-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
Floral organs display tremendous variation in their exterior that is essential for organogenesis and the interaction with the environment. This diversity in surface characteristics is largely dependent on the composition and structure of their coating cuticular layer. To date, mechanisms of flower organ initiation and identity have been studied extensively, while little is known regarding the regulation of flower organs surface formation, cuticle composition, and its developmental significance. Using a synthetic microRNA approach to simultaneously silence the three SHINE (SHN) clade members, we revealed that these transcription factors act redundantly to shape the surface and morphology of Arabidopsis flowers. It appears that SHNs regulate floral organs' epidermal cell elongation and decoration with nanoridges, particularly in petals. Reduced activity of SHN transcription factors results in floral organs' fusion and earlier abscission that is accompanied by a decrease in cutin load and modified cell wall properties. SHN transcription factors possess target genes within four cutin - and suberin-associated protein families including, CYP86A cytochrome P450s, fatty acyl-CoA reductases, GSDL-motif lipases, and BODYGUARD1-like proteins. The results suggest that alongside controlling cuticular lipids metabolism, SHNs act to modify the epidermis cell wall through altering pectin metabolism and structural proteins. We also provide evidence that surface formation in petals and other floral organs during their growth and elongation or in abscission and dehiscence through SHNs is partially mediated by gibberellin and the DELLA signaling cascade. This study therefore demonstrates the need for a defined composition and structure of the cuticle and cell wall in order to form the archetypal features of floral organs surfaces and control their cell-to-cell separation processes. Furthermore, it will promote future investigation into the relation between the regulation of organ surface patterning and the broader control of flower development and biological functions.
Published in the journal: . PLoS Genet 7(5): e32767. doi:10.1371/journal.pgen.1001388
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001388Summary
Floral organs display tremendous variation in their exterior that is essential for organogenesis and the interaction with the environment. This diversity in surface characteristics is largely dependent on the composition and structure of their coating cuticular layer. To date, mechanisms of flower organ initiation and identity have been studied extensively, while little is known regarding the regulation of flower organs surface formation, cuticle composition, and its developmental significance. Using a synthetic microRNA approach to simultaneously silence the three SHINE (SHN) clade members, we revealed that these transcription factors act redundantly to shape the surface and morphology of Arabidopsis flowers. It appears that SHNs regulate floral organs' epidermal cell elongation and decoration with nanoridges, particularly in petals. Reduced activity of SHN transcription factors results in floral organs' fusion and earlier abscission that is accompanied by a decrease in cutin load and modified cell wall properties. SHN transcription factors possess target genes within four cutin - and suberin-associated protein families including, CYP86A cytochrome P450s, fatty acyl-CoA reductases, GSDL-motif lipases, and BODYGUARD1-like proteins. The results suggest that alongside controlling cuticular lipids metabolism, SHNs act to modify the epidermis cell wall through altering pectin metabolism and structural proteins. We also provide evidence that surface formation in petals and other floral organs during their growth and elongation or in abscission and dehiscence through SHNs is partially mediated by gibberellin and the DELLA signaling cascade. This study therefore demonstrates the need for a defined composition and structure of the cuticle and cell wall in order to form the archetypal features of floral organs surfaces and control their cell-to-cell separation processes. Furthermore, it will promote future investigation into the relation between the regulation of organ surface patterning and the broader control of flower development and biological functions.
Introduction
In contrast to other plant cell layers, the epidermis develops a unique cell wall that not merely constitutes of cellulose, hemicelluloses, pectins, and proteins but also of a cuticular matrix, which is largely composed of cutin embedded and overlaid with waxes [1]. Cutin, an insoluble cuticular polymer, is largely composed of interesterified hydroxy and hydroxy epoxy fatty acids and is attached to the outer epidermal layer of cells by a pectinaceous layer [2]. As the epidermal cell grows, the cuticle merges gradually with the cell wall components [3]. Although the role of the epidermis layer in regulating organ growth has remained controversial [4]–[5], it is clear that it is vital for plant survival, development and the interaction with the environment [6]–[7]. Cutin and wax are synthesized exclusively in the epidermis [8] and a massive flux of lipids occurs from the sites of lipid synthesis in the plastid and the endoplasmic reticulum (ER) to the plant surface during cuticle deposition [9]. Significant progress has been made over the past decade in identifying genes involved in the biosynthesis and secretion of cuticular lipids [10]–[11] and in the metabolism and assembly of primary cell wall components [12]–[14]. Despite the close connection between the cell wall and the cuticular matrix, mutants and phenotypes in one of these processes were rarely examined for alteration in the other. Furthermore, to our knowledge, co-regulation of these two processes at the molecular genetic level was overlooked up to now.
Biosynthesis of plant cuticle components and their secretion to the extracellular matrix involve the coordinated induction of several metabolic pathways, in which transcription factors may play a key role [9], [15]. The Arabidopsis SHINE1/WAX INDUCER1 (SHN1/WIN1) AP2-domain protein was the first transcription factor reported to control metabolic pathways generating cuticular waxes [16]–[17]. A subsequent study [18] indicated that SHN1/WIN1 controls cuticle permeability by regulating the expression of cutin biosynthesis genes, particularly LACS2 (LONG CHAIN ACYL-COA SYNTHETASE 2). The induction of wax formation in leaves by over expression of individual SHINE clade genes was suggested to be a second step, possibly an indirect process following cutin biosynthesis [18]. Nevertheless, our current knowledge is limited with respect to the SHN1/WIN1 protein's mode of action and the involvement in particular developmental processes.
Arabidopsis SHN1/WIN1 transcription factor belongs to a small distinct clade of three proteins [16]. They all share two unique conserved motifs outside the AP2 domain, and all three proteins display the same shiny phenotype upon overexpression, suggesting their functional redundancy in cuticular lipid biosynthesis. Additional evidence for functional redundancy among the SHN clade members in cuticular lipid biosynthesis was provided by silencing SHN1/WIN1 [18]. In these plants, floral morphology was not altered and the subtle reduction in the levels of cutin detected in entire flower extracts was enhanced in isolated petals. Besides, their notable expression patterns in reproductive organs suggested that they are probably redundant in function. The expression of SHN1/WIN1 and SHN3 overlapped in various flower organs including in the abscission zones while SHN2 and SHN3 were both expressed in the silique dehiscence zones. Interestingly, expression of SHN2 was very specific to cell separation regions in the anthers and siliques. These expression profiles indicated that SHN transcription factors may also act in a combinatorial manner to secure reproductive organ development, protecting the exterior layers of the plants from environmental stresses. On the other hand, these three clade members differ in their spatial and temporal expression patterns, which suggests that each of them may play specific roles in various organs or under different conditions, and that the actual redundancy between the SHN factors is most probably in their target genes [16]. Further elucidation of the mode of SHN action, their target genes, and their precise connection to plant cuticle formation and plant development requires in-depth characterization of the SHN clade factors, which can be achieved by using double, possibly triple mutants to eliminate redundant activities [16]–[18]. In contrast to Arabidopsis, mutation in the barley SHN1/WIN1 ortholog (Nud) was sufficient to generate a severe morphological change in which the typically hulled caryopses developed into naked ones [19]. Nud was suggested to direct the deposition of a lipidic matter on the pericarp epidermis that adheres the hull to the caryopsis in a way similar to postgenital fusions displayed by numerous cuticular mutants [20]–[21].
In this study we have co-silenced the three SHN clade members in order to decipher their modes of action and resolve their biological roles. We revealed that SHN clade genes regulate the elongation and decoration (i.e. nanoridges formation) of reproductive organ epidermal cells, particularly in the petal surface. They also emerge as mediators of cell adhesion and separation during abscission and dehiscence. Additionally, the results suggest that beside their function in the cutin pathway, these transcription factors possess putative downstream target genes that are involved in cell wall configuration through pectin modifying enzymes and structural proteins. Thus, the study of SHN transcription factors provides novel insight to the transcriptional control that mediates the patterning of reproductive organs surfaces and their associated separation processes in between cell layers.
Results
Co-silencing of the three SHINE clade genes results in severe morphological and surface phenotypes in floral organs
To circumvent the likely functional redundancy between the Arabidopsis SHN clade members we generated plants in which they were simultaneously silenced through an artificial microRNA approach (Figure S1A and Text S1). The presence of cleaved products and transcriptional downregulation of all three SHN genes was confirmed in the 35S:miR-SHN1/2/3 plants (Figure 1A and Figure S1B–S1C). No visual change was observed in these plants during vegetative growth and cuticle permeability of their rosette leaves was normal (Figure S1D–S1G). However, reproductive organs, particularly petals, were severely affected (Figure 1C–1D). This was evident already in buds that displayed postgenital fusions between petals and other floral organs at their tops (Figure 1H–1I). The expansion of petals and elongation of the carpels were restrained and they were curved and/or twisted (Figure 1I and Figure S1L–S1M). The changes in flower organ morphology also impinged on self-pollination and semi-sterility was occasionally detected (Figure 1B). Interestingly, mutant flower organs abscised earlier (Figure 1E and Figure S1J–S1K), and in some cases the abscised flower parts stayed attached to the top of the silique due to the postgenital organ fusion between them (Figure 1F–1G).
Fig. 1. Co-silencing of the three SHN clade genes impacts reproductive organ morphology and cell type–specific characteristics. 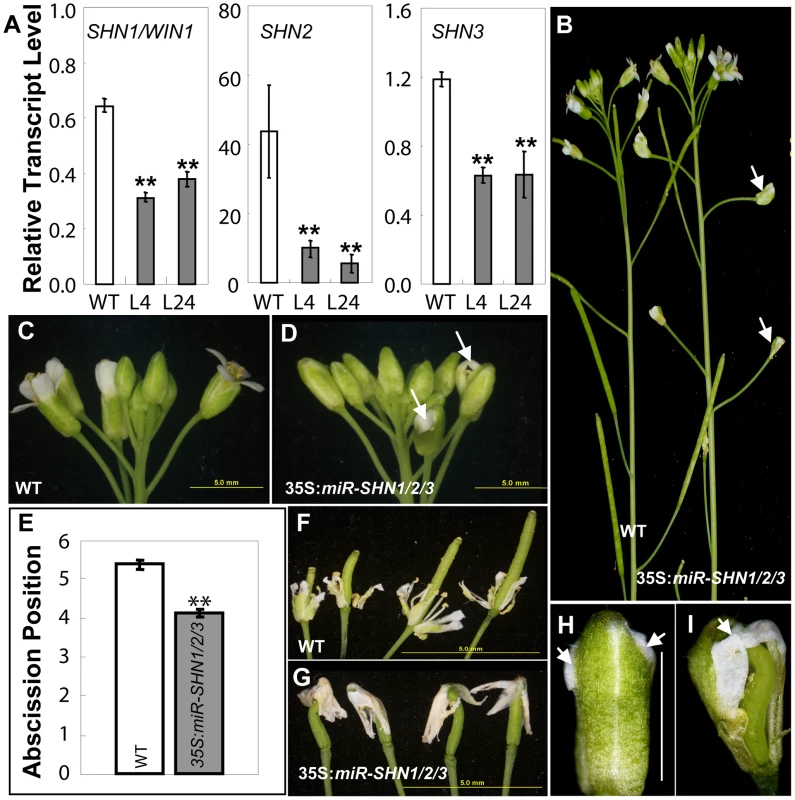
(A) Co-silencing of the three SHN genes as determined by real time RT-PCR in 35S:miR-SHN1/2/3 young buds (n = 3). (B) 35S:miR-SHN1/2/3 plants developed semi-sterile siliques (arrows). (C–D) 35S:miR-SHN1/2/3 inflorescences displayed abnormal buds (arrows). (E) 35S:miR-SHN1/2/3 floral organs (n = 50) abscised earlier than WT ones (n = 34). (F) Abscising WT flower organs. (G) Abscised 35S:miR-SHN1/2/3 flower organs remain attached to the siliques. (H–I) An unopened bud and a bud with sepals and petals removed of 35S:miR-SHN1/2/3 flowers, respectively. Arrows indicate organ fusion sites. In (A) and (E), means and standard errors are presented (**, p<0.01, Student's t-test). Microscopic observation of floral organs surfaces in the 35S:miR-SHN1/2/3 plants revealed extensive alterations to their archetypal epidermal cells (Figure 2 and Figure S2). Both abaxial and adaxial conical cells of petals appeared less elongated, more spherical and compact in addition to being separated with wider spaces as compared to the wild-type (WT) cells (Figure 2). Remarkably, nanoridges, typically displayed on WT petal epidermis [22]–[23], were either absent (adaxial) or significantly reduced (abaxial) in the 35S:miR-SHN1/2/3 petal cells (Figure 2A–2F). Altered epidermis cell size, shape and nanoridge decoration was also observed in surfaces of additional floral organs such as sepals, styles, filaments, nectaries, and pedicles (Figure S1N–S1Q and Figure S2). The observed phenotypes provided evidence that the SHN clade genes function redundantly in cell elongation, separation and nanoridge formation of reproductive organs. In contrast to the 35S:miR-SHN1/2/3 floral organs, silencing SHN1/WIN1 alone did not cause any visible morphological changes in floral organs, particularly in petal surfaces (Figure S3).
Fig. 2. Changes in petal morphology and surface characteristics as observed with electron microscopy. 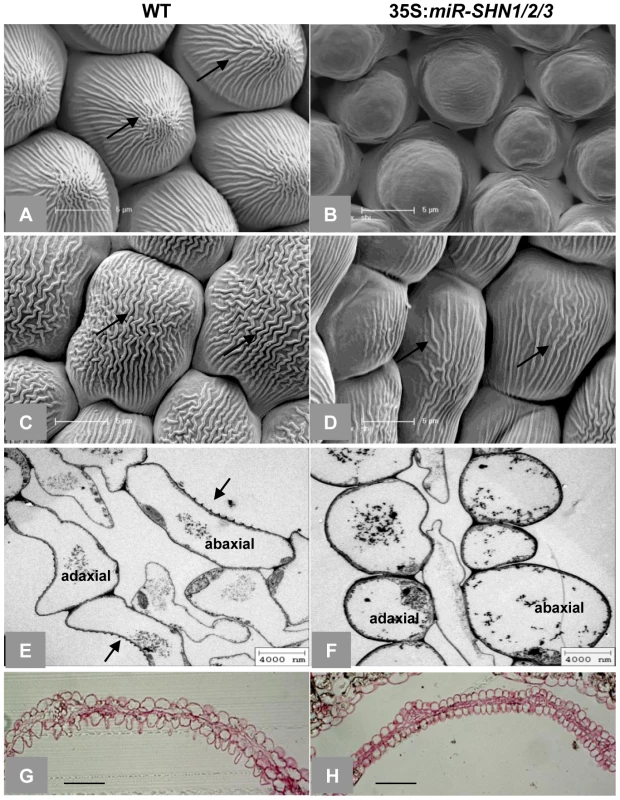
(A–D) SEM images of adaxial (A–B) and abaxial (C–D) petal epidermis, respectively, arrows indicate nanoridges. (E–F) TEM images of petal cross sections, arrows indicate nanoridges. (G–H) Light microscopy images of Rethinium Red stained petal cross sections. Scale bars: A–D: 5 µm; E–F: 4 µm; G–H: 50 µm. Cutin and cell wall–related genes are likely downstream targets of SHINE transcription factors
In order to unravel the molecular mechanism by which the SHN factors regulate the patterning of reproductive organ surfaces we compared the transcriptome of 35S:miR-SHN1/2/3 flower buds to the one of WT. A modest set of 38 differentially expressed genes was detected; 30 transcripts including SHN1 and SHN3 (SHN2 was not represented in the array) were downregulated while 8 others were upregulated in 35S:miR-SHN1/2/3 buds (Table 1).
Tab. 1. A list of genes that displayed up- or down-regulated expression in flower buds of the 35S:miR-SHN1/2/3 plants as compared with wild-type ones. 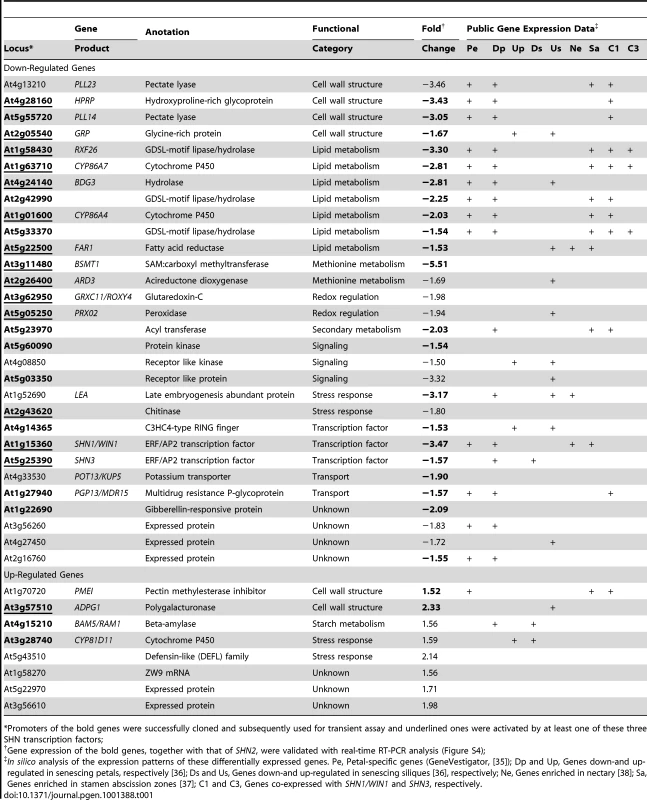
*Promoters of the bold genes were successfully cloned and subsequently used for transient assay and underlined ones were activated by at least one of these three SHN transcription factors; Interestingly, one of the two main functional categories that dominated the differential genes represented six cell wall related genes (Table 1). Four of them corresponded to enzymes associated with pectin degradation or modification, including two pectate lyases (PLL14 and PLL23), a polygalacturonase (ADPG1) and a pectin methylesterase inhibitor (PMEI). Two additional genes putatively encode cell wall structural proteins: a hydroxyproline-rich glycoprotein (HRGP) and a glycine-rich protein (GRP). The second major category consisted of seven genes that putatively encode cuticular lipids (mainly cutin) related proteins, including 2 cytochrome P450s (CYP86A4 and CYP86A7) implicated in flower cutin biosynthesis [18], [23], three GDSL-motif lipase/hydrolases (RXF26, At2g42990, and At5g33370) that are highly similar to the reported cutin related lipase At2g04570 [18], and one hydrolase (BODYGUARD 3, BDG3), the closest homolog of BDG1, an epidermis-specific extracellular protein associated with cuticle formation [24]. Fatty Acyl-CoA Reductase 1 (FAR1), the seventh gene was associated with primary fatty alcohol production [25]; its additional and/or alternative function with relation to surface lipids will be discussed below.
Two downregulated genes encoded a potassium transporter (KUP5) and an ABC transporter (PGP13/MDR15); both are involved in cell growth [26]–[28]. Additional three downregulated genes encoded kinase and/or kinase like proteins, that are potentially involved in reporting sensing aspects of cell wall structure and function [29]. Differential expression of 24 genes including the three SHN genes was subsequently validated using realtime RT-PCR assays (Figure S4 and Text S1). Altogether, gene expression analysis results indicated that the phenotype observed in 35S:miR-SHN1/2/3 reproductive organs probably result from the altered expression of their target genes, particularly those related to cutin and cell wall remodeling and function.
Silencing SHINE clade genes reduces flower cutin load and modifies petal cell wall structure
Because plant organ fusion and separation have been reported to be associated with cuticle [19]–[20], [22], we subsequently examined the changes in cuticular lipids in leaf and flower tissues of the 35S:miR-SHN1/2/3 plants. While the amount of leaf cutin was not significantly changed (Figure S5A), the amount of flower cutin in the 35S:miR-SHN1/2/3 plants was reduced to 48.4% of the wild-type (Figure 3A). The changes in flower cutin loads reflected the changes in the cuticle permeability in flower tissues (Figure S1F–S1I). The substantial decrease of dioic acids (DFA, particularly C16, C18 : 2 and C18 : 1), ω-hydroxy fatty acids (ω-HFA, particularly C16 and C18 : 3), 9/10,16-dihydroxy hexadecanoic acid (C16-9/10,16-DHFA) and 9(10)-hydroxy-hexadecanedioic acid (C16-9/10-HDFA) largely contributed to the reduced flower cutin in the 35S:miR-SHN1/2/3 plants. Levels of cuticular waxes in either leaves or flowers were not significantly altered in the 35S:miR-SHN1/2/3 lines (Figure S5B–S5C).
Fig. 3. Altered flower cutin monomers levels and petal cell wall structure in 35S:miRSHN1/2/3 plants. 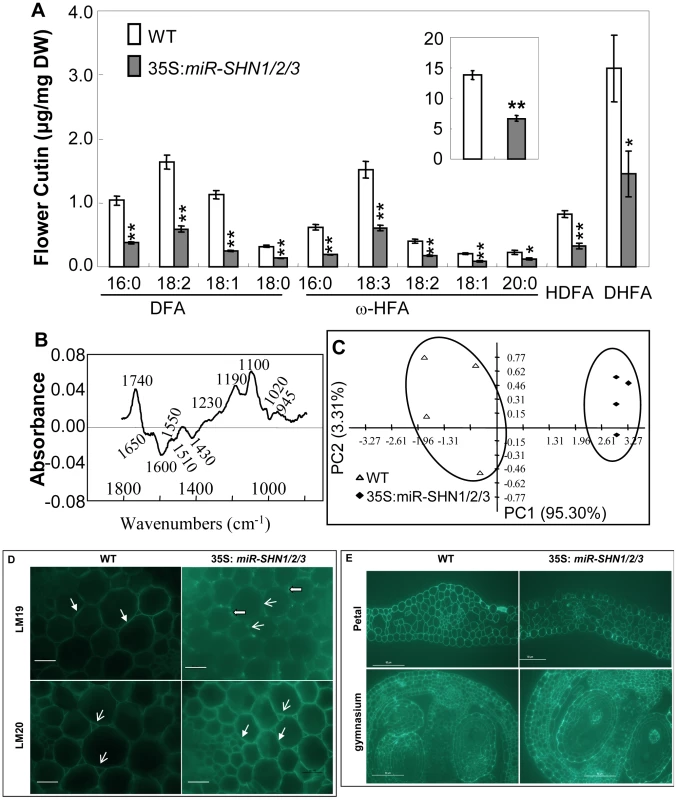
(A) Cutin monomer content in flowers of WT and 35S:miRSHN1/2/3 plants (total flower cutin in the insert). FA, fatty acids; DFA, α,ω-dicarboxylic FA; 2-HFA, 2-hydroxy FA; ω-HFA, ω-hydroxy FA; HDFA, C16-9/10-hydroxy DFA; DHFA, C16-9/10,16-HFA. Values are means and standard errors (n = 3; *, p<0.05; **, p<0.01, Student's t-test). (B–C) Comparison of 35S:miR-SHN1/2/3 and WT petal cell wall by FTIR analysis. In (B), difference spectra obtained by digital subtraction from the WT average spectrum of the 35S:miR-SHN1/2/3 average spectrum, peaks below and above the zero are enriched in the 35S:miR-SHN1/2/3 and WT, respectively. In (C), principle component analysis (PCA) displaying the separation of WT and 35S:miR-SHN1/2/3 FTIR spectra. (D–E) Indirect immunofluoresence detection of the localization of monoantibodies LM19 and LM20 to cell walls of transverse sections of WT and 35S:miR-SHN1/2/3. D. Hand section of inflorescence stems. The fluorescence of LM19, localizing pectin to the middle lamella, became weaker but aggregated along the middle lamella in the 35S:miR-SHN1/2/3 line; the fluorescence of LM20, localizing pectin to the air spaces, became stronger not only in the air spaces but also in the middle lamella in the 35S:miR-SHN1/2/3 line. E. Microtome section of petals and the gynoecium. The fluorescence of LM20 became stronger in petals and the developing seed coats in the 35S:miR-SHN1/2/3 line. Head-filled arrows indicate meddle lamella, arrows point to air spaces, and block arrows designate aggregations. The finding that co-silencing the three SHN genes affected the expression of pectin modifying genes prompted us to analyze the cell wall pectin composition in the seed mucilage and buds. GC-MS analysis did not reveal any significant compositional changes in seed mucilage and the bud cell wall pectic monosaccharides (Figure S5D–S5E and Text S1). We next used Fourier transform infrared (FTIR) spectroscopy to examine if petals of the 35S:miR-SHN1/2/3 plants exhibited structural changes in their cell walls. Principal component analysis (PCA) showed a clear separation of the petal FTIR spectra between 35S:miR-SHN1/2/3 petals and WT ones (Figure 3C). The difference spectrum (Figure 3B) generated by digitally subtracting the average 35S:miR-SHN1/2/3 spectrum from the average WT petals spectrum showed that WT petal cell wall had more acyl esters (1740 cm−1) [30]–[31], amide III proteins (1230 cm−1) [32], and non-cellulosic carbohydrates (1100 to 900 cm−1) [33]. In contrast, 35S:miR-SHN1/2/3 petal cell walls contained more salt-form of pectin (1430 and 1600 cm−1, respectively) [32], amide I and amide II proteins (1650 and 1550 cm−1, respectively) [32]–[33], and phenolic esters or aromatic lignins (1635 and 1510 cm−1) [32]–[33].
To localize the pectic polysaccharides in the cell walls, two novel rat monoclonal antibodies LM19 and LM20, which recognize pectic homogalacturonan (HG) epitopes [34], were used to hybridize transverse sections of inflorescence stems (pith parenchyma) and flowers. Similar to an earlier observation in tobacco plants [34], LM19 localized pectin to junctures (middle lamella) while LM20 localized pectin to the intercellular spaces (air spaces) in both WT and 35S:miR-SHN1/2/3 inflorescence stems (both antibodies appeared as green fluorescence) (Figure 3D). However, the florescence of LM19 in transverse sections of the 35S:miR-SHN1/2/3 samples became weaker and they were aggregated along the middle lamella line. Moreover, the florescence of LM20 in 35S:miR-SHN1/2/3 was enhanced not only in the air spaces but also in the middle lamella. In addition, the florescence of LM20 binding to air spaces become stronger in microtome sections of 35S:miR-SHN1/2/3 petals and developing seed coats, as compared to WT ones (Figure 3E). Because the binding of both LM19 and LM20 to pectin is sensitive to pectate lyase treatment and they bind preferably to HG [35], these results indicated alteration to HG distribution in the mutants. Therefore, silencing the SHN clade genes not only affected the cutin matrix of the cuticle but also the cell wall matrix of the cell.
Characterization of the putative SHINE transcription factors target genes
Remarkably, in silico analysis (Table 1) showed that as SHN1/WIN1, 13 of the differentially expressed genes (12 downregulated and one up regulated in the 35S:miR-SHN1/2/3 plants) display a petal-specific expression pattern [35]. Moreover, all those 12 petal-specific downregulated genes, together with SHN1/WIN1, SHN3, and 3 more genes display decreased expression in senescing petals [36]. Furthermore, 9 of the differential genes in addition to SHN1/WIN1 are expressed in the stamen abscission zone (AZ) [37] while 2 genes and SHN1/WIN1 are enriched in the nectary [38], and 13 genes and SHN3 are differentially expressed in senescing siliques [36]. These results provided evidence that both the SHN factors and their putative targets are associated with reproductive organ development (i.e. petals and siliques) and possibly cell separation as well. The series of genes altered in the 35S:miR-SHN1/2/3 plants were also strongly co-expressed with the SHN factors (Figure S6 and Table S2), further indicating the functional link between the groups of genes we have identified in the array analysis.
In order to examine whether loss of function of the putative SHN clade proteins target genes results in alteration to petal surface we screened for T-DNA insertions in the entire set of 28 downregulated genes. Homozygous knockout lines could be identified for thirteen of them and their petals surface was examined using scanning electron microscopy (Figure S7). Petals of the At5g23970 (a putative acyltransferase) and At5g33370 (a putative GDSL-lipase) knockout plants exhibited collapsed conical cells, while those of At4g24140 (bodyguard3/bdg3), At5g03350 (a receptor like protein) and At1g01600 (cyp86a4) displayed abnormal abaxial nanoridges (Figure 4A–4D).
Fig. 4. Putative downstream target genes of SHN transcription factors in patterning floral organs. 
(A–D) SEM images of defective petal epidermis observed in knock-out mutants of four putative SHN transcription factors target genes: A and B, Adaxial epidermis of knock out lines of At5g23970 (a BAHD family member acyltransferase) and At5g33370 (a GDSL-motif lipase/hydrolase), respectively; (C) and (D), Abaxial epidermis of bdg3 (At4g24140; a hydrolase) and rlp (At5g03350; a legume lectin protein), respectively. See images of WT petal epidermis in Figure 2 and Figure S7. (E–F) Phenotypes observed in 35S:miR-CYP86A4/A7 (cutin-related cytochrome P450s) transgenic plants: (E) A bud showing the fusion between petals and sepals and (F) Collapsed adaxial petal epidermis cells. (G–H) Phenotypes observed in 35S:miR-At5g33370/At3g04290 (GDSL-motif lipase/hydrolases) transgenic plants: (G) A flower showing fused and folded petals and (H) Abnormal adaxial petal epidermis cells. Some differential genes identified in microarray analysis belong to large multi-gene families as for example lipases and cytochrome P450s. This suggested that they might be functionally redundant with other family members. We therefore co-silenced the CYP86A4 with CYP86A7, and the GDSL-lipase At5g33370 with its closest homolog At3g04290, LTL1 [39], via the artificial microRNA method. Plants co-silenced for either one of these pairs of genes displayed severe floral organ fusion and alteration in the conical cell shape and/or epidermis cell decoration (Figure 4E–4H). These results from single knockouts and the co-silenced lines provided additional evidence for the functional link between the putative SHN proteins target genes and the patterning of the petal surface.
SHINE proteins activate promoters of their putative target genes
We subsequently examined the activation of promoters of genes that were differentially expressed in the 35S:miR-SHN1/2/3 plants by the SHN transcription factors using a dual luciferase assay system [40]. Promoter regions of 23 putative targets and the 3 SHN clade genes were examined. Thirteen out of 23 were significantly activated by at least one of the three SHN transcription factors (Figure 5). Promoter regions of seven genes were activated by all three factors including the ones of RXF26, CYP86A4, CYP86A7, BDG3, FAR1, GRP, and GRXC11. The promoters of PRX02 (a peroxidase), ARD3 (an acireductone dioxygenase), and At2g43620 (a chitinase) were only activated by SHN1/WIN1, SHN2, and SHN3, respectively. Interestingly, SHN1/WIN1 and SHN2 were able to activate each other's promoter, while SHN3 was able to activate all three SHN genes promoters. We included LACS2 promoter as a positive control [18], however, activation of this gene promoter by the SHN transcription factors was not detected in our assay. These results further confirmed the functional redundancy of SHN transcription factors in cuticle and cell wall metabolism by acting directly on common targets and by regulating each other and possibly their own transcription.
Fig. 5. Transient expression assays of SHN transcription factors putative target gene promoter regions. 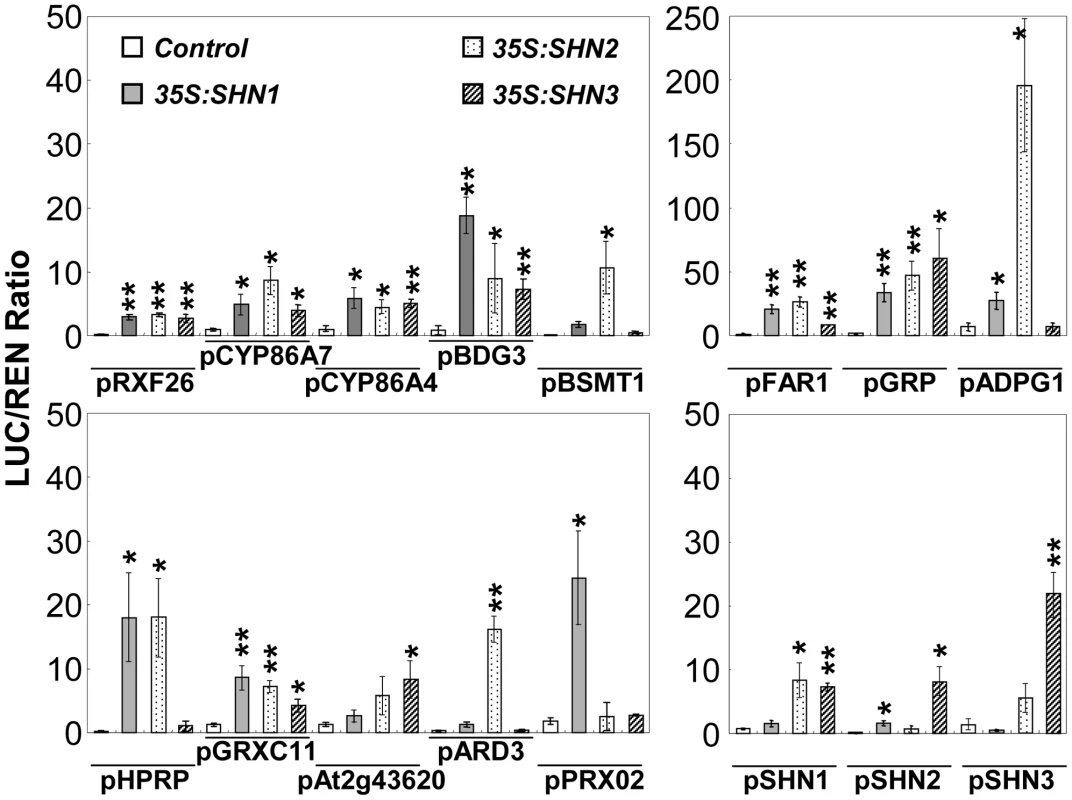
Those promoter regions were co-infiltrated with plasmids containing SHN transcription factors fused to the 35S promoter. Promoters of SHN genes were also included. LUC/REN (firefly luciferase/renilla Luciferase) values represent means and standard errors (n = 4; *, p<0.05; **, p<0.01, Student's t-test). GA modulates the expression of SHINE clade genes
Gibberellins (GAs) are a class of plant hormones involved in the regulation of flower development in Arabidopsis. GA promotes the expression of floral homeotic genes APETALA3 (AP3), PISTILLATA (PI), and AGAMOUS (AG) by antagonizing the effects of DELLA proteins, thereby allowing continued flower development [41]. Publically available array data suggested that GA promotes the expression of SHN1/WIN1 while DELLA suppresses SHN1/WIN1 expression, which was examined in the ga1-3 and the ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1 (i.e. penta) [35]. Remarkably, in young flower buds, GA promotes the expression of thirteen of the putative SHN target genes identified in this study while it down regulates the expression of another four putative target genes, all of them in a DELLA dependent manner ([42], Figure S8A–S8B). In addition, GA regulates another two putative SHN target genes, AT4G27450 and AT1G27940, in a DELLA-independent way [42]. The results described above led us to suggest that GA might be involved in cuticle assembly during flower organ development via modulating the expression (directly or indirectly) of the SHN transcription factors and their downstream target genes.
To test this assumption, we examined the expression of SHN genes in different GA biosynthesis or signaling mutants (Figure 6A). Quantitative RT-PCR analysis showed that expression of SHN1/WIN1 is downregulated in the ga1-3 mutant that is defective in GA biosynthesis. It also showed that DELLA significantly suppressed SHN1/WIN1 expression, since the expression of SHN1/WIN1 in the double (rga-t2 rgl2-1; partial loss of DELLA signaling) and quadruple DELLA (gai-t6 rga-t2 rgl1-1 rgl2-1) mutants in the ga1-3 background was recovered to equal and even much higher levels than that of the wild type, respectively. Knockout of SPY4, another repressor of GA signaling, also enhanced SHN1/WIN1 expression as compared to the wild type. As compared to SHN1/WIN1, SHN2 showed the opposite expression pattern in the background of the various GA biosynthesis and signaling mutants. Expression of SHN2 was upregulated in the ga1-3 background while it was significantly downregulated in the penta and spy4 mutant backgrounds. Interestingly, neither GA biosynthesis nor the signaling mutants significantly altered SHN3 expression.
Fig. 6. Giberellin (GA) biosynthesis and signaling affects the expression of SHN clade genes in flower buds. 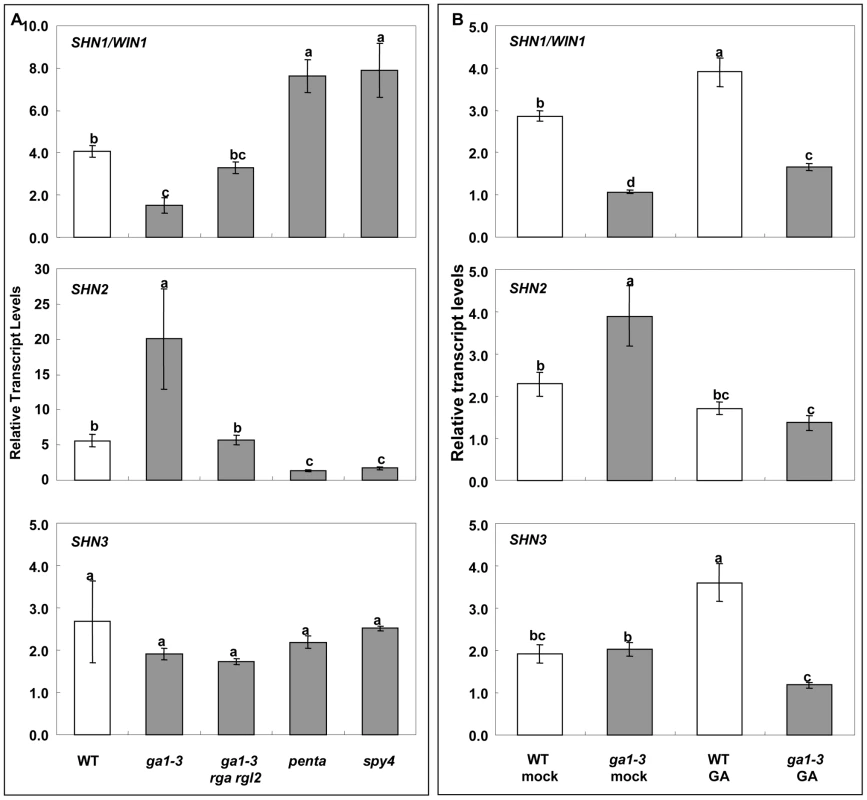
(A) Expression of SHN clade genes in GA biosynthesis and signaling mutants; (B) Expression of SHN clade genes as affected by the application of 100 µM exogenous GA. Values represent means and standard errors (n = 3). Different letters between different columns indicate the level of significance (p<0.05) obtained with a Student's t test. ga1-3 (GA biosynthesis mutant); rga rgl2, double DELLA mutant (RGA, repressor of ga1-3; RGL2, RGA like 2) in the ga1-3 background; penta, gai-t6 rga-t2 rgl1-1 rgl2-1 in the ga1-3 background; spy4 (spindly4) (repressor of GA signaling). We also examined the expression of SHN clade genes in both the WT and ga1-3 flower buds in response to exogenous GA application (Figure 6B). Quantitative RT-PCR analysis showed that GA application to the ga1-3 mutant increased the levels of SHN1/WIN1 and decreased the levels of SHN2 expression as compared to ga1-3 alone, as does the endogenous GA (Figure 6A). The response of SHN3 might be different between endogenous and externally applied GA as its expression did not change significantly in the ga1-3 background alone while it was altered upon GA supplementation in either the WT or ga1-3 (Figure 6B).
Finally, we also carried out GC-MS analysis of the flower cuticular lipids of the GA biosynthesis and signaling mutants. While flower waxes were not significantly altered in the ga1-3 and penta mutant flowers, the total cutin load, particularly of the 9/10,16-dihydroxy hexadecanoic acid (C16-9/10,16-DHFA), the predominant monomer of the Arabidopsis flower cutin, was significantly different between WT and ga1-3 and between ga1-3 and the penta mutant (Figure S9). Nevertheless, SEM observation did not reveal any significant changes in the petal surface of the open flowers in the mutant plants (Figure S9). Since we applied exogenous GA to ga1-3 plants to induce flowering [43] prior to the SEM observation, this might explain the absence of a surface phenotype in mutant petal surface. All together, these results suggest that SHN transcription factors might play a key role in the GA-mediated flower organ development regulatory network.
Discussion
Aerial plant organs display tremendous variation in their surface topography and composition of the cuticular layer covering their outer epidermis. This diversity in the exterior layer is essential for both organogenesis and the interaction with the environment. In flowers for instance, the typical surface of organs is vital for their function as it ensures their proper development by preventing postgenital fusions while at the same time mediating the interaction with insect pollinators [44]–[45]. Whereas many molecular components of pathways determining flower organ initiation and identity have been characterized to date [46], our knowledge regarding formation and function of their outer surface, namely the cuticle, is limited. Here, in-depth analysis of Arabidopsis plants in which the three SHN transcription factors were co-silenced revealed that these regulators play a prominent role in patterning floral organ surface by controlling metabolism of cuticular lipids and possibly the associated cell wall components.
SHINE transcription factors act redundantly to ensure proper floral organ morphology and surface formation
The lack of any visual phenotype in floral organs of SHN1/WIN1 silenced plants ([18]; Figure S3), pointed to functional redundancy among the 3 SHN clade members. Even though expression of either one of the three SHN genes was not entirely reduced, the use of an artificial microRNA targeting the entire clade was sufficient to obtain several, striking, visual phenotypes that matched the previously described SHN genes expression patterns [16]. Floral organs were affected, likely as a result of altered cuticle composition, structure and consequently permeability. However, cuticle alteration might not be the only explanation to the defects observed in organ formation since they might also be a result of SHN genes effect on the process of epidermal cell differentiation and development. This was evidenced in the altered epidermal cells size and shape in petals and sepals of the 35S:miR-SHN1/2/3 plants. These strong epidermis phenotypes (in pavement cells, trichomes and stomata) observed previously in plants overexpressing either one of the three SHN genes support this proposal [16].
Down regulation of the SHN clade genes had an additional effect on floral organs as SEM and transmission electron microscopy (TEM) revealed changes in nanoridges that typically decorate surfaces of flower organs [44]. Formation of nanoridges in Arabidopsis flowers was recently associated with cutin, particularly with C16-9/10,16-DHFA, the major monomer of Arabidopsis petal cutin [22]–[23], that was also dramatically reduced in the 35S:miR-SHN1/2/3 plants. However, the absence of nanoridges on the surface of tomato fruit that also contains C16-9/10,16-DHFA as a major monomer, suggests additional factors including polymer structure and distribution that mediate nanoridge formation [23], [47].
SHINE transcription factors mediate floral organ adhesion and separation
Earlier work using promoter-reporter assays suggested that SHN transcription factors act not only in the interface between the plant and its environment but also at the interface between cells and cell layers [16]. Of particular interest was SHN2 that showed strict expression in the anther and silique dehiscence zones upon organ maturation. The proposed role of SHN transcription factors in the adhesion of cell layers was strongly corroborated by the recent finding that an SHN-like gene in barley (Nud) mediates the contact of the caryopsis surface to the inner side of the hull by forming a specialized lipid layer [19]. In this study we detected earlier abscission of floral organs in the silenced lines which corresponded well with SHN genes expression in the base of sepals, petals, stamens and siliques in the abscission region. Organ separation events including pod shatter, seed detachment from the maternal plant, pollen separation after meiosis, anther dehiscence and floral organ abscission, are thought to be associated with alterations to properties of the cell wall matrix, mainly pectins and wall proteins [1], [48]–[49]. The pectin degradation activity of polygalacturonases (PGs) has been linked with all separation events described above. Recently, three Arabidopsis PGs have been associated with cell separation during reproductive development [50]. One of these, ADPG1, displayed altered expression in the 35S:miR-SHN1/2/3 plants and its promoter was shown here to be activated by SHN1/WIN1 and SHN2. Thus, SHN action on organ adhesion/separation possibly combines modification to cuticular lipids (i.e. cutin) as well as pectins of the cell wall.
Cutin, cell wall, and possibly suberin-associated genes are downstream targets of SHINE transcription factors
Array analysis revealed a concise set of genes that are putative downstream targets of the SHN transcription factors in flower buds, only two out of them (CYP86A4 and CYP86A7) overlapped with the previously reported group of 11 SHN1/WIN1 putative targets [18]. This could be explained by the fact that while Kannagara et al. (2007) detected genes that were upregulated following induction of SHN1/WIN1 in fully expanded leaves [18], we examined flower buds in which the SHN clade genes were co-silenced. Thus, genes from these two experiments most likely represent downstream targets in either leaves or flowers or both tissues. Together, these studies also demonstrated that wax load changes in the SHN overexpression lines were probably an indirect effect.
SHN transcription factors emerge as regulators of genes derived from four prominent families associated with the cuticle including two cytochrome P450s of the CYP86A clade (CYP86A4 and CYP86A7), BDG3, encoding one of the five BDG1-like proteins [24], three genes of the large family of GDSL-motif lipase/hydrolases [39] and one of the eight-member clade of fatty acyl-CoA reductases [25]. Apart from the latter, these genes or their family members have been reported to be involved in either cutin biosynthesis or polymer assembly in the extracellular matrix in plant reproductive organs [10], [23], [51]–[53]. FAR1 has been recently associated with formation of suberin, a polymer that is structurally related to cutin and is often deposited following cell to cell separation in aerial organs to form a protection layer that will shield against penetration of pathogens and dehiscence [25], [54]. Below ground, endodermal suberin is thought to regulate the apoplastic movement of water and solutes into the stele [55]–[56]. The SHN3 expression in roots ([16], Figure S10) and the endodermal expression of FAR1, BDG3, CYP86A4 and At1g16760 (Figure S10) suggested that the latter 4 genes are targets of SHN transcription factors both above and below ground. Hence, SHN transcription factors and their targets are not only involved in cutin assembly in reproductive organs but are likely to play a role in root suberin deposition. CYP86A4 was suggested to provide ω-hydroxylation activity that is complementary to CYP86A1 in the biosynthesis of suberin [57] and FAR1 was recently reported to be associated with generating primary fatty alcohols for suberin deposition [25]. However, the role of BDG3 and At1g16760 in root suberin remains to be determined.
SHINE transcription factors and the GA-mediated flower development network
Previous reports regarding the SHN clade members highlighted their role in regulating the biosynthesis of cuticular lipids for surface formation [16]–[18]. However, the results of the present study imply that activity of these factors goes beyond regulating a single metabolic pathway (i.e. cutin) for cuticle formation and they take part in the genetic program that mediates floral organ morphogenesis, more specifically in determining organ size and shape as well as the formation of specialized epidermis cell types (e.g. the petal conical cells). Related to this, gene expression changes detected in the 35S:miR:SHN1/2/3 flower buds strikingly resemble the ones implicated in the formation of the single epidermis cotton fiber cell during its elongation. These include altered expression of genes associated with cell wall loosening through modification of pectin [58], genes associated with the build-up of a higher turgor by increased accumulation of the major osmoticum such as soluble sugars, K+, and malate [27], redox-related genes [59]–[60], genes related to phytohormone biosynthesis and signaling cascades [61].
Flowering in Arabidopsis consists of three distinct phases: floral initiation, floral organ initiation and floral organ growth. Earlier studies on GA signaling revealed that GA promotes Arabidopsis petal, stamen, and anther development by opposing the function of the DELLA proteins [62] and that GA signaling is not required for floral organ specification but essential for the normal growth and development of these organs [63]. Different combinations of DELLA proteins are key to floral organ development (RGA, RGL1, RGL2), because individual DELLA proteins have different temporal and spatial expression patterns [62]. The unique temporal and spatial expression patterns of SHN clade genes in the flower tissues [16] and their distinct expression patterns in response to the alteration of the GA signaling reported here suggest that SHNs might be part of GA floral regulatory networks. In this context, GA might act as a positive regulator of SHN1/WIN1 in the regulation of floral organs development (i.e. elongation of petal, stamen, and anther) [37], [62] in the early stages of flower development. In addition, GA emerges as a negative regulator of SHN2 in modulating the cell separation processes related to silique and anther dehiscence, floral organ abscission in the later stages of flower development. Hence, GA might be involved in cuticle assembly during the expansion of petals and other floral organs. The growth and elongation of organs requires the interaction between the outer and inner cell layers, which is coordinated by hormonal signals [4]–[5]. GA has been shown to promote cutin synthesis during other growth related processes including the rapidly growing internodes of deep-water rice [64], in extending stems of peas [65], and in developing tomato fruit [66]. Similarly, in this study, GA application resulted in a significant increase in the cutin load of ga1-3 mutant flowers. Future studies positioning the SHN proteins in the wide genetic network that controls flower development will shed light on how cuticle and cell wall metabolism is coordinated with the processes of flowering and fertility.
Materials and Methods
Plant material
All Arabidopsis plants used in miR-SHN1/2/3 experiment were in the Col-0 genetic background, while those used for DELLA or GA experiment were in Ler genetic background. Plants were grown on a soil mixture in a growth room at 20°C, 70% relative humidity, a 16/8-h light/dark cycle at a fluorescent light intensity of 100 µmol m−2s−1. All knock out lines were bought from either ABRC or NASC, while GA biosynthesis and signaling mutant were kind gifts from Hao Yu (National University of Singapore, Singapore) and David Weiss (The Hebrew University, Israel). Exogenous GA application was carried out as described [67] with minor modifications. 100 mM GA3 or ethanol containing water was fine sprayed daily for 6 days on 6-week-old plants, and the buds were collected for analysis.
Generation of transgenic plants
For the 35S:miR-SHN1/2/3 construct, the designed artificial miR-SHN1/2/3 sequence was directly synthesized from BIO S&T (Bio S&T Inc., Montreal, Canada). After being sequenced, it was put into pART7 vector, and finally subcloned to pART27. Transformation to Agrobacterium tumefaciens strain GV3101 was done via electroporation and planta transformation was done via floral dipping as described [68]. Promoter sequences of the putative SHN target genes (approximately 2 kb upstream of the start codon) were cloned from WT genomic DNA, and coding sequences of the three members of SHN clade were cloned from WT flower cDNA, using yellow Taq DNA polymerase (Roboklon Gmbh, Berlin, Germany) with corresponding gene specific primer pairs (Table S1). Those promoters and TFs were cloned into pGreen II 0800-LUC vector and pBIN plus vector, respectively, and then transformed to Agrobacterium tumefaciens strain GV3101. All DNA sequence cloned were examined by direct sequencing.
Histological observations
Toluidine blue examination of cuticle permeability was performed as previously described [69]. For Rethinium red staining, the inflorescences of 7-week-old plants were fixed and embedded in LR White resin (London Resin Co., Basingstoke, UK) as described previously [70]. Sections were cut to a thickness of 0.5–1 mm using a diamond knife on an Ultracut microtome (Leica) and sections were collected on glass slides. The slides were stained with 0.1% Rethinium red for 5 min and washed with double distilled water, and then observed with Nikon ECLIPSE E800 microscope.
Electron microscopy
All electron microscopy works were done as previously described [22]. For scanning electron microscopy (SEM), flowers from 7-week-old plants were collected, fixed with glutaraldehyde using standard SEM protocol [71], dried using critical point drying (CPD), mounted on aluminum stubs and sputter-coated with gold. SEM was performed using an XL30 ESEM FEG microscope (FEI) at 5–10 kV. For TEM, flowers from 7-week-old plants were collected and processed using a standard protocol [72]. The Epon-embedded samples were sectioned (70 nm) using an ultramicrotome (Leica) and observed with a Technai T12 transmission electron microscope (FEI).
RNA extraction and microarray analysis
Total RNA was extracted from closed buds from 7-weeks-old WT and homozygous 35S:miRSHN1/2/3 T3 plants using RNeasy Plant Mini Kit (Qiagen) with an on column DNAse treatment. The subsequent microarray analysis and qRT-PCR analysis were performed as described previously [21]. For microarray analysis, the double-stranded cDNA was purified and served as a template in the subsequent in-vitro transcription reaction for complementary RNA (cRNA) amplification and biotin labeling. The biotinylated cRNA was cleaned, fragmented and hybridized to Affymetrix ATH1 Genome Array chips. Statistical analysis of microarray data was performed using the Partek® Genomics Suite (Partek Inc., St. Louis, Missouri) software. CEL files (containing raw expression measurements) were imported to Partek GS. The data was preprocessed and normalized using the RMA (Robust Multichip Average) algorithm [73]. The normalized data was processed by PCA (Principal Component Analysis) and hierarchical clustering to detect batch or other random effects. To identify differentially expressed genes one-way ANOVA analysis of variance was applied. Gene lists were created by filtering the genes based on: fold change, p<0.01, and signal above background in at least one microarray. Up-regulated genes were defined as those having a greater than or at least 1.5-fold linear intensity ratio while down-regulated genes were defined as those having a less than or at most −1.5-fold linear intensity ratio. The experiment was performed in duplicate, preparing two independent biological replicates from 5–6 plants each.
Wax and cutin analysis
Waxes were extracted and analyzed as described [22]. For cutin analysis, soluble lipids were extracted from leaf and closed buds by dipping them in 10 ml of a methanol/chloroform (1∶1, v/v) mixture for 14 days (solvent changed daily). The tissues were dried, weighed (about 10–20 mg) and kept in N2 till analysis. The cutin was depolymerized and analyzed as described previously [22], [54].
Fourier transform infrared (FTIR) spectroscopy
Petals from 7-week-old flowers were collected (60 petals each sample, n = 8), cleared with chloroform and methanol (1∶1), and then air-dried overnight [74]. Samples were ground with solid crystalline KBr to fine powder and pressed to 1-mm tablelets. FTIR spectra were acquired in the absorbance mode at a resolution of 4 cm−1 with 32 co-added scans at wave number range 4000 to 250 cm−1 using a NICOLE1 380 FITR Spectrometer (Thermo Electron Corporation). Each spectrum was baseline corrected and spectral area normalized prior to generating average spectra and digital subtraction spectra. Primary component analysis was performed using Multiple Experiment Viewer.
Transverse section preparation and immunocytochemistry
Inflorescence stems transverse sections were prepared according to Willats et al [75]. Regions (0.5 cm long) of 7-week-old Arabidopsis stem (3th internodes from the bottom) were excised and sectioned by hand to a thickness of ∼100–300 µm. Sections were immediately placed in fixative consisting of 4% paraformaldehyde in 50 mM PIPES, 5 mM MgSO4, and 5 mM EGTA. Following 30 min of fixation, sections were washed in the PIPES buffer, and then in 1× PBS buffer. Petals and gynoecium transverse section were prepared as described [65] and In vitro immunocytochemistry was carried out as described by Verhertbruggen et al [34]. Sections were incubated for 1.5 h in 5-fold dilution of two new rat monoclonal antibody hybridoma supernatant (LM19 and LM20) diluted in 5% Milk/PBS, respectively. After being washed by gently rocking in PBS at least three times, sections were incubated with a 100-fold dilution of anti-rat IgG (whole molecule) linked to fluorescein isothiocyanate (FITC) in 5% Milk/PBS for 1.5 h in darkness. After washing in PBS for at least 3 times, sections were mounted in a glycerol∶PBS (vol∶vol, 1∶1) solution. Immunofluorescence was observed with Nikon ECLIPSE E800 microscope equipped with epifluorescence irradiation and DIC optics. Images were captured with a camera and NIS-Elements BR30 software.
Dual luciferase assay
Transient assay was carried out as described [40] with the exception that 150 µg/ml instead of acetosyringone was included in the infiltration media [76]. Luminescence was measured using Modulus Microplate Luminometer (Turner Biosystems, Sunnyvale, CA) by mixing 20 µl sample extract with 80 µl Luciferase assay reagent or Renillase assay reagent, respectively, and the data was collected as ratio. Background controls were run with only the transcription factor, promoter-LUC, and pBIN Plus empty vector, and pBIN Plus empty vector with promoter-LUC in the preliminary assay, and pBIN Plus empty vector with promoter-LUC was chosen later for background control in all experiments due to its relatively higher induction of Luciferase activity than other plasmid tested.
Supporting Information
Zdroje
1. TaizL
ZeigerE
2002 Plant Physiology Sunderland, MA Sinauer Associates, Inc 672
2. KolattukudyPE
2001 Polyesters in higher plants. Adv Biochem Eng Biotechnol 71 1 49
3. JeffreeCE
1996 Structure and ontogeny of plant cuticle Oxford BIOS Scientific Publishers 33 83
4. KutscheraU
NiklasKJ
2007 The epidermal-growth-control theory of stem elongation: An old and a new perspective. J Plant Physiol 164 1395 1409
5. Savaldi-GoldsteinS
PetoC
ChoryJ
2007 The epidermis both drives and restricts plant shoot growth. Nature 446 199 202
6. BachL
FaureJD
2010 Role of very-long-chain fatty acids in plant development, when chain length does matter. Comptes Rendus Biologies 333 361 370
7. Reina-PintoJJ
YephremovA
2009 Surface lipids and plant defenses. Plant Physiol Biochem 47 540 549
8. NawrathC
2002 The biopolymer cutin and suberin.
SomervilleCR
MyerowitzEM
The Arabidopsis Book Rockville American Society of Plant Biologists
9. SuhMC
SamuelsAL
JetterR
KunstL
PollardM
2005 Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol 139 1649 1665
10. PollardM
BeissonF
LiYH
OhlroggeJB
2008 Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13 236 246
11. SamuelsL
KunstL
JetterR
2008 Sealing plant surfaces: Cuticular wax formation by epidermal cells. Ann Rev Plant Biol 59 683 707
12. SandhuAP
RandhawaGS
DhuggaKS
2009 Plant cell wall matrix polysaccharide biosynthesis. Mol Plant 2 840 850
13. AndersonCM
WagnerTA
PerretM
HeZH
HeDZ
2001 WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Mol Biol 47 197 206
14. CaffallKH
MohnenD
2009 The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohyd Res 344 1879 1900
15. BrounP
2004 Transcription factors as tools for metabolic engineering in plants. Curr Opin Plant Biol 7 202 209
16. AharoniA
DixitS
JetterR
ThoenesE
van ArkelG
2004 The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16 2463 2480
17. BrounP
PoindexterP
OsborneE
JiangCZ
RiechmannJL
2004 WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci USA 101 4706 4711
18. KannangaraR
BraniganC
LiuY
PenfieldT
RaoV
2007 The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. Plant Cell 19 1278 1294
19. TaketaS
AmanoS
TsujinoY
SatoT
SaishoD
2008 Barley grain with adhering hulls is controlled by an ERF family transcription factor gene regulating a lipid biosynthesis pathway. Proc Natl Acad Sci USA 105 4062 4067
20. LolleSJ
HsuW
PruittRE
1998 Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics 149 607 619
21. PanikashviliD
ShiJX
BocobzaS
FrankeRB
SchreiberL
2010 The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol Plant 3 563 575
22. PanikashviliD
ShiJX
SchreiberL
AharoniA
2009 The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol 151 1773 1789
23. Li-BeissonY
PollardM
SauveplaneV
PinotF
OhlroggeJ
2009 Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc Natl Acad Sci USA
24. KurdyukovS
FaustA
NawrathC
BarS
VoisinD
2006 The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18 321 339
25. DomergueF
VishwanathSJ
JoubesJ
OnoJ
LeeJA
Three Arabidopsis fatty acyl-CoA reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol 153 1539 1554
26. AhnSJ
ShinR
SchachtmanDP
2004 Expression of KT/KUP genes in arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 134 1135 1145
27. RuanYL
LlewellynDJ
FurbankRT
2001 The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell 13 47 60
28. GeislerM
MurphyAS
2006 The ABC of auxin transport: The role of p-glycoproteins in plant development. FEBS Lett 580 1094 1102
29. SeifertGJ
BlaukopfC
2010 Irritable walls: The plant extracellular matrix and signaling. Plant Physiol 153 467 478
30. GouJY
ParkS
YuXH
MillerLM
LiuCJ
2008 Compositional characterization and imaging of “wall-bound” acylesters of Populus trichocarpa reveal differential accumulation of acyl molecules in normal and reactive woods. Planta 229 15 24
31. FellahA
AnjukandiP
WaterlandMR
WilliamsMAK
2009 Determining the degree of methylesterification of pectin by ATR/FT-IR: Methodology optimisation and comparison with theoretical calculations. Carbohyd Polym 78 847 853
32. SeneC
McCannMC
WilsonRH
GrinterR
1994 Fourier-transform raman and fourier-transform infrared spectroscopy (an investigation of five higher plant cell walls and their components). Plant Physiol 106 1623 1631
33. YuP
2005 Molecular chemistry imaging to reveal structural features of various plant feed tissues. J Struct Biol 150 81 89
34. VerhertbruggenY
MarcusSE
HaegerA
Ordaz-OrtizJJ
KnoxJP
2009 An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohyd Res 344 1858 1862
35. ZimmermannP
Hirsch-HoffmannM
HennigL
GruissemW
2004 GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621 2632
36. WagstaffC
YangTJW
SteadAD
Buchanan-WollastonV
RobertsJA
2009 A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J 57 690 705
37. CaiSQ
LashbrookCC
2008 Stamen abscission zone transcriptome profiling reveals new candidates for abscission control: Enhanced retention of floral organs in Transgenic plants overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiology 146 1305 1321
38. KramBW
XuWW
CarterCJ
2009 Uncovering the Arabidopsis thaliana nectary transcriptome: investigation of differential gene expression in floral nectariferous tissues. BMC Plant Biol 9 92
39. NaranjoMA
FormentJ
RoldanM
SerranoR
VicenteO
2006 Overexpression of Arabidopsis thaliana LTL1, a salt-induced gene encoding a GDSL-motif lipase, increases salt tolerance in yeast and transgenic plants. Plant Cell Environ 29 1890 1900
40. HellensRP
AllanAC
FrielEN
BolithoK
GraftonK
2005 Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1 13
41. YuH
ItoT
ZhaoYX
PengJR
KumarPP
2004 Floral homeotic genes are targets of gibberellin signaling in flower development. Proc Natl Acad Sci USA 101 7827 7832
42. CaoDN
ChengH
WuW
SooHM
PengJR
2006 Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol 142 509 525
43. WilsonRN
HeckmanJW
SCR
1992 Gibberelellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100 403 408
44. MartinC
GloverBJ
2007 Functional aspects of cell patterning in aerial epidermis. Curr Opin Plant Biol 10 70 82
45. WhitneyHM
ChittkaL
BruceTJ
GloverBJ
2009 Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr Biol 19 948 953
46. IrishVF
2010 The flowering of Arabidopsis flower development. Plant J 61 1014 1028
47. SaladieM
MatasAJ
IsaacsonT
JenksMA
GoodwinSM
2007 A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol 144 1012 1028
48. WolfS
MouilleG
PellouxJ
2009 Homogalacturonan methyl-esterification and plant development. Mole Plant 2 851 860
49. FarrokhiN
BurtonRA
BrownfieldL
HrmovaM
WilsonSM
2006 Plant cell wall biosynthesis: genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol J 4 145 167
50. OgawaM
KayP
WilsonS
SwainSM
2009 ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21 216 233
51. DobritsaAA
ShresthaJ
MorantM
PinotF
MatsunoM
2009 CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151 574 589
52. LiH
PinotF
SauveplaneV
Werck-ReichhartbD
DiehlP
2010 Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 22 173 190
53. PinotF
BeissonF
2011 Cytochrome P450 metabolizing fatty acids in plants: characterization and physiological roles. FEBS J 278 195 205
54. FrankeR
BriesenI
WojciechowskiT
FaustA
YephremovA
2005 Apoplastic polyesters in Arabidopsis surface tissues–a typical suberin and a particular cutin. Phytochemistry 66 2643 2658
55. FrankeR
HoferR
BriesenI
EmsermannM
EfremovaN
2009 The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J 57 80 95
56. BaxterI
HosmaniPS
RusA
LahnerB
BorevitzJO
2009 Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5 e1000492 doi:10.1371/journal.pgen.1000492
57. HoeferR
BriesenI
BeckM
PinotF
SchreiberL
2008 The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J Exp Bot 59 2347 2360
58. LeeEJ
MatsumuraY
SogaK
HosonT
KoizumiN
2007 Glycosyl hydrolases of cell wall are induced by sugar starvation in Arabidopsis. Plant Cell Physiol 48 405 413
59. HovavR
UdallJA
HovavE
RappR
FlagelL
2008 A majority of cotton genes are expressed in single-celled fiber. Planta 227 319 329
60. ChaudharyB
HovavR
FlagelL
MittlerR
WendelJF
2009 Parallel expression evolution of oxidative stress-related genes in fiber from wild and domesticated diploid and polyploid cotton (Gossypium). BMC Genomics 10 378
61. LeeJJ
WoodwardAW
ChenZJ
2007 Gene expression changes and early events in cotton fibre development. Ann Bot 100 1391 1401
62. ChengH
QinLJ
LeeSC
FuXD
RichardsDE
2004 Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131 1055 1064
63. Mutasa-GottgensE
HeddenP
2009 Gibberellin as a factor in floral regulatory networks. J Exp Bot 60 1979 1989
64. HoffmannbenningS
KendeH
1994 Cuticle Biosynthesis in rapidly growing internodes of deep-water rice. Plant Physiol 104 719 723
65. BowenDJ
WaltonTJ
1988 Cutin Composition and Biosynthesis during Gibberellic Acid-Induced Stem Extension of Pisum-Sativum Var Meteor. Plant Sci 55 115 127
66. KnocheM
BeyerM
PeschelS
OparlakovB
BukovacMJ
2004 Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol Plantarum 120 667 677
67. HisamatsuT
KingRW
2008 The nature of floral signals in Arabidopsis. II. Roles for FLOWERING LOCUS T (FT) and gibberellin. Journal of Experimental Botany 59 3821 3829
68. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J16 735 743
69. TanakaT
TanakaH
MachidaC
WatanabeM
MachidaY
2004 A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37 139 146
70. McCartneyL
Steele-KingCG
JordanE
KnoxJP
2003 Cell wall pectic (1–4)-beta-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J 33 447 454
71. WeigelD
GlazebrookJ
2002 Arabidopsis: A Laboratory Manual: Cold Spring Harbor Laboratory Press
72. ChuartzmanSG
NevoR
ShimoniE
CharuviD
KissV
2008 Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20 1029 1039
73. IrizarryRA
HobbsB
CollinF
Beazer-BarclayYD
AntonellisKJ
2003 Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249 264
74. VogelJP
RaabTK
SchiffC
SomervilleSC
2002 PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14 2095 2106
75. WillatsWGT
GilmartinPM
MikkelsenJD
KnoxJP
1999 Cell wall antibodies without immunization: generation and use of de-esterified homogalacturonan block-specific antibodies from a naive phage display library. Plant J 18 57 65
76. VoinnetO
RivasS
MestreP
BaulcombeD
2003 An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33 949 956
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 5
-
Všechny články tohoto čísla
- Structural and Functional Differences in the Long Non-Coding RNA in Mouse and Human
- Identification, Replication, and Functional Fine-Mapping of Expression Quantitative Trait Loci in Primary Human Liver Tissue
- A −436C>A Polymorphism in the Human Gene Promoter Associated with Severe Childhood Malaria
- A Decline in p38 MAPK Signaling Underlies Immunosenescence in
- The Operon Balances the Requirements for Vegetative Stability and Conjugative Transfer of Plasmid R388
- Novel and Conserved Protein Macoilin Is Required for Diverse Neuronal Functions in
- Ixr1 Is Required for the Expression of the Ribonucleotide Reductase Rnr1 and Maintenance of dNTP Pools
- Genome of Strain SmR1, a Specialized Diazotrophic Endophyte of Tropical Grasses
- A Deficiency of Ceramide Biosynthesis Causes Cerebellar Purkinje Cell Neurodegeneration and Lipofuscin Accumulation
- A Latent Pro-Survival Function for the Mir-290-295 Cluster in Mouse Embryonic Stem Cells
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time
- Prion Formation and Polyglutamine Aggregation Are Controlled by Two Classes of Genes
- Integrated Genome-Scale Prediction of Detrimental Mutations in Transcription Networks
- Post-Embryonic Nerve-Associated Precursors to Adult Pigment Cells: Genetic Requirements and Dynamics of Morphogenesis and Differentiation
- A Novel Mouse Synaptonemal Complex Protein Is Essential for Loading of Central Element Proteins, Recombination, and Fertility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
- A Genetic and Structural Study of Genome Rearrangements Mediated by High Copy Repeat Ty1 Elements
- A Missense Mutation in Causes a Major QTL Effect on Ear Size in Pigs
- A Flurry of Folding Problems: An Interview with Susan Lindquist
- Meiotic Recombination Intermediates Are Resolved with Minimal Crossover Formation during Return-to-Growth, an Analogue of the Mitotic Cell Cycle
- A Nervous Origin for Fish Stripes
- The ISWI Chromatin Remodeler Organizes the hsrω ncRNA–Containing Omega Speckle Nuclear Compartments
- The Telomerase Subunit Est3 Binds Telomeres in a Cell Cycle– and Est1–Dependent Manner and Interacts Directly with Est1
- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



