-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAdaptation and Preadaptation of to Bile
Bile possesses antibacterial activity because bile salts disrupt membranes, denature proteins, and damage DNA. This study describes mechanisms employed by the bacterium Salmonella enterica to survive bile. Sublethal concentrations of the bile salt sodium deoxycholate (DOC) adapt Salmonella to survive lethal concentrations of bile. Adaptation seems to be associated to multiple changes in gene expression, which include upregulation of the RpoS-dependent general stress response and other stress responses. The crucial role of the general stress response in adaptation to bile is supported by the observation that RpoS− mutants are bile-sensitive. While adaptation to bile involves a response by the bacterial population, individual cells can become bile-resistant without adaptation: plating of a non-adapted S. enterica culture on medium containing a lethal concentration of bile yields bile-resistant colonies at frequencies between 10−6 and 10−7 per cell and generation. Fluctuation analysis indicates that such colonies derive from bile-resistant cells present in the previous culture. A fraction of such isolates are stable, indicating that bile resistance can be acquired by mutation. Full genome sequencing of bile-resistant mutants shows that alteration of the lipopolysaccharide transport machinery is a frequent cause of mutational bile resistance. However, selection on lethal concentrations of bile also provides bile-resistant isolates that are not mutants. We propose that such isolates derive from rare cells whose physiological state permitted survival upon encountering bile. This view is supported by single cell analysis of gene expression using a microscope fluidic system: batch cultures of Salmonella contain cells that activate stress response genes in the absence of DOC. This phenomenon underscores the existence of phenotypic heterogeneity in clonal populations of bacteria and may illustrate the adaptive value of gene expression fluctuations.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002459
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002459Summary
Bile possesses antibacterial activity because bile salts disrupt membranes, denature proteins, and damage DNA. This study describes mechanisms employed by the bacterium Salmonella enterica to survive bile. Sublethal concentrations of the bile salt sodium deoxycholate (DOC) adapt Salmonella to survive lethal concentrations of bile. Adaptation seems to be associated to multiple changes in gene expression, which include upregulation of the RpoS-dependent general stress response and other stress responses. The crucial role of the general stress response in adaptation to bile is supported by the observation that RpoS− mutants are bile-sensitive. While adaptation to bile involves a response by the bacterial population, individual cells can become bile-resistant without adaptation: plating of a non-adapted S. enterica culture on medium containing a lethal concentration of bile yields bile-resistant colonies at frequencies between 10−6 and 10−7 per cell and generation. Fluctuation analysis indicates that such colonies derive from bile-resistant cells present in the previous culture. A fraction of such isolates are stable, indicating that bile resistance can be acquired by mutation. Full genome sequencing of bile-resistant mutants shows that alteration of the lipopolysaccharide transport machinery is a frequent cause of mutational bile resistance. However, selection on lethal concentrations of bile also provides bile-resistant isolates that are not mutants. We propose that such isolates derive from rare cells whose physiological state permitted survival upon encountering bile. This view is supported by single cell analysis of gene expression using a microscope fluidic system: batch cultures of Salmonella contain cells that activate stress response genes in the absence of DOC. This phenomenon underscores the existence of phenotypic heterogeneity in clonal populations of bacteria and may illustrate the adaptive value of gene expression fluctuations.
Introduction
Bile is a fluid containing bile salts, cholesterol, and a variety of proteins and electrolytes [1]. Bile is synthesized by parenchymal cells (hepatocytes) in the liver. In mammals with a gall bladder, a fraction of bile is stored in the gall bladder while another fraction flows directly into the small intestine [1]. When food passes by the small intestine, gall bladder contraction releases bile into the duodenum. Bile aids in the digestion of fats, facilitates absorption of fat-soluble vitamins in the intestine, and contributes to the elimination of excess cholesterol and waste metabolic products produced in the liver [1].
About two thirds of bile (dry weight) are made of bile salts, a family of molecules with steroid structure which derive from cholesterol [2]. Bile salts dissolve membrane lipids and cause dissociation of integral membrane proteins. Inside the cell, the detergent activity of bile salts causes misfolding and denaturation of proteins [3], [4]. Chelation of calcium and iron by bile salts is also a source of physiological perturbations [3], [4]. Furthermore, bile salts have DNA damaging capacity, stimulate DNA rearrangements, and induce plasmid curing [4], [5], [6], [7]. However, certain bacterial species are resistant to the antibacterial activities of bile salts [3], [8]. This trait has been exploited for the design of selective microbiological media such as the one-century-old MacConkey agar used in the identification of genera of the family Enterobacteriaceae. On the other hand, bile salts regulate the expression of specific bacterial genes, some of them necessary for bile resistance and others involved in pathogenesis [3], [8]. Bile salts may thus be viewed both as environmental signals used by bacteria to identify bile-containing animal environments and as antibacterial compounds [8].
An extreme example of bile-resistant pathogen is Salmonella enterica, which colonizes the hepatobiliary tract during systemic infection and persists in the gall bladder during chronic infection [9], [10]. Salmonella survival in the mammalian gall bladder seems to involve several strategies. Invasion of the gall bladder epithelium may permit escape from the extremely high concentrations of bile salts present in the gall bladder lumen [11]. Formation of biofilms on gallstones may also protect Salmonella from the bactericidal activities of bile salts [12], [13]. However, planktonic Salmonella cells are also found at high numbers in the bile-laden gall bladder lumen, and the mechanisms employed to thrive in such a harsh environment remain to be identified.
Bile resistance can be studied under laboratory conditions by adding ox bile or individual bile salts to microbiological media [14]. Genetic and biochemical analysis in E. coli and S. enterica in the laboratory has permitted the identification of cell functions and mechanisms involved in bile resistance [3], [8], [14]. The relevance of these reductionist studies is supported by the fact that mutations that cause bile sensitivity in vitro often result in virulence attenuation in the mouse model of S. enterica infection [14]. The list of bile resistance factors in Salmonella and other enteric species includes envelope barriers such as the lipopolysaccharide [15], [16] and the enterobacterial common antigen [17], the outer membrane [18], [19], the cytoplasmic membrane [20], efflux pump systems [21], genes of the multiple antibiotic resistence (mar) and PhoPQ regulons [22], [23], and DNA repair functions [5], [6]. Genetic analysis has also identified cell functions whose loss increases bile resistance, probably by activating cell defense responses [20].
The bile resistance level of wild type Salmonella can be increased over the customary minimal inhibitory concentration by growth in the presence of sublethal concentrations of bile [8], [24]. This phenomenon, henceforth called “adaptation”, is easily observed in the laboratory and may be relevant during Salmonella colonization of the hepatobiliary tract. Below we describe studies of Salmonella adaptation to bile in vitro, and show that growth of S. enterica on sublethal concentrations of bile is accompanied by dramatic changes in gene expression. We also report that batch Salmonella cultures contain cells that show high levels of bile resistance without previous adaptation. This phenomenon, henceforth called “preadaptation”, seems to involve two unrelated processes. One is mutation in specific loci, often related to lipopolysaccharide transport; another is activation of bile resistance responses in a subpopulation of bacterial cells. The latter phenomenon fits well in current views indicating that bacterial populations are heterogeneous, and that fluctuations in gene expression can have adaptive value [25], [26].
Results
Viability of S. enterica SL1344 in the presence of sodium deoxycholate
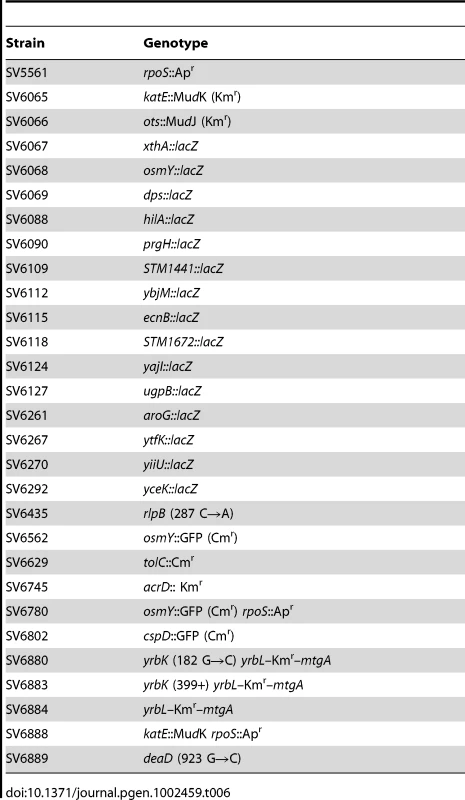
The minimal inhibitory concentration (MIC) of sodium deoxycholate for S. enterica strain SL1344 grown in LB is 7%, and the MIC of ox bile is 12% under the same conditions. These MICs are similar to those previously reported for strain ATCC 14028 [5], [6]. To ascertain whether inhibition of bacterial growth by bile salts involves bacterial death or merely growth arrest, we performed viability tests to distinguish live and dead Salmonella cells in the presence of DOC. Aliquots from Salmonella exponential cultures grown in LB were treated with various concentrations of DOC (1%, 3%, 5%, 7%, and 9%) for 30 min. Examination under the microscope using a commercial live/dead color-based kit was then performed, and the numbers of live/dead cells were counted. Cell counting was randomly performed, and the minimal number of bacterial cells counted was 1,700. A representative experiment is shown in Figure 1, top panel. A direct correlation was found between the percentage of dead Salmonella cells and the concentration of DOC.
Fig. 1. Percentages of live and dead bacteria and relative numbers of colony-forming units. 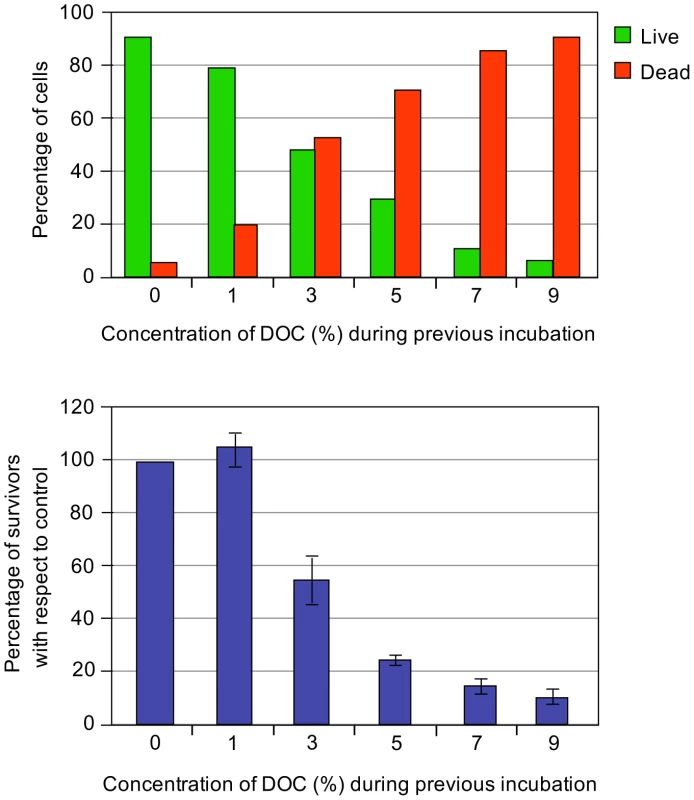
Top panel: Percentages of live and dead bacteria (green and red histograms, respectively) found in 1 ml aliquots of an exponential culture of S. enterica SL1344 incubated in the presence of different concentrations of sodium deoxycholate (1%, 3%, 5%, 7% and 9%) during 30 minutes at 37°C. Bottom panel: Relative numbers of colony forming-units (CFU) after incubation of S. enterica SL1344 in the presence of different concentrations of sodium deoxycholate (1%, 3%, 5%, 7% and 9%) during 30 minutes at 37°C. The number of CFU in the absence of DOC is shown as 100%. The bactericidal capacity of bile was also monitored by plaque counts of colony-forming-units. Aliquots from Salmonella exponential cultures grown in LB were treated with various concentrations of DOC (1%, 3%, 5%, 7%, and 9%) for 30 min. The cultures were then diluted, plated on LB, and incubated overnight at 37°C. Colony counts confirmed that exposure to DOC renders S. enterica cells inviable in a dose-dependent maner (Figure 1, bottom panel).
Adaptation of S. enterica to lethal concentrations of sodium deoxycholate
Although the level of resistance to bile is fairly constant under given conditions, Salmonella can be adapted to grow at higher concentrations of bile by previous growth in the presence of sublethal concentrations [8]. To determine the concentration(s) of DOC that permit adaptation in strain SL1344, S. enterica cultures were grown in LB containing different concentrations of DOC (from 1% to 7%). Aliquots from the cultures were then transferred to microtiter plates containing DOC at concentrations ranging from 1% to 14%. As shown in the diagram of Figure 2, growth at concentrations slightly lower than the MIC (4% and 5%) increased the MIC of DOC to ≥14%. A smaller increase of the MIC was likewise observed after growth in 3% DOC.
Fig. 2. Minimal inhibitory concentrations (MICs) of sodium deoxycholate (DOC) for <i>Salmonella</i> cultures pre-exposed to various concentrations of DOC, and MICs for the same cultures after overnight growth in LB. 
The bacterial cells that had survived bile in the microtiter plate were transferred to LB and cultured overnight. Aliquots from these cultures were then used to determine the MIC of DOC in microtiter plates. As shown in Figure 2, a MIC of 7% DOC was determined for all cultures, indicating that resistance had decreased back to the level characteristic of strain SL1344. Hence, adaptation to DOC by growth at concentrations of 3–5% is reversible, and does not involve selection of bile-resistant mutants, at least under the conditions tested.
Transcriptomic analysis of S. enterica gene expression during growth on sublethal concentrations of sodium deoxycholate
The capacity of bile salts to induce changes in gene expression is well known in S. enterica [22], [27] and in other bacteria [3], as well as in eukaryotes [28]. On these grounds, we hypothesized that the reversible increase of MIC observed when S. enterica is grown in the presence of sublethal concentrations of DOC might involve changes in gene expression. This hypothesis was tested by transcriptomic analysis using the Salgenomics microarray [29].
RNA extraction was performed in exponential and stationary cultures grown in LB with and without 5% DOC (O.D.600 = 0.4 and O.D.600≥1, respectively). S. enterica grew at slower rates in LB+DOC than in LB, and the concentration of bacterial cells in LB+DOC reached a plateau well below the LB control. However, growth did occur, thus indicating that 5% is a sublethal concentration of DOC under such conditions (LB medium, 37°C). Note that these conditions are different from those used in the viability tests described above. This does not exclude, of course, that a fraction of bacterial cells may have been killed, and that the culture derives from the surviving subpopulation. Under these conditions, a large number of Salmonella loci showed differences in their RNA levels depending on whether DOC was present or absent. Raw data from transcriptomic analysis in the presence of 5% DOC have been deposited at the Array Express database (http://www.ebi.ac.uk/miamexpress) with accession number E-MTAB-637. Relevant data are summarized in Table 1 and Table 2. The main conclusions from these experiments can be summarized as follows:
-
The RpoS-dependent genes osmY, dps, uspB, and ecnB [30] were found to be strongly upregulated by DOC in exponential cultures. The poorly characterized RpoS-dependent gene ybiF, located next to dps on the Salmonella chromosome, showed also >3 fold upregulation. In stationary cultures, dps and osmY were also upregulated by DOC. Their lower upregulation under such conditions is consistent with the fact that the RpoS-dependent general stress response is activated in non dividing cells [31]. These observations suggested that activation of the RpoS-dependent general stress response might play a role in adaptation to bile. A previous transcriptomic study in strain ATCC 14028 did not detect RpoS activation upon exposure to DOC [22]. However, the concentration of DOC used by Prouty et al. (3% ox bile) [22] may have been insufficient to trigger RpoS activation. In fact, under the conditions used in our study, concentrations of DOC much higher than those expected to be present in 3% ox bile (around 1% bile salts [1]) did not adapt S. enterica to bile (Figure 2).
-
The general stress response is not the only stress response activated by sublethal concentrations of bile salts: the stress-inducible cspD gene [32], [33] was found to be upregulated by DOC in both exponential and stationary cultures, and the uspA gene [34] in stationary cultures only.
-
The outer membrane protein (OMP) genes ompC and ompD [35] were downregulated by DOC during exponential growth. Because porins provide passage to bile salts [8], downregulation of ompC and ompD may be tentatively interpreted as a defensive modification of the outer membrane to decrease uptake of bile salts.
-
Modification of the cell envelope may not be limited to outer membrane remodeling. Downregulation of mltB, a gene involved in peptidoglycan recycling [36], may provide evidence for cell wall changes produced in response to DOC. The crucial role of the cell envelope in bile resistance is well established in the literature [15], [17], [18], [19], [37].
-
A sublethal concentration of DOC upregulated acrD, which encodes a component of a multidrug resistance efflux pump [38]. Because efflux systems are known to transport bile salts outside the cell [21], upregulation of acrD may be viewed as another defensive response. Upregulation of other transport genes (ugpB and pnuC) was also observed but it is difficult to interpret.
-
Salmonella pathogenicity islands SPI-1 and SPI-2 were strongly downregulated by DOC, as previously described by other authors [27], [39]. Downregulation was observed in stationary cultures only, a result consistent with the fact that neither SPI-1 nor SPI-2 are expressed in exponential cultures [40].
-
Miscellaneous gene expression changes of difficult interpretation are also presented in Table 1 and Table 2. Changes in the synthesis of cytochrome components may suggest alterations in electron transport. The possibility that chemotaxis is altered in the presence of DOC may be also considered, as previously proposed [22]. Altered expression of numerous metabolic genes is also observed.
Tab. 1. <i>S. enterica</i> loci showing altered expression (>3 fold) in the presence of 5% DOC during exponential growth. 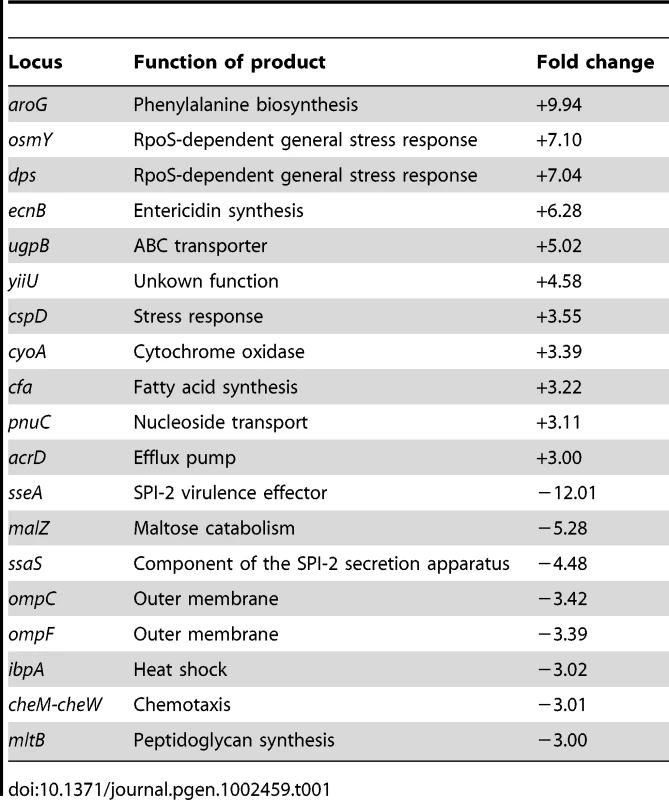
Tab. 2. <i>S. enterica</i> loci showing altered expression (>3 fold) in the presence of 5% DOC during stationary phase. 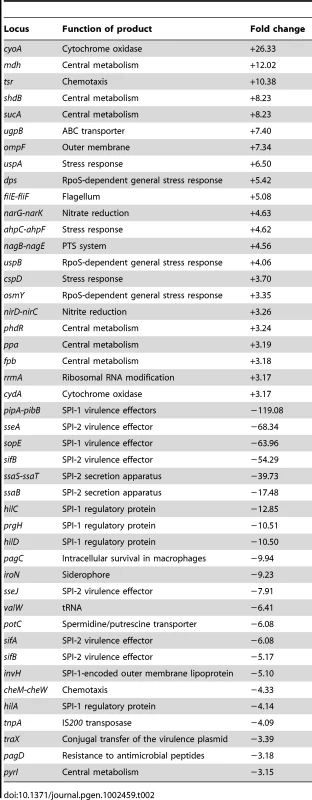
Validation of microarray analysis using lac fusions
Data provided by transcriptomic analysis were validated by monitoring the effect of DOC on the expression of transcriptional lac fusions in 14 S. enterica genes. As a general rule, we chose genes which had shown strong expression changes in the presence of DOC. The sample included genes of the RpoS regulon (osmY, dps, and ecnB), a transport gene (ugpB), a metabolic gene (aroG), and two SPI-1 genes (hilA and prgH). Genes of unknown function, present in all enterics (yiiU, yceK, ytfK, ybjM, and yajI) or Salmonella-specific (STM1441 and STM1672), were also included. The selection of these loci was based on the bile-sensitive phenotype of their mutants (e. g., yiiU), their DNA sequence relatedness to known bile resistance genes (e. g., STM1441, encoding a putative efflux pump) or the cellular location of their products (e. g., yceK, yajI and STM1642, which may encode outer membrane proteins, and ybjM, which may encode a cytoplasmic membrane protein). ß-galactosidase activities were measured in LB and in LB containing 5% DOC. Raw data are shown in Tables S1 and S2. Figure 3 is an elaboration that compares the ß-galactosidase activities of the lac fusions and the expression levels of the corresponding mRNAs detected by microarray analysis. Although differences in expression levels are observed, a correlation between mRNA content and ß-galactosidase activity is found in all cases.
Fig. 3. Validation of transcriptomic analysis: comparison of gene expression differences between LB and LB+5% deoxycholate as measured by RNA content (microarray analysis) and activity of <i>lac</i> fusions. 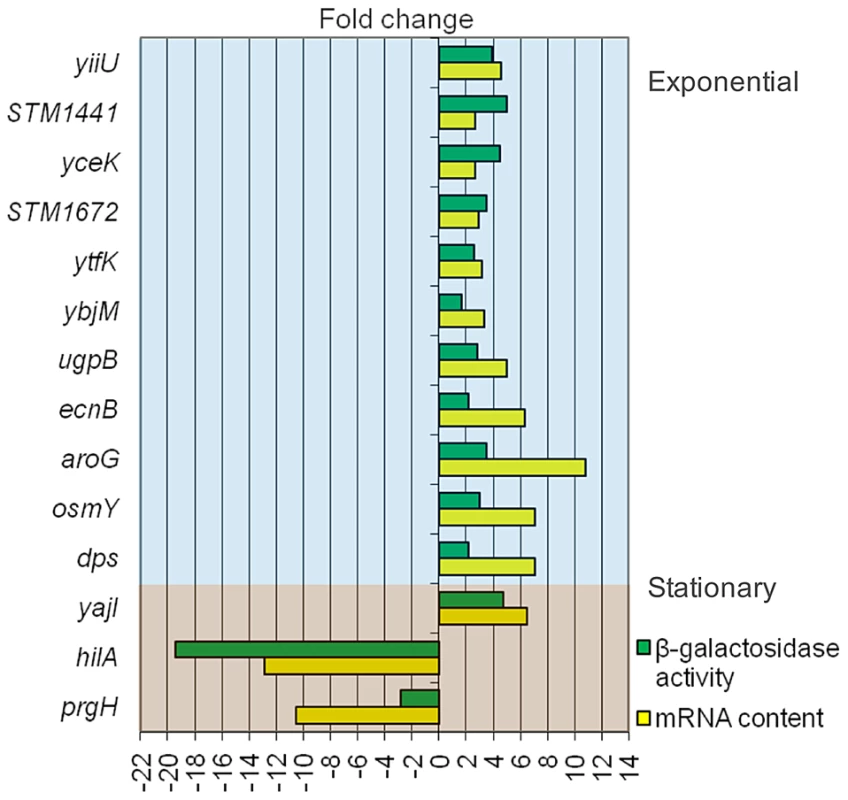
Activation of the RpoS-dependent general stress response in the presence of sublethal concentrations of DOC was further analyzed by monitoring expression of RpoS-dependent genes other than dps and osmY. An additional goal of these experiments was to confirm that DOC upregulated the RpoS regulon in both exponential and stationary cultures. For these experiments, lac fusions in katE, xthA, ots, dps, and osmY were used. ß-galactosidase activities were measured in LB and in LB containing 5% DOC. Expression in exponential cultures was tested at an O.D.600 = 0.4. Stationary cultures were tested at an O.D.600≥1. Data shown in Table 3 and Table 4 confirmed that the RpoS regulon is upregulated by DOC. Upregulation occurs in both exponential and stationary cultures, and the induction ratios vary depending on the gene under study.
Tab. 3. ß-galactosidase activities of lac fusions in RpoS-regulated genes in the presence and in the absence of 5% sodium deoxycholate during exponential growth. 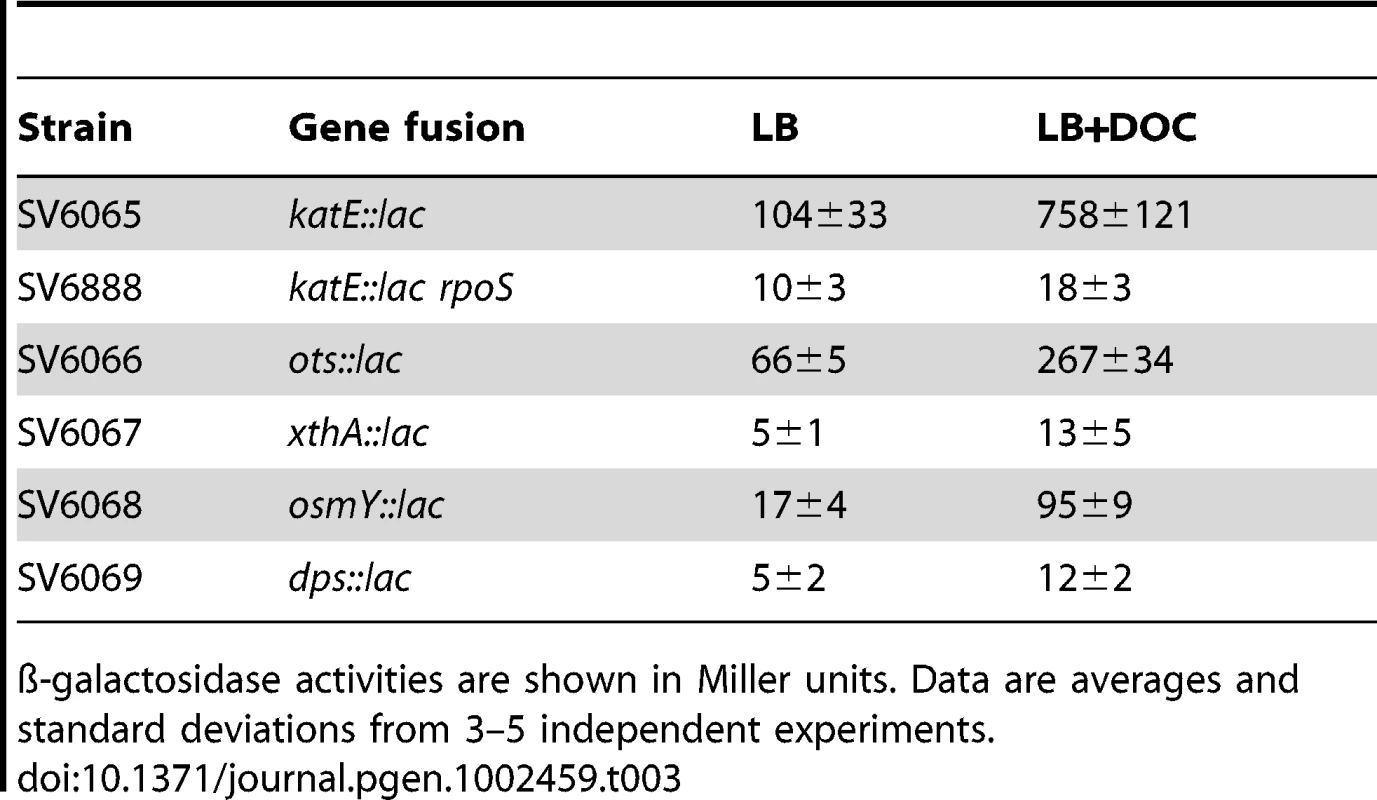
ß-galactosidase activities are shown in Miller units. Data are averages and standard deviations from 3–5 independent experiments. Tab. 4. ß-galactosidase activities of lac fusions in RpoS-regulated genes in the presence and in the absence of sodium deoxycholate in stationary phase. 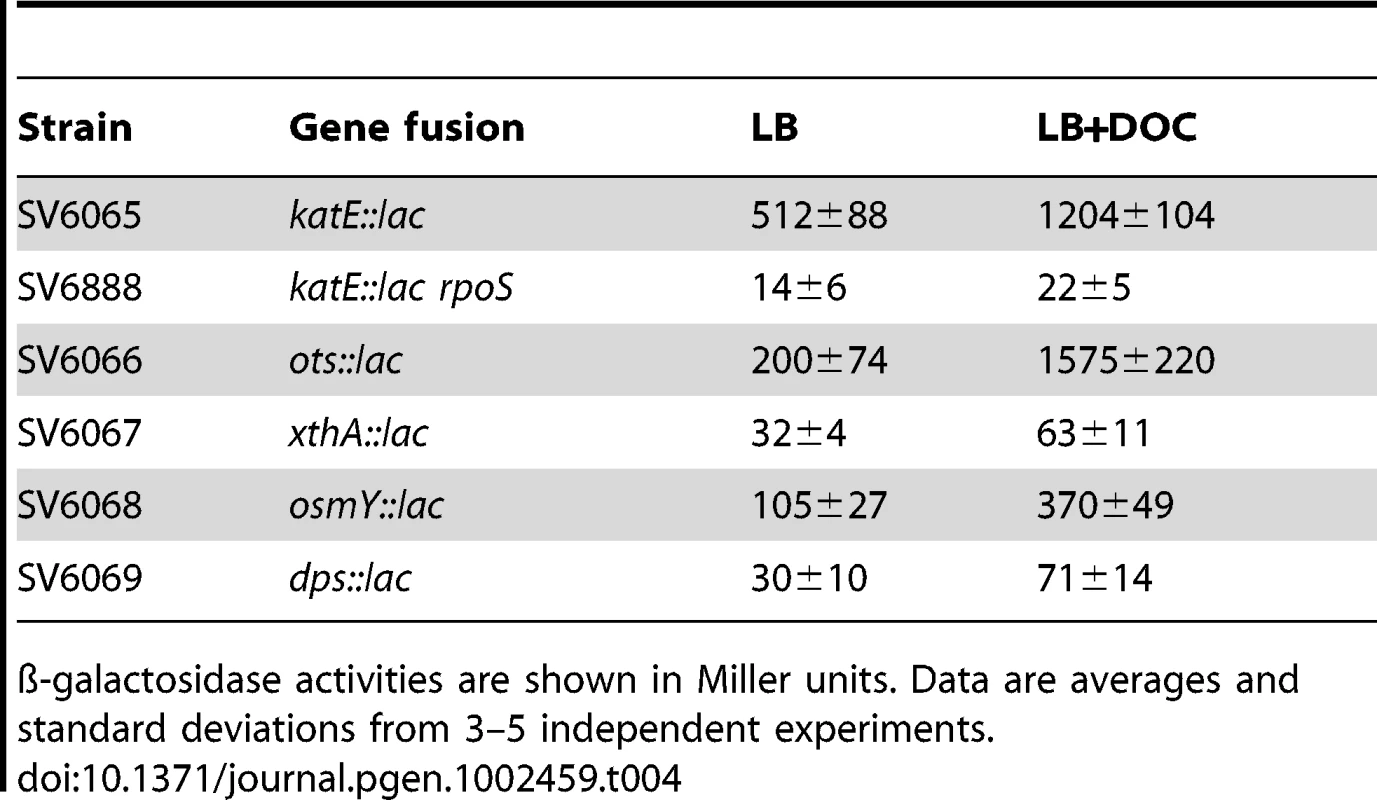
ß-galactosidase activities are shown in Miller units. Data are averages and standard deviations from 3–5 independent experiments. Identification of bile-responsive genes necessary for bile resistance
The identification of genes whose expression was altered in the presence of sublethal concentrations of DOC raised the possibility that such loci might be necessary for bile resistance. We thus tested the MIC of sodium deoxycholate for mutants carrying loss-of-function mutations in 16 genes identified above as upregulated by bile: acrD, yiiU, STM1441, yceK, STM1672, ytfK, ybjM, ugpB, ecnB, aroG, osmY, dps, yajI, katE, ots, and xthA. Results from these trials are shown in Table S3, and can be summarized as follows:
-
The only bile-sensitive mutant was YiiU− (MIC of DOC≅1.5%, compared with 7% in the wild type). Because this locus is virtually unknown, no explanation can be offered for its role in bile resistance.
-
The observation that the AcrD− mutant was not bile-sensitive is in agreement with previous observations made in strain ATCC 14028 [41], and can be explained by redundancy: S. enterica possesses multiple efflux systems, many of them versatile and with overlapping substrate specificity. Hence, bile sensitivity occurs only if multiple efflux systems are eliminated [41]. In agreement with this view, a TolC− mutant (strain SV6629), which lacks an outer membrane protein of all RND efflux pumps [38], [41], showed extreme sensitivity to DOC (MIC≅0.02%, 350 fold lower than the MIC for the wild type). Extreme bile sensitivity of a TolC− mutant has been likewise described in strain ATCC 14028 [41].
-
Redundancy may also explain why mutants lacking individual RpoS-dependent genes (osmY, dps, xthA, katE, and ots) are not bile-sensitive. However, an RpoS− derivative of SL1344 (strain SV5561) showed a MIC of DOC≅3%. Hence, an active RpoS regulon appears to be necessary for bile resistance but the individual RpoS-dependent functions tested in this study (OsmY, Dps, XthA, KatE, and Ots) are dispensable.
Isolation of bile-resistant derivatives of S. enterica SL1344
Non adapted Salmonella populations (e. g., laboratory cultures in LB) are unable to grow on lethal concentrations of bile. However, bile resistance can be acquired by mutation, and bile-resistant mutants can be easily isolated upon plating on lethal concentrations of bile [14], [37]. However, previous descriptions of bile-resistant mutants had involved transposon insertions, which usually cause loss of function and are lethal if they occur in essential genes. To avoid these constraints, we isolated bile-resistant mutants of S. enterica of spontaneous origin. Aliquots from a S. enterica culture grown in LB were plated on LB supplemented with a lethal concentration of ox bile (180 g/l). Use of ox bile instead of DOC was justified by the fact that high concentrations of DOC prevent agar solidification. Bile-resistant colonies appeared at frequencies ranging between 10−6 and 10−7 per cell and generation. Because high concentrations of bile salts are bactericidal (Figure 1), we expected that bile-resistant colonies would derive from bile-resistant cells present in the previous culture. This hypothesis was supported by Luria-Delbrück fluctuation analysis [42]: the averages of bile-resistant colonies obtained from independent cultures showed a variance much higher than the averages from a single culture (Table S4).
Bile-resistant colonies were purified in LB and plated again on LB+bile to confirm bile resistance. During these routine purification procedures, we made the unexpected observation that a relatively large number of bile-resistant isolates had become bile-sensitive. A systematic analysis of the phenomenon was then carried out. Bile resistant isolates were obtained by plating strain SL1344 on LB+ox bile. Colonies were transferred to LB and grown overnight. The MIC of DOC for each isolate was then determined in microtiter plates. These trials confirmed that bile-resistant isolates were of two types: (i) stable, putatively carrying mutations that confer bile resistance; (ii) unstable isolates that lose bile resistance, either partially or completely, upon nonselective growth in LB. The frequencies of mutants and unstable isolates varied from one trial to another (data not shown). A representative experiment involving 59 independent bile-resistant isolates is shown in Figure 4. In this case, 10 isolates turned out to be mutants while the other 49 were unstable bile-resistant isolates.
Fig. 4. Minimal inhibitory concentration of sodium deoxycholate for bile resistant isolates after non-selective growth in LB. 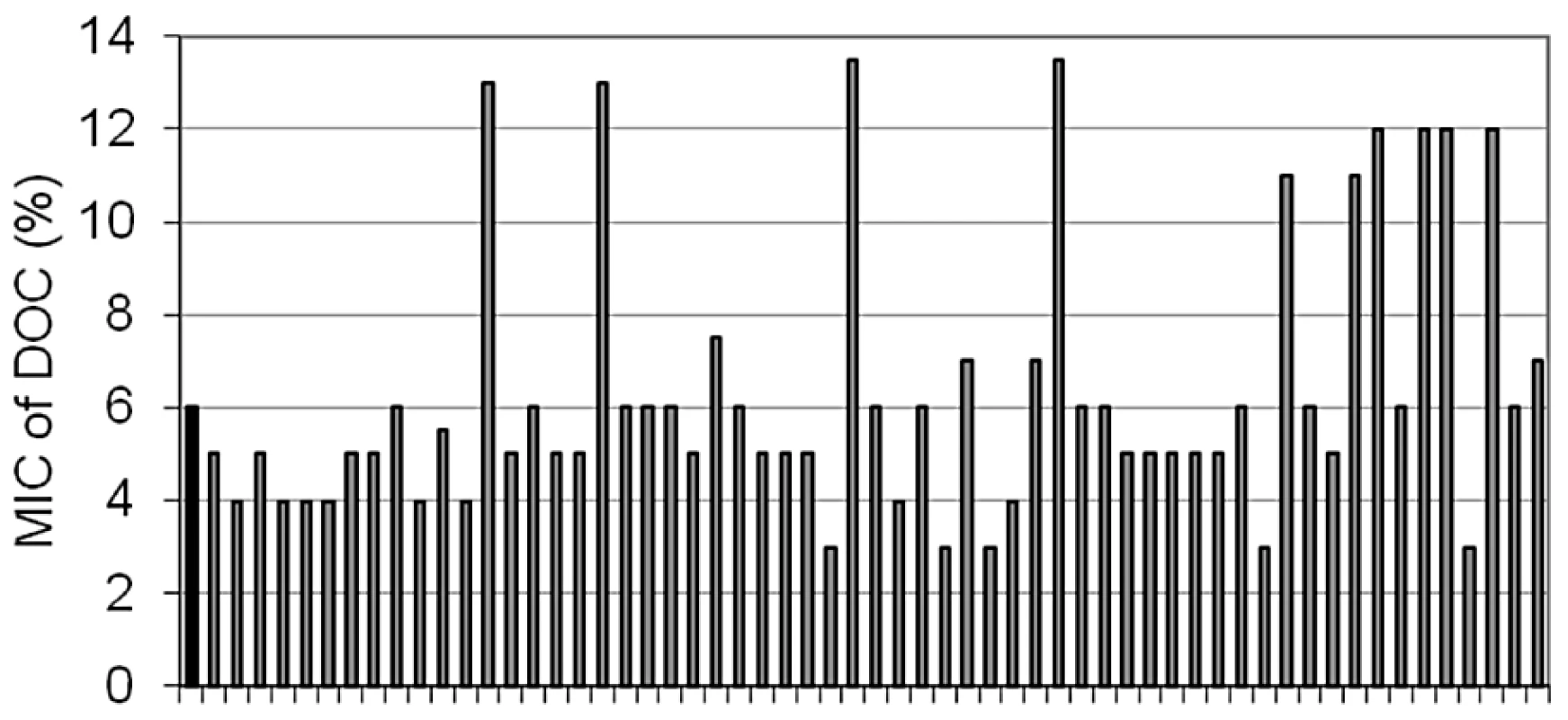
The isolates had been originally obtained on plates containing 18% ox bile. Characterization of bile-resistant mutant derivatives of S. enterica SL1344
Six spontaneous bile-resistant mutants (MIC of DOC ≥14%) of independent origin were chosen for full genome sequencing with the SOLiD platform [43]. Genome sequencing was followed by alignment with the S. enterica SL1344 genome sequence (ftp://ftp.sanger.ac.uk/pub/pathogens/Salmonella/STmSL1344.dbs) to identify DNA sequence differences. The genome of the laboratory stock of S. enterica SL1344 was also sequenced as a control. The mutations found are described in Table 5. Relevant observations are as follows:
-
Four bile-resistant strains were single mutants. Mutants #1 and #6 harbored a nucleotide substitution and an in-frame deletion, respectively, in the S. enterica yrbK gene. YrbK (recently renamed LptC) is a lipopolysaccharide transport protein in E. coli [44]. Mutant #3 harbored a nucleotide substitution in the rlpB gene (also known as lptE), which encodes the Salmonella homolog of E. coli RlpB, a lipopolysaccharide assembly protein [45]. Mutant #4 harbored a nucleotide substitution in the poorly known deaD gene, which in E. coli encodes a putative ATP-dependent RNA helicase [46].
-
Mutants #2 and #5 were double mutants. Interestingly, one of the mutations found in strain #2 mapped in the lipopolysaccharide transport gene yrbK. Strain #2 carried an additional mutation in a putative intergenic region of plasmid 2. In turn, mutant #5 carried frameshifts in two putative loci of unknown function (annotated as strA and sul2) on plasmid 3. These plasmids are specific of strain SL1344 and have not been described in other strains of serovar Typhimurium. A potential role of these plasmids in adaptation to bile seems unlikely, because strain ATCC 14028, which lacks the plasmids, shows MICs of DOC and bile virtually identical to those of SL1344 [5], [17], [19]. The occurrence of plasmid-borne mutations that cause bile resistance is thus interesting but difficult to interpret.
Tab. 5. Mutations present in the genomes of bile-resistant derivatives of S. enterica SL1344. 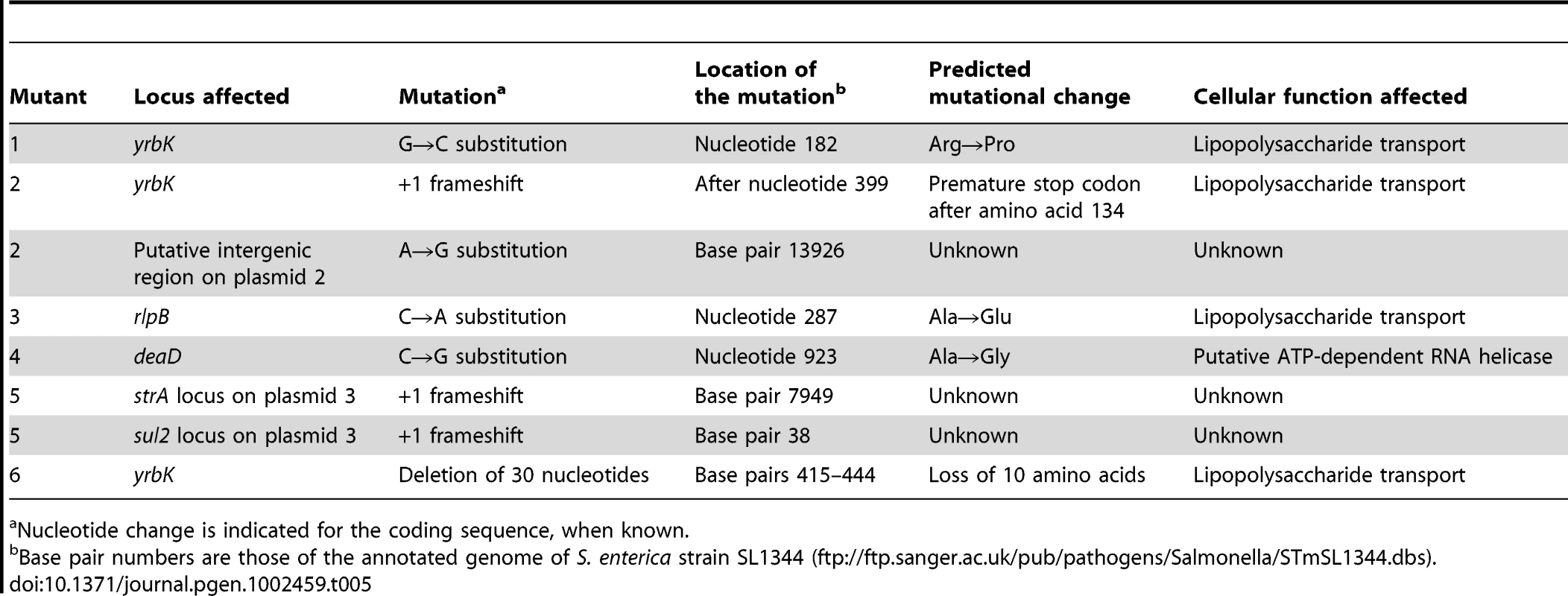
Nucleotide change is indicated for the coding sequence, when known. Bile-resistant mutants carrying mutations in known genes (yrbK, rlpB, and deaD) were reconstructed (see Materials and Methods for reconstruction procedures). All reconstructed mutants showed a MIC of DOC ≥14%, thus confirming that single mutations in yrbK, rlpB, and deaD caused the bile-resistant phenotype of these isolates. The cause of bile resistance in the DeaD− mutant was not further investigated since deaD is a poorly known gene [46]. In contrast, the high frequency of mutations found in lipopolysaccharide transport genes (3 in yrbK and 1 in rlpB) provided evidence that alterations in lipopolysaccharide transport can cause bile resistance. LPS transport genes are known to be essential in E. coli [47], [48]. If such is also the case in S. enterica, the mutations detected must be leaky. Leakiness may seem normal for the G→C (yrbK) and C→A (rlpB) substitutions detected in mutants #1 and #3, and even for the yrbK in-frame deletion detected in mutant #6. The mutation detected in strain #2 (yrbK), however, is a frameshift, a mutation type that often causes loss of function. In fact, the frameshift consists of a C insertion, and results in the formation of a premature stop codon (TAA) eight nucleotides downstream. However, these changes map near the C-terminal region, suggesting that a truncated YrbK protein may be leaky. The view that the C-terminal region of YrbK is dispensable is further supported by the observation that the in-frame deletion found in mutant #6 maps in the same region (Table 5).
Analysis of lipopolysaccharide in bile-resistant mutants
Because the rlpB and yrbK genes have been described in E. coli as involved in LPS transport across the periplasm and LPS assembly at the outer membrane [49], we examined whether the bile-resistant mutants under study showed LPS alterations. The LPS of the DeaD− mutant was also examined. Migration of the LPS in polyacrylamide gel is known to be affected by the number and size of repeating oligosaccharide units in long-chain LPS, such that bands in the profile represent progressively larger concatemers of the repeating oligosaccharide units [45]. Comparison of the LPS profiles of the mutants and the wild type shows that the rlpB, yrbK, and deaD mutations under study do not visibly alter the amount of LPS. However, structural differences are clearly observed between the wild type and mutants #1, #2, and #6, which carry yrbK mutant alleles (Figure 5). Reconstructed YrbK− mutants showed LPS profiles identical to those of their parental mutants (Figure S1). The profiles found in these mutants may indicate differences in the oligosaccharide units that form long-chain LPS [48].
Fig. 5. Lipopolysaccharide profiles of bile-resistant derivatives of S. enterica SL1344, as observed by electrophoresis and silver staining. 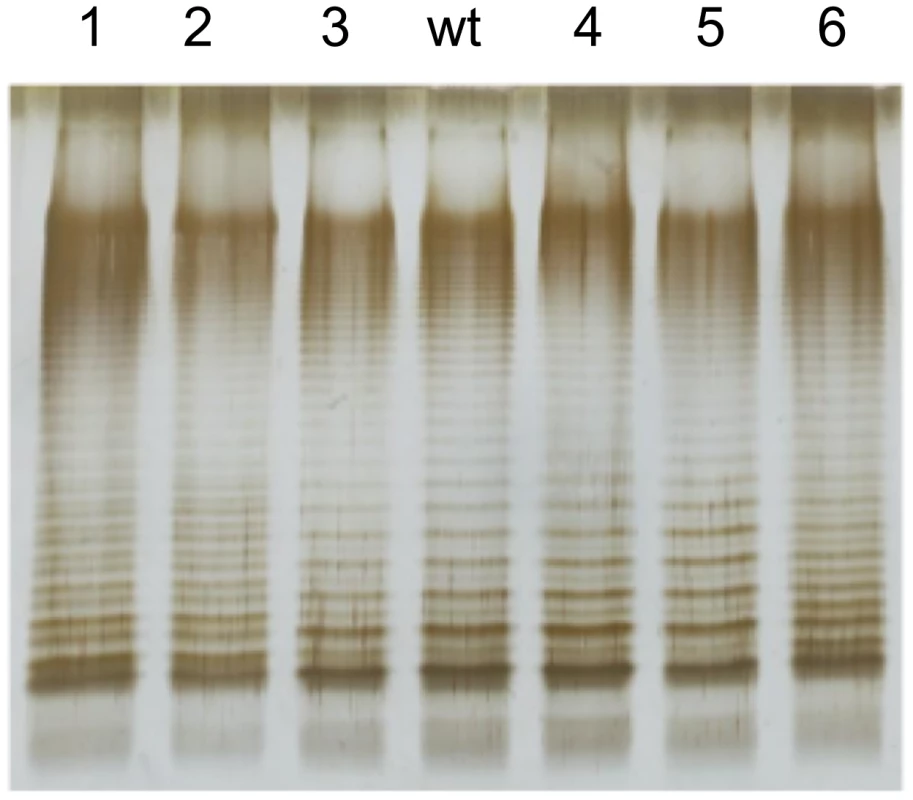
The lane marked “wt” shows the LPS profile of the wild type strain. Lanes 1–6 show the LPS profiles of bile-resistant mutants #1, #2, #3, #4, #5, and #6. Single cell analysis of gene expression in the presence and in the absence of sodium deoxycholate
The observation that preadaptation of S. enterica to bile can occur by reversible, non mutational mechanisms raised the possibility that the bacterial population might contain cells which activate bile resistance responses in the absence of bile. This hypothesis was initially tested by examining the expression level of osmY, a gene of the RpoS regulon, in single Salmonella cells grown in the presence and in the absence of a sublethal concentration of DOC (5%, the same concentration used for transcriptomic analysis). To monitor osmY expression, a green fluorescent protein (GFP) fusion was constructed at the 3′ end of the osmY coding sequence (strain SV6562). Expression of the osmY::GFP fusion in individual Salmonella cells was monitored using a microscope automated fluidic system [50]. These experiments were of two kinds:
-
Single point experiments: S. enterica cultures grown in LB and LB+5% DOC were diluted and transferred to agar pads. Ten fields, each containing ≥30 cells, were manually defined, and the fluorescence level of individual cells was measured [50]. Two representative, independent experiments are shown in Figure 6A and 6B. Exposure to 5% DOC increased osmY::GFP expression in an heterogeneous but consistent manner (red histograms). Cells grown in the absence of DOC (grey histograms) showed lower and more homogeneous levels of osmY::GFP expression. However, a significant degree of heterogeneity was observed, indicating that some cells activate the RpoS general stress response in the absence of DOC. Comparison of panels A and B reveals that the number of cells that activate the RpoS general response in the absence of DOC varies from one experiment to another, thus providing further evidence that phenotypic heterogeneity occurs in batch cultures. As a control, we analyzed osmY::GFP expression in an RpoS− background (strain SV6780). As expected, the level of expression of osmY was significantly lower in the absence of RpoS, and osmY::GFP induction by DOC was very modest in the RpoS− mutant (Figure 6C).
-
Time lapse experiments: S. enterica cultures grown in LB were diluted, transferred to microscope slides, and covered with agar pads containing either LB or LB+5% DOC. The fluorescence of individual cells was then monitored during 90 minutes. A representative experiment involving 14 Salmonella cells is shown in Figure 7. The presence of DOC increased the level of fluorescence, albeit at different levels in different cells (perhaps reflecting differences in the initial level of osmY::GFP induction). Lysis of some cells was observed in the presence of DOC. In the absence of DOC, the fluorescence levels were lower. However, heterogeneous expression of osmY::GFP was observed in the absence of DOC, as in the single point experiments shown in Figure 6.
Fig. 6. Levels of osmY gene expression in individual bacterial cells. 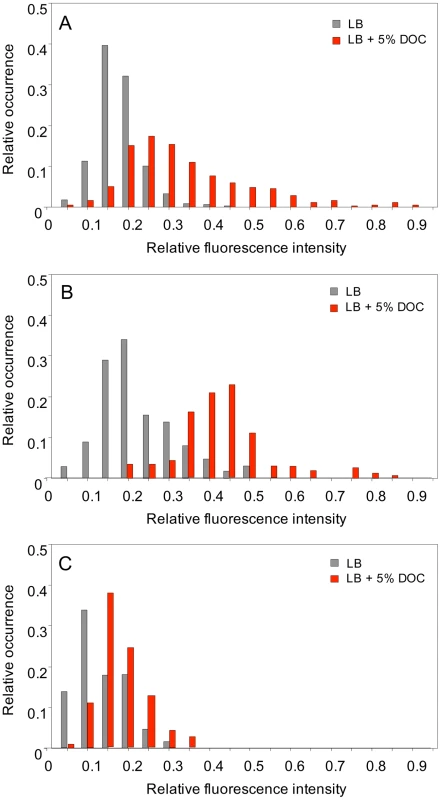
Panels A and B show the distribution of fluorescence intensity in individual cells (N>300) of S. enterica SV6562 (osmY::GFP) in two independent experiments. In both cases, strain SV6562 was grown during 5 h in LB with or without 5% sodium deoxycholate. Histograms represent the proportion of bacterial cells showing distinct fluorescence levels in LB (grey) and LB+DOC (red). Fluorescence intensities are shown in an arbitrary scale (0–1). Panel C shows the distribution of fluorescence intensity in individual cells (N>300) of S. enterica SV6780 (osmY::GFP RpoS−) under conditions identical to those of experiments A and B. Fig. 7. Time course of osmY::GFP expression in individual cells in the presence and in the absence of DOC. 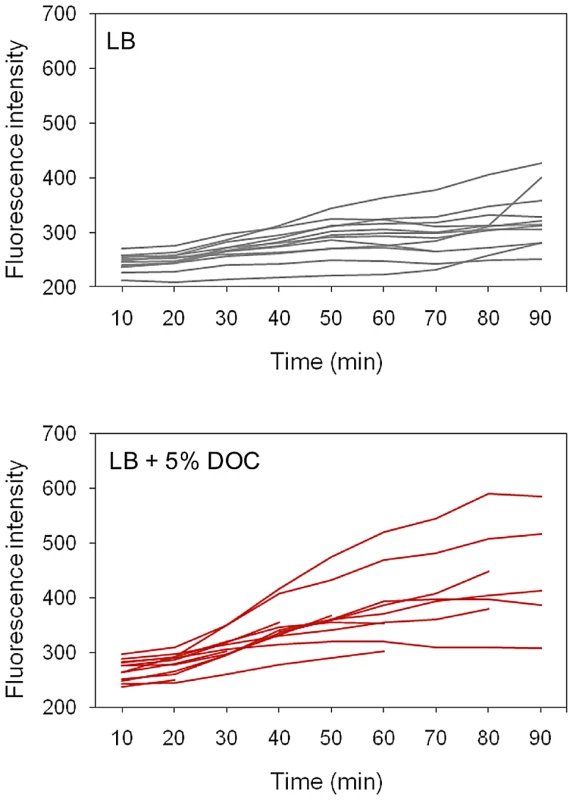
Aliquots from an exponential culture (O.D.600 = 0.5) of S. enterica SV6562 (osmY::GFP) grown in LB were transferred to agar pads containing or not 5% sodium deoxycholate. Bacterial cells were fixed in situ, and GFP fluorescence intensity was measured at 10 min intervals during 90 min. These experiments suggest that S. enterica cultures grown in LB contain cells with elevated expression of the RpoS-dependent general stress response. This observation offers a tentative explanation for non mutational preadaptation to bile: when an aliquot from an LB culture is plated on a lethal concentration of bile, sustainment and/or amplification of the general stress response in certain cells may permit the formation of bile-resistant colonies.
To investigate whether heterogeneous gene expression occurred also in bile-responsive loci that do not belong to the RpoS regulon, we monitored expression of cspD [32], [33], a stress response gene which is upregulated by exposure to a sublethal concentration of DOC (Table 1 and Table 2). For this purpose, a GFP fusion was constructed at the 3′ end of the cspD coding sequence (strain SV6802). Single point experiments in LB and LB+5% DOC were carried out as above, and the fluorescence level of individual cells was measured [50]. Exposure to 5% DOC increased cspD::GFP expression in an heterogeneous but consistent manner (Figure 8). However, heterogeneity was also observed in LB (Figure 8), indicating that some cells activate cspD expression in the absence of DOC. Hence, phenotypic heterogeneity in gene expression is not restricted to RpoS regulon. This observation suggests that non mutational preadaptation to bile may require specific gene expression patterns, perhaps involving multiple loci.
Fig. 8. Levels of cspD gene expression in individual bacterial cells. 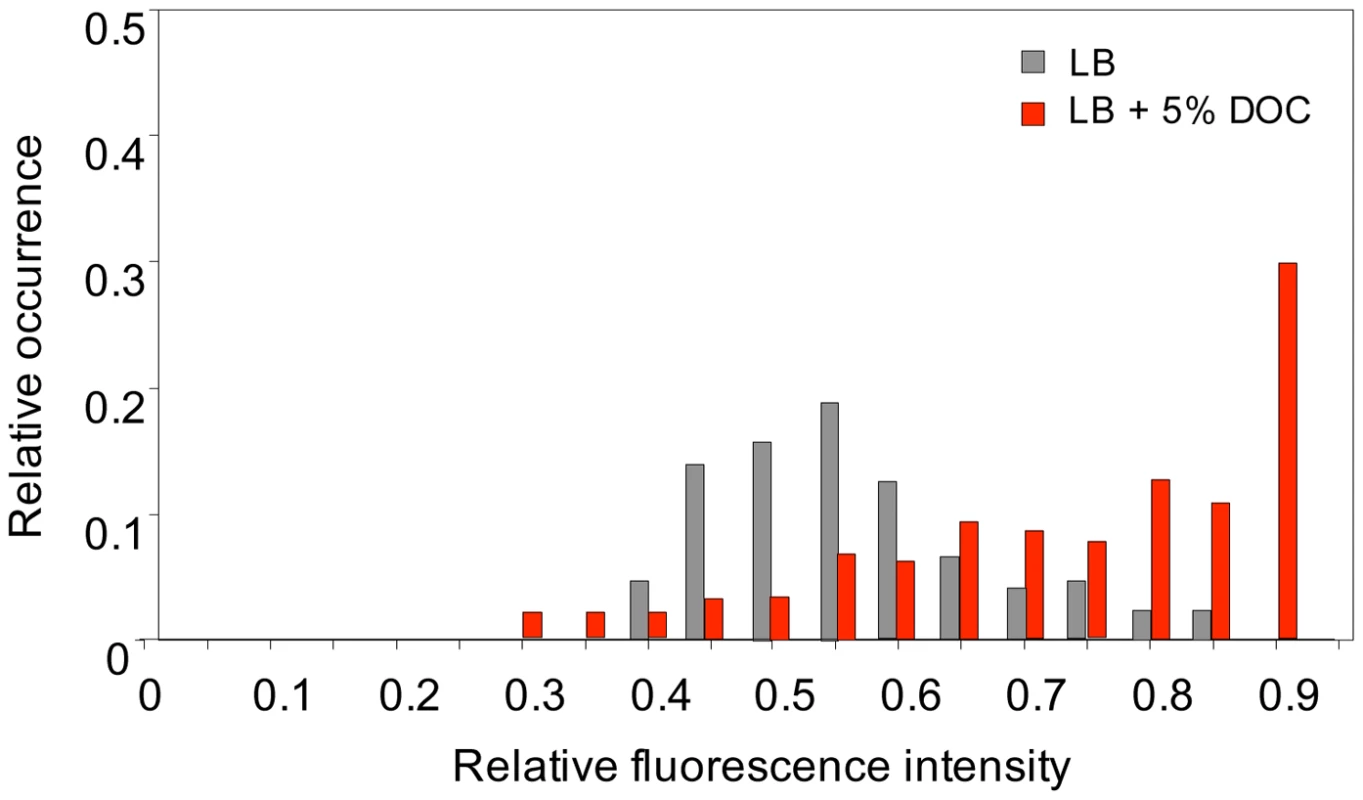
The distribution of fluorescence intensity was measured in individual cells (N>300) of S. enterica SV6802 (cspD::GFP) after growth during 5 h in LB with or without 5% sodium deoxycholate. Histograms represent the proportion of bacterial cells showing distinct fluorescence levels in LB (grey) and LB+DOC (red). Fluorescence intensities are shown in an arbitrary scale (0–1). Discussion
Bacteria live in a changing environment, devoid of the homeostatic mechanisms that create stable conditions in the tissues of multicellular eukaryotes. Except for obligate parasites that have adapted to stable environments, survival of bacteria depends on ceaseless adaptation. This study describes mechanisms employed by Salmonella enterica to survive bile, a fluid with antibacterial capacity due to the presence of bile salts [3], [4], [8]. The adaptation mechanisms have been investigated using either ox bile, which contains a mixture of bile salts [1], or sodium deoxycholate, the most abundant and well known bile salt [2].
The concentrations of bile and DOC that inhibit S. enterica growth depend on the conditions used. For instance, it is well known that dividing cells are more sensitive to bile salts than non dividing cultures [19]. Furthermore, even under specific conditions, the minimal inhibitory concentration of bile increases if the S. enterica culture is previously grown on sublethal concentrations [8]. For instance, minimal inhibitory concentration analyses shown in Figure 2 indicate that growth on 4–5% DOC, a concentration which does not inhibit Salmonella growth, increases the MIC to ≥14%. Growth at DOC concentrations below 3% does not increase the MIC, and concentrations higher than 5% are inhibitory (Figure 2). Hence, sublethal (but relatively high) concentrations of DOC are necessary to increase the MIC above the standard inhibitory concentration.
A conceivable explanation for Salmonella adaptation to bile was that sublethal concentrations of bile might trigger changes of gene expression that could facilitate survival. Transcriptomic analysis in the presence of 5% DOC revealed indeed multiple changes in gene expression, some of which provided tentative explanations for the observed increase in bile resistance. Especially relevant was the observation that exposure to DOC activates the RpoS-dependent general stress response (Table 1, Table 2, Table 3, Table 4, and Figure 3). This response facilitates survival of E. coli and other gamma-proteobacteria under conditions that do not support active growth, and is also known to be activated by multiple stress conditions [31]. Hence it is not surprising that bile salts, which denature proteins and cause DNA damage [3], [4], can trigger the general stress response. The conclusion that the RpoS-dependent general stress response plays a crucial role in bile resistance is supported by two additional observations: (i) Lack of RpoS causes bile sensitivity (Table S3); (ii) S. enterica stationary cultures, in which the RpoS response is physiologically active, are more resistant to both DOC and ox bile [19]. The occurrence of extreme levels of resistance (≥14% DOC) in adapted cultures admits several explanations (not mutually exclusive). Activation of the RpoS regulon by sublethal concentrations of bile may be stronger than physiological activation in stationary cells. It is also possible that high levels of bile resistance result from simultaneous activation of more than one bile-resistance response. This latter possibility is supported by the observation that stress-inducible genes (cspD, uspA, aphC, etc.) that do not belong to the RpoS regulon are also activated by exposure to 5% DOC (Table 1 and Table 2). Furthermore, exposure to bile upregulates the expression of genes of unknown function, some of which might be part of stress response networks. An example is yiiU, which is activated by DOC (Table 1 and Figure 3) and is essential for bile resistance (Table S3).
High throughput analyses of gene expression also indicated that adaptation to bile may involve downregulation of porin genes and other genes encoding envelope structures, as well as upregulation of efflux pumps (Table 1 and Table 2). All these gene expression changes fit well in the literature: porins provide passage to bile salts [8], envelope structures are major barriers for bile salt uptake [8], and efflux pumps can transport bile salts outside the cell [21]. Hence, growth on sublethal concentrations of bile may permit Salmonella adaptation to lethal concentrations by triggering multiple changes in gene expression. Downregulation of pathogenicity island 1 by sublethal concentrations of DOC (Table 2), a phenomenon previously described [27], may be viewed as a signalling system used by Salmonella to identify environments that are not appropriate for epithelial cell invasion (e. g., the duodenum). In turn, downregulation of pathogenicity island 2 in the presence of bile (Table 2) may be viewed as a response that prevents activation of Salmonella functions involved in intracellular survival.
Preadaptation of S. enterica to bile is a completely different phenomenon, which does not pertain to Salmonella populations but to individual bacterial cells. Preadaptation is easily observed under laboratory conditions: when an aliquot of a S. enterica batch culture is plated on LB agar containing a lethal concentration of ox bile, bile-resistant colonies appear at frequencies ranging from 10−6 to 10−7 per cell and generation. These numbers fall in the known range of bacterial mutation frequencies [51]. Not surprisingly, Luria-Delbrück fluctuation analysis confirms that such colonies arise from bile-resistant cells found in the culture aliquot used for inoculation (Table S4). However, we were surprised to find that the majority of bile-resistant isolates obtained under such conditions were unstable, and lost bile resistance if grown overnight in LB without bile. Hence, preadaptive bile resistance seems to involve two distinct phenomena: mutation and non mutational preadaptation.
Full genome sequencing of 6 bile-resistant mutants revealed that 3 mutants carried mutations in yrbK and one in rlpB. The high frequency of mutations in lipopolysaccharide transport genes leaves little doubt that altered LPS transport can cause bile resistance. However, the identification of such mutants has intriguing aspects. One comes from the fact that LPS transport genes are known to be essential in E. coli [47], [48]. If such is also the case in Salmonella, the mutations found (two nucleotide substitutions, one in-frame deletion and one premature stop codon relatively close to the 3′ end of the coding sequence) must all be leaky. Another intriguing question is related to the fact that LPS is a major barrier against bile salts [15]. However, it is conceivable that transport of LPS components across the envelope might sensitize the cell to bile salts, thus explaining why altered transport may confer bile resistance. An alternative, speculative explanation is that the LPS transport proteins altered in bile-resistant mutants might be also involved in LPS assembly and/or modification, and that specific mutations might boost bile resistance. This view may be tentatively supported by the observation that some of the bile-resistant mutants described in this study have altered LPS profiles (Figure 5 and Figure S1).
Non mutational preadaptation to bile was at first sight an intriguing phenomenon. How can a Salmonella batch culture contain cells that are bile-resistant without previous adaptation by growth at sublethal concentrations? However, it is well known that bacterial cultures, albeit genetically clonal, can contain subpopulations of cells with distinct patterns of gene expression [25], either as a consequence of epigenetic control [52] or as the result of stochastic fluctuations in gene expression [25], [53]. We thus hypothesized that non mutational preadaptation might be caused by activation of bile-resistance responses in the absence of bile. This hypothesis was tested by analyzing expression of osmY, an RpoS-dependent gene, and cspD, a stress response gene that does not belong to the RpoS regulon [32], [33], in individual Salmonella cells grown in the presence and in the absence of a sublethal concentration of DOC. Microscopic microfluidics [50] confirmed that exposure to DOC activates osmY and cspD expression in most Salmonella cells (Figure 6 and Figure 7). However, upregulation of osmY and cspD expression was also observed in subpopulations of Salmonella cells in the absence of DOC (Figure 6, Figure 7, and Figure 8). Non mutational preadaptation may thus result from activation of the RpoS-dependent general stress response and/or other stress responses in a cell subpopulation. Stress response activation may either be triggered by a stress situation encountered by individual cells (e. g., increased concentrations of harmful metabolic products) or be accidental. Repression of specific loci, which has not been addressed in this study, may also contribute to preadaptation. It is possible that non mutational preadaptation to environmental challenges is a common phenomenon, and that the acquisition of bile resistance described in this study is merely one example among many others. The widespread occurrence of phenotypic polymorphism in clonal populations of bacteria [25], [52], [53] may support this possibility.
At this stage, it is impossible to ascertain whether the three distinct modes of Salmonella adaptation to bile described in this study may occur or not upon infection of animals. Current evidence obtained in the mouse model of typhoid fever indicates that Salmonella cells can escape the high concentrations of bile salts found in the gall bladder lumen by invading the gall bladder epithelium [11] and by forming biofilms on the surface of gallstones [12], [13]. However, planktonic Salmonella cells are also found in the gall bladder lumen, and little is known about the mechanisms that permit their survival and multiplication. Our model envisions that planktonic Salmonella cells may adapt to the gall bladder lumen by changing their gene expression pattern. Passage by the small intestine and the liver, which contain bile concentrations much lower than those found in the gall bladder [1], [2], might facilitate adaptation in an analogous manner as growth on sublethal concentrations of DOC in the laboratory. Phenotypic heterogeneity and subpopulation formation may additionally contribute to adaptation by activating bile resistance responses prior to colonization of the hepatobiliary tract. Lastly, appearance of bile-resistant mutants may provide an alternative mechanism for Salmonella adaptation to the gall bladder. Because bile salts are mutagenic [5], [6] and the dose of a mutagen is the product of its concentration by the time of exposure [54], mutational adaptation of Salmonella to bile might be speeded up by bile itself, especially during long term infection of the bile-laden gall bladder. This phenomenon might be relevant during persistent and chronic infections, as found for instance in human carriers of Salmonella Typhi [10].
Materials and Methods
Bacterial strains, plasmids, bacteriophages, media, and culture conditions
Strains of Salmonella enterica serovar Typhimurium (often abbreviated as S. enterica) used in this study (Table 1) derive from the mouse-virulent strain SL1344. Strains SV6065, SV6066, SV6067, SV6068, SV6069, SV6629, and SV6745 were constructed by transducing alleles from ATCC 14028 or LT2 to SL1344. Transduction was performed with phage P22 HT 105/1 int201 ([55] and G. Roberts, unpublished data). The P22 HT transduction protocol was described elsewhere [56]. To obtain phage-free isolates, transductants were purified by streaking on green plates, prepared according to Chan et al. [57], except that methyl blue (Sigma Chemical Co., St. Louis, Missouri) substituted for aniline blue. Phage sensitivity was tested by cross-streaking with the clear-plaque mutant P22 H5. Luria-Bertani broth (LB) was used as standard rich medium and E medium [58] as minimal medium. Solid media contained agar at 1.5% final concentration. Cultures were grown at 37°C. Aeration of liquid cultures was obtained by shaking in an orbital incubator. Deoxycholic acid (sodium salt) and sodium choleate (ox bile extract) were both from Sigma. Antibiotics were used at the final concentrations described previously [5]. A strain list is provided as Table 6.
Gene disruption and directed construction of lac fusions
Targeted gene disruption was achieved using plasmids pKD3, pKD4, and pKD13 [59]. Antibiotic resistance cassettes introduced during strain construction were excised by recombination with plasmid pCP20 [59]. The oligonucleotides used for disruption (labeled “FOR” and “REV”) are listed in Table S5 together with the oligonucleotides (labeled “E”) used for allele verification by the polymerase chain reaction. For the construction of transcriptional and translational lac fusions in the Salmonella chromosome, FRT sites generated by excision of Kmr cassettes were used to integrate either plasmid pCE36 or pCE40 [60].
Construction of osmY::GFP and cspD::GFP fusions
An osmY::gfp fusion was constructed as described by Hautefort et al. [61]. A fragment containing the promoterless green fluorescent protein (gfp) gene and the chloramphenicol resistance cassette was amplified from the pZEP07 plasmid with primers 5′ AAG CCG TTG ATG GCG TAA AAA GTG TTA AAA ACG ATC TGA AAG TTC AGT AAT AAG AAG GAG ATA TAC ATA TGA G 3′, and 5′ GGT GCA CAT TAC GCC TCC CGA CAA ACG TCG GGA GGA CGA ATT ACG ACG AAT TAT CAC TTA TTC AGG CGT A 3′. Primers used for cspD amplification were 5′ GCA ATC ACG CCA GCG TCA TCG TGC CCA TCG AAG CAG AGG CCG TTG CAT AGT AAG AAG GAG ATA TAC ATA TGA G 3′, and 5′ CGA TCG GGC TGG CAT TTT GCC TCC TGG ATG TAC ACA ATG AGA CAG AGG AGT TAT CAC TTA TTC AGG CGT A 3′. The 5′ regions of these primers are homologous to the 3′ end of the osmY and cspD coding sequences, so that the fusion is formed immediately after the osmY and cspD stop codons. The constructs were integrated into the chromosome of S. enterica using the Lambda Red recombination system [59].
Directed construction of rlpB and deaD point mutations
The rlpB allele from mutant #3 and the deaD allele from mutant #4 were PCR-amplified using pairs of 30-nucleotide primers that contained XbaI and SacI targets. The primers for rlpB amplification were 5′ TTT TGA GCT CGA AGG TGA TAT CGA CAA CGC 3′, and 5′ TTT TTC TAG ACT CAT TCA TTG CCG CGT TAG 3′; for deaD amplification, 5′ TTT TGA GCT CCG TCT GCT TGA TCA CTT AAA 3′, and 5′ TTT TTC TAG AAC GAC GTT CAC GAC GCG GAC 3′. The resulting fragments were digested with XbaI and SacI, cloned onto pDMS197 [61] and propagated in E. coli CC118 lambda pir [62]. Plasmids derived from pMDS197 were transformed into E. coli S17-1 lambda pir [63]. The resulting strains were used as donors in matings with S. enterica SL1344 as recipients, selecting Tcr transconjugants on E plates supplemented with tetracycline and histidine. Several Tcr transconjugants were grown in nutrient broth (without NaCl) containing 5% sucrose. Individual tetracycline-sensitive segregants were then examined for the incorporation of the mutant allele by DNA sequencing.
Reconstruction of bile-resistant mutants by P22-mediated transduction of a linked marker
The kanamycin-resistant cassette of plasmid pKD4 was inserted at a region close to the mutation under study, using lambda Red recombination [59]. The oligonucleotides employed for gene targeting are listed in Table S5. To reconstruct yrbK mutations, the Kmr cassette was inserted at an intergenic region between yrbL and mtgA, 37 bp downstream the yrbL stop codon and 28 bp downstream the mtgA stop codon (note that yrbL and mtgA undergo divergent transcription). The distance from the Kmr cassette and the yrbK mutations under study is 6,135 bp for mutant #1, and 5,918 bp for mutant #2. To reconstruct the rlpB mutation of mutant #3, the Kmr cassette was introduced at an intergenic region between the putative ORFs ybeL and ybeQ (21 bp downstream the putative ybeL stop codon, and 8,253 bp away from the rlpB mutation). DNA sequence analysis employed the S. Typhimurium SL1344 genome database (ftp://ftp.sanger.ac.uk/pub/pathogens/Salmonella/STmSL1344.dbs).
The presence of the original mutation in the reconstructed mutants was verified by PCR amplification using primers designed ad hoc (Table S5), followed by DNA sequencing. In all constructions, the distance between the Kmr cassette and the mutation under study permitted >90% cotransduction of the Kmr cassette and the point mutation under study, fulfilling calculations made with the formula of Wu [62].
Determination of minimal inhibitory concentrations of sodium deoxycholate
Exponential cultures in LB broth were prepared, and samples containing around 3×102 colony-forming-units (CFU) were transferred to polypropylene microtiter plates (Soria Genlab, Valdemoro, Spain) containing known amounts of sodium deoxycholate (DOC). After 12 h incubation at 37°C, growth was visually monitored. Assays were carried out in triplicate. Student's t-test was used to analyze every MIC. The null hypothesis was that MICs were not significantly different from the MIC for the wild-type. P values of 0.01 or less were considered significant.
Assessment of bacterial viability using a cell staining kit
One ml aliquots of an exponential culture of S. enterica SL1344 grown in LB were incubated in the presence of different concentrations of sodium deoxycholate (1%, 3%, 5%, 7% and 9%) during 30 minutes at 37°C. The cells were then washed three times with 0.85% NaCl and stained using the Viability/Cytotoxicity Assay Kit for Bacteria (Biotium Inc., Hayward, California). Control suspensions of live and dead cells were prepared as described in the kit protocol. Live and dead cells were distinguished using a Leica DMR 020-525.024 fluorescence microscope (Leica Camera AG, Solms, Germany). Live and dead bacteria were counted as the green and red cells (respectively) found in randomly selected 5×5 mm squares painted on a micro cover glass.
Assessment of bacterial viability by plate counts
Aliquots of Salmonella exponential cultures grown in LB, each containing 2×106 cells, were treated with various concentrations of DOC (1%, 3%, 5%, 7%, and 9%) for 30 min. The cultures were then diluted, plated on LB and incubated at 37°C. Counts of colony-forming-units were performed after overnight growth.
Isolation of bile-resistant mutants and Luria-Delbrück fluctuation assays
Bile-resistant derivatives of S. enterica SL1344 were isolated by plating 0.1 ml aliquots (approximately, 2×108 cells) from an overnight LB culture onto LB plates containing 18% ox bile (Sigma-Aldrich, St. Louis, Missouri). Fluctuation analysis was performed as described by Luria and Delbrück [42], and the number of independent cultures was 40.
DNA isolation and full-genome sequencing
Whole genome DNA samples from bile-resistant mutants and from the parent strain SL1344 were prepared by phenol extraction and ethanol precipitation. Whole genome sequencing was performed using the oligonucleotide ligation and detection (SOLiD, v2) platform [43] at the facilities of Sistemas Genómicos S.L., Parque Tecnológico de Valencia, Paterna, Spain, using mate-pair libraries and reads of 25 nucleotides [63]. DNA sequences were aligned with the genome sequence of Salmonella Typhimurium SL1344 available at the Wellcome Trust Sanger Institute, Hinxton, England (ftp://ftp.sanger.ac.uk/pub/pathogens/Salmonella/STmSL1344.dbs). Two mismatches or mispairs per reading were permitted.
RNA isolation, microarray procedures, and data analysis
To prepare cells for RNA extraction, 25 ml of fresh LB and LB+DOC 5% in a 250 ml flask was inoculated with a 1∶100 dilution from an overnight bacterial culture, and incubated with shaking at 250 rpm in a New Brunswick Innova 3100 waterbath at 37°C. Three biological replicates were performed for each strain, and RNA was extracted at an optical density (OD600)∼0.4 (exponential phase) and >1 (stationary phase). RNA extractions were performed as described by Mangan et al. [64], and their quality was assessed on an Agilent 2100 Bioanalyzer. Transcriptomic analyses were performed with the Salgenomics microarray [29]. Hybridization and microarray scanning were performed at the Genomics Service of the Centro Nacional de Biotecnología, C.S.I.C., Cantoblanco, Madrid, Spain (http://www.cnb.uam.es/content/services/genomics). For normalization of the two-color microarray data, LiMMA software [65] was used. Further bioinformatic analysis was carried out with the FIESTA programme (http://bioinfogp.cnb.csic.es/tools/FIESTA/index.php). Raw transcriptomic data were deposited at the Array Express database (http://www.ebi.ac.uk/miamexpress) under accession number E-MTAB-637.
ß-galactosidase assays
Levels of ß-galactosidase activity were assayed as described by Miller [66], using the CHCl3-sodium dodecyl sulfate permeabilization procedure.
Electrophoretic visualization of lipopolysaccharide profiles
To investigate lipopolysaccharide (LPS) profiles, bacterial cultures were grown in LB. Bacterial cells were harvested and washed three times with 0.9% NaCl. The O.D.600 of the washed bacterial suspension was measured to calculate cell concentration. A bacterial mass containing about 3.2×108 cells was pelleted by centrifugation. Treatments applied to the bacterial pellet, electrophoresis of crude bacterial extracts, and silver staining procedures were performed as described by Buendía-Clavería et al. [67]. Three replicates per strain were performed.
Microscopy
Cells were inoculated onto a microscope cover slip and covered with a thin (2 mm thick) semisolid LB agar (1.5%) matrix with or without DOC. In the DOC-containing samples, the final concentration of DOC was 5%. The cover slip and the agar pad were then mounted in a seal flow chamber allowing constant aeration and reduced desiccation. Flow chambers were incubated in a temperature-controlled automated microscope (Nikon TE2000-E-PFS, Nikon, Champigny-sur-Marne, France) at 37°C. For single point experiments, 10 fields, each containing at least 30 cells treated or not with DOC, were manually defined. For 90 min time lapse experiments, a single field was examined, and time points were taken every 10 min under agar pads with or without DOC. Images were recorded using a CoolSNAP HQ2 high resolution camera (Roper Scientific, Evry, France) and a 100x/1.4 DLL objective. Digital analysis and image treatment were performed with Metamorph software 7.5 (Molecular Devices, Sunnyvale, California) as previously described [50].
Supporting Information
Zdroje
1. HofmannAF 1998 Bile secretion and enterohepatic circulation of bile acids. FeldmanMScharschmidtBFSleisengerWB Sleisenger and Fordtran's Gastrointestinal Disease, 6th ed Philadelphia, PA W. B. Saunders & Co 937 948
2. HofmannAFHageyLR 2008 Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci 65 2461 2483
3. BegleyMGahanCGHillC 2005 The interaction between bacteria and bile. FEMS Microbiol Rev 29 625 651
4. MerrittMEDonaldsonJR 2009 Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol 58 1533 1541
5. PrietoAIRamos-MoralesFCasadesusJ 2004 Bile-induced DNA damage in Salmonella enterica. Genetics 168 1787 1794
6. PrietoAIRamos-MoralesFCasadesusJ 2006 Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics 174 575 584
7. Garcia-QuintanillaMPrietoAIBarnesLRamos-MoralesFCasadesusJ 2006 Bile-induced curing of the virulence plasmid in Salmonella enterica serovar Typhimurium. J Bacteriol 188 7963 7965
8. GunnJS 2000 Mechanisms of bacterial resistance and response to bile. Microbes Infect 2 907 913
9. Andrews-PolymenisHLBaumlerAJMcCormickBAFangFC 2010 Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect Immun 78 2356 2369
10. Gonzalez-EscobedoGMarshallJMGunnJS 2011 Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol 9 9 14
11. MenendezAArenaETGuttmanJAThorsonLVallanceBA 2009 Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis 200 1703 1713
12. ProutyAMSchwesingerWHGunnJS 2002 Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70 2640 2649
13. CrawfordRWRosales-ReyesRRamirez-Aguilar MdeLChapa-AzuelaOAlpuche-ArandaC 2010 Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A 107 4353 4358
14. CasadesusJHernandezSBCotaIRamos-MoralesF 2010 Of bacteria and bile. MaloySRHughesKTCasadesusJ The Lure of Bacterial Genetics: A Tribute to John Roth Washington, D. C. ASM Press 153 162
15. PickenRNBeachamIR 1977 Bacteriophage-resistant mutants of Escherichia coli K12. Location of receptors within the lipopolysaccharide. J Gen Microbiol 102 305 318
16. MurataTTsengWGuinaTMillerSINikaidoH 2007 PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar typhimurium. J Bacteriol 189 7213 7222
17. Ramos-MoralesFPrietoAIBeuzonCRHoldenDWCasadesusJ 2003 Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J Bacteriol 185 5328 5332
18. ProutyAMvan VelkinburghJCGunnJS 2002 Salmonella enterica serovar Typhimurium resistance to bile: identification and characterization of the tolQRA cluster. J Bacteriol 184 1270 1276
19. PucciarelliMGPrietoAICasadesúsJGarcía-del-PortilloF 2002 Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148 1171 1182
20. Lopez-GarridoJChengNGarcia-QuintanillaFGarcia-del PortilloFCasadesusJ 2010 Identification of the Salmonella enterica damX gene product, an inner membrane protein involved in bile resistance. J Bacteriol 192 893 895
21. ThanassiDGChengLWNikaidoH 1997 Active efflux of bile salts by Escherichia coli. J Bacteriol 179 2512 2518
22. ProutyAMBrodskyIEManosJBelasRFalkowS 2004 Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol Med Microbiol 41 177 185
23. van VelkinburghJCGunnJS 1999 PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun 67 1614 1622
24. PrietoAIHernandezSBCotaIPucciarelliMGOrlovY 2009 Roles of the outer membrane protein AsmA of Salmonella enterica in the control of marRAB expression and invasion of epithelial cells. J Bacteriol 191 3615 3622
25. VeeningJWSmitsWKKuipersOP 2008 Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62 193 210
26. DavidsonCJSuretteMG 2008 Individuality in bacteria. Annu Rev Genet 42 253 268
27. ProutyAMGunnJS 2000 Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect Immun 68 6763 6769
28. MitroNGilardiFGodioCScottiEDe FabianiE 2008 Bile acids and gene regulation: from nuclear receptors to chromatin. Front Biosci 13 6276 6288
29. MariscottiJFGarcia-del PortilloF 2009 Genome expression analyses revealing the modulation of the Salmonella Rcs regulon by the attenuator IgaA. J Bacteriol 191 1855 1867
30. VijayakumarSRKirchhofMGPattenCLSchellhornHE 2004 RpoS-regulated genes of Escherichia coli identified by random lacZ fusion mutagenesis. J Bacteriol 186 8499 8507
31. HenggeR 2007 The two-component network and the general stress sigma factor RpoS (sS) in Escherichia coli. UtsumiR Bacterial Signal Transduction: Networks and Drug Targets Austin, Texas Landes Bioscience and Springer Science Business Media 40 53
32. YamanakaKZhengWCrookeEWangYHInouyeM 2001 CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol Microbiol 39 1572 1584
33. YamanakaKInouyeM 1997 Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J Bacteriol 179 5126 5130
34. SiegeleDA 2005 Universal stress proteins in Escherichia coli. J Bacteriol 187 6253 6254
35. RuizNKahneDSilhavyTJ 2006 Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol 4 57 66
36. SuvorovMLeeMHesekDBoggessBMobasheryS 2008 Lytic transglycosylase MltB of Escherichia coli and its role in recycling of peptidoglycan strands of bacterial cell wall. J Am Chem Soc 130 11878 11879
37. PrietoAIHernándezSBCotaIPucciarelliMGOrlovY 2009 Roles of the outer membrane protein AsmA of Salmonella enterica in control of marRAB expression and invasion of epithelial cells. J Bacteriol 191 3615 3622
38. PiddockLJ 2006 Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol 4 629 636
39. ProutyAMBrodskyIEManosJBelasRFalkowS 2004 Transcriptional regulation fo Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol Med Microbiol 41 177 185
40. PapezovaKGregorovaDJonuschiesJRychlikI 2007 Ordered expression of virulence genes in Salmonella enterica serovar Typhimurium. Folia Microbiol (Praha) 52 107 114
41. NishinoKLatifiTGroismanEA 2006 Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol Microbiol 59 126 141
42. LuriaSEDelbruckM 1943 Mutations of bacteria from virus sensitivity to virus Resistance. Genetics 28 491 511
43. MardisER 2008 Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 9 387 402
44. ChngSSRuizNChimalakondaGSilhavyTJKahneD 2010 Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc Natl Acad Sci U S A 107 5363 5368
45. WuTMcCandlishACGronenbergLSChngSSSilhavyTJ 2006 Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A 103 11754 11759
46. TooneWMRuddKEFriesenJD 1991 deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol 173 3291 3302
47. RuizNKahneDSilhavyTJ 2009 Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat Rev Microbiol 7 677 683
48. SperandeoPDehoGPolissiA 2009 The lipopolysaccharide transport system of Gram-negative bacteria. Biochim Biophys Acta 1791 594 602
49. SperandeoPLauFKCarpentieriADe CastroCMolinaroA 2008 Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J Bacteriol 190 4460 4469
50. DucretAMaisonneuveENotareschiPGrossiAMignotT 2009 A microscope automated fluidic system to study bacterial processes in real time. PLoS ONE 4 e7282 doi:10.1371/journal.pone.0007282
51. DrakeJWCharlesworthBCharlesworthDCrowJF 1998 Rates of spontaneous mutation. Genetics 148 1667 1686
52. CasadesusJLowD 2006 Epigenetic gene regulation in the bacterial world. Microbiol Mol Biol Rev 70 830 856
53. SatoryDGordonAJHallidayJAHermanC 2011 Epigenetic switches: can infidelity govern fate in microbes? Curr Opin Microbiol 14 212 217
54. Jimenez-SanchezACerda-OlmedoE 1975 Mutation and DNA replication in Escherichia coli treated with low concentrations of N-methyl-N′-nitro-N-nitrosoguanidine. Mutat Res 28 337 345
55. SchmiegerH 1972 Phage P22 mutants with increased or decreased transducing abilities. Mol Gen Genet 119 75 88
56. GarzonACanoDACasadesusJ 1995 Role of Erf recombinase in P22-mediated plasmid transduction. Genetics 140 427 434
57. ChanRKBotsteinDWatanabeTOgataY 1972 Specialized transduction of tetracycline by phage P22 in Salmonella typhimurium. II. Properties of a high frequency transducing lysate. Virology 50 883 898
58. VogelHBonnerD 1956 Acetylornithase of Escherichia coli: partial purification and some properties. J Biol Chem 218 97 106
59. DatsenkoKAWannerBL 2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 90 6640 6645
60. EllermeierCDJanakiramASlauchJM 2002 Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290 153 161
61. HautefortIProencaMJHintonJC 2003 Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol 69 7480 7491
62. WuTT 1966 A model for three-point analysis of random general transduction. Genetics 54 405 410
63. FullwoodMJWeiCLLiuETRuanY 2009 Next-generation DNA sequencing of paired-end tags (PET) for transcriptome and genome analyses. Genome Res 19 521 532
64. ManganMWLucchiniSDaninoVCroininTOHintonJC 2006 The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol Microbiol 59 1831 1847
65. SmythGKSpeedT 2003 Normalization of cDNA microarray data. Methods 31 265 273
66. MillerJH 1972 Experiments in Molecular Genetics Cold Spring Harbor, N. Y. Cold Spring Harbor Laboratory Press
67. Buendia-ClaveriaAMMoussaidAOlleroFJVinardellJMTorresA 2003 A purL mutant of Sinorhizobium fredii HH103 is symbiotically defective and altered in its lipopolysaccharide. Microbiology 149 1807 1818
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant ImmunityČlánek Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání