-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
The prevalence of type 2 diabetes in the United States is projected to double or triple by 2050. We reasoned that the genes that modulate insulin production might be new targets for diabetes therapeutics. Therefore, we developed an siRNA screening system to identify genes important for the activity of the insulin promoter in beta cells. We created a subclone of the MIN6 mouse pancreatic beta cell line that expresses destabilized GFP under the control of a 362 base pair fragment of the human insulin promoter and the mCherry red fluorescent protein under the control of the constitutively active rous sarcoma virus promoter. The ratio of the GFP to mCherry fluorescence of a cell indicates its insulin promoter activity. As G protein coupled receptors (GPCRs) have emerged as novel targets for diabetes therapies, we used this cell line to screen an siRNA library targeting all known mouse GPCRs. We identified several known GPCR regulators of insulin secretion as regulators of the insulin promoter. One of the top positive regulators was Gpr27, an orphan GPCR with no known role in beta cell function. We show that knockdown of Gpr27 reduces endogenous mouse insulin promoter activity and glucose stimulated insulin secretion. Furthermore, we show that Pdx1 is important for Gpr27's effect on the insulin promoter and insulin secretion. Finally, the over-expression of Gpr27 in 293T cells increases inositol phosphate levels, while knockdown of Gpr27 in MIN6 cells reduces inositol phosphate levels, suggesting this orphan GPCR might couple to Gq/11. In summary, we demonstrate a MIN6-based siRNA screening system that allows rapid identification of novel positive and negative regulators of the insulin promoter. Using this system, we identify Gpr27 as a positive regulator of insulin production.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002449
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002449Summary
The prevalence of type 2 diabetes in the United States is projected to double or triple by 2050. We reasoned that the genes that modulate insulin production might be new targets for diabetes therapeutics. Therefore, we developed an siRNA screening system to identify genes important for the activity of the insulin promoter in beta cells. We created a subclone of the MIN6 mouse pancreatic beta cell line that expresses destabilized GFP under the control of a 362 base pair fragment of the human insulin promoter and the mCherry red fluorescent protein under the control of the constitutively active rous sarcoma virus promoter. The ratio of the GFP to mCherry fluorescence of a cell indicates its insulin promoter activity. As G protein coupled receptors (GPCRs) have emerged as novel targets for diabetes therapies, we used this cell line to screen an siRNA library targeting all known mouse GPCRs. We identified several known GPCR regulators of insulin secretion as regulators of the insulin promoter. One of the top positive regulators was Gpr27, an orphan GPCR with no known role in beta cell function. We show that knockdown of Gpr27 reduces endogenous mouse insulin promoter activity and glucose stimulated insulin secretion. Furthermore, we show that Pdx1 is important for Gpr27's effect on the insulin promoter and insulin secretion. Finally, the over-expression of Gpr27 in 293T cells increases inositol phosphate levels, while knockdown of Gpr27 in MIN6 cells reduces inositol phosphate levels, suggesting this orphan GPCR might couple to Gq/11. In summary, we demonstrate a MIN6-based siRNA screening system that allows rapid identification of novel positive and negative regulators of the insulin promoter. Using this system, we identify Gpr27 as a positive regulator of insulin production.
Introduction
Nearly 13% of American adults have diabetes and these numbers continue to rise, mostly from an increase in type 2 diabetes [1], [2]. Although insulin resistance is a cardinal feature of type 2 diabetes, most people with insulin resistance do not develop diabetes because their pancreatic beta cells are able to compensate by increasing insulin production. However, if insulin production cannot match the increased demand imposed by insulin resistance, hyperglycemia and frank diabetes ensues. Over time, beta cell function further declines in most people with type 2 diabetes, resulting in the eventual failure of oral medications and the necessity of insulin therapy [3].
Improving insulin production and beta cell function is therefore a universal goal of diabetes therapeutics. We reasoned that an unbiased search for regulators of insulin production might reveal new diabetes drug targets. Therefore, we constructed a novel screening system to screen for genes important for insulin promoter activity. By screening siRNAs targeting all GPCRs, we identify several GPCRs that regulate insulin promoter activity and specifically characterize Gpr27 as a novel regulator of insulin production.
Results
Generation of an insulin promoter reporter beta cell line
To allow rapid evaluation of insulin promoter activity, the MIN6 mouse beta cell line was infected with a lentivirus that stably expresses destabilized GFP under the control of the proximal 362 base pairs of the human insulin promoter (Figure 1A) [4]. This insulin promoter fragment maintains a substantial proportion of promoter activity and tissue specificity while being compact enough to allow lentiviral delivery [5].
Fig. 1. siRNA screening system to identify regulators of insulin promoter activity. 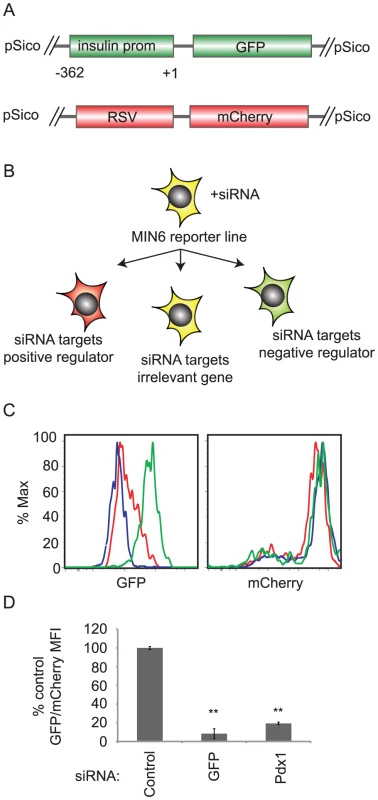
A. Schema of reporter constructs integrated into the screening MIN6 cell line. These are pSico lentiviruses containing the proximal 362 bases of the human insulin promoter driving destabilized GFP and the rous sarcoma virus (RSV) promoter driving mCherry. B. After transfection with siRNAs, GFP and mCherry mean fluorescence intensity (MFI) are measured by flow cytometry. If the siRNA targets a positive regulator, GFP/mCherry MFI decreases. If the siRNA targets a negative regulator, the GFP/mCherry MFI increases. C. Control (green), Pdx1(red), or GFP(blue) siRNAs were transfected into the MIN6 reporter cell line. After 5 days, GFP (left panel) and mCherry (right panel) fluorescence were measured by flow cytometry. D. As in C. The ratio of GFP geometric mean fluorescence intensity (MFI) to mCherry MFI was calculated for each sample. The ratio was normalized to that of control siRNA transfected cells. Data shown are averages and standard error (n = 3). ** p<0.01 versus control siRNA. To favor single copy integration, the construct was delivered at a low multiplicity of infection (MOI) and a clonal line was selected. To generate an internal control reporter, the GFP positive subline was subsequently infected at a low MOI with a second lentivirus containing mCherry under the control of the constitutive rous sarcoma virus promoter (RSV) (Figure 1A). A stable clone expressing both constructs was isolated. In these cells, the ratio of GFP to mCherry fluorescence indicates human insulin promoter activity.
When transfected into this reporter line, siRNAs targeting activators of insulin gene transcription would be expected to reduce insulin promoter activity and reduce the GFP/mCherry ratio, while siRNAs targeting negative regulators of the insulin promoter should increase the GFP/mCherry ratio (Figure 1B). Indeed, transfection of an siRNA targeting the insulin gene transcription factor Pdx1 reduced the GFP/mCherry ratio by 80% as compared to a non-targeting siRNAs (Figure 1C and 1D) [6].
siRNA screen for non-odorant GPCR regulators of the insulin promoter
An RNAi library containing four independent siRNAs targeting the mouse GPCR-ome and selected GPCR related genes was transfected into the reporter cell line. The ratio of the GFP to mCherry fluorescence five days after transfection was calculated for each siRNA and the data were then analyzed using the redundant siRNA analysis (RSA) software [7]. To avoid off-target effects, each siRNA was transfected separately and only genes with more than one siRNA hit were selected for further analysis.
The top genes judged by RSA were then ranked by unsupervised clustering of each gene's RSA p value and its expression level in primary mouse islets, since only those genes expressed in primary cells are of biological interest (Figure 2A). Two publically available mouse islet mRNA-Seq data sets were used. One of these data sets has been previously published and consists of approximately four million mapped reads from islets isolated from female non-pregnant mice and approximately four million mapped reads from islets isolated from pregnant mice [8]. The second, submitted to the NCBI Short Read Archive by Merck, contains approximately 120 million reads from mouse islets (see methods). Because of these modest read numbers, some low abundance transcripts may be erroneously reported as being not expressed using this analysis [9].
Fig. 2. siRNA screen hit selection and initial confirmation. 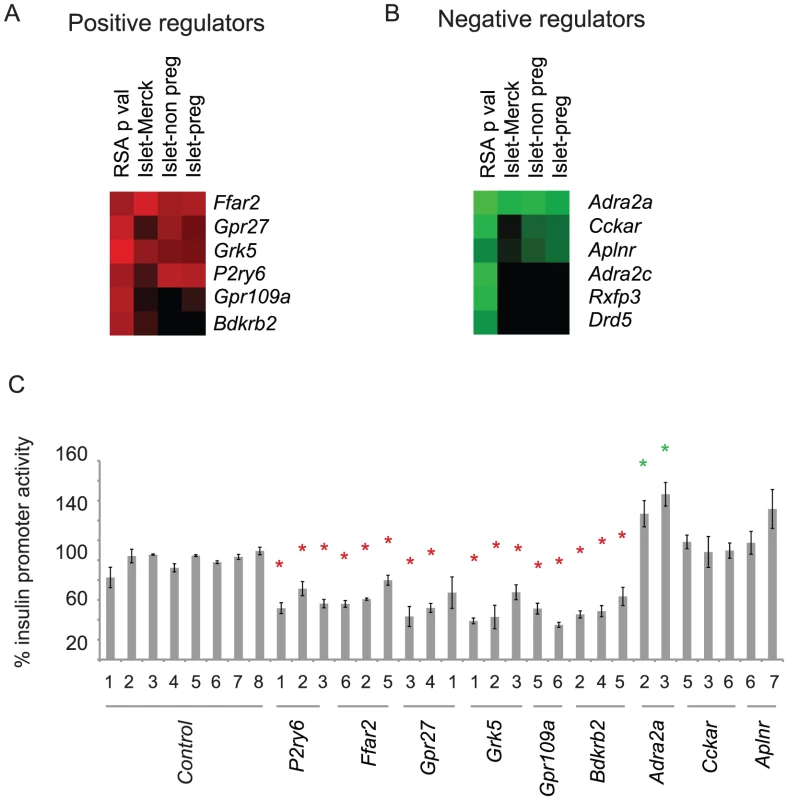
A. Heat map showing the top six putative positive regulators of the insulin promoter clustered based on RSA determined p value, and the base 10 log of the fragments per kilobase of exon model per million mapped reads (FPKM) value from three independent mRNA-seq data sets. B. As in B, but analyzed for negative regulators of insulin promoter activity. Islet-Merck refers to SRA008619 submitted by Merck, Islet-non preg refers to islets from non-pregnant mice, and Islet-preg refers to islets from pregnant mice [8]. C. Confirmation of hits from A and B. The indicated siRNAs were transfected into the screening cell line. GFP and mCherry was measured by flow cytometry. Error bars show standard error of three biological replicates performed (n = 3). * indicates p<0.05 versus >6 of the negative control siRNAs. siRNAs to the top six genes (Ffar2, Gpr27, Grk5, p2ry6, Gpr109a, Bdkrb2) that reduced insulin promoter activity and had detectable expression in primary mouse islets were transfected into the screening cell line for confirmation (Figure 2B). All six genes had at least 2 siRNAs confirm. For the siRNAs that increased GFP/mCherry, we retested the top three genes with high RSA scores and detectable expression in mouse primary islets – Adra2a, Cckar, and Aplnr. Of these three, only the known negative regulator of insulin secretion, Adra2a, confirmed with two independent siRNAs (Figure 2C).
Several of the positive regulators of the insulin promoter we identified were already known to stimulate insulin release in beta cells. Of particular interest was the orphan GPCR, Gpr27, which had no known role in insulin production but was previously found to be enriched in the mouse and human pancreatic islet [10], [11]. We subsequently tested all four siRNAs targeting Gpr27 in the library set on an independently generated MIN6 reporter line expressing stable GFP under the control of the insulin promoter and mCherry under the control of the RSV promoter. All four siRNAs reduced GFP/mCherry fluorescence (Figure S1A). Furthermore, all 4 siRNAs efficiently reduced expression of the Gpr27 mRNA (Figure S1B). We also confirmed that Gpr27 is enriched in beta cell lines (beta TC and MIN6) compared to an alpha cell line (alpha TC) (Figure S2A) and is expressed in primary mouse beta cells (Figure S2B).
Knockdown of Gpr27 reduces the activity of the endogenous mouse insulin promoter in cultured cells and primary islets
Since the screen was based on a human insulin promoter fragment, we measured the effect of Gpr27 knockdown on the endogenous mouse Ins2 and Ins1 genes. Because mature insulin mRNAs have a half-life of nearly 80 hours, we measured insulin pre-mRNAs as previously described [6], [12]. MIN6 cells infected with a Gpr27 shRNA expressing adenovirus (Ad-shGpr27) had a 40–60% reduction in pre-ins2 and pre-ins1 levels compared to control adenovirus (Ad-control) (Figure 3A). To confirm these findings in primary beta cells, we infected intact primary mouse islets with these same adenoviruses. At a high MOI, we were only able to obtain 50% infection rates as measured by flow cytometry, presumably reflecting poor adenovirus penetration into the core of the mouse islet [13]. Therefore, intact islets were dissociated prior to adenovirus infection. Three days after infection, cells were isolated by flow cytometric sorting for GFP and RT-qPCR was performed. Knockdown of Gpr27 produced a significant ∼30% reduction of pre-ins2 (p = 0.03). Concomitantly, there was a nearly significant 30% reduction in the less abundant pre-ins1 message (p = 0.055) (Figure 3B).
Fig. 3. Gpr27 is required for mouse insulin promoter activity and glucose stimulated insulin secretion. 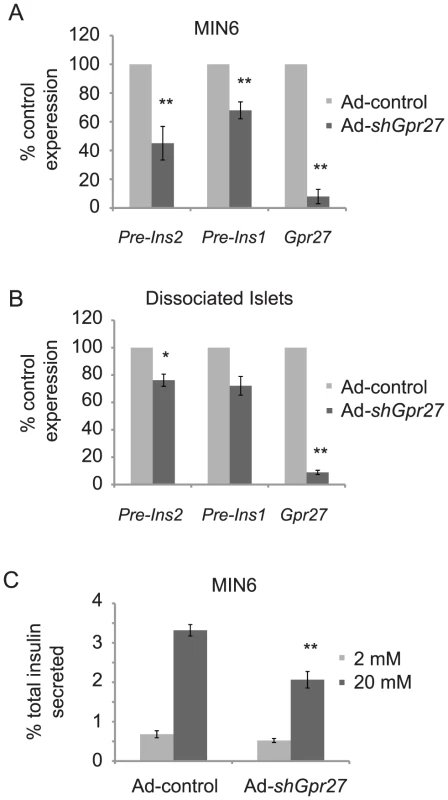
A. MIN6 cells were infected with either Ad-control or Ad-shGpr27. Three days after infection, RT-qPCR was performed for the indicated genes. The data are plotted as % expression compared to control adenovirus with standard error (n = 3). B. As in A, but dispersed primary mouse islets were infected with Ad-control or Ad-shGpr27. After 3 days, infected cells were sorted by flow cytometry of GFP positive cells and RT-qPCR was performed for the indicated genes (n = 3). C. MIN6 cells were infected with either Ad-control or Ad-shGpr27. Three days after infection, glucose stimulated insulin secretion was measured by ELISA after 1 hour of static incubation at either 2 mM or 20 mM glucose. Data are represented as the average of fractional insulin secretion with standard error (n = 9). *p<0.05, **p<0.005 versus Ad-control. Knockdown of Gpr27 impairs glucose stimulated insulin secretion
While insulin production requires insulin promoter activity, minute-to-minute changes in plasma insulin levels are controlled by insulin secretion. Therefore, we asked if Gpr27 knockdown would affect glucose stimulated insulin secretion. Infection of MIN6 cells with Ad-control at an MOI necessary to get >90% infection inhibited glucose stimulated insulin secretion (data not shown). Therefore, we infected MIN6 cells at a lower MOI to achieve approximately 60% infection and measured glucose stimulated insulin secretion from this mixed population by batch incubation. Ad-shGpr27 infected MIN6 cells secreted ∼40% less insulin at 20 mM glucose compared to Ad-control infected cells (Figure 3C). There was no statistically significant difference at 2 mM glucose. Notably, we did not detect a difference in total insulin as normalized to total protein concentration (Ad-control = 27.9+/−1.1 mg insulin per g of total protein; Ad-shGpr27 = 29.4+/−0.94 mg insulin per g of total protein, p value = 0.13). This was not unexpected since the half-life of insulin mRNA is ∼80 hours and the knockdown of Gpr27 was limited to 72 hours due to adenovirus toxicity after that time point. We conclude that Gpr27 plays a measurable role in insulin secretion in addition to insulin promoter activity.
Gpr27's effect on the insulin promoter and insulin secretion requires Pdx1
To define the mechanism of Gpr27 action, we measured transcript levels of selected regulators of the insulin promoter by RT-QPCR in MIN6 cells after Ad-shGpr27 infection. Glis3, Pax6, Nkx6.1, HNF4a, and Pdx1 were reduced after Gpr27 knockdown while others including MafA, NeuroD1, and Pax4 were unchanged (Figure 4A). Concordant with this expression data, Gpr27 knockdown reduced the transcriptional activity of mini-enhancers that bind to Glis3 and Pdx1 (Z, E1/A1, E2/A3) while Gpr27 knockdown had no effect on mini-enhancers that bind to MafA and NeuroD1 (C1/E1) (Figure 4B and 4C).
Fig. 4. Gpr27 knockdown affects multiple transcription factors and requires Pdx1 for its effect on the insulin promoter. 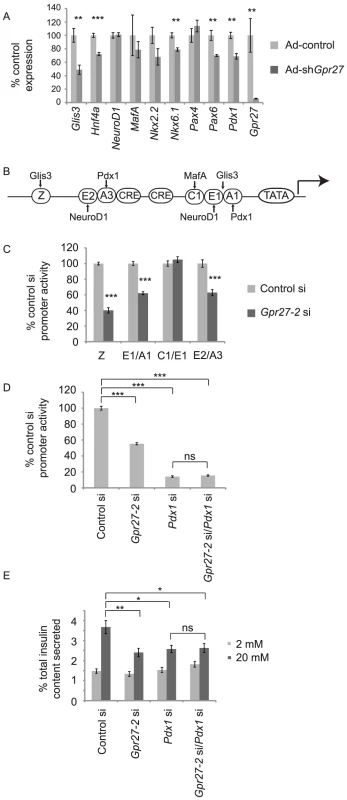
A. MIN6 cells were infected with Ad-control or Ad-shGpr27. Three days after infection RT-qPCR was performed for the indicated genes. Expression level normalized to that of Ad-control are plotted with standard error (n = 6). B. Schematic of the human insulin promoter with selected regulatory sequences and transcription factors that bind to these elements. C. MIN6 cells were cotransfected with the indicated siRNA, insulin promoter firely luciferase construct and thymidine kinase renilla luciferase construct. Two days after transfection, firefly and renilla luciferase activity were measured. The ratio of firefly to renilla luciferase was normalized to the control siRNA. Average and standard error are plotted (n = 6–9) D. As in C using human insulin promoter −362 firefly luciferase (n = 6–9). E. MIN6 cells were transfected with the indicated siRNAs and glucose stimulated insulin secretion was measured after 5 days. Fractional insulin secretion is shown (n = 12). * p<0.05 ** p<0.005 *** p<0.0005 versus control siRNA or control adenovirus (at high glucose part E). Since Pdx1 is required for insulin promoter activity and insulin secretion [14], [15], we asked if Pdx1 is required for the effect of Gpr27's on the insulin promoter. By luciferase assay, we found that the single knockdown of Pdx1 reduced insulin promoter activity by 90% and Gpr27 knockdown alone reduced insulin promoter activity by 40%. However, the knockdown of both Gpr27 and Pdx1 had no additional effect over the single knockdown of Pdx1, showing that Pdx1 is important for the effect of Gpr27 on the insulin promoter (Figure 4D). Importantly, double knockdown of both Gpr27 and Pdx1 was as efficient as single knockdown (Figure S3).
We then asked if Pdx1 was required for the effect of Gpr27 on insulin secretion. The knockdown of Pdx1 reduced fractional insulin secretion at 20 mM glucose and total insulin content (Figure 4E and Figure S4). As with the adenoviral knockdown of Gpr27, an siRNA to Gpr27 reduced glucose stimulated insulin secretion. However, the knockdown of Gpr27 in addition to Pdx1 did not further reduce insulin secretion at 20 mM glucose. We conclude that Gpr27 plays a measurable role in insulin secretion and insulin promoter activity via a mechanism involving Pdx1.
Gpr27 increases IP1 levels
G protein coupling software analysis predicts that Gpr27 could function via Gi or Gq/11 signaling pathways [16]. Since Gpr27 is already expressed in MIN6 cells, we ectopically expressed mouse Gpr27 in HEK293T cells. Robust expression of FLAG-tagged Gpr27 was detected by 24 hours on the surface of the majority of cells (Figure 5B). We then measured cAMP and IP1 – higher cAMP would indicate Gs coupling, lower cAMP would indicate Gi coupling and higher IP1 would indicate Gq/11 coupling (Figure 5A). Gpr27 expression resulted in a 2-fold elevation of IP1 levels while leaving cAMP levels unchanged (Figure 5C and 5D) showing that in this heterologous cell type, Gpr27 may activate the Gq/11 pathway.
Fig. 5. Gpr27 positively regulates inositol phosphate levels. 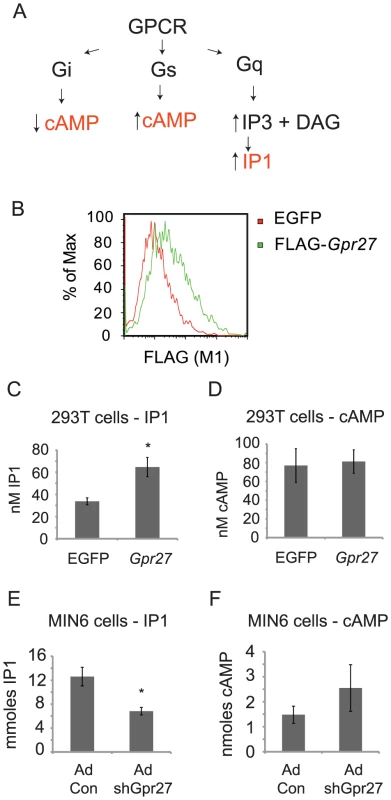
A. Schema of canonical GPCR signaling pathways and resulting expected changes in second messengers cAMP and IP3. B. HEK293T cells were transiently transfected with either GFP plasmid (control) or FLAG-Gpr27 plasmid. 24 hours after transfection cells were analyzed by flow cytometry for extracellular FLAG. C. As in B, but cells were lysed and assayed for IP1. D. As in C, but lysates were analyzed for cAMP. E. MIN6 cells were infected with control or Gpr27 knockdown adenovirus and 3 days later, cells were lysed and IP1 was measured. F. As in E, but cAMP was measured. For C–F, average and standard error is plotted (n = 9); * p<0.005 versus control. If Gpr27 activates Gq/11 in beta cells, then IP1 levels should be reduced in MIN6 cells after knockdown of Gpr27. Therefore, we measured IP1 levels and cAMP levels in MIN6 cells after Gpr27 knockdown. Indeed, knockdown of Gpr27 resulted in reduced IP1 levels while cAMP levels were not significantly changed (Figure 5E and 5F). Taken together, these data show that Gpr27 positively regulates inositol phosphate levels, supporting a role for Gpr27 in activating the Gq/11 pathway.
Discussion
To identify new regulators of the insulin promoter, we developed a novel siRNA screening system in MIN6 cells that allows rapid measurement of insulin promoter activity. As an initial test of the system, an siRNA screen of the GPCR-ome was performed. The RSA algorithm was used to select hits in order to capitalize on the four fold redundancy of the siRNA library [7]. To further increase the specificity of the screen, at least 2 siRNAs must have been identified for a gene to be a hit. The top RSA hits were then prioritized by expression level in mouse primary islets. Besides filtering out genes expressed in MIN6 but not in primary islets, this step also eliminates off-target hits. On the other hand, hit genes with low expression may have been erroneously eliminated because they were below the limit of detection of the mRNA-seq data available at this time [17]. Nonetheless, this filtering step allowed us to focus on genes with reasonable expression in primary cells.
While the confirmation rate for siRNAs to positive regulators was 100%, the confirmation rate for negative regulators was only 33%. This is likely due, in part, to the more modest effect of these siRNAs (∼20–30% increase in GFP/mCherry ratio) as compared to the reconfirmed Adra2a(∼50%), a known negative regulator of insulin secretion.
We identified several other known regulators of insulin secretion as regulators of the insulin promoter. The bradykinin receptor 2 mediates increases in insulin secretion in beta cells [18], [19]. Pyrimidinergic receptor 6 (p2yr6) agonists augment insulin release and this receptor participates in an autocrine feedback loop that potentiates insulin secretion [20], [21]. The free fatty acid receptor 2, which has been hypothesized to play a role in beta cells, was also identified as a positive regulator of the insulin promoter in our screen [22]. Several other receptors were identified in the screen that have no known role in beta cells and these may merit further investigation. Of note, Glp1r was not identified in this screen for a trivial reason; siRNAs targeting this gene were not included in the commercial screening set. Given the nature of our screen, hits would be predicted to either have basal activity or have ligand present in the culture conditions as has been described for p2yr6 [21].
We were most intrigued by the orphan GPCR, Gpr27 [23]. Previous studies have shown that it is enriched in the pancreatic islets of both human and mouse [10], [11]. Detailed mouse tissue profiling of Gpr27 expression by RT-QPCR shows high expression in the mouse brain with lower expression in the islet and heart [24]. Furthermore, Gpr27 mRNA is up-regulated in Neurogenin3 positive endocrine precursors in the developing mouse pancreas [11]. Conversely, Gpr27 is 8-fold down regulated in the Neurogenin3 knockout pancreas [25], [26]. Taken together, these data suggest Gpr27 is an endocrine pancreas specific gene.
We confirmed that knockdown of Gpr27 reduces the activity of human insulin promoter reporters, levels of endogenous mouse Ins2 pre-mRNA, and glucose stimulated insulin secretion. Importantly, Gpr27 knockdown also reduces the levels of endogenous Ins2 pre-mRNA in dissociated primary mouse islets. We also found that the mRNAs for multiple transcription factors that activate the insulin promoter (Glis3, Pdx1, HNF4a) were reduced by Gpr27 knockdown. Other transcription factors critical for beta cell development were also reduced including Nkx6.1 and Pax6. In agreement with the reduction in their expression, only Pdx1 and Glis3 binding mini-enhancers were affected by Gpr27 knockdown (Figure 4C). Finally, there was no further reduction in insulin promoter activity when adding Gpr27 knockdown to Pdx1 knockdown. A limitation of this double knockdown experiment is that given the very strong effect of Pdx1 knockdown alone on insulin promoter activity, a further reduction with Gpr27/Pdx1 double knockdown may be either below our limit of detection or simply reflect no remaining insulin promoter activity.
How might Gpr27 affect both insulin transcription and glucose stimulated insulin secretion? The Gq/11 pathway was an obvious candidate as the expression of Gpr27 in HEK 293T cells increased IP1 levels while the knockdown of Gpr27 reduced IP1 levels in MIN6 cells. Furthermore, triggering of an engineered Gq/11-coupled GPCR in beta cells increases steady state insulin mRNA levels and insulin secretion [27]. However, Pdx1 levels did not change after triggering this Gq/11-coupled GPCR [27] and Gq/11 knockout beta cells have normal levels of Ins1 and beta cell transcription factor mRNAs [21]. Therefore, even if Gpr27 directly couples to Gq/11, Gpr27 may affect insulin secretion and insulin promoter activity independent of Gq/11 as has recently been demonstrated for the M3 receptor [28].
Another candidate for mediating the effects of Gpr27 on insulin promoter and insulin secretion was Pdx1 since it is known to positively regulate both insulin transcription and insulin secretion [14], [15]. We found that the double siRNA knockdown of Gpr27 and Pdx1 produced no further reduction in insulin secretion over Pdx1 knockdown alone, suggesting that Pdx1 is important for Gpr27's effect on insulin secretion. In combination with the reduction in Pdx1 mRNA by Gpr27 knockdown, these data suggest Gpr27 functions upstream of Pdx1. However, the double knockdown data do not exclude the possibility that a Pdx1 lies in a parallel pathway to Gpr27 and these two pathways intersect upstream of insulin secretion.
Taken together, these data suggest that a linear pathway connecting Gpr27 to a single G protein and a single regulatory element in the insulin promoter is overly simplistic. Indeed, a single GPCR can trigger multiple G proteins (reviewed by [29]), can trigger a combination of G protein dependent and independent pathways [30], and can function as heterodimers [31]. Likewise, the insulin promoter contains multiple elements that are both redundant and cooperative [32]. The complexity of these systems highlights the advantage of using a broad, unbiased approach to finding new and unexpected regulators of the insulin promoter. Here, we used such a system to identify a novel GPCR regulator of both insulin secretion and insulin promoter activity – Gpr27. Based on its islet expression and its positive effects on the insulin promoter and insulin secretion, we suggest that Gpr27 may be a novel target for diabetes therapies.
Materials and Methods
Cell culture
MIN6 cells were a gift from Dr. Miyazaki. Alpha TC and beta TC were a gift from Dr. Hanahan. Cells were maintained in high glucose DMEM with 10% fetal bovine serum, and 71.5 mM beta-mercaptoethanol. Sublines were isolated by limiting dilution. Original passage lines were used between passage 25–40. Sublines were used at passages 5–10.
Promoter constructs
Human insulin promoter deletions have been previously described [5]. Promoters were subcloned from pFoxCAT into pFoxLuc [33]. For the lentiviral reporter, the human −362 promoter region was cloned upstream of destabilized GFP or GFP and this cassette was used to replace the U6/CMV-EGFP in pSicoR. pSicoR-RSV-mCherry was created by replacing the U6/CMV of pSicoR mCherry with the RSV promoter [5]. Mini-enhancer reporter constructs have also been previously described [5], [34]. They were subcloned upstream of a minimal thymidine kinase promoter-firefly luciferase reporter.
siRNA transfection
Approximately 5,000 MIN6 cells were transfected in 96 well plates using HiPerfect (Qiagen) with a final siRNA concentration of 25 nM. Cells were analyzed by flow cytometry (LSRII, BD) 5 days after transfection and the geometric mean fluorescence intensity of GFP was normalized to that of mCherry. If the knockdown of GFP by an anti-GFP siRNA was not >80%, the transfection of that plate was considered to be a technical failure and the plate was discarded. This occurred on 1 out of 20 plates and for this reason some genes were only targeted by 3 siRNAs (including Gpr27). Each well was normalized to the negative control siRNA on that 96 well plate. For the confirmation assay for Gpr27 siRNAs, a distinct MIN6 human insulin promoter-GFP/RSV-mCherry reporter line was transfected with the indicated siRNAs with Lipofectamine RNAiMax for 5 days and GFP and mCherry were measured.
GPCR expression analysis and hierarchical clustering
Mouse islet mRNA-seq data was downloaded from the NCBI Short Read Archive (SRP000752 and SRP002569) and FPKM values were calculated using the TopHat and Cufflinks software using the NCBI RefSeq as the reference. Log FPKM and negative log RSA p values were clustered using Cluster 3.0 and heat maps were plotted with JavaTreeView.
siRNA and qPCR probes
siRNAs were obtained from Qiagen. All custom Taqman probes had a confirmed PCR efficiency of between 95–110%. Samples without reverse transcriptase did not amplify. See Text S1 for sequences of custom probes. Taqman probes to mouse Glis3, MafA, Pdx1, NeuroD1, Pax4, Nkx6.1, HNF4a were obtained from Applied Biosystems. Negative control siRNAs for the reconfirmation assay were All-Stars Negative Control (1027280), Negative Control (1022076), Unspecific-Luciferase-1 (1022070), Unspecific-Luciferase-2 (1022073), Hs_LMNA_11 (1022050), Mm_Lmna_5 (SI02655450), Hs_GAPD_5 (SI0253266), Hs_ACTB_1 (1022168).
RT–qPCR
Total RNA was isolated by Trizol (Invitrogen). The RNA was DNase I treated (Turbo DNase, Ambion) and reverse transcription was performed (Superscript III, Invitrogen) using a combination of random hexamers and oligo dT primers. For cell line experiments, each qPCR reaction used between 10–30 ng of total RNA equivalent. To convert to arbitrary linear units, the following formula was used: (2∧15)*(2∧(deltaCT to beta-glucuronidase).
Isolation of MIP-GFP positive cells
Islets from 12–30 week old MIP-GFP mice were isolated by the UCSF Islet Production Core. Islets were digested with trypsin until single cell suspensions were obtained. Cells were sorted by flow cytometry (Aria II, BD or MoFlo, DakoCytomation) into GFP positive and negative fractions and total RNA was isolated. 20 ng of total RNA equivalent was loaded per QRT-PCR reaction.
Luciferase assays
140,000 MIN6 cells were transiently transfected in 24 well plates with the relevant siRNA (5 pmoles), the indicated insulin reporter firefly luciferase plasmid (100 ng), and pRL-TK(Promega) (25 ng) using Lipofectamine 2000 (Invitrogen). For double siRNA knockdowns, 5 pmoles of each siRNA or 10 pmoles of control (anti-GFP) were used. Two days after transient transfection, firely and renilla luciferase were measured using the Dual Luciferase Assay (Promega).
Gpr27 expression in HEK 293T
Gpr27 was cloned by PCR from mouse genomic DNA downstream of a viral signal sequence and amino terminal FLAG epitope tag [35]. This cassette was used to replace the EGFP in pSicoR. HEK 293T cells were transiently transfected with either pSicoR-EGFP or pSicoR-FLAG-Gpr27 using LT1 (Mirus).
FLAG flow cytometry
293T cells were dissociated with PBS without Ca or Mg, stained with M1 anti-FLAG antibody and a Goat anti-mouse secondary antibody coupled to Alexa-594 (Invitrogen).
IP1 and cAMP assays
For 293T, one day after transient transfection in 24 well plates, cells were placed in stimulation buffer (HTRF) for 30 minutes at 37 degrees. The stimulation buffer was then removed and the cells were lysed using the kit lysis buffer. IP1 and cAMP were then measured as directed by the protocol in 384 well plates (HTRF). IP1 and cAMP levels were normalized to live cells numbers counted from duplicate wells. Viable cells counts from Gpr27 transfection were within 20% of control plasmid transfection. For MIN6 cells, 125,000 cells were infected with Gpr27 shRNA or control adenovirus at an MOI of 200 and grown in 24 well dishes (resulting in nearly ∼95% infection). Three days after infection, the cells were placed in stimulation buffer (HTRF) for 30 minutes at 37 degrees. The stimulation buffer was then removed and the cells were lysed in 1% Triton-X100, 50 mM HEPES pH 7.0, NaF 15 mM. The lysate was pre-cleared by centrifugation at 14,000 rpm for 10 minutes. A fraction of the lysate was taken for protein quantitation(micro-BCA, Pierce), IP1 or cAMP measurement (HTRF). Data were normalized to total protein content.
shRNA adenovirus construction
The Gpr27 shRNA was cloned into a modified version of pSicoR with a BstXI site replacing the HpaI site. The mouse U6 promoter and Gpr27 shRNA were then subcloned from pSicoR and placed upstream of the CMV-GFP marker in pAdTrack [36], [37]. Adenovirus was prepared and tittered as previously described [38].
Knockdown in primary mouse islets
Islets were isolated by the UCSF Islet Production Core Facility from 8–12 week old C57Bl/6 male mice. After 24 hours of culture in RPMI and 10% FBS, islets were trypsinized until single cell suspensions were obtained. The dissociated islet cells were resuspended in RPMI+10% FBS and infected with adenovirus at multiplicity of infection (MOI) of 25. Three days after infection, the cells were sorted by flow cytometry (Aria II, BD) for GFP positive cells (50–75% of the live population) and RT-qPCR was performed. The knockdown of pre-ins2, pre-ins1 or Gpr27 from Gpr27 shRNA adenovirus infected cells was calculated by the delta-delta CT method compared to the control adenovirus infection.
Insulin secretion assays
For the adenovirus assays, approximately 500,000 MIN6 cells were infected with the indicated adenoviruses at an MOI of 100 in 6 cm dishes in complete media. Three days after infection, the infection rate was ∼60% by FACS for GFP. Cells were washed 5 times in KRBH buffer (10 mM HEPES pH 7.4, 130 mM NaCl, 5 mM KCl, 1.25 mM KH2PO4, 1.25 mM MgSO4, 2.68 mM CaCl2, 5.26 mM NaHCO3) with 2 mM glucose and rested for 2 hours at 37 degrees. Cells were then washed an additional 3 times with 2 mM glucose KRBH and incubated in 3 mL of 2 mM glucose KRBH for 1 hour at 37 degrees. This supernatant was collected and replaced with 20 mM glucose KRBH for 1 hour at 37 degrees. Cells were washed with PBS before lysis in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Triton X-100 with protease inhibitors. Lysates were spun at 14,000 rpm for 10 minutes and supernatants were spun at 5000 rpm for 5 minutes before analysis by an Ultrasensitive Insulin ELISA (Mercodia). Total protein was measured by Micro-BCA (Pierce). Total insulin was normalized to total protein in the lysate. For the siRNA transfections, 20,000 MIN6 cells were transfected per well of a Corning CellBIND 96 well plate with 25 nM of each siRNA (or 50 nM of control siRNA) using Lipofectamine RNAiMax. 5 days after transfection, cells were washed in KRBH with 2 mM glucose twice, then incubated for 2 hours at 37 degrees, then washed again with KRBH 2 mM glucose twice, then incubated for one hour with KRBH 2 mM, then KRBH with 20 mM glucose for 1 hour. Lysates were prepared in 75 uL of lysis buffer as above. Due to the lower cell numbers in the 96 well plate assay, total insulin was normalized to total genomic DNA measured by Qubit High Sensitivity DNA kit (Life Technologies).
Statistical analysis
For siRNA primary confirmation assay, an independent, two sample, one tailed t-test was used. For the primary islet adenovirus knockdown of Gpr27 an independent, one sample, two tailed t-test was used. All other p values were calculated with an independent, two sample, two tailed t-test.
Ethics statement
Animal experiments were approved by the UCSF Institutional Animal Care and Use Committee (Protocol AN082433-02) with care taken to avoid any unnecessary suffering. Animals were maintained in accordance with the applicable portions of the Animal Welfare act and the DHHS Guide for the Care and Use of Laboratory Animals.
Supporting Information
Zdroje
1. NarayanKMBoyleJPThompsonTJSorensenSWWilliamsonDF 2003 Lifetime risk for diabetes mellitus in the United States. JAMA 290 1884 1890
2. CowieCCRustKFFordESEberhardtMSByrd-HoltDD 2009 Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 32 287 294
3. UKPDS 1998 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352 837 853
4. MiyazakiJArakiKYamatoEIkegamiHAsanoT 1990 Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127 126 132
5. OdagiriHWangJGermanMS 1996 Function of the human insulin promoter in primary cultured islet cells. J Biol Chem 271 1909 1915
6. IypeTFrancisJGarmeyJCSchislerJCNesherR 2005 Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J Biol Chem 280 16798 16807
7. KonigRChiangCYTuBPYanSFDeJesusPD 2007 A probability-based approach for the analysis of large-scale RNAi screens. Nat Methods 4 847 849
8. KimHToyofukuYLynnFCChakEUchidaT Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 16 804 808
9. GriffithMGriffithOLMwenifumboJGoyaRMorrissyAS Alternative expression analysis by RNA sequencing. Nat Methods 7 843 847
10. GaultonKJNammoTPasqualiLSimonJMGiresiPG A map of open chromatin in human pancreatic islets. Nat Genet 42 255 259
11. RegardJBKataokaHCanoDACamererEYinL 2007 Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest 117 4034 4043
12. WelshMNielsenDAMacKrellAJSteinerDF 1985 Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem 260 13590 13594
13. TakahashiRIshiharaHTakahashiKTamuraAYamaguchiS 2007 Efficient and controlled gene expression in mouse pancreatic islets by arterial delivery of tetracycline-inducible adenoviral vectors. J Mol Endocrinol 38 127 136
14. BrissovaMShiotaMNicholsonWEGannonMKnobelSM 2002 Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 277 11225 11232
15. GauthierBRWiederkehrABaquieMDaiCPowersAC 2009 PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab 10 110 118
16. SgourakisNGBagosPGHamodrakasSJ 2005 Prediction of the coupling specificity of GPCRs to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics 21 4101 4106
17. GriffithMTangMJGriffithOLMorinRDChanSY 2008 ALEXA: a microarray design platform for alternative expression analysis. Nat Methods 5 118
18. YangCLeeBChenTHHsuWH 1997 Mechanisms of bradykinin-induced insulin secretion in clonal beta cell line RINm5F. J Pharmacol Exp Ther 282 1247 1252
19. YangCHsuWH 1995 Stimulatory effect of bradykinin on insulin release from the perfused rat pancreas. Am J Physiol 268 E1027 1030
20. ParandehFAbaravicieneSMAmistenSErlingeDSalehiA 2008 Uridine diphosphate (UDP) stimulates insulin secretion by activation of P2Y6 receptors. Biochem Biophys Res Commun 370 499 503
21. SassmannAGierBGroneHJDrewsGOffermannsS The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest 120 2184 2193
22. KebedeMAAlquierTLatourMGPoitoutV 2009 Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab 11 Suppl 4 10 20
23. MatsumotoMSaitoTTakasakiJKamoharaMSugimotoT 2000 An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem Biophys Res Commun 272 576 582
24. RegardJBSatoITCoughlinSR 2008 Anatomical profiling of G protein-coupled receptor expression. Cell 135 561 571
25. JuhlKSarkarSAWongRJensenJHuttonJC 2008 Mouse pancreatic endocrine cell transcriptome defined in the embryonic Ngn3-null mouse. Diabetes 57 2755 2761
26. GuGWellsJMDombkowskiDPrefferFAronowB 2004 Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131 165 179
27. GuettierJMGautamDScarselliMRuiz de AzuaILiJH 2009 A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A 106 19197 19202
28. KongKCButcherAJMcWilliamsPJonesDWessJ M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci U S A
29. MillarRPNewtonCL The year in G protein-coupled receptor research. Mol Endocrinol 24 261 274
30. WeiHAhnSShenoySKKarnikSSHunyadyL 2003 Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A 100 10782 10787
31. LevoyeADamJAyoubMAGuillaumeJLJockersR 2006 Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep 7 1094 1098
32. OhnedaKEeHGermanM 2000 Regulation of insulin gene transcription. Semin Cell Dev Biol 11 227 233
33. JohnsonJDZhangWRudnickARutterWJGermanMS 1997 Transcriptional synergy between LIM-homeodomain proteins and basic helix-loop-helix proteins: the LIM2 domain determines specificity. Mol Cell Biol 17 3488 3496
34. SanderMGriffenSCHuangJGermanMS 1998 A novel glucose-responsive element in the human insulin gene functions uniquely in primary cultured islets. Proc Natl Acad Sci U S A 95 11572 11577
35. ChangWCNgJKNguyenTPellissierLClaeysenS 2007 Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PLoS ONE 2 e1317 doi:10.1371/journal.pone.0001317
36. VenturaAMeissnerADillonCPMcManusMSharpPA 2004 Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A 101 10380 10385
37. HeTCZhouSda CostaLTYuJKinzlerKW 1998 A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A 95 2509 2514
38. LuoJDengZLLuoXTangNSongWX 2007 A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2 1236 1247
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant ImmunityČlánek Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



