-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
Chemical signals are prevalent in sexual communication systems. Mate recognition has been extensively studied within the Lepidoptera, where the production and recognition of species-specific sex pheromone signals are typically the defining character. While the specific blend of compounds that makes up the sex pheromones of many species has been characterized, the molecular mechanisms underpinning the evolution of pheromone-based mate recognition systems remain largely unknown. We have focused on two sets of sibling species within the leafroller moth genera Ctenopseustis and Planotortrix that have rapidly evolved the use of distinct sex pheromone blends. The compounds within these blends differ almost exclusively in the relative position of double bonds that are introduced by desaturase enzymes. Of the six desaturase orthologs isolated from all four species, functional analyses in yeast and gene expression in pheromone glands implicate three in pheromone biosynthesis, two Δ9-desaturases, and a Δ10-desaturase, while the remaining three desaturases include a Δ6-desaturase, a terminal desaturase, and a non-functional desaturase. Comparative quantitative real-time PCR reveals that the Δ10-desaturase is differentially expressed in the pheromone glands of the two sets of sibling species, consistent with differences in the pheromone blend in both species pairs. In the pheromone glands of species that utilize (Z)-8-tetradecenyl acetate as sex pheromone component (Ctenopseustis obliquana and Planotortrix octo), the expression levels of the Δ10-desaturase are significantly higher than in the pheromone glands of their respective sibling species (C. herana and P. excessana). Our results demonstrate that interspecific sex pheromone differences are associated with differential regulation of the same desaturase gene in two genera of moths. We suggest that differential gene regulation among members of a multigene family may be an important mechanism of molecular innovation in sex pheromone evolution and speciation.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002489
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002489Summary
Chemical signals are prevalent in sexual communication systems. Mate recognition has been extensively studied within the Lepidoptera, where the production and recognition of species-specific sex pheromone signals are typically the defining character. While the specific blend of compounds that makes up the sex pheromones of many species has been characterized, the molecular mechanisms underpinning the evolution of pheromone-based mate recognition systems remain largely unknown. We have focused on two sets of sibling species within the leafroller moth genera Ctenopseustis and Planotortrix that have rapidly evolved the use of distinct sex pheromone blends. The compounds within these blends differ almost exclusively in the relative position of double bonds that are introduced by desaturase enzymes. Of the six desaturase orthologs isolated from all four species, functional analyses in yeast and gene expression in pheromone glands implicate three in pheromone biosynthesis, two Δ9-desaturases, and a Δ10-desaturase, while the remaining three desaturases include a Δ6-desaturase, a terminal desaturase, and a non-functional desaturase. Comparative quantitative real-time PCR reveals that the Δ10-desaturase is differentially expressed in the pheromone glands of the two sets of sibling species, consistent with differences in the pheromone blend in both species pairs. In the pheromone glands of species that utilize (Z)-8-tetradecenyl acetate as sex pheromone component (Ctenopseustis obliquana and Planotortrix octo), the expression levels of the Δ10-desaturase are significantly higher than in the pheromone glands of their respective sibling species (C. herana and P. excessana). Our results demonstrate that interspecific sex pheromone differences are associated with differential regulation of the same desaturase gene in two genera of moths. We suggest that differential gene regulation among members of a multigene family may be an important mechanism of molecular innovation in sex pheromone evolution and speciation.
Introduction
Variation is the raw material of evolution; however the nature of this variation remains a topic of much discussion amongst evolutionary biologists [1], [2], [3]. The potential role in evolution of mutations that impact gene regulation rather than the amino acid sequence of a protein was initially proposed in the 1970s. King & Wilson [4] noticed that orthologous proteins between humans and chimpanzees were very similar at the amino acid level compared to the phenotypic differences between the two species and suggested that gene regulation could play an important role in explaining some of the phenotypic differences between the two primates. Since then the relative importance in evolution of regulatory mutations (those affecting gene expression) versus structural mutations (those resulting in amino acid substitutions within the coding region of a protein) has been debated (e.g., Hoekstra and Coyne [1] and references therein).
While structural mutations between orthologous proteins are easy to identify, mutations that affect the regulation of a gene are more difficult to recognize. Regulatory mutations can fall close to the gene in its promoter region (cis-regulatory mutations) or act at a distance from the gene (trans-regulatory mutations). Cis-regulatory mutations usually result in the gain or loss of a site involved in binding a regulatory factor, such as a transcription factor, whereas trans-acting regulatory mutations typically affect the transcription factors themselves. Interestingly, trans-acting regulatory mutations can involve both regulatory and structural mutations of the transcription factor. The relative importance of cis - versus trans-regulatory mutations in the course of evolution is also predicted to be influenced by the degree of pleiotropy [5]. Since transcription factors generally influence the expression of multiple genes, trans-regulatory mutations are more likely to affect a number of traits. On the other hand, cis-regulatory mutations are more likely to impact the target gene alone [6]. In spite of this, both types of regulatory mutations have been identified in the evolution of distinct cases of beneficial traits within species [1] and in the evolution of major morphological innovations at higher taxonomic levels [7]. Despite these examples, there remains little evidence regarding the nature of molecular innovations that underpin the evolution of new species. Few genes involved in speciation have been identified to date [8], and many causal mutations associated with species differences remain unknown. This makes the topic of great interest to further investigate the role of structural mutations and gene regulation in traits that can lead to speciation. Finally, whether these mutations are present as standing variation in the ancestral species or if the process of speciation is limited by the rate of acquisition of newly arising mutations has only recently attracted attention, with most discussion restricted to the acquisition of beneficial traits within species [9], [10].
Speciation is often associated with changes in mate recognition systems [11]. Mate recognition has been extensively studied within the Lepidoptera where the production of long-range species-specific sex pheromone signals by females and their recognition by conspecific males are critical steps. Furthermore, for many species within the Lepidoptera, sex pheromones are often the defining character for biological species [12]. The sex pheromones of many species of moths have been identified [13], and in some systems, enzymes involved in the biosynthesis of pheromone components have been characterized [14], [15]. An important structural characteristic of moth sex pheromone components is the position of double bond(s) along the fatty acid backbone of the molecule. These double bonds are introduced at specific carbon positions by distinct members of the fatty-acyl desaturase family that have evolved a role in pheromone biosynthesis from an ancestral function in essential fatty-acid biosynthesis [16]. A core set of desaturase transcripts is typically found in the pheromone glands of female moths of a given species [16], including two Δ9-desaturases, one with a preference for 16 carbon (16C) fatty acids over 18 carbon (18C) fatty acids and a second with the opposite preference, together with one or several members of the so-called Δ11-desaturase clade that includes enzymes that are increasingly being shown to possess a wide range of desaturation abilities (e.g. Liénard et al. [17] and references therein).
Changes in enzymes involved in pheromone biosynthesis, including desaturases, have been implicated in pheromone differences in both moths and flies. In the Lepidoptera, these include examples of gene neofunctionalisation, where new desaturases have arisen by gene duplication and then diverged to evolve a new function [18], [19]. In contrast, there is a single example where differential expression of desaturase genes in the Asian corn borer is responsible for producing distinct pheromone blends [20]. In Drosophila differential regulation of desaturase F is implicated in the production of distinct cuticular hydrocarbon pheromones between species [21]. With only a few cases to draw from, the importance of structural versus regulatory changes involved in mate recognition and speciation is an open question and more examples are required to form a consensus of their relative contribution.
To investigate the role of desaturases in changes in sex pheromone blends, we have studied the mode of evolution in these enzymes within two genera of leafroller moths, Ctenopseustis (brown-headed leafrollers) and Planotortrix (green-headed leafrollers). Both genera are endemic to New Zealand, with species within the two genera widely distributed across the two main islands [22]. Sequence divergence at the COI locus averages 10%, suggesting that the two genera diverged around 5 million years ago [23]. Although some species develop on specific host plants, such as P. aviciennae on mangroves (Avicienna marina) or C. fraterna on silver fern (Cyathea dealbata), most of the species within the genera are polyphagous, and can develop on angiosperms or gymnosperms, including a number of horticultural and silvicultural crops. Many of the sibling species are difficult to differentiate using classical morphology [22] or mitochondrial bar-coding DNA markers [23], suggesting that they have diverged in the last 500,000 years. Despite this recent divergence, the different sibling species use distinct sex pheromones. Their sex pheromone compounds are tetradecenyl acetates that differ primarily in the position of a single double bond within a fourteen carbon fatty acid backbone. They are biosynthesized from fatty acids (myristic, palmitic or stearic acid), which are desaturated at specific positions, chain-shortened via β-oxidation, reduced to fatty alcohols, and acetylated to give the final products. Within the genera sex pheromone blends contain up to three components in specific ratios (Table S1) that are desaturated at the Δ5, Δ7, Δ8, Δ9 or Δ10 positions, all in the Z configuration [24]. Each genus contains a pair of sibling species that utilize distinct sex pheromone blends. In Planotortrix, P. excessana uses a blend of (Z)-5-tetradecenyl acetate (Z5-14:OAc) and (Z)-7-tetradecenyl acetate (Z7-14:OAc) [25], [26], while its sibling species P. octo, utilizes (Z)-8-tetradecenyl acetate (Z8-14:OAc) and trace amounts (2%) of (Z)-10-tetradecenyl acetate (Z10-14:OAc) [25]. In Ctenopseustis, C. obliquana uses a blend of Z5-14:OAc and Z8-14:OAc [27], while its sibling species C. herana utilizes a sex pheromone consisting solely of Z5-14:OAc [28]. Thus each species pair is characterized by a gain or loss of a particular pheromone component that differs only in the position of a double bond.
Sex pheromones are central to mate recognition in moths and form barriers to gene flow among the Lepidoptera. Species within Ctenopseustis and Planotortrix are no exception. Males of these species are specifically attracted to the sex pheromone blend of their conspecific females, but not to that produced by the respective sibling species, restricting gene flow between sibling species (see Foster et al. [24] for a review). Briefly, Clearwater et al. [29] investigated the cross responses of C. obliquana and C. herana males to conspecific and sibling species sex pheromone blends, as well as females, in wind tunnel and field cage experiments. They found strong preferences for the conspecific pheromone in both experimental formats. Furthermore, Foster et al. [30] tested the attractiveness of a range of ratios of Z5-14:OAc and Z8-14:OAc to C. obliquana and C. herana in a wind tunnel. C. obliquana never landed on lures containing only Z5-14:OAc, while C. herana males never landed on lures containing Z5-14:OAc and Z8-14:OAc. Similarly, field cage cross attraction experiments using P. excessana and P. octo showed high species specificity in male mating behaviour [25]. The lack of interbreeding in the wild is also supported by isozyme-based population genetics, with at least one fixed difference identified for each sibling species pair [31], [32].
The biosynthesis of the compounds found in the pheromone blends of the Ctenopseustis and Planotortrix species have been studied by Fatty Acid Methyl Ester (FAME) analysis of pheromone glands [33] and by monitoring the incorporation of labelled precursors [34], [35], [36]. The Z8-14:OAc used by P. octo is the product of Δ10-desaturation of palmitic acid followed by chain shortening, reduction and acetylation [35], while the Z5-14:OAc and Z7-14:OAc used by P. excessana are the products of Δ9-desaturase activity [36]. The Δ10 - and Δ9-desaturases responsible for these activities have been isolated and characterized from P. octo [37]. The biosynthesis of Z5-14:OAc used by Ctenopseustis species was investigated by labelling experiments in C. herana, where unlike in Planotortrix, FAME analysis implicated the action of a specific Δ5-desaturase [34]. Therefore at least four desaturases are thought to potentially contribute to pheromone biosynthesis in these two genera: two Δ9-desaturases, a Δ10-desaturase and a Δ5-desaturase (Figure 1).
Fig. 1. Schematic outlining the likely biosynthetic routes of the sex pheromone components of C. obliquana, C. herana, P. octo, and P. excessana. 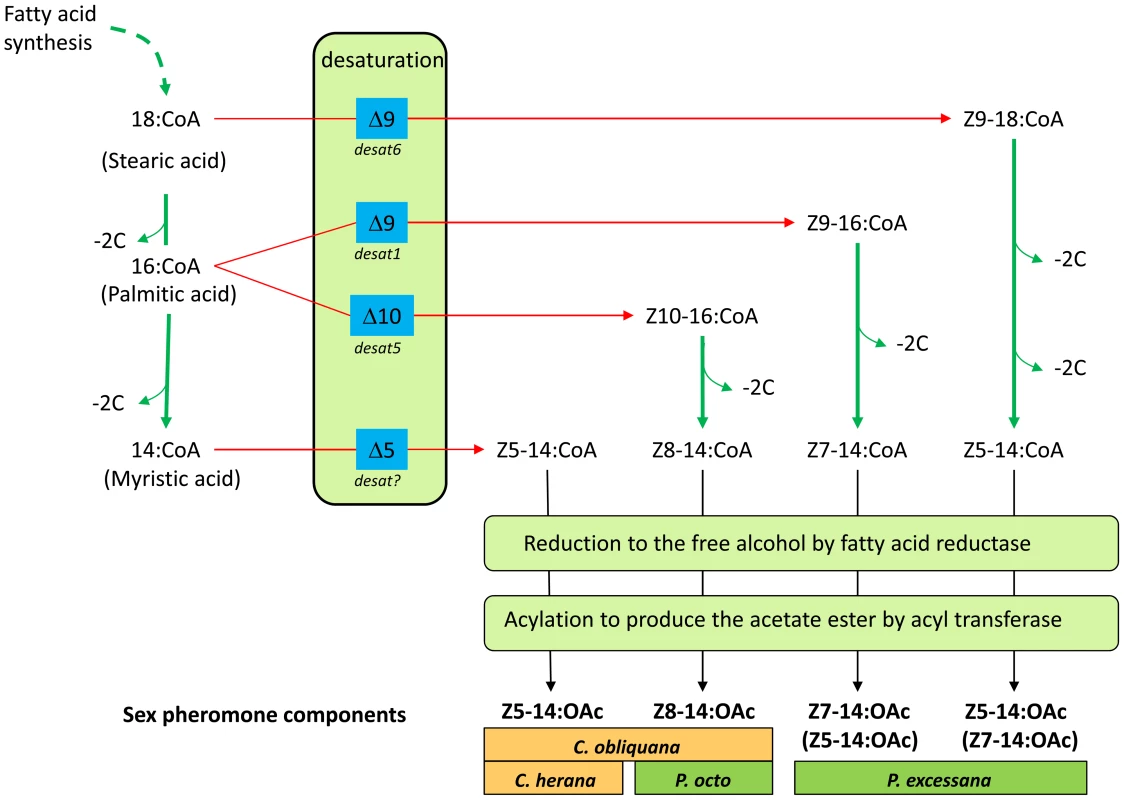
Desat1, desat5 and desat6 correspond to the desaturase genes encoding a Δ9 desaturase with a preference for 16>18 carbon fatty acids, a Δ10-desaturase and a Δ9-desaturase with a preference for 18>16 carbon fatty acids, respectively. Desat? refers to a yet to be identified Δ5-desaturase. Chain shortening by β-oxidation is indicated by ‘−2C’. The minor products of the two Δ9-desaturases in P. excessana (desat1 and desat6) are indicated in brackets. We also note that Z10-14:OAc is a very minor (2%) component of the pheromone blend of P. octo (not shown). We set out to investigate whether structural mutations within coding regions of these desaturases or differential regulation of a standing set of desaturase genes are responsible for the diversity in sex pheromone components used by species within the genera Ctenopseustis and Planotortrix. To obtain a general picture of the set of genes present in these leafroller moths, we first isolated and characterized desaturases from genomic DNA and cDNA from the pheromone glands of C. obliquana, C. herana, P. octo and P. excessana. We then performed functional assays and quantitative real-time PCR to identify the desaturases likely to have a role in sex pheromone biosynthesis in these species. Finally, we compared coding region sequences and the expression of the genes in the pheromone glands of the sibling species to address our question.
Results
Isolation and phylogenetic analysis of desaturase genes
Initially we isolated as many desaturase-encoding sequences as possible from two sets of sibling species in the genera Ctenopseustis and Planotortrix. By Polymerase Chain Reaction (PCR) using degenerate primers designed to target conserved regions of lepidopteran fatty acyl desaturases, we isolated 29 desaturase-like sequences from genomic DNA and/or pheromone gland cDNA of C. obliquana, C. herana, P. excessana, P. octo and in some instances, also from the more distantly related Planotortrix species, P. notophaea. For many of these genes, sequences were recovered from two strains of P. excessana (the North Island and South Island strains). The sequences fall into six groups of desaturase-like genes (desat1-6). Rapid Amplification of cDNA Ends (RACE) PCR was used together with genome walking and analysis of preliminary whole genome sequencing assemblies of C. obliquana and P. octo (unpublished data) to construct predicted coding regions for each ortholog of each desaturase-like gene. The resulting gene contigs were confirmed by PCR and sequencing from pheromone gland cDNA wherever possible. Coding regions were obtained for all genes from all species except for desat3 and desat5 from C. herana, and the final 3′ ∼100 bp of desat5 from C. obliquana. Desat1 and desat5 of P. octo were isolated previously by Hao et al. [37]. All sequences isolated during this study have been deposited on GenBank (accession numbers JN022472–JN022498). An amino acid alignment derived from the 29 sequences grouped into each of the six desaturase-like genes is presented in Figure S1.
The predicted desaturases for which we obtained full length coding regions ranged in size from 331 to 358 amino acids (Table 1, Text S1, S2, S3, S4, S5, S6). Between species (excluding P. notophaea), amino acid sequence identities were highest for desat6 (98.6%–100%) followed by desat1 (97.4%–99.4%) and desat2 (96.1%–99.4%), with the lowest displayed by desat3 (90.4%–95.5%) and desat4 (90.2%–96.8%). The full length sequence of the desat5 was only obtained for Planotortrix species, where the amino acid identity between sibling species was 99.4%. Intron positions were inferred by PCR from genomic DNA or in the case of C. obliquana and P. octo by direct observation from genome scaffolds. Where determined, intron positions were conserved among orthologous genes. Desat2 contains no introns, desat4 contains two introns and the remaining desaturase-like genes, desat1, desat3, desat5 and desat6 each contain three introns. The relative positions and phase of the introns are indicated on the alignments presented in Text S1, S2, S3, S4, S5, S6.
Tab. 1. Summary statistics for desaturases from Ctenopseustis and Planotortrix species. 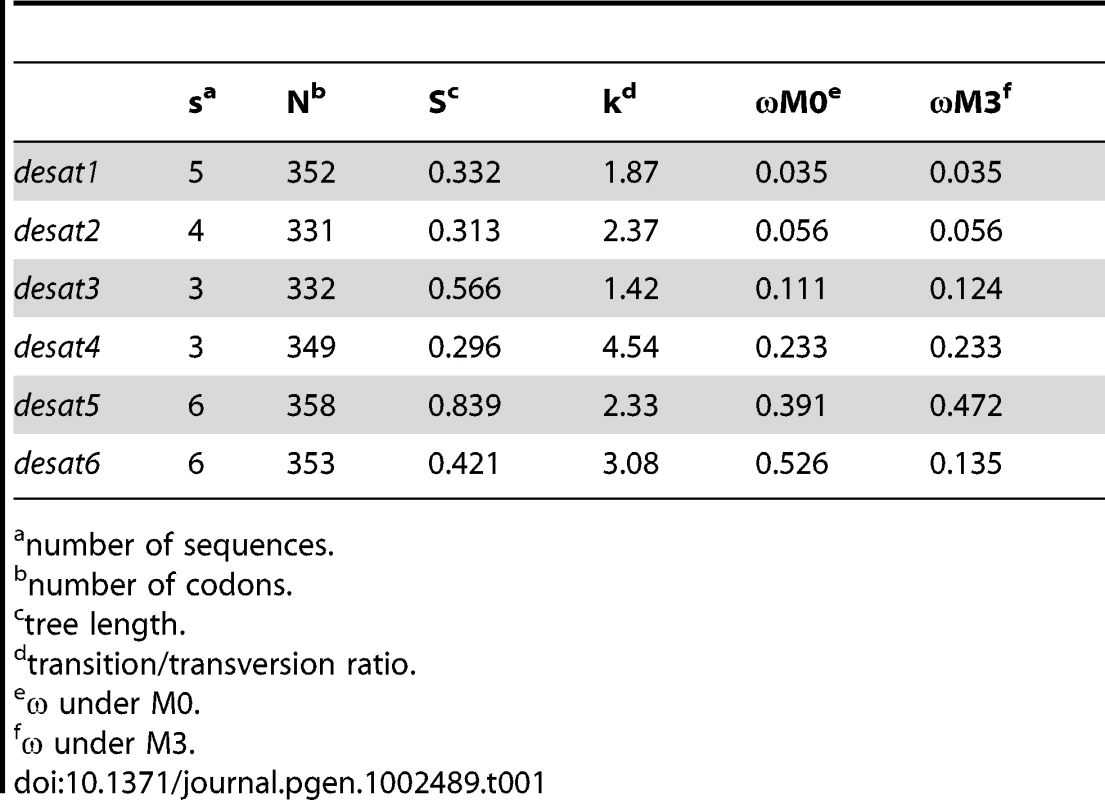
number of sequences. Phylogenetic analysis was conducted on all predicted desaturases from Ctenopseustis and Planotortrix reported above or previously [37], as well as a set of currently available lepidopteran desaturase sequences (Figure 2). Three well-supported clades were observed including a Δ9-desaturase (16C>18C) clade into which the predicted protein desat1 falls, a Δ9-desaturase (18C>16C) clade into which desat6 falls and a so-called Δ11-desaturase clade into which all the remaining Ctenopseustis and Planotortrix desaturases (desat2, desat3, desat4 and desat5) fall. In each case, the Ctenopseustis and Planotortrix orthologs group together, well supported by high bootstrap values. Moreover, some Ctenopseustis and Planotortrix desaturases group closely with previously characterized desaturases from other species. First, the Ctenopseustis and Planotortrix desat2 orthologs group closely with a non-functional desaturase from Choristoneura rosaceana [38]; second, the desat6 orthologs group with a Δ9-desaturase from Epiphyas postvittana [39]; and third, even though less closely related, desat3 groups with a terminal desaturase from Operophtera brumata [40]. Finally, the six predicted desaturases from Ctenopseustis and Planotortrix are all well separated from each other in the phylogenetic tree, with many other lepidopteran desaturases inter-dispersed between them.
Fig. 2. Phylogeny of 86 lepidopteran desaturases including those encoded by desat1-6 from Ctenopseustis and Planotortrix. 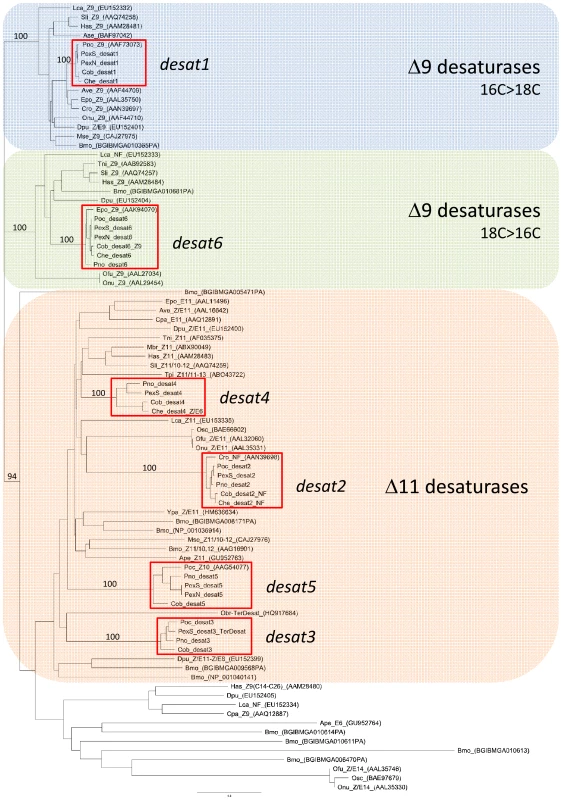
The phylogeny was constructed from protein sequences using PHYML implemented within Geneious using JTT distances. Complete amino acid sequence information was obtained from GenBank, along with desaturases predicted from the genomic sequence of Bombyx mori from the Silkmoth database. Sequences are abbreviated as following: Ape, Antherea pernyi; Ase, Ascotis selenaria; Ave, Argyrotaenia velutinana; Bmo, Bombyx mori; Cpa, Choristoneura parallela; Cro, Choristoneura rosaceana; Che, Ctenopseustis herana; Cob, Ctenopseustis obliquana; Cpo, Cydia pomonella; Epo, Epiphyas postvittana; Has, Helicoverpa assulta; Hze, Helicoverpa zea; Lca, Lampronia capitella; Mbr, Mamestra brassicae; Mse, Manduca sexta; Pex, Planotortrix excessana; Poc, Planotortrix octo; Pno, Planotortrix notophaea; Onu, Ostrinia nubilalis; Ofu, Ostrinia furnacalis; Osc, Ostrinia scapulalis: Obr, Operophtera brumata; Sli, Spodoptera littoralis; Tni, Trichoplusia ni; Tpi, Thaumetopoea pityocampa; Ypa: Yponomeuta padellus. After the abbreviated species name are the desaturase activity if known with NF = non-functional in pheromone biosynthesis; TerDesat = terminal desaturase activity; Z or E, geometry of the double bond. The GenBank accession numbers are given in brackets for previously described desaturases. Bootstrap values in percentages from 1000 bootstrap replicates supporting the three major clades (Δ9-desaturase 16C>18C, Δ9-desaturase 18C>16C, and Δ11-desaturase) and the groups containing the Ctenopseustis and Planotortrix desaturases, indicated by the red outline boxes, are given above the relevant branches. We looked for evidence of nonsense or missense mutations in the sequence of the desaturase-like genes that might impact function and explain differences in the pheromone components used by the different species. No amino acid substitutions were found in the active site regions, such as the histidine-rich regions involved in ion coordination, and no premature stops or frame-shift mutations could be identified. We then undertook likelihood ratio tests using PAML to look for evidence of selection acting on the coding regions of the six desaturases [41]. There was some evidence for positive selection in one of three model comparisons for desat3 and desat5 (Table 2), together with some significantly selected sites identified in desat3 (7), desat4 (2), desat5 (1) and desat6 (1) (Table 3). However, the ratio of non-synonymous to synonymous (dN/dS) nucleotide substitutions (M0) were less than one for all genes, indicative of overall purifying selection and suggestive of conserved function (Table 1).
Tab. 2. Likelihood ratio tests between nested site-specific models.a 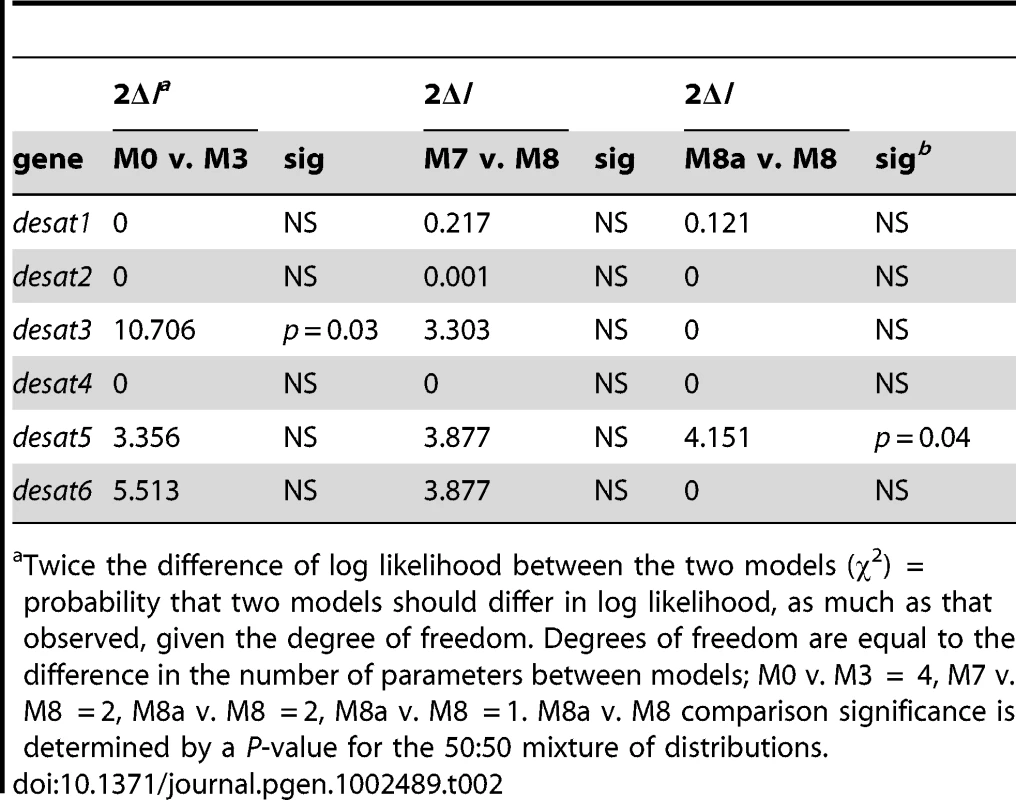
Twice the difference of log likelihood between the two models (χ2) = probability that two models should differ in log likelihood, as much as that observed, given the degree of freedom. Degrees of freedom are equal to the difference in the number of parameters between models; M0 v. M3 = 4, M7 v. M8 = 2, M8a v. M8 = 2, M8a v. M8 = 1. M8a v. M8 comparison significance is determined by a P-value for the 50∶50 mixture of distributions. Tab. 3. Putative positively selected sites and posterior probabilities under M8. 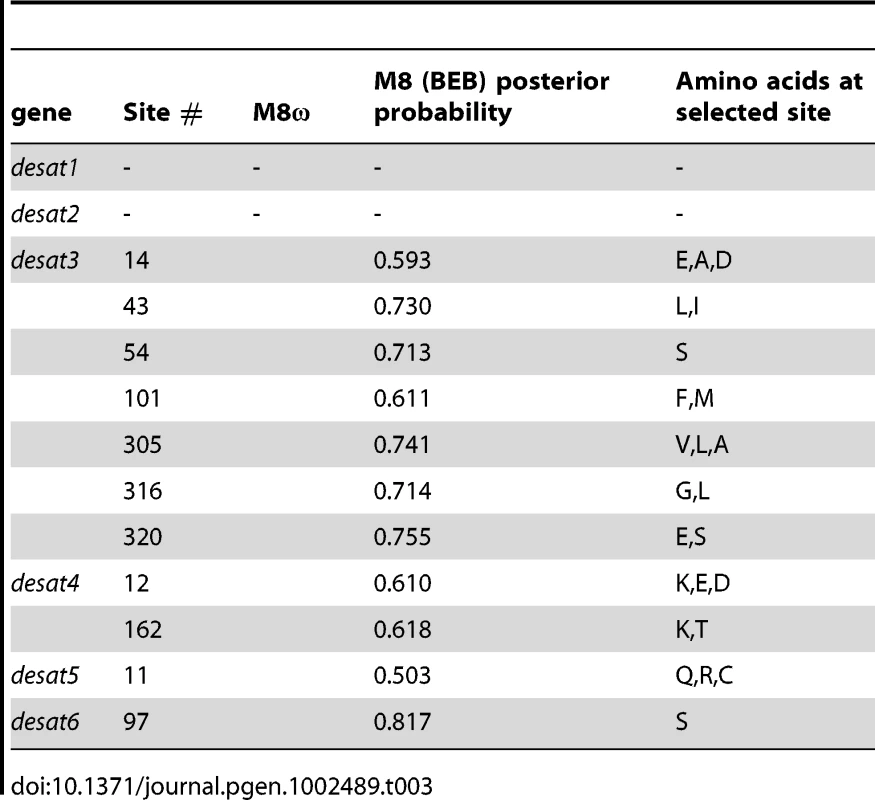
Functional analysis of desaturases in yeast
We then examined the function of the predicted desaturases, or more specifically substrate preference and desaturation specificity within the fatty acid precursors. We investigated sufficient desaturases so that at least one ortholog of each of the six predicted desaturases was characterized. The open reading frames of predicted desaturases were subcloned into the YEpOLEX or pYEX-expression vectors and functional expression was conducted in desaturase-deficient yeast strains. FAME extracts from transformed yeast were analysed to infer the ability of each desaturase to introduce double bonds to pheromone precursors at specific positions and DiMethyl-DiSulphide (DMDS) derivatization was performed to verify the structural identity of the unsaturated products. Since functional analyses from P. octo have shown that desat1 has Δ9-desaturase activity with a preference for 16C over 18C precursors, and desat5 is a Δ10-desaturase [37], we focused on orthologs of desat2, desat3, desat4 and desat6.
Desat2 from C. obliquana or C. herana had the same profile as Cu2+-induced yeast transformed with the pYEX-CHT-only vector (Figure 3A), indicating that they were unable to utilize typical sex pheromone precursors as substrates.
Fig. 3. GC-MS analyses. 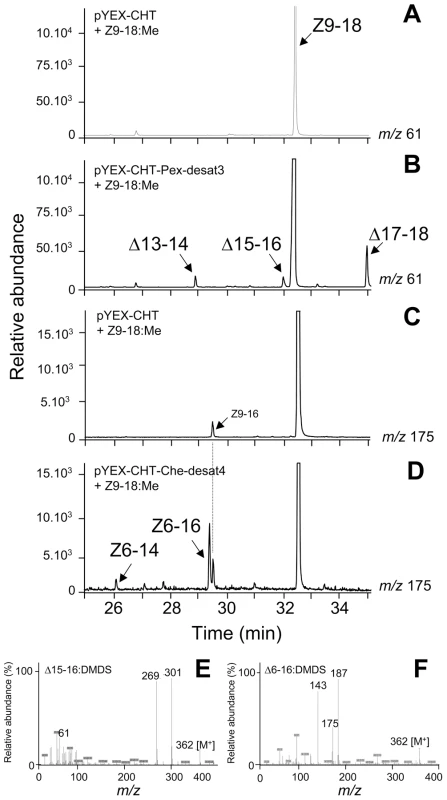
GC-MS analyses of DMDS derivatives from methanolysed Cu2+-induced ole1 elo1 S. cerevisiae yeast supplemented with Z9-18:Me and transformed with (A and C) control pYEX-CHT vector, (B) pYEX-CHT-Pex-desat3 and (D) pYEX-CHT-Che-desat4. The chromatogram traces represent the ion currents obtained by selection of the characteristic ion of terminal and Δ6-DMDS adducts at m/z 61 (A and B) and m/z 175 (C and D), respectively. (E) and (F) represent the mass spectra for terminal C16 DMDS adducts (Δ15-16) (m/z 362 [M+], 61, 301 (A+) and 269 (A+-32)) and Δ6-16 DMDS adducts (m/z 362 [M+], 187, 175 (A+) and 143 (A+-32), respectively. The mass spectra for other DMDS adducts present in the extracts are not shown and displayed a spectrum with the characteristic ions at m/z 334 [M+], 61 and 273 for Δ13-14, at m/z 390 [M+], 61 and 329 for Δ17-18 and at m/z 334 [M+], 175 and 159 for Δ6-14, respectively. FAME analyses from yeast transformed with desat3 from P. excessana and desat4 from C. herana revealed the presence of three and two additional mono-unsaturated products, respectively. In the case of desat3, all DMDS adducts exhibited a diagnostic ion at m/z 61, corresponding to FAMEs with a terminal double bond. These were subsequently identified as the Δ13-14:Me (m/z 334 [M+], 61 and 273), the Δ15-16:Me (m/z 362 [M+], 61 and 301) (Figure 3B–3E) and the Δ17-18:Me (m/z 390 [M+], 61 and 329) (Figure 3B). The DMDS adducts for desat4 exhibited a diagnostic ion at m/z 175, which is characteristic of FAMEs with a double bond at the sixth carbon position (Δ6) and was absent in control samples (Figure 3C). The DMDS adducts corresponded to the Δ6-14:Me (m/z 334 [M+], 175 and 159) (Figure 3D) and Δ6-16:Me (m/z 362 [M+], 175 and 187) (Figure 3D, 3F). No other characteristic ions for mono-unsaturated compounds were detected.
YEpOLEX-Cob-desat6 yeast transformants were able to grow on media lacking Unsaturated Fatty-Acids (UFAs), indicating that desat6 from C. obliquana encodes a functional desaturase that could complement the UFA auxotrophic ole1 strain. Methylated fatty-acid extracts from yeast bearing desat6 transformants showed two major peaks with retention times corresponding to Z9-16:Me and Z9-18:Me (Figure 4A). DMDS analyses revealed a diagnostic ion at m/z 217, confirming the identity of unsaturated FAMEs with a double bond at the Δ9 position. In addition, more Z9-18:Me than Z9-16:Me was produced, with small amounts of Z9-14:Me (m/z 334 [M+], 217 and 117), Z9-15:Me (m/z 348 [M+], 217 and 131) and Z9-17:Me (m/z 376 [M+], 217 and 159) also detected (Figure 4B).
Fig. 4. GC-MS analysis. 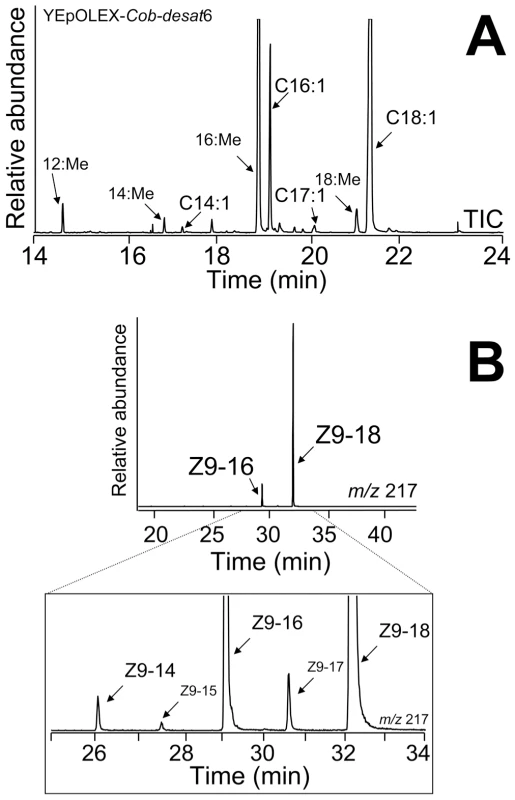
GC-MS analysis of methanolysed total lipid extracts from ole1 S. cerevisiae yeast transformed with YEpOLEX-Cob-desat6. (A) Total ion current (TIC) chromatogram of fatty acid methyl esters of yeast expressing the Cob-desat6 gene. (B) DMDS derivatives of methanolyzed YEpOLEX-Cob-desat6 yeast extracts. The chromatogram traces represent the ion current obtained by selection of the characteristic ion of Δ9-adducts at m/z 217. We are therefore able to summarise the activities of the six predicted desaturase groups, assuming that their function is conserved among orthologs. Desat1 encodes a Δ9-desaturase, with a preference for 16C over 18C precursors, desat2 encodes an enzyme for which an activity has not yet been detected, desat3 encodes a terminal desaturase, desat4 encodes a Δ6-desaturase, desat5 a Δ10 desaturase and desat6 a Δ9-desaturase with a preference for 18C over 16C precursors. Based on these functional analyses we concluded that desat1, desat5 and desat6 are likely to play a role in sex pheromone biosynthesis in these species.
Gene expression analysis of desaturase genes
Initial gene expression analysis using quantitative real-time PCR conducted from cDNA from pooled tissue samples (approx. 100 pheromone glands) by species was undertaken on all six desaturases to examine relative gene expression in larval fat bodies, adult male and female abdomens and adult female pheromone glands. The apparently non-functional desat2 was highly expressed only in larval fat bodies, while all other desaturases had detectable levels of expression across all tissues with desat1, desat5 and desat6, showing high levels of expression in the pheromone gland (data not shown). Because of these results and the unlikely involvement of desat2, desat3 and desat4 in pheromone biosynthesis based on their desaturase activities, we focused on the analysis of the expression levels of desat1, desat5 and desat6, comparing gene expression from pheromone glands of individual female moths. Our hypothesis was that expression differences among the desaturases are responsible for the observed pattern of sex pheromone component differences between the sibling species.
The most striking expression differences in the pheromone glands compared with adult abdomens of the different species among the three desaturases were observed for desat5 (Figure 5). Levels of gene expression were higher in C. obliquana (34-fold) and P. octo (273-fold) pheromone glands compared with abdomens than in C. herana and P. excessana, respectively, where no significant differences in expression were observed between both tissues. Differences in expression of desat1 and desat6 between species and tissues were also observed, but were far less striking than for desat5. Desat1 was more highly expressed in the pheromone glands than in abdomens of P. excessana (3.7-fold) and P. octo (3.3-fold), while for desat6 P. octo showed higher levels of expression than P. excessana both in the gland and the abdomen (1.8-fold). No significant differences in desat1 and desat6 expression were found in the two Ctenopseustis species. To verify that the primers used for quantitative RT-PCR were able to amplify the appropriate desaturase gene with low or barely detectable levels of expression in a particular species (for example desat5 from C. herana and P. excessana), PCRs were conducted using genomic DNA or plasmids containing the relevant desaturase cDNA. In all cases, melting-curve analysis confirmed the presence of a single product of the expected size and/or sequence and negative controls contained no product (data not shown).
Fig. 5. Gene expression of desat1, desat5, and desat6 in the pheromone gland and abdomen of virgin females in Ctenopseustis obliquana, C. herana, P. excessana, and P. octo relative to housekeeper genes. 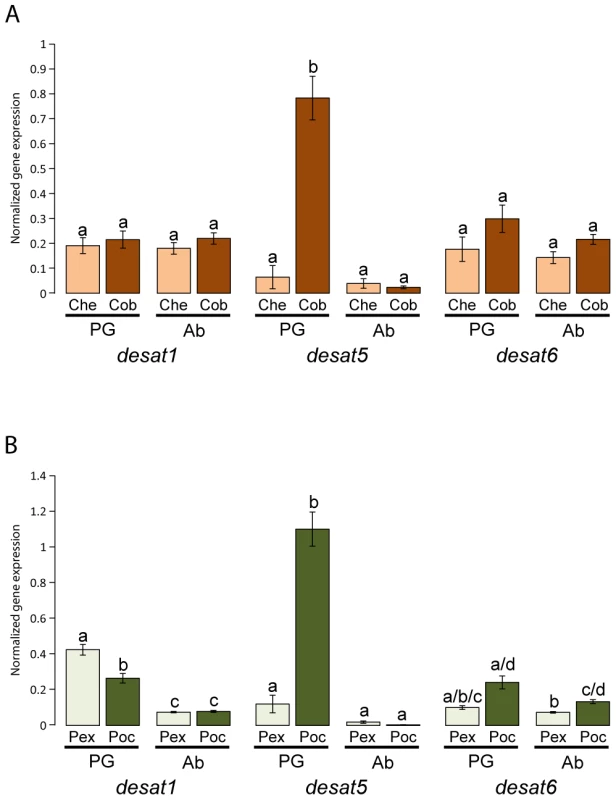
In panel (A) the normalised expression levels in the pheromone gland [PG] of C. herana (Che; light brown; n = 20) and C. obliquana (Cob; dark brown; n = 21) was compared with those in the abdomen [Ab] of C. herana (n = 20) and C. obliquana (n = 21), while in panel (B) the normalised expression levels in the pheromone gland of P. excessana (Pex; light green; n = 39) and P. octo (Poc; dark green; n = 24) are compared with those in the abdomen of P. excessana (n = 39) and P. octo (n = 25). Bars are the mean normalized gene expression, with error bars representing SEMs. Different small case letters indicate significant differences between tissues and/or species at the 95% level using the Bonferroni correction for each desaturase gene. Discussion
The molecular mechanisms involved in the production of the variants that underpin the evolution of new mate recognition systems and new species remain poorly understood. Toward addressing this, we have investigated the molecular differences in desaturase genes involved in pheromone biosynthesis in sibling species within the Ctenopseustis and Planotortrix genera of leafroller moths. We were particularly interested in whether differential regulation of a standing set of desaturase genes within a multigene family is involved in the observed differences in sex pheromone composition between the sibling species, C. obliquana vs. C herana and P. octo vs P. excessana.
Initially, we set out to identify the sequences encoding the four desaturases previously identified biochemically within the pheromone glands of these species. In total we isolated 27 new lepidopteran desaturase-like sequences from five species within the leafroller moth genera Ctenopseustis and Planotortrix (C. obliquana, C. herana, P. octo, P. excessana and P. notophaea). Together with the two desaturases previously isolated and characterized from P. octo [37], these 29 sequences represent six orthologous desaturases. We examined the ability of a representative subset of these predicted desaturases to introduce double bonds in sex pheromone precursors such that at least one member of each set of six orthologous desaturases has now been functionally characterized.
Two of the six desaturases from Ctenopseustis and Planotortrix encode Δ9-desaturases (desat1 and desat6) that display highly conserved functions across all lepidopteran insects investigated to date [17], [19], [37], [38], [39], [42]. For example, desat6 from C. obliquana and its ortholog from E. postvittana (Epo-Z9) encode a Δ9-desaturase with a preference for 18 carbon fatty acid precursors over 16 carbon precursors [39]. The remaining four desaturases all fall into the so-called Δ11 clade. While desat2 from C. obliquana and C. herana have no activity on typical sex pheromone fatty acid precursors, similar to desaturases identified previously from Choristoneura rosaceana and Ch. parallela [38], [43], we identified two desaturases showing interesting activities: desat3 and desat4. Desat3 from P. excessana encodes a desaturase with terminal desaturase activity, an activity that has recently been implicated in pheromone biosynthesis in the winter moth, Operophtera brumata [40]. In contrast with Ctenopseustis and Planotortrix, however, the sex pheromone of the winter moth is not derived from saturated fatty acids, but from linolenic acid. Desat4 from C. herana on the other hand encoded an enzyme with Δ6-desaturase activity. Like the terminal desaturase activity, this desaturation ability has only recently been observed in pheromone biosynthesis within the Lepidoptera. Wang et al. [18] described a Δ6-desaturase from the Chinese tussah silkworm, Antheraea pernyi, involved in the biosynthesis of the (E)-6-hexadecenoic acid as an intermediate in the pathway to producing the dienoic sex pheromone composed of (E,Z)-6,11-hexadecadienal, (E,Z)-6,11-hexadecadienyl acetate and (E,Z)-4,9-tetradecadienyl acetate. Despite these interesting associations with pheromone biosynthesis in other Lepidoptera, it is unlikely that desat3 and desat4, as well as desat2, are involved in pheromone biosynthesis in Ctenopseustis and Planotortrix because of their inability to produce desaturated precursors of the sex pheromone components found in the respective species, and their low level of gene expression levels in pheromone glands of all four species.
There are a number of examples of desaturase orthologs within the Lepidoptera that share a conserved biological function, especially within the two highly conserved Δ9-desaturase subfamilies (desat1 and desat6) (e.g., Roelofs et al. [19], Hao et al. [37]). Within the Δ11-like clade, in which the desat2, desat3, desat4 and desat5 orthologs all fall, the most widespread function is Δ11-desaturase activity. Members of this gene subfamily have been shown to evolve more rapidly than the Δ9-clades [19], which results in Δ11-like orthologs from distantly related species being less conserved (50–60%) and displaying more variable activities (i.e., Δ10, Z/E11, terminal desaturase activity, bifunctional Δ10/12 or Δ11/13). However, in closely related species the function of orthologs is usually conserved. This is the case for the Z/E11-desaturases from Ostrinia spp. [19], non-functional desaturases (e.g. desat2 orthologs) from Choristoneura spp. [38], [43] or Z11-desaturases from Helicoverpa spp. [42], [44]. Only in the case of Choristoneura parallela and Ch. rosaceana did two Δ11-orthologs that shared 92% protein identity differed slightly in activity, producing E11-14:acid or a mixture of Z/E11-14:acids, respectively [38], [43]. Still both enzymes introduced double bonds at the 11th carbon position, only differing in their isomeric preference. Of course one cannot rule out that some of the amino acid differences identified between the different orthologs might encode functional differences. However, we provide circumstantial evidence this is not the case from high sequence conservation (90–99% identity), from phylogenetic relationships within groups (desat2, desat3, desat4 and desat5, respectively), from tests of selection and overall evidence of purifying selection on coding region sequences (Table 1, Table 2, Table 3), and from the position of the amino acid differences being outside the active site regions. We also have tested some orthologous desaturases and do not find differences in activity. Altogether, evidence from previous desaturase studies together with our sequence, phylogenetic and functional analyses provide strong support that significant functional differences between desat3, desat4 and especially desat5 orthologs are unlikely.
Desat1, desat5 and desat6 have biological activities that are sufficient to explain all but one of the observed sex pheromone components and their routes of biosynthesis (Figure 1). Foster [36] provided evidence for the role of two distinct Δ9-desaturases in the biosynthesis of the Z5-14:OAc (desat6) and Z7-14:OAc (desat1) components of the P. excessana sex pheromone blend and a Δ10-desaturase (desat5) in the biosynthesis of Z8-14:OAc in P. octo [35] and C. obliquana [33]. The exception is the biosynthesis of Z5-14:OAc in C. herana and C. obliquana that is thought to be produced directly from myristic acid by a Δ5-desaturase [34], rather than from palmitic or stearic acid via desat1 or desat6, respectively, followed by rounds of chain shortening as found in P. excessana [35]. Despite intensive efforts, we are yet to identify another member of the fatty acyl desaturase family displaying Δ5-desaturase activity from any of the four species.
We then examined whether differential gene regulation of desat1, desat5 and desat6 could be responsible for the different sex pheromone components produced by the sibling species pairs C. obliquana vs C. herana and P. octo vs P. excessana. Interestingly, whereas desat1 and desat6 were expressed at largely similar levels in the four species, desat5 showed substantial differences in gene expression in the pheromone gland of female moths between C. obliquana vs C. herana, and P. octo vs P. excessana (Figure 5). C. obliquana had higher levels of expression of desat5 in female pheromone glands compared with C. herana and similarly, P. octo had higher levels of expression of desat5 compared with P. excessana, which can be associated with the presence (in C. obliquana and P. octo) or absence (in C. herana and P. excessana) of the Z8-14:OAc sex pheromone component.
While we have found evidence for the role of differential gene regulation in the evolution of new pheromone blends, structural mutations have been implicated in this process in genes immediately downstream of the desaturation step within the Lepidoptera. In the European corn borer, Ostrinia nubilalis, a fatty-acyl reductase has been identified that alters the specificity for the desaturated precursors, leading to the production of distinct sex pheromone blends between the so-called Z and E races [45]. Since only a single reductase allele has been identified that is active in pheromone biosynthesis in these moths, with the two alleles diverging at more than 30 amino acid positions (8%) together with several coding regions being under positive selection, only structural mutations are postulated to be responsible for sex pheromone diversity at the reduction step in this species [45]. In contrast, there was no strong evidence for structural mutations being responsible for the production of different sex pheromone components by the sibling species pairs P. octo vs P. excessana, since the desat5 genes are highly conserved (99% identity) and neither candidate loss of function mutations nor any evidence for selection acting on the coding regions of the orthologous desaturases were identified (Table 1, Table 2, Table 3). Although we miss the comparison between complete desat5 cDNA sequences for C. obliquana and C. herana, P. octo and P. excessana differ by only two amino acid differences at the desat5 locus. The first difference is a single amino acid deletion that occurs only in P. octo with respect to other Planotortrix desat5 orthologs, and still confers this species with an intact Δ10-desaturase activity [37]. The second difference is a glutamic acid to lysine substitution two amino acids towards the C terminus from the indel. Despite the possibility that one or both of these substitutions might impact the desaturase activity of desat5 in P. excessana, the fact that they lie outside active site regions suggests this is unlikely. Together, these changes in gene expression strongly suggest that a differential gene regulation of desat5 controls the presence/absence of the Z8-14:OAc in the sex pheromone of these sibling species pairs. These results also suggest that the same molecular mechanism may have been involved independently in pheromone evolution in the two genera.
In both genera a major change in expression of desat5 in the pheromone gland has occurred. The evolutionary event may have been either a loss of expression event from an ancestor expressing high levels of desat5 in their pheromone glands, or a gain event with the ancestor expressing little desat5 in their pheromone gland. We can reasonably infer the order of these evolutionary events by looking at sex pheromone composition in basal species in the two genera (see Newcomb and Gleeson [46] for discussion). Both Z8-14:OAc and Z10-16:OAc are synthesised from palmitic acid by a Δ10-desaturase (desat5), with Z8-14:OAc being produced following a round of β-oxidation, before reduction and acetylation (see Figure 1). Within Ctenopseustis, the basal species, C. servana, does not use any pheromone components that contain a double bond in an even position, suggesting that there has been a gain of the use of Z8-14:OAc or Z10-16:OAc early in the evolution of the genus after the split from C. servana. The use of these components presumably through the gain of expression of desat5 in their pheromone glands, then become widespread, used by both C. obliquana (Z8-14:OAc) and C. filicis (Z10-16:OAc). Subsequently South Island populations of a C. obliquana-like ancestor lost the expression of desat5 in the pheromone gland to give rise to C. herana, which only produces Z5-14:OAc. A similar loss of desat5 expression may also have occurred in the formation of the C. obliquana type II that occurs in a highly restricted North Island population and also only uses Z5-14:OAc as its sex pheromone [29], [47]. The most parsimonious scenario that explains sex pheromone evolution within Ctenopseustis thus suggests that desat5 pheromone gland expression was lost in C. herana following divergence from its C. obliquana-like ancestor. In contrast, the evolution of Δ10 - and Δ8-unsaturated sex pheromone components within Planotortrix likely derives from an evolutionary scenario involving a gain of expression of desat5 in the pheromone glands of P. octo. Hence in this genus, components such as Z5-14:OAc and Z7-14:OAc that are derived from the action of Δ9-desaturases are widespread within the group and thus probably represent the ancestral pheromone blend. The use of Z8-14:OAc is restricted to just two species within the genera, P. octo and its geographically isolated relative P. octoides, the latter of which is only found on the Chathman Islands. Therefore, the presence of Z8-14:OAc is a derived condition within the genus and indicates that desat5 expression in the pheromone glands of P. octo is likely a gain of function event. The alternative scenario that all species but two in the Planotortrix genus have lost expression of desat5 independently is a less parsimonious explanation. Simultaneously or subsequently to producing Z8-14:OAc P. octo must have also lost the ability to produce Z5-14:OAc and Z7-14:OAc. This may have come about through a change in one or both of the Δ9-desaturases.
Apart from this study, sex pheromone evolution through regulatory changes in desaturases has been investigated only in the corn borer moths, Ostrinia furnacalis and O. scapulalis [20]. In this example a Δ11 - and Δ14-desaturase show alternate expression in the pheromone gland of the two species, with the Δ11-desaturase being expressed in the pheromone gland of O. scapulalis but not in O. furnacalis and vice versa for the Δ14-desaturase. Together these examples point to differential gene regulation among a standing set of desaturase genes as a mechanism involved in producing novel sex pheromone components and blends within the Lepidoptera. A further example comes from Drosophila melanogaster where differences in mating ability between African and cosmopolitan populations are caused by sex pheromone differences and have been suggested as a case of incipient speciation [48]. Here, a Δ9-desaturase is differentially regulated in the population through a cis-regulatory deletion within the promoter of the gene resulting in cuticular hydrocarbon sex pheromone differences that may ultimately promote the speciation of the two D. melanogaster races.
Differential gene regulation can result from two classes of mutation; either changes in the regulatory region of the differentially expressed gene (cis-regulatory mutation) or changes in transcriptions factors that bind to the promoter (trans-regulatory mutation). Trans-regulatory mutations are often associated with pleiotropic impacts on the regulation of other genes due to transcription factors typically acting on several promoters. Therefore it is perhaps more likely that cis-regulatory mutations are responsible for the differential regulation of desat5 in Ctenopseustis and Planotortrix, an hypothesis which we are currently in the process of testing. In conclusion we show that interspecific pheromone differences between sibling species are determined by parallel changes in desaturase gene expression in two sister genera. This case study suggests that differential regulation within large multigene families may be an important process in speciation, with changes in gene expression underpinning the evolution of novel mating systems.
Materials and Methods
Insects
Ctenopseustis herana, C. obliquana, Planotortrix excessana, P. octo and P. notophaea were obtained from the Plant & Food Research insect rearing facility at the Mt Albert Research Centre, Auckland, New Zealand. The history of these strains is reported in Newcomb and Gleeson [46], except that an additional strain of P. excessana derived from material caught in the South Island of New Zealand was used to generate a P. excessana South Island strain and the original P. excessana strain is now known as P. excessana North Island. Insects were reared on a 16∶8 light cycle, with larvae reared at 20°C and pupae and adults at 18°C.
Gene isolation
Genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen). Total RNA was extracted from two distinct regions of the abdomen of 2–3 day old virgin adult females and from fat bodies of 5th instar larvae. From the adult females the pheromone gland, located within the dorsal region of the 8th and 9th abdominal segments (denoted ‘pheromone gland’) was dissected. As a control, a lateral region of the 4th to 6th abdominal segments of the same adult females (denoted ‘abdomen’) was also dissected. RNA was isolated from dissected tissue using 800 µl of Trizol (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The expression of desaturase genes was initially characterized from RNA generated from pools of 100 pheromone glands, while RNA from single pheromone glands was used for subsequent Quantitative Real-Time PCR experiments. After DNase treatment (DNaseI amplification grade, Invitrogen), the cDNA synthesis was carried out using the iScript cDNA Synthesis Kit (Bio-Rad) from 1 µg of total RNA or approximately 100 ng of total RNA for single samples, and incubated at 50°C for 1 hr, followed by 70°C for 15 mins.
In order to identify the different desaturases involved in sex pheromone biosynthesis, a progressive approach using successive and complementary methods was used for each species. Initially, degenerate primers were applied to genomic and pheromone gland cDNA using primers designed to conserved amino acid motifs found in lepidopteran desaturases. Sequences of the coding region of desaturase genes were then extended by means of 5′ and 3′ Rapid Amplification of cDNA ends (RACE), genome walking, or Inverse PCR. All the primers used are listed in Table S2. All PCR amplifications were performed on a GeneAmp 9700 (Applied Biosystems). The fragments of interest were cloned into pGEM-T Easy Vector System (Promega) and transformed into JM109 competent E. coli cells, according to the manufacturer's instructions. Sequencing was performed at the Allan Wilson Centre Genome Service (AWCGS) at Massey University, Palmerston North, New Zealand or Macrogen in South Korea.
Degenerate PCR was performed for each species on the genomic DNA or pheromone gland cDNA using 0.2 µl of Platinum Taq DNA polymerase (5 units/µl, Invitrogen), 1.5 mM Mg2+, 0.2 mM of each dNTP and 2 µM of each degenerate PCR primer (Table S2). Cycling conditions were 2 min at 94°C, 35 cycles of 94°C for 10 s, 50°C for 10 s and 72°C for 1 min, and a final extension of 72°C for 10 min.
The 3′ ends of the coding regions were obtained using a modified version of the 3′ RACE System for Rapid Amplification of cDNA ends (Invitrogen). First strand cDNA synthesis was carried out in a final volume of 14 µl using 1–2 µg total RNA, 1 µl 3′AP (or RoRidT16) primer (10 µM), 1 µl of 10 mM dNTPs, and incubated at 65°C for 5 min, and placed on ice for 1 min. Then, 4 µl of 5× first strand buffer, 1 µl of 0.1 M DTT and 1 µl of Superscript III (200units/µl, Invitrogen) were added to the mixture. The reactions were incubated at 50°C for 1 hr, followed by 70°C for 15 min. The 3′-tagged products were detected by PCR amplification using forward desaturase group-specific primers (3′ RACE-F primers) and the 3′AUAP (or Ri) primer, with 0.2 µl of Platinum Taq DNA polymerase (5units/µl, Invitrogen), 1.5 mM Mg2+, 0.2 mM of each dNTP and 0.2 µM of each primer. Cycling conditions were 2 min at 94°C, 30 cycles of 94°C for 10 s, 55°C for 30 s and 72°C for 1 min, and a final extension of 72°C for 10 min.
The 5′ ends of cDNAs were amplified using the 5′ RACE System for Rapid Amplification of cDNA ends kit (Invitrogen). Oligo-dC tails were added to purified 3′ RACE cDNA in a final volume of 20 µl with 4 µl of 5× first tailing buffer, 2 µl of 2 mM dCTP, 3 µl of 5 mM CoCl2. Reactions were incubated at 94°C for 3 min and placed on ice for 1 min. Then, 1 µl of TdT (400units/µl) was added, and the reaction was incubated at 37°C for 10 min and stopped at 65°C for 10 min. Oligo-dC-tailed products were amplified by normal PCR (30 cycles), and 1 µl of the later reaction was used for a nested-PCR (25 cycles), using the 5′ RACE-F and 5′ RACE-R primers (Table S2).
Inverse PCR was performed by digesting genomic DNA overnight at 37°C with NdeI or SalI, BSA (100 µg/ml) and Spermidine (2 mM). Classical phenol/chloroform extraction and ethanol precipitation were used to purify the digested products. These were then circularized by ligation overnight at 16°C with T4 DNA ligase (400 units/µl, New England Biolabs), and purified as previously described. The 3′ ends of two distinct fragments were amplified in C. herana using the Inverse PCR primers (Table S2) under the same conditions as described for the 3′ RACE.
In some cases, genome walking was used to extend the coding sequences of desaturase genes. For this approach genomic DNA was digested overnight at 37°C with DraI, EcoRV, PvuII and StuI, separately, and the products purified with the DNA Isolation Kit for Cells and Tissues (Roche). GenomeWalker adapters were then ligated to both ends of the digests by incubating 3 µl of template overnight at 16°C with 2 µl of adapter primers (100 µM) and 1 µl of T4 DNA ligase (400 units/µl, New England Biolabs) in a final volume of 20 µl. Specific tagged-products were amplified by normal and nested-PCR (see 5′ RACE), using the GW1-F and GW1-R primers (Table S2). Gene-specific PCR was conducted using highly specific PCRs (Gene-specific primers, Table S2) to verify all contigs of the coding regions.
Bioinformatics
Sequences were analysed using Geneious Pro v5.3.4 (Biomatters). Sequences were aligned using ClustalX [49] and codon aligned nucleotide alignments were produced using RevTrans version 1.4.1 (www.cbs.dtu.dk/services/RevTrans/). For phylogenetic analyses, explicit models of evolution were determined using Modeltest [50] and GTR+I+Γ was implemented for likelihood and Bayesian analyses. Parsimony and maximum likelihood analyses were performed in PAUP v4.0b (Sinauer Associates, Sunderland, Massachusetts), and Bayesian inference was implemented in MrBayes v3.0b4 [51]. Evidence for selection was tested by looking for deviations from neutral expectations using the CODEML program in the PAML package [41]. Multiple models were run (M0, M3, M7, M8 and M8a) to assess selection pressures. Comparisons of nested models were used to assess heterogeneous selective pressure amongst sites (M0–M3) or positive selection (M7–M8). Nested models were compared by the implementation of a likelihood-ratio test (LRT), where the LRT is twice the log-likelihood difference of the nested models. Significance was tested using a χ2 test with degrees of freedom equal to the difference in the number of parameters between the two models.
Quantitative real-time RT–PCR
Pheromone glands were dissected from two-day-old virgin adult females of C. herana, C. obliquana, P. excessana and P. octo. Pheromone glands were pooled in lots of 100 or used singly in RNA extractions. Fat bodies were dissected from 5th instar larvae, and pooled in lots of five, while abdomens were extracted singly. RNA and cDNA were extracted as described in the gene isolation section.
The expression of the desaturases together with the housekeeper genes actin, α-tubulin and elongation factor 1 α were determined using primers described in Table S2. All quantitative real-time PCRs were performed for the pheromone gland and the abdomen, for each specimen, in duplicate. Experiments were performed on the LightCycler480 Real-Time Instrument (Roche Diagnostics, Basel, Switzerland), in a final reaction volume of 10 µL, with 80 ng of cDNA, 5 µL 2× SYBR Green Mix (Bio-Rad), and 0.5 µM of each primer. The PCR cycling conditions were set to 2 min at 95°C followed by 45 cycles of 15 s at 95°C, 30 s at 60°C and 30 s at 72°C. A final dissociation curve analysis was added (15 s at 95°C, 15 s at 60°C, and a gradual heating to 95°C at 0.01°C/s) to confirm the presence of a single amplicon.
Relative expression levels were calculated according to the ΔΔCp method [52], [53]. The amplification efficiency was calculated for each PCR using the LinRegPCR software [54]. For each sample, the average Threshold Cycle values were extracted, and a normalization factor, based on the geometric averaging of the reference gene expression levels, was determined using geNorm [55]. Normalization factors allowed correction for PCR efficiency and normalization of the gene expression levels. To test for differences in levels of normalised relative expression between species and tissues one way ANOVAs were conducted using GraphPad Prism 5, with individual comparisons made using Bonferroni-corrected t-tests at the 95% significance level.
Desaturase activity assessment in yeast
Functional assays were performed with at least one ortholog of each of the desaturases. The Cob-desat2, Cher-desat2, Pex-desat3 and Cher-desat4 ORFs were cloned into pYEX-CHT vectors and transformed into the desaturase - and elongase-deficient (elo1 ole1) strain of Saccharomyces cerevisiae (MATa elo1::HIS3 ole1::LEU2 ade2 his3 leu2 ura3) [52]. In the same way, the Cob-desat6 ORF was cloned into the YEpOLEX vector and transformed in the desaturase-deficient (ole1) strain of S. cerevisiae (MATα ole1Δ::LEU2 leu2-3 leu2-112 trp1-1 ura3-52 his4) [56], [57]. Functional assays were performed with the S.c. Easy Transformation kit (Invitrogen AB, Lidingö, Sweden). pYEX-CHT and YEpOLEX vectors only were used as negative control.
Transformed ole1 elo1 or ole1 yeast cells were incubated for 4 days at 30°C on selective medium plates containing 0.7% YNB (w/o amino acids, with ammonium sulphate) and a complete drop-out medium lacking uracil and leucine (ForMedium LTD, Norwich, England), 2% glucose, 0.01% adenine, 1% tergitol (type Nonidet NP-40, Sigma-Aldrich Sweden AB, Stockholm, Sweden) and 0.5 mM unsaturated oleic acid (Larodan Fine Chemicals, Malmö, Sweden). Note that in addition to Z9-18:Acid, ole1 elo1 yeast contained residual traces of Z9-16:Acid because of supplementation during the earlier procedure of making cells competent.
Individual ole1 elo1 colonies were selected and incubated in 10 ml fresh selective medium and cultures were incubated in inclined position at 30°C for 48 hr and 250 rpm (Innova 42, New Brunswick Scientific). Yeast cultures were diluted to an OD600 = 0.4 in 10 ml fresh SC-U medium and supplemented with 25 µl CuSO4 1 M in water (final concentration: 2.5 mM). After 48 hr of incubation at 250 rpm in presence of copper, yeast cells were collected by centrifugation at 2,000× g (Labofuge 200, Heraeus Instruments) and washed with sterile water. The total yeast lipid fraction was extracted with chloroform∶methanol (2∶1, v∶v) and the extracts were base-methanolyzed according to standard protocols [17], [58]. Double bond localization in methyl esters was determined by dimethyl disulfide (DMDS) derivatization [59] before GC-MS analysis.
To test for genetic complementation of the ole1 auxotrophy by the YEpOLEX-Cob-desat6, individual SC-U-Leu yeast colonies were selected and patched onto YPAD plates lacking Fatty Acids (FA) and incubated for 4 days at 30°C. Positive transformants were subsequently grown for 48 hr at 30°C and 300 rpm in 10 ml SC medium without FA, recovered by centrifugation and washed with water, followed by base methanolysis and DMDS derivatization.
Before analysis by GC-MS analyses, samples were concentrated under a gentle flow of pure nitrogen to a final volume of approx. 50 µl. For analysis of fatty acid methyl esters (FAMEs), 3 µl was injected on a gas chromatograph (Hewlett Packard HP 5890II GC system) coupled to a mass selective detector (HP 5972) and equipped with a polar INNOWAX column (100% polyethylene glycol, 30 m×0.25 mm×0.25 µm, Agilent Technologies). The GC-MS was operated in electron impact mode (70 eV) and the injector was configured in splitless mode at 220°C with helium used as carrier gas (velocity: 30 cm/s). The oven temperature was maintained for 2 min at 50°C and increased at a rate of 10°C/min up to 220°C, held for 20 min.
For analysis of DMDS adducts, 2 µl was injected on a GC (Hewlett Packard HP 6890, Agilent Technologies) equipped with an HP-5MS capillary column (5% Phenyl Methyl Siloxane; 30 m×250 µm: df = 0.25 µm; carrier gas: helium; velocity: 30 cm/s), an automatic injector (HP-7683), and coupled to a HP 5973 mass selective detector. The injector was configured in splitless mode at 250°C. The oven temperature was maintained for 2 min at 80°C, increased at a rate of 15°C/min up to 140°C, increased at a rate of 5°C/min up to 280°C, and held for 10 min.
Supporting Information
Zdroje
1. HoekstraHECoyneJA 2007 The locus of evolution: evo devo and the genetics of adaptation. Evolution 61 995 1016
2. CarrollSB 2005 Evolution at two levels: on genes and form. PLoS Biol 3 e245 doi:10.1371/journal.pbio.0030245
3. WrayGA 2007 The evolutionary significance of cis-regulatory mutations. Nature Reviews Genetics 8 206 216
4. KingM-CWilsonAC 1975 Evolution at two levels in humans and chimpanzees. Science 188 107 116
5. KirschnerMGerhartJ 1998 Evolvability. Proceedings of the National Academy of Sciences USA 95 8420 8427
6. SternDL 2000 Perspective: evolutionary developmental biology and the problem of variation. Evolution 54 1079 1091
7. CarrollSB 2008 Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134 25 36
8. NosilPSchluterD 2011 The genes underlying the process of speciation. Trends in Ecology and Evolution 26 160 167
9. HartleyCJNewcombRDRussellRJYongCGStevens 2006 Amplification of DNA from preserved specimens shows blowflies were preadapted for the rapid evolution of insecticide resistance. Proceedings of the National Academy of Sciences USA 103 8757 8762
10. KarasovTMesserPWPetrovDA 2010 Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genet 6 e1000924 doi:10.1371/journal.pgen.1000924
11. SmadjaCButlinRK 2009 On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102 77 97
12. LinnCEJrRoelofsWL 1995 Pheromone communication in moths and its role in the speciation process. LambertDMSpencerHG Speciation and the recognition concept: theory and application Baltimore and London 263 300
13. El-SayedAM 2008 The Pherobase: database of insect pheromones and semiochemicals. http://www.pherobase.com
14. TillmanJASeyboldSJJurenkaRABlomquistGG 1999 Insect pheromones - an overview of biosynthesis and endocrine regulation. insect Biochemistry and Molecular Biology 29 481 514
15. MatsumotoS 2010 Molecular mechanisms underlying sex pheromone production in moths. Bioscience Biotechnology & Biochemistry 74 223 231
16. KnippleDCRosenfieldC-LNielsenRYouKMJeongSE 2002 Evolution of the integral membrane desaturase gene family in moths and flies. Genetics 162 1737 1752
17. LiénardMAStrandhMHedenströmEJohanssonTLöfstedtC 2008 Key biosynthetic gene subfamily recruited for pheromone production prior to the extensive radiation of Lepidoptera. BMC Evolutionary Biology 8 270
18. WangH-LLiénardMAZhaoC-HWangC-ZLöfstedtC 2010 Neofunctionalization in an ancestral insect desaturase lineage led to rare Δ6 pheromone signals in the Chinese tussah silkworm. Insect Biochemistry and Molecular Biology 40 742 751
19. RoelofsWLLiuWHaoGJiaoHRooneyAP 2002 Evolution of moth sex pheromones via ancestral genes. Proceedings of the National Academy of Sciences USA 99 13621 13626
20. SakaiRFukuzawaMNakanoRTatsukiSIshikawaY 2009 Alternative suppression of transcription from two desaturase genes is the key for species-specific sex pheromone biosynthesis in two Ostrinia moths. Insect Biochemistry and Molecular Biology 39 62 67
21. ShirangiTRDufourHDWilliamsTMCarrollSB 2009 Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 7 e1000168 doi:10.1371/journal.pbio.1000168
22. DugdaleJS 1990 Reassessment of Ctenopseustis Meyrick and Planotortrix Dugdale with descriptions of two new genera (Lepidoptera: Tortricidae). New Zealand Journal of Zoology 17 437 465
23. LanghoffPAuthierABuckleyTRDugdaleJSRodrigoA 2009 DNA barcoding of the endemic New Zealand leafroller moth genera, Ctenopseustis and Planotortrix. Molecular Ecology Resources 9 691 698
24. FosterSPDugdaleJSWhiteCS 1991 Sex pheromones and the status of greenheaded and brown headed leafroller moths in New Zealand. New Zealand Journal of Zoology 18 63 74
25. GalbreathRABennMHYoungHHoltVA 1985 Sex pheromone components in the New Zealand greenheaded leafroller Planotortrix excessana (Lepidoptera: Tortricidae). Zeitschrift fur Naturforschung 40 266 271
26. FosterSPClearwaterJRMugglestonSJShawPW 1990 Sex pheromone of a Planotortrix excessana sibling species and reinvestigation of related species. Journal of Chemical Ecology 16 2461 2474
27. YoungHGalbreathRABennMHHoltVAStrubleDL 1996 Sex pheromone components in New Zealand brownheaded leafroller Ctenopseustis obliquana (Lepidoptera: Tortricidae). Zeitschrift fur Naturforschung 40 262 265
28. FosterSPRoelofsWL 1987 Sex pheromone differences in populations of the brownheaded leafroller, Ctenopseustis obliquana. Journal of Chemical Ecology 13 623 629
29. ClearwaterJRFosterSPMugglestonSJDugdaleJSPriesnerE 1991 Intraspecific variation and interspecific differences in sex pheromones of sibling species in Ctenopseustis obliquana complex. Journal of Chemical Ecology 17 413 429
30. FosterSPMugglestonSJLöfstedtCHanssonB 1997 A genetic study on pheromonal communication in two Ctenopseustis moths. CardéRTMinksA Insect Pheromones: New Directions 514 524
31. WhiteCSLambertDM 1994 Genetic differences among pheromonally distinct New Zealand leafroller moths. Biochemical Systematics and Ecology 22 329 339
32. WhiteCSLambertDM 1995 Genetic continuity within, and discontinuities among, populations of leafroller moths with distinct sex-pheromones. Heredity 75 243 255
33. LöfstedtCRoelofsWL 1985 Sex pheromone precursors in two primitive New Zealand tortricid moth species. Insect Biochemistry 15 729 734
34. FosterSPRoelofsWL 1996 Sex pheromone biosynthesis in the tortricid moth, Ctenopseustis herana (Felder & Rogenhofer). Archives of Insect Biochemistry and Physiology 32 135 147
35. FosterSPRoelofsWL 1988 Sex pheromone biosynthesis in the leafroller moth Planotortix excessana by Δ10 desaturation. Archives of Insect Biochemistry and Physiology 8 1 9
36. FosterSP 1998 Sex pheromone biosynthesis in the tortricid moth Planotortrix excessana (Walker) involves chain-shortening of palmitoleate and oleate. Archives of Insect Biochemistry and Physiology 37 158 167
37. HaoGLiuWO'ConnorMRoelofsWL 2002 Acyl-CoA Z9 - and Z10-desaturase genes from a New Zealand leafroller moth species, Planotortrix octo. Insect Biochemistry and Molecular Biology 32 961 966
38. HaoGO'ConnorMLiuWRoelofsWL 2002 Characterization of Z/E11 - and Z9-desaturases from the obliquebanded leafroller moth, Choristoneura rosaceana. Journal of Insect Science 2 11 Available online: insectscience.org/12.11
39. LiuWJiaoHMurrayNCO'ConnorMRoelofsWL 2002 Gene characterized for membrane desaturase that produces (E)-11 isomers of mono - and diunsaturated fatty acids. Proceedings of the National Academy of Sciences USA 99 620 624
40. DingB-JLiénardMAWangH-LZhaoC-HLöfstedtC 2011 Terminal fatty-acyl-CoA desaturase involved in sex pheromone biosynthesis in the winter moth (Operophtera brumata). Insect Biochemistry and Molecular Biology 41 715 722
41. YangZ 1997 PAML: a program for package for phylogenetic analysis by maximum likelihood. CABIOS 15 555 556
42. RosenfieldC-LYouKMMarsella-HerrickPRoelofsWLKnippleDC 2001 Structural and functional conservation and divergence among acyl-CoA desaturases of two noctuid species, the corn earworm, Helicoverpa zea, and the cabbage looper, Trichoplusia ni. Insect Biochemistry and Molecular Biology 31 949 964
43. LiuWRooneyAPXueBRoelofsWL 2004 Desaturases from the spotted fireworm moth (Choristoneura parallela) shed light on the evolutionary origins of novel moth sex pheromone desaturases. Gene 342 303 311
44. JeongSERosenfieldC-LMarsella-HerrickPYouKMKnippleDC 2003 Multiple acyl-CoA desaturase-encoding transcripts in pheromone glands of Helicoverpa assulta, the oriental tobacco budworm. Insect Biochemistry and Molecular Biology 33 609 622
45. LassanceJ-MGrootATLiénardMAAntonyBBorgwardtC 2010 Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature 466 486 489
46. NewcombRDGleesonDM 1998 Pheromone evolution within the genera Ctenopseustis and Planotortrix (Lepidoptera: Tortricidae) inferred from a phylogeny based on cytochrome oxidase I gene variation. Biochemical Systematics and Ecology 26 473 484
47. FosterSPClearwaterJRMugglestonSJDugdaleJS 1986 Probable sibling species complexes within two described New Zealand leafroller moths. Naturwissenschaften 73 156 158
48. DalleracRLabeurCJallonJ-MKnippleDCRoelofsWL 2000 A Δ9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proceedings of the National Academy of Sciences USA 97 9449 9454
49. ThompsonJDGibsonTJPlewniakFJeanmouginFHigginsDG 1997 The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25 4876 4882
50. PosadaDCrandallKA 1998 Modeltest: testing the model of DNA substitution. Bioinformatics 14 817 818
51. RonquistFHuelsenbeckJP 2003 MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572 1574
52. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. METHODS 25 402 408
53. PfafflMW 2006 Relative quantification. DorakT Real-time PCR La Jolla, CA, USA International University Line 63 82
54. RamakersCRuijterJMLekanne DeprezRHMoormanAFM 2003 Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters 339 62 66
55. VandesompeleJDe PreterKPattynFPoppeBVan RoyN 2002 Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3 RESEARCH0034
56. SchneiterRTatzerVGoggGLeitnerEKohlweinS-D 2000 Elo1-dependent carboxy-terminal elongation of C14 : 1 Δ9 to C16 : 1Δ11 fatty acids in Saccharomyces cerevisiae. Journal of Bacteriology 182 3655 3660
57. StuckeyJEMcDonoughVMMartinCE 1990 The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. The Journal of Biological Chemistry 265 20144 20149
58. KnippleDCRosenfieldC-LMillerSJLiuWTangJ 1998 Cloning and functional expression of a cDNA encoding a pheromone gland-specific acyl-CoA Δ11-desaturase of the cabbage looper moth, Trichoplusia ni. Proceedings of the National Academy of Sciences USA 95 15287 15292
59. BuserHRArnHGuerinPRauscherS 1983 Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Analytical Chemistry 55 818 822
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant ImmunityČlánek Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



