A Nervous Origin for Fish Stripes
article has not abstract
Published in the journal:
. PLoS Genet 7(5): e32767. doi:10.1371/journal.pgen.1002081
Category:
Perspective
doi:
https://doi.org/10.1371/journal.pgen.1002081
Summary
article has not abstract
The current excitement about adult stem and progenitor cells is an opportunity for developmental biologists to shed new light on old problems. Where do differentiated cells in the adult come from, and what else can their progenitor cells do? These questions are especially accessible in the zebrafish, where there is a deep experimental and genetic toolbox, and a developmental pathway with a well-defined metamorphic transition from larvae to adult. One of the premier model systems in this premier-league model organism is pigmentation, since pigment cells in fish—including black melanocytes (known as melanophores in fish), yellow xanthophores, and reflective iridophores—are organized into an array of beautiful, accessible, and evolutionarily diverse patterns that have helped motivate their study [1], [2]. Adult zebrafish show a longitudinally striped pigment pattern, distinct from the embryonic one. The adult pigment cells derive primarily from dormant stem cells, not embryonic pigment cells. But the cellular identity and location of these adult pigment cell progenitors has remained mysterious.
In this issue of PLoS Genetics, Budi et al. [3] provide an answer to the cryptic origin of adult pigment cells, concluding that they originate from cells associated with the peripheral nervous system (Figure 1). The findings of this study are remarkably similar to recent work on the developmental origin of mammalian melanocytes [4], demonstrating (once again) the conservation of developmental principles across large evolutionary distances.
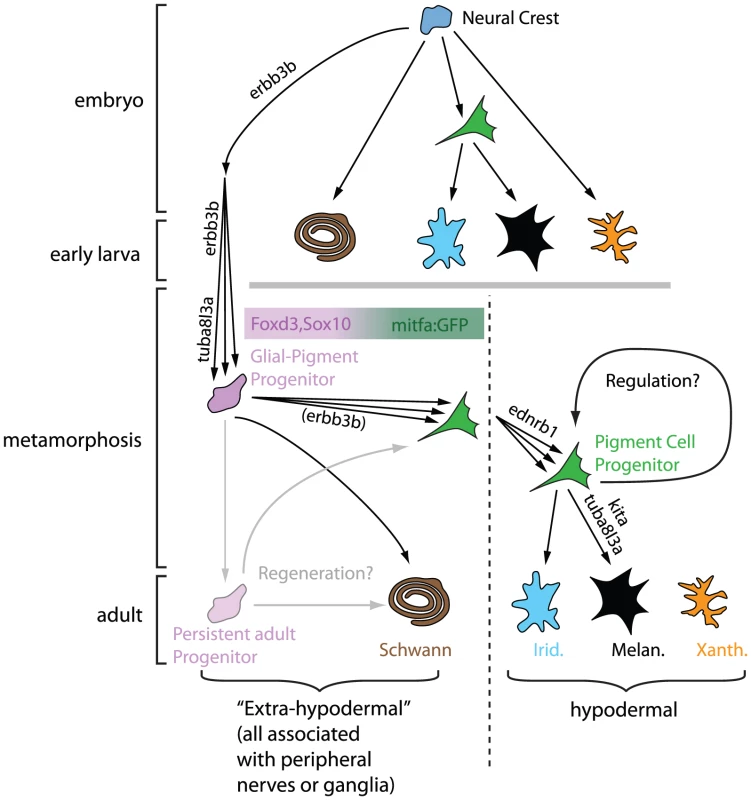
Cryptic, but Now Revealed
Budi et al. begin their studies by showing that cells that express foxd3 and sox10, markers of both glial and early neural crest cells [5]–[7], are seen in mid-metamorphic stage larvae, in dorsal root ganglia, myotomes, and the base of the dorsal fin. They define these cells as “extra-hypodermal”, since all are outside of the skin, where adult pigment cells are normally found. They noticed that a transgene, mitfa::GFP, which in embryos marks pigment cell precursors and differentiating melanocytes [8], is expressed by numerous extra-hypodermal cells, but not before metamorphic stages. Double-labelling experiments suggest that mitfa::GFP cells derive from foxd3- and sox10-expressing cells and that some cells expressing these markers become proliferative prior to and during early metamorphosis. All these progenitor cells are strongly associated with peripheral nerves. Crucially, DiI labelling and time-lapse analysis of mitfa::GFP fish demonstrate that a proportion of labelled cells move into the skin, where some differentiate into melanocytes or iridophores.
Further support for the authors' model came from exquisitely detailed quantitative comparisons of progenitor behaviour in key patterning mutants. Many fewer mitfa::GFP progenitors arrive in the skin in erbb3b and tuba813a mutants, which are known to be required for formation of melanocyte progenitors [9], [10]; once there, the mutant cells show abnormalities of differentiation. Previous studies have distinguished early (Kit-dependent) and late (Csfr1- and Ednrb1-dependent) melanophores, differing in where they form and how they contribute to adult stripes [11]–[14]. The authors also show differences in proliferative and differentiation behaviour of mitfa::GFP progenitors in mutants for these genes. To what extent these differences can explain the distinctive phenotypes remains to be established, and will likely require detailed mathematical modelling of the patterning process. Nevertheless, this study provides an unprecedented amount of quantitative data on which to base modelling efforts.
An interesting observation emerging from this work comes in particular from the erbb3b (also known as picasso) mutant studies. Although this mutation severely reduces the number of pigment cell precursors (as defined by expression of mitfa::GFP) during metamorphosis, the few mutant cells that do survive to reach the hypodermis actually exhibit a greater degree of proliferation and differentiation relative to wild type. This observation seems to demonstrate the often assumed, but rarely shown, regulative behaviour of pigment cells in vivo.
Finally, the fish's ability to regenerate pigment cells is another fascinating aspect of this model system [15]. It is intriguing that Budi et al. show foxd3 or mitfa::GFP-expressing extra-hypodermal cells persisting in adults since this suggests that they may contribute to regeneration of melanocytes. They use an elegant genetic approach to show that adults have limited regenerative capacity for pigment cells, at least in the double-mutant background used. Although not assessed here, it would be interesting to examine whether extra-hypodermal progenitor cell numbers become correspondingly reduced during this experiment.
Answers and More Questions
This study provides compelling evidence for a neural origin of adult pigment cells and reveals the mitfa::GFP transgene as an invaluable marker for the early stages of their development. These breakthroughs inevitably suggest new questions. Are these progenitor cells neural crest-derived? While overwhelmingly likely, this still needs to be formally shown. Do all adult pigment cells arise from these progenitor cells? The authors have not been able to identify the origin of xanthophores, and the possibility that some melanophores and iridophores derive from other cells remains. Where are the pigment cell stem cells? The authors show that progenitors for mitfa::GFP cells express foxd3 and sox10 and reside within the peripheral nervous system. This may suggest a dispersed, axon-associated source of pigment cell progenitors. However, these latter cells themselves may arise from spatially more localized stem cells, perhaps in ganglia or specific niches of the nerves, which simply use the peripheral nerves as convenient tramways for dispersal. Even if the stem cells are dispersed and axon-associated, what do they look like? Do differentiated satellite glia and/or Schwann cells have latent potency to form pigment cells? Or are there distinct, dormant stem cells intermingled with them? For now, a Schwann cell precursor, as in mammals [4], is the most parsimonious hypothesis, since both Sox10 and Foxd3 are involved in maintaining multipotency [16]–[18] and are expressed in Schwann cell precursors [5], [6]. Answering these questions will require intensive lineage-tracing studies of single cells at different stages prior to and during metamorphosis.
The progenitor populations identified here are abundant during metamorphosis, and it is far from clear that all will form pigment cells. While many may remain as a source of regenerative cells, what cell types can they form? The authors are understandably cautious, but propose that the foxd3/sox10-expressing cells are tripotent (glia/melanocyte/iridophore), while the mitfa::GFP-expressing cells may be bipotent (melanophore/iridophore), although separate unipotent progenitors for each cell type are not yet ruled out. Detailed lineage studies will address these issues, and may reveal other, unexpected, derivatives.
This landmark study provides compelling evidence for the location and identity of a cellular source of fish adult pigment cells. We can now anticipate comprehensive examination of these cells in the many available mutants, promising mechanistic insight into the enduring problem of adult pigment pattern formation. Beyond this, the identification of the cryptic progenitors of adult pigment cells should make the fish pigmentary system another tractable model in which to explore general principles of adult stem cell biology. For example, issues such as how stem cell potency is controlled and develops, how multiple cell types are generated in appropriate numbers from them, and how these cells are activated during regeneration can all be explored with this system. It is now just a matter of time before fish pigmentation helps colour our understanding of adult stem cell biology!
Zdroje
1. KelshRNHarrisMLColanesiSEricksonCA 2008 Stripes and belly-spots-A review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol
2. ParichyDM 2006 Evolution of danio pigment pattern development. Heredity 97 200 210
3. BudiEHPattersonLBParichyDM 2011 Post-embryonic nerve-associated precursors to adult pigment cells: genetic requirements and dynamics of morphogenesis and differentiation. PLoS Genet 7 5 e1002044 doi:10.1371/journal.pgen.1002044
4. AdameykoILallemendFAquinoJBPereiraJATopilkoP 2009 Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139 366 379
5. DuttonKAPaulinyALopesSSElworthySCarneyTJ 2001 Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128 4113 4125
6. KelshRNDuttonKMedlinJEisenJS 2000 Expression of zebrafish fkd6 in neural crest-derived glia. Mech Dev 93 161 164
7. OdenthalJNusslein-VolhardC 1998 fork head domain genes in zebrafish. Dev Genes Evol 208 245 258
8. CurranKRaibleDWListerJA 2009 Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev Biol 332 408 417
9. BudiEHPattersonLBParichyDM 2008 Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development 135 2603 2614
10. ParichyDMTurnerJMParkerNB 2003 Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish. Dev Biol 256 221 241
11. JohnsonSLAfricaDWalkerCWestonJA 1995 Genetic control of adult pigment stripe development in zebrafish. Dev Biol 167 27 33
12. ParichyDMMellgrenEMRawlsJFLopesSSKelshRN 2000 Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish danio rerio. Dev Biol 227 294 306
13. ParichyDMRansomDGPawBZonLIJohnsonSL 2000 An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development 127 3031 3044
14. ParichyDMRawlsJFPrattSJWhitfieldTTJohnsonSL 1999 Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development 126 3425 3436
15. O'Reilly-PolTJohnsonSL 2009 Melanocyte regeneration reveals mechanisms of adult stem cell regulation. Semin Cell Dev Biol 20 117 124
16. KimJLoLDormandEAndersonDJ 2003 SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38 17 31
17. MundellNALaboskyPA 2011 Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development 138 641 652
18. LiuYLaboskyPA 2008 Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells 26 2475 2484
Štítky
Genetika Reprodukční medicínaČlánek vyšel v časopise
PLOS Genetics
2011 Číslo 5
Nejčtenější v tomto čísle
- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
- A Nervous Origin for Fish Stripes
