-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFound: The Elusive ANTAR Transcription Antiterminator
article has not abstract
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002773
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002773Summary
article has not abstract
Regulated transcription termination provides an efficient and responsive means to control gene expression [1]. Intrinsic terminators, which consist of an RNA stem-loop followed by a poly-U tract, catalyze termination by disrupting the RNA polymerase elongation complex [2]. In antitermination, an antiterminator stem-loop is mutually exclusive with the terminator stem-loop [1], [3]. In different contexts, formation of the antiterminator stem-loop is governed by a translating ribosome [1], a ligand-binding riboswitch [4], [5], or a signal-responsive RNA–binding protein [3]. This latter mechanism is illuminated by Ramesh et al. [6], who studied antitermination by a broadly distributed class of signal-responsive RNA-binding proteins containing the ANTAR domain.
AmiR and NasR Define the ANTAR Regulators
Studies conducted in the 1990s characterized two related antitermination proteins: AmiR, which regulates aliphatic amide catabolism in Pseudomonas aeruginosa (amiECBRS operon) [7], [8], and NasR, which regulates nitrate assimilation in Klebsiella oxytoca (nasFEDCBA operon) [9], [10]. RNA binding occurs through the carboxyl-terminal ANTAR domain (AmiR and NasR transcription antitermination regulator) [11]. AmiR and NasR RNA binding responds to signal input mediated by the amide-binding AmiC protein [12], [13] and the nitrate-binding NIT domain [14], [15], respectively.
AmiR and NasR target the transcribed leader RNA upstream of the amiE and nasF operons, respectively [12], [14]. Both leaders encode two obvious stem-loop secondary structures: the distal intrinsic terminator (including a poly-U tract) [2], and P1, a proximal structure essential for antitermination (Figure 1A). Further analysis of the nasF operon leader identified three subelements essential for NasR binding and antitermination: the P1 stem; residues A1 and G4 in the P1 loop; and part of the linker region that connects the P1 and terminator stem-loop structures [16]. However, the RNA secondary structure(s) formed during antitermination remained a mystery.
Fig. 1. RNA-binding proteins stabilize antiterminator structures. 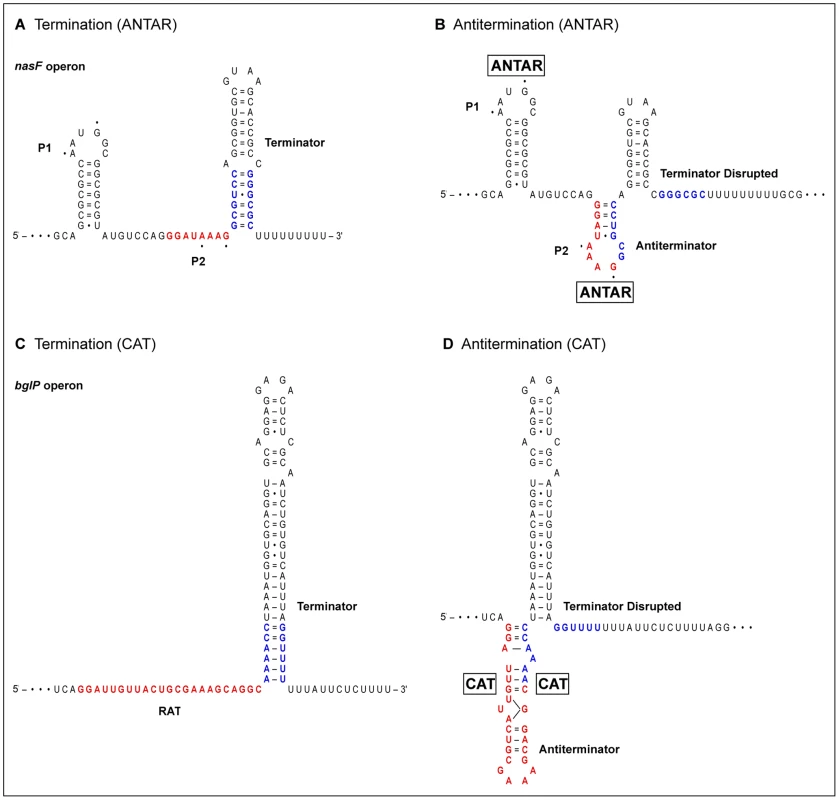
Alternative stem-loop structures in transcribed leader regions from representative operons are shown. Terminator stem regions that participate in alternative structures are in blue, whereas antiterminator regions are in red. (A) NasR-responsive nasF operon leader in termination conformation. Dots indicate the critical residues A1 and G4 in the P1 and P2 loops. (B) Each ANTAR monomer is hypothesized to bind one of the loops, P1 and P2. Stabilizing the P2 antiterminator structure permits transcription readthrough [6] by shortening the terminator stem and separating it from the poly-U tract [2]. (C) LicT-responsive bglP operon leader in termination conformation. RAT is the ribonucleic antiterminator. (D) Each CAT (co-antiterminator) monomer binds to the antiterminator stem. Unusual base-pairing within the antiterminator stem is depicted schematically [25]. Stablilizing the antiterminator structure permits transcription readthrough [22] by shortening the terminator stem and separating it from the poly-U tract. Although different ANTAR proteins use a variety of signal input domains, at least half contain a two-component signal transduction receiver domain [6], [11]. Receiver function is governed through phosphorylation and dephosphorylation by a cognate signal-responsive sensor kinase [17]. For example, the ethanolamine-responsive EutW sensor kinase controls the EutV response regulator, which mediates antitermination control of ethanolamine catabolism (eut operons) in Enterococcus faecalis [18]–[20]. Other ANTAR proteins, including AmiR, contain a non-phosphorylated pseudo-receiver domain [13], [17].
New Progress with EutV
Ramesh et al. [6] now report a substantial advance toward understanding ANTAR-mediated transcription antitermination. The eut gene cluster contains intrinsic terminators upstream of four genes (eutP, eutG, eutS, and eutA), facilitating the identification of shared sequence features. Each of these leaders contains a terminator along with two upstream stem-loop structures, P1 and P2 [6] (Figure 1B). The eutG leader P2 structure was noted independently by Baker and Perego [20]. Similar structures are present in the amiE and nasF leaders. Strikingly, both the P1 and P2 loops contain the critical residues A1 and G4, identified as essential for NasR-mediated antitermination [16]. Moreover, the distal stem of P2 overlaps part of the terminator proximal stem [6], [20], and thereby forms an antiterminator structure (Figure 1B). This suggests a general model for ANTAR-mediated transcription termination, in which the dimeric ANTAR protein binds simultaneously to structures P1 and P2, stabilizing the P2 antiterminator to enable transcription readthrough. (Indeed, restriction sites introduced into the nasF operon leader [16] destroy the P2 structure, explaining the resultant uninducible phenotype.)
To test this model, Ramesh et al. first constructed a variety of site-specific alterations in the eutP leader, and confirmed the subelements important for EutV binding and antitermination: the P1 and P2 structures; their relative spacing; and the P1 and P2 loop residues A1 and G4 [6]. Ramesh et al. then evaluated the EutV oligomeric states, finding that the wild-type protein required phosphorylation to dimerize. Truncated proteins lacking the receiver domain were isolated as dimers, although the most efficient RNA binding was observed with truncated protein that retains the central coiled-coil. This suggests that the coiled-coil enforces proper spatial orientation between the two ANTAR monomers. Presumably, each ANTAR monomer binds one of the two structures, P1 or P2.
Finally, Ramesh et al. conducted bioinformatic analyses to identify ANTAR target leaders in bacterial genomes. Examples were found in a broad range of species from the Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. Some of the leaders are adjacent to genes of unknown or uncertain function, whereas many others are adjacent to genes involved in inorganic nitrogen acquisition (ammonium, nitrate, or dinitrogen) or assimilation (glutamine synthetase and associated regulators). Many ethanolamine utilization clusters also were identified.
Together, these results provide concrete hypotheses for the structure and function of both ANTAR domain proteins and their leader RNA targets. Moreover, the association of many ANTAR regulators with aspects of nitrogen metabolism provokes intriguing questions about their physiology and evolution.
Comparison to CAT Domain Antitermination
Proteins of the BglG-LicT-SacY family, present in both gram-negative and gram-positive lineages, control genes for sugar catabolism. These homodimeric proteins contain PRD domains (PTS regulation domain) that place them under control of the phosphotransferase system for sugar uptake [21]. The carboxyl-terminal CAT (co-antiterminator) domain interacts with the RAT (ribonucleic antiterminator) element in the transcribed leader region. The CAT-stabilized antiterminator is mutually exclusive with the terminator structure [22] (Figure 1C and 1D).
The CAT domain is a homodimer of four-stranded β-sheets [23]. Phosphorylation results in massive structural changes [24] that bring the two CAT monomers into proper alignment to interact with a distorted minor grove in the antiterminator hairpin stem [25]. This stem is interrupted by two non-identical asymmetric internal loops, which are recognized similarly by the protein dimer. Sequence analysis suggests that this RNA-binding mode likely is conserved in homologous systems [25].
How Does ANTAR Bind RNA?
X-ray structures are known for three ANTAR domain proteins, each with a different amino-terminal input domain: AmiR, pseudo-receiver [13]; Mycobacterium tuberculosis Rv1626, receiver [26]; and NasR, NIT [27]. Each protein contains an α-helical coiled-coil connecting the two domains, but each structure reveals a different spatial orientation of the ANTAR monomers and a different configuration of the coiled-coil. It is likely that signal is propagated through the coiled-coil to bring the ANTAR monomers into proper alignment for RNA binding [6].
The ANTAR domain adopts a helix-turn-helix conformation, but how it binds RNA is unknown. RNA-binding helix-turn-helix proteins include σ70 region 4.2, which binds the −35 region of promoter DNA as well as 6S RNA [28], and Ffh, which binds 4.5 S RNA in the signal recognition particle [29]. Both of these RNA targets form bulged stem-loop structures, so the binding sites have characteristics of dsRNA.
Future progress in understanding ANTAR-mediated antitermination requires knowledge of ANTAR domain conformation and interaction with its RNA target; conformational shifts mediated through the coiled-coil; and kinetics of RNA binding in relation to transcription-driven formation of the P1, P2, and terminator structures.
Zdroje
1. YanofskyC 2007 RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 13 1141 1154
2. PetersJMVangeloffADLandickR 2011 Bacterial transcription terminators: the RNA 3′-end chronicles. J Mol Biol 412 793 813
3. HenkinTMYanofskyC 2002 Regulation by transcription attenuation in bacteria: how RNA provides instructions for transcription termination/antitermination decisions. Bio Essays 24 700 707
4. RothABreakerRR 2009 The structural and functional diversity of metabolite-binding riboswitches. Annu Rev Biochem 78 305 334
5. Gutiérrez-PreciadoAHenkinTMGrundyFJYanofskyCMerinoE 2009 Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol Mol Biol Rev 73 36 61
6. RameshADebRoySGoodsonJRFoxKAFazH 2012 The mechanism for RNA recognition by ANTAR regulators of gene expression. PLoS Genet 8 e1002666 doi:10.1371/journal.pgen.1002666
7. DrewRLoweN 1989 Positive control of Pseudomonas aeruginosa amidase synthesis is mediated by a transcription anti-termination mechanism. J Gen Microbiol 135 817 823
8. WilsonSAWachiraSJMNormanRAPearlLHDrewRE 1996 Transcription antitermination regulation of the Pseudomonas aeruginosa amidase operon. EMBO J 15 5907 5916
9. GoldmanBSLinJTStewartV 1994 Identification and structure of the nasR gene encoding a nitrate - and nitrite-responsive positive regulator of nasFEDCBA (nitrate assimilation) operon expression in Klebsiella pneumoniae M5al. J Bacteriol 176 5077 5085
10. LinJTStewartV 1996 Nitrate and nitrite-mediated transcription antitermination control of nasF (nitrate assimilation) operon expression in Klebsiella pneumoniae M5al. J Mol Biol 256 423 435
11. ShuCJZhulinIB 2002 ANTAR: an RNA-binding domain in transcription antitermination regulatory proteins. Trends Biochem Sci 27 3 5
12. NormanRAPohCLPearlLHO'HaraBPDrewRE 2000 Steric hindrance regulation of the Pseudomonas aeruginosa amidase operon. J Biol Chem 275 30660 30667
13. O'HaraBPNormanRAWanPTRoeSMBarrettTE 1999 Crystal structure and induction mechanism of AmiC-AmiR: a ligand-regulated transcription antitermination complex. EMBO J 18 5175 5186
14. ChaiWStewartV 1998 NasR, a novel RNA-binding protein, mediates nitrate-responsive transcription antitermination of the Klebsiella oxytoca M5al nasF operon leader in vitro. J Mol Biol 283 339 351
15. ShuCJUlrichLEZhulinIB 2003 The NIT domain: a predicted nitrate-responsive module in bacterial sensory receptors. Trends Biochem Sci 28 121 124
16. ChaiWStewartV 1999 RNA sequence requirements for NasR-mediated, nitrate-responsive transcription antitermination of the Klebsiella oxytoca M5al nasF operon leader. J Mol Biol 292 203 216
17. BourretRB 2010 Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol 13 142 149
18. Del PapaMFPeregoM 2008 Ethanolamine activates a sensor histidine kinase regulating its utilization in Enterococcus faecalis. J Bacteriol 190 7147 7156
19. FoxKARameshAStearnsJEBourgogneAReyes-JaraA 2009 Multiple posttranscriptional regulatory mechanisms partner to control ethanolamine utilization in Enterococcus faecalis. Proc Natl Acad Sci USA 106 4435 4440
20. BakerKAPeregoM 2011 Transcription antitermination by a phosphorylated response regulator and cobalamin-dependent termination at a B12 riboswitch contribute to ethanolamine utilization in Enterococcus faecalis. J Bacteriol 193 2575 2586
21. van TilbeurghHDeclerckN 2001 Structural insights into the regulation of bacterial signalling proteins containing PRDs. Curr Opin Struct Biol 11 685 693
22. HoumanFDiaz-TorresMRWrightA 1990 Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell 62 1153 1163
23. van TilbeurghHManivalXAymerichSLhosteJMDumasC 1997 Crystal structure of a new RNA-binding domain from the antiterminator protein SacY of Bacillus subtilis. EMBO J 16 5030 5036
24. GrailleMZhouCZReceveur-BréchotVCollinetBDeclerckN 2005 Activation of the LicT transcriptional antiterminator involves a domain swing/lock mechanism provoking massive structural changes. J Biol Chem 280 14780 14789
25. YangYDeclerckNManivalXAymerichSKochoyanM 2002 Solution structure of the LicT-RNA antitermination complex: CAT clamping RAT. EMBO J 21 1987 1997
26. MorthJPFengVPerryLJSvergunDITuckerPA 2004 The crystal and solution structure of a putative transcriptional antiterminator from Mycobacterium tuberculosis. Structure 12 1595 1605
27. BoudesMLazarNDurandDGaidenkoTAStewartV 2012 Structure of the NasR transcription antiterminator reveals a one-component system with a NIT nitrate-receptor coupled to an ANTAR RNA-binding effector. Mol Microbiol In press
28. KlockoADWassarmanKM 2009 6S RNA binding to Esigma(70) requires a positively charged surface of sigma(70) region 4.2. Mol Microbiol 73 152 164
29. BateyRTSagarMBDoudnaJA 2001 Structural and energetic analysis of RNA recognition by a universally conserved protein from the signal recognition particle. J Mol Biol 307 229 246
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



