-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRibosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
The ribosome is critical for all aspects of cell growth due to its essential role in protein synthesis. Paradoxically, many Ribosomal proteins (Rps) act as tumour suppressors in Drosophila and vertebrates. To examine how reductions in Rps could lead to tissue overgrowth, we took advantage of the observation that an RpS6 mutant dominantly suppresses the small rough eye phenotype in a cyclin E hypomorphic mutant (cycEJP). We demonstrated that the suppression of cycEJP by the RpS6 mutant is not a consequence of restoring CycE protein levels or activity in the eye imaginal tissue. Rather, the use of UAS-RpS6 RNAi transgenics revealed that the suppression of cycEJP is exerted via a mechanism extrinsic to the eye, whereby reduced Rp levels in the prothoracic gland decreases the activity of ecdysone, the steroid hormone, delaying developmental timing and hence allowing time for tissue and organ overgrowth. These data provide for the first time a rationale to explain the counter-intuitive organ overgrowth phenotypes observed for certain members of the Minute class of Drosophila Rp mutants. They also demonstrate how Rp mutants can affect growth and development cell non-autonomously.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002408
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002408Summary
The ribosome is critical for all aspects of cell growth due to its essential role in protein synthesis. Paradoxically, many Ribosomal proteins (Rps) act as tumour suppressors in Drosophila and vertebrates. To examine how reductions in Rps could lead to tissue overgrowth, we took advantage of the observation that an RpS6 mutant dominantly suppresses the small rough eye phenotype in a cyclin E hypomorphic mutant (cycEJP). We demonstrated that the suppression of cycEJP by the RpS6 mutant is not a consequence of restoring CycE protein levels or activity in the eye imaginal tissue. Rather, the use of UAS-RpS6 RNAi transgenics revealed that the suppression of cycEJP is exerted via a mechanism extrinsic to the eye, whereby reduced Rp levels in the prothoracic gland decreases the activity of ecdysone, the steroid hormone, delaying developmental timing and hence allowing time for tissue and organ overgrowth. These data provide for the first time a rationale to explain the counter-intuitive organ overgrowth phenotypes observed for certain members of the Minute class of Drosophila Rp mutants. They also demonstrate how Rp mutants can affect growth and development cell non-autonomously.
Introduction
One of the early phenotypic classes identified in Drosophila was the Minutes, which were classified based on the heterozygous adults having short slender bristles on the body, a generally smaller body size and a delay in the onset of metamorphosis [1]. It has long been considered that understanding the basis for these phenotypes will provide fundamental clues to the mechanisms underlying the control of cell growth and proliferation as well as of tissue and organ size [2]. In 1976 it became apparent that many Minute genes encode Ribosomal proteins (Rps) [3] and by 2007 most of the Minutes were confidently ascribed to the Rp genes [4]. In all organisms, Rps are essential for the assembly and optimal functioning of the ribosome and are, therefore, obligate for protein synthesis and cell growth (reviewed in [5]–[6]). Due to their essential role in ribosome biogenesis, mutations that reduce Rp expression would be expected to limit cell growth. This cell intrinsic requirement for Rps explains many aspects of the Minute phenotype, such as the thin bristles and reduced body size in some Minutes. In contrast, other aspects of the Minute phenotype have remained enigmatic.
Paradoxically, reduced levels of some Drosophila Rps result in overgrowth of specific tissues. For example, RpS6 mutant larvae have overgrown lymph glands, due to increased growth and over-proliferation of the lymph gland cells [7], and develop melanotic masses [8]–[9], a characteristic feature of over-proliferation of hemocytes [10]. Thus reduced RpS6 expression results in tissue overgrowth, consistent with RpS6 having a tumour suppressor like function. Similarly, we have shown that RpL5 or RpL38 heterozygous adult flies exhibit significant increases in the size of the wings due to increased cell growth [11]. Rps have also been implicated as tumour suppressors in the vertebrate zebrafish model, where a genetic screen identified a link between malignant peripheral nerve sheath tumours and heterozygosity for several loss-of-function Rp mutations [12].
In mammalian systems, there is also evidence that Rp heterozygosity is frequently associated with tissue overgrowth and predisposition to cancer. For example, mutations in RpS19, RpS17, RpS24, RpL35a, RpS7, RpL5, RpL11, RpS10 and RpS26 have been associated with the human disease Diamond Blackfan Anemia (DBA), a dominant autosomal bone marrow failure syndrome, characterised by hypoplastic anemia with a predisposition to leukemia [13]–[19]. Mutations in RpS14 are also associated with 5q - syndrome and predisposition to acute myeloid leukemia [20]–[21]. Although RpS19 heterozygosity disrupts ribosome biogenesis 22–24, how reduced levels of Rps promote the excessive proliferation associated with progression to leukemia remains unclear and whether the mechanism is related to tissue overgrowth of Minutes has not been investigated.
Defining the mechanisms by which Rp heterozygosity results in tissue overgrowth and how reduction in a certain Rp gene predisposes a specific tissue to overproliferation in Drosophila is critical to understanding the processes linking growth and proliferation with tissue homeostasis. Furthermore, the insight provided by the Drosophila system may provide important clues in understanding how Rp mutations can promote cancer in humans.
Development of the Drosophila eye has been extensively used to identify and characterise regulators of growth and proliferation [25]–[26]. The Drosophila eye is composed of a highly organised array of photoreceptor clusters or ommatidia, which develop from an epithelial monolayer known as the eye imaginal disc. Differentiation of the ommatidia occurs in a wave that moves from the posterior toward the anterior. The anterior cells divide asynchronously and are separated from the differentiated posterior compartment by the morphogenetic furrow (MF) [27]. Mitotic division cycles become synchronized in the MF where cells are paused in G1 and a subset of photoreceptor cells are specified. The remaining retinal cells synchronously re-enter the cell cycle in the “Second Mitotic Wave” (SMW), which is composed of a tight band of DNA synthesis and mitosis. These final cell divisions provide the cells required for differentiation of the ommatidial structures that form the adult eye [28].
A hypomorphic mutation of cycE, cycEJP [29], reduces cycE expression during eye imaginal disc development to result in decreased S phases and small, rough adult eyes due to fewer cells (Figure 1A, compare i with ii) [29]. cycEJP therefore provides a sensitised genetic background to identify modifiers of eye proliferation, with suppressors of the phenotype being classed as “tumour suppressors” and predicted to normally function as cell cycle inhibitors [26]. To examine the mechanism(s) underlying the overgrowth phenotypes exhibited by some Minutes we have taken advantage of the unexpected observation that mutant RpS6 suppresses the hypo-proliferative, small eye phenotype of cycEJP mutants [26]. The data presented here confirm that reduced function of RpS6 suppresses the cycEJP small eye phenotype and we further demonstrate that this is not associated with restored proliferation in the SMW. Suppression of the cycEJP adult eye phenotype was observed with Rp mutants for both the small subunit (RpS12 and RpS19) and the large subunit (RpL38), which suggests the ability to restore eye size may be a more general property of reduced Rp abundance. Further investigation revealed that reduced RpS6 does not, however, lead to increased levels of CycE protein in the eye and that reduction of RpS6 specifically in the eye does not suppress the cycEJP small eye phenotype. Instead we demonstrate that reduced Rp levels in the prothoracic gland in RpS6 mutants decreased the activity of steroid hormone ecdysone, delayed development and hence allowed additional time for restoration of growth in the cycEJP mutants.
Fig. 1. RpS6 mutant suppresses the small rough eye phenotype of cycEJP, but not through restoring CycE protein levels. 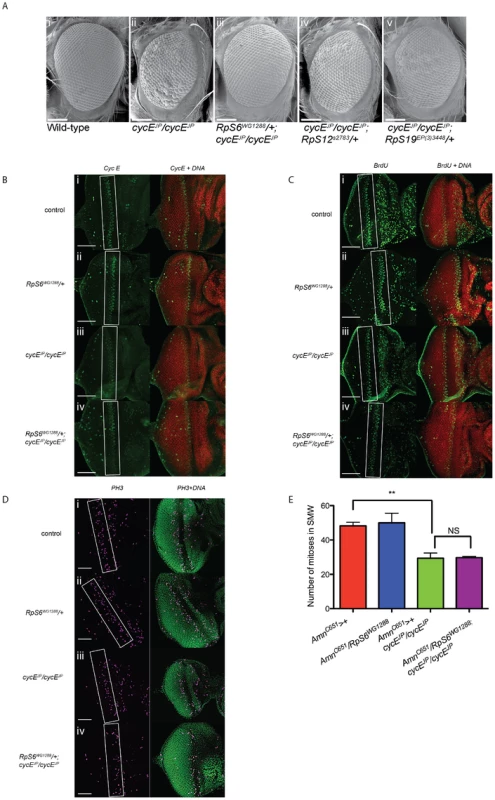
(A) Scanning electron micrographs (SEM) of female adult eyes with genotypes as indicated. Orientation of eyes: anterior (left) posterior (right). Scale bar 100 µm. (B) Confocal images of 3rd instar eye imaginal discs stained for CycE and DNA with genotypes as indicated. White boxes mark the band of cycE cells. Images were taken at 40× magnification. Orientation of eye discs: anterior (left), posterior (right). Scale bar equals 50 µm. (C) Confocal images of 3rd instar eye imaginal discs stained for BrdU incorporation and DNA with genotypes as indicated. White boxes mark the band of S phase cells. Images were taken at 40× magnification. Orientation of eye discs: anterior (left), posterior (right). Scale bar equals 50 µm. (D) Confocal images of 3rd instar eye imaginal discs stained for cells in the SMW (PH3) and DNA with genotypes as indicated. White boxes mark the band of cells in SMW. Images were taken at 40× magnification with 0.7× optical zoom. Orientation of eye discs: anterior (left), posterior (right). Scale bar equals 50 µm. (E) Graph quantifying the number of cells in the SMW. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where ** = p<0.01, NS = not significant and n = 3. Results
Rp mutants suppress the cycEJP hypomorphic small eye phenotype
Mammalian cyclin E (cycE) is a well-characterised oncogene and, like the Drosophila homolog, regulates G1 - to S-phase progression [30]–[32]. The cycEJP hypomorphic mutant has reduced cycE expression predominantly in the developing eye imaginal disc and, as a result, fewer S phases and small, rough adult eyes (Figure 1A ii and [29]). Previously a genetic screen for modifiers of the cycEJP phenotype identified the RpS6 mutant RpS6air8, which reduces RpS6 expression, as a suppressor of the cycEJP small eye phenotype [26]. This observation is consistent with previous observations that reduced RpS6 expression can promote proliferation in RpS6 mutant larvae [7]–[9].
We utilised the cycEJP small eye phenotype to examine the mechanisms by which reducing Rp levels can result in tissue overgrowth. As the original RpS6air8 line was no longer available to confirm the previous findings [26], we demonstrated suppression of cycEJP using an alternate RpS6 mutation, RpS6WG1288 [8]–[9], which also exhibits the classic Minute phenotype of slender bristles (not shown) and a developmental delay (Figure 3C, red data points). RpS6WG1288/+ restored the eye size and reduced roughness in the cycEJP background to give adult eyes with a more wild-type appearance (Figure 1A, compare i and ii with iii). Thus, two independent RpS6 mutations (RpS6air8 and RpS6WG1288) suppress the cycE hypomorphic small eye phenotype, consistent with reduced RpS6 function leading to increased proliferation in the cycEJP mutant.
Fig. 2. Reducing RpS6 by RNAi in the whole fly, but not specifically in the eye, suppresses cycEJP. 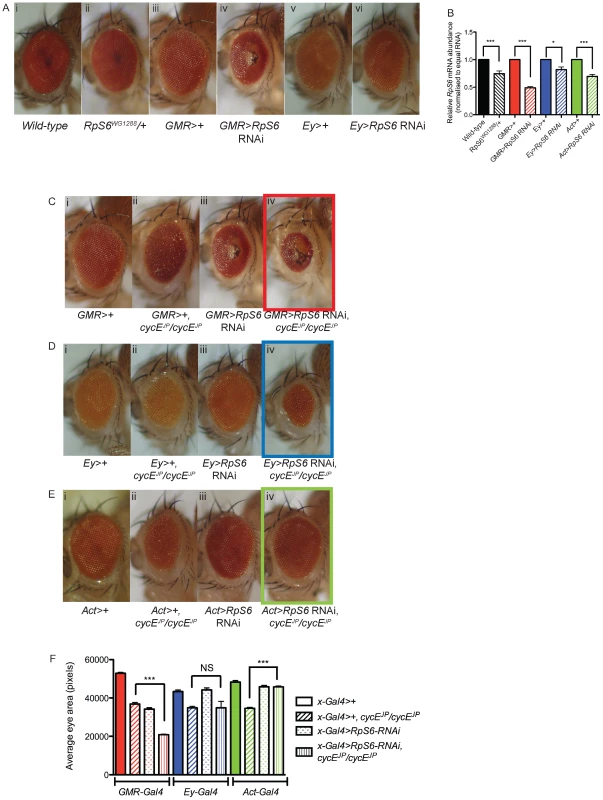
(A) Light micrographs of female adults bearing the genotypes as indicated. GMR-Gal4 drives expression in differentiated eye photoreceptor cells. Ey-Gal4 drives expression in all eye cells. (B) Graph showing the relative mRNA levels of RpS6 from the RpS6 mutant, eye specific reductions of RpS6 (GMR-Gal4 and Ey-Gal4) and ubiquitous reductions of RpS6 (Actin-Gal4) as measured by qRT-PCR. RNA samples were extracted from ten 3rd instar larvae or thirty 3rd instar eye imaginal discs. Samples were normalised to equal amounts of RNA (1 µg). Results are represented as the mean +/− standard error (n = 3). Statistical analysis applied: One-way ANOVA, where * = p<0.05, *** = p<0.001. (C–E) Light micrographs of female adult eyes bearing the genotypes indicated. Act-Gal4 drives expression in all cells. Orientation of eyes: anterior (left), posterior (right). (F) Graph of average eye area. (GMR>+) n = 13, (GMR>+, cycEJP/cycEJP) n = 13, (GMR>RpS6 RNAi) n = 15, (GMR>RpS6 RNAi; cycEJP/cycEJP) n = 19, (Ey>+) n = 21, (Ey>+; cycEJP/cycEJP) n = 11, (Ey>RpS6 RNAi) n = 17, (Ey>RpS6 RNAi; cycEJP/cycEJP) n = 12, (Act>+) n = 16, (Act>+; cycEJP/cycEJP) n = 42, (Act>RpS6 RNAi) n = 31, (Act>RpS6 RNAi; cycEJP/cycEJP) n = 49. Results are represented as the mean +/− standard error. Statistical analysis applied: One-way ANOVA, where *** = p<0.001 and NS = not significant. In order to test whether suppression of cycEJP was specific to mutation of RpS6 or was potentially a more general consequence of reducing Rp levels, we tested two other Rp mutants that give Minute phenotypes, RpS12s2783 and RpS19bEP3448. Reducing RpS12 and RpS19 levels, with the mutant alleles RpS12s2783 [33] and RpS19bEP3448 (http://flybase.org/reports/FBrf0104946.html) resulted in a moderate suppression of cycEJP (Figure 1A iv and v, respectively). The cycEJP eye phenotype was also suppressed with a large subunit Rp mutant, RpL382b1 [11] (Figure S1). The finding that mutations in four different Rps from both subunits suppress the cycEJP phenotype suggests that this may be a common feature of Minutes.
RpS6 does not suppresses cycEJP by restoring Cyclin E protein levels in the eye
The majority of the suppressors examined in detail from the original cycEJP screen demonstrated the ability to restore CycE protein towards wild-type levels and an associated increase in S phase progression [26]. Thus we examined whether RpS6WG1288 might similarly restore CycE levels in the eye. However, examination of CycE levels in eye discs from 3rd instar larvae revealed that this was not the case (Figure 1B, compare i and iii with iv). As reported previously [29] and consistent with the reduced CycE levels, S phase cells were also reduced in eye discs of cycEJP (Figure 1C iii). In line with the finding that CycE was not altered, the reduced S phases in the SMW of cycEJP were not obviously increased by reducing RpS6 (Figure 1C iv). Thus suppression of the cycEJP phenotype occurs in the absence of obvious changes to CycE abundance and S phase progression.
To monitor whether there was an overall change to cell cycle progression in the eye, we carried out anti-phosphohistone H3 staining to identify cells in mitosis as an alternative measure of cell cycles in the SMW (Figure 1D and quantified in 1E). The SMW of cycEJP mutants exhibited a significant reduction in their mitotic index as expected (Figure 1D iii and 1E). Importantly however the mitotic index was not restored in cycEJP eyes by the RpS6 mutant (Figure 1D iv and 1E). Therefore in these animals there is not a significant increase in the rate of cell cycle progression in the SMW, which suggests that this is unlikely to be the mechanism underlying suppression of cycEJP by the RpS6 mutant.
Specific reduction of RpS6 in the eye does not suppress cycEJP
The findings above suggested that the suppression of cycEJP by the RpS6 mutant was not associated with either restoration of CycE or with altered cell cycle progression. As the cycEJP hypomorph predominantly affects the eye, we sought to test whether specific reduction of RpS6 in the cycEJP eye could suppress the phenotype. Using the eye specific GMR-Gal4 to drive expression of a UAS-RpS6 RNAi transgene, in both the SMW and differentiated cells posterior to the morphogenetic furrow [34]–[35], resulted in a smaller eye with a glassy appearance and necrotic patches (Figure 2A, compare iii with iv) [36] and 50% reduction in RpS6 mRNA in eye-antennal discs (Figure 2B). We then tested whether specific reduction of RpS6 in the eye could suppress the cycEJP phenotype. Reducing RpS6 with GMR-Gal4, which results in a small eye phenotype alone, was unable to suppress the cycEJP phenotype, and rather resulted in an additive reduction in eye size (Figure 2C, compare ii with iv). Due to the severity of the GMR>RpS6 RNAi phenotype we also tested knockdown with an alternate eye driver Ey-Gal4, which is expressed in all eye cells [37]–[38]. This resulted in ∼20% reduction in RpS6 mRNA in eye-antennal discs (Figure 2B) and did not produce an obvious adult eye phenotype alone (Figure 2A, compare v with vi). Thus like heterozygous RpS6WG1288/+, Ey>RpS6 RNAi does not result in an obvious eye phenotype (Figure 2A, compare i with ii). However, in direct contrast to RpS6WG1288/+, Ey>RpS6 RNAi enhanced rather than suppressed the cycEJP rough eye phenotype (Figure 2D, compare ii with iv). Together these data demonstrated that reducing the abundance of RpS6 in the eye, either robustly or modestly, was unlikely to be the mechanism underlying suppression of the cycE hypomorphic phenotype by the RpS6 mutant.
Fig. 3. RpS6 mutant larvae have smaller prothoracic glands and an ecdysone dependent developmental delay. 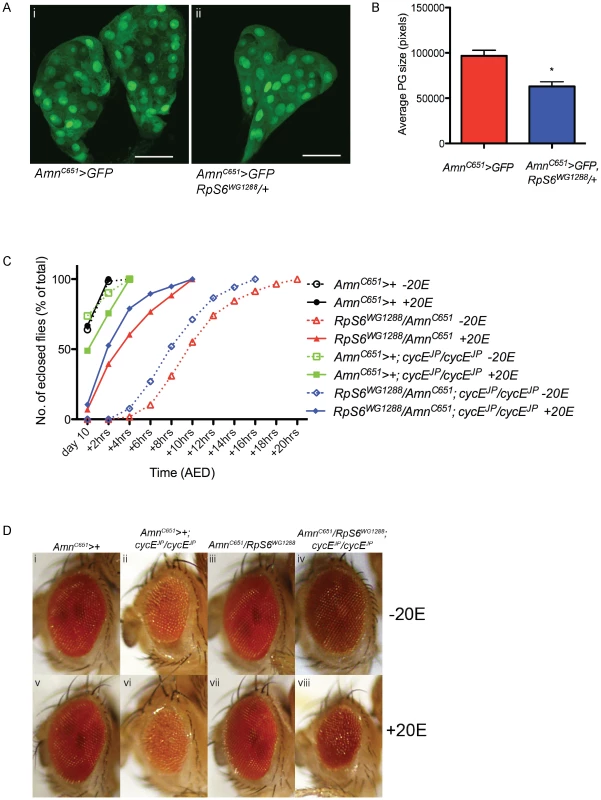
(A) Confocal images of 3rd instar prothoracic glands marked with GFP with genotypes indicated. Magnification 40×. Scale bar 50 µM. (B) Graph of average PG size. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where * = p<0.05 (n = 3). (C) Graph representing the time to eclosion after egg deposition (AED) of genotypes indicated raised in the presence or absence of ecdysone (20E). AmnC651-Gal4 drives expression in the prothoracic gland. (D) Light micrographs of female adult eyes bearing the genotypes indicated raised in the presence or absence of ecdysone (20E). Orientation of eyes: anterior (left), posterior (right). RpS6 suppresses cycEJP in an eye tissue non-autonomous manner
Because specifically reducing RpS6 in the eye did not suppress the cycEJP small eye phenotype, we considered the possibility that the interaction between RpS6 and cycEJP might be mediated by a mechanism extrinsic to the eye. To test this we placed UAS-RpS6 RNAi expression under the control of a range of ubiquitous Gal4 drivers in an effort to replicate the environment of the RpS6 mutant, by reducing RpS6 in the whole fly. Knockdown of RpS6 with the strong ubiquitous drivers Daughterless-Gal4 or Tubulin-Gal4 resulted in either early larval or embryonic lethality (Table S1). This is likely to be a result of RpS6 levels dropping below the threshold required for sufficient ribosome assembly and thus protein synthesis to support cell growth and proliferation. Consistent with this observation, reduction of RpS6 mRNA levels with strong drivers expressed in specific embryonic segments or larval domains also resulted in lethality (Engrailed-Gal4, Patched-Gal4) or shrivelled, stumpy wings (MS1096-Gal4) (Table S1 and Figure S2, compare iii with iv).
In contrast to the strong Gal4 drivers, reducing RpS6 mRNA levels with the relatively weaker ubiquitous driver, Actin-Gal4, resulted in viable flies (Figure S2, compare i with ii), which had a reduction in RpS6 mRNA similar to the levels seen in RpS6WG1288/+ larvae (Figure 2B, compare striped black and striped green bars). Importantly, this low-level reduction of RpS6 throughout the fly resulted in suppression of the cycEJP eye phenotype (Figure 2E, compare ii with iv) and a significant increase in eye size (Figure 2F, green bars). These data suggested that factors extrinsic to the eye were essential for suppression of cycEJP by the RpS6 mutant, consistent with our inability to detect changes in CycE activity or protein levels in the eye in the RpS6 mutant background.
Suppression of the cycEJP phenotype by the RpS6 mutant is reversed by Ecdysone
As Rp mutations are associated with a developmental delay, we considered the possibility that the cell non-autonomous mechanism by which mutant RpS6WG1288 and RpS6 RNAi suppressed cycEJP might involve, at least in part, the ecdysone pathway, which is known to control timing of development and thus the growth period of the larvae. Specifically, release of ecdysone from the prothoracic gland (PG) dictates the timing of the metamorphosis from larvae to pupae (reviewed in [39]). As adult fly size is determined by the size of the larva at the time of pupal molt, the timing of ecdysone release plays a vital role in the growth of the fly [40]. We therefore examined whether RpS6WG1288/+ might suppress the cycEJP eye phenotype via an ecdysone-dependent, cell non-autonomous mechanism.
Previous studies have reported a role for the PG as a size-assessment organ [41]–[43]. Inhibiting the growth of the PG causes an underestimation of body size and results in pupation at a larger size. Conversely, promoting the growth of the PG results in smaller flies [41]–[43]. For example, overexpression of a dominant negative isoform of PI3 Kinase (Dp110DN) specifically in the PG blocks insulin pathway signalling and PG growth [41]. The smaller PG and associated reduction in ecdysone levels in these animals results in larger pupae and adults due to an extended larval growth period [41]–[43].
We therefore tested if the RpS6 mutant might suppress the cycEJP phenotype by impairing PG growth and, as a consequence, affecting the level of ecdysone. During eye disc development the morphogenetic furrow moves forward by one row of ommatidia (3–4 cell rows) every 70 minutes [44] and the doubling time for cells in the proliferating, anterior portion of the eye disc is approximately 12 hours [45]. Thus a developmental delay would provide the anterior asynchronously dividing cells and the cells comprising the second mitotic wave of the eye imaginal disc extra time to grow and divide in order to compensate for the proliferation rate defect resulting from reduced CycE activity.
First, examination of heterozygous RpS6 (RpS6WG1288/+) PGs, marked by expression of GFP, revealed that the glands were 35% smaller than GFP marked control PGs at the same time after egg deposition (AED) (Figure 3A, compare i with ii and quantified in 2B). This is also consistent with reports of RpS6air8 mutant larvae having small, abnormal PGs [7]. As a direct consequence of reduced PG growth, it would also be expected that RpS6WG1288/+ larvae should be developmentally delayed. Examination of developmental timing in RpS6WG1288/+ heterozygotes revealed that reducing the levels of RpS6 resulted in a delay in eclosion of up to 18 hours, compared to wild type (Figure 3C, compare open black circle with open red triangle). Importantly, the delay associated with the RpS6 mutant is reduced by addition of the active form of ecdysone, 20-hydroxyecdysone (20E) (Figure 3C, red data points and statistical analysis shown in Table S3), which suggests the delay in the RpS6 mutant is dependent on ecdysone levels.
The observation that the number of SMW divisions in the RpS6WG1288/+; cycEJP/cycEJP eyes were not significantly different to cycEJP alone suggests that the developmental delay and associated extra time for more cell divisions might underlie suppression of cycEJP. To investigate this possibility we tested whether suppression of cycEJP by the RpS6 mutant was impaired when the developmental delay is reduced by addition of 20E (Figure 3D). First we demonstrated that the RpS6WG1288/+; cycEJP/cycEJP animals had a developmental delay comparable to that for the RpS6 mutant alone, which could be reduced by the addition of ecdysone (Figure 3C, blue data points and statistical analysis shown in Table S3). Importantly, acceleration of development by the addition of 20E to the RpS6WG1288/+; cycEJP/cycEJP larvae resulted in a failure to suppress the small eye phenotype (Figure 3D, compare iv with viii). Thus suppression of the cycEJP phenotype by the RpS6 mutant is dependent on a developmental delay, which is sensitive to the level of ecdysone.
Reducing RpS6 specifically in the prothoracic gland impairs growth and causes a developmental delay
To further test our hypothesis that reduced levels of individual Rps in the PG of Minute mutants might restore proliferation in the cycEJP eye by inducing a developmental delay, we sought to reduce Rp expression in the PG using AmnC651-Gal4 which drives expression in the PG [41] and UAS-Rp RNAi for RpS6, RpS13 or RpL38. We first demonstrated the RNAi was able to reduce RpS6 protein by knocking down specifically in the PG, and staining with an anti-RpS6 antibody (Figure S3A). Consistent with the importance of Rps for growth, reducing Rps in the PG resulted in much smaller PGs in these larvae compared with the control at the equivalent time point of 5 days AED (Figure 4A ii–iv). Moreover, reduction of RpS6 levels resulted in PGs that were smaller than for the RpS6WG1288/+ PGs, suggesting a greater reduction in RpS6 (compare Figure 4A ii to Figure 3A ii). Examination of the AmnC651>RpS6 RNAi PGs at 12 days AED revealed that the size of the gland was still considerably smaller than the control PG (data not shown). As a smaller PG would be predicted to result in less ecdysone synthesis and release, we examined if the reduction in PG size affected ecdysone activity in the larvae. qRT-PCR was performed on whole larvae to measure ecdysone activity indirectly by quantifying the mRNA levels of an ecdysone responsive gene, E74B [41]. E74B levels were normalised to Actin-5C, a non-ecdysone responsive gene. RNAi-mediated reduction of RpS6, RpS13 or RpL38 in the PG resulted in up to 90% decrease in E74B expression (Figure 4B), suggesting strongly reduced ecdysone activity, reflecting the small size of the PG.
Fig. 4. Reducing Rps by RNAi in the PG results in developmental delay and small prothoracic glands. 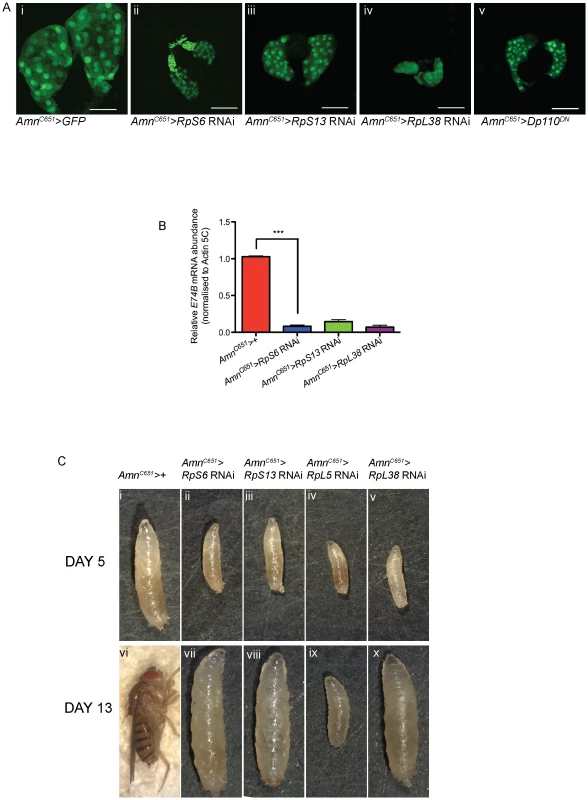
(A) Confocal images of 3rd instar prothoracic glands marked with GFP at day 5 with genotypes indicated. AmnC651-Gal4 drives expression in the prothoracic gland. Dp110DN is a dominant-negative form of PI3K. Magnification 40×. Scale bar 50 µM. (B) qRT-PCR of relative mRNA levels of an ecdysone responsive gene E74B. RNA samples were extracted from 3rd instar larvae. Samples were normalised to Actin5C mRNA levels. (AmnC651>RpS6 RNAi) n = 4, (AmnC651>RpS13 RNAi) n = 2, (AmnC651>RpL38 RNAi) n = 2. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where *** = p<0.001. (C) Light micrographs of 5 days AED larvae (i–v) or 13 days AED adult (vi) or delayed larvae (vii–x) with genotypes marked. Consistent with the robust reduction in PG size and reduced ecdysone activity, we observed an extreme developmental delay in the larvae with RNAi-mediated knockdown of RpS6, RpS13 or RpL38 in the PG. At day 5, these larvae were smaller in size compared with control larvae (Figure 4C, compare i with ii–v). While the control larvae underwent pupation as normal at day 5, larvae with reduction of RpS6, RpS13 or RpL38 specifically in the PG continued to feed and grow beyond day 10 to become giant larvae, which fail to pupate (Figure 4C, compare vi with vii–viii, x). The phenotype for the RpL5 knockdown in the PG was even more dramatic, being 2nd instar larval lethal (Figure 4C ix), suggesting that RpL5 was knocked down below the threshold required for cell intrinsic growth [36], [46]–[47] and, therefore, development of the PG gland. This is consistent with the lethality that results when strong drivers are used to express RNAi transgenes targeting the Rps investigated here (Table S2).
The AmnC561-Gal4 insertion is not expressed solely in the PG, being expressed throughout the ring gland early, in some cells in the ventral ganglion and in neurosecretory cells of the brain [41]. As the neurosecretory cells of the brain can also play a role in developmental timing and growth [48], we addressed the possibility that RpS6 knockdown in these cells might be responsible for the overgrowth by using another driver, P0206-Gal4 [43], that also expresses in the PG, but not in the neurosecretory cells. Consistent with the effect being mediated through defects in PG development, knockdown of either RpS6 or RpL38 using P0206-Gal4 also resulted in an extreme developmental delay whereby larvae continue to feed for greater than 20 days and fail to pupate, which was associated with a smaller PG (Figure S4A).
The impaired growth and developmental delay is mediated by ecdysone
To assess whether the reduced ecdysone production was the cause of the developmental delay and larval overgrowth resulting from Rp knockdown in the PG, 20E was introduced to the food of AmnC651>RpS6 RNAi larvae (Figure 5A). The addition of 20E resulted in a variable restoration of pupariation, which ranged from progression towards cuticle darkening in larvae to cuticle development and early pupal morphology (Figure 5A, compare v with vi–vii and Figure 5B green bars). Although the AmnC651>RpS6 RNAi larvae were able to pupate, the ectopic addition of 20E was unable to initiate the final steps of metamorphosis, including the formation of adult structures. This suggests that ∼30% of the endogenous 20E activity achieved by feeding the larvae (Figure 5B) is sufficient to trigger pupariation, but is below the threshold required for adult metamorphosis. The failure of metamorphosis may be confounded by the fact that pupae, unlike larvae, can no longer take up 20E by feeding. Indeed, the largest peak of endogenous ecdysone release occurs after cuticle formation and is required for the formation of adult structures [39].
Fig. 5. Addition of 20-hydroxyecdysone can partially rescue Amn>RpS6 RNAi larval lethality. 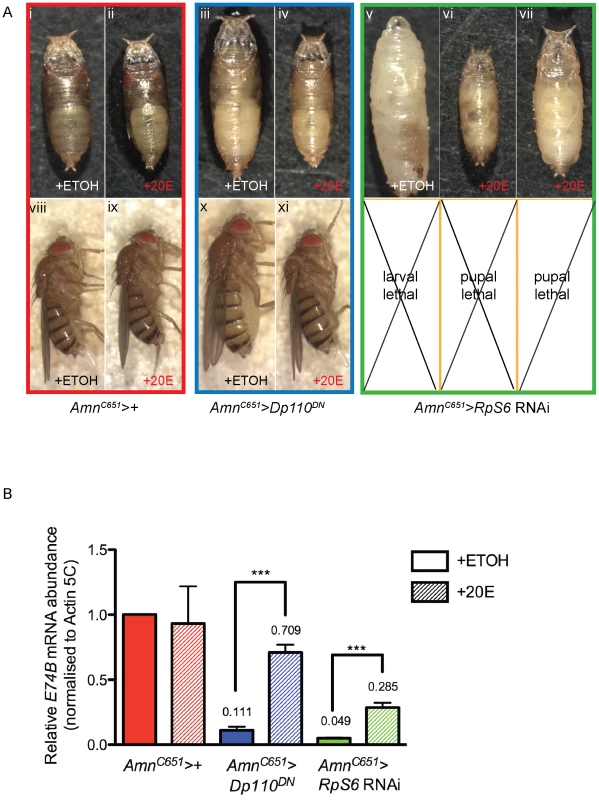
(A) Light micrographs of day 8 pupae/larvae or day 15 female adults bearing the genotypes indicated. The larvae were fed 0.75 mg/mL of 20E or equivalent concentration of 7.5% (v/v) ETOH. (B) qRT-PCR of relative mRNA levels of an ecdysone responsive gene E74B from larvae with or without 0.75 mg/mL of 20E. RNA samples were extracted from 3rd instar larvae. Samples were normalised to Actin5C mRNA levels. Results are represented as the mean +/− standard error (n = 3). Statistical analysis applied: unpaired t-test, where *** = p<0.001. To confirm this failure to restore pupation was not due to insufficient 20E in the food we carried out a control rescue experiment with an alternate growth regulator, PI3K, which has previously been shown to modulate PG size and development [41]. Despite having a PG size similar to that of AmnC651>RpS6 RNAi (Figure 4A, compare ii with v) and associated extreme developmental delay, the AmnC651>Dp110DN (dominant negative PI3K) larvae were only moderately delayed and pupated, but eclosed as larger flies (Figure 5A, compare viii with x, and [41]). We demonstrated that feeding 20E to larvae overexpressing dominant negative PI3K in the PG (AmnC651>Dp110DN) restored the time of pupation back to day 5, the adults eclosed at a normal size (Figure 5A, compare iii and x with iv and xi), and E74B levels were significantly increased compared to that of control (Figure 5B, blue bars). This restoration of timing and size toward control suggested that the 20E was successfully taken up and processed by the AmnC651>Dp110DN larvae. The difference in the severity of the phenotypes in terms of developmental delay, strongly suggested that ecdysone levels are more sensitive to disruption of Rps and ribosome biogenesis than to disruption of insulin pathway-dependent growth in the PG
Reducing RpS6 levels using RNAi in the prothoracic gland in cycEJP background suppresses the cycEJP phenotype
As RpS6 knockdown in the PG gland resulted in a failure to undergo pupation, in order to carry out further studies we examined whether we could reduce the severity of the phenotype and facilitate development into adult stages using a temperature sensitive isoform of the Gal4 repressor, Gal80 (Gal80TS [49]) that allows temporal control of the induction of RpS6 knockdown by RNAi in the PG. Thus, knockdown of RpS6 was delayed until late 2nd instar and although this still resulted in large, developmentally delayed larvae (Figure 6A, compare i with v), these larvae were able to undergo pupation and eclosed as large adults (Figure 6A, compare ii with vi). In addition, we observed increases in the eye size (Figure 6A, compare iii to vii) and statistically significant increase in the wing size (Figure 6A, compare iv to viii, quantified in 6B), in the AmnC651;Gal80TS>RpS6 RNAi adults compared with control.
Fig. 6. Reducing RpS6 in the PG is associated with tissue overgrowth and suppresses cycEJP. 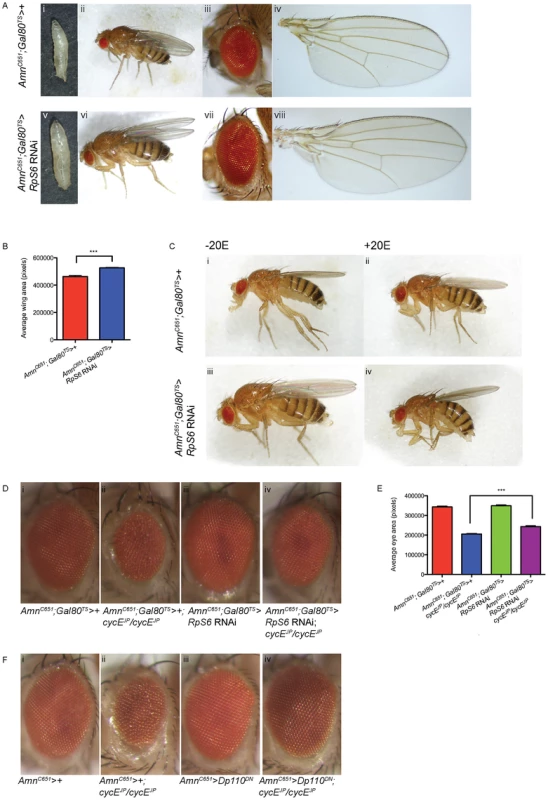
(A) Light micrographs of larvae, whole adult flies, adult eyes and wings bearing the genotypes: (i–iv) control (AmnC651; Tubulin-Gal80TS>+) and (v–viii) delaying reduction of RpS6 in the PG until 2nd instar (AmnC651; Tubulin-Gal80TS>RpS6 RNAi). (B) Graph of average wing area. (AmnC651; Tubulin-Gal80TS>+) n = 7, (AmnC651; Tubulin-Gal80TS>RpS6 RNAi) n = 10. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where *** = p<0.001. (C) Light micrographs of female adult flies bearing the genotypes indicated raised in the presence or absence of ecdysone (20E). (D) Light micrographs of female adult eyes bearing the genotypes indicated. Orientation of eyes: anterior (left), posterior (right). (E) Graph of average eye area. (AmnC651; Tubulin-Gal80TS>+) n = 26, (AmnC651; Tubulin-Gal80TS>+; cycEJP/cycEJP) n = 26, (AmnC651; Tubulin-Gal80TS>RpS6 RNAi) n = 31, (AmnC651; Tubulin-Gal80TS>RpS6 RNAi; cycEJP/cycEJP) n = 19. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where *** = p<0.001. (F) Light micrographs of female adult eyes bearing the genotypes indicated. Orientation of eyes: anterior (left), posterior (right). We then tested whether we were able to alter this overgrowth by the addition of ecdysone. Indeed, addition of 20E to the AmnC651;Gal80TS>RpS6 RNAi restores the adults to a similar size to the AmnC651;Gal80TS control animals (Figure 6C, compare ii to iv). This suggests that the overgrowth also depends on reduced levels of ecdysone activity, as observed for the AmnC651>Dp110DN animals (shown in Figure 5A xi where body size is similar to control in AmnC651>Dp110DN +20E, Figure 5A ix). Thus the overgrowth phenotype resulting from reduction of RpS6 in the PG was sensitive to the level of 20E, which supports the hypothesis that the developmental delay associated with knockdown of RpS6 specifically in the PG is due to impaired ecdysone release and delayed metamorphosis.
Most importantly, reduction of RpS6 in the PG resulted in suppression of the cycEJP eye phenotype, with a statistically significant increase in adult eye size (Figure 6D, compare ii with iv, and quantified in 6E). Thus, the ability of the RpS6 mutant to suppress the cycEJP phenotype occurs, at least in part, through a defect in PG growth and the associated delay in development. The suppression by PG-driven RpS6 knockdown was not as strong as observed for the RpS6 mutant, which could be a consequence of the severe reduction in 20E activity in these animals (Figure 4B). As ecdysone release is required for proper morphogenetic furrow progression in eye discs [50], the drastic reduction in 20E levels in the PG-driven RpS6 RNAi animals, specifically in a background of diminished CycE levels, might also delay furrow progression. Thus, even though extra time is spent during the larval growth period, the suppression is incomplete because of the role of 20E in controlling the developmental signals required for furrow progression [50]–[51].
These data strongly support a model whereby RpS6WG1288/+ suppresses the small rough eye phenotype of cycEJP via a cell non-autonomous mechanism. Reduced abundance of RpS6 in the PG of cycEJP animals decreases PG size, ecdysone activity and consequently results in a developmental delay and time for additional growth of the eye. To definitively test this model, we examined the effect of restoring RpS6 expression in the PG of RpS6WG1288/+; cycEJP/cycEJP flies. According to the model above, if the decrease in RpS6 expression specifically in the PG is responsible for the ability of RpS6WG1288/+ to suppress the small cycEJP eye phenotype, then we would predict that restoring RpS6 expression specifically in the PG in the RpS6WG1288/+; cycEJP/cycEJP flies would prevent the developmental delay and inturn prevent the suppression of the small eye phenotype. Consistent with this, expression of RpS6 using the Phantom-Gal4 (Phm-Gal4) driver [43], a PG specific driver, resulted in ectopic expression of RpS6 in the PG (Figure S3B). Similar results were shown for enforced expression of RpS6 in the PG using PG driver AmnC651-Gal4. Restoration of expression of RpS6 in the PGs of RpS6WG1288/+; cycEJP/cycEJP flies using either the AmnC651-Gal4 (Figure 7A, compare iii with iv) or Phm-Gal4 driver (Figure 7B, compare iii with iv) prevented RpS6WG1288 from suppressing the cycEJP eye phenotype (quantified in Figure 7C). Subsequent studies demonstrated this was because enforced expression of RpS6 in the PG's of RpS6WG1288 animal prevented the developmental delay (Figure 7D, green data points and statistical analysis shown in Table S4). Together these data are consistent with the above model and unequivocally demonstrate that the ability of the RpS6WG1288/+ mutant to suppress the cycEJP phenotype is due to reduction of RpS6 abundance specifically in the PG.
Fig. 7. Restoring RpS6 expression in the PG inhibits the suppression of cycEJP by the RpS6WG1288 mutant. 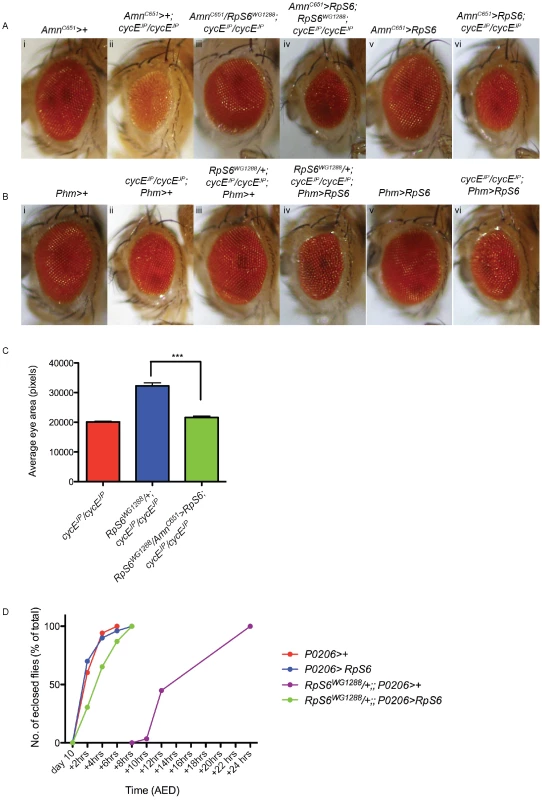
(A,B) Light micrographs of female adult eyes bearing the genotypes indicated. Orientation of eyes: anterior (left), posterior (right). AmnC651-Gal4 and Phm-Gal4 drive expression in the prothoracic gland. (C) Graph of average eye area. (AmnC651>+; cycEJP/cycEJP) n = 7, (RpS6WG1288/+; cycEJP/cycEJP; Phm>+) n = 6, (RpS6WG1288/AmnC651>RpS6; cycEJP/cycEJP) n = 14. Results are represented as the mean +/− standard error. Statistical analysis applied: unpaired t-test, where *** = p<0.001. (D) Graph representing the time to eclosion after egg deposition (AED) of genotypes indicated. P0206-Gal4 is a ring gland specific driver. In summary, these data strongly support the hypothesis that the ability of the RpS6 mutant to suppress the cycEJP small rough eye phenotype is, in large part, due to a reduction of PG size and an associated decrease in ecdysone activity, which results in an extended larval growth period that allows the eye discs extra time to grow. This model predicts that manipulation of other growth modulatory genes in the PG would also suppress the cycEJP phenotype. Indeed, consistent with this model, overexpression of UAS-Dp110DN in the PG was also able to suppress the cycEJP small rough eye phenotype (Figure 6F, compare ii with iv). As observed for the RpS6 mutant, CycE protein levels, BrdU and PH3 in the AmnC651>Dp110DN, cycEJP/cycEJP eye imaginal discs were not altered compared with cycEJP alone (Figure S5). As we do not see a significant increase in the SMW divisions in these animals, when compared with cycEJP alone, this further supports the idea that the increased time spent in the larval growth stage allows more time for division, which leads to suppression of the small eye phenotype.
Discussion
Since the Minutes were first described in 1929 [2], geneticists have sought to understand the mechanisms underlying these phenotypes as an avenue toward elucidating the complex mechanisms controlling body size. More recently, heterozygous mutations in multiple Rp genes have been associated with overgrowth phenotypes [11]–[12], [20], but the underlying mechanism has remained poorly understood. We addressed this question here taking advantage of a genetic screen for modifiers of a cycE hypomorph, which identified an RpS6 mutant as a suppressor [26], to investigate possible mechanisms by which Rp mutations may contribute to overgrowth.
The cell non-autonomous model for suppression of cycEJP and overgrowth phenotypes in Minutes
Our data demonstrate that Rp mutants suppress the cycE phenotype via a mechanism extrinsic to the eye, involving control of developmental timing though the PG. We propose the following model to explain this phenomenon (Figure 8). Firstly, reduced Rp levels in the PG of Rp mutant flies decreases ribosome biogenesis thus inhibiting PG growth, which in turn results in reduced ecdysone synthesis and a subsequent delay in development (Figure 8A). The extended growth period resulting from the developmental delay allows time for more cell divisions and growth in the eye, thereby allowing the eye imaginal disc to achieve normal size prior to pupation, thus suppressing the cycEJP small eye phenotype (Figure 8B). In support of the tissue extrinsic component of PG-ecdysone model, we have demonstrated that reducing RpS6 specifically in the PG suppresses cycEJP (Figure 6D), and conversely overexpression of RpS6 in the PG prevents suppression of the cycEJP by mutant RpS6 (Figure 7A–7B).
Fig. 8. The ecdysone model of cycEJP suppression and Minute overgrowth phenotype. 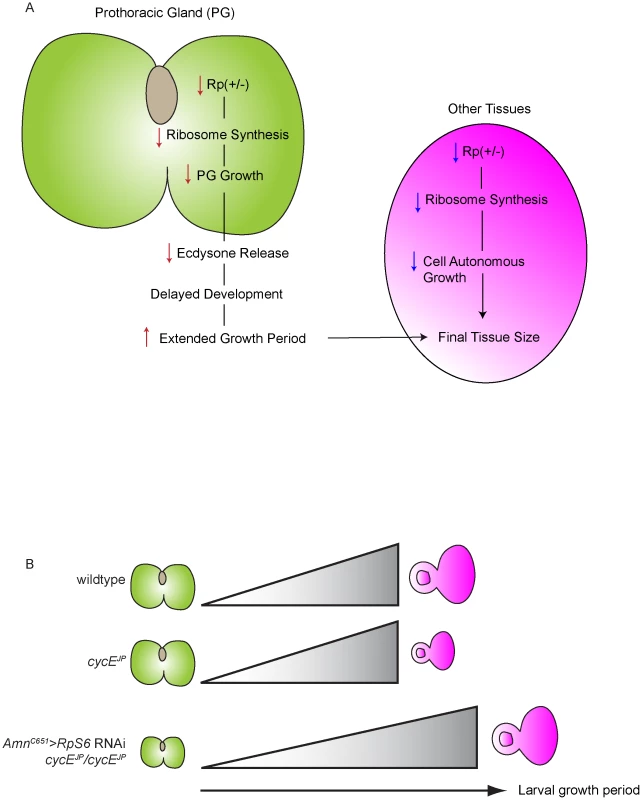
(A) Diagram of the two effects of Rp reductions in Drosophila. First is the intrinsic effect of reducing Rps in the prothoracic gland (PG). The second is an extrinsic effect on the target tissue. The final size of the adult fly is the net consequence of both effects. (B) Model for suppression of cycEJP via altered PG size and ecdysone activity. In wild-type PGs, ecdysone titres accumulate and allow normal growth of the eye imaginal disc (depicted by the grey gradient). In cycEJP eye discs, while the PG size is normal, the eye discs have reduced proliferation/growth due to the cycEJP mutation. Reduction of RpS6 reduces PG size and ecdysone activity to cause an extended larval growth period, allowing extra time for the cycEJP eye discs to grow. As a developmental delay is a consistent feature of Minutes, it was speculated by Brehme in 1939 that this aspect of the phenotype might be due to insufficient ecdysone (as reviewed in [47]). Our work confirms this hypothesis and importantly, also provides a framework for how the Rp Minute collection of mutants are associated with both impaired growth and, counter-intuitively, tissue overgrowth (Figure 8A). In essence final tissue/body size in a Minute fly is a product of interplay between the tissue intrinsic effect of altering Rp levels in the cells of individual tissues and the extrinsic effects of Rp mutants on hormone release (Figure 8A) and thus developmental timing. As Rps and the rRNAs are required in equimolar amounts to form functional ribosomes, the relative contribution of tissue intrinsic versus extrinsic growth requirements to final tissue/body size would be dependent on the expression level and stability of each Rp, which will dictate whether levels of the specific Rp are rate-limiting for ribosome biogenesis in a given tissue. Enlargement of tissues for any given Minute would only occur if reduction of the Rp in the affected tissue did not reduce levels below those required for tissue growth. If Rp levels were below the threshold in a particular tissue, its growth would be inhibited, effectively negating the effects of an increased larval growth period provided by the developmental delay. This is consistent with the observation that expression of a given Rp mRNA varies between tissues [52]–[54], indicating that a particular Rp may be rate limiting for proliferative growth in one tissue but not in another. For example, while all of the Minutes are developmentally delayed, wing overgrowth has not been widely described, suggesting that the reduced levels of the relevant Rp associated with the Minute in question are limiting in both the wing and PG. In contrast, RpL382b1/+ and RpL52d2/+ flies have overgrown wings [11] which suggests that the reduced level of RpL38 associated with RpL382b1/+ flies is not limiting for proliferative growth in wing discs but is limiting for PG growth, thus the extended growth period results in larger adult wings. Therefore the final size of the Minute and its individual tissues is the net effect of both the tissue extrinsic effects of reducing Rps in the PG, and the tissue intrinsic effects of reducing Rps in the cells of other tissues (Figure 8A).
The mechanisms behind maintaining body/organ size are complex, and in addition to intrinsic cellular growth rate and the time spent in the growth phase prior to pupation described above, recent studies of imaginal disc regeneration reveal that the final size of Drosophila imaginal tissues is sensitive to an overarching mechanism that slows the division rate of the non-regenerating compartments even in the event of developmental delay [55]. This may explain why the RpS6WG1288/+ mutant is able restore eye size back toward the wild type size in a background sensitised to impaired eye growth, ie., the cycEJP background, but does not normally lead to eye overgrowth or overgrowth of other tissue compartments, despite being associated with a developmental delay.
Clearly however, these final size constraints can be overridden or are not triggered in certain Minutes eg., RpL382b1/+ and RpL52d2/+ flies which have overgrown wings. In these cases the ongoing wing imaginal disc growth occurring during the extended larval period appears to be sufficient to overcome the normal size control checkpoints that normally restrict overgrowth of this tissue. Consistent with this model, knockdown of RpS6 or RpL38 specifically in the PG rather than the whole fly using the ring gland driver (P0206-Gal4) results in a smaller PG and developmental delay, which is associated with overgrown larvae (Figure S4A) and for RpL38 with significantly increased wing imaginal disc size (Figure S4B–S4C).
Together these findings demonstrate the complexities of the cell non-autonomous effects of Rp reduction on tissue growth, which has implications for many of the experimental manipulations carried out by Drosophila researchers. For example if mosaic clones are generated in the whole animal using the Minute technique to maximize size of mosaic clonal tissue, this might also impact on PG growth and have unforeseen cell non-autonomous effects on the tissue of interest, which will need to be taken into consideration.
The relationship between overgrowth in Minutes and predisposition to cancer associated with Rp haploinsufficiency in vertebrates
Our studies also raise the interesting question of whether the cell non-autonomous mechanisms underlying tissue overgrowth phenotypes of Minutes described here are relevant to the mechanisms responsible for tissue-specific phenotypes associated with Rp mutations in vertebrates. These ribosomopathies [56] include abnormal erythrocyte maturation, thrombocytosis and a predisposition to leukemia, associated with Rp haploinsufficiency syndromes such as the 5q - syndrome and Diamond-Blackfan anaemia (DBA) in humans [13], [20] or nerve sheath tumours in fish [12]. We think the cell non-autonomous mechanism described herein is unlikely at least for the 5q - syndrome, as the pathogenesis of ribosomal protein-mediated bone marrow failure appears to be largely cell intrinsic involving ribosomal stress mediated activation of p53 and defective development of haematopoietic system [57]. This is not to say that cell extrinsic effects of ribosomopathies may not contribute to development defects and disease at some level in vertebrates, for example, through defective growth of tissues important for release of paracrine or endocrine acting hormones. Clearly additional studies are required to determine to what extent altered Rp gene dosage contributes to human disease other than bone marrow failure and whether they are mediated by cell intrinsic or extrinsic mechanism or, indeed both.
In summary, our findings establish that suppression of cycEJP by the RpS6 mutant is exerted via a mechanism wherein reduced Rp levels in the prothoracic gland decreases abundance of the steroid hormone ecdysone, delaying development and hence allowing additional time for tissue and organ overgrowth. These data provide for the first time a rationale to explain the counter-intuitive organ overgrowth phenotypes observed for certain Drosophila Rp mutants. Furthermore, they provide new insight into mechanisms governing tissue size homeostasis, suggesting that different tissues may exhibit distinct thresholds of expression of individual Rps. Thus, regulated expression of individual Rps could exert tissue specific effects on cell growth and organ size.
Materials and Methods
Drosophila stocks and culture
Unless otherwise stated the fly strains used were obtained from the Bloomington Stock Center and are described in FlyBase (http://flybase.org). The UAS-RpS6 transgenic lines for overexpression were generated by cloning the full-length RpS6 cDNA into pUAST and then injected into Drosophila embryos, as previously described in [58]. The following strains were described in: w−;cycEJP [29], AmnC651-Gal4 [41], Phm-Gal4 [43], P0206-Gal4 [42]–[43], UAS-Dp110DN [59], UAS-RasV12 [60], UAS-Cyclin E [61], UAS-p35 [62], GMR-p21 [63], UAS-cycD and UAS-cdk4 [64].
Generation of ribosomal protein RNAi transgenic flies
RpS6 RNAi construct: the longest open reading frame for RpS6 (654 bp) was PCR amplified with primers 5′-CTGCAGGAATTCGGACAGGTTGTGGAGGCCGAT-3′ and 5′-GGTACCGAATTCTTACTTCTTGTCGCTGGAGACAG-3′ (EcoRI sequence underlined) and PCR products were digested with EcoRI and ligated into the SYMpUAST vector [65].
RpS13, RpL5, RpL30 and RpL38 RNAi constructs: products were digsted with XbaI and inserted into pWIZ as inverted repeats in a two–step cloning process [66]. RpS13: the 302 bp coding region of the 3rd exon was PCR amplified with primers 5′-ATATTCTAGAGCATCATCCTGCGTGACTCGC-3′ and 5′-ATATTCTAGAGGCAACCAGGGCGGAGGC-3′ (XbaI sequence underlined). RpL5: the 264 bp coding region of the 2nd exon PCR amplified with primers 5′-GCGCTCTAGAGGTTTCGTTAAGGTAGTC-3′ and 5′-GCATTCTAGACTGGATCCCGTATTTGGG-3′. RpL30: the 199 bp 5′UTR and coding region of the 1st exon was PCR amplified with primers 5′-GCATTCTAGATCGCCTGCAGTGCTTTAACC-3′and 5′-ATATTCTAGACTCAGGGCGGGCGTGTTGC-3′. RpL38: the 213 bp coding region of the 2nd exon was PCR amplified with primers 5′-GCGCTCTAGAATGCCACGGGAAATTAAAG-3′ and 5′-GCGCTCTAGATTATTTCACCTCCTTTAC-3′. All constructs were injected into Drosophila embryos, as previously described in [58].
Temperature shift experiments with Gal80TS
Conditional expression of UAS-RpS6 RNAi was carried out using a temperature sensitive isoform of Gal80, the repressor of Gal4 (Gal80TS [49]). Larvae were raised at the permissive temperature of 18°C and shifted at late 2nd instar to the restrictive temperature of 25°C.
Assessing developmental delay
For each experiment, forty 1st instar larvae were collected 24 hour AED (0–4 hour collections) from lay cages with grape agar plates. To measure time of eclosion, vials were checked for the number of eclosed adults every 2 hours from 10 days AED until adult flies no longer emerged. For 20-hydroxyecdysone treatment twenty 1st instar larvae were collected 24 hour AED (0–4 hour collections) and transferred into vials containing yeast paste supplemented daily with 0.75 mg/ml of 20-hydroxyecdsyone (Sigma).
Microscopy and imaging
Antibody staining, BrdU labelling and quantification were carried out as described previously [67]–[68]. Antibodies used were the anti-RpS6 polyclonal (raised in mice), anti-bromodeoxyuridine (Becton Dickinson), PH3 (Upstate) and anti-cycE (rat) (a gift from Helena Richardson). Serial sections of eye imaginal discs or prothoracic glands were taken on a Zeiss Imager Z1 using the LSM 510 Meta software. Image preparation and analysis were conducted in Adobe Photoshop CS2 v9.0 and ImageJ v1.37.
For light microscopy images were captured on an Olympus DP11 camera. Female adult eyes were imaged at 5.6× magnification, and larvae or adult flies were imaged at 1.6× magnification. All images were processed using Adobe Photoshop. Eye area was measured by tracing around the perimeter of the photoreceptor cells of cropped images using Metamorph Offline version 7.6.3.0 software.
For electron microscopy female adult flies were progressively fixed in 25% (v/v) acetone for 1 hour nutation at room temperature, 50% (v/v) acetone for 1 hour nutation at room temperature, 75% (v/v) acetone for 1 hour nutation at room temperature, and finally stored in 100% acetone. The sample was then critical point dried on a Balters CPD 030 Critical Point Dryer and coated with gold particles in an Edwards 6150B Gold Sputter Coater. Images were recorded on a Phillips XL30 FEG Field Emission Electron Microscope.
Prothoracic gland size measurements
For measurements of prothoracic gland (PG) area size, confocal images of PGs taken at 40× magnification were quantified with BB Thermometer v1.1 Software (BenBritten.com).
Wing size measurements
Adult wings were mounted into Canada Balsam and xylene. Images were taken at 4.5× magnification. Whole wing area was measured using the magnetic lasso tool and record measurement function of Adobe Photoshop.
Reverse Transcriptase–PCR (RT–PCR)
Total RNA was isolated from ten 3rd instar larvae or thirty 3rd instar eye imaginal discs with TRIzol (GibcoBRL) following manufacturer's instructions. cDNA was synthesised from 1 µg RNA using the Superscript First Strand synthesis system for RT-PCR (Invitrogen) following the manufacturer's guidelines. qRT-PCR was carried out with SYBR Green under standard conditions in the Step One Plus Real Time PCR system (Applied Biosystems)
Primer sequences were as follows:
RpS6 forward (TGTTCCAGCTCAACGTTTCCT)
RpS6 reverse (TCGTCGACCACTTCGAATAGC)
Actin 5C forward (CCCCAAGGCCAACCGTGAGA)
Actin 5C reverse (ACCCGAAGCGTACAGCGAGAGC)
E74B primers as published in [41].
Amplicon specificity was verified by melting curve analysis (65 to 90°C) after 40 cycles. An average Ct value for the three technical replicates was calculated for each sample. The fold change expression of RpS6 mRNA levels was normalised to equal RNA and determined using the 2−ΔΔCT method. E74B mRNA levels were normalised to Actin 5C mRNA levels of untreated control cells and determined using the 2−ΔΔCT method [69].
Statistical analysis
Statistical analysis was performed in GraphPad Prism software using either Unpaired t-test or One-way ANOVA, with Tukey's test for multiple comparisons, as stated in figure legends.
Supporting Information
Zdroje
1. BridgesCBMorganTH 1923 The third-chromosome group of mutant characters of Drosophila melanogaster. Carnegie Institution of Washington 327 1 251
2. SchultzJ 1929 The Minute Reaction in the Development of DROSOPHILA MELANOGASTER. Genetics 14 366 419
3. HuangSLBakerBS 1976 The mutability of the minute loci of Drosophila melanogaster with ethyl methanesulfonate. Mutat Res 34 407 414
4. MarygoldSJRooteJReuterGLambertssonAAshburnerM 2007 The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol 8 R216
5. SteitzTA 2008 A structural understanding of the dynamic ribosome machine. Nat Rev Mol Cell Biol 9 242 253
6. WarnerJRMcIntoshKB 2009 How common are extraribosomal functions of ribosomal proteins? Mol Cell 34 3 11
7. WatsonKLJohnsonTKDenellRE 1991 Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev Genet 12 173 187
8. WatsonKLKonradKDWoodsDFBryantPJ 1992 Drosophila homolog of the human S6 ribosomal protein is required for tumor suppression in the hematopoietic system. Proc Natl Acad Sci U S A 89 11302 11306
9. StewartMJDenellR 1993 Mutations in the Drosophila gene encoding ribosomal protein S6 cause tissue overgrowth. Mol Cell Biol 13 2524 2535
10. EvansCJHartensteinVBanerjeeU 2003 Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell 5 673 690
11. MarygoldSJCoelhoCMLeeversSJ 2005 Genetic analysis of RpL38 and RpL5, two minute genes located in the centric heterochromatin of chromosome 2 of Drosophila melanogaster. Genetics 169 683 695
12. AmsterdamASadlerKCLaiKFarringtonSBronsonRT 2004 Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2 e139 doi:10.1371/journal.pbio.0020139
13. DraptchinskaiaNGustavssonPAnderssonBPetterssonMWilligTN 1999 The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 21 169 175
14. CmejlaRCmejlovaJHandrkovaHPetrakJPospisilovaD 2007 Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum Mutat 28 1178 1182
15. GazdaHTGrabowskaAMerida-LongLBLatawiecESchneiderHE 2006 Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am J Hum Genet 79 1110 1118
16. FarrarJENaterMCaywoodEMcDevittMAKowalskiJ 2008 Abnormalities of the large ribosomal subunit protein, Rpl35A, in diamond-blackfan anemia. Blood
17. GazdaHTSheenMRVlachosAChoesmelVO'DonohueMF 2008 Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet 83 769 780
18. CmejlaRCmejlovaJHandrkovaHPetrakJPetrtylovaK 2009 Identification of mutations in the ribosomal protein L5 (RPL5) and ribosomal protein L11 (RPL11) genes in Czech patients with Diamond-Blackfan anemia. Hum Mutat 30 321 327
19. DohertyLSheenMRVlachosAChoesmelVO'DonohueMF 2010 Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet 86 222 228
20. EbertBLPretzJBoscoJChangCYTamayoP 2008 Identification of RPS14 as a 5q - syndrome gene by RNA interference screen. Nature 451 335 339
21. PellagattiAHellstrom-LindbergEGiagounidisAPerryJMalcovatiL 2008 Haploinsufficiency of RPS14 in 5q - syndrome is associated with deregulation of ribosomal - and translation-related genes. Br J Haematol 142 57 64
22. Leger-SilvestreICaffreyJMDawalibyRAlvarez-AriasDAGasN 2005 Specific Role for Yeast Homologs of the Diamond Blackfan Anemia-associated Rps19 Protein in Ribosome Synthesis. J Biol Chem 280 38177 38185
23. CmejlovaJDolezalovaLPospisilovaDPetrtylovaKPetrakJ 2006 Translational efficiency in patients with Diamond-Blackfan anemia. Haematologica 91 1456 1464
24. DanilovaNSakamotoKMLinS 2008 Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood
25. HalfarKRommelCStockerHHafenE 2001 Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development 128 1687 1696
26. BrumbyASecombeJHorsfieldJCoombeMAminN 2004 A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in Drosophila. Genetics 168 227 251
27. ThomasBJZipurskySL 1994 Early pattern formation in the developing Drosophila eye. Trends Cell Biol 4 389 394
28. ReadyDFHansonTEBenzerS 1976 Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53 217 240
29. SecombeJPispaJSaintRRichardsonH 1998 Analysis of a Drosophila cyclin E hypomorphic mutation suggests a novel role for cyclin E in cell proliferation control during eye imaginal disc development. Genetics 149 1867 1882
30. KoffAGiordanoADesaiDYamashitaKHarperJW 1992 Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 257 1689 1694
31. DulicVLeesEReedSI 1992 Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257 1958 1961
32. KnoblichJASauerKJonesLRichardsonHSaintR 1994 Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77 107 120
33. SpradlingACSternDBeatonARhemEJLavertyT 1999 The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153 135 177
34. EllisMCO'NeillEMRubinGM 1993 Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development 119 855 865
35. FreemanM 1996 Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87 651 660
36. EnerlyELarssonJLambertssonA 2003 Silencing the Drosophila ribosomal protein L14 gene using targeted RNA interference causes distinct somatic anomalies. Gene 320 41 48
37. HazelettDJBourouisMWalldorfUTreismanJE 1998 decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125 3741 3751
38. HauckBGehringWJWalldorfU 1999 Functional analysis of an eye specific enhancer of the eyeless gene in Drosophila. Proc Natl Acad Sci U S A 96 564 569
39. ThummelCS 2001 Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 1 453 465
40. King-JonesKThummelCS 2005 Developmental biology. Less steroids make bigger flies. Science 310 630 631
41. CaldwellPEWalkiewiczMSternM 2005 Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol 15 1785 1795
42. ColombaniJBianchiniLLayalleSPondevilleEDauphin-VillemantC 2005 Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310 667 670
43. MirthCTrumanJWRiddifordLM 2005 The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol 15 1796 1807
44. BaslerKHafenE 1989 Dynamics of Drosophila eye development and temporal requirements of sevenless expression. Development 107 723 731
45. XinSWengLXuJDuW 2002 The role of RBF in developmentally regulated cell proliferation in the eye disc and in Cyclin D/Cdk4 induced cellular growth. Development 129 1345 1356
46. CramtonSELaskiFA 1994 string of pearls encodes Drosophila ribosomal protein S2, has Minute-like characteristics, and is required during oogenesis. Genetics 137 1039 1048
47. LambertssonA 1998 The minute genes in Drosophila and their molecular functions. Adv Genet 38 69 134
48. McBrayerZOnoHShimellMParvyJPBecksteadRB 2007 Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13 857 871
49. ZeidlerMPTanCBellaicheYCherrySHaderS 2004 Temperature-sensitive control of protein activity by conditionally splicing inteins. Nat Biotechnol 22 871 876
50. BrennanCAAshburnerMMosesK 1998 Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development 125 2653 2664
51. BrennanCALiTRBenderMHsiungFMosesK 2001 Broad-complex, but not ecdysone receptor, is required for progression of the morphogenetic furrow in the Drosophila eye. Development 128 1 11
52. BortoluzziSd'AlessiFRomualdiCDanieliGA 2001 Differential expression of genes coding for ribosomal proteins in different human tissues. Bioinformatics 17 1152 1157
53. IshiiKWashioTUechiTYoshihamaMKenmochiN 2006 Characteristics and clustering of human ribosomal protein genes. BMC Genomics 7 37
54. ThorrezLVan DeunKTrancheventLCVan LommelLEngelenK 2008 Using ribosomal protein genes as reference: a tale of caution. PLoS ONE 3 e1854 doi:10.1371/journal.pone.0001854
55. Smith-BoltonRKWorleyMIKandaHHariharan 2009 Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell 16 797 809
56. NarlaAEbertBL 2010 Ribosomopathies: human disorders of ribosome dysfunction. Blood 115 3196 3205
57. BarlowJLDrynanLFTrimNLErberWNWarrenAJ 2010 New insights into 5q - syndrome as a ribosomopathy. Cell Cycle 9 4286 4293
58. SpradlingAC 1986 P element-mediated transformation. Drosophila: A practical approach IRL Press, Oxford 175 197
59. LeeversSJWeinkoveDMacDougallLKHafenEWaterfieldMD 1996 The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J 15 6584 6594
60. KarimFDRubinGM 1998 Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125 1 9
61. CrackDSecombeJCoombeMBrumbyASaintR 2002 Analysis of Drosophila cyclin EI and II function during development: identification of an inhibitory zone within the morphogenetic furrow of the eye imaginal disc that blocks the function of cyclin EI but not cyclin EII. Dev Biol 241 157 171
62. HayBAWolffTRubinGM 1994 Expression of baculovirus P35 prevents cell death in Drosophila. Development 120 2121 2129
63. de NooijJCHariharanIK 1995 Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science 270 983 985
64. DatarSAJacobsHWde la CruzAFLehnerCFEdgarBA 2000 The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J 19 4543 4554
65. GiordanoERendinaRPelusoIFuriaM 2002 RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics 160 637 648
66. LeeYSCarthewRW 2003 Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30 322 329
67. MitchellNCrannaNRichardsonHQuinnL 2008 The Ecdysone-inducible zinc-finger transcription factor Crol regulates Wg transcription and cell cycle progression in Drosophila. Development 135 2707 2716
68. MitchellNCJohansonTMCrannaNJErALRichardsonHE 2010 Hfp inhibits Drosophila myc transcription and cell growth in a TFIIH/Hay-dependent manner. Development 137 2875 2884
69. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25 402 408
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



