-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIxr1 Is Required for the Expression of the Ribonucleotide Reductase Rnr1 and Maintenance of dNTP Pools
The Saccharomyces cerevisiae Dun1 protein kinase is a downstream target of the conserved Mec1-Rad53 checkpoint pathway. Dun1 regulates dNTP pools during an unperturbed cell cycle and after DNA damage by modulating the activity of ribonucleotide reductase (RNR) by multiple mechanisms, including phosphorylation of RNR inhibitors Sml1 and Dif1. Dun1 also activates DNA-damage-inducible genes by inhibiting the Crt1 transcriptional repressor. Among the genes repressed by Crt1 are three out of four RNR genes: RNR2, RNR3, and RNR4. The fourth RNR gene, RNR1, is also DNA damage-inducible, but is not controlled by Crt1. It has been shown that the deletion of DUN1 is synthetic lethal with the deletion of IXR1, encoding an HMG-box-containing DNA binding protein, but the reason for this lethality is not known. Here we demonstrate that the dun1 ixr1 synthetic lethality is caused by an inadequate RNR activity. The deletion of IXR1 results in decreased dNTP levels due to a reduced RNR1 expression. The ixr1 single mutants compensate for the reduced Rnr1 levels by the Mec1-Rad53-Dun1-Crt1–dependent elevation of Rnr3 and Rnr4 levels and downregulation of Sml1 levels, explaining why DUN1 is indispensible in ixr1 mutants. The dun1 ixr1 synthetic lethality is rescued by an artificial elevation of the dNTP pools. We show that Ixr1 is phosphorylated at several residues and that Ser366, a residue important for the interaction of HMG boxes with DNA, is required for Ixr1 phosphorylation. Ixr1 interacts with DNA at multiple loci, including the RNR1 promoter. Ixr1 levels are decreased in Rad53-deficient cells, which are known to have excessive histone levels. A reduction of the histone gene dosage in the rad53 mutant restores Ixr1 levels. Our results demonstrate that Ixr1, but not Dun1, is required for the proper RNR1 expression both during an unperturbed cell cycle and after DNA damage.
Published in the journal: . PLoS Genet 7(5): e32767. doi:10.1371/journal.pgen.1002061
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002061Summary
The Saccharomyces cerevisiae Dun1 protein kinase is a downstream target of the conserved Mec1-Rad53 checkpoint pathway. Dun1 regulates dNTP pools during an unperturbed cell cycle and after DNA damage by modulating the activity of ribonucleotide reductase (RNR) by multiple mechanisms, including phosphorylation of RNR inhibitors Sml1 and Dif1. Dun1 also activates DNA-damage-inducible genes by inhibiting the Crt1 transcriptional repressor. Among the genes repressed by Crt1 are three out of four RNR genes: RNR2, RNR3, and RNR4. The fourth RNR gene, RNR1, is also DNA damage-inducible, but is not controlled by Crt1. It has been shown that the deletion of DUN1 is synthetic lethal with the deletion of IXR1, encoding an HMG-box-containing DNA binding protein, but the reason for this lethality is not known. Here we demonstrate that the dun1 ixr1 synthetic lethality is caused by an inadequate RNR activity. The deletion of IXR1 results in decreased dNTP levels due to a reduced RNR1 expression. The ixr1 single mutants compensate for the reduced Rnr1 levels by the Mec1-Rad53-Dun1-Crt1–dependent elevation of Rnr3 and Rnr4 levels and downregulation of Sml1 levels, explaining why DUN1 is indispensible in ixr1 mutants. The dun1 ixr1 synthetic lethality is rescued by an artificial elevation of the dNTP pools. We show that Ixr1 is phosphorylated at several residues and that Ser366, a residue important for the interaction of HMG boxes with DNA, is required for Ixr1 phosphorylation. Ixr1 interacts with DNA at multiple loci, including the RNR1 promoter. Ixr1 levels are decreased in Rad53-deficient cells, which are known to have excessive histone levels. A reduction of the histone gene dosage in the rad53 mutant restores Ixr1 levels. Our results demonstrate that Ixr1, but not Dun1, is required for the proper RNR1 expression both during an unperturbed cell cycle and after DNA damage.
Introduction
Cells experiencing DNA damage or replication blocks activate stress response pathways, or checkpoints, that arrest the cell cycle and facilitate DNA repair. In budding yeast, the key checkpoint protein kinases are Mec1 (homolog of human ATR) and Rad53 (homolog of CHK2 and functional homolog of CHK1 in human), reviewed in [1], [2]. In human cells, ATR and CHK2 are upstream regulators of p53 and are inactivated in many cancers.
In Saccharomyces cerevisiae, Mec1 and Rad53 are essential and control phosphorylation and activation of the checkpoint kinase Dun1 [3] (Figure 1). Dun1 shares homology with Rad53 and Chk2 and is required for the DNA damage response. The essential function of the Mec1-Rad53-Dun1 pathway is to maintain an adequate supply of dNTPs by regulating the activity of ribonucleotide reductase (RNR) during the normal cell cycle [4]–[6]. RNR catalyzes the rate-limiting step in the biosynthesis of all four dNTPs and maintains both their balance and appropriate concentration. Full activation of RNR in S. cerevisiae by the Mec1-Rad53-Dun1 checkpoint in response to DNA damage results in a 6 - to 8-fold increase in dNTP concentration [7]. Such increases in dNTP concentration during DNA damage correlate with DNA damage tolerance. Four genes encode yeast RNR: RNR1 and RNR3 encode the large subunit [8], [9], and RNR2 and RNR4 encode the small subunit [10]–[13].
Fig. 1. Mec1-Rad53-Dun1–dependent regulation of S. cerevisiae ribonucleotide reductase. 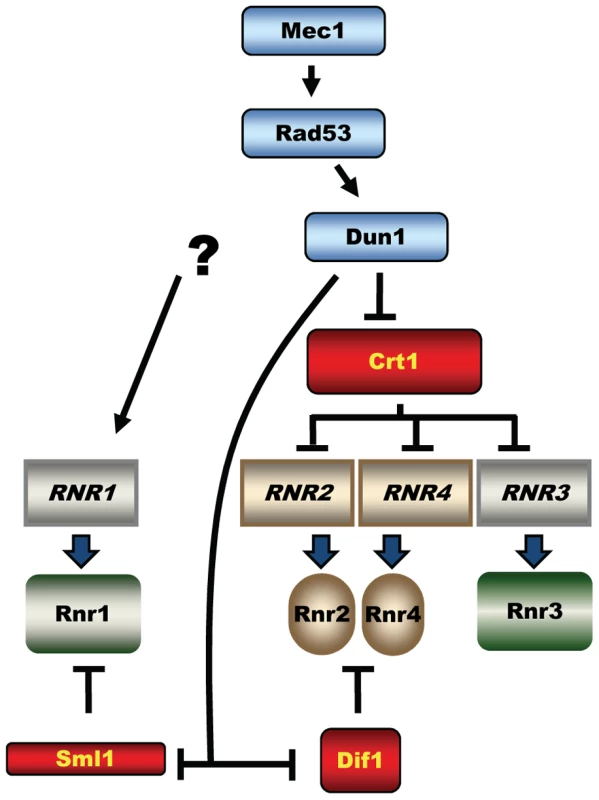
The activated Dun1 kinase relieves inhibition of RNR by targeting the transcriptional repressor Crt1(Rfx1) and protein inhibitors Sml1 and Dif1. The three key targets of the Mec1-Rad53-Dun1 pathway are Sml1, a protein inhibitor of RNR; Crt1 (Rfx1), a transcription factor; and Dif1, a protein that regulates the nuclear retention of Rnr2 and Rnr4 (Figure 1). Phosphorylation of Sml1 during S phase or after DNA damage by Dun1 targets Sml1 for proteolysis, which relieves the inhibition of RNR activity [14]. Phosphorylation of Dif1 releases Rnr2 and Rnr4 into the cytoplasm, where they combine with Rnr1 to form an active RNR complex [15], [16]. Crt1 blocks transcription at target promoters through recruitment of the general repressors Tup1 and Ssn6 [17]. Phosphorylation of Crt1 in a Mec1-Rad53-Dun1–dependent manner after DNA damage or replication stress promotes its dissociation from target promoters and activation of transcription. Crt1 represses RNR2, RNR3, and RNR4 [12], [17]. RNR3 is not essential and is normally expressed at very low levels, but is highly induced by DNA damage and has been used in genetic screens for the identification of both DUN1 and CRT1 [3], [17]. Interestingly, the fourth RNR gene, RNR1, is also DNA damage inducible but does not contain the Crt1-binding sites in its promoter and consequently is not repressed by Crt1. The mechanism of RNR1 activation by DNA damage remains unknown [17], [18].
The lethality of mec1 and rad53 mutants can be rescued either by deletion of SML1 (suppressor of mec1 lethality) [5], CRT1 [17], DIF1 [15], [16], or by overexpression of RNR1 or RNR3 [6], all resulting in increased RNR activity. In contrast, the deletion of DUN1 is not lethal and does not cause any obvious proliferation defects except for a slightly prolonged S phase, defects in mitochondrial propagation and decreased dNTP levels [3], [14], [19]. It is therefore possible that another pathway exists downstream of Mec1 and Rad53, functioning in parallel with Dun1. Alternatively, the elevation of dNTP levels by the Mec1-Rad53-Dun1 pathway is essential only in mec1 or rad53 mutants, because Mec1 and Rad53, in contrast to Dun1, are involved in a plethora of important chromosomal transactions, reviewed in [1].
Others have performed large-scale analyses of synthetic genetic interactions, in which the DUN1 gene was one of the baits. In two of such screens, DUN1 mutants were found to be synthetic lethal with a gene encoding the intrastrand cross-link recognition protein (Ixr1), but the reason for this synthetic interaction remains unknown [20], [21]. Ixr1 is a high mobility group (HMG) transcription factor first identified by its ability to bind DNA modified by the anticancer drug cisplatin (cis-diamminedichloriplatinum(II)) [22]. Very little is known about the cellular function of Ixr1. In addition to the two HMG boxes, Ixr1 has several polyglutamine regions, important for protein–protein interactions. Its closest homolog in yeast is Abf2 (TFAM in human), a mitochondrial DNA-binding protein important for replication and transcription [23]. Earlier, Ixr1 was implicated in aerobic transcriptional repression of COX5b, which encodes a subunit of mitochondrial cytochrome c oxidase [24].
Here we demonstrate that Ixr1 is required for the maintenance of the Rnr1 levels. In the absence or Ixr1, Rnr1 levels are decreased and became even lower after DNA damage, instead of increasing as in the wild-type. This observation explains the sensitivity of ixr1 mutants to hydroxyurea (HU), an inhibitor of RNR. In contrast, the levels of Rnr3 and Rnr4 in ixr1 mutants are increased due to the activation of the Mec1-Rad53-Dun1-Crt1 pathway, and increase even further after DNA damage and replication blocks, similar to wild-type. We show that deletion of SML1 or overexpression of RNR1 or RNR3 elevates dNTP pools and rescues the ixr1 dun1 synthetic lethality. The requirement for RNR activation in ixr1 via Dun1 explains why Dun1 is indispensible in ixr1 mutants.
Results
dun1Δ is synthetic lethal with ixr1-S366F
Earlier screens for synthetic genetic interactions between dun1Δ and other genes used a collection of yeast strains with null alleles in all nonessential genes. To facilitate identification of synthetic genetic interactions of DUN1 with essential genes we performed a colour-based synthetic lethal screen using a dun1Δ strain as described in the Materials and Methods. Briefly, the ade2 ade3 yeast strains are white unless a plasmid with ADE3 is present, conferring red color. Three mutations resulting in synthetic lethality with dun1Δ (first designated as mut1, mut2, and mut3) were isolated based on the inability of the ade2 ade3 dun1Δ mut strains to lose the pK503 plasmid carrying DUN1 and ADE3. MUT1 was identified as RAD53, MUT2 as WHI3, and MUT3 as IXR1. Sequencing of the rad53 mutant allele identified a single point mutation changing His 622 to Tyr. The H622 residue of Rad53 was identified before as crucial for the interaction of Rad53 with Rad9 [25], but the synthetic lethality of rad53-H622Y was not known. Sequencing of whi3 identified a single point mutation changing Gln in position 481 to a stop codon. Consistent with this observation, deletion of WHI3 showed a synthetic growth defect with dun1Δ in a large-scale analysis [20]. Finally, sequencing of the ixr1 mutant allele identified a single point mutation that changed Ser 366 to Phe in the first of the protein's two HMG boxes (Figure 2A). This highly conserved serine residue (Figure 2B) forms water-mediated hydrogen bonds to DNA bases and interacts with DNA [26]. In this study, we concentrated our efforts on the genetic interaction of DUN1 with IXR1.
Fig. 2. dun1 ixr1 synthetic lethality is rescued by increased RNR activity. 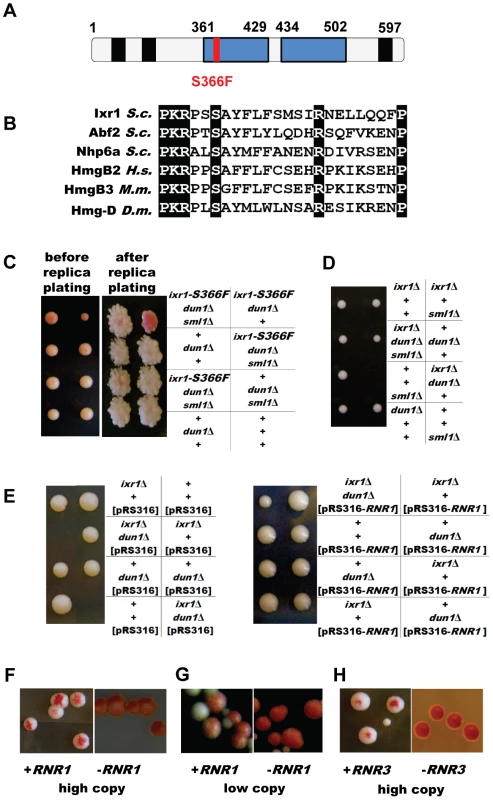
(A) Schematic representation of the Ixr1 protein. HMG boxes are shown in blue and polyQ regions in black. (B) Alignment of homologous HMG proteins close to the conserved S366. (C) Tetrad analysis demonstrating that deletion of SML1 rescues the dun1Δ ixr1-S366F synthetic lethality. The dun1Δ ixr1-S366F strain (TOY544) carries a DUN1-containing plasmid, pK503, which confers a red color; this plasmid is lost in ixr1-S366F dun1Δ sml1Δ colonies (TOY544×TOY588). (D) Tetrad analysis demonstrating that deletion of SML1 rescues the ixr1Δ dun1Δ synthetic lethality (TOY604×TOY527). (E) Tetrad analysis demonstrating that a low-copy RNR1 vector rescues the ixr1Δ dun1Δ synthetic lethality. The diploid strain ixr1/IXR1 dun1/DUN1 (TOY527×TOY603) was transformed with pRS316 and pBJ6 (pRS316-RNR1), sporulated and tetrad analysis was performed. (F) Overexpression of RNR1 rescues the ixr1-S366F dun1Δ synthetic lethality. The pK503 plasmid, containing the DUN1 gene and conferring red color, is lost on YP-Gal medium in the ixr1-S366F dun1Δ strain transformed with pESC-URA-pGAL1-RNR1 plasmid (left panel, sectoring phenotype), but not when transformed with pESC-URA-pGAL1 (right panel). (G) Low-copy RNR1 vector rescues the ixr1-S366F dun1Δ synthetic lethality. The pK503 plasmid, containing the DUN1 gene and conferring red color, is lost in the ixr1-S366F dun1Δ strain transformed with the pBJ6 plasmid (left panel, sectoring phenotype), but not when transformed with pRS316 (right panel). (H) Overexpression of RNR3 rescues the ixr1-S366F dun1Δ synthetic lethality. The pK503 plasmid, containing the DUN1 gene and conferring red color, is lost in the ixr1-S366F dun1Δ strain transformed with pBAD79 plasmid (left panel, sectoring phenotype), but not when transformed with pRS414 (right panel). Deletion of SML1 or elevated expression of RNR genes rescues dun1Δ ixr1 synthetic lethality
A well-established role of Dun1 is to increase RNR activity by targeting Sml1 and Dif1 for degradation and by transcriptional activation of RNR2, RNR3 and RNR4. Therefore, we asked whether deletion of SML1 or elevated expression of RNR genes rescues the lethality of dun1Δ ixr1. The dun1Δ ixr1-S366F [pK503] strain, originally identified in the screen, was crossed with a dun1Δ sml1Δ strain. Both strains are ade2 ade3 mutants. The unstable pK503 plasmid was lost in dun1Δ sml1Δ ixr1-S366F colonies, based on their white color, but not in dun1Δ ixr1-S366F colonies (Figure 2C). Next, we crossed ixr1Δ sml1Δ with dun1Δ. Tetrad analysis confirmed that dun1Δ ixr1Δ sml1Δ spores were viable while dun1Δ ixr1Δ were unable to germinate (Figure 2D). An additional copy of RNR1 rescues the dun1 ixr1 synthetic lethality, as demonstrated by sporulation and tetrad analysis of the ixr1/IXR1 dun1/DUN1 diploid strain transformed with a centromeric plasmid pBJ6 containing RNR1 under the control of the native promoter (Figure 2E). Finally, after transformation with plasmids overexpressing RNR1 (Figure 2F) or RNR3 (Figure 2H) or with the centromeric pBJ6 plasmid containing an additional copy of RNR1 (Figure 2G), we also observed the loss of the pK503 plasmid from the dun1Δ ixr1-S366F strain, leading to the sectoring phenotype.
Rnr3 and Rnr4 levels are increased in ixr1 mutants, while Sml1 levels are decreased
The requirement of Dun1 for ixr1 viability suggests that the Mec1-Rad53-Dun1 pathway is activated in ixr1 strains. A highly sensitive readout of this pathway's activation is induction of Crt1-controlled RNR2, RNR3, and RNR4. Indeed, Rnr3 and Rnr4 levels were higher in ixr1-S366F and ixr1Δ strains compared to wild-type (Figure 3A, lanes 1–3). Rnr2 levels were not significantly changed in ixr1Δ compared to wild-type (Figure 3B). Activation of the Mec1-Rad53-Dun1 pathway in ixr1 strains was not maximal; exposure to DNA damaging agents further increased Rnr2, Rnr3 and Rnr4 levels (Figure 3A and 3B). We were able to detect an increase in Rnr3-HA levels in the undamaged ixr1Δ strain only with the more sensitive anti-HA antibodies, but not with the polyclonal anti-Rnr3 antibodies, also indicating that the Mec1-Rad53-Dun1 pathway activation is low (Figure S1A). Importantly, the elevation of Rnr2, Rnr3 and Rnr4 in response to DNA damage was identical in the ixr1 and wild-type strains, indicating that the Mec1-Rad53-Dun1 pathway was not compromised in ixr1 mutants. Sml1 levels were decreased in ixr1, again suggesting that the Mec1-Rad53-Dun1 pathway was activated (Figure 3C). We did not observe a mobility shift of the Rad53 band in ixr1Δ, indicating low activation of the Mec1-Rad53-Dun1 pathway (Figure 3D). The full activation of the checkpoint by DNA damage resulted in hyperphosphorylation of Rad53 both in ixr1Δ and wild-type, leading to a shift of the Rad53 band (Figure 3D).
Fig. 3. Deletion of IXR1 leads to increased Rnr3 and Rnr4 levels and decreased Sml1 levels. 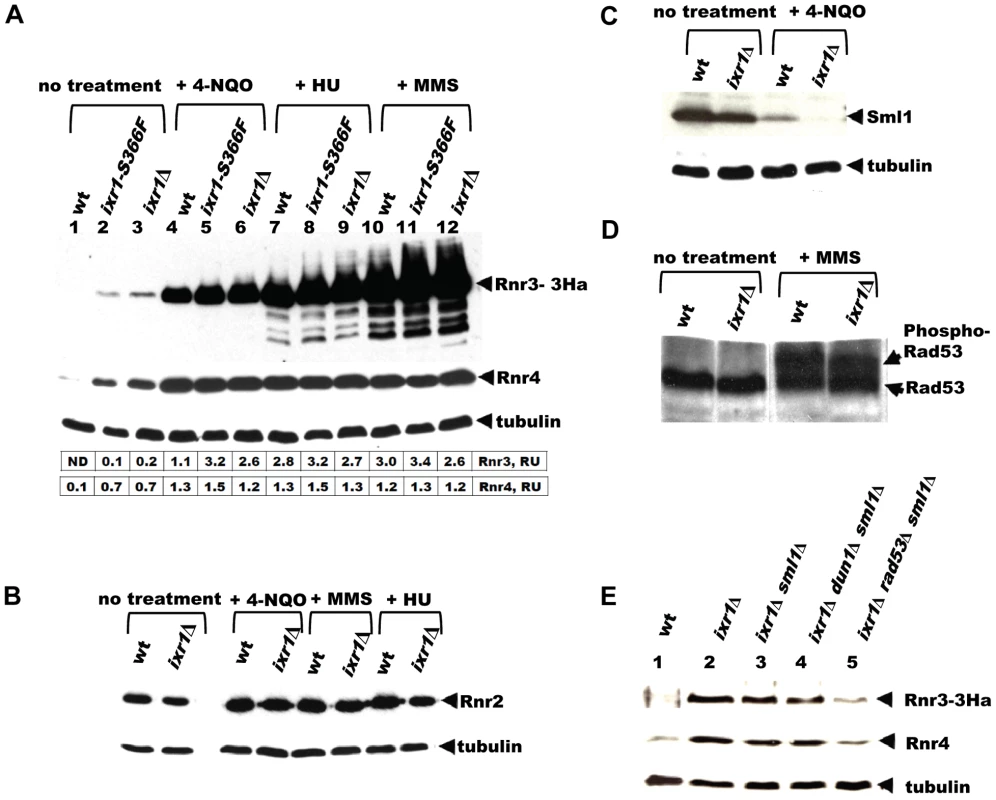
(A) Western blot analysis of Rnr3-HA and Rnr4 levels in wild-type (AC447-2A), ixr1-S366F (TOY619), and ixr1Δ (TOY621) strains before and after 2 hours treatment with 0.2 mg/L 4-nitroquinoline 1-oxide (4-NQO), 200 mM HU, or 0.02% methyl methanesulfonate (MMS). Rnr3 and Rnr4 levels were quantified in relative units (RU, levels of Rnr3 or Rnr4 divided by the levels of tubulin in corresponding sample) as described in Materials and Methods. ND – not detected. (B) Western blot analysis of Rnr2 levels in wild-type (AC447-2A) and ixr1Δ (TOY621) strains before treatment and after 2 hours treatment with 0.2 mg/L 4-NQO, 0.02% MMS, or 200 mM HU. (C) Western blot analysis of Sml1 levels in wild-type (W1588-4C) and ixr1Δ (TOY736) strains before treatment and after 2 hours treatment with 0.02% MMS. (D) Western blot analysis of Rad53 phosphorylation status in wild-type (W1588-4C) and ixr1Δ (TOY736) strains before treatment and after 2 hours treatment with 0.02% MMS. (E) Deletion of RAD53 but not of SML1 or DUN1 abolishes the upregulation of Rnr3 and Rnr4 levels in ixr1Δ. Western blot analysis of Rnr3-HA and Rnr4 levels. The following strains were analyzed: wt (AC447-2A), ixr1Δ (TOY732), ixr1Δ sml1Δ (TOY778), ixr1Δ dun1Δ sml1Δ (TOY772), and ixr1Δ rad53Δ sml1Δ (TOY781). Nevertheless, the increased Rnr3 and Rnr4 levels in ixr1Δ are Rad53 dependent. Deletion of RAD53, but not of DUN1, abolished upregulation of Rnr3 and Rnr4 in the ixr1Δ strain (Figure 3E). We interpret this result to mean that Rad53 can take over the Dun1 function and activate Rnr3 and Rnr4 expression in ixr1Δ when DUN1 is deleted, but not vice versa because Dun1 functions downstream of Rad53. A DUN1-independent pathway for RNR transcriptional induction was observed earlier, and it was suggested that Rad53 can directly recognize Dun1 substrates [12]. Alternatively, Rad53, and not Dun1, could be the main activator of Rnr3 and Rnr4 expression in the ixr1Δ strain in the absence of DNA damage.
dNTP levels are lower in the ixr1Δ strain compared to wild-type
Because the levels of several RNR proteins are increased (Rnr3, Rnr4) or unchanged (Rnr2) in ixr1Δ, while Sml1 levels are decreased, the dNTP levels might have been elevated in the ixr1 strains; however, dNTP levels were lower in ixr1Δ compared to wild-type (Figure 4A). Similarly, dNTP levels in ixr1Δ sml1Δ were lower than in sml1Δ alone (Figure 4A). DNA damage induction by 4-nitroquinoline 1-oxide (4-NQO) resulted in an increase in dNTP concentration in ixr1Δ, but to a lower level compared to wild-type (Figure 4B). The ixr1Δ mutant exhibited an increased frequency of petite formation (Figure 4C) and sensitivity to HU (Figure 4D), two phenotypes associated with decreased dNTP production [5]. Interestingly, although the dNTP levels were higher in ixr1Δ sml1Δ than in wild type, Rnr3 and Rnr4 in ixr1Δ sml1Δ were still elevated to the same levels as in the ixr1Δ single mutant (Figure 3E). This observation indicates that activation of the Mec1-Rad53-Dun1 pathway in ixr1Δ is not only due to a decreased dNTP production, but also due to some other defects.
Fig. 4. Deletion of IXR1 leads to decreased dNTP levels. 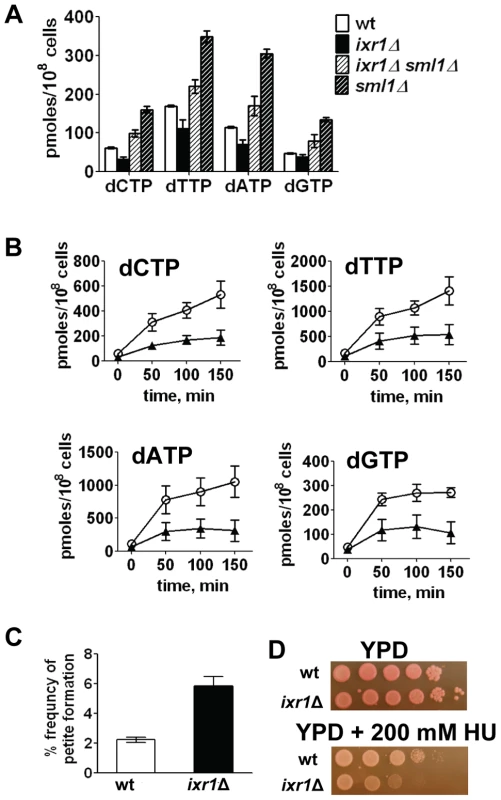
(A) dNTP levels are decreased in ixr1Δ (TOY736), increased in ixr1Δ sml1Δ (TOY714) compared to wild-type (W1588-4C), but lower than in sml1Δ (U952-3B). Values shown are the average from two independent experiments with the minimum and maximum values represented as error bars. (B) dNTP levels increase in ixr1Δ (TOY736, black triangles) during the treatment with 0.2 mg/L 4-NQO, but less than in a wild-type strain (W1588-4C, open circles). Values shown are the average from two independent experiments with the minimum and maximum values represented as error bars. (C) ixr1Δ (TOY736) has a higher frequency of petite formation than a wild-type strain (W1588-4C). (D) ixr1Δ (TOY736) is more sensitive to HU than a wild-type strain (W1588-4C). Levels of Rnr1 in ixr1 decrease after DNA damage
The paradoxical finding that dNTP pools decreased despite the activation of the Mec1-Rad53-Dun1 pathway in ixr1 strains indicates a deficiency in another component(s) of the RNR machinery. Analysis of Rnr1 steady state levels demonstrated moderately decreased levels in ixr1Δ and in ixr1-S366F compared to wild-type (∼64%, and ∼62% respectively) (Figure 5A, 5B, and 5C (0 min lanes)). As expected, incubation of wild-type cells in the presence of 4-NQO or HU for two hours led to an increase in Rnr1 levels (∼39% and 57%, respectively) (Figure 5A and 5B). Interestingly, the same treatment of ixr1-S366F and ixr1Δ led to a further reduction of Rnr1 to ∼19% and ∼37%, respectively, after 4-NQO treatment, and to ∼56% and ∼46%, respectively, after the HU treatment (Figure 5A and 5B). This reduction was continuous; Rnr1 levels remained lower in ixr1Δ throughout a 12-hour incubation with 4-NQO (Figure 5C). Based on flow-cytometric analysis, the ixr1Δ strain had a slightly greater proportion of cells in S phase compared to wild-type both before and during 4-NQO treatment (Figure 5C), although the overall proliferation rate was similar between ixr1Δ and wild-type (Figure 5D).
Fig. 5. Rnr1 levels are reduced in ixr1 after DNA damage. 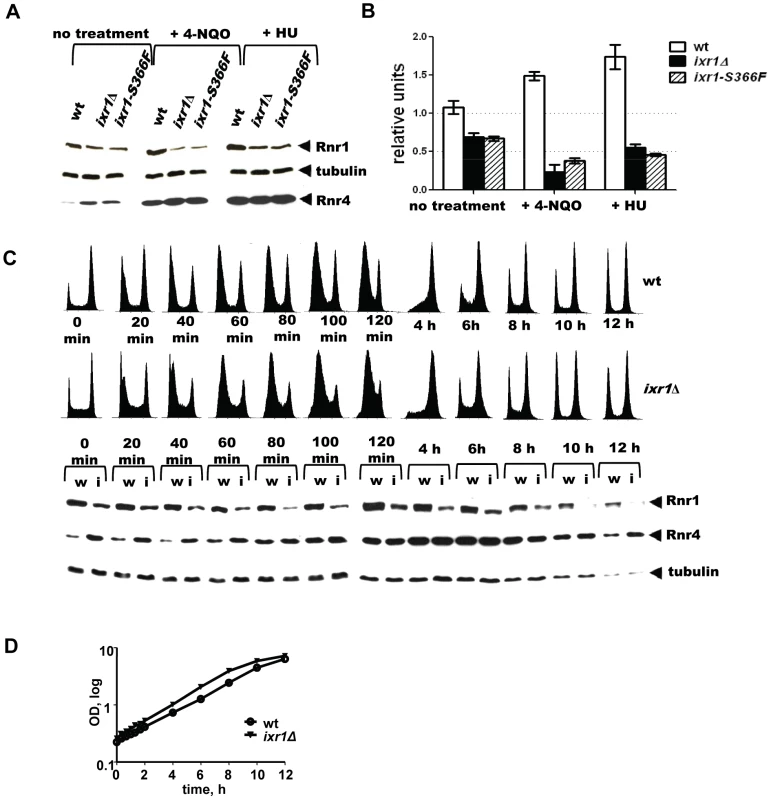
(A) Western blot analysis of Rnr1 levels in wild-type (wt) (W1588-4C), ixr1-S366F (TOY734), and ixr1Δ (TOY736) strains before and after 2 hours treatment with 0.2 mg/L 4-NQO or 200 mM HU. (B) Quantification of Rnr1 levels in wild-type (wt) (W1588-4C), ixr1Δ (TOY736) and ixr1-S366F (TOY734) strains before and after 2 hours treatment with 0.2 mg/L 4-NQO or 200 mM HU. Tubulin was used as the internal control (see Materials and methods). Error bars represent standard error of the mean (SEM). (C) Lower panel, dynamics of Rnr1 decrease and Rnr4 increase in wt (W1588-4C, lanes marked with “w”) and ixr1Δ (TOY736, lanes marked with “i”) strains during a 12-hour time-course incubation with 0.2 mg/L 4-NQO. Upper panel, the corresponding flow-cytometric histograms. (D) Proliferation curves for the experiment in Figure 4C. The decreased Rnr1 levels caused by ixr1Δ provide an explanation for the synthetic lethality displayed by the ixr1Δ dun1Δ double mutant strain: the dun1Δ strains are defective in relieving the inhibition of RNR imposed by Sml1, Dif1, and Crt1.
Deletion of IXR1 negatively affects RNR1 transcription
Using a β-galactosidase assay we demonstrated that the decreased Rnr1 levels in ixr1Δ are due to a lower RNR1 promoter activity, which indicates that Ixr1 directly or indirectly regulates RNR1 transcription (Figure 6A). Deletion of IXR1 also caused a concomitant increase in RNR3 and RNR4 promoter activities (Figure 6A), in agreement with the observed increase in Rnr3 and Rnr4 protein levels (Figure 3A). To gain further insight into the mechanism of RNR1 regulation by Ixr1, we performed chromatin immunoprecipitation (ChIP) experiments followed by qPCR using Ixr1-9xMyc fusion protein and 9E10 antiserum. We analyzed binding of Ixr1 to the RNR1 promoter (pRNR1) region. In addition, we analyzed the DSF2 promoter (pDSF2) region earlier identified as an Ixr1-interacting locus [27] and the actin (ACT1) open reading frame, a commonly used negative control. As another control, we used an untagged congenic IXR1 strain. Ixr1 interacted with all three loci (Figure 6B): relative to the input DNA we recovered 0.46%, 0.35% and 0.79% of pRNR1, pDSF2 and ACT1 loci, respectively, in the IXR1-9Myc strain. The interaction of Ixr1 with the RNR1 promoter did not change after the treatment of cells with 4-NQO (0.46% and 0.48%, respectively). In the untagged strain, DNA recovery was at background levels as judged by the ChIP samples where 9E10 antiserum was omitted. The precipitation of the ACT1 ORF locus indicates that Ixr1 binds to many loci in the genome.
Fig. 6. Ixr1 regulates RNR1 promoter activity. 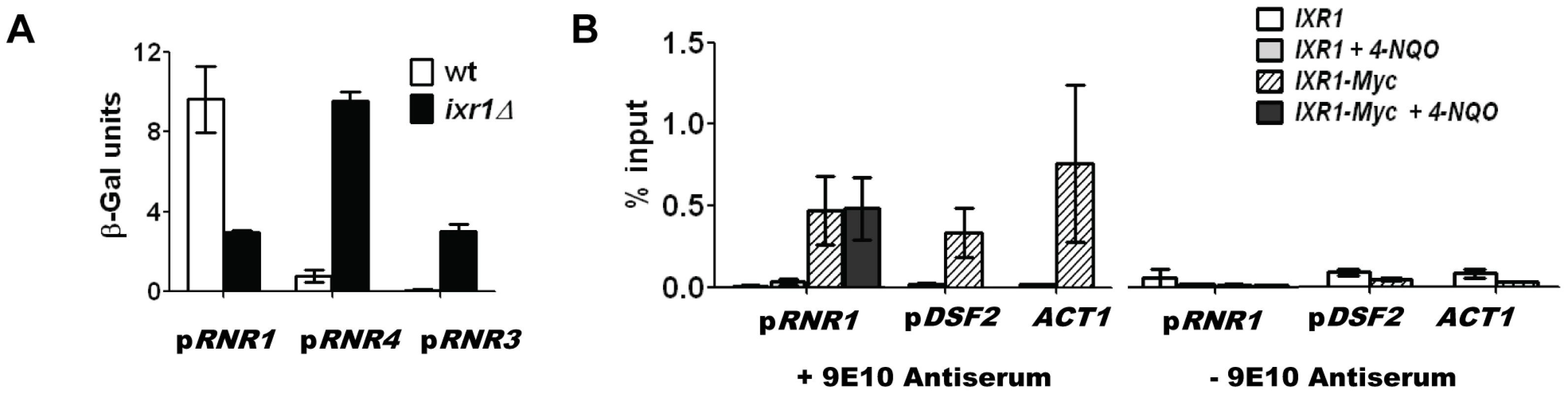
(A) β-Galactosidase assay of RNR1, RNR3, and RNR4 promoter activity in wild-type (W1588-4C) and ixr1Δ (TOY736) strains. Promoters of the analyzed genes were fused with the lacZ repoter gene and the respective plasmids were transformed in the wild type and ixr1Δ strains. β-Gal units were quantified as described in Materials and Methods. (B) Analysis of DNA associated with Ixr1 was performed by chromatin immunoprecipitation (ChIP) followed by qPCR using locus-specific primers for the RNR1 promoter (pRNR1), the DSF2 promoter (pDSF2) and the ACT1 gene. ChIP was performed with IXR1 (W1588-4C) and IXR1-9MYC (TOY836) strains using anti-Myc antiserum 9E10 or mock-antiserum. For pRNR1, ChIP was performed both without and with addition of 4-NQO (0.2 mg/L). Y axis represents the amount of precipitated DNA relative to the input DNA. Values shown are the average from two independent experiments with the minimum and maximum values represented. Elevation of Rnr1 levels in response to DNA damage depends on Rad53, but not Dun1
The DNA-damage-inducible genes become damage uninducible in dun1 mutants, because Dun1 is required to relieve the inhibition imposed by the transcriptional inhibitor Crt1. Indeed, induction of Rnr4 in response to 4-NQO is less pronounced in the dun1Δ sml1Δ strain compared to wt (Figure 7A). The RNR1 promoter, however, does not contain Crt1 sites and the expression of RNR1 is not affected by the CRT1 deletion [17], [18]. In Figure 7A, we demonstrate that the elevation of Rnr1 levels in response to DNA damage does not depend on DUN1, but does depend on Rad53 and Mec1. All checkpoint mutant strains in this experiment contained sml1Δ, because mec1Δ and rad53Δ are inviable otherwise. As a control, we demonstrate that the deletion of SML1 by itself has little effect on Rnr1 and Rnr4 levels in the wild type and ixr1Δ strains treated by 4-NQO (Figure S1C).
Fig. 7. Elevation of Rnr1 levels in response to DNA damage requires MEC1, RAD53, and IXR1, but not DUN1. 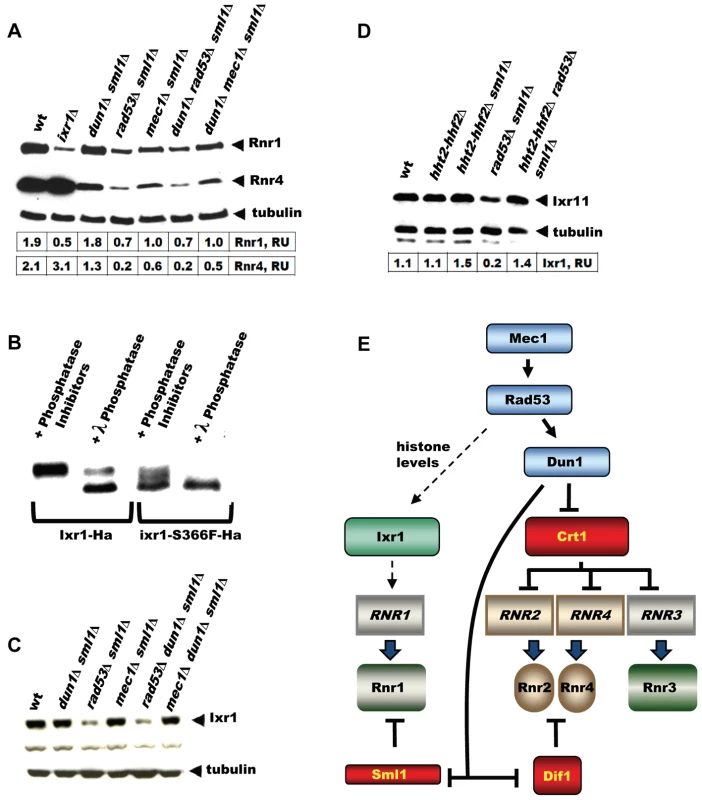
(A) Western blot analysis of Rnr1 and Rnr4 levels. The following strains were incubated with 0.2 mg/L 4-NQO for 2 hours: wt (W1588-4C), ixr1Δ (TOY736), dun1Δ sml1Δ (TOY728), rad53Δ sml1Δ (TOY782), mec1Δ sml1Δ (TOY711), dun1Δ rad53Δ sml1Δ (TOY786), and dun1Δ mec1Δ sml1Δ (TOY774). Rnr1 and Rnr4 levels were quantified in relative units (RU, levels of Rnr1 or Rnr4 divided by the levels of tubulin in corresponding sample) as described in Materials and Methods. (B) Western blot analysis of λ-phosphatase treated extracts from Ixr1-Ha (TOY655) and Ixr1-S366F-Ha (TOY650) strains. (C) Western blot analysis of Ixr1 levels in wt (W1588-4C), dun1Δ sml1Δ (TOY728), rad53Δ sml1Δ (TOY782), mec1Δ sml1Δ (TOY711), rad53Δ dun1Δ sml1Δ (TOY786) and mec1Δ dun1Δ sml1Δ (TOY774). (D) Western blot analysis of Ixr1 levels in wild-type (W1588-4C), hht2-hhf2Δ (TOY806), hht2-hhf2Δ sml1Δ (TOY821), rad53Δ sml1Δ (TOY782) and hht2-hhf2Δ rad53Δ sml1Δ (TOY819). Ixr1 levels were quantified in relative units (RU, levels of Ixr1 divided by the levels of tubulin in corresponding sample) as described in Materials and Methods. (E) Mec1-Rad53-Dun1-dependent regulation of S. cerevisiae ribonucleotide reductase. Expression of RNR1 depends on Mec1, Rad53 and Ixr1, but does not depend on Dun1 or Crt1. Ixr1 levels depend on histone dosage
Because Mec1, Rad53 and Dun1 are protein kinases, it was possible that they directly phosphorylate Ixr1 and modulate its function. We analyzed the phosphorylation status of Ixr1 and Ixr1-S366F proteins and found that Ixr1 is a phosphoprotein most likely phosphorylated at several residues (Figure 7B). Serine 366 is important for Ixr1 phosphorylation, as Ixr1-S366F separated by SDS-PAGE as several bands with higher mobility compared to Ixr1. Treatment of Ixr1 with λ-phosphatase increased the mobility of Ixr1 to that of Ixr1-S366F. However, we did not observe changes in Ixr1 mobility in mec1, rad53, or dun1 mutants (Figure 7C). Interestingly, Ixr1 levels were significantly lower in rad53Δ, but not in mec1Δ or dun1Δ, compared to wt (Figure 7C). Rad53 is known to regulate histone levels, and rad53 strains have increased amount of histones [28], [29]. Lowering histone dosage in the rad53 mutant strains by deleting copies of histone 3 and histone 4 genes (HHT2 and HHF2) restored Ixr1 to wild-type levels (Figure 7D).
Discussion
The Dun1 kinase is a downstream target of the Mec1-Rad53 checkpoint, which monitors the genome integrity. In S. cerevisiae, the Mec1-Rad53-Dun1 pathway also regulates the RNR activity both during the normal cell cycle and after DNA damage [4]. RNRs are instrumental in controlling dNTP balance and concentration [30]. Deletion of DUN1 is synthetic lethal with the deletion of many genes involved in DNA replication and DNA repair [20], [21]. Synthetic lethality of dun1 with a number of other genes remains unexplained. Here, we demonstrate that IXR1, deletion of which is synthetic lethal with dun1, is required for the normal expression of the RNR1 gene and maintenance of the dNTP pools.
In the absence of DNA damage, the deletion of IXR1 leads to a moderate decrease of Rnr1 and dNTP levels. This decrease is partially compensated by the activation of the Mec1-Rad53-Dun1 pathway. We base this conclusion on the following observations. First, Rnr3 and Rnr4, whose levels are controlled by the Mec1-Rad53-Dun1 pathway, are upregulated in ixr1, and Rad53 is required for this upregulation. Second, RNR inhibitor Sml1, whose levels are also controlled by the Mec1-Rad53-Dun1 pathway, is downregulated in ixr1. Third, DUN1 is indispensable in ixr1, but ixr1 dun1Δ synthetic lethality is rescued by elevated dNTP levels. We note that elevation of dNTP levels in ixr1Δ caused by the SML1 deletion does not eliminate the checkpoint activation (Figure 3E), indicating that deletion of IXR1 leads to replication stress not only because of the decreased dNTP levels expression but also because of other defects. It is conceivable that in addition to RNR1 some other genes involved in the DNA biosynthesis are regulated by Ixr1. The reported synthetic lethality of ixr1Δ with the origin recognition complex mutant orc2-1 [31] and synthetic sickness with the thymidylate kinase mutant cdc8-2 [32] indicate the importance of Ixr1 for the processes involved in DNA replication.
RNR expression increases in response to DNA damage in most organisms. In E. coli, nrdA and nrdB (encoding the large and the small RNR subunits, respectively) are among the most highly induced genes following UV exposure (induced ∼20 - and ∼7-fold, respectively) [33], [34]. In mammalian cells, DNA damage induces the p53R2 protein, an alternative small RNR subunit, about 4-fold in a p53-dependent manner [35]–[37]. Similarly, the Drosophila large RNR subunit, RnrL, is induced by ionizing radiation in wild-type, but not p53-deficient strains [38]. In the yeast Schizosaccharomyces pombe, RNR genes are among the most robustly induced genes following DNA damage [39]. All four S. cerevisiae RNR genes are activated by DNA damage and replication blocks [8], [10], [12]. The pathway involved in the activation of RNR2, RNR3 and RNR4 is well understood and requires the Mec1-Rad53-Dun1 kinase cascade, which targets Crt1, transcriptional inhibitor of DNA-damage-inducible genes (Figure 1A). Here we demonstrate that elevation of Rnr1 in response to DNA damage requires Mec1 and Rad53, but not Dun1 (Figure 7A). Earlier, it has been shown that RNR1 expression does not depend on Crt1 [17], [18]. Thus, the downstream Dun1-Crt1 part of the Mec1-Rad53-Dun1-Crt1 pathway, which is known to control the DNA-damage-inducible genes in yeast, is not involved in the regulation of Rnr1. Instead, the elevation of Rnr1 levels in response to DNA damage requires Ixr1 (Figure 7E).
In addition to Crt1, transcription of RNR2, RNR3 and RNR4 genes is also controlled by Rox1 and Mot3, the DNA binding proteins that repress the hypoxic genes by recruiting the Ssn6/Tup1 general repression complex. Again, in contrast to RNR2, RNR3, and RNR4 genes, no Rox1 or Mot3 sites are present in the RNR1 promoter [18]. Transcription of RNR1 is controlled by MBF, a dimeric transcription factor composed of Swi6 and Mbp1 [40]–[42]. Interestingly, Swi6 is directly phosphorylated by Rad53 in response to DNA damage [43]. It will be interesting to investigate whether Ixr1 is important for MBF-dependent regulation of the RNR1 promoter. Earlier, Ixr1 was implicated in controlling the levels of the hypoxic gene COX5b [24]. Currently, we do not know whether Ixr1 is involved in the activation of RNR1 expression in response to oxygen deprivation.
In contrast to many other HMG-box proteins, Ixr1 is rather large (68 kDa) and contains several polyglutamine repeats, which are often involved in protein-protein interactions and are present in many transcription factors. The HMG box is a conserved domain of ∼80 amino acids, binding to the minor groove of DNA. Proteins containing two or more HMG boxes usually recognize structural features of DNA without sequence specificity, while proteins containing one HMG box can recognize DNA in a sequence specific manner. In S. cerevisiae, there are two proteins containing two HMG boxes (Ixr1 and Abf2) and five proteins containing one HMG box (Nhp6A, Nhp6B, Nhp10, Hmo1 and Rox1). The closest homolog of Ixr1, Abf2, binds to many loci in the mitochondrial genome [44]. Yet, the HMG-box proteins with two or more HMG boxes can bind to specific loci in the genome. For example, human transcription factor UBF, which has 6 HMG boxes and belongs to the sequence-nonspecific class of HMG-box proteins, binds specifically to rDNA or to heterologous UBF-binding sequences from Xenopus integrated into ectopic sites on human chromosomes [45]. Our ChIP analysis of Ixr1 identified the RNR1 promoter as a binding locus. However, Ixr1 bound equally well at two other tested loci, the DSF2 promoter and the ACT1 open reading frame. Still, it is possible that, in the context of the RNR1 promoter, Ixr1 together with other proteins directly regulates RNR1 gene expression.
Interestingly, the mutation in the Ixr1 S366 residue that is important for interaction of HMG boxes with DNA results in the same phenotype as the deletion of IXR1 gene. We show that this serine residue is required for the phosphorylation of several amino acid residues in Ixr1. Currently we do not know the phosphorylation status of S366. It is possible that S366 itself is not phosphorylated, but its interaction with the DNA or other proteins is required for the phosphorylation of other residues in Ixr1. Multiple phosphorylation of Ixr1 causes an increase in the apparent molecular weight of the protein: Ixr1 separates by SDS-PAGE as a ∼85 kDa protein (not as predicted 68 kDa). We demonstrate that neither Mec1, nor Rad53, nor Dun1 are responsible for the phosphorylation of Ixr1, as its mobility is not affected in the respective mutants. The region of the HMG domain around the Ser366 residue has been shown to affect DNA binding specificity. All sequence-specific HMG proteins have an asparagine at this position, whereas all non-sequence-specific HMG proteins have a serine (e.g., Ser10 in the D. melanogaster HMG-D box co-crystallized with DNA) [26]. To our knowledge, crystal structures analyzing the interaction of HMG boxes and DNA were solved with the non-phosphorylated proteins. It would be interesting to investigate whether S366 is phosphorylated in Ixr1, whether the corresponding serine residues are phosphorylated in other HMG proteins in other species, and whether Ser366 phosphorylation affects DNA binding and/or makes binding of the HMG box sequence specific.
Although the mobility of Ixr1 is not changed, its levels are significantly reduced in the rad53Δ strain (Figure 7C). rad53 mutant strains are known to have increased histone levels due to a defect in histone degradation [28]. Increased histone levels presumably lead to decreased Ixr1 levels, because we show that decreasing histone dosage in the rad53 strain restores Ixr1 levels (Figure 7D). There are at least two possibilities explaining this interplay between Ixr1 and histone levels. Because Ixr1 contains two HMG boxes and therefore binds DNA presumably without sequence specificity, it might compete with histones for DNA binding. Increased histones in rad53Δ might displace Ixr1 from the IXR1 promoter, where it was shown to bind and regulate its own expression [46]. Alternative, but not exclusive possibility is that Ixr1 displaced by histones from DNA undergoes degradation.
In summary, we identify Ixr1 as a novel factor involved in regulation of dNTP pools and RNR1, a gene that, in contrast to all other known DNA-damage inducible genes, is not controlled by Dun1 and Crt1.
Materials and Methods
Yeast strains and primers
All yeast strains used in this study are congenic to W1588-4C [5]. Table 1 gives only the allele(s) that differ from the W1588-4C genotype. Table S1 lists primers used for strain construction. DUN1 was deleted using the KanMX4 cassette PCR-amplified with primers F_Dun1 and R_Dun1 from the dun1Δ::KanMX4 Y03798 strain (Euroscarf). The CY1263 ade3::HISG strain [47] was crossed with W1588-4C to select ade2 ade3 clones (TOY502). The resulting strain was crossed with dun1Δ::KanMX4 to create the strain used for the synthetic lethality screen (TOY527). The TOY836 (IXR1-9MYC) strain was generated by amplifying and introducing the 9MYC-TRP1 cassette from the Z1580 strain [48] into W1588-4C.
Tab. 1. Yeast strains used in this study. 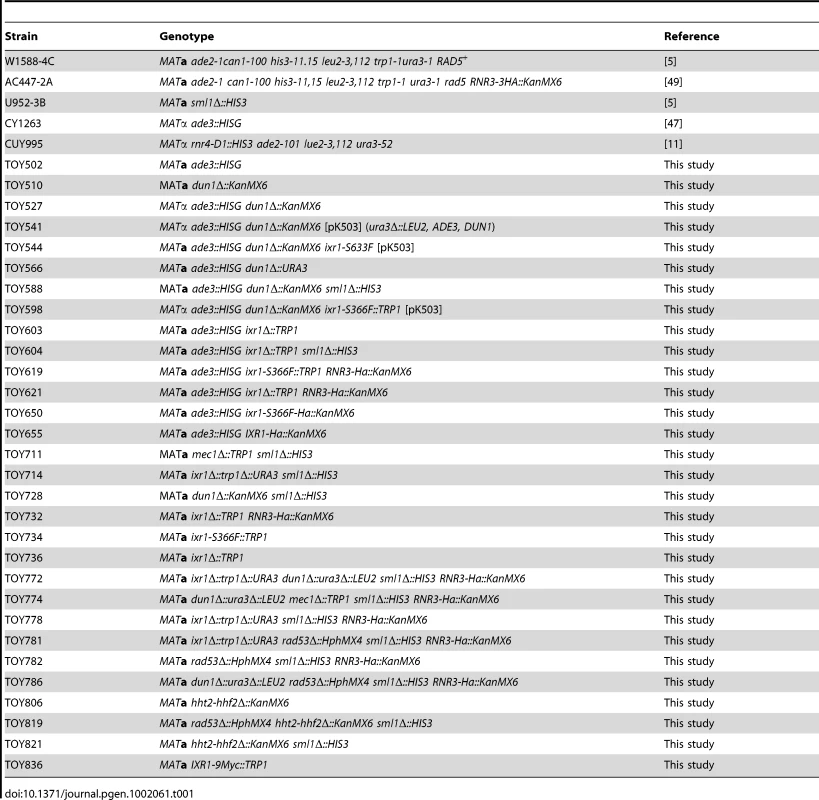
Plasmids
To overexpress Rnr1 or Rnr3, the previously described pESC-pGAL1-RNR1 or pBAD79 plasmids were used [6], [49]. To express RNR1 under its own promoter from a low-copy centromeric vector, the pBJ6 (pRS316-RNR1) plasmid was used (gift of Anders Byström, Umeå University). To construct pK503 (ura3Δ::LEU2, ADE3, DUN1), the DUN1 gene including the promoter region was PCR-amplified using primers Dun1_F and Dun1_rev. The PCR product was cloned into the SalI site of p2013 [50], and the URA3 gene in the resulting plasmid was then replaced by LEU2. To construct pK521 (TRP1, DUN1) a SalI/SalI fragment of DUN1 from pK503 was cloned into the SalI site of pRS414 [51]. To construct plasmids for the β-Galactosidase assay, the RNR1, RNR3 and RNR4 promoters were PCR amplified from the W1588-4C genomic DNA using primers pR1-F, pR1-R, pR3-F, pR3-R, pR4-F and pR4-R. The RNR3 promoter was cloned in the BamHI site of pJO20, and the RNR1 and RNR4 promoters were cloned in the BamHI site of pJO21 [52], resulting in plasmids pK505, pK504 and pK506, respectively. β-Galactosidase levels were assayed as described [52].
Synthetic lethal screen
To identify mutations synthetic lethal with dun1Δ, we used a color-based synthetic lethal screen [53]. The TOY541 strain carrying pK503 was grown in selective medium to ∼2×107 cells/ml. Cells were spun down, resuspended in water, plated onto YPD plates at 1500 cells/plate, and UV-mutagenized with a dose of 150 J/m2, resulting in 30% survival. Plates were placed in the dark and incubated for 3 days at 30°C. Non-sectoring red colonies were re-streaked twice on YPD, and those retaining the red color were selected for further analysis. Candidate mutants were transformed with pK521 to exclude mutants synthetically lethal with the plasmid-borne ADE3 or LEU2 genes. Transformants were grown on –Trp medium, and strains with a sectoring phenotype were selected. The candidate mutants were crossed with TOY566 to test recessiveness/dominance, and tetrad analysis was performed to select mutations with monogenic inheritance. Selected strains were mated in all possible combinations to establish complementation groups. In total, we isolated 4 mutants falling into three complementation groups and identified them as one ixr1, one rad53 and two whi3 mutants as outlined below.
One strain from each complementation group was transformed with a pRS314-based yeast genomic DNA library [54], and transformants were selected on –Trp plates. Clones that showed a sectoring phenotype were re-streaked onto –Trp plates, and plasmids were isolated from these clones and partially sequenced using T3 and T7 standard primers. The obtained sequences were subjected to BLAST homology searches using the S. cerevisiae genome database, and genomic regions were retrieved. One of the regions contained the IXR1 ORF (TOY544). A TRP1 cassette was inserted downstream of the IXR1 ORF creating TOY598, which was crossed with TOY566 to verify the co-segregation of a genomic marker and the non-sectoring phenotype. Then, the genomic region retrieved from the DNA library in pRS314 was shortened, and resulting plasmids were re-transformed in TOY544. Plasmids lacking the full-length IXR1 ORF failed to recover the sectoring phenotype. IXR1 and ixr1 ORFs were PCR amplified from the genomic DNA of TOY502 and TOY544, respectively, using primers F_ixr and R_ixr, and sequenced using the same primers. The mutations in RAD53 and WHI3 genes were identified by the same procedure and PCR amplified followed by sequencing using primers F_rad53, R_rad53, F_whi3 and R_whi3.
Western blotting and antibodies
Protein samples for Western blotting were prepared as described [55]. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Protran BA 85, Whatman, USA) using the Minigel System (C.B.S. Scientific Co., USA).
Rabbit polyclonal anti-Rnr1 (AS09 576), anti-Rnr2 (AS09 575), anti-Rnr3 (AS09 574), and anti-Sml1 (AS10 847) antibodies were produced by Agrisera, Sweden (peptides used for immunization are listed in Table S2). For the detection of Ixr1 we used rabbit polyclonal antibodies produced by Agrisera, Sweden (Table S2). For the detection of the HA-tag, mouse monoclonal 12CA5 antibodies were used (1∶5000). For the detection of both Rnr4 and α-tubulin [56], we used YL1/2 rat monoclonal antibodies (Sigma) at 1∶2500. These antibodies recognize C-termini of α-tubulin and small RNR subunits from different species. The absence of the Rnr4 band on a Western blot with an extract from an rnr4Δ strain (CUY995, [11]) confirmed that YL1/2 antibodies specifically recognize yeast Rnr4 (Figure S1B). For the detection of Rad53, we used yC-19 goat polyclonal antibodies at 1∶2000 (Santa Cruz Biotechnology, USA).
Quantification of protein levels was performed using ImageJ software (http://rsbweb.nih.gov/ij). Protein levels were calculated as relative units (RU, levels of the particular protein divided by the levels of tubulin in corresponding sample). To quantify Rnr1 levels three independent clones were analyzed on the same membrane.
Chromatin immunoprecipitation and quantative PCR
Chromatin immunoprecipitation followed by qPCR was performed as previously described (Barsoum et al., 2010). DNA damaging agent 4-NQO was added to the cells to final concentration 0.25 mg/L at OD ∼0.5 and cells were grown 2 hours to OD ∼1.2–1.5. To amplify RNR1 promoter, DSF2 promoter and ACT1 open reading frame ChIP_pRNR1, ChIP_pDSF2 and ChIP_ACT1 primers were used (Table S1).
Treatment with λ Phosphatase
9×107 cells were collected, vortexed with glass beads in 10% w/v trichloroacetic acid and spun down 10 min in microcentrifuge in cold room. Pellet was re-suspended in 150 µl of λ-Phosphatase buffer, pH was adjusted to 7.5 with basic 1 M Tris and 15 µl of 10× Complete Protease Inhibitor Cocktail (Roche Applied Biosystems) was added to the samples. 60 µl of 10× PhosStop Phosphatase Inhibitor Cocktail (Roche Applied Biosystems) or 6 µl of λ Phosphatase (New England Biolabs) was added to the respective samples and all samples were incubated 1 hour at 30°C. Then, samples were boiled 10 min with Laemmli buffer and analyzed by SDS PAGE followed by the Western blotting.
Measurement of dNTP levels
NTP and dNTP extraction and quantification were performed as previously described [7]. Nucleotides were analyzed by HPLC on a Partisphere SAX-5 HPLC column (4.6 mm×125 mm, Whatman International Ltd.) using a UV-2075 Plus detector (Jasco, Tokyo, Japan).
Analysis of HU tolerance and measurement of the frequency of petite formation
Mid-log phase cells were collected, sonicated, and plated at appropriate dilutions. For spot assays, 2 µl of 10-fold serial dilutions were spotted onto YPD plates or YPD plates containing 200 mM HU. Cells were grown at 30°C for 3 days. Measurement of the frequency of petite formation was done as described before [5].
Supporting Information
Zdroje
1. ZegermanPDiffleyJF 2009 DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst)
2. Navadgi-PatilVMBurgersPM 2009 A tale of two tails: Activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair (Amst)
3. ZhouZElledgeSJ 1993 DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell 75 1119 1127
4. ZhaoXChabesADomkinVThelanderLRothsteinR 2001 The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J 20 3544 3553
5. ZhaoXMullerEGRothsteinR 1998 A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell 2 329 340
6. DesanyBAAlcasabasAABachantJBElledgeSJ 1998 Recovery from DNA replicational stress is the essential function of the S-phase checkpoint pathway. Genes Dev 12 2956 2970
7. ChabesAGeorgievaBDomkinVZhaoXRothsteinR 2003 Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112 391 401
8. ElledgeSJDavisRW 1990 Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev 4 740 751
9. YagleKMcEnteeK 1990 The DNA damage-inducible gene DIN1 of Saccharomyces cerevisiae encodes a regulatory subunit of ribonucleotide reductase and is identical to RNR3. Mol Cell Biol 10 5553 5557
10. ElledgeSJDavisRW 1987 Identification and isolation of the gene encoding the small subunit of ribonucleotide reductase from Saccharomyces cerevisiae: DNA damage-inducible gene required for mitotic viability. Mol Cell Biol 7 2783 2793
11. WangPJChabesACasagrandeRTianXCThelanderL 1997 Rnr4p, a novel ribonucleotide reductase small-subunit protein. Mol Cell Biol 17 6114 6121
12. HuangMElledgeSJ 1997 Identification of RNR4, encoding a second essential small subunit of ribonucleotide reductase in Saccharomyces cerevisiae. Mol Cell Biol 17 6105 6113
13. HurdHKRobertsCWRobertsJW 1987 Identification of the gene for the yeast ribonucleotide reductase small subunit and its inducibility by methyl methanesulfonate. Mol Cell Biol 7 3673 3677
14. ZhaoXRothsteinR 2002 The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc Natl Acad Sci U S A 99 3746 3751
15. LeeYDWangJStubbeJElledgeSJ 2008 Dif1 is a DNA-damage-regulated facilitator of nuclear import for ribonucleotide reductase. Mol Cell 32 70 80
16. WuXHuangM 2008 Dif1 controls subcellular localization of ribonucleotide reductase by mediating nuclear import of the R2 subunit. Mol Cell Biol 28 7156 7167
17. HuangMZhouZElledgeSJ 1998 The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell 94 595 605
18. KlinkenbergLGWebbTZitomerRS 2006 Synergy among differentially regulated repressors of the ribonucleotide diphosphate reductase genes of Saccharomyces cerevisiae. Eukaryot Cell 5 1007 1017
19. FasulloMTsaponinaOSunMZChabesA 2010 Elevated dNTP levels suppress hyper-recombination in Saccharomyces cerevisiae S-phase checkpoint mutants. Nucleic Acids Research 38 1195 1203
20. PanXYePYuanDSWangXBaderJS 2006 A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124 1069 1081
21. CostanzoMBaryshnikovaABellayJKimYSpearED 2010 The genetic landscape of a cell. Science 327 425 431
22. BrownSJKellettPJLippardSJ 1993 Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science 261 603 605
23. DiffleyJFStillmanB 1991 A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A 88 7864 7868
24. LambertJRBilanchoneVWCumskyMG 1994 The ORD1 gene encodes a transcription factor involved in oxygen regulation and is identical to IXR1, a gene that confers cisplatin sensitivity to Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 91 7345 7349
25. SunZHsiaoJFayDSSternDF 1998 Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science 281 272 274
26. MurphyFVtSweetRMChurchillME 1999 The structure of a chromosomal high mobility group protein-DNA complex reveals sequence-neutral mechanisms important for non-sequence-specific DNA recognition. EMBO J 18 6610 6618
27. WorkmanCTMakHCMcCuineSTagneJBAgarwalM 2006 A systems approach to mapping DNA damage response pathways. Science 312 1054 1059
28. GunjanAVerreaultA 2003 A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 115 537 549
29. SinghRKKabbajMHPaikJGunjanA 2009 Histone levels are regulated by phosphorylation and ubiquitylation-dependent proteolysis. Nat Cell Biol 11 925 933
30. ReichardP 1988 Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem 57 349 374
31. SuterBTongAChangMYuLBrownGW 2004 The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae. Genetics 167 579 591
32. TongAHEvangelistaMParsonsABXuHBaderGD 2001 Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294 2364 2368
33. GibertICaleroSBarbeJ 1990 Measurement of in vivo expression of nrdA and nrdB genes of Escherichia coli by using lacZ gene fusions. Mol Gen Genet 220 400 408
34. CourcelleJKhodurskyAPeterBBrownPOHanawaltPC 2001 Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158 41 64
35. TanakaHArakawaHYamaguchiTShiraishiKFukudaS 2000 A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404 42 49
36. NakanoKBalintEAshcroftMVousdenKH 2000 A ribonucleotide reductase gene is a transcriptional target of p53 and p73. Oncogene 19 4283 4289
37. HakanssonPHoferAThelanderL 2006 Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem 281 7834 7841
38. AkdemirFChristichASogameNChapoJAbramsJM 2007 p53 directs focused genomic responses in Drosophila. Oncogene
39. Fernandez SarabiaMJMcInernyCHarrisPGordonCFantesP 1993 The cell cycle genes cdc22+ and suc22+ of the fission yeast Schizosaccharomyces pombe encode the large and small subunits of ribonucleotide reductase. Mol Gen Genet 238 241 251
40. IyerVREisenMBRossDTSchulerGMooreT 1999 The transcriptional program in the response of human fibroblasts to serum. Science 283 83 87
41. de BruinRAKalashnikovaTIChahwanCMcDonaldWHWohlschlegelJ 2006 Constraining G1-specific transcription to late G1 phase: the MBF-associated corepressor Nrm1 acts via negative feedback. Mol Cell 23 483 496
42. DirickLMollTAuerHNasmythK 1992 A Central Role for Swi6 in Modulating Cell-Cycle Start-Specific Transcription in Yeast. Nature 357 508 513
43. SidorovaJMBreedenLL 2003 Rad53 checpoint kinase phosphorylation site preference identified in the Swi6 protein of Saccharomyces cerevisiae. Molecular and Cellular Biology 23 3405 3416
44. KucejMKucejovaBSubramanianRChenXJButowRA 2008 Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J Cell Sci 121 1861 1868
45. MaisCWrightJEPrietoJLRaggettSLMcStayB 2005 UBF-binding site arrays form pseudo-NORs and sequester the RNA polymerase I transcription machinery. Genes Dev 19 50 64
46. Castro-PregoRLamas-MaceirasMSoengasPCarneiroIGonzalez-SisoI 2010 Regulatory factors controlling transcription of Saccharomyces cerevisiae IXR1 by oxygen levels: a model of transcriptional adaptation from aerobiosis to hypoxia implicating ROX1 and IXR1 cross-regulation. Biochemical Journal 425 235 243
47. ZhongTArndtKT 1993 The yeast SIS1 protein, a DnaJ homolog, is required for the initiation of translation. Cell 73 1175 1186
48. LeeTIRinaldiNJRobertFOdomDTBar-JosephZ 2002 Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298 799 804
49. ChabesAStillmanB 2007 Constitutively high dNTP concentration inhibits cell cycle progression and the DNA damage checkpoint in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104 1183 1188
50. JohanssonMJBystromAS 2004 The Saccharomyces cerevisiae TAN1 gene is required for N4-acetylcytidine formation in tRNA. RNA 10 712 719
51. SikorskiRSHieterP 1989 A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 19 27
52. OstlingJCarlbergMRonneH 1996 Functional domains in the Mig1 repressor. Mol Cell Biol 16 753 761
53. BenderAPringleJR 1991 Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol 11 1295 1305
54. JauertPAJensenLEKirkpatrickDT 2005 A novel yeast genomic DNA library on a geneticin-resistance vector. Yeast 22 653 657
55. PeterMGartnerAHoreckaJAmmererGHerskowitzI 1993 FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell 73 747 760
56. StandartNMBraySJGeorgeELHuntTRudermanJV 1985 The small subunit of ribonucleotide reductase is encoded by one of the most abundant translationally regulated maternal RNAs in clam and sea urchin eggs. J Cell Biol 100 1968 1976
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 5
-
Všechny články tohoto čísla
- Structural and Functional Differences in the Long Non-Coding RNA in Mouse and Human
- Identification, Replication, and Functional Fine-Mapping of Expression Quantitative Trait Loci in Primary Human Liver Tissue
- A −436C>A Polymorphism in the Human Gene Promoter Associated with Severe Childhood Malaria
- A Decline in p38 MAPK Signaling Underlies Immunosenescence in
- The Operon Balances the Requirements for Vegetative Stability and Conjugative Transfer of Plasmid R388
- Novel and Conserved Protein Macoilin Is Required for Diverse Neuronal Functions in
- Ixr1 Is Required for the Expression of the Ribonucleotide Reductase Rnr1 and Maintenance of dNTP Pools
- Genome of Strain SmR1, a Specialized Diazotrophic Endophyte of Tropical Grasses
- A Deficiency of Ceramide Biosynthesis Causes Cerebellar Purkinje Cell Neurodegeneration and Lipofuscin Accumulation
- A Latent Pro-Survival Function for the Mir-290-295 Cluster in Mouse Embryonic Stem Cells
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time
- Prion Formation and Polyglutamine Aggregation Are Controlled by Two Classes of Genes
- Integrated Genome-Scale Prediction of Detrimental Mutations in Transcription Networks
- Post-Embryonic Nerve-Associated Precursors to Adult Pigment Cells: Genetic Requirements and Dynamics of Morphogenesis and Differentiation
- A Novel Mouse Synaptonemal Complex Protein Is Essential for Loading of Central Element Proteins, Recombination, and Fertility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
- A Genetic and Structural Study of Genome Rearrangements Mediated by High Copy Repeat Ty1 Elements
- A Missense Mutation in Causes a Major QTL Effect on Ear Size in Pigs
- A Flurry of Folding Problems: An Interview with Susan Lindquist
- Meiotic Recombination Intermediates Are Resolved with Minimal Crossover Formation during Return-to-Growth, an Analogue of the Mitotic Cell Cycle
- A Nervous Origin for Fish Stripes
- The ISWI Chromatin Remodeler Organizes the hsrω ncRNA–Containing Omega Speckle Nuclear Compartments
- The Telomerase Subunit Est3 Binds Telomeres in a Cell Cycle– and Est1–Dependent Manner and Interacts Directly with Est1
- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



