-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
RNA–directed DNA methylation (RdDM) is an epigenetic control mechanism driven by small interfering RNAs (siRNAs) that influence gene function. In plants, little is known of the involvement of the RdDM pathway in regulating traits related to immune responses. In a genetic screen designed to reveal factors regulating immunity in Arabidopsis thaliana, we identified NRPD2 as the OVEREXPRESSOR OF CATIONIC PEROXIDASE 1 (OCP1). NRPD2 encodes the second largest subunit of the plant-specific RNA Polymerases IV and V (Pol IV and Pol V), which are crucial for the RdDM pathway. The ocp1 and nrpd2 mutants showed increases in disease susceptibility when confronted with the necrotrophic fungal pathogens Botrytis cinerea and Plectosphaerella cucumerina. Studies were extended to other mutants affected in different steps of the RdDM pathway, such as nrpd1, nrpe1, ago4, drd1, rdr2, and drm1drm2 mutants. Our results indicate that all the mutants studied, with the exception of nrpd1, phenocopy the nrpd2 mutants; and they suggest that, while Pol V complex is required for plant immunity, Pol IV appears dispensable. Moreover, Pol V defective mutants, but not Pol IV mutants, show enhanced disease resistance towards the bacterial pathogen Pseudomonas syringae DC3000. Interestingly, salicylic acid (SA)–mediated defenses effective against PsDC3000 are enhanced in Pol V defective mutants, whereas jasmonic acid (JA)–mediated defenses that protect against fungi are reduced. Chromatin immunoprecipitation analysis revealed that, through differential histone modifications, SA–related defense genes are poised for enhanced activation in Pol V defective mutants and provide clues for understanding the regulation of gene priming during defense. Our results highlight the importance of epigenetic control as an additional layer of complexity in the regulation of plant immunity and point towards multiple components of the RdDM pathway being involved in plant immunity based on genetic evidence, but whether this is a direct or indirect effect on disease-related genes is unclear.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002434
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002434Summary
RNA–directed DNA methylation (RdDM) is an epigenetic control mechanism driven by small interfering RNAs (siRNAs) that influence gene function. In plants, little is known of the involvement of the RdDM pathway in regulating traits related to immune responses. In a genetic screen designed to reveal factors regulating immunity in Arabidopsis thaliana, we identified NRPD2 as the OVEREXPRESSOR OF CATIONIC PEROXIDASE 1 (OCP1). NRPD2 encodes the second largest subunit of the plant-specific RNA Polymerases IV and V (Pol IV and Pol V), which are crucial for the RdDM pathway. The ocp1 and nrpd2 mutants showed increases in disease susceptibility when confronted with the necrotrophic fungal pathogens Botrytis cinerea and Plectosphaerella cucumerina. Studies were extended to other mutants affected in different steps of the RdDM pathway, such as nrpd1, nrpe1, ago4, drd1, rdr2, and drm1drm2 mutants. Our results indicate that all the mutants studied, with the exception of nrpd1, phenocopy the nrpd2 mutants; and they suggest that, while Pol V complex is required for plant immunity, Pol IV appears dispensable. Moreover, Pol V defective mutants, but not Pol IV mutants, show enhanced disease resistance towards the bacterial pathogen Pseudomonas syringae DC3000. Interestingly, salicylic acid (SA)–mediated defenses effective against PsDC3000 are enhanced in Pol V defective mutants, whereas jasmonic acid (JA)–mediated defenses that protect against fungi are reduced. Chromatin immunoprecipitation analysis revealed that, through differential histone modifications, SA–related defense genes are poised for enhanced activation in Pol V defective mutants and provide clues for understanding the regulation of gene priming during defense. Our results highlight the importance of epigenetic control as an additional layer of complexity in the regulation of plant immunity and point towards multiple components of the RdDM pathway being involved in plant immunity based on genetic evidence, but whether this is a direct or indirect effect on disease-related genes is unclear.
Introduction
RNA-directed DNA methylation (RdDM) is an epigenetic modification mechanism driven by noncoding small interfering RNAs (siRNAs) [1], [2]. siRNAs are present in most eukaryotic organisms, are highly developed in plants and regulate gene expression at the transcriptional and posttranscriptional level in a sequence-specific manner. In contrast to microRNAs (miRNAs) that are derived from the transcripts of miRNA genes generated by RNA Polymerase II, production of RdDM-associated siRNAs requires RNA Polymerase IV (Pol IV) complex activity which includes, among other constituents, the largest and second largest subunits, NRPD1 and NRPD2, respectively [3]–[5]. Upon the action of Pol IV, the resulting single-stranded RNAs are used as templates for RNA-dependent RNA polymerase 2 (RDR2) generating double-stranded RNAs, which are processed by DICER-LIKE 3 (DCL3) [6], [7]. Subsequently, RNA methyltransferase HUA ENHANCER-1 (HEN1) generates functional siRNAs that are recruited by ARGONAUTE4 (AGO4) to form the AGO4-RISC multiprotein complex guided to siRNA-complementary genome sequences [8]–[10]. AGO4-siRNA complexes interact with the RNA Polymerase V (Pol V) complex, which includes the largest and second largest subunits, NRPE1 and NRPD2, respectively. Pol V is somehow required to recruit DRM2 methyltransferase as well as histone-modifying complexes to finally establish the methylation pattern in the siRNA-complementary genome sequences; however, the details of this recruitment are unknown. This process results in the methylation of certain genome repeat regions and their subsequent transcriptional silencing [2]. Among the different classes of siRNA, the 24 nt in lenght hetrocromatic siRNAs (hc-siRNAs) and repeat-associated siRNAs (ra-siRNAs), primarily derived from transposons, repeated elements and heterochromatin regions, are those functioning in the RdDM pathway by mediating DNA methylation and/or histone modification at the target sites [2].
Small RNAs regulate a multitude of biological processes in plants, including sustaining genome integrity, development, metabolism and responses to changing environmental conditions and abiotic stress [11]. Increasing evidences also indicate that plant endogenous small RNAs, including miRNAs and siRNAs, are integral regulatory components of plant defense machinery against microbial pathogens [12]. The Arabidopsis miR393 imparts basal resistance to the bacterial pathogen Pseudomonas syringae DC3000 by targeting the auxin receptors TIR1, ABF2 and ABF3 [13]. Besides miR393, two other miRNA families, miR160 and miR167, are upregulated following PsDC3000 inoculation and target members of auxin-response factors (ARF) [14]. Thus, in response to bacterial infection, plants suppress multiple components of the auxin signaling pathway. In turn, bacteria have developed type III secretion effectors that repress transcription of miRNA genes, the host RNA silencing machinery is suppressed and therefore disease susceptibility increase [15]. Similarly, Lu et al. [16] identified a series of 10 miRNAs families in loblolly pine whose expression were suppressed, and the transcript levels of their target genes increased, upon infection with the rust fungus Cromartium quercuum f. sp. fusiforme. Likewise, upon infection of Brasica rapa with Turnip mosaic virus (TuMV) the miR1885 is upregulated, and its target is predicted to be a member of the TIR-NBS-LRR class of disease-resistance proteins [17]. Thus, it appears that following detection of pathogen-associated molecules, plant cells undergo changes in miRNA global profiles that mediate the establishment of a specific defense response [12], [18].
Although plants contain only several hundred miRNAs, they contain huge numbers of endogenous siRNAs but only in a few cases the involvement of siRNAs in plant immunity has been described. In Arabidopsis, the natural antisense transcript (NAT)-derived nat-siRNAAATGB2 and the long siRNA lsiRNA-1, which specifically targets the mitochondrial pentatricopeptide protein(PPR)-like gene PPRL and the RNA-binding protein gene AtRAP, respectively, are induced by the bacterial pathogen PsDC3000(avrRpt2) and contribute to plant antibacterial immunity [19], [20]. The endogenous siRNAs generated at disease resistance RPP4 locus, which impart resistance to both the bacterial Ps pv. maculicola and the oomycetes Hyaloperonospora arabidopsidis, constitute a third example for siRNA-mediated resistance responses [21]. However, it remains unclear how RdDM participates in this type of processes.
The understanding of the overall contribution and requirement of the different components that conform the RdDM pathway, and how important they are in the regulation of the RdDM-mediated processes, particularly in relation to plant immunity, is an issue that still remains to be fully understood. Previously we described a genetic screen in Arabidopsis design to identify mutants (ocp mutants) with altered immune responses [22]. This allowed identifying AGO4, through the characterization of its mutant allele ago4-2/ocp11, as an important component of the RdDM pathway in mediating plant immune responses towards PsDC3000 [23]. Towards characterizing the contribution of other components of the RdDM pathway in plant immunity, we report here on the isolation and characterization of ocp1, a recessive mutant allele of NRPD2. Our results support that RdDM, through the action of RNA Pol V, is pivotal in modulating immune responses towards pathogens.
Results/Discussion
Characterization of ocp1 Plants
The Arabidopsis ocp mutants were identified previously in a genetic screen [22] designed to isolate negative regulators of pathogen-induced defense responses. The H2O2-responsive and defense-related Ep5C gene promoter fused to GUS was used as reporter [24]. Here we described the characterization of the ocp1 mutant. Figure 1A shows the constitutive Ep5C::GUS expression in rosette leaves from ocp1 plants compared with its parental Col-0 line (line 5.2). ocp1 plants exhibited similar plant architecture and growth habit to the wild-type plants (Figure 1B). F1 hybrids from a backcross between parental and ocp1 plants showed the absence of GUS activity, and GUS activity segregated in the F2 progeny as a single recessive Mendelian locus [OCP1:ocp1, 111 : 33 (P<0.05, χ2 test)].
Fig. 1. Characterization of ocp1 plants. 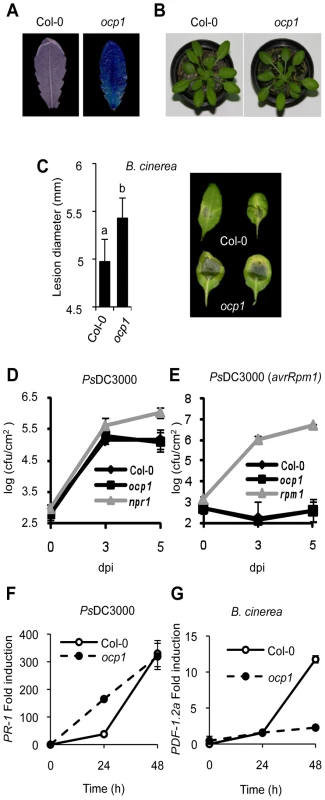
(A) Comparative histochemical analysis of GUS activity in rosette leaves from a parental wild-type plant carrying the PEp5C:GUS transgene (left), and ocp1 mutant plant (right). (B) Macroscopic comparison of 3-week-old wild-type (left) and ocp1 plants (right). (C) Resistance response of wild-type and ocp1 plants to virulent B. cinerea. Lesion size was measured 5 days after inoculation (dpi). Data points represent average lesion size ± SE (n≥30 lesions). Representative leaves from wild-type and ocp1 plants 4 dpi. (D–E) Growth rates of virulent PsDC3000 (D) and avirulent PsDC3000 (AvrRpm1) (E) in Col-0, ocp1 and npr1 or rpm1 plants. (F–G) RT-qPCR expression analysis of PR-1 (F) and PDF1.2a (G) in wild-type and ocp1 plants at different times following inoculation with PsDC3000 (F) and B. cinerea (G). Data represent the mean ± SD; n = 3 biological replicates. We hypothesize that the constitutive expression of Ep5C::GUS observed in ocp1 plants might be accompanied by an altered disease resistance response to pathogens as previously revealed in ocp3 and ocp11 plants [22], [23], [25], [26]. Therefore, we inoculated ocp1 plants with the virulent necrotrophic fungal pathogen Botrytis cinerea and monitored the disease response in leaves in comparison with the parental line. Disease was scored by recording the extent of necrosis. Wild-type plants exhibited normal susceptibility to B. cinerea (Figure 1C), with inoculated leaves showing necrosis accompanied by extensive proliferation of the fungal mycelia. In contrast, ocp1 plants showed increased susceptibility to B. cinerea distinguished by moderate but statistical significant enlargement of necrotic areas at inoculation sites (Figure 1C).
Susceptibility of ocp1 plants to pathogens was also investigated with the bacterial pathogen PsDC3000. The npr1 mutant, which is compromised in resistance towards this pathogen [27] was used as a control. Resulting bacterial growth in inoculated leaves is shown in Figure 1D and indicates the wild-type and ocp1 mutant susceptibility was unchanged towards virulent PsDC3000. In addition, plants were inoculated with an avirulent strain of PsDC3000 carrying the avrRpm1 gene that triggers a hypersensitive cell death response in the plant that stops bacterial growth. The rpm1 mutant, compromised in the hypersensitive response and consequently hypersusceptible to the pathogen, was used as a control. Results showed the growth of PsDC3000 (avrRpm1) in ocp1 plants was not significantly different to that observed in wild-type plants (Figure 1E). These results were consistent with normal accumulation of transcripts of the salicylic acid (SA)-responsive gene PR-1 at 48 h following inoculation with PsDC3000 (Figure 1F), however induction occurs earlier in ocp1 plants. Interestingly, induction of the jasmonic acid (JA)-responsive gene PDF1.2a, a characteristic molecular response of plants to fungal attack, was compromised in ocp1 plants following inoculation with B. cinerea (Figure 1G). This later observation is congruent with the observation that ocp1 plants showed enhanced disease susceptibility to this pathogen (Figure 1C).
OCP1 Is At3g23780 and Encodes NRPD2, the Second Largest Subunit of the RNA Pol IV and Pol V
The genetic lesion carried by ocp1 plants was identified by positional cloning (Figure S1). A single nucleotide deletion was detected on locus At3g23780, particularly in the third exon of the transcribed gene encoding NRPD2, the second largest subunit of the RNA Pol IV and Pol V protein complexes (Figure 2A and Figure S1C). The loss of a nucleotide residue created a change in the NRPD2 open reading frame that leads to a frame shift starting at residue 595 (Figure 2A) followed by an incorrect 22 amino acid C-terminal tail sequence before an in-frame stop codon (Figure S2). The mutation renders a protein of 616 amino acid residues, instead of the 1172 contained in NRPD2, that thus has lost almost half of the protein sequence, including the amino acids that contribute to the active site of RNA polymerases [28].
Fig. 2. ocp1 is a mutant allele of NRPD2. 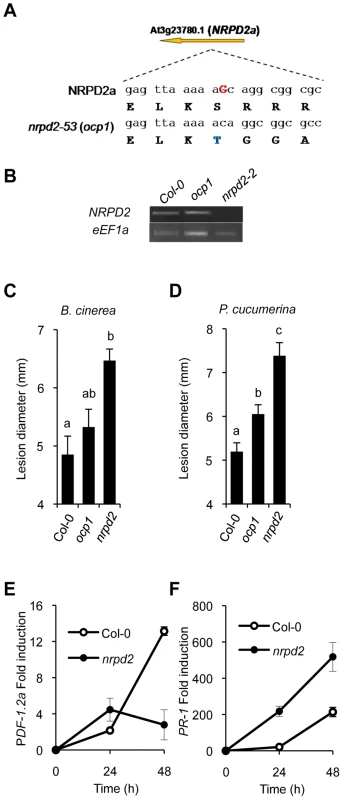
(A) OCP1 corresponds to At2g27040 encoding NRPD2. The G nucleotide residue deleted in the ocp1 allele is indicated in red bold uppercase letters in the wild-type sequence. Deduced amino acid sequences are indicated below each nucleotide triplet, and the first amino acid change (S to T) where the frameshift of the OCP1 protein starts is shown in blue. (B) NRPD2 expression level by RT-PCR in mRNAs derived from Col-0, nrpd2-2 and ocp1 plants. The eEF1a house-keeping gene was used as a control. (C–D) nrpd2 plants show enhanced susceptibility to fungal pathogens. Lesion size was measured in Col-0, ocp1 and nrpd2-2 plants after inoculation with B. cinerea (C) or P. cucumerina (D). Data points represent average lesion size ± SE (n≥30 lesions). ANOVA detected significant differences at the P<0.05 level. (E–F) RT-qPCR determination of PDF1.2a (E) and PR-1 (F) transcript levels following inoculation with P. cucumerina. Data represent the mean ± SD; n = 3 biological replicates. The result obtained in our mapping strategy was corroborated with a test of allelism between ocp1 plants and plants carrying a null allele of NRPD2, in particular with nrpd2-2 plants which carry a T-DNA insertion (SALK_046208) [3]. Analysis of GUS expression driven by the Ep5C gene promoter in 20 F1 plants derived from a cross between homozygous ocp1 plants with homozygous nrpd2-2 plants or, alternatively, from a reversed cross between nrpd2-2 plants with ocp1 plants, revealed that all F1 plants showed constitutive GUS expression (Figure S3). Conversely, control crosses between the parental Col-0 plants carrying the Ep5C::GUS gene construct (line 5.2) with either ocp1 plants or nprd2-2 plants revealed no GUS expression in any of the F1 22 plants analyzed (Figure S3). The result indicates that the ocp1 and nrpd2-2 are mutant alleles of the same NRPD2 gene and supported the conclusion that the ocp1 mutation represents a loss of function allele. Hence, the ocp1 mutation will be referred also as ocp1/nrpd2-53.
From the type of mutation found, we cannot exclude the possibility that ocp1 plants are still able to produce a truncated version of the NRPD2 protein with a residual ability to interact with other components of the RNA polymerase complexes. Since Pol IV and Pol V complexes are comprised of a variety of interacting subunits, some being polymerase-specific while other subunits shared (i.e., NRPD2) [5], [29], [30], and with some cross-talk described for some of their subunits (i.e., between NRPD2 and NRPE1; [4]), we can not discard the possibility that the relationships between the different components of the two RNA polymerase complexes may become differentially altered in the ocp1 mutant. In this respect, the availability of the ocp1 allele may represent a valuable experimental tool to approach the biochemical regulation of the RdDM mechanism.
Interestingly, RT-PCR analyses of NRPD2 transcript levels in ocp1 plants revealed the absence of notable changes in gene expression compared with Col-0 plants (Figure 2B). This is in marked contrast with the expression observed in nrpd2-2 null mutant plants where no transcript amplification products can be obtained (Figure 2B). A comparison of the disease resistance response between ocp1 and nrpd2-2 plants revealed that while the ocp1 plants showed a moderate increase in susceptibility to B. cinerea, the nrpd2-2 null mutant responded to B. cinerea infection with a remarkable enhancement in susceptibility (Figure 2C). The enhanced susceptibility phenotype of nrpd2-2 plants was further corroborated by recording the susceptibility towards Plectosphaerella cucumerina, a different fungal necrotroph (Figure 2D). Consistent with the observed increase in disease susceptibility to P. cucumerina, RT-qPCR experiments revealed that induction of the JA-responsive PDF1.2a gene was disabled in nrpd2-2 plants compared to Col-0 (Figure 2E). These results mirror what occurs in ocp1 plants following B. cinerea infection (Figure 1F). Of importance for understanding the immune-related phenotype of nrpd2-2 plants is the observation that expression of the SA-responsive PR-1 gene was clearly enhanced following fungal inoculation in the mutant when compared to wild-type plants (Figure 2F). Since nrpd2-2 plants show an enhanced disease susceptibility of bigger magnitude than that observed in ocp1/nrpd2-53 plants, subsequently, the experiments related to disease resistance/susceptibility will be carried out employing the nrpd2-2 allele.
SUPERMAN, 5S Genes, and the AtSN1 Retroelement Are Hypomethylated in ocp1 Plants
To further substantiate the molecular phenotype of ocp1 plants in relation to RdDM, we checked if the methylation status of different RdDM target sequences could be similarly affected in ocp1 and nrpd2-2 plants. We analyzed the methylation status in ocp1 plants of the RdDM pathway DNA target sequences SUPERMAN, ribosomal 5S genes and the retrotransposon AtSN1 [31]. We used methylation tests employing the methylation-sensitive restriction endonuclease HaeIII (where HaeIII will not cut DNA if methylated), with subsequent amplification by PCR [32]. Initial experiments revealed that ocp1, as well as ago4-2/ocp11 plants used as controls, exhibit a higher degree of hypomethylation in SUPERMAN gene compared to Col-0 plants (Figure 3A). Analyses were extended to the ribosomal 5S genes and the AtSN1 retrotransposon and we incorporated nrpd2-2, nrpd1-3 and nrpe1-1 mutants for comparison. Figure 3B shows mutants demonstrated higher degrees of hypomethylation in the sequences analyzed. DNA samples derived from ocp1 plants exhibited decreased amplification for the 5S and AtNS1 loci, confirming a clear DNA methylation deficiency in this mutant. The ABI5 gene, whose sequence contains no restriction sites for HaeIII, was used as a control. Methylation tests were also used to ascertain whether or not the enhanced induction observed for the PR-1 gene, or the repression of PDF1.2a, in the nrpd2 mutant following fungal infection correlated with defects in the DNA methylation of their promoter regions. Since both genes contain a large number of recognition sites for the methylation-sensitive restriction enzymes FspEI, MspJI and AvaII (168 target sequences in the PR-1 gene and 298 targets in the PDF1.2a gene), and where FspEI and MspJI sites must be methylated for the enzymes to cleave the DNA, we used restriction analysis with these enzymes with subsequent amplification by PCR to check the methylation status of the PR-1 and PDF1.2a genes. The results shown in Figure S4 and Figure S5 revealed that none of the promoters appear methylated, not even in Col-0 plants. Conversely, the sensitivity of the methylated 5S ribosomal DNA (Figure S4) to the aforementioned enzymes revealed the appropriateness of the method used to identify methylation of cytosine residues. The lack of a methylation footprint in the DNA of the defense-related PR-1 and PDF1.2a genes might suggest that the abnormal expression patterns concurring in nrpd2 mutant plants must obey not to a direct modification of cytosine residues but to other type of chromatin modification or mechanism similarly controlled either directly or indirectly by the RdDM pathway.
Fig. 3. ocp1 plants show hypomethylation in RdDM target DNA sequences. 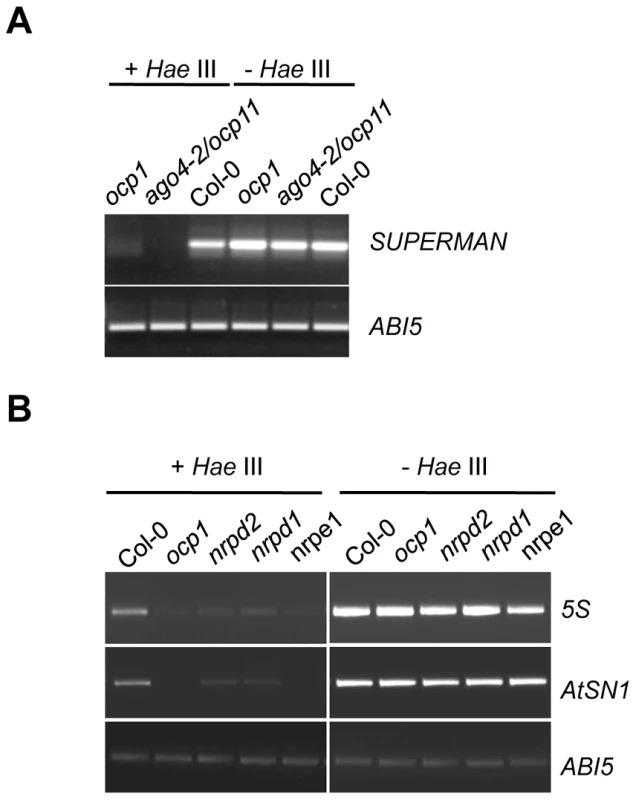
Genomic DNA isolated from Col-0, ocp1 and ago4-2/ocp11 plants (A) and nrpd2, nrpd1 and nrpe1 (B) was digested (+) or not (−) with HaeIII and amplified by PCR for SUPERMAN promoter (A), the ribosomal 5S genes and the retrotransposon AtSN1 (B). ABI5 contains no target sequences for HaeIII and was used as a control. The Pol V Complex, But Not Pol IV, Is Required for the Correct Immune Response against B. cinerea and P. cucumerina
As for NRPD2, we addressed if other RdDM pathway components are similarly engaged in plant immunity. A comparative analysis of the disease resistance response of nrpd1, nrpe1, and ago4 mutant plants due to inoculation by B. cinerea was performed in relationship to nrpd2. Figure 4A shows an increase in nrpe1 disease susceptibility to B. cinerea; the susceptibility being of a magnitude similar to that attained in nrpd2 plants. This enhancement in susceptibility was comparatively greater than that observed in ocp1 plants but less than in ago4-2/ocp11 plants. Conversely, nrpd1 plants did not exhibit a significant deviation from the normal disease response observed in Col-0 plants. This differential behavior was further corroborated in the Pol IV and Pol V defective mutants by challenging with P. cucumerina (Figure 4B). The nrpd1 nrpe1 double mutant that would be defective in both Pol IV and Pol V activities was incorporated in this experiment for comparison. nrpd1 nrpe1 plants showed an enhanced disease susceptibility of a magnitude similar to that attained in nrpe1 or nrpd2 plants. Furthermore, fungal biomass determination in leaves inoculated with P. cucumerina, as an alternative method for recording disease resistance, also revealed that the single nrpd2 and nrpe1 mutants, as well as the double nrpd1 nrpe1 mutant support significantly more fungal growth than Col-0 and the nrpd1 mutant (Figure S6). Therefore, the Pol V complex participates in the regulation of the immune response to necrotrophs while the Pol IV complex appears at least partially dispensable. This is sustained also by the observation that expression patterns of the PDF1.2a and the PR-1 genes in nrpd1 plants are dissimilar to that commonly attained in both nrpe1 and nrpd2 plants, either in the course of infection with P. cucumerina (Figure 4C–4D) or upon chemical induction by treating plants with a solution of either 0.5 mM SA (Figure S7A) or 0.1 mM JA (Figure S7B). Notorious is the higher JA-triggered PDF1.2 gene induction in nrpd1 plants in comparison to Col-0 (Figure S7B). Conversely, this JA-triggered PDF1.2a gene induction is notably repressed in the Pol V defective mutants (Figure 4C and Figure S7B). This is in marked contrast with the altered expression pattern observed in nrpd2 plants where induction of PR-1 gene expression showed enhancement following inoculation with P. cucumerina (Figure 4D) or upon external application of SA (Figure S7A). Importantly, this pattern of gene expression was reproduced in nrpe1 plants (Figure 4D and Figure S7A). Moreover, the transcription factors WRKY6 and WRKY53 that bind W-box and transcriptionally regulate gene expression of SA-related genes, including PR-1 [33], and are themselves induced by pathogen infection [34], show similar enhanced level of induction following SA application in nrpd2 and nrpe1 plants when compared to Col-0 or nrpd1 plants (Figure S7C and S7D).
Fig. 4. Comparative immune responses of RdDM mutants to inoculation with B. cinerea and P. cucumerina. 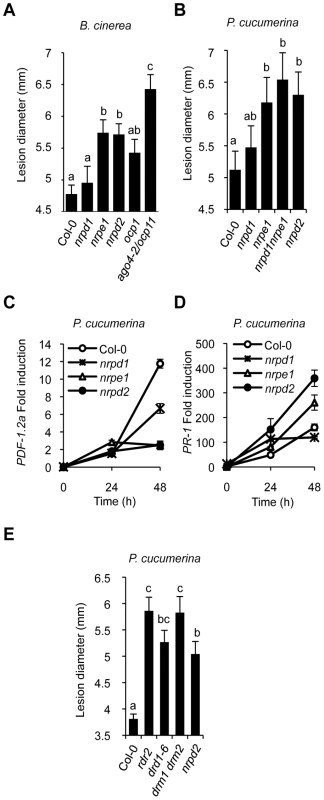
(A) Disease susceptibility of Col-0, nrpd1, nrpe1, nrpd2, ocp1 and ago4-2/ocp11 plants to B. cinerea. (B) Comparative disease susceptibility of the Pol IV and Pol V defective mutants to P. cucumerina. (C–D) RT-qPCR of PDF1.2a (C) and PR-1 (D) transcript levels following inoculation with P. cucumerina in Col-0, nrpd1, nrpe1 and nrpd2 plants. Data represent the mean ± SD; n = 3 biological replicates. (E) Comparative disease susceptibility of rdr2, drd1, drm1drm2 and nrpd2 mutants to P. cucumerina. ANOVA detected significant differences at the P<0.05 level. drd1, rdr2, and drm1drm2 Mutants Show Compromised Immune Responses to P. cucumerina
To further extent the implication of RdDM mechanism in plant immunity we inoculated the drm1drm2 double mutant plants, which is compromised in de novo DNA methylation [35], with P. cucumerina and recorded the disease response. Results in Figure 4E reveal that loss of the functional RDM methyltransferase compromise disease resistance and results in plants showing enhanced susceptibility to P. cucumerina to levels even higher than in nrpd2 plants. This reinforces the proposal that RdDM is pivotal for plant immunity. Likewise, we observed that the chromatin-remodeling factor DRD1, which is required for the association of NRPE1 with chromatin [36], is also pivotal for plant immunity. Figure 4E shows that drd1-6 plants phenocopy Pol V defective mutants. The RNA-dependent RNA polymerase 2 (RDR2), which functions early in the RdDM pathway by generating dsRNA from the ssRNA transcripts thought to emanate presumably from the the Pol IV complex was also entertained in these experiments. Intriguingly, rdr2 plants also show a strikingly enhancement in susceptibility to fungal infection (Figure 4E), achieving highest levels of susceptibility to P. cucumerina. This observation strongly argues in favor of RDR2 as required for plant immunity. Then, how can a mechanism explain the exclusion of RNA Pol IV in mediating plant immunity while the rest of downstream components of the RdDM pathway are engaged in this biological process? There are previous evidences where Pol V has been described to operate independently of Pol IV, such as in the mechanism for maintaining the methylation status of target sequences [37], and thus for some processes Pol IV and Pol V act not in concert [38]. A hypothesis that could help explain the paradoxical observations indicating that Pol V, RDR2, AGO4, DRD1 and DRM2, but not Pol IV, are required for plant immunity to fungal pathogens could be one where RDR2 can accept RNA transcripts derived from the action of RNA Pol V, and not necessarily only from RNA Pol IV. These putative transcripts thought to be acted upon by RDR2, which generates dsRNA, will be processed into siRNAs and feed into the RdDM pathway. In support for the existence of Pol V-dependent transcripts required for DNA methylation and silencing is the recent identification of low-abundance intergenic non-coding (IGN) transcripts [36]. It could be that a similar situation is on the basis to explain the requirement of RdDM for plant immune responses. This possibility merits future research approaches.
SA–Mediated Defense Genes Are Poised for Enhanced Activation by Histone Modifications in Pol V Defective Mutants
The previous results suggest that in Pol V defective mutants SA-related defense genes are poised for enhanced activation following perception of pathogenic cues and concurrently JA-related defenses appear impeded for induction. This will be congruent with a notion where Pol V may regulate a priming phenomenon for SA-mediated defense responses that ultimately would modulate the speed and extent of gene activation. However, the lack of a methylation footprint in the DNA of the defense-related PR-1 and PDF1.2a genes (Figure S5 and Figure S6) suggest that the observed abnormal gene expression patterns concurring in the Pol V defective mutants is not to be due to an altered DNA methylation pattern resulting from a defective RdDM pathway. However, one could still entertained the possibility that changes in chromatin structure such as those obeying to covalent modification of histones, which are also under the control of the RdDM pathway, may be on the basis for the enhanced expression observed for PR-1 and, therefore, for the altered resistance phenotypes in the mutant plants. This would be congruent with the recent identification of a mechanism linking chromatin modification in wild type plants, through the differential modification of histones in several genes encoding WRKY transcription factors (i.e. WRKY6, WRKY29 or WRKY53), with priming of a defense response following pharmacological treatment with the SA analogue acidobenzolar S-methyl (BTH) which functions as a priming agent in plants [39]. Thus, we hypothesized that in Pol V defective mutants PR-1 could be poised for enhanced activation of gene expression by a differential modification of histones.
By using chromatin immunoprecipitation (ChIP) we analyzed trimethylation of histone H3 Lys4 (H3K4me3) and acetylation of histone H3 Lys9 (H3K9ac) on the promoter of the PR-1 gene. For comparison, the promoter of the JA-inducible PDF1.2a gene, that of the constitutively expressed Actin2 gene and also those of the WRKY6 and WRKY53 genes were similarly studied. The specificity of the ChIP reaction was evaluated in advance by measuring histone modifications on these genes in Col-0 plants treated with BTH (Figure S8A and S8B). On the PR-1 promoter H3K4me3 and H3K9ac marks increased after BTH application while these marks did not change in the promoters of Actin2 or PDF1.2a (Figure S8A and S8B). As for PR-1, these chromatin marks were similarly increased in the promoters of WRKY6 and WRKY53 upon treatment with BTH (Figure S8C). Thus chromatin marks normally associated with active genes [39], [40] are set in the promoters of SA-related defense genes by the priming stimulus of BTH. Interestingly, determination of H3K4me3 (Figure 5A) and H3K9ac (Figure 5B) chromatin marks in the PR-1 promoter in ChIP samples derived from nrpd2 and nrpe1 plants, revealed that these marks are already set in these two mutants, although PR-1 gene activation does not take place. Thus, Pol V defective mutants mimic Col-0 plants treated with the priming agent BTH. This reconciles with the idea that the PR-1 gene is switch on for priming in the Pol V defective mutant and explains why this gene shows enhanced induction upon pathogenic attack in the same mutants (Figure 4D). In the nrpd1 mutant only a moderate increase in the setting of these chromatin marks in the promoter of PR-1 was detected (Figure 5A and 5B). No variation in similar activation marks was observed in the promoters of the Actin2 and PDF1.2a genes (Figure 5A and 5B). Other histone marks, such as H3K9me2 and H3K27me3, both of which repressive marks normally associated with heterochromatin and established through the RdDM pathway [41], appear notably reduced in the PR-1 promoter in ChIP samples derived from nrpd2 and nrpe1 plants, and much less reduced in nrpd1 plants, when compared to Col-0 plants (Figure S9A and S9B). Moreover, Col-0 plants respond to P.cucumerina infection with reduction in the setting of these two repressive histone marks in the PR-1 gene promoter but not in the promoters of the PDF1.2a or Actin2 genes (Figure S9C). The dismantling of histone repressive marks in infected plants, along with the concurring increase in histone activation marks and decrease in repressive marks in the promoter of the PR-1 gene, as observed in nrpd2 and nrpe1 plants, gives further support to the implication of Pol V in regulating defense gene activation.
Fig. 5. Histone H3 modifications. 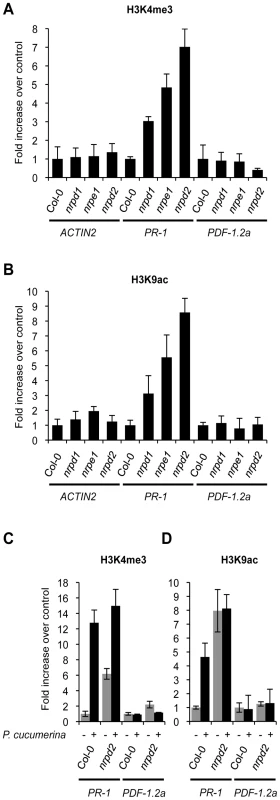
Comparative level of histone modifications of PR-1, PDF1.2a and Actin2 gene promoters as present in leaf samples from Col-0, nrpd1, nrpe1 and nrpd2 plants. (A) Histone H3 Lys4 trimethylation (H3K4me3) on the indicated gene promoters. (B) Histone H3 K9 acetylation (H3K9ac) on the indicated gene promoters. Data are standardized for Col-0 histone modification levels. (C–D) H3K4me3 (C) and H3K9ac (D) modifications on PR-1 and PDF1.2a gene promoters in Col-0 and nrpd2 plants 48 h after inoculation with P. cucumerina. (D) (−) mock inoculated plants, (+) P. cucumerin inoculated plants. Data are standardized for mock inoculated Col-0 histone modification levels. Data represent the mean ± SD; n = 3 biological replicates. As for PR-1, H3K4me3 activation marks are also constitutively set in the promoters of the WRKY6 and WRKY53 genes in healthy nrpd2 and nrpe1 plants (Figure S8C), again mirroring the effect carried out by BTH on Col-0 for these promoters (Figure S8C). Further analysis demonstrated that Col-0 plants respond to P. cucumerina infection with a drastic increase in the setting of H3K4me3 and H3K9ac activation marks in the promoters of PR-1 (Figure 5C and 5D). In nprd2 plants, in which these chromatin marks are already set in PR-1, P. cucumerina inoculation further increase H3K4me3 marks on the PR-1 promoter to levels that are even higher than those attained in Col-0 (Figure 5C). However, for H3K9ac marks no further increase was observed in nrpd2 plants, suggesting that this type of mark is completely set in the mutant. In contrast, no variation in the setting of these chromatin marks was detected in the PDF1.2a promoter upon fungal infection (Figure 5C and 5D). For WRKY6 and WRKY53 gene promoters, Col-0 plants respond to P. cucumerina infection by similarly increasing H3K4me3 mark setting in both promoters (Figure S10). Compared to Col-0, nrpd2 plants constitutively carry increased H3K4me3 mark setting in WRKY6 and WRKY53 gene promoters and do not show further increases upon inoculation, but instead slightly decrease (Figure S9). Together, these data imply that Pol V, either directly or indirectly, regulates the extent of chromatin modifications on SA defense-related gene promoters, and may be the underlying mechanism controlling priming marks facilitating the more rapid activation of gene expression observed upon perception of pathogenic cues. As reported for other genes, the observed covalent modifications in chromatin might provoke increases in the accessibility of DNA or perhaps in the provision of docking sites for gene activators [42], [43].
nrpd2 and nrpe1 Plants Show Enhanced Resistance to PsDC3000
Enhanced activation of SA-mediated defenses is characteristic of plants resistant to biotrophic pathogens, like PsDC3000, and is on the basis for a systemic type of immunity known as systemic acquired resistance (SAR) [44]. Our results on a priming effect for enhanced expression of SA defense-related genes in nrpd2 and nrpe1 plants suggest these mutants may be altered in the resistance to PsDC3000. Consequently, we addressed Pol IV and Pol V defective mutants in search for defects in the immune response to PsDC3000. We used ago4-2/ocp11 and npr1 plants as controls, both exhibiting heightened PsDC3000 disease susceptibility [23], [27]. Interestingly, a significant enhanced disease resistance to PsDC3000 was observed in nrpd2, nrpe1, and in nrpd1 nrpe1 plants, when compared to Col-0 plants (Figure 6). In contrast, statistically significant effects were not observed in nrpd1 plants relative to Col-0 in response to PsDC3000, giving further support to the idea that RNA Pol IV seems not engaged in plant immunity. The observed heightened resistance towards PsDC3000 in nrpd2 and nrpe1 plants indicated that in wild-type plants Pol V is required for susceptibility to this pathogen. However, in ago4-2/ocp11 plants resistance to PsDC3000 is severely compromised. Although there is no obvious explanation for this contrasting effect, as previously stated [23] one can speculate that AGO4 can serve a novel function, and while required for an effective defense response it may operate independently of the RdDM pathway.
Fig. 6. Comparative immune responses of Pol IV and Pol V defective mutants to inoculation with Pseudomonas syringae DC3000. 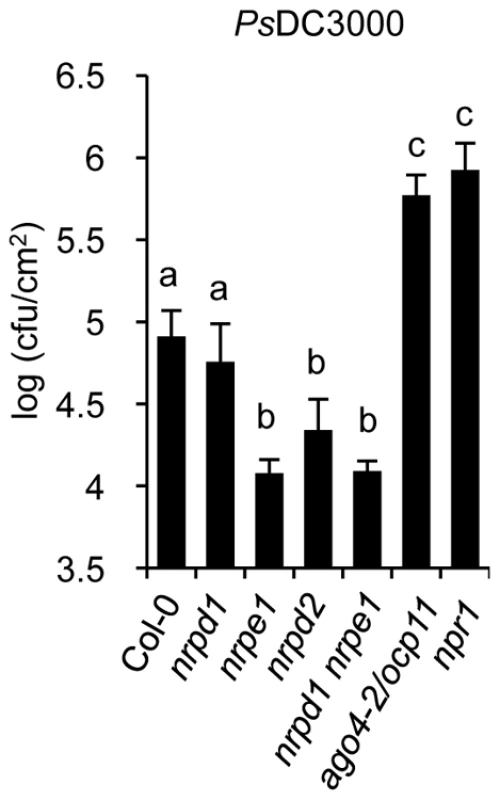
Growth rates of PsDC3000 in Col-0, nrpd1, nrpe1, nrpd2, and nrpd1 nrpe1 plants. The PsDC3000 disease susceptible mutants ago4-2/ocp11 and npr1 were included for comparison. Data represent the mean ± SD; n = 3 biological replicates. An important observation derived from the results presented is the co-existence of an enhanced disease resistance to a biotrophic bacteria, like PsDC3000, with an enhanced susceptibility to necrotrophic fungi in Pol V defective mutants. This reveals an underlying complexity in the control of disease resistance by RdDM. The SA and JA signal pathways are under an antagonistic equilibrium that occasionally culminates with the partial inhibition of one pathway when the other is facilitated. Consequently the interaction between pathways serves to optimize responses to a specific type of pathogenic insult [45]. Our results demonstrated that nrpd2 and nrpe1 plants are poised for enhanced activation of SA defense-related genes and respond to pathogen attack with a marked enhancement in the induced expression of marker genes, which suggests these plants are more prone to mobilize the defense arsenal controlled by SA. A simpler explanation for these observations is that in wild type plants Pol V negatively regulates a priming mechanism for SA-mediated disease resistance while keeping intact a JA-mediated disease resistance. Defects in Pol V function, such as those observed in nrpd2 and nrpe1 mutants, de-repress the priming mechanism for SA-mediated resistance through pertinent chromatin modifications, and renders enhanced resistance to PsDC3000. As a tradeoff, presumably mediated through endogenous antagonistic cross talk mechanisms, mis-regulation of the JA-mediated disease resistance occurs. This thus explaining the repressed expression of JA-marker gene and the heightened susceptibility of nrpd2 and nrpe1 plants to fungal pathogens. However, although this mechanism seems very likely, we still cannot disregard the possibility that RdDM may be similarly required for normal expression of one or more unknown genes involved in JA signaling. Disruption of RdDM could thus lead to a disruption of JA signaling which would in turn result in hyper-activation of SA signaling. In fact, mutant plants with JA-mediated signaling pathway defects and hypersensitivity to fungal necrotrophs concurrently present a less repressed SA-mediated signaling pathway, resulting in a more efficient defense response when challenged with biotrophic pathogens [45], [46]. Experiments directed towards identification of an epigenetic footprint associated to the JA pathway merits future reach and will help clarify the complexity of the antagonistic cross-talk mechanism between the SA and the JA signal transduction pathways.
A deeper understanding on how the RdDM and associated chromatin modification acts as a mechanism controlling gene priming and induced immune responses in plants, and how pathogens may counteract this epigenetic regulation for their own benefit will open new avenues for the a better knowledge on how plant immunity is orchestrated.
Materials and Methods
Plant Material and Growth Conditions
Arabidopsis were grown in a growth chamber (19 to 23°C, 85% relative humidity, 100 µE m−2 s−1 fluorescent illumination) under a 10/14 h light/dark photoperiod. All mutants are in Col-0 background. ago4-2/ocp11, npr1, rpm1-1, rdr2, drd1-6 and drm1/drm2 plants were previously described [23]. nrpd2-2 (SALK_046208); nrpe1-11 (SALK_029919) and nrpd1-3 (SALK_128428) were obtained from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/). nrpd1 nrpe1 double mutant was obtained from T. Lagrange.
GUS Staining
Plant leaves were incubated overnight at 37°C in GUS staining buffer as previously described [22].
The ocp1 mutant was backcrossed twice to the PEp5C:GUS line to confirm its recessive inheritance. ocp1 plants were crossed to Ler, and F1 plants were allowed to self. F2 plants were scored for co-segregation of high constitutive GUS activity with simple sequence length polymorphisms (SSLP) [40]. Molecular markers were derived from the polymorphism database between the Ler and Col-0 ecotypes (http://www.arabidopsis.org).
PCR-Based Methylation Assays
Methylation tests using the methylation-sensitive endonuclease HaeIII, FspEI, AvaII and MspJI were performed as described [32]. The relative DNA fragment amounts corresponding to SUPERMAN, 5S and AtSN1 were obtained after 30, 25 and 35 respective PCR cycles. For ABI5, 30 (A) or 26 (B) PCR cycles were used. PR-1 and PDF1.2a methylation assays are provided in a supplemental file.
Expression Analysis
Gene expression analysis, by either RT-PCR or RT-qPCR was performed as described previously [23]. The primers used to amplify the different genes and DNA regions, and the PCR conditions employed for genotyping T-DNA insertions, and RT-PC and qRT-PC experiments are provided in the supporting information file Text S1.
Bacterial and Fungal Bioassays
Bacterial strains were grown overnight and used to infect 5-week-old Arabidopsis leaves by infiltration and bacterial growth determined as described [23]. Twelve samples were used for each data point and represented as the mean ± SEM of log c.f.u./cm2. B. cinerea and P. cucumerina bioassays were performed as previously described [24]. Fungal disease symptoms were evaluated by determining the lesion diameter (in mm) of a minimum of 30 lesions. All experiments were repeated at least three times with similar results.
Chromatin Immunoprecipitation
Chromatin isolation and immunoprecipitation were performed as described [47]. Chip samples, derived from three biological replicates, were amplified in triplicate and measured by quantitative PCR using primers for PR-1, WRKY6, WRKY53 and Actin2 as reported [39]. The rest of primers are described in Text S1. All ChIP experiments were performed in three independent biological replicates. The antibodies used for immunoprecipitation of modified histones from 2 g of leaf material were antiH3K4m3 (#07-473 Millipore), antiH3K4ac (#07-352 Millipore), antiH3K9me2 (ab1772 Abcam) and antiH3K27me3 (ab6002 Abcam).
Supporting Information
Zdroje
1. MatzkeMKannoTDaxingerLHuettelBMatzkeAJ 2009 RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol [doi:10.1016/j.ceb.2009.01.025]
2. LawJAJacobsenSE 2010 Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Rev Gent 11 204 220
3. OnoderaYHaagJRReamTNunesPCPontesO 2005 Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120 613 622
4. PontierDYahubyanGVegaDBulskiASaez-VasquezJ 2005 Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev 19 2030 2040
5. ReamTSHaagJRWierzbickiATNicoraCDNorbeck 2009 Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA Polymerase II. Mol Cell 33 192 203
6. ChanSWZilbermanDXieZJohansenLKCarringtonJC 2004 RNA silencing genes control de novo DNA methylation. Science 303 1336
7. XieZJohansenLKGustafsonAMKasschauKDLellisAD 2004 Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2 e104 doi:10.1371/journal.pbio.0020104
8. ZilbermanDCaoXJacobsenSE 2003 ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299 716 719
9. ZilbermanDCaoXJohansenLKXieZCarringtonJC 2004 Role of Arabidopsis ARGONAUTE4 in RNA-directed DNA methylation triggered by inverted repeats. Curr Biol 14 1214 1220
10. QiYHeXWangXJKohanyOJurkaJ 2006 Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature 443 1008 1012
11. ChinnusamyVZhuJK 2009 Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12 133 139
12. Katiyar-AgarwalSJinH 2010 Role of Small RNAs in Host-Microbe Interactions. Annu Rev Phytopathol 48 225 46
13. NavarroLDunoyerPJayFArnoldBDharmasiriN 2006 A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312 436 439
14. FahlgrenNHowellMDKasschauKDChapmanEJSullivanCM 2007 High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE 2 e219 doi:10.1371/journal.pone.0000219
15. NavarroLJayFNomuraKHeSYVoinnetO 2008 Suppression of the microRNA pathway by bacterial effector proteins. Science 321 964 967
16. LuSSunYHAmersonHChiangVL 2007 MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. Plant J 51 1077 1098
17. HeX-FFangY-YFengLGuoH-S 2008 Characterization of conserved and novel microRNAs and their targets, including aTuMV-induced TIR-NBS-LRR class R gene-derived novel miRNA in Brassica. FEBS Lett 582 2445 2452
18. Ruiz-FerrerVVoinnetO 2009 Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60 485 510
19. Katiyar-AgarwalSGaoSVivian-SmithAJinH 2007 A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev 21 3123 3134
20. Katiyar-AgarwalSMorganRDahlbeckDBorsaniOVillegasAJr 2006 A pathogen-inducible endogenous siRNA in plant immunity. Proc Natl Acad Sci USA 103 18002 18007
21. YiHRichardsEJ 2007 A cluster of disease resistance genes in Arabidopsis is coordinately regulated by transcriptional activation and RNA silencing. Plant Cell 19 2929 2939
22. CoegoARamírezVGilMJFlorsVMauch-ManiB 2005 An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17 2123 2137
23. AgorioAVeraP 2007 ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 19 3778 3790
24. CoegoARamírezVEllulPMaydaEVeraP 2005 The H2O2-regulated Ep5C gene encodes a peroxidase required for bacterial speck susceptibility in tomato. Plant J 42 283 293
25. RamírezVCoegoALópezAAgorioAFlorsV 2009 Drought tolerance in Arabidopsis is controlled by the OCP3 disease resistance regulator. Plant J 58 578 591
26. RamírezVVan der EntSGarcía-AndradeJCoegoAPieterseCM 2010 OCP3 is an important modulator of NPR1-mediated jasmonic acid-dependent induced defenses in Arabidopsis. Plant Biol 10 199
27. CaoHGlazebrookJClarkeJDVolkoSDongX 1997 The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57 63
28. HaagJRPontesOPikaardCS 2009 Metal A and metal B sites of nuclear RNA polymerase Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing. PLoS ONE 4 e4110 doi:10.1371/journal.pone.0004110
29. GaoZLiuH-LDaxingerLPontesOHeX 2010 An RNA polymerase II - and AGO4-associated protein acts in RNA-directed DNA methylation. Nature 465 106 109
30. LawJAAusinIJohnsonLMVashishtAAZhuJ-K 2010 A protein complex required for Polymerase V transcripts and RNA directed DNA methylation in Arabidopsis. Curr Biol 20 951 956
31. BenderJ 2004 DNA methylation and epigenetics. Annu Rev Plant Biol 55 41 68
32. HamiltonAVoinnetOChappellLBaulcombeD 2002 Two classes of short interfering RNA in RNA silencing. EMBO J 21 4671 4679
33. RushtonPJSomssichIERinglerPShenQJ 2010 WRKY transcription factors. Trends Plant Sci 15 247 258
34. DongJChenCChenZ 2003 Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51 21 37
35. CaoXJacobsenSE 2002 Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc Natl Acad Sci USA 99 16491 16498
36. WierzbickiATHaagJRPikaardCS 2008 Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135 635 648
37. PontesOCosta-NunesPVithayathilPPikaardCS 2009 RNA polymerase V functions in Arabidopsis interphase heterochromatin organization independently of the 24-nt siRNA-directed DNA methylation pathway. Mol Plant 2 700 710
38. DouetJTutoisSTourmenteS 2009 A Pol V – Mediated Silencing, Independent of RNA – Directed DNA Methylation, Applies to 5S rDNA. PLoS Genet 5 e1000690 doi:10.1371/journal.pgen.1000690
39. JaskiewiczMConrathUPeterhänselC 2011 Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12 50 55
40. PokholokDKHarbisonCTLevineSColeMHannettNM 2005 Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122 517 27
41. PontvianneFBlevinsTPikaardCS 2010 Arabidopsis histone lysin methyltransferases. Adv Bot Res 1; 53 1 22
42. KannoTKannoYSiegelRMJangMKLenardoMJ 2004 Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol Cell 13 33 43Kanno et al, 2004;
43. VermeulenMMulderKWDenissovSPijnappelWWvan SchaikFM 2007 Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 131 58 69
44. DurrantWEDongX 2004 Systemic acquired resistance. Annu Rev Phytopathol 42 185 209
45. KoornneelAPieterseCMJ 2008 Cross Talk in Defense Signaling. Plant Physiol 146 839 844
46. SpoelSHJohnsonJSDongX 2007 Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104 18842 18847
47. HaringMOffermannSDankerTHorstIPeterhanselC 2007 Chromatin immunoprecipitation: quantitative analysis and data normalization. Plant Methods 2 11
48. BellCJEckerJR 1994 Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19 137 144
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



