-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInterspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
The grass smuts comprise a speciose group of biotrophic plant parasites, so-called Ustilaginaceae, which are specifically adapted to hosts of sweet grasses, the Poaceae family. Mating takes a central role in their life cycle, as it initiates parasitism by a morphological and physiological transition from saprobic yeast cells to pathogenic filaments. As in other fungi, sexual identity is determined by specific genomic regions encoding allelic variants of a pheromone-receptor (PR) system and heterodimerising transcription factors. Both operate in a biphasic mating process that starts with PR–triggered recognition, directed growth of conjugation hyphae, and plasmogamy of compatible mating partners. So far, studies on the PR system of grass smuts revealed diverse interspecific compatibility and mating type determination. However, many questions concerning the specificity and evolutionary origin of the PR system remain unanswered. Combining comparative genetics and biological approaches, we report on the specificity of the PR system and its genetic diversity in 10 species spanning about 100 million years of mating type evolution. We show that three highly syntenic PR alleles are prevalent among members of the Ustilaginaceae, favouring a triallelic determination as the plesiomorphic characteristic of this group. Furthermore, the analysis of PR loci revealed increased genetic diversity of single PR locus genes compared to genes of flanking regions. Performing interspecies sex tests, we detected a high potential for hybridisation that is directly linked to pheromone signalling as known from intraspecies sex. Although the PR system seems to be optimised for intraspecific compatibility, the observed functional plasticity of the PR system increases the potential for interspecific sex, which might allow the hybrid-based genesis of newly combined host specificities.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002436
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002436Summary
The grass smuts comprise a speciose group of biotrophic plant parasites, so-called Ustilaginaceae, which are specifically adapted to hosts of sweet grasses, the Poaceae family. Mating takes a central role in their life cycle, as it initiates parasitism by a morphological and physiological transition from saprobic yeast cells to pathogenic filaments. As in other fungi, sexual identity is determined by specific genomic regions encoding allelic variants of a pheromone-receptor (PR) system and heterodimerising transcription factors. Both operate in a biphasic mating process that starts with PR–triggered recognition, directed growth of conjugation hyphae, and plasmogamy of compatible mating partners. So far, studies on the PR system of grass smuts revealed diverse interspecific compatibility and mating type determination. However, many questions concerning the specificity and evolutionary origin of the PR system remain unanswered. Combining comparative genetics and biological approaches, we report on the specificity of the PR system and its genetic diversity in 10 species spanning about 100 million years of mating type evolution. We show that three highly syntenic PR alleles are prevalent among members of the Ustilaginaceae, favouring a triallelic determination as the plesiomorphic characteristic of this group. Furthermore, the analysis of PR loci revealed increased genetic diversity of single PR locus genes compared to genes of flanking regions. Performing interspecies sex tests, we detected a high potential for hybridisation that is directly linked to pheromone signalling as known from intraspecies sex. Although the PR system seems to be optimised for intraspecific compatibility, the observed functional plasticity of the PR system increases the potential for interspecific sex, which might allow the hybrid-based genesis of newly combined host specificities.
Introduction
Sexual reproduction affords important benefits owing to an accelerated adaptive evolution and the efficient elimination of deleterious mutations [1], [2]. As a result of the evolutionary struggle for life sexual reproduction became prevalent in most organisms [3]–[5]. However, sexually reproducing organisms have to ensure the maintenance of individual sexual identities and the prevention of selfing and hybridisation, all linked to increased costs. The functional and genetic aspects of these trade-offs have been broadly studied in many organismic groups such as mammals, plants and fungi [6]–[10].
Fungi are excellent model systems to study sex determination, mate recognition and mating type evolution [5], [11], [12]. The fruiting bodies of agaricomycetes are the most prominent sexual structures in fungi giving rise to comprehensive studies on sex in this subgroup of basidiomycetes [13]–[18]. Strikingly, most basidiomycetes are stringently heterothallic and sexual identity is determined by two specific mating type gene clusters that encode a pheromone-receptor (PR) system and heterodimerising homeodomain (HD) transcription factors. Their components are functionally conserved even across phyla [19]–[21] and transspecific polymorphism of mating type alleles has been preserved since the last common ancestor of basidiomycetes and ascomycetes [22], [23].
Depending on the chromosomal independence or linkage of both mating loci, meiosis segregates either four or two different mating types referred to as tetrapolarity and bipolarity, respectively [12], [14]. In the tetrapolar agaricomycetes Coprinopsis cinereus and Schizophyllum commune each allele of the multiallelic PR locus contains several receptors and pheromones giving rise to thousands of sexes [24]. By contrast, PR loci of bipolar species are biallelic, either due to suppressed recombination within the large mating type region [25] or due to the loss of their mating type-specific pheromone receptor function [26]. Interestingly, there are intermediate states of less strict bipolarity and partially preserved recombination as shown in Sporidiobolus salmonicolor, a member of Puccinomycotina [27]. However, mating type loci of different phylogenetic groups underwent individual genetic transitions. A clear basidiomycete-wide survey regarding the diversity of those regions and their origin is still missing.
Basidiomycete pheromones and receptors are both allelic variants of a single gene each [15]. Pheromone genes encode precursors of lipopeptide pheromones that are proteolytically processed as well as S-farnesylated and -carboxymethylated at their C-terminal CAAX-motif [15], [28]. After secretion pheromones are recognised by their cognate G protein-coupled receptors (GPCRs), which represent the largest family of transmembrane receptors in eukaryotes. GPCRs are believed to have a conserved tertiary structure and serve as potential targets for antifungal drug development [29]. Pheromone-activated receptors trigger an intracellular signal transduction network that involves a specific signal transduction cascade, the mitogen-activated protein kinase (MAPK) module [30].
The functionality of the PR system relies on the simple principle of only allowing the combination of proteins from different mates to initiate sexual development [11]. This restriction makes demands on the specificity of both receptors and pheromones in a co-evolutionary manner. Single amino acid changes in pheromone receptors altered their specificity and enabled the sensing of different non-self pheromones [31]–[33]. Furthermore, studies applying synthetic pheromone derivatives of both Ustilago maydis and U. hordei revealed a qualitative and quantitative correlation between pheromones and pheromone-dependent mating responses [34], [35]. This functional plasticity of the PR system corresponds to observations of interspecific sexual compatibility in Ustilaginaceae encompassing merely fusing sporidia up to completely fertile F1 hybrids with mixed host preferences (summarised in [36]).
Among basidiomycetes the plant biotrophic grass smuts are of special interest since in their life cycle mating is directly linked to parasitism. They belong to a speciose monophyletic group of plant biotrophic parasites that are specifically adapted to hosts of the sweet grasses, the Poaceae [37], [38]. Research on its model species U. maydis, U. hordei and Sporisorium reilianum revealed first insights into their complex and diverse mating biology [39]–[42]. U. maydis is a particularly good example with respect to mating genetics, physiology and pheromone signalling [43]–[45]. Its parasitic phase is initiated by a morphological and physiological transition from haploid saprobic yeast cells to dikaryotic infectious filaments. To this end compatible mating partners have to find each other and fuse. During this process pheromone signalling triggers the formation of conjugation hyphae, their directed growth towards the source of compatible pheromone and their final fusion [40]. On the molecular level pheromone perception triggers the phosphorylation of the HMG box transcription factor Prf1 (pheromone response factor 1) via a MAPK cascade. Subsequently, Prf1 specifically activates a set of pheromone-responsive genes including the mating type genes by binding to pheromone response elements (PRE) [46], [47].
Upon plasmogamy, pathogenic development and the maintenance of the dikaryon are mediated by the heterodimerising transcription factors bW and bE that originate from the HD mating type loci of both mating partners [12], [48]. Thus, the sexual life cycle can only proceed if mating partners are heteroallelic in both mating loci. This dependence on mating imposes strong selection pressure towards a fully compatible mating system and obviously favoured HD allele radiation to at least 19 functionally different HD alleles in U. maydis and five in S. reilianum (J. Kämper, personal communication; [42], [49]).
Unlike multiallelic HD loci, the PR loci of grass smuts were long thought to be biallelic, e.g. in U. maydis and U. hordei with each PR allele a1 and a2 encoding one receptor and one pheromone flanked by two species-specific genes, lba and rba [21],[39]. The a2 allele encodes two additional pheromone-induced genes, lga2 and rga2, that are involved in the uniparental inheritance of mitochondria in U. maydis [50]. Interestingly, further studies on the PR system of additional grass smut species revealed a large diversity showing three different molecular organisations in the corresponding genomic region. In particular, U. maydis is tetrapolar using two PR alleles [39], U. hordei is bipolar using two PR alleles [41] and S. reilianum is tetrapolar using three PR alleles [42]. Furthermore, the a2 locus of U. maydis contains a pheromone-encoding pseudogene, encouraging speculations about a more complex ancestral mating type system [39]. These observations raised questions about their ancient genetic structure and the subsequent evolutionary transitions of the mating type system in smut fungi and furthermore, challenged the idea of a species-specific PR system. In order to re-evaluate current findings and to round up our perspective on fungal mating in a broader genetic and evolutionary context, we focused on the specificity of the PR system and its genetic diversity in non-model species. In this evolutionary approach of 10 different species spanning about 100 million years of Ustilaginaceae evolution, we sequenced 11 novel PR loci including complete gene sequences of 10 fungal pheromone receptors and 21 lipopeptide pheromones. Combining sequence comparisons and interspecies mating assays, we assessed the probability of hybridisation in Ustilaginales and its potential role in evolution.
Results
Phylogenetic backbone of Ustilaginales
To understand genetic transitions of mating type loci in a broader evolutionary context, we investigated a representative world-wide set of 25 Ustilaginales species (Table S1, shaded in grey). 18 of these species either collected on field trips (6 specimens) or originating from herbarium material (12 specimens) were cultured for further investigation (Tables S1, S2). From 22 species (Table S1) we amplified the well-established marker genes ef1-α, rpb1, lsu rDNA, ssu rDNA and ITS containing 5.8S rDNA encoding elongation factor 1-alpha, RNA polymerase II subunit 1, large subunit rDNA, small subunit rDNA and internal transcribed spacer containing 5.8S rDNA, respectively. Together with the reference sequences of Cintractia limitata, Malassezia globosa, Mal. pachydermatis, Schizonella melanogramma, Sporisorium reilianum, Ustanciosporium standleyanum and Ustilago maydis (Table S1) we calculated a robust multi-gene phylogeny that represents all major groups of Ustilaginales. The phylogeny was rooted with the non-grass smuts Mal. globosa, Mal. pachydermatis, Melanotaenium euphorbiae and Urocystis eranthidis (Figure 1). Bayesian Markov chain Monte Carlo and Maximum Likelihood (ML) analyses revealed identical topologies supporting the monophyly of Ustilaginaceae as well as Ustilaginales with 1.0 posterior probabilities and 100% bootstrap support each. Within Ustilaginaceae, we found one clade dominated by Sporisorium species including S. reilianum and U. maydis (Figure 1, coloured in red), a second clade dominated by Ustilago species including U. hordei (Figure 1, coloured in green), a third clade that consists of S. consanguineum and U. spermophora (Figure 1, coloured in blue) and a forth clade that consists of Tranzscheliella hypodytes and U. williamsii (Figure 1, coloured in yellow). Macalpinomyces eriachnes is resolved as a sister taxon of the ingroup species of the first three clades (Figure 1). Thus, at least four different clades could be defined in the Ustilaginaceae.
Fig. 1. Multi-gene phylogeny and interspecific sexual compatibility of Ustilaginales. 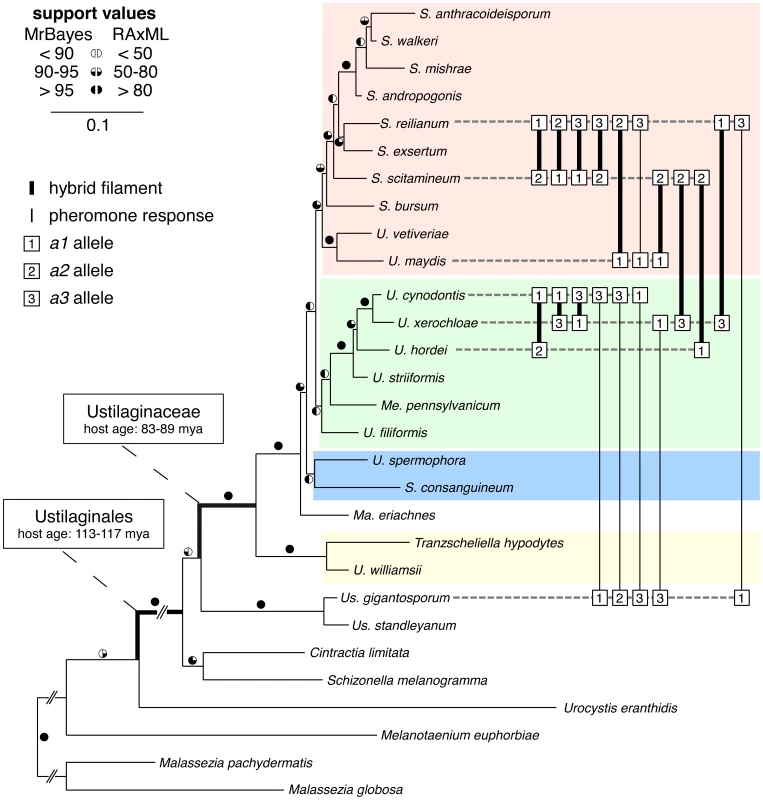
Concatenated Maximum Likelihood (ML) analysis of 2571 bp of ssu, ITS, lsu rDNA, ef1-α and rpb1. Circles next to branches indicate bootstrap support values and a posteriori probabilities of Bayesian and ML analyses, respectively. Branch lengths correspond to substitutions per site and abbreviated branches indicate longer branches. Connected squares illustrate hybrid filament formation (bold lines) or pheromone response (thin lines). Numbers in squares represent respective a mating types. Coloured boxes depict different phylogenetic clades (see text). Host ages refer to [60]. Diversity of the PR system of Ustilaginales
To analyse the diversity of the PR system, we pursued two sequencing strategies. We first assessed the occurrence of the pheromone receptor genes pra1, pra2 and pra3 in a set of 104 different species of Ustilaginaceae using PCR amplification. To this end, we designed allele-specific degenerated primers based on available sequences of pheromone receptors of U. maydis, U. hordei and S. reilianum. Primers for pra1, pra2 and pra3 were directed against conserved regions overlapping with trans-membrane domain (TMD) 1 and TMD6, TMD2 and the inner loop between TMD5 and TMD6, as well as TMD1 and the inner loop between TMD5 and TMD6, respectively (see Materials and Methods, Table S3). This initial approach revealed fragments of the expected sizes of about 780 bp, 620 bp and 680 bp from three pra1, five pra2 and two pra3 receptor genes, respectively. Subsequently, these sequences were used in addition to the initial reference sequences to design nested degenerated primers, which were again allele specific and directed against conserved regions (for details see Table S3). Thereby, 20 additional PCR fragments were obtained resulting in a dataset of 36 partial sequences of pheromone receptor genes containing 30 novel sequences (Table 1) and six known sequences.
Tab. 1. Species collection as well as accession numbers of the 5-gene phylogeny and PR loci-associated genes. 
CBS: Centraalbureau for Schimmelcultures, DB: Dominik Begerow, HAJB - Herbarium Havanna Jardín botánico, hmk: Herbarium Martin Kemler, HRK: Herbarium Ronny Kellner, HUV: Herbarium Ustilaginales Vánky, JG: Herbarium J. Gossmann, KVU: Kálmán Vánky Ustilaginales, M: Botanische Staatssammlung München, MP: Herbarium Meike Piepenbring, RK: strain collection Ronny Kellner, n.a.: not available, (1): [37], (2): personal communication. Sequences obtained in this study are shown in boldface. Species in boldface were used in the 5-gene phylogeny. In a second approach, we sequenced complete PR loci of U. cynodontis, U. filiformis, U. xerochloae, Me. pennsylvanicum, S. walkeri and the non-grass smut Us. gigantosporum. To this end, we performed genome walks starting either from genes of PR locus-flanking regions or from the pheromone receptor sequences obtained in the degenerated primer approach. Within flanking regions, we chose the highly conserved genes lba and panC (left border a locus and probable pantoate-beta-alanine ligase). For this purpose, we designed gene-specific degenerated primers based on available sequences of S. reilianum, U. hordei and U. maydis (see Materials and Methods). Since degenerated primers directed against flanking genes were applicable for all tested strains we were able to sequence PR loci of Me. pennsylvanicum (a1 locus), S. walkeri (a1 locus), U. filiformis (a1 locus) and Us. gigantosporum (a1, a2, a3 locus) that escaped the described initial approach. Applying BLAST [51] we predicted complete coding sequences of 10 pheromone receptors within these mating type loci. In sum, the two strategies revealed 42 novel sequences of pra receptors from 34 species (Table S4).
To assess the number of different a alleles in our dataset we performed ML analyses of two pheromone receptor sequence alignments comprising either complete coding sequences of 17 pheromone receptors or all available partial sequences, including trimmed sequences from genome walks and published sequences (Table S1). Both phylogenies resolve three mating type-specific clades with 100% bootstrap support for full length sequences and 83, 99 and 100% bootstrap support for partial sequences, showing a very high consistency between the different datasets (Figure 2, Figure S1). Furthermore, each novel gene encoding pheromone receptors, that has been sequenced by use of primers non-specific for certain alleles, groups with sequences of one of the three pra alleles. This suggests that the existence of a fourth PR allele is highly unlikely. In sum, 21 sequences could be assigned to pra1, 13 to pra2 and 13 to pra3. Together with the observed conservation of one receptor per locus this indicates the presence of only three pra alleles in Ustilaginaceae.
Fig. 2. Phylogeny of mating type-specific pheromone receptors. 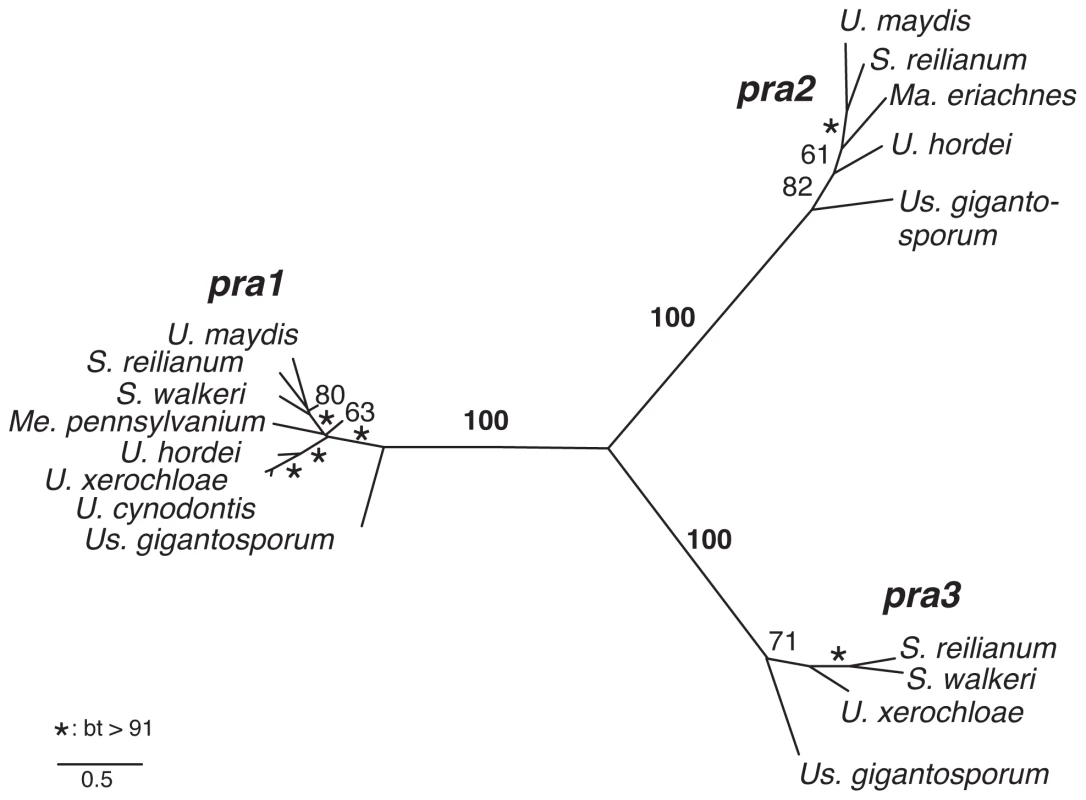
Maximum Likelihood analysis of complete pheromone receptor-coding sequences. Numbers and asterisks next to branches indicate bootstrap (bt) support values and branch lengths correspond to substitutions per site. To support this observation, we also identified pheromone precursor genes in our genome walk data by performing sequence comparisons to mfa genes of U. maydis, S. reilianum and U. hordei. This resulted in the identification of 21 pheromone precursor genes with, at most, two genes per locus. A ML analysis of a pheromone precursor alignment including all 28 available coding sequences confirmed three mating type-specific clades albeit the support in single clades was weaker due to the sparse sequence information of short pheromone sequences (Figure S2). In essence, three pra and three mfa alleles are ancient and unique to Ustilaginales.
To evaluate the occurrence of pra alleles in a phylogenetic background, we mapped species-specific information on a ML phylogeny from partial rDNA sequences (lsu and ITS containing 5.8S) of 108 species of Ustilaginomycotina containing all 104 species that were tested in the degenerated primer approach (Figure S3). All three pra alleles are present in the three major clades of Ustilaginaceae as well as in the non-Ustilaginaceae species Us. gigantosporum showing that these three pra receptors are not restricted to S. reilianum but are apparent in many species. In addition, they do not correlate with phylogenetic groupings. Thus, these data strongly support the hypothesis that the last common ancestor of the Ustilaginaceae had a triallelic PR system whose three alleles are conserved and which gave rise to convergent evolution of biallelic states.
Organisation and genetic diversity of genes at the PR locus of Ustilaginales
So far, we focused on the pheromone receptor and pheromone precursor genes. To examine the precise organisation of the PR locus we analysed 11 a loci of Ustilaginales spanning at least one border gene (Figure 3). Remarkably, there is a high degree of synteny between PR loci of different species regarding genes for pheromones and receptors as well as PRE (pheromone response element) sites. The latter suggests a conserved regulation of pheromone and receptor gene expression via Prf1 homologs. In contrast, the genetic organisation of border genes flanking the PR locus is less conserved. For example, the border genes rba and panC are missing in Us. gigantosporum. In addition, the a1 locus of U. xerochloae is flanked by an inverted homologous gene of um02342 and sr13546 encoding two proteins of unknown function. They locate 106.2 kb upstream on the same chromosome in U. maydis and 43.1 kb downstream in S. reilianum (Figure 3). The first right border a locus genes of Us. gigantosporum represent an inverted sr13582 homolog (protein of unknown function) and two homologous genes that locate at the same chromosome 81.1 kb downstream of the a1 locus of U. maydis (um02414 and um02415; related to dihydrouridine synthase and related to anti-silencing protein 1) and 92.5 kb upstream of the a2 locus of S. reilianum (sr10827 and sr10828; related to tRNA dihydrouridine synthase and related to anti-silencing protein 1). A left border gene of the S. walkeri a3 locus preserved only the first of three introns that were observed in the homologous genes um02380, sr13588 and in a respective homolog of Us. gigantosporum (protein of unknown function). Furthermore, the panC homolog of S. walkeri is inverted (Figure 3). These differences between inner and outer regions of PR loci provide evidence for differential constraints on recombination comprising strong conservation of mating type regions and weak dynamics in the evolutionary history of flanking regions.
Fig. 3. Genetic structure of mating type a gene clusters of Ustilaginales. 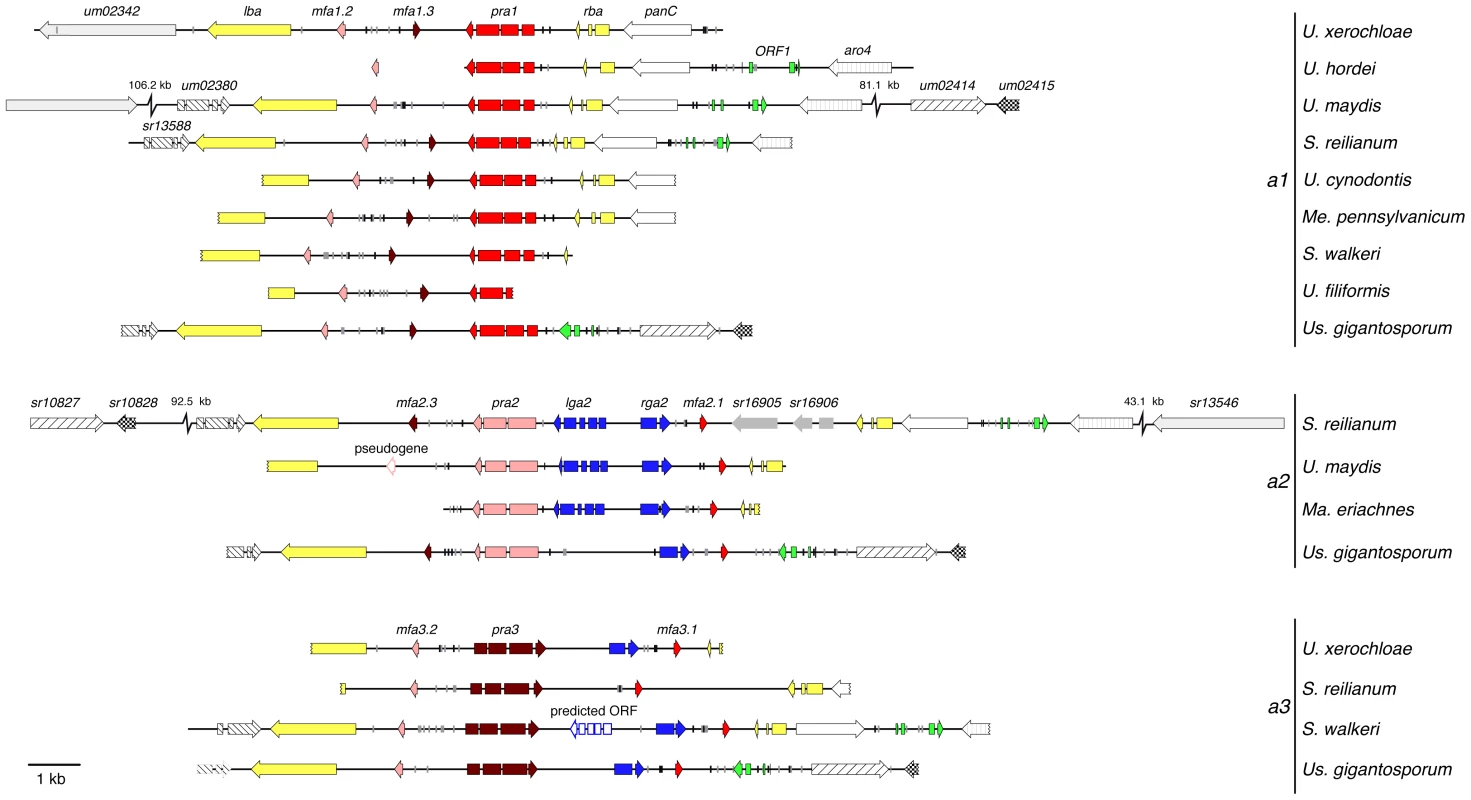
Shown are three a locus alleles of different Ustilaginales species. Arrows indicate coding regions of respective genes and lines represent non-coding or intron regions. Pheromones and cognate pheromone receptors are depicted in red shades. Homologous border genes are depicted in identical colours or patterns. Strokes represent pheromone response element sites (ACAAAGGGA) with no (black) or one mismatch (grey). Abbreviation signs depict connected regions on respective chromosomes. um and sr gene numbers correspond to gene identifications on MUMDB [104] and MSRDB [105]. We next addressed whether interspecific genetic diversity of single genes reflects the differential conservation of gene organisation between PR loci and their flanking regions. For this purpose, we calculated the nucleotide diversity π from all genes of the PR locus and its flanking regions. Since single gene datasets each contain sequences of different species, we considered their individual phylogenetic diversity (pd) based on the five-gene phylogeny described above and divided π by pd. The pd index indicates the proportional branch length in relation to the total branch length of the phylogeny [52]. Genes within the PR locus, namely lga2, rga2 and genes for pheromone receptors and pheromones show significantly increased nucleotide diversity π in comparison with the flanking genes lba, rba, aro4, coding for a probable phospho-2-dehydro-3-deoxyheptonate aldolase, ORF1, panC, as well as the house-keeping genes rpb1, ef1-α, lsu rDNA, ssu rDNA and ITS rDNA including 5.8S (Figure 4). The diversity of the pheromone genes is most probably even higher since gap positions in their alignment are not considered in DnaSP diversity calculations (Figure S4). Unlike other flanking genes, homologs of um02380 revealed increased nucleotide diversity similar to pra3 (Figure 4). Hence, the increased nucleotide diversity of mating type genes and PR locus-flanking genes contrasts the conservation of their gene organisation and suggests accelerated mutation rates for the highly syntenic PR locus genes.
Fig. 4. Nucleotide diversity of PR loci-associated and house-keeping genes. 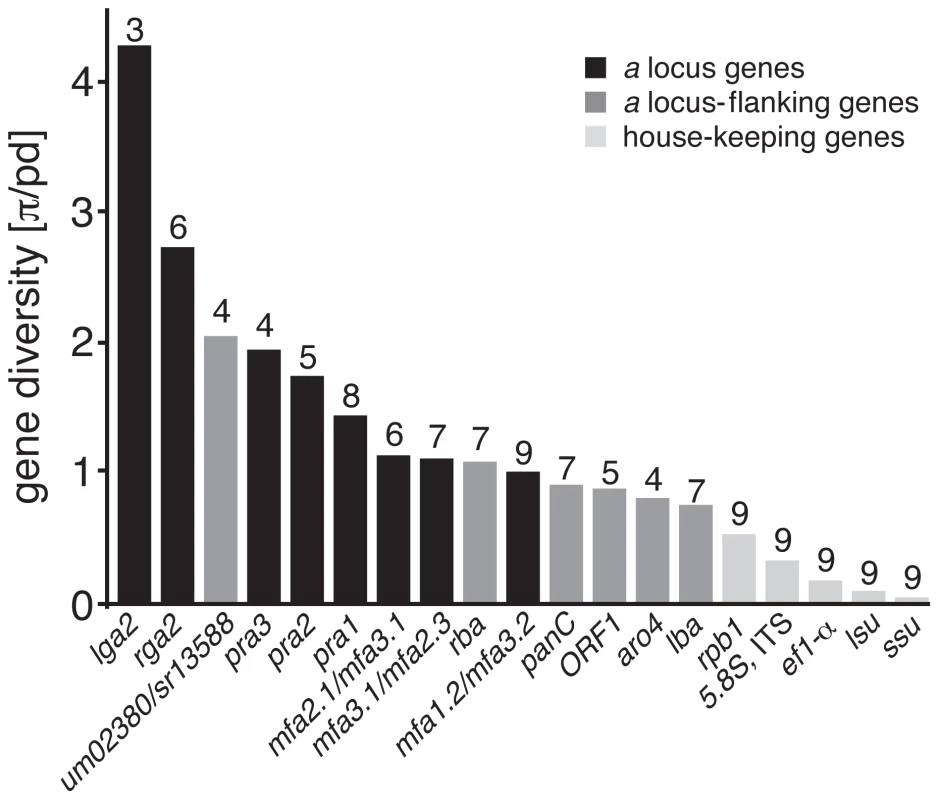
Bars indicate nucleotide diversity (π) estimates divided by the phylogenetic diversity (pd) of respective datasets. Black bars: a locus genes, dark grey bars: a locus-flanking genes, light grey bars: house-keeping genes. Numbers above bars indicate the quantity of analysed sequences. Increased nucleotide diversity could relate to adaptive changes that were driven by specific evolutionary constraints. In order to compare the evolutionary constraints of PR locus-associated genes we used seven codon site models of variable ratios of ω values across sites, which are implemented in PAML v4.3 (see Material and Methods), and calculated likelihood ratio statistics for each dataset of Figure 4. In addition, we analysed datasets from partial sequences of lba and panC. Datasets of lga2 and the pheromones were excluded from the analysis because of the small dataset with only three sequences for lga2 and shortness of the pheromone sequences. The analysis revealed that in each gene ω varied among codons (except ORF1) as the Nsites Model M3 rejects M0 (Table S4). For the datasets of pra2 and panC model M8 (beta&ω), which allows for positive selection, fitted the data better than model M7 (beta), which does not allow for positive selection. As model M8a is not rejected by M8 the identified divergence of both genes rather accounts to relaxed purifying selection than positive selection. In summary, these results support the hypothesis that the investigated PR-flanking regions do freely recombine (except one flanking region of Ustilago hordei).
To investigate whether the increase in nucleotide diversity is linked to specific sites within the encoded pheromone receptors, we predicted transmembrane domains for pheromone receptor sequences and performed sliding window analyses of the nucleotide diversity π and the ratio of non-synonymous and synonymous substitutions (dN/dS ratios) for each allele-specific pheromone receptor alignment. The analyses revealed several diversity peaks within pra1, pra2 and pra3 that slightly resemble each other but neither nucleotide diversity nor dN/dS ratios suggest prominent sites (Figure S5). This shows that diversity peaks and species-specific substitutions scatter almost randomly on the pra genes without hints to differential selection of single sites. In summary, we observed strong synteny of PR loci whose genes accumulated significantly more substitutions than PR locus-flanking and house-keeping genes.
Homologs of lga2 and rga2 in Ustilaginaceae
Besides pheromone - and receptor-encoding genes, a2 loci of U. maydis and S. reilianum contain two additional genes, namely lga2 and rga2. As shown for U. maydis they encode mitochondrial proteins, whose concerted action is responsible for the uniparental inheritance of mitochondria [50]. Sequence comparison applying BLAST [51] furthermore identified homologs of rga2 in respective regions of a2 loci of Ma. eriachnes and Us. gigantosporum. Surprisingly, a3 loci of U. xerochloae, S. walkeri and Us. gigantosporum also encode a homolog of rga2 that locates between homologs of pra3 and mfa3.1 (Figure 3). To assess the homology of these putative rga2 genes, we performed a multiple amino acid alignment of all predicted Rga2 proteins and the reference proteins of U. maydis and S. reilianum (Figure S6). All predicted genes of different species revealed the same intron structure and encoded proteins comprised comparable amino acid sequence identities of 30 to 53% in relation to Rga2 of U. maydis and S. reilianum. Additionally, we applied iPSORT prediction [53] revealing mitochondrial target signals for the putative Rga2 proteins as was reported for Rga2 of U. maydis [54].
Compared to rga2, lga2 is significantly less conserved between U. maydis and S. reilianum. To identify homologs within respective regions of a2 and a3 loci we conducted gene predictions based on U. maydis intron characteristics using the Augustus prediction server [55]. To verify homology of the identified genes to lga2, we furthermore predicted targeting peptide signals in the respective proteins applying iPSORT prediction [53] and screened for functional domains applying SMART [56], [57]. Since lga2 is a direct target of the bW/bE homeodomain transcription factor we additionally searched for promoter sequence identity upstream of the putative lga2 genes. Importantly, in the a2 locus of Ma. eriachnes we found a putative lga2 gene that showed homology to known sequences. This gene displays 32% sequence identity to lga2 of S. reilianum, shows the same intron structure and the gene product contains a mitochondrial target signal and an F-box-like motif. Although this domain does not completely overlap with the predicted F-box-like motif of Lga2 of U. maydis [54] (Figure 5A), both F-box-like motifs are located within a protein region that contains the most shared amino acids (12 out of 20) for all three species (Figure 5A). Based on information of the promoter sequence of lga2 in U. maydis [58], [59], we identified a sequence with high similarity to the His-Kon8 binding site within the 5′ region of lga2 of S. reilianum and Ma. eriachnes indicating the same regulation via bW/bE transcription factors (Figure 5B). In particular, out of 29 nucleotides 18 and 15 nucleotides overlap in S. reilianum and Ma. eriachnes, respectively. However, even lowering stringency and gene predictions based on intron characteristics did not identify a clear lga2 homolog in other species. We only identified an ORF with four introns and a mitochondrial target signal in the a3 locus of S. walkeri. Thus, lga2 homologs are likely lacking in the a loci of Us. gigantosporum. In conclusion, rga2 is not restricted to the a2 locus but also occurs in the a3 locus, where it does not pair with lga2. This indicates a complex mechanism of parental inheritance of mitochondria within Ustilaginales.
Fig. 5. Multiple alignment of Lga2 homologs and their regulatory regions. 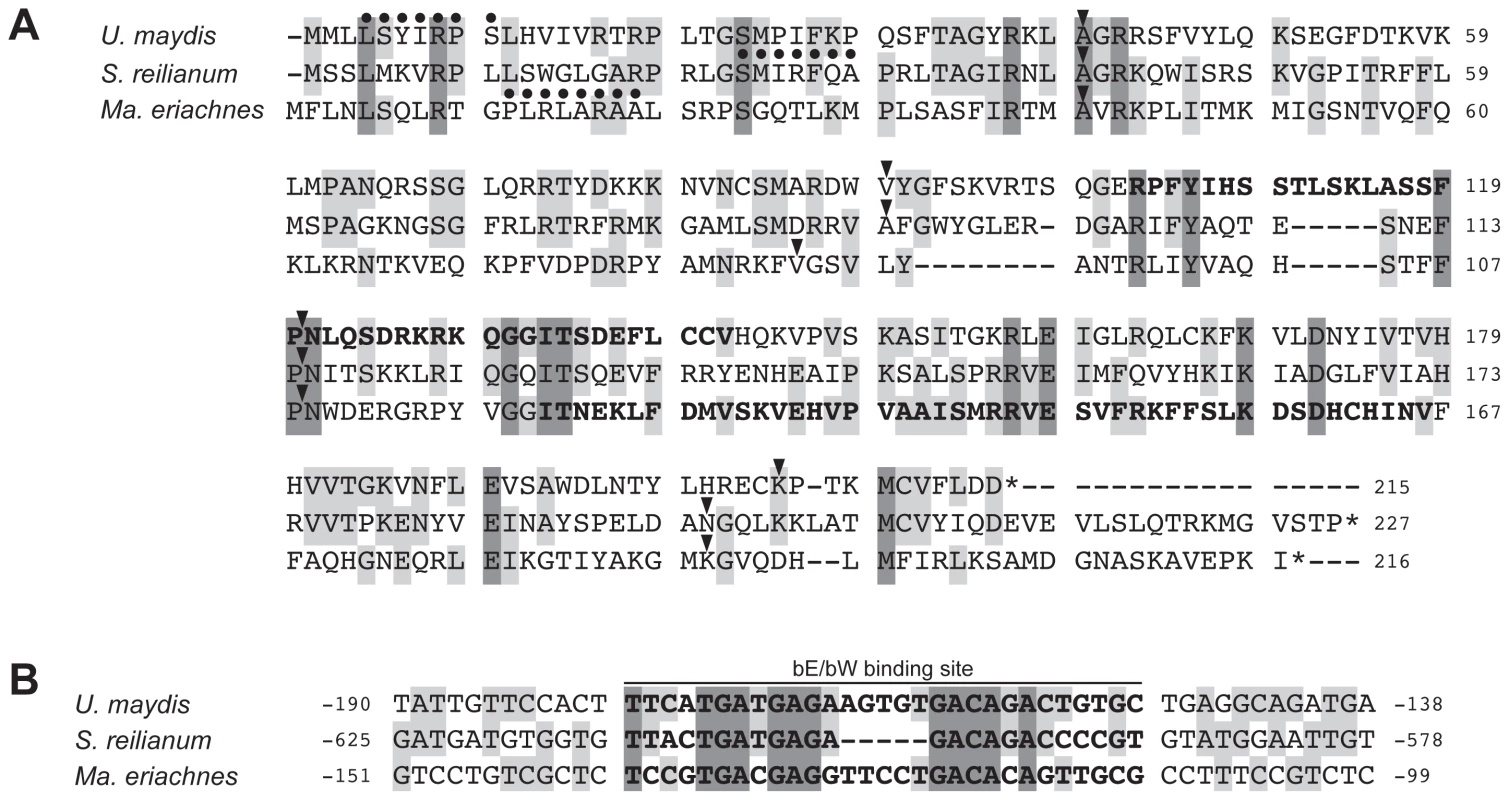
(A) Amino acid alignment of Lga2 sequences from reference species (S. reilianum and U. maydis) and proposed sequences of Ma. eriachnes. Dots indicate predicted mitochondria target signals. Arrowheads indicate positions of introns in the respective genes. Dashes represent alignment indels. Grey shades mark positions with two (light grey) or three (dark grey) identical amino acid residues. Bold letters indicate predicted F-box-like motifs. (B) Nucleotide sequence alignment of the lga2 b-binding site and its flanking regions of U. maydis with 5′ sites of lga2 of S. reilianum and Ma. eriachnes. Grey shades mark sites with two (light grey) or three (dark grey) identical aminoacids and nucleotides, respectively. Interspecific compatibility in Ustilaginales
The dimension of intercompatibility within grass smuts is still unclear and a representative dataset that gives an overview of the whole Ustilaginaceae and beyond is currently missing. Consequently, we screened a representative set of seven species for interspecies sexual compatibility (summarised in Figure 1). Firstly, we monitored the development of conjugation hyphae in liquid media indicating an active PR locus-dependent pheromone response. Secondly, we examined the formation of hybrid filaments on plates containing potato dextrose and charcoal (PD-CC), indicating plasmogamy and the activity of compatible HD alleles. Finally, we illustrated interspecific sexual fusion and filament formation for two examples using scanning electron microscopy (SEM).
Initially, we optimised mating conditions under which all tested species showed an adequate intraspecific mating behaviour. Whereas each species efficiently formed filaments on PD-CC plates, the mating reaction in liquid media distinctly varied between species. Although most compatible strains of one species developed mating structures in water and liquid PD, the reaction in PD was significantly weaker (data not shown). However, since U. cynodontis and U. xerochloae only mated in liquid PD, each mating assay applying liquid media was performed in water and in liquid PD (Table S5).
In the first two series, 720 single mating tests were performed comprising two replicates of 120 different mating tests under the three conditions described above (water, liquid PD, PD-CC plates). The 120 different mating tests consisted of 11 intraspecific and 109 interspecific confrontations. From 109 different interspecific confrontations 18 resulted in a distinct mating reaction (Figure 1, Tables S5 and S6). Figure 6A and 6B exemplify the three recognised interaction types comprising conjugation hyphae formation (Figure 6A v) followed by filament formation (Figure 6B v), conjugation hyphae formation (Figure 6A vi) without filament formation (Figure 6B vi) and yeast-like growth without any reaction (Figure 6A, 6B vii). In three cases, namely S. scitamineum MAT2 confronted with U. xerochloae a1 or a3 and S. reilianum a1 confronted with U. xerochloae a3, we detected only very few hybrid filaments without respective observations of conjugation hyphae in liquid media (Tables S5, S6) which, most probably, is the result of a very low mating rate.
Fig. 6. Interspecific mating reactions between different species of Ustilaginales. 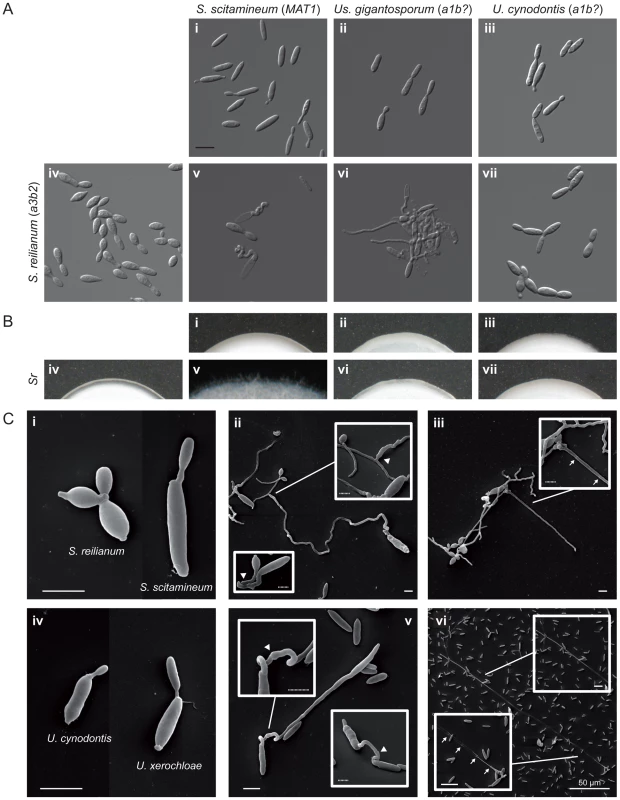
(A) Differential interference contrast (DIC) images of mating assays in liquid potato dextrose. Images i–iv show yeast cultures of respective species. Images v, vi and vii show confrontations of S. reilianum with S. scitamineum, Us. gigantosporum and U. cynodontis, respectively. All figures are scaled equally. bar: 10 µm, b?: unknown b allele. (B) Filament formation on charcoal-containing potato dextrose media. Images i–vii correspond to sample descriptions in A. Figure width represents 3 mm. (C) SEM images of mating assays of S. reilianum and S. scitamineum (i–iii) and U. cynodontis and U. xerochloae (iv–vi). Single yeast cells (i, iv) form conjugation hyphae that fuse (arrowheads in ii, v), expand and form empty sections by the insertion of basal septa (arrows in iii, vi). bar: 4 µm, dotted bar: 1 µm. Each tested species revealed intercompatibility at least with two other species including matings between pairs of closely and distantly related species. All five interspecies matings with Us. gigantosporum, that means across the Ustilaginaceae family border, stimulated the formation of conjugation hyphae that did not fuse (Figure 1, Tables S5 and S6). Within Ustilaginaceae hybrid filament formation was observed for all interspecific crossings except for the crossing of S. reilianum a3b1 with U. maydis a1b1.
SEM revealed that haploid sporidia of different species (Figure 6C i, iv) form conjugation hyphae that fuse through a thickened fusion site (arrowheads in Figure 6C ii, v). Conjugation hyphae of S. scitamineum are significantly thicker than those of S. reilianum (Figure 6C ii). Upon fusion, hybrids of S. reilianum and S. scitamineum as well as U. cynodontis and U. xerochloae form filaments that expand at the apical growth cone and form characteristic empty sections via insertion of retraction septa at the basal pole (arrows in Figure 6C iii, vi). This clearly confirms the sexual compatibility between different grass smut species and emphasises their increased potential for hybridisation that, considering the phylogenetic background of their hosts [60], has been preserved for more than 100 million years of evolution.
To find out whether the development of interspecific mating structures is directly linked to pheromone signalling we used two haploid strains of U. maydis (a1b1 and a2b2) that express Gfp under the control of the mfa1 promoter. In these strains Gfp expression is specifically increased in response to pheromone recognition ([46], Materials and Methods), thereby serving as a molecular readout for active pheromone signalling. Both Gfp strains were confronted with 14 different haploid strains of six Ustilaginales species and screened for Gfp fluorescence. As a positive control, we used two haploid wild type strains of U. maydis (a1b2 and a2b1) and the respective compatible Gfp strain. For quantification of Gfp expression three independent experiments were performed. Consistent with the results of mating assays described above, only combinations of the U. maydis a1b1 Gfp reporter strain with S. scitamineum and S. reilianum induced mating structures (Figure 7A). The quantification of the fluorescence revealed that interspecific confrontations with S. scitamineum (MAT2) and S. reilianum (a2+a3) induced significantly less fluorescence than intraspecific confrontations with compatible wild type strains of U. maydis (Figure 7B). These differences are consistent with the quantitative differences of sexual structures observed in interspecific matings. Thus, reporter gene expression illustrates that similar to intraspecific crossings interspecific mating also induces pheromone signalling, indicating the deployment of the same physiological and molecular network in both events.
Fig. 7. Interspecific induction of mating via pheromone signalling in U. maydis. 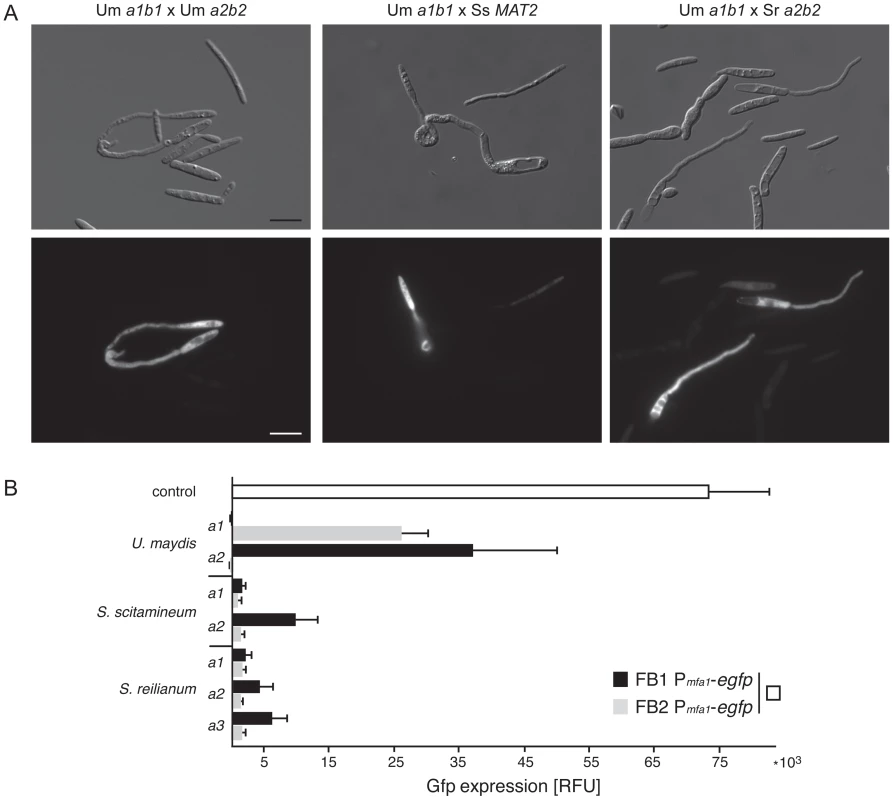
(A) Differential interference contrast (DIC) and fluorimetric images from positive pheromone response reactions in liquid potato dextrose. Conjugation hyphae are formed by both mating partners (DIC images). All figures are scaled equally. bar: 10 µm. (B) The diagram illustrates fluorimetric measurements (relative fluorescence units, RFU) from mating assays of U. maydis Pmfa1-egfp strains FB1 (a1b1) and FB2 (a2b2) confronted with different mating types (a1, a2 and a3) of different smut species in water. Black and grey bars refer to RFUs of confrontations with strain FB1 Pmfa1-egfp and strain FB2 Pmfa1-egfp, respectively. U. maydis wild type strains FB6a (a2b1) and FB6b (a1b2) were used as positive controls. The white bar depicts RFU of the mating of FB1 Pmfa1-egfp and FB2 Pmfa1-egfp. Error bars indicate standard deviations of three independent experiments. Discussion
Genetic organisation and evolution of a mating type loci in Ustilaginales
Sexual identity in basidiomycetes is determined by a few genes that reside at two specific genomic regions, the so-called mating type loci [61]. Studies on many model organisms, e.g. Coprinopsis cinerea [16], Cryptococcus sp. [62], Microbotryum violaceum [23], Schizophyllum commune [63] and Ustilago maydis [45], revealed that the mating type genes and the mating-dependent signalling network are conserved across large phylogenetic distances. By contrast, the genetic structure of both sex-determining regions is remarkably diverse resulting in bipolar and tetrapolar species with two or multiple alleles of mating type loci [18]. The mating type locus encoding the pheromone-receptor (PR) system shows diverse genetic determinations that have supposedly evolved from rather simple ancestral types via individual translocations, gene duplications and fusions to the second mating type locus [61].
Studying a representative set of different species of Ustilaginales we could show that three PR alleles, which were until now only described for S. reilianum [42], are conserved among members of Ustilaginaceae and most likely represent the plesiomorphic character state of this group (Figure 1 and Figure 2). In consequence, the less frequently observed biallelic states as reported for S. scitamineum [36], U. maydis [39] or for species of the conspecific group of U. hordei and its close relatives [36] should have evolved independently from triallelic states at least three times. With opposite mating obligatory for the pathogenic development of grass smuts it is highly unlikely that the genetic transitions of their PR system from tetrapolarity to bipolarity occurred as a result of loss of function as shown for Coprinellus disseminatus [26]. However, it remains unknown whether those transitions in the mating type loci followed degeneration processes as proposed for U. maydis [39] or whether the linkage of the PR and HD locus as shown for U. hordei [41] predominates in Ustilaginaceae. Since most basidiomycetes with a biallelic PR locus are bipolar and several fungal examples propose an evolutionary trajectory from tetrapolarity to bipolarity via chromosomal linkage of both mating type loci [64]–[66], we propose that most of the biallelic species of Ustilaginaceae have chromosomally linked mating type loci.
The necessity for Ustilaginales to mate in order to conserve their parasitical niche as well as to assure sexual recombination imposes strong selection pressure towards successful mating. In general, diversity levels of reproductive genes in many taxonomic groups show rapid diversification of sex-related genes [67]. Although the precise selective forces driving this diversification and their functional consequences for mating biology are poorly understood, accumulating evidence suggests an adaptive co-evolutionary process as a main driving force for increased diversification of reproductive genes [67]–[69]. Consistently, in Ustilaginales the mating type-specific genes pra1 to 3, mfa1 to 3, rga2 and lga2 revealed increased interspecific diversity compared to either a locus-flanking or house-keeping genes (Figure 4). At least for pheromones and their cognate receptors a co-evolutionary scenario is likely since interacting genes reside on different alleles and their expression patterns are similar [70]. This would suggest similar constraints on their evolutionary rate as shown for Saccharomyces cerevisiae [71]. An additional aspect that could promote diversification of mating type genes is the functional plasticity and broad specificity of the PR system. Since small changes within pheromone and receptor genes do not necessarily lead to loss of function, they are rather under relaxed than strict purifying selection favouring their rapid diversification.
Lga2 - and Rga2-dependent inheritance of mitochondria
In most sexually reproductive eukaryotes, stochastic and deterministic processes induce uniparental inheritance (UPI) of mitochondria. Both UPI and biparental inheritance (BPI) of mitochondria entail advantages and disadvantages regarding mitochondrial recombination, evolutionary conflicts and energy balance [72]–[75]. In U. maydis, UPI is a deterministic process depending on the interplay of the mating type-specific proteins Lga2 and Rga2 [76]. Whereas Lga2 blocks the fusion of parental mitochondria and mediates their uniparental elimination, Rga2 protects against Lga2-dependent elimination [50]. In S. reilianum and U. maydis lga2 and rga2 genes are restricted to the a2 allele [39], [42]. We could show that in the case of Ustilaginales with three PR alleles this restriction to the a2 allele rather constitutes an exception since rga2 genes of S. walkeri, U. xerochloae and Us. gigantosporum additionally reside within a3 alleles (Figure 3). Since a homolog of lga2 is missing in the three mating type loci of Us. gigantosporum and the a3 allele of S. walkeri contains a predicted gene coding for a novel protein with a mitochondrial targeting signal, our data strongly suggest a more complex or even species-specific mechanism of mitochondrial inheritance in grass smuts, involving different combinations of a mating type-specific genes. Referring to the role of Lga2 and Rga2 in U. maydis [50] and based on our sequence data (Figure 3), one mechanism could encompass sexual fusions of species with three PR alleles resulting in either UPI or BPI of mitochondria depending on the combination of the two a mating types. In particular, mating of a1 and a2 strains would result in UPI whereas mating of a1 and a3 strains as well as a2 and a3 strains would result in BPI, thereby uniting uniparental and biparental inheritance of mitochondria in one species.
Interspecific sex and hybridisation-based speciation in Ustilaginales
Hybridisation can lead to substantial genomic changes and thereby gives rise to various novel phenotypes [77]. In consequence, hybridisation has been frequently discussed with regard to its role in evolutionary adaptation and diversification for various organismic groups (reviewed in [78], [79]) including fungi [80]. In order to hybridise parental species have to overcome pre - and postzygotic barriers requiring interspecific sexual compatibility [81]. Using a set of seven species we demonstrated that sexual intercompatibility up to the stage of plasmogamy (Figure 6) is common within Ustilaginales bridging more than 100 million years of evolutionary differentiation (Figure 1). In addition, in the investigated crossings interspecific sex activates the same signalling machinery as intraspecific sex (Figure 7), emphasising the functional redundancy of self and non-self pheromones and their cognate receptors. Referring to studies in various animals and plants (reviewed in [82]), this broad intercompatibility between closely as well as distantly related species of Ustilaginales could lead to hybridisation events more frequently than previously expected. In most cases, hybridisation effects introgression but sometimes it also initiates hybrid speciation [83]. The more frequent emergence of newly combined genotypes would increase the probability for one genotype to arise that exhibits a higher fitness compared to its parental species or that enables the exploitation of a novel ecological niche [78]. Such niche differentiation is known from homoploid hybrids of several smut species including closely related species, e.g. the conspecific group of U. hordei and its close relatives as well as distantly related species like S. reilianum and S. cruentum [36]. In addition, co-phylogenetic studies of Ustilaginaceae and their hosts revealed evidence for hybridisation events in Ustilaginaceae. In particular, there is much incongruity between both topologies [84] that, referring to the strong host specificity of grass smuts, was assumed to result from common host jumps and/or hybridisation events [38]. Although it is not clear how these host jumps occurred, as in highly adapted species this might involve complex genetics, our data and several observations of natural hybrids (summarised in [36]) highlight the potential relevance of hybridisation in grass smut speciation. Nevertheless, there are reproductive barriers between intercompatible grass smut species as shown for U. maydis and S. reilianum that independently established on the same host and coexist without evidence for “natural” hybrids [85].
Thus, it remains unclear whether mating specificities are directly linked to host specificities or if mating specificities and mating efficiency change after the establishment of new host specificities. However, single outbreaks, as the rust fungus hybrid Melampsora xcolumbiana on Populus hosts [86], emphasise the ecological relevance of novel hybrid-based genotypes. Hence, the future challenge will be to track the distribution of hybrids among natural populations and to examine their individual ecological potential.
Materials and Methods
Species selection, fungal cultures, and growth conditions
For phylogenetic analyses 104 species of Ustilaginales were analysed in total (Table S1, Figure S3). Seven of the species, namely Melanopsichium pennsylvanicum, Urocystis eranthidis, Ustilago avenae, U. cynodontis, U. filiformis, U. williamsii and Ustanciosporium gigantosporum were collected in field trips for this study. For Cintractia limitata, Malassezia globosa, Mal. pachydermatis and Schizonella melanogramma we used sequence information from GenBank [87].
From 24 species we used cultures that either originate from collaborators (5 species) or were cultured from herbarium material in this work (19 species, Table S2). The strains of 22 species were deposited at CBS. To increase the germination success of spores and to separate sporidia, spores were germinated in three different liquid media (complete media (CM) [88]; potato dextrose (PD); water) with shaking at 16°C and 28°C. If necessary, kanamycin was added to the media (100 µg/ml). Single haploid yeast cultures were isolated from streak plates (PD) of liquid cultures with germinated spores. U. maydis strain FB2 Pmfa1-egfp was constructed by transformation of progenitor strain FB2 (a2b2) with linearised plasmid pmfa1-egfp-cbx [46]. Homologous integration event at the ip locus was verified by Southern analysis [89].
Species identity of new cultures was checked by ITS rDNA sequencing (Table S1, see below). In mating assays we used only verified single yeast cultures of 18 haploid cultures of 7 different species in total, namely Sporisorium reilianum, S. scitamineum, U. cynodontis, U. hordei, U. maydis, U. xerochloae and Ustanciosporium gigantosporum (Table S2). For further experiments isolated strains were stored at −80°C in PD-glycerine and re-grown at 28°C on PD or CM agar plates.
PCR conditions and sequencing
Genomic DNA from yeast cultures was isolated by the method of [90]. Genomic DNA from herbarium material was isolated with DNeasy96 Plant Kit (Qiagen, Hilden). ITS rDNA containing 5.8S was amplified using the primers ITS1 and ITS4 [91]. Partial ssu rDNA, lsu rDNA, rpb1 and ef1-α were amplified using the primers NS23 and NS24 [92], LR0R and LR6 [93], [94], RoK157 and RoK158 (Table S3) and 987F and 1567R [95], respectively. Detailed primer descriptions are given in Table S3. Primer properties were evaluated with OligoCalc [96], [97] or Clonemanager v9.0 (Sci-Ed Software). Primers were obtained from SIGMA-ALDRICH (Hamburg). All PCR amplifications were performed on a PTC-200 Thermo Cycler (MJ Research). For DNA amplification ≤5 kb Phusion® High-Fidelity Polymerase (Finnzymes, Espoo) or peqGold Taq DNA Polymerase (Peqlab) and for >5 kb KOD Xtreme™ Polymerase (Merck Biosciences, Nottingham) were used following manufacturer's instructions. PCR products were purified directly or through gel purification using my-Budget Double Pure Kit (Bio-Budget). Purified fragments were sequenced on an Abi 3130XL sequencer (Applied Biosystems) by the sequencing service of the Biochemistry department at the Ruhr-Universität Bochum or by GATC Biotech AG Konstanz. Nucleotide sequences of ITS, lsu, ssu, ef1-α, rpb1, pra1, pra2, pra3 and mating type loci have been deposited at GenBank under the accession numbers JN367287 - JN367447 as listed in Tables S1 and S4.
Genome walking procedure
In order to obtain complete a loci sequences, degenerated primers were used to amplify pra1, pra2, pra3, lba and panC in different species. Initial primer design was based on published sequences of U. maydis (MUMDB), Sporisorium reilianum (MSRDB) and U. hordei (GenBank; [87]). Genome walks started from amplified regions applying the GenomeWalker™ Kit (Clontech Laboratories, Mountain View) and following manufacturer's instructions. Completed loci were checked by long-range PCR and enzymatic digestion. A detailed primer list is given in Table S3.
Phylogenetic reconstructions
Sequences were quality checked and hand edited using Sequencher 4.8 (Gene Codes Corporation). Nucleotide alignments were performed with MAFFT 6.707 [98] in default mode using a maximum number of 1000 iterations. Amino acid alignments were performed with BioEdit [99] applying ClustalW [100]. Afterwards, leading and trailing gaps were removed manually from the alignments except for ITS alignments which were trimmed using Gblocks v0.91 on the MABL server (http://www.phylogeny.fr) applying all less stringent settings. Maximum Likelihood (ML) [101] analyses were performed with RAxML 7.0.4 [102]. RAxML 7.0.4 conducted 1000 bootstrap replicates using a rapid bootstrap algorithm [103] applying GTRMIX approximation. The more accurate GTRCAT approximation was applied in the subsequent ML search for the best scoring ML tree starting from each 5th bootstrap tree. Bootstrap support values were drawn at the best scoring ML tree. In multi-gene ML analyses, sequences were concatenated. For each partition RAxML estimated and optimised individual α-shape parameters, GTR-rates and empirical base frequencies. With the partitioned multiple alignment of ITS, lsu, ssu, ef1-α and rpb1 we additionally performed a Bayesian analysis using MrBayes v3.1 [104]. In order to allow the overall evolutionary rate to be different across partitions, the evolutionary model was applied individually and parameter estimations were unlinked. Monte Carlo Markov chains (MCMC) were run over one million generations under the GTR+I+G model. Trees were sampled every 100 generations leading to 10,000 trees. To check for overall convergence, this approach was repeated four times with random starting trees. After examination in Tracer v1.5 (http://tree.bio.ed.ac.uk/software/tracer), a burn-in of 2500 was chosen for each run. Out of the remaining trees a majority rule consensus was calculated to obtain estimates for a posteriori probabilities. All trees were visualised and edited in FigTree v1.3.1.
Gene identification and sequence analyses
In order to identify homologous genes, sequences were compared with GenBank [87] and the genome databases from Ustilago maydis (MUMDB, [105]) and Sporisorium reilianum (MSRDB, [106]) applying BLAST [51]. Furthermore, SMART [56], [57] and iPSORT [53] were used to identify functional domains and subcellular localisation signals in the corresponding amino acid sequences. Since lga2 homology between U. maydis and S. reilianum is weak except for the number of introns and BLAST [51] did not identify homologs, we ran gene predictions in respective mating loci regions using Augustus (http://augustus.gobics.de). Coding sequences of homologous genes were determined manually according to reference sequences from U. maydis, S. reilianum and U. hordei.
Completely sequenced pheromone receptors and the deduced protein sequences were characterised with respect to their predicted transmembrane domains, nucleotide diversity and their dN/dS ratios along the protein sequence. Transmembrane domains were predicted using TMpred [107], as well as MEMSAT and MEMSAT-SVM [108] on the PSIPRED server (http://bioinf.cs.ucl.ac.uk/psipred, [109]). Nucleotide diversity π was calculated with DnaSP v5.10.01 [110] applying Jukes-Cantor correction. To compare nucleotide diversity of different gene datasets, values were divided by the phylogenetic diversity (pd) of respective species subsets. pd was calculated with phylocom 4.1 [52] based on the multi-gene phylogeny (Figure 1).
To analyse how genetic variation distributes along pheromone receptor genes, we performed sliding window analyses of π in DnaSP v.5.10.01 (windowlength: 25, step size: 5). Differential selection of single sites within pheromone receptors was tested applying codeml (implemented in PAML v4.3 [111]–[113]). Since we found no significance for positive selection, we illustrated the proportion of non-synonymous substitutions along the receptor alignments. Therefore, we estimated the average behaviour of each codon for all pairwise comparisons for synonymous and non-synonymous mutations using SNAP of the HIV database website (http://www.hiv.lanl.gov, [114]) and illustrated the ratio of non-synonymous and synonymous values along the amino acid sequence.
Mating assays on PD-CC and in liquid media
Mating assays were performed with haploid strains that were isolated from different species. To determine the mating type and to validate haploidy, we performed mating tests, amplified pheromone receptors and stained nuclei with DAPI. Mating tests were performed with cultures of the same species and with cultures of different species. Densely grown liquid pre-cultures (PD, 200 rpm shaking at 28°C) were diluted in liquid media (PD, pH 8.0) and grown over night (28°C, 200 rpm,) to an optical density OD600 between 0.4 and 0.8. Cells were harvested by centrifugation (1000 g, 5 min at room temperature) and pellets were resuspended in distilled water (pH 8.0) or PD (pH 8.0) to a final OD600 of 1.0. 150 µl cell suspensions of each strain were mixed and added into 24 well plates. a mating type compatibility and conjugation hyphae formation were screened after 6 and 12 hours of incubation at 28°C using a Zeiss Axiostar microscope. To test for b mating type compatibility, 3 µl cell suspensions were dropped on PD charcoal plates, incubated at 28°C and screened for filament formation after 18 hours using a Zeiss Stemi 2000-C binocular. Mating type-specific primers that locate within pheromone receptors were used to identify and validate opposite mating types of fungal strains (Table S3). For DAPI staining, cells were fixed in 2% formaldehyde for 30 min, transferred to mounting media containing DAPI (Linaris, Wertheim-Bettingen) and analysed using a Zeiss Axio Observer microscope.
Light microscopy
Cell suspensions were dropped on glass slides that were covered with a thin layer of agarose (2% w/v) and analysed using a Zeiss Axio Observer microscope equipped with objective lenses of 40-fold (Plan-Neofluar, 1.3 NA), 63-fold (Plan-Apochromat, 1.4 NA) and 100-fold (Plan-Apochromat, 1.4 NA) magnification. Epifluorescence microscopy was conducted using Gfp filter sets (ET470/40BP, ET495LP, BP525/50) and DAPI filter sets (HC 387/11, HC 447/60, BS 409). Filters were obtained from AHF Analysentechnik (Tübingen). Frames were taken with a CCD camera CoolSNAP HQ2 (Photometrics, Tucson). Microscope and camera were controlled by MetaMorph 7.5 (Molecular Devices, Ismaning). The same software was used for measurements and image processing including adjustment of brightness, contrast and γ values, as well as correction of background unevenness.
SEM microscopy
Cell suspensions were dropped on Poly-L-Lysine coated glass slides. Immediately after drying samples were fixed with 4% formaldehyde - 1% glutaraldehyde (v/v) in 0.2 M phosphate buffer (pH 7.4, modified from [115]) for one hour. Afterwards, samples were rinsed three times in 0,2 M phosphate buffer (pH 7.4), dehydrated in ethanol (50/75/100/100%), transferred to formaldehyde dimethyl acetal (FDA), critical-point dried, sputter-coated with gold-palladium for 200 s and analysed using a DSM 950 scanning electron microscope (Zeiss, Oberkochen, Germany).
Fluorimetric measurements of pheromone induced Gfp
U. maydis mutant strains expressing Gfp under the mfa1 promoters were confronted with a collection of haploid strains from different species and screened for Gfp fluorescence. Due to differences in mating behaviour of different species, matings were performed under two different conditions, in distilled water (pH 8.0) and in liquid PD (pH 8.0), for six hours at 28°C in 24 well plates. After incubation, 200 µl cell suspension was transferred to black-walled 96 well plates and relative fluorescence units (RFU) were measured at room temperature with excitation and emission wavelengths of 485 nm and 520 nm, respectively (bandwidth 9 nm and 20 nm, respectively) using a monochromator fluorescence reader (Tecan, Männedorf). Three independent experiments were performed. In order to compare different measurements of one experiment and due to differences in base fluorescence and optical densities between different fungal species and strains, OD600 dependent base fluorescence was subtracted from measured RFUs.
Supporting Information
Zdroje
1. WeismannA 1886 Zur Frage nach der Vererbung erworbener Eigenschaften. Biologisches Zentralblatt 6 33 48
2. HamiltonWDAxelrodRTaneseR 1990 Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci U S A 87 3566 3573
3. WilhelmDPalmerSKoopmanP 2007 Sex determination and gonadal development in mammals. Physiol Rev 87 1 28
4. JohnsonMTSmithSDRausherMD 2009 Plant sex and the evolution of plant defenses against herbivores. Proc Natl Acad Sci U S A 106 18079 18084
5. LeeSCNiMLiWShertzCHeitmanJ 2010 The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 74 298 340
6. SekidoRLovell-BadgeR 2009 Sex determination and SRY: down to a wink and a nudge? Trends Genet 25 19 29
7. BarrettSC 2010 Understanding plant reproductive diversity. Philos Trans R Soc Lond B Biol Sci 365 99 109
8. HarshmanLGZeraAJ 2007 The cost of reproduction: the devil in the details. Trends Ecol Evol 22 80 86
9. GoddardMRGodfrayHCBurtA 2005 Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434 636 640
10. AanenDKHoekstraRF 2007 Why Sex Is Good: On Fungi and Beyond. HeitmanJKronstadJWTaylorDRCasseltonLA Sex in fungi - Molecular Determination and Evolutionary Implications Washington D.C. ASM Press 527 534
11. CasseltonLA 2002 Mate recognition in fungi. Heredity 88 142 147
12. HeitmanJKronstadJWTaylorDRCasseltonLA 2007 Sex in fungi - Molecular Determination and Evolutionary Implications Washington ASM Press 572
13. KniepH 1928 Die Sexualität der niederen Pflanzen Jena Fisher 544
14. RaperJ 1966 Genetics of Sexuality in Higher Fungi New York The Ronald Press Co 283
15. CasseltonLAOlesnickyNS 1998 Molecular genetics of mating recognition in basidiomycete fungi. Microbiol Mol Biol Rev 62 55 70
16. KüesU 2000 Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Rev 64 316 353
17. KotheEGolaSWendlandJ 2003 Evolution of multispecific mating-type alleles for pheromone perception in the homobasidiomycete fungi. Curr Genet 42 268 275
18. RaudaskoskiMKotheE 2010 Basidiomycete mating type genes and pheromone signaling. Eukaryot Cell 9 847 859
19. HuGKampALinningRNaikSBakkerenG 2007 Complementation of Ustilago maydis MAPK mutants by a wheat leaf rust, Puccinia triticina homolog: potential for functional analyses of rust genes. Mol Plant Microbe Interact 20 637 647
20. FowlerTJDeSimoneSMMittonMFKurjanJRaperCA 1999 Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol Biol Cell 10 2559 2572
21. BakkerenGKronstadJW 1996 The pheromone cell signaling components of the Ustilago a mating-type loci determine intercompatibility between species. Genetics 143 1601 1613
22. MayGShawFBadraneHVekemansX 1999 The signature of balancing selection: fungal mating compatibility gene evolution. Proc Natl Acad Sci U S A 96 9172 9177
23. DevierBAguiletaGHoodMEGiraudT 2008 Ancient Trans-specific Polymorphism at Pheromone Receptor Genes in Basidiomycetes. Genetics 181 209 223
24. CasseltonLAKüesU 2007 The Origin of Multiple Mating Types in the Model Mushrooms Coprinopsis cinerea and Schizophyllum commune. HeitmanJKronstadJWTaylorDRCasseltonLA Sex in fungi - Molecular Determination and Evolutionary Implications Washington D.C. ASM Press 283 300
25. FraserJADiezmannSSubaranRLAllenALengelerKB 2004 Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biol 2 e384 doi:10.1371/journal.pbio.0020384
26. JamesTYSrivilaiPKüesUVilgalysR 2006 Evolution of the bipolar mating system of the mushroom Coprinellus disseminatus from its tetrapolar ancestors involves loss of mating-type-specific pheromone receptor function. Genetics 172 1877 1891
27. CoelhoMASampaioJPGoncalvesP 2010 A deviation from the bipolar-tetrapolar mating paradigm in an early diverged basidiomycete. PLoS Genet 6 e1001052 doi:10.1371/journal.pgen.1001052
28. SpelligTBölkerMLottspeichFFrankRWKahmannR 1994 Pheromones trigger filamentous growth in Ustilago maydis. EMBO J 13 1620 1627
29. XueCHsuehYPHeitmanJ 2008 Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol Rev 32 1010 1032
30. FeldbrüggeMBölkerMSteinbergGKämperJKahmannR 2006 Regulatory and structural networks orchestrating mating, dimorphism, cell shape, and pathogenesis in Ustilago maydis. KüesUFisherR The Mycota I: growth, differentiation and sexuality Heidelberg Springer-Verlag 375 391
31. FowlerTJMittonMFVaillancourtLJRaperCA 2001 Changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune. Genetics 158 1491 1503
32. OlesnickyNSBrownAJHondaYDyosSLDowellSJ 2000 Self-compatible B mutants in Coprinus with altered pheromone-receptor specificities. Genetics 156 1025 1033
33. GolaSKotheE 2003 The little difference: in vivo analysis of pheromone discrimination in Schizophyllum commune. Curr Genet 42 276 283
34. SzaboZTönnisMKesslerHFeldbrüggeM 2002 Structure-function analysis of lipopeptide pheromones from the plant pathogen Ustilago maydis. Mol Genet Genomics 268 362 370
35. KostedPJGerhardtSAAndersonCMStierleASherwoodJE 2000 Structural requirements for activity of the pheromones of Ustilago hordei. Fungal Genet Biol 29 107 117
36. FisherGWHoltonCS 1957 Biology and Control of the Smut Fungi New York The Ronald Press Company 622
37. StollMBegerowDOberwinklerF 2005 Molecular phylogeny of Ustilago, Sporisorium, and related taxa based on combined analyses of rDNA sequences. Mycol Res 109 342 356
38. BegerowDStollMBauerR 2006 A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia 98 906 916
39. UrbanMKahmannRBölkerM 1996 The biallelic a mating type locus of Ustilago maydis: remnants of an additional pheromone gene indicate evolution from a multiallelic ancestor. Mol Gen Genet 250 414 420
40. SnetselaarKMBölkerMKahmannR 1996 Ustilago maydis Mating Hyphae Orient Their Growth toward Pheromone Sources. Fungal Genet Biol 20 299 312
41. BakkerenGJiangGWarrenRLButterfieldYShinH 2006 Mating factor linkage and genome evolution in basidiomycetous pathogens of cereals. Fungal Genet Biol 43 655 666
42. SchirawskiJHeinzeBWagenknechtMKahmannR 2005 Mating type loci of Sporisorium reilianum: novel pattern with three a and multiple b specificities. Eukaryot Cell 4 1317 1327
43. KämperJKahmannRBölkerMMaLJBrefortT 2006 Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 97 101
44. BrefortTDoehlemannGMendoza-MendozaAReissmannSDjameiA 2009 Ustilago maydis as a Pathogen. Annu Rev Phytopathol 47 423 445
45. VollmeisterESchipperKBaumannSHaagCPohlmannT 2011 Fungal development of the plant pathogen Ustilago maydis. FEMS Microbiol Rev DOI:10.1111/j.1574-6976.2011.00296.x
46. KaffarnikFMüllerPLeibundgutMKahmannRFeldbrüggeM 2003 PKA and MAPK phosphorylation of Prf1 allows promoter discrimination in Ustilago maydis. EMBO J 22 5817 5826
47. ZarnackKEichhornHKahmannRFeldbrüggeM 2008 Pheromone-regulated target genes respond differentially to MAPK phosphorylation of transcription factor Prf1. Mol Microbiol 69 1041 1053
48. KahmannRKämperJ 2004 Ustilago maydis: how its biology relates to pathogenic development. New Phytologist 164 31 42
49. RowellJBDeVayJE 1954 Genetics of Ustilago zeae in relation to basic problems of its pathogenicity. Phytopathology 44 356 362
50. FedlerMLuhKSStelterKNieto-JacoboFBasseCW 2008 The a2 Mating-type Locus Genes lga2 and rga2 Direct Uniparental mtDNA Inheritance and Constrain Mitochondrial DNA Recombination During Sexual Development of Ustilago maydis. Genetics 181 847 860
51. AltschulSFMaddenTLSchäfferAAZhangJZhangZ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
52. WebbCOAckerlyDDKembelSW 2008 Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24 2098 2100
53. BannaiHTamadaYMaruyamaONakaiKMiyanoS 2002 Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18 298 305
54. BortfeldMAuffarthKKahmannRBasseCW 2004 The Ustilago maydis a2 mating-type locus genes lga2 and rga2 compromise pathogenicity in the absence of the mitochondrial p32 family protein Mrb1. Plant Cell 16 2233 2248
55. StankeMDiekhansMBaertschRHausslerD 2008 Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24 637 644
56. SchultzJMilpetzFBorkPPontingCP 1998 SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A 95 5857 5864
57. LetunicIDoerksTBorkP 2009 SMART 6: recent updates and new developments. Nucleic Acids Res 37 D229 32
58. RomeisTBrachmannAKahmannRKämperJ 2000 Identification of a target gene for the bE-bW homeodomain protein complex in Ustilago maydis. Mol Microbiol 37 54 66
59. BrachmannAWeinzierlGKämperJKahmannR 2001 Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol Microbiol 42 1047 1063
60. PrasadVStrombergCAAlimohammadianHSahniA 2005 Dinosaur coprolites and the early evolution of grasses and grazers. Science 310 1177 1180
61. CasseltonLAFeldbrüggeM 2010 Mating and sexual morphogenesis in basidiomycete fungi. BorkovichKEbboleD Cellular and Molecular Biology of Filamentous Fungi Washington D.C. ASM Press 536 551
62. LiWMetinBWhiteTCHeitmanJ 2010 Organization and evolutionary trajectory of the mating type (MAT) locus in dermatophyte and dimorphic fungal pathogens. Eukaryot Cell 9 46 58
63. OhmRAde JongJFLugonesLGAertsAKotheE 2010 Genome sequence of the model mushroom Schizophyllum commune. Nat Biotechnol 28 957 963
64. HsuehYPFraserJAHeitmanJ 2008 Transitions in sexuality: recapitulation of an ancestral tri - and tetrapolar mating system in Cryptococcus neoformans. Eukaryot Cell 7 1847 1855
65. XuJSaundersCWHuPGrantRABoekhoutT 2007 Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci U S A 104 18730 18735
66. MetinBFindleyKHeitmanJ 2010 The mating type locus (MAT) and sexual reproduction of Cryptococcus heveanensis: insights into the evolution of sex-determining chromosomal regions in fungi. PLoS Genet 6 e1000961 doi:10.1371/journal.pgen.1000961
67. SwansonWJVacquierVD 2002 The rapid evolution of reproductive proteins. Nat Rev Genet 3 137 144
68. ClarkNLAagaardJESwansonWJ 2006 Evolution of reproductive proteins from animals and plants. Reproduction 131 11 22
69. WikLKarlssonMJohannessonH 2008 The evolutionary trajectory of the mating-type (mat) genes in Neurospora relates to reproductive behavior of taxa. BMC Evol Biol 8 109
70. MüllerPWeinzierlGBrachmannAFeldbrüggeMKahmannR 2003 Mating and pathogenic development of the Smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot Cell 2 1187 1199
71. HakesLLovellSCOliverSGRobertsonDL 2007 Specificity in protein interactions and its relationship with sequence diversity and coevolution. Proc Natl Acad Sci U S A 104 7999 8004
72. CosmidesLMToobyJ 1981 Cytoplasmic inheritance and intragenomic conflict. J Theor Biol 89 83 129
73. BirkyCW 1995 Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci U S A 92 11331 11338
74. PartridgeLHurstLD 1998 Sex and conflict. Science 281 2003 2008
75. XuJ 2005 The inheritance of organelle genes and genomes: patterns and mechanisms. Genome 48 951 958
76. BasseCW 2010 Mitochondrial inheritance in fungi. Current Opinion in Microbiology 13 712 719
77. BaackEJRiesebergLH 2007 A genomic view of introgression and hybrid speciation. Curr Opin Genet Dev 17 513 518
78. BurkeJMArnoldML 2001 Genetics and the fitness of hybrids. Annu Rev Genet 35 31 52
79. MalletJ 2008 Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Philos Trans R Soc Lond B Biol Sci 363 2971 2986
80. GiraudTRefregierGLe GacMde VienneDMHoodME 2008 Speciation in fungi. Fungal Genet Biol 45 791 802
81. QvarnströmABaileyRI 2008 Speciation through evolution of sex-linked genes. Heredity 102 4 15
82. MalletJ 2005 Hybridization as an invasion of the genome. Trends Ecol Evol 20 229 237
83. MalletJ 2007 Hybrid speciation. Nature 446 279 283
84. BegerowDGökerMLutzMStollM 2004 On the evolution of smut fungi on their hosts. AgererRPiepenbringMBlanzP Frontiers in basidiomycote mycology Eching IHW Verlag 81 98
85. MunkacsiABStoxenSMayG 2007 Domestication of maize, sorghum, and sugarcane did not drive the divergence of their smut pathogens. Evolution 61 388 403
86. NewcombeGStirlingBBradshawHD 2001 Abundant Pathogenic Variation in the New Hybrid Rust Melampsora xcolumbiana on Hybrid Poplar. Phytopathology 91 981 985
87. BensonDAKarsch-MizrachiILipmanDJOstellJSayersEW 2011 GenBank. Nucleic Acids Res 39 D32 7
88. BanuettFHerskowitzI 1989 Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci U S A 86 5878 5882
89. BrachmannAKönigJJuliusCFeldbrüggeM 2004 A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol Genet Genomics 272 216 226
90. HoffmanCSWinstonF 1987 A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57 267 272
91. WhiteTJBrunsTDLeeSTaylorDR 1990 Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. InnisMAGelfandDHSninskyJWhiteTJ PCR Protocols: a Guide to Methods and Amplification San Diego Academic Press 315 322
92. GargasATaylorJW 1992 Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84 589 592
93. MoncalvoJMWangHHseuRS 1995 Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87 223 238
94. VigalysRHesterM 1990 Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172 4238 4246
95. RehnerSABuckleyE 2005 A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97 84 98
96. Oligo-Calc: Oliginucleotide Properties Calculator. Available: http://basic.northwestern.edu/biotools/OligoCalc.html via the Internet
97. KibbeWA 2007 OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35 W43 46 doi:10.1093/nar/gkm234
98. KatohKAsimenosGTohH 2009 Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537 39 64
99. HallTA 1999 BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41 95 98
100. ThompsonJDHigginsDGGibsonTJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
101. FelsensteinJ 1981 Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17 368 376
102. StamatakisALudwigTMeierH 2005 RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21 456 463
103. StamatakisAHooverPRougemontJ 2008 A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57 758 771
104. RonquistFHuelsenbeckJP 2003 MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572 1574
105. MUMDB MIPS Ustilago Maydis DataBase. Available: http://mips.helmholtz-muenchen.de/genre/proj/ustilago via the Internet
106. MSRDB MIPS Sporisorium Reilianum DataBase. Available: http://mips.helmholtz-muenchen.de/genre/proj/sporisorium/ via the Internet
107. HofmannKStoffelW 1993 TMbase - A database of membrane spanning proteins segments. Biol Chem 374 166
108. NugentTJonesDT 2009 Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics 10 159
109. BuchanDWWardSMLobleyAENugentTCBrysonK 2010 Protein annotation and modelling servers at University College London. Nucleic Acids Res 38 W563 8
110. LibradoPRozasJ 2009 DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25 1451 1452
111. YangZ 1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 555 556
112. YangZ 2007 PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol Biol Evol 24 1586 1591
113. YangZWongWSNielsenR 2005 Bayes empirical bayes inference of amino acid sites under positive selection. Mol Biol Evol 22 1107 1118
114. KorberB 2000 Computational Analysis of HIV Molecular Sequences. RodrigoAGLearnGH HIV Signature and Sequence Variation Analysis Netherlands Kluwer Academic Publishers 55 72
115. McDowellEMTrumpBF 1976 Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100 405 414
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



