-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDistinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
Oomycetes in the class Saprolegniomycetidae of the Eukaryotic kingdom Stramenopila have evolved as severe pathogens of amphibians, crustaceans, fish and insects, resulting in major losses in aquaculture and damage to aquatic ecosystems. We have sequenced the 63 Mb genome of the fresh water fish pathogen, Saprolegnia parasitica. Approximately 1/3 of the assembled genome exhibits loss of heterozygosity, indicating an efficient mechanism for revealing new variation. Comparison of S. parasitica with plant pathogenic oomycetes suggests that during evolution the host cellular environment has driven distinct patterns of gene expansion and loss in the genomes of plant and animal pathogens. S. parasitica possesses one of the largest repertoires of proteases (270) among eukaryotes that are deployed in waves at different points during infection as determined from RNA-Seq data. In contrast, despite being capable of living saprotrophically, parasitism has led to loss of inorganic nitrogen and sulfur assimilation pathways, strikingly similar to losses in obligate plant pathogenic oomycetes and fungi. The large gene families that are hallmarks of plant pathogenic oomycetes such as Phytophthora appear to be lacking in S. parasitica, including those encoding RXLR effectors, Crinkler's, and Necrosis Inducing-Like Proteins (NLP). S. parasitica also has a very large kinome of 543 kinases, 10% of which is induced upon infection. Moreover, S. parasitica encodes several genes typical of animals or animal-pathogens and lacking from other oomycetes, including disintegrins and galactose-binding lectins, whose expression and evolutionary origins implicate horizontal gene transfer in the evolution of animal pathogenesis in S. parasitica.
Published in the journal: . PLoS Genet 9(6): e32767. doi:10.1371/journal.pgen.1003272
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003272Summary
Oomycetes in the class Saprolegniomycetidae of the Eukaryotic kingdom Stramenopila have evolved as severe pathogens of amphibians, crustaceans, fish and insects, resulting in major losses in aquaculture and damage to aquatic ecosystems. We have sequenced the 63 Mb genome of the fresh water fish pathogen, Saprolegnia parasitica. Approximately 1/3 of the assembled genome exhibits loss of heterozygosity, indicating an efficient mechanism for revealing new variation. Comparison of S. parasitica with plant pathogenic oomycetes suggests that during evolution the host cellular environment has driven distinct patterns of gene expansion and loss in the genomes of plant and animal pathogens. S. parasitica possesses one of the largest repertoires of proteases (270) among eukaryotes that are deployed in waves at different points during infection as determined from RNA-Seq data. In contrast, despite being capable of living saprotrophically, parasitism has led to loss of inorganic nitrogen and sulfur assimilation pathways, strikingly similar to losses in obligate plant pathogenic oomycetes and fungi. The large gene families that are hallmarks of plant pathogenic oomycetes such as Phytophthora appear to be lacking in S. parasitica, including those encoding RXLR effectors, Crinkler's, and Necrosis Inducing-Like Proteins (NLP). S. parasitica also has a very large kinome of 543 kinases, 10% of which is induced upon infection. Moreover, S. parasitica encodes several genes typical of animals or animal-pathogens and lacking from other oomycetes, including disintegrins and galactose-binding lectins, whose expression and evolutionary origins implicate horizontal gene transfer in the evolution of animal pathogenesis in S. parasitica.
Introduction
Saprolegnia species are watermolds or oomycetes that are endemic to probably all fresh water ecosystems. These understudied pathogens can cause destructive diseases of amphibians, crustaceans, fish and insects in aquaculture and in natural environments worldwide [1], [2]. With the rise of fish as a principal source of animal protein, and the decline of wild fish stocks, aquaculture production has increased on average by 11% per year worldwide over the past ten years (FAO Fishery Information). Intensive aquatic farming practices have resulted in explosive growth in pathogen populations, which has been exacerbated by the ban of malachite green as a pesticide. Losses due to microbial, parasitic and viral infections are the largest problem in fish farms nowadays, and have a significant effect on animal welfare and sustainability of the industry. The salmon farming industry is particularly affected by Saprolegnia parasitica. This pathogen causes Saprolegniosis (also known as Saprolegniasis), a disease characterized by visible grey or white patches of mycelium on skin and fins, and subsequent penetration of mycelium into muscles and blood vessels [1], [3]. It is estimated that 10% of all hatched salmon succumb to Saprolegnia infections and losses are estimated at tens of millions of dollars annually [2]. In addition to the damage to the aquaculture industry, the declines of natural salmonid populations have also been attributed to Saprolegnia infections [1]. More in-depth knowledge of the epidemiology, biology and pathology of the pathogen is urgently needed. A draft genome sequence of S. parasitica provides an excellent starting point to investigate the disease process at the molecular and cellular level and may lead to novel avenues for sustainable control of Saprolegniosis.
Animal pathogens have evolved independently multiple times in lineages such as Stramenopila, Alveolata, Amebozoa, Euglenozoa and Mycota, as well as in numerous bacterial lineages. Oomycetes such as Saprolegnia belong to the kingdom Stramenopila (Patterson, 1989), syn. Straminipila (Dick, 2001), that includes photosynthetic algae such as kelp and diatoms, ubiquitous saprotrophic flagellates such as Cafeteria roenbergensis, and obligate mammalian parasites such as Blastocystis [4], [5]. Although many Saprolegnia and related species are capable of causing diseases on a wide range of animal hosts including humans, relatively little is known about their mechanisms of pathogenicity. Among the oomycetes, most animal pathogens including S. parasitica belong to the class Saprolegniomycetidae (Figure 1A). The oomycetes also include many plant pathogens and these are mainly concentrated within the class Peronosporomycetidae. There are a small number of interesting exceptions to this otherwise sharp dichotomy, including the mammalian pathogen Pythium insidiosum (Peronosporomycetidae) and the plant pathogens Aphanomyces euteiches and Aphanomyces cochlioides (Saprolegniomycetidae) [1], [6], [7].
Fig. 1. Taxonomy and ancestral genomic features in S. parasitica. 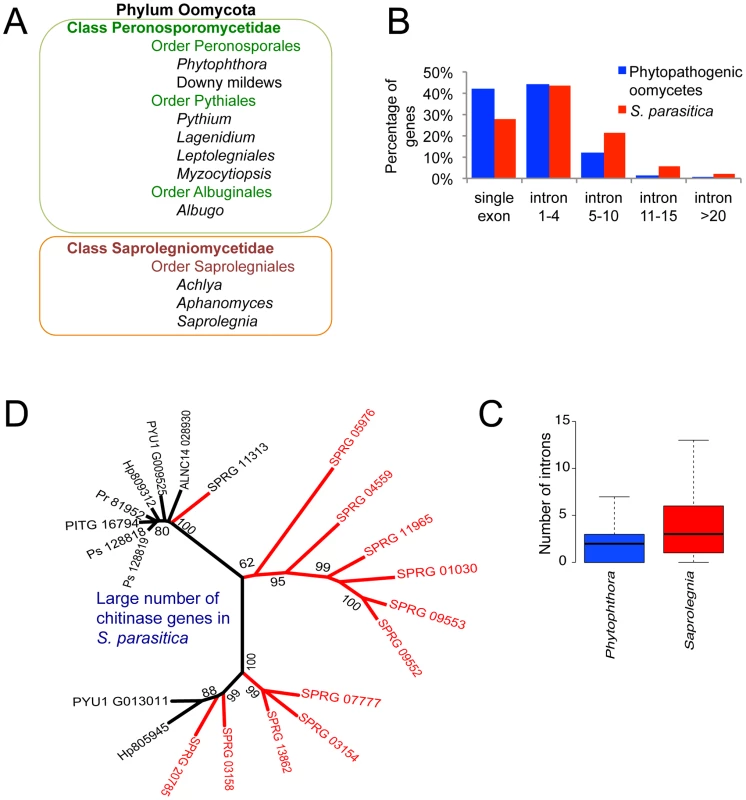
(A) Animal pathogenic and plant pathogenic oomycetes reside in different taxonomic units. (B) Comparison of intron number in phytopathogenic oomycetes (the average count from the total genes of P. infestans, P. ramorum, P. sojae, Py. ultimum and H. arabidopsidis) and S. parasitica among all genes. (C) Significant difference in intron number in 4008 orthologous genes shared by S. parasitica and Phytophthora species (average intron count of P. infestans, P. sojae and P. ramorum). (Wilcoxon test, p<0.001). (D) Large number of chitinase genes belonging to CAZy family GH-18 in S. parasitica (red) compared to other oomycetes (black; Ps = P. sojae, Pr = P. ramorum, PITG = P. infestans, Hp = H. arabidopsidis, Pyu = Py. ultimum, ALNC = A. laibachii). The phylogenetic tree was constructed with chitinase genes from oomycetes using Maximum likelihood method. Plant pathogens in the class Peronosporomycetidae include Phytophthora and Pythium species, downy mildew pathogens and white rusts (Figure 1A). Well-known examples are the potato late blight pathogen Phytophthora infestans that caused the great Irish famine in the 1840s [8], the soybean root rot pathogen Phytophthora sojae [9], and the sudden oak death pathogen Phytophthora ramorum [10]. Sequenced and assembled genomes have been generated for P. sojae and P. ramorum [11], P. infestans [12], and for several relatives including the broad host range plant pathogens Phytophthora capsici [13] and Pythium ultimum [14], the downy mildew pathogen of Arabidopsis, Hyaloperonospora arabidopsidis [15], and the white blister rust of Brassicaceae, Albugo candida [16]. Genome analyses have revealed a bi-partite genome organization in these pathogens, in which gene dense regions containing clusters of orthologs with well-conserved sequences and gene order are interspersed with repeat-rich regions containing rapidly evolving families of virulence genes and numerous transposons [12], [17], [18].
The virulence gene families of plant pathogenic oomycetes encode numerous hydrolytic enzymes for degradation of plant carbohydrates, extracellular toxins such as NLP and PcF toxins, and at least three families of cell-entering effector proteins, RXLR effectors, CHXC effectors and Crinkler proteins [7], [11], [19], [20]. These classes of effector proteins contain amino acid sequence motifs (RXLR, CHXC, and LFLAK respectively) that are involved in entry into the host plant cell [12], [21], [22], [23], [24], [25] and effector domains that target diverse host physiological processes to suppress immunity and promote infection [17]. In the genome of the broad host-range necrotrophic oomycete Py. ultimum, which primarily targets immature or stressed plant tissues, enzyme families are expanded that enable degradation of readily accessible carbohydrates such as pectins, starch, and sucrose, while RXLR effectors appear to be completely absent [14]. In the obligate biotroph H. arabidopsidis, most gene families are smaller, especially those encoding hydrolytic enzymes [15]. Interestingly, EST libraries from the saprolegniomycete plant pathogen A. euteiches revealed the presence of Crinkler effectors (but neither NLP toxins nor RXLR effectors) [26], suggesting that Crinklers are ancestral to oomycete pathogens [23].
So far, only limited genomic resources are available for animal pathogenic oomycetes. Analysis of small sets of EST data of S. parasitica [27], [28] and Py. insidiosum [29], revealed the presence of secreted protein families with potential roles in virulence such as glycosyl hydrolases, proteases, and protease inhibitors, as well as proteins involved in protection against oxidative stress. The S. parasitica data set included a host-targeting protein SpHtp1 (S. parasitica host-targeting protein 1) that was subsequently demonstrated to enter fish cells through binding to a tyrosine-O-sulfated fish cell surface ligand [30].
Here we report the genome sequence and transcriptome analysis of S. parasitica, the first genome sequenced from an animal pathogenic oomycete. We compared the genome to those of plant pathogenic oomycetes, revealing distinctive genome expansions and adaptations tailored to the physiology of the respective hosts. This study adds to our understanding of mechanisms for invasion and colonization of animal host cells by eukaryotic pathogens.
Results
A compact, highly polymorphic genome exhibiting extensive loss of heterozygosity
The strategy of whole genome shotgun sequencing was applied to S. parasitica strain CBS223.65, which is a strain isolated from infected pike (Esox lucius). A combination of 454 (fragment and 3 kb jumping libraries) and Sanger (Fosmid library) sequencing data (∼50-fold average read coverage) were used to assemble the genome, yielding an initial assembly length of 53 Mb and a scaffold N50 length of 281 kb (see Table S1 for additional assembly statistics). Read coverage and rates of polymorphism were computed based on alignments of Illumina data (generated for polymorphism discovery) to the genome assembly. Based on the distribution of coverage and polymorphisms (Figure S1A and S1B), we conclude that the assembly represents a composition of regions of diploid consensus (76% of the assembly) with the remainder corresponding to separately assembled haplotypes, resulting from the high polymorphism rate with a peak of 2.6% (Table S2 and Figure S1C). Taking into account the 24% of the assembly corresponding to separately-assembled haplotypes, the total assembled haploid genome size was adjusted to 42.3 Mb (see Text S1). Based on read coverage we estimated the total genome size to be 62.8 Mb. The remaining 20.4 Mb of genomic sequence were estimated to correspond to collapsed tandem repeat content in the genome assembly (Text S1). Read coverage analysis of the separated haplotypes and of regions showing loss of heterozygosity (see below) produced an overall genome size estimate of 63 Mb (Text S1), consistent with our effective assembled genome size estimate. The difference between the assembly size and the read coverage estimate likely results from tandem repeats collapsed in the assembly and uncertainties in read alignments, as observed in other oomycete genome sequence assemblies (Table 1). More than 98% of Trinity [31] de novo assembled transcripts from in vitro growth RNA-Seq data (described in Material and Methods) mapped to the genome assembly, indicating that the expressed gene content is very well represented by the assembled genome. The genome size of S. parasitica is consistent with the size range of most previously sequenced oomycete genomes, which rank from 45 Mb (Py. ultimum) [14] to 65 Mb (P. ramorum) [11], far below the outlier of 240 Mb (P. infestans) [12] (Table 1).
Tab. 1. Genome statistics and intron features of oomycetes. 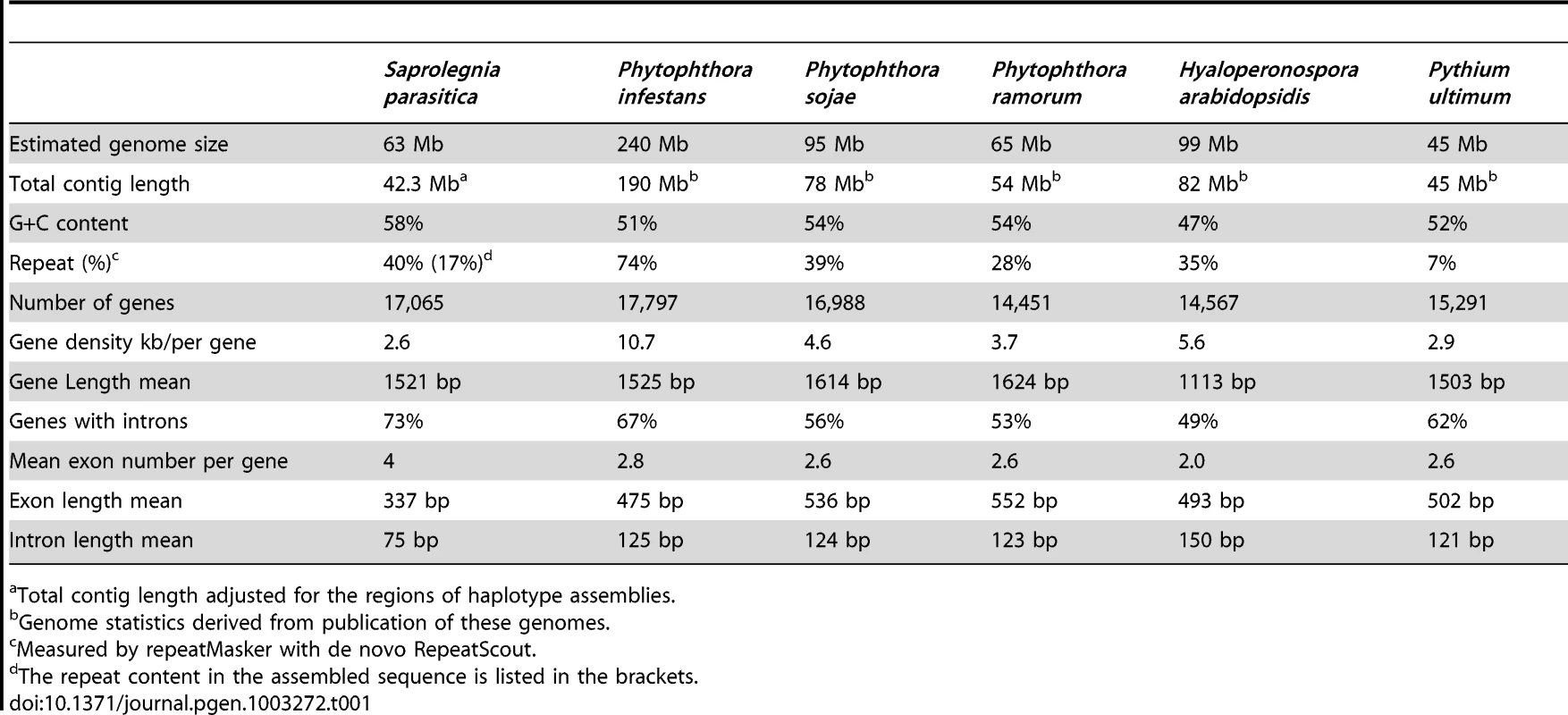
Total contig length adjusted for the regions of haplotype assemblies. Approximately one-third of the assembled S. parasitica genome was found to correspond to regions exhibiting loss of heterozygosity (LOH) (Figure S1C and Text S1). LOH resulting from mitotic instability has been observed in other oomycete genomes [13], [32], [33], and provides a potential adaptive mechanism that promotes expression of genetic diversity within a clonal pathogen population [13]. The prevalence of LOH within a S. parasitica population and the role LOH plays in S. parasitica evolution remain to be determined.
A total of 20,113 coding gene models were computationally predicted, with 3,048 pairs of coding genes assigned as alleles within separately assembled haplotype regions (Table S3), yielding an adjusted coding gene count of 17,065, similar in magnitude to counts of genes identified in other sequenced oomycete genomes (Table 1). There are 5,291 coding genes (31% of total) found to reside within regions of LOH (Table S3). No obvious enrichment or depletion of biologically relevant gene functions could be detected within the defined regions of LOH that would suggest that the LOH observed had resulted from selection in the individual strain sequenced.
The gene density found in S. parasitica is one of the highest reported so far for oomycetes, with one gene per 2.6 kb (Table 1). This is slightly denser than in Py. ultimum (one gene per 2.9 kb) and A. candida (2.9 kb), and much denser than in P. infestans (one gene per 10.7 kb). Many of the S. parasitica genes are novel; only 40% of the S. parasitica predicted proteins have homologs with more than 50% sequence similarity to those from other organisms, including oomycetes. A conserved core-proteome of 4,215 proteins can be identified for plant pathogenic peronosporomycetes based on genomes from multiple Phytophthora species, H. arabidopsidis and Py. ultimum [14]. Of these 4215, S. parasitica shares only 3518 orthologs (Figure S2A), of which only about 40% show strong sequence similarity (>50%). 20% of the core set is not detectable in the S. parasitica proteome (Figure S2B). Although the genomes of the peronosporomycetes show substantial conservation of gene order (synteny), little of this synteny is preserved in S. parasitica, as was observed for A. candida [16].
Interestingly, compared to other oomycetes, S. parasitica genes contain a larger number of introns (Figure 1B, Table 1). More than 73% of the S. parasitica genes contain at least one intron, compared to 50–60% in other oomycete species (Table 1). Among 4008 orthologs shared between S. parasitica and three Phytophthora species, the majority of the genes have different numbers of introns. For example, more than half of the S. parasitica genes have more introns than their orthologs in Phytophthora, and 15% of the S. parasitica genes have 5 or more additional exons compared to their Phytophthora orthologs (Figure 1C). The intron abundance in S. parasitica potentially more closely matches the ancestral state, assuming a trend of intron reduction as found in animal and fungal lineages [34].
The S. parasitica genome has very few known mobile elements, which is consistent with its smaller size compared to the transposon-rich Phytophthora genomes. Of the 160 repeat families identified among all sequenced Phytophthora species, only one LTR retrotransposon family was found in the S. parasitica genome (Figure S3A). This group of LTR elements, which occur at low copy numbers (<20) in known oomycete genomes (Figure S3B), is thus ancient. The largest transposon family in S. parasitica (approx. 50 copies in the assembled sequence, and an estimated a few hundred copies in the genome) belongs to the LINE repeat group (Text S1). LINE elements are abundant in animal genomes and play roles in genome evolution and modulation of gene expression [35]. Curiously, the S. parasitica LINE element family is absent from the Phytophthora genomes but shares sequence similarity with the LINE elements from animal genomes (Figure S3C), raising the possibility that this family was acquired from an animal host.
Saprolegnia parasitica has a very large kinome
Eukaryotic protein kinases (ePKs) regulate a myriad of cellular activities by phosphorylating target proteins in response to internal or external signals. S. parasitica has one of the largest kinomes that have been identified to date, with 543 predicted ePKs. For example, S. parasitica has 65 more ePKs than the human kinome by the same prediction criteria (Figure S4A). Eukaryotic protein kinases have been classified into eight major groups based on sequence similarity. We classified members of the S. parasitica ePK superfamily using a set of HMMs based on previously identified kinases (Figure S4A). S. parasitica has a large expansion of tyrosine kinase-like proteins with 298 members and a large number of unclassified kinases (114), suggesting novel functions performed by the S. parasitica kinome. Interestingly, the S. parasitica kinome contains several kinase families that have typically not been seen outside the metazoan clade, for example CAMK2, NUAK, SNRK, and PHK from the Ca2+ calmodulin-dependent kinase group. Some of these “metazoan” kinases are shared with plant pathogenic oomycetes [36], substantially pushing back the evolutionary origin of these kinases. We found 131 kinases containing predicted transmembrane helices (Figure S4B), suggesting that S. parasitica has a large number of protein kinases that may function as cell surface receptors with roles in signaling. Such a large receptor repertoire may facilitate the recognition of stimuli from extracellular abiotic and biotic environments.
Chitin metabolism
The cell wall of a pathogen plays a central role at the host-pathogen interface. In particular, cell wall related proteins and polysaccharides are a large source of PAMPs (Pathogen Associated Molecular Patterns) of both animal and plant pathogens [37], [38]. In addition, cell wall carbohydrate biosynthetic enzymes represent a potential target of antimicrobial compounds when similar activities are not encountered in the host. This is illustrated by the demonstration that the specific inhibition of chitin synthase (CHS) in Saprolegnia leads to cell death although chitin represents no more than 1% of the total cell wall carbohydrates of the pathogen [39], [40].Chitin is a structural crystalline polymer typically associated with the fungal cell wall. Historically, the absence of chitin in Phytophthora [41] has led to the general concept that oomycetes are devoid of chitin and that cellulose, the major load-bearing structural polysaccharide in oomycete walls, is a key feature distinguishing oomycetes from true fungi. However, the occurrence of chitin has since been demonstrated in various oomycete species belonging to the Saprolegniales [39], [40], [42]. In addition, GlcNAc-based carbohydrates that do not seem to correspond to crystalline chitin, but whose biosynthesis is most likely performed by putative chitin synthase gene products are present in the walls of Aphanomyces euteiches [43].The S. parasitica genome appears to contain genes that encode enzymes involved in chitin biosynthesis, modification and degradation (Table S4). Out of these, six genes correspond to putative chitin synthases (SPRG_02074, SPRG_02554, SPRG_04151, SPRG_09812, SPRG_06131 and SPRG_19383). Interestingly, this number is higher than in other oomycetes where only one or two CHS putative genes have been detected [39], [43] (Table S4). The only oomycete CHS gene product for which the function has been unequivocally demonstrated through heterologous expression and in vitro biochemical assays is CHS2 from Saprolegnia monoica [39]. Thus, as for most CHS genes from other oomycetes, the function of the S. parasitica genes annotated here as CHS remains to be demonstrated. As reported earlier in S. monoica [39], two of the newly identified S. parasitica CHS gene products (SPRG_09812 and SPRG_04151) contain a so-called MIT (Microtubule Interacting and Trafficking (or Transport)) domain. These MIT domains are possibly involved in the trafficking and delivery of the corresponding CHS at the apex of the hyphal cells, as previously suggested for S. monoica [39]. There are also a large number (14) of chitinase genes in S. parasitica compared to other oomycetes, which could represent many ancestral forms of oomycete chitinases (Figure 1D).
Adaptations of metabolism to animal pathogenesis
There are major structural and physiological differences between plant and animal cells, and thus the metabolisms of plant and animal pathogens have likely adapted accordingly to the respective host cellular environments. Pectin is a major constituent of plant cell walls and a target for extracellular enzymes produced by pathogenic and saprophytic microorganisms. Plant pathogenic fungi and oomycetes produce a large array of enzymes to degrade pectin, including polygalacturonase (PG), pectin and pectate lyases (PL), and pectin methylesterases (PME). Animal cells lack a cell wall, and as might be expected, the pathogen S. parasitica encodes very few cell wall degrading enzymes in its genome. Genes encoding hydrolytic enzymes such as cutinase and pectin methyl esterases appear to be absent, and PL and PG genes are greatly reduced in numbers as compared to plant pathogenic oomycetes (Table 2). The remaining small numbers of PLs and PGs may play a role in the saprophytic life stage of S. parasitica in the aquatic environment outside of fish hosts.
Tab. 2. Gene families potentially involved in pathogenesis in Saprolegnia parasitica. 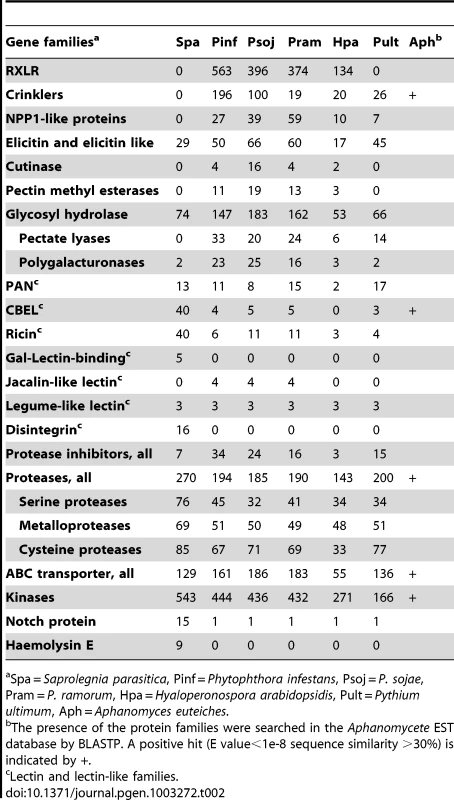
Spa = Saprolegnia parasitica, Pinf = Phytophthora infestans, Psoj = P. sojae, Pram = P. ramorum, Hpa = Hyaloperonospora arabidopsidis, Pult = Pythium ultimum, Aph = Aphanomyces euteiches. S. parasitica proliferates in host tissue rich in proteins and ammonium. Concomitantly, its pathways involved in inorganic nitrogen and sulfur assimilation have degenerated (Figure 2A). The loss of these metabolic capabilities has occurred independently in the obligate oomycete plant pathogen H. arabidopsidis [15], as well as within several lineages of obligate fungal plant pathogens, presumably due to the high level of parasitic adaptation in these organisms. Strikingly the same physical clusters of genes have been lost in each lineage, namely the genes encoding nitrate reductase, nitrite reductase, sulfite reductase and nitrate transporters (Table S5). Also in line with a protein-rich environment that is a major source of both carbon and nitrogen, the S. parasitica genome contains 56 genes predicted to encode amino acid transporters. Most of the S. parasitica transporters appear to be novel because less than 20 of the predicted amino acid transporters have orthologs or closely related paralogs in other oomycete genomes. Phylogenetic analysis shows there are lineage-specific expansions of amino acid transporter genes in the different oomycete genomes, with recently duplicated S. parasitica genes forming the largest group (Figure 2B).
Fig. 2. Metabolic adaptations to animal pathogenesis. 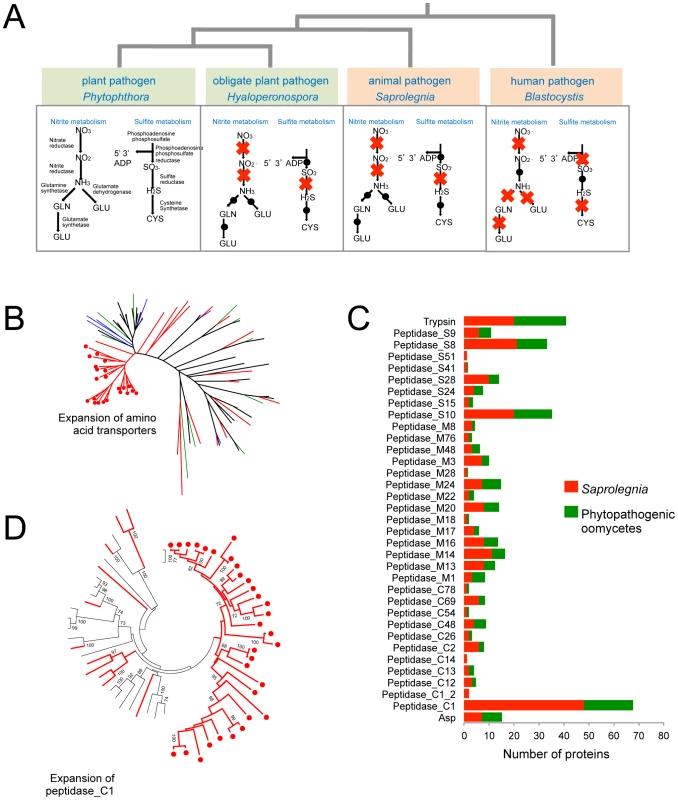
(A) Independent degeneration of nitrite and sulfite metabolic pathways in animal pathogens and obligate biotrophic plant pathogens. Red cross indicates the gene encoding the enzyme is absent in the genome. (B) Lineage specific expansion of amino acid transporters. Members from Pythium (black), Hyaloperonospora (green), Albugo (blue) and S. parasitica (red) are included. - The S. parasitica-specific clade is marked with red dots. (C) Secreted peptidase families in S. parasitica and phytopathogenic oomycetes (the average count from the total peptidase genes of P. infestans, P. ramorum, P. sojae, Py. ultimum and H. peronospora) . Peptidase_C1, Peptidase_S8 and Peptidase_S10 are the largest families in S. parasitica. (D) Lineage-specific expansion of peptidase_C1 family. Members from P. sojae, P. ramorum and P. infestans (black) and S. parasitica (red) are included. The S. parasitica-specific clade is marked with red dots. Other metabolic differences with oomycete plant pathogens
The gene for phospholipase C (PLC) is absent in all of the sequenced peronosporomycete plant pathogens, but is present in S. parasitica (SPRG_04373). Phylogenetic analysis groups the S. parasitica PLC gene with that of other heterokont species (Figure S5, Table S6). This shows that the S. parasitica PLC is most likely to be ancestral and that the absence of PLC in other oomycetes is due to gene loss.
Peronosporomycete plant pathogens are sterol auxotrophs and their genomes are missing most genes encoding enzymes involved in sterol biosynthesis [44]. In contrast, analysis of the EST collection from A. euteiches and the S. parasitica genome predicts the existence of enzymes that function in a novel sterol biosynthetic pathway [26] which has been shown to lead to the synthesis of fucosterol in A. euteiches [45]. Importantly, one of the genes SPRG_09493 encodes a CYP51 sterol-demethylase (Figure S6), a major target of antifungal chemicals that could perhaps also be used to combat Saprolegniomycetes.
Candidate virulence proteins
Like plant pathogens, S. parasitica presumably secretes a battery of virulence proteins to promote infection. Due to co-evolution with the host, virulence proteins are typically rapidly evolving and may appear to be unique to the species, or encoded by recently expanded gene families [17], [19]. The S. parasitica genome contains a large number of genes (11,825) that are not orthologous to any known genes in other species (Figure S2A and S2B), and many recently expanded gene families. There are at least 87 pfam domains that are either unique or show recent expansions in S. parasitica as compared to other oomycete species (Table S7). An estimated 970 proteins (Table S8) were predicted to be extracellular based on previously established bioinformatics criteria [11], [12], such as the presence of a eukaryotic signal peptide, and lack of targeting signals to organelles or membranes. Many of the expanded families appear to function at the exterior or cell surface of the pathogens, such as proteins containing CBM1 (Carbohydrate Binding Module Family I according to the CAZy database (http://www.cazy.org/; [46]), ricin B lectin, Notch domains, and also numerous peptidases. Among the proteins that are unique to S. parasitica compared to plant pathogenic oomycetes, the largest families have similarities to animal-pathogenesis-associated proteins, such as disintegrins, ricin-like galactose-binding lectins and bacterial toxin-like proteins (haemolysin E).
Oomycetes contain an unusually large number of proteins with novel domain combinations, recruited from common metabolic, regulatory and signaling domains [47], [48]. S. parasitica contains in total 169 novel domain combinations that are specific to this pathogen (Table S9). As described above, some of the lineage-expanded domains such as CBM and ricin are used for novel combinations to form composite proteins. Additional domains used for novel combinations are the cytochrome p450 and tyrosinase domains. Proteins carrying S. parasitica-specific domain combinations are significantly enriched (hypergeometric test, p<0.001) in predicted secreted proteins (3.6% of secretome), whereas only 1.2% of total proteins have S. parasitica-specific novel combinations. The enrichment in secreted proteins is strongly suggestive of a role for the novel domain combinations in pathogenesis.
There are about 1000 proteins that are predicted to be secreted by S. parasitica, based on criteria used for secretome prediction in other oomycetes [11], [12]. Two groups of proteins dominate the secretome of S. parasitica: proteases and lectins (Figure 3A, Table S8). There are over a hundred members in the each of the two groups. In the proteome, S. parasitica has one of the largest repertoires of proteases (270) known to date compared to most other single cell or filamentous eukaryotic pathogens (such as most sequenced fungi species) that typically have between 70 and 150 proteases. In almost every family of proteases, including cysteine-, serine - and metallo-proteases (Table 2), there is an expansion in S. parasitica compared to P. sojae (Figure 2C). The most relatively abundant family of proteases is the papain-like peptidase_C1 proteins, comprising 48 proteins; the other oomycetes contain only about 20 proteins. The majority of papain-like peptidase_C1 genes (80%) have been generated by S. parasitica lineage-specific gene duplications and form a lineage-specific clade in the phylogenetic reconstruction (Figure 2D). Amongst the cell-surface associated proteins, ricin_B_lectin-like proteins and CBM1 domain-containing proteins are most abundant (Table 2). There are 40 ricin-like and 40 CBM1 genes in S. parasitica, a large expansion compared to other known oomycetes. In some cases, these domains are fused to other secreted protein domains having catalytic activities (protease and cellulose) to form novel proteins unique to oomycetes (Figure 3B). However, typical peronosporomycete proteins were not found, such as CBEL (Cellulose Binding Elicitor Lectin), which contains a CBM1-PAN domain association and mediates the binding of mycelium to cellulosic substrates [49]. The saprolegniomycete plant pathogen Aphanomyces euteiches also lacked CBEL proteins [26].
Fig. 3. Specialized proteins in the secretome of S. parasitica. 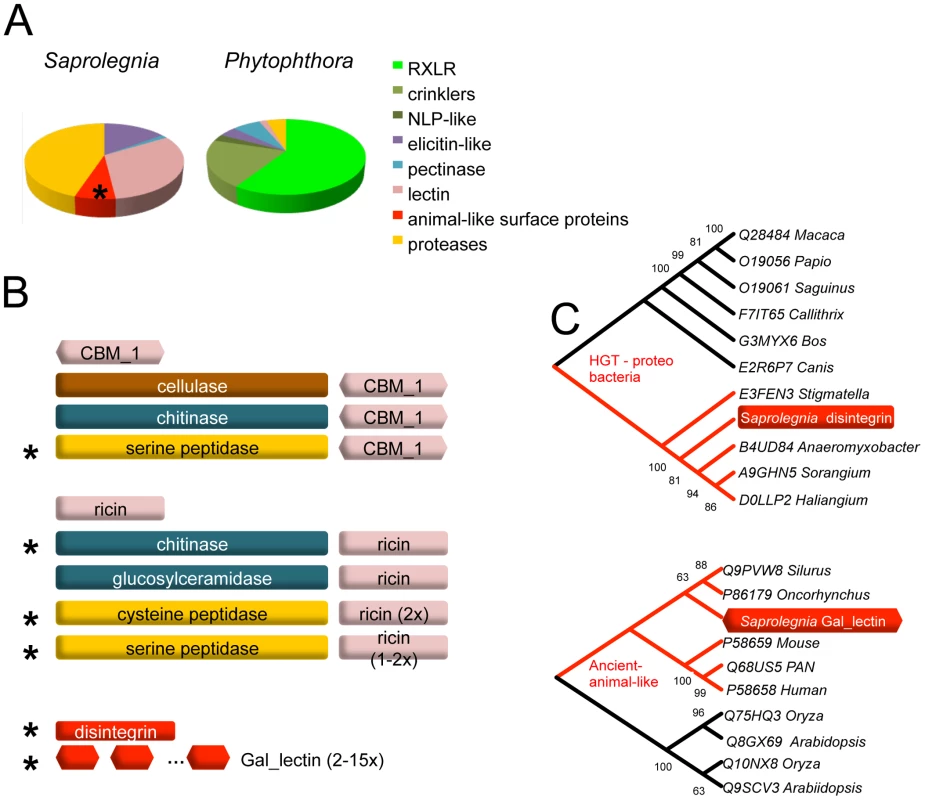
(A) Distributions of major classes of specialized secreted proteins compared between animal and plant pathogenic oomycetes. P. infestans represents Phytophthora species. (B) S. parasitica secreted proteins that carry various lectin domain fusions are schematically drawn. Domains or domain architectures unique to S. parasitica are marked with an asterisk. Proteins containing single domains are also listed. (C) Phylogenetic relationship of lectins. The S. parasitica disintegrin gene (SPRG_01285 groups with bacterial homologs; gal_lectin gene (SPRG_05731)) groups with animal species. All other paralogous S. parasitica disintegrin and gal_lectin genes group closely with these two representatives, respectively, and are not shown. Protease activities secreted by Saprolegnia parasitica
We investigated whether culture filtrates containing secreted proteins from S. parasitica could degrade trout immunoglobulin M (IgM) as previously found for bacterial fish pathogens [50]. Although no effect was observed when supernatants of two-day old cultures were incubated with an IgM enriched fraction (data not shown), the 7-day post-inoculation supernatant degraded the IgM protein fraction within several hours (Figure 4A). No degradation of trout IgM was detected when heat-treated 7-day post-inoculation supernatant was used. The protease inhibitors EDTA (a metalloproteinase inhibitor) and PMSF (serine protease inhibitor) showed partial inhibition of the IgM-degrading activity while E-64 (a cysteine protease inhibitor) did not show any inhibition. The combination of EDTA and PMSF prevented IgM degradation and the detection was similar to the pea broth control.
Fig. 4. Rainbow trout IgM proteolysis by S. parasitica secreted proteases. 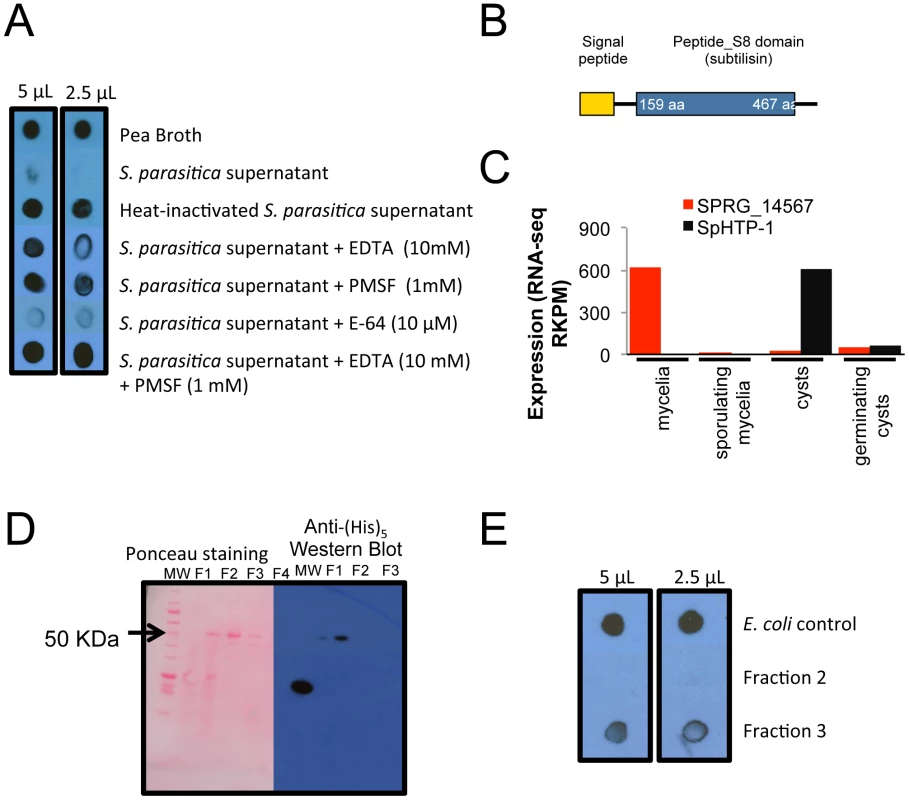
(A) 7-day old culture filtrates were capable of degrading rainbow trout IgM after an overnight incubation at 10°C. (B) Schematic drawing of the domains present in the protease SPRG_14567 (C) Expression pattern of SPRG_14567 in different life stages. The RKPM of RNA-seq data is plotted, and the previously identified effector SpHTP-1 is plotted to show contrasting expression patterns. (D) The recombinant subtilisin-like protease SPRG_14567 was partially purified through tandem ion exchange (SO3−) and nickel affinity columns (Fractions 1 to 4) following detection in a Western blot using anti-(His)5 HRP antibody. (E) Fractions 2, 3 and soluble proteins from untransformed E. coli were tested for IgM-degrading activity with only the fraction containing the recombinant SPRG_14567 exhibiting proteolysis. These results suggest that secreted proteases from S. parasitica could degrade fish IgM and that metalloproteinases and serine proteases may be the classes involved in this process. To further characterize the IgM-degrading properties of S. parasitica proteases, a serine protease (SPRG_14567) was selected from the S. parasitica genome based on our observation that this protease possesses a secretory signal peptide (Figure 4B) and is highly expressed (RNA-Seq data) (Figure 4C). Interestingly, SPRG_14567 showed strong degrading activity towards trout IgM while no activity was detected when control E. coli soluble proteins were used (Figure 4B and 4E). This indicates that this serine protease is capable of degrading fish IgM and could be a virulence factor that combats the activity of fish immunoglobulins against Saprolegnia.
Candidate effector proteins
To establish a successful infection, pathogens often deliver effector proteins and toxins into the host cytoplasm to manipulate host immunity [7], [24], [51]. Analogous to bacterial Type II secreted toxins that enter host cells via lipid-receptor-mediated endocytosis, plant pathogenic oomycetes utilize a host-targeting domain to deliver effectors into plant cells. Hundreds of effectors carrying the host targeting motifs of RXLR and LFLAK have been identified in plant pathogenic oomycetes [11], [12], [22]. However, these large families of Crinkler and RXLR effectors appear to be absent in S. parasitica (Table 2). Using sensitive BLAST and HMM searches based on the RXLR domain and C-terminal domains of effectors, no plant pathogen-like RXLR effectors could be detected in the genome. Bioinformatic searches with the de novo motif-finding program MEME did not identify other putative host-targeting motifs. Despite the absence of large RXLR effector families, S. parasitica does have a small family of host targeting proteins related to SpHtp1, which do contain an N-terminal RXLR sequence. Interestingly one of these proteins was shown to translocate into fish cells [28] and entry required the N-terminal leader sequence [30]. The lack of sequence similarity between any part of SpHtp1 and RXLR-proteins from plant pathogen oomycetes, except for the three proximally located amino acid residues, suggests that the presence of the RXLR-sequence in SpHtp1 is currently unclear. No significant matches were found to Crinkler effectors, suggesting they are absent from S. parasitica. Using search criteria that do not rely on sequence homology, such as induction of expression during the pre-infection stage, presence of secretion signals, and signatures of fast evolution, several candidates (Table S10) for host-targeting proteins (including SpHtp1) were identified in S. parasitica. None of these candidates have homology to known proteins, and their functions are currently unknown.
The commonalities between animal and plant pathogenic oomycetes are highlighted by their shared PAMPs. Proteinaceous PAMPs found in plant pathogenic oomycetes, namely CBM1 [37], elicitins, and Cys-rich-family-3 proteins [14] are found in S. parasitica (Figure S7A). Elicitins are extracellular lipid transfer proteins that elicit defense responses in some species of plants, especially Nicotiana [52]. There are 29 elicitin-like proteins in S. parasitica. Phylogenetic reconstruction shows that the majority of the S. parasitica elicitins form three lineage-specific clades, distant from the canonical elicitin group [53] (Figure S7B). We also detected six YxSL[RK] containing secreted proteins, previously identified as candidate effectors [14], that also show sequence divergence from known members of this family. It is an intriguing question whether animal innate immune systems can detect these potential PAMPs, as does the plant innate immune system. For example it seems unlikely that CBM1 can act as immune elicitor for animal cells since binding to the plant cell wall cellulose is required to induce immune responses in plants [37].
Elevated nucleotide substitution rate of pathogenesis associated gene families
To study the polymorphism and evolutionary rate of S. parasitica genes, we sequenced a related strain, VI-02736, which was isolated from an infected Atlantic salmon, for comparison. More than of 90% of the CBS223.65 reference sequence was covered by VI-02736 reads, allowing us to identify 1,467,567 SNPs between the two strains, giving an average SNP rate of 3.3% (Text S1, Table S11 and Figure S8A and S8B). We have also determined that LOH is unique to the CBS strain, as it is apparently absent in the sequenced VI-02736 strain (Text S1, Figure S8C and S8D).
The Ka/Ks ratio (Ka - number of non-synonymous substitutions per non-synonymous site, Ks - number of synonymous substitutions per synonymous site) was calculated for the annotated S. parasitica gene set. The set of 3518 oomycete core ortholog genes gives a median Ka/Ks ratio of 0.05, a rate comparable to previously published Ka/Ks rate of the conserved gene dense region of Phytophthora sibling species and related strains [54]. Non-parametric Z tests between the core ortholog group and a particular gene family (listed in Table 2) were performed to determine which family shows elevated substitution rates. Among the pathogenesis-related gene families, we identified four groups, namely elicitins, disintegrins, host targeting proteins and haemolysins, that have significantly elevated median Ka/Ks ratios as compared to the core-ortholog groups (Figure S9A). Between the two S. parasitica strains, the highest Ka/Ks ratio was observed in the haemolysin E family, a group of toxin-like genes that were possibly horizontally acquired from bacteria (see below). Interestingly, similar patterns of elevated Ka/Ks ratios were also found between haplotypes of the reference strain (Figure S9B). Three families, the disintegrins, elicitins and haemolysins, show significant elevations in Ka/Ks as compared to the core ortholog group (Figure S9B).
Potential role of horizontal gene transfer in development of pathogenicity
Horizontal gene transfer events from bacteria and archaea appear to have contributed to some of the novel biosynthetic pathways found in the oomycetes [47]. By utilizing codon usage and protein domain classification (see Methods), at least 100 genes could be identified with a potential phylogenetic origin outside the super-kingdom of Chromalveolates by using the program Alien_hunter [55] and by interrogating Pfam search results. Among these, we further identified around 40 genes belonging to 5 families that could potentially be associated with pathogenesis (Table 3). Many of these genes appear to have been acquired from bacterial species, in particular from Proteobacteria. Some potential acquisitions may have occurred relatively recently as they only occur in Saprolegnia and seem to have nucleotide compositions predictive of foreign acquisition (Alien_hunter score >50). A caveat for our analysis is that S. parasitica is the only available genome so far outside of the plant pathogenic oomycetes. There are a great variety of basal oomycetes [56], [57] that do not have genome sequence information and have not been investigated. Therefore, it could be that these HGT events could have occurred in some ancestral oomycetes .
Tab. 3. Predicted horizontally transferred genes that may be associated with pathogenesis in Saprolegnia parasitica. 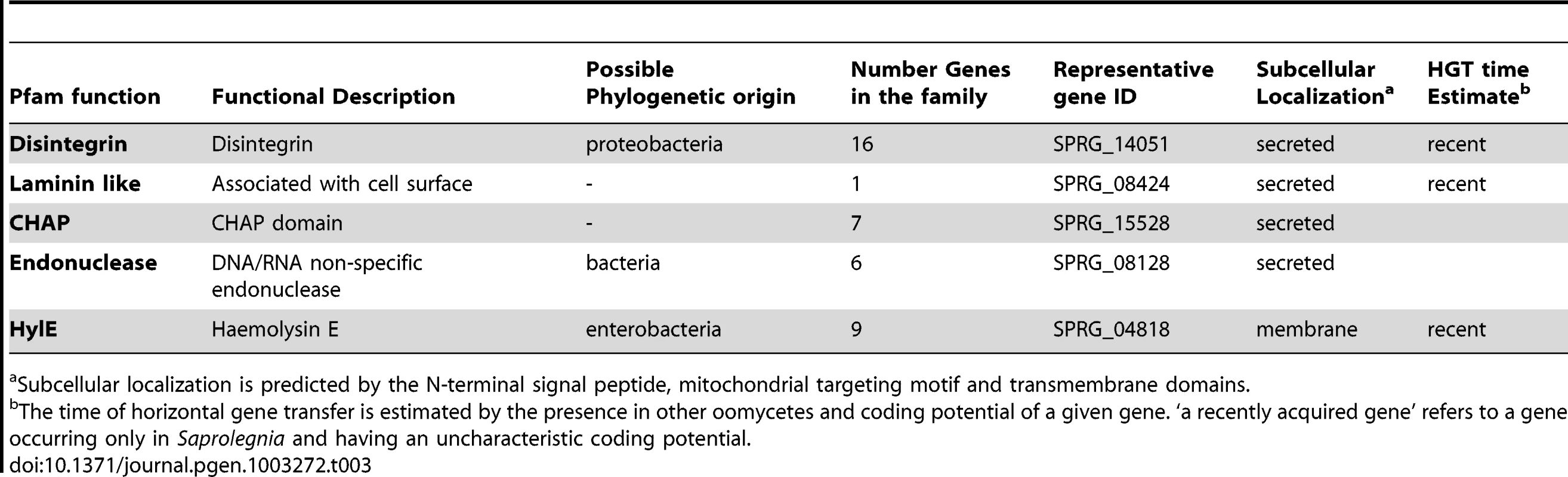
Subcellular localization is predicted by the N-terminal signal peptide, mitochondrial targeting motif and transmembrane domains. Several groups of extracellular enzymes were potentially acquired from bacteria; for example, the CHAP (cysteine, histidine-dependent amidohydrolases/peptidases) family and a family of secreted nucleases (Table 3). The presence of the CHAP family (pfam hit E value<1e-5) is unexpected in S. parasitica, because it is commonly associated with bacterial physiology and metabolism [58]. These genes have undergone repeated duplications in S. parasitica, resulting in an expansion of gene numbers. Another potential bacterial acquisition is a family of toxin-like proteins similar to haemolysin (HlyE), a pore-forming toxin from enterobacteria such as Salmonella [59]. These genes have also undergone recent duplication resulting in nine copies in S. parasitica. Two members (SPRG_03140, SPRG_20514) of the HlyE family are expressed during infection.
Another distinctive feature of the S. parasitica secretome is the presence of animal-like surface proteins. The phylogenetic affinities of the two groups, distinguished by gal_lectin-like and disintegrin-like domains, suggest a possible origin via HGT, but from different sources. Gal_lectin refers to the D-galactoside binding lectin initially purified from the eggs of sea urchin [59], [60], [61], representing a group of lectins that occurs widely on fish eggs and skins [62]. Phylogenetic analysis shows that the closest homologs are animal gal_lectins (Figure 3C). The S. parasitica gal_lectin genes are among the most highly induced and highly expressed genes in pre-infection and infection stages, suggesting that gal_lectin may facilitate adhesion and invasion of fish cells. The gal_lectin genes contain an unusually large number of introns (7 introns), as do mammalian and fish gal_lectin genes, further suggesting a common origin. The S. parasitica genes have a codon usage similar to core orthologs, in contrast to other candidate HGT genes, suggesting they have been in the S. parasitica genome long enough to be largely assimilated. The S. parasitica disintegrin genes were potentially acquired from bacteria (Figure 3C, Table 3) and have since expanded. Disintegrins were initially identified as proteins preventing blood clotting in viper venoms [63]. In animals, disintegrins inhibit aggregation of the platelets by binding to the integrin/glycoprotein IIb-IIIa receptor. The crucial amino acid motif CRxxxxxCDxxExC, mediating ligand binding, is conserved in the S. parasitica disintegrins. All 16 disintegrin genes in S. parasitica are expressed in the pre-infection stages (see below), with several members belonging to the top 1% most highly expressed genes, suggesting that they may play a role interacting with animal hosts. However, we have not been able to experimentally demonstrate the role of disintegrins in pathogenesis so far (Text S1 and Figure S10).
Tissue-specific and host-induced gene expression
S. parasitica has, like most other oomycetes, clearly defined life stages, including motile zoospores that are able to swim, encyst and germinate upon attachment to its host tissue. We performed strand-specific Illumina RNA-Seq analysis of 4 developmental stages (mycelium, sporulating mycelium, cysts, and germinating cysts [3–5 hours]) of S. parasitica, as well as a time course analysis (0, 8 and 24 hours) of a rainbow trout fibroblast cell-line (RTG-2) challenged with cysts of S. parasitica. RNA-Seq reads were mapped to the S. parasitica genome and gene annotations, and transcript abundance values were quantified as RPKM (reads per kilobase transcript length per million reads mapped).
Large numbers of genes were differentially expressed in the various life stages and conditions. Cyst and germinating-cyst stages showed similar expression profiles (linear correlation R2 = 0.93, p<0.001), with less than 6% of all the genes showing >4-fold expression differences (p<0.001; Figure 5A). In contrast, the other developmental life stages and infection time course showed somewhat larger differences (R2 of 0.84–0.88), with up to 27% of genes differentially expressed between cysts and mycelia (> = 4-fold difference; p< = 0.001). The time course experiment shows the relative abundance of the host and pathogen transcripts changing as infection progressed (Figure 5B). At the 0 hour and 8 hours time points, very few pathogen transcripts were detected (less than 1% and 3%, respectively) whereas at 24 hours, 63% of the transcripts were derived from the pathogen. At 24 hours, the transcript profile of the pathogen closely resembled that of in vitro-grown mycelia, with only 3.5% of all genes differentially induced between the two stages (>4 fold differences, p<0.001). Compared to the cysts used as inoculum, 7.2% of genes were induced after 8 hours of infection and 10% of genes were induced by 24 hours (>4 fold differences, p<0.001) (Figure S11A). We have defined the stage prior to host tissue colonization (germinating cyst) as the pre-infection stage. In plant pathogenic oomycetes, germinating cysts express many pathogenesis-associated genes [12], [64], [65], [66], [67]. The previously characterized S. parasitica gene encoding the host targeting protein SpHtp1 [28] is induced more than 100-fold in the pre-infection stage as compared to other stages. At the pre-infection stage, 10% of all genes were induced as compared to the mycelial stage (>4 fold differences, p<0.001) (Figure 5C; Figure S11B; Table S12). The profile of the germinating cysts - resembled that of the 8 hours fish cell infection (R2 = 0.88, p<0.001), with only 4% of genes showing induction (>4-fold differences, p<0.001).
Fig. 5. Differentially expressed genes detected by RNA-Seq. 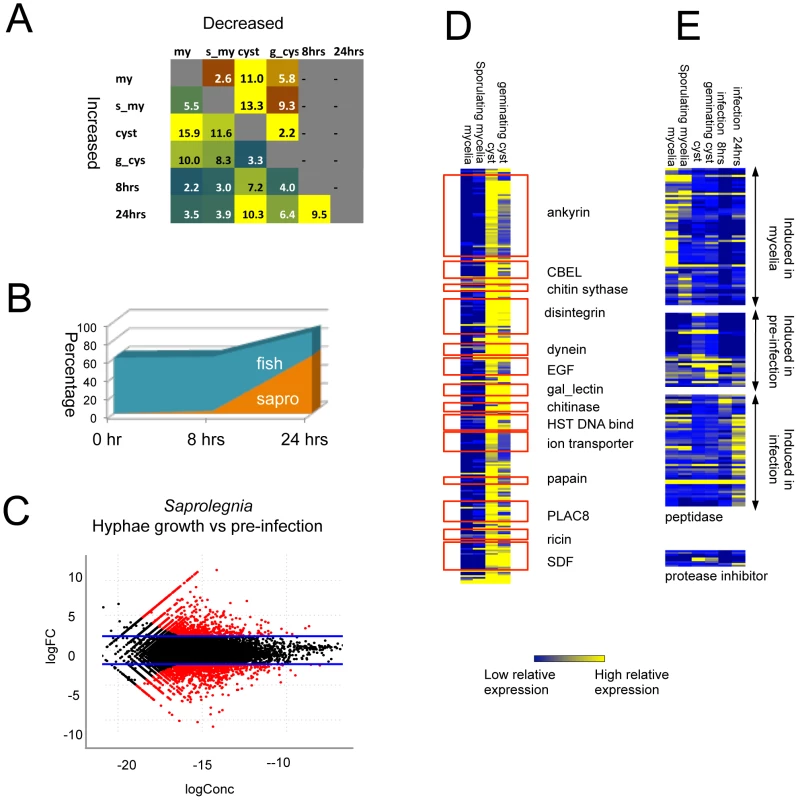
(A) Percentage of differentially expressed genes in pair-wise comparisons of tissue types. Genes with 4 fold RPKM (reads per kilobase per million) differences were considered to be differentially expressed (negative binomial exact test p<0.001, p value adjusted with Benjamini & Hochberg correction, Table S12) (B) Gene families showing differential expression between vegetative tissue (mycelia and sporulating mycelia) and pre-infection tissues (cysts and germinating cysts). CBEL:fungal Cellulose Binding Domain Like protein, EGF:(Epidermal Growth Factor, gal_lectin: Galactose binding Lectin domain, HST: Heat shock factors, PLAC8: Placenta-specific gene 8 protein, SDF: Sodium Dicarboxylate symporter Family (Table S13). (C) Growth phase specific expression of peptidases and protease inhibitors (Table S14). (D) Relative abundance of S. parasitica and fish transcripts during interaction. (E). S. parasitica transcript distribution in pre-infection versus vegetative tissue. logFC = log2(pre-infection/vegetative/pre-infection); logConc = the log2 of average reads counts per million of each gene in the two tissue types,). Red dots indicate significant differences (p<0.001; negative binomial test). For genes that encode proteins specific to or expanded in S. parasitica as compared to other oomycetes, around 25% were induced in germinating cysts compared to mycelia (>4-fold differences, p<0.001) (Figure S11C, Table S13). A total of 14 protein families showed both lineage-specific domain expansion and up-regulation in germinating cysts (Figure 5D). The largest groups are proteins carrying ankyrin domains or lectin domains, which suggests the importance of protein-protein and protein-carbohydrate interactions in the initial stages of host colonization. Another group of proteins belonging to this category are transporters such as ion transporters and sodium symporters, suggesting that metabolic exchange processes are active during the establishment of early infection.
The RNA-Seq data also suggest that the very large S. parasitica kinome plays an important role during the infection process. Around 10% of all kinase genes, spanning all major kinase classification groups, showed 4-fold or more induction in germinating cysts compared to mycelia (>4-fold differences, p<0.001) (Figure S11C). Many of the numerous S. parasitica proteases were expressed in a specific life stage or distinct point during the infection process (Figure 5D/E, Table S14). One group of peptidases (6%) was expressed in germinating cysts. These include subtilase proteins (SPRG_15005) that carry ricin lectin domains and are highly expressed in germinating cysts. A large group of peptidases (19%) were induced during the interaction with fish cells. There are 7 protease-inhibitors encoded in the genome; and they also showed patterns of differential expression (Figure 5C). The Kazal peptidase inhibitor (SPRG_09563) was most highly expressed in cysts and germinating cysts.
To confirm the RNA-seq data, we have also performed qPCR for a set of disintegrin genes, showing expression in cysts and germinating cysts, but no detection in mycelium and sporulating mycelium as seen for the RNA-seq data (Figures S11 C,D).
Taken together, the RNA-seq data reveal that members of the kinases, proteases, disintegrins and gal_lectins show upregulated expression in the pre-infection stage (Table S13 and S14), which warrants using these candidate genes for future pathogenesis related studies.
Discussion
The genome sequence of S. parasitica, together with transcriptome and polymorphism data, reveals a predicted core proteome very similar to those of plant pathogenic oomycetes, but an adapted proteome strongly aligned to its animal-pathogenic lifestyle.
From the genome sequence and the RNA-Seq data we could identify several groups of proteins predicted to be secreted at the pre-infection stages that might facilitate early interactions with the hosts of S. parasitica. Several of these proteins seem to be unique to S. parasitica and may have evolved to interact specifically with fish cells. They are predicted to be targeted to the extracellular environment or incorporated into the exterior surface of the pathogen. Several of the proteins contain CBM domains (fungal cellulose binding domain), ricin-like domains, Notch-like domains and/or various peptidase domains. Most putative early interaction proteins have potential roles in pathogenesis, such as animal-cell-surface-like proteins (disintegrin, gal_lectin) and haemolysin E toxin-like proteins. Lectins could help cysts to bind to host skin. Since the lectins are highly expressed during the initial and later infection stages, we hypothesize that they play an important role in cell-cell contact throughout the interaction. This would also suggest that an intimate contact with the host cell is required for pathogenesis, suppression of host defence processes, and/or nutrient uptake. Following attachment, the pathogen engages a large arsenal of potential virulence factors in the form of proteases to attack the host tissue. Interestingly, S. parasitica has one of the largest numbers of proteases found in any organism. At least one protease (SPRG_14567) was found to be able to degrade IgM, suggesting an active role in suppressing initial immune responses, as fish IgM's are able to bind to infection related antigens, even in the absence of prior immunization.
In comparison to plant pathogenic oomycetes, S. parasitica has no canonical RXLR or Crinkler effector genes, nor NLP toxin genes in its genome. Nevertheless, one small protein family has been found for which one member was shown to translocate inside trout cells [2], [30], which suggests that the interaction of S. parasitica with its hosts is more subtle than a simple necrotrophic interaction based on secretion of toxins and protein degradation. In fact, we speculate that the initial stages of the interaction may involve a more ‘biotrophic’ approach by the pathogen, whereby the immune response of the host is avoided or even suppressed during initial colonization via, for example, proteases or effector proteins. Following this biotrophic stage, the host tissue is bombarded with proteases, lipases, and lysing enzymes. If S. parasitica was a plant pathogen, it would thus have been classified as a ‘hemi-biotroph’ and not a saprotroph as its name would suggest. Further experiments are required to demonstrate that this is indeed the case.
Pathogenicity towards animals has evolved independently in both the fungi and oomycetes. It has also evolved at least three additional times within the kingdom Stramenopila: within the Pythiales (Figure 1A), within the genus Aphanomyces [6] and in the non-oomycete Blastocystis. Analyses of the genomes or sequenced ESTs of these other pathogens reveals some interesting parallels. For example, based on only a small set of ESTs of the oomycete human pathogen Py. insidiosum [29], an identified expressed lectin CBM-encoded transcript has been implicated in the process of pathogenesis. Blastocystis hominis is a Stramenopile human pathogen with a very small genome of 19 Mb encoding only 6020 genes, and is phylogenetically very divergent from oomycetes [4]. Despite the large evolutionary distance, the genome of Blastocystis shows reduction of nitrogen and sulfite metabolic pathways, similar to what is seen in obligate plant pathogens. In addition, similar to the acquired lectins in S. parasitica, Blastocystis has also horizontally transferred genes with animal like features, such as genes encoding Ig domains [4]. The fungal pathogen Batrachochytrium dendrobatidis that causes global amphibian decline, inhabits an aquatic environment like S. parasitica, and also causes diseases on animal skin and tissues. Analysis of its genome revealed patterns of expansion of protease families [68]. Although the particular families are different, B. dendrobatidis possesses several families of lineage expanded proteases such as metallopeptidase (M36).
The extensive LOH observed in S. parasitica, covering approximately one-third of the genome and also the high rate of polymorphism (2.6%) in the remainder of the genome are similar to recent observations in the genome of the oomycete plant pathogen Phytophthora capsici, where LOH was found to be associated with changes in mating type and pathogenicity [13]. We speculate that, as in P. capsici, LOH may provide a mechanism in S. parasitica for rapidly expressing diversity within a population, fixing alleles, and enabling rapid adaptation to its environment.
The evolution of plant and animal pathogenesis in oomycetes has been associated with several major molecular events (Figure 6). Since both Phytophthora and Saprolegnia have large kinomes, the expansion of kinases is likely a relatively early event in oomycete evolution. The comparison of the animal pathogen S. parasitica with plant pathogens with different lifestyles has shown that surviving on ammonium rich tissue has led to a reduction of metabolic pathways independently in S. parasitica and obligate oomycete plant pathogens. Similar reductions have also occurred in obligate fungal pathogens [69] and in the distantly related stramenopile human pathogen Blastocystis [4]. The evolution of plant pathogenicity has been associated with a series of reduction events such as intron loss, chitin loss and sterol loss. Some of these losses may be due to evasion of plant immunity; for example chitin can act as a PAMP to trigger plant defense responses. The evolution of plant pathogens has been accompanied by expansions of large repertoires of effectors, which have been shown to modulate plant host physiology. In contrast, the development of animal pathogenesis has been facilitated by expansion of proteases and horizontally acquired lectins and toxins.
Fig. 6. Molecular genetic events associated with the evolution of animal and plant pathogenesis in oomycetes. 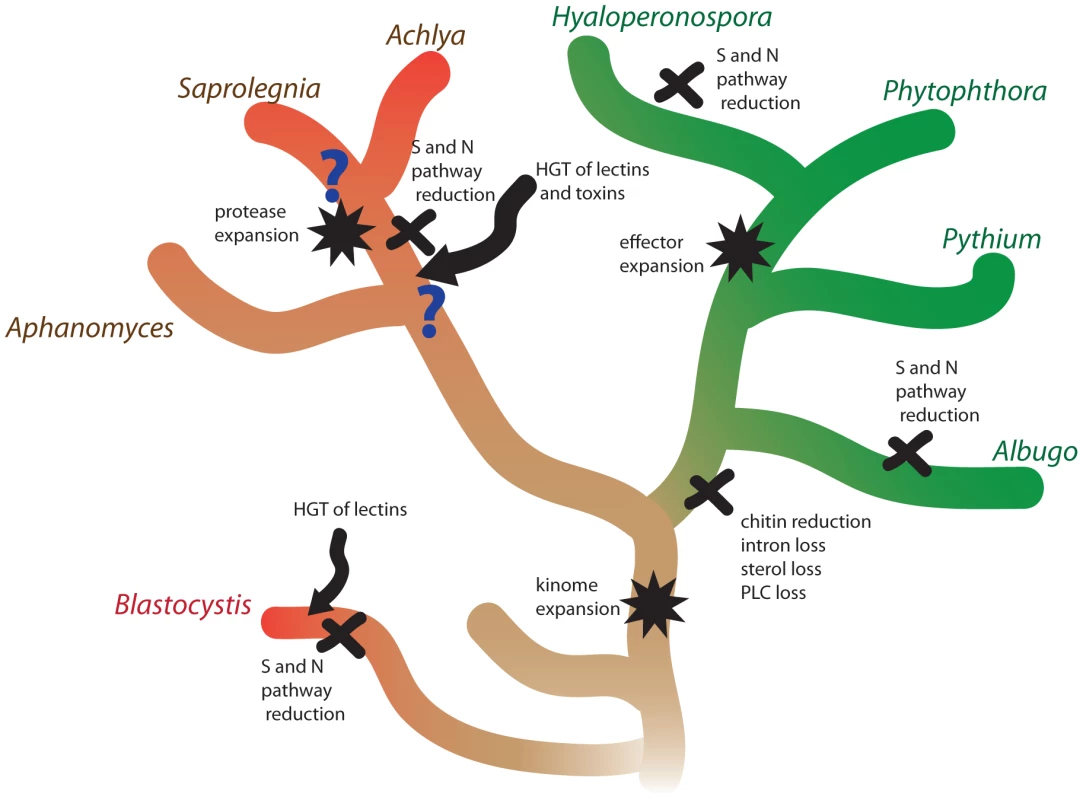
The lineages of animal pathogens are colored red and the lineages of plant pathogens are colored green. The basal lineage is colored brown. S and N pathways refer to sulfite and nitrite assimilation, respectively. Methods
Strain selection and genomic DNA isolation
Saprolegnia parasitica isolate CBS223.65 was isolated from young pike (Esox lucius) in 1965 and obtained from Centraal Bureau voor Schimmelcultures (CBS), the Netherlands. Saprolegnia parasitica isolate VI-02736 (N12) was obtained from parr of Atlantic salmon in Scotland in 2002 (Lochailort) [70] and kindly provided by Dr. Ida Skaar (Norwegian Veterinary Institute). Both isolates were maintained on potato dextrose agar (Fluka). For genomic DNA isolation, S. parasitica was grown for three days at 24°C in pea broth (125 g L−1 frozen peas, autoclaved, filtered through cheese cloth, volume adjusted to 1 L and autoclaved again). Genomic DNA extraction was adapted from Haas et al [12]. Fresh mycelia (∼1 g) were ground to a fine powder under liquid nitrogen, mixed with 10 mL of extraction buffer (0.2 M TrisHCl pH 8.5, 0.25 M NaCl, 25 mM EDTA, 0.5% SDS), 7 mL Tris-equilibrated phenol and 3 mL of chloroform∶isoamyl alcohol (24∶1), incubated at room temperature for 1 hr and centrifuged at 6,000 g for 30 m. The aqueous phase was extracted with equal volume of chloroform∶isoamyl alcohol (24∶1) and centrifuged at 10,000 g for 15 min. 50 µl of 10 mg/ml RNase A was added to the aqueous phase and incubated for 30 min at 37°C. Isopropanol (0.6 volumes) was added, mixed gently, and then the DNA was precipitated on ice for 30 min. DNA was collected by centrifugation at 10,000 g for 20 min, washed with 70% ethanol, dried and resuspended in DNase/RNase-free water. DNA was checked for quality and RNA contamination by gel electrophoresis using 0.8% agarose.
Collection of samples and RNA isolation
The life stages of S. parasitica were harvested as described by van West et al. [28]. Zoospores and cysts were collected by pouring the culture filtrate through a 40–70 µm cell strainer and concentrated by centrifugation for 5 min at 1500 g. RNA was isolated from mycelia and sporulating mycelia using the RNeasy kit (Invitrogen) according to the manufacturer's protocol. RNA from zoospores, cysts and germinating cysts was resuspended in TRIzol (Invitrogen) and aliquoted as 1 ml portions in 2-ml screw-cap tubes containing 10–35 glass 1 mm diameter beads (BioSpec) and immediately frozen in liquid nitrogen. Frozen cells were processed with a FastPrep machine (ThermoSavant) and shaken several times at a speed of 5.0 for 45 sec until defrosted and homogenized. TRIzol RNA isolation was then performed according to manufacturer's recommendations (Invitrogen).
Host-induced gene expression was measured in rainbow trout (Oncorhynchus mykiss) gonadal tissue continuous cell line RTG-2, obtained from the American Type Culture Collection (ATCC CCL-55) [71]. RTG-2 cells were grown to a monolayer at 24°C (no CO2) and washed twice with Hank's Balanced Salt Solution (HBSS). Cells were harvested in approximately 5 ml of HBSS and S. parasitica cysts were added (5×104 cysts per 75 cm flask) and the mixture was then incubated at room temperature for 15 min. At this time, the time 0 hr (T0) stage sample was collected, by gently pouring off medium (and also loose/non-attached cysts) and then adding TRIzol reagent for RNA extraction. Other samples were incubated for 8 hr (T8) or 24 hr (T24) at 24°C (no CO2) in Leibovitz (L-15) growth medium (Gibco) supplemented with 10% foetal calf serum (Biosera), 200 U ml−1 penicillin and 200 µg ml−1 streptomycin (Fisher). Cells were harvested for RNA extraction by pouring off medium and adding TRIzol. Cells were loosened using a cell scraper (Fisher) and the suspension was aliquoted as 1 ml portions into 2-ml screw-cap tubes containing 10–35 glass 1 mm diameter beads (Biospec) and immediately frozen in liquid nitrogen. Frozen cells were processed using a FastPrep machine and shaken several times at a speed of 5.0 for 45 sec until defrosted and homogenized. TRIzol RNA isolation was then performed according to manufacturer's recommendations.
Infected and uninfected fish tissue was collected from a trout (O. mykiss, caught in a commercial Scottish hatchery) that showed lesions caused by S. parasitica infection. Tissue was collected from the lesion (body) and from an infected anal fin. As an uninfected control, similar tissue not showing symptoms of infection was collected from the same fish. Tissue was ground up in liquid N2 and RNA was isolated using the RNeasy kit (QIAGEN) according to manufacturer's recommendations.
Infected and uninfected Atlantic salmon (Salmo salar) egg samples were collected from a commercial Scottish hatchery. Five eggs per sample were ruptured using a needle and placed into 5 ml of TRIzol and RNA was isolated according to manufacturer's recommendations with an additional phenol-chloroform extraction step. Multiple sample batches were pooled to obtain sufficient material for RNA-Seq library construction.
All RNA samples were resuspended in DNase/RNase-free water and treated with Turbo DNA-free DNase (Ambion) according to manufacturer's recommendations. RNA was checked for quantity and purity using a Nanodrop spectrophotometer (Thermo Scientific) and 1% agarose gel electrophoresis. Samples were stored at −80°C and were not defrosted until use.
454 and Sanger sequencing for genome assembly
454 fragment and 3 kb jumping whole genome shotgun libraries were generated for isolate CBS 223.65 as previously described [72]. Libraries were sequenced with ∼400 base single end reads (Titanium chemistry) using a 454 GS FLX sequencer following the manufacturer's recommendations (454 Life Sciences/Roche). Approximately a total of 22-fold sequence coverage was generated from fragment and 3 kb jumping libraries combined. A 40 kb insert Fosmid whole genome shotgun library from isolate CBS 223.65 was generated using the EpiFOS fosmid cloning system following manufacturer's recommendations (Epicentre). The Fosmid library was end-sequenced with ∼700 bp reads using Sanger technology to approximately 0.3-fold coverage using a 3730xl DNA analyzer following manufacturer's recommendations (Applied Biosystems/Life Technologies).
Illumina sequencing for variant detection and RNA-Seq
For variant calling, Illumina whole genome shotgun fragment libraries were generated for isolates CBS 223.65 and VI-02736 as previously described [73] and sequenced with 76 base paired-end reads to a minimum of 70-fold sequence coverage using an Illumina Genome Analyzer II (Illumina) following the manufacturer's recommendations. Illumina strand-specific dUTP RNA-Seq libraries were generated for all RNA samples as previously described [74] with the following modifications. The mRNA was processed using the Dynabeads mRNA purification kit (Invitrogen) and incubated with RNA fragmentation buffer (Affymetrix) at 80°C for 1.5 to 3.5 minutes depending sample quality. Indexed adaptors for Illumina sequencing were ligated onto end-repaired, A-tailed cDNA fragments by incubation with 4,000 units of T4 DNA ligase (New England Biolabs) in a 20 ml reaction overnight at 16°C. 16 to 21 cycles of PCR were used to amplify sequencing libraries. Libraries were purified using 1–3 rounds of AMPure beads (Beckman Coulter Genomics) following manufacturer's recommendations. Libraries were sequenced with 76 base paired-end reads using an Illumina Genome Analyzer II following manufacturer's recommendations (Illumina) generating a total of 124 million reads.
Saprolegnia parasitica CBS 223.65 de novo genome assembly
Sanger Fosmid paired-end reads were quality trimmed using the ARACHNE ‘Assemblez’ module (http://www.broadinstitute.org/crd/wiki/index.php/Assemblez). Read headers were amended to contain template and pairing information. Sanger, 454 fragment and 3 kb jumping reads were assembled using 454's Newbler assembler version 04292009 (454 Life Sciences/Roche). The resulting assembly was further processed using the ARACHNE ‘HybridAssemble’ module (http://www.broadinstitute.org/crd/wiki/index.php/HybridAssemble) using the 454 assembly, Sanger and 454 read data as input with option ‘RecycleBadContigs’ turned off allowing for extra copies of repeat sequences to be assembled. The Arachne ‘AddReadsAsContigs’ module was run to allow assembly of additional repetitive sequence. The assembly was screened for known sequencing vector sequences using BLAST and contigs with hits to known sequence vectors were removed. The assembly was further post-processed by removing contigs and scaffolds less than 200 bp and 2 kb in length respectively.
SNP calling
Illumina shotgun sequence data were used to identify polymorphisms in two S. parasitica strains (CBS 223.65 and VI-02736). Illumina paired-end fragment reads from strains CBS 223.65 and VI-02736 were independently aligned to the S. parasitica CBS 223.65 reference assembly using the BWA aligner [75] using default settings. Read alignments were sorted by scaffold and position along the reference assembly. SNP calling was performed using the GATK Unified Genotyper module [76]. Variant Call Format (VCF) files containing SNP calls were filters for low quality using parameters: AB>0.75 && DP>40 ∥ DP>500 ∥ MQ0>40 ∥ SB>−0.10. SNP calls for CBS 223.65 and VI-02736 can be retrieved from the Broad Institute Saprolegnia parasitica genome database website (http://www.broadinstitute.org/annotation/genome/Saprolegnia_parasitica/MultiDownloads.html).
Loss of heterozygosity and haplotype region identification
SNP calls and depth of read coverage information were parsed from the VCF file described above and analyzed in non-overlapping 5 kb windows (Figure S1). Using this information genome segments were partitioned into three groups: separated haplotypes (coverage depth ranging 20–55-fold and SNP rate <1%), diploid homozygous involving LOH (coverage depth ranging 56–90-fold and SNP rate <1%), and diploid heterozygous (coverage depth ranging 40–90-fold and SNP rate > = 1%). Coverage of separated haplotype regions peaks at ∼40-fold and the regions are mostly devoid of SNPs. The region corresponding to the diploid consensus exhibits ∼60-fold coverage and nearly a 3% SNP rate. The peaks in Figure S1B corresponding to the separated haplotype and consensus diploid regions are connected by a small ridge, which correspond to windows spanning boundaries between the different kinds of regions. The coverage for the diploid consensus regions is not exactly double as compared to the predicted separately assembled haplotype regions, and is less than the diploid homozygous regions; most likely this results from the relative difficulty of aligning short Illumina reads to diploid consensus sequences in the context of the high polymorphism rate observed.
Individual genes located in haplotype contigs were assigned as likely allelic pairs based on SNP rate, depth of coverage, and taking into consideration best reciprocal blast matches and synteny between separately assembled haplotype contigs.
Genome annotation
Gene finding used both evidence-based (including EST, RNA-Seq and homology data) and ab initio methods. Gene-finding algorithms FGenesH, GeneID and GeneMark were trained for S. parasitica using existing gene and EST datasets. Then a statistical sampling of gene calls as well as genes of interest were manually curated, and the results were used to validate gene calls and fine-tune the gene caller. RNA-Seq data was incorporated into gene structure annotations using PASA [77] as described in Rhind et al. [78]. Subsequently, the annotated total gene set was subjected to Pfam domain analysis, OrthoMCL clustering analysis and KEGG metabolic pathway analysis.
Expression analysis with RNA-Seq
Illumina RNA-Seq data was processed as follows. Sequencing adaptors were identified and removed from reads by exact match to adaptor sequences. Reads were aligned to S. parasitica gene transcripts using Bowtie (allowing up to 2 mismatches per read, and up to 20 alignments per read). Transcript levels were calculated as FPKM (fragments per kilobase cDNA per million fragments mapped). The program EdgeR [79] was used to identify differentially expressed transcripts. Transcripts with significantly different levels (p< = 0.001 and over 4-fold difference) were identified, and p-values were adjusted for multiple testing by using the Benjamini & Hochberg [80] correction.
Metabolism analysis
The predicted proteomes of S. parasitica and representative plant pathogenic oomycetes were annotated by mapping against a reference set of metabolic pathways from KEGG (Kyoto Encyclopedia of Genes and genomes) [81]. The method used, KAAS (KEGG Automated Annotation Server), utilizes bidirectional best hits to assign pathways. Subsequently, the metabolic genes and pathways were manually annotated and compared to other oomycete pathogens. Known genes involved in nitrogen and sulfur metabolism were used to search the S. parasitica genome by TBLASTN search; genes were considered to be candidates if a positive hit was found (E value<1e-5). For chitin metabolism analysis, genes were annotated based on Pfam homology (E value<1e-5) with the exception of chitin synthase genes, which were identified after Blastp analysis against a set of oomycete and fungal CHS. A total of twelve GH18 genes were identified, of which six are arranged in small clusters of two paralogous genes.
Horizontal gene transfer analysis
Two different approaches were used to screen S. parasitica genes for candidate HGT origins. In the first approach, the genome sequence was screened with the program Alien_hunter [55]. The program utilizes an interpolated variable order motif method to determine horizontally transferred events, purely based on compositional difference between a region and the whole genome framework. Because the methodology is independent of any existing datasets, we used it to examine the S. parasitica genome. Genomic regions were identified as alien when the Alien_hunter score was above 50. Out of 1442 S. parasitica supercontigs, 206 supercontigs had distinct regions marked as alien after running Alien_hunter. Subsequently, the 1616 gene models that lay within the candidate alien regions were extracted and compared with other oomycete genomes (P. sojae, P. ramorum, P. infestans, H. arabidopsidis and Py. ultimum). In the second approach, the entire proteome of each oomycete was scanned for homology to Pfam-A protein families using the hmmscan algorithm from Hmmer 3.0 applied to the Hidden Markov Model dataset (Pfam-A.hmm v.24). A cut-off e-value threshold of 1e-3 was applied. From the Pfam domain analysis we obtained 307 sequences that had distinct domains not found in any of the Phytophthora species, and 31 of the candidates derived from the Pfam analysis overlapped with the results from Alien_hunter. We then blast-searched all 1616 genes from Alien_hunter and the 307 genes from the domain analysis against the NCBI non-redundant database (nr) to obtain the primary functions. Phylogenetic analysis using neighbor joining was then performed on the final set of genes.
Analysis of secreted proteases
S. parasitica CBS 223.65 mycelium plugs were grown in pea broth for 2 or 7 days. The culture supernatants were harvested, centrifuged at 5000× g for 10 min (4°C) and the soluble fraction used as a source of secreted proteases. The ammonium sulfate precipitated fraction from rainbow trout serum [82] was used as source of fish IgM (10 mg/mL of total protein concentration). For protease activity experiments, 50 µL of the culture supernatants were incubated overnight at 10°C with 5 µL of the IgM enriched fraction. Pea broth was used as a negative control. Heat-inactivated supernatant was obtained by incubating the culture supernatants for 15 min at 95°C. The protease inhibitors EDTA (10 mM), PMSF (1 mM) and E-64 (10 µM) were used to identify classes of proteases involved in IgM degradation.
Two samples volumes (2.5 and 5 µL) were spotted onto nitrocellulose membranes, allowed to dry for 45 min, blocked with milk-PBS (5% dry-milk) and remaining IgM was detected using a monoclonal anti-trout/salmon HRP conjugated antibody (Aquatic Diagnostics). SPRG_14567 was cloned from S. parasitica CBS223.65 cDNA into the pET21B vector. E. coli Rossetta-gami B competent cells were transformed with the expression vector by heat-shock. Transformed cells were grown in modified LB media (100 mM NaHPO4, pH 7.4; 2 mM MgSO4, glucose 0.05% w/v; and NaCl 0.5% w/v). When E. coli cells reached an OD600 of 0.8, cultures were induced with IPTG (10 mM) and incubated at 200 rpm at 37°C for 12 hr. The soluble fraction was purified from French press supernatant by tandem ion exchange (on SO3−) and nickel affinity columns. Chromatography fractions were submitted tor 1D SDS-gel electrophoresis (NuPAGE Electrophoresis System, Invitrogen) and then transferred to nitrocellulose membranes (XCell II Blot module, Invitrogen). Membranes were blocked with milk-PBS-T (0.02% v/v Tween, 10% dry milk) for 20 min. Anti-(His)5 HRP conjugate (QIAGEN) was added to a final dilution of 1∶20,000 and incubated at room temp for 1 hr for detection of the recombinant protein. Chromatography fractions were tested for IgM degrading activity as previously described [50]. Briefly, 50 µL (5 µg/mL of total protein concentration) of the fractions were incubated overnight at 10°C with 5 µL of the IgM enriched fraction. Untransformed E. coli soluble proteins were used as a control. Remaining IgM was then detected in a dot-blot as described above.
Phylogeny reconstruction and domain combination analysis
Gene models were analyzed using TMHMM and ClustalX. For phylogeny reconstruction, sequences were aligned using ClustalX and aligned sequences were subjected to phylogenetic analysis (NJ) using PAUP. The predicted proteome of S. parasitica was compared to that of six pathogenic oomycetes together with sixty-four other eukaryotic species covering all major groups of the eukaryotic tree of life, as previously used by Seidl et al. [48]. We used hmmer-3 [http://hmmer.org] and a local Pfam-A database to predict 1,798,601 domains in 862,909 proteins of which 19,896 domains in 10,887 proteins are found in S. parasitica. The architectures of multi-domain proteins were analyzed from the N - to the C-terminus, which identified 18,512 domain combinations consisting of two contiguous domains. Of these there were 1120 oomycete-specific domain combinations encoded by 2286 proteins. The great majority of combinations were specific to a single species, and only 58 combinations were found in more than one oomycete species. S. parasitica contained 338 domain combinations that are specific for oomycetes including 169 domain combinations encoded by 215 genes that are specific for this species (Table S9).
Data deposition
Saprolegnia parasitica CBS 223.65: Sanger sequence data were submitted to the NCBI Trace Archive (http://www.ncbi.nlm.nih.gov/Traces/trace.cgi) and can be retrieved using query: CENTER_NAME = “BI” and CENTER_PROJECT = “G1848”. 454 and Illumina sequence data were submitted to the NCBI Short Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) and can be retrieved using the following accession numbers: 454 fragment reads (SRX007896, SRX007901, SRX007898, SRX007895, SRX005344, SRX007971, SRX007958, SRX005346); 454 3 kb jumping reads (SRX007902, SRX007899, SRX007937, SRX007574, SRX007903); Illumina fragment reads (SRX022535); Illumina RNA-Seq reads (BioProject 164643: mycelium SRX155934, sporulation mycelium SRX155933, cysts SRX155932, germinating cysts SRX155938, infected fish cell-line t = 0 SRX155937, infected fish cell-line t = 8 SRX155936 and infected fish cell-line t = 24 SRX155935. BioProject 167986: infected fish tissue SRX155944, uninfected fish tissue SRX155942, infected salmon eggs SRX155943, SRX155940 and uninfected salmon eggs SRX155941). Draft genome assembly sequence was submitted to GenBank (BioProject ID 36583, accession ADCG00000000).
Saprolegnia parasitica VI-02736 : Illumina sequence data were submitted to the SRA (BioProject 164645, accession SRX155939).
SNP calls for strains CBS 223.65 and VI-02736 can be downloaded from the Broad Institute Saprolegnia parasitica genome database website (http://www.broadinstitute.org/annotation/genome/Saprolegnia_parasitica/MultiDownloads.html).
Supporting Information
Zdroje
1. PhillipsAJ, AndersonVL, RobertsonEJ, SecombesCJ, van WestP (2008) New insights into animal pathogenic oomycetes. TIM 16 : 13–19.
2. Van WestP (2006) Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challenges for an old problem. Mycologist 20 : 99–104.
3. Bruno DW, van West P, GW B (2009) Saprolegnia and other oomycetes. In: Bruno DW, , editors. In: Fish Diseases and Disorders, 2nd edition. CABI. pp. 669–720.
4. DenoeudF, RousselM, NoelB, WawrzyniakI, Da SilvaC, et al. (2011) Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol 12(3): R29.
5. PattersonDJ (1999) The diversity of eukaryotes. American Naturalist 154: S96–S124.
6. Dieguez-UribeondoJ, GarciaMA, CereniusL, KozubikovaE, BallesterosI, et al. (2009) Phylogenetic relationships among plant and animal parasites, and saprotrophs in Aphanomyces (Oomycetes). Fungal Genet.Biol 46 : 365–376.
7. Tyler BM (2009) Effectors. In: Oomycete Genetics and Genomics. John Wiley & Sons, Inc. pp. 361–385.
8. Van West P, Vleeshouwers VG (2004) The Phytophthora infestans -host interaction. In: Talbot NJ, editor. In: Plant Pathogen Interactions, Annual Plant Reviews. Blackwell Scientific Publishers. pp. 219–242.
9. TylerBM (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8 : 1–8.
10. GruenwaldNJ, GarbelottoM, GossEM, HeungensK, ProsperoS (2012) Emergence of the sudden oak death pathogen Phytophthora ramorum. TIM 20 : 131–138.
11. TylerBM, TripathyS, ZhangX, DehalP, JiangRHY, et al. (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313 : 1261–1266.
12. HaasBJ, KamounS, ZodyMC, JiangRHY, HandsakerRE, et al. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461 : 393–398.
13. LamourK, MudgeJ, GobenaD, Hurtado-GonzalesOP, SchmutzJ, et al. (2012) Genome sequencing and mapping reveal loss of heterozygosity as a mechanism for rapid adaptation in the vegetable pathogen Phytophthora capsici. Mol Plant-Microbe Interact 25 : 1350–1360.
14. LevesqueCA, BrouwerH, CanoL, HamiltonJP, HoltC, et al. (2010) Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol 11(7): R73.
15. BaxterL, TripathyS, IshaqueN, BootN, CabralA, et al. (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330 : 1549–1551.
16. LinksMG, HolubE, JiangRHY, SharpeAG, HegedusD, et al. (2011) De novo sequence assembly of Albugo candida reveals a small genome relative to other biotrophic oomycetes. BMC Genomics 12 : 503.
17. JiangRHY, TylerBM (2012) Mechanisms and evolution of virulence in oomycetes. Annu Rev Phytopathol 50 : 295–318.
18. RaffaeleS, KamounS (2012) Genome evolution in filamentous plant pathogens: why bigger can be better. Nature Rev Microbiol 10 : 417–430.
19. KamounS (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44 : 41–60.
20. SchornackS, HuitemaE, CanoLM, BozkurtTO, OlivaR, et al. (2009) Ten things to know about oomycete effectors. Mol Plant Pathol 10 : 795–803.
21. GrouffaudS, van WestP, AvrovaAO, BirchPRJ, WhissonSC (2008) Plasmodium falciparum and Hyaloperonospora parasitica effector translocation motifs are functional in Phytophthora infestans. Microbiology-Sgm 154 : 3743–3751.
22. JiangRHY, TripathyS, GoversF, TylerBM (2008) RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. PNAS 105 : 4874–4879.
23. SchornackS, van DammeM, BozkurtTO, CanoLM, SmokerM, et al. (2010) Ancient class of translocated oomycete effectors targets the host nucleus. PNAS 107 : 17421–17426.
24. WhissonSC, BoevinkPC, MolelekiL, AvrovaAO, MoralesJG, et al. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450 : 115–118.
25. DouD, KaleSD, WangX, JiangRHY, BruceNA, et al. (2008) RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. The Plant Cell 20 : 1930–1947.
26. GaulinE, MadouiM-A, BottinA, JacquetC, MatheC, et al. (2008) Transcriptome of Aphanomyces euteiches: new oomycete putative pathogenicity factors and metabolic pathways. Plos One 3: e1723.
27. Torto-AlaliboT, TianMY, GajendranK, WaughME, van WestP, et al. (2005) Expressed sequence tags from the oomycete fish pathogen Saprolegnia parasitica reveal putative virulence factors. BMC Microbiology 5 : 46.
28. Van WestP, de BruijnI, MinorKL, PhillipsAJ, RobertsonEJ, et al. (2010) The putative RxLR effector protein SpHtp1 from the fish pathogenic oomycete Saprolegnia parasitica is translocated into fish cells. FEMS Microbiology 310 : 127–137.
29. KrajaejunT, KhositnithikulR, LerksuthiratT, LowhnooT, RujirawatT, et al. (2011) Expressed sequence tags reveal genetic diversity and putative virulence factors of the pathogenic oomycete Pythium insidiosum. Fungal Biol 115 : 683–696.
30. WawraS, BainJ, DurwardE, de BruijnI, MinorKL, et al. (2012) Host-targeting protein 1 (SpHtp1) from the oomycete Saprolegnia parasitica translocates specifically into fish cells in a tyrosine-O-sulphate-dependent manner. PNAS 109 : 2096–2101.
31. GrabherrMG, HaasBJ, YassourM, LevinJZ, ThompsonDA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotech 29 : 644–U130.
32. ChamnanpuntJ, ShanWX, TylerBM (2001) High frequency mitotic gene conversion in genetic hybrids of the oomycete Phytophthora sojae. PNAS 98 : 14530–14535.
33. MacGregorT, BhattacharyyaM, TylerB, BhatR, SchmitthennerAF, et al. (2002) Genetic and physical mapping of Avrla in Phytophthora sojae. Genetics 160 : 949–959.
34. SlamovitsCH, KeelingPJ (2009) Evolution of ultrasmall spliceosomal introns in highly reduced nuclear genomes. Mol Biol Evol 26 : 1699–1705.
35. MuotriAR, MarchettoMCN, CoufalNG, GageFH (2007) The necessary junk: new functions for transposable elements. Human Mol Gen 16: R159–R167.
36. JudelsonHS, Ah-FongAMV (2010) The kinome of Phytophthora infestans reveals oomycete-specific innovations and links to other taxonomic groups. BMC Genomics 11 : 700.
37. GaulinE, DrameN, LafitteC, Torto-AlaliboT, MartinezY, et al. (2006) Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. The Plant Cell 18 : 1766–1777.
38. Schneider DJ, Collmer A (2010) Studying plant-pathogen interactions in the genomics era: beyond molecular Koch's Postulates to systems biology. In: VanAlfen NK, Bruening G, Leach JE, editors. Annu. Rev. Phytopathol. Vol 48. pp. 457–479.
39. GuerrieroG, AvinoM, ZhouQ, FugelstadJ, ClergeotP-H, et al. (2010) Chitin synthases from Saprolegnia are involved in tip growth and represent a potential target for anti-oomycete drugs. PLoS Pathogens 6: e1001070–e1001070.
40. MélidaH, Sandoval-SierraJV, Diéguez-UribeondoJ, BuloneV (2013) Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryotic Cell 12 : 194–203.
41. Bartnicki-GarciaS (1968) Cell wall chemistry morphogenesis and taxonomy of fungi. Annu Rev Microbiol 22 : 87–108.
42. BuloneV, ChanzyH, GayL, GirardV, FevreM (1992) Characterization of chitin and chitin synthase from the cellulosic cell-wall fungus Saprolegnia monoica. Experimental Mycology 16 : 8–21.
43. BadreddineI, LafitteC, HeuxL, SkandalisN, SpanouZ, et al. (2008) Cell wall chitosaccharides are essential components and exposed patterns of the phytopathogenic oomycete Aphanomyces euteiches. Eukaryotic Cell 7 : 1980–1993.
44. GaulinE, BottinA, DumasB (2010) Sterol biosynthesis in oomycete pathogens. Plant signaling & behavior 5 : 258–260.
45. MadouiM-A, Bertrand-MichelJ, GaulinE, DumasB (2009) Sterol metabolism in the oomycete Aphanomyces euteiches, a legume root pathogen. New Phytologist 183 : 291–300.
46. CantarelBL, CoutinhoPM, RancurelC, BernardT, LombardV, et al. (2009) The Carbohydrate-active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Research 37: D233–D238.
47. MorrisPF, SchlosserLR, OnaschKD, WittenschlaegerT, AustinR, et al. (2009) Multiple horizontal gene transfer events and domain fusions have created novel regulatory and metabolic networks in the oomycete genome. Plos One 4: e6133.
48. SeidlMF, Van den AckervekenG, GoversF, SnelB (2011) A domain-centric analysis of oomycete plant pathogen genomes reveals unique protein organization. Plant Physiology 155 : 628–644.
49. GaulinE, JauneauA, VillalbaF, RickauerM, Esquerre-TugayeMT, et al. (2002) The CBEL glycoprotein of Phytophthora parasitica var. nicotianae is involved in cell wall deposition and adhesion to cellulosic substrates. Journal of Cell Science 115 : 4565–4575.
50. BjornsdottirB, FridjonssonOH, MagnusdottirS, AndresdottirV, HreggvidssonGO, et al. (2009) Characterisation of an extracellular vibriolysin of the fish pathogen Moritella viscosa. Veterinary Microbiology 136 : 326–334.
51. LuY-J, SchornackS, SpallekT, GeldnerN, ChoryJ, et al. (2012) Patterns of plant subcellular responses to successful oomycete infections reveal differences in host cell reprogramming and endocytic trafficking. Cellular Microbiology 14 : 682–697.
52. HuitemaE, VleeshouwersV, CakirC, KamounS, GoversF (2005) Differences in intensity and specificity of hypersensitive response induction in Nicotiana spp. by INN, INF2A, and INF2B of Phytophthora infestans. Molecular Plant-Microbe Interactions 18 : 183–193.
53. JiangRHY, TylerBM, WhissonSC, HardhamAR, GoversF (2006) Ancient origin of elicitin gene clusters in Phytophthora genomes. Molecular Biology and Evolution 23 : 338–351.
54. RaffaeleS, FarrerRA, CanoLM, StudholmeDJ, MacLeanD, et al. (2010) Genome evolution following host jumps in the Irish potato famine pathogen lineage. Science 330 : 1540–1543.
55. VernikosGS, ParkhillJ (2006) Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands. Bioinformatics 22 : 2196–2203.
56. BeakesGW, GlocklingSL, SekimotoS (2012) The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 249 : 3–19.
57. KüpperFC, MaierI, MüllerG, Loiseaux-De GoerS, GuillouL (2006) Phylogenetic affinities of two eukaryotic pathogens of marine macroalgae, Eurychasma dicksonii (Wright) Magnus and Chytridium polysiphoniae Coh. Cryptogamie, Algologie 27 : 165–184.
58. BatemanA, RawlingsND (2003) The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends in Biochemical Sciences 28 : 234–237.
59. Hunt S, Green J, Artymiuk PJ (2010) Hemolysin E (HlyE, ClyA, SheA) and related toxins. In: Anderluh G, Lakey J, editors. Proteins: Membrane Binding and Pore Formation. pp. 116–126.
60. OzekiY, MatsuiT, SuzukiM, TitaniK (1991) Amino acid sequence and molecular characterization of a D-galactoside -specific lectin purified from sea-urchin (Anthocidaris crassipina) eggs. Biochemistry 30 : 2391–2394.
61. OzekiY, YokotaY, KatoKH, TitaniK, MatsuiT (1995) Developmental expression of D-galactoside-binding lectin in sea urchin (Anthocidaris crassipina) eggs. Experimental Cell Research 216 : 318–324.
62. OgawaT, WatanabeM, NaganumaT, MuramotoK (2011) Diversified carbohydrate-binding lectins from marine resources. Journal of amino acids 2011 : 838914–838914.
63. McLaneM, SanchezE, WongA, Paquette-StraubC, PerezJC (2004) Disintegrins. Curr Drug Targets Cardiovasc Haematol Disord 4 : 327–355.
64. EbstrupT, SaalbachG, EgsgaardH (2005) A proteomics study of in vitro cyst germination and appressoria formation in Phytophthora infestans. Proteomics 5 : 2839–2848.
65. ShanWX, MarshallJS, HardhamAR (2004) Gene expression in germinated cysts of Phytophthora nicotianae. Molecular Plant Pathology 5 : 317–330.
66. Torto-AlaliboTA, TripathyS, SmithBM, ArredondoFD, ZhouL, et al. (2007) Expressed sequence tags from Phytophthora sojae reveal genes specific to development and infection. Molecular Plant-Microbe Interactions 20 : 781–793.
67. YeW, WangX, TaoK, LuY, DaiT, et al. (2011) Digital gene expression profiling of the Phytophthora sojae transcriptome. Molecular Plant-Microbe Interactions 24 : 1530–1539.
68. RosenblumEB, StajichJE, MaddoxN, EisenMB (2008) Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences of the United States of America 105 : 17034–17039.
69. McDowellJM (2011) Genomes of obligate plant pathogens reveal adaptations for obligate parasitism. Proceedings of the National Academy of Sciences of the United States of America 108 : 8921–8922.
70. StuelandS, HataiK, SkaarI (2005) Morphological and physiological characteristics of Saprolegnia spp. strains pathogenic to Atlantic salmon, Salmo salar L. Journal of Fish Diseases 28 : 445–453.
71. WolfK, QuimbyMC (1962) Established eurythermic line of fish cell in vitro. Science 135 : 1065–1066.
72. LennonNJ, LintnerRE, AndersonS, AlvarezP, BarryA, et al. (2010) A scalable, fully automated process for construction of sequence-ready barcoded libraries for 454. Genome Biology 11: R15.
73. FisherS, BarryA, AbreuJ, MinieB, NolanJ, et al. (2011) A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biology 12: R1.
74. LevinJZ, YassourM, AdiconisX, NusbaumC, ThompsonDA, et al. (2010) Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nature Methods 7 : 709–U767.
75. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
76. McKennaA, HannaM, BanksE, SivachenkoA, CibulskisK, et al. (2010) The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20 : 1297–1303.
77. HaasBJ, DelcherAL, MountSM, WortmanJR, SmithRK, et al. (2003) Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Research 31 : 5654–5666.
78. RhindN, ChenZ, YassourM, ThompsonDA, HaasBJ, et al. (2011) Comparative functional genomics of the fission yeasts. Science 332 : 930–936.
79. RobinsonMD, McCarthyDJ, SmythGK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 : 139–140.
80. BenjaminiY, HochbergY (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological 57 : 289–300.
81. KanehisaM, GotoS, KawashimaS, OkunoY, HattoriM (2004) The KEGG resource for deciphering the genome. Nucleic Acids Research 32: D277–D280.
82. Hudson L, Hay F (1980) Practical Immunology. London, UK: Blackwell Scientific Publications. pp. 1–9, 113–137, 227–229.
Štítky
Genetika Reprodukční medicína
Článek PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant TransformationČlánek The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene ClusterČlánek USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and TranscriptionČlánek Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA EditingČlánek Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 6
-
Všechny články tohoto čísla
- BMS1 Is Mutated in Aplasia Cutis Congenita
- High Trans-ethnic Replicability of GWAS Results Implies Common Causal Variants
- How Cool Is That: An Interview with Caroline Dean
- Genetic Architecture of Vitamin B and Folate Levels Uncovered Applying Deeply Sequenced Large Datasets
- Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle,
- Meiosis-Specific Stable Binding of Augmin to Acentrosomal Spindle Poles Promotes Biased Microtubule Assembly in Oocytes
- Environmental Dependence of Genetic Constraint
- H3.3-H4 Tetramer Splitting Events Feature Cell-Type Specific Enhancers
- Network Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
- Effectively Identifying eQTLs from Multiple Tissues by Combining Mixed Model and Meta-analytic Approaches
- Altered Splicing of the BIN1 Muscle-Specific Exon in Humans and Dogs with Highly Progressive Centronuclear Myopathy
- The NADPH Metabolic Network Regulates Human Cardiomyopathy and Reductive Stress in
- Negative Regulation of Notch Signaling by Xylose
- A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
- Transcriptome-Wide Mapping of 5-methylcytidine RNA Modifications in Bacteria, Archaea, and Yeast Reveals mC within Archaeal mRNAs
- Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen
- Latent Effects of Hsp90 Mutants Revealed at Reduced Expression Levels
- Impact of Natural Genetic Variation on Gene Expression Dynamics
- DeepSAGE Reveals Genetic Variants Associated with Alternative Polyadenylation and Expression of Coding and Non-coding Transcripts
- The Identification of -acting Factors That Regulate the Expression of via the Osteoarthritis Susceptibility SNP rs143383
- Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs
- The RNA Export Factor, Nxt1, Is Required for Tissue Specific Transcriptional Regulation
- Inferring Demographic History from a Spectrum of Shared Haplotype Lengths
- Histone Acetyl Transferase 1 Is Essential for Mammalian Development, Genome Stability, and the Processing of Newly Synthesized Histones H3 and H4
- PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant Transformation
- DNA Methylation Restricts Lineage-specific Functions of Transcription Factor Gata4 during Embryonic Stem Cell Differentiation
- The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Deregulation of the Protocadherin Gene Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy
- Evidence for Two Different Regulatory Mechanisms Linking Replication and Segregation of Chromosome II
- USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and Transcription
- Methylation of Histone H3 on Lysine 79 Associates with a Group of Replication Origins and Helps Limit DNA Replication Once per Cell Cycle
- A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue
- The Gene Desert Mammary Carcinoma Susceptibility Locus Regulates Modifying Mammary Epithelial Cell Differentiation and Proliferation
- Hooked and Cooked: A Fish Killer Genome Exposed
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
- Mutations in Cause Autosomal Recessive Congenital Ichthyosis in Humans
- Integrated Transcriptomic and Epigenomic Analysis of Primary Human Lung Epithelial Cell Differentiation
- RSR-2, the Ortholog of Human Spliceosomal Component SRm300/SRRM2, Regulates Development by Influencing the Transcriptional Machinery
- Comparative Polygenic Analysis of Maximal Ethanol Accumulation Capacity and Tolerance to High Ethanol Levels of Cell Proliferation in Yeast
- SPO11-Independent DNA Repair Foci and Their Role in Meiotic Silencing
- Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery
- Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA Editing
- Functional Analysis of Neuronal MicroRNAs in Dauer Formation by Combinational Genetics and Neuronal miRISC Immunoprecipitation
- DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
- Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
- Heritable Change Caused by Transient Transcription Errors
- From Many, One: Genetic Control of Prolificacy during Maize Domestication
- Neuronal Target Identification Requires AHA-1-Mediated Fine-Tuning of Wnt Signaling in
- Loss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
- H-NS Can Facilitate Specific DNA-binding by RNA Polymerase in AT-rich Gene Regulatory Regions
- Prophage Dynamics and Contributions to Pathogenic Traits
- Global DNA Hypermethylation in Down Syndrome Placenta
- Fragile DNA Motifs Trigger Mutagenesis at Distant Chromosomal Loci in
- Disturbed Local Auxin Homeostasis Enhances Cellular Anisotropy and Reveals Alternative Wiring of Auxin-ethylene Crosstalk in Seminal Roots
- Causes and Consequences of Chromatin Variation between Inbred Mice
- Genome-scale Analysis of FNR Reveals Complex Features of Transcription Factor Binding
- Distinct and Atypical Intrinsic and Extrinsic Cell Death Pathways between Photoreceptor Cell Types upon Specific Ablation of in Cone Photoreceptors
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMS1 Is Mutated in Aplasia Cutis Congenita
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



