotates the phenotypes of mouse models, is organised at its highest level into 30 over-arching phenotypes [21]. Of these, three categories (Behavior/Neurological, Hearing/Vestibular/Ear and Lethality-Postnatal) were significantly over-represented by AGP ASD dn CNV genes compared to expectation by random chance, thus associated with AGP ASD dn CNV genes (BH-adjusted one-sided Fisher's test p<5%; Table S3). Importantly, these significant associations are all specific to Gain CNVs (2.0–2.7-fold increases) whilst observed counts for Loss CNVs differ little from expected values (data not shown).
The significant enrichments of these three overarching categories with the AGP ASD dn CNVs then provided the rationale necessary for testing of all their finer-scale phenotypic terms for association (162, 218 and 2 terms, for Behaviour/Neurological, Hearing/Vestibular/Ear and Lethality-Postnatal categories, respectively; see Materials and Methods). Although the Nervous System phenotypic category was not significantly over-represented among ASD dn CNV genes (All AGP ASD dn CNVs: 1.3-fold increase, p = 0.03, BH-adjusted p>5%), the behavioural presentations of ASD are likely to be manifestations of nervous system abnormalities. Consequently, we also tested for significant enrichments of finer-scale phenotypes within this category (282 terms). Subsequently, 23 behavioural, 21 nervous system, 27 hearing and 1 postnatal-lethality phenotypes were identified as being significantly enriched among the AGP ASD dn CNV genes (BH-adjusted p<5%; Figure 1 and Table S3). For 3 CNV sets, namely AGP, Marshall et al. and Levy et al., we also identified a significant excess of genes associated with abnormal CNS synaptic transmission phenotypes in mice, thereby triplicating this association (AGP 3.0-fold enrichment, p = 7×10−5, BH-adjusted p<5%; Marshall et al. 2.1-fold enrichment, p = 1×10−4, BH-adjusted p<5%; Levy et al. AGP 2.2-fold enrichment, p = 5×10−5, BH-adjusted p<5%; Sanders et al. 1.6-fold enrichment, p = 0.008, BH-adjusted p>5%; Table 1, Table S3).
Fig. 1. Relationships of mouse model phenotypic terms enriched among genes overlapped by de novo CNVs identified in individuals with ASD. 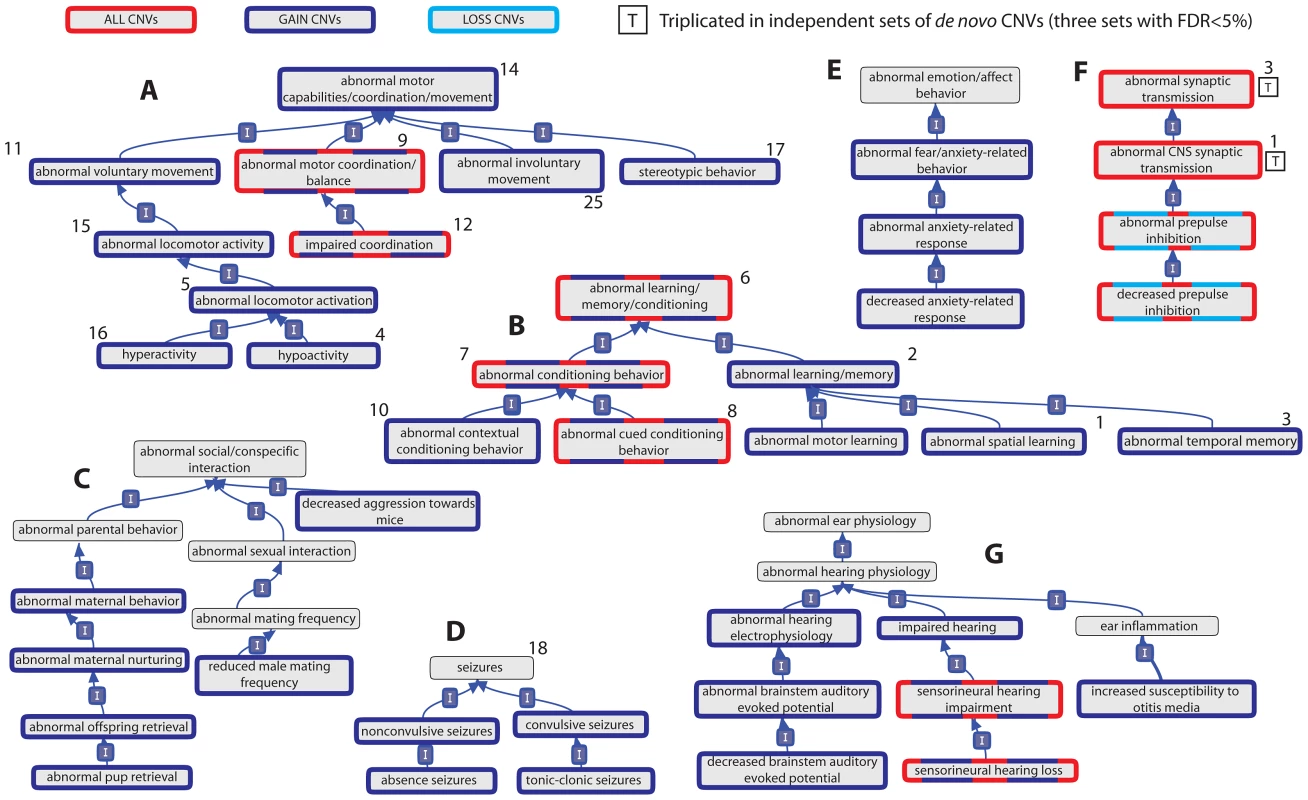
Relationships between phenotypic terms within the Mammalian Phenotype Ontology are indicated by a blue arrow running from the child term to the parent term. Terms are significant (BH-adjusted p<5%) in at least one of 4 sets of de novo CNVs identified in individuals with autism if they are shown with a coloured border (red, dark and light blue). Those terms whose significant enrichment is observed in three independent sets, and thus triplicated, are marked with a boxed letter “T”. Panels A–E show representative clusters of Behaviour/Neurological phenotypic category, while Panel F shows the enriched phenotypes from the Nervous System phenotypic category and Panel G shows representative enrichments from the Hearing/Vestibular/Ear phenotypic category. The number adjacent to the phenotypic terms indicates the rank of that phenotypic term among those phenotypes significantly enriched among a set of 22 disease genes previously implicated in ASD (see Results). Tab. 1. Triplicated mouse model phenotype associations among genes overlapped by sets of de novo CNVs identified in individuals with ASD. 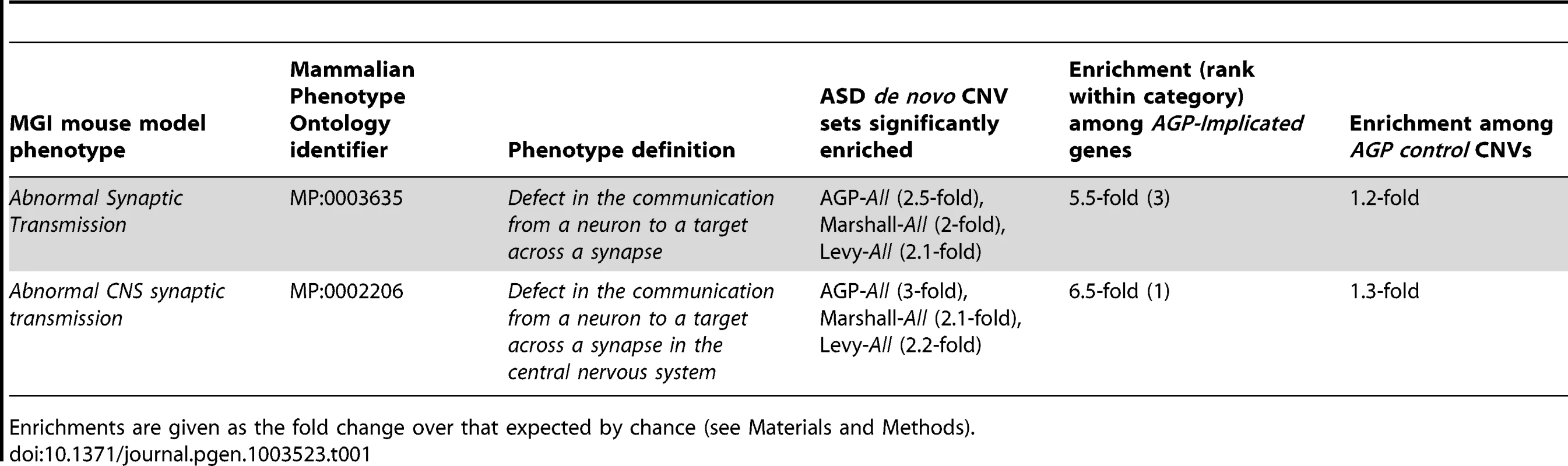
Enrichments are given as the fold change over that expected by chance (see Materials and Methods). Genes previously implicated in ASD are also associated with these model phenotypes
We next sought to determine whether model phenotypes enriched among the mouse orthologues of genes previously implicated in ASD are equivalent to those we now associate with ASD dn CNVs. Of 36 genes that had been causally-implicated in ASD by previous studies, as defined previously [6], phenotypic annotations were available for the unique mouse orthologues of 26 (see Materials and Methods). We removed 4 genes that were also overlapped by an ASD dn CNV to form a wholly independent set of 22 genes herein termed ASD-Implicated genes (Table S4).
We observed a striking concordance between the model phenotypes associated with the ASD-Implicated genes and those associated with the ASD dn CNV genes despite these sets' complete independence: the two abnormal synaptic phenotypes with triplicated associations to ASD dn CNVs ranked 1st and 3rd among those Nervous System-category phenotypes that were most significantly associated with ASD-Implicated genes, while 15 of the top 18 behavioural-category phenotypic associations among ASD-Implicated genes were among those independently associated with the AGP dn CNVs (Figure 1, Table 1, Table S4).
The protein products of genes that contribute to these phenotypic associations interact
Given the repeated enrichment within independent CNV sets of genes whose mouse orthologues are associated with abnormal synaptic transmission phenotypes, we asked whether the protein products of the 59 synaptic phenotype CNV genes taken from across all sets might interact within common processes or pathways. Even after correcting for the increased likelihood that the products of genes with behavioural or neurological associations interact, our analysis showed that the number of these proteins' interactions is unexpectedly high (3.75-fold over-representation, p = 0.006; Figure 2, Table 2 and Table S5; see Materials and Methods). When we then added the set of 36 ASD-Implicated genes, the number of direct protein interactions increased yet further (3.2-fold over-representation, p<0.002). Cumulatively, our results show that many of the 59 synaptic phenotype CNV genes and 36 ASD-Implicated genes function in concert and yield similar consequences when disrupted (Figure 2, Table 2).
Fig. 2. An ASD-associated interaction network. 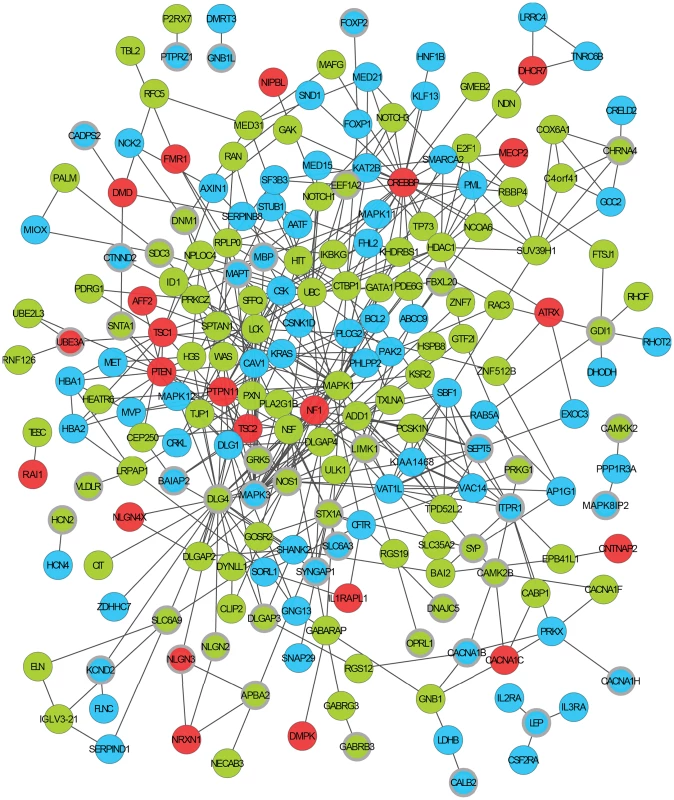
The network is formed from direct protein-protein interactions between the products of ASD dn CNV genes that are associated with synaptic phenotypes (shown with thicker grey border), genes previously implicated in ASD (ASD-implicated genes), and other ASD dn CNV genes whose products directly interact with these gene's products. Physical interactions between two proteins are shown as an edge connecting two circles representing each gene. Genes found to be duplicated in autistic patients in this study are shown in green, deleted genes in blue, and ASD-implicated genes in red. An alternative and more detailed view of this network is shown in Figure S6. Tab. 2. Protein-Protein Interaction (PPI) network enrichments. 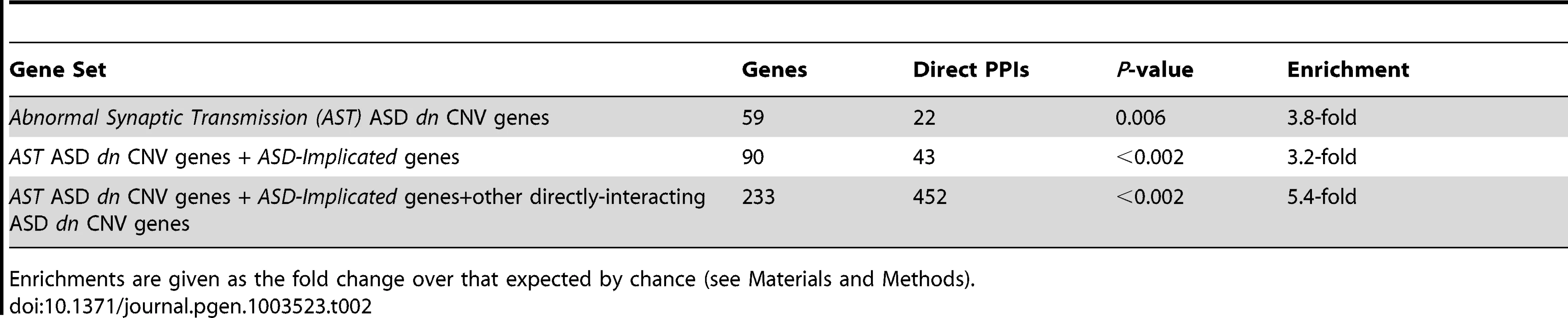
Enrichments are given as the fold change over that expected by chance (see Materials and Methods). Mouse model phenotypic information is available only for the orthologues of fewer than a quarter of human genes (see Materials and Methods). It is thus expected that not all genes within CNVs that are causally associated with synaptic abnormalities can be identified using this resource alone. To identify additional ASD candidate genes, we sought all those ASD dn CNV genes whose protein products were known to directly interact with the products of any of the 59 synaptic phenotype CNV genes or 36 ASD-Implicated genes. This identified an additional 174 CNV genes that form an expanded network with a 5.4-fold interaction over-representation (p<0.002; herein termed the ASD-associated network; Figure 2, Table S5). Of these 174 additional interacting proteins, the mouse orthologues of 74 (43%) do not yet have phenotypic information. Of the 100 additional interacting genes with mouse model phenotypes, 44 are known to exhibit behavioural phenotypes, and of these 35 exhibit one or more of the significantly associated behavioural phenotypes identified above (Figure 1 and Table S5). Examining the more general functional annotations of genes within the ASD-associated network using Gene Ontology (GO) identifies convergent functional themes that are consistent with broad synaptic functioning, organisation and maintenance (Table S6; Summarised using REVIGO in Figures S1, S2 and S3 [22]). This functional coherence is supported by the observation that 192 of the 210 (91%) proteins within the ASD-associated network reside in a single inter-connected cluster, thereby also providing known interactions that provide pathways through which effects originating from distinct mutations can aetiologically converge (Figure 2). Despite their known functional interconnections, the vast majority of these ASD candidate genes are novel (Table S5).
The 203 CNV genes singled-out through the synaptic mouse phenotype associations and the ASD-associated network provide a causal hypothesis for 81 (45%) of the patients considered. The median number of candidate genes per patient is 3 (mean 3.8, s.d. 3.2) suggesting a substantial role for epistasis in ASD. The network identified here provides not only the means for mediating epistatic interactions but is also indicative of the deleteriousness of copy change: Among the 22 patients that have only a single copy-changed candidate gene, that candidate gene has on average 3 times the number of interaction partners in the network as compared to the candidate genes from patients with multiple candidate genes (medians 3 vs. 1, respectively, Mann-Whitney U test p = 0.002). Thus, given the known deleteriousness of disrupting highly interacting “hub” genes within biological networks [23], we propose that the disruption of multiple non-hub genes within the autism network may be required to elicit an autistic phenotype comparable to the singular disruption of a hub gene within the same network.
Duplicated and deleted ASD candidate genes converge on common aetiologies
Of the 203 CNV genes identified through the synaptic mouse phenotype associations and the ASD-associated network, 110 (54%) are found only in duplications while 91 (45%) are only in deletions. We next investigated how the two directions – duplications or deletions – of copy number change might reflect common or divergent aetiologies. To achieve this we analysed the GO biological process annotations assigned to duplicated and, separately, to deleted genes for significantly over-represented terms (Table S6). While many of the over-represented annotation terms are shared between the deleted and duplicated gene sets, we noted a striking difference: The deleted candidate genes are significantly enriched only in genes that are positive regulators of biological processes (GO:0048518, 35/82 annotated genes, 2.4-fold enrichment, BH-adjusted p = 3×10−4) while, conversely, an enrichment of genes that are negative regulators of biological processes is only observed amongst the duplicated candidate gene set (GO:0048519, 34/105 annotated genes, 2-fold enrichment, BH-adjusted p = 0.006; Table 3). Each of the 4 CNV set's candidate genes contribute to each of these enrichments with many sets nominally significant individually (Table S7). Furthermore, reclassifying the partially duplicated, and therefore likely-disrupted, genes as deletions enhances these enrichments further (Table S8). These enrichments are complementary and thus immediately suggest a convergent model of action in which the duplication of negative regulator genes or the deletion of positive regulator genes both act to perturb a common target process and affect the same outcome. The unusually frequent deletions of positive regulators and duplications of negative regulators enable specific and biologically-meaningful interpretations of the ASD-associated network (see Figure 3 and Discussion).
Fig. 3. Distinct duplications and deletions of genes whose proteins interact within the ASD-associated network perturb pathways in the same direction. 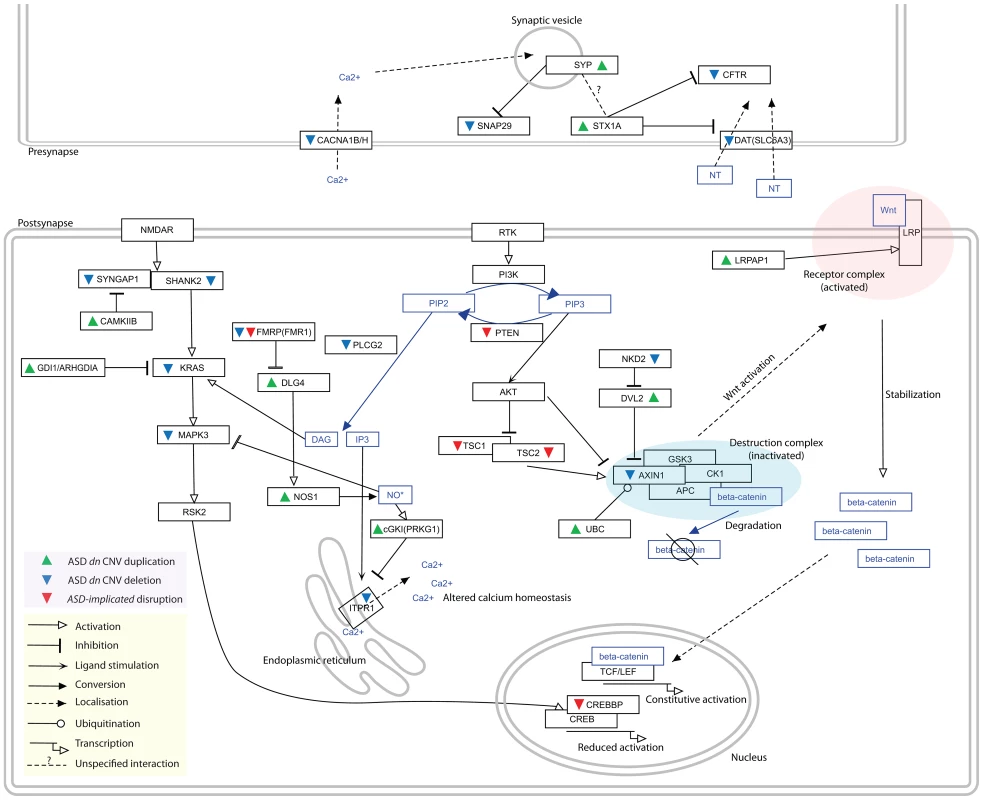
Genes duplicated within ASD dn CNVs are indicated with green upwards arrows while those deleted are denoted by blue downwards arrows. Previously identified ASD-Implicated genes found to be disrupted in autism patients are denoted with red downwards arrows. The nature of the interactions/regulations between proteins/molecules are shown with different edge types (see in-figure legend). The ASD-associated network (Figure 2) identifies several deletion/duplication pathway cascades, for example the MAPK3 pathway (see Discussion for additional examples). Here, deletions of the MAPK3 pathway components (i.e. SYNGAP1, SHANK2, KRAS, MAPK3, PAK2, and CREBBP) and duplications of their negative regulators (i.e. FMR1, GDI1, ARHGDIA, CAMK2B, and CAMKK2) found in autistic patients identify converging effects on the MAPK pathway, specifically reduced CREB-dependent transcription [9], [62], [63], [64]. CREB-dependent transcription has been implicated in neuroadaptation [20]. In addition, increased NO* production leads to the inhibition of MAPK1/3 activity [65], which fits well with the observed CNV duplications of both NOS1 and DLG4, the latter gene promoting recruitment of NOS1 [66]. Similarly, duplication of PRKG1, which is up-regulated by NO* and expresses a product that inhibits IP3 production, is predicted to reduced activation of the calcium-releasing IP3-receptor ITPR1 [67], which is in turn found to be deleted. Tab. 3. Regulatory GO enrichments amongst ASD dn CNVs candidate genes. 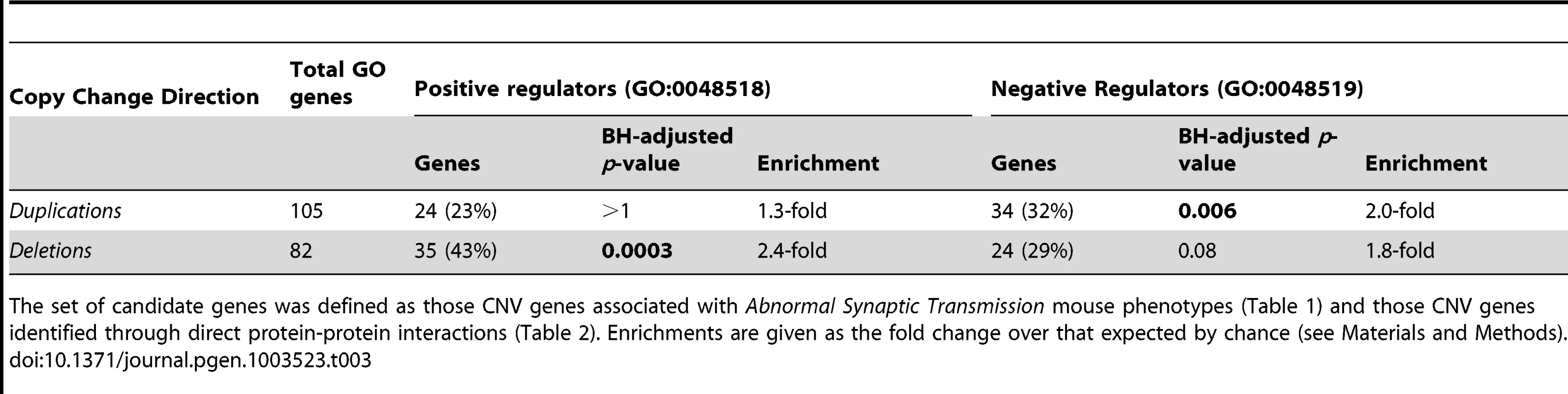
The set of candidate genes was defined as those CNV genes associated with Abnormal Synaptic Transmission mouse phenotypes (Table 1) and those CNV genes identified through direct protein-protein interactions (Table 2). Enrichments are given as the fold change over that expected by chance (see Materials and Methods). Discussion
This study has (i) identified among 3 independent sets of ASD dn CNVs, and therefore triplicated, an enrichment of genes whose mouse orthologues, when disrupted, yield an abnormal synaptic transmission phenotype; (ii) shown that these genes' protein products exhibit a significantly high number of interactions between themselves and to the products of genes previously implicated in ASD; (iii) that this interaction network extends directly to include many more proteins of genes affected by the ASD dn CNVs of almost half of the cohort; (iv) that the gene products in this ASD-associated network possess roles in synaptic function, organisation and maintenance; (v) that many individuals with ASD possess multiple copy changed genes from the ASD-associated network; (vi) that genes that are highly connected within the network (“hub genes”) are significantly enriched among patients that possess only a single ASD-associated network gene; and, finally (vii) that this network's genes that are deleted are significantly enriched in genes that act to positively regulate biological processes while those that are duplicated are significantly enriched in negative regulators.
An association of ASD CNVs with genes that yield synaptic phenotypes when disrupted in mice has been reported before in rare CNVs but replication was not achieved [13]. Here, despite little overlap between the 3 CNV sets involved (Table S2E), we are able to triplicate this association. These synaptic associations provide aetiological insight that accords well with the emerging neurophysiological view of a strong role for synaptic dysfunction in autism [24]. It is also further strengthened by the over-represented functions among genes within the broader ASD-associated network, whose functions include vesicle transport, cell junction organisation and calcium transport (Figures S1, S2 and S3, Table S6). However, the breadth of dysfunction suggested by the roles of these physically-interacting proteins implicate other, more intracellular processes, such as the cytoskeletal and cellular transport processes, that may affect synapse formation, structure and/or maintenance (Figure S1, Table S6).
The known physical interactions between these genes' products provide pathways through which separate genetic perturbations can converge functionally (Figure 2, Table S5) while the importance of a gene within the ASD-associated network, as specified by the degree of connectivity, appears to be an indicator of ASD-relevant deleteriousness (see Results). Recently, two large-scale studies examining the exomes of autistic patients also identified an excess of protein-protein interactions between genes harbouring suspected causative mutations, reporting smaller networks with 49 [15] and 45 [14] participating genes of which 3 genes and 2 genes, respectively, are also identified through our network. After excluding overlapping genes and compared to random gene sets of equivalent size, the number of connections between gene products in each of the O'Roak et al. and Neale et al. reported networks to the network we identify here are 12-fold and 38-fold over-represented, respectively (p<0.002 for both). Thus, despite little overlap in genes, the strong interconnectedness between these networks identifies pathways through which cellular perturbations arising from distinct mutations identified in separate studies may converge. The single nucleotide variants (SNVs) detected in these two published exome studies are largely predicted to be harmful to the function of the encoded proteins, and therefore comparable to the copy number deletion events in our study. Corroborating our finding of an enrichment of genes that positively regulate among deletions, we also observe a highly significant enrichment of positive regulators among the more strongly-interconnected set of genes identified by Neale et al. (2.7-fold enrichment, p = 3.8×10−6, BH-adjusted p<0.05) which, while enriched, is not significant among the less well-connected genes reported by O'Roak et al. (1.4-fold enrichment, p>0.05).
Despite chronologically limiting our mouse phenotypic dataset to avoid bias (see Materials and Methods), the similarities between the behavioural mouse phenotypes associated with the AGP dn CNVs and human ASD presentations appear clear, with abnormal social/conspecific interaction, stereotypic behaviour and abnormal memory/learning/conditioning phenotypes all over-represented (Figure 1). Many of our study's ASD-associated phenotypes bear a striking resemblance to other frequently co-occurring symptoms, such as impaired coordination [25], [26], [27], anxiety-related phenotypes [28], and absence and tonic-clonic seizures [29], [30], [31] (Figure 1). Finally, we observe a strong enrichment of genes whose disruption yields hearing phenotypes in mice. This observation accords well with estimates in the literature that hearing abnormalities (including sensorineural hearing disorders) affect between 33–46% of ASD cases (Figure 1G) [32], [33]. Many of the associated hearing, and some nervous system, mouse phenotypes are related to peripheral hearing abnormalities, particularly concerning the cochlea and mechanoreception (Figure S4 and Table S3). Inner ear mechanoreception abnormalities appear to have received little attention compared to other regions involved in auditory reception and processing [33]. This is despite improvements in hearing following cochlear implants in individuals with ASD [34] and the knowledge that rare mutations in several genes implicated in ASD (including CHD7, NIPBL, PTPN11 and TBX1) can cause inner ear abnormalities in humans [35], [36], [37], [38].
The enrichment of deleted genes in the network that positively regulate biological processes and a complementary enrichment of duplicated genes that negatively regulate biological processes suggest the occurrence of convergent aetiologies whereby both deletions and duplication act to perturb biological processes relevant to autism in the same direction (Figures 2 and 3). Indeed, the interactions within the ASD-associated network reveal this proposition to be consistent with the experimental literature. For example, considering the STX1A/CFTR/SLC6A3 (aka. DAT) interactions (Figure 3), over-expression of STX1A decreases DAT dopamine transport activity [39], and reduces the CFTR-mediated chloride current by inhibiting trafficking of CFTR to the cell surface [40]. These findings predict that over-expression of STX1A yields an effect similar to the deletion of DAT or CFTR and, concordantly, whereas STX1A lies within a duplication, CFTR and DAT are each deleted. Furthermore, STX1A also interacts with SYP (also duplicated), which negatively regulates SNAP proteins (SNAP29 is deleted) [41], [42]. SNAP proteins are key to presynaptic exocytosis, a process also likely to be disturbed by altered calcium homeostasis resulting from the array of deleted voltage-dependent calcium channels (CACNA1B [deletion], CACNA1C [ASD-implicated deletion], and CACNA1H [deletion]) [43] (Figure 3).
Another example of apparent convergence in aetiology and outcome are the copy number changes affecting the PI3K/Wnt pathways (Figure 3). Here, many copy number changes are predicted to converge to reduce or disrupt the action of the β-catenin destruction complex in the Wnt/β-catenin signalling pathway; the deletion of AXIN1, the increased ubiquitination of AXIN1 by duplication of UBC [44] or the disruption of the PI3K pathway due to mutations in PTEN, TSC1, or TSC2 [45] (Figure 3). Perturbations affecting AXIN1in ASD include the duplication of DVL2 which inhibits AXIN1 (deleted) function [46]. Furthermore, as NKD family proteins promote the degradation of DVL proteins [47], [48], the deletion of NKD2 may increase the activities of DVL2 and thereby also inhibit AXIN1. Concordant with a decrease in β-catenin degradation, an increase in β-catenin stabilization could result from LRPAP1, NKD2 and DVL2 copy number changes. LRPAP1 is thought to have protective roles in LRP1 trafficking and its duplication may therefore increase LRP1 availability [49]. The outcome of the copy number change and disruption of each of these genes is likely to up-regulate the Wnt-stimulated TCF/LEF-dependent transcription, a pathway whose down-regulation has been proposed to have therapeutic benefits in ASD models [50], [51], [52].
Given the ever-increasing number of genetic variants that thus far have been implicated in ASD, the focus will inevitably shift from enumeration towards understanding how these variants contribute to the common pathways and processes underlying this complex disease. Here we have identified a large network of interacting proteins affected by copy number variants identified in patients with ASD, and shown how the network topology and direction of copy number change can be used to interpret these variants' pathway perturbations. Therapeutically targeting molecules at the ends of pathologically-perturbed regulatory cascades may provide more broadly-applicable treatments, while pathological gene duplications may identify attractive targets for knock-down therapeutics as a means of ameliorating perturbed pathways.
Materials and Methods
De novo CNVs identified in individuals with ASD
Four sets of de novo CNVs were employed in this study (Table S1), of which two are drawn from the Simons Simplex Collection and thus overlap [53]. The largest set consists of 73 de novo CNVs identified in 54 (out of 996) individuals with strict autism by the Autism Genome Project (AGP; Table S1) [6]. Of these, 39 CNVs had been confirmed as de novo by independent methods while 34 were considered likely to be de novo by the CNV calling algorithms. The second set consisted of 28 de novo CNVs identified in 24 patients reported in a study by Marshall et al. [54]; two patients who had been reanalysed by the AGP have been removed; Table S2. The third set consisted of 94 de novo CNVs identified in 82 patients reported in a study by Levy et al. [20] and the fourth set consisted of 67 de novo CNVs identified in 63 patients reported in a study by Sanders et al. [55]. Forty two patients examined by Levy et al. were also present in the study by Sanders et al. but this does not affect our findings; As the synaptic phenotype associations that we report and take forward in the Results were identified amongst both the AGP and the Marshall et al. sets, neither the Levy et al. nor Sanders et al. sets were required for replication and thus these latter sets non-independence from each other does not undermine this association. For all sets, contributing patients have been evaluated as having ASD according to ADI-R and/or ADOS criteria. Herein, de novo CNVs identified in patients with ASD is termed “ASD dn CNVs”.
Assigning genes to CNVs
Human genes were assigned to ASD dn CNVs according to Ensembl Ensmart54 [56]. To be confident that the expressed coding sequence of a gene is affected by the copy number change, we conservatively required at least one coding exon of every known transcript of a gene to be overlapped by a CNV for that gene to be deemed overlapped (Table S2). Particular consideration was given to showing that our gene assignment procedure and statistical over-representation analyses did not yield any functional bias under the null hypothesis (see Figure S5 and Methods S1). Genes observed to be copy number variable in the same direction (gain/loss) within a set of CNVs employed by the AGP as a control, i.e. identified from individuals with no obvious psychiatric history in a previous study were removed from the ASD dn CNV gene lists because these are less likely to be associated with ASD [6]. Although it remains possible that common variants contribute to ASDs, our study focuses on genes affected by rare, de novo variants (see Introduction).
Mouse Genome Informatics (MGI) phenotypes
Annotations of phenotypes resulting from disruptions of mouse orthologues of these affected genes were obtained from the Mouse Genome Informatics (MGI) resource (http://www.informatics.jax.org) and interpolated as described previously [12], [57], [58], [59]. Using simple, unambiguous, 1∶1 gene orthology relationships from the MGI resource, 5,283 distinct MGI phenotypic terms were mapped to 5,671 human genes. Each phenotype belongs to one or more of 33 over-arching categories. We considered only 4,055 reasonably populated phenotypes, defined as those with at least 1% of all genes associated with the relevant phenotypic category, thereby reducing uninformative results and improving methodological power. As an unreplicated association between genetic variants in autism patients and a mouse model phenotype was reported in April 2010 [13], we employed only those phenotypes reported in the MGI resource prior to this date, thereby reducing any subsequent phenotyping bias or consequential circularity in discovery. However, our findings remain, or are strengthened by, those more recently reported mouse model phenotypes (data not shown).
Protein-protein interactions
We employed DAPPLE: Disease Association Protein-Protein Link Evaluator [60] to identify direct protein-protein interactions among the protein products of the genes contributing to functional enrichments. A protein-protein interaction network's connectivity was calculated as published previously [60]. Enrichment analysis was carried out by comparing the number of identified direct protein interactions with the average of those identified from 500 gene sets, in which genes were randomly sampled while matched in set size. To account for the increased likelihood that genes that share behavioural associations are more likely to interact than randomly selected genes, we randomly selected sets of orthologues from 1,766 genes annotated with behaviour and neurological phenotypes in the MGI.
Statistical analysis
Due to the small numbers of de novo CNVs considered here and a lack of a control set of de novo CNVs, performing a case-control comparison is not possible. Thus, employing the one-sided Fisher's exact test, we tested the null hypothesis that a (mouse) phenotype associated with (human) Ensembl genes overlapping a set of ASD-associated CNV genomic intervals occurs at a frequency that is no different from that expected from the genome as a whole. Randomisations confirmed that this approach did not yield artefactual bias (see Methods S1 and Figure S5). A multiple testing correction, BH-adjusted p<5%, was applied to account for number of functional terms (phenotypes or GO terms) tested when examining a given gene set [61].
Supporting Information
Zdroje
1. KoganMD, BlumbergSJ, SchieveLA, BoyleCA, PerrinJM, et al. (2009) Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics 124 : 1395–1403.
2. Veenstra-VanderweeleJ, ChristianSL, CookEHJr (2004) Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet 5 : 379–405.
3. ChakrabartiS, FombonneE (2005) Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 162 : 1133–1141.
4. BaileyA, Le CouteurA, GottesmanI, BoltonP, SimonoffE, et al. (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25 : 63–77.
5. StankiewiczP, LupskiJR (2010) Structural variation in the human genome and its role in disease. Annu Rev Med 61 : 437–455.
6. PintoD, PagnamentaAT, KleiL, AnneyR, MericoD, et al. (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 : 368–372.
7. LarsenFW, MouridsenSE (1997) The outcome in children with childhood autism and Asperger syndrome originally diagnosed as psychotic. A 30-year follow-up study of subjects hospitalized as children. Eur Child Adolesc Psychiatry 6 : 181–190.
8. ReichenbergA, GrossR, WeiserM, BresnahanM, SilvermanJ, et al. (2006) Advancing paternal age and autism. Arch Gen Psychiatry 63 : 1026–1032.
9. WebberC (2011) Functional Enrichment Analysis with Structural Variants: Pitfalls and Strategies. Cytogenet Genome Res 135 : 277–85.
10. StateMW, LevittP (2011) The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci 14 : 1499–1506.
11. WebberC, Hehir-KwaJY, NguyenDQ, de VriesBB, VeltmanJA, et al. (2009) Forging links between human mental retardation-associated CNVs and mouse gene knockout models. PLoS Genet 5: e1000531.
12. ShaikhTH, Haldeman-EnglertC, GeigerEA, PontingCP, WebberC (2011) Genes and biological processes commonly disrupted in rare and heterogeneous developmental delay syndromes. Hum Mol Genet 20 : 880–893.
13. GaiX, XieHM, PerinJC, TakahashiN, MurphyK, et al. (2012) Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry 17 : 402–411.
14. NealeBM, KouY, LiuL, Ma'ayanA, SamochaKE, et al. (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485 : 242–245.
15. O'RoakBJ, VivesL, GirirajanS, KarakocE, KrummN, et al. (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485 : 246–250.
16. Ben-DavidE, ShifmanS (2012) Networks of neuronal genes affected by common and rare variants in autism spectrum disorders. PLoS Genet 8: e1002556.
17. GilmanSR, IossifovI, LevyD, RonemusM, WiglerM, et al. (2011) Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70 : 898–907.
18. BasuSN, KolluR, Banerjee-BasuS (2009) AutDB: a gene reference resource for autism research. Nucleic Acids Res 37: D832–836.
19. SandersSJ, MurthaMT, GuptaAR, MurdochJD, RaubesonMJ, et al. (2012) De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485 : 237–241.
20. LevyD, RonemusM, YamromB, LeeYH, LeottaA, et al. (2011) Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70 : 886–897.
21. SmithCL, EppigJT (2009) The Mammalian Phenotype Ontology: enabling robust annotation and comparative analysis. Wiley Interdiscip Rev Syst Biol Med 1 : 390–399.
22. SupekF, BosnjakM, SkuncaN, SmucT (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6: e21800.
23. JeongH, MasonSP, BarabasiAL, OltvaiZN (2001) Lethality and centrality in protein networks. Nature 411 : 41–42.
24. BourgeronT (2009) A synaptic trek to autism. Curr Opin Neurobiol 19 : 231–234.
25. GreenD, CharmanT, PicklesA, ChandlerS, LoucasT, et al. (2009) Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol 51 : 311–316.
26. MingX, BrimacombeM, WagnerGC (2007) Prevalence of motor impairment in autism spectrum disorders. Brain Dev 29 : 565–570.
27. StaplesKL, ReidG (2010) Fundamental movement skills and autism spectrum disorders. J Autism Dev Disord 40 : 209–217.
28. WhiteSW, OswaldD, OllendickT, ScahillL (2009) Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev 29 : 216–229.
29. Giovanardi RossiP, PosarA, ParmeggianiA (2000) Epilepsy in adolescents and young adults with autistic disorder. Brain Dev 22 : 102–106.
30. SteffenburgS, GillbergC, SteffenburgU (1996) Psychiatric disorders in children and adolescents with mental retardation and active epilepsy. Arch Neurol 53 : 904–912.
31. TuchmanRF, RapinI, ShinnarS (1991) Autistic and dysphasic children. II: Epilepsy. Pediatrics 88 : 1219–1225.
32. KlinA (1993) Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J Autism Dev Disord 23 : 15–35.
33. HitoglouM, VerveriA, AntoniadisA, ZafeiriouDI (2010) Childhood autism and auditory system abnormalities. Pediatr Neurol 42 : 309–314.
34. DonaldsonAI, HeavnerKS, ZwolanTA (2004) Measuring progress in children with autism spectrum disorder who have cochlear implants. Arch Otolaryngol Head Neck Surg 130 : 666–671.
35. BosmanEA, PennAC, AmbroseJC, KettleboroughR, StempleDL, et al. (2005) Multiple mutations in mouse Chd7 provide models for CHARGE syndrome. Hum Mol Genet 14 : 3463–3476.
36. VitelliF, ViolaA, MorishimaM, PramparoT, BaldiniA, et al. (2003) TBX1 is required for inner ear morphogenesis. Hum Mol Genet 12 : 2041–2048.
37. MoySS, NadlerJJ (2008) Advances in behavioral genetics: mouse models of autism. Mol Psychiatry 13 : 4–26.
38. GotzJ, IttnerLM (2008) Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci 9 : 532–544.
39. CervinskiMA, FosterJD, VaughanRA (2010) Syntaxin 1A regulates dopamine transporter activity, phosphorylation and surface expression. Neuroscience 170 : 408–416.
40. TangBL, GeeHY, LeeMG (2011) The cystic fibrosis transmembrane conductance regulator's expanding SNARE interactome. Traffic 12 : 364–371.
41. EdelmannL, HansonPI, ChapmanER, JahnR (1995) Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J 14 : 224–231.
42. McMahonHT, MisslerM, LiC, SudhofTC (1995) Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83 : 111–119.
43. HauckeV, NeherE, SigristSJ (2011) Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci 12 : 127–138.
44. KimS, JhoEH (2010) The protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2). J Biol Chem 285 : 36420–36426.
45. MakBC, KenersonHL, AicherLD, BarnesEA, YeungRS (2005) Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol 167 : 107–116.
46. ZhangY, NeoSY, HanJ, LinSC (2000) Dimerization choices control the ability of axin and dishevelled to activate c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem 275 : 25008–25014.
47. WhartonKAJr, ZimmermannG, RoussetR, ScottMP (2001) Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev Biol 234 : 93–106.
48. SchneiderI, SchneiderPN, DerrySW, LinS, BartonLJ, et al. (2010) Zebrafish Nkd1 promotes Dvl degradation and is required for left-right patterning. Dev Biol 348 : 22–33.
49. WillnowTE, RohlmannA, HortonJ, OtaniH, BraunJR, et al. (1996) RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J 15 : 2632–2639.
50. ZhangY, SunY, WangF, WangZ, PengY, et al. (2012) Downregulating the Canonical Wnt/beta-catenin Signaling Pathway Attenuates the Susceptibility to Autism-like Phenotypes by Decreasing Oxidative Stress. Neurochem Res 37 : 1409–1419.
51. OkerlundND, CheyetteBN (2011) Synaptic Wnt signaling-a contributor to major psychiatric disorders? J Neurodev Disord 3 : 162–174.
52. MinesMA, YuskaitisCJ, KingMK, BeurelE, JopeRS (2010) GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One 5: e9706.
53. FischbachGD, LordC (2010) The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron 68 : 192–195.
54. MarshallCR, NoorA, VincentJB, LionelAC, FeukL, et al. (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82 : 477–488.
55. SandersSJ, Ercan-SencicekAG, HusV, LuoR, MurthaMT, et al. (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70 : 863–885.
56. HubbardTJ, AkenBL, AylingS, BallesterB, BealK, et al. (2009) Ensembl 2009. Nucleic Acids Res 37: D690–697.
57. EppigJT, BultCJ, KadinJA, RichardsonJE, BlakeJA, et al. (2005) The Mouse Genome Database (MGD): from genes to mice–a community resource for mouse biology. Nucleic Acids Res 33: D471–475.
58. EppigJT, BlakeJA, BultCJ, RichardsonJE, KadinJA, et al. (2007) Mouse genome informatics (MGI) resources for pathology and toxicology. Toxicol Pathol 35 : 456–457.
59. BultCJ, EppigJT, KadinJA, RichardsonJE, BlakeJA (2008) The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic Acids Res 36: D724–728.
60. RossinEJ, LageK, RaychaudhuriS, XavierRJ, TatarD, et al. (2011) Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet 7: e1001273.
61. BenjaminiY, HochbergY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 57 : 289–300.
62. MuddashettyRS, NalavadiVC, GrossC, YaoX, XingL, et al. (2011) Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell 42 : 673–688.
63. ChenHJ, Rojas-SotoM, OguniA, KennedyMB (1998) A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20 : 895–904.
64. HartMJ, MaruY, LeonardD, WitteON, EvansT, et al. (1992) A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science 258 : 812–815.
65. RainesKW, CaoGL, LeeEK, RosenGM, ShapiroP (2006) Neuronal nitric oxide synthase-induced S-nitrosylation of H-Ras inhibits calcium ionophore-mediated extracellular-signal-regulated kinase activity. Biochem J 397 : 329–336.
66. NikonenkoI, BodaB, SteenS, KnottG, WelkerE, et al. (2008) PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol 183 : 1115–1127.
67. RuthP, WangGX, BoekhoffI, MayB, PfeiferA, et al. (1993) Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A 90 : 2623–2627.
Štítky
Genetika Reprodukční medicína
Článek PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant TransformationČlánek The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene ClusterČlánek Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish PathogenČlánek USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and TranscriptionČlánek Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA EditingČlánek Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 6
-
Všechny články tohoto čísla
- BMS1 Is Mutated in Aplasia Cutis Congenita
- High Trans-ethnic Replicability of GWAS Results Implies Common Causal Variants
- How Cool Is That: An Interview with Caroline Dean
- Genetic Architecture of Vitamin B and Folate Levels Uncovered Applying Deeply Sequenced Large Datasets
- Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle,
- Meiosis-Specific Stable Binding of Augmin to Acentrosomal Spindle Poles Promotes Biased Microtubule Assembly in Oocytes
- Environmental Dependence of Genetic Constraint
- H3.3-H4 Tetramer Splitting Events Feature Cell-Type Specific Enhancers
- Network Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
- Effectively Identifying eQTLs from Multiple Tissues by Combining Mixed Model and Meta-analytic Approaches
- Altered Splicing of the BIN1 Muscle-Specific Exon in Humans and Dogs with Highly Progressive Centronuclear Myopathy
- The NADPH Metabolic Network Regulates Human Cardiomyopathy and Reductive Stress in
- Negative Regulation of Notch Signaling by Xylose
- A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
- Transcriptome-Wide Mapping of 5-methylcytidine RNA Modifications in Bacteria, Archaea, and Yeast Reveals mC within Archaeal mRNAs
- Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen
- Latent Effects of Hsp90 Mutants Revealed at Reduced Expression Levels
- Impact of Natural Genetic Variation on Gene Expression Dynamics
- DeepSAGE Reveals Genetic Variants Associated with Alternative Polyadenylation and Expression of Coding and Non-coding Transcripts
- The Identification of -acting Factors That Regulate the Expression of via the Osteoarthritis Susceptibility SNP rs143383
- Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs
- The RNA Export Factor, Nxt1, Is Required for Tissue Specific Transcriptional Regulation
- Inferring Demographic History from a Spectrum of Shared Haplotype Lengths
- Histone Acetyl Transferase 1 Is Essential for Mammalian Development, Genome Stability, and the Processing of Newly Synthesized Histones H3 and H4
- PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant Transformation
- DNA Methylation Restricts Lineage-specific Functions of Transcription Factor Gata4 during Embryonic Stem Cell Differentiation
- The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Deregulation of the Protocadherin Gene Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy
- Evidence for Two Different Regulatory Mechanisms Linking Replication and Segregation of Chromosome II
- USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and Transcription
- Methylation of Histone H3 on Lysine 79 Associates with a Group of Replication Origins and Helps Limit DNA Replication Once per Cell Cycle
- A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue
- The Gene Desert Mammary Carcinoma Susceptibility Locus Regulates Modifying Mammary Epithelial Cell Differentiation and Proliferation
- Hooked and Cooked: A Fish Killer Genome Exposed
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
- Mutations in Cause Autosomal Recessive Congenital Ichthyosis in Humans
- Integrated Transcriptomic and Epigenomic Analysis of Primary Human Lung Epithelial Cell Differentiation
- RSR-2, the Ortholog of Human Spliceosomal Component SRm300/SRRM2, Regulates Development by Influencing the Transcriptional Machinery
- Comparative Polygenic Analysis of Maximal Ethanol Accumulation Capacity and Tolerance to High Ethanol Levels of Cell Proliferation in Yeast
- SPO11-Independent DNA Repair Foci and Their Role in Meiotic Silencing
- Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery
- Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA Editing
- Functional Analysis of Neuronal MicroRNAs in Dauer Formation by Combinational Genetics and Neuronal miRISC Immunoprecipitation
- DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
- Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
- Heritable Change Caused by Transient Transcription Errors
- From Many, One: Genetic Control of Prolificacy during Maize Domestication
- Neuronal Target Identification Requires AHA-1-Mediated Fine-Tuning of Wnt Signaling in
- Loss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
- H-NS Can Facilitate Specific DNA-binding by RNA Polymerase in AT-rich Gene Regulatory Regions
- Prophage Dynamics and Contributions to Pathogenic Traits
- Global DNA Hypermethylation in Down Syndrome Placenta
- Fragile DNA Motifs Trigger Mutagenesis at Distant Chromosomal Loci in
- Disturbed Local Auxin Homeostasis Enhances Cellular Anisotropy and Reveals Alternative Wiring of Auxin-ethylene Crosstalk in Seminal Roots
- Causes and Consequences of Chromatin Variation between Inbred Mice
- Genome-scale Analysis of FNR Reveals Complex Features of Transcription Factor Binding
- Distinct and Atypical Intrinsic and Extrinsic Cell Death Pathways between Photoreceptor Cell Types upon Specific Ablation of in Cone Photoreceptors
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMS1 Is Mutated in Aplasia Cutis Congenita
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



