-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAncestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
The telomerase reverse transcriptase synthesizes new telomeres onto chromosome ends by copying from a short template within its integral RNA component. During telomere synthesis, telomerase adds multiple short DNA repeats successively, a property known as repeat addition processivity. However, the consequences of defects in processivity on telomere length maintenance are not fully known. Germline mutations in telomerase cause haploinsufficiency in syndromes of telomere shortening, which most commonly manifest in the age-related disease idiopathic pulmonary fibrosis. We identified two pulmonary fibrosis families that share two non-synonymous substitutions in the catalytic domain of the telomerase reverse transcriptase gene hTERT: V791I and V867M. The two variants fell on the same hTERT allele and were associated with telomere shortening. Genealogy suggested that the pedigrees shared a single ancestor from the nineteenth century, and genetic studies confirmed the two families had a common founder. Functional studies indicated that, although the double mutant did not dramatically affect first repeat addition, hTERT V791I-V867M showed severe defects in telomere repeat addition processivity in vitro. Our data identify an ancestral mutation in telomerase with a novel loss-of-function mechanism. They indicate that telomere repeat addition processivity is a critical determinant of telomere length and telomere-mediated disease.
Published in the journal: . PLoS Genet 7(3): e32767. doi:10.1371/journal.pgen.1001352
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001352Summary
The telomerase reverse transcriptase synthesizes new telomeres onto chromosome ends by copying from a short template within its integral RNA component. During telomere synthesis, telomerase adds multiple short DNA repeats successively, a property known as repeat addition processivity. However, the consequences of defects in processivity on telomere length maintenance are not fully known. Germline mutations in telomerase cause haploinsufficiency in syndromes of telomere shortening, which most commonly manifest in the age-related disease idiopathic pulmonary fibrosis. We identified two pulmonary fibrosis families that share two non-synonymous substitutions in the catalytic domain of the telomerase reverse transcriptase gene hTERT: V791I and V867M. The two variants fell on the same hTERT allele and were associated with telomere shortening. Genealogy suggested that the pedigrees shared a single ancestor from the nineteenth century, and genetic studies confirmed the two families had a common founder. Functional studies indicated that, although the double mutant did not dramatically affect first repeat addition, hTERT V791I-V867M showed severe defects in telomere repeat addition processivity in vitro. Our data identify an ancestral mutation in telomerase with a novel loss-of-function mechanism. They indicate that telomere repeat addition processivity is a critical determinant of telomere length and telomere-mediated disease.
Introduction
Telomerase is a specialized DNA polymerase that synthesizes new telomere repeats onto chromosome ends [1], [2]. Telomerase has two essential conserved components, a catalytic reverse transcriptase, hTERT, and an RNA component, hTR [3], [4]. The RNA component of telomerase contains a template sequence for the addition of new telomere repeats [5]. In order to synthesize long telomere tracts, hTERT copies from the RNA template once, translocates, and then iteratively adds successive repeats [6]. This property is known as telomere repeat addition processivity [7]. Functional domains within hTR and hTERT, as well as telomerase-extrinsic factors, have been implicated in repeat addition processivity [8]–[10]. However, whether repeat addition processivity is critical for telomere length maintenance in vivo is not fully known.
Germline mutations in the essential telomerase components hTERT and hTR lead to a clinical spectrum of syndromes of telomere shortening (reviewed in [11]). Affected individuals suffer from degenerative organ failure in the bone marrow, lung and liver. In adulthood, syndromes of telomere shortening most commonly manifest as progressive and irreversible scarring of the lung in an age-related disorder known as idiopathic pulmonary fibrosis (IPF) [11]. Mutations in hTERT or hTR underlie the inheritance in 8–15% of familial forms of pulmonary fibrosis and 1–3% of sporadic cases [12]–[15]. This mutation frequency along with the common prevalence of IPF make pulmonary disease the most common manifestation of germline defects in telomerase [11]. In severe forms, syndromes of telomere shortening are clinically recognized in the premature aging syndrome dyskeratosis congenita where aplastic anemia is the most common cause of mortality and where there is an increased incidence of acute myeloid leukemia (AML) [16], [17]. AML, both de novo, and in the setting of myelodysplasia, has also been reported as a first manifestation of germline mutant telomerase genes [18], [19]. A subset of pulmonary fibrosis patients and families with short telomeres suffer complications from aplastic anemia and cryptogenic liver cirrhosis [12], [13], [20], [21]. Insights into telomerase genetics as well as their consequences on telomerase function have therefore become intimately connected with the pathophysiology of several age-related disorders.
Heterozygous mutations in hTERT and hTR cause telomere shortening through haploinsufficiency [20]–[23]. Previously identified mutations in telomerase have been shown to cause loss-of-function due to defects in hTR stability, essential catalytic function, and ribonucleoprotein assembly, but not repeat addition processivity [13], [20], [22], [24], [25]. We identified two families with familial pulmonary fibrosis where each of the affected index cases carried two heterozygous variants that predicted non-synonymous amino acid substitutions in the reverse transcriptase domain of hTERT. We show that these two families share a common founder, identifying an ancestral mutation in telomerase. Although the double heterozygote hTERT did not drastically affect telomerase's capacity to add nucleotides within a single repeat, it had severe defects in repeat addition processivity and was associated with telomere shortening. Our data indicate that inherited defects in telomerase processivity may be sufficient to contribute to telomere shortening and to a familial telomere-mediated syndrome.
Results
Non-synonymous variants in the reverse-transcriptase domain of hTERT segregate with the pulmonary fibrosis phenotype
In a screen of 75 familial pulmonary fibrosis probands for telomerase mutations, we identified a proband from a family designated number 13 who carried two single nucleotide variants in hTERT. The first was a c.2371G→A transition in exon 7, and the second was c.2599G→A transition in exon 10 (Figure 1A, 1B). These predicted two non-synonymous substitutions in the reverse transcriptase domain: V791I and V867M, respectively (Figure 1C). The single nucleotide variants were absent in 200 ethnically matched controls, as well as in a multi-ethnic control panel examining the hTERT gene sequence [23]. To determine whether these nucleotide substitutions were on the same allele (i.e. in cis) and whether they were associated with the pulmonary fibrosis phenotype, we sequenced genomic DNA from affected family members and examined the segregation. hTERT V791I and V867M were always present together (11 of 11 individuals across 3 generations) suggesting that they were on the same hTERT haplotype (Figure 2). The hTERT variants predicting V791I and V867M segregated with the pulmonary fibrosis diagnosis across four generations in all the individuals we examined [n = 7, 5 directly sequenced, 1 obligate carrier (13.II.5) and 1 probable carrier (13.I.1), Figure 2]. The log of the odds ratio (LOD) score of the mutant hTERT allele segregating with the pulmonary fibrosis was significant at 3.3. The segregation of the mutations with the disease phenotype indicated that this double mutant hTERT was likely disease causing.
Fig. 1. Position and conservation of non-synonymous variants in hTERT shared by pulmonary fibrosis families 13 and 143 probands. 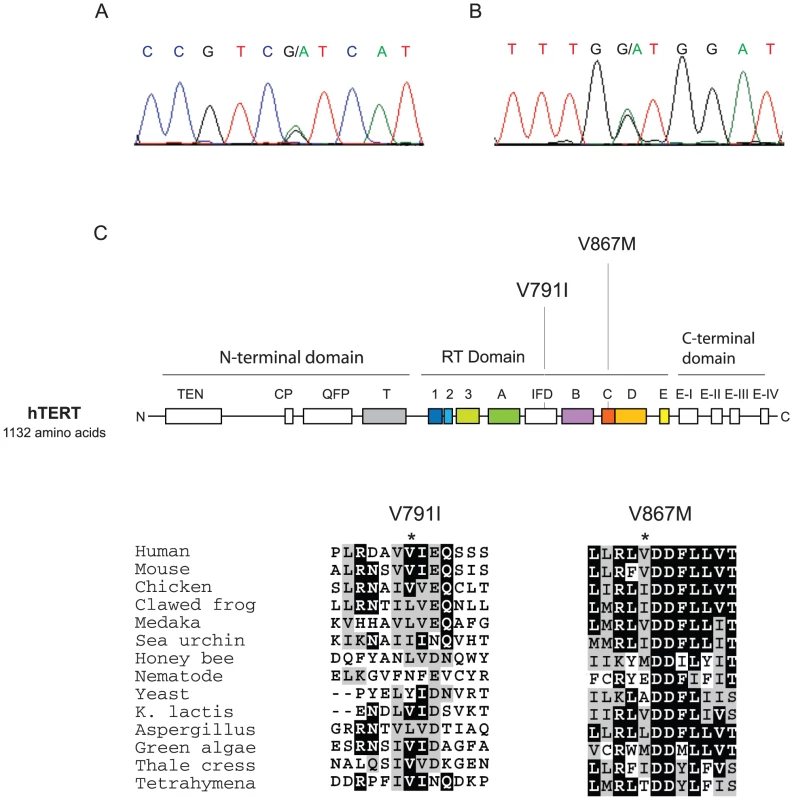
A,B. Chromatograms of single nucleotide variants predict non-synonymous amino acid substitutions. The first was a c.2371G→A transition in exon 7 (A), and the second was c.2599G→A transition in exon 10 (B). C. Panel shows conserved hTERT motifs shared with other TERTs. The non-synonymous amino acid variant residues are indicated within the reverse transcriptase domain. hTERT V791 falls in the IFD domain between the A and B motifs, and hTERT V867 is adjacent to the invariant motif C aspartic acid residues which are essential for reverse transcriptase function and are indicated by *. Alignment of TERT sequence across 14 species indicates that both V791 and V867 fall within conserved motifs. Fig. 2. Four generation pedigrees of pulmonary fibrosis probands from Families 13 and 143 of the Vanderbilt Registry. 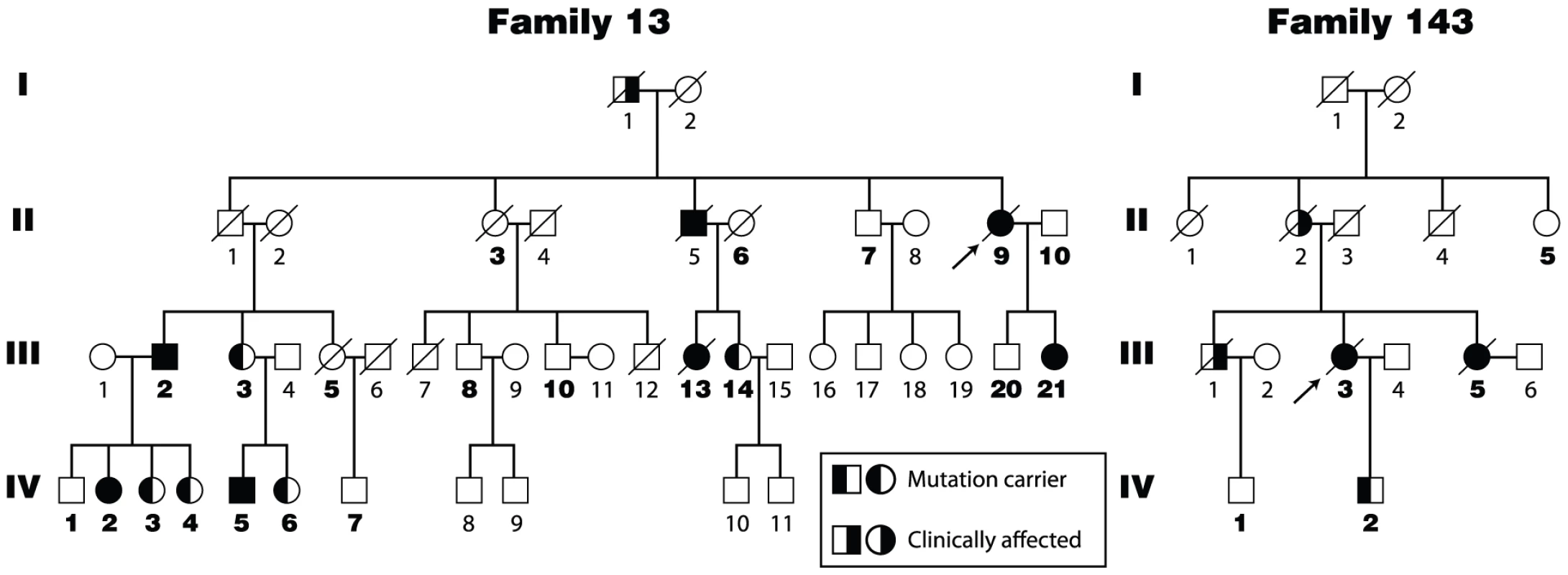
Mutation and affected status are indicated by symbols shown in the key and individuals in whom DNA was available are noted by the pedigree number in bold text. In both families, the hTERT 791I and 867 M variants co-segregate, consistent with the fact that these heterozygous substitutions are on the same allele in cis. hTERT 791I-867M also segregates with the pulmonary fibrosis phenotype in all the individuals in whom DNA was available. The symbols are identified in the key, and completely filled symbols indicate clinically affected individuals who carry the double mutant TERT. Two pulmonary fibrosis families share common ancestry
In an independent screen of 24 pulmonary fibrosis families, we identified a second kindred, designated family 143, whose proband carried the identical substitutions in hTERT. In this family, hTERT V791I and hTERT V867M also co-segregated with the pulmonary fibrosis phenotype (Figure 2). Since the two variants were in cis and were rare, we reasoned that Families 13 and 143 may have a single common ancestor. To address this, we carefully queried the genealogy. Independently, members of the two families reported lineage to an individual of the same surname who was born in 1808 in the United States. According to public census records, this ancestor had grandparents who emigrated in the eighteenth century from the British Isles. The genealogy suggested that Families 13 and 143 may be related and that the putative mutation(s) have been present for at least 6 generations, possibly with ancestry as far back as the early nineteenth century. To determine whether Families 13 and 143 shared a common founder, we genotyped polymorphic microsatellite and minisatellite sequences that flank as well as fall within the hTERT gene (Figure S1A, S1B). In all the individuals who carried the hTERT substitutions at 791 and 867 positions, we identified a shared haplotype block which was both within and flanked hTERT (Figure S1A, S1B). These data, together with the family histories, indicated that Families 13 and 143 shared a common ancestor who carried the double mutant hTERT allele.
hTERT 791I-867M causes defects in repeat addition processivity in vitro
To determine the functional significance of the hTERT 791 and 867 variants, we first examined the evolutionary conservation of the hTERT V791 and V867 residues. hTERT V791 fell within the insertion in finger domain (IFD) between the A and B motifs of the reverse transcriptase domain [10], [26], a telomerase specific motif (Figure 1B, 1C). hTERT V867 fell within the universal reverse transcriptase motif C, and was adjacent to the invariant aspartic acid residues which are essential for the catalytic function of telomerase and other reverse transcriptases [4] (Figure 1B, 1C). The sequence alignment from representative species showed that these two residues are generally conserved as hydrophobic amino acids in most organisms, and are therefore potentially important for telomerase function.
To directly examine whether the variant hTERT affects telomerase activity, we reconstituted the mutant telomerase and measured enzyme activity in vitro. At standard assay conditions of 1 mM nucleotide concentrations [8], [12], [13], [20], [27], the 791I alone did not have obvious defects in activity or processivity (Figure 3A, lane 3). A minor inter-repeat pause was present for 867M; this has been previously suggested to be due to nucleotide affinity defects [28] (Figure 3A, lane 4). This pause was also present in the 791I-867M double mutant (Figure 3A, lane 5). However, the overall activity and processivity of 867M and 791I-867M were not affected (Figure 3A, lanes 4 and 5). For comparison, we measured the activity of a known hTERT mutation L55Q previously identified in a family with pulmonary fibrosis [13]. This mutant showed a significant decrease in overall activity (Figure 3A, lane 2). Since the segregation and the genetic evidence supported the 791I-867M being a pathogenic allele, we assayed its function at nucleotide concentrations that are closer to the estimated Km for telomerase [29], [30]. The lower concentrations also more closely mimic estimates of intra-nuclear nucleotide concentrations (10 µM) [31]–[33]. Under these conditions, there was little effect on the synthesis of the first repeat compared with wildtype telomerase (Figure 3A–3D). We next measured repeat addition processivity. Notably, hTERT 867M and the double mutant hTERT had significant decreases in repeat addition processivity (Figure 3A and 3E). The decreased repeat addition processivity was not seen for the 55Q and 791I alleles (Figure 3A and 3E). This decrease was evident by the lower intensity of high molecular weight repeat products relative to the first product (Figure 3A, lane 6 compared with lanes 9 and 10). For example, by the fourth repeat, both hTERT 867M and 791I-867M had approximately a 10-fold reduction in telomere product compared with wildtype telomerase (Figure 3E). The decrease in processivity was independent of the hTERT epitope tag used in affinity purification, as we saw the same degree of impairment for 867M and 791I-867M whether a C-terminal HA or N-terminal FLAG epitope was used (not shown). To exclude the possibility that the double mutant hTERT may have dominant negative effects, we performed mixing studies of wildtype and 791I-867M and found no additional decreases in activity or processivity (not shown). These data indicated that although hTERT 791I and 867M co-segregate with the disease phenotype, the 867M mutation appears to be the pathogenic variant and predominantly affects telomere repeat addition processivity in vitro.
Fig. 3. The ancestral mutant telomerase affects repeat addition processivity in vitro. 
A. Telomerase activity assay of non-synonymous hTERT variants identified in family 13 and 143 probands. L55Q was previously identified in a pulmonary fibrosis family and known to compromise catalysis. Telomerase activity assay at high nucleotide (1 mM) concentrations on the left shows no defects in catalytic activity or processivity for hTERT V791I and V867M or the double mutant. At lower nucleotide concentration (10 µM), hTERT 867M and hTERT V791I-V867M both show defects in repeat addition processivity as evidenced by the decreased intensity of the high molecular weight products relative to the first repeat. Low exposure image of the internal loading control is shown below. B. Low exposure image of the gel shown in (A) is shown to visualize the +1, +2, and +3 nucleotide bands clearly. C. SDS-PAGE of 35S labeled hTERT used in (A) to monitor the expression of in vitro synthesized hTERTs. D. Quantitation of first repeat addition as measured by the total intensity of the +1, +2, +3, and +4 nucleotide bands. Quantitation is based on 3 independent experiments. * Indicates P-value <0.01 and error bars indicate standard error of the mean. E. Quantitation of processivity across the first four repeats (R1, R2, R3 and R4) is shown by the linear regression line. Mutant TERT is associated with short telomere length
Telomerase haploinsufficiency causes telomere shortening and the severity of the consequent phenotypes correlates with the telomere length [20], [34]. To examine if the mutant telomerase is associated with telomere shortening, we measured telomere length in family members using combined flow cytometry and fluorescence in situ hybridization (flow-FISH) [12], [13]. In all cases, mutation carriers had lymphocyte telomere lengths below the 10th percentile of a normal distribution compared with age-matched controls (Figure 4). In 6 of 9 mutation carriers, the telomere length fell below the 1st percentile, a range that is highly specific for the presence of a germline telomere maintenance defect [12], [13], [35] (Figure 4, P<0.001, paired t-test). Therefore the mutant hTERT is associated with telomere shortening in mutation carriers.
Fig. 4. Telomere length in mutation carriers in families 13 and 143 have short telomeres compared to age-matched controls. 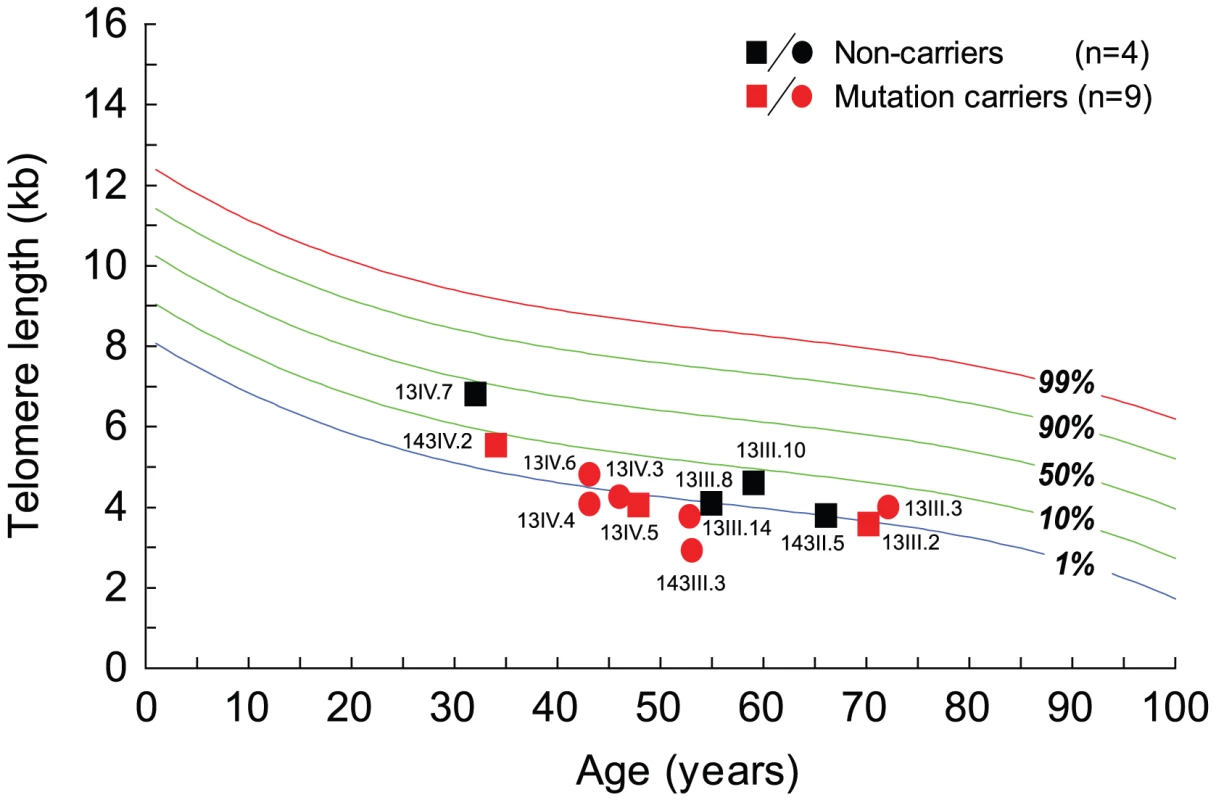
Panel shows telomere length as measured in lymphocytes by flow-FISH compared to normal distribution of age-matched controls. Percentiles are based on telomere length data from 400 controls. Squares refer to males and circles refer to females. Individuals refer to pedigree position in Figure 2. Individual 13III.8 has short telomeres and was diagnosed with an overlap syndrome of emphysema and pulmonary fibrosis. A spectrum of telomere-mediated disease is associated with ancestral TERT mutation
Syndromes of telomere shortening manifest as degenerative disease in the lung, liver and bone marrow, and a subset of pulmonary fibrosis families falls on this spectrum [11], [13]. To examine whether the mutant TERT leads to the full spectrum of telomere-mediated disease, we characterized the clinical phenotypes in families 13 and 143. Of the 18 genetically affected individuals in the two pedigrees, 11 had pulmonary disease. In the majority of cases (7 of 11, 64%), the interstitial lung disease met the criteria for usual interstitial pneumonia/IPF (Figure 5A, 5B and Table S1). In affected individuals, the onset of disease was in adulthood with a mean age of 56 (range 32–67). There was very subtle genetic anticipation for the age at death across the generations we could examine (e.g. age 61 and mean age 56 for generations I and II respectively in family 13, Table S1). Two individuals at the age of 50 and 51 reported chronic liver function abnormalities that were unexplained after a thorough work-up. We identified subclinical cytopenias in one individual (age 50), and one individual was diagnosed with AML at age 67 and subsequently died from complications of interstitial lung disease (Table S1). We clinically examined the probands and their relatives for the typical mucocutaneous features of dyskeratosis congenita but did not identify any signs of skin hyperpigmentation, nail dystrophy or oral leukoplakia. Therefore the telomerase defect we identified in families 13 and 143 appears to primarily cause adult-onset phenotypes. These phenotypes are clinically most prominent in the lung, but features of the full spectrum of a telomere syndrome manifest at lower frequency including subclinical cytopenias, liver function abnormalities and AML.
Fig. 5. CAT scans from pulmonary fibrosis probands and non-carrier sibling with short telomeres. 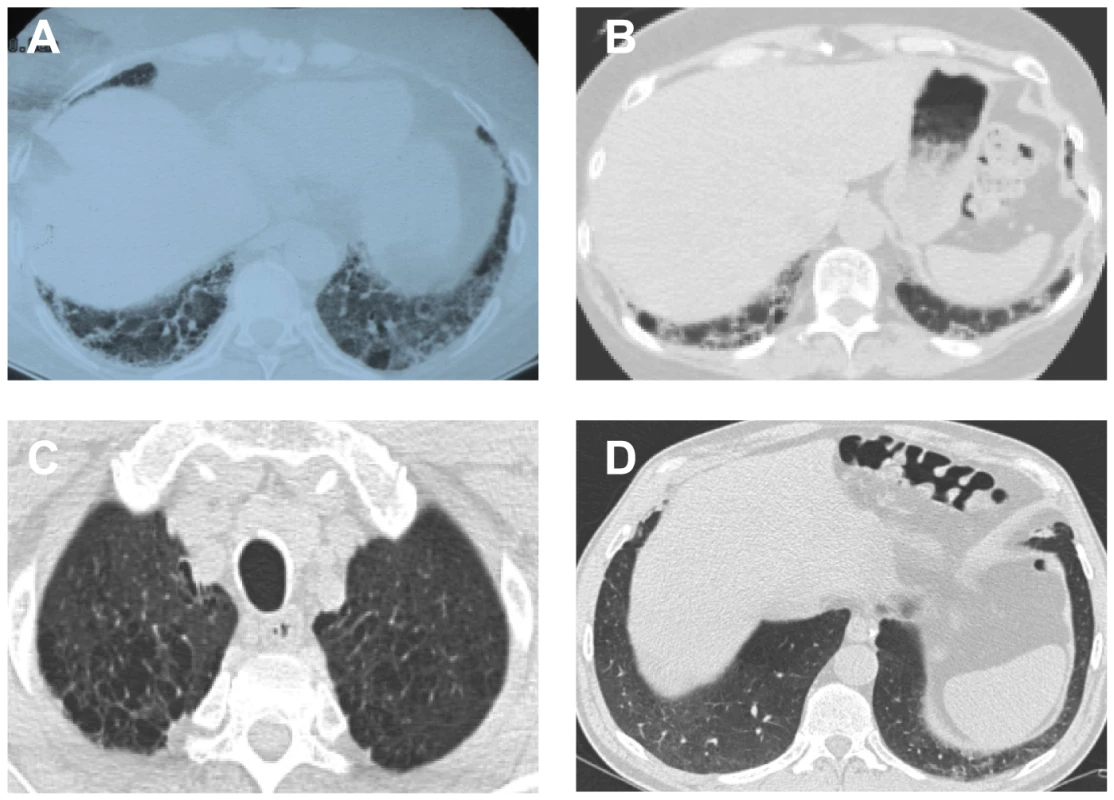
A,B show lower thoracic CAT scan images from the probands in family 13 and 143. Both images show basilar honeycombing typical of idiopathic pulmonary fibrosis. C,D are apical and lower thoracic CAT scan images respectively of sibling with short telomere who does not carry the mutant hTERT (Individual designated 13III.8 in Figure 2 and Figure 3). This individual has apical changes consistent with centrilobular emphysema as well as lower thoracic ground glass changes consistent with an interstitial process. Unaffected siblings of mutation carriers have short telomeres
Telomere length is a heritable trait and parental telomere length determines offspring telomere length even when telomerase is wildtype [34], [36], [37]. In a large dyskeratosis congenita family, siblings of mutation carriers were shown to have short telomeres; however telomere-related phenotypes in these individuals have not been previously reported [38]. Since the hTERT 791I-867M mutation was associated with short telomeres, we examined whether their non-mutation carrier relatives may also have short telomeres. In 4 individuals we examined, the lymphocyte telomere length was below the 10th percentile compared with age-matched controls, and in two individuals, the telomere length fell at or below the 1st percentile (Figure 4, P = 0.039, paired t-test). To determine whether short telomeres may be a risk factor for developing telomere-mediated disease, we examined the clinical phenotypes of the individuals who did not carry the hTERT 791I-867M allele but who had short telomeres. We identified one patient who presented to our clinic with shortness of breath at the age of 53. His history was significant for a lifelong history of cigarette use (greater than 50 pack-years). The patient's CAT scan showed a mixed picture of interstitial lung disease with ground glass infiltrates and emphysema (Figure 5C, 5D), and lung biopsy confirmed the presence of interstitial fibrosis on the background of bronchiolitis. Although cigarette smoke has been shown to be associated with short telomeres and is known to contribute to the risk of both emphysema and pulmonary fibrosis [39], it is intriguing to consider the possibility that parental telomere length may have contributed to the telomere shortening in this individual and to his risk of lung disease.
Discussion
Repeat addition processivity is a unique biochemical attribute of the telomerase reverse transcriptase, and here we show it may be critical for telomere maintenance in vivo. Telomerase-intrinsic and extrinsic factors have been implicated in repeat addition processivity [8]–[10], and our study suggests that inherited defects that affect this unique property of the telomerase enzyme may contribute to telomere length heterogeneity and to telomere-mediated disease. Although individuals in the two kindreds we describe carried two in cis variants in hTERT, our biochemical studies suggest that hTERT 867M is likely the functionally important mutation. As such, the hTERT 791I rare variant may serve as a useful genetic marker and, along with the 867M, can identify other families with shared ancestry to the families we report herein. Single nucleotide titration studies have implicated hTERT V867 to be important in telomerase function [28]. Studies of the Tetrahymena thermophila TERT have also implicated the orthologous residue adjacent to V867 in repeat addition processivity [40]. These observations, along with our findings, indicate that residues within motif C of the telomerase reverse transcriptase domain are important determinants of telomere repeat addition processivity. Several mechanisms of telomerase haploinsufficiency have been previously reported for disease causing mutations including loss of hTR stability, impaired association of hTR with hTERT, and loss of catalytic function [13], [20], [22], [24], [25]. In this study, the strong genetic evidence linking the mutant TERT to a known telomere-mediated disease, and the evidence of telomere shortening in vivo, indicate that the mutant TERT affects telomere maintenance. Our in vitro biochemical studies show that the mutant TERT is defective in repeat addition processivity, pointing to this as the likely mechanism for the loss of telomerase function and the consequent organ failure.
We report on an ancestral mutation in hTERT which manifested independently in two pulmonary fibrosis families. To our knowledge, hTERT 791I-867M is the most ancient telomerase mutation, and it is likely that other kindreds with familial pulmonary fibrosis and other features of telomere syndromes will be subsequently found to share ancestry with these pedigrees. In contrast to a family with a functionally null hTERT mutation where genetic anticipation was striking and caused a two decade earlier onset of disease across each generation [20], hTERT 791I-867M causes only subtle anticipation across the generation spans we studied. This observation suggests that the extent of genetic anticipation may correlate with the degree of telomerase loss-of-function thus making genetic anticipation more difficult to detect across consecutive generations in families that carry hypomorphic mutations. Telomerase preferentially elongates the shortest telomeres [41], [42], and our functional studies which show intact single repeat synthesis, point to the fact that the ancestral hTERT 791I-867M may have the capacity to add initial telomere tracts, thus healing the shortest telomeres. However, with successive telomere repeats, telomere addition is less efficient. Loss of telomere repeat addition processivity may therefore be a manifestation of more hypomorphic mutations, and therefore adult-onset phenotypes, as the shortest telomeres may still be elongated, albeit with shorter telomere tracts.
In this multi-generation study, although pulmonary fibrosis was the most common manifestation in mutation carriers, several individuals had extra-pulmonary manifestations of telomere-mediated disease. One individual had avascular necrosis and macrocytosis, two individuals reported history of cryptogenic liver function abnormalities, and one patient had a history of AML prior to the diagnosis of interstitial lung disease. Bone marrow failure, avascular necrosis and cryptogenic liver cirrhosis are all known complications of dyskeratosis congenita [17], and pulmonary fibrosis families with mutant telomerase genes have been known to have an increased incidence of aplastic anemia, a common complication of dyskeratosis congenita [13]. AML, often arising in the setting of myelodysplasia, has been recently reported as a first manifestation of mutant telomerase genes [18], [19], and it is possible that families with pulmonary fibrosis due to telomerase deficiency also have an increased incidence of AML. In 8 consecutive pulmonary fibrosis families with known hTR or hTERT mutations, including the 2 we report herein, there was a total of 3 first degree relatives of IPF probands who died with AML at ages 25, 59, 68. Dyskeratosis congenita patients are known to have an increased incidence of AML [16]. These observations highlight the fact that a subset of families with pulmonary fibrosis falls on the same spectrum as dyskeratosis congenita and that the diagnosis of telomere syndrome in these patients is relevant to their clinical work-up and surveillance. Pulmonary fibrosis patients should be queried about a personal or family history of AML, along with aplastic anemia, as part of the screening history for a telomere syndrome.
In summary, an ancestral mutation within the reverse transcriptase domain of telomerase manifests as familial pulmonary fibrosis and causes defects in telomere repeat addition processivity. Genetic factors that affect repeat addition processivity may be important determinants of telomere length heterogeneity across populations, and can contribute to understanding the inherited basis of telomere-mediated disease.
Methods
Subjects and ethics statement
Subjects were recruited through the Vanderbilt Familial Pulmonary Fibrosis Registry and gave written informed consent [13]. The study was approved by the institutional review boards of both Vanderbilt and Johns Hopkins Universities. The probands from Families 13 and 143, and the majority of mutation carrier and non-carrier relatives were clinically evaluated. Primary medical records were used to document the diagnoses listed in Table S1. We used the consensus classification to phenotype the idiopathic interstitial lung disease [43].
Genotyping and telomere length measurement
Genomic DNA was extracted from peripheral blood using standard methods. We sequenced hTERT [13] and confirmed variants bidirectionally. Control DNA was obtained from Corriel Repository with self-reported Northern European ethnicity, similar to the families we studied. We used Merlin to calculate a single point LOD score [44], under the assumption of autosomal dominant inheritance and a 1/10,000 population frequency of idiopathic interstitial lung disease. We determined allele size of microsatellites D5S1981 (Forward-cctgtaccaatccatgc, Reverse-gagccatgtgagtgtcc) and D5S2005 (Forward-cctcaggtgggttattgac, Reverse-cccagggctttacgagt) using fluorescent labeled forward primers obtained from Qiagen (Valencia, CA). PCR products were analyzed on the ABI Genome Analyzer instrument (Applied Biosystems). Pherograms were interpreted manually to determine allele size. We amplified and genotyped mini-satellites/variable number of tandem repeats within hTERT: 2–2 (intron 2) and 6–1 (intron 6) as published [45]. We determined the number of tandem repeats using gel electrophoresis. Telomere length was measured by flow-FISH on peripheral blood mononuclear cells [13].
Telomerase activity assay
TERT protein alignment was generated using CLUSTALW followed by BoxShade analysis (v.3.21), and we used NP_937983 for the hTERT protein sequence. TERT sequences were obtained from http://telomerase.asu.edu [46]. To test the activity and processivity of the telomerase mutants, we expressed each of them in vitro and quantified function using a direct telomerase activity assay as previously described [10]. All telomerase variants were reconstituted using the TNT (transcription and translation) Quick Coupled rabbit reticulocyte lysate system (Promega) following manufacturer's instructions. Briefly, recombinant N-FLAG tagged hTERT was expressed in 10 µL of TNT lysate with 35S labeled methionine at 30°C for 60 minutes. To obtain active telomerase, in vitro transcribed hTR pseudoknot (nt 32–195) and CR4-CR5 (nt 239–328) fragments were each added to a concentration of 8 µM and incubated at 30°C for 30 minutes. To avoid variations in the quality of telomerase reconstituted, the wildtype and variant hTERT proteins were all expressed from the same batch of TNT lysate and the reconstituted enzymes were assayed immediately without freezing. To assay the activity and processivity of each telomerase variant, a 10 µl reaction was carried out using 3 µl of in vitro reconstituted telomerase in the presence of 1x PE buffer (50 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM DTT, 3 mM MgCl2, and 1 mM spermidine) and 2 pmol of 5′-32P end-labeled (TTAGGG)3 telomere primer at 30°C for 1 h. Deoxynucleotides (dATP, dTTP, and dGTP) were also included at concentrations of either 1 mM or 10 µM, as indicated. Reactions were terminated by phenol-chloroform extraction followed by ethanol precipitation before being resolved on a 10% denaturing polyacrylamide gel. To quantitate the first repeat product, we measured product intensity at low exposure to clearly visualize the +1, +2, +3, +4 nucleotides (as shown in Figure 3B), and normalized to the amount of unpolymerized oligonucleotide loading control after subtracting background. Telomerase processivity was calculated by measuring the intensity of each repeat band, normalized to the intensity of the first repeat, and plotted against the repeat number [9].
Supporting Information
Zdroje
1. GreiderCW
BlackburnEH
1985
Identification of a specific telomere terminal transferase activity in Tetrahymena extracts.
Cell
43
405
413
2. GreiderCW
BlackburnEH
1987
The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity.
Cell
51
887
898
3. FengJ
FunkWD
WangSS
WeinrichSL
AvilionAA
1995
The RNA component of human telomerase.
Science
269
1236
1241
4. LingnerJ
HughesTR
ShevchenkoA
MannM
LundbladV
1997
Reverse transcriptase motifs in the catalytic subunit of telomerase.
Science
276
561
567
5. GreiderCW
BlackburnEH
1989
A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis.
Nature
337
331
337
6. AutexierC
LueNF
2006
The structure and function of telomerase reverse transcriptase.
Annu Rev Biochem
75
493
517
7. GreiderCW
1991
Telomerase is processive.
Mol Cell Biol
11
4572
4580
8. ChenJL
GreiderCW
2003
Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility.
Embo J
22
304
314
9. WangF
PodellER
ZaugAJ
YangY
BaciuP
2007
The POT1-TPP1 telomere complex is a telomerase processivity factor.
Nature
445
506
510
10. XieM
PodlevskyJD
QiX
BleyCJ
ChenJJ
A novel motif in telomerase reverse transcriptase regulates telomere repeat addition rate and processivity.
Nucleic Acids Res
38
1982
1996
11. ArmaniosM
2009
Syndromes of telomere shortening.
Annu Rev Genomics Hum Genet
10
45
61
12. AlderJK
ChenJJ
LancasterL
DanoffS
SuSC
2008
Short telomeres are a risk factor for idiopathic pulmonary fibrosis.
Proc Natl Acad Sci U S A
105
13051
13056
13. ArmaniosMY
ChenJJ
CoganJD
AlderJK
IngersollRG
2007
Telomerase mutations in families with idiopathic pulmonary fibrosis.
N Engl J Med
356
1317
1326
14. CronkhiteJT
XingC
RaghuG
ChinKM
TorresF
2008
Telomere shortening in familial and sporadic pulmonary fibrosis.
Am J Respir Crit Care Med
178
729
737
15. TsakiriKD
CronkhiteJT
KuanPJ
XingC
RaghuG
2007
Adult-onset pulmonary fibrosis caused by mutations in telomerase.
Proc Natl Acad Sci U S A
104
7552
7557
16. AlterBP
GiriN
SavageSA
RosenbergPS
2009
Cancer in dyskeratosis congenita.
Blood
113
6549
6557
17. KirwanM
DokalI
2008
Dyskeratosis congenita: a genetic disorder of many faces.
Clin Genet
73
103
112
18. CaladoRT
RegalJA
HillsM
YewdellWT
DalmazzoLF
2009
Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia.
Proc Natl Acad Sci U S A
106
1187
1192
19. KirwanM
VulliamyT
MarroneA
WalneAJ
BeswickR
2009
Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia.
Hum Mutat
30
1567
1573
20. ArmaniosM
ChenJL
ChangYP
BrodskyRA
HawkinsA
2005
Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita.
Proc Natl Acad Sci U S A
102
15960
15964
21. Diaz de LeonA
CronkhiteJT
KatzensteinAL
GodwinJD
RaghuG
2010
Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations.
PLoS ONE
5
e10680
doi:10.1371/journal.pone.0010680
22. VulliamyT
MarroneA
GoldmanF
DearloveA
BesslerM
2001
The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita.
Nature
413
432
435
23. YamaguchiH
CaladoRT
LyH
KajigayaS
BaerlocherGM
2005
Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia.
N Engl J Med
352
1413
1424
24. LyH
CaladoRT
AllardP
BaerlocherGM
LansdorpPM
2005
Functional characterization of telomerase RNA variants found in patients with hematologic disorders.
Blood
105
2332
2339
25. RobartAR
CollinsK
Investigation of human telomerase holoenzyme assembly, activity, and processivity using disease-linked subunit variants.
J Biol Chem
285
4375
4386
26. LueNF
LinYC
MianIS
2003
A conserved telomerase motif within the catalytic domain of telomerase reverse transcriptase is specifically required for repeat addition processivity.
Mol Cell Biol
23
8440
8449
27. ChenJ
AstleCM
HarrisonDE
1999
Development and aging of primitive hematopoietic stem cells in BALB/cBy mice.
Exp Hematol
27
928
935
28. DrosopoulosWC
PrasadVR
2007
The active site residue Valine 867 in human telomerase reverse transcriptase influences nucleotide incorporation and fidelity.
Nucleic Acids Res
35
1155
1168
29. JarstferMB
CechTR
2002
Effects of nucleotide analogues on Euplotes aediculatus telomerase processivity: evidence for product-assisted translocation.
Biochemistry
41
151
161
30. MaineIP
ChenSF
WindleB
1999
Effect of dGTP concentration on human and CHO telomerase.
Biochemistry
38
15325
15332
31. ArionD
BorkowG
GuZ
WainbergMA
ParniakMA
1996
The K65R mutation confers increased DNA polymerase processivity to HIV-1 reverse transcriptase.
J Biol Chem
271
19860
19864
32. GolinelliMP
HughesSH
2002
Nontemplated nucleotide addition by HIV-1 reverse transcriptase.
Biochemistry
41
5894
5906
33. SkoogL
BjursellG
1974
Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells.
J Biol Chem
249
6434
6438
34. HaoLY
ArmaniosM
StrongMA
KarimB
FeldserDM
2005
Short telomeres, even in the presence of telomerase, limit tissue renewal capacity.
Cell
123
1121
1131
35. AlterBP
BaerlocherGM
SavageSA
ChanockSJ
WekslerBB
2007
Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita.
Blood
110
1439
1447
36. NjajouOT
CawthonRM
DamcottCM
WuSH
OttS
2007
Telomere length is paternally inherited and is associated with parental lifespan.
Proc Natl Acad Sci U S A
104
12135
12139
37. ArmaniosM
AlderJK
ParryEM
KarimB
StrongMA
2009
Short telomeres are sufficient to cause the degenerative defects associated with aging.
Am J Hum Genet
85
823
832
38. GoldmanF
BouarichR
KulkarniS
FreemanS
DuHY
2005
The effect of TERC haploinsufficiency on the inheritance of telomere length.
Proc Natl Acad Sci U S A
102
17119
17124
39. ValdesAM
AndrewT
GardnerJP
KimuraM
OelsnerE
2005
Obesity, cigarette smoking, and telomere length in women.
Lancet
366
662
664
40. BryanTM
GoodrichKJ
CechTR
2000
A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity.
J Biol Chem
275
24199
24207
41. HemannMT
StrongMA
HaoLY
GreiderCW
2001
The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability.
Cell
107
67
77
42. TeixeiraMT
ArnericM
SperisenP
LingnerJ
2004
Telomere length homeostasis is achieved via a switch between telomerase - extendible and -nonextendible states.
Cell
117
323
335
43. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias
2002
This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001.
Am J Respir Crit Care Med
165
277
304
44. AbecasisGR
ChernySS
CooksonWO
CardonLR
2002
Merlin – –rapid analysis of dense genetic maps using sparse gene flow trees.
Nat Genet
30
97
101
45. LeemSH
Londono-VallejoJA
KimJH
BuiH
TubacherE
2002
The human telomerase gene: complete genomic sequence and analysis of tandem repeat polymorphisms in intronic regions.
Oncogene
21
769
777
46. PodlevskyJD
BleyCJ
OmanaRV
QiX
ChenJJ
2008
The telomerase database.
Nucleic Acids Res
36
D339
343
Štítky
Genetika Reprodukční medicína
Článek Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis inČlánek c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and PhosphodiesterasesČlánek Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 3
-
Všechny články tohoto čísla
- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- Testing for an Unusual Distribution of Rare Variants
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities!
- Initial Mutations Direct Alternative Pathways of Protein Evolution
- Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in
- Sensing of Replication Stress and Mec1 Activation Act through Two Independent Pathways Involving the 9-1-1 Complex and DNA Polymerase ε
- Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in
- Identification of a Novel Type of Spacer Element Required for Imprinting in Fission Yeast
- Chiasmata Promote Monopolar Attachment of Sister Chromatids and Their Co-Segregation toward the Proper Pole during Meiosis I
- Global Analysis of the Relationship between JIL-1 Kinase and Transcription
- H3K9me2/3 Binding of the MBT Domain Protein LIN-61 Is Essential for Vulva Development
- REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock
- A Novel Unstable Duplication Upstream of Predisposes to a Breed-Defining Skin Phenotype and a Periodic Fever Syndrome in Chinese Shar-Pei Dogs
- Polycomb Repressive Complex 2 Controls the Embryo-to-Seedling Phase Transition
- A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Germ Line
- Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits
- A Genome-Wide Association Study of Upper Aerodigestive Tract Cancers Conducted within the INHANCE Consortium
- Ancestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
- Ultra-Deep Sequencing of Mouse Mitochondrial DNA: Mutational Patterns and Their Origins
- Phenotype Restricted Genome-Wide Association Study Using a Gene-Centric Approach Identifies Three Low-Risk Neuroblastoma Susceptibility Loci
- The Toll-Like Receptor Gene Family Is Integrated into Human DNA Damage and p53 Networks
- Polycomb Targets Seek Closest Neighbours
- Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis
- c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and Phosphodiesterases
- Estimating Divergence Time and Ancestral Effective Population Size of Bornean and Sumatran Orangutan Subspecies Using a Coalescent Hidden Markov Model
- Rif1 Supports the Function of the CST Complex in Yeast Telomere Capping
- A Tradeoff Drives the Evolution of Reduced Metal Resistance in Natural Populations of Yeast
- Quantifying the Underestimation of Relative Risks from Genome-Wide Association Studies
- Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in
- Triplet Repeat–Derived siRNAs Enhance RNA–Mediated Toxicity in a Drosophila Model for Myotonic Dystrophy
- The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
- Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
- Environmental Sex Determination in the Branchiopod Crustacean : Deep Conservation of a Gene in the Sex-Determining Pathway
- Systematic Detection of Polygenic Regulatory Evolution
- The SUMO Isopeptidase Ulp2p Is Required to Prevent Recombination-Induced Chromosome Segregation Lethality following DNA Replication Stress
- Uncoupling Antisense-Mediated Silencing and DNA Methylation in the Imprinted Cluster
- Role of the Drosophila Non-Visual ß-Arrestin Kurtz in Hedgehog Signalling
- Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti–dsDNA Autoantibody Production
- COMPASS-Like Complexes Mediate Histone H3 Lysine-4 Trimethylation to Control Floral Transition and Plant Development
- H3 Lysine 4 Is Acetylated at Active Gene Promoters and Is Regulated by H3 Lysine 4 Methylation
- Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches
- A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in
- Roles of () in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- A Molecular Phylogeny of Living Primates
- Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice
- A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



