-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
The RRM-type RNA-binding protein Mei2 is a master regulator of meiosis in fission yeast, in which it stabilizes meiosis-specific mRNAs by blocking their destruction. Artificial activation of Mei2 can provoke the entire meiotic process, and it is suspected that Mei2 may do more than the stabilization of meiosis-specific mRNAs. In our current study using a new screening system, we show that Mei2 genetically interacts with subunits of CTDK-I, which phosphorylates serine-2 residues on the C-terminal domain of RNA polymerase II (Pol II CTD). Phosphorylation of CTD Ser-2 is essential to enable the robust transcription of ste11, which encodes an HMG-type transcription factor that regulates the expression of mei2 and other genes necessary for sexual development. CTD Ser-2 phosphorylation increases under nitrogen starvation, and the stress-responsive MAP kinase pathway, mediated by Wis1 MAPKK and Sty1 MAPK, is critical for this stress response. Sty1 phosphorylates Lsk1, the catalytic subunit of CTDK-I. Furthermore, a feedback loop stemming from activated Mei2 to Win1 and Wis4 MAPKKKs operates in this pathway and eventually enhances CTD Ser-2 phosphorylation and ste11 transcription. Hence, in addition to starting meiosis, Mei2 functions to reinforce the commitment to it, once cells have entered this process. This study also demonstrates clearly that the stress-responsive MAP kinase pathway can modulates gene expression through phosphorylation of Pol II CTD.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002387
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002387Summary
The RRM-type RNA-binding protein Mei2 is a master regulator of meiosis in fission yeast, in which it stabilizes meiosis-specific mRNAs by blocking their destruction. Artificial activation of Mei2 can provoke the entire meiotic process, and it is suspected that Mei2 may do more than the stabilization of meiosis-specific mRNAs. In our current study using a new screening system, we show that Mei2 genetically interacts with subunits of CTDK-I, which phosphorylates serine-2 residues on the C-terminal domain of RNA polymerase II (Pol II CTD). Phosphorylation of CTD Ser-2 is essential to enable the robust transcription of ste11, which encodes an HMG-type transcription factor that regulates the expression of mei2 and other genes necessary for sexual development. CTD Ser-2 phosphorylation increases under nitrogen starvation, and the stress-responsive MAP kinase pathway, mediated by Wis1 MAPKK and Sty1 MAPK, is critical for this stress response. Sty1 phosphorylates Lsk1, the catalytic subunit of CTDK-I. Furthermore, a feedback loop stemming from activated Mei2 to Win1 and Wis4 MAPKKKs operates in this pathway and eventually enhances CTD Ser-2 phosphorylation and ste11 transcription. Hence, in addition to starting meiosis, Mei2 functions to reinforce the commitment to it, once cells have entered this process. This study also demonstrates clearly that the stress-responsive MAP kinase pathway can modulates gene expression through phosphorylation of Pol II CTD.
Introduction
The cell cycle programs for mitosis and meiosis appear to be strictly segregated from each other, although they are likely to have molecular mechanisms in common. Analyses in lower eukaryotes have shown that factors required exclusively for meiosis, generated through the transcriptional activation of meiosis-specific genes, are largely responsible for the segregation of these two processes [1], [2]. In addition, we have reported previously in fission yeast that meiosis-specific mRNAs transcribed at the wrong time during the mitotic cell cycle are removed selectively by nuclear exosomes, thereby preventing the inappropriate expression of the meiotic program in mitotic cells [3], [4]. The master meiotic regulator in fission yeast, Mei2, an RNA-binding protein with three RRM domains [5]–[7], suppresses the function of this selective removal system by sequestering a key component Mmi1, which is an RNA-binding protein of the YTH family [3]. Mei2 thus ensures full expression of meiosis-specific genes and facilitates execution of the meiotic program (reviewed in [8]). However, it is unlikely that the function of Mei2 in meiosis is confined to the tethering of Mmi1 as the artificial inactivation of Mmi1 does not induce the full meiotic program, whereas the experimental induction of the activated form of Mei2 does so [3], [6]. The mechanisms and pathways by which Mei2 promotes the entire meiotic program is therefore a subject of considerable interest.
To identify possible upstream or downstream effectors of Mei2, we devised a new screening system and found that a subunit of CTDK-I, which is a CDK-like kinase complex that phosphorylates the C-terminal repeat domain of the largest subunit of RNA polymerase II (Pol II CTD) [9], [10], could genetically interact with Mei2. More specifically, the phosphorylation of Pol II CTD by CTDK-I was found to affect the expression of ste11, which encodes a key transcription factor that regulates the mei2 gene. Pol II CTD serves as a binding scaffold for a variety of nuclear factors, and its phosphorylation status has been implicated in regulation of an ever-increasing number of functions necessary to execute complex transcriptional processes [9], [10]. Our aforementioned findings indicate that the phosphorylation of Ser-2 residues on Pol II CTD in fission yeast is unique in that it is required mainly for the meiotic program, via the activation of ste11 transcription, but is not absolutely necessary for the mitotic program. Essentially the same conclusions have been reached independently by others, through global gene expression analysis [11]. Here we further show that the stress-responsive MAP kinase cascade is crucial for the phosphorylation of Ser-2 residues under nutrient starvation, which is a condition suitable for meiosis. We also show that artificially activated Mei2 has the potential to promote the phosphorylation of Ser-2 residues on Pol II CTD via the stress-responsive MAP kinase cascade, irrespective of the nutrient conditions.
Taken together, the results of our present study demonstrate a new regulatory paradigm for meiosis by Mei2 in fission yeast, i.e., that this master meiotic regulator ensures the commitment to meiosis by strengthening the transcription of ste11 via a feedback loop comprising the stress-responsive MAP kinase cascade and the phosphorylation of Pol II CTD by CTDK-I.
Results
Isolation of lsg1 as a suppressor of the ectopic meiosis induced by the artificial activation of the meiotic regulator Mei2
The haploid fission yeast strain JV312 harbors the mei2-L-SATA allele driven by the authentic mei2 promoter. This allele contains a combination of two mutations, mei2-L and mei2-SATA. The former mutation confers temperature-sensitivity to the Mei2 protein (our unpublished results), whereas the latter activates this gene constitutively, overriding the inhibitory phosphorylation by Pat1 kinase [6]. JV312 cells arrest during vegetative growth and induce ectopic meiosis at 25°C because the Mei2-L-SATA protein is functional at this temperature. However, these cells continue vegetative growth at 32°C because Mei2-L-SATA is then inert and does not interfere with cell growth pathways. To identify novel upstream regulators or downstream effectors of Mei2, we screened for suppressor mutants that could grow at 25°C by insertional mutagenesis of JV312 (see Materials and Methods). Several suppressor mutants were thereby isolated, one of which was found to contain an insertion in SPBC4B3.08, which is annotated in the fission yeast database (http://old.genedb.org/genedb/pombe/) to encode a homologue of the γ subunit of RNA polymerase II C-terminal domain kinase I (CTDK-I). CTDK-I belongs to the CDK family, but in addition to the catalytic subunit α and the cyclin-like regulatory subunit β conserved among these family members, it contains a third γ subunit [12], [13]. In fission yeast, the lsk1 and lsc1 genes encode the α and β subunits of the CDK proteins, respectively [14], [15]. Hereafter, we designate SPBC4B3.08 as lsg1.
Because the level of homology between fission yeast Lsg1 and Saccharomyces cerevisiae CTDK-I γ (CTK3) was found not to be high (a 24% amino acid identity; Figure S1), we examined whether Lsg1 was indeed a functional homolog of CTDK-I γ. We constructed the lsg1-deletion strain by replacing the entire lsg1 ORF with a drug-resistant cassette, and compared its phenotype with that of lsk1Δ and lsc1Δ. The lsg1Δ strain exhibited no significant defects in mitotic growth, like the lsk1Δ and lsc1Δ strains previously analyzed [14], [15] (Figure 1A). The doubling time in liquid YE medium at 30°C was 2.1 h for the wild-type, 2.2 h for lsg1Δ, 2.3 h for lsk1Δ, and 2.2 h for lsc1Δ, respectively. However, lsg1Δ cells showed hypersensitivity to Latrunculin A, an inhibitor of actin polymerization, which was a phenotype reported previously for lsk1Δ and lsc1Δ [14], [15] (Figure 1A). In addition, both lsk1Δ and lsc1Δ could suppress the growth defect of mei2-L-SATA at 25°C as efficiently as lsg1Δ (Figure 1B). These observations confirmed that lsg1 indeed encodes the CTDK-I γ subunit, and indicated that loss of CTDK-I activity is responsible for the suppression of mei2-L-SATA.
Fig. 1. Phenotypes of the mutants defective in each CTDK-I subunit. 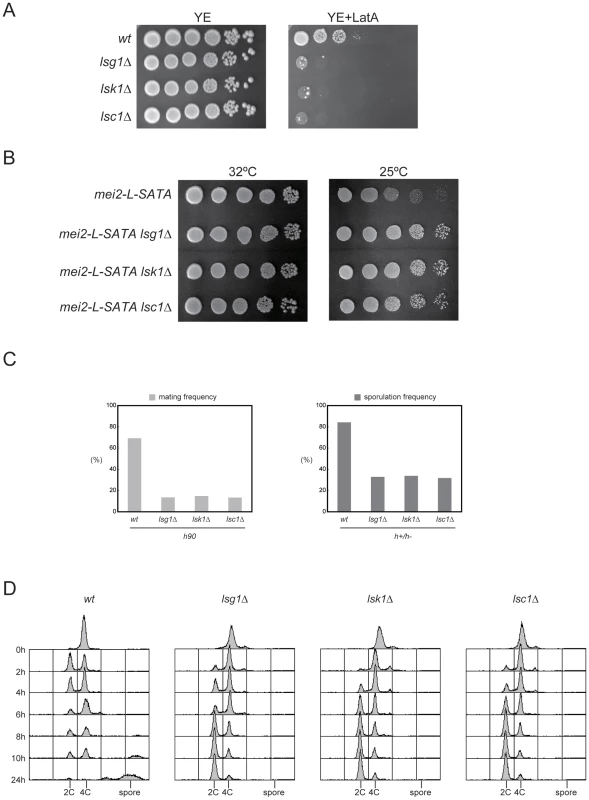
(A) Sensitivity to Latrunculin A. Growth of haploid strains JY450 (wild-type), JT659 (lsg1Δ), JT660 (lsk1Δ) and JT661 (lsc1Δ) was examined on YE plates with or without addition of 0.5 µM Latrunculin A. Ten-fold serial dilutions of each strain were spotted and incubated at 30°C for four days. (B) Suppression of mei2-L-SATA by lsg1Δ, lsk1Δ, or lsc1Δ. Ten-fold serial dilutions of haploid strains JV312 (mei2-L-SATA), JT662 (mei2-L-SATA lsg1Δ), JT663 (mei2-L-SATA lsk1Δ), and JT664 (mei2-L-SATA lsc1Δ) were spotted onto SD plates and incubated either at 32°C or 25°C for four days. (C) Reduced mating and sporulation frequencies in the CTDK-I deletion mutants. Cells of the homothallic (h90) haploid strains JY450 (wild-type), JT659 (lsg1Δ), JT660 (lsk1Δ), and JT661 (lsc1Δ) were examined microscopically for their conjugation frequency after incubation on SSA plates at 30°C for three days (left panel). Cells of heterozygous diploid (h+/h−) strains JY362 (wild-type), JT665 (lsg1Δ/lsg1Δ), JT666 (lsk1Δ/lsk1Δ), and JT667 (lsc1Δ/lsc1Δ) were examined microscopically for their sporulation frequency after incubation on SSA plates at 30°C for two days (right panel). (D) DNA content of the diploid strains JY362, JT665, JT666, and JT667 exposed to nitrogen starvation. Cells were cultured in liquid MM medium to mid-log phase and then shifted to MM-N medium. Aliquots were taken at the indicated intervals and the cellular DNA content was determined by FACS analysis. Deletion mutants of the CTDK-I subunits are defective in sexual development
Although deletion of the gene encoding each CTDK-I subunit led to no obvious defect under normal growth conditions, these deletion mutants all showed impairments in conjugation and sporulation under starved conditions. Under these conditions, haploid lsg1Δ, lsk1Δ or lsc1Δ cells conjugated at a lower frequency than wild-type cells, and diploid lsg1Δ, lsk1Δ or lsc1Δ cells underwent azygotic meiosis and sporulation at a lower frequency than wild-type cells (Figure 1C). We further found that the progression of the meiotic cell cycle was significantly retarded in the CTDK-I subunit mutants. Fluorescence-activated cell sorting (FACS) analysis indicated that diploid lsg1Δ, lsk1Δ or lsc1Δ cells began to arrest in G1 phase as late as eight hours after the shift to nitrogen starvation and showed minimal premeiotic DNA synthesis even after 24 hours. In contrast, wild-type cells began to arrest in G1 phase after two hours and completed premeiotic DNA synthesis at between 2 and 6 hours (Figure 1D).
The loss of ste11 expression is the major cause of the mating and sporulation deficiency in the CTDK-I mutants
Our observations that the CTDK-I deletion mutants were defective in sexual development and could suppress growth deficiency, evoked by the mei2-L-SATA allele, led us to speculate that the expression of ste11, which encodes an HMG-family transcription factor, might be impaired in these mutants. Our reasoning was that 1) Ste11 regulates the transcription of many genes essential for sexual development, including mei2 [16]; 2) the deletion of ste11 has been shown to suppress ectopic meiosis induced by the pat1 mutation and restore vegetative growth, by blocking the expression of mei2 [17], [18]; and 3) we had noticed that ste11Δ cells show G1 arrest retardation under conditions of nitrogen starvation, even more extensively than lsg1Δ, lsk1Δ or lsc1Δ cells, while mei2Δ cells are not so much affected (Figure S2A). We thus analyzed the transcription of ste11 in lsg1Δ cells and found that it was significantly suppressed (Figure S2B). Because requirement of CTDK-I for the expression of ste11 has been independently discovered and already reported by Hermand and his colleagues [11], we briefly summarize our data that confirm their conclusions in Figures S2 and S3. We tested whether the forced expression of ste11 could recover sexual development in the CTDK-I deletion mutants. The overexpression of ste11 from the nmt1 promoter, which is roughly four to five times as strong as the physiological expression, effectively recovered conjugation and subsequent meiosis in lsg1Δ, lsk1Δ and lsc1Δ homothallic haploid cells (Figure S2C), indicating that the loss of ste11 expression is a major cause of the mating and sporulation deficiency in the CTDK-I mutants. We then determined the range of genes whose expression is regulated by CTDK-I, by comparing the gene expression profiles between lsg1Δ and wild-type cells starved of nitrogen for 2.5 hours. Genome-wide microarray analysis indicated that the expression of 64 genes was downregulated more than two-fold in the lsg1Δ mutant, whereas 22 genes showed upregulation by more than two-fold in the mutant (Figure S3A). Notably, 33 out of the 64 downregulated genes identified, including ste11 itself, have been shown previously to be controlled by Ste11 [19]. These genes are listed in Table S1. In contrast, the expression of atf1, pcr1, rst2, and other genes that also encode an upstream regulator of ste11 transcription [20]–[25], was not significantly affected by the deletion of lsg1 (Figure S3B), suggesting that CTDK-I may exert its effects on ste11 transcription directly.
Ser-2 of the Pol II CTD is phosphorylated by CTDK-I in the course of meiosis
Previous work has shown that Lsk1 is involved in the phosphorylation of Ser-2 residues within the heptad repeats of the carboxy terminal domain (CTD) of RNA polymerase II [15]. To determine whether the Pol II CTD phosphorylation status might be changed by the induction of sexual development, we analyzed phosphorylation of Ser-2 and Ser-5 residues within the CTD before and after the shift to nitrogen-depleted medium. Extracts were prepared from wild-type and lsg1Δ homothallic haploid cells, either growing or shifted to nitrogen-free minimal medium, and the phosphorylation of CTD was examined using monoclonal antibodies that recognize either phospho-Ser-2, phospho-Ser-5, or unphosphorylated CTD. As shown in Figure 2A, the phosphorylation of Ser-2 residues on the CTD repeats was increased by nitrogen starvation in wild-type cells, but not in lsg1Δ cells. The level of phospho-Ser-5 was unaffected by nitrogen starvation in both strains. These results suggest that nitrogen starvation induces the phosphorylation of CTD Ser-2 residues by CTDK-I.
Fig. 2. The phosphorylation of Ser-2 residues on Pol II CTD is required for ste11 expression. 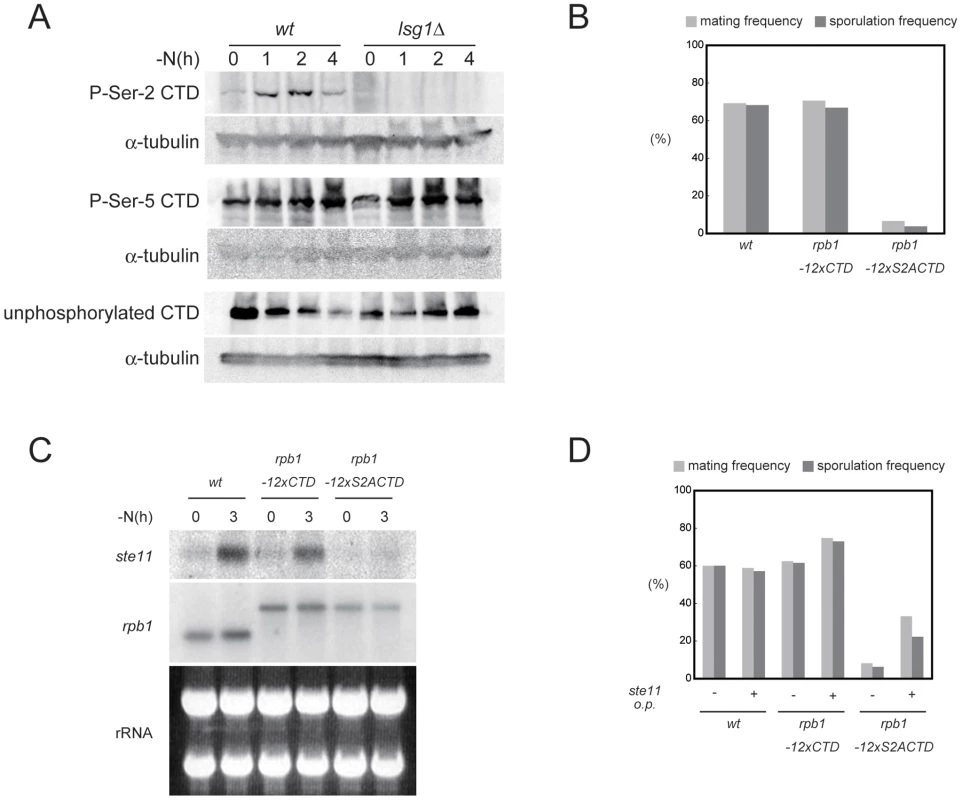
(A) Nitrogen starvation induces the phosphorylation of Ser-2 residues on Pol II CTD in wild-type (JY450) but not in lsg1Δ (JT659) cells. Cells of the two strains were subjected to nitrogen starvation for the indicated periods and analyzed by immunoblotting with antibodies against Ser-2 phosphorylated CTD, Ser-5 phosphorylated CTD, or unphosphorylated CTD. α-tubulin is shown as a loading control. (B) Comparison of the mating and sporulation frequencies among wild-type (JY450), rpb1-12×CTD (JT668), and rpb1-12×S2ACTD (JT669) strains. Cells were incubated on SSA plates at 30°C for three days, and the frequencies were determined microscopically. (C) Expression of ste11 in cells examined in (B). Cells were grown to mid-log phase and shifted to nitrogen-free medium. They were then harvested right before and at 4 hours after this shift, and subjected to northern blot analysis. rRNAs stained with ethidium bromide are shown as a loading control. Expression of rbp1, which was not affected by nitrogen starvation, is also shown for comparison. The rbp1 transcripts in JT668 and JT669 were larger than the authentic transcript due to a vector sequence inserted during the strain construction [15]. (D) Effects of ste11 overexpression on mating and sporulation in the rpb1-12×CTD and rpb1-12×S2ACTD strains. JY450, JT668, and JT669 cells harboring either pREP41-ste11 or pREP41 were examined for their mating and sporulation frequencies after incubation on SSA plates at 30°C for three days. We next evaluated the possibility that the insufficient phosphorylation of CTD Ser-2 residues in the CTDK-I mutants underlies their sexual development deficiency. For this purpose we examined the phenotypes caused by two rpb1 alleles (reported by J. Karagiannis and kindly provided to us), namely rpb1-12×CTD and rpb1-12×S2ACTD. The former allele produces Rpb1 carrying a CTD that consists of 12 copies of the authentic heptad repeat (YSPTSPS), whereas the latter produces Rpb1 with 12 copies of a mutant heptad repeat in which Ser-2 is substituted by alanine (YAPTSPS) [15]. Wild-type Rpb1 carries 29 repeats of the heptad [26], but the previous work has shown that 12 repeats are sufficient for cell viability [15]. Cells carrying the rpb1-12×S2ACTD allele were impaired severely in terms of conjugation and sporulation (Figure 2B), and the transcription of ste11 was greatly reduced in them (Figure 2C). Furthermore, the sterility of the rpb1-12×S2ACTD strain was rescued, although not completely, by the overexpression of ste11 (Figure 2D). These results strongly suggest that CTDK-I facilitates the transcription of ste11 by phosphorylating Ser-2 residues on Pol II CTD. In general, the rpb1-12×S2ACTD strain showed severer phenotypes than the CTDK-I mutants with regard to sexual development, probably because CTD Ser2 could also be phosphorylated supplementarily by Cdk9 [11].
The stress-responsive MAP kinase pathway is required for the phosphorylation of CTD Ser-2 residues
We wished to determine the mechanism by which nitrogen starvation caused the increased phosphorylation of CTD Ser-2 by CTDK-I. The concentration of CTDK-I subunits per cell was not found to be significantly altered upon nitrogen starvation (Figure S4A). We also measured the levels of Fcp1, a phosphatase that has been shown to preferentially remove phosphate groups from synthetic CTD peptides phosphorylated on Ser-2 [27], [28]. However, the levels of this protein were also not changed significantly upon nitrogen starvation (Figure S4B).
It has been reported in S. cerevisiae that CTD Ser-2 phosphorylation increases both upon heat shock and during the diauxic shift [29]. The phosphorylation of CTD Ser-2 is also known to be elevated by an exposure to hydroxyurea or UV irradiation [30]. We speculated therefore that nitrogen starvation may be recognized as a stress, which could then affect the phosphorylation status of the CTD in fission yeast. We hence examined the possible involvement of Sty1 (also called Spc1/Phh1), a MAP kinase known to be crucial to the response to stress [31]–[33], in CTD phosphorylation. As shown in Figure 3A, the phosphorylation of CTD Ser-2 in response to nitrogen starvation was dramatically reduced in sty1Δ cells compared with wild-type cells. Deletion of the atf1 gene, which encodes a target of Sty1 MAPK, also significantly affected Ser-2 phosphorylation, whereas the ste11 and mei2 genes appeared to be dispensable for this phosphorylation event in response to nitrogen starvation (Figure 3A). Deletion of pcr1, which encodes a bZIP protein that forms a heterodimer with Atf1 [21], [23], did not affect Ser-2 phosphorylation significantly (Figure S5), and produced a much less severe phenotype compared with mutants lacking atf1, as observed previously for other features [23], [34]. The deletion of rst2, which encodes a transcription factor necessary to activate ste11 in response to glucose starvation and cAMP reduction [24], [25], also had no affect on Ser-2 phosphorylation (Figure S5).
Fig. 3. The stress-responsive MAP kinase Sty1 is essential for the phosphorylation of CTD Ser-2 residues. 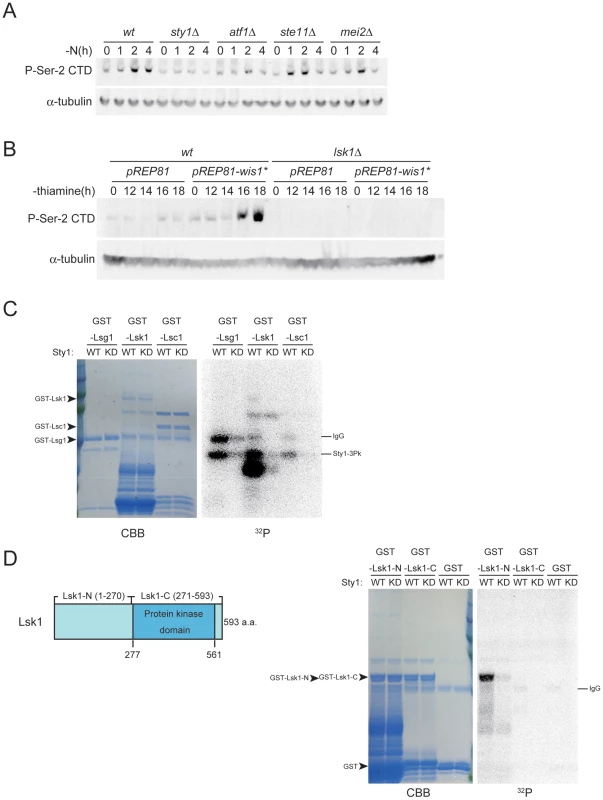
(A) CTD Ser-2 phosphorylation was examined in JY450 (wild-type), JT674 (sty1Δ), JX303 (atf1Δ), JZ496 (ste11Δ), and JZ127 (mei2Δ) cells. The cultures were subjected to nitrogen starvation, sampled at the indicated intervals, and analyzed by immunoblotting with antibodies specific for Ser-2 phosphorylated CTD. α-tubulin was detected as a loading control. (B) JY450 and JT660 (lsk1Δ) cells were transformed with either pREP81 or pREP81-wis1*, the latter of which expresses a constitutively active form of MAPKK Wis1. Each transformant was cultured in liquid MM without thiamine for 12 to 18 hours to derepress the weakened nmt1 promoter on pREP81 (nmt1-81). Cells were harvested at the indicated times and analyzed by immunoblotting as in (A). (C) Phosphorylation of GST-Lsg1, GST-Lsk1 and GST-Lsc1 by Sty1 was examined in vitro (right panel, 32P). Substrates were stained with Coomassie brilliant blue to indicate their quantities (left panel, CBB). (D) GST-Lsk1-N (1–270), GST-Lsk1-C (271–593), and GST as a control were analyzed for phosphorylation (32P) and quantities (CBB) as in (C). A schematic illustration of the structure of Lsk1 is also shown. We then examined the effects of a forced activation of the Sty1 MAPK pathway, by expressing a constitutively active form of Wis1 MAPKK in the yeast cells. Phosphorylation of Ser-2 was induced by expression of the active MAPKK from a plasmid, even in the presence of ample nitrogen (Figure 3B). However, this ectopic phosphorylation was not observed in lsk1Δ cells (Figure 3B), indicating that the observed phosphorylation was mediated by CTDK-I. These results suggest that the activation of Sty1 MAP kinase in response to nitrogen starvation is pivotal to the promotion of CTD Ser-2 phosphorylation by CTDK-I.
Sty1 MAP kinase phosphorylates Lsk1, the catalytic subunit of CTDK-I in vitro
To examine if the stress-responsive MAPK Sty1 directly phosphorylates CTDK-I, we prepared an in vitro phosphorylation system as detailed in Materials and Methods. Each subunit of CTDK-I, namely Lsk1, Lsc1 or Lsg1, was fused with GST, and the fusion proteins were affinity-purified. Pk-tagged Sty1 MAPK (Sty1-Pk) and its kinase-dead form (Sty1-KD-Pk) were prepared respectively from S. pombe strains NJ761 and NJ767, provided kindly by N. Jones, as described previously [34]. The kinase preparation and each GST-fusion protein were mixed and incubated in the kinase reaction buffer supplemented with [γ-32P]-ATP. As shown in Figure 3C, GST-Lsk1 appeared to be phosphorylated by Sty1, although the full-length protein apparently underwent extensive proteolysis and a possible degradation product was the most heavily labeled. GST-Lsc1 and GST-Lsg1, as well as the control GST, did not appear to be a good substrate of Sty1 in this analysis (Figure 3C). To confirm that Sty1 could phosphorylate Lsk1, we divided Lsk1 into two parts, the N - and C-terminal halves, and fused each of them to GST (Figure 3D). These fusion proteins were relatively stable, and when mixed with active Sty1, the N-terminal half was significantly phosphorylated (Figure 3D). Moreover, our preliminary analysis has shown that at least serine 109 on Lsk1, which constitutes a MAPK substrate consensus sequence PGSP, is a preferred phosphorylation site for Sty1 (data not shown). Analysis of Lsg1 dissected into two parts confirmed that it was not likely to be a substrate of Sty1 (data not shown). These results indicate that Sty1 MAPK is likely to phosphorylate Lsk1 directly and thereby activate CTDK-I, which in turn phosphorylates CTD Ser-2 residues.
The phosphorylation of CTD Ser-2 is regulated by a feedback system during meiosis
We made a surprising observation when we analyzed the status of CTD Ser-2 phosphorylation in cells undergoing ectopic meiosis induced by artificial expression of the activated form of Mei2, i.e., Mei2-SATA. As we reported previously [6], these cells underwent meiosis in the presence of ample nitrogen, a condition that does not stimulate the stress-responsive Sty1 MAP kinase cascade. However, the phosphorylation of CTD Ser-2 was observed in these meiotic cells (Figure 4A). Given this finding, we speculated as to whether the phosphorylation of CTD Ser-2 during Mei2-SATA-induced meiosis was dependent on CTDK-I and/or Sty1. We further tested relevant mutant strains and found that the Mei2-SATA-induced Ser-2 phosphorylation was abolished in lsk1Δ and reduced dramatically in sty1Δ, indicating its stringent dependency on both of these factors (Figure 4B). Sty1 has been positioned upstream of mei2 expression in the stress-responsive signal transduction pathway and in cooperation with a chromatin-remodeling factor Atf1, activates the transcription of ste11 [20]–[22]. The produced Ste11 in turn binds to the upstream region of mei2 and activates the transcription of this gene [16]. We thus hypothesized that activated Mei2 can affect its upstream factors through a feedback regulation.
Fig. 4. The activation of Mei2 leads to elevated CTD Ser-2 phosphorylation. 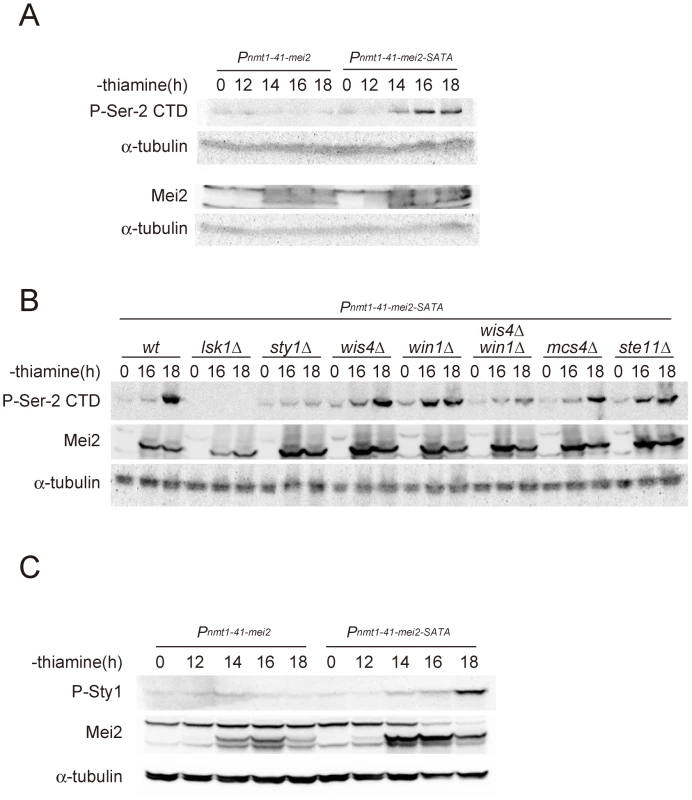
(A) Cells of the JX382 fission yeast strain, which carries a mei2 ORF driven by the attenuated nmt1 promoter (nmt1-41) on the chromosome, and of the JX383 strain, which carries the mei2-SATA ORF but is otherwise identical to JX382, were cultured in liquid MM with no supplementation of thiamine. The nmt1-41 promoter was therefore derepressed under these growth conditions. Cells were sampled at the indicated times and the phosphorylation of Ser-2 residues within the CTD repeats was examined by immunoblotting. These samples were also examined for the expression of Mei2 protein by western blot. α-tubulin is shown as a loading control. (B) The Pnmt1-41-mei2-SATA allele in JX383 was combined with either lsk1Δ (JT675), sty1Δ (JT676), wis4Δ (JT677), win1Δ (JT678), wis4Δ win1Δ (JT679), mcs4Δ (JT680), or ste11Δ (JT681). Cells of each strain were cultured in liquid MM with no thiamine addition for 16 and 18 hours, and harvested. Lysates were prepared and then analyzed by immunoblotting with anti-phospho-Ser-2 CTD. The production of Mei2 protein was also evaluated by immunoblotting with α-tubulin detected as a loading control. (C) Cells of the JX382 and JX383 strains were cultured and processed as described in (A). Immunoblotting was performed using antibodies specific for the phosphorylated form of Sty1 MAPK. The Mei2 protein and a loading control α-tubulin were also immunoblotted. To identify the component of the stress-responsive signaling pathway that is feedback-regulated by Mei2, we examined mutants that are defective in components of the pathway that function upstream of Sty1. Sty1 MAPK is activated by Wis1 MAPKK [31], [32], [35], [36], which in turn is activated by either Wis4/Wak1 MAPKKK or Win1 MAPKK [37]–[39]. A response regulator protein, Mcs4, associates with Wis4/Wak1, and probably also with Win1, to regulate the MAPKKK activity [38], [40]. We investigated the phosphorylation of Ser-2 during Mei2-SATA-induced meiosis in mcs4Δ, wis4Δ, win1Δ, and wis4Δ win1Δ mutant strains, together with control wild-type, lsk1Δ, sty1Δ, and ste11Δ strains. As summarized in Figure 4B, the phosphorylation of Ser-2 was observed in mcs4Δ and ste11Δ cells, indicating that Mcs4 and Ste11 are not directly involved in the feedback activation of Ser-2 phosphorylation. Ser-2 phosphorylation was observed also in the wis4Δ and win1Δ mutants but was found to be greatly reduced in the wis4Δ win1Δ double mutant. These results indicated that the feedback signals from activated Mei2 might ultimately merge with the stress-responsive MAPK cascade at the Wis4/Wak1 and Win1 MAPKKKs, although there could be a third target because Ser-2 phosphorylation was not completely abolished in wis4Δ win1Δ (Figure 4B). We observed that the level of Sty1 MAPK phosphorylation increased during Mei2-SATA-induced meiosis (Figure 4C), which reinforces the presence of a signaling pathway from Mei2 to the MAPK cascade.
Physiological significance of the feedback
To evaluate physiological significance of the feedback, we examined whether activation of Mei2 would result in enhancement of ste11 expression during meiosis. Firstly, we induced ectopic meiosis by shifting the mei2-L-SATA strain from 32°C to 25°C in the presence of rich nutrition. As shown in Figure 5A, expression of ste11 was evident in this strain but not in the wild-type, and this expression was dependent on lsk1. Secondly, we induced ectopic meiosis by shifting the temperature-sensitive pat1-114 mutant from 25°C to 34°C. Again, expression of ste11 was induced significantly in pat1-114 cells under rich nutrition, in an lsk1-dependent manner (Figure 5B). Deletion of mei2 blocked ste11 expression in these cells. The temperature-shift did not induce ste11 expression in wild-type (Figure 5B) or mei2Δ cells (not shown). These results indicate clearly that activation of Mei2 can stimulate expression of ste11 through phosphorylation of PolII CTD.
Fig. 5. Activated Mei2 enhances expression of ste11 via CTDK-I. 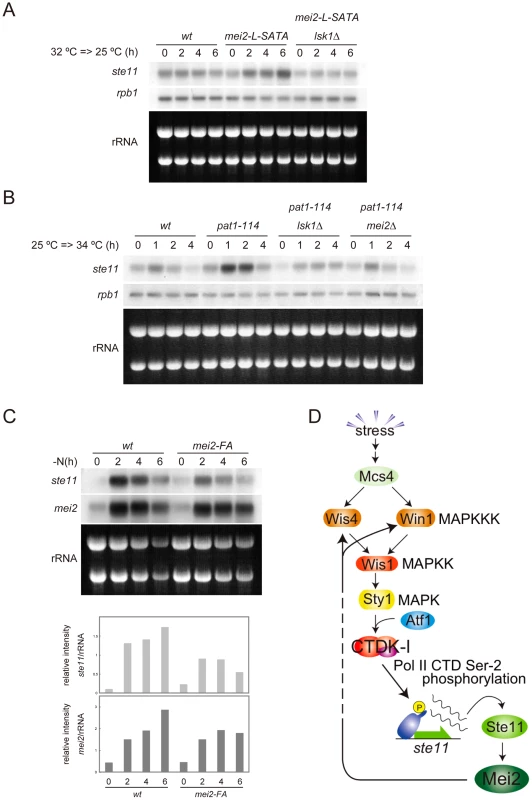
(A) Northern blot analysis of ste11 expression in JY741 (wild-type), JV312 (mei2-L-SATA) and JT663 (mei2-L-SATA lsk1Δ). Cells were grown to the mid-log phase in MM at 32°C, shifted to 25°C, and sampled at the indicated intervals. Total RNA (10 µg) from each sample was resolved by gel electrophoresis and subjected to northern blot analysis to detect transcripts of ste11 and rpb1. rRNAs stained with ethidium bromide are shown as a loading control. (B) Northern blot analysis of ste11 expression in JY333 (wild-type), JZ409 (pat1-114), JT915 (pat1-114 lsk1Δ) and JW92 (pat1-114 mei2Δ). Cells were grown to the mid-log phase in MM at 25°C, shifted to 34°C, sampled at the indicated intervals, and analyzed as in (A). (C) Northern blot analysis of ste11 and mei2 expression in heterozygous diploid (h+/h−) strains JY362 (wild-type) and JT908 (mei2-FA/mei2-FA). Cells were grown to the mid-log phase at 30°C, shifted to nitrogen-free medium, and sampled at the indicated intervals. Total RNA (10 µg) from each sample was resolved by gel electrophoresis and subjected to northern blot analysis to detect ste11 and mei2 transcripts. The level of their expression was normalized by the amount of rRNAs stained with ethidium bromide, and is displayed in a graph for quantitative comparison (lower panel). (D) A diagram of the regulatory pathway leading to the activation of CTDK-I and expression of ste11, which incorporates a feedback loop from Mei2 to MAPKKK Wis4 and Win1. We finally evaluated the contribution of the feedback regulation to the expression of ste11 during meiosis under physiological conditions. To do so, we used the mei2-FA allele, which produces inactive Mei2 protein [5], [6]. We compared expression of ste11 and mei2 in wild-type and mei2-FA cells subjected to nitrogen starvation. As shown in Figure 5C, the level of ste11 mRNA, normalized by ribosomal RNA, and that of mei2 mRNA also, were higher in wild-type cells than in mei2-FA cells, and the difference became greater in later stages. This suggests that activated Mei2 protein in wild-type cells indeed enhances ste11 expression via feedback.
Taken together, we propose that fission yeast possess a regulatory circuit, as depicted in Figure 5D, which is likely to be crucial in ensuring an irreversible commitment to meiosis and a strict differentiation of the mitotic and meiotic cell cycle programs.
Discussion
In our present study, we have demonstrated that a genetic interaction exists between the subunits of CTDK-I, a protein kinase complex that phosphorylates RNA polymerase II CTD, and the master meiotic regulator in fission yeast, Mei2. Furthermore, our analyses indicate that a loss of CTDK-I function impairs the transcription of the ste11 gene, which encodes a transcription activator essential for the expression of mei2 and other genes crucial for sexual development. However, this loss of function does not significantly affect the gene expression required for vegetative growth. In an independent study, Hermand and colleagues have performed genome-wide mapping of three kinds of CTD kinases and also of serine 2 - and 5-phosphorylated Pol II in fission yeast to investigate the link between CTD phosphorylation and specific cellular events [11]. Consequently they have found that the CTDK-I catalytic subunit Lsk1 and Ser-2-phosphorylated Pol II associate with a rather limited number of transcription units and play only minor roles during vegetative growth, but become essential during sexual development. These authors have further reported that nitrogen starvation enhances recruitment of Lsk1 to the ste11 gene, and remarked that the phosphorylation of CTD Ser-2 plays a highly specialized role in gene regulation in fission yeast, unlike in other organisms, and is virtually confined to the regulation of a single key gene controlling sexual differentiation. Our study fully supports this notion. While a subsequent study [26] suggests that the deleterious effects of loss of Ser-2 phosphorylation on ste11 transcrition can be compensated partially by loss of Ser-7 phosphorylation, the nature of such extreme specification and its evolution is an intriguing enigma.
Our present data have further shown that the stress-responsive MAP kinase pathway is crucial for the activation of CTDK-I under conditions of nitrogen starvation. The requirement for Sty1 MAPK and its target Atf1 for the expression of ste11 has been known for some time [20]–[22], but the details of the molecular mechanisms involved have remained unknown. It now appears that CTD Ser-2 phosphorylation is a key step in the activation of ste11 expression by the Sty1 MAPK cascade. It has been shown that when phosphorylated and activated by Wis1 MAPKK, the Sty1 protein migrates to the nucleus and resides on the promoter regions of stress-responsive genes [31], [34], [41]. This is also the case for the Sty1 ortholog in S. cerevisiae Hog1 [42], [43]. As shown above, Sty1 can directly phosphorylate Lsk1 in vitro. While the phosphorylation of Lsk1 in vivo remains to be confirmed, it appears to be conceivable that Sty1 may also be recruited to the ste11 promoter and phosphorylate CTDK-I staying there, which in turn phosphorylates CTD and licenses RNA polymerase II to transcribe the gene. In this regard, it is noteworthy that hsp9, which encodes a small heat-shock protein [44] and is one of the genes responsible for the “core environmental stress response” or CESR in fission yeast [45], was detected among our possible target genes upregulated by CTD Ser-2 phosphorylation (Table S1). Interestingly, Reiter et al. have shown previously that Sty1 MAPK is recruited to the promoter of hsp9 and other CSRE genes upon osmotic stress in an Atf1-dependent manner, but does not necessarily phosphorylate Atf1 as a substrate [34]. This suggests that ste11 and hsp9 may be similarly regulated by the Sty1 – CTDK-I – CTD phosphorylation system. However, conventional Chip analyses have not provided convincing evidence for the association of Sty1 with the ste11 promoter, and we are conducting further experiments to scrutinize this possible scheme.
The results of our present analyses demonstrate unambiguously that a feedback-regulatory system operates in fission yeast during the meiotic cell cycle. In this feedback loop, the active form of Mei2 can eventually stimulate the stress-responsive MAPKKKs and enhance the transcription of ste11 through the Sty1 – CTDK-I – CTD phosphorylation system. From our findings we can outline a framework of the molecular mechanisms that differentiate the mitotic and meiotic programs in fission yeast as in Figure 5D. However, it remains currently unknown how the RNA-binding protein Mei2 can fulfill such a never-anticipated task and how many steps may mediate between Mei2 and the MAPKKKs, raising another challenging scientific query as represented by the broken line in Figure 5D.
Materials and Methods
Fission yeast strains, genetic procedures, and media
The S. pombe strains used in this study are listed in Table S2. The general genetic procedures used in the S. pombe experiments were as described previously [46]. Complete medium YE, minimal medium SD, minimal medium MM and its nitrogen-free derivative MM-N [47], synthetic sporulation medium SSA [48] were used to culture the cells. Transformation of S. pombe was performed using the lithium acetate method [49].
Genetic screen
The ura4+ cassette used for insertion mutagenesis was amplified by PCR using the primers N18AGCTTAGCTACAAATCCCACTGGCT and N18TGTGATATTGACGAACTTTTTGAC (N18: 18b random DNA sequence). The PCR products were then introduced into JV312 (mei2-L-SATA ura4-D18) cells, and transformants were plated onto SD lacking uracil and incubated at 25°C. Colonies were selected, and the site of ura4+ integration was determined via the sequencing of inverse PCR products [50].
Flow cytometric analysis
Samples were prepared for flow cytometry essentially as described previously [51] and then analyzed using a FACScan (Becton-Dickinson, San Jose, CA).
Microarray analysis
JY450 (wild-type) and JT659 (lsg1Δ) cells were grown to mid-log phase in MM medium and shifted to MM-N medium. The cells were collected 2.5 h after the shift, and total RNA was extracted as described previously [52]. Data acquisition and normalization were performed by Roche Applied Science, Japan. The microarray data was deposited to the GEO database under the accession number of GSE32516.
Northern blot analysis
Northern blot analysis was performed as described [53]. DNA fragments used to probe for transcripts of ste11, rpb1 and mei2 were labeled with [α-32P] dCTP using random primers.
Western blot analysis
Cell extracts were prepared and separated essentially as described earlier [54]. Briefly, cells grown to the mid-log phase were shifted to nitrogen-free medium, and sampled at various intervals. Total lysates were extracted and resolved by SDS-PAGE. Immunoblotting was performed using primary antibodies specific to unphosphorylated CTD (8WG16, Covance, Princeton, NJ, used at 1∶2000), Ser-5 phosphorylated CTD (H14, Covance, used at 1∶2000), Ser-2 phosphorylated CTD (H5, Covance, used at 1∶1000), Mei2 (Our lab preparation, used at 1∶1000), the phosphorylated form of Sty1 MAPK (P-p38 MAPK, Cell Signaling Technology, Danvers, MA, used at 1∶500), or GFP (clones 7.1 and 13.1, Roche Applied Science, Indianapolis, IN, used at 1∶1000). As secondary antibodies, donkey anti-rabbit IgG conjugated with horseradish peroxidase (GE Healthcare, Waukesha, WI) was used for the Mei2 and P-p38 MAPK antibodies at a dilution of 1∶2000. Sheep anti-mouse IgG conjugated with horseradish peroxidase (GE Healthcare) was used to detect all other primary antibodies at a dilution of 1∶2000. Immunoblotting with a monoclonal antibodies specific for α-tubulin, either TAT-1 (a gift from Dr. Keith Gull, University of Birmingham) [55], or Clone B-5-1-2 (Sigma Aldrich, St. Louis, MO), was performed as a loading control.
In vitro phosphorylation assay
Cells expressing chromosomally tagged Sty1-3Pk (NJ761), or Sty1KD-3Pk (NJ767) were subjected to nitrogen starvation for 1 h. Extracts were prepared, protein immunoprecipitated, and the immuno-complexes tested for kinase activity as described [34]. Affinity purified GST-fusion proteins were used as substrates.
Supporting Information
Zdroje
1. MataJLyneRBurnsGBahlerJ 2002 The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet 32 143 147
2. KassirYAdirNBoger-NadjarERavivNGRubin-BejeranoI 2003 Transcriptional regulation of meiosis in budding yeast. Int Rev Cytol 224 111 171
3. HarigayaYTanakaHYamanakaSTanakaKWatanabeY 2006 Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature 442 45 50
4. YamanakaSYamashitaAHarigayaYIwataRYamamotoM 2010 Importance of polyadenylation in the selective elimination of meiotic mRNAs in growing S. pombe cells. EMBO J 29 2173 2181
5. WatanabeYYamamotoM 1994 S.pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell 78 487 498
6. WatanabeYYabanaSChikashigeYHiraokaYYamamotoM 1997 Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 386 187 190
7. YamashitaAWatanabeYNukinaNYamamotoM 1998 RNA-assisted nuclear transport of the meiotic regulator Mei2p in fission yeast. Cell 95 115 123
8. YamamotoM 2010 The selective elimination of messenger RNA underlies the mitosis-meiosis switch in fission yeast. Proc Jpn Acad Ser B Phys Biol Sci 86 788 797
9. HiroseYManleyJL 2000 RNA polymerase II and the integration of nuclear events. Genes Dev 14 1415 1429
10. PhatnaniHPGreenleafAL 2006 Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev 20 2922 2936
11. CoudreuseDvan BakelHDewezMSoutourinaJParnellT 2010 A gene-specific requirement of RNA polymerase II CTD phosphorylation for sexual differentiation in S. pombe. Curr Biol 20 1053 1064
12. LeeJMGreenleafAL 1991 CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr 1 149 167
13. SternerDELeeJMHardinSEGreenleafAL 1995 The yeast carboxyl-terminal repeat domain kinase CTDK-I is a divergent cyclin-cyclin-dependent kinase complex. Mol Cell Biol 15 5716 5724
14. KaragiannisJBimboARajagopalanSLiuJBalasubramanianMK 2005 The nuclear kinase Lsk1p positively regulates the septation initiation network and promotes the successful completion of cytokinesis in response to perturbation of the actomyosin ring in Schizosaccharomyces pombe. Mol Biol Cell 16 358 371
15. KaragiannisJBalasubramanianMK 2007 A cyclin-dependent kinase that promotes cytokinesis through modulating phosphorylation of the carboxy terminal domain of the RNA Pol II Rpb1p sub-unit. PLoS ONE 2 e433 doi:10.1371/journal.pone.0000433
16. SugimotoAIinoYMaedaTWatanabeYYamamotoM 1991 Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev 5 1990 1999
17. SipiczkiM 1988 The role of sterility genes (ste and aff) in the initiation of sexual development in Schizosaccharomyces pombe. Mol Gen Genet 213 529 534
18. WatanabeYIinoYFuruhataKShimodaCYamamotoM 1988 The S.pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J 7 761 767
19. MataJBahlerJ 2006 Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc Natl Acad Sci U S A 103 15517 15522
20. TakedaTTodaTKominamiKKohnosuAYanagidaM 1995 Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J 14 6193 6208
21. KanohJWatanabeYOhsugiMIinoYYamamotoM 1996 Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells 1 391 408
22. ShiozakiKRussellP 1996 Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev 10 2276 2288
23. WatanabeYYamamotoM 1996 Schizosaccharomyces pombe pcr1+ encodes a CREB/ATF protein involved in regulation of gene expression for sexual development. Mol Cell Biol 16 704 711
24. KunitomoHHiguchiTIinoYYamamotoM 2000 A zinc-finger protein, Rst2p, regulates transcription of the fission yeast ste11+ gene, which encodes a pivotal transcription factor for sexual development. Mol Biol Cell 11 3205 3217
25. HiguchiTWatanabeYYamamotoM 2002 Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol Cell Biol 22 1 11
26. SchwerBShumanS 2011 Deciphering the RNA polymerase II CTD code in fission yeast. Mol Cell 43 311 318
27. KimuraMSuzukiHIshihamaA 2002 Formation of a carboxy-terminal domain phosphatase (Fcp1)/TFIIF/RNA polymerase II (pol II) complex in Schizosaccharomyces pombe involves direct interaction between Fcp1 and the Rpb4 subunit of pol II. Mol Cell Biol 22 1577 1588
28. HausmannSShumanS 2002 Characterization of the CTD phosphatase Fcp1 from fission yeast. Preferential dephosphorylation of serine 2 versus serine 5. J Biol Chem 277 21213 21220
29. PatturajanMSchulteRJSeftonBMBerezneyRVincentM 1998 Growth-related changes in phosphorylation of yeast RNA polymerase II. J Biol Chem 273 4689 4694
30. OstapenkoDSolomonMJ 2003 Budding yeast CTDK-I is required for DNA damage-induced transcription. Eukaryot Cell 2 274 283
31. ShiozakiKRussellP 1995 Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378 739 743
32. MillarJBBuckVWilkinsonMG 1995 Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev 9 2117 2130
33. KatoTJrOkazakiKMurakamiHStettlerSFantesPA 1996 Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett 378 207 212
34. ReiterWWattSDawsonKLawrenceCLBahlerJ 2008 Fission yeast MAP kinase Sty1 is recruited to stress-induced genes. J Biol Chem 283 9945 9956
35. DegolsGShiozakiKRussellP 1996 Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol 16 2870 2877
36. DegolsGRussellP 1997 Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol 17 3356 3363
37. WilkinsonMGMillarJB 1998 SAPKs and transcription factors do the nucleocytoplasmic tango. Genes Dev 12 1391 1397
38. ShiehJCWilkinsonMGMillarJB 1998 The Win1 mitotic regulator is a component of the fission yeast stress-activated Sty1 MAPK pathway. Mol Biol Cell 9 311 322
39. ShiozakiKShiozakiMRussellP 1998 Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol Biol Cell 9 1339 1349
40. BuckVQuinnJSoto PinoTMartinHSaldanhaJ 2001 Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell 12 407 419
41. GaitsFDegolsGShiozakiKRussellP 1998 Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev 12 1464 1473
42. AlepuzPMJovanovicAReiserVAmmererG 2001 Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol Cell 7 767 777
43. AlepuzPMde NadalEZapaterMAmmererGPosasF 2003 Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J 22 2433 2442
44. OrlandiICavadiniPPopoloLVaiM 1996 Cloning, sequencing and regulation of a cDNA encoding a small heat-shock protein from Schizosaccharomyces pombe. Biochim Biophys Acta 1307 129 131
45. ChenDTooneWMMataJLyneRBurnsG 2003 Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell 14 214 229
46. GutzHHeslotHLeupoldULoprienoN 1974 Schizosaccharomyces pombe. KingRD Handbook of Genetics New York Plenum Publishing Corporation 395 446
47. MorenoSKlarANurseP 1991 Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194 795 823
48. EgelREgel-MitaniM 1974 Premeiotic DNA synthesis in fission yeast. Exp Cell Res 88 127 134
49. OkazakiKOkazakiNKumeKJinnoSTanakaK 1990 High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res 18 6485 6489
50. ChuaGTaricaniLStangleWYoungPG 2000 Insertional mutagenesis based on illegitimate recombination in Schizosaccharomyces pombe. Nucleic Acids Res 28 E53
51. ImaiYYamamotoM 1994 The fission yeast mating pheromone P-factor: its molecular structure, gene structure, and ability to induce gene expression and G1 arrest in the mating partner. Genes Dev 8 328 338
52. MatsuoTOtsuboYUranoJTamanoiFYamamotoM 2007 Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol 27 3154 3164
53. YamashitaAWatanabeYYamamotoM 1997 Microtubule-associated coiled-coil protein Ssm4 is involved in the meiotic development in fission yeast. Genes Cells 2 155 166
54. KohdaTATanakaKKonomiMSatoMOsumiM 2007 Fission yeast autophagy induced by nitrogen starvation generates a nitrogen source that drives adaptation processes. Genes Cells 12 155 170
55. WoodsASherwinTSasseRMcRaeTHBainesAJ 1989 Definition of individual components within the cytoskelton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci 93 491 500
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



