-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDiverse CRISPRs Evolving in Human Microbiomes
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) loci, together with cas (CRISPR–associated) genes, form the CRISPR/Cas adaptive immune system, a primary defense strategy that eubacteria and archaea mobilize against foreign nucleic acids, including phages and conjugative plasmids. Short spacer sequences separated by the repeats are derived from foreign DNA and direct interference to future infections. The availability of hundreds of shotgun metagenomic datasets from the Human Microbiome Project (HMP) enables us to explore the distribution and diversity of known CRISPRs in human-associated microbial communities and to discover new CRISPRs. We propose a targeted assembly strategy to reconstruct CRISPR arrays, which whole-metagenome assemblies fail to identify. For each known CRISPR type (identified from reference genomes), we use its direct repeat consensus sequence to recruit reads from each HMP dataset and then assemble the recruited reads into CRISPR loci; the unique spacer sequences can then be extracted for analysis. We also identified novel CRISPRs or new CRISPR variants in contigs from whole-metagenome assemblies and used targeted assembly to more comprehensively identify these CRISPRs across samples. We observed that the distributions of CRISPRs (including 64 known and 86 novel ones) are largely body-site specific. We provide detailed analysis of several CRISPR loci, including novel CRISPRs. For example, known streptococcal CRISPRs were identified in most oral microbiomes, totaling ∼8,000 unique spacers: samples resampled from the same individual and oral site shared the most spacers; different oral sites from the same individual shared significantly fewer, while different individuals had almost no common spacers, indicating the impact of subtle niche differences on the evolution of CRISPR defenses. We further demonstrate potential applications of CRISPRs to the tracing of rare species and the virus exposure of individuals. This work indicates the importance of effective identification and characterization of CRISPR loci to the study of the dynamic ecology of microbiomes.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002441
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002441Summary
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) loci, together with cas (CRISPR–associated) genes, form the CRISPR/Cas adaptive immune system, a primary defense strategy that eubacteria and archaea mobilize against foreign nucleic acids, including phages and conjugative plasmids. Short spacer sequences separated by the repeats are derived from foreign DNA and direct interference to future infections. The availability of hundreds of shotgun metagenomic datasets from the Human Microbiome Project (HMP) enables us to explore the distribution and diversity of known CRISPRs in human-associated microbial communities and to discover new CRISPRs. We propose a targeted assembly strategy to reconstruct CRISPR arrays, which whole-metagenome assemblies fail to identify. For each known CRISPR type (identified from reference genomes), we use its direct repeat consensus sequence to recruit reads from each HMP dataset and then assemble the recruited reads into CRISPR loci; the unique spacer sequences can then be extracted for analysis. We also identified novel CRISPRs or new CRISPR variants in contigs from whole-metagenome assemblies and used targeted assembly to more comprehensively identify these CRISPRs across samples. We observed that the distributions of CRISPRs (including 64 known and 86 novel ones) are largely body-site specific. We provide detailed analysis of several CRISPR loci, including novel CRISPRs. For example, known streptococcal CRISPRs were identified in most oral microbiomes, totaling ∼8,000 unique spacers: samples resampled from the same individual and oral site shared the most spacers; different oral sites from the same individual shared significantly fewer, while different individuals had almost no common spacers, indicating the impact of subtle niche differences on the evolution of CRISPR defenses. We further demonstrate potential applications of CRISPRs to the tracing of rare species and the virus exposure of individuals. This work indicates the importance of effective identification and characterization of CRISPR loci to the study of the dynamic ecology of microbiomes.
Introduction
CRISPRs, together with cas genes (CRISPR-associated genes), provide acquired resistance against viruses and conjugative plasmids [1], [2], and are found in most archaeal (∼90%) and bacterial (∼40%) genomes [3], [4], [5]. CRISPR arrays consist of 24–47 bp direct repeats, separated by unique sequences (spacers) that are acquired from viral or plasmid genomes [6]. Even though some CRISPR arrays may contain hundreds of spacers (an extreme case is the CRISPR array in the Haliangium ochraceum DSM 14365 genome, which has 588 copies of its repeat), they tend to be much smaller, generally with dozens of spacers. The repeat sequences of some CRISPRs are partially palindromic, and have stable, highly conserved RNA secondary structures, while others lack detectable structures [7].
CRISPR arrays are usually adjacent to cas genes, which encode a large and heterogeneous family of proteins with functional domains typical of nucleases, helicases, polymerases, and polynucleotide-binding proteins. CRISPR/Cas systems commonly use repeat and spacer-derived short guide CRISPR RNAs (crRNAs) to silence foreign nucleic acids in a sequence-specific manner [8], . CRISPR/Cas defense pathways involve several steps, including integration of viral or plasmid DNA-derived spacers into the CRISPR array, expression of short crRNAs consisting of unique single repeat-spacer units, and interference with invading foreign genomes at both the DNA and RNA levels, by mechanisms that are not yet fully understood [8], [10]. The diversity of cas genes suggests that multiple pathways have arisen to use the basic information contained in the repeat-spacer units in diverse defense mechanisms. The CRISPR components are evolutionarily closely linked and potentially evolve simultaneously as an intact locus—sequence analysis reveals that the direct repeats in CRISPR locus and the linked cas genes co-evolve under analogous evolutionary pressure [11].
Previous studies have shown that CRISPR loci are very diverse and abundant in the genomes of bacteria and archaea. In addition, it has been shown that CRISPR loci with the same repeat sequence and cas gene set can be found in multiple bacterial species, implying horizontal gene transfer (HGT) [12]. Moreover, CRISPR loci can change their spacer content rapidly, as a result of interactions between viruses (or plasmids) and bacteria: several metagenomic studies investigating host-virus population dynamics have shown that CRISPR loci evolve in response to viral predation and that CRISPR spacer content and sequential order provide both historically and geographically insights [13], [14], [15], [16]—essentially, epidemiology.
As a reflection of the infectious dynamics of microbial communities, the study of CRISPRs is an essential compliment to the study of the human microbiome, encompassing both disease ecology and ecological immunology [17]. Infectious disease works to maintain both species diversity [18], [19] and genotypic diversity [20] within a species, as has recently been shown for marine microbiomes [21], [22]. As such, infectious agents may be at least partially responsible for the amazing species diversity and turnover found throughout the human microbiome [23]. The ability of CRISPR loci to prevent plasmid spread is medically relevant, in that the exchange of conjugative elements is perhaps the dominant mechanism by which antibiotic resistance genes (notably multi-drug resistance) move within a biome, and by which pathogens acquire resistance [24]; CRISPR activities could be expected to retard this exchange (e.g. [25]).
CRISPR composition in human microbial communities, the relative rate of CRISPR locus change, or how CRISPR loci vary between different body sites and between the microbiota of different individuals are less studied, as compared to other environments. A recent analysis of streptococcal CRISPRs from human saliva, in which CRISPR spacers and repeats were amplified from salivary DNA, using the conserved streptococcal CRISPR repeat sequence for priming, revealed substantial spacer sequence diversity within and between subjects over time [26], which is imagined to reflect the dynamics of phage and other infectious agents in the human mouth [2].
The availability of more than 700 shotgun metagenomic datasets from the Human Microbiome Project (HMP) enables us to explore the distribution and diversity of many more CRISPRs, and to discover new ones, across different body sites, in a systematic manner. We developed a targeted assembly strategy (see Figure 1) to better identify CRISPRs in shotgun metagenomic sequences, as whole-metagenomic assembly failed to reconstruct many CRISPRs that otherwise could be identified. All of the programs available to date [27], [28], [29], [30] are designed to find CRISPRS from assembled contigs that are sufficiently long to contain at least partial CRISPR loci; however, it is very difficult to assemble metagenome reads into contigs containing CRISPR loci, because of their repeated structures. We thus needed to collect sequencing reads associated with CRISPRs and assemble them specifically. For known CRISPRs (identified in reference genomes), we identified consensus sequences of CRISPR repeats, collected the reads containing these sequences, and assembled these reads into CRISPR contigs. We also identified CRISPRs from the whole-metagenome assemblies, and for the novel CRISPRs or new CRISPR variants (that are not seen in the reference genomes), applied the same assembly strategy to achieve a more comprehensive identification of the novel CRISPRs across the samples. This approach allows us to study the evolution of CRISPRs in human microbiomes.
Fig. 1. A diagram of the targeted assembly approach for CRISPR. 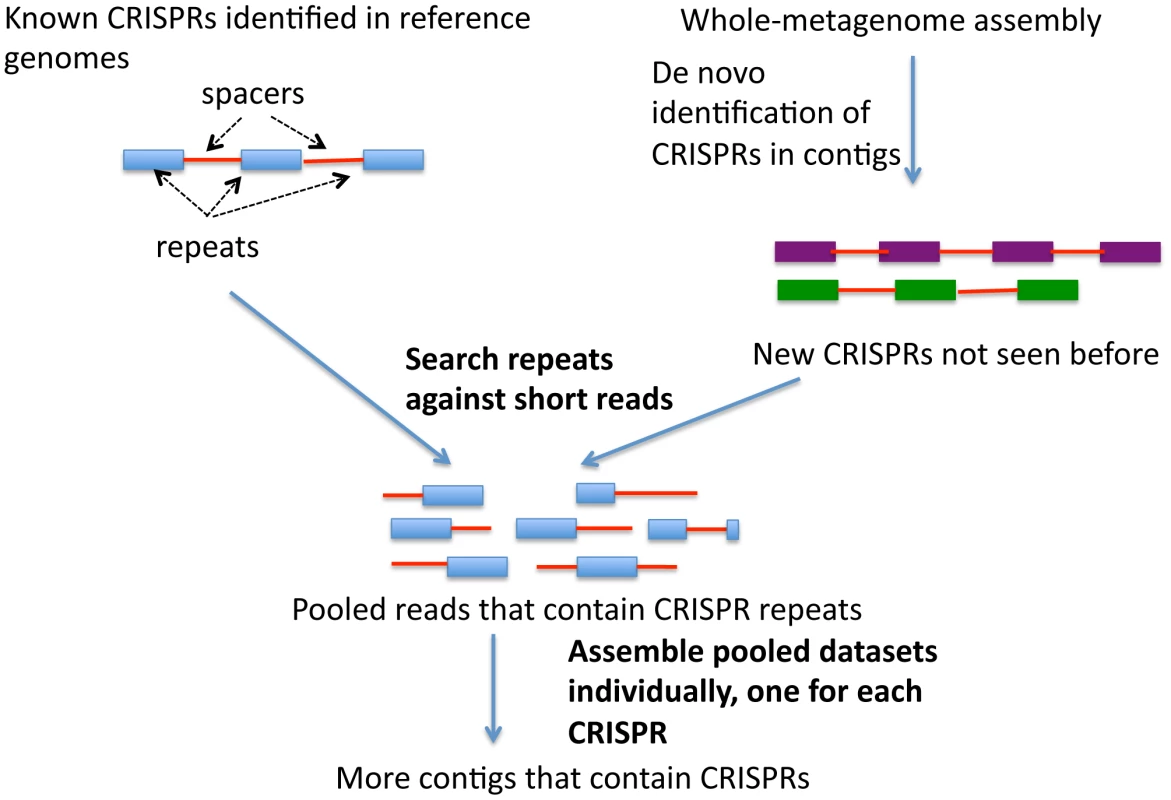
Results
We identified and selected 64 known CRISPRs—including the streptococcal CRISPR—from complete and draft bacterial genomes and 86 novel CRISPRs from the 751 HMP whole-metagenome assemblies, using metaCRT and CRISPRAlign (see Methods). For each selected CRISPR, we then applied the targeted assembly approach (for each CRISPR, first pool the reads that contain the repeat, and then assemble the pooled reads only; see Methods for a validation of the targeted assembly approach using simulated datasets) to achieve a more comprehensive characterization of the CRISPR loci in the human microbiome shotgun datasets. Below we provide detailed analysis of the targeted assembly approach, and the resulting CRISPR loci (listed in Table 1 and Tables S1 and S2).
Tab. 1. List of selected CRISPRs discussed in the paper. 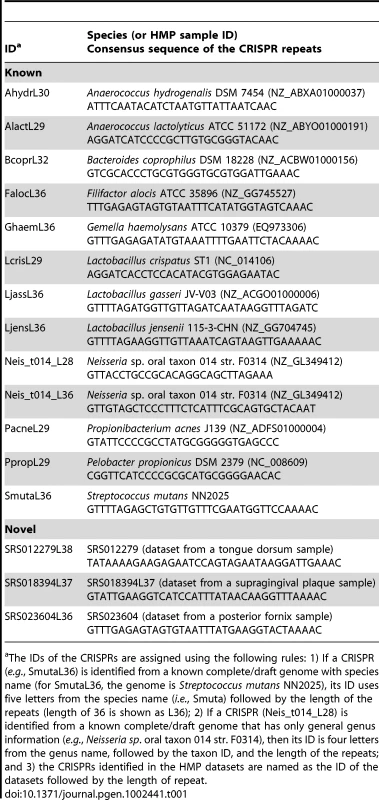
The IDs of the CRISPRs are assigned using the following rules: 1) If a CRISPR (e.g., SmutaL36) is identified from a known complete/draft genome with species name (for SmutaL36, the genome is Streptococcus mutans NN2025), its ID uses five letters from the species name (i.e., Smuta) followed by the length of the repeats (length of 36 is shown as L36); 2) If a CRISPR (Neis_t014_L28) is identified from a known complete/draft genome that has only general genus information (e.g., Neisseria sp. oral taxon 014 str. F0314), then its ID is four letters from the genus name, followed by the taxon ID, and the length of the repeats; and 3) the CRISPRs identified in the HMP datasets are named as the ID of the datasets followed by the length of repeat. Targeted assembly improves the characterization of CRISPRs
We first asked if our targeted assembly strategy helps to identify CRISPR elements from metagenomic datasets, and found that it greatly improved detection (see comparison in Table 2). The improvements are twofold. First, the targeted assembly approach identifies known CRISPRs in more human microbiome datasets, as compared to the annotation of CRISPRs using whole-metagenome assemblies. Second, targeted assembly resulted in longer CRISPR arrays, from which we can extract many more diverse spacers for analyzing the evolution of the CRISPRs and other purposes. Here we use three examples to demonstrate the performance of the targeted assembly.
Tab. 2. Comparison of CRISPR identification using whole-metagenome assembly and targeted assembly. 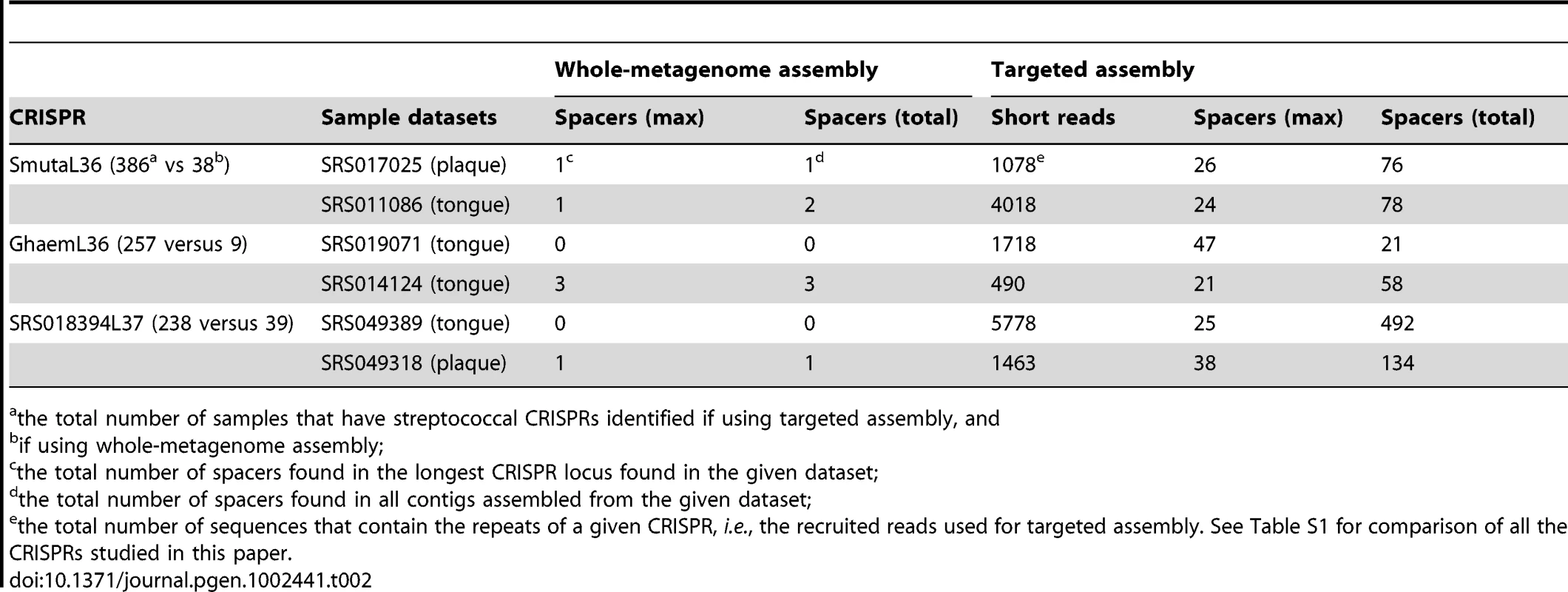
the total number of samples that have streptococcal CRISPRs identified if using targeted assembly, and The first example is the streptococcal CRISPR SmutaL36 (see Table 1), a CRISPR that is conserved in streptococcal species such as Streptococcus mutans [26]. This CRISPR was observed only in a limited number of samples (38 out of 751 datasets) when using contigs from whole-metagenome assembly. But our targeted CRISPR assembly identifies instances of CRISPR SmutaL36 in ∼10 times more (386) datasets. Consistent with the distribution of streptococcus across body sites, most of the 386 datasets are from oral samples: 120 of 128 supragingival plaques (94%), 128 of 135 tongue dorsum samples (95%), and 97 of 121 buccal mucosa samples (80%) (see Table 3). CRISPR SmutaL36 was only found in a small proportion of samples from other body locations, where streptococcus rarely exists (e.g., 4 of 148 stool samples, and none of the posterior fornix datasets). Table 2 shows the details of targeted assembly of this CRISPR in two datasets.
Tab. 3. Distribution of selected CRISPRs across body sites. 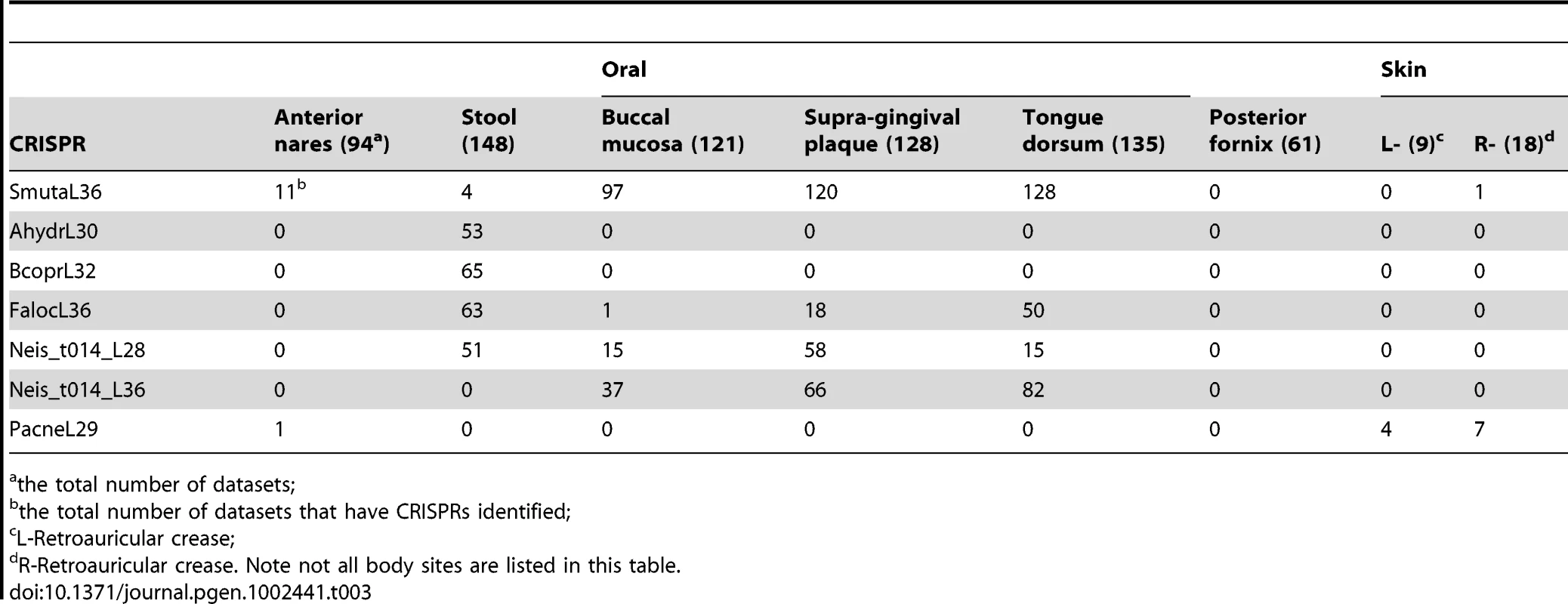
the total number of datasets; The other two examples are GhaemL36 and SRS018394L37 (see details in Table 2). CRISPR GhaemL36 was initially identified from the genome of Gemella haemolysans ATCC 10379 using metaCRT. Targeted assembly further identified instances of this CRISPR in 258 oral-associated samples. The longest contig—of 3121 bases—was assembled from the SRS019071 dataset. This CRISPR array has even more repeats (48; i.e., 47 spacers) than the CRISPR array in the Gemella haemolysans reference genome, which has 29 repeats. CRISPR SRS018394L37 (currently not yet associated with a host genome) was initially identified from the whole-metagenome assembly of SRS018394, but targeted assembly reveals the presence of this CRISPR in 238 oral-associated microbiomes. The contig that was assembled in SRS049389 is the longest one (2014 bps), containing 25 spacers.
In most cases we have tested, targeted assembly dramatically improves the identification of CRISPRs in the HMP datasets: for 142 CRISPRs (out of 150), targeted assembly resulted in CRISPR identification in more HMP samples as compared to using whole-metagenome assemblies, and for 36 CRISPRs, targeted assembly identified instances of the corresponding CRISPR in at least 10 times more datasets (see Table S1). It suggests that specifically designed assembly approaches, such as the targeted assembly approach for CRISPR assembly presented here, are important for the characterization of functionally important repetitive elements that otherwise may be poorly assembled in a whole-metagenome assembly (which tends to be confused by repeats), and such a comprehensive identification is important for deriving an unbiased distribution of these functional elements across different body sites among individuals.
Novel CRISPRs are found in human microbiome samples
In order to identify novel CRISPR loci, with which to seed further targeted assemblies, we set out to find loci based simply on the structural patterns of CRISPR loci, using the program metaCRT, which we modified from CRT (see Methods). As a result, we found and selected 86 different types of novel CRISPR repeats in metagenomic samples, which could not be found in reference genomes, for further targeted assembly (see Methods). Table 1 lists selected examples of novel CRISPRs that we identified in HMP datasets (see Table 1 for naming conventions). A full list of CRISPRs (including the number of CRISPR contigs assembled in each metagenomic dataset) is available as Table S1. In this section, we highlight two examples of novel CRISPRs.
CRISPR SRS012279L38 was identified from a whole-metagenome assembly contig of dataset SRS012279 (derived from a tongue dorsum sample; see Figure 2A). The identified CRISPR contig has 6 copies of a 38-bp repeat (the last copy is incomplete; see Table 1 for the consensus sequence of the repeats). De novo gene prediction by FragGeneScan [31] reveals 10 protein-coding genes in this contig, among which 9 share similarities with cas genes from other genomes, including Leptotrichia buccalis DSM 1135 (NC_013192, an anaerobic, gram-negative species, which is a constituent of normal oral flora [32]) and Fusobacterium mortiferum ATCC 9817, by BLASTP search using the predicted protein sequences as queries (see Figure 2B). (By contrast, BLASTX search of this contig against nr database only achieved annotations for 7 genes). In addition, similarity searches revealed a single identical copy of this repeat in the genome of Leptotrichia buccalis DSM 1135 (from 1166729 to 1166764; de novo CRISPR prediction shows that this genome has several CRISPR arrays, including an array that has 84 copies of a 29-bp repeat, but none of the CRISPRs have the same repeat sequence as SRS012279L38). These two lines of evidence (similar cas genes, and an identical region in the genome) suggest that the SRS012279L38 CRISPR we found in the human microbiomes could have evolved from Leptotrichia buccalis or a related species.
Fig. 2. A potentially novel CRISPR array identified in a contig (9848 bases) from sample SRS012279. 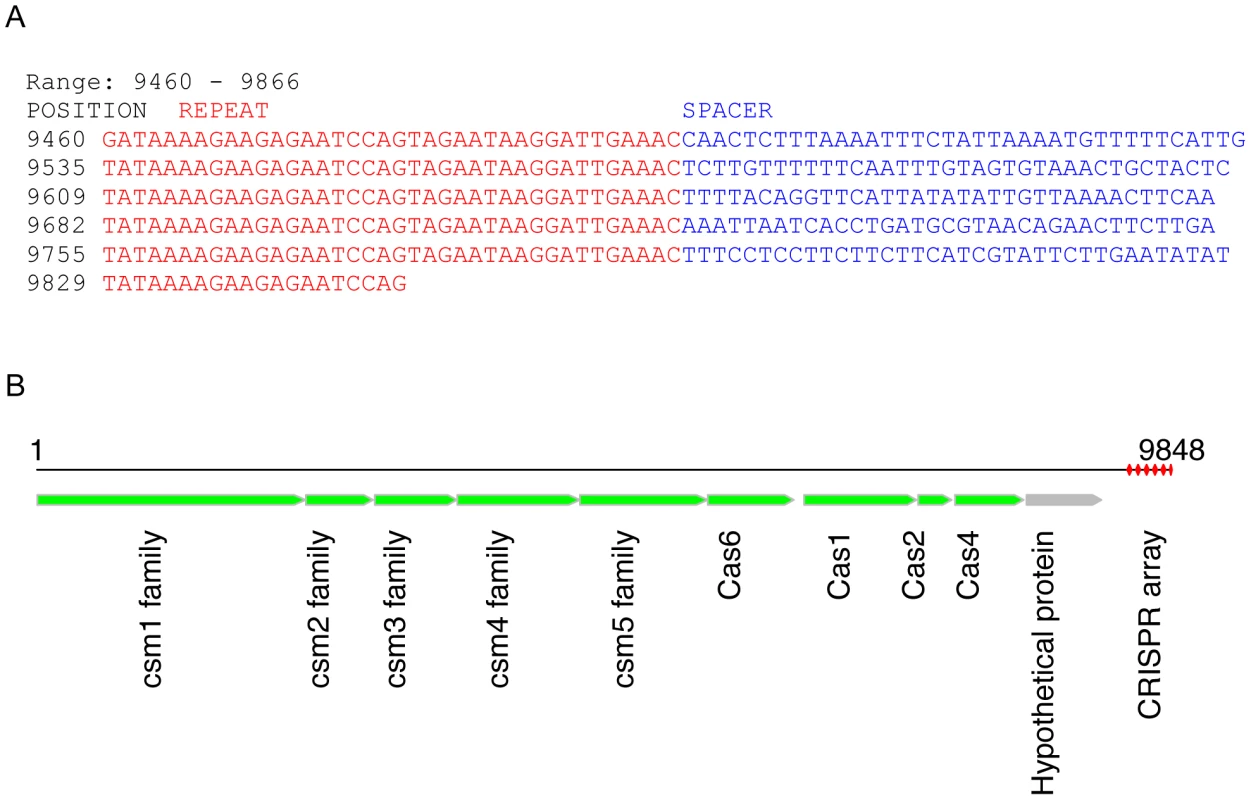
(A) This CRISPR array has 6 copies of the repeat (repeat sequences shown in red font and spacer shown in blue). (B) shows our annotation of this contig, in which the CRISPR array is highlighted in red. We first predicted ORFs in this contig using FragGeneScan [31], and then blasted predicted proteins against the nr protein database to retrieve annotations; for example, the predicted Cas1 is similar to the Cas1 protein identified in Leptotrichia buccalis C-1013-b (accession ID: YP_003163976), with 60% sequence identify and 80% sequence similarity. Targeted assembly of this novel CRISPR (SRS012279L38) in HMP datasets resulted in 278 contigs from 97 datasets, confirming the presence of this CRISPR in human microbiomes. In particular, the CRISPR fragments (407 bps) identified from the whole-metagenome assembly of SRS012279 were assembled into a longer CRISPR contig (890 bps) by targeted assembly. A total of 14 unique but related repeat sequences were identified from 278 CRISPR contigs, and two of them (which differ at 3 positions) are dominant, constituting 71% of the repeats in the CRISPR contigs. Notably, all the repeats could be clustered into a single consensus sequence with an identity threshold of 88%. By contrast, the spacer sequences are very diverse across different samples. For example, we obtained a total of 352 unique spacer sequences, which were clustered into 345 consensus sequences with an identity threshold of 90%. Among 352 unique spacers, 114 spacer sequences were shared by multiple samples—a single spacer was shared by at most eight samples.
The second example is CRISPR SRS023604L36, initially identified in a whole-metagenome assembly contig of dataset SRS023604 (derived from posterior fornix), which has 5 copies of a 36 bp repeat (see consensus sequence of the CRISPR repeat in Table 1). Targeted assembly of this CRISPR across all HMP metagenomic datasets revealed further instances of this CRISPR in several other datasets, including two from stool, and two from posterior fornix. Moreover, the CRISPR contig was assembled into a longer contig of 778 bps containing 12 copies of the CRISPR repeat. BLAST search of the CRISPR repeat against the nr database did not reveal any significant hits.
Expanding the CRISPR space by human microbiomes
To investigate how much the CRISPRs identified in the HMP datasets can expand the CRISPR space (sequence space of the CRISPR repeats), we built a network of CRISPRs, based on the sequence similarity between CRISPR repeats. An edge in the network between two CRISPR repeats, each represented by a node, indicates that the two repeats can be transformed from one to another by at most 10 operations (including mutations, insertions, and deletions). Since it is difficult to determine the direction of CRISPR repeats [7] (especially for the CRISPR arrays that have incomplete structures), given two repeats, we calculated two edit distances—one is the distance between the two repeats, and the other one is between one repeat and the reverse complement of the other—and used the smaller value as the edit distance between the two repeats. The global network (Figure 3A; see Figure S1 with node labels) shows that most of the novel CRISPRs identified in the human microbiomes are remotely related to ones identified in complete (or draft) genomes. Still, there are small clusters that contain only novel HMP CRISPRs, indicating that these CRISPRs are substantially different from ones identified in the reference genomes. In Figure 3B, we have colored nodes by body site: while specific CRISPR repeats can be highly specific to body site (see below), the larger families of repeats shown in Figure 3B do not appear to cluster based on body site.
Fig. 3. Visualizations of the CRISPR network of 150 CRISPRs, each represented as a node. 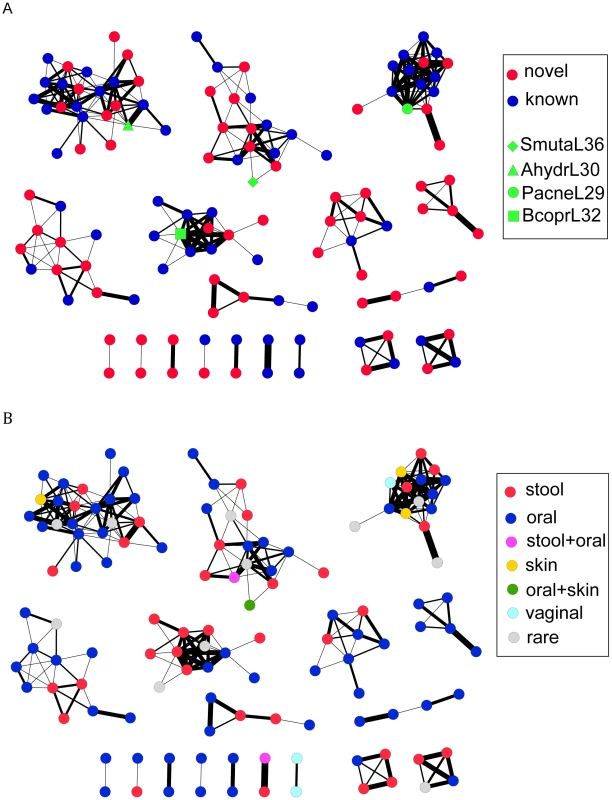
There is an edge between two nodes, if the edit distance between the consensus sequences of the repeats of the corresponding CRISPRs is <10, with edges of small edit distances (i.e., the two CRISPRs share more similar repeats) shown in thick lines and edges of larger edit distances in thin lines. In (A), the known CRISPRs are shown as blue nodes (except for several CRISPRs highlighted in green), and the novel CRISPRs identified in the HMP datasets are shown as red nodes. In (B), the nodes are colored based on body site, in which the CRISPRs are most frequently found. CRISPRs are assigned as rare if they were found in <5 samples; otherwise, they are assigned to particular body site(s) if they are found in more than 10 percent of the samples for that particular body site (e.g., stool+skin). The figures were prepared using Cytoscape [43]. We further studied the sequence patterns of the repeats for each group and our results show 1) distinct patterns among the groups, and 2) high conservation around the stem and start/end positions in CRSIPR repeats of each group (see sequence logos—for the large groups—in Figure S2). The consensuses revealed by the logos show consistencies with the results in a previous study, which used a similar approach, based on alignments of CRISPR repeats, for classification of CRISPR repeats [7].
CRISPRs have diverse distributions across human body sites and individuals
Overall, the distributions of CRISPRs are largely body-site specific (see Figure 4 and Figure S3; the name of CRISPR and the number of samples in which the CRISPR was found are listed in Table S3). For example, CRISPRs AhydrL30 ad BcoprL32 are only found in stool samples (see Table 3). Exceptions include two CRISPRs that were found from both a significant number of gut - and oral-associated samples: Neis_t014_L28 were found in 51 gut samples and 92 oral-associated samples; FalocL36 identified from Filifactor alocis ATCC 35896 were found in 63 gut samples and 72 oral-associated samples, including 50 tongue dorsum samples (see Table 3).
Fig. 4. Distribution of CRISPRs across body sites. 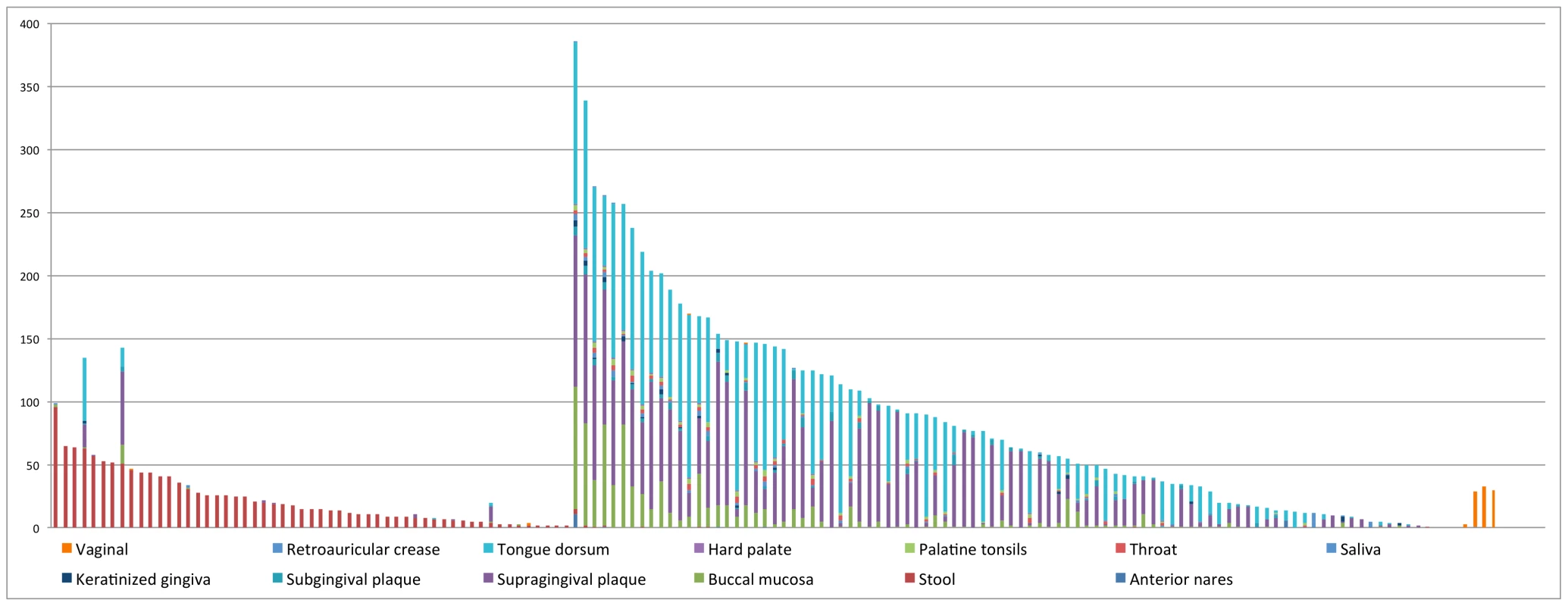
In this figure, the x-axis represents 150 CRISPRs and the y-axis represents the total number of samples in which instances of each CRISPR are found. Note that there are roughly one third as many stool samples as oral samples, probably explaining the apparently smaller number of CRISPRs in the gut microbiome. See Table S3 for details of the distribution of CRISPRs across body sites. The first 50 CRISPRs shown in Figure 4 are mainly found in stool samples. AshahL36, which was initially identified from Alistipes shahii WAL 8301, was found in more than half of gut-related samples (96 out of 147 samples). On the other hand, 99 CRISPRs are mainly found in oral samples, in particular, tongue dorsum, supragingival plaque, and buccal mucosa. We found 5 CRISPRs that exist in more than half of the oral-associated samples (out of 417): SmutaL36, KoralL32 from Kingella oralis ATCC 51147, Veil_sp3_1_44_L36 and Veil_sp3_1_44_L35 from Veillonella sp. 3_1_44, and SoralL35 from Streptococcus oralis ATTC 35037. 4 CRISPRs are mostly found in vaginal samples (AlactL29, LjensL36, LjassL36, and LcrisL29). 1 CRISPR is skin-specific (PacneL29), found mainly in skin samples. Below we discuss the body-site distributions of a few examples.
Neis_t014_L28 and Neis_t014_L36 are inferred from a single genome, Neisseria sp. oral taxon 014 str. F0314, but these two CRISPRs show distinct absence/presence profiles across body sites (see Table 3). For stool samples, there exists only CRISPR Neis_t014_L28 in 51 datasets, but not Neis_t014_L36. And Neis_t014_L36 is relatively more prevalent in oral-associated samples as compared to Neis_t014_L28. The different body site distributions can be explained by the fact that these two CRISPRs are found in different sets of genomes (although both can exist in a common genome, Neisseria sp. oral taxon 014 str. F0314). Neis_t014_L36 has been identified in multiple Neisseria genomes, including Neisseria meningitidis ATCC 13091, Neisseria meningitidis 8013 (so Neis_t014_L36 belongs to the Nmeni subtype among the 8 subtypes defined by Haft et al [33]), Neisseria flavescens SK114, and Actinobacillus minor NM305. Neis_t014_L28, however, was only found in Neisseria sp. oral taxon 014 str. F0314. On the other hand, even though we could not find any CRISPRs containing the exactly same repeat as Neis_t014_L28 in the complete/draft genomes other than Neisseria sp. oral taxon 014 str. F0314, many CRISPRs, when a few mismatches are allowed, were found in diverse genomes (for example, Crenothrix polyspora, Legionella pneumophila 2300/99 Alcoy, and Thioalkalivibrio sp. K90mix) from environmental samples.
Four CRISPRs (AlactL29, LjensL36, LjassL36, and LcrisL29) exist mostly in vaginal samples. AlactL29, initially identified from the Anaerococcus lactolyticus genome, was found only in 3 vaginal samples. Notably, LjensL36 was found in 28 vaginal samples (which comprise 43% of vaginal samples collected) and 1 skin sample. This observation is consistent with a previous study showing that Lactobacillus constitutes over 70% of all bacteria sampled from vaginas of healthy, fertile women, and Lactobacillus jensenii is one of the major genomes [34]. Interestingly, we could find evidence of adaptation in the LjensL36 spacer against Lactobacillus phage Lv-1 (NC_011801) (see below). LjassL36 was found in 33 vaginal samples by targeted assembly. We confirmed that it is in different Lactobacillus genomes, such as Lactobacillus gasseri and Lactobacillus crispatus, by BLAST search. CRISPR LcrisL29, which was identified in the Lactobacillus crispatus genome, was found in 31 vaginal samples, and we found the same repeat sequence in the Lactobacillus helveticus genome.
PacneL29 was the only skin-specific CRISPR we found in the HMP datasets. Interestingly, instances of PacneL29 are found in Propionibacterium acnes HL110PA4 and Propionibacterium acnes J139, but not other P. acnes isolates (including KPA171202, SK137, J165, and SK187). This indicates a potential application of CRISPRs in the characterization of specific stains for a species in human microbiomes.
CRISPRs have very diverse spacers
The HMP project enables us to explore the diversity of streptococcal CRISPRs (and others) at a much broader scale (with 751 samples from 104 healthy individuals). The CRISPRs that we identified in human microbiomes exhibited substantial sequence diversity in their spacers among subjects. Targeted assembly of the streptococcal CRISPRs (SmutaL36) in HMP datasets resulted in a total of 15,662 spacers identified from 386 samples, among which 7,815 were unique spacers (clustering of the spacers at 80% identify resulted in a non-redundant collection of 7,436 sequences). See Figure S4 for the sharing of the spacers in streptococcal CRISPRs among all individuals, which shows several large clusters of spacers that are shared by multiple individuals (for clarity, we only keep spacers that were shared by more than eight samples in this figure). In particular, the most common spacer is shared by 25 individuals (in 32 samples).
More importantly, we could check the sharing of CRISPR spacers across different body sites and sub-body sites (e.g., multiple oral sites) using HMP datasets (Pride et al. examined streptococcal CRISPRs in saliva samples from 4 individuals [26]). Figure 5 shows the spacer sharing among 6 selected individuals, each of whom has multiple samples with identified streptococcal CRISPRs from multiple body sites (see Figure S5 for the spacer sharing with spacers clustered at 80% sequence identify). By examining the distribution of the spacers across samples, we observed that samples re-sampled from the same individual and oral site shared the most spacers, different oral sites from the same individual shared significantly fewer, while different individuals had almost no common spacers, indicating the impact of subtle niche differences and histories on the evolution of CRISPRs. Our observation is largely consistent with the conclusion from Pride et al. [26]. But our study showed that different samples from the same oral site of the same person, even samples collected many months apart, could still share a significant number of spacers (e.g., the supragingival plaque samples from individual 1 in visit 1 and visit 2, with 238 days between the two visits, and the tongue dorsum samples from individual 5 in visit 1 and visit 3, with 336 days between the two visits; as shown in Figure 5). Our study also showed that although the different oral sites of the same individual share similar spacers, this sharing (e.g., between the supragingival plaque sample and the buccal mucosa sample for individual 1) is minimal, as compared to the spacer sharing between samples collected in different visits but from the same oral site (e.g., between the supragingival plaque samples from visit 1 and visit 2 for individual 1). Finally, our study shows that the spacer turnover varies among individuals—for the 6 selected individuals, individual 3 shows significantly higher turnover of the spacers between visits, as compared to other individuals.
Fig. 5. Sharing of streptococcal CRISPR spacers among samples from 6 individuals. 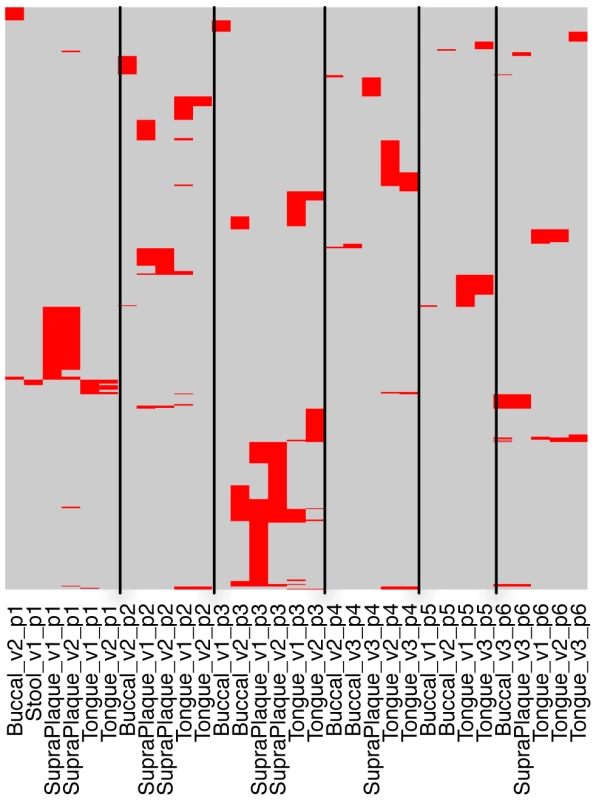
In this map, the rows are the 761 spacers (clustered at 98% identify) identified in one or more of these 6 individuals, and the columns are samples (e.g., Stool_v1_p1 indicates a sample from stool of individual 1, in visit 1; Tongue_v2_p1 indicates dataset from tongue, individual 1, in visit 2). Buccal stands for buccal mucosa, and SupraPlaque stands for supragingival plaque. The red lines indicate the presence of spacers in each of the samples. Multiple lines in the same row represent a spacer that is shared by multiple samples. We also checked the spacer diversity for the CRISPR KoralL32, since it and its variants are one of the most abundant CRISPRs. This CRISPR was assembled from 339 samples: 327 from oral sites and 2 from gut. The targeted assembly of KoralL32 found 7282 unique spacers, among which the most commonly shared spacer is shared by 35 individuals (in 58 samples). Figure S6 shows the sharing of the spacers among the individuals for this CRISPR, which shows similar spacer-sharing patterns as those found in the streptococcal CRISPRs.
The similarity between spacers from the same individual suggests that we may still be able to trace the evolution of CRISPRs, especially in the same body site of the same individual, even though the CRISPR loci tend to have extremely high turnover of their spacers.
CRISPR spacer sequences can be used to trace the viral exposure of microbial communities
As a consequence of CRISPR adaptation, the spacer contents in CRISPR arrays reflect diverse phages and plasmids that have passed through the host genome [1], [35], [36], [37]. However, previous studies have shown that only 2% of the spacer sequences have matches in GenBank, which is probably due to the fact that bacteriophage and plasmids are still poorly represented in databases [13], [14]. Similarity searches of identified spacers against viral genomes enable identification of the viral sources of the spacers (i.e., proto-spacers) captured in each CRISPR locus. For example, similarity searches of the 7,815 unique spacers in the streptococcal CRISPR against viral genomes revealed similarities between streptococcal spacers and 22 viral genomes (species names and accession IDs are listed in Table S4), and the two most prevalent viruses are Streptococcus phage PH10 (NC_012756) and Streptococcus phage Cp-1 (NC_001825) (see Figure 6A). Figure 6B suggests that the potential proto-spacers are rather evenly distributed along the phage genomes (except for a few regions, including a region that encodes for an integrase, which is highlighted in red in Figure 6B). Although the positional distribution of the proto-spacers is close to random, the sequences adjoining the proto-spacers for streptococcal CRISPR we identified in the virus genomes showed conserved short sequence motifs (GG) (see Figure S7 for the sequence logo), which is also the most common proto-spacer adjacent motif (PAM) shared by several CRISPR groups, as reported in [38].
Fig. 6. Traces of viral sequences in the streptococcal CRISPRs in human microbiomes. 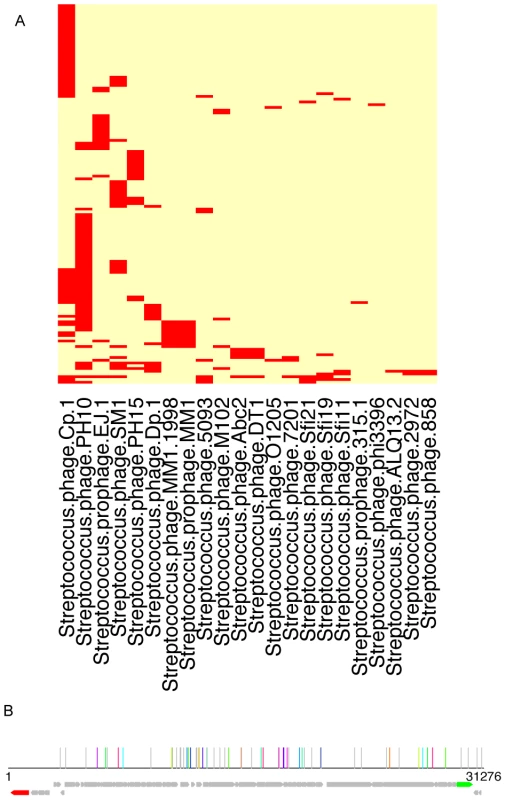
(A) A two-way clustering of viral genomes and the HMP datasets based on the presence patterns of viral sequences in the CRISPR loci identified in the HMP datasets: the columns are the viral genomes, and the rows are HMP datasets. It shows that the genome of Streptococcus phage PH10 (NC_012756) has the most regions that are similar to the spacers in streptococcal CRISPRs. This figure was prepared using the heatmap function in R, with the default clustering method (hclust) and distance measure (Euclidean). (B) Mapping of the spacers onto the 31,276 base genome of Streptococcus phage PH10; in this figure, each vertical line shows a potential proto-spacer, a region in the virus genome that is similar to a spacer found in HMP datasets; lines of the same color show sets of proto-spacers identified from the same HMP dataset (other individual proto-spacers are shown in gray lines); the ORFs are shown in arrows (the red arrow is an integrase and the green arrow is annotated as endolysin). Another example is CRISPR PacneL29, which is mainly found in skin-associated microbiomes. BLAST search of the identified spacers against the virus genome dataset revealed similarity between the spacers and several regions in Propionibacterium phage PA6 (NC_009541). We also found evidence of adaptation in LjensL36 against Lactobacillus phage Lv-1 (NC_011801): BLAST search shows significant matches to a total of 38 regions in the phage genome. Overall we found 23 CRISPRs that have spacers with high sequence similarities (≥90% over 30 bps) with virus genomes collected from the NCBI ftp site (Table S5).
We also searched the spacers against plasmid sequences (collected in the IMG database). For example, matches were found between the detected streptococcal CRISPR spacers and more than 10 plasmid sequences (including Streptococcus thermophilus plasmid pER35, pER36, pSMQ308, and pSMQ173b; Bacillus subtilis plasmid pTA1040; and Streptococcus pneumoniae plasmids pSMB1, pDP1 and pSpnP1). See Table S6 for a summary of the plasmids that share high homology with the CRISPR spacers.
The CRISPER spacers can also be used to identify viral contigs in metagenome assemblies that contain proto-spacers. As an example, similarity searches of identified streptococcal CRISPR spacers against the HMP assemblies revealed 37 potential viral contigs (of lengths from 2,134 to 56,413 bp): these contigs show high homology (>80% sequence similarity) with known viral genomes. The largest contig (of 56,413 bps) is similar to the genome of Streptococcus phage Dp-1 (NC_015274), with 88% sequence identify, and covers almost the entire viral genome (of 59,241 bps). A future paper will fully explore this approach.
Conserved CRISPR repeat sequences can be used to reveal rare species in human microbiome
Because of the large number of repeats that many CRISPR loci contain, CRISPR repeats of rare species with low sequence coverage in a community can still be found. It was reported that repeat-based classification [7] corresponds to a cas gene-based classification of CRISPRs [33], which revealed several subtypes of CRISPRs largely constrained within groups of evolutionarily related species (e.g., the Ecoli subtype). As such, we may use the presence of the repeats of a particular CRISPR as a first indication of the presence of related genome(s) in a microbiome, even though CRISPR locus has been found transferred horizontally as a complete package among genomes [11].
We use CRISPR PpropL29 as an example to demonstrate this potential application, as PpropL29 was identified in only a small proportion of the HMP samples (11 datasets): including 7 supragingival plaque samples (out of 125) and 4 tongue dorsum samples (out of 138). All the PpropL29-related repeats identified in these samples can be clustered into 7 unique sequences. In order to find the most likely reference genomes for these 7 unique repeat sequences, we blasted these repeat sequences against the human microbiome reference genomes and found 100% identity matches in the Lautropia mirabills genome. To investigate the overall coverage of this genome by the reads (not only the CRISPR regions), we mapped the entire collection of reads from four samples: SRS019980 and SRS021477 (both are from supragingival plaque, and have an 100% identity match with the CRISPR repeat in the Lautropia mirabills genome); SRS019974 (from tongue dorsum, with a slightly different CRISPR repeat sequence with 3 differences); and SRS019906 (which does not contain any CRISPR repeats similar to PpropL29, used as a control). The mapping results show the reads from two samples SRS019980 and SRS021477 each cover ∼80% of the Lautropia mirabills genome, which is very significant evidence that these two microbiomes include Lautropia mirabills. But the other two samples have only a limited number of reads mapped to the genome (e.g., only 3089, reads in SRS019906 were mapped into Lautropia mirabills). This contrast suggests that identification of CRISPRs by targeted assembly could provide significant evidence for the existence of certain rare genomes.
Discussion
We have applied a targeted assembly approach to CRISPR identification, to characterize CRISPRs across body sites in different individuals. Our studies show that a directed approach—such as our targeted assembly approach—is important for a comprehensive (thus less biased) estimation of the distribution of CRISPRs across body sites and individuals, and their dynamics. Note that in this study, we only focused on CRISPRs identified in eubacterial genomes, since archaea are rare in human microbiomes (we looked for, but did not find, archaeal CRISPRs in the HMP assemblies). Also for the sake of simplicity, we derived a non-redundant list of CRISPRs based on the similarity of the CRISPR repeats (see Methods), and detailed targeted assembly was only applied to these CRISPRs.
Although many CRISPR arrays may be missed by whole-metagenome assembly, we show that whole-metagenome assemblies are useful for identifying novel CRISPRs (as de novo prediction of CRISPRs relies on sequence features of CRISPRs that do not exist in short reads). Once seeding CRISPRs are identified from whole-metagenome assemblies, we can go back to the original short read datasets, and pursue a comprehensive characterization of the CRISPRs, using the targeted assembly approach. Also, we did not fully utilize the presence of cas genes for identification of novel CRISPRs in our study, since in many cases we can identify arrays of repeats, but not their associated cas genes. A future direction is to combine targeted assembly of CRISPRs and whole-metagenome assembly, aiming to achieve even better assembly of the CRISPR loci with more complete structures, including cas genes and the arrays of repeats and spacers. Such an improvement is necessary to achieve a more comprehensive characterization of especially the novel CRISPRs discovered in metagenomes, and the temporal order of spacer addition to arrays.
The immediate utility of this study is to provide more complete inventories of CRISPR loci in human microbiomes and their distributions in different human body sites, and the spacer content of these loci. The identification of CRISPR spacers opens up several potential applications, including tracing the viral exposure of the hosts, studying the sequence patterns of the regions adjoining the spacer precursors in viral genomes, and discovering viral contigs in metagenome assemblies. It has been shown that short sequence motifs found in the regions adjacent to the spacer precursors in the viral genomes determine the targets of the CRISPR defense system [38], and we were able to analyze the sequence patterns of regions adjacent to spacer precursors for several CRISPRs with the most spacers identified in the HMP datasets (including SmutaL36, LjensL36, and KoralL32; see sequence logos in Figure S7). When more metagenomic datasets become available, we will extend the analysis to more CRISPRs, which may provide insights into the mechanism of the CRISPR defense system (including the turnover patterns of the CRISPR spacers, and the target recognition of the CRISPR defense systems). Our preliminary exploration of viral contigs—by searching CRISPR spacers against whole-metagenome assemblies—suggests that we can identify new virus genomes in metagenome assemblies; further computational and experimental analysis will be needed to confirm these contigs.
We look forward to being able to utilize CRISPR spacer sequences to understand human and human microbiome biology better, utilizing the metadata associated with the HMP datasets. This awaits a more complete sampling of individuals over time, and of known relationships; and a far better characterization of bacteriophage and other selfish genetic elements in the human biome (our inventory of spacers is a standard against which phage and plasmid collections can be judged).
Methods
De novo identification of CRISPRs
CRT [28] is a tool for fast, de novo identification of CRISPRs in long DNA sequences. CRT works by first detecting repeats that are separated by a similar distance, and then checking for other CRISPR specific requirements (e.g., the spacers need to be non-repeating and similarly sized). We modified CRT to consider incomplete repeats at the ends of contigs from whole-metagenome assembly, and call the modified program metaCRT.
Identification of CRISPRs by similarity search
We implemented CRISPRAlign for identifying CRISPRs in a target sequence (a genome or a contig) that has repeats similar to a given CRISPR (query CRISPR). CRISPRAlign works by first detecting substrings in the target sequence (or its reverse complement) that are similar to the repeat sequence of a query CRISPR, and then checking for other requirements, as in metaCRT. Both metaCRT and CRISPRAlign are available for download at http://omics.informatics.indiana.edu/CRISPR/.
Selection of known and novel CRISPRs for targeted assembly in HMP datasets
Using metaCRT and CRISPRAlign, we prepared a list of known CRISPRs repeats (identified from complete/draft bacterial genomes) and a list of potentially novel ones (identified only in the whole-metagenome assemblies from the HMP datasets) for further detailed study of their distributions among the HMP datasets. As we show in Results, the targeted assembly strategy is important for an efficient and comprehensive characterization of these CRISPRs in human microbiome datasets.
Known CRISPRs were first identified from the bacterial genomes (or drafts) collected in the IMG dataset (version 3.3), using metaCRT. We then selected a subset of the identified CRISPRs that meet the following requirements: direct repeats are of length 24–40 bps, there are a minimum of 4 copies of the direct repeats, and the individual repeats each differ by at most one nucleotide from the repeat consensus sequence, on average. The parameters were chosen to minimize false CRISPRs, considering that a CRISPR array typically contains 27 repeats, with an average repeat length of 32 base pairs [28]. We only kept CRISPRs that can be found in at least one of the whole-metagenome assemblies, using CRISPRAlign. We further reduced the number of candidate CRISPRs by keeping only those that share at most 90% sequence identity along their repeats by CD-HIT [39], as there are CRISPRs that share very similar repeats, and our targeted assembly strategy can recover the CRISPRs with slight repeat differences. To avoid including a repeat and its reverse complete (metaCRT does not consider the orientation for the repeats) in the non-redundant list, we included reverse complement sequences of the CRISPR repeats in the clustering process. Therefore, a repeat would be classified into two clusters by CD-HIT (the reverse complete of the repeat would be classified into a different cluster), one of which was removed to reduce redundancy.
We consider that a CRISPR identified in the HMP assemblies is novel if we find no instances of this CRISPR in the IMG bacterial genomes and the HMP reference genomes, with at most 4 mismatches using CRISPRAlign. Similarly, we only kept a non-redundant list of the novel candidates.
In total, we selected a collection of non-redundant CRISPRs—including 64 known CRISPRs and 86 novel ones—for further targeted assembly from HMP shotgun reads. The detailed information for these CRISPRs (repeat sequences, and their resources, and the references for the CRISPRs already collected in the CRISPRdb database http://crispr.u-psud.fr/ [6]), is provided in Tables S1 and S2.
Targeted assembly of CRISPRs
For the targeted assembly of CRISPRs, we first carried out a BLASTN search with each putative CRISPR repeat sequence as the query, to collect reads that contain the repeat sequence (see Figure 1). In order to make the similarity search tolerant to sequencing errors and genomic variations that are observed among the multiple copies of a CRISPR repeat (in one CRISPR locus or between different CRISPR loci), we allowed three mismatches over the entire CRISPR repeat sequence: we retained only the reads that are aligned with the entire CRISPR repeat sequence with a maximum of three mismatches. With these reads containing CRISPR repeat sequences, we ran SOAPdenovo [40] with k-mers of 45 bps, which are sufficiently long to assemble reads with the repetitive sequences found in CRISPRs. In general, whole-metagenome contigs are assembled using shorter k-mers (for example, 21–23 bps in MetaHit [41] and 25 bps in HMP assembly [42]), as longer k-mers often fragment assemblies into short contigs. After CRISPR contigs were assembled, the exact boundaries of the repeats and spacers were obtained using CRISPRAlign.
Validation of the targeted assembly approach using simulated datasets
We simulated short reads from 6 reference genomes (Azospirillum B510, Streptococcus mutans NN2025, Deferribacter desulfuricans SSM1, Dehalococcoides GT, Erwinia amylovora ATCC 49946, and Escherichia coli K12 MG1655), and applied our method to attempt to assemble the 10 known CRISPRs in these genomes. All 54 contigs assembled by our targeted assembly approach match perfectly to known CRISPRs in the reference genomes. We listed the genome names, the CRISPR repeats, the coordinates of the known CRISPRS in the reference genomes, and the coordinates of the contigs aligned on the reference genomes in Table S7.
Datasets
We used the dataset Human Microbiome Illumina WGS Reads (HMIGWS) Build 1.0 available at http://hmpdacc.org/HMIWGS, and the whole-metagenome assemblies from the HMP consortium (http://www.hmpdacc.org/). The bacterial genomes were downloaded from the IMG database (http://img.jgi.doe.gov/cgi-bin/m/main.cgi), NCBI ftp site (ftp://ftp.ncbi.nih.gov/genomes), and human microbiome project website (http://www.hmpdacc.org/data_genomes.php). The viral genomes were downloaded from the NCBI ftp site (http://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid=10239). Additional phage genomes were downloaded from the PhAnToMe database site (http://www.phantome.org/Downloads/DNA/all_sequences/).
Supporting Information
Zdroje
1. BarrangouRFremauxCDeveauHRichardsMBoyavalP 2007 CRISPR provides acquired resistance against viruses in prokaryotes. Science 315 1709 1712
2. HorvathPBarrangouR 2010 CRISPR/Cas, the immune system of bacteria and archaea. Science 327 167 170
3. JansenREmbdenJDGaastraWSchoulsLM 2002 Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43 1565 1575
4. SorekRKuninVHugenholtzP 2008 CRISPR–a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol 6 181 186
5. van der OostJJoreMMWestraERLundgrenMBrounsSJ 2009 CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci 34 401 407
6. GrissaIVergnaudGPourcelC 2007 The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinformatics 8 172
7. KuninVSorekRHugenholtzP 2007 Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol 8 R61
8. DeltchevaEChylinskiKSharmaCMGonzalesKChaoY 2011 CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471 602 607
9. DeveauHGarneauJEMoineauS 2010 CRISPR/Cas System and Its Role in Phage-Bacteria Interactions. Annual Review of Microbiology 64 475 493
10. DeveauHBarrangouRGarneauJELabontéJFremauxC 2008 Phage Response to CRISPR-Encoded Resistance in Streptococcus thermophilis. Journal of Bacteriology 190 1390 1400
11. ChakrabortySSnijdersAPChakravortyRAhmedMTarekAM 2010 Comparative network clustering of direct repeats (DRs) and cas genes confirms the possibility of the horizontal transfer of CRISPR locus among bacteria. Mol Phylogenet Evol 56 878 887
12. GoddeJBickertonA 2006 The Repetitive DNA Elements Called CRISPRs and Their Associated Genes: Evidence of Horizontal Transfer Among Prokaryotes. Journal of Molecular evolution 62 718 729
13. AnderssonAFBanfieldJF 2008 Virus population dynamics and acquired virus resistance in natural microbial communities. Science 320 1047 1050
14. HeidelbergJFNelsonWCSchoenfeldTBhayaD 2009 Germ warfare in a microbial mat community: CRISPRs provide insights into the co-evolution of host and viral genomes. PLoS ONE 4 e4169 doi:10.1371/journal.pone.0004169
15. HeldNLWhitakerRJ 2009 Viral biogeography revealed by signatures in Sulfolobus islandicus genomes. Environ Microbiol 11 457 466
16. KuninVHeSWarneckeFPetersonSBGarcia MartinH 2008 A bacterial metapopulation adapts locally to phage predation despite global dispersal. Genome Research 18 293 297
17. HawleyDMAltizeSM 2011 Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. . Functional Ecology 25 48 60
18. KeesingFHoltRDOstfeldRS 2006 Effects of species diversity on disease risk. Ecol Lett 9 485 498
19. WolinskaJSpaakP 2009 The cost of being common: evidence from natural Daphnia populations. Evolution 63 1893 1901
20. HamiltonWDAxelrodRTaneseR 1990 Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci USA 87 3566 3573
21. AnglyFEFeltsBBreitbartMSalamonPEdwardsRA 2006 The marine viromes of four oceanic regions. PLoS Biol 4 e368 doi:10.1371/journal.pbio.0040368
22. ParadaVBaudouxACSintesEWeinbauerMGHerndlGJ 2008 Dynamics and diversity of newly produced virioplankton in the North Sea. ISME J 2 924 936
23. VenturaMSozziTTurroniFMatteuzziDvan SinderenD 2010 The impact of bacteriophages on probiotic bacteria and gut microbiota diversity. Genes Nutr
24. WoodfordNTurtonJFLivermoreDM 2011 Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev
25. GarneauJEDupuisMEVillionMRomeroDABarrangouR 2010 The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468 67 71
26. PrideDTSunCLSalzmanJRaoNLoomerP 2011 Analysis of streptococcal CRISPRs from human saliva reveals substantial sequence diversity within and between subjects over time. Genome Research 21 126 136
27. GrissaIVergnaudGPourcelC 2007 CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res 35 W25 57
28. BlandCRamseyTSabreeFLoweMBrownK 2007 CRISPR Recognition Tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics 8 209
29. EdgarR 2007 PILER-CR: Fast and accurate identification of CRISPR repeats. BMC Bioinformatics 8 18
30. RousseauCGonnetMLe RomancerMNicolasJ 2009 CRISPI: a CRISPR interactive database. Bioinformatics 25 3317 3318
31. RhoMTangHYeY 2010 FragGeneScan: predicting genes in short and error-prone reads. Nucl Acids Res doi:10.1093/nar/gkq747
32. BhallyHSLemaCRomagnoliMBorekAWakefieldT 2005 Leptotrichia buccalis bacteremia in two patients with acute myelogenous leukemia. Anaerobe 11 350 353
33. HaftDHSelengutJMongodinEFNelsonKE 2005 A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1 e60 doi:10.1371/journal.pcbi.0010060
34. MartínREscobedoSSuárez JuanE 2010 Induction, structural characterization, and genome sequence of Lv1, a prophage from a human vaginal Lactobacillus jensenii strain. Int Microbiol 13 113 121
35. MarraffiniLASontheimerEJ 2010 CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet 11 181 190
36. MojicaFJMDíez-VillaseñorCsGarcía-MartínezJsSoriaE 2005 Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. Journal of Molecular evolution 60 174 182
37. MarraffiniLASontheimerEJ 2008 CRISPR interference limits horizontal gene transfer in Staphylococci by targeting DNA. Science 322 1843 1845
38. MojicaFJDiez-VillasenorCGarcia-MartinezJAlmendrosC 2008 Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155 733 740
39. LiWJaroszewskiLGodzikA 2001 Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics 17 282 283
40. LiRZhuHRuanJQianWFangX 2010 De novo assembly of human genomes with massively parallel short read sequencing. Genome Res 20 265 272
41. QinJLiRRaesJArumugamMBurgdorfKS 2010 A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464 59 65
42. The Human Microbiome Consortium 2012 Structure, Function and Diversity of Human Microbiome in an Adult Reference Population. Nature: doi:10.1038/nature11234
43. SmootMEOnoKRuscheinskiJWangPLIdekerT 2011 Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27 431 432
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



