-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMaize Is Required for Maintaining Silencing Associated with Paramutation at the and Loci
To understand the molecular mechanisms underlying paramutation, we examined the role of Unstable factor for orange1 (Ufo1) in maintaining paramutation at the maize pericarp color1 (p1) and booster1 (b1) loci. Genetic tests revealed that the Ufo1-1 mutation disrupted silencing associated with paramutation at both p1 and b1. The level of up regulation achieved at b1 was lower than that at p1, suggesting differences in the role Ufo1-1 plays at these loci. We characterized the interaction of Ufo1-1 with two silenced p1 epialleles, P1-rr′ and P1-prTP, that were derived from a common P1-rr ancestor. Both alleles are phenotypically indistinguishable, but differ in their paramutagenic activity; P1-rr′ is paramutagenic to P1-rr, while P1-prTP is non-paramutagenic. Analysis of cytosine methylation revealed striking differences within an enhancer fragment that is required for paramutation; P1-rr′ exhibited increased methylation at symmetric (CG and CHG) and asymmetric (CHH) sites, while P1-prTP was methylated only at symmetric sites. Both silenced alleles had higher levels of dimethylation of lysine 9 on histone 3 (H3K9me2), an epigenetic mark of silent chromatin, in the enhancer region. Both epialleles were reactivated in the Ufo1-1 background; however, reactivation of P1-rr′ was associated with dramatic loss of symmetric and asymmetric cytosine methylation in the enhancer, while methylation of up-regulated P1-prTP was not affected. Interestingly, Ufo1-1–mediated reactivation of both alleles was accompanied with loss of H3K9me2 mark from the enhancer region. Therefore, while earlier studies have shown correlation between H3K9me2 and DNA methylation, our study shows that these two epigenetic marks are uncoupled in the Ufo1-1–reactivated p1 alleles. Furthermore, while CHH methylation at the enhancer region appears to be the major distinguishing mark between paramutagenic and non-paramutagenic p1 alleles, H3K9me2 mark appears to be important for maintaining epigenetic silencing.
Published in the journal: . PLoS Genet 8(10): e32767. doi:10.1371/journal.pgen.1002980
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002980Summary
To understand the molecular mechanisms underlying paramutation, we examined the role of Unstable factor for orange1 (Ufo1) in maintaining paramutation at the maize pericarp color1 (p1) and booster1 (b1) loci. Genetic tests revealed that the Ufo1-1 mutation disrupted silencing associated with paramutation at both p1 and b1. The level of up regulation achieved at b1 was lower than that at p1, suggesting differences in the role Ufo1-1 plays at these loci. We characterized the interaction of Ufo1-1 with two silenced p1 epialleles, P1-rr′ and P1-prTP, that were derived from a common P1-rr ancestor. Both alleles are phenotypically indistinguishable, but differ in their paramutagenic activity; P1-rr′ is paramutagenic to P1-rr, while P1-prTP is non-paramutagenic. Analysis of cytosine methylation revealed striking differences within an enhancer fragment that is required for paramutation; P1-rr′ exhibited increased methylation at symmetric (CG and CHG) and asymmetric (CHH) sites, while P1-prTP was methylated only at symmetric sites. Both silenced alleles had higher levels of dimethylation of lysine 9 on histone 3 (H3K9me2), an epigenetic mark of silent chromatin, in the enhancer region. Both epialleles were reactivated in the Ufo1-1 background; however, reactivation of P1-rr′ was associated with dramatic loss of symmetric and asymmetric cytosine methylation in the enhancer, while methylation of up-regulated P1-prTP was not affected. Interestingly, Ufo1-1–mediated reactivation of both alleles was accompanied with loss of H3K9me2 mark from the enhancer region. Therefore, while earlier studies have shown correlation between H3K9me2 and DNA methylation, our study shows that these two epigenetic marks are uncoupled in the Ufo1-1–reactivated p1 alleles. Furthermore, while CHH methylation at the enhancer region appears to be the major distinguishing mark between paramutagenic and non-paramutagenic p1 alleles, H3K9me2 mark appears to be important for maintaining epigenetic silencing.
Introduction
Paramutation, originally described at the r1 (red1) locus in maize [1], refers to the exchange of epigenetic information between two alleles in a heterozygote that leads to heritable change in expression of one of the alleles. In maize, regulatory genes involved in the synthesis of flavonoids -anthocyanins and phlobaphenes - have been extensively used to study paramutation. Thus far, paramutation has been described for four loci involved in flavonoid biosynthesis: r1 (red1) and b1 (booster1) encode basic helix-loop-helix (bHLH) transcription factors while pl1 (purple plant1) and p1 (pericarp color1) encode R2R3 Myb transcription factors [2]. Of these r1, b1, and pl1 regulate biosynthesis of anthocyanins while p1 regulates biosynthesis of phlobaphenes. Paramutation-like phenomena have also been reported for another gene in maize [3], and other plants and animals [4], [5].
Detailed characterization of paramutation at the b1 locus demonstrated that seven 853-bp tandem repeats located ∼100-kb upstream of the transcription start site are required for paramutation [6], [7]. Cloning of mediator of paramutation1 (mop1) revealed that an RNA-dependent RNA polymerase (RDR), most similar to Arabidopsis RDR2, is required for establishment and maintenance of paramutation [8] and indicated that RNA-mediated chromatin silencing regulates paramutation [9]. The role of RNA-mediated silencing mechanisms in paramutation has been further strengthened by the cloning of two additional maize genes. Of these, required to maintain repression6 (rmr6) encodes largest subunit of RNA polymerase IV (Pol IV) similar to Arabidopsis NRPD1 [10] and Mop2/rmr7 encodes second-largest subunit similar to Arabidopsis NRPD2/E2 [11], [12] which functions in both Pol IV and Pol V complexes [13]. Pol IV and Pol V are plant-specific polymerases which function in the biogenesis of small RNAs and in the RNA-mediated chromatin silencing pathway [13]–[15].
The p1 locus has been used as a model system to understand molecular and epigenetic mechanisms that regulate tissue-specific gene expression. p1-controlled red phlobaphene pigments accumulate in pericarp, cob glumes, husk, silk and tassel glumes. Alleles of p1 are identified based on a two-letter suffix, which denotes their expression in the pericarp and cob glume, respectively [16], [17]. Variable pigmentation patterns of several p1 alleles have been attributed not only to DNA sequence differences, but also to differential epigenetic states [18]–[21]. For instance, P1-rr and P1-wr, two prototype p1 alleles have distinct phenotypes; P1-rr produces red pericarp and red cob glume while P1-wr specifies white pericarp and red cob glume phenotypes. These alleles have major structural differences: P1-rr is a single copy gene while P1-wr is composed of six or more gene copies tandemely arranged in a head-to-tail fashion [18]. Both alleles have similar coding and regulatory sequences and functional analyses have identified similar basal promoter and proximal enhancer regions [22], [23]. However, P1-rr has fully pigmented pericarp whereas P1-wr accumulates no pigment in this tissue. The difference in pericarp expression pattern has been attributed to higher DNA methylation within the regulatory sequences of P1-wr in comparison to P1-rr which displays very low levels of DNA methylation [18]. Several p1 alleles that show a P1-rr-like pericarp and cob glume pigmentation phenotype and carry a P1-wr mutlicopy gene structure have been shown to be hypomethylated [21]. Likewise, loss of pericarp and cob glume pigmentation of P1-wr*, a silent epiallele of P1-wr, has been attributed to even denser cytosine methylation than that of P1-wr [20].
Presence of Unstable factor for orange1 (Ufo1), an un-cloned trans-acting dominant modifier, induces loss of cytosine methylation from P1-wr and P1-wr*, thereby relieving epigenetic suppression and leading to ectopic gain of phlobaphene pigmentation in various plant organs including dried silk, tassel glume, husk, and leaf sheath [20], [24], [25]. Ufo1-induced phenotypes show incomplete penetrance (some progeny carrying the mutation completely lack the mutant phenotype) and poor expressivity (the extent of mutant phenotype is variable).
Paramutation at the p1 locus was observed due to the interactions of the endogenous P1-rr allele with a transgene carrying fragments of the P1-rr regulatory region. The transgene was composed of a 1.2-kb P1-rr distal enhancer fragment (P1.2) located 5-kb upstream of P1-rr transcription start site, a basal (b) p1 promoter fragment (−236 to +326 untranslated leader), GUS coding region, and PinII terminator [26]. When plants with this transgene (P1.2b::GUS) were crossed with those carrying the P1-rr allele, a subset of the transgenic progeny showed a striking reduction in pericarp and cob glume pigmentation. The silenced state of P1-rr, designated as P1-rr′, was inherited independent of the transgene, and it could silence the naïve P1-rr allele. Importantly, the silenced, paramutagenic state of P1-rr′ was associated with increased DNA methylation within the P1.2 fragment that is required for paramutation [26]. Additional transgenic experiments revealed that the P1.2 fragment is required and sufficient for paramutation [27]. The mop1-1 and Mop2-1 mutations disrupt paramutation of P1-rr to P1-rr′ demonstrating that RNA mediated mechanisms are involved in establishment of silencing associated with p1 paramutation [11], [27]. Maintenance of silencing is less dependent on RNA mediated mechanisms, as up to three consecutive generations of exposure to the mop1-1 mutation were required for P1-rr′ up regulation while Mop2-1 had no effect on P1-rr′ silencing even after three generations of continuous exposure [11].
To further elucidate the mechanism(s) underlying epigenetic regulation of paramutation, we characterized involvement of Ufo1 in the regulation of silencing associated with b1 and p1 paramutation. We compared effects of the Ufo1-1 mutation on paramutagenicity and densities of DNA methylation within the P1.2 fragment in P1-rr′ and another spontaneous epimutation of P1-rr, P1-prTP (patterned pericarp and red cob glume). This study highlights the role of histone modifications and DNA methylation and relationship between origin, epigenetic state and differential paramutagenic behavior of these epialleles derived from the common progenitor P1-rr allele. Possible mechanisms dictating different effects of Ufo1-1 on paramutation at b1 and p1 loci are discussed.
Results
Ufo1 reactivates the silent P1-rr′ allele
To test if Ufo1-1 reactivates single-copy silenced p1 alleles, P1-rr′ ufo1 plants were crossed with p1-ww Ufo1-1 (Figure 1A). Of 239 F1 plants screened, 62 (26%) showed gain of pericarp pigmentation while 177 (74%) remained silent as indicated by a colorless pericarp except red pigmentation at the point where silk attaches to pericarp during seed development (hereafter referred to as silk scarred phenotype). Similar to earlier published results [20], [24], the effect of Ufo1-1 on P1-rr′ silencing was not fully penetrant as only a subset of the F1 progeny showed gain of pigmentation with pericarp phenotypes varying from uniformly red or orange to red/orange variegation of pericarps. To test the heritability of reactivated P1-rr′ phenotypes, F1 plants showing gain of pericarp pigmentation were crossed with p1-ww[4Co63] (Figure 1B). As expected, approximately half of the progeny (54.4%) had colorless pericarp and cob glume specified by the homozygosity for the recessive p1-ww allele ears (χ2 = 0.79; P = 0.38). If Ufo1-1 mediated P1-rr′ up regulation were not heritable then 25% of total progeny is expected to carry Ufo1-1 and P1-rr′ and have up regulated pericarp and cob glume pigmentation phenotype, while 25% of progeny carrying P1-rr′ and a wild type ufo1 allele should have silenced silk scarred ears. Results of the analysis demonstrated that 37.8% of ears had silk scarred P1-rr′ phenotype and 7.8% had up regulated red or orange pericarp and cob glume pigmentation phenotypes. This segregation ratio indicates that the reactivated P1-rr′ state is not heritable and reverts back to silenced state after segregation of Ufo1-1.
Fig. 1. Ufo1-1 mediates reactivation of P1-rr′. 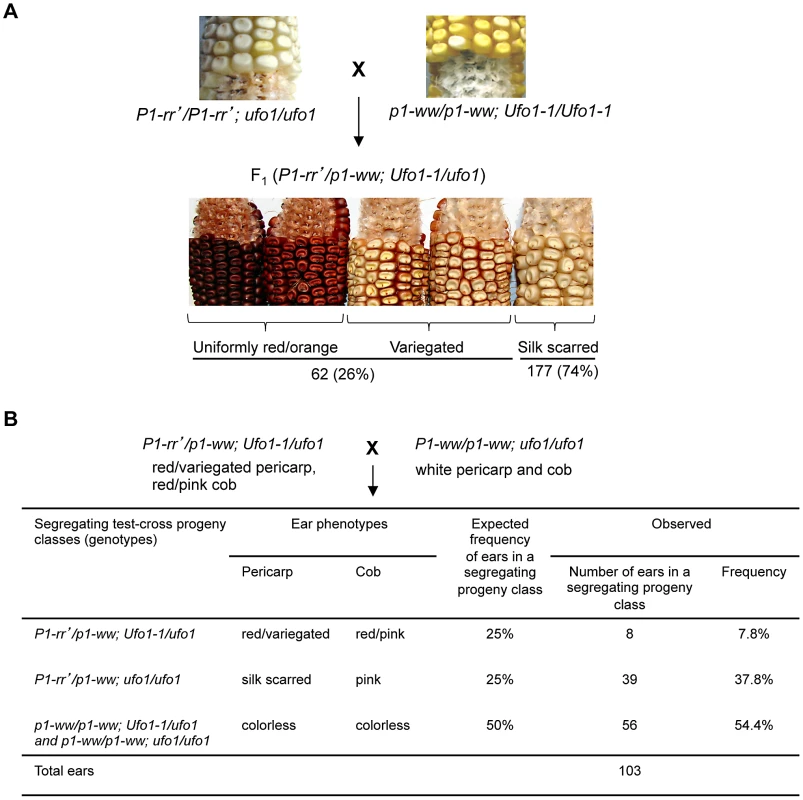
A. Pericarp and cob glume phenotypes of F1 progeny plants obtained from a cross between highly suppressed P1-rr′ ufo1 and p1-ww Ufo1-1. B. Heritability of Ufo1-1-induced reactivation of P1-rr′. F1 plants were crossed with p1-ww[4Co63] and progeny was scored for pericarp pigmentation. Expected segregation frequencies are based on the assumption that P1-rr′ reverts back to silenced state after segregation of Ufo1-1. The paramutagenic activity of individual P1-rr′ families is highly variable and the suppression of naive P1-rr by P1-rr′ can range from 0 to >90% [26]. We tested if P1-rr′ families with differential paramutagenic activity also differ for their extent of activation by Ufo1. Representative P1-rr′ plants were crossed with p1-ww Ufo1-1 and each P1-rr′ plant was also crossed with the paramutable naïve P1-rr allele (Figure 2). Scoring the resultant progeny for frequency of paramutation revealed that P1-rr′ paramutagenicity varied between 40 and 81.6%, while reactivation by Ufo1-1 varied between 5.2% and 62.9%. Interestingly, frequency of reactivation by Ufo1-1 was lower in the progeny of the highly paramutagenic P1-rr′ (families 3 and 4) as compared to the progeny of the low paramutagenic P1-rr′ (families 1 and 2). Statistical analysis revealed that high paramutagenic ability negatively correlated with frequency of reactivation by Ufo1-1 (R2 = −0.86). This result demonstrates that the mechanism(s) governing the paramutagenic activity also interfere with the effect of the Ufo1-1 mutation on P1-rr′.
Fig. 2. Paramutagenicity of P1-rr′ inversely correlates with frequency of reactivation by Ufo1-1. 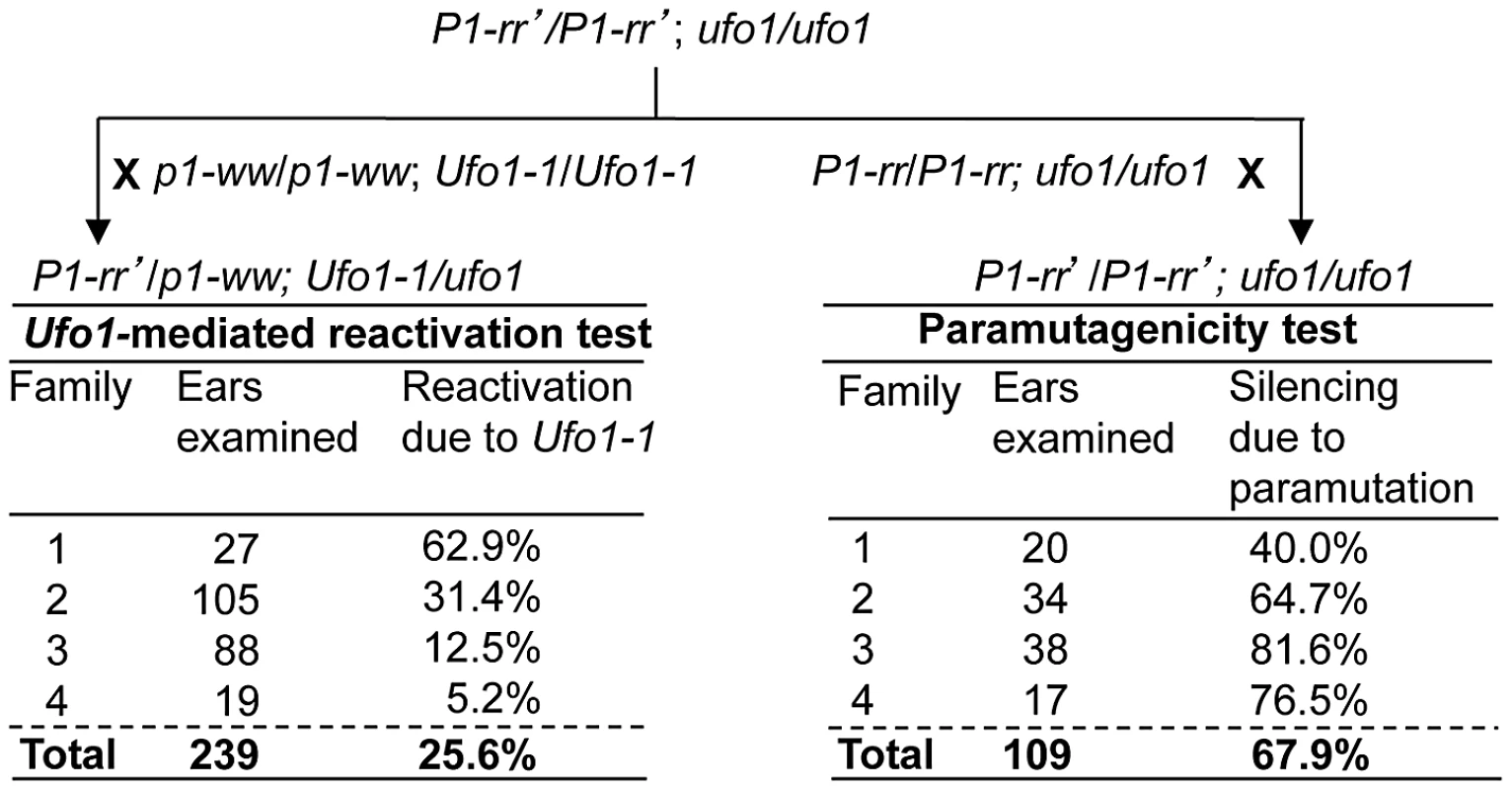
Crossing scheme is shown at the top. Tables show results of tests for P1-rr′ paramutagenicity (on the right) and Ufo1-1 mediated P1-rr′ reactivation (on the left) for the same P1-rr′ plants. Ufo1-1–induced reactivation is associated with hypomethylation of P1-rr′
The silenced state of P1-rr′ is characterized by increased methylation of the P1.2 enhancer element [26]. To test if the reactivation of P1-rr′ by Ufo1-1 involved hypomethylation within the P1.2 enhancer sequence, p1 fragment 15 was used as a probe to hybridize gel blots carrying leaf genomic DNA digested with methylation sensitive endonuclease HpaII (Figure 3) and with a combination of HpaII and methylation insensitive endonuclease DraI (data not shown). In P1-rr, two major fragments of approximately 1.2 and 1.1-kb are observed (Figure 3A) which originate from unmethylated HpaII sites flanking the fragment 15 in the upstream promoter and downstream of 3′ untranslated region (UTR) (Figure 3B). Most of these sites are methylated in P1-rr′ resulting in the loss of 1.2 and 1.1-kb bands and appearance of higher molecular weight bands indicating increased DNA methylation at these HpaII sites (Figure 3B). Examination of restriction patterns revealed loss of DNA methylation in all reactivated (R) and non-activated (N) P1-rr′/p1-ww; Ufo1-1/+ plants, as seen from the loss of high (8.1 and 6.5-kb) molecular weight bands. However, additional loss of DNA methylation from HpaII sites that flank fragment 15 are observed in the R plants which results in the reappearance of 1.2 and 1.1-kb bands. This result demonstrates that partial loss of DNA methylation occurs in all reactivated P1-rr′/p1-ww; Ufo1-1/+ plants, but greater hypomethylation is observed in plants with up regulated pericarp and cob glume pigmentation phenotype.
Fig. 3. Ufo1-1-mediated loss of cytosine methylation in P1-rr′. 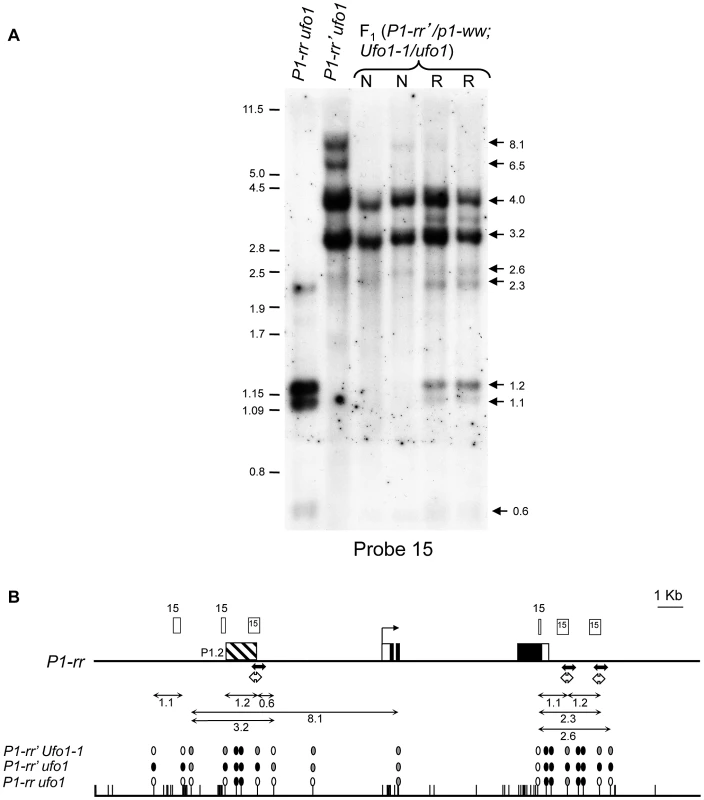
A. DNA gel blot showing methylation differences among P1-rr ufo1, P1-rr′ufo1, two F1 sibling plants not reactivated by Ufo1-1 (marked as N), and two F1 sibling plants reactivated by Ufo1-1 (marked as R). Leaf genomic DNA was digested with HpaII endonuclease and hybridized with probe fragment 15. Molecular weight marker in kilobase pair is shown on the left, and arrows on the right show positions and sizes of the hybridizing bands. B. Methylation map of P1-rr ufo1, P1-rr′ ufo1 and P1-rr′ Ufo1-1. Gene structure is shown at the top. A solid black line represents P1-rr sequence with black boxes denoting exons and open boxes indicating UTR's. Transcription start site is indicated as a bent arrow. Positions and relative sizes of the probe 15 fragments are shown as open boxes above the gene map; three relatively smaller boxes labeled as 15 are the truncated copies of probe 15. Striped box to the left of transcription start site represents the P1.2 enhancer fragment. Solid double-headed arrows below the gene map indicate the region examined by genomic bisulfite sequencing while hollow double-headed arrows represent the region analyzed by ChIP assay. Methylation map of HpaII sites is shown below the gene structure. Solid line represents the P1-rr sequence while the short vertical lines represent HpaII sites. Ovals above the HpaII sites represent methylation status of individual sites; open, grey and black ovals represent non-methylated, partially methylated, and completely methylated HpaII sites, respectively. Lines with double arrowheads above the methylation map represent the HpaII fragments observed on the DNA gel blot; only fragments and HpaII sites resolved by the restriction analysis are shown. To assay methylation of individual cytosine residues, we performed genomic bisulfite sequencing on the upper strand of a 443-bp fragment of the P1.2 enhancer region, which is required for paramutation. Since P1-rr is expressed in pericarp and Ufo1-1 induced gain of pigmentation is more pronounced in this tissue, we used pericarp DNA for the methylation assay. In the functional P1-rr allele, almost all of the symmetric (CG and CHG; H is A, T, or C), and asymmetric (CHH) cytosine sites were un-methylated (Figure 4). Silencing of P1-rr′ was associated with hypermethylation of most symmetric sites, and to a lesser extent, with that of asymmetric sites (Figure 4, Figure S1). Additionally, symmetric cytosine methylation was higher in the 5′ end and it was reduced toward the 3′ end of the P1.2 fragment. Analysis of reactivated P1-rr′Ufo1-1 plants revealed that DNA methylation was dramatically reduced at all symmetric and asymmetric cytosines to a level comparable with that of the P1-rr ufo1 plants. Therefore, the DNA gel blot and bisulfite sequencing analyses demonstrated that Ufo1-1-mediated reactivation of P1-rr′ correlates with reduction of methylation within the P1.2 enhancer fragment.
Fig. 4. Ufo1-1-induced methylation modifications of individual cytosine residues in the 443-bp fragment of the P1.2 enhancer of P1-rr′ and P1-prTP. 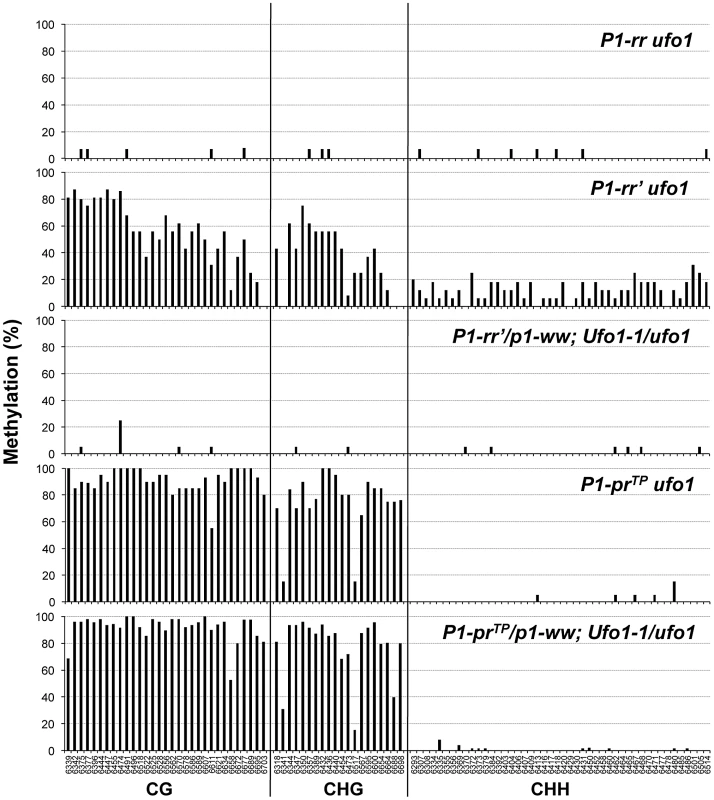
P1-rr′/p1-ww; Ufo1-1/ufo1 and P1-prTP/p1-ww; Ufo1-1/ufo1 refer to plants that showed gain of pigmentation. Bisulfite sequencing was performed on genomic DNA extracted from pericarp tissue. Genomic DNA from two independent plants per genotype was used for bisulfite sequencing. For each genotype, the percent methylation is shown on the y-axis while the position of cytosine residues in CG, CHG, and CHH context is shown at the x-axis of the bottom graph. P1-prTP is a silenced, hypermethylated epiallele of P1-rr
In an earlier study, Das and Messing [19] described a P1-pr (patterned pericarp and red cob glume) epiallele of P1-rr with reduced pericarp pigmentation. P1-pr silencing correlated with increased DNA methylation and failure of the P1.2 enhancer [28] to undergo tissue-specific chromatin remodeling in pericarp [19], [29]. We characterized another independently isolated spontaneous epiallele of P1-rr, P1-prTP, which is phenotypically similar to P1-pr [19] and P1-rr′ [26] with colorless or silk scarred pericarp and light pink to light red cob glume (Figure 5A). The silenced P1-prTP state is very stable as out of ∼1,000 plants screened, none showed any spontaneous gain of pericarp pigmentation (data not shown).
Fig. 5. Silencing of P1-prTP, an epiallele of P1-rr, is correlated with DNA methylation. 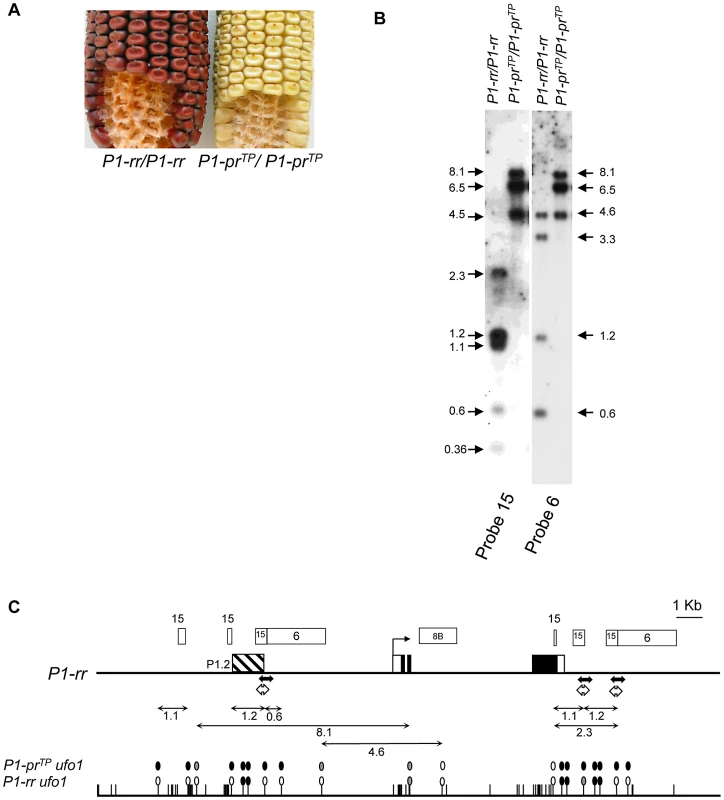
A. Loss of pericarp and cob glume pigmentation phenotype of P1-prTP. The pericarp pigmentation is present only at the silk attachment point while the cob glumes are pink. B. P1-prTP is highly methylated as compared to P1-rr. Leaf genomic DNA of P1-rr and P1-prTP was digested with HpaII and sequentially hybridized with p1 probe fragments 15 and 6 (See Figure 5C for location of probes). Size and location of hybridizing bands in kilobase pair is indicated by arrows on the left for probe 15 and on the right for probe 6. C. Methylation map of P1-rr ufo1 and P1-prTP ufo1. Gene structure is shown at the top and the rest of the figure description is same as in Figure 3B. Extensive DNA gel blot analysis and partial sequencing revealed that the P1-prTP sequence is identical to P1-rr (not shown). To test if P1-prTP silencing is associated with epigenetic modification, we compared DNA methylation of HpaII-digested P1-prTP and P1-rr. In P1-rr, the probe fragment 15 hybridized to five restriction fragments including the 1.2 kb and 1.1 kb bands (Figure 5B). Loss of these bands and appearance of higher molecular weight bands in P1-prTP showed that the region around the P1.2 enhancer was methylated (Figure 5C). Similarly, upon hybridization with the probe fragment 6, P1-prTP produced higher molecular weight bands as compared to P1-rr indicating methylation within the promoter region. However, P1-prTP and P1-rr had similar methylation levels in the coding region as shown by hybridization with probe fragment 8B (Figure S2). Taken together, these results demonstrate that P1-prTP is an epiallele of P1-rr and that silencing of P1-prTP is associated with DNA methylation of upstream regulatory regions including the P1.2 enhancer.
P1-prTP is not paramutagenic to P1-rr
To test if P1-prTP participates in paramutation, it was crossed with P1-rr and resulting F1 progenies were scored for pericarp pigmentation. All 300 F1 plants screened showed red pericarp and red cob glume phenotype indicating that P1-prTP is non-paramutagenic to P1-rr and behaves as a recessive allele. Moreover, in the F2 generation, P1-rr and P1-prTP phenotypes segregated in the expected 3∶1 ratio further supporting that P1-prTP does not participate in paramutation (data not shown). To summarize, genetic data indicated that P1-prTP is not paramutagenic to a naive P1-rr.
Ufo1-1 disrupts the suppressed state of P1-prTP
To test if the Ufo1-1 mutation can disrupt the silenced state of P1-prTP, genetic crosses were performed (Materials and Methods, Figure 6A). Analysis of 155 F1 plants revealed that P1-prTP was upregulated by the Ufo1-1 mutation and, similar to P1-rr′, frequency of up regulation was low as only 21% of plants exhibited gain in pericarp and cob glume pigmentation (Figure 6A). Further, the F2 progeny of Ufo1-1 expressing plants did not produce the expected phenotypic ratio of 9∶3∶3∶1 (data not shown). To assay whether Ufo1-1-mediated reactivation of P1-prTP was heritable, F1 plants with an increased pericarp and cob glume pigmentation were crossed with colorless p1-ww[4Co63] plants carrying a wild type ufo1 allele (Figure 6B). As expected, 50% of the progeny was homozygous for p1-ww and therefore produced colorless and pericarp and cob glume (χ2 = 1.25; P = 0.263). If the activated state of P1-prTP returned to a silenced state after segregation of Ufo1-1, 25% of progeny was expected to have a silenced phenotype, while the remaining 25%, still carrying Ufo1-1, would have increased pigmentation. There were 28.2% of silk scarred and 17% of red/variegated pericarp and cob glume individuals indicating that, similar to P1-rr′, P1-prTP up regulation was not heritable in the absence of Ufo1-1. Overall, these observations demonstrate that Ufo1-1 temporarily disrupts the silenced epigenetic state of P1-prTP leading to increased pericarp pigmentation. Non-heritable P1-prTP gain of pigmentation in the mutant Ufo1-1 background is reminiscent of Ufo1-1 interaction with other p1 alleles [20], [24].
Fig. 6. Representative data showing Ufo1-1–induced reactivation of P1-prTP. 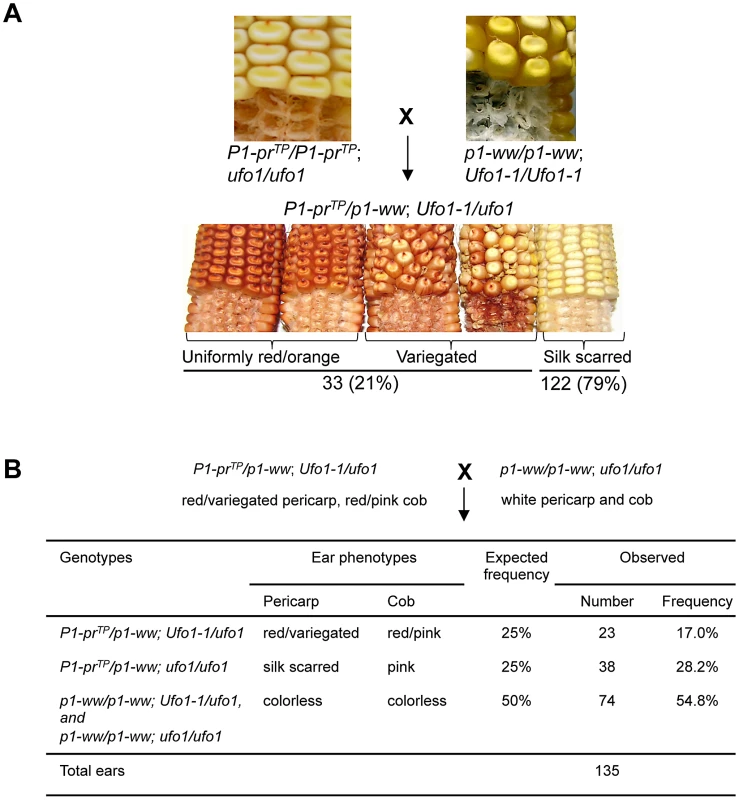
A. Pericarp and cob glume pigmentation phenotypes of F1 progeny obtained from a cross between P1-prTP and p1-ww Ufo1-1 plants. B. Heritability of Ufo1-1-induced reactivation of P1-prTP. F1 plants showing gain of pericarp pigmentation (red/variegated pericarps) were crossed with p1-ww[4Co63] and test-cross progenies were examined for pericarp and cob glume pigmentation. Expected segregation frequencies are based on assumption that increased pigmentation of P1-prTP allele is not heritable in the absence Ufo1-1. Ufo1-1–induced reactivation of P1-prTP occurs in the presence of DNA methylation within the P1.2 enhancer
Gel blot analysis involving HpaII-digested leaf genomic DNA hybridized with p1 probe fragments did not detect any methylation differences between the silenced and reactivated P1-prTP plants (Figure S2). We also performed genomic bisulfite sequencing of the 443 bp of the P1.2 enhancer in pericarp of P1-prTP ufo1 and P1-prTP/p1-ww; Ufo1-1/ufo1 plants. In comparison to P1-rr′, which is hypermethylated in symmetric and asymmetric contexts, P1-prTP is hypermethylated only in symmetric contexts (Figure 4). No decrease in DNA methylation was observed in pericarps of P1-prTP/p1-ww plants that were strongly up regulated by Ufo1-1. These results are in striking contrast with P1-rr′ where Ufo1-1-up regulation correlated with dramatic reduction of cytosine methylation and demonstrates that similar phenotypes of P1-rr′ and P1-prTP are specified by distinct molecular mechanisms.
Ufo1-1–induced reactivation is associated with depletion of suppressive histone marks on P1-rr′ and P1-prTP
Histone 3 lysine 9 dimethylation (H3K9me2) is a histone modification associated with heterochromatin assembly and transcriptional silencing. To test if histone modifications are involved in the silencing of P1-rr′ and P1-prTP, chromatin immunoprecipitation (ChIP) followed by quantitative real-time PCR (qPCR) was performed to determine the H3K9me2 enrichments at the P1.2 region. The ChIP assays showed that the chromatin encompassing the 1.2 kb distal enhancer is significantly enriched for H3K9me2 in P1-rr′ and P1-prTP plants as compared to P1-rr (Figure 7). Thus, irrespective of their involvement in paramutation, silenced state of both alleles is associated with enrichment of the suppressive H3K9me2 mark.
Fig. 7. Comparison of H3K9-dimethylation (H3K9me2) levels in P1-rr′ and P1-prTP in the absence and presence of Ufo1-1. 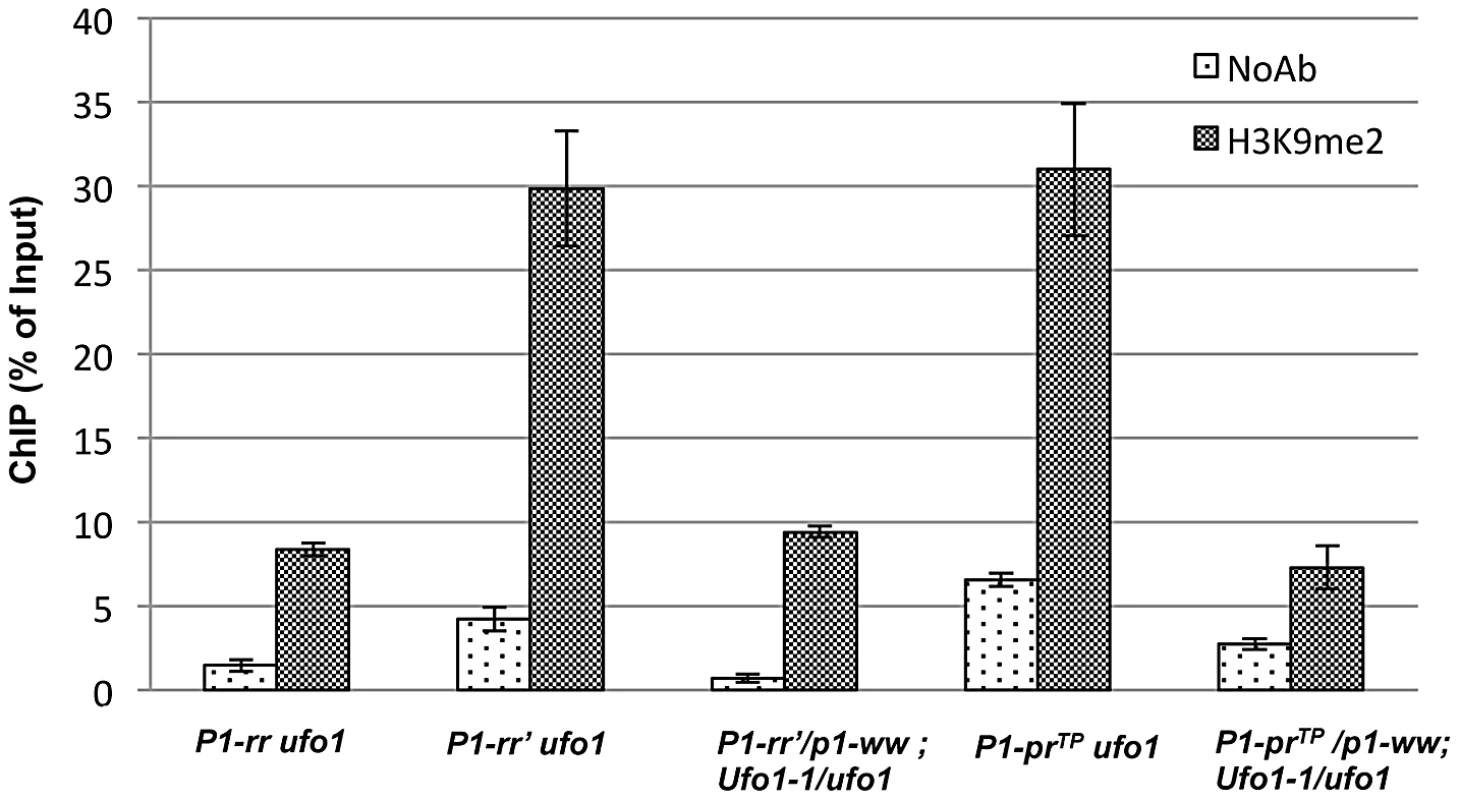
ChIP assay was performed using pericarp tissues. Chromatin complex was immunoprecipitated with antibodies against H3K9me2. Mouse IgG was used as a negative control (NoAb). Quantitative PCR was performed to quantify the DNA enrichments at the P1.2 kb distal enhancer. The data presented here is the mean ± SE from three biological replicates of three independent ChIP experiments. To further investigate if ufo1 is involved in maintenance of H3K9me2, ChIP assays were performed at P1-rr′ and P1-prTP in the presence of Ufo1-1. Interestingly, in the presence of Ufo1-1, there is a dramatic reduction of H3K9me2 within the 1.2 kb distal enhancer region of P1-rr′ and P1-prTP (Figure 7). To summarize, these results demonstrate that ufo1 plays a role in maintaining repressive H3K9me2 histone marks at P1-rr′ and P1-prTP.
Ufo1-1 disrupts silencing of the paramutated b1 allele
Paramutation at the b1 locus occurs when the highly expressing, darkly pigmented B-I allele is exposed to a low expressing, lightly pigmented B′ allele in heterozygote [4]. While paramutable B-I is unstable and spontaneously reverts to B′ at variable frequencies, paramutagenic B′ is stable in wild type genetic backgrounds. To test if Ufo1-1-mediated disruption of epigenetic silencing associated with paramutation extends beyond the p1 locus, the silenced B′ allele was introgressed into the Ufo1-1 background and B′-specified plant pigmentation was evaluated (Figure S3). Examination of the segregating progeny revealed that B′-specified pigmentation increased in the mutant (Ufo1-1/ufo1), as evident from multiple, wide and darkly pigmented sectors on sheaths (Figure 8A), husks, and tassels (not shown). Extent of B′ pigmentation in the Ufo1-1 background was moderate and never reached solid dark purple plant pigment observed in B-I or dark pigmentation phenotypes observed for B′ in mop1-1 [8], [9] and mop2-1 [11] mutant backgrounds. The Ufo1-1 mutation was penetrant in about half of F1 plants, and this number, but not intensity of B′ pigmentation, increased in later backcrosses (BC2 and BC3) (Figure 8B). Overall, these results show that the Ufo1-1 mutation disrupts the silenced state of B′ allele and therefore ufo1 is involved in the pathway that regulates silencing associated with paramutation at multiple loci.
Fig. 8. Ufo1-1 increases B′ pigmentation. 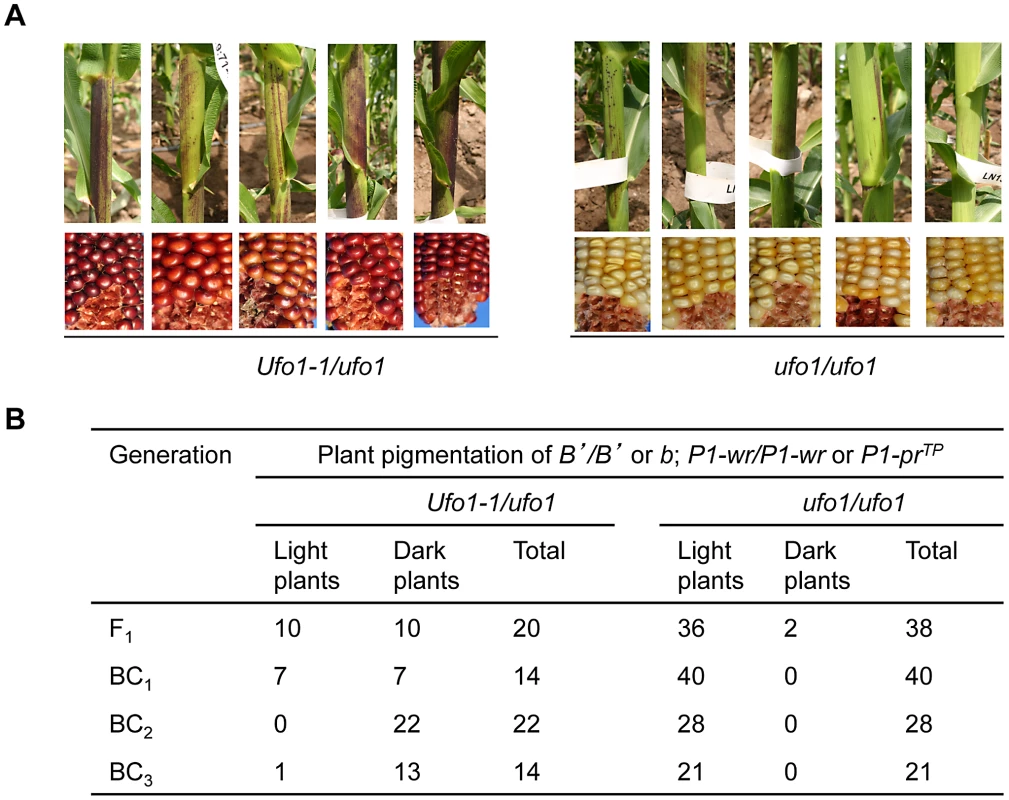
Effect of Ufo1-1 was assayed in four consecutive backcrosses as shown in the crossing scheme in Figure S3. A. Photo panels from BC2 generation showing B′ plant pigmentation and corresponding P1-wr pericarp and cob glume phenotypes, which were used as phenotypic indicators of Ufo1-1 presence. The P1-wr has colorless pericarp and red cob and P1-prTP has silk-scarred pericarp and pink cob glume in the ufo1 genetic background. In the mutant Ufo1-1/ufo1 background P1-wr and P1-prTP exhibit dramatic increase in pigmentation and have very dark red pericarp and cob glume. In the wild type ufo1 background, the B′ allele has light streaky plant pigmentation while in the Ufo1-1/ufo1 background B′ plants have increased pigmentation as evidenced by the presence of broad darkly pigmented sectors. This increase of B′ pigmentation is significant because B′ never displays any increase in pigmentation in wild type genetic backgrounds. B. Summary of the results from F1 and three generations of introgression in the mutant Ufo1-1/ufo1 background are shown. Discussion
This study provides further insights into the mechanisms underlying epigenetic silencing and paramutation in maize. Despite sharing a common P1-rr progenitor allele, the two epialleles described here are distinct in that P1-rr′ is paramutagenic while P1-prTP is neutral and does not participate in paramutation. Silencing of both these alleles is associated with increased cytosine methylation within the P1.2 distal enhancer. Hypermethylation in P1-rr′ is observed in all cytosine contexts while the methylation in P1-prTP is restricted to CG and CHG contexts. Both alleles, however, have enrichment in H3K9me2 in this region suggesting that this suppressive mark is involved in maintenance of silencing in both alleles. Presence of Ufo1-1 alleviates epigenetic suppression which is associated with dramatic reduction of H3K9me2. We also show here that Ufo1-1 disrupts the paramutation-associated silencing of the B′ allele. Thus ufo1 is involved in the maintenance of epigenetic silencing originating spontaneously (epimutation), and that during paramutation.
Unique cytosine methylation patterns of P1-rr′ and P1-prTP indicate involvement of distinct mechanisms in silencing of these alleles
Given the fact that P1-rr′ and P1-prTP are epialleles of a common progenitor allele (P1-rr), their differential paramutagenic ability is intriguing; while P1-rr′ is able to communicate its chromatin state in trans to P1-rr, P1-prTP fails to do so. A notable difference between these epialleles is the nature of their origin: P1-rr′ silencing was induced by a transgene carrying P1.2 distal enhancer sequence, while P1-prTP originated spontaneously and causal factors for its origin are unknown. Establishment of transgene-mediated silencing at P1-rr′ requires RNA-dependent RNA polymerase activity of mop1 [27] and plant-specific RNA polymerase IV/V activity as evidenced by requirement for mop2 [11] implicating RNA mediated mechanism in the origin of this epiallele. The extent and distribution of cytosine methylation in the distal enhancer region of P1-rr′ and P1-prTP are strikingly different. In P1-rr′, this region is methylated in all sequence contexts (CG, CHG, and CHH), and CG and CHG methylation levels are higher in the 5′ and decrease toward the 3′ end, while CHH methylation is evenly distributed throughout the assayed region. These high and low methylation regions correspond, respectively, to the middle and the end of the sequence homology between the endogenous P1.2 enhancer and the transgene fragment that caused the initial silencing. This pattern of symmetric cytosine methylation and increased levels of asymmetric CHH methylation further points to the role of RNA-mediated mechanisms in P1-rr′ silencing. In P1-prTP, however, only CG and CHG sites are methylated and the methylation is uniformly high throughout the assayed region. Furthermore, while P1-rr′ can, on rare occasions, show spontaneous gain of function [26], silenced state of P1-prTP is very stable. While it is possible that initial events leading to the silencing of P1-prTP involved RNA mediated mechanism, based on a lack of asymmetric DNA methylation, RNA signals do not appear to contribute to the maintenance of DNA methylation. In summary, difference in the levels of CHH methylation, a hallmark of RdDM, seems to be the only epigenetic mark that correlates with differential paramutagenic ability of the two alleles.
Loss of suppressive histone mark but not cytosine methylation correlates with Ufo1-1–mediated reactivation of silenced alleles
While the paramutagenic (P1-rr′) and non-paramutagenic (P1-prTP) alleles differ for their cytosine methylation patterns within the P1.2 enhancer, both display enrichment in H3K9me2. Furthermore, while symmetric methylation persists at the enhancer in Ufo1-reactivated P1-prTP, levels of H3K9me2 are decreased in both reactivated alleles. Thus, H3K9me2 appears to be an indispensable repressive epigenetic mark for maintaining silencing at the paramutagenic and non-paramutagenic p1 epialleles. However, we cannot rule out that, both H3K9me2 and DNA methylation play important and mutually reinforcing roles in maintenance of silencing at both P1-prTP and P1-rr′. In a similar study at the b1 locus, tandem repeats critical for paramutation exhibited tissue-independent DNA methylation, while enrichment in H3K9me2 and H3K27me2 was tissue-specific [30]. Based on these results, authors concluded that H3K9me2 does not play a role in the mitotic heritability of the silenced B′ state, but rather serves to reinforce silencing in a tissue-specific manner [30]. In our study, H3K9me2 enrichment was observed in pericarp tissue of P1-rr′ and P1-prTP pericarps, and loss of silencing in Ufo1-1 correlated with loss of H3K9me2. However, it remains unclear whether this mark is involved in tissue-specific regulation and reinforcement of silencing, or it also plays a role in tissue-independent maintenance of silencing.
Several studies have reported a positive correlation between cytosine, especially CHG, methylation and H3K9me2 marks [31]-[34]. In Arabidopsis, loss of KRYPTONITE, an H3K9-specific methyltransferase, results in a loss of both H3K9me2 and CHG methylation [35] and a genome-wide survey of H3K9me2 and CHG methylation has shown very high correlation between the two epigenetic marks [32]. In our study, CHG methylation and not H3K9me2 persists in the enhancer of reactivated P1-prTP Ufo1-1 plants indicating that H3K9me2 and CHG methylation may exist independent of each other. However, our data does not exclude the possibility that CHG methylation is crucial for establishment of silencing in P1-PrTP.
Activation of P1-rr′ by Ufo1-1 is inversely correlated with paramutagenicity
The paramutagenic ability of P1-rr′ is highly variable; silencing of a naive P1-rr allele by P1-rr′ in independent families varied between 0–95% in the current and an earlier study [26]. We demonstrate that the paramutagenic ability of P1-rr′ was inversely correlated with Ufo1-1-induced reactivation. Thus, highly paramutagenic P1-rr′ stocks interfere with Ufo1-1-mediated reactivation in a manner not currently understood. Variable levels of p1 alleles reactivation have been attributed to incomplete penetrance of Ufo1-1, and to the extent of epigenetic silencing of the p1 allele involved [20], [24]. Ufo1-1 induces pericarp and cob glume pigmentation in moderately methylated P1-wr plants, but only cob glume pigmentation in a highly methylated P1-wr*. Interestingly, repeated back crosses of P1-wr* Ufo1-1 with p1-ww Ufo1-1 stock eventually leads to a gain of pericarp pigmentation (R. Sekhon, P. Wang and S. Chopra, unpublished data). Thus, the highly silenced epigenetic states (P1-wr* and highly paramutagenic P1-rr′ families) may not show immediate gain of pericarp pigmentation in the presence of Ufo1-1 while moderately silenced states (P1-wr) can be readily perturbed.
ufo1 has a broader role in diverse epigenetic pathways
The Ufo1-1 mutant allele perturbs the organ-specific expression patterns of the multicopy p1 alleles P1-wr and P1-wr* [20], [24]. These alleles do not participate in paramutation [20], [26]. Our finding that presence of Ufo1-1 leads to reactivation of the paramutagenic P1-rr′ and B′ alleles indicates that the wild type factor is involved in maintenance of silencing imposed by paramutation. While the absence of RNA-dependent RNA polymerase MOP1 also leads to reactivation of silenced P1-rr′ and a gain of pericarp pigmentation in the P1-wr allele, several generations of absence of MOP1 are required for such activation [27]. In contrast, lack of ufo1 for one generation is sufficient to abolish silencing at the p1 locus. On the other hand, lack of ufo1 leads only to a partial reactivation of B′ while lack of MOP1 results in immediate disruption of silencing. It thus appears that epigenetic suppression at these two loci is mediated by distinct but overlapping pathways.
Reactivation of the silenced loci in the absence of ufo1 suggests that it is directly involved in the epigenetic mechanism(s) responsible for the silencing. Ufo1-1-mediated reactivation of P1-rr′ and also in P1-wr [20], [24] is associated with the loss of DNA methylation within regulatory regions whereas the de-repression of P1-prTP does not seem to involve any methylation modifications at the region tested. These results support our argument [20] that ufo1 may not be directly involved in establishing and/or maintaining DNA methylation. Given that reactivation of both P1-rr′ and P1-prTP in the Ufo1-1 background was associated with a loss of H3K9me2 marks, it appears that ufo1 is involved in maintaining these heterochromatic marks. Future studies to examine the role of Ufo1-1 in establishment and maintenance of silencing associated with paramutagenic and non-paramutagenic systems, and cloning of the gene will provide insights into ufo1-dependent mechanisms in epigenetic regulation of gene expression.
Materials and Methods
Genetic stocks
The maize P1-rr allele used in this study is derived from the P1-rr-4B2 genetic stock [36]. Origin of the P1-rr′ stock has been previously described [26]. The P1-rr′ allele used in this study was progeny of a homozygous (P1-rr′/P1-rr′) plant that showed strong silencing and had colorless pericarp and light pink cob glume. The P1-prTP, a spontaneous epiallele of P1-rr, has been previously reported [26] and this epiallele is distinct from the previously characterized P1-pr allele [19]. A stock carrying Ufo1-1 has been described previously [24]. The Ufo1-1 was introgressed into the inbred line 4Co63 (National Seed Storage Laboratory, Fort Collins, CO), which carries a null p1-ww allele. Since the p1-ww Ufo1-1 plants do not produce phlobaphene pigmentation, presence of Ufo1-1 was tested by crossing individual plants with P1-wr[W23] [17]. Ectopic gain of pigmentation in pericarp and other organs in the resulting F1 progeny confirmed the presence of Ufo1-1 in the p1-ww Ufo1-1 stock [24]. The stock carrying B′ allele of the b1 gene was obtained from E.H. Coe, Jr. (University of Missouri, Columbia) and this stock carries the Pl-sr allele of the pl gene that does not impart b1-specified pigmentation to the plant body. All plant stocks used in this study carry functional alleles for the structural genes required for anthocyanin and/or phlobaphene biosynthesis.
DNA gel blot analysis
Genomic DNA was isolated from the fifth or sixth leaf using CTAB method [37]. Genomic DNA was digested to completion using restriction enzymes, reagents, and protocols from Promega (Madison, WI). DNA gel blot was performed as described previously [20]. DNA fragments of p1 used as probes in DNA gel blot analysis have been described previously [36], [38].
Genomic bisulfite sequencing
The upper DNA strand of a 443-bp sub-fragment of the P1.2 fragment required for paramutation, was assayed by genomic bisulfite sequencing. Pericarp tissues were collected 18 days after pollination (DAP) from individual plants and genomic DNA was extracted using modified CTAB method [37]. For each genotype, DNA from two plants was subjected to genomic bisulfite sequencing and pooled results from the assay are presented. Eight micrograms of DNA was completely digested with suitable restriction enzymes that cut outside the amplified fragment of interest. The digested DNA was treated with sodium bisulfite following a previously published protocol [39] with modifications [20]. The promoter region was amplified using nested PCR primers [25]. The resulting PCR products were gel purified, cloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA), and 20–30 clones/ligation/genotype were sequenced. Due to the complex P1-rr locus structure, the region studied by bisulfite sequencing is present in three places (see Figure 3B for details). The sequenced region is also present in p1-ww[4Co63] albeit with multiple indels and single nucleotide polymorphisms (R.S. Sekhon and S. Chopra, unpublished). These sequence differences were used to omit the clones that originated from the null p1-ww allele. Percent methylation at each cytosine residue was calculated by dividing the number of clones methylated for the residue by the total number of clones for that residue for all of the amplified P1-rr′ and P1-prTP regions.
Chromatin immunoprecipitation assay and quantitative real-time PCR (ChIP-qPCR)
ChIP assays were performed using pericarp tissues following a modified protocol as described previously [40], [41]. Briefly, pericarp tissues were harvested at 18 DAP and cross-linked with 3% formaldehyde. The chromatin complex was then extracted and sheared to a size range of 0.5 to 1 kb fragments using a Bioruptor (Diagenode, Denville, NJ). The anti-H3K9me2 antibody used for ChIP was kindly provided by Dr. Hiroshi Kimura [41]. This antibody was coupled with sheep anti-mouse IgG Dynabeads M-280 (Invitrogen, Grand Island, NY) and incubated with the sheared chromatin. A normal mouse IgG was used as no antibody control (NoAb). The ChIPed DNA was further purified using QIAquick PCR Purification Kit (QIAGEN, Valencia, CA) and quantified with qPCR. The relative enrichment of H3K9me2 modification was normalized to the input DNA loaded in the ChIP reaction as described previously [40]. The primers specific to the P1.2 kb distal enhancer region used in this study are PW_RTF15-2F (5′-GACGTCTCACCGGCTCACA-3′) and PW_RTF15-2R (5′-ATGCAACGCAACGCTTTG-3′). The relative differences between ChIP assay and input sample were determined using the percentage-of-input method (see ChIP analysis; http://www.invitrogen.com/site/us/en/home/Products-and-Services/Applications/RNAi-Epigenetics-and-Gene-Regulation/Chromatin-Remodeling/Chromatin-Immunoprecipitation-ChIP/chip-analysis.html). Data shown in this study are representative result of three independent experiments.
Supporting Information
Zdroje
1. BrinkRA (1956) A genetic change associated with the R locus in maize which is directed and potentially reversible. Genetics 41 : 872–890.
2. ChandlerVL, StamM (2004) Chromatin conversations: mechanisms and implications of paramutation. Nature Reviews Genetics 5 : 532–544.
3. PiluR, PanzeriD, CassaniE, Cerino BadoneF, LandoniM, et al. (2009) A paramutation phenomenon is involved in the genetics of maize low phytic acid1-241 (lpa1-241) trait. Heredity 102 : 236–245.
4. ChandlerVL (2007) Paramutation: from maize to mice. Cell 128 : 641–645.
5. ChandlerV, AllemanM (2008) Paramutation: epigenetic instructions passed across generations. Genetics 178 : 1839–1844.
6. StamM, BeleleC, DorweilerJE, ChandlerVL (2002) Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes and Development 16 : 1906–1918.
7. StamM, BeleleC, RamakrishnaW, DorweilerJE, BennetzenJL, et al. (2002) The regulatory regions required for B′ paramutation and expression are located far upstream of the maize b1 transcribed sequences. Genetics 162 : 917–930.
8. DorweilerJE, CareyCC, KuboKM, HollickJB, KermicleJL, et al. (2000) mediator of paramutation1 is required for establishment and maintenance of paramutation at multiple maize loci. Plant Cell 12 : 2101–2118.
9. AllemanM, SidorenkoL, McGinnisK, SeshadriV, DorweilerJE, et al. (2006) An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 : 295–298.
10. ErhardKFJr, StonakerJL, ParkinsonSE, LimJP, HaleCJ, et al. (2009) RNA polymerase IV functions in paramutation in Zea mays. Science 323 : 1201–1205.
11. SidorenkoL, DorweilerJE, CiganAM, Arteaga-VazquezM, VyasM, et al. (2009) A dominant mutation in mediator of paramutation2, one of three second-largest subunits of a plant-specific RNA polymerase, disrupts multiple siRNA silencing processes. PLoS Genet 5: e1000725 doi:10.1371/journal.pgen.1000725.
12. StonakerJL, LimJP, ErhardKFJr, HollickJB (2009) Diversity of Pol IV function is defined by mutations at the maize rmr7 locus. PLoS Genet 5: e1000706 doi:10.1371/journal.pgen.1000706.
13. PikaardCS, HaagJR, ReamT, WierzbickiAT (2008) Roles of RNA polymerase IV in gene silencing. Trends in Plant Science 13 : 390–397.
14. HerrAJ, JensenMB, DalmayT, BaulcombeDC (2005) RNA polymerase IV directs silencing of endogenous DNA. Science 308 : 118–120.
15. OnoderaY, HaagJR, ReamT, Costa NunesP, PontesO, et al. (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120 : 613–622.
16. BrinkRA, StylesED (1966) A collection of pericarp factors. Maize Genetics Cooperation Newsletter 40 : 149–160.
17. ChopraS, AthmaP, PetersonT (1996) Alleles of the maize P gene with distinct tissue specificities encode Myb-homologous proteins with C-terminal replacements. Plant Cell 8 : 1149–1158.
18. ChopraS, AthmaP, LiXG, PetersonT (1998) A maize Myb homolog is encoded by a multicopy gene complex. Molecular and General Genetics 260 : 372–380.
19. DasOP, MessingJ (1994) Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics 136 : 1121–1141.
20. SekhonRS, PetersonT, ChopraS (2007) Epigenetic modifications of distinct sequences of the p1 regulatory gene specify tissue-specific expression patterns in maize. Genetics 175 : 1059–1070.
21. CoccioloneSM, ChopraS, Flint-GarciaSA, McMullenMD, PetersonT (2001) Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant Journal 27 : 467–478.
22. SidorenkoLV, LiX, CoccioloneSM, ChopraS, TaglianiL, et al. (2000) Complex structure of a maize Myb gene promoter: functional analysis in transgenic plants. Plant Journal 22 : 471–482.
23. CoccioloneSM, SidorenkoLV, ChopraS, DixonPM, PetersonT (2000) Hierarchical patterns of transgene expression indicate involvement of developmental mechanisms in the regulation of the maize P1-rr promoter. Genetics 156 : 839–846.
24. ChopraS, CoccioloneSM, BushmanS, SangarV, McMullenMD, et al. (2003) The maize Unstable factor for orange1 is a dominant epigenetic modifier of a tissue specifically silent allele of pericarp color1. Genetics 163 : 1135–1146.
25. SekhonRS, ChopraS (2009) Progressive loss of DNA methylation releases epigenetic gene silencing from a tandemly repeated maize Myb gene. Genetics 181 : 81–91.
26. SidorenkoLV, PetersonT (2001) Transgene-induced silencing identifies sequences involved in the establishment of paramutation of the maize p1 gene. Plant Cell 13 : 319–335.
27. SidorenkoL, ChandlerV (2008) RNA-dependent RNA polymerase Is required for enhancer-mediated transcriptional silencing associated with paramutation at the maize p1 gene. Genetics 180 : 1983–1993.
28. SidorenkoL, LiX, TaglianiL, BowenB, PetersonT (1999) Characterization of the regulatory elements of the maize P-rr gene by transient expression assays. Plant Molecular Biology 39 : 11–19.
29. LundG, MessingJ, ViottiA (1995) Endosperm-specific demethylation and activation of specific alleles of alpha-tubulin genes of Zea mays L. Mol Gen Genet 246 : 716–722.
30. HaringM, BaderR, LouwersM, SchwabeA, van DrielR, et al. (2010) The role of DNA methylation, nucleosome occupancy and histone modifications in paramutation. The Plant Journal 63 : 366–378.
31. JohnsonLM, BostickM, ZhangX, KraftE, HendersonI, et al. (2007) The SRA methyl-cytosine-binding domain links DNA and histone methylation. Current Biology 17 : 379–384.
32. BernatavichuteYV, ZhangX, CokusS, PellegriniM, JacobsenSE (2008) Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS ONE 3: e3156 doi:10.1371/journal.pone.0003156.
33. EstèveP-O, ChinHG, SmallwoodA, FeeheryGR, GangisettyO, et al. (2006) Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes and Development 20 : 3089–3103.
34. MalagnacF, BarteeL, BenderJ (2002) An Arabidopsis SET domain protein required for maintenance but not establishment of DNA methylation. EMBO Journal 21 : 6842–6852.
35. JacksonJP, LindrothAM, CaoX, JacobsenSE (2002) Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416 : 556–560.
36. GrotewoldE, AthmaP, PetersonT (1991) Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proceedings of the National Academy of Sciences of the United States of America 88 : 4587–4591.
37. Saghai-MaroofMA, SolimanKM, JorgensenRA, AllardRW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proceedings of the National Academy of Sciences of the United States of America 81 : 8014–8018.
38. LecheltC, PetersonT, LairdA, ChenJ, DellaportaSL, et al. (1989) Isolation and molecular analysis of the maize P locus. Molecular and General Genetics 219 : 225–234.
39. JacobsenSE, SakaiH, FinneganEJ, CaoX, MeyerowitzEM (2000) Ectopic hypermethylation of flower-specific genes in Arabidopsis. Current Biology 10 : 179–186.
40. HaringM, OffermannS, DankerT, HorstI, PeterhanselC, et al. (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3 : 11.
41. KimuraH, Hayashi-TakanakaY, GotoY, TakizawaN, NozakiN (2008) The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell structure and function 33 : 61–73.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 10
-
Všechny články tohoto čísla
- The Germline Genome Provides a Niche for Intragenic Parasitic DNA: Evolutionary Dynamics of Internal Eliminated Sequences
- Classical Genetics Meets Next-Generation Sequencing: Uncovering a Genome-Wide Recombination Map in
- Calpain-5 Mutations Cause Autoimmune Uveitis, Retinal Neovascularization, and Photoreceptor Degeneration
- Cofilin-1: A Modulator of Anxiety in Mice
- The Date of Interbreeding between Neandertals and Modern Humans
- Embryos of Robertsonian Translocation Carriers Exhibit a Mitotic Interchromosomal Effect That Enhances Genetic Instability during Early Development
- Viral Evasion of a Bacterial Suicide System by RNA–Based Molecular Mimicry Enables Infectious Altruism
- Phosphatase-Dead Myotubularin Ameliorates X-Linked Centronuclear Myopathy Phenotypes in Mice
- Full-Length Synaptonemal Complex Grows Continuously during Meiotic Prophase in Budding Yeast
- MOV10 RNA Helicase Is a Potent Inhibitor of Retrotransposition in Cells
- A Likelihood-Based Framework for Variant Calling and Mutation Detection in Families
- The Contribution of RNA Decay Quantitative Trait Loci to Inter-Individual Variation in Steady-State Gene Expression Levels
- New Partners in Regulation of Gene Expression: The Enhancer of Trithorax and Polycomb Corto Interacts with Methylated Ribosomal Protein L12 Its Chromodomain
- Mining the Unknown: A Systems Approach to Metabolite Identification Combining Genetic and Metabolic Information
- Mutations in (Hhat) Perturb Hedgehog Signaling, Resulting in Severe Acrania-Holoprosencephaly-Agnathia Craniofacial Defects
- The Many Landscapes of Recombination in
- Faster-X Evolution of Gene Expression in
- Loss of Slc4a1b Chloride/Bicarbonate Exchanger Function Protects Mechanosensory Hair Cells from Aminoglycoside Damage in the Zebrafish Mutant
- Regulation of ATG4B Stability by RNF5 Limits Basal Levels of Autophagy and Influences Susceptibility to Bacterial Infection
- and the BTB Adaptor Are Key Regulators of Sleep Homeostasis and a Dopamine Arousal Pathway in Drosophila
- Mutation and Fetal Ethanol Exposure Synergize to Produce Midline Signaling Defects and Holoprosencephaly Spectrum Disorders in Mice
- Specific Missense Alleles of the Arabidopsis Jasmonic Acid Co-Receptor COI1 Regulate Innate Immune Receptor Accumulation and Function
- Deep Genome-Wide Measurement of Meiotic Gene Conversion Using Tetrad Analysis in
- Mismatch Repair Balances Leading and Lagging Strand DNA Replication Fidelity
- Distinguishing between Selective Sweeps from Standing Variation and from a Mutation
- Cytokinesis-Based Constraints on Polarized Cell Growth in Fission Yeast
- Deposition of Histone Variant H2A.Z within Gene Bodies Regulates Responsive Genes
- Functional Antagonism between Sas3 and Gcn5 Acetyltransferases and ISWI Chromatin Remodelers
- The SET-Domain Protein SUVR5 Mediates H3K9me2 Deposition and Silencing at Stimulus Response Genes in a DNA Methylation–Independent Manner
- Morphogenesis and Cell Fate Determination within the Adaxial Cell Equivalence Group of the Zebrafish Myotome
- Muscle-Specific Splicing Factors ASD-2 and SUP-12 Cooperatively Switch Alternative Pre-mRNA Processing Patterns of the ADF/Cofilin Gene in
- Maize Is Required for Maintaining Silencing Associated with Paramutation at the and Loci
- Increasing Signal Specificity of the TOL Network of mt-2 by Rewiring the Connectivity of the Master Regulator XylR
- Use of Pleiotropy to Model Genetic Interactions in a Population
- RAB-Like 2 Has an Essential Role in Male Fertility, Sperm Intra-Flagellar Transport, and Tail Assembly
- Variants Affecting Exon Skipping Contribute to Complex Traits
- Topoisomerase II– and Condensin-Dependent Breakage of -Sensitive Fragile Sites Occurs Independently of Spindle Tension, Anaphase, or Cytokinesis
- Comparison of Family History and SNPs for Predicting Risk of Complex Disease
- Recovery of Arrested Replication Forks by Homologous Recombination Is Error-Prone
- A Mutation in the Gene Causes Alternative Splicing Defects and Deafness in the Bronx Waltzer Mouse
- Comparative Genomics Suggests an Independent Origin of Cytoplasmic Incompatibility in
- It Was Heaven: An Interview with Evelyn Witkin
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Mutation in the Gene Causes Alternative Splicing Defects and Deafness in the Bronx Waltzer Mouse
- Mutations in (Hhat) Perturb Hedgehog Signaling, Resulting in Severe Acrania-Holoprosencephaly-Agnathia Craniofacial Defects
- Classical Genetics Meets Next-Generation Sequencing: Uncovering a Genome-Wide Recombination Map in
- Regulation of ATG4B Stability by RNF5 Limits Basal Levels of Autophagy and Influences Susceptibility to Bacterial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



