-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
Fossil records indicate that life appeared in marine environments ∼3.5 billion years ago (Gyr) and transitioned to terrestrial ecosystems nearly 2.5 Gyr. Sequence analysis suggests that “hydrobacteria” and “terrabacteria” might have diverged as early as 3 Gyr. Bacteria of the genus Azospirillum are associated with roots of terrestrial plants; however, virtually all their close relatives are aquatic. We obtained genome sequences of two Azospirillum species and analyzed their gene origins. While most Azospirillum house-keeping genes have orthologs in its close aquatic relatives, this lineage has obtained nearly half of its genome from terrestrial organisms. The majority of genes encoding functions critical for association with plants are among horizontally transferred genes. Our results show that transition of some aquatic bacteria to terrestrial habitats occurred much later than the suggested initial divergence of hydro - and terrabacterial clades. The birth of the genus Azospirillum approximately coincided with the emergence of vascular plants on land.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002430
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002430Summary
Fossil records indicate that life appeared in marine environments ∼3.5 billion years ago (Gyr) and transitioned to terrestrial ecosystems nearly 2.5 Gyr. Sequence analysis suggests that “hydrobacteria” and “terrabacteria” might have diverged as early as 3 Gyr. Bacteria of the genus Azospirillum are associated with roots of terrestrial plants; however, virtually all their close relatives are aquatic. We obtained genome sequences of two Azospirillum species and analyzed their gene origins. While most Azospirillum house-keeping genes have orthologs in its close aquatic relatives, this lineage has obtained nearly half of its genome from terrestrial organisms. The majority of genes encoding functions critical for association with plants are among horizontally transferred genes. Our results show that transition of some aquatic bacteria to terrestrial habitats occurred much later than the suggested initial divergence of hydro - and terrabacterial clades. The birth of the genus Azospirillum approximately coincided with the emergence of vascular plants on land.
Introduction
Fossil records indicate that life appeared in marine environments ∼3.5–3.8 billion years ago (Gyr) [1] and transitioned to terrestrial ecosystems ∼2.6 Gyr [2]. The lack of fossil records for bacteria makes it difficult to assess the timing of their transition to terrestrial environments; however sequence analysis suggests that a large clade of prokaryotic phyla (termed “terrabacteria”) might have evolved on land as early as 3 Gyr, with some lineages later reinvading marine habitats [3]. For example, cyanobacteria belong to the terrabacterial clade, but one of its well-studied representatives, Prochlorococcus, is the dominant primary producer in the oceans [4].
Bacteria of the genus Azospirillum are found primarily in terrestrial habitats, where they colonize roots of important cereals and other grasses and promote plant growth by several mechanisms including nitrogen fixation and phytohormone secretion [5], [6]. Azospirillum belong to proteobacteria, one of the largest groups of “hydrobacteria”, a clade of prokaryotes that originated in marine environments [3]. Nearly all known representatives of its family Rhodospirillaceae are found in aquatic habitats (Figure 1 and Table S1) suggesting that Azospirillum represents a lineage which might have transitioned to terrestrial environments much later than the Precambrian split of “hydrobacteria” and “terrabacteria”. To obtain insight into how bacteria transitioned from marine to terrestrial environments, we sequenced two well studied species, A. brasilense and A. lipoferum, and a third genome of an undefined Azospirillum species became available while we were carrying out this work [7].
Fig. 1. Habitats of Azospirillum and its closest aquabacterial relatives. 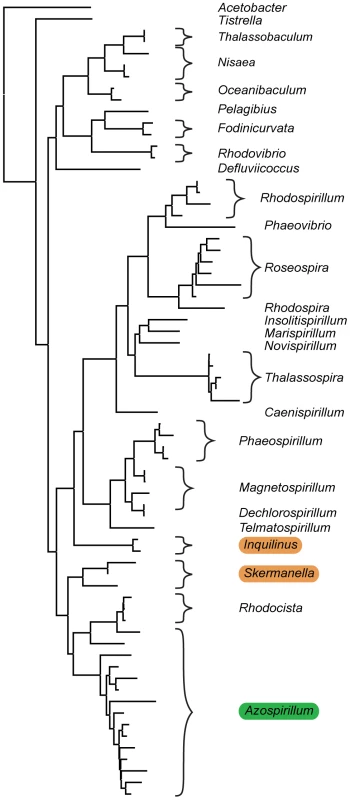
A maximum-likelihood tree built from 16S rRNA sequences from members of Rhodospirillaceae. Acetobacter acetii, a member of the same order Rhodospirillales, but a different family, Acetobacteriaceae, is shown as an outgroup. Aquatic inhabitants are not highlighted; terrestrial are highlighted in brown and plant-associated Azospirillum is highlighted in green. See Table S1 for details. Results/Discussion
In contrast to the genomes of their closest relatives (other Rhodospirillaceae), the three Azospirillum genomes are larger and are comprised of not one, but seven replicons each (Figure S1 and Table 1). Multiple replicons have been previously suggested for various Azospirillum strains [8]. The largest replicon in each genome has all characteristics of a bacterial chromosome, whereas the smallest is a plasmid. Five replicons in the genomes of A. lipoferum and Azospirillum Sp. 510 can be defined as “chromids” (intermediates between chromosomes and plasmids [9]), whereas in A. brasilense only three replicons are “chromids” (Tables S2 and S3). While multiple replicons, and chromids specifically, are not unusual in proteobacteria [9], [10], Azospirillum lipoferum has the largest number of chromids among all prokaryotes sequenced to date [9] indicating a potential for genome plasticity.
Tab. 1. General features of <i>Azospirillum</i> genomes. 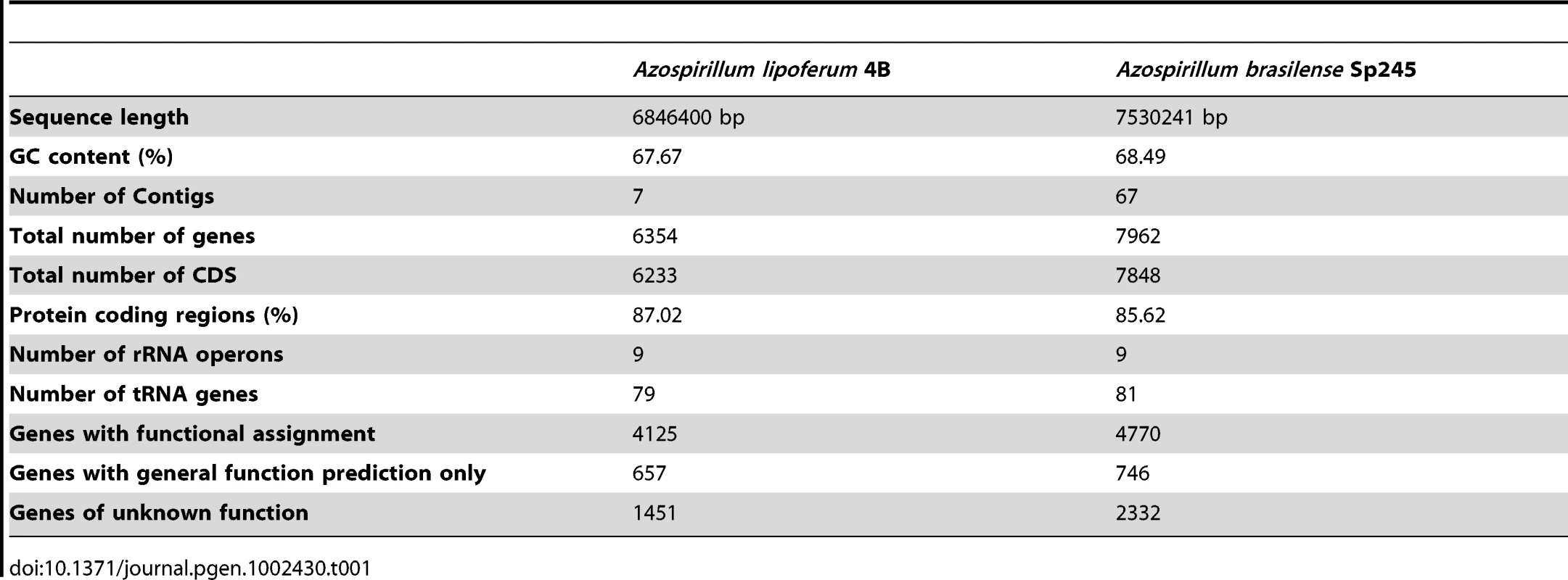
Comparisons among the three genomes reveal further evidence of extraordinary genome plasticity in Azospirillum, a feature that has also been suggested by some experimental data [11]. We found very little synteny between replicons of Azospirillum species. The genetic relatedness among Azospirillum strains is comparable to that of rhizobia, other multi-replicon alpha-proteobacteria (Table S4). Surprisingly, we found substantially more genomic rearrangement within Azospirillum genomes than within rhizobial genomes (Figure 2) that are suggested to exemplify genome plasticity in prokaryotes [10]. This could be a consequence of many repetitive sequences and other recombination hotspots (Tables S4 and S5), although the detailed mechanisms underlying such extraordinary genome plasticity remain incompletely understood.
Fig. 2. Whole-genome alignments for Azospirillum and related multi-replicon rhizobial species. 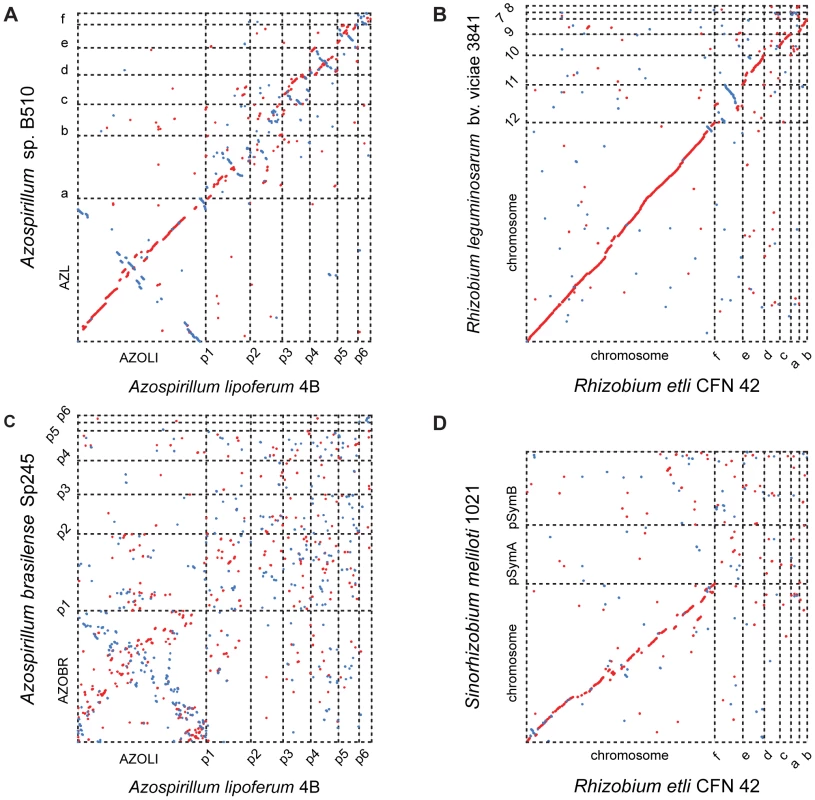
Relative distances between genomes (calculated from a concatenated ribosomal protein tree): A. lipoferum 4B to Azospirillum sp.510 – 0.01; Rhizobium etli to Rhizobium leguminosarum – 0.02; A. lipoferum 4B to A. brasilense Sp245 – 0.10; Rhizobium etli to S. meliloti – 0.11. Which genes does Azospirillum share with its aquatic relatives, and what is the origin of its additional genes? To answer this question, we developed a robust scheme for detecting ancestral and horizontally transferred (HGT) genes (Figure 3) using bioinformatics tools, then classified most protein coding genes in the Azospirillum genomes as ancestral or horizontally transferred with quantified degrees of confidence (Figure 4A and Table S6). Remarkably, nearly half of the genes in each Azospirillum genome whose origins can be resolved appeared to be horizontally transferred. As a control, we subjected the genomes of other Rhodospirillaceae to the same analysis, finding a substantially lower HGT level in aquatic species, while the number of ancestral genes in all organisms was comparable (Figure 4B). Horizontally transferred genes are frequently expendable, whereas ancestral genes usually serve ‘house-keeping’ functions and are conserved over long evolutionary distances [12]. To further validate our classifications, we determined functional assignments of genes in each of the two categories using the COG database [13]. The ‘ancestral’ set primarily contained genes involved in ‘house-keeping’ functions such as translation, posttranslational modification, cell division, and nucleotide and coenzyme metabolism (Figure 5). The HGT set contained a large proportion of genes involved in specific dispensable functions, such as defense mechanisms, cell wall biogenesis, transport and metabolism of amino acids, carbohydrates, inorganic ions and secondary metabolites (Figure 5 and Table S6). This is consistent with the role of HGT in adaptation to the rhizosphere, an environment rich in amino acids, carbohydrates, inorganic ions and secondary metabolites excreted by plant roots [14].
Fig. 3. Scheme for detecting ancestral and horizontally transferred genes. 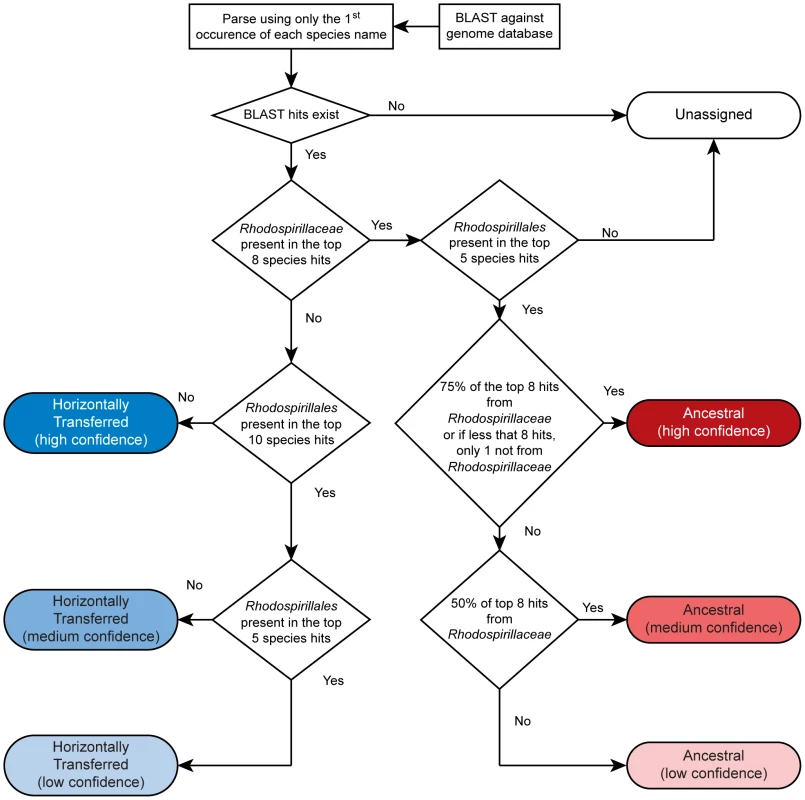
See Materials and Methods for details. Fig. 4. Ancestral (red) and horizontally transferred (blue) genes in Azospirillum. 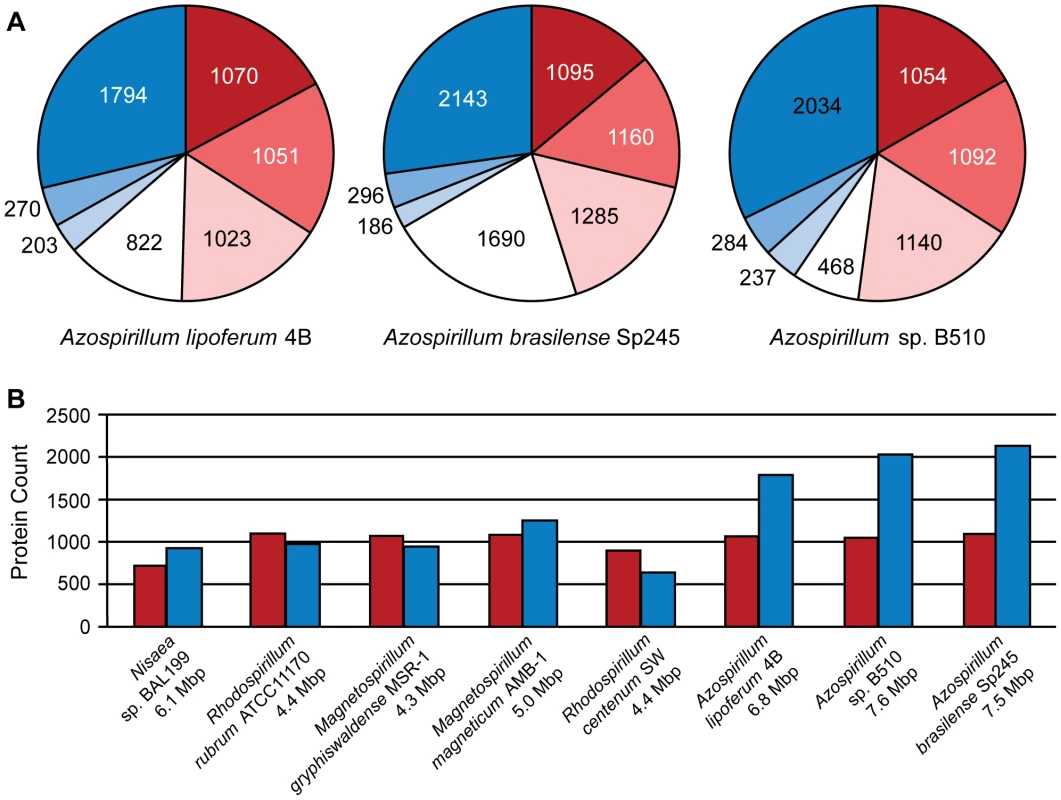
(A) Proportion of ancestral and horizontally transferred genes predicted in three Azospirillum genomes with varying confidence: intensity of color shows high (dark), medium (medium) and low (light) levels of confidence for predictions (see Materials and Methods). Genes that cannot be assigned using this protocol are shown in white. Majority of these genes are unique to each species and have no identifiable homologs; thus, they are likely the result of HGT. (B) Proportion of ancestral and horizontally transferred genes in genomes of Rhodospirillaceae. Only genes that were predicted with high confidence are shown. Fig. 5. Functional categories for A. lipoferum 4B genes enriched in ancestral (top) and horizontally transferred (bottom) genes. 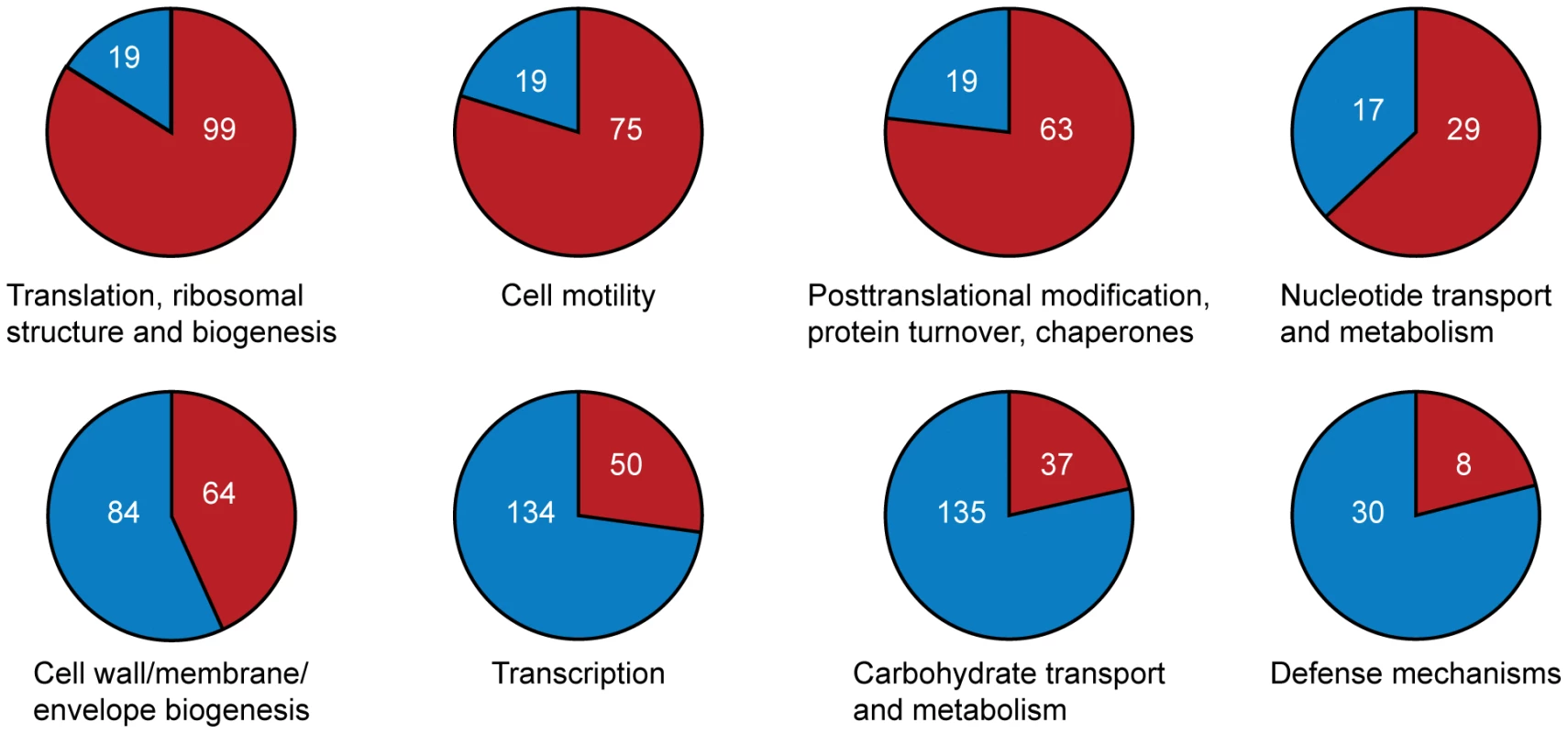
Only genes that were predicted with high confidence are shown. Such an extraordinary high level of HGT in Azospirillum genomes leads us to hypothesize that it was a major driving force in the transition of these bacteria from aquatic to terrestrial environments and adaptation to plant hosts. This process was likely promoted by conjugation and transduction, as Azospirillum hosts phages and notably a Gene Transfer Agent [15]; this should have also resulted in loss of ancestral ‘aquatic’ genes that are not useful in the new habitat. Indeed, one of the defining features of Rhodospirillaceae, photosynthesis (responsible for the original taxonomic naming of these organisms – purple bacteria) is completely absent from Azospirillum. We have analyzed origins of genes that are proposed to be important for adaptation to the rhizosphere and interactions with the host plant [6], [16]. Consistent with our hypothesis, the majority of these genes were predicted to be horizontally transferred (Figure 6 and Table S7). It is important however to stress that plant-microbe interactions involve a complex interplay of many functions that are determined by both ancestral and horizontally acquired genes.
Fig. 6. Proportion of ancestral (red) and horizontally transferred (blue) genes involved in adaptation of Azospirillum to the rhizosphere and its interaction with host plants (see Table S7 for details). 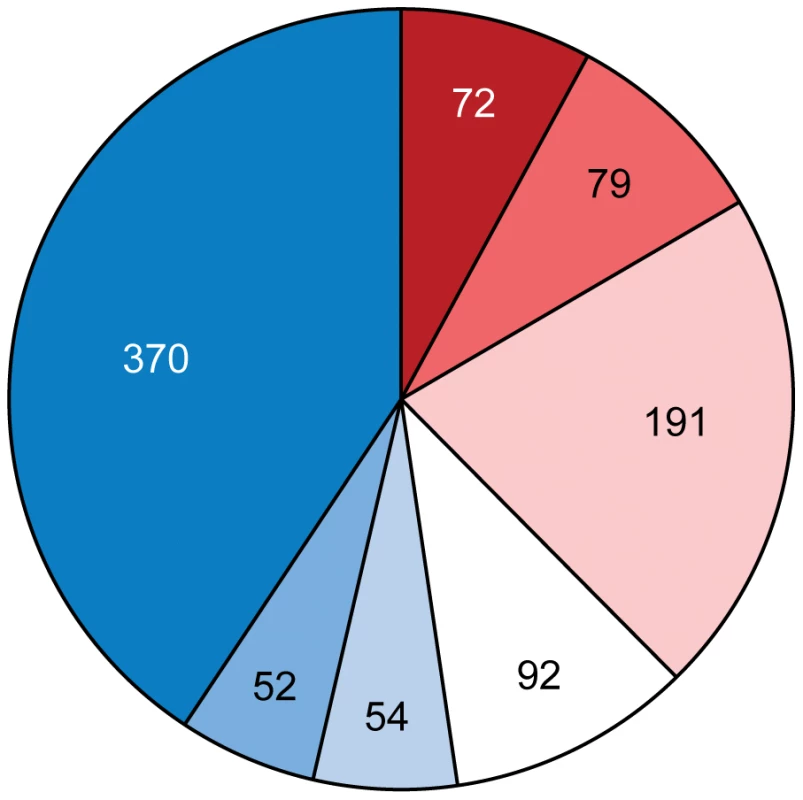
Color intensity indicates high (dark), medium (medium) and low (light) confidence levels for prediction (see Materials and Methods for details). What was the source of horizontally transferred genes? A large proportion of genes that we assigned as HGT show relatedness to terrestrial proteobacteria, including representatives of Rhizobiales (distantly related alpha-proteobacteria) and Burkholderiales (beta-proteobacteria) (Figure 7) that are soil and plant-associated organisms. In the absence of fossil data, it is nearly impossible to determine the time of divergence for a specific bacterial lineage; however, a rough approximation (1–2% divergence in the 16S rRNA gene equals 50 Myr [17]) suggests that azospirilla might have diverged from their aquatic Rhodospirillaceae relatives 200–400 Myr (Table S8). This upper time limit coincides with the initial major radiation of vascular plants on land and evolution of plant roots, to 400 Myr [18], [19]. Grasses, the main plant host for Azospirillum, appeared much later, about 65–80 Myr [20], which is consistent with reports that azospirilla can also colonize plants other than grasses.
Fig. 7. Taxonomic distribution of the best BLAST hits for predicted HGT in <i>Azospirillum</i>. 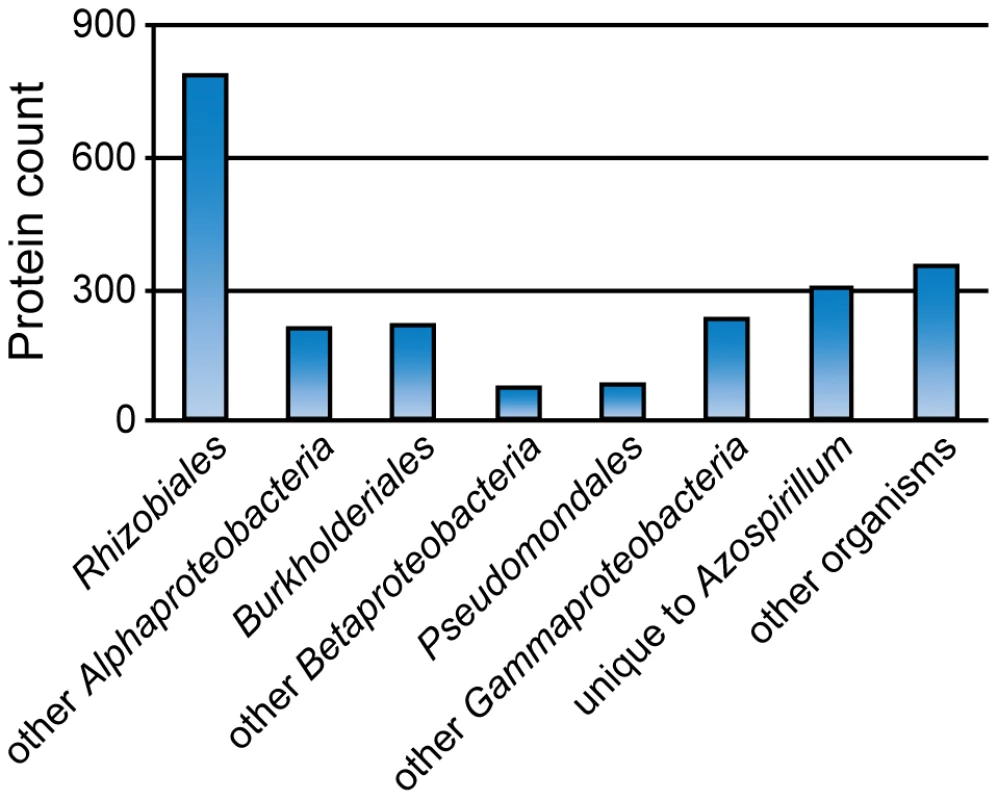
Using a global proteomics approach we have found that many HGT genes including nearly 1/3 of those that are common to all three Azospirillum genomes were expressed under standard experimental conditions and under nitrogen limitation, a condition usually encountered in the rhizosphere of natural ecosystems (Figure 8 and Table S9).
Fig. 8. Proportion of ancestral (red) and horizontally transferred (blue) genes in the proteomics data for A. lipoferum 4B. 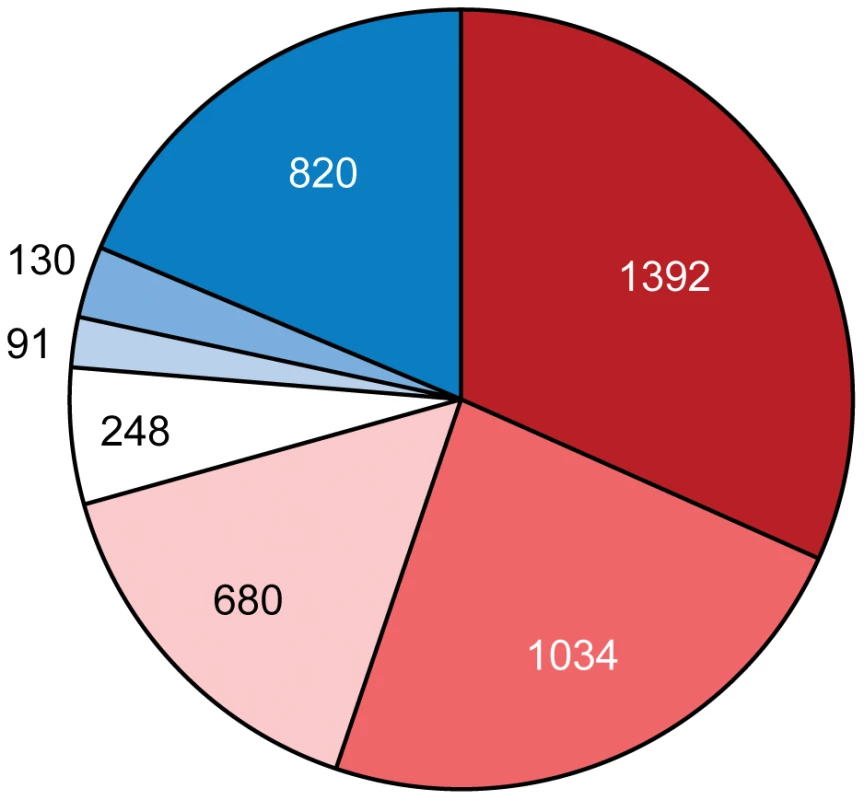
Color intensity indicates high (dark), medium (medium) and low (light) confidence levels for prediction. See Table S9 for details. Genes that differentiated the Azospirillum species from one another and from their closest relatives are implicated in specializations, such as plant colonization. Azospirillum and closely related Rhodospirillum centenum both possess multiple chemotaxis operons and are model organisms to study chemotaxis [21], [22]. Interestingly, operon 1, which controls chemotaxis in R. centenum [22], plays only a minor role in chemotaxis of A. brasilense [23]. All three Azospirillum species possess three chemotaxis operons that are orthologous to those in R. centenum; however, they also have additional chemotaxis operons that are absent from their close aquatic relative (Figure S2 and Tables S6 and S10). Additional chemotaxis operons have been acquired by azospirilla prior to each speciation event yielding 4, 5 and 6 chemotaxis systems in A. brasilense Sp245, A. lipoferum 4B and Azospirillum sp. 510, respectively. These stepwise acquisitions have made the latter organism an absolute “chemotaxis champion”, with 128 chemotaxis genes, more than any other prokaryote sequenced to date (data from MiST database [24]). Recent analysis showed the prevalence of chemotaxis genes in the rhizosphere [25]. We have determined that the dominant chemotaxis genes in this dataset belong to a specific chemotaxis class F7 [26] (Figure S3 and Table S11). Strikingly, it is this F7 system that is shared by all Azospirillum and is predicted to have been transferred horizontally into their common ancestor.
Cellulolytic activity may be crucial to the ability of some azospirilla to penetrate plant roots [27]. All Azospirillum genomes encode a substantial number of glycosyl hydrolases that are essential for decomposition of plant cell walls (Figure 9). The total number of putative cellulases and hemicellulases in azospirilla is comparable to that in soil cellulolytic bacteria (Table S12) and most of them are predicted to be acquired horizontally (Table S6). We tested three Azospirillum species and found detectable cellulolytic activity in A. brasilense Sp245 (Figure 10). The A. brasilense Sp245 genome contains three enzymes encoded by AZOBR_p470008, AZOBR_p1110164 and AZOBR_150049 (Figure 11) that are orthologous to biochemically verified cellulases. We propose that these and other horizontally transferred genes (e.g. glucuronate isomerase, which is involved in pectin decomposition) contributed to establishing A. brasilense Sp245 as a successful endophyte [27]. Interestingly, another successful endophytic bacterium, Herbaspirillum seropedicae, lacks the genes coding for plant cell wall degradation enzymes [28] indicating that endophytes may use very different strategies for penetrating the plant.
Fig. 9. Glycoside hydrolases in Azospirillum with a potential to degrade the plant cell wall. 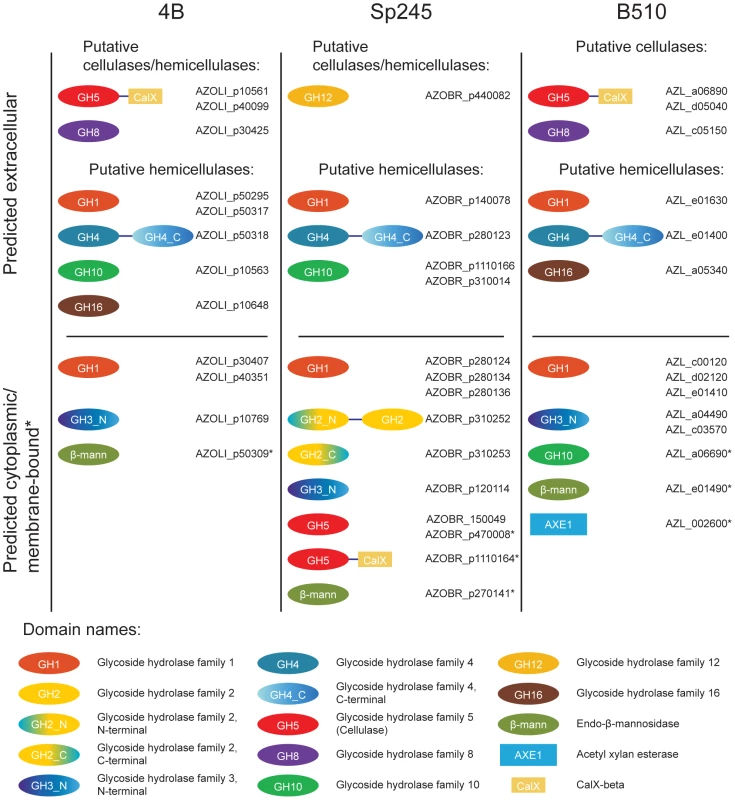
The genomes of Azospirillum encode from 26 to 34 glycoside hydrolases that belong to various CAZy [54] families (Table S12). Total number of glycoside hydrolases in Azospirillum species is similar to that in a soil cellulolytic bacterium Thermobifida fusca [61]. All three species have orthologs of putative cellulases (AZOLI_p10561, AZOLI_p40099; AZOBR_p1110164; AZL_a06890; AZL_d05040) with unique domain architecture: GH_5 – CalX-β. The other two putative cellulases (AZOBR_150049, AZOBR_p470008) are found only in A. brasilense. In addition to putative cellulases, Azospirillum species encode putative extracellular endoglucanases that may be involved in cellulose/hemicellulose degradation. For example, glycoside hydrolases that belong to family GH8 (AZOLI_p30425, AZL_c05150), which are known for a wide range of cellulose-containing substrates [62]–[64] and family GH12 (AZOBR_p440082). All three species are predicted to secrete a number of putative hemicellulases, that belong to glycoside hydrolase families GH1 (β-glycosidases), GH4 (glucuronidase/galactosidase), GH10 (endo-xylanases) and GH16 (licheninases) (Table S12). CAZy families were assigned as described in Materials and Methods. Fig. 10. Cellulolytic activity of A. brasilense Sp245 cells. 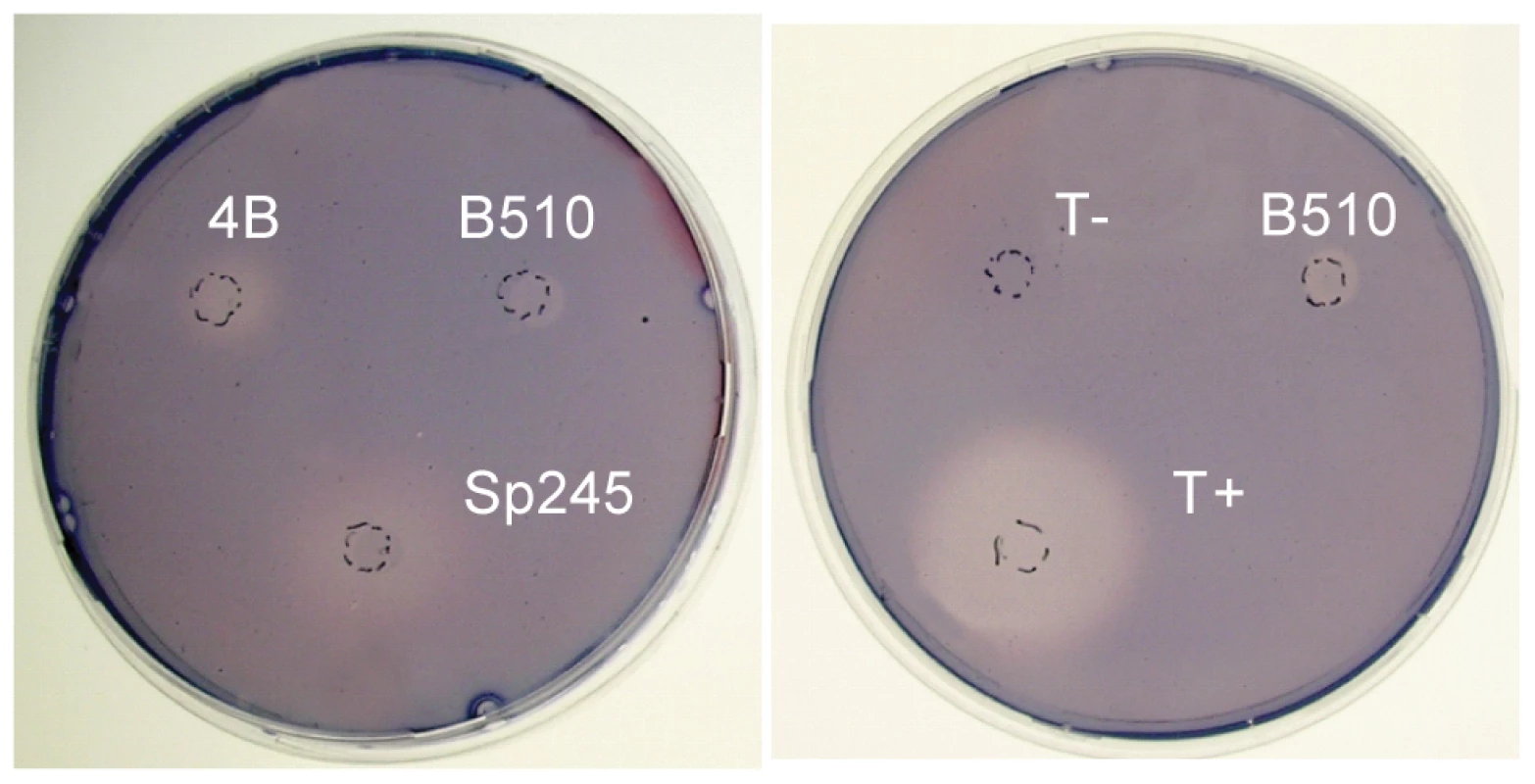
All three Azospirillum species are shown on the left panel. Known cellulose degrader (Dickeya dadantii 3937, T+) and non-degrader (Agrobacterium tumefaciens NT1, T-) are shown as positive and negative controls, respectively. Fig. 11. Phylogenetic trees for thiamine synthetase (left) and cellulase (right). 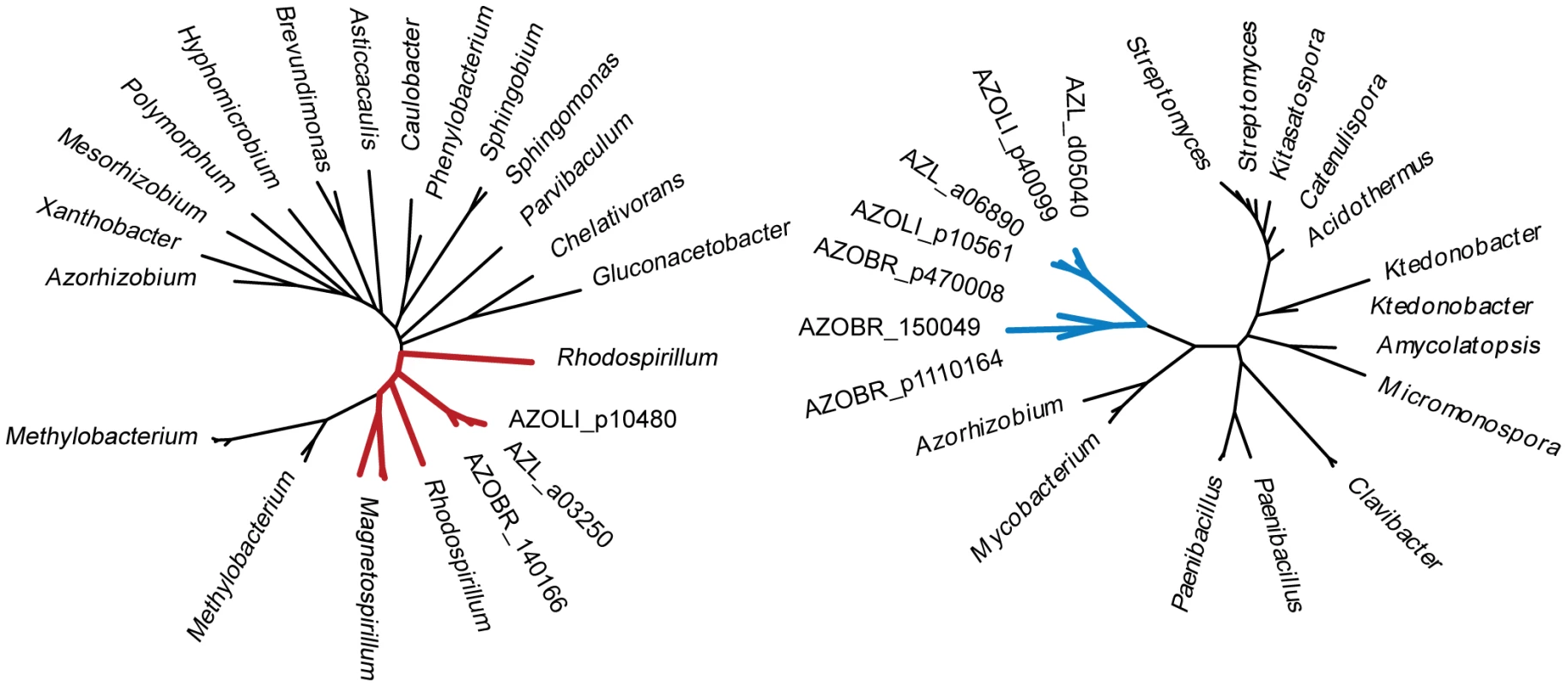
The trees exemplify ancestral and HGT relationships, respectively, that were predicted with high confidence. Trees were built from aligned sequences of the A. brasilense Sp245 query and twenty most similar sequences determined by BLAST. The thiamine synthetase set contains only representatives of alpha-proteobacteria including Rhodospirillaceae (shown in red). The cellulase set consists of representatives of Actinobacteria, Firmicutes, and Chloroflexi with only one representative of alpha-proteobacteria other than Azospirillum (that are shown in blue, highlighting their HGT origin), Azorhizobium. Attachment, another function important for plant association by Azospirillum, was also acquired horizontally. Type IV pili is a universal feature for initiating and maintaining contact with the plant host [29], [30]. The genome of A. brasilense Sp245 lacks genes coding for Type IV pili, but encodes a set of genes for TAD (tight adhesion) pili that are known to be HGT prone [31]. In our analysis, TAD pili were confidently predicted to be a result of HGT (Table S6). We show that a mutant deficient in TAD pili had a severe defect in attachment and biofilm formation (Figure 12) suggesting a role for TAD in plant-microbe association.
Fig. 12. TAD pili in A. brasilense are required for biofilm formation. 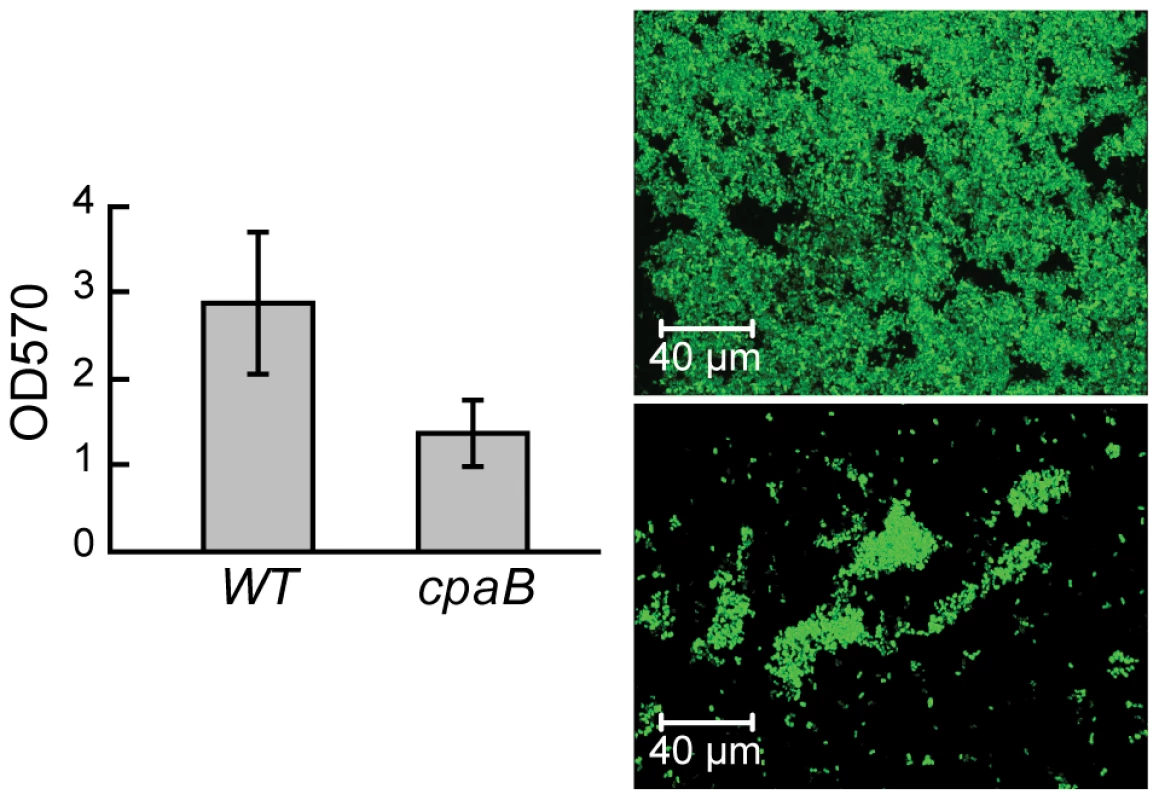
Quantification of biofilm formed by wild type (wt) and a pili mutant (cpaB) on glass using crystal violet staining (left panel) and 3-D-reconstruction of the biofilm formed by wild type (top) and a pili mutant (bottom) by confocal microscopy (right panel). Concluding remarks
Horizontal gene transfer has been long recognized as a major evolutionary force in prokaryotes [12]. Its role in the emergence of new pathogens and adaptation to environmental changes is well documented [32]. While other recent studies indicate that HGT levels in natural environments may reach as much as 20% of a bacterial genome [33], our data suggest that HGT has affected nearly 50% of the Azospirillum genomes, in close association with dramatic changes in lifestyle necessary for transition from aquatic to terrestrial environments and association with plants. Emergence of these globally distributed plant-associated bacteria, which appear to coincide with radiation of land plants and root development, likely has dramatically changed the soil ecosystem.
Materials and Methods
Genome sequencing and assembly
The genome of Azospirillum lipoferum 4B was sequenced by the whole random shotgun method with a mixture of ∼12X coverage of Sanger reads, obtained from three different libraries, and ∼18X coverage of 454 reads. Two plasmid libraries of 3 kb (A) and 10 kb (B), obtained by mechanical shearing with a Hydroshear device (GeneMachines, San Carlos, California, USA), were constructed at Genoscope (Evry, France) into pcDNA2.1 (Invitrogen) and into the pCNS home vector (pSU18 modified, Bartolome et al.[34]), respectively. Large inserts (40 kb) (C) were introduced into the PmlI site of pCC1FOS. Sequencing with vector-based primers was carried out using the ABI 3730 Applera Sequencer. A total of 95904 (A), 35520 (B) and 15360 (C) reads were analysed and assembled with 504591 reads obtained with Genome Sequencer FLX (Roche Applied Science). The Arachne “HybridAssemble” version (Broad institute, MA) combining 454 contigs with Sanger reads was used for assembly. To validate the assembly, the Mekano interface (Genoscope), based on visualization of clone links inside and between contigs, was used to check the clones coverage and misassemblies. In addition, the consensus was confirmed using Consed functionalities (www.phrap.org), notably the consensus quality and the high quality discrepancies. The finishing step was achieved by PCR, primer walks and transposon bomb libraries and a total of 5460 sequences (58, 602 and 4800 respectively) were needed for gap closure and quality assessment.
The genome of strain Azospirillum brasilense Sp245 was sequenced by the whole random shotgun method with a mixture of ∼10X coverage of Sanger reads obtained from three different libraries and ∼25X coverage of 454 reads. A plasmid library of 3 kb, obtained by mechanical shearing with a Hydroshear device (GeneMachines, San Carlos, California, USA), were constructed at Plant Genome Mapping Laboratory (University of Georgia, USA) into pcDNA2.1 vector (Invitrogen). Large inserts (40 kb) were introduced into the PmlI site of pCC1FOS. Sequencing with vector-based primers was carried out using the ABI 3730 Applera Sequencer. The Arachne “HybridAssemble” version combining 454 contigs with Sanger reads was used for assembly. Contig scaffolds were created using Sequencher (Gene Codes) and validated using clone link inside and between contigs.
Genome annotation
AMIGene software [35] was used to predict coding sequences (CDSs) that were submitted to automatic functional annotation [36]. The resulting 6233 A. lipoferum 4B CDSs and 7848 A. brasilense Sp245 CDSs were assigned a unique identifier prefixed with “AZOLI” or “AZOBR” according to their respective genomes. Putative orthologs and synteny groups were computed between the sequenced genomes and 650 other complete genomes downloaded from the RefSeq database (NCBI) using the procedure described in Vallenet et al. [36]. Manual validation of the automatic annotation was performed using the MaGe (Magnifying Genomes) interface. IS finder (www-is.biotoul.fr) was used to annotate insertion sequences [37]. The A. lipoferum 4B nucleotide sequence and annotation data have been deposited to EMBL databank under accession numbers: FQ311868 (chromosome), FQ311869 (p1), FQ311870 (p2), FQ311871 (p3), FQ311872 (p4), FQ311873 (p5), FQ311874 (p6). The A. brasilense Sp245 nucleotide sequence and annotation data have been deposited at EMBL databank under accession numbers: HE577327 (chromosome), HE577328 (p1), HE577329 (p2), HE577330 (p3), HE577331 (p4), HE577332 (p5), HE577333 (p6). In addition, all the data (i.e., syntactic and functional annotations, and results of comparative analysis) were stored in a relational database, called AzospirilluScope [36], which is publicly available at http://www.genoscope.cns.fr/agc/mage/microscope/about/collabprojects.php?P_id=39.
Computational genomics/bioinformatics
BLAST searches were performed using NCBI toolkit version 2.2.24+ [38]. Multiple sequence alignments were built using the L-INS-i algorithm of MAFFT [39] with default parameters. Phylogenetic tree construction was performed using PhyML [40] with default parameters unless otherwise specified. 16S rRNA sequences were retrieved from the Ribosomal Database Project [41].
A concatenated ribosomal protein tree was constructed from sequenced members of alpha-proteobacteria with a 98% 16S rRNA sequence identity cutoff to limit overrepresentation. The following ribosomal proteins were used: L3, L5, L11, L13, L14, S3, S7, S9, S11, and S17. The proteins were identified using corresponding Pfam models and HMMER [42] searches against the genomes of sequenced alpha-proteobacteria selected above. The sequences were aligned and concatenated. GBlocks [43] with default parameters was used to reduce the number of low information columns. The tree was constructed using PhyML with the following options: empirical amino acid frequencies, 4 substitution categories, estimated gamma distribution parameter, and NNI tree topology search.
Assignment of gene ancestry
Protein sequences queries from all 3 Azospirillum genomes were used in BLAST searches against the non-redundant microbial genome set constructed by Wuichet and Zhulin [26] supplemented with sequenced members of Rhodospirillales absent in the original set (Acetobacter pasteurianus IFO 3283-01, alpha proteobacterium BAL199, Magnetospirillum gryphiswaldense MSR-1, and Magnetospirillum magnetotacticum MS-1). E-value cutoff of 10∧−4 was used.
Only the first occurrence of each species was used in ancestry assignment. Proteins were assigned as being ancestral or horizontally transferred, with varying degrees of confidence, based on the presence of members of Rhodospirillales and Rhodospirillaceae in the top eight BLAST hits. Ancestral assignment was based on the top 8 hits, based on the number of Rhodospirillaceae genomes in the database: 2 Azospirillum, 3 Magnetospirillum, 2 Rhodospirillum, and Nisaea sp. BAL199, excluding the organism on which ancestry assignment is being performed. High confidence ancestral proteins have at least 6 of the top 8 species belonging to Rhodospirillales or all but 1, if the BLAST result had less than 8 species. This rule allows for 1–2 independent events of HGT from Rhodospirillales to other distantly related species. Medium confidence ancestral proteins have at least 4 Rhodospirillaceae in the top 8. Low confidence ancestral proteins have at least 1 Rhodospirillaceae in the top 8, excluding hits to other Azospirillum genomes. High confidence horizontally transferred proteins have 0 hits to Rhodospirillales in the top 10, excluding hits to other Azospirillum genomes. Medium confidence horizontally transferred proteins have 0 hits to Rhodospirillales in the top 5, excluding hits to other Azospirillum genomes. Low confidence horizontally transferred proteins have 0 hits to Rhodospirillaceae in the top 8, excluding hits to other Azospirillum genomes. Unassigned proteins either have no BLAST hits outside Azospirillum, or simultaneously classify as medium confidence horizontally transferred and medium or low confidence ancestral.
Proteomics
Cell growth
Azospirillum brasilense strain Sp245: Overnight starter cultures (5 mL) were inoculated from fresh plates. Starter cultures were grown overnight at 27°C in a shaking water bath in minimal media containing malate as carbon source and ammonium chloride as nitrogen source. Cells were pelleted from starter cultures and washed with appropriate growth media. Base media for all cultures was minimal media (MMAB) [44] with 20 mM malate as carbon source, ammonium chloride as nitrogen source where appropriate, and molybdate. Starter cultures were resuspended with appropriate media and used to inoculate 250 mL cultures for nitrogen-fixing growth, or 500 ml cultures for non-nitrogen-fixing growth. Nitrogen fixation requires a great deal of energy and continuous optimal oxygen concentration, so growth of nitrogen fixing cells is slower than those growing in nitrogen sufficient conditions. Cells grown under nitrogen fixing conditions exhibit a doubling time of 170 minutes while control (non nitrogen fixing) cells have a doubling time of 120 minutes [21]. Further, OD of cells grown under nitrogen fixing cultures never reaches high levels, tending to level off at or below an OD600 of 0.2–0.3 [21]. Therefore, each growth condition was optimized as follows. For nitrogen-fixing cultures, nitrogen gas was sparged through the head space of the media bottle through the serum port, and sufficient air was injected to give a final oxygen content in the head space of 2%; cultures were grown at 25°C without shaking to early log phase (OD600 = 0.1–0.2) to minimize exposure to high levels of oxygen, as Azospirillum species are microaerophilic diazotrophs. Non-nitrogen fixing cultures were grown under optimum growth conditions (shaking and in presence of ammonium) at 25°C on an orbital shaker to mid-log phase (OD600 = 0.5–0.6). Cells were harvested by centrifugation at 8000 rpm for 10 minutes, washed twice with 50 mM Tris (pH 7.9), then pelleted by centrifugation at 8000 rpm for 10 minutes, and stored at −80 C. Cell pellets from two biological replicates were pooled for subsequent proteome preparation. Azospirillum lipoferum: Growth conditions were as described above for A. brasilense Sp245, except that cells were grown in MMAB media supplemented with 1 mg/L D-biotin.
Proteome preparation for LC/LC-MS/MS
Frozen cell pellets (0.1 g for each sample) were resuspended at a rate of 500 µl lysis buffer/0.1 g wet cell pellet weight in lysis buffer of 6 M guanidine hydrochloride, 10 mM DTT solubilized in 50 mM Tris-HCl, 10 mM CaCl2 [45]. Resuspended cells were then further lysed by sonication. Lysate was centrifuged at 18,000 g for 20 minutes to clear cellular debris. Supernatant was collected for tryptic digestion. 10 mM DTT was added and lysate was incubated at 60°C for 1 hour. Lysate was then diluted 6-fold with trypsin digestion buffer (50 mM Tris-HCl, 10 mM CaCl2, 10 mM DTT, pH 7.9) and 20 µg sequencing-grade trypsin (Promega, Madison, WI) was added to each sample. Samples were incubated overnight at 37°C with gentle rotation. An additional 20 µg of trypsin was added the following morning and samples were subsequently incubated for an additional 5–6 hours at 37°C with gentle rotation. Digestion was halted by addition of 5 µl formic acid to the 5 ml lysate. Samples were then desalted using Sep-Pak Plus C-18 solid phase extraction (Waters, Milford, MA) following manufacturer's recommendations, and subsequently concentrated and solvent-exchanged into 100% HPLC-grade H2O, 0.1% formic acid using vacuum centrifugation (Savant, Thermo Scientific). Samples were aliquoted into 40 µL volumes and stored at −80°C until analysis.
LC/LC-MS/MS analysis
Proteome samples were analyzed via Multi-dimensional Protein Identification Technology (MudPIT) [46]–[48] with triphasic columns. Columns were individually packed using a pressure cell (New Objective, Woburn, MA). Back columns were loaded in 150 µm ID fused silica capillary tubing first with 3 cm of Luna 5 µm particle diameter strong cation exchange (SCX) resin (Phenomenex, Torrance, CA) followed by 3 cm of Aqua 5 µm C-18 reverse phase resin (Phenomenex). Proteome aliquots (40 µl) were loaded directly onto the back column via pressure cell and subsequently coupled to the front column. Front columns were pulled from 100 µm ID fused silica capillary tubing to a tip with an inside diameter of 5 µm using a P-2000 laser puller (Sutter Instruments, Novato, CA), and packed with a 17 cm long bed of Aqua 5 µm diameter C-18 reverse phase resin. This column acts as the resolving column for peptides eluted from the back column. For analysis, the combined columns were placed directly in-line with an LTQ mass spectrometer (ThermoScientific, San Jose, CA) using a Proxeon source.
Chromatographic separation was accomplished with an Ultimate HPLC system (LC Packings, a division of Dionex, San Francisco, CA) providing a flow rate of 100 µl/minute which was split prior to the resolving column such that the final flow rate through the resolving column was ∼300 nl/minute. Twelve two-dimensional (2D) chromatographic steps were done. An initial 1 hour gradient from buffer A (95% water, 5% acetonitrile, 0.1% formic acid) to buffer B (70% acetonitrile, 0.1% formic acid) bumped the peptides from the initial reverse phase column onto the strong cation exchange column. Subsequent cycles included 2 minute salt pulses with varying percentages of 500 mM ammonium acetate (10, 15, 20, 25, 30, 35, 40, 45, 50, 60%) to first elute subsets of peptides from the SCX column according to charge, followed by a 2 hour gradient from buffer A to buffer B, to further separate peptides by hydrophobicity. The final chromatographic step consisted of a 20 minute salt pulse of 100% 500 mM ammonium acetate, followed by a 2 hour A-to-B gradient.
Data collection was controlled by Xcaliber software (ThermoScientific). Data was collected in data-dependent mode with one full scan followed by 6 dependent scans, each with 2 microscans. Dynamic exclusion was employed with a repeat count of 1, repeat duration of 60 s and exclusion list size of 300 and duration of 180 s. Isolation mass width was set at 3 m/z units.
Data analysis
The Sp245 protein database was constructed from translated CDSs called in the draft genome sequence (http://genome.ornl.gov/microbial/abra/19sep08/). The 4B protein database was constructed from translated CDSs called in the complete genome sequence. A list of common contaminants was appended to the gene call sequences, and all coding sequences, including contaminant sequences, were reversed and appended to the forward sequences in order to serve as distractors. From the number of identifications in the reverse direction, peptide false positive (FP) rates were determined using the formula %FP = 2[No. reverse ID/(no. reverse ID+no. real ID)] [49]; FP rates ranged from 1.4%–4.3%. All MS/MS spectra were searched against the corresponding database using SEQUEST [50], specifying tryptic digestion, peptide mass tolerance of 3 m/z and a fragment ion tolerance of 0.5 m/z. Additionally, search parameters included two dynamic modifications: 1. methylation represented by a mass shift of +14 m/z on glutamate residues, and 2. deamidation followed by methylation represented by a mass shift of +15 m/z on glutamine residues. Output data files were sorted and filtered with DTASelect [51], specifying XCorr filter levels of 1.8 for peptides with a charge state of +1, 2.5 for those with charge state +2 and 3.5 for charge state +3, minimum delta CN of 0.08, semi-tryptic status and 2 peptides per protein identification. In order to determine relative abundance of a given protein in a sample, normalized spectral abundance factors (NSAF) were calculated for each individual protein k using the formula NSAFk = (SpC/L)k/Σ (SpC/L)n, where SpC is the total spectral count for all peptides contributing to protein k, L is the length of protein k, and n is the total number of proteins detected in the sample [52].
Identification of glycoside hydrolases
Bidirectional BLAST was used to identify orthologs of the putative glycoside hydrolase (GH) genes. Phyml package was used to confirm evolutionary relationships and visualize the results. Domain architectures were obtained through Pfam [53] search for each protein. Then information from CAZy [54] and recent analysis [55] was used to assign putative activities of the predicted GHs.
Classification of chemotaxis systems in the rhizosphere
Chemotaxis proteins were identified in genomic datasets as previously described [56]. Using CheA sequences from a recent chemotaxis system classification analysis [26], alignments of the P3–P5 regions of CheA were built for each class and for the entire set of CheA sequences. Each alignment was made non-redundant so that no pair of sequences shared more than 80% sequence identity. Hidden Markov Models (HMMs) were built from each non-redundant alignment and used to create library via the HMMER3 software package (version HMMER 3.0b3) [42] and default parameters.
The rhizosphere CheA sequences from a recent study [25] were run against the CheA HMM library. Unclassified sequences (Unc) are those with top hits to the full CheA set HMM rather than a class-specific HMM. The remaining sequences were assigned to the class of the top scoring HMM.
Cellulase assay
Azospirillum strains and control strains (Dickeya dadantii 3937 as a positive control, A. tumefaciens NT1 as a negative control) were cultured for 16 h in liquid AB minimal medium [57] containing 0.2% malate and 1 mg/L biotin. An aliquot of 107 cells (for Dickeya dadantii 3937) or 2.107 cells (for all other strains) was deposited on top of AB plates containing 0.1% carboxymethylcellulose instead of malate. Plates were incubated for 5 days before being stained as previously described [58].
Pili mutant and attachment assay
A 211-bp cpaB (AZOBR_p460079) internal fragment was amplified by PCR with primers F6678 (GCGTGGACCTGATCCTGAC) and F6679 (GTGACCGTCTCGCTCTGAC) and subcloned into pGEM-T easy (Promega). White colonies were screened by PCR with primers F6678 and F6679 for correct insertion in pGEM-T easy, resulting in pR3.37. The insert of plasmid pR3.37 was digested with NotI and cloned into the NotI site of pKNOCK-Km [59], resulting in pR3.39 after transfer into chemically-competent cells of E. coli S17.1 λpir. pR3.39 was introduced into A. brasilense Sp245 by biparental mating. Transconjugants resulting from a single recombination event of pR3.39 were selected on AB medium containing 0.2% malate, ampicillin (100 mg/mL) and kanamycin (40 mg/mL). The correct insertion of pKNOCK into cpaB was confirmed by PCR with primers (F6678 and F5595 TGTCCAGATAGCCCAGTAGC, located on pKNOCK) and sequencing of the PCR amplicon.
Sp245 and Sp245cpaB were labelled with pMP2444 [60] allowing the constitutive expression of EGFP. The strains were grown in NFB* (Nitrogen free broth containing 0.025% of LB) with appropriate antibiotics in glass tubes containing a cover-slide, under a mild lateral agitation for 6 days. After the incubation, the liquid and the cover-slide were removed from the tubes and the biofilm formed at the air/liquid interface was colored by 0.1% crystal violet. After two washings with distilled water, crystal violet was solubilized by ethanol and quantified by spectrophotometry at 570 nm. The experiment was performed twice in triplicate. In parallel, the colonization of the glass cover-slide was monitored by confocal laser scanning microscopy (510 Meta microscope; Carl Zeiss S.A.S.) equipped with an argon-krypton laser, detectors, and filter sets for green fluorescence (i.e., 488 nm for excitation and 510 to 531 nm for detection). Series of horizontal (x-y) optical sections with a thickness of 1 µm were taken throughout the full length of the Sp245 and Sp245cpaB biofilms. Three dimensional reconstructions of biofilms were performed using LSM software release 3.5 (Carl Zeiss S.A.S.).
Supporting Information
Zdroje
1. MojzsisSJArrheniusGMcKeeganKDHarrisonTMNutmanAP 1996 Evidence for life on Earth before 3,800 million years ago. Nature 384 55 59
2. WatanabeYMartiniJEOhmotoH 2000 Geochemical evidence for terrestrial ecosystems 2.6 billion years ago. Nature 408 574 578
3. BattistuzziFUHedgesSB 2009 A major clade of prokaryotes with ancient adaptations to life on land. Mol Biol Evol 26 335 343
4. KettlerGCMartinyACHuangKZuckerJColemanML 2007 Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet 3 e231 doi:10.1371/journal.pgen.0030231
5. OkonYLabandera-GonzalezCA 1994 Agronomic applications of Azospirillum: An evaluation of 20 years worldwide field inoculation. Soil Biol Biochem 26 1591 1601
6. SteenhoudtOVanderleydenJ 2000 Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24 487 506
7. KanekoTMinamisawaKIsawaTNakatsukasaHMitsuiH 2010 Complete genomic structure of the cultivated rice endophyte Azospirillum sp. B510. DNA Res 17 37 50
8. Martin-DidonetCCChubatsuLSSouzaEMKleinaMRegoFG 2000 Genome structure of the genus Azospirillum. J Bacteriol 182 4113 4116
9. HarrisonPWLowerRPKimNKYoungJP 2010 Introducing the bacterial ‘chromid’: not a chromosome, not a plasmid. Trends Microbiol 18 141 148
10. GonzalezVSantamariaRIBustosPHernandez-GonzalezIMedrano-SotoA 2006 The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci U S A 103 3834 3839
11. VialLLavireCMavinguiPBlahaDHauratJ 2006 Phase variation and genomic architecture changes in Azospirillum. J Bacteriol 188 5364 5373
12. KooninEVMakarovaKSAravindL 2001 Horizontal gene transfer in prokaryotes: quantification and classification. Annu Rev Microbiol 55 709 742
13. TatusovRLNataleDAGarkavtsevIVTatusovaTAShankavaramUT 2001 The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res 29 22 28
14. DennisPGMillerAJHirschPR 2010 Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72 313 327
15. BoyerMHauratJSamainSSegurensBGavoryF 2008 Bacteriophage prevalence in the genus Azospirillum and analysis of the first genome sequence of an Azospirillum brasilense integrative phage. Appl Environ Microbiol 74 861 874
16. GiraudEMoulinLVallenetDBarbeVCytrynE 2007 Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316 1307 1312
17. KuoCHOchmanH 2009 Inferring clocks when lacking rocks: the variable rates of molecular evolution in bacteria. Biol Direct 4 35
18. KenrickPCranePR 1997 The origin and early evolution of plants on land. Nature 389 33 39
19. RavenJAEdwardsD 2001 Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52 381 401
20. PrasadVStrombergCAAlimohammadianHSahniA 2005 Dinosaur coprolites and the early evolution of grasses and grazers. Science 310 1177 1180
21. XieZUlrichLEZhulinIBAlexandreG 2010 PAS domain containing chemoreceptor couples dynamic changes in metabolism with chemotaxis. Proc Natl Acad Sci U S A 107 2235 2240
22. JiangZYBauerCE 1997 Analysis of a chemotaxis operon from Rhodospirillum centenum. J Bacteriol 179 5712 5719
23. BibleANStephensBBOrtegaDRXieZAlexandreG 2008 Function of a chemotaxis-like signal transduction pathway in modulating motility, cell clumping, and cell length in the alphaproteobacterium Azospirillum brasilense. J Bacteriol 190 6365 6375
24. UlrichLEZhulinIB 2010 The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res 38 D401 407
25. BuchanACrombieBAlexandreGM 2010 Temporal dynamics and genetic diversity of chemotactic-competent microbial populations in the rhizosphere. Environ Microbiol 12 3171 3184
26. WuichetKZhulinIB 2010 Origins and diversification of a complex signal transduction system in prokaryotes. Sci Signal 3 ra50
27. AssmusBHutzlerPKirchhofGAmannRLawrenceJR 1995 In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl Environ Microbiol 61 1013 1019
28. PedrosaFOMonteiroRAWassemRCruzLMAyubRA 2011 Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet 7 e1002064 doi:10.1371/journal.pgen.1002064
29. DorrJHurekTReinhold-HurekB 1998 Type IV pili are involved in plant-microbe and fungus-microbe interactions. Mol Microbiol 30 7 17
30. RameyBEKoutsoudisMvon BodmanSBFuquaC 2004 Biofilm formation in plant-microbe associations. Curr Opin Microbiol 7 602 609
31. TomichMPlanetPJFigurskiDH 2007 The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol 5 363 375
32. HandelsmanJTiedjeJAlvarez-CohenLAshburnerMCannIKO 2007 The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet. National Academies Press, Washington, DC 158
33. Caro-QuinteroADengJAuchtungJBrettarIHofleMG 2011 Unprecedented levels of horizontal gene transfer among spatially co-occurring Shewanella bacteria from the Baltic Sea. ISME J 5 131 140
34. BartolomeBJubeteYMartinezEde la CruzF 1991 Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102 75 78
35. BocsSCruveillerSVallenetDNuelGMedigueC 2003 AMIGene: Annotation of MIcrobial Genes. Nucleic Acids Res 31 3723 3726
36. VallenetDLabarreLRouyZBarbeVBocsS 2006 MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res 34 53 65
37. SiguierPPerochonJLestradeLMahillonJChandlerM 2006 ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34 D32 36
38. AltschulSFMaddenTLSchafferAAZhangJZhangZ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
39. KatohKKumaKTohHMiyataT 2005 MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33 511 518
40. GuindonSDufayardJFLefortVAnisimovaMHordijkW 2010 New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59 307 321
41. ColeJRWangQCardenasEFishJChaiB 2009 The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37 D141 145
42. EddySR 1998 Profile hidden Markov models. Bioinformatics 14 755 763
43. TalaveraGCastresanaJ 2007 Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56 564 577
44. HauwaertsDAlexandreGDasSKVanderleydenJZhulinIB 2002 A major chemotaxis gene cluster in Azospirillum brasilense and relationships between chemotaxis operons in alpha-proteobacteria. FEMS Microbiol Lett 208 61 67
45. ThompsonMRChoureyKFroelichJMEricksonBKVerBerkmoesNC 2008 Experimental approach for deep proteome measurements from small-scale microbial biomass samples. Anal Chem 80 9517 9525
46. McDonaldWHOhiRMiyamotoDTMitchisonTJYatesJR 2002 Comparison of three directly coupled HPLC MS/MS strategies for identification of proteins from complex mixtures: single-dimension LC-MS/MS, 2-phase MudPIT, and 3-phase MudPIT. Int J Mass Spectrom 219 245 251
47. WashburnMPWoltersDYatesJR 2001 Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19 242 247
48. WoltersDAWashburnMPYatesJR 2001 An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem 73 5683 5690
49. PengJMEliasJEThoreenCCLickliderLJGygiSP 2003 Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: The yeast proteome. J Proteome Res 2 43 50
50. EngJKMccormackALYatesJR 1994 An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectr 5 976 989
51. TabbDLMcDonaldWHYatesJR 2002 DTASelect and contrast: Tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1 21 26
52. WashburnMPFlorensLCarozzaMJSwansonSKFournierM 2006 Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40 303 311
53. FinnRDMistryJTateJCoggillPHegerA 2010 The Pfam protein families database. Nucleic Acids Res 38 D211 222
54. CantarelBLCoutinhoPMRancurelCBernardTLombardV 2009 The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37 D233 238
55. SukharnikovLOCantwellBJPodarMZhulinIB 2011 Cellulases: ambiguous nonhomologous enzymes in a genomic perspective. Trends Biotechnol 29 473 479
56. WuichetKAlexanderRPZhulinIB 2007 Comparative genomic and protein sequence analyses of a complex system controlling bacterial chemotaxis. Methods Enzymol 422 1 31
57. ShawPDPingGDalySLChaCCronanJEJr 1997 Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci U S A 94 6036 6041
58. ParkSRChoSJYunHD 2000 Cloning and sequencing of pel gene responsible for CMCase activity from Erwinia chrysanthemi PY35. Biosci Biotechnol Biochem 64 925 930
59. AlexeyevMF 1999 The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26 824 826, 828
60. BloembergGVWijfjesAHLamersGEStuurmanNLugtenbergBJ 2000 Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol Plant Microbe Interact 13 1170 1176
61. LykidisAMavromatisKIvanovaNAndersonILandM 2007 Genome sequence and analysis of the soil cellulolytic actinomycete Thermobifida fusca YX. J Bacteriol 189 2477 2486
62. QiMJunHSForsbergCW 2007 Characterization and synergistic interactions of Fibrobacter succinogenes glycoside hydrolases. Appl Environ Microbiol 73 6098 6105
63. FierobeHPBagnara-TardifCGaudinCGuerlesquinFSauveP 1993 Purification and characterization of endoglucanase C from Clostridium cellulolyticum. Catalytic comparison with endoglucanase A. European journal of biochemistry/FEBS 217 557 565
64. OguraJToyodaAKurosawaTChongALChohnanS 2006 Purification, characterization, and gene analysis of cellulase (Cel8A) from Lysobacter sp. IB-9374. Biosci Biotechnol Biochem 70 2420 2428
65. BerlemanJEBauerCE 2005 Involvement of a Che-like signal transduction cascade in regulating cyst cell development in Rhodospirillum centenum. Mol Microbiol 56 1457 1466
66. BerlemanJEBauerCE 2005 A che-like signal transduction cascade involved in controlling flagella biosynthesis in Rhodospirillum centenum. Mol Microbiol 55 1390 1402
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



