-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
For the yeast Saccharomyces cerevisiae, nutrient limitation is a key developmental signal causing diploid cells to switch from yeast-form budding to either foraging pseudohyphal (PH) growth or meiosis and sporulation. Prolonged starvation leads to lineage restriction, such that cells exiting meiotic prophase are committed to complete sporulation even if nutrients are restored. Here, we have identified an earlier commitment point in the starvation program. After this point, cells, returned to nutrient-rich medium, entered a form of synchronous PH development that was morphologically and genetically indistinguishable from starvation-induced PH growth. We show that lineage restriction during this time was, in part, dependent on the mRNA methyltransferase activity of Ime4, which played separable roles in meiotic induction and suppression of the PH program. Normal levels of meiotic mRNA methylation required the catalytic domain of Ime4, as well as two meiotic proteins, Mum2 and Slz1, which interacted and co-immunoprecipitated with Ime4. This MIS complex (Mum2, Ime4, and Slz1) functioned in both starvation pathways. Together, our results support the notion that the yeast starvation response is an extended process that progressively restricts cell fate and reveal a broad role of post-transcriptional RNA methylation in these decisions.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002732
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002732Summary
For the yeast Saccharomyces cerevisiae, nutrient limitation is a key developmental signal causing diploid cells to switch from yeast-form budding to either foraging pseudohyphal (PH) growth or meiosis and sporulation. Prolonged starvation leads to lineage restriction, such that cells exiting meiotic prophase are committed to complete sporulation even if nutrients are restored. Here, we have identified an earlier commitment point in the starvation program. After this point, cells, returned to nutrient-rich medium, entered a form of synchronous PH development that was morphologically and genetically indistinguishable from starvation-induced PH growth. We show that lineage restriction during this time was, in part, dependent on the mRNA methyltransferase activity of Ime4, which played separable roles in meiotic induction and suppression of the PH program. Normal levels of meiotic mRNA methylation required the catalytic domain of Ime4, as well as two meiotic proteins, Mum2 and Slz1, which interacted and co-immunoprecipitated with Ime4. This MIS complex (Mum2, Ime4, and Slz1) functioned in both starvation pathways. Together, our results support the notion that the yeast starvation response is an extended process that progressively restricts cell fate and reveal a broad role of post-transcriptional RNA methylation in these decisions.
Introduction
Upon nutrient limitation, diploid cells of the yeast Saccharomyces cerevisiae can undergo two distinct developmental responses. In the presence of a fermentable carbon source (e.g., dextrose or galactose), starvation induces a modified mitotic division that produces elongated daughter progeny termed pseudohyphae (PH) [1]. Reiterated cell division under this PH program forms chains of elongated cells on solid agar, allowing yeast to forage for nutrients [2], [3]. In contrast, cells engage in meiotic development and sporulation if starved for nitrogen in the presence of a non-fermentable carbon source (e.g., acetate or glycerol). Under this program, the diploid genome is duplicated (2C to 4C) and then segregated into four haploid (1C) meiotic products encased in a spore wall. This spore structure protects haploid progeny until favorable nutrient conditions are available.
Recent findings suggest that the two developmental responses to nitrogen deprivation, PH development and meiotic sporulation, are not entirely separate pathways. First, cells that are returned to mitotic growth from meiotic prophase produce elongated buds, reminiscent of PH cells [4]. Second, PH development and sporulation share a set of regulatory factors. Genes that are necessary for meiotic induction, IME1 and IME2 (Inducer of Meiosis 1 and 2, respectively) [5], [6], [7], [8], are also necessary for PH development and the subsequent formation of filaments on solid agar [9]. Furthermore, strains lacking the function of the early meiotic gene IME4 display both meiotic defects and an increased ability to adhere to agar, a phenotype associated with PH development [10], [11].
In yeast, IME4 encodes the sole functional member of a class of RNA-modifying enzymes conserved throughout eukaryotes [12]. These enzymes, identified by homology to the N6-adenosyl methyltransferase in humans, MT-A70, catalyze the post-transcriptional methylation of adenosine (to form N6-methyladenosine—m6A) in RNA. The function of this modification on mRNA is as yet unclear. In vitro work suggests that m6A enhances the translational activity of modified messages [13], whereas in vivo experiments suggest that this modification may play an additional role in message stability and processing [14], [15], [16]. Although this form of RNA methylation is barely detectable in yeast undergoing mitotic growth, m6A accumulates on mRNA molecules during meiosis [17], [18]. Strains encoding catalytically inactive alleles of IME4 do not accumulate m6A and display defects in meiotic entry [18]. Ime4 modifies the transcripts of IME1 and IME2 under these conditions, which may explain these defects upon nutrient starvation [17].
Here, we investigated the role of mRNA methylation in the programmed response to nutrient starvation. Because earlier work had shown that cells remain capable of resuming mitotic growth until the exit from meiotic G2 (reviewed in [19]), we examined the early starvation response in temporal detail. We found that cells were already committed to a starvation response after meiotic cell cycle entry, because they formed PH buds when returned to nutrient-rich conditions. Lineage restriction during this period was partially dependent on the RNA methylation activity of Ime4, which had separable roles in promoting meiosis and inhibiting PH growth. Both functions also required Mum2 and Slz1, two poorly understood meiotic proteins that interacted with Ime4 and, like Ime4, were necessary for maintaining normal levels of meiotic mRNA methylation. These results point to a central role of mRNA methylation in coordinating starvation-induced developmental decisions in yeast.
Results
Return to mitotic growth from pre-meiotic S or G2 results in PH development
We investigated the relationship between meiotic induction and PH growth in SK1, a strain that efficiently undergoes both the PH and meiotic developmental programs [9], [20]. To analyze the early starvation response in a controlled and synchronous manner, we employed a Return-To-Growth (RTG) assay [19], [21], in which diploid cells were incubated for a given amount of time in extremely nutrient-poor liquid medium (SPO) before being returned to rich medium (YPD). Previous studies used this approach to show that cells exiting from meiotic G2/prophase are committed to meiosis and will complete sporulation even in rich medium [22], [23], suggesting that analysis of cell morphology after RTG provides a measure for the developmental potential of nutrient-starved cells. By analyzing morphological changes at hourly intervals in an RTG time course, we confirmed that cells become committed to meiosis as they exit from meiotic G2/prophase and enter the meiotic divisions. This commitment occurred 5 to 7 hours after inoculation in SPO in our strains (Figure 1A, 1B).
Fig. 1. RTG from starvation results in three distinct cell morphologies. 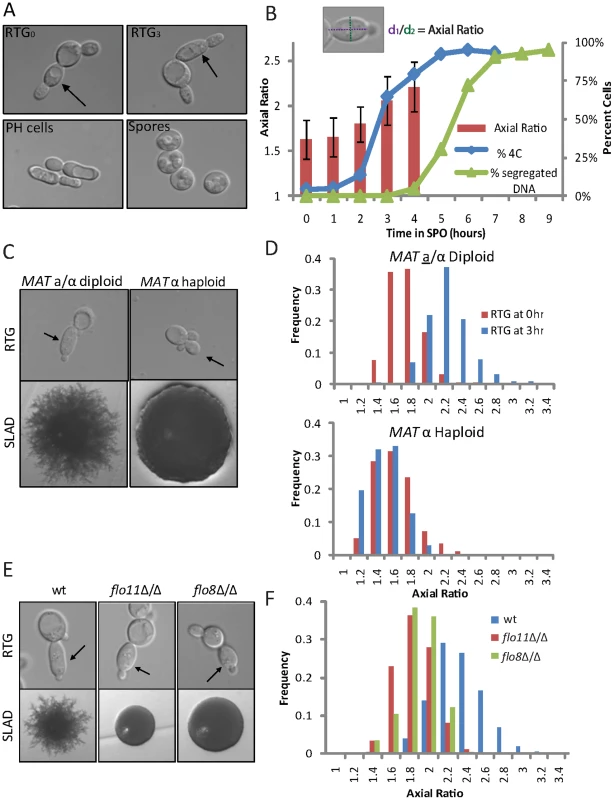
(A) Representative morphologies of daughter cells following RTG from SPO throughout a meiotic time course. Top left panel: RTG at 0 hours, i.e., after growth in BYTA, (RTG0); top right panel: RTG at 3 hours (RTG3), which is comparable to PH cells from solid nitrogen medium (bottom left panel). Bottom right panel: RTG at 6 hours (RTG6). RTG0 and RTG3 cells were photographed after one complete cell cycle, corresponding to 160 minutes and 220 minutes after shift to rich medium, respectively (see Figure S1A). RTG6 cells were photographed three hours after shift to rich medium, at which point the majority of cells (>95%) had formed spores. Arrows indicate primary daughter cells upon RTG0 and RTG3. B) Quantification of axial ratio in wild-type (SAy821) cells upon RTG throughout a meiotic time course (red bars, left axis; n = 200 cells/time point) relative to percent of cells undergoing pre-meiotic DNA synthesis (i.e., 4C cells) (blue diamonds, right axis, quantified by FACS, 3×104 cells/time point) and percent cells undergoing meiotic divisions as assayed by DAPI staining (green triangles, right axis). Schematic at top defines axial ratio. The majority (>80%) of RTG5, RTG6, RTG7, and RTG9 cells either formed spores or remained unbudded three hours after shift to rich medium; axial ratio was therefore not quantified for these time points. C) MAT a/α diploids (SAy821) (top left panel) or MAT α haploids (H224) (top right panel) were returned to growth in rich medium after meiotic induction. Arrows indicate primary buds. Bottom panels represent colony morphologies after growth on SLAD for 6 days. D) Distribution of axial ratios of primary daughter cells upon RTG3 for strains in (C): wild-type diploid (SAy821 top panel), haploid MAT α (H224 bottom panel) (n = 200 cells/strain). RTG0 is represented in red bars, RTG3 in blue bars. E) Wild-type (SAy821), flo11Δ/Δ (SAy789) and flo8Δ/Δ (SAy905) daughter cell morphologies upon RTG3 (top panels). Arrows indicate primary daughter cells. The same strains were photographed after growth on SLAD for 6 days (bottom panels). Axial ratios are quantified in (F) (n = 200 cells/strain). Analysis of cell morphology from the RTG experiment also revealed an earlier decision point, when cells became committed to a starvation response. Cells returned to growth after short starvation (0–1 hours) formed the ovoid buds characteristic of vegetative growth. However, cells that had largely completed pre-meiotic S phase or were in meiotic G2/prophase (3–4 hours after shift to SPO) were unable to do so. Instead, these cells formed elongated daughter cells that resembled PH cells (Figure 1A, 1B). Similar elongated buds are also apparent in images of RTG cells published recently by Dayani and colleagues [4]. Further analysis revealed that formation of these elongated buds paralleled PH development on solid nitrogen starvation medium (SLAD) in all aspects tested. Specifically, RTG3 bud elongation occurred only in diploid cells and was dependent on FLO11, which encodes a cell surface protein necessary for PH development, and its transcriptional regulator, FLO8 (Figure 1C–1F) [24], [25], [26]. Furthermore, buds formed after DNA replication and mother and daughter pairs re-budded synchronously after cytokinesis (Figure S1) [3]. We termed this process RTG-PH development and conclude that, after meiotic entry, yeast cells are restricted to either meiosis or PH development. This finding suggests that nutrient-deprived yeast pass a decision point that commits them to starvation-induced differentiation.
RTG-PH development requires IME1 and IME2, but not pre-meiotic S phase
Because this developmental restriction coincided with pre-meiotic S phase (Figure 1B), we investigated whether pre-meiotic DNA replication was required for RTG-PH development. Pre-meiotic DNA synthesis in meiosis was prevented either chemically, using hydoxyurea, or genetically, by deleting the S phase cyclins CLB5 and CLB6 [27]. Neither condition inhibited PH development during RTG or on solid medium (Figure S2). In fact, when pre-meiotic DNA synthesis was prevented, cells instead replicated the genome upon RTG (Figure S2). These observations indicate that pre-meiotic S phase is not itself necessary for RTG-PH development. Notably, RTG-PH developmental potential followed the “readiness-for-sporulation" period as defined by Simchen as colleagues, which occurred earlier in meiosis (1 hour after induction into SPO) under our sporulation conditions (Figure S2).
To probe the genetic underpinnings of RTG-PH development, we investigated the roles of factors known to regulate both meiosis and PH growth. The transcription factor Ime1 is essential for entry into the meiotic program [5]. Similarly, loss of the meiosis-specific CDK-like kinase Ime2 leads to an extreme delay in meiotic entry [28]. Both factors are also required for PH development on SLAD medium [5], [6], [7]. We found that in the absence of either IME1 or IME2, cells failed to enter RTG-PH development and instead formed ovoid buds upon RTG3 (Figure 2A, 2B), although it should be noted that ime2Δ/Δ cells were able to form elongated RTG-PH buds after extended periods in SPO (Figure S3). These data suggest that, upon severe starvation, Ime1 and Ime2 promote both RTG-PH development and meiosis.
Fig. 2. IME1 and IME2 are necessary for RTG PH development. 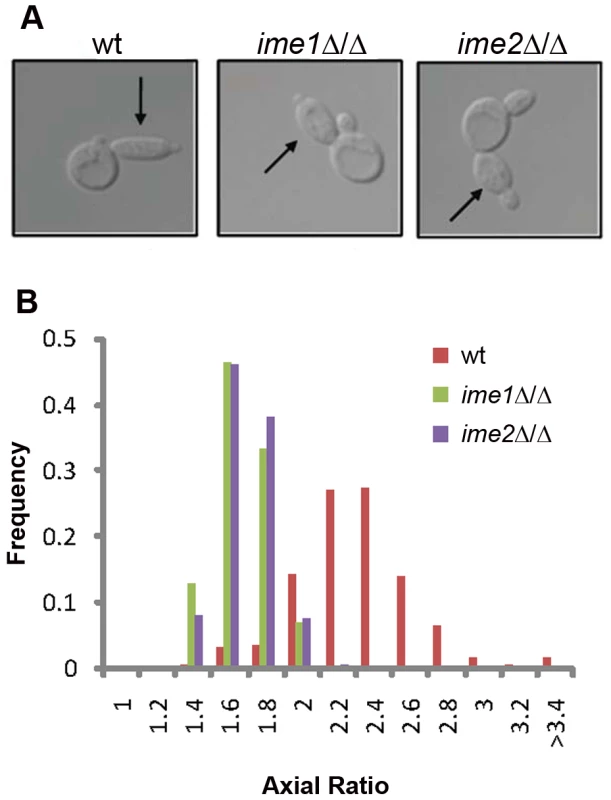
A) Representative bud morphology after RTG3 in wild-type (SAy821), ime1Δ/Δ (SAy834) and ime2Δ/Δ (SAy859). Arrows indicate primary buds. The same strains were photographed after growth on SLAD for 6 days (bottom panels). B) Axial ratio quantification of RTG3 cells for strains in (A) (n = 200 cells/strain). Separable roles for IME4 in controlling meiosis and PH development
Because IME1 and IME2 are both regulated by the RNA methyltransferase Ime4, we also analyzed meiosis and RTG-PH development in ime4Δ/Δ mutants. Previous work demonstrated that IME4 promotes efficient entry into meiosis, although the severity of the meiotic entry defect of ime4Δ/Δ mutants varies considerably between strain backgrounds [17], [18]. In SK1, ime4Δ/Δ mutants exhibited only a minor delay in the initiation of meiotic DNA replication (Figure 3A), but were severely delayed in exit from meiotic G2/prophase as determined by monitoring the induction of the middle-meiotic transcription factor NDT80 and the kinetics of meiotic DNA segregation (Figure 3B, 3C). Additionally, spore formation was substantially reduced in ime4Δ/Δ cells (Figure 3D). However, the viability of those spores that formed was comparable to that of wild-type cells, indicating that, although the meiotic divisions were delayed, chromosome segregation was not affected in these strains (Figure 3E). A strain carrying point mutations in the evolutionarily-conserved catalytic motif IV of Ime4 (ime4-D349A, W351A—referred as ime4-cat) [12], [18] recapitulated these phenotypes, albeit with reduced severity (Figure 3A–3E), indicating that the RNA methyltransferase activity of Ime4 contributes to the efficient progression through meiosis.
Fig. 3. Cells encoding IME4 mutant alleles show developmental defects. 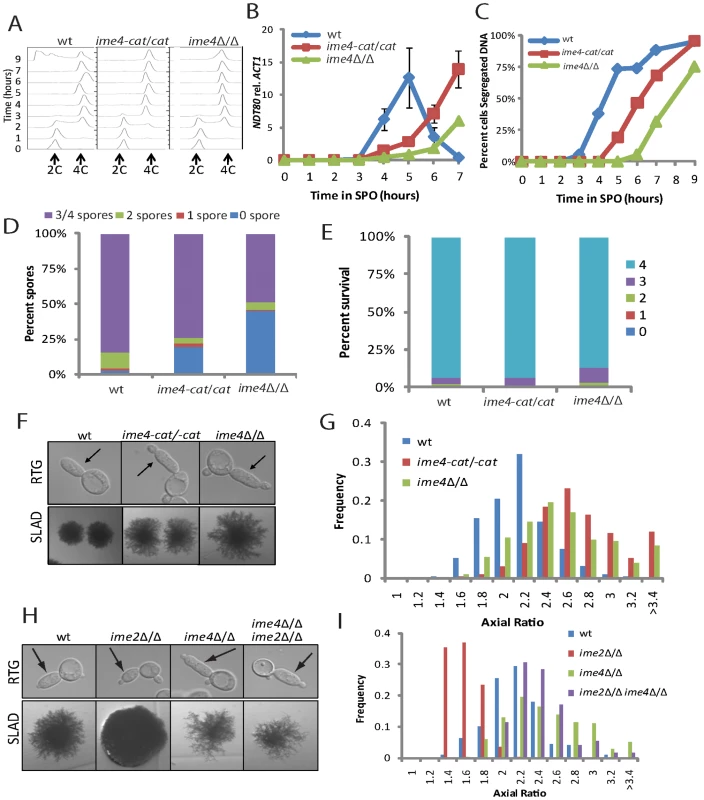
A) FACS analysis of DNA synthesis in wild-type (SAy821), ime4-cat/cat (SAy1086) and ime4Δ/Δ (SAy771) strains throughout a meiotic time course (n = 3×104 cells/strain/time point). DNA content of diploid cells before DNA replication (2C) and after DNA replication (4C) is indicated. B) NDT80 transcript levels in the strains from (A) during a meiotic time course. Transcript levels were determined by RT-PCR and normalized to ACT1 transcript levels. C) Kinetics for meiotic nuclear divisions as assayed by DAPI DNA staining in the strains from (A) (n = 200 cells/strain). D) Number of asci with one, two, three, four, or no spores in the strains from (A) after 24 hours in SPO medium (n = 200 cell/strain). E) Spore viability in the strains from (A); legend indicates number of surviving spores upon dissection (n = 187 tetrads/strain). F) Representative images of cells from strains in (A) after RTG3 (top panels—arrows indicate primary buds) and colonies grown on SLAD for 6 days (bottom panels). G) Quantification of axial ratios of RTG3 cells shown in (F), (n = 200 cells/strain). H) Representative images of cells after RTG3 (top panels—arrows indicate primary buds) and colonies grown on SLAD for 6 days (bottom panels) of wild-type (SAy821), ime2Δ/Δ (SAy859), ime4Δ/Δ (SAy771) or ime2Δ/Δ ime4Δ/Δ (SAy1123) strains. Arrows indicate primary daughter cells. I) Quantification of axial ratios of RTG3 cells shown in (H), (n = 200 cells/strain). Unlike IME1 or IME2, loss of IME4 leads to increased agar adhesion [10], a phenotype associated with increased PH development. Moreover, deletion of IME4 or mutation of its RNA methyltransferase domain resulted in bud hyper-elongation upon RTG3 and the formation of hyper-filamentous colonies on SLAD medium (Figure 3F, 3G). These phenotypes represented genuine forms of PH development because they were dependent on FLO11 and FLO8 (Figure S4). ime4Δ/Δ cells lacking FLO11 or FLO8 failed to form elongated daughter cells upon RTG and were deficient for PH colony development on nitrogen starvation SLAD medium. Thus, IME4 acts as an inhibitor of PH development.
To test whether inhibition of PH development occurred independently of the role Ime4 plays in regulating IME1 and IME2, we analyzed double mutants. As shown in Figure 3H and 3I, ime4Δ/Δ ime2Δ/Δ double mutants were hyper-elongated in both the RTG and SLAD contexts, even when compared to a time point at which ime2Δ/Δ cells are capable of forming RTG-PH cells (Figure S2). The hyper-elongation of ime4Δ/Δ cells even in the absence of IME2 suggests that IME4 regulates PH growth in part independently or downstream of IME2-dependent meiotic initiation and thus indicates separable functions for IME4 in promoting meiosis and inhibiting PH development.
Ime4 protein and mRNA methylation peak in pre-meiotic S and G2
To determine the period during starvation when Ime4 is most abundant, we analyzed Ime4 protein levels by Western blotting in a synchronous time course after inoculation in SPO. As shown in Figure 4A, Ime4 levels increased soon after the shift to starvation conditions and peaked during pre-meiotic S and G2/prophase. Once cells entered into the meiotic divisions, full-length Ime4 disappeared rapidly. We also observed the accumulation of a faster-migrating band that may represent a carboxy-terminal cleavage product of Ime4 (Figure 4A).
Fig. 4. m6A accumulates prior to meiotic divisions. 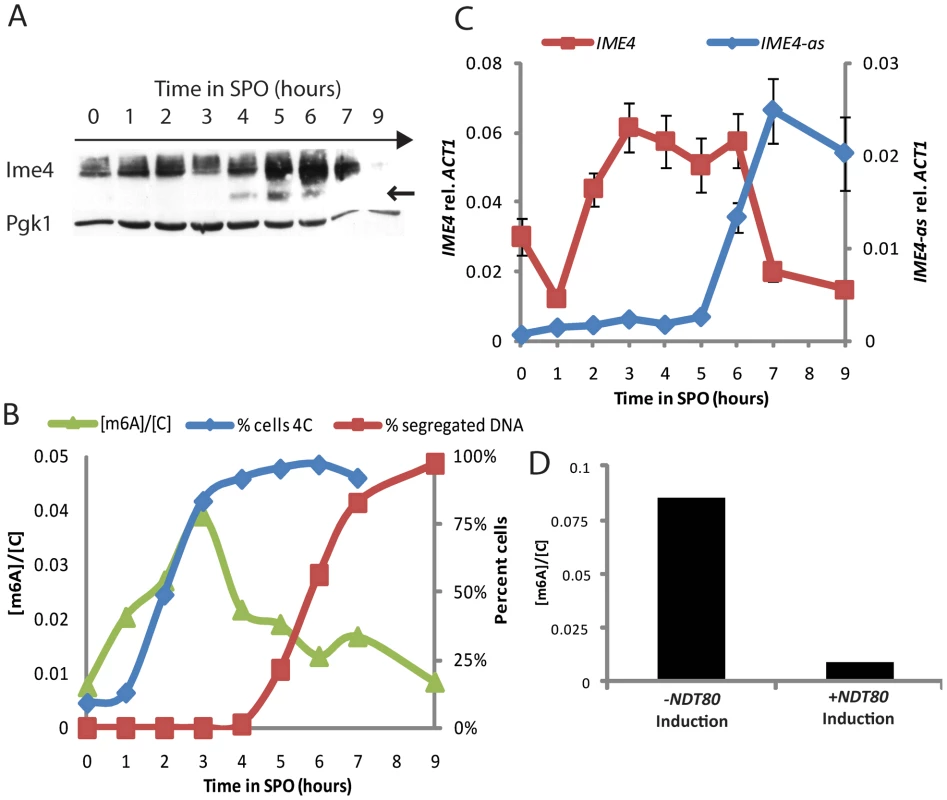
A) Western analysis for 3x-myc-tagged Ime4 protein (SAy914) throughout meiosis; Pgk1 protein serves as loading control. B) Quantification of m6A abundance relative to cytosine throughout meiosis (green triangles, left axis) in a wild-type strain (SAy821). Percent of 4C cells as quantified by FACS (3×104 cells/time point—blue diamonds, right axis) and percent cells undergoing nuclear divisions as assayed by DAPI staining (200 cells/time point—red squares, right axis) are shown as references for meiotic progression. C) Strand-specific qPCR for sense IME4 (red squares, left axis) and antisense transcript (IME4-as) (blue diamonds, right axis) transcript throughout meiosis. D) m6A relative to cytosine quantification in cells carrying an estradiol-inducible NDT80 construct as their sole source of NDT80 (SAy995). Cells were treated with β-estradiol or vehicle 6 hours after meiotic induction and monitored at 9 hours. To investigate whether Ime4 protein levels correlated with activity we followed the kinetics of m6A accumulation on polyadenylated RNA by two-dimensional thin-layer chromatography. This analysis revealed that m6A accumulation tightly matched the levels of full-length Ime4 during the starvation time course. m6A accumulated soon after the shift to SPO, peaked during pre-meiotic S and G2/prophase, and decreased as cells entered into the meiotic divisions (Figure 4B). Entry into the meiotic divisions resulted in a decrease in the IME4 sense transcript and the concomitant accumulation of the IME4 regulatory antisense transcript [10], [29] (Figure 4C), suggesting that IME4 expression may be regulated both at the protein and transcriptional levels to ensure tight repression of IME4.
To determine more precisely the point at which m6A was lost we employed cells encoding an estradiol-inducible allele of NDT80, the transcription factor necessary for exit from meiotic G2/prophase [8], [30], [31]. In the absence of estradiol, these cells arrest at the end of G2/prophase [8], [32]. Under these conditions, cells continued to produce methylated transcripts even after 9 hours of starvation (Figure 4D). By contrast, cells that were induced to express NDT80 displayed reduced levels of m6A at this time point. Thus, NDT80 activation and exit from meiotic G2/prophase is necessary for the down-regulation of RNA methylation activity.
Ime4 interacts with Mum2 and Slz1 to form a methyltransferase complex
To identify regulators of Ime4, we conducted a two-hybrid screen using full-length Ime4 as bait [33]. The two most abundant hits isolated from this screen were MUM2 (MUddled Meiosis 2) and SLZ1 (Sporulation-specific Leucine Zipper 1) both of which have previously been implicated in meiotic progression [34], [35], [36]. Our screen identified 28 independent clones of MUM2 and 8 independent clones of SLZ1. All 28 clones spanned the 3′ region of MUM2 and all 8 clones spanned the 3′ region of SLZ1, suggesting that the respective carboxy-terminal regions of these proteins are sufficient for conferring interaction with Ime4 (Figure S5). In support of the physical interactions revealed by two-hybrid analysis, Ime4 efficiently co-immunoprecipitated with Mum2 and, to a lesser extent, Slz1 (Figure 5A). Like Ime4, Mum2 and Slz1 were induced during starvation in SPO as determined by Western blotting (Figure 5B).
Fig. 5. Mum2 and Slz1 interact with Ime4 and are required for m6A formation. 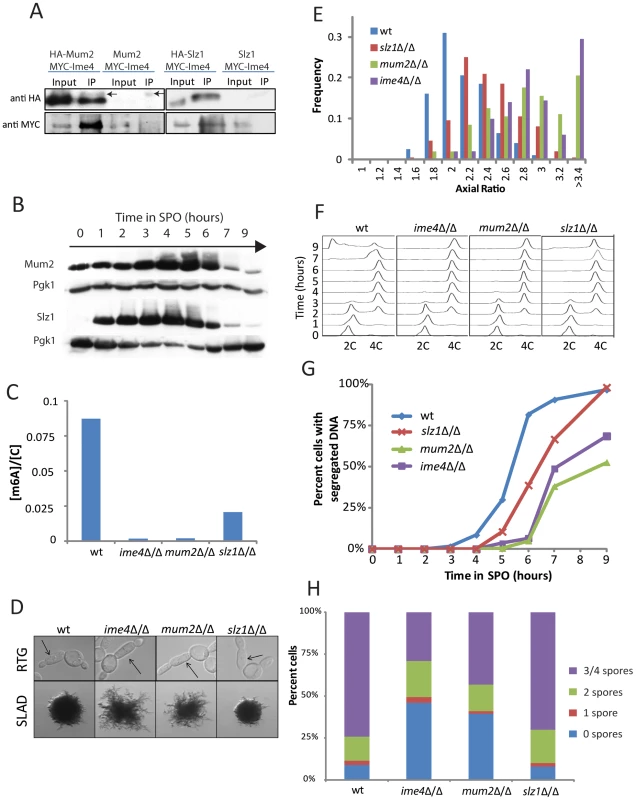
A) Western analysis for co-immunoprecipitation of Mum2 (left panels) and Slz1 (right panels) with Ime4. HA-tagged Mum2 or Slz1 was immunoprecipitated from cellular extracts 3 hours after induction of meiosis and probed for interaction with myc-tagged Ime4 (SAy1232, SAy1253, respectively). A myc-Ime4 (SAy914) strain without HA-tags served as a control. Arrows in the IP lanes indicate IgG bands. B) Western analysis for HA-tagged Mum2 protein (SAy1235) or HA-tagged Slz1 protein (SAy1254) throughout meiosis; Pgk1 protein serves as loading control. C) Quantification of m6A on mRNA three hours after meiotic starvation, when m6A accumulation is maximal in wild-type cells (SAy821). Deleting any one of ime4Δ/Δ (SAy771), mum2Δ/Δ (SAy1196) and slz1Δ/Δ (SAy1206) results in a reduction in m6A levels. D) Wild-type (SAy821), ime4Δ/Δ (SAy771), mum2Δ/Δ (SAy1196) and slz1Δ/Δ (SAy1206) daughter cell morphology upon RTG3 (top panels). Arrows indicate primary buds. The same strains were photographed after growth on SLAD for 6 days (bottom panels). E) Axial ratio quantification of primary daughter cells upon RTG3 for strains in (D). (F) FACS analysis of DNA synthesis in strains from (D) throughout a meiotic time course (n = 3×104 cells/strain/time point). DNA content of diploid cells before DNA replication (2C) and after DNA replication (4C) is indicated. G) Kinetics for meiotic nuclear divisions as assayed by DNA staining by DAPI in the strains from (C) (n = 200 cells/strain/time point). H) Number of asci with one, two, three/four, or no spores in strains from (D) after 24 hours in SPO medium (n = 200 cell/strain). To test whether Mum2 and Slz1 acted together with Ime4 in controlling mRNA methylation during starvation, we quantified m6A levels in meiotic G2/prophase in cells lacking IME4, MUM2 or SLZ1. As previously reported, ime4Δ/Δ cells did not accumulate m6A in meiosis [18]. Importantly, mum2Δ/Δ mutants also failed to accumulate m6A mRNA in pre-meiotic G2 and m6A in slz1Δ/Δ cells accumulated to substantially lower levels than in wild type cells (Figure 5C). Taken together, these findings indicate that Ime4, Mum2, and Slz1 bind to each other and function together to mediate m6A RNA methylation during starvation. We term this protein complex the MIS (Mum2, Ime4, Slz1) complex.
Consistent with their shared functions in mRNA methylation, mum2Δ/Δ and slz1Δ/Δ cells recapitulated the defects of ime4Δ/Δ mutants with respect to meiotic progression and PH development. Like ime4Δ/Δ, deletion of either MUM2 or SLZ1 resulted in hyper-filamentation upon RTG3 and on SLAD plates. The hyper-filamentation phenotype appeared less pronounced for slz1Δ/Δ cells (Figure 5D, 5E). Similarly, like ime4Δ/Δ cells, both mum2Δ/Δ and slz1Δ/Δ mutants replicated DNA under starvation conditions with essentially wild-type kinetics, but were delayed in progressing into the meiotic divisions (Figure 5F, 5G). Again, the delay was less severe for slz1Δ/Δ mutants than for ime4Δ/Δ or mum2Δ/Δ mutants, mirroring the less dramatic effect of SLZ1 deletion on m6A levels. The loss of function mutations in MUM2 and SLZ1 had meiotic defects consistent with the other phenotypes: deletion of MUM2 resulted in a spore formation defect similar to loss of IME4, whereas deletion of SLZ1 did not appreciably affect spore formation (Figure 5H). We conclude that the MIS complex controls m6A RNA methylation and the yeast starvation response.
Expression of the MIS complex is sufficient to confer mRNA methylation
The necessity of IME4, MUM2, and SLZ1 for the methylation of mRNA during meiosis raised the question of whether expression of these genes was sufficient to induce the methylation of mRNA. To test this, one copy of each gene was placed under control of the inducible CUP1 promoter in diploid cells while the other copy remained unaltered. Expression of these genes was induced by the addition of cupric sulfate in rich medium, a condition in which m6A does not normally accumulate on mRNA (Figure 6A). Under these growth conditions, neither Mum2 nor Ime4 were expressed at levels close to those found in meiosis (Figure 6B); Slz1 did not accumulate in cells until induction of meiotic development (Figure 5B). We found that inducing expression of IME4, MUM2, or SLZ1 singly in these conditions was not sufficient to induce m6A accumulation on mRNA (Figure 6A). By contrast, induction of both IME4 and MUM2 resulted in a strong accumulation of m6A. Induction of all three MIS components (MUM2, IME4, and SLZ1) further elevated m6A, albeit only by a small fraction, consistent with the role of SLZ1 as a non-essential component of the MIS complex (Figure 6A). Notably, none of the strains that express m6A in rich conditions exhibited any obvious morphological or growth differences as compared to un-induced control cells (data not shown). These data suggest that the restriction of mRNA methylation to times of starvation is largely a result of the starvation-specific expression of the MIS complex. Taken together, these phenotypes suggest a model in which Mum2 and Ime4 are essential components of an RNA methyltransferase complex, with Slz1 providing an accessory role necessary for optimal function.
Fig. 6. MIS complex expression is sufficient to induce m6A accumulation on mRNA. 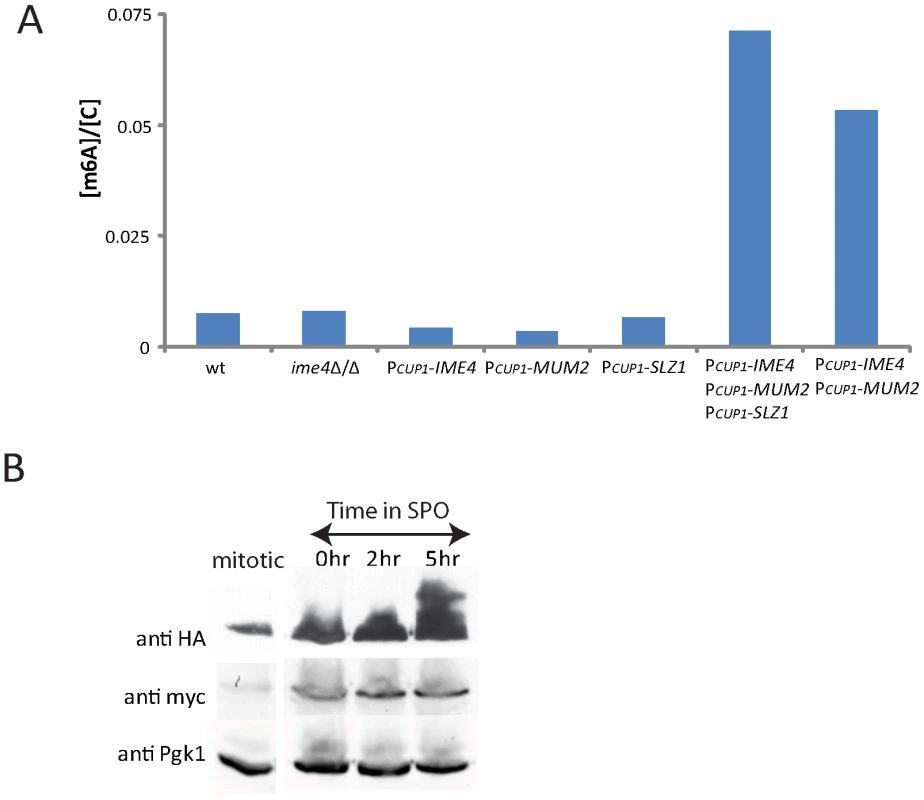
A) m6A accumulation on mRNA was quantified in rich conditions in wild-type (SAy821), ime4Δ/Δ (SAy771), PCUP1-IME4 (SAy1249), PCUP1-MUM2 (SAy1251), PCUP1-SLZ1 (SAy1250), PCUP1-IME4 PCUP1-MUM2 PCUP1-SLZ1 (SAy1248) and PCUP1-IME4 PCUP1-IME4 (SAy1252) after 150 minutes of mitotic growth in the presence of cupric sulfate. B) Western analysis for expression of Ime4 and Mum2. Cells encoding epitope-tagged Ime4 and Mum2 (SAy1232) were collected either from the rich cupric-sulfate media conditions in (A) (first column) or at 0, 2, and 5 hours in meiosis (as labeled), then subjected to Western analysis for either Ime4 (anti-myc) or Mum2 (anti-HA). Pgk1 serves as a loading control. Images across each row come from the same exposure. m6A down-regulation is necessary for RTG-PH development
The MIS complex acts as an inhibitor of PH development, which raises the question of how cells are able to enter RTG-PH development during meiotic G2/prophase when the levels of MIS complex components and m6A are high. To answer this question, we monitored m6A levels upon RTG3. This analysis revealed that m6A levels dropped rapidly after RTG3, approaching the level of vegetative cells by 75 minutes after RTG3, shortly before bud formation was initiated in RTG cultures (Figure 7A). These observations suggest that MIS-dependent inhibition of PH development is alleviated in time to allow elongated bud growth in RTG3 cultures.
Fig. 7. m6A formation inhibits filamentation. 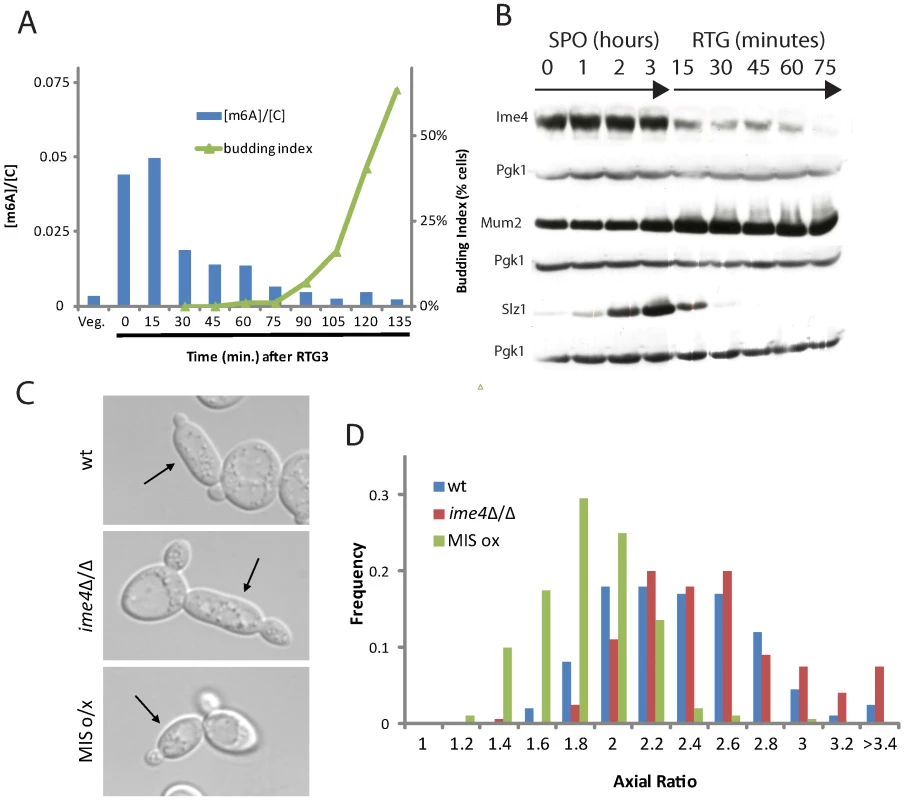
A) Quantification of m6A abundance relative to cytosine (blue bars, left axis) and budding index (green triangles, right axis) upon RTG3. B) Western analysis for 3x-myc-tagged Ime4 protein (SAy914), 3x-HA-tagged Mum2 protein (SAy1235) or 3x-HA-tagged Slz1 protein (SAy1254) throughout RTG3 (i.e., following the shift to YPD after 3 hours in SPO); Pgk1 protein serves as loading control. C) Representative images of cells from wild-type (SAy821), ime4Δ/Δ (SAy771) and a strain induced to express the three components, IME4, MUM2 and SLZ1 (SAy1248) from PCUP1 after RTG3. All strains were treated with cupric sulfate upon RTG3 into YPD. D) Axial ratio quantifications of RTG3 cells from cells in (C) (n = 200 cells/strain). The drop in m6A levels upon RTG3 was accompanied by modification of MIS complex components. Western analysis revealed that Ime4 protein levels gradually decreased and were undetectable by 75 minutes after RTG3 (Figure 7B). Concomitantly, a fraction of Mum2 accumulated in a higher molecular-weight form, a modification that was also apparent when cells entered the meiotic divisions (Figure 5B). Similar to Ime4, Slz1 was quickly degraded and was no longer detectable by 30 minutes after RTG (Figure 7B). These data indicate that before initiating the PH developmental program, cells remove existing methylated RNA and, in parallel, deactivate the mRNA methyltransferase program by modifying or degrading components of the MIS complex before initiating the PH developmental program.
To test whether this down-regulation of the mRNA methyltransferase program is necessary for PH development upon RTG, we ectopically expressed the MIS complex components in RTG3 cells. As shown in Figure 7C and 7D, RTG3 cells expressing the MIS complex failed to form PH cells and instead developed ovoid buds. These data support the conclusion that mRNA methylation is inhibitory to PH development and suggest that the decrease in m6A expression upon RTG is necessary for PH cell development.
Discussion
Control of cell fate during starvation
Our results are consistent with a progressive restriction of diploid cell fate in response to severe nutrient deprivation. During the initial lineage restriction, which coincides with pre-meiotic DNA replication, cells commit to starvation-induced differentiation. In this state, cells remain bipotential, as they can either form spores or engage in PH development, depending on nutrient availability. Only after starvation conditions have persisted long enough to initiate the middle-meiotic program do cells become committed to meiosis and sporulation [22], [23]. The timing of the bipotential stage occurred after readiness-to-sporulate and before meiotic commitment, as defined by Simchen and colleagues [22].
We propose that the bipotential state is geared toward balancing the need to proliferate with survival under nutrient deprivation. Although the formation of protective spores provides better odds of survival for individual cells (or their meiotic offspring) in a harsh environment, sporulation is very time-intensive, which represents a competitive disadvantage if neighboring cells continue to proliferate. By maintaining the option to enter PH development while completing the early stages of sporulation, cells are primed to forage the environment and proliferate, should nutrient deprivation turn out to be transient.
RTG as a tool for studying yeast development
The RTG regimen has proven a powerful tool for probing the yeast starvation response and has provided important insights into a variety of meiotic processes including meiotic commitment and DNA repair [4], [21], [22], [23], [37], [38]. Importantly, the RTG-PH procedure also offers a new avenue for dissecting the kinetics and biochemistry of PH development. Previous assays analyzed PH development in S. cerevisiae on solid medium or liquid suspension [3], [39], [40]. Under these conditions, PH cells form colonies comprised of a mixture of yeast form and PH cells that divide asynchronously with respect to each other and invade into agar. The asynchronous population of multiple cell types prohibited a biochemical analysis of gene expression in PH cells. Other studies have utilized butanol in liquid culture to study PH development [41], [42]. Although growth in butanol results in elongated cells, formation of those cells does not require FLO11 or its regulators. By contrast, the genetic and morphological features of RTG-PH are indistinguishable from PH development on solid agar. The RTG-PH method thus provides an opportunity to study PH development in homogeneous and synchronous cultures.
RNA methylation restricts cell fate
The RTG procedure enabled the discovery that a tightly controlled m6A mRNA methylation program governs cell fate restriction during starvation. m6A is an enigmatic mRNA modification that is highly increased during starvation in diploid yeast [11], [18] and may function to enhance translational activity [13], [16] or to regulate message processing and stability [14], [15]. Previous work had identified the meiotic inducer Ime4 as the enzyme necessary for m6A formation [18]. Our experiments show that Ime4 binds two additional proteins, Mum2 and Slz1, to form a protein complex, which we termed the MIS complex. All three proteins are required for efficient RNA methylation, indicating that Mum2 and Slz1 promote the methyltransferase activity of Ime4. There are no obvious protein domains that would indicate a specific function of either Mum2 or Slz1 other than the Slz1 leucine-zipper motif, a domain type that often functions in protein dimerization. However, based on the severity of the phenotypes associated with loss of these two proteins, we predict that Mum2 forms an integral activator of the MIS complex, possibly by activating Ime4 catalytic activity or by targeting Ime4 to mRNA substrates, whereas Slz1 likely only has accessory functions.
Our results suggest that accumulation of m6A mRNA is largely governed by regulating the abundance of MIS complex components. All three proteins are specifically expressed during pre-meiotic S and G2/prophase and are sufficient to induce m6A methylation in rich medium. Moreover, concomitant with the loss of m6A mRNA during the meiotic divisions or during return to growth, expression of MIS complex components drops and the proteins are rapidly modified or degraded. However, given the rapid drop in m6A mRNA observed after return to growth in particular, m6A mRNA may also become actively demethylated. In human cells, the protein FTO was recently shown to act as an mRNA demethylase [43], but homologues of FTO have thus far not been identified in yeast.
MIS-complex-dependent RNA methyltransferase activity governs multiple developmental processes during nutrient starvation. All three MIS complex components are required for efficient sporulation and may act at multiple steps during this process [18], [34], [36]. Ime4 and Mum2 had been shown to promote entry into the meiotic program and premeiotic DNA replication, respectively [18], [35], [36], although our results as well as previous observation suggest that the importance of these early roles varies with strain background [18]. Our findings indicate that RNA methylation also functions later in the meiotic program to promote the expression of the middle-meiosis transcription factor NDT80 and thus meiotic commitment. Ime4 may activate NDT80 expression either directly, by methylating the NDT80 transcript, or indirectly, through the previously characterized modification of IME2 transcript [17], as Ime2 activity is necessary for expression of Ndt80 protein [8]. Interestingly, NDT80 is, in turn, required to down-regulate of mRNA methylation during the meiotic divisions revealing a negative feedback loop in switching off mRNA methylation.
MIS-complex activity suppresses PH development in a manner that is at least partially separable from its function in promoting meiosis and that involves down-regulation of the key PH factor, FLO11. Inhibition of FLO11 expression may occur through methylation (and hence, activation) of SFL1, a well-characterized inhibitor of FLO11 and the PH program [44]. Identification of specific substrates for mRNA methylation will be necessary to determine how Ime4 regulates these developmental decisions.
Conservation of the MIS complex
Sequence and functional comparisons suggest that Ime4 and Mum2 are conserved throughout eukaryotic evolution. Previous biochemical studies in human cells isolated two independent components, with a possible third additional factor, as necessary for catalyzing mRNA methylation [45], [46]. Of these three components, only MT-A70, the human homolog of IME4, has been cloned [46]. This subunit is conserved throughout virtually all eukaryotes, including Arabidopsis thaliana and Drosophila melanogaster [12], [14], [46], [47]. Mum2 is similarly conserved; the Arabidopsis MUM2 homolog, AtFIP37, was previously found to interact with the Arabidopsis IME4 homolog, MTA, although its role in RNA methylation was not determined [14]. MUM2 also bears homology to Drosophila melanogaster Fl(2)d and human WTAP-1, the latter of which therefore is a strong candidate to be the cognate human mRNA methyltransferase component. Alignment of MUM2, Fl(2)d, AtFIP37 and WTAP-1 protein sequences revealed that MUM2 is the most diverged from the other homologues, although all four genes have conserved residues near the C-terminus, a region we suggest is necessary for interaction with Ime4 (Figure S6). Intriguingly, MUM2 and AtFIP37 homologues in metazoans have been shown to play a role in mRNA splicing [48], [49], [50], [51]. Although the yeast genome is generally intron-poor, introns are strongly over-represented in early meiotic transcripts [52], [53], raising the possibility that mRNA methylation may influence the splicing of these transcripts to regulate the starvation-induced development in yeast. The homologues of IME4 and MUM2 are highly expressed in the reproductive organs as well as other developing tissues in higher eukaryotes [14], [47], [50], [54]. Thus, the control of developmental decisions by mRNA methylation may be widely conserved.
Methods
Strains and growth conditions
Strain genotypes are shown in Table S1. To induce synchronous meiotic entry, cells were pre-selected on 1% yeast extract, 2% peptone, 3% glycerol, 2% agar for 24 hours at 30°C, grown for 24 hr in 1% yeast extract, 2% peptone, 4% dextrose at 30°C, diluted in BYTA (1% yeast extract, 2% tryptone, 1% potassium acetate, 50 mM potassium phthalate) to OD600 = 0.2 and grown for another 16 hr at 30°C, 300 rpm. Cells were then washed once with water and re-suspended in SPO (0.3% potassium acetate) at OD600 = 2.0 and incubated at 30°C at 190 rpm. For RTG experiments, cells were removed from SPO at the indicated times, collected by centrifugation, re-suspended in pre-warmed 1% yeast extract, 2% peptone, 2% dextrose and incubated at 30°C at 190 rpm. Pseudohyphal growth was assayed after 6 days of growth on synthetic low-ammonium dextrose (SLAD) medium described in [2] containing 0.5% glucose. The CUP1 promoter was induced with 100 µM CuSO4 in rich media. RTG cells were photographed and measured after one complete cell cycle, when the daughter cell initiated budding, in order to gauge maximal length of daughter buds. Two hybrid analysis was performed as in [33]. Full-length IME4 was expressed as a fusion to the Gal4 DNA-binding domain and was transformed into a bait strain that was mated with the two-hybrid library. Plasmids from colonies that showed growth on auxotrophic media and expressed LacZ were further purified and sequenced.
Cell morphology quantification
Cells were photographed under 40× magnification and primary bud morphology was quantified using ImageJ (Rasband W., National Institutes of Health, http://rsb.info.nih.gov/ij/index.html).
Quantitative PCR
Total RNA was obtained by standard phenol∶chloroform∶isoamyl alcohol extraction. cDNA was generated using random hexamers or strand-specific primers and the Qiagen QuantiTect Reverse Transcription Kit. Transcript abundance was quantified using reagents from Applied Biosystems and the ABI 7500 real-time PCR system. Primer sequences are provided in Table S2.
Co-immunoprecipitation
50 ml of meiotic culture was harvested 3 hours after meiotic induction in the presence of protease inhibitors (Complete protease inhibitors, Roche). Cells were washed once with 1M Tris-HCl, pH 7.5 and snap-frozen. Frozen pellets were resuspended in lysis buffer (150 mM NaCl, 50 mM Tris pH 7.5, 1% NP40, 10 mM PMSF, Complete mini protease inhibitors (Roche) at 2× concentration) and glass-bead homogenized three times for 5 minutes at 4°C. Debris was pelleted by centrifugation for 10 minutes, and supernatant was incubated with HA-conjugated agarose beads (Pierce) with head-over-tail rotation for three hours. Beads were washed 5times in lysis buffer and boiled in reducing loading buffer, followed by the standard protocol for Western analysis as described below.
Other techniques
Flow cytometric analysis of DNA content, 4′,6-diamidino-2-phenylindole (DAPI) staining for DNA segregation analysis and cell staging by spindle morphology using tubulin indirect immunofluorescence were performed as described in [55]. Western analyses were performed as described in [56], with anti-c-myc (9E10, Covance) or anti-HA (HA.11, Covance) at a concentration of 1∶1000. TLC analysis was carried out as in [14]. RNA was extracted from cells using a standard hot acid phenol protocol; poly(A) RNA was purified from total RNA with the Dynabeads mRNA purification system (Invitrogen) and analyzed on cellulose plates (20 cm×20 cm) from EMD.
Supporting Information
Zdroje
1. CullenPJSpragueGFJr 2012 The regulation of filamentous growth in yeast. Genetics 190 23 49
2. GimenoCJLjungdahlPOStylesCAFinkGR 1992 Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68 1077 1090
3. KronSJStylesCAFinkGR 1994 Symmetric cell division in pseudohyphae of the yeast Saccharomyces cerevisiae. Mol Biol Cell 5 1003 1022
4. DayaniYSimchenGLichtenM 2011 Meiotic recombination intermediates are resolved with minimal crossover formation during return-to-growth, an analogue of the mitotic cell cycle. PLoS Genet 7 e1002083 doi:10.1371/journal.pgen.1002083
5. KassirYGranotDSimchenG 1988 IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52 853 862
6. MitchellAPDriscollSESmithHE 1990 Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol Cell Biol 10 2104 2110
7. FoianiMNadjar-BogerECaponeRSageeSHashimshoniT 1996 A meiosis-specific protein kinase, Ime2, is required for the correct timing of DNA replication and for spore formation in yeast meiosis. Mol Gen Genet 253 278 288
8. BenjaminKRZhangCShokatKMHerskowitzI 2003 Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev 17 1524 1539
9. StrudwickNBrownMParmarVMSchroderM 2010 Ime1 and Ime2 are required for pseudohyphal growth of Saccharomyces cerevisiae on nonfermentable carbon sources. Mol Cell Biol 30 5514 5530
10. HongayCFGrisafiPLGalitskiTFinkGR 2006 Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell 127 735 745
11. ShahJCClancyMJ 1992 IME4, a gene that mediates MAT and nutritional control of meiosis in Saccharomyces cerevisiae. Mol Cell Biol 12 1078 1086
12. BujnickiJMFederMRadlinskaMBlumenthalRM 2002 Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m(6)A methyltransferase. J Mol Evol 55 431 444
13. TuckMTWiehlPEPanT 1999 Inhibition of 6-methyladenine formation decreases the translation efficiency of dihydrofolate reductase transcripts. Int J Biochem Cell Biol 31 837 851
14. ZhongSLiHBodiZButtonJVespaL 2008 MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20 1278 1288
15. CarrollSMNarayanPRottmanFM 1990 N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol Cell Biol 10 4456 4465
16. HeilmanKLLeachRATuckMT 1996 Internal 6-methyladenine residues increase the in vitro translation efficiency of dihydrofolate reductase messenger RNA. Int J Biochem Cell Biol 28 823 829
17. BodiZButtonJDGriersonDFrayRG 2010 Yeast targets for mRNA methylation. Nucleic Acids Res 38 5327 5335
18. ClancyMJShambaughMETimpteCSBokarJA 2002 Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res 30 4509 4518
19. SimchenG 2009 Commitment to meiosis: what determines the mode of division in budding yeast? Bioessays 31 169 177
20. MagwenePMKayikciOGranekJAReiningaJMSchollZ 2011 Outcrossing, mitotic recombination, and life-history trade-offs shape genome evolution in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 108 1987 1992
21. HonigbergSMEspositoRE 1994 Reversal of cell determination in yeast meiosis: postcommitment arrest allows return to mitotic growth. Proc Natl Acad Sci U S A 91 6559 6563
22. SimchenGPinonRSaltsY 1972 Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res 75 207 218
23. EspositoREEspositoMS 1974 Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci U S A 71 3172 3176
24. KobayashiOSudaHOhtaniTSoneH 1996 Molecular cloning and analysis of the dominant flocculation gene FLO8 from Saccharomyces cerevisiae. Mol Gen Genet 251 707 715
25. LoWSDranginisAM 1998 The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol Biol Cell 9 161 171
26. RuppSSummersELoHJMadhaniHFinkG 1999 MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J 18 1257 1269
27. StuartDWittenbergC 1998 CLB5 and CLB6 are required for premeiotic DNA replication and activation of the meiotic S/M checkpoint. Genes Dev 12 2698 2710
28. Guttmann-RavivNBoger-NadjarEEdriIKassirY 2001 Cdc28 and Ime2 possess redundant functions in promoting entry into premeiotic DNA replication in Saccharomyces cerevisiae. Genetics 159 1547 1558
29. GelfandBMeadJBruningAApostolopoulosNTadigotlaV 2011 Regulated antisense transcription controls expression of cell-type-specific genes in yeast. Mol Cell Biol 31 1701 1709
30. ChuSHerskowitzI 1998 Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell 1 685 696
31. XuLAjimuraMPadmoreRKleinCKlecknerN 1995 NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol 15 6572 6581
32. CarlileTMAmonA 2008 Meiosis I is established through division-specific translational control of a cyclin. Cell 133 280 291
33. JamesPHalladayJCraigEA 1996 Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144 1425 1436
34. BrizaPBogengruberEThurARutzlerMMunsterkotterM 2002 Systematic analysis of sporulation phenotypes in 624 non-lethal homozygous deletion strains of Saccharomyces cerevisiae. Yeast 19 403 422
35. DavisLBarberaMMcDonnellAMcIntyreKSternglanzR 2001 The Saccharomyces cerevisiae MUM2 gene interacts with the DNA replication machinery and is required for meiotic levels of double strand breaks. Genetics 157 1179 1189
36. EngebrechtJMasseSDavisLRoseKKesselT 1998 Yeast meiotic mutants proficient for the induction of ectopic recombination. Genetics 148 581 598
37. ZenvirthDLoidlJKleinSArbelAShemeshR 1997 Switching yeast from meiosis to mitosis: double-strand break repair, recombination and synaptonemal complex. Genes Cells 2 487 498
38. FriedlanderGJoseph-StraussDCarmiMZenvirthDSimchenG 2006 Modulation of the transcription regulatory program in yeast cells committed to sporulation. Genome Biol 7 R20
39. PrinzSAvila-CampilloIAldridgeCSrinivasanADimitrovK 2004 Control of yeast filamentous-form growth by modules in an integrated molecular network. Genome Res 14 380 390
40. GimenoCJFinkGR 1994 Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol 14 2100 2112
41. LorenzMCCutlerNSHeitmanJ 2000 Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol Biol Cell 11 183 199
42. ZeitlingerJSimonIHarbisonCTHannettNMVolkertTL 2003 Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell 113 395 404
43. JiaGFuYZhaoXDaiQZhengG 2011 N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7 885 7
44. RobertsonLSFinkGR 1998 The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci U S A 95 13783 13787
45. BokarJARath-ShambaughMELudwiczakRNarayanPRottmanF 1994 Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269 17697 17704
46. BokarJAShambaughMEPolayesDMateraAGRottmanFM 1997 Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3 1233 1247
47. HongayCFOrr-WeaverTL 2011 Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc Natl Acad Sci U S A 108 14855 14860
48. GranadinoBCampuzanoSSanchezL 1990 The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J 9 2597 2602
49. PenalvaLORuizMFOrtegaAGranadinoBVicenteL 2000 The Drosophila fl(2)d gene, required for female-specific splicing of Sxl and tra pre-mRNAs, encodes a novel nuclear protein with a HQ-rich domain. Genetics 155 129 139
50. VespaLVachonGBergerFPerazzaDFaureJD 2004 The immunophilin-interacting protein AtFIP37 from Arabidopsis is essential for plant development and is involved in trichome endoreduplication. Plant Physiol 134 1283 1292
51. SmallTWPickeringJG 2009 Nuclear degradation of Wilms tumor 1-associating protein and survivin splice variant switching underlie IGF-1-mediated survival. J Biol Chem 284 24684 24695
52. MundingEMIgelAHShiueLDorighiKMTrevinoLR 2010 Integration of a splicing regulatory network within the meiotic gene expression program of Saccharomyces cerevisiae. Genes Dev 24 2693 2704
53. JuneauKPalmCMirandaMDavisRW 2007 High-density yeast-tiling array reveals previously undiscovered introns and extensive regulation of meiotic splicing. Proc Natl Acad Sci U S A 104 1522 1527
54. OrtegaA 2005 Localization of the Drosophila protein FL(2)D in somatic cells and female gonads. Cell Tissue Res 320 361 367
55. VisintinRHwangESAmonA 1999 Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398 818 823
56. FalkJEChanACHoffmannEHochwagenA 2010 A Mec1 - and PP4-dependent checkpoint couples centromere pairing to meiotic recombination. Dev Cell 19 599 611
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



