-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
Plants under pathogen attack produce high levels of ethylene, which plays important roles in plant immunity. Previously, we reported the involvement of ACS2 and ACS6, two Type I ACS isoforms, in Botrytis cinerea–induced ethylene biosynthesis and their regulation at the protein stability level by MPK3 and MPK6, two Arabidopsis pathogen-responsive mitogen-activated protein kinases (MAPKs). The residual ethylene induction in the acs2/acs6 double mutant suggests the involvement of additional ACS isoforms. It is also known that a subset of ACS genes, including ACS6, is transcriptionally induced in plants under stress or pathogen attack. However, the importance of ACS gene activation and the regulatory mechanism(s) are not clear. In this report, we demonstrate using genetic analysis that ACS7 and ACS11, two Type III ACS isoforms, and ACS8, a Type II ACS isoform, also contribute to the B. cinerea–induced ethylene production. In addition to post-translational regulation, transcriptional activation of the ACS genes also plays a critical role in sustaining high levels of ethylene induction. Interestingly, MPK3 and MPK6 not only control the stability of ACS2 and ACS6 proteins via direct protein phosphorylation but also regulate the expression of ACS2 and ACS6 genes. WRKY33, another MPK3/MPK6 substrate, is involved in the MPK3/MPK6-induced ACS2/ACS6 gene expression based on genetic analyses. Furthermore, chromatin-immunoprecipitation assay reveals the direct binding of WRKY33 to the W-boxes in the promoters of ACS2 and ACS6 genes in vivo, suggesting that WRKY33 is directly involved in the activation of ACS2 and ACS6 expression downstream of MPK3/MPK6 cascade in response to pathogen invasion. Regulation of ACS activity by MPK3/MPK6 at both transcriptional and protein stability levels plays a key role in determining the kinetics and magnitude of ethylene induction.
Published in the journal: . PLoS Genet 8(6): e32767. doi:10.1371/journal.pgen.1002767
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002767Summary
Plants under pathogen attack produce high levels of ethylene, which plays important roles in plant immunity. Previously, we reported the involvement of ACS2 and ACS6, two Type I ACS isoforms, in Botrytis cinerea–induced ethylene biosynthesis and their regulation at the protein stability level by MPK3 and MPK6, two Arabidopsis pathogen-responsive mitogen-activated protein kinases (MAPKs). The residual ethylene induction in the acs2/acs6 double mutant suggests the involvement of additional ACS isoforms. It is also known that a subset of ACS genes, including ACS6, is transcriptionally induced in plants under stress or pathogen attack. However, the importance of ACS gene activation and the regulatory mechanism(s) are not clear. In this report, we demonstrate using genetic analysis that ACS7 and ACS11, two Type III ACS isoforms, and ACS8, a Type II ACS isoform, also contribute to the B. cinerea–induced ethylene production. In addition to post-translational regulation, transcriptional activation of the ACS genes also plays a critical role in sustaining high levels of ethylene induction. Interestingly, MPK3 and MPK6 not only control the stability of ACS2 and ACS6 proteins via direct protein phosphorylation but also regulate the expression of ACS2 and ACS6 genes. WRKY33, another MPK3/MPK6 substrate, is involved in the MPK3/MPK6-induced ACS2/ACS6 gene expression based on genetic analyses. Furthermore, chromatin-immunoprecipitation assay reveals the direct binding of WRKY33 to the W-boxes in the promoters of ACS2 and ACS6 genes in vivo, suggesting that WRKY33 is directly involved in the activation of ACS2 and ACS6 expression downstream of MPK3/MPK6 cascade in response to pathogen invasion. Regulation of ACS activity by MPK3/MPK6 at both transcriptional and protein stability levels plays a key role in determining the kinetics and magnitude of ethylene induction.
Introduction
The gaseous phytohormone ethylene profoundly impacts plant growth, development, and response to environmental stimuli [1]–[7]. Studies from a number of labs have defined a signaling pathway—from ethylene receptors to downstream signaling components to transcription factors—that alters gene expression and leads to ethylene-induced phenotypes (reviewed in [1], [2], [5], [8], [9]). Ethylene-regulated responses are suppressed in the absence of ethylene, and such suppression is released upon plant sensing of ethylene. As a result, all ethylene-regulated processes begin with the induction of ethylene biosynthesis [10]. Plants under stress, including wounding, flooding, drought, osmotic shock, ozone, and pathogen/insect invasion, produce elevated levels of ethylene [1], [6], [7], [11]. For this reason, ethylene is also known as a plant stress hormone. The biosynthetic pathway of ethylene has been fully elucidated for over two decades. Two enzymatic steps are unique to ethylene biosynthesis: conversion of S-adenosyl-methionine (SAM), a common metabolic precursor, to 1-amino-cyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) and oxidative cleavage of ACC to form ethylene by ACC oxidase (ACO) [1], [4], [12]. ACS activity is very low in tissues that do not produce a large amount of ethylene and is enhanced under conditions that promote ethylene formation [1], [4], [12]–[14]. In contrast, ACO is constitutively present in most vegetative tissues. As a result, ACS is believed to be the committing and generally rate-limiting enzyme in ethylene biosynthesis.
ACS is encoded by a small gene family in plants. In Arabidopsis, there are nine ACS members. Based on the presence/absence of phosphorylation sites in their C-termini, ACS isoforms are classified into three types [15]. Type I ACS isoforms, which include Arabidopsis ACS1, ACS2, and ACS6, have phosphorylation sites by both mitogen-activated protein kinases (MAPKs) and calcium-dependent protein kinases (CDPKs) [16], [17]. Type II ACS isoforms, which include Arabidopsis ACS4, ACS5, ACS8, and ACS9, only have putative CDPK phosphorylation sites. In contrast, Type III ACS isoforms have shorter C-terminal extension and lack both phosphorylation sites. ACS7 and ACS11 are the two Type III ACS isoforms in Arabidopsis. ACS1 has a short deletion with the highly conserved tripeptide Thr-Asn-Pro (TNP) missing. It is enzymatically inactive as a homodimer, but can form functional heterodimers with other Type I isoforms and may contribute to ethylene biosynthesis [18], [19]. ACS isoforms show cell - and tissue-specific expression and are developmentally regulated. In addition, expression of some members is highly responsive to extracellular stimuli [1], [20].
More recent studies have highlighted the importance of ACS protein stability regulation by protein phosphorylation and dephosphorylation. MAPK cascades are signaling modules downstream of sensors/receptors that transduce extracellular stimuli into intracellular responses in eukaryotes. A basic MAPK cascade is composed of three interconnected kinases. MAPKs function at the bottom of the three-kinase cascade and are activated by MAPK kinases (MAPKKs) through phosphorylation on the Thr and Tyr residues in their activation motif between the kinase subdomain VII and VIII. The activity of MAPKKs is, in turn, regulated by MAPKK kinases (MAPKKKs) via phosphorylation of two Ser/Thr residues in the activation loop of MAPKKs. MAPKKKs receive signals from upstream receptors/sensors, most of the time indirectly with additional components involved [21], [22]. The outputs of a MAPK cascade are dependent on the substrates of the MAPK(s) in the cascade. A subset of MAPKs in plants, represented by tobacco SIPK/Ntf4/WIPK and Arabidopsis MPK3/MPK6, is activated under various stress conditions that elevate ethylene production (reviewed in [21], [23]–[26]). A gain-of-function analysis in tobacco revealed that activation of SIPK/WIPK induces high levels of ethylene production [27]. More detailed analyses in Arabidopsis have demonstrated that ACS2 and ACS6, two Type I ACS isoforms, are substrates of MPK3 and MPK6 [16], [28]. Phosphorylation of ACS2/ACS6 by MPK3 and MPK6 stabilizes the ACS protein in vivo, resulting in increases in cellular ACS activity and in ethylene production. The degradation machinery targets the C-terminal, non-catalytic domain of ACS6 and possibly ACS2 because of their sequence similarity [29]. Phosphorylation of ACS6 introduces negative charges to its C-terminus, which reduces the turnover of ACS6 by the ubiquitin-proteasome degradation machinery.
In addition to protein phosphorylation, protein dephosphorylation also plays critical role in ACS stability regulation. Recently, it was demonstrated that protein phosphatase 2A dephosphorylates ACS2/ACS6 and destabilizes them, a critical process that counteracts with MAPK phosphorylation [30]. Members of the Type II group, including ACS5 and ACS9, are also regulated at protein stability levels, possibly by protein phosphorylation as well [15], [31]–[33]. However, the kinase(s) involved remain unidentified. Because of the complex regulation of ACS protein/activity at multiple levels, many details about the up-regulation of ethylene biosynthesis remain unclear, including the specific ACS isoforms involved in the ethylene induction in response to a specific stimulus, the regulatory pathways that control the expression of ACS genes, and the components involved in the regulation of ACS protein stability. It has been known for decades that a subset of ACS genes, including Arabidopsis ACS6, is transcriptionally activated in plants under stress or pathogen attack. However, the importance of this transcriptional activation and the underlying regulatory mechanism are not known. Furthermore, ethylene induction by different stimuli exhibits different kinetics and magnitude. The underlying molecular mechanism of such differential induction is also unclear.
We are interested in the regulation of ethylene biosynthesis in plants infected by pathogens. ACS2 and ACS6, two Type I ACS isoforms, are involved in Botrytis-induced ethylene production [28]. The residual levels of ethylene induction in the acs2/acs6 double mutant suggest involvement of additional ACS isoforms. In this study, we investigated (1) the potential involvement of all ACS isoforms in ethylene induction triggered by B. cinerea infection, (2) the importance of transcriptional activation of ACS gene expression, (3) the signaling pathways involved in the ACS gene activation, and (4) the molecular mechanism underlying the differential kinetics and magnitude of ethylene induction by different stimuli. We found that members in all three ACS groups are involved in pathogen-induced ethylene production, with ACS2, ACS6, and ACS7 contributing the most to B. cinerea-induced ethylene production. Based on analyses of an ACS6 knockdown mutant and of conditional gain-of-function ACS6 transgenic lines, we also can conclude that the transcriptional activation of the ACS6 gene plays a critical role in sustaining high levels of ethylene induction. Interestingly, MPK3 and MPK6 not only function in the phosphorylation-induced stabilization of ACS2/ACS6 proteins, but also signal the ACS2 and ACS6 gene activation after B. cinerea infection. WRKY33, a MPK3/MPK6 substrate that regulates camalexin biosynthesis [34], is also responsible for turning on ACS2/ACS6 expression downstream of MPK3/MPK6 cascade. WRKY33 binds to the W-boxes in the ACS2/ACS6 promoters in vivo and is directly involved in MPK3/MPK6-induced ACS2/ACS6 gene expression. The duration and magnitude of MPK3/MPK6 activation vary with different stimuli and correlate well with the duration and magnitude of ethylene induction. Regulation of ACS activity at multiple levels by the MPK3/MPK6 cascade is an important mechanism by which the levels/kinetics of ethylene production are regulated during plant stress/defense response.
Results
Activation of ACS gene expression in B. cinerea–infected Arabidopsis
Our previous research demonstrated involvement of ACS2 and ACS6 in ethylene induction in B. cinerea-infected Arabidopsis [28]. This research also implicated the involvement of additional ACS genes since there was still an approximately 25% residual level of ethylene induction in the acs2/acs6 double mutant. To identify the ACS isoforms involved, we profiled the expression of all nine ACS genes in Arabidopsis infected with B. cinerea. As shown in Figure 1A, transcripts of ACS2, ACS6, ACS7, and ACS8 accumulated approximately 1600, 200, 50, and 1200 fold, respectively, over their basal levels. ACS11 transcript also accumulated about 6 fold. ACS5 and ACS9 transcripts could be reliably detected, but no increases were observed. In contrast, ACS1 and ACS4 transcripts were not detectable. To better assess the potential contribution of each ACS gene to ethylene production, we also calculated their expression levels relative to that of EF1α (Figure 1B). This calculation allowed us to compare the relative levels of expression between different ACS genes. From this dataset, we found that the expression levels of ACS2, ACS6, and ACS7 were among the highest. ACS8 and ACS11 had lower levels of expression after induction, while ACS5 and ACS9 expression remained very low. The expression of ACS8 increased more than 1200 fold relative to its basal level (Figure 1A). However, because of its low basal level expression, the induced level of ACS8 transcript was still much lower than those of ACS2, ACS6, and ACS7 (Figure 1B). If regulation at other levels is the same, ACS8 is likely a minor contributor despite the high-fold induction. In contrast, levels of ACS7 transcript were considerably elevated (Figure 1B), despite a relatively low fold induction (Figure 1A), a result of a relatively high basal level. Based on these results, we speculated that ACS7 might be a major contributor to ethylene induction after plant sensing of pathogen invasion besides ACS2 and ACS6.
Fig. 1. Activation of ACS gene expression in Arabidopsis after B. cinerea infection. 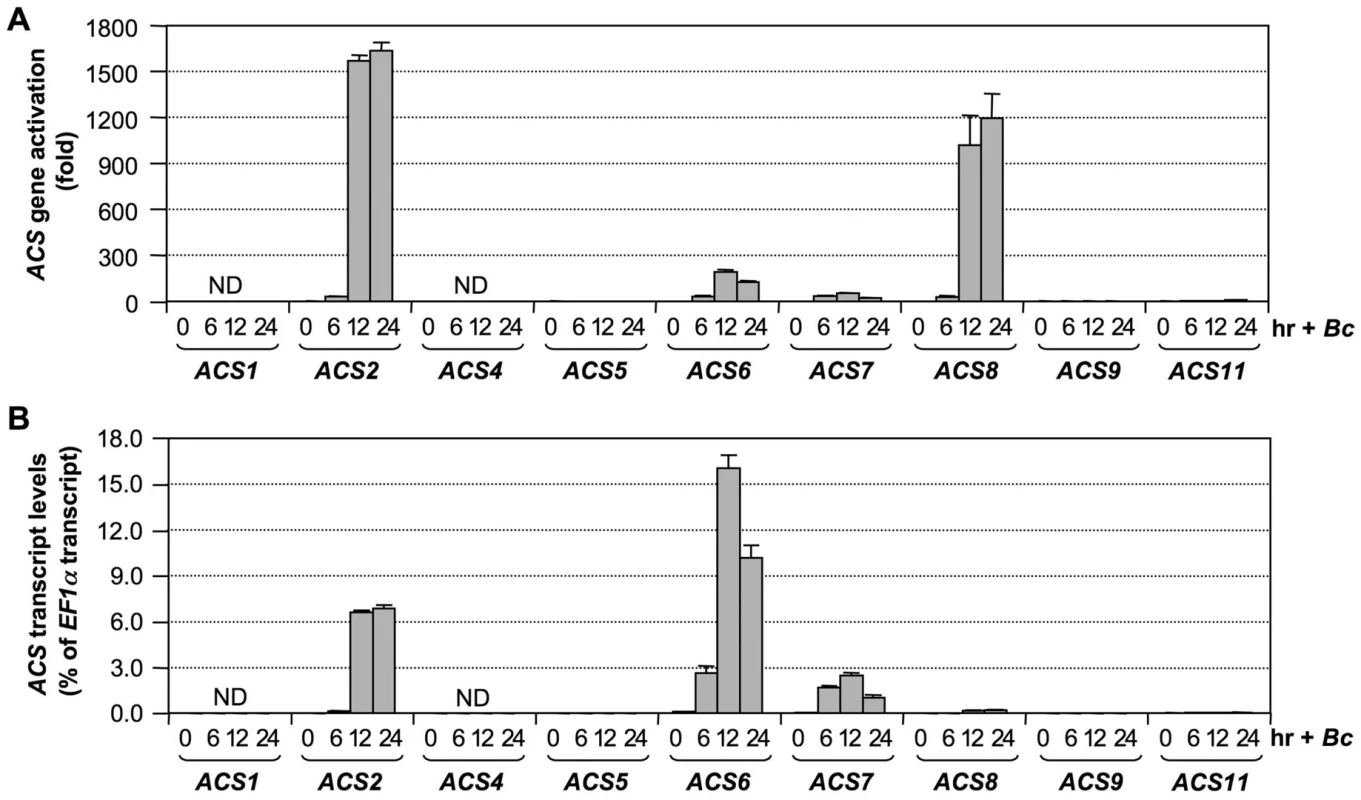
(A) Twelve-day-old Arabidopsis seedlings grown in GC vials were inoculated with B. cinerea spores. Samples were collected at indicated times for total RNAs isolation. Expressions of all nine ACS genes were quantified by real-time PCR. Induction of ACS gene expression (fold of induction relative to the level before inoculation) was calculated by the double ΔCt method. (B) ACS transcript levels are expressed as percentage of EF1α transcript, which allows comparison of expression levels between different ACS genes. In both calculations, the expression of EF1α was used as a reference. Error bars indicate standard deviations (n = 3). ND, not detectable. B. cinerea–induced ethylene production involves ACS isoforms in all three groups
To establish the involvement of ACS7, we identified two null mutant alleles of ACS7, acs7-1 (FLAG_431D05, in Ws-0 background) and acs7-2 (CSHL_ET5768, in Ler-0 background). In both mutant alleles, B. cinerea-induced ethylene production is slightly reduced (Figure S1), similar to that in the acs2 or acs6 single mutant [28]. This result suggests that ACS7 also contributes to B. cinerea-induced ethylene production. In our previous publications [16], [28], we did not assign allele numbers to the acs2 and acs6 mutants. To be consistent with the nomenclature used in Dr. Theologis's lab [35], the acs6 allele (Salk_090423) was given an allele number of acs6-2. This allele turned out to be a knockdown mutant (more discussion later). In contrast, the acs6-1 mutant allele (SALK_025672) in the study by Tsuchisaka et al. (2009) is a null mutant with a T-DNA insertion in the open reading frame (ORF) [35]. We failed to identify any plant with a T-DNA insertion when we initially ordered this line from the Arabidopsis Biological Resource Center (ABRC) in 2003. The acs2 and acs7-1 mutant alleles we used [16], [28](Figure S1) are the same as the acs2-1 and acs7-1 alleles, respectively, in Tsuchisaka et al. (2009) report [35].
We then crossed acs7-1 into the acs2-1/acs6-2 double mutant background and identified an acs2-1/acs6-2/acs7-1 triple mutant in the F3 generation. As shown in Figure 2, only about 10% residual ethylene production was observed in the acs2-1/acs6-2/acs7-1 triple mutant, confirming the importance of ACS7 in B. cinerea-induced ethylene production. Residual ethylene induction in the acs2-1/acs6-2/acs7-1 triple mutant again points to involvement of additional ACS members. To identify them, we utilized the high-order acs mutants generated in Dr. Theologis' lab [35]. We found that acs2-1/acs6-1 seedlings produced a lower level of ethylene than our acs2-1/acs6-2 double mutant after challenged with B. cinerea (Figure 3 and Figure S2), which is consistent with the knockdown nature of our acs6-2 allele. Additional mutation of ACS4, ACS5, and ACS9 genes, either one at a time or all three at once, in the acs2-1/acs6-1 background did not further reduce the ethylene induction. This finding is consistent with our previous conclusion that ACS5 and ACS9 are not involved in the ethylene induction triggered by B. cinerea infection [28]. In contrast, mutation of the ACS7 gene in the acs2-1/acs6-1/acs4-1/acs5-2/acs9-1 background resulted in further reduction in the ethylene induction. In this sextuple mutant (acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1) background, mutation of ACS11, but not ACS1, slightly reduced the ethylene production. The very low level of ethylene induction in the acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1/acs11-1 plants also implicates the involvement of ACS8. In the absence of B. cinerea infection, seedlings of all genotypes produced less than 15 nL ethylene per gram of seedlings within 24 hours, a very low level in comparison to the B. cinerea-induced ethylene production (Figure S3). From this dataset, we can also conclude that ACS7 contributed the most to the basal level ethylene production. Seedlings without a functional ACS7 gene including the acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1/acs11-1 failed to produce a detectable level of ethylene. As a result, the low-level ethylene production in this octuple acs mutant in response to B. cincerea infection is indeed a contribution of ACS8 gene.
Fig. 2. ACS7 also contributes to B. cinerea-induced ethylene production in Arabidopsis. 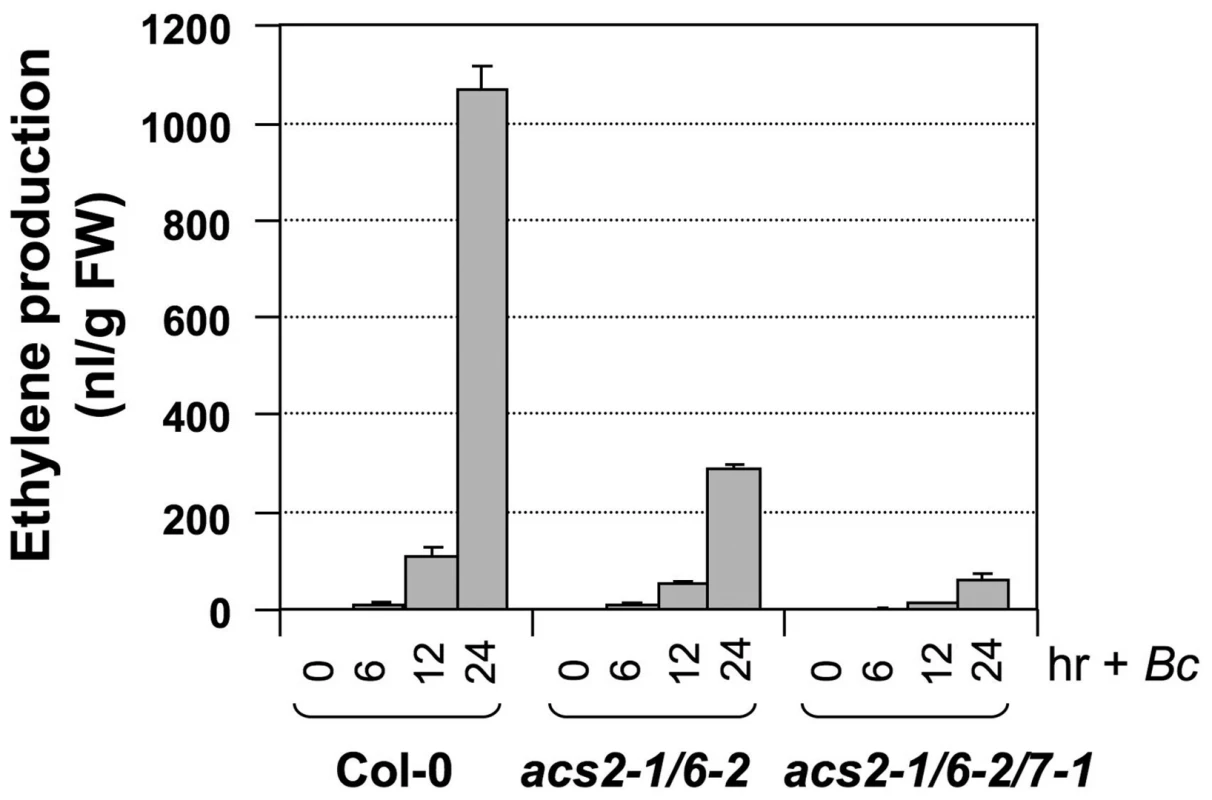
Twelve-day-old wild type (Col-0), acs2-1/acs6-2 double mutant, and acs2-1/acs6-2/acs7-1 triple mutant Arabidopsis seedlings grown in GC vials were inoculated with B. cinerea. Ethylene levels in the headspace were determined at indicated times. Error bars indicate standard deviations (n = 3). Fig. 3. Ethylene induction in high-order acs mutants after B. cinerea infection. 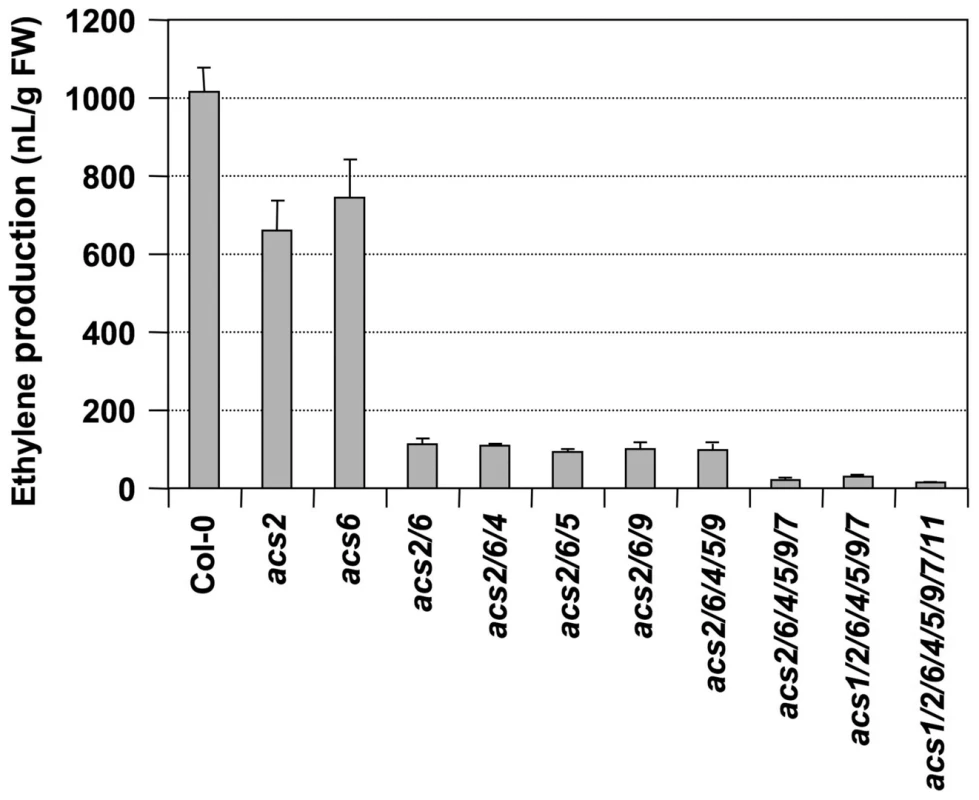
Twelve-day-old wild type (Col-0) and acs mutants generated in Dr. Theologis' lab were inoculated with B. cinerea. Ethylene levels in the headspace were determined at 24 hrs after spore inoculation. Error bars indicate standard deviations (n = 3). The allele numbers are omitted for easy labeling. They are acs1-1, acs2-1, acs4-1, acs5-2, acs6-1, acs7-1, acs9-1, and acs11-1. In summary, we can conclude that ACS2 and ACS6 are major contributors of ethylene induction and that ACS7, ACS8, and ACS11 contribute less, with a total of ∼15% of the ethylene induction in B. cinerea-infected Arabidopsis. Among the minor contributors, the role of ACS7 and ACS8 in B. cinerea-induced ethylene production is clear. In contrast, the contribution of ACS11 is somewhat uncertain because of the conclusion of its involvement is based on a small quantitative difference. One potential mechanism underlying each ACS isoform's contribution to ethylene induction is through the up-regulation of their gene expression (Figure 1). There is a good correlation between the transcriptional activation of ACS gene expression (Figure 1) and involvement in B. cinerea-induced ethylene production (Figure 3).
Induction of ACS2 and ACS6 gene expression is dependent on MPK3/MPK6 pathway
Previously, we demonstrated the importance of phosphorylation regulation of ACS2 and ACS6 by MPK3 and MPK6 in Arabidopsis in response to pathogens/pathogen-associated molecular patterns (PAMPs) [16], [28], [29]. It is well known that the ACS6 gene is highly induced by stress, including wounding and pathogen infection [1], [4], [6], [11], [36], [37]. However, the importance of ACS gene activation in pathogen-induced ethylene production remains unclear. After our discovery that phosphorylation of ACS2 and ACS6 proteins by MPK3/MPK6 is required for ACS2/ACS6 protein stabilization and accumulation, we started to explore the potential contribution of ACS gene activation. Theoretically, an increase in ACS transcript levels is likely to increase the rate of de novo ACS protein synthesis, which, in turn, will increase the net ACS protein/activity after MAPK phosphorylation and protein stabilization.
To determine whether ethylene induction in the conditional gain-of-function GVG-NtMEK2DD (DD, for short) plants [16], [28] is associated with ACS gene activation, we profiled the expression of ACS genes in DD plants after dexamethasone (DEX) treatment. As shown in Figure 4, the expressions of ACS2 and ACS6 were highly induced. Different from B. cinerea-infected seedlings, no induction in ACS7, ACS8, and ACS11 expression levels were observed. In addition, we noticed the kinetics of ACS6 induction in the DD plants were different from that in B. cinerea-infected plants (Figure 4 versus Figure 1). This difference is likely a result of the synchronous response in DD plants. In contrast, the infection process of B. cinerea is progressive. As more cells sensed the growing hyphae, higher levels of ACS6 transcript were induced at later time points.
Fig. 4. Activation of ACS gene expression after MPK3/MPK6 activation in the gain-of-function DD Arabidopsis seedlings. 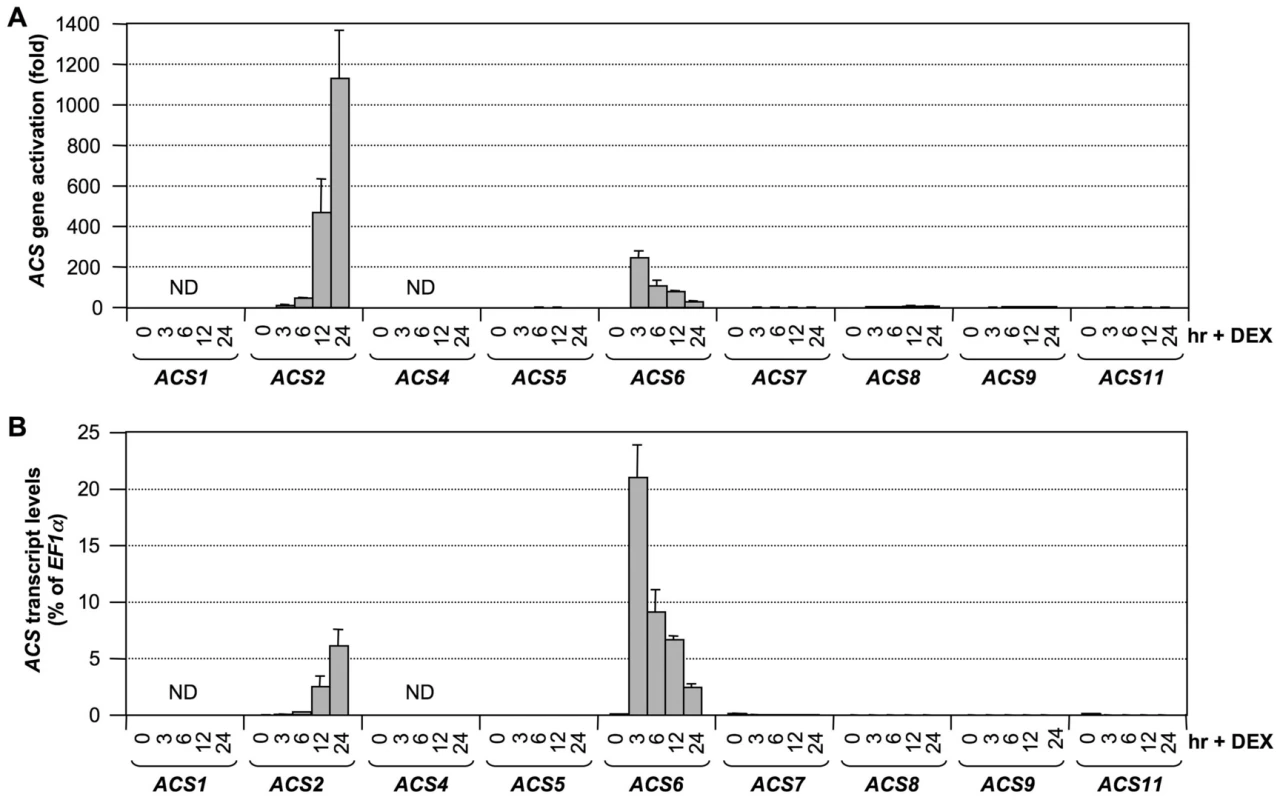
(A) Twelve-day-old conditional gain-of-function DD Arabidopsis seedlings grown in GC vials were treated with 1-µM DEX. Samples were collected at indicated times for total RNA preparation. After reverse transcription, expressions of all nine ACS genes were quantified by real-time PCR. Induction of ACS gene expression (fold of induction relative to the level before inoculation) was calculated by the double ΔCt method. (B) ACS transcript levels expressed as percentage of EF1α transcript, which allows comparison of expression levels among different ACS genes. In both calculations, the expression of EF1α was used as a reference. Error bars indicate standard deviations (n = 3). ND, not detectable. To confirm that MPK3 and MPK6 are responsible for induction of ACS2 and ACS6 expression levels in the gain-of-function DD plants, we examined the expression of ACS2 and ACS6 in DD/mpk3 and DD/mpk6 plants. As shown in Figure 5, induction of ACS2 and ACS6 was compromised in both the mpk3 and mpk6 single mutant backgrounds. This finding demonstrates that induction of ACS2 and ACS6 in DD seedlings after DEX treatment is indeed a result of MPK3/MPK6 activation. Based on the fact that MPK3 and MPK6 are highly activated after B. cinerea infection [28], [38], we speculate that the MPK3/MPK6 cascade is involved in regulating the B. cinerea-induced ACS2/ACS6 gene expression and that induction of ACS7, ACS8, and ACS11 expression is regulated by pathway(s) other than MPK3/MPK6 cascade.
Fig. 5. Activation of ACS2 and ACS6 gene expressions in gain-of-function DD is dependent on downstream MPK3 and MPK6. 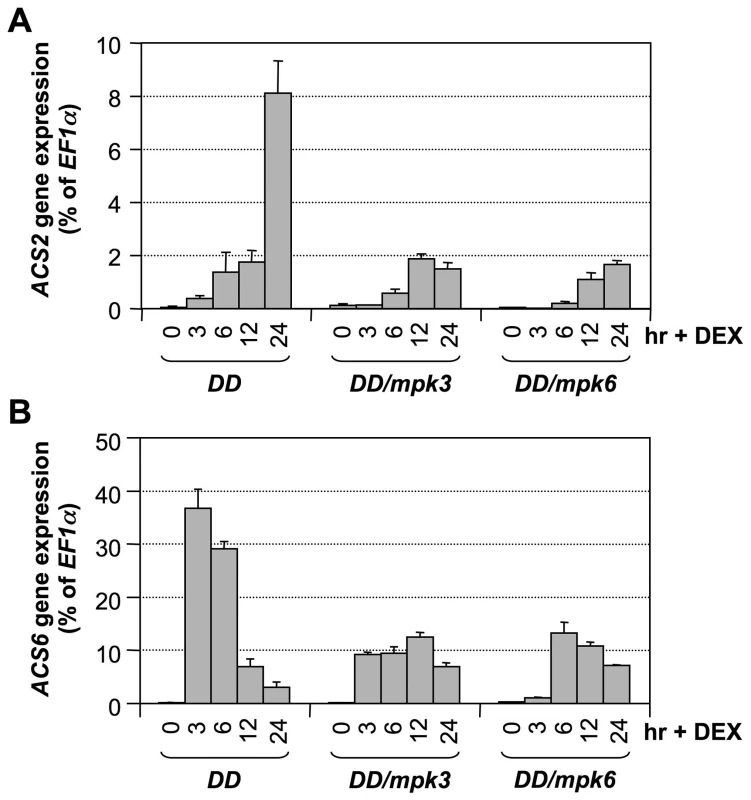
Twelve-day-old DD, DD/mpk3, and DD/mpk6 seedlings grown in GC vials were treated with 1-µM DEX. Samples were collected at indicated times. Total RNAs were extracted and treated with DNase to remove trace genomic DNA contamination. After reverse transcription, expressions of ACS2 (A) and ACS6 (B) genes were quantified by real-time PCR. ACS transcript levels were calculated as a percentage of the EF1α transcript. Error bars indicate standard deviations (n = 3). To provide loss-of-function evidence to support the role of the MPK3/MPK6 cascade in B. cinerea-induced ACS2/ACS6 gene activation, we compared the induction of ACS2 and ACS6 gene expressions in wild type, mpk3 single mutant, mpk6 single mutant, and rescued mpk3/mpk6 double mutant. The rescued mpk3/mpk6 double mutant was obtained by transforming a DEX-inducible promoter-driven MPK6 (GVG-MPK6) into mpk3−/−/mpk6+/− plants. When the T3 mpk3−/−/mpk6+/−/GVG-MPK6+/+ plants began to flower, DEX was sprayed every other day to rescue the embryo lethality of the mpk3−/−/mpk6−/−/GVG-MPK6+/+ zygotes. Progenies with mpk3−/−/mpk6−/−/GVG-MPK6+/+ genotype were called rescued mpk3/mpk6 double mutants [39], and were used for this experiment. As shown in Figure 6, B. cinerea-induced ACS2 and ACS6 expressions were little affected in either the mpk3 or mpk6 single mutant. In the rescued mpk3/mpk6 double mutant, the induction of both genes was dramatically reduced, which supports the conclusion that MPK3 and MPK6 regulate expressions of ACS2 and ACS6 based on the gain-of-function analysis.
Fig. 6. B. cinerea induced ACS2 and ACS6 gene activation is dependent on functional MPK3 and MPK6. 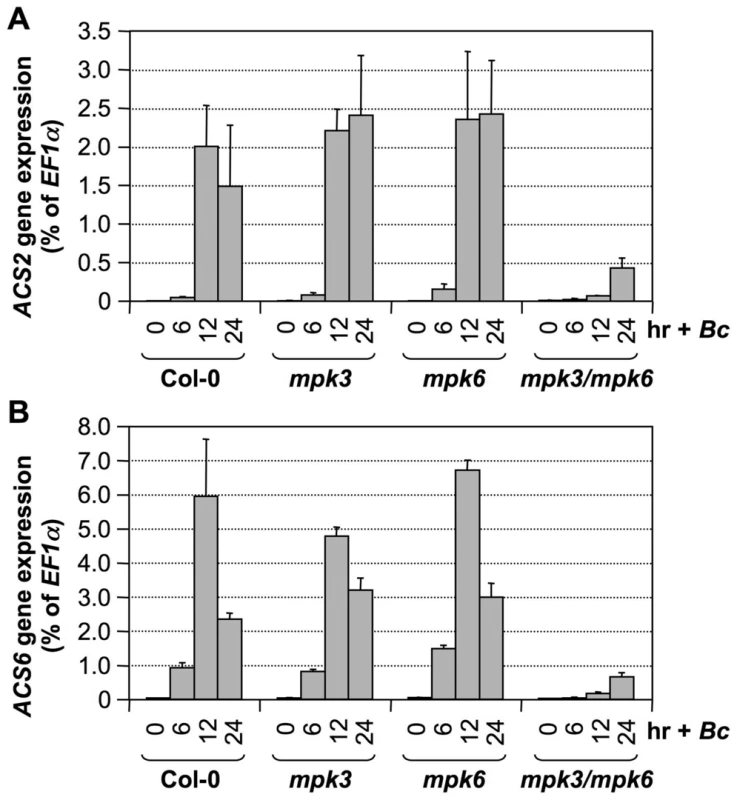
Twelve-day-old wild type (Col-0), mpk3, mpk6, and rescued mpk3/mpk6 double mutant seedlings grown in GC vials were inoculated with B. cinerea spores. Samples were collected at indicated times. Total RNAs were extracted and treated with DNase to remove trace genomic DNA contamination. After reverse transcription, expressions of ACS2 (A) and ACS6 (B) genes were quantified by real-time PCR. ACS transcript levels were calculated as a percentage of the EF1α transcript. Error bars indicate standard deviations (n = 3). Different from B. cinerea-induced ACS2/ACS6 gene activation, gain-of-function DD-induced ACS2/ACS6 gene activation was compromised in either mpk3 or mpk6 single mutant background (Figure 5 and Figure 6). There are several potential explanations for this seemingly contradictory observation. First of all, MAPK-phosphorylation regulation of ACS2/ACS6 gene activation will be affected by both the phosphorylation of the downstream transcription factor(s) such as WRKY33 (more discussion below), and their dephosphorylation by the unidentified phosphatase(s). It is possible that in the gain-of-function DD plants, both MPK3 and MPK6 are needed to overcome the action of the phosphatase(s) to maintain the phosphorylation of there transcription factor(s) and the subsequent up-regulation of ACS2/ACS6 expression. In the absence of either MAPK, the signaling strength is below the threshold to counteract the phosphatases and the activation of ACS2/ACS6 expression is severely compromised. It is possible that, in addition to the activation of MPK3/MPK6 cascade, pathogen infection may also inactivate the dephosphorylation process as a mechanism to promote higher levels of ethylene production. In this situation, the absence of only one MAPK may not be sufficient to block ACS2/ACS6 activation. Alternatively, it is possible that the activation of pathways other than MAPK cascade can compensate the weakened MAPK pathway in the single mpk3 or mpk6 mutant, making it necessary to mutate both MPK3 and MPK6 to see the loss-of-function phenotype in response to B. cinerea infection. Similar phenomenon was also observed in MPK3/MPK6-mediated camalexin induction in response to B. cinerea infection [38].
WRKY33 is involved in the MPK3/MPK6-regulated ACS2/ACS6 gene activation
WRKY33 is a substrate of MPK3/MPK6 in regulating the pathogen-induced phytoalexin biosynthesis [34]. WRKY33 functions as a transcriptional activator downstream of MPK3 and MPK6 in promoting the expression of camalexin biosynthetic genes. To determine whether WRKY33 also is involved in activation of ACS2 and ACS6 genes downstream of the MPK3/MPK6 cascade, we quantified the expression of these two genes in DD and DD/wrky33 plants. As shown in Figure 7B, the induction of ACS2 and ACS6 mRNA by the gain-of-function DD transgene was compromised in wrky33 mutant background. Associated with this, the induction of ethylene biosynthesis was mostly inhibited (Figure 7A). Previously, we showed that DD protein induction and MPK3/MPK6 activation in DD/wrky33 plants are indistinguishable from those in DD plants [34], which strongly supports the conclusion that WRKY33 also functions downstream of MPK3/MPK6 in promoting the expression of ACS2 and ACS6 genes. The low residual levels of ACS2 and ACS6 gene activation is likely to be a result of other WRKY transcription factors that can partially substitute for the loss of WRKY33.
Fig. 7. WRKY33 functions downstream of the MPK3/MPK6 cascade in inducing the expression of ACS2 and ACS6 genes in the gain-of-function DD seedlings. 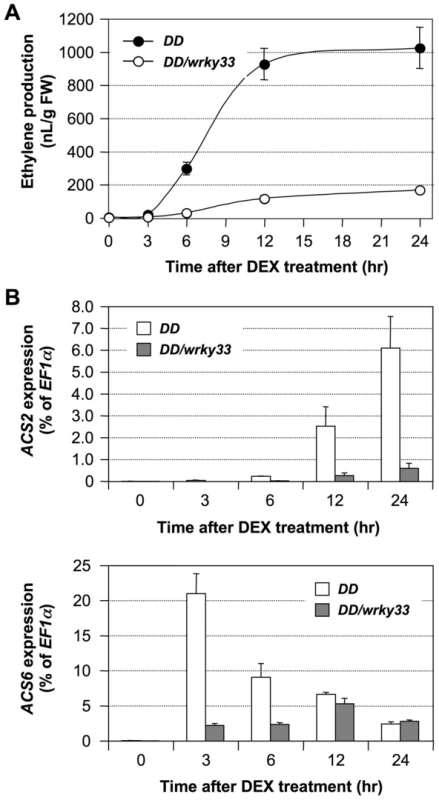
(A) Mutation of WRKY33 compromises ethylene induction in DD seedlings. Twelve-day-old DD and DD/wrky33 seedlings grown in GC vials were treated with 1-µM DEX. Ethylene accumulation in GC vials was monitored at indicated times, and then seedlings were collected for gene expression analysis. Error bars indicate standard deviations (n = 3). (B) MPK3/MPK6-induced ACS2 and ACS6 gene expression in DD plants is dependent on WRKY33. Total RNA was extracted from seedlings collected in (A). Expressions of ACS2 (upper panel) and ACS6 (lower panel) genes were quantified by real-time PCR. ACS transcript levels were calculated as a percentage of the EF1α transcript. Error bars indicate standard deviations (n = 3). In contrast to the gain-of-function DD plants, mutation of the WRKY33 gene had a minor impact on ethylene induction triggered by B. cinerea infection. As shown in Figure 8A, we observed only about a 20% decrease in ethylene production in both alleles of the wrky33 mutant. A comparison of ACS2 and ACS6 gene expressions in the wild type and in the wrky33 mutant revealed that about one-third of the induction in ACS2/ACS6 expression remained in the wrky33 mutant (Figure 8B). This suggests that ACS2 and ACS6 still could be partially activated in the absence of WRKY33. Because of the low residual ACS2/ACS6 gene activation in the mpk3/mpk6 mutant (Figure 6), we speculate that the residual levels seen in the wrky33 mutant are MPK3/MPK6-dependent but WRKY33-independent, again pointing to additional transcription factors, possibly WRKY33 homologs that partially replace WRKY33 in its absence.
Fig. 8. Induction of ACS2 and ACS6 gene expression after B. cinerea infection was partially inhibited in wrky33 mutant. 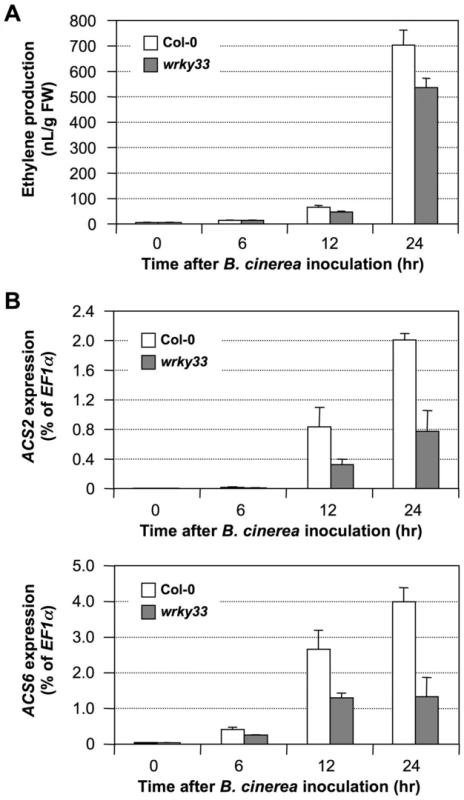
(A) Mutation of WRKY33 partially blocks ethylene induction in Arabidopsis infected by B. cinerea. Twelve-day-old wild type (Col-0) and wrky33 seedlings grown in GC vials were inoculated with B. cinerea spores. Ethylene accumulation in GC vials was monitored at indicated times, and seedlings were collected for gene expression analysis. Error bars indicate standard deviations (n = 3). (B) B. cinerea-induced ACS2 and ACS6 gene expression is compromised in the wrky33 mutant. Total RNA was isolated from the seedlings collected in (A). Expressions of ACS2 (upper panel) and ACS6 (lower panel) genes were quantified by real-time PCR. ACS transcript levels were calculated as a percentage of the EF1α transcript. Error bars indicate standard deviations (n = 3). No major reductions were observed in the induction of ACS7, ACS8, and ACS11 expression in wrky33 infected with B. cinerea (Figure S4), which is consistent with the conclusion that their activation is MPK3/MPK6 and WRKY33 independent. The normal activation of ACS7, ACS8, and ACS11 expressions, together with the residual level of ACS2/ACS6 gene activation and protein phosphorylation stabilization, may explain the observation that the induction of ethylene in the wrky33 mutant was reduced by only about 20% after B. cinerea infection (Figure 8A). In contrast, the wrky33 mutation almost completely blocked induction of ethylene biosynthesis in the gain-of-function DD plants (Figure 7A). B. cinerea can activate multiple signaling pathways in plants. It is possible that pathway(s) other than MPK3/MPK6 cascade are able to partially compensate the loss of WRKY33. It is known that pathogen infection induces a large number of WRKY genes [40], [41], some of which might be able to partially compensate the loss of WRKY33 gene in activating the expression of ACS2/ACS6.
WRKY33 binds to the W-boxes in the promoters of ACS2 and ACS6 genes in vivo
Genetic analysis revealed that WRKY33 is essential for gain-of-function MPK3/MPK6-induced ACS2/ACS6 gene expression (Figure 7B). Examination of the ACS2 and ACS6 promoters revealed the presence of eight and seven W-boxes, respectively (Figure 9A). To further substantiate the role of WRKY33 in the activation of ACS2 and ACS6 gene expression, we performed chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analysis to determine whether ACS2 and ACS6 genes are direct targets of the WRKY33 transcription factor. For this experiment, we used DD/wrky33 mutant plants complemented with a 35S promoter-driven WRKY33 transgene, which contains a four-copy myc epitope tag at the N-terminus (DD/wrky33/35S:4myc-WRKY33) [34]. The presence of the myc tag allowed us to immunoprecipitate the WRKY33-DNA complex by using a commercial anti-myc antibody. As shown in Figure 9B, immunoprecipitation with the anti-myc antibody greatly enriched ACS2 and ACS6 promoter regions containing the W-boxes. In contrast, the IgG control antibody failed to enrich either gene promoter. This result demonstrates that WRKY33 directly binds to the promoters of ACS2 and ACS6 in vivo, suggesting that WRKY33 is the transcription factor downstream of the MPK3/MPK6 cascade involved in the activation of ACS2 and ACS6 expression.
Fig. 9. WRKY33 transcription factor binds to the promoter of ACS2 and ACS6 genes in vivo. 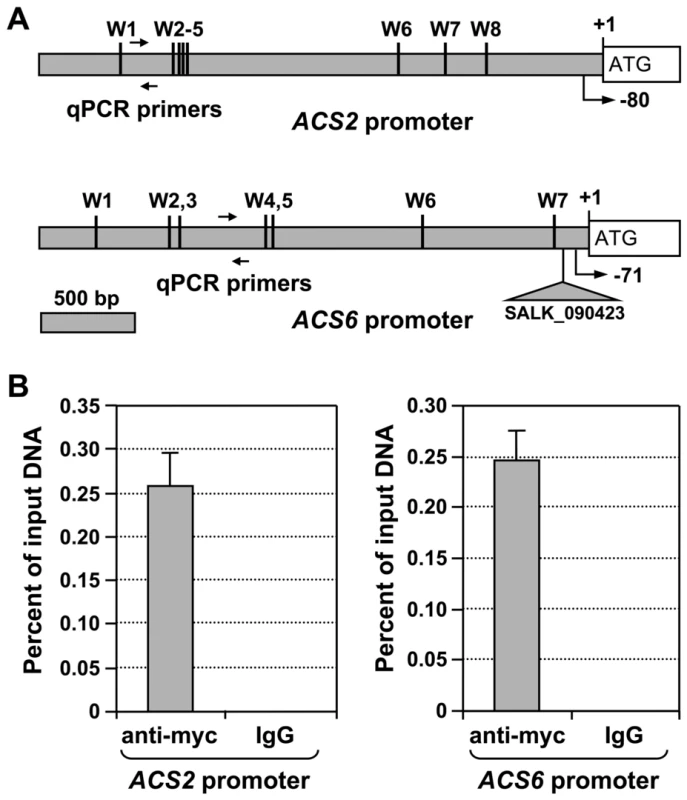
(A) The promoters of ACS2 and ACS6 genes are rich in W-boxes, the cis-element binding sites of the WRKY transcription factor. A diagram indicates the number and relative position of the W-boxes in the promoters of the ACS2 and ACS6 genes. Line arrows indicate the position of primers used for qPCR after chromatin immunoprecipitation (ChIP). Positions of the predicted transcriptional starting sites are indicated by arrows with turning lines and negative numbers. The T-DNA insertion site in the SALK_090423 acs6-2 allele, which locates in the promoter region of ACS6 gene, is also indicated. (B) ChIP-qPCR analysis was performed using DD/4myc-WRKY33WT plants generated from the cross of wrky33/4myc-WRKY33WT with DD lines. Input chromatin was isolated from two-week-old seedlings 12 hr after DEX treatment. Epitope-tagged WRKY33-chromatin complex was immunoprecipitated with an anti-myc antibody. A control reaction was processed side-by-side using mouse IgG. ChIP- and input-DNA samples were quantified by real-time qPCR using primers specific to the promoters of ACS2 (left panel) and ACS6 (right panel) genes. ChIP results are presented as percentage of input DNA. Error bars indicate standard deviations (n = 3). High levels of ACS6 gene expression elevate B. cinerea–induced ethylene production
To provide further direct evidence in support of the role of ACS6 gene activation in B. cinerea-induced ethylene production, we transformed a DEX-inducible promoter-driven ACS6 (GVG-ACS6) construct into the acs2-1/acs6-2/acs7-1 mutant background and then compared the ethylene induction in acs2-1/acs6-2/acs7-1/GVG-ACS6 plants with and without DEX treatment. Two independent lines (#5 and #12) with different levels of ACS6 transgene induction after DEX treatment were used for this experiment to establish a correlation between the levels of ACS6 gene expression and the levels of ethylene induction. As shown in Figure 10A, without DEX treatment, both GVG-ACS6 transgenic lines produced about the same levels of ethylene after B. cinerea treatment in comparison to the acs2-1/acs6-2/acs7-1 triple mutant. In the presence of DEX, which induced ACS6 expression (Figure 10B), the ethylene production was greatly enhanced. The higher level of ACS6 induction in line #5 in the presence of DEX correlated with a higher level of ethylene induction than that in line #12. Furthermore, ethylene induction in Line #5 was higher than that in the wild type, indicating that transgene induction after DEX treatment not only complements the loss of ACS6, but also compensates the loss of ACS2 and ACS7 genes. In the absence of B. cinerea infection, DEX treatment only slightly elevated the ethylene production (Figure S5) to a level similar to the basal level ethylene production of Col-0 (∼10 nL/g FW in 24 hrs). This low-level ethylene production is likely a result of high-level ACS6 gene induction after DEX treatment (and associated higher-level of de novo ACS6 protein synthesis) in combination with the basal level activity of MPK6, which can phosphorylate and stabilize ACS6 protein. MPK6 has very low basal activity even in the absence of stress/pathogen infection [16]. This is consistent with our previous conclusion that the overexpression of ACS6 gene in the absence of MPK3/MPK6 activation is not sufficient to induce ethylene production due to the lack of phosphorylation stabilization [16]. As a result, we can conclude that the high level of ethylene production seen in acs2-1/acs6-2/acs7-1/GVG-ACS6 lines after DEX and B. cinerea treatment is a combination of high level of gene expression (as a result of DEX treatment), and phosphorylation stabilization due to MPK3/MPK6 activation by B. cinerea infection.
Fig. 10. Conditional expression of the ACS6 gene in the acs2-1/acs6-2/acs7-1 mutant background restores ethylene induction triggered by B. cinerea infection. 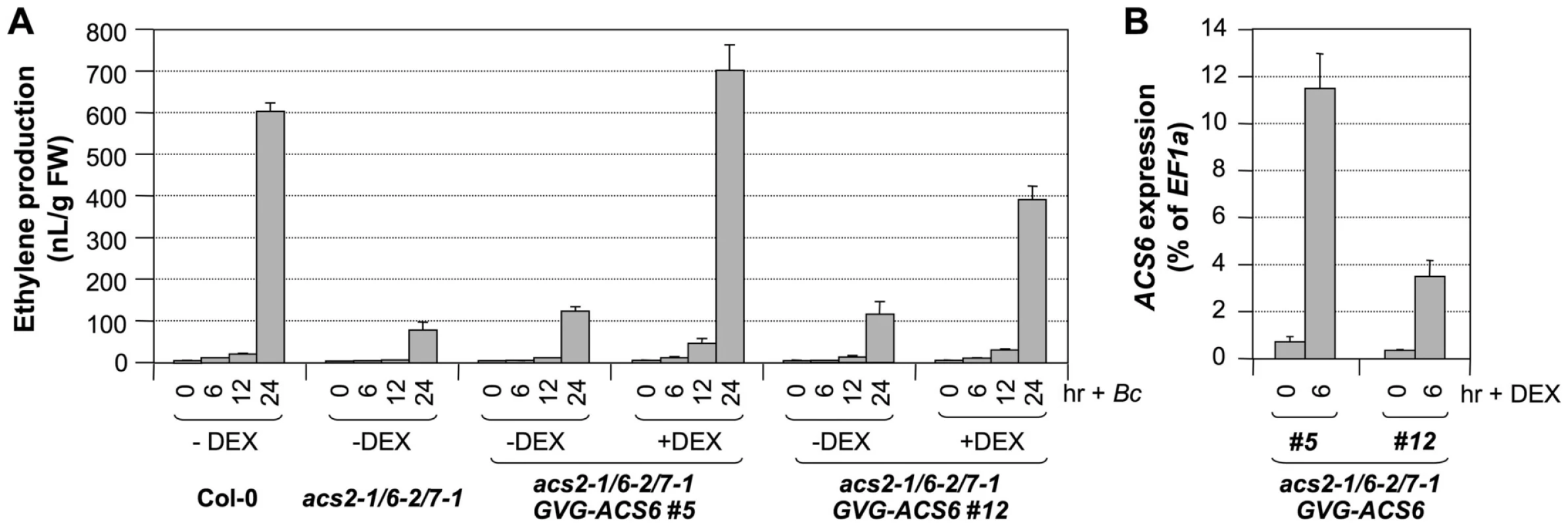
(A) Induction of ACS6 gene expression in the acs2-1/acs6-2/acs7-1/GVG-ACS6 seedlings restores B. cinerea-induced ethylene production. Twelve-day-old seedlings of wild type, acs2-1/acs6-2/acs7-1, and two independent lines of the GVG-ACS6 transgene in the acs2-1/acs6-2/acs7-1 background (#5 and #12) were inoculated with B. cinerea spores. Two groups of acs2-1/acs6-2/acs7-1/GVG-ACS6 seedlings were included with one treated with 1-µM DEX and the other treated with an equal volume of ethanol, the solvent for the DEX stock solution, at the time of B. cinerea spore inoculation. Ethylene accumulation in GC vials was monitored at indicated times. Error bars indicate standard deviations (n = 3). (B) Induction of ACS6 transgene expression in acs2-1/acs6-2/acs7-1/GVG-ACS6 seedlings after DEX treatment. Seedlings were collected before (0 hr) and 6 hr after DEX treatment. Induction of the ACS6 from the GVG-ACS6 transgene was quantified by real-time PCR. ACS6 transcript levels were calculated as a percentage of the EF1α transcript. Error bars indicate standard deviations (n = 3). Our attempts to identify the T-DNA insertion line in the coding region of the ACS6 gene (SALK_025672, acs6-1) failed to reveal a true mutant plant from the seeds received. As a result, we have been using the SALK_090423 line (acs6-2), which has a T-DNA insertion 170 bp upstream of the ATG start codon (Figure 9A)[16], [28]. In the past, we routinely used the double ΔCt method to quantify gene expression in real-time PCR analysis, which indicated the acs6-2 mutant allele as a knockout mutant (Figure 11B, upper panel). However, a more careful analysis performed in this study revealed that it is actually a knockdown mutant with an elevated basal level expression. As shown in Figure 11B (lower panel), acs6-2 seedlings showed a higher basal level of ACS6 expression, but no increase in its transcripts was detected after B. cinerea infection. In contrast, no transcript was detected in the acs6-1 mutant before and after B. cinerea infection. Side-by-side comparison demonstrated that ethylene production levels in acs6-2 and acs6-1 single mutants after B. cinerea inoculation were similar (Figure 11A). In contrast, acs2-1/acs6-2 seedlings produce higher levels of ethylene than acs2-1/acs6-1 (both have the same acs2 mutant allele)(Figure S2). The observable difference between acs6-2 and acs6-1 alleles in the acs2-1 mutant background could be due to the reduction of total ethylene production in the absence of ACS2 gene, which makes it possible to observe a small difference. These results suggest that acs6-2 mutant allele is not a null mutant as acs6-1 allele, and that the high level induction of ACS6 is important to pathogen-induced ethylene production. Together with the gain-of-function evidence shown in Figure 10, we can conclude that ACS6 gene activation plays an essential role in promoting ethylene production in plants challenged by pathogens.
Fig. 11. The acs6-2 mutant allele is a knockdown mutant. 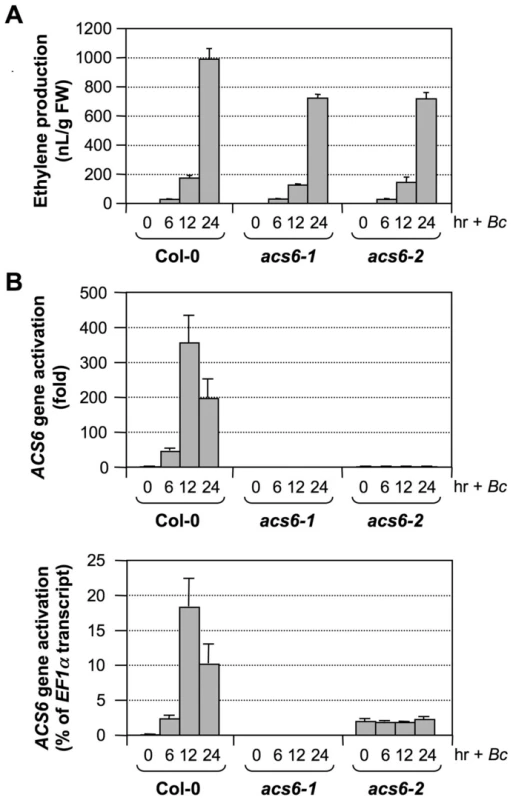
(A) B. cinerea-induced ethylene production in wild type, acs6-2 (SALK_090423), and acs6-1 (SALK_025672) plants. Twelve-day-old seedlings grown in GC vials were inoculated with B. cinerea spores. Ethylene accumulation in GC vials was monitored at indicated times, and seedlings were collected for gene expression analysis. Error bars indicate standard deviations (n = 3). (B) Induction of ACS6 expression in wild type (Col-0), acs6-2, and acs6-1 seedlings after B. cinerea inoculation. Total RNA was isolated from the seedlings collected in (A). Expression of the ACS6 gene was quantified by real-time PCR. ACS6 transcript levels were expressed as fold of induction relative to the zero time point (upper panel) and as a percentage of the EF1α transcript (lower panel). Error bars indicate standard deviations (n = 3). Discussion
Plants challenged by pathogens, especially necrotrophs such as B. cinerea, produce very high levels of ethylene, a critical event in plant disease resistance [6], [7], [35]. In contrast, other stress stimuli, including wounding, only trigger a transient and low-level ethylene biosynthesis. Previously, we reported that MPK3/MPK6 phosphorylation-induced stabilization of ACS2 and ACS6 proteins is an important mechanism in promoting ethylene induction in Arabidopsis [16], [28], [29]. It is also clear from data based on acs2-1/acs6-2 double mutant that ACS2 and ACS6 are not the only contributors in pathogen-induced ethylene production [28]. In this report, we demonstrate the involvement of three additional members of the ACS gene family. Mutation of ACS2 and ACS6, two Type I ACS members, abolishes ∼85% of the ethylene production induced by B. cinerea (Figure 2 and Figure 3) [28]. We failed to detect the transcript from ACS1, the third member of the Arabidopsis Type I ACS isoforms, in the seedlings, and its mutation does not reduce pathogen-triggered ethylene production (Figure 3) [28]. As a result, we believe that ACS1 contributes little, if any, to pathogen-triggered ethylene production. ACS7, a member of the Type III ACS, plays an intermediate role. Its mutation in the acs2-1/acs6-2 background reduces ethylene induction to less than 5% of that observed in the wild type (Figure 2 and Figure 3). This isoform is also the major contributor of the basal level ethylene production in the absence of pathogen infection (Figure S3). The transcripts of ACS2, ACS6, and ACS7 are among the most abundant in B. cinerea-infected Arabidopsis (Figure 1B). The residual levels of ethylene induction in acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1 mutant are likely from ACS8, a Type II ACS isoform, and ACS11, a Type III ACS isoform.
Dual-level regulation of ACS2 and ACS6 by the MPK3/MPK6 cascade in plant stress/defense response
In addition to post-translational regulation, we found that transcriptional activation of the ACS genes is also critical to the high-level of ethylene induction, as depicted in our working model (Figure 12). Stress - and pathogen-activation of ACS genes, such as Arabidopsis ACS6, is well established [1], [36], [42]. In this report, we delineated a signaling pathway involved in the transcriptional activation of ACS2/ACS6 in Arabidopsis after pathogen infection. It is interesting to find that MPK3 and MPK6 not only function in the phosphorylation-induced stabilization of ACS2/ACS6 proteins, but also regulate the expression of ACS2 and ACS6 genes. The MPK3/MPK6 cascade-induced ACS2/ACS6 gene activation is mediated by WRKY33, another MPK3/MPK6 substrate [34]. WRKY33 binds to the W-boxes in the promoters of the ACS2 and ACS6 genes directly in vivo (Figure 9) and is involved in the MPK3/MPK6-induced ACS2/ACS6 gene expression (Figure 7). Mutation of WRKY33 resulted in a smaller reduction (∼60%) in ACS2/ACS6 gene activation in response to B. cinerea infection (Figure 8), possibly due to the presence of other WRKY(s) that can partially compensate the loss of WRKY33. Conditional overexpression of ACS6 in the acs2-1/acs6-2/acs7-1 mutant background greatly enhances the ethylene induction (Figure 10). Furthermore, reduction in ethylene induction in the acs6-2 allele, a knockdown mutant (Figure 11), provides loss-of-function evidence that demonstrates the importance of ACS6 gene activation during pathogen invasion. Transcriptional activation of ACS2 likely has a similar role.
Fig. 12. A model depicting the dual-level regulation of ACS activity by MPK3/MPK6-dependent and independent pathways during pathogen-induced ethylene production. 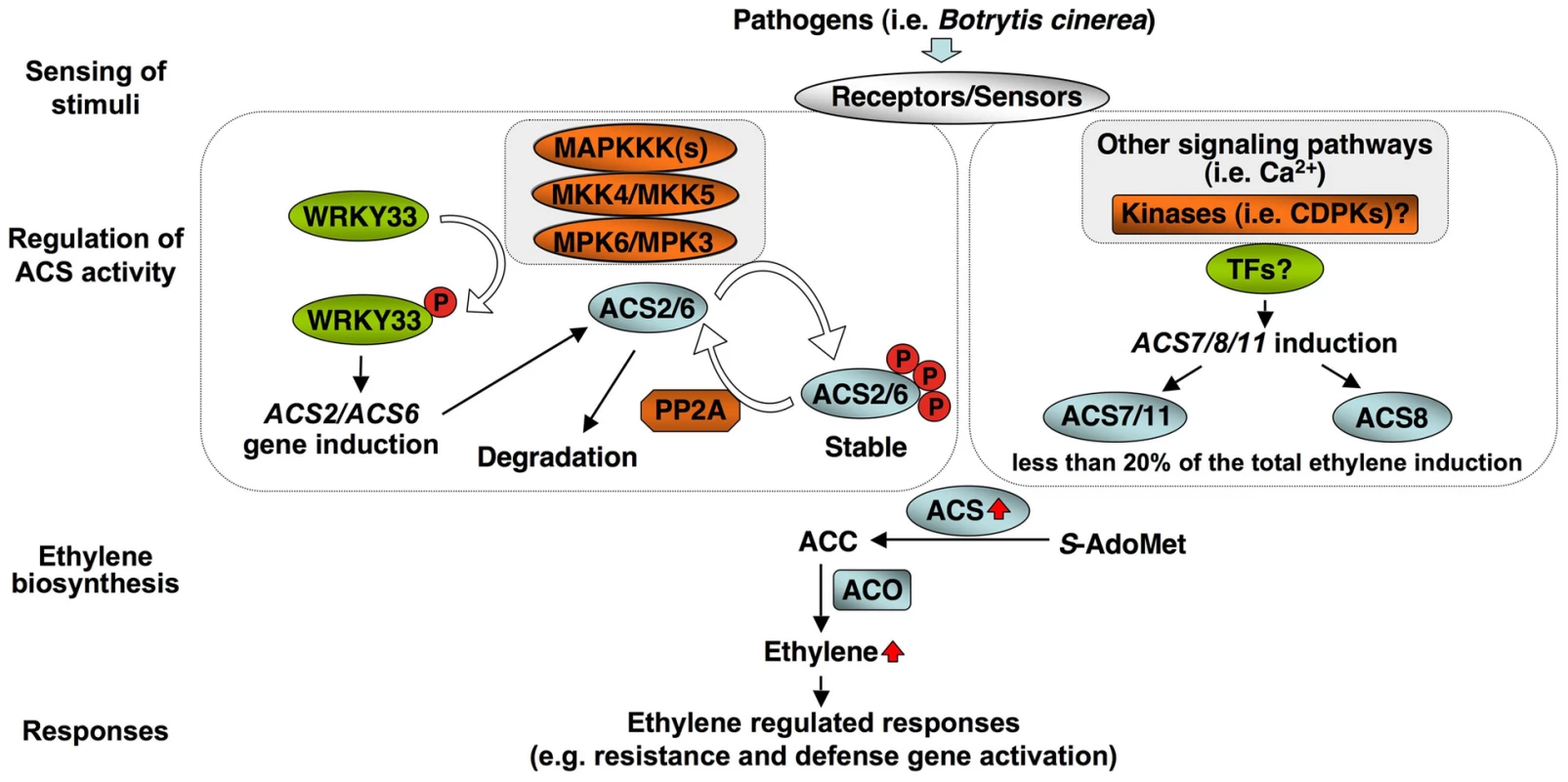
Members of all three types of ACS isoforms are involved in pathogen-induced ethylene production. In B. cinerea-infected plants, Type I (ACS2/ACS6) isoforms contribute the most (∼85%). ACS2 and ACS6 are regulated by the MPK3/MPK6 cascade at both transcriptional and protein stability levels. The transcriptional up-regulation is mediated by WRKY33, a MPK3/MPK6 substrate. Type II (ACS8 and ACS11) and Type III (ACS7) isoforms are activated at the transcriptional level although the regulatory pathway(s) involved is not clear at present. Increase in total cellular ACS activity drives the elevated ethylene production, which triggers downstream responses. Induction of ACS6 expression is associated with stress-induced ethylene production [1], [20], [36], [42]. However, direct evidence supporting the role of ACS gene activation has been lacking. In Arabidopsis, overexpression of wild type ACS6 genes is not sufficient to elevate ethylene production because of the requirement of protein phosphorylation and stabilization [16]. In addition, overexpression of the ACS6 gene in the wild type background fails to enhance ethylene production upon B. cinerea inoculation (Li, G., Liu, Y., and Zhang, S., unpublished data), a result of the high-level gene activation of the endogenous ACS genes (Figure 1). In this study, we expressed the ACS6 gene in an acs2-1/acs6-2/acs7-1 mutant background. The use of a DEX-inducible promoter and two independent lines with different levels of ACS6 gene induction after DEX treatment allowed us to demonstrate the importance of ACS6 gene activation (Figure 10).
Our acs2-1/acs6-2 double mutant produces about 25% of the wild type level of ethylene after B. cinerea infection (Figure 2) [28]. In contrast, the acs2-1/acs6-1 line only produces ∼15% of the wild type ethylene (Figure 3). The difference between these two double mutants is likely a result of different acs6 mutant alleles since both have the same acs2-1 mutant allele. The difference between acs2-1/acs6-2 and acs2-1/acs6-1 also suggests that the acs6-2 allele is not a complete null mutant, which is supported by the presence of ACS6 transcript in acs6-2 allele (Figure 11B). Since the T-DNA insertion in this allele is in front of the transcriptional starting site (Figure 9A), functional transcript is likely to be produced in this mutant allele. Nonetheless, the reduction of ethylene induction in the acs6-2 single mutant or in the acs2-1/acs6-2 double mutant [28](Figure 2) demonstrates the importance of high-level induction of ACS6 expression in pathogen-induced ethylene production. Interestingly, the reduction in ethylene induction in the acs2/acs6 double mutant is always more than the sum of the reduction in each single mutant (Figure 3) [28]. It is possible that the heterodimers of ACS2 and ACS6 are less active than each of homodimer. In the absence of one isoform, only the homodimer can be formed, which partially compensates for the loss of the other isoform.
Importance of dual-level regulation ACS isoforms in determining the levels and kinetics of ethylene induction
Although very inefficient energy-wise, regulation of the ACS protein at the protein stability level by phosphorylation/dephosphorylation allows rapid induction of ethylene biosynthesis, which can occur within minutes after plant sensing of external stimuli [43]–[45]. Such rapid response could be important to plant response to the stress/pathogen stimuli. However, even after phosphorylation stabilization, the ACS6 protein may not be very stable. The half-life of the phospho-mimicking ACS6DDD is only ∼3 hours [29]. In the meantime, protein phosphatase 2A will counteract with the MAPKs by dephosphorylating the phospho-ACS protein [30]. Under this circumstance, transcriptional activation can provide another mechanism to further enhance the ethylene induction in response to pathogen infection. It is well known that stress-induced ethylene production follows different kinetics depending on the stimuli. However, the molecular mechanism underlying this difference is unclear. A good correlation exists between the kinetics of MPK3/MPK6 activation and ethylene induction. For instance, wounding induces a transient ethylene production, which is associated with a transient activation of MAPKs [46], [47]. In contrast, infection of plants by pathogens, especially necrotrophic fungal pathogens, triggers a long-lasting and high-level induction of ethylene biosynthesis, which correlates with a long-lasting and high-magnitude activation of MPK3/MPK6 [28].
As depicted in Figure 12, MPK3 and MPK6 regulate ethylene induction via two different mechanisms: by direct phosphorylation and stabilization of ACS2 and ACS6 proteins [16], [28], [29] and by activation of ACS2 and ACS6 gene expression (this study). Transient activation of MPK3/MPK6 by wounding is also associated with the activation of ACS2/ACS6 gene expression [36]. However, due to the transient nature of MAPK activation, which returns to basal level within ∼0.5 hr to 1 hr [47], the de novo synthesized ACS protein may not have the chance to be phosphorylated and will be degraded quickly in the absence of MAPK phosphorylation. In B. cinerea-infected plants, the induction of ACS2 and ACS6 gene expression will result in high rates of de novo protein synthesis. On top of this, the high-level and long-lasting activation of MPK3/MPK6 [28], [38] can maintain de novo synthesized ACS2 and ACS6 proteins in a phosphorylated state and thereby stabilize the protein against proteasome-mediated degradation [16], [29]. This dual-level regulatory mechanism can maintain a greatly enhanced level of cellular ACS activity and ethylene production in pathogen-infected plants. Recently, it was shown that a PP2A protein phosphatase can counteract with MPK3/MPK6 by dephosphorylating ACS2/ACS6 and can destabilize the ACS protein [30]. In this situation, it is even more important to have the high-level, long-lasting activation of MPK3/MPK6 in order to maintain the ACS2/ACS6 protein in a phosphorylated state to ensure the high rate ethylene biosynthesis observed in plants challenged by pathogens.
WRKY33 is a key transcriptional regulator downstream of MPK3/MPK6 in regulating gene expression in multiple pathways
Activation of MPK3/MPK6 and their orthologs in other plant species induces the expression of large number of stress/defense related genes [48], [49], suggesting the involvement of downstream transcription factors. ERF104 is a substrate of MPK6. Phosphorylation of ERF104 by MPK6 results in release of ERF104 from the complex, which allows ERF104 to activate the expression of genes further downstream [50]. Recently, we identified the transcription factor WRKY33 as the substrate of MPK3 and MPK6 [34]. WRKY33 is involved in the induction of camalexin biosynthesis by promoting the expression of camalexin biosynthetic genes [34], [51]. In this report, we demonstrate that WRKY33 is involved also in activation of ACS2 and ACS6 gene expression and ethylene induction. In the wrky33 mutant background, gain-of-function DD-induced ACS2 and ACS6 gene activation is essentially abolished. Furthermore, a ChIP-qPCR analysis demonstrated that WRKY33 directly binds to the promoters of ACS2 and ACS6 genes (Figure 9). These results reveal that WRKY33 regulates gene expression in multiple stress/defense responses and may function as a master transcriptional regulator downstream of the MPK3/MPK6 cascade.
The expression of both ACS2 and ACS6 genes are regulated by the MPK3/MPK6 cascade and its downstream WRKY33. However, the induction kinetics of ACS2 and ACS6 genes are different in both gain-of-function DD transgenic plants (Figure 4) and wild type plants after pathogen treatment (Figure 1). One possibility is that one or more transcription factors, other than WRKY33, are involved. The differential involvement of these unknown transcription factors could result in the different kinetics observed in the induction of ACS2 and ACS6 genes. These transcription factors may or may not be regulated by the MPK3/MPK6 cascade. The activation of MPK3 and MPK6 proteins in the gain-of-function DD plants is sufficient to induce the expression of ACS2 and ACS6 genes to levels similar to those observed in B. cinerea-infected plants (Figure 1 and Figure 4), suggesting that the transcriptional machinery controlling expression of ACS2 and ACS6 genes is fully turned on in DD plants. On the other hand, mutation of WRKY33 essentially blocks DD-induced ACS2/ACS6 gene activation (Figure 7B) but only partially blocks the induction of ACS2 and ACS6 genes in B. cinerea-infected plants (Figure 8B), suggesting the activation of additional components by B. cinerea that cannot be activated by MPK3/MPK6 cascade alone, possibly homologs of WRKY33 that can partially replace the function of WRKY33 in its absence.
Contribution of MPK3/MPK6-independent pathway(s) to stress-/pathogen-induced ethylene biosynthesis
The aforementioned discussions are focused on the role of the MPK3/MPK6 cascade in regulating ACS2 and ACS6, two major contributors of pathogen-induced ethylene production, as depicted in our working model (Figure 12). A similar level of reduction in ethylene induction in the mpk3/mpk6 and acs2/acs6 double mutants (∼85%) is consistent with our conclusion that the MPK3/MPK6 signaling cascade only controls ACS2 and ACS6. Genetic evidence also supports the involvement of three additional ACS isoforms, ACS7, ACS8, and ACS11, in B. cinerea-induced ethylene production (Figure 2 and Figure 3). ACS7 and ACS11 are the two members in the Type III ACS group in Arabidopsis. ACS8 belongs to the Type II ACS group. Based on mutant analyses, these three ACS genes contribute about 15% of the total ethylene produced in B. cinerea-infected plants (Figure 3) [28]. Transcriptional activation of ACS7, ACS8, and ACS11 is not regulated by the MPK3/MPK6 cascade (Figure 4); the signaling pathway(s) involved in the activation of their expression is unknown. It is also unclear whether ACS7, ACS8, and ACS11 are regulated at the protein stability level. Since ACS7 and ACS11 do not have a typical putative phosphorylation site in their C-termini, they are likely to be regulated at the transcriptional level only. ACS8, similar to ACS5 and ACS9, has a putative CDPK phosphorylation site in its C-terminus. It is possible that phosphorylation by CDPK(s) is involved in its protein stability regulation, similar to ACS5 and ACS9 [32], [33].
Ethylene plays an important role in plant disease resistance. Using a high-order acs mutant, Tsuchisaka et al. (2009) demonstrated that ethylene production is essential to plant resistance against B. cinerea. However, ethylene induction was not examined in the study. In this report, we demonstrate that ethylene induction in acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1/acs11-1 mutant plants after B. cinerea infection is only at ∼2% of that in the wild type (Figure 3). Plant sensing of abiotic stress stimuli or invading pathogens triggers a number of signaling events. Among them, the activation of MAPK cascades and calcium influx are two of the earliest [21], [24], [52]. Our research demonstrates the regulation of ACS2 and ACS6 by a specific MAPK cascade at both transcriptional and post-translational levels. This pathway contributes ∼85% of the total ethylene induction in plants challenged by pathogens. Regulation of the remaining ACS isoforms is unclear at present. Additional studies, including identification of the signaling pathway(s) involved in regulation of ACS7, ACS8, and ACS11 expressions, protein phosphorylation, and protein stability, are needed to further our understanding of the complex regulation of ethylene induction during the plant stress/defense response.
Materials and Methods
Plant growth conditions and treatments
Soil-grown plants were maintained at 22°C in a growth chamber with a 14-hr light cycle (100 µE/m−2 sec−1). For experiments, seeds were surface-sterilized. After imbibition at 4°C for 3–5 days, seeds were sown in petri dishes with liquid half-strength Murashige and Skoog (MS) medium and grown in a growth chamber at 22°C with continuous light (70 µE/m−2 sec−1). Five-day-old seedlings were transferred to 20-ml GC vials with 6 ml of liquid half-strength MS medium (10 seedlings per vial) and maintained under the same growth conditions. Twelve - to fourteen-day-old seedlings grown in GC vials were used for experiments.
Procedures for Botrytis cinerea (Strain: DSM 4709) maintenance and spore preparation were described previously [28]. Twelve-day-old seedlings grown in GC vials were inoculated with B. cinerea spores at a final concentration of 4.0×105 spores/vial. Induction of DD and ACS6 expressions in GVG-NtMEK2DD and GVG-ACS6 transgenic plants was performed by the addition of DEX stock solution (5 mM in ethanol) to a final concentration of 1 µM. An equal volume of ethanol was used as a negative (−DEX) control.
At least two independent repetitions were performed with similar results for experiments with multiple time points. For single time-point experiments, at least three independent repetitions were performed.
Mutant lines and generation of transgenic plants
Arabidopsis thaliana Columbia (Col-0) ecotype was used as the wild-type control, unless stated otherwise. T-DNA insertion mutant alleles of MPK3 (At3g45640), MPK6 (At2g43790), ACS1 (At3g61510), ACS2 (At1g01480), and ACS6 (At4g11280) were described previously [16], [28], [39]. The two ACS7 (At4g26200) mutant alleles, acs7-1 (FLAG_431D05) and acs7-2 (CSHL_ET5768), were obtained from INRA and Cold Spring Harbor Laboratory, respectively. High-order acs mutants generated in Dr. Athanasios Theologis' laboratory [35] were obtained from the Arabidopsis Biological Resource Center (ABRC). The stock numbers are CS16564 (acs2-1), CS16569 (acs6-1), CS16581 (acs2-1/acs6-1), CS16603 (acs2-1/acs6-1/acs4-1), CS16607 (acs2-1/acs6-1/acs5-2), CS16609 (acs2-1/acs6-1/acs9-1), CS16644 (acs2-1/acs6-1/acs4-1/acs5-2/acs9-1), CS16649 (acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1), CS16650 (acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1), and CS16651 (acs1-1/acs2-1/acs6-1/acs4-1/acs5-2/acs9-1/acs7-1/acs11-1). Conditionally rescued mpk3/mpk6 double mutant was generated by transformation of DEX-inducible promoter driven MPK6 cDNA (GVG-MPK6) into mpk3−/−/mpk6+/− plants [39]. Double mutant seedlings were recovered from seeds of mpk3−/−/mpk6+/−/GVG-MPK6 plants sprayed with 30 µM DEX during the flowering stage. GVG-NtMEK2DD (abbreviated as DD), DD/mpk3, and DD/mpk6 lines were previously described [28], [38].
The DEX-inducible promoter driven ACS6 construct (GVG-ACS6) was generated by cloning the ACS6 ORF with a 4xmyc tag [29] into the Xho I/Spe I sites of the pTA7002 vector [53]. The binary vector was transformed into Agrobacterium tumefaciens strain GV3101. Arabidopsis transformation was performed by the floral dip procedure [54], and transformants were identified by screening for hygromycin resistance. Independent lines with ACS6 transgene induction were identified based on real-time qPCR analysis.
Ethylene measurement
GC vials with Arabidopsis seedlings were flushed and capped immediately after treatment. At indicated times, ethylene levels in the headspace of the GC vials were measured by gas chromatography as previously described [16]. Seedlings were then collected, weighed, frozen in liquid nitrogen, and stored at −80°C for future analysis.
RNA extraction and real-time PCR analysis
Total RNA was extracted using the Trizol reagent (Invitrogen). After DNase treatment, RNA (2 µg) was used for reverse transcription. Real-time PCR analysis was performed using an Opticon™ 2 real-time PCR machine as described previously [38]. The transcript of the EF1α gene was used to normalize the samples. Relative gene expression was calculated using two different methods. The first method is the commonly used double ΔCt method, which gives fold of gene induction relative to basal level before treatment (0 hr time point). The second method expresses the transcript level relative to that of the EF1α gene in the same sample, which is a better method when comparison of expression levels of different genes is necessary. The primers used for real-time PCR were ACS1 (At3g61510, 5′-ACGCTTTTCTCGTCCCTACTC-3′ and 5′-GGCCTTAAGGTACGCTGATTC-3′), ACS2 (At1g01480, 5′-GGATGGTTTAGGATTTGCTTTG-3′ and 5′-GCACTCTTGTTCTGGATTACCTG-3′), ACS4 (At2g22810, 5′-AACAACCTTGTGCTCACTGCT-3′ and 5′-AGATCCCTATCAAACCCTGGA-3′), ACS5 (At5g65800, 5′-GACTCTCATGTTTTGCCTTGC-3′ and 5′-TTGGAAGCCATTAGAGCTTGA-3′), ACS6 (At4g11280, 5′-GTTCCAACCCCTTATTATCC-3′ and 5′-CCGTAATCTTGAACCCATTA-3′), ACS7 (At4g26200, 5′-ACGGTACGATACCATTGTGGA-3′ and 5′-GCTCGCCGTCTTTAGTTTTCT-3′), ACS8 (At4g37770, 5′-CCTTCCTTCCTTCAAGAATGC-3′ and 5′-GAGAGTCTCGTTAGCCGGAGT-3′), ACS9 (At3g49700, 5′-CATACCTCGACGAAAACCAGA-3′ and 5′-TCATGTCAACCCAACAGAACA-3′), ACS11 (At4g08040, 5′-CAAACGATGGAGGTTGCTATG-3′ and 5′-TTGGAGACCCATTTGTTGATAAG-3′), and EF1α (At5g60390, 5′-TGAGCACGCTCTTCTTGCTTTCA-3′ and 5′-GGTGGTGGCATCCATCTTGTTACA-3′).
ChIP–qPCR analysis
F1 plants generated from the cross of wrky33/4myc-WRKY33 and DD lines were used for the ChIP assay. Two-week-old seedlings treated with 1-µM DEX for 12 hr were processed as previously described [55]. Briefly, chromatin was isolated from 0.8 g of frozen tissue and sonicated with a Bioruptor sonicator (15 s on and 15 s off cycles, medium-energy settings) for 6 min. Immunoprecipitation was performed by incubating chromatin with 2 µg of anti-myc antibody (Millipore) or mouse IgG (negative control) for 1 hr at 4°C. The protein-chromatin immunocomplex was captured using Protein G-Dynal magnetic beads (Invitrogen). After Proteinase K digestion, the immunoprecipitated DNA was purified using a ChIP DNA Clean and Concentrator kit (Zymo Research Corporation). Immunoprecipitated DNA and input DNA were analyzed by qPCR using primers specific for the promoter regions of PAD3 and WRKY33. The primer pairs (forward and backward) used for ChIP-qPCR were ACS2 (5′-AGGCCATAAGCCCATTCAAA-3′ and 5′-GCCTACAGTGCACGACTTCA-3′) and ACS6 (5′-AAAGTCGTTGAGATTGTGTTGG-3′ and 5′-TGGCAGCCTTAAAGACCAGT-3′), which are in proximity of the W-boxes in the promoters. ChIP results are presented as percentage of input DNA.
Accession numbers
Sequence data for this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: MPK3 (At3g45640), MPK6 (At2g43790), EF1α (At5g60390), ACS1 (At3g61510), ACS2 (At1g01480), ACS4 (At2g22810), ACS5 (At5g65800), ACS6 (At4g11280), ACS7 (At4g26200), ACS8 (At4g37770), ACS9 (At3g49700), ACS11 (At4g08040), and WRKY33 (At2g38470).
Supporting Information
Zdroje
1. WangKL-CLiHEckerJR 2002 Ethylene biosynthesis and signaling networks. Plant Cell 14 S131 S151
2. SchallerGEKieberJJ 2002 Ethylene. SomervilleCRMeyerowitzEM The Arabidopsis Book Rockville, MD American Society of Plant Biologists 1 19 doi/10.1199/tab.0071, http://www.aspb.org.publications/arabidopsis/
3. KleeHJ 2004 Ethylene Signal Transduction. Moving beyond Arabidopsis. Plant Physiol 135 660 667
4. ZarembinskiTITheologisA 1994 Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol 26 1579 1597
5. KendrickMDChangC 2008 Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11 479 485
6. BroekaertWFDelaureSLDe BolleMFCCammueBPA 2006 The role of ethylene in host-pathogen interactions. Ann Rev Phytopathol 44 393 416
7. van LoonLCGeraatsBPJLinthorstHJM 2006 Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11 184 191
8. ChangCBleeckerAB 2004 Ethylene Biology. More Than a Gas. Plant Physiol 136 2895 2899
9. StepanovaANAlonsoJM 2009 Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12 548 555
10. KendeH 2001 Hormone response mutants: a plethora of surprises. Plant Physiol 125 81 84
11. AbelesFBMorganPWSaltveitMEJ 1992 Ethylene in plant biology San Diego Academic Press
12. YangSFHoffmanNE 1984 Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Biol 35 155 189
13. SatoTTheologisA 1989 Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme of ethylene synthesis in plants. Proc Natl Acad Sci USA 86 6621 6624
14. ChaeHSKieberJJ 2005 Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10 291 296
15. YoshidaHNagataMSaitoKWangKEckerJ 2005 Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol 5 14
16. LiuYZhangS 2004 Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16 3386 3399
17. KamiyoshiharaYIwataMFukayaTTatsukiMMoriH 2010 Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J 64 140 150
18. YamagamiTTsuchisakaAYamadaKHaddonWFHardenLA 2003 Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278 49102 49112
19. TsuchisakaATheologisA 2004 Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc Natl Acad Sci USA 101 2275 2280
20. TsuchisakaATheologisA 2004 Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136 2982 3000
21. IchimuraKShinozakiKTenaGSheenJHenryY 2002 Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7 301 308
22. WidmannCGibsonSJarpeMBJohnsonGL 1999 Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev 79 143 180
23. TenaGAsaiTChiuW-LSheenJ 2001 Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4 392 400
24. ZhangSKlessigDF 2001 MAPK cascades in plant defense signaling. Trends Plant Sci 6 520 527
25. JonakCÖkrészLBögreLHirtH 2002 Complexity, crosstalk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5 415 424
26. RodriguezMCSPetersenMMundyJ 2010 Mitogen-activated protein kinase signaling in plants. Ann Rev Plant Biol 61 621 649
27. KimCYLiuYThorneETYangHFukushigH 2003 Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15 2707 2718
28. HanLLiGJYangKYMaoGWangR 2010 Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J 64 114 127
29. JooSLiuYLuethAZhangS 2008 MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J 54 129 140
30. SkottkeKRYoonGMKieberJJDeLongA 2011 Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC Ssynthase isoforms. PLoS Genet 7 e1001370 doi:10.1371/journal.pgen.1001370
31. YoshidaHWangKLCChangC-MMoriKUchidaE 2006 The ACC synthase TOE sequence is required for interaction with ETO1 family proteins and destabilization of target proteins. Plant Mol Biol 62 427 437
32. ChaeHSFaureFKieberJJ 2003 The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15 545 559
33. WangKL-CYoshidaHLurinCEckerJR 2004 Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428 945 950
34. MaoGMengXLiuYZhengZChenZ 2011 Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23 1639 1653
35. TsuchisakaAYuGJinHAlonsoJMEckerJR 2009 A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183 979 1003
36. ArtecaJMArtecaRN 1999 A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsis leaves. Plant Mol Biol 39 209 219
37. PengH-PLinT-YWangN-NShihM-C 2005 Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Mol Biol 58 15 25
38. RenDLiuYYangK-YHanLMaoG 2008 A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105 5638 5643
39. WangHNgwenyamaNLiuYWalkerJCZhangS 2007 Stomatal development and patterning are regulated by environmentally responsive MAP kinases in Arabidopsis. Plant Cell 19 63 73
40. DongJChenCChenZ 2003 Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51 21 37
41. EulgemTSomssichIE 2007 Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10 366 371
42. VahalaJSchlagnhauferCDPellEJ 1998 Induction of an ACC synthase cDNA by ozone in light-grown Arabidopsis thaliana leaves. Physiol Plant 103 45 50
43. CampbellADLabavitchJM 1991 Induction and regulation of ethylene biosynthesis by pectic oligomers in cultured pear cells. Plant Physiol 97 699 705
44. SpanuPGrosskopfDGFelixGBollerT 1994 The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol 106 529 535
45. FelixGGrosskopfDGRegenassMBollerT 1991 Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA 88 8831 8834
46. WuJHettenhausenCMeldauSBaldwinIT 2007 Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19 1096 1122
47. ZhangSKlessigDF 1998 The tobacco wounding-activated MAP kinase is encoded by SIPK. Proc Natl Acad Sci USA 95 7225 7230
48. KimCYZhangS 2004 Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J 38 142 151
49. YangK-YLiuYZhangS 2001 Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98 741 746
50. BethkeGUnthanTUhrigJFPoschlYGustAA 2009 Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci USA 106 8067 8072
51. QiuJ-LFiilBKPetersenKNielsenHBBotangaCJ 2008 Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27 2214 2221
52. BlumeBNürnbergerTNassNScheelD 2000 Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12 1425 1440
53. AoyamaTChuaN-H 1997 A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11 605 612
54. CloughSBentA 1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
55. KaufmannKMuinoJMOsterasMFarinelliLKrajewskiP 2010 Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat Protocols 5 457 472
Štítky
Genetika Reprodukční medicína
Článek Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental InvolvementČlánek Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target GenesČlánek Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early EmbryosČlánek Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2Článek Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human CellsČlánek TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome EndsČlánek Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal CordČlánek Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth ofČlánek MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 6
-
Všechny články tohoto čísla
- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- Mimetic Butterflies Introgress to Impress
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Selection-Driven Gene Loss in Bacteria
- Decreased Mitochondrial DNA Mutagenesis in Human Colorectal Cancer
- Parallel Evolution of Auditory Genes for Echolocation in Bats and Toothed Whales
- Diverse CRISPRs Evolving in Human Microbiomes
- The Rad4 ATR-Activation Domain Functions in G1/S Phase in a Chromatin-Dependent Manner
- Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains
- Uracil-Containing DNA in : Stability, Stage-Specific Accumulation, and Developmental Involvement
- Fuzzy Tandem Repeats Containing p53 Response Elements May Define Species-Specific p53 Target Genes
- Adaptive Introgression across Species Boundaries in Butterflies
- G Protein Activation without a GEF in the Plant Kingdom
- Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes
- An Engineering Approach to Extending Lifespan in
- Incompatibility and Competitive Exclusion of Genomic Segments between Sibling Species
- Effects of Histone H3 Depletion on Nucleosome Occupancy and Position in
- Patterns of Evolutionary Conservation of Essential Genes Correlate with Their Compensability
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
- Preferential Genome Targeting of the CBP Co-Activator by Rel and Smad Proteins in Early Embryos
- A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity
- Protective Coupling of Mitochondrial Function and Protein Synthesis via the eIF2α Kinase GCN-2
- Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis
- Cohesin Proteins Promote Ribosomal RNA Production and Protein Translation in Yeast and Human Cells
- TERRA Promotes Telomere Shortening through Exonuclease 1–Mediated Resection of Chromosome Ends
- Stimulation of Host Immune Defenses by a Small Molecule Protects from Bacterial Infection
- A Broad Requirement for TLS Polymerases η and κ, and Interacting Sumoylation and Nuclear Pore Proteins, in Lesion Bypass during Embryogenesis
- Genome-Wide Identification of Ampicillin Resistance Determinants in
- The CCR4-NOT Complex Is Implicated in the Viability of Aneuploid Yeasts
- Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees
- Genetic Variants on Chromosome 1q41 Influence Ocular Axial Length and High Myopia
- Is a Key Regulator of Pancreaticobiliary Ductal System Development
- The NSL Complex Regulates Housekeeping Genes in
- Attenuation of Notch and Hedgehog Signaling Is Required for Fate Specification in the Spinal Cord
- Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis
- Genome-Wide Functional Profiling Identifies Genes and Processes Important for Zinc-Limited Growth of
- Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia
- MicroRNA93 Regulates Proliferation and Differentiation of Normal and Malignant Breast Stem Cells
- Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family
- Found: The Elusive ANTAR Transcription Antiterminator
- The Mutation in Chickens Constitutes a Structural Rearrangement Causing Both Altered Comb Morphology and Defective Sperm Motility
- Control of CpG and Non-CpG DNA Methylation by DNA Methyltransferases
- Polymorphisms in the Mitochondrial Ribosome Recycling Factor Compromise Cell Respiratory Function and Increase Atorvastatin Toxicity
- Gene Expression Profiles in Parkinson Disease Prefrontal Cortex Implicate and Genes under Its Transcriptional Regulation
- Global Regulatory Functions of the Endoribonuclease III in Gene Expression
- Extensive Evolutionary Changes in Regulatory Element Activity during Human Origins Are Associated with Altered Gene Expression and Positive Selection
- The Regulatory Network of Natural Competence and Transformation of
- Brain Expression Genome-Wide Association Study (eGWAS) Identifies Human Disease-Associated Variants
- Quantifying the Adaptive Potential of an Antibiotic Resistance Enzyme
- Divergence of the Yeast Transcription Factor Affects Sulfite Resistance
- The Histone Demethylase Jhdm1a Regulates Hepatic Gluconeogenesis
- RNA Methylation by the MIS Complex Regulates a Cell Fate Decision in Yeast
- Rare Copy Number Variants Observed in Hereditary Breast Cancer Cases Disrupt Genes in Estrogen Signaling and Tumor Suppression Network
- Genome-Wide Location Analysis Reveals Distinct Transcriptional Circuitry by Paralogous Regulators Foxa1 and Foxa2
- A Mutation Links a Canine Progressive Early-Onset Cerebellar Ataxia to the Endoplasmic Reticulum–Associated Protein Degradation (ERAD) Machinery
- The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression
- Limits to the Rate of Adaptive Substitution in Sexual Populations
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Rumors of Its Disassembly Have Been Greatly Exaggerated: The Secret Life of the Synaptonemal Complex at the Centromeres
- The NSL Complex Regulates Housekeeping Genes in
- Tipping the Balance in the Powerhouse of the Cell to “Protect” Colorectal Cancer
- Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



