-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHistone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
Epigenetic regulation plays critical roles in the regulation of cell proliferation, fate determination, and survival. It has been shown to control self-renewal and lineage differentiation of embryonic stem cells. However, epigenetic regulation of adult stem cell function remains poorly defined. Drosophila ovarian germline stem cells (GSCs) are a productive adult stem cell system for revealing regulatory mechanisms controlling self-renewal and differentiation. In this study, we show that Eggless (Egg), a H3K9 methyltransferase in Drosophila, is required in GSCs for controlling self-renewal and in escort cells for regulating germ cell differentiation. egg mutant ovaries primarily exhibit germ cell differentiation defects in young females and gradually lose GSCs with time, indicating that Egg regulates both germ cell maintenance and differentiation. Marked mutant egg GSCs lack expression of trimethylated H3K9 (H3k9me3) and are rapidly lost from the niche, but their mutant progeny can still differentiate into 16-cell cysts, indicating that Egg is required intrinsically to control GSC self-renewal but not differentiation. Interestingly, BMP-mediated transcriptional repression of differentiation factor bam in marked egg mutant GSCs remains normal, indicating that Egg is dispensable for BMP signaling in GSCs. Normally, Bam and Bgcn interact with each other to promote GSC differentiation. Interestingly, marked double mutant egg bgcn GSCs are still lost, but their progeny are able to differentiate into 16-cell cysts though bgcn mutant GSCs normally do not differentiate, indicating that Egg intrinsically controls GSC self-renewal through repressing a Bam/Bgcn-independent pathway. Surprisingly, RNAi-mediated egg knockdown in escort cells leads to their gradual loss and a germ cell differentiation defect. The germ cell differentiation defect is at least in part attributed to an increase in BMP signaling in the germ cell differentiation niche. Therefore, this study has revealed the essential roles of histone H3K9 trimethylation in controlling stem cell maintenance and differentiation through distinct mechanisms.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002426
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002426Summary
Epigenetic regulation plays critical roles in the regulation of cell proliferation, fate determination, and survival. It has been shown to control self-renewal and lineage differentiation of embryonic stem cells. However, epigenetic regulation of adult stem cell function remains poorly defined. Drosophila ovarian germline stem cells (GSCs) are a productive adult stem cell system for revealing regulatory mechanisms controlling self-renewal and differentiation. In this study, we show that Eggless (Egg), a H3K9 methyltransferase in Drosophila, is required in GSCs for controlling self-renewal and in escort cells for regulating germ cell differentiation. egg mutant ovaries primarily exhibit germ cell differentiation defects in young females and gradually lose GSCs with time, indicating that Egg regulates both germ cell maintenance and differentiation. Marked mutant egg GSCs lack expression of trimethylated H3K9 (H3k9me3) and are rapidly lost from the niche, but their mutant progeny can still differentiate into 16-cell cysts, indicating that Egg is required intrinsically to control GSC self-renewal but not differentiation. Interestingly, BMP-mediated transcriptional repression of differentiation factor bam in marked egg mutant GSCs remains normal, indicating that Egg is dispensable for BMP signaling in GSCs. Normally, Bam and Bgcn interact with each other to promote GSC differentiation. Interestingly, marked double mutant egg bgcn GSCs are still lost, but their progeny are able to differentiate into 16-cell cysts though bgcn mutant GSCs normally do not differentiate, indicating that Egg intrinsically controls GSC self-renewal through repressing a Bam/Bgcn-independent pathway. Surprisingly, RNAi-mediated egg knockdown in escort cells leads to their gradual loss and a germ cell differentiation defect. The germ cell differentiation defect is at least in part attributed to an increase in BMP signaling in the germ cell differentiation niche. Therefore, this study has revealed the essential roles of histone H3K9 trimethylation in controlling stem cell maintenance and differentiation through distinct mechanisms.
Introduction
Histone modification represents one of the most common epigenetic mechanisms for controlling gene expression, and thus cell proliferation, fate determination and survival during development [1]. Histone modification has recently been subjected to extensive investigation for its roles in the control of self-renewal and lineage differentiation of embryonic stem cells (ESCs) by disrupting functions of the enzymes that are important for catalyzing the modifications [2]–[7]. Among different histone modifications, trimethylation of histone 3 lysine 9 (H3K9me3) has been widely studied and is often associated with heterochromatin formation, gene repression and transcriptional elongation in different tissue types and organisms [1]. SETDB1, one of the H3K9 trimethylases in the mouse, was recently shown to be important for maintaining ESC self-renewal [8]. However, its role in adult stem cell regulation remains to be determined.
In the Drosophila ovary, two or three GSCs are located at the tip of the germarium, which is the structure located at the apical end of an ovariole [9], [10]. These GSCs physically interact with cap cells anteriorly and escort cells laterally. The immediate differentiating GSC progeny, known as cystoblasts (CBs), can further divide synchronously without cytokinesis to form 2-cell, 4-cell, 8-cell and 16-cell cysts. CBs, mitotic cysts and newly formed 16-cell cysts are surrounded by escort cells. Cap cells form a niche for maintaining GSC self-renewal by producing BMP-like molecules Dpp and Gbb [11]–[13]. Dpp and Gbb activate BMP signaling in the GSC to directly repress expression of differentiation factors such as bam, and thereby maintain GSC self-renewal [13], [14]. Chromatin remodeling factors, such as ISWI and Stonewall, have been shown to be important for maintaining GSC self-renewal through distinct mechanisms. ISWI is required for repressing bam transcription in GSCs [15], while Stonewall likely represses a Bam-independent pathway [16]. Lsd1 is a H3K4 demethylase in the Drosophila ovary, and its mutations cause upregulation of H3K4 trimethylation and gene activation [17]. Recently, Lsd1 has been shown to be required in escort cells (ECs) to repress dpp expression and promote germ cell differentiation [18]. These findings indicate that epigenetic regulation is important for GSC self-renewal.
In Drosophila, there are three known H3K9 methyltransferases, Su(var)3-9, G9a and eggless (egg, also known as dSETDB1). Su(var)3-9 was the first identified H3K9 methyltransferase in Drosophila [19], and it is responsible for H3K9me3 at the core of the chromocenter, which provides docking sites for HP1 recruitment and thus heterochromatin formation and maintenance [20], [21]. Recently, G9a was also shown to exhibit H3K9-, H3K27 - and H3K4 - methyltransferase activity and localize to the euchromatic region, but it is dispensable for normal Drosophila development [22], [23]. Egg is the histone methyltransferase responsible for maintaining H3K9me3 on the fourth chromosome, and it works with Su(var)3-9 to maintain H3K9me3 in the pericentric heterochromatin of all chromosomes [24]–[27]. Egg is expressed throughout Drosophila development, and is an essential gene because its homozygous deletion causes lethality [25], [26]. In addition, the females carrying homozygous EMS-induced egg mutations do not lay any eggs, and it is this phenotype upon which its name is based [28]. The egg mutant ovaries exhibit defects in follicle cell proliferation and the maintenance or survival of somatic cells and germ cells [28]. Consistently, Windei (Wde), the Drosophila homolog of human MCAF1, is an essential Egg cofactor and is also required for germ cell maintenance [29]. Interestingly, Egg is located in pericentric heterochromatin and catalyzes H3K9 trimethylation in GSCs and their immediate descendants, while SU(VAR)3-9 is primarily in charge of H3K9 trimethylation in more differentiated germline cysts in egg chambers [27]. Mutations in egg and Su(var)3-9 abolish H3K9me3 from germ cells in the germarium and the developing egg chambers, respectively. Although egg is proposed to maintain the survival of germ cells in the Drosophila ovary, it remains unclear whether it is required for GSC maintenance or simply germ cell survival. In this study, we have revealed the essential role of Egg in controlling GSC self-renewal and a novel role of Egg in the regulation of germ cell differentiation.
Results
Egg Is Essential for GSC Maintenance and Germ Cell Differentiation
To investigate how germ cells are lost in egg mutant ovaries, we examined the germ cell phenotypes of different egg mutant allelic combinations. In the Drosophila ovary, somatic cells and different germ cell types can be distinguished using molecular markers. Lamin C can label both TFs and cap cells, which can be easily distinguished by their distinct cellular morphologies [12]: TF cells are disc-like cells lining up in a row, while cap cells are round-shaped cells tightly packing together next to TFs. Vasa can label all the germ cells including GSCs [30], [31], while Hts labels spectrosomes in GSCs and CBs as well as branched fusomes in cystocytes [32] (Figure 1A). In this study, three egg mutants, egg2138 and egg1473 and egg235, were used to investigate its function in female germ cell development. The Egg protein contains two tudor domains and one bifurcated SET domain, which carry out the functions of binding to the methylated H3K9 and catalyzing H3K9 trimethylation, respectively [28]. The mutations in egg1473 and egg235 were previously shown to cause the production of truncated proteins by deletion of the entire SET domain and all the functional domains, respectively (Clough et al., 2007). The egg2138 mutation corresponds to a truncated protein resulting from deletion of the SET domain and part of the second tudor domain and thus is a strong mutation (T. Hazelrigg, personal communications). Heterozygous egg2138 and egg1473 mutant germaria carry two or three GSCs and one CB as in normal wild-type germaria (Figure 1A and 1F). In contrast, egg2138/egg1473, egg2138/egg235, egg1473/egg235 mutant germaria exhibit two defects in germ cell development in addition to the previously reported follicle cell defects. The germaria in the newly eclosed two to three-day-old mutant females contain many spectrosome-containing single germ cells, which are located away from cap cells (Figure 1B). The undifferentiated GSC-like or CB-like cells also persist after they have been packed into egg chambers along with differentiated germ cell cysts (Figure 1C). For quantification, undifferentiated GSC-like or CB-like cells located outside the niche in the germarium are quantified as undifferentiated germ cells (UGCs). For the heterozygous controls, over 95% of the germaria contain one, two or three spectrosome-containing UGCs (Figure 1D). By contrast, 88% of the egg2138/egg1473 mutant germaria harbor more than 4 UGCs, and 30% of them have ten or more UGCs (Figure 1D). These results have revealed that egg is required for germ cell differentiation.
Fig. 1. egg is required for GSC maintenance and germ cell differentiation. 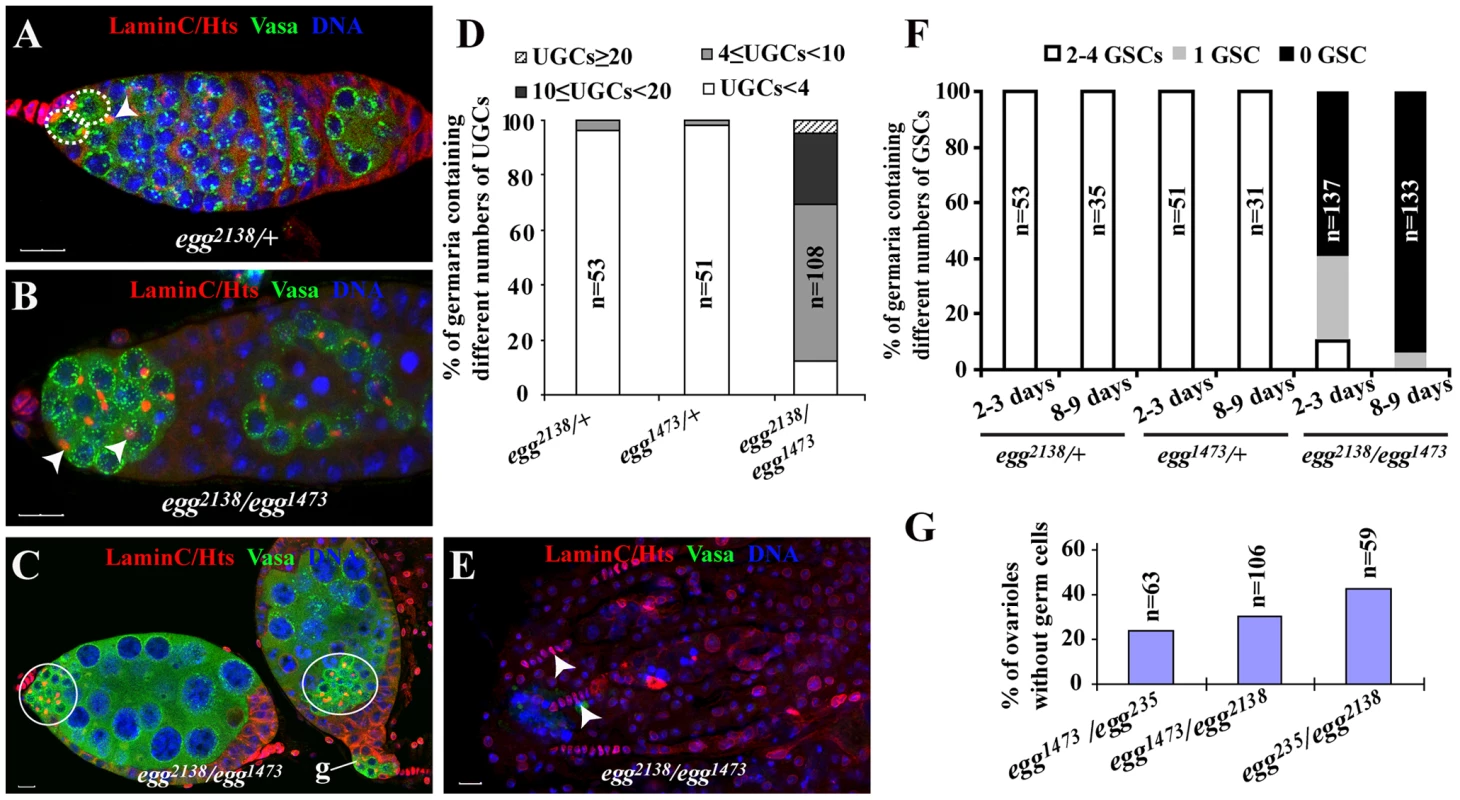
Control and egg mutant germaria are labeled for Lamin C (red, terminal filament and cap cells), Hts (red, spectrosomes and fusomes), Vasa (green, germline cells) and DAPI (blue, nuclei). (A) A week-old egg heterozygous control germarium showing two GSCs (broken circles) and one CB (arrowhead). (B) 3-day-old mutant egg germarium shows the accumulation of spectrosome-containing undifferentiated germ cells (UGCs) (arrowheads), indicative of differentiation defects. (C) The egg mutant chambers following the germarium (indicated by g) contain both differentiated germ cells and a cluster of undifferentiated spectrosome-containing single germ cells (circled). (D) 3-day-old egg mutant germaria contain more UGCs than the heterozygous controls. Germaria are categorized based on the UGC number. “n” indicates the number of germaria examined for each genotype. (E) Week-old egg mutant germaria showing only remaining TFs (arrowheads) and complete absence of germ cells including GSCs, which is indicated by absence of Vasa expression. (F) GSCs are gradually lost in egg mutant germaria. Germaria are categorized by the number of GSCs per germarium. (G) Germless ovarioles in 3–5 day-old ovaries of different egg mutant combinations are consistently observed. Scale bars represent 10 µm. As reported previously, egg is also required for germ cell maintenance [29]. Indeed, egg mutant germaria gradually lose their GSCs and eventually become agametic with time (Figure 1E and 1F). Even at the age of 2 or 3 days, 59% of the egg2138/egg1473 mutant germaria contain no GSCs, and 16% of them completely lose germ cells, including GSCs, indicating that some GSCs have already been lost in young mutant females (Figure 1F). At the age of 8 or 9 days, the GSC loss phenotype becomes more severe. 94% of those mutant germaria contain no GSCs, and 84% of them become completely depleted of germ cells (Figure 1F). Regarding the GSC loss phenotype, other mutant allelic combinations give the comparable GSC loss phenotype at the age of one week old (Figure 1G). These results show that egg is required for GSC maintenance.
Egg Is Required Intrinsically for GSC Self-Renewal and Proliferation
The genes identified so far for GSC regulation are required for either GSC maintenance or differentiation but rarely for both. To further understand how Egg regulates both GSC maintenance and differentiation, we used FLP-mediated FRT recombination to remove egg function intrinsically from arm-lacZ-marked GSCs. In this study, marked GSCs are identified by loss of lacZ (encoding β-galactosidase or β-gal) expression, presence of spectrosome and physical contact with cap cells/the niche as in our previous studies [11], [33]. As shown previously, about 80% of the marked control GSCs detected at the first week after clonal induction (ACI) are still maintained three weeks ACI (Figure 2A–2D). The marked control GSCs still remain in the niche two weeks and three weeks ACI (Figure 2B and 2C). In contrast, less than 20% of the marked egg mutant GSCs for egg1473, egg235 and egg2138 detected at the first week ACI are still maintained three weeks ACI, indicating that Egg is required intrinsically for maintaining GSCs (Figure 2D–2G). Two or three weeks ACI, the lost marked mutant egg GSCs have developed into differentiated 16-cell germline cysts either in the germaria or egg chambers (Figure 2F and 2G). The differentiation of marked egg mutant cysts could be due to perdurance of Egg protein. To rule out this possibility, we examined H3K9me3 in marked egg mutant germline clones. Consistent with the role of Egg in catalyzing H3K9 trimethylation, the marked egg1473, egg235 or egg2138 mutant GSCs and cysts as early as one week ACI have abolished H3K9 trimethylation in comparison with their neighboring control GSCs and cysts (Figure 2H and 2I), but their H3K9 dimethylation remains unchanged (Figure 2J). These results confirm that Egg is a key enzyme responsible for H3K9 trimethylation in GSCs and early germ cell cysts, and that there is not much Egg protein perdurance in the marked egg mutant germline clones one week ACI. However, almost all the germaria carrying a marked mutant GSC do not accumulate marked egg mutant spectrosome-containing single germ cells, indicating that Egg is intrinsically dispensable for germ cell differentiation. These results indicate that Egg is required intrinsically only for controlling GSC maintenance but not for differentiation.
Fig. 2. egg is required intrinsically for GSC maintenance and the survival of late differentiated germ cells. 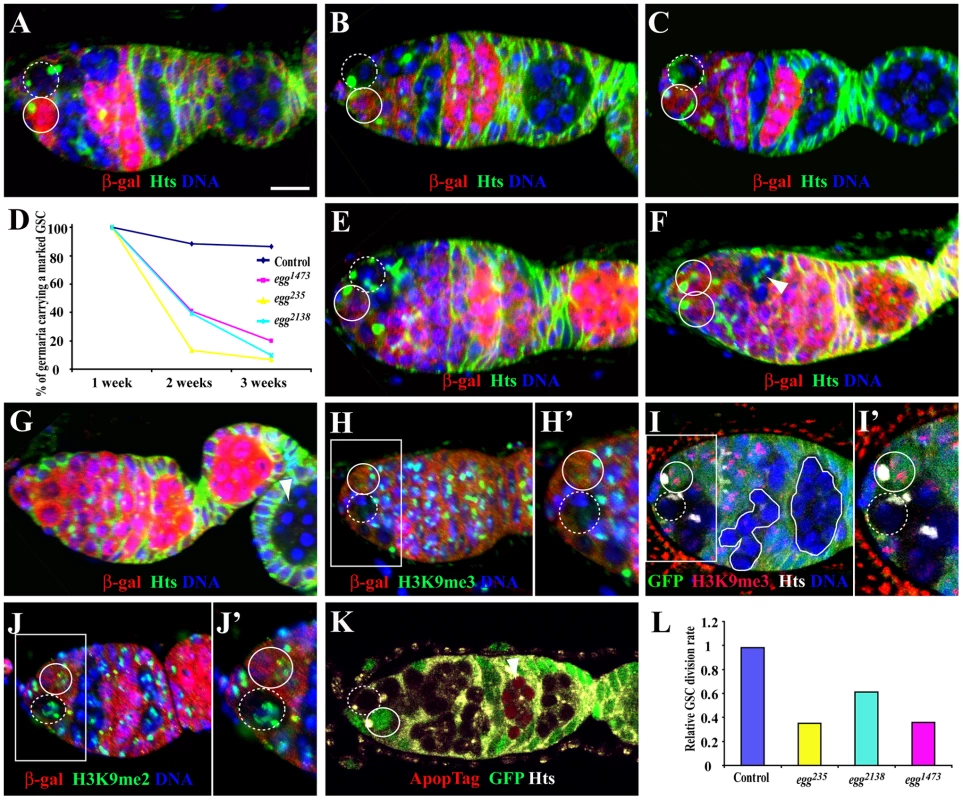
Germaria in A–G are labeled for LacZ (red), Hts (green) and DAPI (blue), while unmarked and marked GSCs are indicated by broken and solid circles, respectively. (A–C) Marked wild-type GSCs are maintained one week (A), two weeks (B) and three weeks (C) after clonal induction (ACI). (D) Percentages of the germaria carrying a marked wild-type or egg mutant GSC clone change with time (one week, two weeks and three weeks ACI). (E) A marked egg mutant GSC still remains in the niche one week ACI. (F, G) The marked mutant GSC is lost from the niche two weeks (F) or three weeks (G) ACI, , which is evidenced by presence of a marked mutant cyst (arrowheads in F and G). (H, H′) A marked egg mutant GSC (broken circle) has lost H3K9me3 staining in comparison with its neighboring unmarked control GSC (circle) one week ACI. (I, I′) A marked egg mutant GSC (broken circle) and mutant 16-cell cysts (solid lines) have no H3K9me3 staining in comparison with its neighboring unmarked control GSC (circle) and cysts twelve days ACI. (J, J′) Marked egg mutant GSC (broken circle) and unmarked control GSC (solid circle) have comparable H3K9me2 staining one week ACI. (K) A marked egg mutant 16-cell cyst (arrowhead), but not the marked egg mutant GSC (broken circle), is apoptotic twelve days ACI. (L) Relative GSC division rates for marked control and egg mutant GSCs. Scale bar: 10 µm. The loss of the marked egg mutant GSCs could be due to their competitive disadvantage over their neighboring control GSCs. We used an RNAi-mediated knockdown approach to inactivate egg function in all the GSCs in the niche using nanos-gal4-driven UAS-RNAi expression. Two independent RNAi lines [eggRNAi-1 (HMS00443) and -2 (HMS00112)], which can be expressed in female germ cells including GSCs using nanos-gal4VP16 [34], were used to knockdown egg function in GSCs. Consistent with egg mutant clonal analysis results, germline expression of eggRNAi-1 leads to almost complete elimination of germ cells including GSCs in one-week old females (Figure S1A–S1C). However, germline expression of eggRNAi-2 results in formation of swollen germaria due to the accumulation of a few more spectrosome-containing single germ cells and differentiated cysts, but only rare GSC loss (Figure S1C–S1E), suggesting that eggRNAi-2 may be less effective in knocking down egg expression. Interestingly, the accumulated single germ cells and germ cell cysts in the germarium are positive for the commonly used DNA damage marker H2AX in comparison with the control that only meiotic germ cells are positive for this marker, indicating that egg is involved in DNA damage control. The accumulated DNA damage could also explain the accumulation of some spectrosome-containing single germ cells due to mitotic arrest. These results support the idea that Egg is required intrinsically for maintaining GSCs, and also suggest that it controls GSC maintenance possibly via maintaining the genome integrity.
Loss of the marked mutant egg GSCs could be due to either defective self-renewal or apoptosis. To rule out the possibility that the mutant egg GSCs are lost due to apoptosis, we performed TUNEL-based ApopTag labeling of the marked mutant egg GSCs and cysts. Interestingly, no marked mutant egg GSCs are apoptotic (total 38 marked egg mutant GSC clones examined), suggesting that DNA damage caused by loss of egg function leads to defective GSC self-renewal but not apoptosis (Figure 2K). Interestingly, egg mutant mitotic cysts and 16-cell cysts in the regions 1 and 2a are not apoptotic. However, among the 38 ovarioles, 9 of them carry at least one apoptotic egg mutant 16-cell cyst in the regions 2b or 3 of the germaria, indicating that DNA damage caused by loss of egg function leads to apoptosis of differentiated 16-cell cysts (Figure 2G and 2K). Because DNA damage often results in cell cycle arrest, we would expect that loss of egg function slows down proliferation of GSCs, CBs and mitotic cysts. To test this idea, we then determined the relative division rates for marked control and egg mutant GSCs as we previously reported [11]. As expected, the relative division rate for marked wild-type control GSCs is close to 1 (Figure 2L). In contrast, the relative division rates for marked egg mutant GSCs are much lower than that for the control, indicating that egg mutant GSCs divide much slower than wild-type controls (Figure 2L). Consistently, only 24.3% of the marked mutant egg1473 GSCs (n = 33) are positive for BrdU in contrast with 32.8% for the unmarked control GSCs (n = 134) in the same population of the germaria. These results could also explain the accumulation of CBs and mitotic cysts in the germarium following the inactivation of egg function in the germline (Figure S1E). These results indicate that Egg is required intrinsically for promoting GSC self-renewal and proliferation.
Egg Controls GSC Self-Renewal by Repressing a Bam-Independent Differentiation Pathway
Niche-activated BMP signaling is known to be necessary and sufficient for GSC self-renewal [11], [12]. In Drosophila ovarian GSCs, active BMP signaling represses bam expression and activates Dad expression, which can be monitored by reporter lines bam-GFP and Dad-lacZ, respectively [13], [14], [35], [36]. In the marked mutant egg1473 GSCs, bam-GFP is still repressed as in the neighboring unmarked control GSCs of the same germaria, indicating that egg is dispensable for BMP signaling-mediated bam repression in GSCs (Figure 3A–3A′). Interestingly, in the marked mutant egg1473 GSCs, Dad-lacZ fails to be activated to similarly high expression levels as those in their neighboring unmarked control GSCs of the same germaria, indicating that egg intrinsically regulates Dad transcription in GSCs (Figure 3B–3B′). Because Egg only regulates transcriptional activation of Dad but not repression of bam in GSCs, we speculate that Egg is dispensable for BMP signaling but indirectly regulates Dad expression.
Fig. 3. egg is required intrinsically for maintaining GSCs by repressing a Bam/Bgcn-independent differentiation pathway. 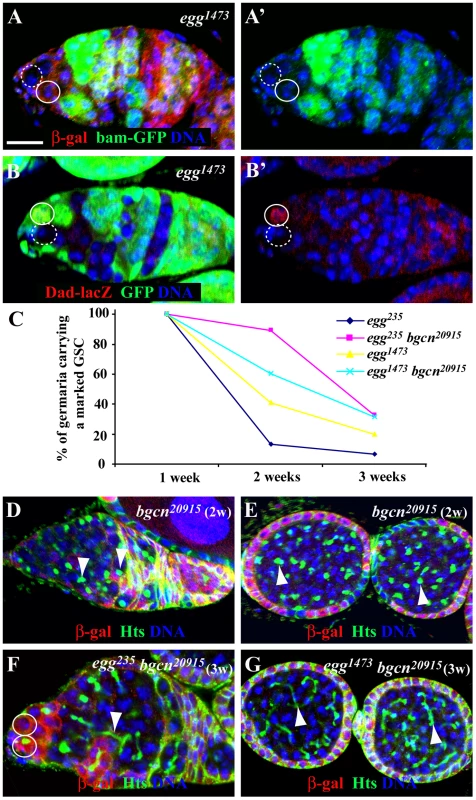
(A and A′) Both lacZ-negative marked egg mutant GSC (broken circle) and lacZ-positive unmarked control GSC repress bam-GFP expression (A′). (B and B′) A GFP-negative marked egg mutant GSC (broken circle) loses its Dad-lacZ expression (B′) in comparison with the neighboring GFP-positive unmarked control GSC (solid circle). (C) Percentages of marked egg mutant GSCs and egg bgcn double mutant GSCs change with time. (D) The marked lacZ-negative bgcn mutant GSC continuously generates spectrosome-containing single germ cells (arrowhead). (E) The marked LacZ-negative bgcn mutant single germ cells in the two egg chambers contain a spectrosome (arrowhead). (F) The marked lacZ-negative egg bgcn mutant GSC is lost from the niche evidenced by the presence of two lacZ-positive GSCs in the niche, but its marked mutant progeny remaining in the germarium contain a branched fusome (arrowhead). (G) The marked lacZ-negative egg bgcn mutant germ cells in the two egg chambers have a branched fusome (arrowhead). Scale bar: 10 µm. To further investigate if Egg controls GSC self-renewal by repressing a Bam-independent pathway, we generated lacZ-marked egg bgcn double mutant GSCs and examined their maintenance and differentiation. Mutations in either bam or bgcn can completely block GSC differentiation, and bam overexpression fails to induce GSC differentiation in the absence of bgcn function, indicating that bam and bgcn function in the same genetic pathway to control GSC differentiation [37]–[39]. Bgcn20915 is a strong or null mutation [40]. Interestingly, those egg bgcn double mutant GSCs are lost much faster than the marked control GSCs, but slower than the marked egg mutant GSCs, indicating that Egg maintains GSC self-renewal at least in part by repressing a Bam/Bgcn-independent pathway (Figure 3C). The partial rescue of the mutant egg GSC loss phenotype by the bgcn mutation further supports the idea that egg is required intrinsically for GSC self-renewal. In contrast with the knowledge that marked bgcn mutant GSCs continuously produce spectrosome-containing single germ cells (Figure 3D and 3E), the marked egg bgcn mutant GSC progeny can differentiate into cysts in the germarium based on the morphology of their branched fusome three weeks ACI (Figure 3F and 3G). These differentiated double mutant cells with a branched fusome can also be found to be packed together in egg chambers, indicating that these double mutant germ cells do not undergo proper terminal differentiation (Figure 3G). These results suggest that egg maintains GSC self-renewal at least partly by repressing a Bam/Bgcn-independent pathway.
Egg Does Not Intrinsically Regulate E-Cadherin Accumulation in the Stem Cell-Niche Junction
E-cadherin is required for anchoring GSCs in the niche for long-term self-renewal by accumulating in the stem cell-niche junction [33]. To investigate if Egg is required for regulating E-cadherin accumulation in GSCs, we examined E-cadherin accumulation in the stem cell-niche junction between a marked GSC and its neighboring control GSC. After carefully examining 10 such egg mutant and control GSC pairs, we did not observe any difference in E-cadherin accumulation in the stem cell-niche junction between them (Figure 4A). In addition, egg mutant and wild-type follicle cells in the egg chamber do not have any obvious difference in E-cadherin accumulation on their apical side (Figure 4B). To further rule out the possibility that E-cadherin is required for Egg-mediated GSC maintenance, we tested if nanos-gal4-driven germ cell-specific UASp-shg (shg encodes E-cadherin in Drosophila) expression could rescue or slow down the loss phenotype of the marked egg mutant GSCs. UASp-shg has been used previously to express E-cadherin in GSCs [40], [41]. Consistent with the idea that Egg does not regulate E-cadherin in GSCs, forced E-cadherin expression shows little rescue effect on the loss phenotype of the mutant egg GSCs (Figure 4C). Taken together, we conclude that Egg does not maintain GSCs via regulation of E-cadherin accumulation in the GSC-niche junction.
Fig. 4. egg is dispensable intrinsically for maintaining E-cadherin accumulation in the GSC-niche junction. 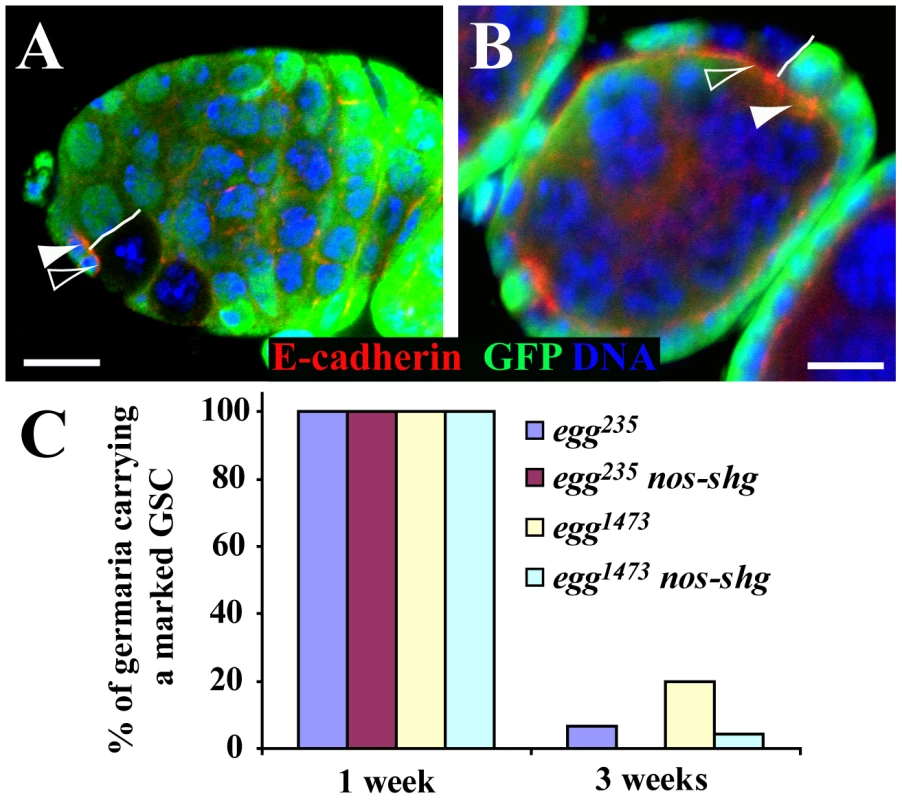
(A) A GFP-negative marked egg mutant GSC (filled arrowhead) has similar E-cadherin accumulation in the GSC-niche junction to its neighboring GFP-positive unmarked control GSC (open arrowhead). The solid line highlights the boundary between the two GSCs. (B) GFP-negative marked egg follicle cells (filled arrowhead) have similar apical E-cadherin accumulation to their neighboring GFP-positive unmarked control follicle cells (open arrowhead). The solid line highlights the boundary between the mutant and control follicle cells. (C) nos-gal4 driven germ cell-specific expression of E-cadherin fails to rescue the stem cell loss phenotype of marked mutant egg GSCs. Scale bars: 10 µm. Egg Is Required in Escort Cells (ECs) for Controlling the Differentiation of GSC Progeny
ECs have recently been shown to control germ cell differentiation by repressing Dally expression through EGFR signaling and thus preventing Dpp diffusion outside the GSC niche [42]–[45]. Thus, we then tested if egg function is required in ECs for controlling germ cell differentiation using EC-specific RNAi-mediated knockdown. C587-gal4 is specifically expressed in ECs and early somatic follicle progenitor cells [13]. To avoid the potential off-target effect of RNAi-mediated knockdown, three more independent RNAi constructs targeting different regions of the egg transcript, [eggRNAi-3(VDRC#101677), -4(VDRC#33730) and -5(VDRC#22172)], were used in this study in addition to eggRNAi-1 and -2. In contrast with the ovaries carrying c587-gal4 or UAS-RNAi alone, which contain a germarium followed by a string of egg chambers (Figure 5A), the ovaries carrying both c587-gal4 and one of the five UAS-RNAi constructs for egg often have their germaria containing a mixture of spectrosome-containing single germ cells and differentiated germ cell cysts, indicative of germ cell differentiation defects (Figure 5B and 5C; Figure S2). Although the germaria and egg chambers contain differentiated cysts evidenced by the presence of a branched fusome, most of the germ cells are spectrosome-containing single germ cells (Figure 5B–5D). The single germ cells in the germaria fail to differentiate further even after they are packed into individual egg chambers (Figure 5B and 5C; Figure S2). The budding defects observed in egg knockdown ovaries are likely caused by disruption of follicle progenitor cell proliferation and differentiation (Figure 5B and 5C; Figure S2). These results demonstrate that egg is required in ECs for controlling CB differentiation and in follicle progenitor cells for their proper differentiation or proliferation.
Fig. 5. egg is required in ECs to control germ cell differentiation. 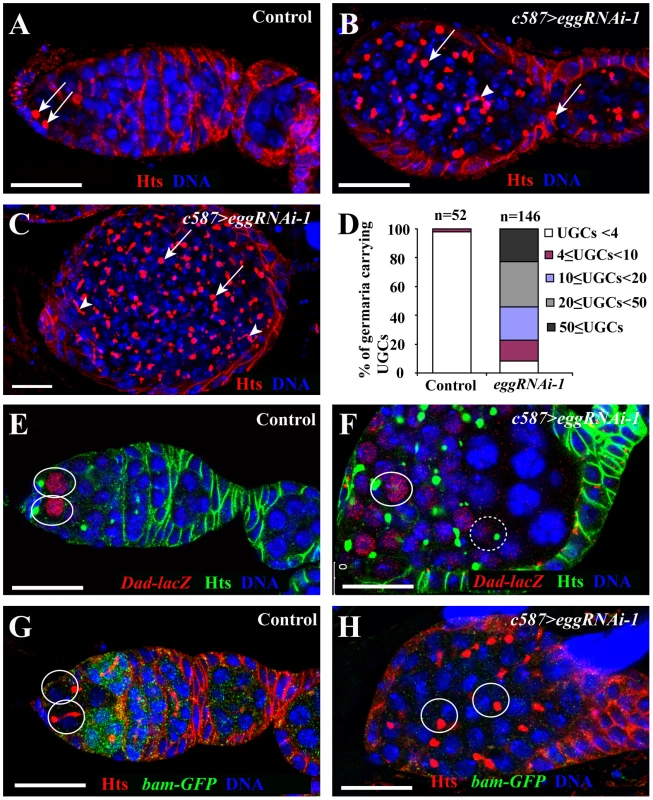
(A) A wild-type germarium showing two GSCs (arrows) tethered to cap cells and one CB containing round spectrosome. (B, C) C587-gal4 driven egg knockdown leads to accumulation of spectrosome-containing cells (arrows) and differentiated cysts containing a branched fusome (arrowhead). (D) Quantification of spectrosome-containing UGCs in week-old egg knockdown ovaries. (E) Dad-lacZ expression is restricted to GSCs (ovals) in a wild-type germarium. (F) In a c587-gal4-driven egg knockdown germarium, some spectrosome-containing UGCs (solid circle) express high Dad-lacZ, while the others (broken circles) express low Dad-lacZ. (G) bam-GFP expression is repressed in GSCs (circles) but upregulated in differentiating cysts in a wild-type germarium. (H) In a c587-gal4-driven egg knockdown germarium, bam-GFP expression is absent from spectrosome-containing UGCs (circles). Scale bars: 20 µm. To further determine if single germ cells accumulated in the germaria are GSC-like or CB-like, we examined the expression of bam-GFP and Dad-lacZ. As mentioned earlier, Dad-lacZ is expressed primarily in the GSCs of the control germaria carrying only c587-gal4 or UAS-RNAi (Figure 5E), and bam-GFP is normally expressed in differentiated germ cells but is repressed in GSCs (Figure 5G). In contrast, in the germaria in which egg function is knocked down in ECs, most of spectrosome-containing single germ cells further away from cap cells still retain Dad-lacZ expression and repress bam-GFP expression similar to endogenous GSCs, (Figure 5F and 5H). These results indicate that the accumulated single germ cells in the germaria behave like GSCs, and also further suggest that in the absence of egg function in ECs, CBs fail to differentiate likely due to upregulation of BMP signaling in germ cells.
Egg Controls BMP Signaling Activity in Differentiated Germ Cells via ECs
To investigate if increased BMP signaling activity is responsible for the germ cell differentiation defect caused by egg knockdown in ECs, we tested if removal of one copy of dpp gene could suppress the germ cell differentiation defect. Interestingly, a copy of dpphr4 or dpphr56 can partially suppress the GSC-like tumorous phenotype caused by egg knockdown in ECs, and consequently more normal-looking germaria can be observed in comparison with egg knockdown alone (Figure 6A–6E). These results indicate that increased BMP signaling is at least in part responsible for the germ cell differentiation defect caused by egg knockdown in ECs. Recently, Lsd1 has been suggested to repress dpp transcription in ECs, thus promoting germ cell differentiation [18]. One of the possibilities is that egg may be involved in repressing dpp transcription in ECs. To test the possibility, we used two independent RNAi strains against different regions of dpp to knock down dpp mRNA expression in the ECs in which eggRNAi is also expressed. dpp knockdown in ECs cannot rescue the germ cell differentiation defect caused by egg knockdown (Figure 6F–6H). In addition, our quantitative RT-PCR results also show that there is no increase in dpp mRNAs in the EC-specific egg knockdown ovaries (Figure S3A). These results indicate that egg is dispensable for dpp repression in ECs.
Fig. 6. egg knockdown in ECs causes EC gradual loss and increases BMP signaling in differentiated germ cells. 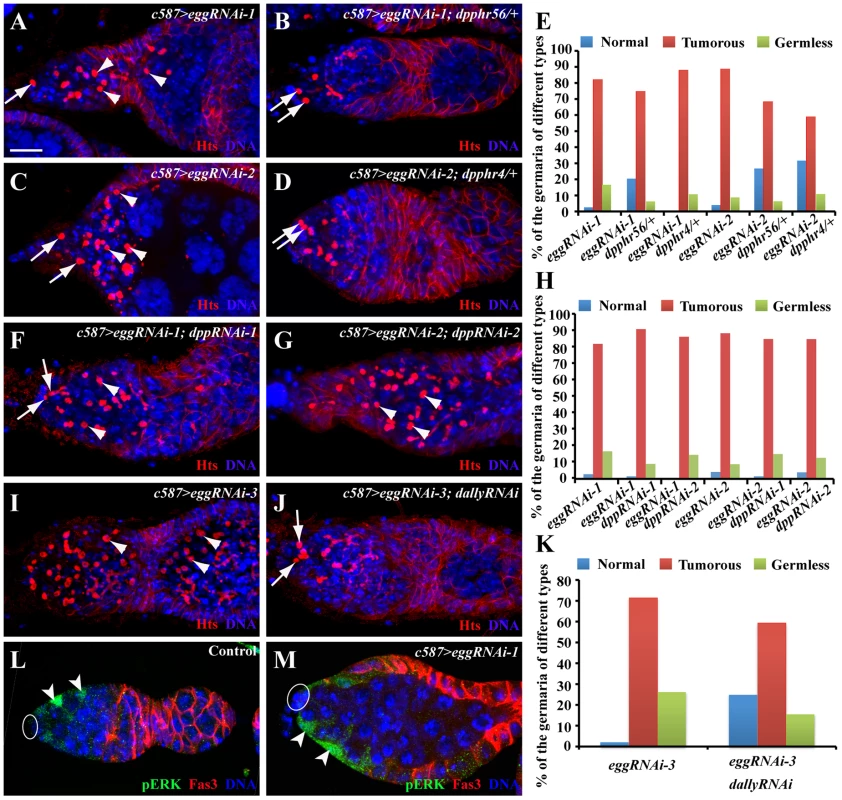
(A–E) RNAi-mediated knockdown of egg causes accumulation of single germ cells containing a spectrosome (arrowhead, A and C) in addition to endogenous GSCs (arrows, A and C), while removal of a copy dpp gene using dpphr4 or dpphr56 mutation can suppress the germ differentiation defect and thus increase normal germaria with two or three GSCs (arrows) and differentiated germ cells (B and D). The quantitative results are summarized in E. (F–H) RNAi-mediated dpp knockdown in ECs fails to suppress the germ cell differentiation defect caused by egg knockdown in ECs. Double knockdown germaria still accumulate excess spectrosome-containing single germ cells (arrowheads, F and G) in addition to endogenous GSCs (arrows, G). The quantitative results are summarized in H. (I–K) dally knockdown in ECs suppresses the germ cell differentiation defect evidenced by a decrease in spectrosome-containing single germ cells (arrowheads in I; endogenous GSCs indicated by arrows in J). K shows quantitative results. (L, M) egg knockdown in ECs (arrowheads, M) does not affect pERK expression in in comparison with control ECs (arrowheads, L). Ovals in L and M indicate cap cells. Scale bars: 10 µm. One recent study has clearly established that MAPK signaling functions downstream of EGFR in ECs to repress the expression of dally, whose gene product facilitates Dpp diffusion [42]. The egg mutant phenotype raises a possibility that egg is involved in the repression of dally expression in ECs. Interestingly, dally knockdown in ECs can partially suppress the germ cell differentiation defect caused by egg knockdown, indicating that dally upregulation in ECs contributes to the germ cell differentiation defect (Figure 6I–6K). To further test if egg is involved in regulation of EGFR signaling in ECs, we examined the expression of pERK, which has been used to monitor EGFR signal transduction in ECs [42], [45]. pERK is preferentially expressed in wild-type ECs as reported [45] (Figure 6L). In the egg knockdown ECs, pERK expression remains normal or close to normal (Figure 6M). These results suggest that egg functions either downstream of or in parallel with EGFR signaling to repress dally expression in escort cells.
Egg Functions in ECs to Control Their Survival
During the characterization of the EC-specific egg knockdown mutant phenotype, we noticed that most of the egg knockdown germaria have smaller regions 1 and 2a than in the control germaria, while others appear to completely lose ECs, suggesting that egg knockdown in ECs leads to gradual EC loss (Figure 7A–7C). In the absence of ECs, germ cells are also depleted from the germaria (Figure 7C; Figure S4), suggesting that ECs are also required for maintaining GSCs. To further investigate if egg knockdown affects EC maintenance, we used the lacZ enhancer trap line PZ1444 to quantify the number of ECs in wild-type and egg knockdown germaria. PZ1444 is known to label both cap cells and ECs in the germarium [12], [46]. In the control germaria, PZ1444 labels 20 to 35 ECs (Figure 7A and 7D). In the egg knockdown germaria, the number of ECs has already decreased at the age of 1–2 days (Figure 7D). At the age of 8 or 9 days, all PZ1444-positive ECs in 63% of the egg knockdown germaria are completely lost, and consequently no germ cells including GSCs exist in the germaria (Figure 7C and 7D). However, the egg chambers associated with those germaria are still full of spectrosome-containing single germ cells (Figure S4), indicating that germ cell differentiation is absolutely dependent on the presence of functional ECs. To further determine if EC loss is caused by apoptosis, we forced expression of p35, an apoptosis inhibitor, in the egg knockdown ECs. Indeed, p35 expression can prevent EC loss and formation of germless germaria, indicating that Egg is required for maintaining EC survival and thus GSCs (Figure 7E and 7F). Interestingly, the germ cell differentiation defect remains, indicating that Egg also functions in ECs to promote germ cell differentiation through modulating BMP or other signaling pathways. These results suggest that egg is required for maintaining EC survival and regulating EC function for promoting germ cell differentiation.
Fig. 7. Egg is required for EC survival. 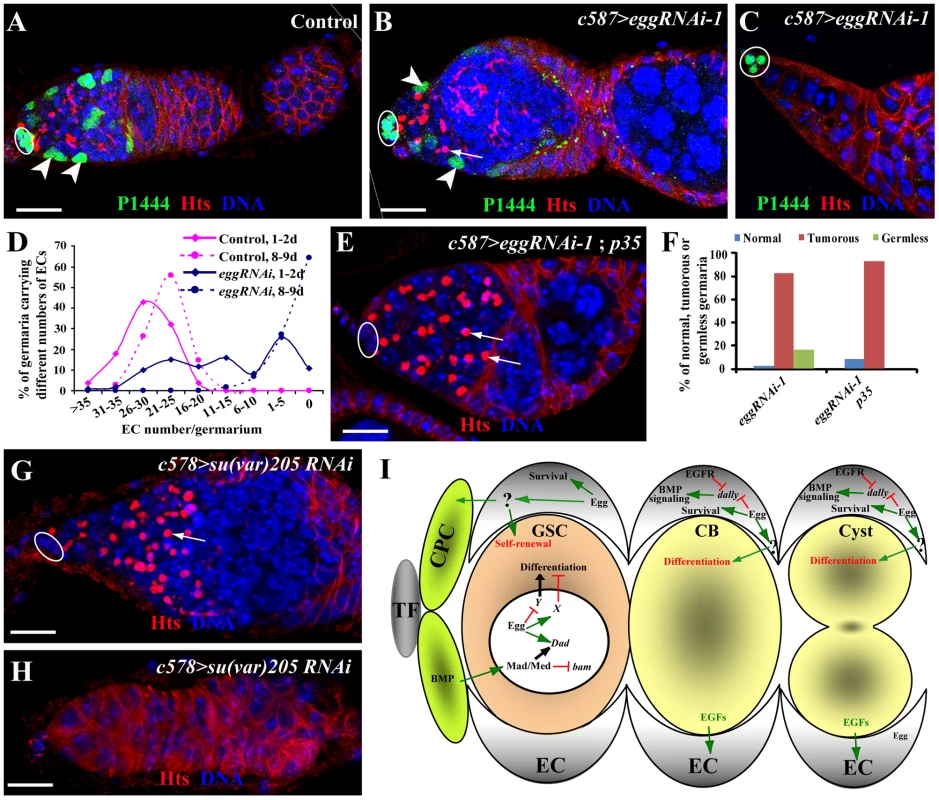
(A) A control PZ1444/+ germarium shows lacZ expression in all the ECs (two by arrowheads) in addition to cap cells (oval). (B–D) c587-gal4-driven egg knockdown in ECs results in reduced ECs (two by arrowheads, B) or the complete absence of ECs (C), which are located posterior to cap cells (oval, B and C). D shows quantitative changes in EC numbers of control and egg knockdown germaria with age. (E, F) Overexpression of p35 suppresses the EC loss caused by RNAi-mediated egg knockdown in ECs (arrows). Quantitative results (F) show that p35 overexpression in ECs suppresses the germless phenotype but not germ cell differentiation defects. (G, H) RNAi-mediated su(var)205 knockdown in ECs causes the accumulation of spectrosome-containing single germ cells (arrow, G) and the complete loss of germ cells (H). (I) A working model for explaining functions of Egg in GSCs and ECs for controlling GSC maintenance and differentiation. In the GSC, Egg may repress the expression of a gene that is important for GSC differentiation or activate expression of a gene that is important for repressing GSC differentiation. In addition, it also directly or indirectly regulates Dad expression along with BMP signaling. In the EC, Egg is required for controlling the survival of the EC, which is important for proper germ cell differentiation and GSC maintenance. Egg may control expression of dally and other BMP regulators in the EC to prevent BMP signaling from spreading to differentiated germ cells. HP1 Is Required in ECs to Regulate Germ Cell Differentiation and Control EC Survival
To further determine if any other chromatin regulators are also required in ECs to regulate germ cell differentiation, we sought to use the same RNAi approach to knock down expression of sin3A, su(z)12 and su(var)205 genes, which encode general factors regulating heterochromatin formation or repressing gene transcription [29], [47], [48]. Interestingly, knockdown of sin3A and su(z)12 in ECs fails to yield any discernible phenotype on germ cell differentiation. In contrast, knockdown of su(var)205 in ECs leads to the germ cell differentiation defect and the EC loss phenotype, which is identical to those in egg knockdown (Figure 7G and 7H). This is consistent with the biochemical function of Su(var)205, a HP1 protein binding to H3K9me3 [19], [49], [50], [51]. These results suggest that HP1 and Egg, but not other general transcriptional repressors, function specifically in ECs to control EC survival and regulate germ cell differentiation.
Discussion
Although the mouse H3K9 trimethylase SETDB1 was recently shown to be important for maintaining ESC self-renewal by repressing the expression of developmentally regulated genes [8], its role in regulation of adult stem cells has not yet been established. In this study, we show that the Drosophila SETDB1 homolog, Egg, is required intrinsically for controlling GSC self-renewal and extrinsically for controlling GSC differentiation in the Drosophila ovary. The egg mutant ovaries exhibit both GSC loss and germ cell differentiation defects. We further demonstrate that Egg controls GSC self-renewal by repressing a Bam/Bgcn-independent pathway. In addition, EC-specific RNAi-mediated knockdown of egg function leads to gradual EC loss and germ cell differentiation defects, indicating that Egg is required for EC maintenance and germ cell differentiation. Recently, we have proposed that ECs function as a niche for promoting germ cell differentiation [52]. Furthermore, Egg functions in ECs to control germ cell differentiation at least in part by preventing BMP signaling from spreading to the differentiation niche and regulating EC survival. Therefore, we propose that Egg is a key H3K9 trimethylase in the Drosophila ovary, which is required intrinsically for controlling GSC self-renewal via repressing a Bam/Bgcn-independent differentiation pathway and in ECs for controlling germ cell differentiation by preventing BMP signaling spreading to the differentiation niche (Figure 7I). The findings from this study have further supported the idea that ECs function as a germ cell differentiation niche. It will be of great interest to test if SETDB1 is also important for controlling adult stem cell self-renewal and differentiation in mammalian systems.
Egg Is Required Intrinsically for Controlling GSC Self-Renewal by Repressing a Bam/Bgcn-Independent Differentiation Pathway
In the previous study [28], Egg was shown to be a primary H3K9 trimethylase in follicle progenitor cells for maintaining H3K9me3 and regulating their proliferation and survival [28]. Egg and its co-factor Wde were also shown to be required for maintaining H3K9me3 in early germ cells and regulating their survival [29]. This study has further demonstrated that Egg is required intrinsically for controlling GSC self-renewal and proliferation. Consistent with the previous finding [28], we have shown that H3K9me3 but not H3K9me2 is eliminated in marked egg mutant GSCs. In addition, marked egg mutant GSCs are lost rapidly from the niche in comparison with the marked control GSCs, further supporting the idea that Egg is required for GSC maintenance. Moreover, the marked egg mutant GSCs and mitotic cysts are negative for TUNEL-based ApopTag labeling, but the marked 16-cell cysts in the regions 2b and 3 of the germarium are observed to be positive, indicating that Egg is dispensable for the survival of GSCs and early mitotic cysts but is required for the survival of 16-cell cysts. Finally, marked egg mutant GSCs appear to proliferate slower than the control GSCs based on cyst production and BrdU labeling. We used RNAi-mediated knockdown to show that loss of Egg function from GSCs and their progeny leads to the accumulation of DNA damage, suggesting that Egg is required for maintaining genome integrity. The accumulated DNA damage could also explain retarded GSC proliferation and increased 16-cell cyst apoptosis. These results demonstrate that Egg is required intrinsically for GSC self-renewal and proliferation and for the survival of 16-cell cysts.
BMP signaling and E-cadherin-mediated cell adhesion are essential for maintaining GSCs in the Drosophila ovary [11], [13], [14], [33]. BMP signaling represses bam-GFP expression and activates Dad-lacZ expression in GSCs [13], [14], [35], [36]. H3K9me3 is thought to be a histone marker for heterochromatin formation and transcriptional repression [1]. Surprisingly, in marked egg mutant GSCs, bam-GFP remains repressed as in wild-type GSCs, but Dad-lacZ expression fails to be activated, indicating that Egg, and presumably H3K9me3, is dispensable for BMP signaling-mediated transcriptional repression of bam. The requirement of Egg for transcriptional activation of Dad could be indirect, but the detailed mechanism awaits further investigation. We have further demonstrated functionally that Egg controls GSC self-renewal by repressing a Bam/Bgcn-independent pathway by showing that marked bgcn egg double mutant GSCs are still lost at a much faster rate than marked control GSCs. Previously, Pumilio and Pelota were proposed to control GSC self-renewal by repressing a Bam/Bgcn-independent differentiation pathway as mutations for either factor can drive differentiation of bam mutant germ cells [53]–[55]. Interestingly, mutations in egg can also cause differentiation of bgcn mutant germ cells, further supporting the idea that Egg represses a Bam/Bgcn-independent differentiation pathway to maintain GSC self-renewal. There are two possible strategies for Egg to repress differentiation and thus maintain GSC self-renewal: Egg represses the expression of a gene(s) important for GSC differentiation or activates the expression of a gene(s) critical for repressing GSC differentiation (Figure 7I). Unfortunately, it remains unclear how Egg represses GSC differentiation to maintain self-renewal. Therefore, the identification of Egg target genes in GSCs will help define the unknown GSC differentiation pathway along with the identification of target genes of Pumilio and Pelota in order to gain a deeper understanding of GSC self-renewing mechanisms.
During the revision of this manuscript, a study was published to propose that Egg is required for H3K9me3 and heterochromatin formation in CBs and differentiated cysts, and is required for expression of piRNA genes and thus repression of transposable elements (TEs) [56]. Loss of piRNAs in germ cells is known to cause the activation of transposable elements (TEs) and consequently an increase in DNA damage [57]. Consistently, our study shows that loss of egg function in germ cells leads to the accumulation of DNA damage. The regulation of piRNA by Egg offers mechanistic insight into why Egg is required for GSC maintenance and proliferation [56]. However, our study has two different findings. One is that H3K9me3 establishment begins from GSCs, but not from CBs as the published study proposed [56]. The other is that Egg is also required intrinsically for GSC maintenance and proliferation, but not for CB differentiation. The published study showed that spectrosome-containing single germ cells accumulate following germline-specific egg knockdown [56]. In our study, germline-specific expression of eggRNAi-1 leads to GSC loss, which is consistent with our mutant clonal analysis results, whereas the expression of eggRNAi-2 results in swollen germaria containing a few more spectrosome-containing CBs and cysts than control. The accumulation of the few more single germ cells is likely due to DNA damage-caused slowdown of mitotic progression. The difference between the published study and our study could be simply caused by different egg knockdown efficiencies.
Egg Controls Germ Cell Differentiation by Regulating EC Survival and BMP Signaling
egg homozygous ovaries accumulate more undifferentiated germ cells and gradually lose their GSCs, which appear to be paradoxical. The egg mutant GSC loss phenotype can be attributed to the intrinsic requirement for GSC self-renewal. Our further genetic analysis has revealed the requirement of Egg in ECs for controlling GSC differentiation by EC-specific RNAi-mediated egg knockdown. In the absence of Egg function from ECs, GSC progeny fail to differentiate and continuously proliferate as single germ cells, indicative of differentiation defects. In addition, loss of Egg function in ECs also causes EC loss, and in the complete absence of ECs, the progeny that have been generated before GSC loss also accumulate as single germ cells, further supporting that ECs are required for CB differentiation. Some of the accumulated single germ cells appear to upregulate Dad-lacZ expression and repress bam-GFP expression, suggesting that BMP signaling spreads to the germ cell differentiation niche, thereby interfering with germ cell differentiation. These findings suggest that Egg is required in ECs to promote germ cell differentiation at least in part by preventing self-renewal-promoting BMP signaling from spreading to the germ cell differentiation niche.
EFGR signaling has been suggested to act in ECs to control germ cell differentiation by repressing expression of Dally, a protein important for facilitating BMP diffusion [42]. Interestingly, in the egg knockdown ECs, the expression of pERK, an EGFR signaling indicator, still remains normal, indicating that Egg is not essential for EGFR signaling in ECs. However, dally knockdown in ECs can partially suppress the egg knockdown mutant germ cell tumor phenotype, indicating that upregulation of dally in egg knockdown ECs contributes to BMP upregulation in the differentiation niche and to germ cell differentiation defects. The regulation of dally in ECs by Egg could be direct or indirect. The newly published study on Egg has shown that loss of Egg function in ECs leads to defective piRNA production and germ cell differentiation defects [56]. Consistently, we also confirmed that egg knockdown in ECs results in dramatically increased expression of TEs (Figure S3B and S3C). The germ cell differentiation defect can be rescued by a mutation in one of the DNA damage checkpoint genes, suggesting that DNA damage in ECs affects their ability to regulate germ cell differentiation [56]. It will be of great interest to investigate if the mutation in the checkpoint gene also rescues defective BMP signaling in differentiated cells. Based on our findings from this study, we propose that Egg functions downstream of or in parallel with EGFR signaling to repress dally expression in ECs, thereby preventing BMP signaling from spreading to the differentiation niche (Figure 7I). Because the signal(s) from ECs to control germ cell differentiation has not been identified yet, it remains unclear whether Egg also regulates additional factors independent of BMP signaling in ECs to control germ cell differentiation.
GSC-Contacting ECs Function as an Integral Part of the GSC Niche
In this study, we have also shown that the egg knockdown ECs are gradually lost, and that GSCs cannot be maintained in the complete absence of ECs. This is consistent with our recently published finding that disruption of Rho function in ECs also cause EC loss and thus GSC loss [58]. Because 5 to 6 most anteriorly localized ECs directly contact cap cells and GSCs, we propose that these ECs function as a part of the GSC niche to promote self-renewal by directly providing signals or indirectly by regulating cap cells function (Figure 7I). One previous study suggests that JAK-STAT signaling functions in ECs to control GSC maintenance indirectly [59]. How these GSC-contacting ECs contribute to GSC regulation remains to be further investigated.
Materials and Methods
Drosophila Stocks
Flies were maintained at 25°C on molasses-based media supplied with live yeast unless otherwise specified. The strains used in this study include: egg235, egg2138 and egg1473 [28](kindly provided by T. Hazelrigg); Dad-lacZ [60], bam-GFP [61], PZ1444 [46], nos-gal4 [62], c587-gal4 [63], dpphr56 [11], dpphr4 [11], UAS-p35 [64] and w1118 (control).
RNAi-Mediated egg Knockdown in ECs
For egg knockdown in ECs, the c587-gal4; UAS-dcr2 line was used to drive the expression of five independent eggless RNAi constructs, HMS00443 and HMS00112 from Harvard Medical School (kindly provided by Dr. N. Perrimon), and three other lines (#21172, #33730 and #101677) from the Vienna Drosophila RNAi Center (VDRC). UAS-RNAi lines for dpp (dppRNAi-1: JF01090; dppRNAi-2: JF01371)and dally [52] were kindly provided by Dr. N. Perrimon and Dr. X. Lin (Cincinnati Children's Hospital Medical Center), respectively. After eclosion, their progeny were collected and reared at 29°C for several days as described in the text for each experiment.
Generation of Marked egg Mutant GSC Clones
The marked control and egg mutant GSC clones were generated using the FLP-mediated FRT recombination technique as described previously [11], [33]. H3K9me3, H3K9me2 and TUNEL staining were performed 7 or 12 days after clone induction (ACI). The genotypes used for clonal analysis were: (1) hs-flp/+; FRT42D/FRT42D ubiGFP; (2) hs-flp/+; FRT42D egg235/FRT42D ubiGFP; (3) hs-flp/+; FRT42D egg1473/FRT42D ubiGFP, (4) hs-flp/+; FRT42D egg2138/FRT42D ubiGFP, (5) hs-flp/+;FRT42D/FRT42D arm-lacZ; (6) hs-flp/+; FRT42D egg235/FRT42D arm-lacZ;(7) hs-flp/+; FRT42D egg1473/FRT42D arm-lacZ and (8) hs-flp/+; FRT42D egg2138/FRT42D arm-lacZ. For generating bgcn egg double mutant clones, the following genotypes were used: (1) hs-flp/+; FRT42D bgcn20915 egg235/FRT42D arm-lacZ; (2) hs-flp/+; FRT42D bgcn20915 egg1473/FRT42D arm-lacZ.
Immunohistochemistry and Fluorescent Microscopy
Ovaries were dissected, fixed and stained according to the method described previously [33]. The following primary antibodies were used: monoclonal mouse anti-Hts (1B1, 1∶4, DSHB), mouse anti-Lamin C (LC28.26, 1∶4, DSHB), mouse anti-Orb (4H8, 1∶4, DSHB), mouse anti-Fas3 (7G10, 1∶3, DSHB), rat anti-Vasa (1∶10, DSHB), rat anti-E-cadherin DCAD2 (1∶3, DSHB), polyclonal rabbit anti-GFP (1∶100, Molecular Probes), chicken anti-GFP antibody (1∶200, Invitrogen), rabbit anti-β-galactosidase (1∶100, Molecular Probes), rabbit anti-H3K9me3 (1∶200, Abcam ab8898) and rabbit anti-phosphorylated ERK1/2 (1∶200, a gift from Dr. Y. Cai). Secondary antibodies used were: goat anti-rabbit, goat anti-rat and goat anti-mouse antibodies conjugated to Alexa 488, Alexa 568 or Cy5 (1∶100, Molecular Probes) and Dylight 488 donkey anti-chicken antibody (1∶200, Jackson ImmunoResearch Laboratories). TUNEL staining was performed using the ApopTag Red In Situ Apoptosis Detection Kit (Chemicon, S7165) according to the manufacturer's protocol. All micrographs were taken using an inverted Leica TCS SP5 confocal microscope. For the experiments involving comparison between mutants and wild type, the same parameters were used for confocal imaging.
Supporting Information
Zdroje
1. LessardJACrabtreeGR 2010 Chromatin regulatory mechanisms in pluripotency. Annu Rev Cell Dev Biol 26 503 532
2. AdamoASeseBBoueSCastanoJParamonovI 2011 LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 13 652 660
3. LohYHZhangWChenXGeorgeJNgHH 2007 Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev 21 2545 2557
4. FazzioTGHuffJTPanningB 2008 An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell 134 162 174
5. PasiniDHansenKHChristensenJAggerKCloosPA 2008 Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev 22 1345 1355
6. ShenXKimWFujiwaraYSimonMDLiuY 2009 Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell 139 1303 1314
7. BuszczakMSpradlingAC 2006 Searching chromatin for stem cell identity. Cell 125 233 236
8. BilodeauSKageyMHFramptonGMRahlPBYoungRA 2009 SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev 23 2484 2489
9. KirillyDXieT 2007 The Drosophila ovary: an active stem cell community. Cell Res 17 15 25
10. XieTSpradlingA 2001 The Drosophila Ovary: An In Vivo Stem Cell System. MarshakDRGardnerRLGottliebD Stem Cell Biology Cold Spring Harbor, , N.Y. Cold Spring Harbor Laboratory Press 129 148
11. XieTSpradlingAC 1998 decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94 251 260
12. XieTSpradlingAC 2000 A niche maintaining germ line stem cells in the Drosophila ovary. Science 290 328 330
13. SongXWongMDKawaseEXiRDingBC 2004 Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131 1353 1364
14. ChenDMcKearinD 2003 Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol 13 1786 1791
15. XiRXieT 2005 Stem cell self-renewal controlled by chromatin remodeling factors. Science 310 1487 1489
16. MainesJZParkJKWilliamsMMcKearinDM 2007 Stonewalling Drosophila stem cell differentiation by epigenetic controls. Development 134 1471 1479
17. Di StefanoLJiJYMoonNSHerrADysonN 2007 Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr Biol 17 808 812
18. EliazerSShalabyNABuszczakM 2011 Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc Natl Acad Sci U S A 108 7064 7069
19. BannisterAJZegermanPPartridgeJFMiskaEAThomasJO 2001 Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410 120 124
20. EbertASchottaGLeinSKubicekSKraussV 2004 Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev 18 2973 2983
21. SchottaGEbertAKraussVFischerAHoffmannJ 2002 Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. Embo J 21 1121 1131
22. MisJNerSSGrigliattiTA 2006 Identification of three histone methyltransferases in Drosophila: dG9a is a suppressor of PEV and is required for gene silencing. Mol Genet Genomics 275 513 526
23. StabellMEskelandRBjorkmoMLarssonJAalenRB 2006 The Drosophila G9a gene encodes a multi-catalytic histone methyltransferase required for normal development. Nucleic Acids Res 34 4609 4621
24. Brower-TolandBRiddleNCJiangHHuisingaKLElginSC 2009 Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of Drosophila melanogaster. Genetics 181 1303 1319
25. SeumCReoEPengHRauscherFJ3rdSpiererP 2007 Drosophila SETDB1 is required for chromosome 4 silencing. PLoS Genet 3 e76 doi:10.1371/journal.pgen.0030076
26. TzengTYLeeCHChanLWShenCK 2007 Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc Natl Acad Sci U S A 104 12691 12696
27. YoonJLeeKSParkJSYuKPaikSG 2008 dSETDB1 and SU(VAR)3-9 sequentially function during germline-stem cell differentiation in Drosophila melanogaster. PLoS ONE 3 e2234 doi:10.1371/journal.pone.0002234
28. CloughEMoonWWangSSmithKHazelriggT 2007 Histone methylation is required for oogenesis in Drosophila. Development 134 157 165
29. KochCMHonemann-CapitoMEgger-AdamDWodarzA 2009 Windei, the Drosophila homolog of mAM/MCAF1, is an essential cofactor of the H3K9 methyl transferase dSETDB1/Eggless in germ line development. PLoS Genet 5 e1000644 doi:10.1371/journal.pgen.1000644
30. LaskoPFAshburnerM 1988 The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335 611 617
31. HayBJanLYJanYN 1988 A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 55 577 587
32. LinHYueLSpradlingAC 1994 The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120 947 956
33. SongXZhuCHDoanCXieT 2002 Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296 1855 1857
34. NiJQZhouRCzechBLiuLPHolderbaumL 2011 A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8 405 407
35. CasanuevaMOFergusonEL 2004 Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development 131 1881 1890
36. KaiTSpradlingA 2003 An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A 100 4633 4638
37. McKearinDMSpradlingAC 1990 bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev 4 2242 2251
38. OhlsteinBMcKearinD 1997 Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 124 3651 3662
39. OhlsteinBLavoieCAVefOGateffEMcKearinDM 2000 The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics 155 1809 1819
40. JinZKirillyDWengCKawaseESongX 2008 Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 2 39 49
41. ChenSKanekoSMaXChenXIpYT 2010 Lissencephaly-1 controls germline stem cell self-renewal through modulating bone morphogenetic protein signaling and niche adhesion. Proc Natl Acad Sci U S A 107 19939 19944
42. LiuMLimTMCaiY 2010 The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal 3 ra57
43. GuoZWangZ 2009 The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136 3627 3635
44. AkiyamaTKamimuraKFirkusCTakeoSShimmiO 2008 Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol 313 408 419
45. SchultzCWoodCGJonesDLTazukeSIFullerMT 2002 Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129 4523 4534
46. MargolisJSpradlingA 1995 Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121 3797 3807
47. PennettaGPauliD 1998 The Drosophila Sin3 gene encodes a widely distributed transcription factor essential for embryonic viability. Dev Genes Evol 208 531 536
48. MullerJHartCMFrancisNJVargasMLSenguptaA 2002 Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111 197 208
49. EissenbergJCMorrisGDReuterGHartnettT 1992 The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131 345 352
50. EissenbergJCJamesTCFoster-HartnettDMHartnettTNganV 1990 Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci U S A 87 9923 9927
51. LachnerMO'CarrollDReaSMechtlerKJenuweinT 2001 Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 116 120
52. KirillyDWangSXieT 2011 Self-maintained escort cells form a germline stem cell differentiation niche. Development 138 5087 5097
53. ChenDMcKearinD 2005 Gene circuitry controlling a stem cell niche. Curr Biol 15 179 184
54. SzakmaryACoxDNWangZLinH 2005 Regulatory Relationship among piwi, pumilio, and bag-of-marbles in Drosophila Germline Stem Cell Self-Renewal and Differentiation. Curr Biol 15 171 178
55. XiRDoanCLiuDXieT 2005 Pelota controls self-renewal of germline stem cells by repressing a Bam-independent differentiation pathway. Development 132 5365 5374
56. RanganPMaloneCDNavarroCNewboldSPHayesPS 2011 piRNA Production Requires Heterochromatin Formation in Drosophila. Curr Biol 21 1373 1379
57. KlattenhoffCBratuDPMcGinnis-SchultzNKoppetschBSCookHA 2007 Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell 12 45 55
58. KirillyDWangSXieT 2011 Self-Maintained Escort Cells Form a Germline Stem Cell Differentiation Niche. Development (In press)
59. DecottoESpradlingAC 2005 The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell 9 501 510
60. TsuneizumiKNakayamaTKamoshidaYKornbergTBChristianJL 1997 Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389 627 631
61. ChenDMcKearinDM 2003 A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130 1159 1170
62. Van DorenMWilliamsonALLehmannR 1998 Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol 8 243 246
63. ManseauLBaradaranABrowerDBudhuAElefantF 1997 GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn 209 310 322
64. HayBAWolffTRubinGM 1994 Expression of baculovirus P35 prevents cell death in Drosophila. Development 120 2121 2129
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



