-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaComparative Genomics of and the Bacterial Species Concept
The importance of host-specialization to speciation processes in obligate host-associated bacteria is well known, as is also the ability of recombination to generate cohesion in bacterial populations. However, whether divergent strains of highly recombining intracellular bacteria, such as Wolbachia, can maintain their genetic distinctness when infecting the same host is not known. We first developed a protocol for the genome sequencing of uncultivable endosymbionts. Using this method, we have sequenced the complete genomes of the Wolbachia strains wHa and wNo, which occur as natural double infections in Drosophila simulans populations on the Seychelles and in New Caledonia. Taxonomically, wHa belong to supergroup A and wNo to supergroup B. A comparative genomics study including additional strains supported the supergroup classification scheme and revealed 24 and 33 group-specific genes, putatively involved in host-adaptation processes. Recombination frequencies were high for strains of the same supergroup despite different host-preference patterns, leading to genomic cohesion. The inferred recombination fragments for strains of different supergroups were of short sizes, and the genomes of the co-infecting Wolbachia strains wHa and wNo were not more similar to each other and did not share more genes than other A - and B-group strains that infect different hosts. We conclude that Wolbachia strains of supergroup A and B represent genetically distinct clades, and that strains of different supergroups can co-exist in the same arthropod host without converging into the same species. This suggests that the supergroups are irreversibly separated and that barriers other than host-specialization are able to maintain distinct clades in recombining endosymbiont populations. Acquiring a good knowledge of the barriers to genetic exchange in Wolbachia will advance our understanding of how endosymbiont communities are constructed from vertically and horizontally transmitted genes.
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003381
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003381Summary
The importance of host-specialization to speciation processes in obligate host-associated bacteria is well known, as is also the ability of recombination to generate cohesion in bacterial populations. However, whether divergent strains of highly recombining intracellular bacteria, such as Wolbachia, can maintain their genetic distinctness when infecting the same host is not known. We first developed a protocol for the genome sequencing of uncultivable endosymbionts. Using this method, we have sequenced the complete genomes of the Wolbachia strains wHa and wNo, which occur as natural double infections in Drosophila simulans populations on the Seychelles and in New Caledonia. Taxonomically, wHa belong to supergroup A and wNo to supergroup B. A comparative genomics study including additional strains supported the supergroup classification scheme and revealed 24 and 33 group-specific genes, putatively involved in host-adaptation processes. Recombination frequencies were high for strains of the same supergroup despite different host-preference patterns, leading to genomic cohesion. The inferred recombination fragments for strains of different supergroups were of short sizes, and the genomes of the co-infecting Wolbachia strains wHa and wNo were not more similar to each other and did not share more genes than other A - and B-group strains that infect different hosts. We conclude that Wolbachia strains of supergroup A and B represent genetically distinct clades, and that strains of different supergroups can co-exist in the same arthropod host without converging into the same species. This suggests that the supergroups are irreversibly separated and that barriers other than host-specialization are able to maintain distinct clades in recombining endosymbiont populations. Acquiring a good knowledge of the barriers to genetic exchange in Wolbachia will advance our understanding of how endosymbiont communities are constructed from vertically and horizontally transmitted genes.
Introduction
The increasing availability of genomic data for closely related strains and species enables bacterial population sizes and structures to be explored in far greater detail than was possible until now. A major question is whether asexually reproducing bacterial cells are organized into “clusters” that contain genetic diversity, yet are distinguishable from each other [1]–[4]. Such clusters can arise through geographic isolation or extreme habitat specialization [5]. Whether bacteria that are not separated by any physical or geographic barriers can evolve into distinct groups is less clear, but studies of free-living bacteria such as Vibrio, Synechococcus and Bacillus have suggested that the formation of sequence clusters correlate with ecological specialization [6]–[8]. Likewise, a recent study of thermophilic archaea indicated ongoing speciation and suggested that these species are maintained by ecological differentiation within hot springs [9]. Studying the mechanisms and selective forces that influence the organization of genetic diversity in unicellular organisms is important for our understanding of speciation processes.
In bacteria, recombination between incipient species can potentially be an important factor affecting speciation. In a speciation model whereby populations diverge mainly through neutral processes alone, sequence divergence depends on the ratio of recombination to mutation [10]–[12]. In an ecological model of speciation, adaptive and ecological divergence of incipient species instead depends on the ratio of the selection intensity against recombined, niche-determining genes from the other population to the recombination rate between these populations [13]–[16]. If however, the populations are geographically isolated they may diverge regardless of their potential to recombine. In any of these models, the distinctness of sequences of incipient species can be enhanced by periodic selection, the success of which depends on the rate of recombination within populations. Finally, recombination can be a source of adaptation whereby one species can acquire an adaptive gene from another species.
The rate at which substitutions are introduced into a genome by recombination relative to mutation events (r/m) varies by more than two orders of magnitude in bacteria [17]. The highest r/m ratios (>50) have been observed for oceanic bacteria of the SAR11 clade [18], which are the most abundant bacteria in the upper surface waters of the oceans and have been shown to lack the mismatch repair system [19]. The lowest r/m ratios (<0.5) have been associated with obligate host-associated bacteria, such as Buchnera aphidicola [20] and other endosymbionts, that have co-evolved with their hosts for hundreds of millions of years. Such long-term co-evolution serves as a strong physical barrier to gene exchange between bacteria adapted to different hosts. In effect, these highly specialized endosymbiont populations are perhaps best described as distinct taxonomic units, or species.
Wolbachia is an obligate intracellular symbiont infecting various species of arthropods and filarial nematodes, where it is maternally inherited through the germ line cells [21]. In arthropods, Wolbachia is most known for the ability to manipulate the reproduction of their hosts in various ways, which include induction of parthenogenesis, feminization, male killing, and cytoplasmic incompatibility (CI) [21]. In filarial nematodes Wolbachia is mutualistic and necessary for normal development and fertility [22]. In addition to these roles, several studies have emerged in recent years indicating that Wolbachia may also have other functions, such as providing ATP for the host [23], improving longevity [23] or fecundity [24], protection against viruses [25], [26] and uptake of iron [27]. Unlike maternally inherited mutualistic endosymbionts that have been co-evolving with their hosts, arthropod Wolbachia can be lost and gained from the host population and they show high recombination frequencies [17].
Wolbachia is currently defined as a single species, which is further classified into a number of divergent supergroups (A–N). The most well studied supergroups are A and B that infect arthropods and C and D that infect filarial nematodes [28], [29]. The supergroup classification scheme was originally proposed based on single-gene phylogenies [30], and more recently supported by multi-locus sequence typing [31]. Since these analyses suggested that Wolbachia supergroups represent genetically distinct clades, it is debated whether some or all of these groups should be designated different species [32]. However, due to high levels of recombination between super-groups in a few marker genes such as the surface protein wsp [33] and frequent exchange of phage DNA [34], it is unclear whether the super-group classification scheme is representative of the genomes overall. Moreover, no phenotypic traits have been identified that correlate with the separation of arthropod-infecting strains into different supergroups. On the contrary, strains of different supergroup affiliation may display similar phenotypic traits and host ranges. For example, double infections with super-group A and B strains have been found in many insects, and the induction of cytoplasmic incompatibility is common in both supergroups. The distribution of other phenotypic traits is less well investigated [23]–[27]. In the absence of strong host-specialization patterns, niche partitioning within hosts provides an alternative mechanism of speciation.
To evaluate the extent of recombination and identify the ecological and physiological features that may explain the separation into supergroups, genome data is required. However, the sequencing of obligate endosymbionts such as Wolbachia is not trivial, since these bacteria are often present in low abundance in their hosts and cannot be cultivated outside of their hosts. Some protocols specifically designed to extract DNA from Wolbachia have been developed in recent years [35], [36] but the preparation of enough DNA for sequencing is still very time-consuming for obligate host-associated bacteria with low infection densities.
Because of these challenges, genomic data is currently only available for a few Wolbachia strains. These are the two supergroup A strains, wMel infecting Drosophila melanogaster [37] and wRi infecting Drosophila simulans [38] and the genomes of one supergroup B strain, wPip, from the mosquito Culex quinquefasciatus [39], and one supergroup D strain wBm isolated from the nematode Brugia malayi [40]. Early draft genomes have also been presented for two other supergroup B strains, namely wAlbB infecting the mosquito Aedes albopictus [41] and wVitB infecting the parasitic wasp Nasonia vitripennis [42]. Genome sizes are small, in the range of 1.5 Mb. Recombination has been shown to be prevalent between strains that belong to supergroup A, suggesting that Wolbachia is a highly recombining intracellular community [38]. Genomes in the A and B-supergroups contain between 20 to 60 ankyrin repeat genes. Although it is generally thought that these genes play a key role in host-interaction processes and may be involved in the reproductive phenotypes, it has been difficult to pinpoint the particular functions of these genes.
Wolbachia strains wHa and wNo are especially interesting in the context of this discussion since they share several phenotypic traits, but belong to different supergroups. Importantly, both strains cause CI in their host Drosophila simulans, where they occur as natural double infections in populations on the Seychelles and in New Caledonia [43]. Strain wHa has also been found as a single infection on Hawaii and in French Polynesia, but natural populations of D. simulans infected only with wNo are very rare [44]. Several studies support the hypothesis that the double infection originated on the Seychelles and spread east to the Indo-Pacific islands, where after wNo was lost from some populations [45]–[47]. Notably, a double-infection very similar to the one found for D. simulans is also found in the sister species Drosophila sechellia, which is endemic to the Seychelles. Furthermore, D. simulans and D. sechellia have very similar mitochondrial genomes, despite significant divergence in the nuclear genome. It therefore seems likely that the Wolbachia double-infection preceded the speciation event between D. simulans and D. sechellia. A recent study has estimated the time to a common ancestor of the D. simulans subcomplex (including D. simulans, D. sechellia and D. mauritiana) to be ∼242.000 years ago [48], suggesting that the co-infection originated at least a few hundred thousand years ago.
In this study, we present a new method for the preparation of DNA from Wolbachia, based on multiple-displacement amplification that enables genome data to be collected for Wolbachia strains with low infection densities. We have applied this protocol to the sequencing of the genomes of Wolbachia strains wHa and wNo that are co-infecting D. simulans. By comparative analysis of these and previously sequenced Wolbachia genomes, we have analyzed whether genomic features such as recombination, genome rearrangements and gene acquisitions could explain the separation of Wolbachia strains into distinct supergroups. The findings are discussed in light of the species concept for bacteria.
Results
Sequencing the Wolbachia wHa and wNo genomes
We developed a novel procedure for the isolation and amplification of Wolbachia DNA present in low quantity in the insect hosts. In brief, Wolbachia cells were purified from embryos of Drosophila simulans and multiple-displacement amplification (MDA) was performed directly on the isolated bacterial cells (see Materials and Methods). Single and 3 kb paired-end sequence reads were collected from the amplified DNA using the 454 sequencing technology and assembled de novo. The sequence coverage obtained from each data set was very large, and we therefore only used 10% to 30% of the data for assembly with Mira (Table 1). The proportion of single and paired-end reads that assembled was estimated to between 96–97% and 86–88%, respectively (Table S1). The 454 sequence reads in the assembly had mean and median sizes of 300 to 400 bp, whereas the median length of the 454 sequence reads that did not assemble was less than 100 bp (Table S1) and of lower quality (Figure S1).
Tab. 1. Sequencing data and purity. 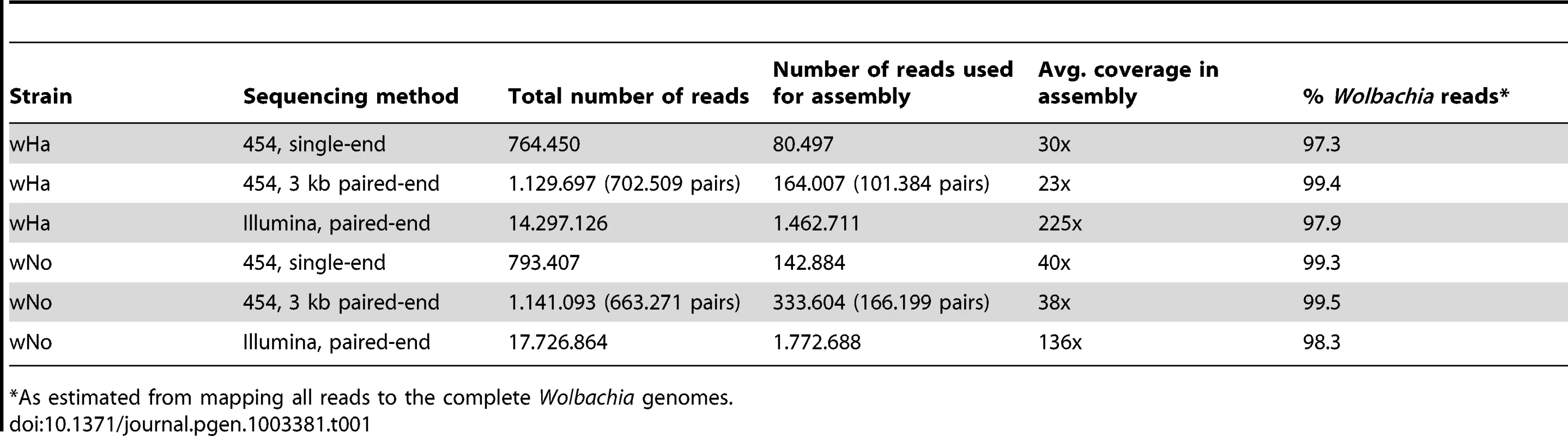
As estimated from mapping all reads to the complete Wolbachia genomes. Illumina paired-end reads were mapped onto the assembly to correct for frameshift errors generated by the 454 technology. The overall coverage of the wHa and wNo genomes in the final assemblies was about 40 to 80-fold for the 454 data and 100 to 200-fold for the Illumina data (Table 1). All gaps were closed by PCR on non-amplified DNA, confirming the reliability of the scaffolds obtained from the sequence data of the amplified DNA. In two positions in the wHa genome, located 20 kb apart and containing a long repeat of 7.5 kb with 5 genes, the PCR reactions failed from one side. However, single reads and read pairs supported the connection between the repeats and the unique sequences flanking each of the two copies.
This is the first demonstration that the MDA method can be applied in order to generate complete genome sequences from a small number of starting cells of uncultivable bacterial endosymbionts. The MDA method is known to produce amplification bias and chimeric reads when applied to single cells, which prevents genome closure. We considered the risk that such artifacts could have influenced the final genome sequence, but found these artifacts to be less dominant when multiple endosymbiont cells were used to start the reaction. Importantly, the entire Wolbachia genomes were represented by the sequence data in the final assemblies although coverage was unevenly distributed across the genome (Figure S2). The same coverage pattern was observed irrespectively of the method used for sequencing (Figure S2A–S2C), suggesting that the amplification bias is not random, but probably determined by the primer sets included in the amplification kit.
The fraction of chimeric reads in the single-end 454 sequence library was about 1%, which is considered normal according to the Newbler manual. As expected, the percentage was higher for the 454 paired-end reads, about 13–14% (Figure S3), but part of these chimeric read pairs might have been generated during library preparation rather than during the MDA reaction. Even though some regions have a higher amount of chimeric reads, we do not believe that they have had a significant effect on the assembly, since the coverage of these putative chimeric reads closely follow the coverage distribution of non-chimeric reads and hence regions with high amounts of chimeric reads also have high amounts on non-chimeric reads (Figure S3). We conclude that the overall fraction of chimeric reads was too low to have an effect on the assembly.
In retrospect, we mapped the individual sequence reads back to the Wolbachia genome and estimated that more than 97% of all reads represented Wolbachia DNA (Table 1). The remaining few percent was mostly derived from mitochondrial DNA from Drosophila simulans, with little or no nuclear DNA in the preparation. However, a manual search in the non-assembled sequences produced by the Mira assembly software of the wNo sample revealed the presence of wHa reads in low quantities (Figure S2D). Since most of the wHa genome was covered but no nuclear DNA was detected, it is unlikely that these reads were derived from bacterial sequences integrated into the host nuclear genome. Rather, we believe that there may have been a slight contamination of wHa during sample preparation and sequencing, or that the double-infected line from which wNo was generated was not completely cured of wHa. No wNo reads were found in any of the amplified DNA samples for wHa. In conclusion, the large majority of sequence data generated by the MDA method was of good quality, not chimeric and covered the entire genome with little or no contamination of nuclear DNA.
Genome features
The wNo and wHa genomes are 1.3 Mb in size and contain circa 1,000 genes, which corresponds to a coding density of about 80% (Table 2). This is comparable to the fraction of coding DNA in the previously sequenced Wolbachia genomes with the exception of wMel, in which a larger fraction of short open reading frames were identified as genes, resulting in a higher estimated coding density of 94% (Table 2). As in all previously sequenced genomes of arthropod Wolbachia, several phage-derived fragments were identified. The wHa genome contains two such regions, one of which encodes a nearly complete WO-phage. The wNo genome contains four segments of putative phage origin, of which the two larger fragments together contain all conserved parts of the WO-phage. Pseudogenes were identified in all four phage segments in the wNo genome, making it unlikely that any of them could individually produce phage particles. We observed a similar number of putatively functional IS elements in the two genomes, 12 in wHa and 14 in wNo (Table S2). Additionally, we identified 58 defective IS elements in wHa, of which 17 were defective IS3 elements. No defective IS3 elements were present in the wNo genome, which only contained a total of 14 defective IS elements.
Tab. 2. General features of <i>Wolbachia</i> genomes. 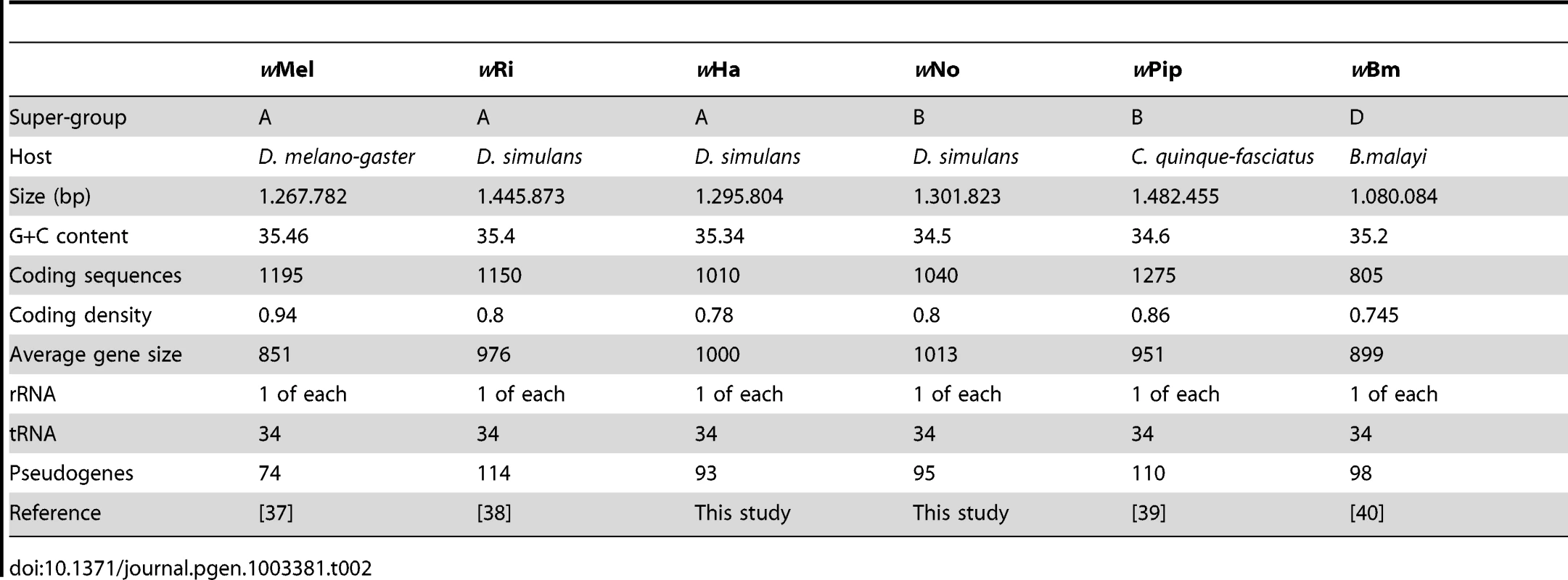
The Wolbachia core genome phylogeny
Adding the wHa and wNo genomes to the previously produced draft and complete Wolbachia genome sequences, we tested the robustness of the supergroup classification scheme using three A-group (wHa, wRi, wMel) and three B-group (wNo, wPip, wAlbB) strains. We identified 660 orthologous core genes present in all six genomes. A phylogenetic analysis with the maximum likelihood method based on a concatenated alignment of the core genes supported the separation of the two supergroups with 100% bootstrap support, and further suggested that wRi and wHa are most closely related within the A-group, and that wPip and wAlbB are sister taxa within the B-group (Figure 1). Consistently, gene order structures were largely conserved within supergroups, but highly scrambled in all pair-wise comparisons of A - and B-group genomes (Figure 2). Thus, the classification of these strains into two supergroups is strongly supported by both the sequences and the architectures of the Wolbachia genomes.
Fig. 1. Phylogenetic relationships of six Wolbachia strains. 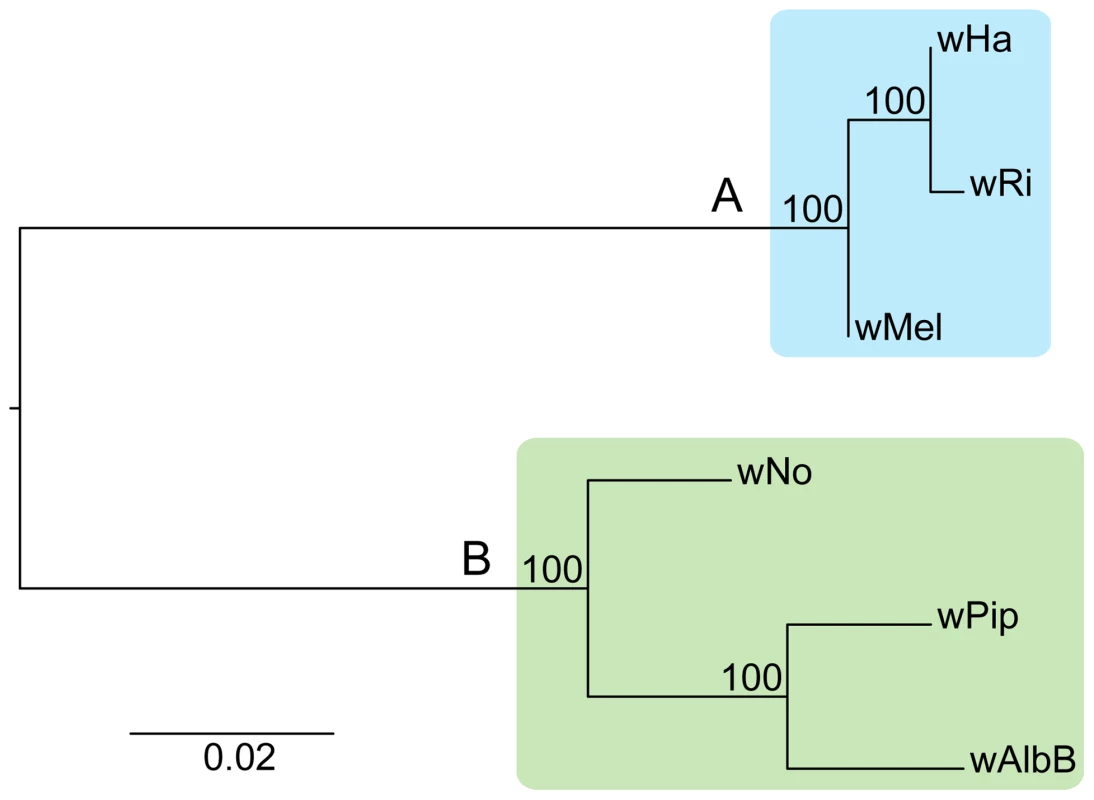
The phylogenetic tree was inferred using the maximum likelihood method from a concatenated alignment of 660 single copy orthologous genes. Numbers on the branches represent the support from 1000 bootstrap replicates. The blue box indicates the strains from supergroup A and the green box indicates the strains from supergroup B. Fig. 2. Genomic overview of the similarity between completely sequenced Wolbachia strains. 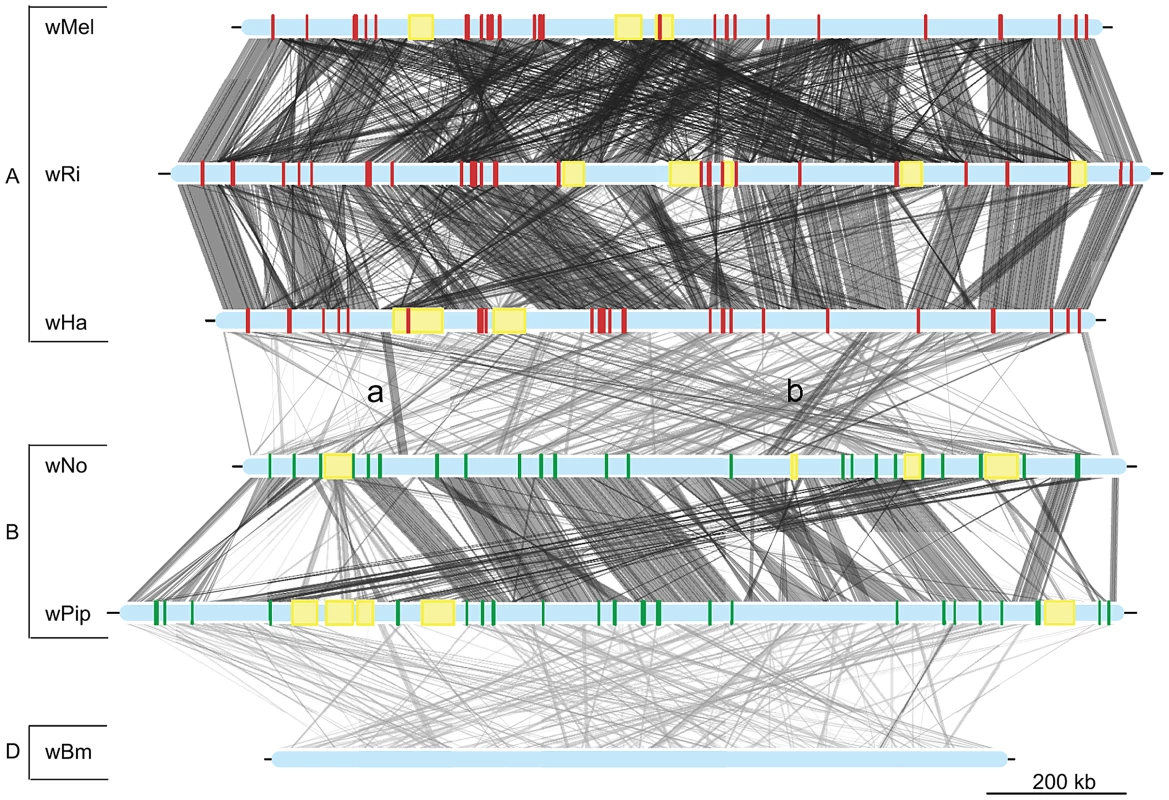
Red boxes show the location of supergroup A specific genes (only in the supergroup A strains, wMel, wRi and wHa), whereas green boxes indicate the position of supergroup B specific genes (only in the supergroup B strains wNo and wPip). The yellow boxes represent the location of prophage elements in supergroup A and B strains. The supergroup D strain wBm is included for comparison. Similarity between sequences is indicated by the intensity of the grey lines, where darker is more similar. The lines between the wNo and wHa genomes with the small letters “a” and “b” indicate two regions with atypical synteny and sequence conservation. Recombination within and between Wolbachia supergroup A and B
To test the hypothesis that recombination mediates cohesion within supergroups but is reduced between supergroups, we examined the topologies of single gene trees, studied the spread of sequence divergence estimates, and inferred the relative fraction of intragenic recombination events both within and across the supergroup boundaries.
Single gene trees
We first constructed single gene trees with the maximum likelihood method and clustered the tree topologies based on pair-wise weighted Robinson-Fould distances. For 652 of the 660 trees, the separation of the A - and B-group strains was consistent, but among these the 9 possible topologies were represented in almost equal abundance (Figure 3). Additionally, each of the three possible topologies within each supergroup accounted for about one third (29% to 40%) of the 660 trees (Figure 3), which is close to 33% as expected if the taxa are randomly clustered within the two supergroups. However, a few more trees indicated a clustering of wRi and wHa to the exclusion of wMel (240 trees, 36%), and of wPip and wAlbB to the exclusion of wNo (262 trees, 40%) (Figure 3). Thus, the clustering of wRi and wHa, and of wPip and wAlbB in the concatenated tree (Figure 1) may be due to a higher fraction of genes that support these relationships rather than a higher overall sequence similarity. In a more stringent analysis, we analyzed a subset of 260 trees with bootstrap support values above 75% for all nodes (for a few example trees, see Figure S4). We saw that the percentage of trees representing each topology remained similar to those in the total data set (Figure 3) and thus no particular topology was an artifact of low resolution. Taken together, our results indicate that the supergroup division is highly robust, but that about two thirds of the trees have a topology within supergroups that does not reflect evolution through vertical decent, as expected if the strains belonging to the same supergroup are highly recombinogenic (Figure 3).
Fig. 3. Schematic figure of individual tree topologies inferred from single-copy orthologs. 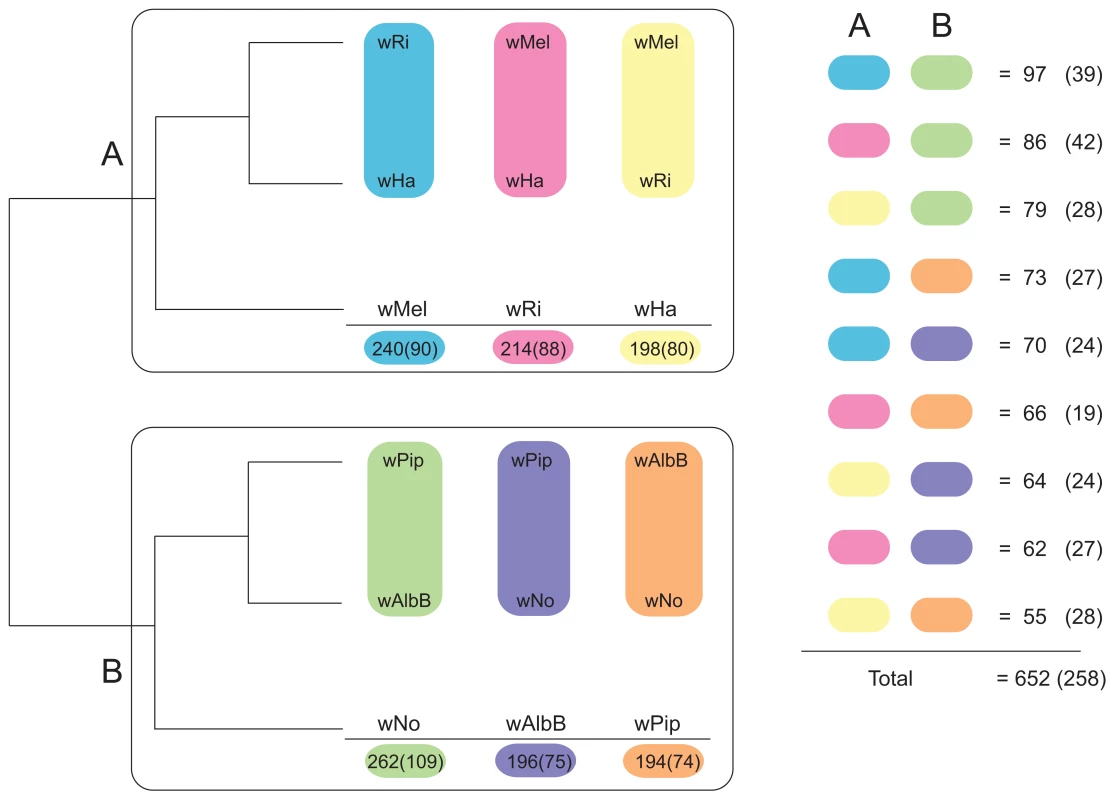
Phylogenetic trees were inferred using the maximum likelihood methods from 660 single-copy gene orthologs, 652 of which supported the division between super-group A and B. The schematic tree summarizes the distribution of the 652 gene trees, where the number of trees supporting a topology within each super-group is shown. The coloured blocks on the right show the number of trees found for each of the 9 possible topologies with separation of the supergroups. The numbers were obtained by clustering individual trees based on weighted Robinson-Fould distances. Numbers in parenthesis represent the trees where all nodes have a bootstrap value higher than 75. In contrast, only sporadic cases of recombination between strains of different supergroups were detected in the analysis. Of the 660 core genes, 8 genes generated a topology that was incongruent with the supergroup classification scheme. Five of these genes (nuoJKLN and ccmE) code for proteins involved in the respiratory chain complex and are co-located in a long syntenic segment that is highly similar in sequence between the A and B-supergroup strains (marked as b in Figure 2). In E. coli, all 14 nuo genes are located in the same cluster [49], but in Wolbachia they are split up into 7 different clusters: nuoABC, nuoD, nuoE, nuoF, nuoGH, nuoI and nuoJKLN. The three additional core genes that yielded a topology that conflicted with the supergroups were coxB, which code for cytochrome oxidase subunit B in the respiratory chain complex, mutS and ribE.
Additionally, we identified a cluster of genes encompassing 13 kb that showed an atypically high sequence similarity between wHa and wNo (marked as a in Figure 2). This segment is not present in the wPip genome and was therefore not included in the set of 660 single gene orthologs. A separate phylogenetic analysis revealed near identity for six co-located genes in wNo and the three supergroup A genomes, which contrasts with the clear separation of the A and B-group strains in trees produced from the flanking genes (Figure 4). The high sequence similarity for these genes provides indirect evidence for a recent transfer of a long DNA segment between supergroups.
Fig. 4. Recombination of chromosomal genes between the supergroup A and B strains. 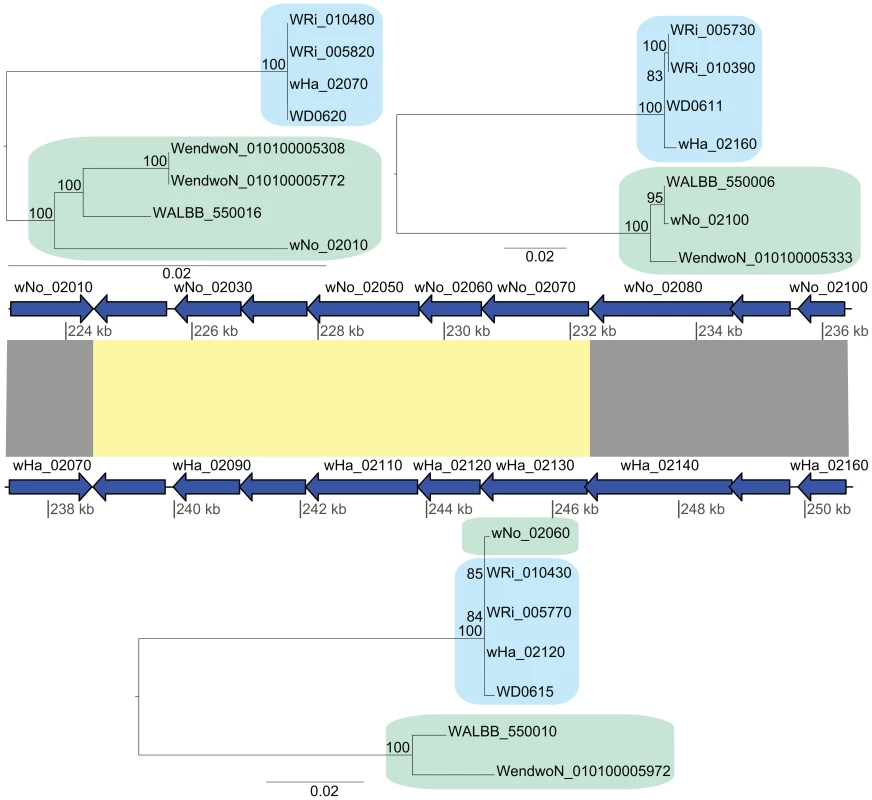
The yellow block indicates the six genes that produce a gene topology indicative of recombination between wNo and the A-group strains (tree topology shown below the gene order comparison plot). The upstream and downstream flanking genes show the normal separation of the strains into two super-groups (tree topologies shown above the gene order comparison plot). Finally, we inferred several cases of gene transfers of phages between strains of different supergroups. All genomes contain prophages of similar organization (Figure S5) and our single gene phylogenies of phage genes revealed a mosaic pattern, consistent with repeated recombination of phage genes within and across the supergroup boundary. A transfer of the orf7 phage gene between wHa and wNo was previously indicated by PCR analysis [34]. However, none of the orf7 sequences in the wHa and wNo genomes were identical to the PCR-amplified sequences. Instead, we observed near sequence identity of a few other phage genes in the wHa and wNo genomes. For example, the head genes of wHa-WO2 were most closely related to prophage genes from the Wolbachia B-group strains wNo and wPip (Figure 5C), whereas the baseplate and tail genes were most closely related to the homologous genes in the WO-A and WO-B prophages in the A-group strain wMel (Figure 5A and 5B). We also noted a close relationship of the wNo-WO4 genes to the wRi-WOC prophage (Figure 5D), consistent with previous analyses of a smaller gene set [38].
Fig. 5. Horizontal transfer of bacteriophage genes between the supergroup A and B strains. 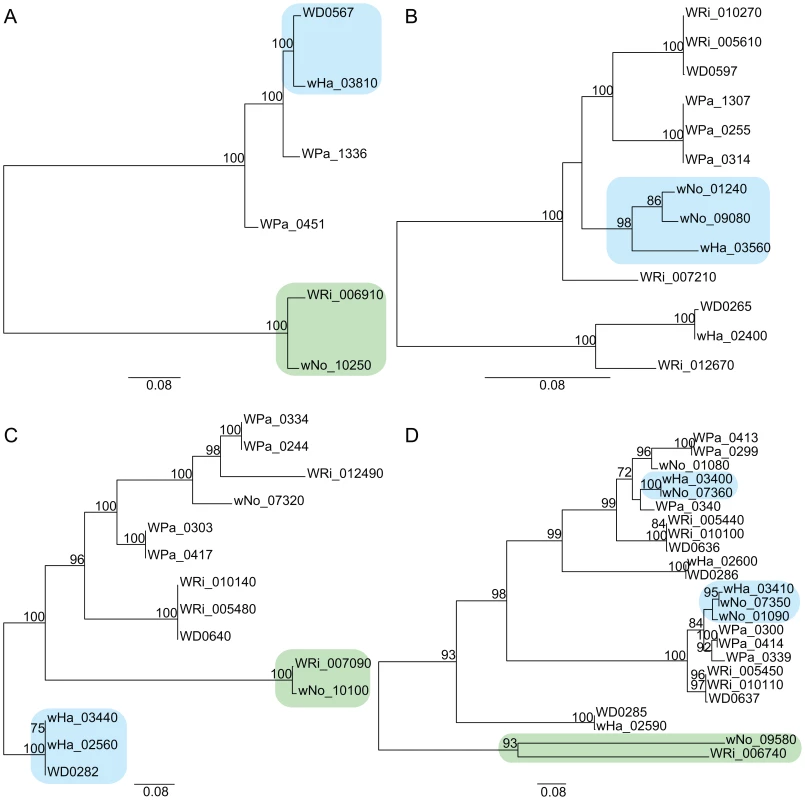
Phylogenetic trees were inferred using the maximum likelihood methods from four prophage-associated genes A) “Late control D”, B) “Terminase”, C) “Baseplate W”, D) “ANK.”. Large spread of sequence divergence levels
To quantify the heterogeneity in sequence divergences for the individual genes, we determined substitution frequencies at synonymous sites (dS) for all pairwise combinations of the 660 orthologs. For genes that undergo high frequencies of recombination, the dS value does not necessarily reflect the frequency of mutations, but it can still be used as a proxy of sequence divergence levels. According to this analysis, the A-group strains (median dS-values = 0.03) were less divergent than the B-group strains (median dS-values = 0.06–0.08) (Table S3).
We plotted the relative pairwise dS-values for each of the 660 genes in a ternary plot for supergroup A and B separately to illustrate the consistency of the divergence estimates (Figure 6A and 6B). If two out of three strains are consistently more similar to each other than what either is to the third strain, the dots will be concentrated in one spot of the triangle (the average of the relative dS-values), as previously observed for Rickettsia (see Fig. 2B in [38]). In contrast, if there is no consistent signal in the data, as observed for the highly recombinogenic species Neisseria meningitidis, the dots will be distributed across the triangle (see Fig. 2C in [38]). For Wolbachia, the relative dS values were widely distributed across the triangle, with the spread estimated to 0.43 and 0.32 for the A and B-group strains, respectively (calculated as the distance from the average point in the plot as described by [38]) which is similar to a previous estimate of 0.42 for the A-group strains wRi, wMel and wUni [38]. The large spread of the values indicate that the individual dS-values are in many cases far off from the average relative value in both the A and B-group strains, as expected when homologous recombination overrides the vertical inheritance pattern of single nucleotide substitutions.
Fig. 6. Ternary plot of sequence divergence levels at synonymous sites. 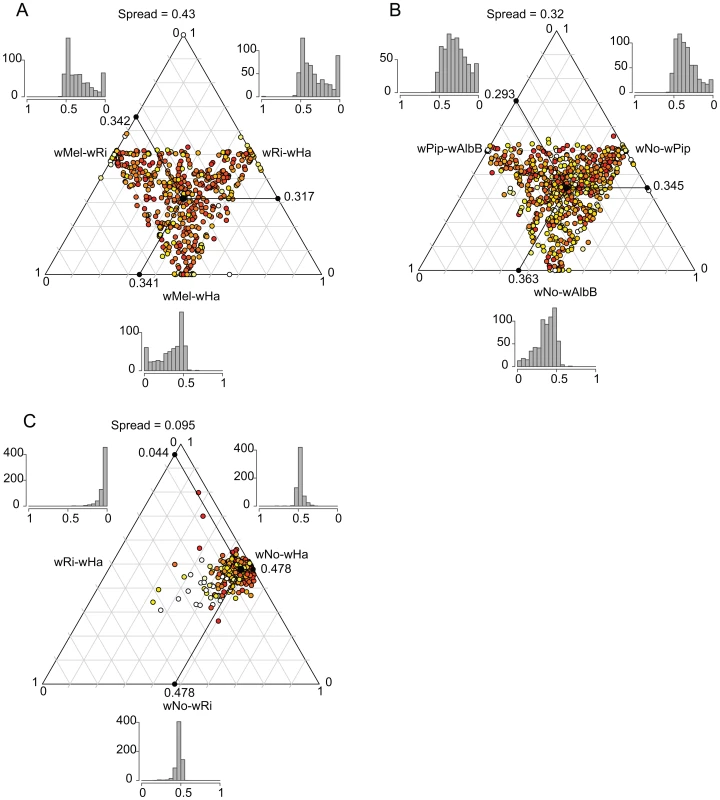
The plot shows the variation in synonymous substitution frequencies for the 660 single-copy orthologs in A) supergroup A Wolbachia strains, B) supergroup B Wolbachia strains and C) D. simulans infecting Wolbachia strains. Each dot in the plot represents one gene. Absolute dS-values have been transformed to relative values between 0 and 1 and the mean relative dS-value for each pair is shown on each axis. The spread represents the median distance to the mean point. The color of each dot represents the maximum absolute dS-value among the 3 pairs, ranging from light yellow (low values) to red (high values). The histograms show the frequency of relative dS-values within each pair. For comparison, we also included a plot of the dS-values for the 660 genes in the three Wolbachia strains infecting D. simulans (wHa, wRi and wNo). The dS values for wHa and wRi was 20-fold lower than the dS value for pairs that include wNo (dS = 0.57–0.58, Table S3), and the spread of the dS values was estimated to only 0.095 (Figure 6C). This suggests that the two supergroups diverged from each other mainly by the accumulation of nucleotide substitutions, since only very few genes show a pattern of divergence that conflict with the overall divergence pattern between the strains.
Relative frequency of recombination to mutation
We used ClonalFrame to estimate the overall ratio (r/m) at which recombination and mutation events generate a substitution, for the concatenated alignment of all 660 single copy orthologs present in all six Wolbachia strains. Overall, the r/m ratio was estimated to 3.57 (95% credibility region 3.43–3.71). We also quantified the r/m ratio for each branch in the tree using ClonalFrame, including only positions where the probability of substitution via mutation or recombination was higher or equal to 0.95. The ratios were consistently high, ranging from 2.33 on the branch to wMel to 8.82 on the branch to wRi (Table 3). This suggests that the likelihood that a base difference is due to a recombination event is on the average 2 to 8 times higher than the likelihood for a mutation event to cause a substitution, in any of the strains in our analysis.
Tab. 3. Estimates of recombinations and mutations occurring in each strain. 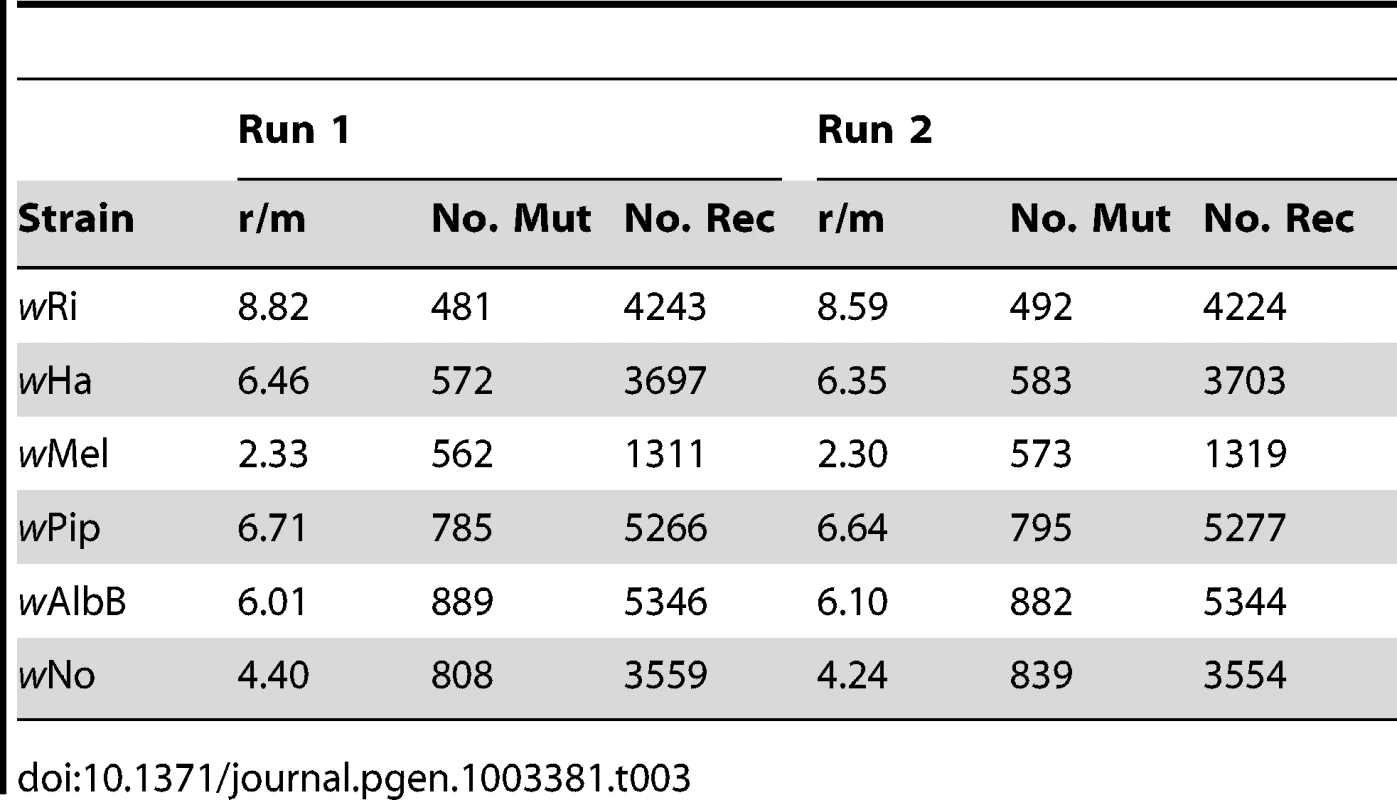
Intragenic recombination events
While our analyses of single-gene phylogenies and divergences at synonymous sites indicated very little recombination between supergroup A and B, we considered the possibility that smaller fragments of genes could be exchanged. To estimate the number of genes affected by recombination events within and between supergroups, we used four different methods (NSS, MaxChi, Phi and geneconv) to reduce the risk of identifying false positives. A total of 120 of the 660 single copy orthologs gave significant results with all four methods and an additional 73 genes gave significant results with three of the methods. Thus, about one third of all genes carried a signal of past recombination events according to this analysis. This represents a conservative estimate since events that span across the length of the gene will not be detected by these methods.
In this analysis, geneconv is the only program that identifies which sequences in the alignment took part in the recombination event. We therefore evaluated the 176 genes for which recombination was detected with geneconv and at least two additional methods, in order to determine whether the intragenic recombination events had occurred within or between supergroups. Further, we ran geneconv with different mismatch penalty settings, to optimize for detection of recombination of both highly similar and more divergent pairs of sequences (see materials and methods). While the number of genes in which recombination was detected was relatively similar regardless of the geneconv settings, the sizes of the recombining fragments were more variable (Table S4, Figure S6). Recombining fragments were in the range of 300 to 700 bp for the A-group strains and 200 to 600 bp for the B-group strains. The inferred recombination fragments between supergroups were much shorter, in the range of 70 to 180 bp (Table S4).
Regardless of recombination detection method, short recombination events may go undetected in comparisons of highly similar sequences, such as of strains within supergroups. The number of intragenic recombination events within supergroups is therefore likely to have been underestimated. In contrast, the power to detect recent recombination events is relatively higher between strains of different supergroups due to the higher level of sequence divergence, particularly for long fragments. Therefore, we can confidently conclude that recombination between supergroups does take place, but appears to be restricted to small fragments. In effect, the total number of basepairs affected by recombination events across supergroups is several fold smaller than those affected by recombination events within supergroups. Moreover, since recombination events between supergroups have accumulated during a longer time period, we also conclude that the rate of recombination between supergroups is lower than within.
Recombination, sequence divergence, and functional categories
There may be several reasons why the frequencies of recombination were reduced between strains of different supergroups, one of which is that the high levels of divergence between the supergroups could serve as a barrier to recombination and restrict recombination events to a few highly conserved genes or domains. To test this hypothesis, we examined whether the 111 genes affected by recombination events across supergroups (Table S4) were less divergent than genes in which no such events had been detected. To this end, we estimated the pairwise genetic divergence between wNo and all other strains for genes where recombination was detected between at least one of the other two B-supergroups strains (wPip and wAlbB) and at least one of the three supergroup A strains (n = 51).
We found that the divergence between wNo and the A supergroup strains was not significantly different for these genes compared to the genes (n = 549) not affected by such recombination events (Mann-Whitney test, wMel p = 0.389, wRi p = 0.3622, wHa p = 0.475). However, the pairwise divergences between wNo and each of the B-supergroup strains were significantly larger for the genes with recombination across the supergroups compared to the rest of the data set (Mann-Whitney test, p<<0.01). Similar results were obtained when any of the other strains were used in the same manner as wNo (data not shown). These results suggest that recombination across supergroups does not only occur in genes with low divergence between supergroups. However, when such events occur they give rise to higher divergence between strains from the same supergroup and they can lead to an increased overall divergence measure of the genes even though many of the recombination tracts are short in size.
Additionally, we could not detect a bias towards certain functional categories for genes affected by recombination events between supergroups (Figure S7). We conclude that recombination between strains of different supergroups is not restricted to highly conserved genes or targeted to particular gene functions.
Horizontal gene transfers and functional novelty
Novel gene acquisitions may confer the ability to inhabit new niches. In the case of endosymbionts, the acquisition of a new gene might potentially broaden the host range, but could also lead to ecological specialization within the existing host. For example, the uptake of a novel gene might contribute to the physical separation of strains with and without the new gene, leading to speciation. To investigate this hypothesis, we examined gene content differences between the two supergroups.
In total, we identified 33 and 24 protein clusters that were specific to the A - and B-group genomes, respectively (Tables S5, S6). A comparison of the number of protein clusters solely present in the A - or B-supergroup strains to the number of protein clusters found in any other combination of three strains showed that the supergroup specific protein clusters are largely over-represented (Figure S8).
Functional categorization of these clusters identified a few particularly interesting acquisitions in the A-group strains of genes putatively involved in the regulation of arginine transport systems (argR), stress response (cydAB) and modulation of host cellular functions (fic). Phylogenetic analyses revealed sequence similarities to several other intracellular bacteria, such as Legionella, Rickettsia and Chlamydia, indicating that these genes may serve a role for the intracellular lifestyle (Figure 7, Figure S9).
Fig. 7. Phylogenetic analysis of the ArgR repressor gene. 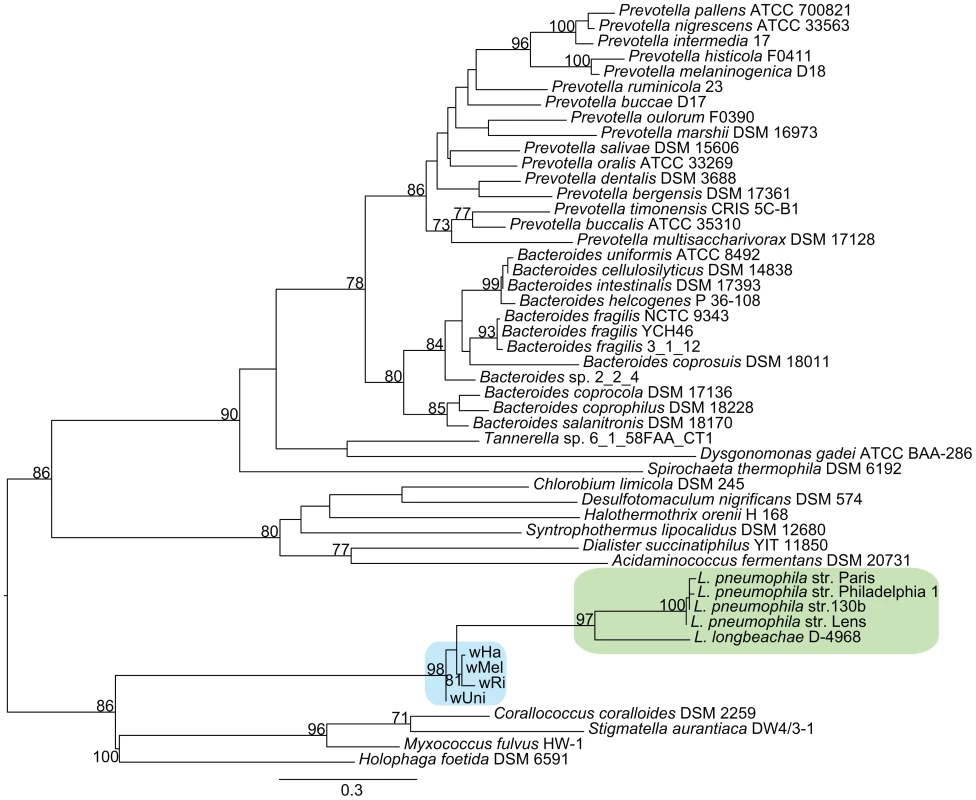
The phylogenetic tree was inferred using the maximum likelihood method based on a protein alignment of the ArgR repressor from many different bacterial species. Numbers on the branches represent the support from 1000 bootstrap replicates. The blue box indicates the Wolbachia strains from the supergroup A strains, and the green box indicates Legionella species. As in Legionella pneumophila, the gene for the arginine repressor ArgR is co-located with three genes for an arginine ABC transporter and phylogenetic reconstruction confirmed the close affiliation between Wolbachia and Legionella of the entire cluster of four genes (Figure 7, data not shown). Previous studies of other pathogenic bacteria have shown that arginine may be associated with virulence. Additionally, arginine can be converted to nitric oxide by the host as part of the innate immune response. In Legionella, the expression of the genes for the arginine repressor and transporter is sensitive to the presence of L-arginine and derepression is observed during intracellular growth [50]. In analogy, we infer that the Wolbachia ABC-transporters are expressed when the concentration of arginine is low, stimulating uptake of arginine through the ABC-transporters.
The Fic domain proteins solely present in the A-group strains are particularly interesting since their homologs in other bacteria have been shown to be secreted into the host cell cytoplasm to modify host regulatory GTPases [51], thereby causing the disruption of the host actin cytoskeleton [52], [53] or host cellular rearrangements [54]. The ability to manipulate host GTPases is most likely a general feature of all proteins containing this protein domain since it has also been reported in the distantly related Fic-domain containing human HYPE protein [53].
The comparison of gene contents also indicates possible differences in cell division and lipid II biosynthesis due to gene loss in the B-group strains. For example, the ftsWIBL genes, which are involved in these processes in E. coli are present in the A-group strains, but absent from the B-group strains and present only as pseudogenes in wBm. Additionally, the murC gene, which catalyses the attachment of the first amino acid to the glycan, has been split into two genes located distantly from each other in the genomes of all B-group strains, including wVitB. One of the two genes encodes the N-terminal domain and the other encodes the C-terminal domain fused to a recombinase zinc beta ribbon domain (PFAM: PF13408) (Figure S10). Interestingly, experimental evidence has shown that a lipid-II-like molecule is synthesized in the supergroup B Wolbachia strain wAlbB [55], suggesting that the murC gene function is present despite the separation of the sequences encoding the functional domains into two genes.
Uniquely present in the B-group strains is a cluster of genes encoding outer membrane proteins which are found in two to three copies in each of the supergroup B genomes, including wVitB. Located at the corresponding genomic position in the A-group strains is a non-coding region of approximately 1 kb, which does not show any significant sequence similarity to genes in the B-group strains (Figure S11A). Eight of the nine proteins in this cluster contain PFAM domains annotated as outer-surface proteins, including the family to which the Wolbachia surface protein (wsp) belongs (PF01617). A phylogenetic analysis revealed a clustering of genes between the strains, rather than within the genome of one strain, suggesting that gene duplication occurred before divergence of these B-group strains (Figure S11B). Short sequence fragments with significant similarity to these surface proteins were identified in the wBm genome, indicating loss from supergroup A. However, since no homologs outside Wolbachia supergroup B could be identified, the origin and function of this outer membrane protein family remain to be determined.
Discussion
Evolutionary and functional studies of adaptations to the intracellular environment have previously been hampered by technical challenges associated with the inability to cultivate these bacteria outside their host cells, making it difficult to obtain DNA in large quantities and of enough purity for genome sequencing projects. To bridge this gap, we present a protocol with the potential to enable genomic studies of endosymbiotic bacteria without the need for laborious cultivation methods. Our approach relies on the purification of endosymbionts from their hosts, followed by whole genome amplification to increase the quantity of DNA. We have shown that although only small quantities of bacterial cells were used as the starting material, assembly and gap closure was not hindered by the drawbacks normally encountered with whole-genome amplification of single bacterial cells. Using this method, we have sequenced the genomes of two Wolbachia strains, wHa and wNo, that co-infect D. simulans. In a comparative genome analysis that included these and other Wolbachia genomes, we have corroborated the genetic separation of the Wolbachia supergroups A and B by a phylogenetic analysis of 660 concatenated core genes, suggesting that they should indeed be considered different species.
Discussions about the bacterial species concept have largely focused on the criteria used to define the species boundary [56], [57]. The most commonly used species definition is based on 16S rRNA sequence similarity, with the cut-off arbitrarily set to 97%, although 99% identity has also been suggested as a criterion of taxonomic species demarcation [58]. The Wolbachia strains investigated here are 97–98% identical in the 16S rRNA gene between supergroups and 99% identical within supergroups, thus representing a border-line case based on the 16S rRNA species definition.
Conceptually, bacterial species should also (i) fall into well-supported sequence clusters, (ii) evolve under cohesive processes within the species, (iii) be ecologically distinct and (iv) be irreversibly separated from each other [15], [59]. Our comparative genomics study shows clearly that Wolbachia supergroup A and B strains evolve as distinct clusters. Below, we discuss whether any of the other three concepts are also applicable to Wolbachia.
Are Wolbachia strains within each supergroup evolving under cohesive processes?
High recombination frequencies were previously estimated for strains belonging to super-group A [38], and confirmed in this study in both supergroup A and B. Single-gene phylogenies showed all possible divergence patterns for strains within each supergroup in nearly equal proportions, and the spread of the relative dS values for individual genes within supergroups was very high (0.3–0.4), which is in the range of the naturally competent and highly recombinogenic bacterial pathogen Neisseria meningitidis (Spread = 0.34) [38]. Thus, there is a very strong bias for substitutions caused by recombination within Wolbachia supergroups, which suggests that there is very little selection against recombination within Wolbachia supergroups, consistent with the species concept.
Are Wolbachia strains from supergroups A and B ecologically distinct?
For endosymbionts, co-evolution with hosts is thought to generate a physical barrier that leads to the evolution of ecologically distinct species. This is exemplified by a strong congruence of Wolbachia and host phylogenies for nematode-infecting strains [60], [61]. However, for Wolbachia strains infecting insects, host and endosymbiont phylogenies are generally not congruent [30], [31], [62]. Strains of different supergroups can infect the same host species, as exemplified by wHa and wNo in this study, just as strains of the same supergroup, such as wHa and wMel, can infect different host species. Furthermore, there is no simple association between supergroup affiliation and reproductive disorders, since strains of both supergroups are capable of inducing for example cytoplasmic incompatibility.
Yet, our analysis shows that there are differences in gene content between supergroup A and B, which are likely to influence the interactions with the host and the surrounding environment. Notable among the A-group specific functions are genes for uptake of arginine, tolerance to stress and secretion of proteins involved in the modulation of host cellular functions, whereas the B-group specific gene set included genes for outer surface structures. Thus, our data raise the possibility that the supergroups might have evolved into distinct ecotypes within the same host species, potentially avoiding competition through niche partitioning and thereby achieving a stable co-existence.
Although niche partitioning has not yet been investigated for hosts infected with multiple Wolbachia strains, Veneti et al. [63] demonstrated that Wolbachia strains of different supergroups show distinct localization patterns within the host embryo. The A-group strains (with the exception of wRi) were localized to the posterior part of the embryo, whereas the B-group strains were observed in the anterior part during the syncytial blastoderm stage. However, only a few highly similar strains of each supergroup were included in the analysis, and it remains to be determined whether the observed patterns are characteristic of a broader selection of strains from the two supergroups. Physical separation of endosymbionts within hosts does not necessarily have to be absolute to allow for speciation, since quantitative differences in associations with different habitats might also generate ecologically distinct species [6]. In analogy, differences in abundances and/or compartmentalization within the host could potentially lead to ecologically distinct species. Indeed, distinct localization patterns of endosymbionts within hosts have for example been observed for different genera of whiteflies [64].
While the current overlapping host ranges of supergroup A and B and the occurrence of multiple infections with strains from both groups appears to contradict the possibility of host specialization, several studies have provided some evidence for specialization to hosts and/or habitats [65]–[68]. However, these studies were based small gene datasets, such as the wsp gene that code for a hypervariable surface protein and/or core genes used in multi locus sequence typing. If these genes are as recombinogenic in all Wolbachia strains as reported here, sequence similarity measures within supergroups will reflect gene recombination histories rather than strain relationships. Correlations between genotypes and host-association patterns within supergroups will thus mostly depend more on the gene sets selected for the analyses.
In conclusion, both experimental evidence and additional genome data is needed in order to evaluate the ecological distinctness of Wolbachia strains both within and between supergroups. Now that a supergroup specific gene repertoire has been identified, it should be possible to investigate both the ecological roles of these genes, as well as the strain localization at various stages of host development.
Are Wolbachia strains from super-groups A and B irreversibly separated?
All evidence gathered in this study indicates that strains from different supergroups represent distinct clusters, but are they irreversibly separated or do they still exchange genetic material? Importantly, our analyses have shown that recombination events between supergroup A and B have occurred, but that the fragments are of shorter sizes and have had a much lower impact on the genomes than recombination events within the groups. Recombination events that span over all or most of a gene are very rare since only 8 of the 660 gene trees did not provide support for the supergroup division. Consistently, we only identified a few long recombination tracts between the supergroups. These few transfers of co-located genes might thus exemplify how one organism can acquire another population's adaptation while the integrity of its own niche-defining characteristics is still preserved.
The wHa and wNo genomes are thought to have co-infected D. simulans for at least 200,000 years, which is a relatively short time period compared to at least a few million years since the divergence of the A and B-groups (as inferred from a few % difference in their 16S rRNA genes). Hence, even though we do not find more recombination between wHa and wNo than between other strains belonging to different supergroups, we cannot exclude the possibility that the exchange of genetic material between them would increase given longer time. Alternatively, there is some form of barrier to genetic exchange between strains of supergroup A and B.
The simplest form of barrier to gene transfer is the presence of incompatible mobile elements. However, we do not think that this is the case in Wolbachia since the gene phylogenies indicated transfer of phage genes across the supergroup boundary. Moreover, transfer of a complete bacteriophage genome between strains of different supergroups was recently discovered in Nasonia vitripennis [42]. Even so, there is no concrete evidence that these phages regularly transfer genetic material other than their own genomes, and thus there could still be differences in the frequencies at which genetic material is transferred between the two supergroups.
Another form of barrier is that the sequence divergence per se limits recombination. The mismatch repair system has been seen to prevent homologous recombination between divergent sequences and loss of the mutSL genes for the mismatch repair system is known to cause dramatic increases in both mutation and homologous recombination frequencies. However, even though we found that the mutS gene is full-length and probably functional in both the A and B-group Wolbachia strains, and that all genomes except the wRi genome have two copies of the mutL gene, we found no inverse correlation between sequence divergence levels and recombination frequencies for individual genes. In natural populations, the mutS gene recombines and is gained and lost in a cyclic manner in response to environmental changes, leading to altered mutation and recombination rates. The resulting mutator phenotypes are selected during periods of environmental fluctuations and then restored by recombination with a functional copy from another strain [69]. We saw that in Wolbachia, one of the mutL genes is associated with a prophage element in wPip and wHa and located near to a previously detected insertion in wMel that might stem from a phage, indicative of horizontal gene transfer. Additionally, we detected intra-genic recombination in both the mutS and mutL genes. Thus, the presence of a seemingly functional mismatch repair system all strains analyzed does not preclude that recombination frequencies could have fluctuated in the past due to gains and losses of these genes.
A recent model suggests that almost identical sequences between the donor and recipient are required at one or both ends of a recombination fragment in order for recombination to occur and that the imported fragments are digested until a good enough match is obtained [70]. Consistent with our data, this model predicts that shorter recombination tracts will be found between more divergent sequences, since more cuts are required in order for the ends to match. Essentially, if true, this implies that when two genomes have diverged enough only short fragments can recombine between them. As a consequence, it is unlikely that recombination events are sufficient to invoke convergence between the supergroups even though they share the same habitat for a long period of time, as is the case with the Wolbachia strains wHa and wNo. Although we did not see a correlation between sequence diversity and intragenic recombination, this model cannot be ruled out since the end points of each recombination fragment were not investigated.
Genome rearrangements present yet another barrier to recombination and is thereby an important factor in speciation processes in eukaryotic organisms, mainly because of suppressed recombination at rearranged sites during meiosis in heterozygous individuals [71]. Although bacteria do not evolve by sexual reproduction, homologous recombination could be suppressed in chromosomal regions that are not co-linear because of rearrangements or insertions of genes in one of the two genomes. Indeed, a recent study showed that recombination frequencies are suppressed close to lineage-specific genes, which might lead to higher divergence levels in their vicinity [72]. Furthermore, long recombination events can only occur if the target genome has a similar gene order. Since a single long recombination event can override several shorter intra-genic recombination fragments, extensive rearrangements could contribute to the separation of the lineages. The genomes of Wolbachia strains that belong to the same supergroup show much higher colinearity than strains of different supergroups, potentially contributing to the observed lower frequency of recombination events between the A and B supergroups.
In summary, a number of different explanations could account for the observed reduced level of recombination between supergroup A and B. Although we do not know whether there has been selection against recombination between supergroups or if the reduced levels of recombination was driven by neutral processes alone, our results strongly suggest that the A and B supergroups have now become irreversibly separated.
What started the speciation process in Wolbachia?
The acquisition of advantageous novel genes or mutations is hypothesized to trigger speciation events according to the ecotype model of speciation, which has so far only been evaluated for free-living bacteria [73]. In this context, it is notable that we have identified supergroup-specific genes sets that appear to be the result of horizontal gene transfers. Although it is too early to speculate about the functions of these group-specific genes, it is quite possible that their acquisitions induced significant phenotypic changes. Selective advantages associated with any of these phenotypes could have purged diversity within the groups, thereby contributing to the genetic separation of the two lineages.
Another scenario could be that the loss or gain of genes in one strain of Wolbachia resulted in reproductive isolation between infected hosts, for example through CI [74], [75]. However, it is difficult to evaluate the likelihood for such a scenario, since multiple infections and recent horizontal transmission of Wolbachia strains between different host-species have blurred the ancestral patterns of infections.
Alternatively, the speciation event may have been triggered or enhanced by extensive rearrangements, due to a burst in the activity of IS-elements. All Wolbachia genomes from supergroup A and B sequenced to date contain an unusually high level of IS-elements. For example, 11% of the genome of Wolbachia strain wRi was estimated to consist of IS-elements, and 17 of the 35 identified breakpoints between the genomes of wMel and wRi are located at IS-elements [38]. Additionally, many of the IS elements in Wolbachia genomes carry mutations that are likely to have rendered these elements non-functional, which is an unusual feature of bacterial IS-elements since they are commonly believed to have a rapid turnover rate within genomes [76]. Making use of the presence of these degraded IS-elements, a recently published simulation study aiming to explain the distribution of IS copies in the modern Wolbachia genomes suggested two major periods of intense transpositional activity, a very recent burst and an ancient expansion of the most divergent IS copies [77]. Such an expansion could have induced major changes in gene order structures, leading to suppressed recombination close to the breakpoints. Since two rearranged genomes can never converge to the same gene orders again, an ancestral expansion of IS-elements followed by genome rearrangements could have irreversibly separated the two groups. However, since the age of the ancestral expansion is not known, it is difficult to test this hypothesis. The recent expansion of IS-elements in Wolbachia could potentially have lead to similar diversifications in more closely related strains, a hypothesis that could be tested by investigating diverse lineages within the same supergroups.
It is obvious that no single speciation hypothesis will be applicable to all bacteria. Although Wolbachia is an obligate intracellular bacterium, it is atypical in that it is a generalist with a high prevalence and a broad host range in a diverse group of insects. The most remarkable aspect of its evolution is the expansion of the host range, which might have occurred independently in both supergroups after their separation. The acquisition of genes to manipulate the host combined with high recombination frequencies to shuffle beneficial alleles among all members in the group could help explain much of this ability. Many questions remain to be solved, such as for example if there is adaptive selection for ecological divergence within supergroups, and if strains from different supergroups inhabit different niches within their broad range of host species. To further investigate speciation processes in Wolbachia, we need to study the global distribution patterns and population structures of hosts and endosymbionts. The methods developed in this paper offer the possibility to perform such large-scale, whole-genome surveys of Wolbachia and other endosymbionts.
Methods
DNA preparation and sequencing
The wNo-infected fly line was generated by a series of backcrosses on a double-infected fly line collected on Noumea in 1989 [78]. The wHa-infected fly line was collected on Hawaii in 1990, as a natural single-infection [79]. Both Wolbachia-infected fly lines have been kept at the laboratory of Prof. Kostas Bourtzis for over fifteen years and have extensively been used in Wolbachia-related experimental work.
The purification of Wolbachia cells was carried out as in [39], with some modifications. Flies were allowed to oviposit on apple-juice agar for two hours, and 15–30 embryos were collected for the purification. The embryos were dechorionated in bleach, rinsed with water, and homogenized in phosphate-buffered saline (PBS) buffer with a sterile micropestle. The homogenate was centrifuged at 400 x g for 5 min to pellet large debris, including host nuclei. The supernatant was centrifuged at 5,400 x g for 5 min to pellet Wolbachia cells. The pellet was re-suspended in PBS, and another slow centrifugation was carried out (400 x g for 5 min) to remove remaining debris. The supernatant was passed first through a 5 µm pore size filter (Millipore, Bedford, MA), and then through a 2.7 µm pore size filter (Whatman, USA). The filtrate was centrifuged at 6,900 x g for 15 min to pellet the Wolbachia cells. Most of the supernatant was removed, leaving a bacterial pellet in approximately 3–5 µl PBS.
A multiple-displacement amplification (MDA) was carried out directly on the bacterial pellet, using Repli-g midi kit (Qiagen) according to manufacturer's instructions (protocol for Amplification of Genomic DNA from Blood or Cells). The amplified samples were cleaned prior to sequencing with QIAamp DNA mini kit, according to manufacturer's instructions (Qiagen, supplementary protocol for Purification of REPLI-g amplified DNA). Since MDA is known to be extremely sensitive, precautions were taken to avoid contamination during the purification of Wolbachia cells, including sterile-filtering of all solutions, and autoclaving/UV-treatment of plastic utensils.
Three independently amplified samples for each Wolbachia strain were used for library construction and sequencing, so that each genome was sequenced by ½ plate of single-end and 3 kb paired-end 454 and 1/12 lane paired-end Illumina. 454 sequencing was done at SciLifeLab Stockholm on a 454 Roche FLX machine using Titanium chemistry and standard preparations for single-end and 3 kb paired-end libraries. Illumina sequencing was done on a HiSeq2000 instrument at the Uppsala SNP & SEQ platform, using standard Illumina protocols for preparation of paired-end libraries, generating 2×100 bp sequences from each fragment.
Genome assembly and annotation
The 454 datasets were assembled de novo with both Newbler (454 Life Sciences Corp., Roche, Branford, CT 06405, US) and Mira [80]. Assemblies were compared with Mauve [81] and ACT [82] and the discrepancies between the best assemblies and all sequence gaps were resolved with PCR amplification from total fly DNA extractions (DNeasy Blood and Tissue kit, Qiagen) and subsequent direct sequencing of the PCR products. Since the Newbler assembly proved to be generally more correct it was used as a reference to order the contig sequences from MIRA into scaffolds and close the remaining gaps, resulting in two circular Wolbachia genomes. In two positions on the wHa genome PCR-products could not be obtained, but read-pairs that go in and out of the repeat sequence associated with these genome positions support the current arrangement. Gap closure and manual sequence editing of PCR products was done using Consed [83]. Consed was also used to map the Illumina sequences onto the contigs generated using 454 data, in order to correct errors in homopolymer tracts.
To evaluate the purity and quality of the DNA samples used for sequencing, the sequence reads were mapped onto the completed genomes. The Illumina reads were filtered using Trimmomatic [84], and mapped using bwa [85]. The sam-formatted output file from bwa was converted to bam, sorted in coordinates and duplicated reads were marked using Picard tools (http://picard.sourceforge.net). Proper and non-proper read pairs (as set in the sam-file flag by bwa) were extracted with samtools [86]. The single and paired-end 454 reads were mapped separately using the Newbler mapper. For the paired-end 454 reads, true and false pairs (as defined in the output file 454PairStatus.txt by Newbler) were extracted and mapped separately. Coverage was calculated from the bam-files using the depth command in samtools and subsequently plotted using R (R development core team 2011).. The mean quality of assembled and non-assembled 454 reads was plotted with Prinseq [87].
An annotation pipeline was developed using the Diya framework [88]. Prodigal was used for gene prediction [89], GenePrimp for identifying suspicious start/stop codons and pseudogenes [90], and hmmsearch as implemented in pfam_scan.pl was used for domain prediction with the PFAM database [91]. All annotations were manually edited using Artemis [92]. Overview figures of similarity between complete genomes and local genome regions were generated with GenoPlotR [93].
IS-elements were identified based on open-reading frames and a manual search of all repeats. All IS-elements were assigned to an IS family by TBlastX searches against IS-finder [94]. Functional IS-elements were defined as alignments that could be extended to contain the complete annotated IS-element. IS-elements that were truncated compared to their best hit in IS-finder or contained frameshifts were considered non-functional.
Phylogenomics
Homologous genes between six Wolbachia strains (wHa, wNo, wRi, wMel, wPip and wAlbB) were determined using reciprocal protein blast searches between all the protein sequences from the genomes and subsequent clustering with the MCL algorithm [95]. In order for genes to be considered homologous, the shortest protein in a pair needed to be at least 60% of the length of the longer gene and be aligned over at least 80% of its length.
Ortholog clusters containing a single gene from all 6 Wolbachia genomes were aligned on the protein level using mafft [96] and backtranslated to nucleotides. The alignments were pruned to remove gap sites present in 50% or more of the aligned sequences.
A strain phylogeny was inferred on a concatenate alignment of the single gene orthologs in RAxML using the GTRGAMMA model and constructing 1 slow best maximum likelihood tree and 1000 rapid bootstrap replicates. Additionally, phylogenetic trees were inferred independently for each ortholog cluster by RAxML [97] using the GTRCAT model, and constructing 1 slow best maximum likelihood tree and 100 rapid bootstrap replicates. Pairwise Robinson-Fould (R-F) distances were calculated using RAxML by inputting a concatenated file with the 660 individual gene trees. The weighted R-F distances were used to cluster the trees with hclust (method complete and height cutoff of 1) in R. Phylogenetic trees of clusters with members of all strains, but containing paralogous copies and located in prophage regions were inferred by the same method as the single gene orthologs. However, since the current assembly of the wAlbB genome does not contain complete genes for most of the prophage, this strain was excluded from the analysis.
Substitution frequencies
The same 660 single-gene ortholog clusters were used to calculate synonymous substitution rates (dS) between all pairs of genes in the alignment using codeml from the PAML package with the codon-based model of substitutions described in [98] and nucleotide distances with RAxML using the GTR model. The pair-wise dS-values obtained were used to quantify the amount of recombination within supergroup A and B by plotting relative dS - values in a ternary plot and calculating the spread of the values from the mean relative dS-values by using R, as described in [38].
Mutation and recombination
The alignments of the 660 single-gene ortholog clusters were used for recombination detection within genes with PhiPack [99] (which calculates the p-values for three individual methods, Neighbour similarity score (NSS), Maxchi and Phi) and GENECONV [100]. Recombination was inferred for p-values less than 0.01. For counting recombination between vs. within supergroups with geneconv, only global inner fragments with a Bonferroni corrected KA p-value less than 0.05 was used. Additionally, to calculate the r/m parameter, two independent ClonalFrame [101] runs were performed on a concatenated alignment of all the single orthologs as individual blocks using 100.000 iterations, with a burn-in of 50.000 iterations and recording the parameters every 100th iteration. Convergence between the clonal-frame runs was tested using the ClonalFrame graphical user interface. r/m for each node of the tree was calculated from the output file of the two separate ClonalFrame runs. The probability of a substitution generated by mutation was calculated as (1-R)*S and the probability of a substitution being generated by recombination was calculated as R*S, where R is the posterior probability of recombination and S is the posterior probability of substitution. Only positions where the probability of substitution via mutation or recombination was higher or equal to 0.95 were counted. The number of recombination events was calculated by looking at continuous stretches of sites were the posterior probability of recombination was never lower than 0.5 and contained at least one site with a probability of 0.95.
Geneconv was run using three different levels of mismatch penalty, in order to account for differences in divergence between the strains and differences in age of the transferred fragments. The mismatch penalty is inversely proportional to the total number of site differences between two sequences, and directly proportional to the gscale parameter (except when no mismatches are allowed, gscale = 0) according to the formula; mismatch penalty = (number of total polymorphisms in the alignment)* gscale/(number of site differences between each pair of sequences). This means that sequences with a lower number of total differences, will get a higher penalty for a mismatch with the same gscale setting.
Analyses of supergroup specific genes
MCL clusters that contained genes from only super-group A or B were further analyzed by taking the protein sequences from either wHa (representing the A supergroup) and wNo (representing the B supergroup) and blasting (tblastn) them against the complete genomes from the other supergroups, including the supergroup D genome of Wolbachia wBm. Clusters that did not have a match in any of the other genomes with either an e-vale less than e-5 and 60% of the protein aligned or an e-value less than e-20 and 30% of the protein aligned and identity of minimum 35%, were considered supergroup specific. Additionally, if the matches from tblastn contained stop codon or frame-shifts, the hit was called a pseudogene even if the above criteria were met.
The protein sequences of fic domain proteins with known function (FiDo family) as listed in [102] were downloaded from Genbank. Additionally, the protein sequences for the 10 best non-overlapping blastp hits against the nr database when using the three Wolbachia fic genes were downloaded. Similarly, for cydA, cydB, argR and the arginine ABC transporter genes, the top 50 blastp hits against the nr database were downloaded. For the outer membrane proteins specifically found in the B-supergroup, no additional species were found in the database, but the homologous protein sequences from wVitB were included. In all cases, the protein sequences were aligned with mafft and pruned to remove gap sites that were present in 50% or more of the aligned sequences. The phylogenetic trees were inferred with RAxML using the PROTCATWAG model, and constructing 1 slow best maximum likelihood tree and 1000 rapid bootstrap replicates.
Data deposition
The complete sequences of Wolbachia wNo and wHa genomes are deposited in Genbank under accession numbers CP003883 and CP003884, respectively.
Supporting Information
Zdroje
1. MiraA, Martin-CuadradoAB, D'AuriaG, Rodriguez-ValeraF (2010) The bacterial pan-genome:a new paradigm in microbiology. Int Microbiol 13 : 45–57.
2. GeversD, CohanFM, LawrenceJG, SprattBG, CoenyeT, et al. (2005) Opinion: Re-evaluating prokaryotic species. Nat Rev Microbiol 3 : 733–739.
3. HanageWP, FraserC, SprattBG (2005) Fuzzy species among recombinogenic bacteria. BMC Biol 3 : 6.
4. AchtmanM, WagnerM (2008) Microbial diversity and the genetic nature of microbial species. Nat Rev Microbiol 6 : 431–440.
5. WhitakerRJ (2006) Allopatric origins of microbial species. Philos Trans R Soc Lond B Biol Sci 361 : 1975–1984.
6. HuntDE, DavidLA, GeversD, PreheimSP, AlmEJ, et al. (2008) Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science 320 : 1081–1085.
7. BecraftED, CohanFM, KuhlM, JensenSI, WardDM (2011) Fine-scale distribution patterns of Synechococcus ecological diversity in microbial mats of Mushroom Spring, Yellowstone National Park. Appl Environ Microbiol 77 : 7689–7697.
8. ConnorN, SikorskiJ, RooneyAP, KopacS, KoeppelAF, et al. (2010) Ecology of speciation in the genus Bacillus. Appl Environ Microbiol 76 : 1349–1358.
9. Cadillo-QuirozH, DidelotX, HeldNL, HerreraA, DarlingA, et al. (2012) Patterns of gene flow define species of thermophilic Archaea. PLoS Biol 10: e1001265 doi:10.1371/journal.pbio.1001265.
10. FraserC, HanageWP, SprattBG (2007) Recombination and the nature of bacterial speciation. Science 315 : 476–480.
11. FalushD, TorpdahlM, DidelotX, ConradDF, WilsonDJ, et al. (2006) Mismatch induced speciation in Salmonella: model and data. Philos Trans R Soc Lond B Biol Sci 361 : 2045–2053.
12. HanageWP, SprattBG, TurnerKM, FraserC (2006) Modelling bacterial speciation. Philos Trans R Soc Lond B Biol Sci 361 : 2039–2044.
13. CohanFM (2006) Towards a conceptual and operational union of bacterial systematics, ecology, and evolution. Philos Trans R Soc Lond B Biol Sci 361 : 1985–1996.
14. CohanFM, KoeppelAF (2008) The origins of ecological diversity in prokaryotes. Curr Biol 18: R1024–1034.
15. WiedenbeckJ, CohanFM (2011) Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol Rev 35 : 957–976.
16. LuoC, WalkST, GordonDM, FeldgardenM, TiedjeJM, et al. (2011) Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc Natl Acad Sci U S A 108 : 7200–7205.
17. VosM, DidelotX (2009) A comparison of homologous recombination rates in bacteria and archaea. ISME J 3 : 199–208.
18. VerginKL, TrippHJ, WilhelmLJ, DenverDR, RappeMS, et al. (2007) High intraspecific recombination rate in a native population of Candidatus pelagibacter ubique (SAR11). Environ Microbiol 9 : 2430–2440.
19. ViklundJ, EttemaTJ, AnderssonSG (2012) Independent genome reduction and phylogenetic reclassification of the oceanic SAR11 clade. Mol Biol Evol 29 : 599–615.
20. TamasI, KlassonL, CanbackB, NaslundAK, ErikssonAS, et al. (2002) 50 million years of genomic stasis in endosymbiotic bacteria. Science 296 : 2376–2379.
21. WerrenJH, BaldoL, ClarkME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–751.
22. TaylorMJ, BandiC, HoeraufA (2005) Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol 60 : 245–284.
23. DarbyAC, ArmstrongSD, BahGS, KaurG, HughesMA, et al. (2012) Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res
24. WeeksAR, TurelliM, HarcombeWR, ReynoldsKT, HoffmannAA (2007) From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5: e114 doi:10.1371/journal.pbio.0050114.
25. OsborneSE, LeongYS, O'NeillSL, JohnsonKN (2009) Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5: e1000656 doi:10.1371/journal.ppat.1000656.
26. TeixeiraL, FerreiraA, AshburnerM (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e2 doi:10.1371/journal.pbio.0060002.
27. BrownlieJC, CassBN, RieglerM, WitsenburgJJ, Iturbe-OrmaetxeI, et al. (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368 doi:10.1371/journal.ppat.1000368.
28. SaridakiA, BourtzisK (2010) Wolbachia: more than just a bug in insects genitals. Curr Opin Microbiol 13 : 67–72.
29. AugustinosAA, Santos-GarciaD, DionyssopoulouE, MoreiraM, PapapanagiotouA, et al. (2011) Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PLoS ONE 6: e28695 doi:10.1371/journal.pone.0028695.
30. WerrenJH, ZhangW, GuoLR (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Biol Sci 261 : 55–63.
31. BaldoL, Dunning HotoppJC, JolleyKA, BordensteinSR, BiberSA, et al. (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72 : 7098–7110.
32. LoN, ParaskevopoulosC, BourtzisK, O'NeillSL, WerrenJH, et al. (2007) Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int J Syst Evol Microbiol 57 : 654–657.
33. BaldoL, LoN, WerrenJH (2005) Mosaic nature of the wolbachia surface protein. J Bacteriol 187 : 5406–5418.
34. BordensteinSR, WernegreenJJ (2004) Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol 21 : 1981–1991.
35. Iturbe-OrmaetxeI, WoolfitM, RancesE, DuplouyA, O'NeillSL (2011) A simple protocol to obtain highly pure Wolbachia endosymbiont DNA for genome sequencing. J Microbiol Methods 84 : 134–136.
36. MavinguiP, VanVT, LabeyrieE, RancesE, VavreF, et al. (2005) Efficient procedure for purification of obligate intracellular Wolbachia pipientis and representative amplification of its genome by multiple-displacement amplification. Appl Environ Microbiol 71 : 6910–6917.
37. WuM, SunLV, VamathevanJ, RieglerM, DeboyR, et al. (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2: e69 doi:10.1371/journal.pbio.0020069.
38. KlassonL, WestbergJ, SapountzisP, NaslundK, LutnaesY, et al. (2009) The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci U S A 106 : 5725–5730.
39. KlassonL, WalkerT, SebaihiaM, SandersMJ, QuailMA, et al. (2008) Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol 25 : 1877–1887.
40. FosterJ, GanatraM, KamalI, WareJ, MakarovaK, et al. (2005) The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3: e121 doi:10.1371/journal.pbio.0030121.
41. MavinguiP, Valiente MoroC, Tran-VanV, Wisniewski-DyeF, RaquinV, et al. (2012) Whole-genome sequence of Wolbachia strain wAlbB, an endosymbiont of tiger mosquito vector Aedes albopictus. J Bacteriol 194 : 1840.
42. KentBN, SalichosL, GibbonsJG, RokasA, NewtonIL, et al. (2011) Complete bacteriophage transfer in a bacterial endosymbiont (Wolbachia) determined by targeted genome capture. Genome Biol Evol 3 : 209–218.
43. MercotH, CharlatS (2004) Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica 120 : 51–59.
44. JamesAC, DeanMD, McMahonME, BallardJW (2002) Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity (Edinb) 88 : 182–189.
45. SolignacM (2004) Mitochondrial DNA in the Drosophila melanogaster complex. Genetica 120 : 41–50.
46. BallardJW (2004) Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol Biol Evol 21 : 428–442.
47. DeanMD, BallardJW (2005) High divergence among Drosophila simulans mitochondrial haplogroups arose in midst of long term purifying selection. Mol Phylogenet Evol 36 : 328–337.
48. GarriganD, KinganSB, GenevaAJ, AndolfattoP, ClarkAG, et al. (2012) Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res 22 : 1499–1511.
49. AmarnehB, De Leon-RangelJ, VikSB (2006) Construction of a deletion strain and expression vector for the Escherichia coli NADH:ubiquinone oxidoreductase (Complex I). Biochim Biophys Acta 1757 : 1557–1560.
50. Hovel-MinerG, FaucherSP, CharpentierX, ShumanHA (2010) ArgR-regulated genes are derepressed in the Legionella-containing vacuole. J Bacteriol 192 : 4504–4516.
51. AktoriesK (2011) Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol 9 : 487–498.
52. YarbroughML, LiY, KinchLN, GrishinNV, BallHL, et al. (2009) AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323 : 269–272.
53. WorbyCA, MattooS, KrugerRP, CorbeilLB, KollerA, et al. (2009) The fic domain: regulation of cell signaling by adenylylation. Mol Cell 34 : 93–103.
54. PalaniveluDV, GoepfertA, MeuryM, GuyeP, DehioC, et al. (2011) Fic domain-catalyzed adenylylation: insight provided by the structural analysis of the type IV secretion system effector BepA. Protein Sci 20 : 492–499.
55. HenrichfreiseB, SchieferA, SchneiderT, NzukouE, PoellingerC, et al. (2009) Functional conservation of the lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia: why is lipid II needed? Mol Microbiol 73 : 913–923.
56. Rossello-MoraR, AmannR (2001) The species concept for prokaryotes. FEMS Microbiol Rev 25 : 39–67.
57. KonstantinidisKT, TiedjeJM (2005) Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A 102 : 2567–2572.
58. StackebrandtE, EbersJ (2006) Taxonomic parameters revisited: tarnished gold standards. Microbiology Today 152–155.
59. CohanFM (2001) Bacterial species and speciation. Syst Biol 50 : 513–524.
60. BandiC, AndersonTJ, GenchiC, BlaxterML (1998) Phylogeny of Wolbachia in filarial nematodes. Proc Biol Sci 265 : 2407–2413.
61. CasiraghiM, AndersonTJ, BandiC, BazzocchiC, GenchiC (2001) A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 122 Pt 1 : 93–103.
62. BaldoL, BordensteinS, WernegreenJJ, WerrenJH (2006) Widespread recombination throughout Wolbachia genomes. Mol Biol Evol 23 : 437–449.
63. VenetiZ, ClarkME, KarrTL, SavakisC, BourtzisK (2004) Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl Environ Microbiol 70 : 5366–5372.
64. GottliebY, GhanimM, GueguenG, KontsedalovS, VavreF, et al. (2008) Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J 22 : 2591–2599.
65. RussellJA, Goldman-HuertasB, MoreauCS, BaldoL, StahlhutJK, et al. (2009) Specialization and geographic isolation among Wolbachia symbionts from ants and lycaenid butterflies. Evolution 63 : 624–640.
66. StahlhutJK, DesjardinsCA, ClarkME, BaldoL, RussellJA, et al. (2010) The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol Ecol 19 : 1940–1952.
67. BaldoL, AyoubNA, HayashiCY, RussellJA, StahlhutJK, et al. (2008) Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol 17 : 557–569.
68. JigginsFM, BentleyJK, MajerusME, HurstGD (2002) Recent changes in phenotype and patterns of host specialization in Wolbachia bacteria. Mol Ecol 11 : 1275–1283.
69. DenamurE, LecointreG, DarluP, TenaillonO, AcquavivaC, et al. (2000) Evolutionary implications of the frequent horizontal transfer of mismatch repair genes. Cell 103 : 711–721.
70. CohanFM, AracenaS (2012) Prokaryotic Sex: Eukaryote-like Qualities of Recombination in an Archaean Lineage. Current Biology 22: R601–R602.
71. FariaR, NavarroA (2010) Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol Evol 25 : 660–669.
72. RetchlessAC, LawrenceJG (2007) Temporal fragmentation of speciation in bacteria. Science 317 : 1093–1096.
73. CohanFM, PerryEB (2007) A systematics for discovering the fundamental units of bacterial diversity. Curr Biol 17: R373–386.
74. MillerWJ, EhrmanL, SchneiderD (2010) Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog 6: e1001214 doi:10.1371/journal.ppat.1001214.
75. BordensteinSR, O'HaraFP, WerrenJH (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409 : 707–710.
76. WagnerA, LewisC, BichselM (2007) A survey of bacterial insertion sequences using IScan. Nucleic Acids Research 35 : 5284–5293.
77. CerveauN, LeclercqS, LeroyE, BouchonD, CordauxR (2011) Short - and long-term evolutionary dynamics of bacterial insertion sequences: insights from Wolbachia endosymbionts. Genome Biol Evol 3 : 1175–1186.
78. MercotH, LlorenteB, JacquesM, AtlanA, Montchamp-MoreauC (1995) Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141 : 1015–1023.
79. O'NeillSL, KarrTL (1990) Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348 : 178–180.
80. Chevreux B, Wetter T., Suhai S (1999) Genome Sequence Assembly Using Trace Signals and Additional Sequence Information. Computer Science and Biology: Proceedings of the German Conference on Bioinformatics (GCB) 99. pp. 44–56.
81. DarlingAE, MauB, PernaNT (2010) progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5: e11147 doi:10.1371/journal.pone.0011147.
82. CarverTJ, RutherfordKM, BerrimanM, RajandreamMA, BarrellBG, et al. (2005) ACT: the Artemis Comparison Tool. Bioinformatics 21 : 3422–3423.
83. GordonD, AbajianC, GreenP (1998) Consed: a graphical tool for sequence finishing. Genome Res 8 : 195–202.
84. LohseM, BolgerAM, NagelA, FernieAR, LunnJE, et al. (2012) RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res 40: W622–627.
85. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
86. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
87. SchmiederR, EdwardsR (2011) Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS ONE 6: e17288 doi:10.1371/journal.pone.0017288.
88. StewartAC, OsborneB, ReadTD (2009) DIYA: a bacterial annotation pipeline for any genomics lab. Bioinformatics 25 : 962–963.
89. HyattD, ChenGL, LocascioPF, LandML, LarimerFW, et al. (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11 : 119.
90. PatiA, IvanovaNN, MikhailovaN, OvchinnikovaG, HooperSD, et al. (2010) GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 7 : 455–457.
91. BatemanA, BirneyE, CerrutiL, DurbinR, EtwillerL, et al. (2002) The Pfam protein families database. Nucleic Acids Res 30 : 276–280.
92. RutherfordK, ParkhillJ, CrookJ, HorsnellT, RiceP, et al. (2000) Artemis: sequence visualization and annotation. Bioinformatics 16 : 944–945.
93. GuyL, KultimaJR, AnderssonSG (2010) genoPlotR: comparative gene and genome visualization in R. Bioinformatics 26 : 2334–2335.
94. SiguierP, PerochonJ, LestradeL, MahillonJ, ChandlerM (2006) ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34: D32–36.
95. LiL, StoeckertCJJr, RoosDS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189.
96. KatohK, MisawaK, KumaK, MiyataT (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30 : 3059–3066.
97. StamatakisA (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22 : 2688–2690.
98. YangZ (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 : 1586–1591.
99. BruenTC, PhilippeH, BryantD (2006) A simple and robust statistical test for detecting the presence of recombination. Genetics 172 : 2665–2681.
100. PadidamM, SawyerS, FauquetCM (1999) Possible emergence of new geminiviruses by frequent recombination. Virology 265 : 218–225.
101. DidelotX, FalushD (2007) Inference of bacterial microevolution using multilocus sequence data. Genetics 175 : 1251–1266.
102. MattooS, DurrantE, ChenMJ, XiaoJ, LazarCS, et al. (2011) Comparative analysis of Histophilus somni immunoglobulin-binding protein A (IbpA) with other fic domain-containing enzymes reveals differences in substrate and nucleotide specificities. J Biol Chem 286 : 32834–32842.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



