-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPost-Embryonic Nerve-Associated Precursors to Adult Pigment Cells:
Genetic Requirements and Dynamics of Morphogenesis and
Differentiation
The pigment cells of vertebrates serve a variety of functions and generate a
stunning variety of patterns. These cells are also implicated in human
pathologies including melanoma. Whereas the events of pigment cell development
have been studied extensively in the embryo, much less is known about
morphogenesis and differentiation of these cells during post-embryonic stages.
Previous studies of zebrafish revealed genetically distinct populations of
embryonic and adult melanophores, the ectotherm homologue of amniote
melanocytes. Here, we use molecular markers, vital labeling, time-lapse imaging,
mutational analyses, and transgenesis to identify peripheral nerves as a niche
for precursors to adult melanophores that subsequently migrate to the skin to
form the adult pigment pattern. We further identify genetic requirements for
establishing, maintaining, and recruiting precursors to the adult melanophore
lineage and demonstrate novel compensatory behaviors during pattern regulation
in mutant backgrounds. Finally, we show that distinct populations of latent
precursors having differential regenerative capabilities persist into the adult.
These findings provide a foundation for future studies of post-embryonic pigment
cell precursors in development, evolution, and neoplasia.
Published in the journal: . PLoS Genet 7(5): e32767. doi:10.1371/journal.pgen.1002044
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002044Summary
The pigment cells of vertebrates serve a variety of functions and generate a
stunning variety of patterns. These cells are also implicated in human
pathologies including melanoma. Whereas the events of pigment cell development
have been studied extensively in the embryo, much less is known about
morphogenesis and differentiation of these cells during post-embryonic stages.
Previous studies of zebrafish revealed genetically distinct populations of
embryonic and adult melanophores, the ectotherm homologue of amniote
melanocytes. Here, we use molecular markers, vital labeling, time-lapse imaging,
mutational analyses, and transgenesis to identify peripheral nerves as a niche
for precursors to adult melanophores that subsequently migrate to the skin to
form the adult pigment pattern. We further identify genetic requirements for
establishing, maintaining, and recruiting precursors to the adult melanophore
lineage and demonstrate novel compensatory behaviors during pattern regulation
in mutant backgrounds. Finally, we show that distinct populations of latent
precursors having differential regenerative capabilities persist into the adult.
These findings provide a foundation for future studies of post-embryonic pigment
cell precursors in development, evolution, and neoplasia.Introduction
A fundamental challenge for modern developmental biology is to determine how populations of stem and progenitor cells are established, maintained, and recruited to differentiate at particular times and places during post-embryonic development and in the adult organism. The significance of the problem cannot be overstated. Not only are these cells essential for normal development and homeostasis, but understanding their biology has profound translational importance. If we seek to evoke regenerative responses in a clinical content, then post-embryonic stem and progenitor populations may well supply the cells for doing so [1]–[3]. If we hope to delay natural tissue senescence, it is the life cycle of these cells that may need to be manipulated [4]–[7]. And if we aim to control malignancy, these cells or their transformed progeny will often be our targets of choice [8]–[10].
Pigment cells are of great utility for understanding the biology of post-embryonic stem and progenitor cells. Pigment cells are a classic and enduring system for studying morphogenesis and differentiation, and a century of effort has provided a firm understanding of many aspects of pigment cell development in the embryo [11]–[14]. These cells arise from neural crest cells, which migrate from the dorsal neural tube and contribute not only to pigment cells, but also glia and neurons of the peripheral nervous system, bone and cartilage of the craniofacial skeleton, and more. Despite the long-standing interest in these embryonic events, it is now clear that pigment cells of adults derive in large part from post-embryonic stem cells that are themselves of neural crest origin [15]–[18]. We know some of the mechanisms that underlie post-embryonic precursor development yet many outstanding questions remain. Foremost among these concern the genes and cellular behaviors by which pigment stem or progenitor cells are established during early development and subsequently maintained, whether there exist distinct subpopulations of such cells with different genetic requirements and potentials, and how these cells are recruited during normal development and homeostasis.
Answers to these questions will provide insights into the basic biology of the adult pigment cell lineage, and can inform our understanding of post-embryonic neural crest derivatives as well as stem and progenitor cells more generally. These answers are also of enormous biomedical significance, as the skin pigment cell of mammals, the melanocyte, is associated with a variety of human pathologies [19] and transformed cells of this lineage give rise to melanoma, one of the most common cancers [20], [21] and also one of the most deadly [21]–[25]. Poor outcomes reflect the inefficacy of non-surgical treatments and the highly invasive character of melanoma cells [26]–[29]. This invasiveness results in part from neural crest and melanocyte-specific factors that are already expressed by untransformed precursors, as well as lineage-specific factors that are re-expressed upon transformation [30]. Better understanding the genetic requirements and dynamics of melanocyte development and homeostasis can thus provide insights into the behaviors of transformed cells, and may suggest novel strategies for clinical intervention.
In recent years, the zebrafish has proven to be a tractable system for elucidating features of pigment cell development in the embryo and during the larval-to-adult transformation, a period of post-embryonic development analogous to later organogenesis, fetal and neonatal development of mammals [31]–[33]. In the embryo, neural crest cells differentiate into embryonic/early larval melanophores, the zebrafish homologue of mammalian melanocytes. Melanophores and melanocytes depend on many of the same genes and pathways [12], [14], [34], and melanomas with characteristics equivalent to those of human melanomas can be induced in zebrafish [18], [34], [35].
The development of zebrafish adult pigmentation involves a “pigment pattern metamorphosis” in which an embryonic/early larval pigment pattern is transformed into that of the adult [12], [33], [36]–[38]. Whereas the embryonic/early larval pigment cells and pattern develop by 3.6 SSL (standardized standard length [33]; about 4 days post-fertilization), new “metamorphic” melanophores begin to differentiate scattered over the flank by 5.9 SSL and ultimately migrate to form the adult stripes. Simultaneously, additional metamorphic melanophores appear already at sites of stripe formation, and many embryonic/early larval melanophores are lost. These events culminate in a juvenile pigment pattern by 11.0 SSL (4–5 weeks post-fertilization), consisting of two melanophore stripes bounding a lighter “interstripe”. Melanophores comprising these stripes reside in the “hypodermis” between the epidermis and the myotomes [39], [40]. Other adult melanophores are found in the epidermis, the dorsal scales, and the fins. Two additional classes of pigment cells also develop: yellow–orange xanthophores, which populate the interstripe and are required for organizing melanophores into stripes [38], [41], [42]; and iridescent iridophores, which are initially limited to the interstripe but later occupy melanophore stripes as well [33]. During later adult development, additional stripes and interstripes are added as the fish grows.
Mutants with pigment pattern defects limited to post-embryonic stages have suggested a model in which embryonic/early larval melanophores develop directly from the neural crest, whereas metamorphic melanophores develop from latent stem cells of presumptive neural crest origin. For example, picasso and puma mutants have normal embryonic/early larval melanophores, but profound deficiencies in their complements of metamorphic melanophores. picasso encodes the neuregulin receptor erbb3b, which acts both autonomously and non-autonomously to the metamorphic melanophore lineage. Pharmacological inhibition of ErbB signaling further revealed that erbb3b activity is required during neural crest migration for the later development of metamorphic melanophores, suggesting this locus is essential for establishing a pool of precursors that will differentiate only later during the larval-to-adult transformation [43]. By contrast, puma encodes tubulin alpha 8-like 3a (tuba8l3a) and acts autonomously to the metamorphic melanophore lineage. The temperature sensitivity of this allele allowed the identification of a critical period during pigment pattern metamorphosis, suggesting a role in maintaining or expanding a population of latent precursors, or recruiting these cells as melanophores [36], [44], [45].
To date it has not been known where latent precursors to metamorphic melanophores reside, how erbb3b, tuba8l3a or other loci promote the normal morphogenesis and differentiation of these cells and their progeny, or whether pigment cell precursors have indefinite or more limited re-population potential. Here we investigate these issues using molecular marker analyses, transgenesis, vital labeling, and time-lapse imaging in wild-type and mutant backgrounds. We show that during post-embryonic development, proliferative pigment cell precursors are associated with peripheral nerves and ganglia, and migrate to the hypodermis during pigment pattern metamorphosis where they differentiate as melanophores and iridophores. Nerve-associated pigment cell precursors are missing or reduced in ErbB-deficient and tuba8l3a mutant backgrounds. By contrast, these precursors persist in other mutants having less severe metamorphic melanophore deficiencies, though their subsequent development is marked both by defects, and partial regulation, of morphogenesis and differentiation. Finally, we show that latent precursors persist into the adult but that different precursor pools have different regenerative potentials. These findings provide a critical context for understanding the cellular bases of adult melanophore development, the mechanistic underpinnings of mutant phenotypes, and the roles for latent precursors in adult homeostasis, regeneration, and neoplasia.
Results
A melanogenic, erbb3b-dependent population of extra-hypodermal progenitor cells
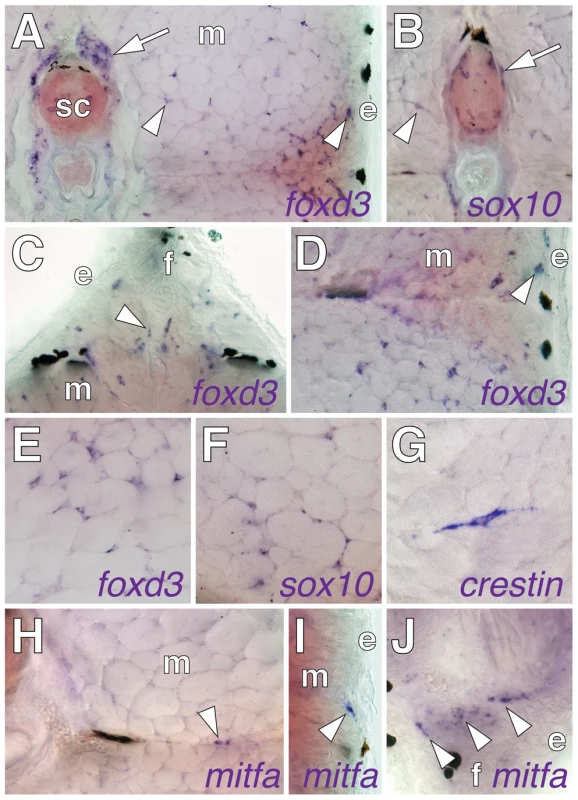
To identify tissues that might harbor latent precursors to adult melanophores, we examined post-embryonic zebrafish for transcripts expressed by embryonic neural crest cells, reasoning that some of the cells expressing such markers might comprise a population of undifferentiated melanophore precursors. We examined foxd3 and sox10, which are expressed by early neural crest cells, subpopulations of neural crest-derived glia, and some other cell types [46]–[49], as well as crestin, which is known only to be expressed by neural crest cells and their derivatives [50]. Cells expressing these loci were present in the hypodermis where the adult pigment pattern forms, but also in the myotomes, adjacent to the spinal cord, and at the bases of the fins (Figure 1A–1G), raising the possibility of both hypodermal and “extra-hypodermal” precursors for metamorphic melanophores.
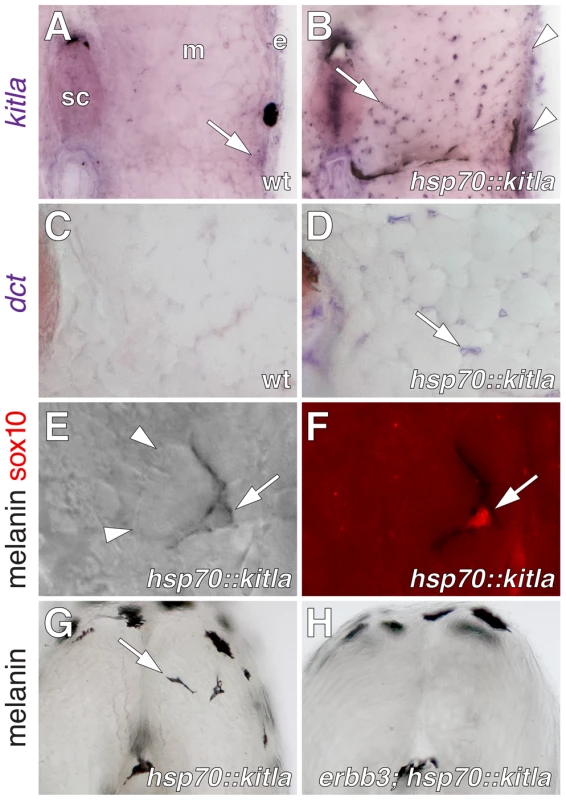
If extra-hypodermal, post-embryonic cells expressing genes typical of early neural crest cells contribute to metamorphic melanophores, we hypothesized that some of these cells should differentiate if supplied with appropriate trophic support. To test this idea, we used a heat-shock inducible transgenic line, Tg(hsp70::kitla)wp.r.t2, to misexpress the melanogenic factor kit ligand-a (kitla) [51] throughout the larva (Figure 2A, 2B). These larvae developed ectopic melanophores deep within the myotomes, which were never found in identically treated siblings lacking the transgene (Figure 2C–2G).
Previously we showed that erbb3b is essential for establishing precursors to metamorphic melanophores [43]. Accordingly, if extra-hypodermal kitla-responsive melanogenic cells and metamorphic melanophores arise from a common precursor pool, then ectopic melanophores should fail to develop in erbb3b mutants misexpressing kitla. Indeed, erbb3b; Tg(hsp70:kitla) larvae exhibited ∼30-fold fewer ectopic melanophores than wild-type Tg(hsp70::kitla) larvae (Figure 2H). A similar outcome was observed when wild-type larvae were treated with the ErbB inhibitor, AG1478, during the embryonic critical period for erbb3b activity in establishing metamorphic melanophore precursors [43]. Together, these results indicated that some extra-hypodermal cells are competent to differentiate as melanophores in the wild-type, but that most of these cells are missing when erbb3b activity is lost.
Extra-hypodermal melanophore precursors are nerve-associated, proliferative, and specified for the melanophore lineage during the larval-to-adult transformation
Our identification of extra-hypodermal cells expressing genes typical of early neural crest and glial cells, as well as kitla-responsive melanogenic cells within the myotomes, led us to ask whether any of these cells embark upon a melanophore differentiation program during normal post-embryonic development. Since precursors to metamorphic melanophores require erbb3b, we further predicted that any candidate extra-hypodermal precursors of these cells should be missing in larvae deficient for erbb3b activity.
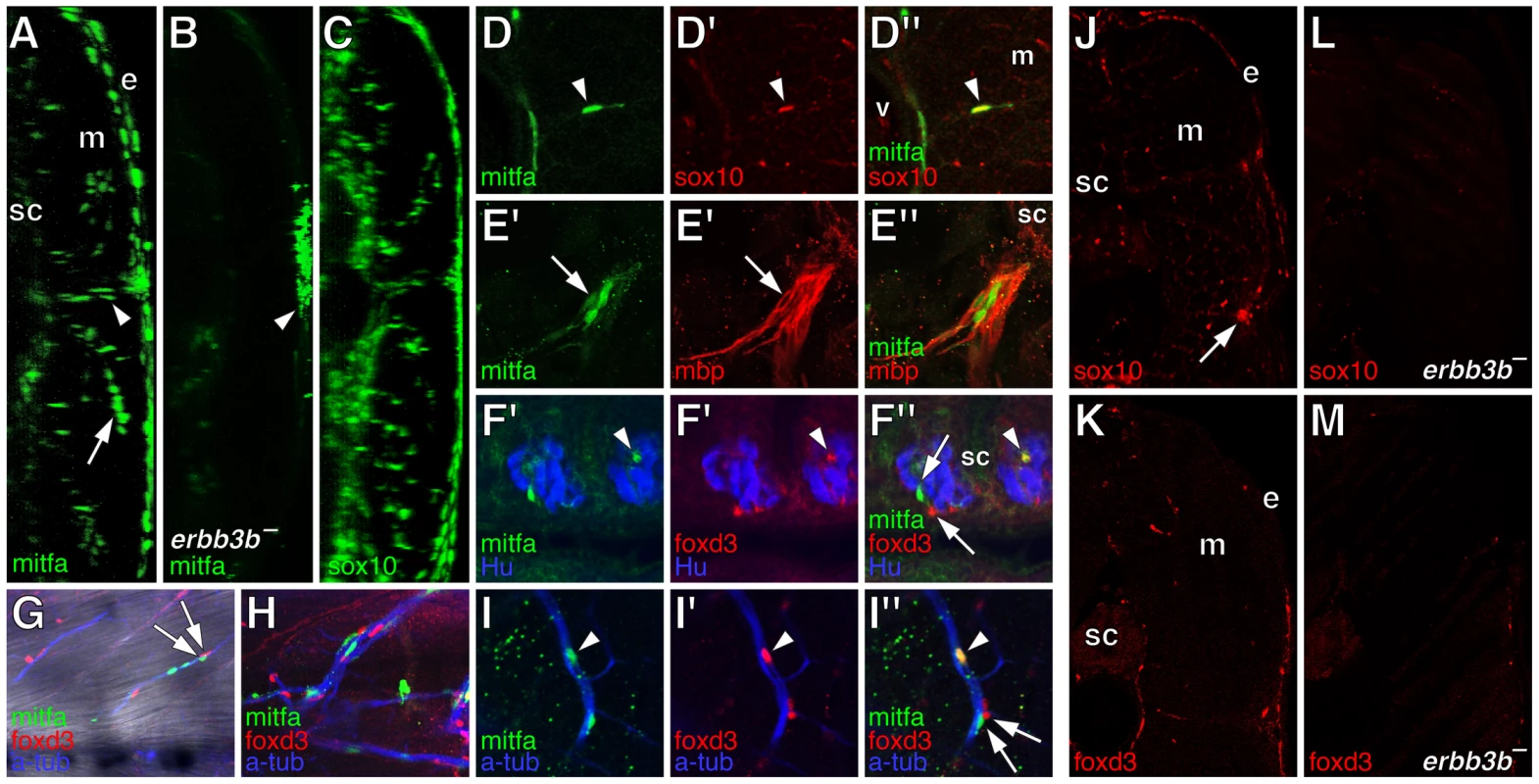
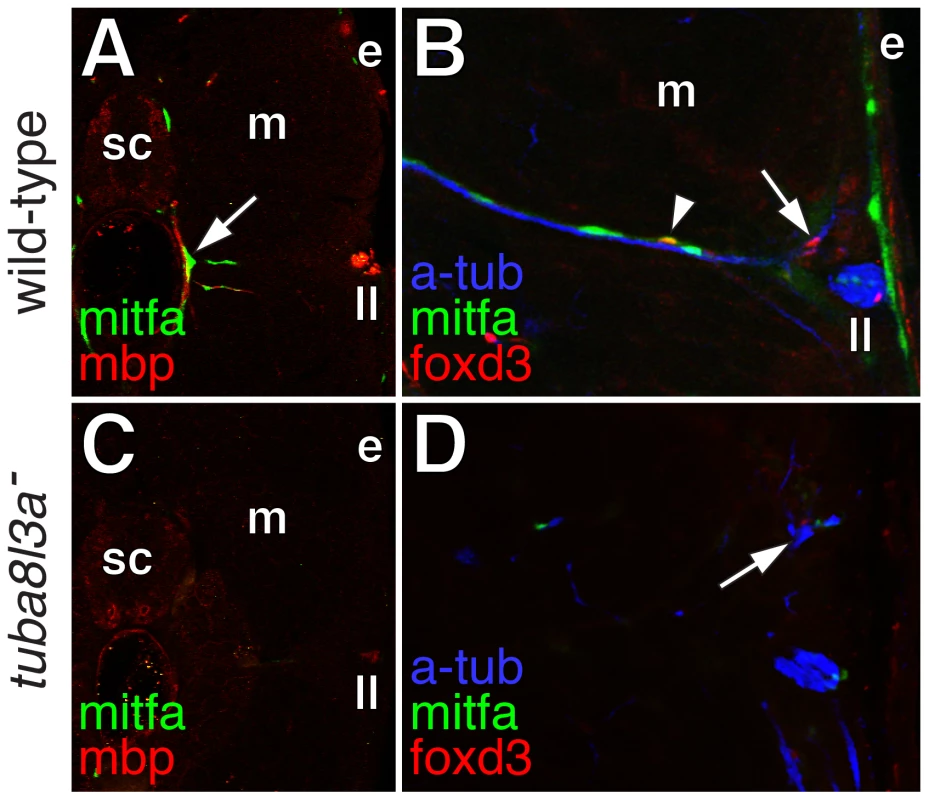
In the wild-type, we found extra-hypodermal cells expressing mitfa, encoding a master regulator of melanophore fate specification (Figure 1H–1J) [52], [53]. Cells expressing mitfa in extra-hypodermal locations typically did so at lower levels than cells within the hypodermis and were at the limit of detection given the sensitivity of in situ hybridization at post-embryonic stages. However, we also identified extra-hypodermal cells expressing GFP driven by the proximal mitfa promoter in the transgenic line, Tg(mitfa::GFP)w47, which faithfully recapitulates mitfa expression in the melanophore lineage and in bipotent precursors to melanophores and iridophores in the embryo [54], [55] (Figure 3A; Videos S1, S2). In contrast to the wild-type, mitfa::GFP+ cells were largely absent from both erbb3b mutants and wild-type larvae treated with AG1478 during the embryonic erbb3b critical period (Figure 3B and see below; Video S3). In neither genetic background could we detect extra-hypodermal cells expressing transcript for dct, encoding a melanin synthesis enzyme.
The many extra-hypodermal mitfa::GFP+ cells in larvae contrasts with embryogenesis [54] and suggests that extra-hypodermal cells may be specified for the melanophore lineage during the larval-to-adult transformation. If so, we predicted that cells expressing markers typical of early neural crest cells (or glia) should occassionally be found to express mitfa::GFP as well. We therefore examined larvae for simultaneous expression of mitfa::GFP, sox10, and foxd3, and, to learn where these cells reside, we examined larvae with cell-type specific markers for surrounding tissues. These analyses revealed numerous mitfa::GFP+ cells associated with peripheral nerves and ganglia, including the doral root ganglia, ventral motor root fibers, lateral line nerve, and nerve fibers coursing through the myotomes (Figure 3E–3H; Figure S1). Ectopic melanophores within the myotomes of wild-type Tg(hsp70:kitla) larvae were likewise nerve-associated (Figure S2).
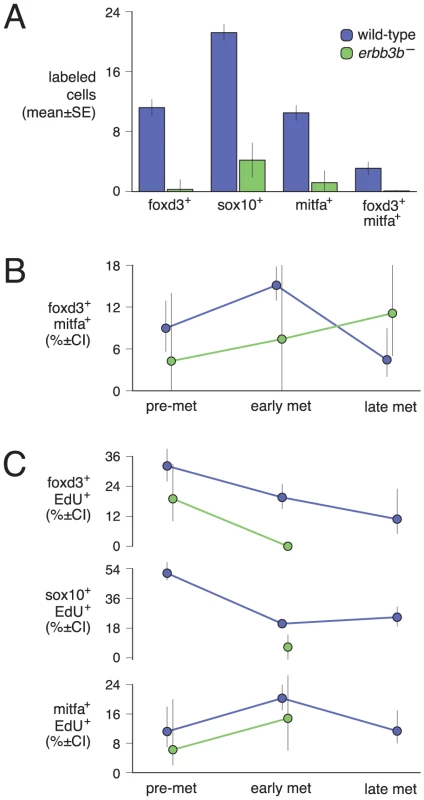
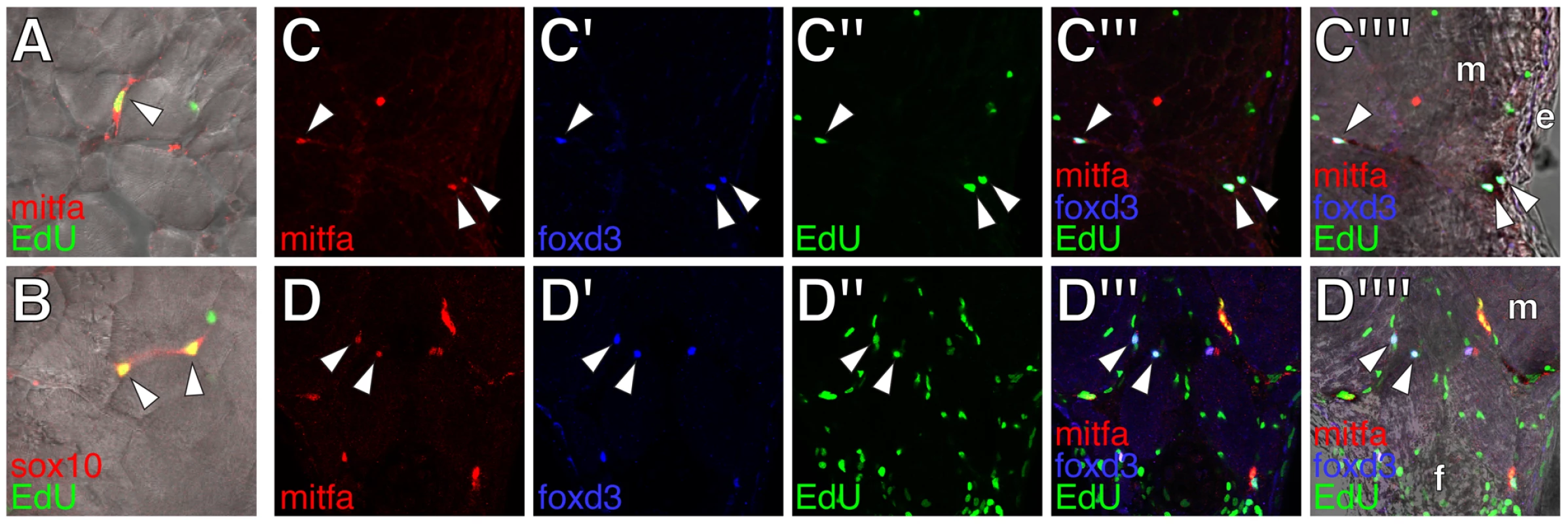
Double label analyses with markers of neural crest, glial, and melanophore lineages revealed that, in wild-type larvae, mitfa::GFP+ cells were often in close proximity to cells expressing sox10 and foxd3, and also co-expressed sox10, as expected given the direct regulation of mitfa by sox10 (Figure 3D, 3G, 3H) [56], [57]. We also found that 4–15% of mitfa::GFP+ cells co-expressed foxd3, with doubly labeled cells occurring most frequently during early metamorphosis, when the rate of melanophore population increase is maximal [36] (Figure 3F, 3I; Figure 4A, 4B). This frequency of double labeling is reminiscent of 15–18 h embryos, in which 9–12% of mitfa::GFP+ cells are foxd3+ [54]. In contrast to the co-labeling of mitfa::GFP and foxd3, we did not find mitfa::GFP expression by myelinating glia expressing myelin basic protein (mbp) (Figure 3E; Figure S1). As anticipated, however, some cells expressing foxd3 or sox10 co-expressed mbp. All of these cell types were deficient in erbb3b mutants and wild-type larvae treated with AG1478 during the embryonic erbb3b critical period (Figure 3J–3M; Figure 4A, 4B; Figure S4), though a few residual mitfa::GFP+ and foxd3+ cells occurred in anterior and posterior regions, corresponding to axial levels where a few residual melanophores develop during metamorphosis [43] (data not shown).
If some foxd3+ and sox10+ cells are progenitors to post-embryonic melanoblasts, we predicted that a period of population expansion could precede the appearance of mitfa::GFP+ cells, which might themselves be proliferative. Consistent with this idea, we found EdU incorporation by all three cell types but EdU-labeling of post-embryonic foxd3+ and sox10+ cells was most frequent prior to pigment pattern metamorphosis, whereas EdU-labeling of mitfa::GFP+ cells was most frequent during the peak of pigment pattern metamorphosis (Figure 4C, Figure 5). Given these findings, we further asked if doubly labeled foxd3+; mitfa::GFP+ cells constitute an especially proliferative population. These analyses revealed EdU-incorporation in 55% of foxd3+; mitfa::GFP+ cells, a significantly higher frequency than for cells expressing only foxd3 (17% EdU+) or only mitfa::GFP (24% EdU+; χ2 = 131.7, d.f. = 2, P<0.0001). The frequency of EdU labeling amongst foxd3+; mitfa::GFP+ cells did not vary significantly across stages (χ2 = 2.3, d.f. = 2, P = 0.3). Finally, in erbb3b mutants sampled at selected stages, we found lower levels of EdU incorporation than in wild-type, though small numbers of cells overall resulted in correspondingly low statistical power (Figure 4C; Figure S4).
The foregoing analyses indicated that, during post-embryonic development, a proliferative population of extra-hypodermal, erbb3b-dependent foxd3+ and sox10+ cells associated with peripheral nerves and ganglia becomes specified as precursors to melanophores (or as bipotential precursors to melanophores and iridophores; see reference [55]).
Extra-hypodermal cells contribute to the hypodermal populations of metamorphic melanophores and iridophores
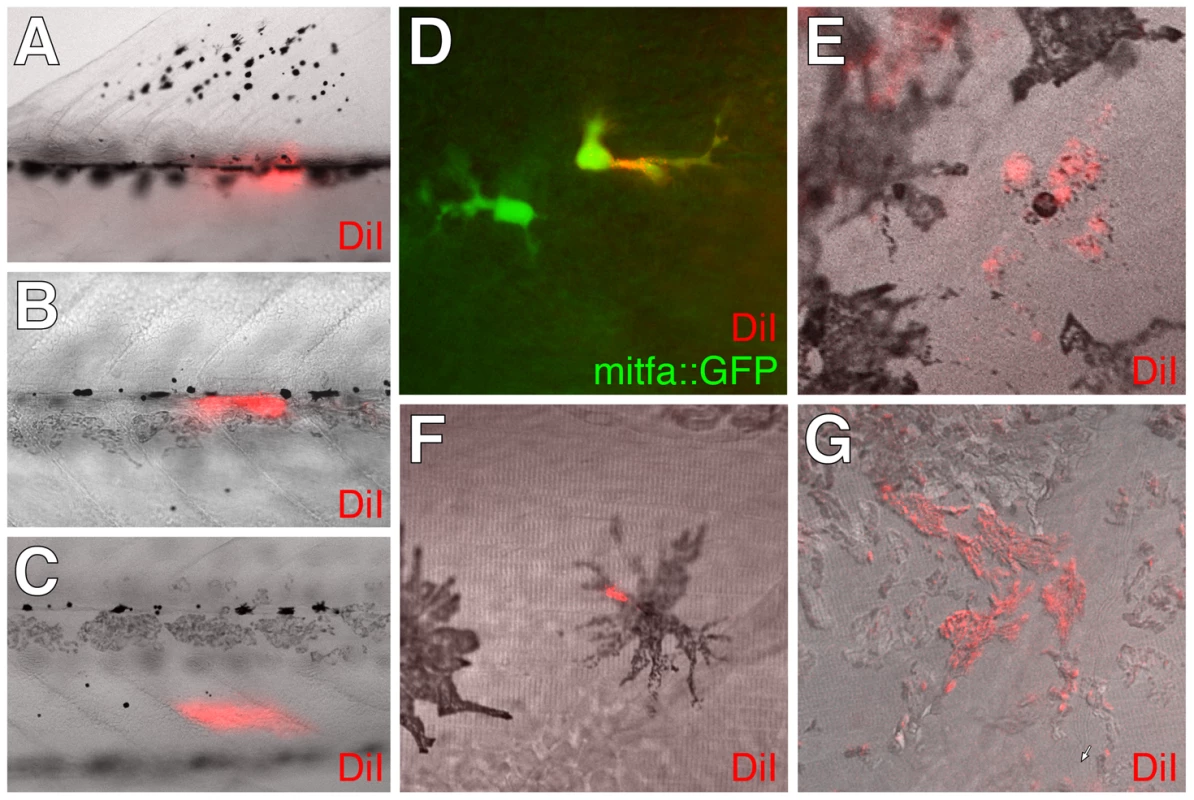
The development of post-embryonic, extra-hypodermal mitfa::GFP+ cells suggested the possibility that such cells migrate to the hypodermis during metamorphosis. To test this idea, we injected DiI into the myotomes, the horizontal myoseptum, or the base of the dorsal fin of wild-type or Tg(mitfa::GFP) larvae and we assessed after ≥4 d whether DiI-labeled cells were present within the hypodermis distant from the injection sites. In 10–30% of injected larvae we found hypodermal DiI-labeled melanized cells, mitfa::GFP+ cells, or iridophores (Figure 6), indicating that some extra-hypodermal cells migrated to the hypodermis during metamorphosis.
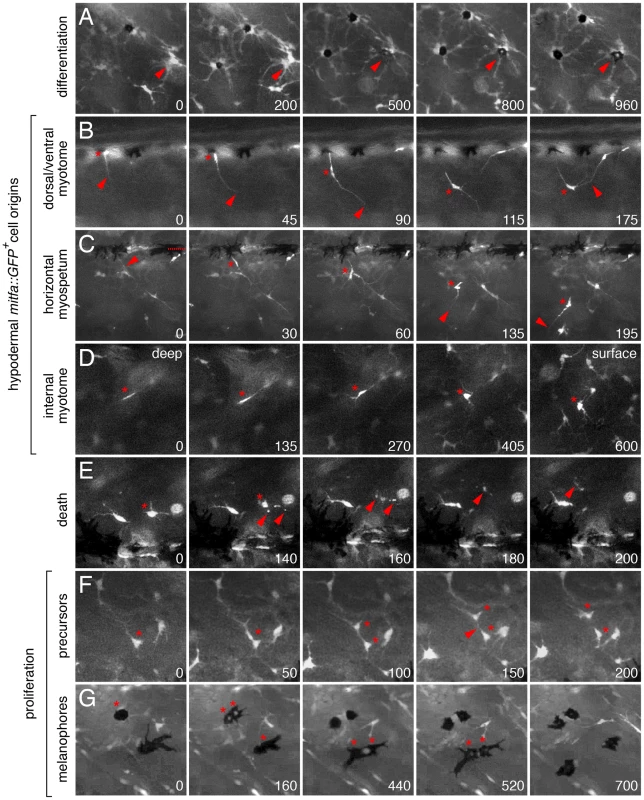
To further test the hypothesis that extra-hypodermal cells contribute to the hypodermal melanophore population, we examined cell behaviors by time-lapse imaging of trunks derived from Tg(mitfa::GFP) larvae. Movies revealed the differentiation of mitfa::GFP+ cells into melanophores as well as their migration (Figure 7A; Videos S4, S5, S6). We therefore assessed the migratory pathways by which mitfa::GFP+ cells had reached the hypodermis. Approximately half of all mitfa::EGFP+ cells arrived within the hypodermis during imaging. Some entered the hypodermis after migrating over the dorsal or ventral margins of the myotomes, whereas others originated from within the myotomes, emerging either from the vicinity of the horizontal myoseptum or along vertical myosepta (Figure 7B–7D, Figure 8; Videos S7, S8, S9, S10). Movies also revealed the movement of mitfa::GFP+ cells along nerves and their detachment from nerves to migrate more broadly through the fish (Videos S11, S12). Together then, DiI labeling and time-lapse imaging indicated that extra-hypodermal cells contribute to hypodermal mitfa::GFP+ cells, melanophores, and iridophores during the larval-to-adult transformation.
Genetic requirements for extra-hypodermal precursor morphogenesis and differentiation
Our findings suggested that a normal complement of adult melanophores depends on contributions from a pool of extra-hypodermal precursors. If this is the case, we predicted that mutants with severe metamorphic melanophore deficiencies should have correspondingly severe deficiencies of extra-hypodermally derived mitfa:GFP+ cells. To test the contributions of extra-hypodermal cells to hypodermal mitfa::GFP+ cells and melanophores, we crossed the Tg(mitfa::GFP) transgene into erbb3b and tuba8l3a mutants, which exhibit severely reduced numbers of metamorphic melanophores [36], [43], [44].
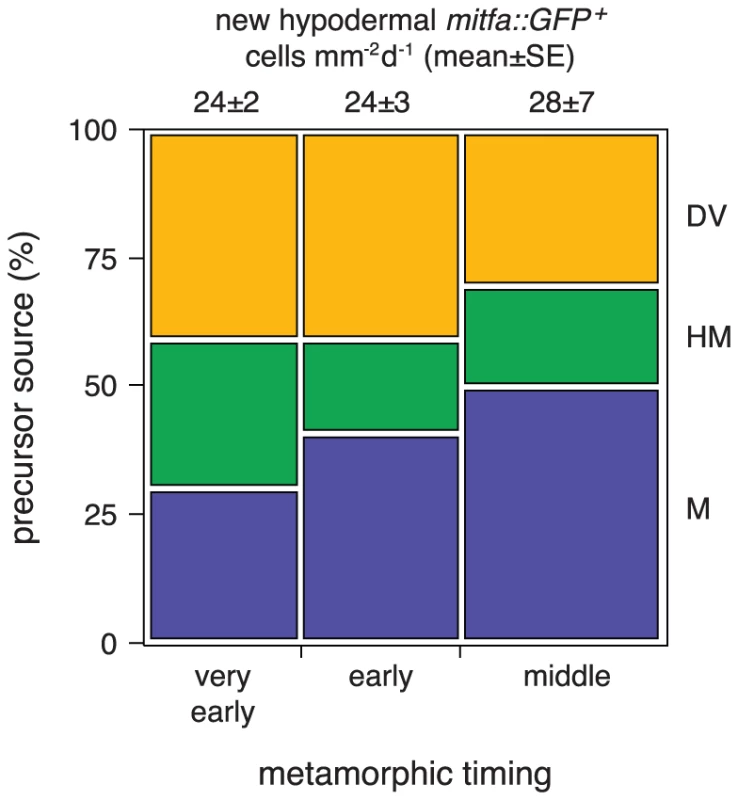
In comparison to the wild-type, and as predicted from the foregoing analyses, erbb3b mutants had dramatically fewer extra-hypodermally derived mitfa::GFP+ cells (Figure 9A; Video S13). erbb3b mutant mifa::GFP+ cells originated from the vicinity of the horizontal myoseptum (Figure 9B), and once in the hypodermis, these cells were more likely to differentiate and to divide (Figure 9C; in contrast to the somewhat reduced rates of EdU incorporation prior to reaching the hypodermis shown in Figure 4C). tuba8l3a mutants also had significantly fewer extra-hypodermally derived mitfa::GFP+ cells. These cells were more likely to differentiate, but divided at only one-third the frequency of the wild-type (Figure 9; Video S14). tuba8l3a mutants exhibit a post-embryonic demyelination of the peripheral nervous system [44], and we found that regions exhibiting mbp+ glial deficiencies and peripheral nerve defasciculation had fewer mitfa::GFP+ and foxd3+ cells (Figure 10). We did not observe cells doubly labeled for foxd3 and mitfa::GFP in tuba8l3a mutants.
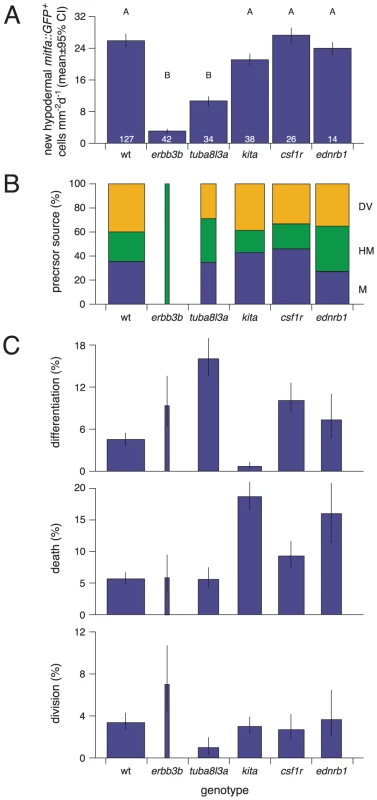
Amongst the erbb3b - and tuba8l3a-dependent metamorphic melanophore populations, are temporally and genetically distinct subpopulations, comprising early metamorphic melanophores that are initially dispersed but later migrate into stripes, and late metamorphic melanophores that develop already at sites of stripe formation [37], [43], [45] (Figure S5). Early metamorphic melanophores are ablated in kita mutants, but persist in colony stimulating factor-1 receptor (csf1r) and endothelin receptor b1 (ednrb1) mutants. By contrast, late metamorphic melanophores persist in kita mutants, but are ablated in csf1r and ednrb1 mutants [37], [38], [58], [59]. To test if these differences reflect differential persistence of distinct precursor pools, or differences in the subsequent morphogenesis and differentiation of cells arising from a common precursor pool, we crossed the Tg(mitfa::GFP) transgene into kitab5, csf1rj4e1 and ednrb1b140 mutants and examined the origins of hypodermal mitfa::GFP+ cells as well as their frequencies of differentiation, death and proliferation.
In contrast to erbb3b and tuba8l3a mutants, kita, csf1r, and ednrb1 mutants did not exhibit significantly fewer extra-hypodermally derived mitfa::GFP+ cells than the wild-type, though cells in kita mutants typically failed to differentiate and instead died at high frequency, whereas cells in csf1r and ednrb1 mutants were more likely both to differentiate and to die (Figure 9; Video S15). We did not observe gross defects in mitfa:GFP+ cell motility in any of the mutant backgrounds. Finally, in contrast to the proliferation of unmelanized mitf::GFP+ cells (Figure 9C), proliferation of differentiated melanophores was rare in the wild-type (0.1%; N = 3822 melanophores observed) and in most of the mutants (0.2–0.4%; N = 4358 melanophore observed). In kita mutants, however, the few melanophores that differentiated divided frequently (14%; N = 35 melanophores observed; variation among genotypes: χ2 = 38.1, d.f. = 5, P<0.0001; Video S16). Together these data show that erbb3b and tuba8l3a each promote the development of extra-hypodermal mitfa::GFP+ cells, whereas all five loci promote the differentiation and morphogenesis of these cells once they reach the hypodermis.
Latent melanophore precursors in adult zebrafish
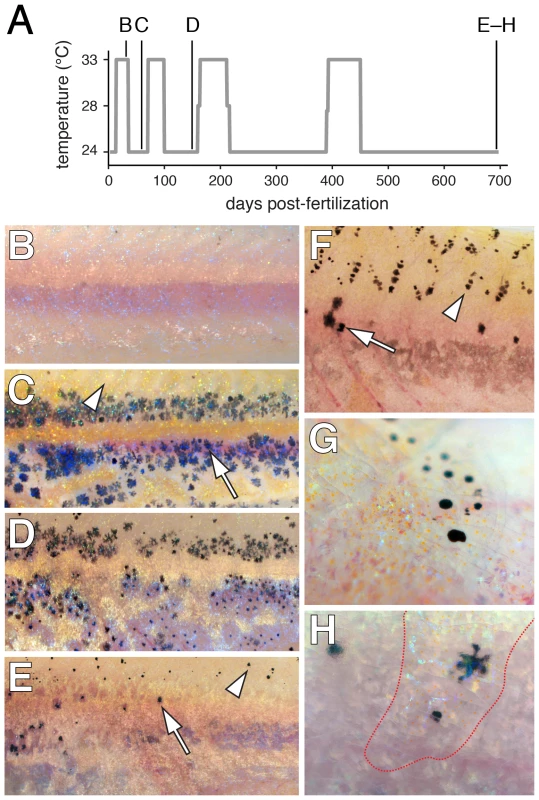
Our demonstration that extra-hypodermal precursors contribute to hypodermal melanophores led us to ask whether latent pigment cell precursors persist into the adult. Extra-hypodermal foxd3+ and mitfa::GFP+ cells were distributed in adult fish similarly to metamorphic stages and also were found associated with the scales (Figure S6). To test the capacity of latent precursors to supply new melanophores, we sought to ablate melanophores with the goal of provoking a regenerative response. Because fish doubly mutant for kitab5 and presumptive null alleles of csf1r lack body melanophores [38], we reasoned that fish doubly mutant for kitab5 and the temperature-sensitive allele csf1rut.r1e174 (csf1rTS) [60] should have fewer melanophores (equivalent to kitab5 single mutants) at permissive temperature, but should lack all melanophores at restrictive temperature. Repeated exposure to restrictive and permissive temperatures should thus allow for repeated cycles of ablation and regeneration of these kita-independent, csf1r-dependent melanophores. As predicted, kita; csf1rTS double mutants that were initially indistinguishable from kita single mutants lost body melanophores when shifted to restrictive temperature (Figure 11A, 11B). After returning to permissive temperature, fish initially recovered kita-independent hypodermal melanophores, though progressively fewer of these cells were regenerated in successive ablation–recovery cycles (Figure 11C, 11D). Surprisingly, ablations also resulted in the de novo development and regeneration of scale melanophores, which are normally absent from kita mutants (Figure 11F, 11G)[37]. The few later hypodermal melanophores that were recovered in kita; csf1rTS mutants were often located beneath scales populated with melanophores, iridophores and xanthophores (Figure 11H), raising the possibility that some of these regenerative hypodermal melanophores may have been scale-derived. Overall, these findings suggest that precursors to kita-independent, csf1r-dependent hypodermal melanophores persist in the adult yet have a finite regenerative potential, whereas an additional precursor pool associated with adult scales has a greater regenerative capability.
Discussion
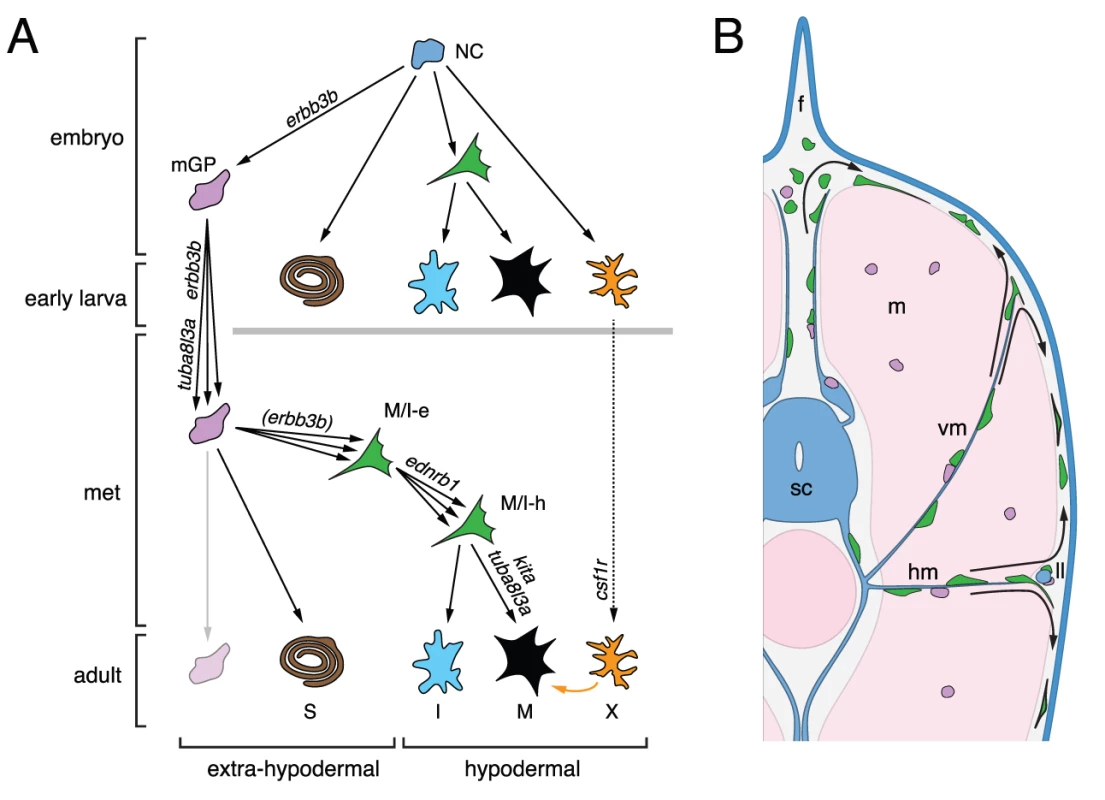
The results of this study and previous analyses [36], [43], [45] suggest a model for the development of adult melanophores in zebrafish (Figure 12). Pluripotent foxd3+ precursors to glia [46], [61], adult melanophores and iridophores are established in an erbb3b-dependent manner during embryogenesis, and thereafter are associated with post-embryonic peripheral nerves and ganglia. This precursor population is expanded and maintained during pre-metamorphic larval development in an erbb3b - and tuba8l3a-dependent manner, and cells within this pool become specified for pigment cell lineages beginning immediately before, and continuing through, pigment pattern metamorphosis. During the larval-to-adult transformation, these extra-hypodermal precursors migrate to the hypodermis, and there contribute to metamorphic melanophores and iridophores. Some enter the hypodermis after migrating over the dorsal or ventral margins of the myotomes, others emerge from vertical or horizontal myosepta; some may emigrate from the lateral line nerve. Once in the hypodermis, these cells require tuba8l3a for their proliferation, as well as kita, ednrb1, and, to a lesser extent, csf1r, for their survival and eventual differentiation. Later in adults, some latent precursors persist and can supply a limited number of new hypodermal melanophores, whereas other precursors associated with scales have a greater regenerative capacity. Below we discuss several aspects and implications of this model.
Extra-hypodermal niches and the erbb3b - and tuba8l3a-dependence of latent precursors to adult melanophores
A major finding of our study is that post-embryonic mitfa::GFP+ cells are associated with peripheral nerves coursing through the myotomes as well as more medial nerves and ganglia. We further showed that nerve-associated cells could be induced to differentiate ectopically as melanophores, and that extra-hypodermal mitfa::GFP+ cells migrate to the hypodermis where some differentiate as melanophores during normal development. These observations suggest that peripheral nerves or ganglia serve as niches for post-embryonic precursors to adult melanophores and are broadly consistent with a recent study demonstrating a peripheral nerve origin for adult skin melanocytes of amniotes [62] as well as analyses revealing interconversion of glial and melanocyte fates in vitro [63]–[65]. Our study complements and extends recent lineage tracing studies of flounder larvae, in which adult pigment cell precursors were found to migrate to the hypodermis from dorsal and ventral regions during pigment pattern metamorphosis [66], [67].
Our analyses also provide insights into the molecular and proliferative phenotypes of metamorphic melanophore precursors. foxd3 often acts as a transcriptional repressor and is associated with the maintenance of pluripotency and pluripotent cells [68]–[71]. In the neural crest lineage, foxd3 is expressed by pluripotent cells and presumptive glia, and can inhibit mitfa transcription, favoring iridophore or glial over melanogenic fates [54], [55], [61], [72]. We found that some nerve-associated foxd3+ cells co-expressed mitfa::GFP just before and continuing through pigment pattern metamorphosis, and that such cells were especially likely to have incorporated EdU. These observations raise the possibility that nerve-associated foxd3+ cells are a pluripotent and proliferative population that can give rise to hypodermal melanophores and iridophores during pigment pattern metamorphosis. Our finding that DiI-labeled, extra-hypodermal tissues give rise to melanophores and iridophores with equal frequency is consistent with this idea. Because the mitfa::GFP transgene we employed is repressible by foxd3, we speculate that co-expression of mitfa::GFP reflects low levels of perduring foxd3 protein as precursors adopt a pigmentary fate, similar to observations of early neural crest morphogenesis [54], or that a balance between the anti-melanogenic and pro-melanogenic effects of foxd3 and mitfa, respectively, prevents specified cells from differentiating prematurely. Nevertheless, we note that our analyses revealed more extra-hypodermal mitfa::GFP+ cells than mitfa+ cells, which differs from the one-to-one corrrespondence of such cells in embryos [54]. Additional mitfa::GFP+ cells could reflect low levels of mitfa expression that fall below the threshold for detection by in situ hybridization at post-embryonic stages, yet are sufficient for accumulating detectable levels of relatively stable GFP. Or, mitfa::GFP expression in some cells could reflect a partial disregulation of the transgene, as might occur if regulatory elements for post-embryonic expression are missing. Distinguishing between these possibilities will require the production and analysis of additional transgenic reporter lines, but whichever the outcome, the mitfa::GFP reporter we have used will be a valuable tool for further dissecting the mechanisms of post-embryonic melanophore development.
Examination of erbb3b and tuba8l3a mutants provides additional support for the idea that extra-hypodermal, nerve-associated precursors are essential for metamorphic melanophore development. We found that presumptive precursors to glia and pigment cells were missing from erbb3b mutants and wild-type larvae in which ErbB activity had been inhibited during the embryonic critical period for adult melanophore development. An on-going requirement for erbb3b is suggested as well, by reduced complements of adult melanophores following acute inhibition of ErbB signaling in sensitized backgrounds during pigment pattern metamorphosis [43], and by reduced rates of EdU incorporation in erbb3b mutants during post-embryonic development (this study). Because ErbB signaling can repress melanocyte differentiation [62], [73], a further role in preventing the premature differentiation of nerve-associated precursors to hypodermal pigment cells seems likely. Notably, our finding that extra-hypodermal, kitla-responsive melanogenic cells were missing in ErbB-deficient backgrounds at post-embryonic stages contrasts with increased numbers of such cells at embryonic/early larval stages [74]. This difference likely reflects the pleiotropic nature of ErbB signals and a difference between the stages examined: blocking ErbB activity in the embryo presumably results in a de-repression of melanophore differentiation amongst transiently persisting precursor cells, whereas by post-embryonic stages, such precursors presumably have been lost.
In contrast to erbb3b mutants, tuba8l3a mutants exhibit a post-embryonic demyelination of peripheral nerves and a corresponding critical period for adult melanophore development [36], [44], [45]. In agreement with our model, tuba8l3a mutant larvae were deficient for mbp+ glia, nerve-associated foxd3+ cells and foxd3+; mitfa::GFP+ cells, and also had reduced rates of division amongst hypodermal mitfa::GFP+ cells. Because tuba8l3a acts autonomously to the metamorphic melanophore lineage [45], these findings suggest a defect in the maintenance or expansion of pluripotent precursors to mbp+ glia and mitfa:GFP+ pigment cell precursors. The post-embryonic onset of these phenotypes further suggests the existence of genetically distinct populations of embryonic and adult glia, analogous to embryonic and metamorphic melanophores.
Pattern development, regulation, and the roles of temporally and genetically distinct metamorphic melanophore populations
Time-lapse imaging comparisons of wild-type and mutant backgrounds further defined roles for genes previously known to function in pigment pattern development: kita, csf1r, and ednrb1 all promoted the survival of mitfa::GFP+ cells whereas kita also promoted the differentiation of these cells as melanophores. Yet, these analyses revealed compensatory responses of pigment cells and their precursors in mutant backgrounds as well. For example, residual mitfa::GFP+ cells in mutants with extra-hypodermal precursor deficiencies (erbb3b, tuba8l3a) or hypodermal defects in cell survival (csf1r, ednrb1) exhibited concomitantly greater rates of differentiation, and, in one instance (erbb3b), an increased rate of proliferation. Moreover, the reduced survival and differentiation of mitfa::GFP+ cells in kita mutants was coupled with a 70-fold increase in melanophore proliferation. These findings highlight the remarkably regulative nature of zebrafish pigment pattern development [36], [41], [42] and also the importance of direct imaging for understanding cellular behaviors that would not be predicted from terminal phenotypes alone.
A particularly dramatic example of pattern regulation occurs during regeneration. Zebrafish larval melanophores regenerate following laser ablation or the administration of melanocytotoxic drugs [74]–[76], adult fin melanophores regenerate along with other tissues after fin amputation [77]–[79], and hypodermal body melanophores regenerate after localized laser ablations [80], [81]. To test the capacity of latent precursors to supply new hypodermal melanophores in the adult, we used fish doubly mutant for kita and csf1rTS in which loss of residual melanophores at restrictive temperature likely reflects the withdrawal of trophic support provided by csf1r-dependent xanthophores [42], [60]. Our finding that progressively fewer hypodermal melanophores were recovered after repeated ablations implies a limited re-population potential for latent precursors that give rise to these kita-independent, csf1r-dependent late metamorphic melanophores. Whether the same is true of kita-dependent early metamorphic melanophores remains to be determined. Nevertheless, our finding that scale melanophores regenerated repeatedly-in contrast to late metamorphic, hypodermal melanophores-suggests a more highly regulative precursor pool associated with the adult scales, and highlights the possibility of spatially and temporally distinct pools of precursors having different morphogenetic and differentiative potentials. That scale melanophores developed de novo in these fish, despite their absence from kita single mutants, likely reflects a priority effect; e.g., initially abundant xanthophores may repress melanophore development in kita mutants [42], [82], [83] but simultaneous development of both melanophores and xanthophores during regeneration allows for a stable pattern comprising both cell types. The similar re-population potential of scale and fin melanophores, and the previous observation that basonuclin-2 mutants, which are deficient for hypodermal melanophores, nevertheless retain both scale and fin melanophores [84], also suggest the possibility of more extensive similarities between scale - and fin-associated precursor pools.
Finally our study provides new insights into the temporally distinct populations of melanophores in zebrafish. We found severe deficiencies in the numbers of extra-hypodermally derived mitfa::GFP+ cells in erbb3b and tuba8l3a mutants, illustrating the critical role of such cells in supplying metamorphic melanophores overall. By contrast, we found no evidence for reduced numbers of extra-hypodermally derived mitfa::GFP+ cells in kita, csf1r, or ednrb1 mutants, indicating that different genetic requirements of early and late metamorphic melanophores do not reflect differences in the establishment or maintenance of these cells. These findings suggest either of two interpretations. Early and late metamorphic melanophores could arise from a common precursor pool with differences in residual melanophore complements among mutants reflecting specific requirements for kita, csf1r, and ednrb1 in downstream events of morphogenesis and differentiation. For example, our data indicate that mitfa::GFP+ cells require kita for their surival, and presumably, terminal differentiation: the development of late metamorphic melanophores in kita mutants could thus reflect the late appearance of factors able to substitute for kita activity in promoting melanophore differentiation. Conversely, we found that mitfa::GFP+ cells were only marginally dependent on csf1r: the failure of late metamorphic melanophores to develop in csf1r mutants could, in turn, reflect a late-arising, post-differentiation requirement for trophic support from csf1r-dependent xanthophores [38], [42], [60]. An alternative interpretation is that early and late metamorphic melanophores do arise from distinct, but still cryptic, precursor pools that simply were not revealed by our methods. In other systems, niches initially assumed to have just one type of stem or progenitor cell have sometimes been found to harbor distinct classes of cells with disparate proliferative or differentiative potentials [85]–[87]. Additional time-lapse imaging analyses at later stages and genetically based lineage analyses that are now being conducted should provide further insights into these possibilities.
Post-embryonic stem cells in development, evolution, and neoplasia
The identification of extra-hypodermal nerve-associated precursors to adult melanophores in zebrafish (this study) and amniotes [62] indicates that a fuller understanding of adult pigment cell development and pattern formation requires a focus on post-embryonic precursors, as distinct from embryonic neural crest cells. The existence of genetically separable populations of embryonic pigment cells, derived directly from neural crest cells, and adult pigment cells, derived from post-embryonic precursors, further suggests that species-differences in adult pigment patterns may be explicable by evolutionary changes in the establishment, maintenance or recruitment of post-embryonic latent precursors, with few if any consequences for earlier pigment patterns [88]. Lastly, a peripheral nerve origin for adult pigment cells also raises the possibility that the frequent and generally fatal metastases of melanoma cells to the central nervous system [26]–[29],[89],[90] may reflect the continued or reiterated expression of genes that favor proliferation and migration in a nerve microenvironment.
Materials and Methods
Fish rearing, staging, and genetic stocks
Fish were reared at 28–29°C, 14L:10D. Post-embryonic stages are reported as standardized standard length (SSL) measurements, which indicate developmental progress of free-feeding larvae more reliably than days post-fertilization [33]. Tg(mitfa::GFP)w47 and Tg(−4.9sox10:egfp)ba2 fish were generously provided by D. Raible and R. Kelsh, respectively. Tg(TrDct::mCherry)wp.r.t3 and Tg(hsp70::kitla)wp.r.t2 fish were produced using tol2kit Gateway vectors and Tol2-mediated transgenesis [91], [92] and heat shocks with the latter strain were administered at 37°C for 1 hr three times daily for two days. Experiments with erbb3b mutants used either of two presumptive null alleles, erbb3but.r2e1 or erbb3bwp.r2e2 [93]. Experiments with csf1r mutants used either the presumptive null allele csf1rj4blue or the temperature-sensitive allele csf1rut.r1e174 [60]. In temperature shift experiments, fish were shifted repeatedly between restrictive temperature (33°C) and permissive temperature (24°C). All experiments with the kita mutant used the presumptive null allele kitab5 [59]. tuba8l3aj115e1 encodes a missense substitution with temperature-sensitive effects. Experiments with this allele were performed at standard rearing temperature, intermediate between restrictive (33°C) and permissive (24°C) temperatures [36], [44], [45], to allow analyses of a fuller complement of mitfa::GFP+ cells. Quantitative analyses here are thus likely to underestimate effects of the tuba8l3a mutation. Animal use conformed to University of Washington IACUC guidelines.
Imaging and image analysis
Fish were viewed with Olympus SZX-12 or Zeiss Discovery epifluorescence stereomicroscopes or with a Zeiss Observer inverted compound epifluorescence microscope with Apotome. Images were collected in Axiovision software using Axiocam HR or MR3 cameras. For thick specimens, stacks of images were collected and processed using Zeiss Axiovision 6D Acquisition or Extended Focus modules and some fluorescence images were deconvolved using the Zeiss Axiovision Deconvolution module. Alternatively, specimens were viewed and images collected on Zeiss 510 META or Olympus FV1000 laser confocal microscopes.
Histology
For immunohistochemistry, larvae were fixed in 4% paraformaldehyde containing 1% DMSO in PBS, rinsed, embedded in agarose, then sectioned by vibratome at 150–200 µm. Sections were washed in PBS/1% DMSO/0.3% Triton-X pH 7.4 (PDTX), blocked in PDTX containing 10–20% heat inactivated goat serum then incubated overnight at 4°C with primary antibody. We used polyclonal antisera raised in rabbit against zebrafish sox10 (1 : 500; provided by B. Appel [94]), zebrafish foxd3 (1 : 400; D. Raible [47]), zebrafish mbp (1 : 50; W. Talbot [95]), and GFP (1 : 200; A11122, Invitrogen) as well as monoclonal antibodies against GFP (1 : 200; 3E6 A11120, Invitrogen) and acetylated α-tubulin (1 : 200; 6-11B-1, T6793 Sigma). After washing, sections were incubated with secondary antibodies (AlexaFluor 405, 488, 568, 647; Invitrogen), washed and imaged.
In situ hybridization of post-embryonic zebrafish followed [44], [84]. For some analyses larvae were sectioned at 100–300 µm by vibratome. Detailed methods for in situ hybridization are available online at http://protist.biology.washington.edu/dparichy/.
DiI injection
Cell Tracker CM-DiI (Invitrogen) was prepared as a stock solution in DMSO then diluted to 0.025–0.05% in 0.3 M sucrose just before use. Larvae were anesthetized briefly and injected with 1–2 nl of DiI using a borosilicate needle, imaged immediately to ascertain the specificity of staining in target tissues, then reared individually until analyzed.
Ex vivo time-lapse imaging
Larvae were rinsed with 10% Hanks medium, anesthetized and then sacrificed by decapitation using a razor blade. After removing the anterior portion of the trunk and discarding the tails, larval trunks were placed on 0.4 µm transwell membranes (Millipore) in glass-bottom dishes containing L-15 medium, 3% fetal bovine serum, and penicillin/streptomycin. Trunks were equilibrated at 28.5°C for 3 h then imaged for 18–24 h (20 or 30 minute intervals between images) on a Zeiss Observer inverted epifluorescence microscope. Comparisons between isolated trunks imaged continuously for 24 h and repeatedly anesthetized intact larvae did not reveal gross differences in the survival of mitfa::GFP+ cells, though average maximal estimated velocities of mitfa::GFP+ cells were reduced by ∼22% in cultured trunks as compared to intact larvae (P<0.05; N = 67 cells examined). Imaging over longer durations resulted in increased rates of cell death throughout the explant and thus were not used for analyses.
Pharmacological inhibition of ErbB activity
A stock solution of AG1478 [4-(3-chloroanilino)-6,7-dimethoxyquinazoline; Calbiochem] was diluted in DMSO. Embryos were treated with 3 µM AG1478 in 10% Hanks for through either 72 or 96 hours post-fertilization. To facilitate penetration, 0.5% DMSO was added to all media and embryos were dechorionated prior to treatment. Fish were reared in glass Petri dishes and solutions were changed daily.
EdU labeling
Larvae were incubated with 0.005% 5-ethynyl-2′-deoxyuridine (EdU; Intvitrogen) in 10% Hank's medium containing 1% DMSO for 36 h. Larvae were then sacrificed, fixed with 4% PFA/1% DMSO, and vibrotome sectioned (150–200 µm) for immunohistochemistry followed by histochemical detection of EdU according to manufacturer's recommendations.
Statistical analyses
Quantitative data were analyzed with JMP 8.0.2 (SAS Institute, Cary NC). Frequency data were examined using multiple logistic regression or contingency table analyses, and tested for effects of genotype, stage, and genotype x stage interactions. Significance of effects were assessed by likelihood ratio tests and non-significant factors were removed from the final models. Analyses of variance were used for continuous variables including counts. Residuals were examined for normality and homogeneity of variances, conditions that were achieved for some variables after transformation by square root or natural logarithm. Further details of statistical analyses are available upon request.
Supporting Information
Zdroje
1. BedelbaevaKSnyderAGourevitchDClarkLZhangXM
2010
Lack of p21 expression links cell cycle control and appendage
regeneration in mice.
Proc Natl Acad Sci U S A
107
5845
5850
2. SalewskiRPEftekharpourEFehlingsMG
2010
Are induced pluripotent stem cells the future of cell-based
regenerative therapies for spinal cord injury?
J Cell Physiol
222
515
521
3. GuoJKCantleyLG
2010
Cellular maintenance and repair of the kidney.
Annu Rev Physiol
72
357
376
4. VoogJJonesDL
2010
Stem cells and the niche: a dynamic duo.
Cell Stem Cell
6
103
115
5. InomataKAotoTBinhNTOkamotoNTanimuraS
2009
Genotoxic stress abrogates renewal of melanocyte stem cells by
triggering their differentiation.
Cell
137
1088
1099
6. MayackSRShadrachJLKimFSWagersAJ
2010
Systemic signals regulate ageing and rejuvenation of blood stem
cell niches.
Nature
463
495
500
7. NishimuraEKGranterSRFisherDE
2005
Mechanisms of hair graying: incomplete melanocyte stem cell
maintenance in the niche.
Science
307
720
724
8. AlisonMRIslamSWrightNA
2010
Stem cells in cancer: instigators and
propagators?
J Cell Sci
123
2357
2368
9. YoussefKKVan KeymeulenALapougeGBeckBMichauxC
2010
Identification of the cell lineage at the origin of basal cell
carcinoma.
Nat Cell Biol
12
299
305
10. LeLQShipmanTBurnsDKParadaLF
2009
Cell of origin and microenvironment contribution for
NF1-associated dermal neurofibromas.
Cell Stem Cell
4
453
463
11. Le DouarinNM
1999
The Neural Crest.
Cambridge
Cambride University Press
12. KelshRNHarrisMLColanesiSEricksonCA
2009
Stripes and belly-spots-A review of pigment cell morphogenesis in
vertebrates.
Semin Cell Dev Biol
20
90
104
13. ThomasAJEricksonCA
2008
The making of a melanocyte: the specification of melanoblasts
from the neural crest.
Pigment Cell Melanoma Res
21
598
610
14. CooperCDRaibleDW
2008
Mechanisms for reaching the differentiated state: Insights from
neural crest-derived melanocytes.
Semin Cell Dev Biol
15. NishimuraEKJordanSAOshimaHYoshidaHOsawaM
2002
Dominant role of the niche in melanocyte stem-cell fate
determination.
Nature
416
854
860
16. WongCEParatoreCDours-ZimmermannMTRochatAPietriT
2006
Neural crest-derived cells with stem cell features can be traced
back to multiple lineages in the adult skin.
J Cell Biol
175
1005
1015
17. RobinsonKCFisherDE
2009
Specification and loss of melanocyte stem cells.
Semin Cell Dev Biol
20
111
116
18. WhiteRMZonLI
2008
Melanocytes in development, regeneration, and
cancer.
Cell Stem Cell
3
242
252
19. NordlundJJBoissyREHearingVJKingRAOettingWS
2006
The Pigmentary System: Physiology and
Pathophysiology.
2nd Edition ed:Blackwell Publishing, Inc
20. RigelDS
1997
Malignant melanoma: incidence issues and their effect on
diagnosis and treatment in the 1990s.
Mayo Clin Proc
72
367
371
21. JemalASiegelRWardEHaoYXuJ
2009
Cancer statistics, 2009.
CA Cancer J Clin
59
225
249
22. BalchCMGershenwaldJESoongSJThompsonJFDingS
2010
Multivariate analysis of prognostic factors among 2,313 patients
with stage III melanoma: comparison of nodal micrometastases versus
macrometastases.
J Clin Oncol
28
2452
2459
23. BalchCMGershenwaldJESoongSJThompsonJFAtkinsMB
2009
Final version of 2009 AJCC melanoma staging and
classification.
J Clin Oncol
27
6199
6206
24. BalchCMSoongSJGershenwaldJEThompsonJFReintgenDS
2001
Prognostic factors analysis of 17,600 melanoma patients:
validation of the American Joint Committee on Cancer melanoma staging
system.
J Clin Oncol
19
3622
3634
25. UongAZonLI
2010
Melanocytes in development and cancer.
J Cell Physiol
222
38
41
26. MouawadRSebertMMichelsJBlochJSpanoJP
2010
Treatment for metastatic malignant melanoma: old drugs and new
strategies.
Crit Rev Oncol Hematol
74
27
39
27. BhatiaSTykodiSSThompsonJA
2009
Treatment of metastatic melanoma: an overview.
Oncology (Williston Park)
23
488
496
28. JilaveanuLBAzizSAKlugerHM
2009
Chemotherapy and biologic therapies for melanoma: do they
work?
Clin Dermatol
27
614
625
29. BarthAWanekLAMortonDL
1995
Prognostic factors in 1,521 melanoma patients with distant
metastases.
J Am Coll Surg
181
193
201
30. GuptaPBKuperwasserCBrunetJPRamaswamySKuoWL
2005
The melanocyte differentiation program predisposes to metastasis
after neoplastic transformation.
Nat Genet
37
1047
1054
31. DevotoSHStoiberWHammondCLSteinbacherPHaslettJR
2006
Generality of vertebrate developmental patterns: evidence for a
dermomyotome in fish.
Evol Dev
8
101
110
32. HawkesJW
1974
The structure of fish skin. I. General
organization.
Cell Tissue Res
149
147
158
33. ParichyDMElizondoMRMillsMGGordonTNEngeszerRE
2009
Normal table of postembryonic zebrafish development: staging by
externally visible anatomy of the living fish.
Developmental Dynamics
238
2975
3015
34. CeolCJHouvrasYWhiteRMZonLI
2008
Melanoma Biology and the Promise of Zebrafish.
Zebrafish
5
247
255
35. PattonEEWidlundHRKutokJLKopaniKRAmatrudaJF
2005
BRAF mutations are sufficient to promote nevi formation and
cooperate with p53 in the genesis of melanoma.
Curr Biol
15
249
254
36. ParichyDMTurnerJM
2003
Zebrafish puma mutant decouples pigment pattern and somatic
metamorphosis.
Developmental Biology
256
242
257
37. JohnsonSLAfricaDWalkerCWestonJA
1995
Genetic control of adult pigment stripe development in
zebrafish.
Dev Biol
167
27
33
38. ParichyDMRansomDGPawBZonLIJohnsonSL
2000
An orthologue of the kit-related gene
fms is required for development of neural crest-derived
xanthophores and a subpopulation of adult melanocytes in the zebrafish,
Danio rerio.
Development
127
3031
3044
39. HirataMNakamuraKKanemaruTShibataYKondoS
2003
Pigment cell organization in the hypodermis of
zebrafish.
Dev Dyn
227
497
503
40. HawkesJW
1974
The structure of fish skin. II. The chromatophore
unit.
Cell Tissue Res
149
159
172
41. ParichyDM
Turner JM. Cellular interactions during adult pigment stripe development
in zebrafish;
2003
Academic Press Inc Elsevier Science
486
42. NakamasuATakahashiGKanbeAKondoS
2009
Interactions between zebrafish pigment cells responsible for the
generation of Turing patterns.
Proc Natl Acad Sci U S A
106
8429
8434
43. BudiEHPattersonLBParichyDM
2008
Embryonic requirements for ErbB signaling in neural crest
development and adult pigment pattern formation.
Development
135
2603
2614
44. LarsonTAGordonTNLauHEParichyDM
2010
Defective adult oligodendrocyte and Schwann cell development,
pigment pattern, and craniofacial morphology in puma mutant zebrafish having
an alpha tubulin mutation.
Dev Biol
346
296
309
45. ParichyDMTurnerJMParkerNB
2003
Essential role for puma in development of postembryonic neural
crest-derived cell lineages in zebrafish.
Dev Biol
256
221
241
46. KelshRNDuttonKMedlinJEisenJS
2000
Expression of zebrafish fkd6 in neural crest-derived
glia.
Mech Dev
161
164
47. ListerJACooperCNguyenKModrellMGrantK
2006
Zebrafish Foxd3 is required for development of a subset of neural
crest derivatives.
Developmental Biology
290
92
104
48. StewartRAArduiniBLBerghmansSGeorgeREKankiJP
2006
Zebrafish foxd3 is selectively required for neural crest
specification, migration and survival.
Developmental Biology
292
174
188
49. DuttonKAPaulinyALopesSSElworthySCarneyTJ
2001
Zebrafish colourless encodes sox10 and specifies
non-ectomesenchymal neural crest fates.
Development
128
4113
4125
50. LuoRAnMArduiniBLHenionPD
2001
Specific pan-neural crest expression of zebrafish Crestin
throughout embryonic development.
Dev Dyn
220
169
174
51. HultmanKABaharyNZonLIJohnsonSL
2007
Gene Duplication of the zebrafish kit ligand and partitioning of
melanocyte development functions to kit ligand a.
PLoS Genet
3
e17
doi:10.1371/journal.pgen.0030017
52. ListerJARobertsonCPLepageTJohnsonSLRaibleDW
1999
nacre encodes a zebrafish microphthalmia-related protein that
regulates neural-crest-derived pigment cell fate.
Development
126
3757
3767
53. HouLPavanWJ
2008
Transcriptional and signaling regulation in neural crest stem
cell-derived melanocyte development: do all roads lead to
Mitf?
Cell Res
18
1163
1176
54. CurranKRaibleDWListerJA
2009
Foxd3 controls melanophore specification in the zebrafish neural
crest by regulation of Mitf.
Dev Biol
332
408
417
55. CurranKListerJAKunkelGRPrendergastAParichyDM
2010
Interplay between Foxd3 and Mitf regulates cell fate plasticity
in the zebrafish neural crest.
Dev Biol
344
107
118
56. ElworthySListerJACarneyTJRaibleDWKelshRN
2003
Transcriptional regulation of mitfa accounts for the sox10
requirement in zebrafish melanophore development.
Development
130
2809
2818
57. HarrisMLBaxterLLLoftusSKPavanWJ
2010
Sox proteins in melanocyte development and
melanoma.
Pigment Cell Melanoma Res
23
496
513
58. ParichyDMMellgrenEMRawlsJFLopesSSKelshRN
2000
Mutational analysis of endothelin receptor b1
(rose) during neural crest and pigment pattern
development in the zebrafish Danio rerio.
Dev Biol
227
294
306
59. ParichyDMRawlsJFPrattSJWhitfieldTTJohnsonSL
1999
Zebrafish sparse corresponds to an orthologue of c-kit and is
required for the morphogenesis of a subpopulation of melanocytes, but is not
essential for hematopoiesis or primordial germ cell
development.
Development
126
3425
3436
60. ParichyDMTurnerJM
2003
Temporal and cellular requirements for Fms signaling during
zebrafish adult pigment pattern development.
Development
130
817
833
61. ThomasAJEricksonCA
2009
FOXD3 regulates the lineage switch between neural crest-derived
glial cells and pigment cells by repressing MITF through a non-canonical
mechanism.
Development
136
1849
1858
62. AdameykoILallemendFAquinoJBPereiraJATopilkoP
2009
Schwann cell precursors from nerve innervation are a cellular
origin of melanocytes in skin.
Cell
139
366
379
63. NicholsDHWestonJA
1977
Melanogenesis in cultures of peripheral nervous tissue. I. The
origin and prospective fate of cells giving rise to
melanocytes.
Dev Biol
60
217
225
64. DupinEGlavieuxCVaigotPLe DouarinNM
2000
Endothelin 3 induces the reversion of melanocytes to glia through
a neural crest-derived glial-melanocytic progenitor.
Proc Natl Acad Sci U S A
97
7882
7887
65. DupinERealCGlavieux-PardanaudCVaigotPLe DouarinNM
2003
Reversal of developmental restrictions in neural crest lineages:
transition from Schwann cells to glial-melanocytic precursors in
vitro.
Proc Natl Acad Sci U S A
100
5229
5233
66. WatanabeKWashioYFujinamiYAritakiMUjiS
2008
Adult-type pigment cells, which color the ocular sides of
flounders at metamorphosis, localize as precursor cells at the proximal
parts of the dorsal and anal fins in early larvae.
Dev Growth Differ
50
731
741
67. YamadaTOkauchiMArakiK
2010
Origin of adult-type pigment cells forming the asymmetric pigment
pattern, in Japanese flounder (Paralichthys olivaceus).
Dev Dyn
239
3147
3162
68. LiuYLaboskyPA
2008
Regulation of embryonic stem cell self-renewal and pluripotency
by Foxd3.
Stem Cells
26
2475
2484
69. HannaLAForemanRKTarasenkoIAKesslerDSLaboskyPA
2002
Requirement for Foxd3 in maintaining pluripotent cells of the
early mouse embryo.
Genes Dev
2650
2661
70. TengLMundellNAFristAYWangQLaboskyPA
2008
Requirement for Foxd3 in the maintenance of neural crest
progenitors.
Development
135
1615
1624
71. MundellNALaboskyPA
2011
Neural crest stem cell multipotency requires Foxd3 to maintain
neural potential and repress mesenchymal fates.
Development
72. IgnatiusMSMooseHEEl-HodiriHMHenionPD
2008
colgate/hdac1 Repression of foxd3 expression is required to
permit mitfa-dependent melanogenesis.
Dev Biol
313
568
583
73. BuacKXuMCroninJWeeraratnaATHewittSM
2009
NRG1/ERBB3 signaling in melanocyte development and melanoma:
inhibition of differentiation and promotion of
proliferation.
Pigment Cell Melanoma Res
22
773
784
74. HultmanKABudiEHTeasleyDCGottliebAYParichyDM
2009
Defects in ErbB-dependent establishment of adult melanocyte stem
cells reveal independent origins for embryonic and regeneration
melanocytes.
PLoS Genet
5
e1000544
doi:10.1371/journal.pgen.1000544
75. YangCTJohnsonSL
2006
Small molecule-induced ablation and subsequent regeneration of
larval zebrafish melanocytes.
Development
133
3563
3573
76. YangCTSengelmannRDJohnsonSL
2004
Larval melanocyte regeneration following laser ablation in
zebrafish.
J Invest Dermatol
123
924
929
77. RawlsJFJohnsonSL
2003
Temporal and molecular separation of the kit receptor tyrosine
kinase's roles in zebrafish melanocyte migration and
survival.
Dev Biol
262
152
161
78. RawlsJFJohnsonSL
2001
Requirements for the kit receptor tyrosine kinase during
regeneration of zebrafish fin melanocytes.
Development
128
1943
1949
79. LeeYNachtrabGKlinsawatPWHamiDPossKD
2010
Ras controls melanocyte expansion during zebrafish fin stripe
regeneration.
Dis Model Mech
3
496
503
80. KondoSShirotaH
2009
Theoretical analysis of mechanisms that generate the pigmentation
pattern of animals.
Semin Cell Dev Biol
20
82
89
81. YamaguchiMYoshimotoEKondoS
2007
Pattern regulation in the stripe of zebrafish suggests an
underlying dynamic and autonomous mechanism.
Proc Natl Acad Sci U S A
104
4790
4793
82. GoodrichHBGreeneJM
1959
An experimental analysis of the development of a color pattern in
the fish Brachydanio albolineatus Blyth.
J exp Zool
141
15
45
83. GoodrichHBMarzulloCMBronsonWR
1954
An analysis of the formation of color patterns in two fresh-water
fish.
J exp Zool
125
487
505
84. LangMRPattersonLBGordonTNJohnsonSLParichyDM
2009
Basonuclin-2 requirements for zebrafish adult pigment pattern
development and female fertility.
PLoS Genet
5
e1000744
doi:10.1371/journal.pgen.1000744
85. GrecoVGuoS
2010
Compartmentalized organization: a common and required feature of
stem cell niches?
Development
137
1586
1594
86. LiLCleversH
2010
Coexistence of quiescent and active adult stem cells in
mammals.
Science
327
542
545
87. ZammitPS
2008
All muscle satellite cells are equal, but are some more equal
than others?
J Cell Sci
121
2975
2982
88. QuigleyIKTurnerJMNuckelsRJManuelJLBudiEH
2004
Pigment pattern evolution by differential deployment of neural
crest and post-embryonic melanophore lineages in Danio
fishes.
Development
131
6053
6069
89. BafaloukosDGogasH
2004
The treatment of brain metastases in melanoma
patients.
Cancer Treat Rev
30
515
520
90. PalmieriDChambersAFFelding-HabermannBHuangSSteegPS
2007
The biology of metastasis to a sanctuary site.
Clin Cancer Res
13
1656
1662
91. UrasakiAMorvanGKawakamiK
2006
Functional dissection of the Tol2 transposable element identified
the minimal cis-sequence and a highly repetitive sequence in the subterminal
region essential for transposition.
Genetics
174
639
649
92. KwanKMFujimotoEGrabherCMangumBDHardyME
2007
The Tol2kit: A multisite gateway-based construction kit forTol2
transposon transgenesis constructs.
Developmental Dynamics
236
3088
3099
93. BudiEHPattersonLBParichyDM
2010
Morphogenesis of post-embryonic neural crest-derived pigment cell
precursors revealed by time lapse imaging during zebrafish adult pigment
pattern formation.
Dev Biol
344
448
94. ParkHCBoyceJShinJAppelB
2005
Oligodendrocyte specification in zebrafish requires
notch-regulated cyclin-dependent kinase inhibitor function.
J Neurosci
25
6836
6844
95. LyonsDAPogodaHMVoasMGWoodsIGDiamondB
2005
erbb3 and erbb2 are essential for schwann cell migration and
myelination in zebrafish.
Curr Biol
15
513
524
96. KelshRNSchmidBEisenJS
2000
Genetic analysis of melanophore development in zebrafish
embryos.
Dev Biol
225
277
293
97. GuyonneauLMurisierFRossierAMoulinABeermannF
2004
Melanocytes and pigmentation are affected in dopachrome
tautomerase knockout mice.
Mol Cell Biol
24
3396
3403
98. CarneyTJDuttonKAGreenhillEDelfino-MachinMDufourcqP
2006
A direct role for Sox10 in specification of neural crest-derived
sensory neurons.
Development
133
4619
4630
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 5
-
Všechny články tohoto čísla
- Structural and Functional Differences in the Long Non-Coding RNA in Mouse and Human
- Identification, Replication, and Functional Fine-Mapping of Expression Quantitative Trait Loci in Primary Human Liver Tissue
- A −436C>A Polymorphism in the Human Gene Promoter Associated with Severe Childhood Malaria
- A Decline in p38 MAPK Signaling Underlies Immunosenescence in
- The Operon Balances the Requirements for Vegetative Stability and Conjugative Transfer of Plasmid R388
- Novel and Conserved Protein Macoilin Is Required for Diverse Neuronal Functions in
- Ixr1 Is Required for the Expression of the Ribonucleotide Reductase Rnr1 and Maintenance of dNTP Pools
- Genome of Strain SmR1, a Specialized Diazotrophic Endophyte of Tropical Grasses
- A Deficiency of Ceramide Biosynthesis Causes Cerebellar Purkinje Cell Neurodegeneration and Lipofuscin Accumulation
- A Latent Pro-Survival Function for the Mir-290-295 Cluster in Mouse Embryonic Stem Cells
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time
- Prion Formation and Polyglutamine Aggregation Are Controlled by Two Classes of Genes
- Integrated Genome-Scale Prediction of Detrimental Mutations in Transcription Networks
- Post-Embryonic Nerve-Associated Precursors to Adult Pigment Cells: Genetic Requirements and Dynamics of Morphogenesis and Differentiation
- A Novel Mouse Synaptonemal Complex Protein Is Essential for Loading of Central Element Proteins, Recombination, and Fertility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
- A Genetic and Structural Study of Genome Rearrangements Mediated by High Copy Repeat Ty1 Elements
- A Missense Mutation in Causes a Major QTL Effect on Ear Size in Pigs
- A Flurry of Folding Problems: An Interview with Susan Lindquist
- Meiotic Recombination Intermediates Are Resolved with Minimal Crossover Formation during Return-to-Growth, an Analogue of the Mitotic Cell Cycle
- A Nervous Origin for Fish Stripes
- The ISWI Chromatin Remodeler Organizes the hsrω ncRNA–Containing Omega Speckle Nuclear Compartments
- The Telomerase Subunit Est3 Binds Telomeres in a Cell Cycle– and Est1–Dependent Manner and Interacts Directly with Est1
- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání