-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaZelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
The earliest stages of development in most metazoans are driven by maternally deposited proteins and mRNAs, with widespread transcriptional activation of the zygotic genome occurring hours after fertilization, at a period known as the maternal-to-zygotic transition (MZT). In Drosophila, the MZT is preceded by the transcription of a small number of genes that initiate sex determination, patterning, and other early developmental processes; and the zinc-finger protein Zelda (ZLD) plays a key role in their transcriptional activation. To better understand the mechanisms of ZLD activation and the range of its targets, we used chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-Seq) to map regions bound by ZLD before (mitotic cycle 8), during (mitotic cycle 13), and after (late mitotic cycle 14) the MZT. Although only a handful of genes are transcribed prior to mitotic cycle 10, we identified thousands of regions bound by ZLD in cycle 8 embryos, most of which remain bound through mitotic cycle 14. As expected, early ZLD-bound regions include the promoters and enhancers of genes transcribed at this early stage. However, we also observed ZLD bound at cycle 8 to the promoters of roughly a thousand genes whose first transcription does not occur until the MZT and to virtually all of the thousands of known and presumed enhancers bound at cycle 14 by transcription factors that regulate patterned gene activation during the MZT. The association between early ZLD binding and MZT activity is so strong that ZLD binding alone can be used to identify active promoters and regulatory sequences with high specificity and selectivity. This strong early association of ZLD with regions not active until the MZT suggests that ZLD is not only required for the earliest wave of transcription but also plays a major role in activating the genome at the MZT.
Published in the journal: . PLoS Genet 7(10): e32767. doi:10.1371/journal.pgen.1002266
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002266Summary
The earliest stages of development in most metazoans are driven by maternally deposited proteins and mRNAs, with widespread transcriptional activation of the zygotic genome occurring hours after fertilization, at a period known as the maternal-to-zygotic transition (MZT). In Drosophila, the MZT is preceded by the transcription of a small number of genes that initiate sex determination, patterning, and other early developmental processes; and the zinc-finger protein Zelda (ZLD) plays a key role in their transcriptional activation. To better understand the mechanisms of ZLD activation and the range of its targets, we used chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-Seq) to map regions bound by ZLD before (mitotic cycle 8), during (mitotic cycle 13), and after (late mitotic cycle 14) the MZT. Although only a handful of genes are transcribed prior to mitotic cycle 10, we identified thousands of regions bound by ZLD in cycle 8 embryos, most of which remain bound through mitotic cycle 14. As expected, early ZLD-bound regions include the promoters and enhancers of genes transcribed at this early stage. However, we also observed ZLD bound at cycle 8 to the promoters of roughly a thousand genes whose first transcription does not occur until the MZT and to virtually all of the thousands of known and presumed enhancers bound at cycle 14 by transcription factors that regulate patterned gene activation during the MZT. The association between early ZLD binding and MZT activity is so strong that ZLD binding alone can be used to identify active promoters and regulatory sequences with high specificity and selectivity. This strong early association of ZLD with regions not active until the MZT suggests that ZLD is not only required for the earliest wave of transcription but also plays a major role in activating the genome at the MZT.
Introduction
Delayed activation of the zygotic genome during the early phases of embryogenesis is a nearly universal phenomenon in metazoans. Immediately following egg activation, the zygotic genome is largely transcriptionally quiescent, with development controlled by maternally contributed mRNAs and proteins [1], [2]. At a point known as the maternal-to-zygotic transition (MZT) the degradation of maternally provided RNAs is tightly coordinated with widespread initiation of zygotic transcription. Despite the ubiquity of these events, we are only beginning to understand how the zygotic genome is activated at this discrete developmental timepoint.
In Drosophila melanogaster, the fertilized egg undergoes a series of replication cycles without cytoplasmic divisions to generate a syncytial blastoderm [3]. During cycle 14, the blastoderm nuclei cellularize and general zygotic transcription is initiated [3]–[5]. However, a subset of genes required for sex determination, pattern formation and cellularization are transcribed as early as cycle 8 [6]. These genes share a common set of related heptameric DNA motifs, CAGGTAG and related “TAGteam” elements, in their regulatory regions, the removal of which abolishes early activation [7].
Several factors present in the early embryo that bind to TAGteam elements have been identified [8]–[10], but accumulated evidence suggests that the zinc-finger transcription factor Zelda (ZLD) is the most important in regulating early gene expression. Mutations in zld lead to defects in early embryonic mitosis and severe cellularization defects by mitotic cycle 14 [10], [11]. A microarray study of ZLD-depleted embryos identified 120 genes whose proper expression during early embryogenesis is dependent on ZLD [10], but the full range of ZLD targets and its mechanisms of action are not known.
We have, for several years, been investigating the genome-wide binding of the transcription factors that regulate the anterior-posterior and dorsal-ventral patterning of transcription during and immediately following the MZT. We used chromatin immunoprecipitation coupled with DNA microarray hybridization (ChIP-chip) to identify the regions bound during mitotic cycle 14 by 21 of these patterning transcription factors. While the regions bound by any particular factor are, predictably, enriched for its target sequence, in virtually every case the most strongly enriched sequence was not the specific target, but CAGGTAG [12]. This striking and unexpected observation suggested that, in addition to its established role in regulating early transcriptional activation, ZLD might play a central role in regulating genome activity at the MZT. Here we investigate this possibility using chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-Seq) to determine the genomic landscape of ZLD binding as the embryo progresses through the MZT.
Results
ChIP-Seq on individually staged and hand-sorted embryos
Although we were particularly interested in the possible role of ZLD at the MZT (mitotic cycle 14), we felt it was essential that we investigate ZLD binding when it is known to activate early zygotic transcription, as well as at the onset of and during the MZT. We therefore collected embryos from population cages and fixed them for chromatin extraction at three timepoints following egg-laying: 60–90 minutes, targeting mitotic cycles 8 and 9 when ZLD levels increase [9], [11] and the earliest zygotic transcription occurs [6]; 120–150 minutes targeting mitotic cycle 13 and early mitotic cycle 14 when widespread zygotic transcription begins; and 180–210 minutes targeting late mitotic cycle 14 when robust zygotic transcription has been established.
In a typical ChIP experiment, chromatin would be prepared directly from these timed embryo collections. However, D. melanogaster females do not always lay eggs immediately following fertilization, meaning that while these bulk embryo collections were timed to target a particular stage, they invariably contained a small number of older embryos. Since, at this stage of development, even moderately older embryos contain substantially more DNA, even a small fraction of contaminating older embryos can represent a substantial fraction of purified chromatin. We therefore hand sorted each pool by individually examining every embryo under a light microscope and removing those that did not have the distinguishing morphological characteristics of the stage that sample was targeting.
Through this laborious procedure we obtained pure pools containing approximately 1, 0.2, and 0.1 g of embryos respectively for cycles 8–9, late cycle 13 and early cycle 14, and late cycle 14 (Figure S1). For simplicity, in the rest of the manuscript, we will refer to these samples as cycle 8, cycle 13 and late cycle 14 respectively, although we want to emphasize that we sorted embryos based on morphology and not directly on mitotic cycle, and each sample contained a mix of embryos at adjacent mitotic cycles.
We performed immunoprecipitations using previously described affinity-purified anti-ZLD antibodies [9]. To avoid possible cross reactivity between these antibodies and other zinc-finger containing transcription factors, we depleted our antibody pool of antibodies that recognize any of the four zinc fingers that comprise the DNA-binding domain. We sequenced immunoprecipitated DNA on an Illumina Genome Analyzer IIx, mapped reads to the D. melanogaster reference sequence using Bowtie [13], and identified peaks using the Grizzly Peak-finding algorithm (see Materials and Methods). These data represent the first genome-wide analysis of ZLD binding, and, to our knowledge, the first genome-wide analysis of transcription factor binding as the embryo proceeds through zygotic genome activation at the MZT.
ZLD is bound to thousands of sites prior to the MZT
Although we used a relatively small amount of input chromatin for each sample, the ChIP-Seq data were of high quality, with well-resolved peaks and high signal-to-noise ratio (Figure 1A). We identified 11,374 peaks at cycle 8, 10,471 peaks at cycle 13, and 9,432 peaks at late cycle 14 (Tables S1, S2, S3).
Fig. 1. ZLD binds to TAGteam elements in promoters and regulatory elements prior to zygotic activation. 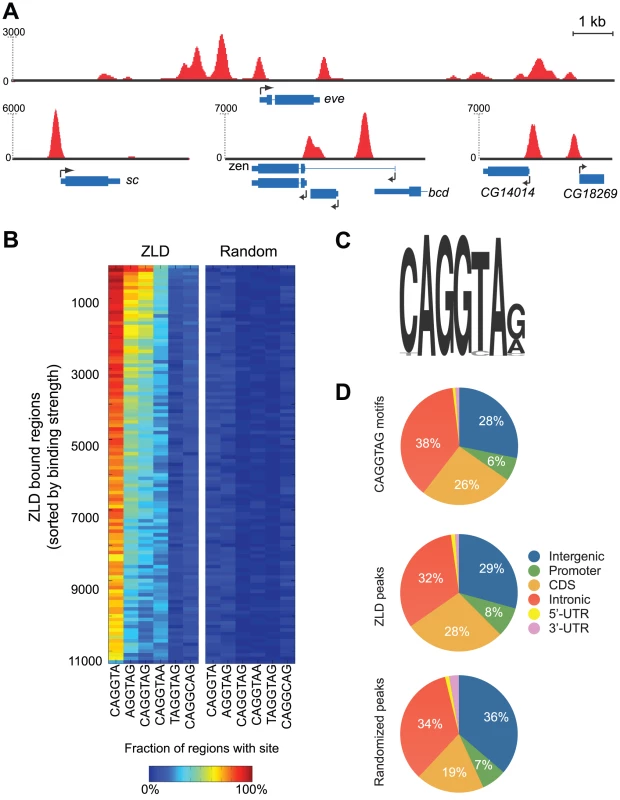
(A) Snapshots of ZLD binding at cycle 8 in the loci containing the early-transcribed genes scute (sc), even-skipped (eve), zerknüllt (zen), CG14014 and CG18269 (B) Enrichment of ZLD binding site variants among ZLD peaks from cycle 8. Peaks were sorted from highest (top) to lowest (bottom) levels of binding and binned into groups of 100. The percent of peaks in each bin containing the appropriate motif within 150 bp of the peak location is indicated by the color of the cell in the left panel, the percent of peaks containing the motif after random repositioning of peaks is shown in the right panel. (C) Sequence motif identified from top 500 ZLD peaks using MEME [14] (D) Distribution of genomic annotations among occurrences of CAGGTAG (top), ZLD peaks at cycle 8 (center), and the expected distribution of annotations (based on their genomic abundance) after randomly repositioning the peaks across the genome (bottom). We analyzed these regions for enriched occurrences of sequence motifs using a variety of computational algorithms [14]–[16], and consistently recovered the previously identified 7-mer CAGGTAG [7], [9], [10] and several variants as the primary determinants of ZLD binding in vivo (Figure 1B and 1C). For example, 66% of the top 1,000 ZLD peaks at cycle 8 contain the CAGGTAG motif at least once, as opposed to the random expectation of 1.8%. Similarly, CAGGTA and AGGTAG, two shorter versions of the motif, appear, respectively, in 94% and 75% of the most highly bound regions. There is also a strong correlation between the number of occurrences of CAGGTAG in a region and the magnitude of ZLD binding (Figure S2). Surprisingly, other TAGteam elements, including TAGGTAG and CAGGCAG, which were shown by mutation analysis to participate in the early expression of scute (sc) [7], and to be bound by ZLD in vitro [9], [10], were not significantly enriched among the top 1,000 regions bound in vivo.
As previous experiments implicated ZLD in the activation of early zygotic expression [10], [11], we focused our attention on binding at cycle 8, when zygotic transcription is initiated. We observed strong ZLD binding to many genes in cycle 8 embryos, including the early-transcribed genes sc, zerknüllt (zen) and even-skipped (eve) (Figure 1A). ZLD was found at the promoters of 1,171 genes at cycle 8. However, promoters (defined here as 500 bp upstream to 150 bp downstream of the transcription start site) represented only eight percent of ZLD-bound regions, with the remainder distributed evenly across gene bodies and non-coding DNA (Figure 1D). The observed distribution of bound regions closely mirrors the distribution of the CAGGTAG motif across the genome (Figure 1D). Indeed, we find that 64% of CAGGTAG sites are bound by ZLD in cycle 8 embryos, indicating that ZLD's inherent affinity for DNA, rather than interactions with other factors or chromatin structure, is the major determinant of its binding at this early stage.
ZLD directly regulates transcriptional activation in the early embryo
In their paper describing ZLD as a CAGGTAG binding protein, Liang et al. [10] used microarrays to measure expression differences between wildtype cycle 8–13 embryos and those lacking maternal ZLD. Although they identified 120 genes down-regulated in ZLD-depleted embryos, they could not determine how much of this effect was directly due to the actions of ZLD. To see if we could resolve this ambiguity, we compared their genome-wide mutant expression data to our ZLD binding data, and found a very strong association between ZLD binding and expression. In particular, most genes strongly bound by ZLD at their promoters during cycle 8, and detectably expressed in cycle 8–13 wildtype embryos, were downregulated in embryos lacking ZLD (Figure 2A). The effect is more pronounced when we exclude maternally deposited mRNAs (using data from [17]), as the expression effect of ZLD binding is restricted to zygotically transcribed genes (Figure 2B). These analyses suggest that the expression effects observed by Liang et al. were largely direct, and that ZLD binding to promoters is required for zygotic activation of the small number of genes transcribed in the early embryo.
Fig. 2. Zygotically transcribed ZLD targets are downregulated in embryos depleted for maternal ZLD. 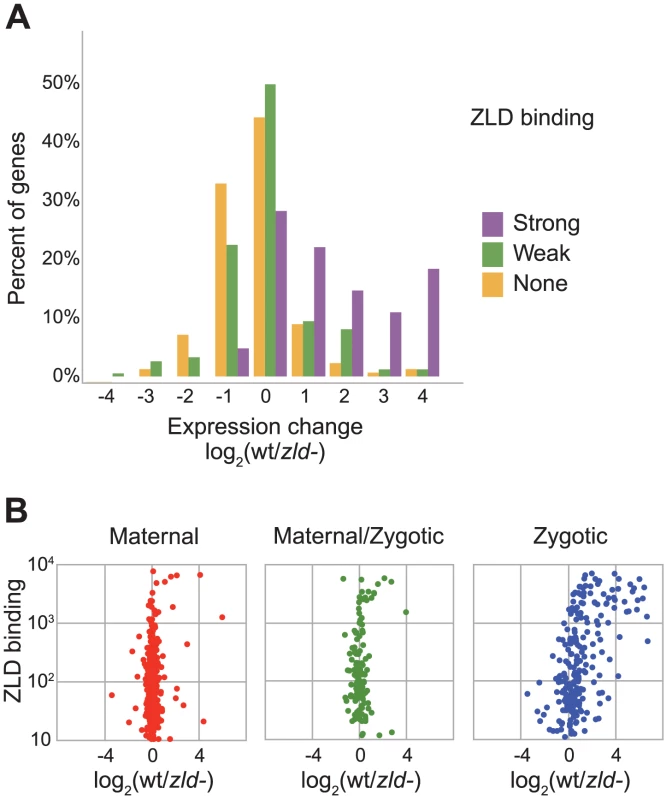
(A) Gene expression differences between wildtype embryos and ZLD-depleted embryos [10], among 101 zygotic genes with strong (greater than 500) ZLD cycle 8 binding at promoters (purple), at 270 zygotic genes with weaker ZLD promoter binding (green), and among 1639 zygotic genes with no ZLD promoter binding (orange). Zygotic genes are as defined in [17] (B) Comparison of effect of ZLD depletion and ZLD promoter binding at cycle 8 among maternally deposited genes with no zygotic expression in early embryo (left), genes that are both maternally deposited and zygotically transcribed (center), and genes with exclusively zygotic transcription (right). Classification of genes is as defined in [17]. ZLD binds early to the promoters of genes subsequently transcribed at the MZT
Given this strong relationship between ZLD promoter binding in cycle 8 embryos and changes in transcription upon ZLD depletion, we next examined the relationship between ZLD binding and the onset of zygotic transcription in wildtype embryos. We took advantage of a recently published high-resolution time course of zygotic gene expression in the early embryo [17], and compared ZLD binding at the promoters of 2,010 genes with exclusively zygotic expression to the time at which the genes are first detectably transcribed (Figure 3).
Fig. 3. At mitotic cycle 8, ZLD marks promoter and non-promoter regions of genes activated at the MZT. 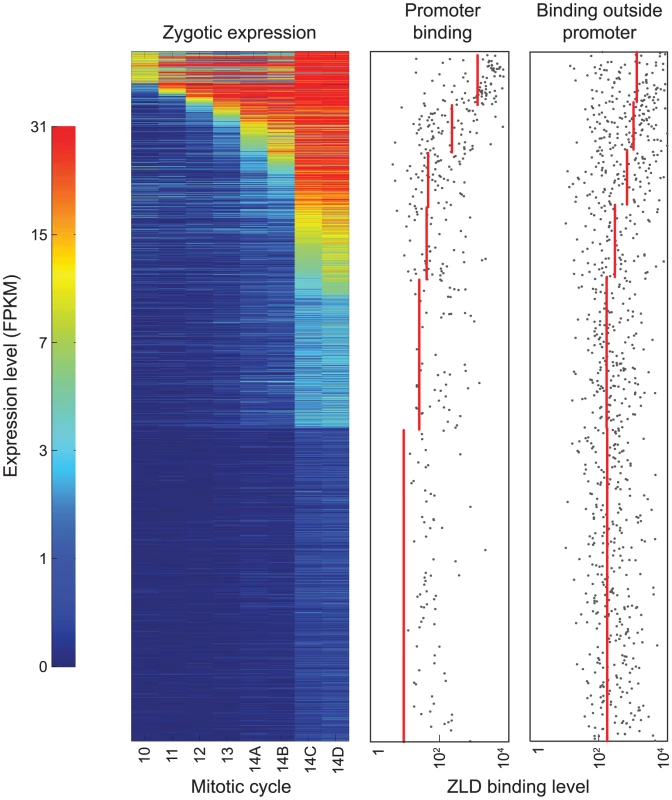
The left panel shows the expression patterns of 2,010 genes with exclusively zygotic expression at eight timepoints before and during the MZT (list of zygotically transcribed genes and expression data from [17]). Genes are sorted from early (top) to late (bottom) onset of zygotic transcription. The two panels on the right show the levels of ZLD binding to promoter and non-promoter regions for each gene (grey dots; regions with no detected binding are not plotted). Red lines show average levels of ZLD binding in groups of genes with similar times of transcription onset. Surprisingly, the promoters of many genes that are not expressed until cycle 14 were already bound by ZLD at cycle 8. For example, the genes odd-paired (opa) and leak (lea) are not expressed until mitotic cycle 14, but were highly bound by ZLD at cycle 8 (Figure S3). More generally, there was a strong correlation between the strength of cycle 8 ZLD promoter binding and the onset and magnitude of gene expression (Figure 3), with higher levels of cycle 8 promoter binding associated with earlier and stronger expression.
Early ZLD binding marks sites later bound by zygotically expressed transcription factors
While ZLD binding at promoters is strongly associated with zygotic transcription, the widespread binding of ZLD to non-promoter regions of genes active in the early embryo suggests a more general role in activating the zygotic genome. As shown in Figure 1D, more than 90 percent of the regions bound by ZLD are outside of promoter regions. And, as with promoter binding, there is a high correlation between ZLD binding in non-promoter regions and the timing and magnitude of zygotic expression (Figure 3).
As discussed in the introduction, many regions bound by the transcription factors that establish anterior-posterior (A-P) and dorsal-ventral (D-V) patterning in the early embryo are significantly enriched for CAGGTAG and other ZLD binding sites [12], [18]. We therefore compared ZLD binding at cycle 8 to genome-wide binding measurements of 21 transcription factors involved in A-P and D-V patterning [12], [18]. A strikingly large fraction of the regions most strongly bound by these factors in the cellular blastoderm at mitotic cycle 14 (which several lines of evidence suggest are functional enhancers [12], [18]) are already bound by ZLD at cycle 8 (Figure 4A). Given that only four of these factors (BCD, CAD, GT and KR) are present in the embryo at cycle 8, ZLD must be bound to this large collection of enhancers prior to the binding of most of these additional transcription factors—at least four nuclear divisions prior in the majority of cases.
Fig. 4. ZLD binding precedes and overlaps transcription-factor binding in regulatory sequences. 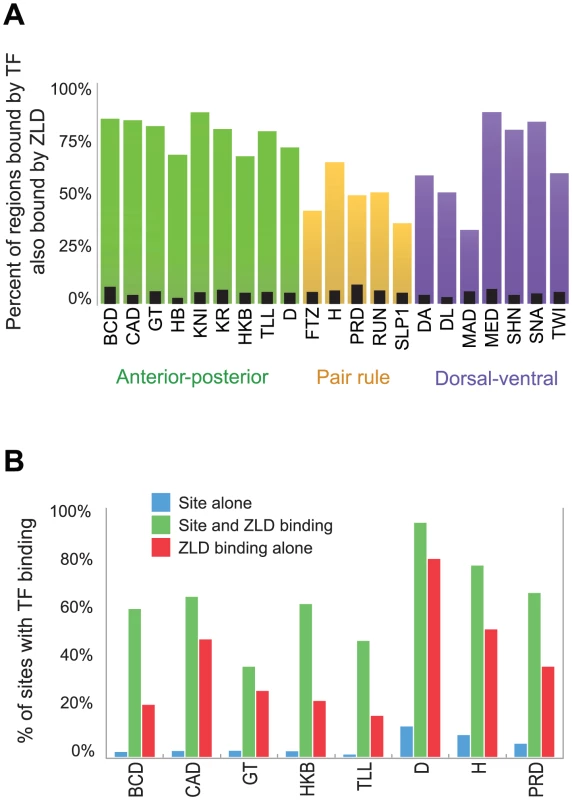
(A) Fraction of regions highly bound (top 300 regions from [18]) by each of 21 transcription factors involved in anterior-posterior or dorsal-ventral patterning at cycle 14 that are bound by ZLD at cycle 8. As a control, narrow black bars show coverage after random reshuffling of ZLD-bound regions (B) For eight factors from [18], we compare the fraction of genome-wide recognition site occurrences that are occupied by each factor (blue), to the fraction of occupied sites that occur in regions that were bound by ZLD in cycle 8 (green). The significant increase in the probability of a factor binding to its own recognition sites in ZLD-bound regions emphasizes the role that ZLD plays in activation of the zygotic genome. Red bars mark the expected percent of TF-occupied sites among ZLD peaks, even in the absence of a TF-recognition element. We analyzed only the eight factors with clear simple recognition elements based on in vitro binding data. To examine whether ZLD binding affects subsequent transcription factor binding or is simply associated with it, we examined the relationship between the presence of transcription factor target sequences, ZLD binding and transcription factor binding for the subset of factors whose binding specificity is known. As expected, the presence of a target sequence alone is a poor predictor of binding of the corresponding factor (Figure 4B, blue bars), presumably because many of these sequences are found in regions of closed chromatin [19]. However, when we restrict this analysis to regions bound early by ZLD the predictive power of these motifs increases dramatically (Figure 4B, green bars), suggesting that ZLD binding plays a significant role in determining which regions of the genome are accessible to transcription factor binding.
We next directly examined the relationship between ZLD binding and chromatin accessibility, using recently published DNAseI accessibility from cycle 14 embryos [20]. We found that ZLD binding at cycle 8 was strongly predictive of DNAseI accessibility at cycle 14, with regions bound strongly by ZLD at cycle 8 highly enriched for regions of open chromatin at cycle 14 (Figure 5A). There is also a strong correlation between the amount of ZLD binding at cycle 8 and regions of high DNA accessibility at cycle 14 (Figure 5B; r = 0.27). The relationship between ZLD binding at late cycle 14 and DNAse accessibility at cycle 14 was even stronger (Figure 5C; r = 0.43).
Fig. 5. Early ZLD binding predicts chromatin state at the MZT. 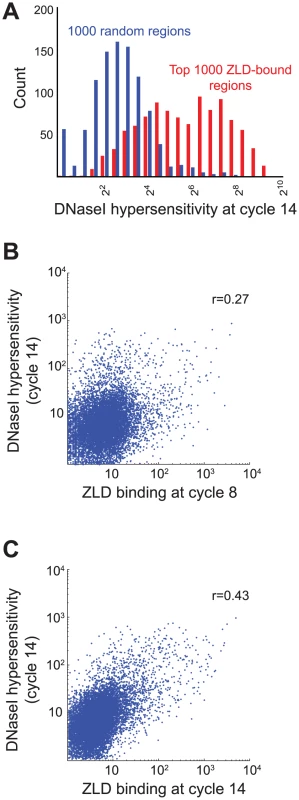
(A) Histogram of DNA accessibility based on DNaseI hypersensitivity from [20] among the most strongly bound 1,000 ZLD peaks at cycle 8 (red) and among randomly shuffled peak positions (blue). (B) Comparison of genome-wide ZLD binding at cycle 8 (x-axis) vs. the DNaseI hypersensitivity values at cycle 14 [20], showing a genome-wide correlation coefficient of 0.27 (C) Same as (B), but using ZLD binding from late cycle 14, showing correlation coefficient of 0.43. The increasing conformity of ZLD binding to chromatin state piqued our interest in the dynamics of individual ZLD binding sites. ZLD binding is fairly stable over time: of 12,135 peaks found in pooled data from the three stages, 10,873 (90%) are found in all three stages (Figure S1B and Table S4). For example, ZLD is bound at all three stages to genes such as sc and eve that are transcribed prior to the MZT, as well as to genes such as lea and opa that are expressed only later. There are, however, clear changes in binding. For example, 775 sites are present in cycle 8 embryos but absent at late cycle 14 (Figure 6A, Figure S1B, and Table S4). This dynamic binding is specific to individual bound regions, as we identified many loci where ZLD binding at one site remained unchanged while binding to a neighboring binding site increased or decreased.
Fig. 6. ZLD binding dynamics through the MZT. 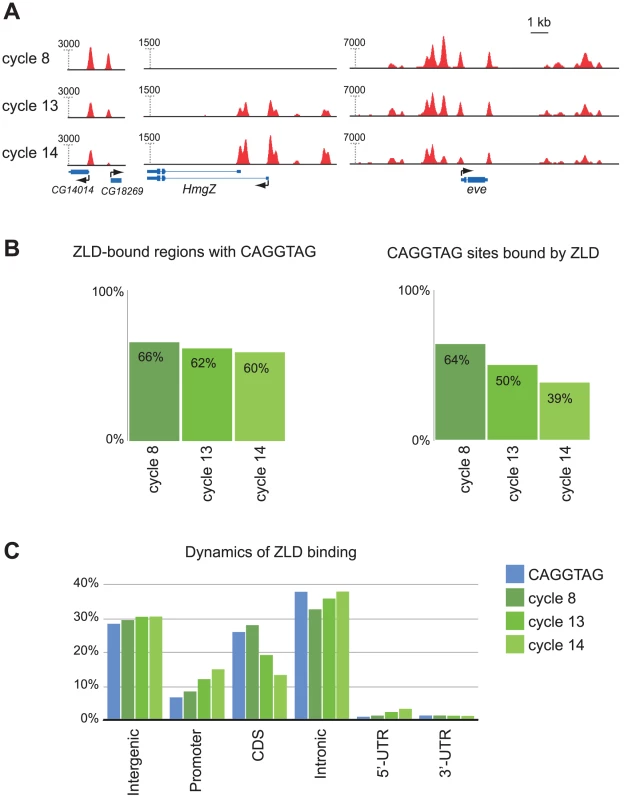
(A) Dynamics of ZLD binding during cycle 8 (top track for each gene), cycle 13 (middle track), and late cycle 14 (bottom track) at three genomic loci: and CG14014/CG18269, HmgZ, and even-skipped (eve) (B) Specificity and sensitivity of the relationship between CAGGTAG and ZLD binding at the three timepoints. Shown are the fractions of the top 1,000 ZLD peaks that contain the motif at the three timepoints (left), and the fraction motif occurrences that are bound by ZLD (right). (C) Distribution of genomic annotations among genomic occurrences of the CAGGTAG motif (blue) and ZLD peaks from three timepoints (green). An interesting pattern emerged when we examined the relationship between ZLD binding, TAGteam sites and gene annotations at the three developmental stages. At cycle 8 there is a very strong relationship between occurrences of CAGGTAG and ZLD binding (Figure 6B): over 66% of the top 1,000 ZLD bound regions contain a CAGGTAG site (compared to 1.8% expected at random), while an astonishing (relative to other studied factors) 64% of genomic CAGGTAG sites are indeed bound by ZLD. The unusually high fraction of CAGGTAG sites bound by ZLD at cycle 8 suggests that chromatin at this stage is in a fairly accessible state. By cycle 13, only 50% of CAGGTAG sites are bound (Figure 6B), as ZLD binding becomes more enriched in promoter sequences and less enriched among coding regions (Figure 6C). And by late cycle 14, only 38.5% of the CAGGTAG sites are bound (Figure 6B) and the shift from coding region to promoter binding continues (Figure 6C). The decreasingly specificity of ZLD for CAGGTAG sites over time suggests that the chromatin landscape is becoming more differentiated, and may also reflect the larger number and greater diversity of DNA-binding proteins present after zygotic transcription begins.
Discussion
A model for ZLD as a pioneer transcription factor that shapes the chromatin landscape at the MZT
ZLD and the TAGteam sequences to which it binds were originally identified as key regulators of the early wave of zygotic transcription that precedes the MZT [7], [10], and our genome-wide measurements of ZLD binding validate this activity. However, we have demonstrated that ZLD is also bound to the promoters and enhancers of more than a thousand genes that are not transcribed until the MZT, and that early ZLD binding is strongly associated with open chromatin and transcription factor binding during the MZT. Thus, rather than being specifically involved in the onset of zygotic transcription, our data indicate that ZLD has a much wider role in activating the zygotic genome, although its specific molecular mechanism remains elusive.
The sequence of ZLD offers few clues to its function. Its roughly 1,600 amino acids contain no known domains besides C2H2 zinc-fingers, and none of its orthologs (found only in arthropods) have been experimentally characterized [10], [11], [21]. That ZLD is important in both promoters and enhancers, and that its binding seems to affect the distribution of a diverse collection of transcription factors, argue against it directly recruiting polymerase and transcription factors. We propose instead that ZLD acts as a generic activator of the zygotic genome by controlling chromatin accessibility and/or histone modifications in the regions where it is bound.
There is increasingly good evidence that difference in chromatin state across the genome at the MZT play a major role in determining which regions are active. We and others have recently assayed the state of chromatin in cycle 14 embryos and shown that regions of concentrated transcription factor binding are strongly associated with regions of “open” chromatin [22], and that temporal changes in DNA accessibility and transcription factor binding are often coordinated [20]. Furthermore, a recent computational analysis from our lab that dissected the factors that influence our ability to predict transcription factor binding offers compelling evidence that, at least in the D. melanogaster blastoderm, the state of chromatin shapes—and does not simply reflect—transcription factor binding [19]. But one important question left unanswered by these studies is how differences in chromatin state are established. Our data and analyses clearly implicate CAGGTAG sites and ZLD.
We already knew that CAGGTAG sites were enriched in active promoters and regions of transcription factor binding at the MZT [7], [10], [12], [18], and that the gain and loss of CAGGTAG sites is a major driver of changes in transcription factor binding at the MZT between different Drosophila species [23]. Here we have shown that ZLD binds to these CAGGTAG sites in vivo; that there is a tight connection between ZLD binding, chromatin state and MZT activity; and, crucially, that ZLD binding precedes, by at least several mitotic cycles, transcription factor binding and transcription at regions active at the MZT. Thus it is in precisely the right places at the right time to act as a generic activator of the MZT.
Although little is known about the chromatin state in the early embryo, our data support a model in which the genome transitions from a fairly uniform open state (in which ZLD binds to 65% of CAGGTAG sites) to the mosaic of open and closed domains known to exist in cycle 14 [22] (and in which ZLD binds to only 39% of CAGGTAG sites). If this is correct, we suggest ZLD likely plays a role in managing this transition, recruiting or repelling chromatin remodeling proteins to the regions where it is bound in uniformly open chromatin at cycle 8 and thereby ensuring they remain open at cycle 14. It is, however, also possible that early ZLD binding to its MZT targets may represent opportunistic binding of the protein to accessible regions containing CAGGTAG sites, with its MZT-specific activity arising from binding closer in time to the MZT.
ZLD shares some compelling similarities with Xenopus β-catenin, which is required for expression of a subset of genes prior to the MZT [24]. At least two genes, siamois and xnr3, require β-catenin for expression, but are not expressed until the MZT. β-catenin is required at or before the 32 cell stage to poise siamois and xnr3 for activation and helps to establish this poised state by recruiting the histone methyltransferase Prmt2 to the promoters of these genes [25]. Thus β-catenin and ZLD are similarly required to drive pre-MZT expression of a subset of genes and also to poise additional genes for activation at the MZT. But unlike the specialized function of β-catenin, our data suggest that ZLD acts globally to activate the zygotic genome.
Our proposed function for ZLD is reminiscent of the so-called “pioneer” transcription factors. This concept was introduced to describe the role of FoxA1 in regulating gene regulation in the developing mammalian liver. In the undifferentiated endoderm, FoxA1 is bound to the enhancer of the hepatocyte-expressed albumin gene (Alb1) before Alb1 is expressed [26]. FoxA1 binding mediates chromatin decondensation, and this modified chromatin environment allows for the subsequent binding of additional transcription factors that drive liver-specific gene expression [27].
However, in contrast to chromatin in multipotent progenitor cells, the chromatin of the totipotent cells of the early embryo are likely to be in a relatively “open” conformation [28]. Thus, ZLD may not actively mediate chromatin decondensation but rather may act to maintain regions of accessible chromatin. There is precedent for chromatin remodeling being involved in the MZT. In mice, the chromatin-remodeling enzyme BRG1 is required for zygotic genome activation [29].
Work in embryonic stem cells and in zebrafish embryos suggests that transcriptional activation at the MZT also involves specific histone modifications. In zebrafish, histones acquire modification patterns reminiscent of pluripotent embryonic stems cells as the embryo progresses through the MZT [30]. Most notably, histone H3 acquires both marks of active transcription, tri-methylation on lysine four (H3K4me3), and of repression, tri-methylation on lysine 27 (H3K27me3). These bivalent histone marks were initially observed in embryonic stem cells and have been shown to poise the genomes of these cells for differentiation [31]–[33].
Such bivalent marks have not been observed in Drosophila. However the earliest embryos examined were 4–12 hours old [34], after the embryo has transitioned through the MZT and its cells are no longer fully pluripotent. Recently, it has been shown that in embryonic stem cells, bivalent domains are resolved as cells differentiate [33], raising the possibility that bivalent domains are present in Drosophila but no longer evident in the post-gastrulation embryos that have been examined. Perhaps ZLD works by recruiting or otherwise influencing the recruitment of proteins that modify chromatin, or by modifying chromatin itself. However, the fact that no bivalent domains have been observed in Drosophila or in Xenopus [35] leaves open the possibility that ZLD is acting through a different mechanism. It is imperative that careful genome-wide analysis of histone modifications be performed in Drosophila and other species as they transition through the MZT to determine whether the formation of bivalent chromatin domains is a common characteristic of pluripotent cells.
What differentiates ZLD target genes expressed prior to the MZT from those genes expressed only later?
The genes most highly-bound by ZLD are transcribed by cycle 10. In one case, it has been shown that increased ZLD binding alone can lead to precocious activation [7], and it is possible that high levels of ZLD binding to promoters and proximal enhancers is sufficient to activate expression. However, most ZLD bound regions are not active until cycle 14. The generally lower levels of ZLD binding to these regions may necessitate the presence of other factors (such as patterning transcription factors or STAT92E [36]) not expressed or activated until closer to the MZT. In this way ZLD would act indirectly to keep chromatin open at these regions until these other factors are able to exert their control. Alternatively, ZLD may act to directly recruit a zygotically expressed coactivator to the regulatory regions of genes expressed at the MZT. For example, ZLD could recruit factors, such as P-TEFb, that work to release stalled RNA polymerase II [37] or, similar to β-catenin, recruit chromatin-modifying enzymes [25]. The ability of ZLD to activate transcription could also be modulated by post-translational modifications to the protein itself.
It is worth noting that before zygotic induction Drosophila embryos are undergoing rapid rounds of DNA replication and ORC, the replication initiator, does not bind to specific sequences [38], but rather depends upon access to open chromatin [39]. Hence ZLD, with its potential role in shaping the chromatin landscape may also play a key role prior to transcription initiation in allowing for the proper assembly and spacing of pre-replication sites, and CAGGTAG may be a good predictor of origins [40]. As the embryo progresses through the MZT, ORC binding becomes less closely spaced and origin firing becomes less synchronous suggesting that DNA replication reflects a changing chromatin environment.
It is noteworthy that ZLD may activate distinct sets of genes by different mechanisms. TAGteam sites were first defined as sequence elements driving the expression of a small number of genes prior to the MZT [7]. It was therefore assumed that the TAGteam-binding protein, ZLD, might function specifically to activate this subset of genes. However, we have shown that ZLD is marking the genome for widespread transcriptional activation of the zygotic genome at cycle 14. Perhaps, ZLD is able to directly activate the small subset of genes expressed prior to the MZT, but that ZLD-mediated gene activation at the MZT requires additional zygotically expressed cofactors or post-translational modifications.
Genome poising as a general feature of animal development
Given the ability of transcription factors such as β-catenin, FoxA1, and ZLD to mark genes for subsequent activation, and the recent evidence that chromatin remodeling, histone modifications and RNA polymerase II occupancy prepare developmental genes for later transcription, we suggest that the poising of genomes for subsequent activation is likely to be a common feature of pluripotent cells. Determining the roles of these mechanisms in regulating gene expression at this important developmental timepoint will be crucial to understanding how these cells are poised for differentiation and how subsequent activation can be regulated to drive specific cell fates.
Materials and Methods
ZLD antibody purification
As described in Harrison et al. [9], rabbits were immunized with GST fused to amino acids 1117–1487 of ZLD and purified against the same portion of the protein fused to maltose binding protein (MBP). As this portion of ZLD includes the zinc-finger DNA-binding domain, we further purified these antibodies using MBP fused to the four zinc fingers, amino acids 1318–1444. For our experiments, we recovered the antibodies that failed to bind to this MBP fusion protein and confirmed by immunoblot that these antibodies could recognize the full-length ZLD, but not the DNA-binding domain alone.
Formaldehyde crosslinking of staged and sorted embryos, and chromatin isolation
D. melanogaster flies were maintained in large population cages in an incubator set at standard conditions (25°C). Embryos were collected for 30 minutes, and then allowed to develop for 60, 120 or 180 additional minutes before being harvested and fixed with formaldehyde. The fixed embryos were staged and hand sorted in small batches using an inverted microscope (Figure S1A) to remove the small number of older contaminating embryos resulting from egg retention, with the sorting first done at 4× and then confirmed at 10× magnification. Our visual inspection of all of the processed embryos gave us great confidence that we had removed later-stage contaminants, a view bolstered by an assessment of the trends in ZLD binding over the three timepoints. In particular, the presence of regions with no binding at cycle 8 but high levels of binding in later stages (Figure 6A) demonstrated that our cycle 8 binding was not simply later stage contamination, as did the enrichment for cases in which cycle 13 binding was intermediate between cycle 8 and late cycle 14 (Figure S1B).
1, 0.2, and 0.1 g of embryos at the three different stages respectively, were used to prepare chromatin for immunoprecipitation following the CsCl2 gradient ultracentrifugation protocol as previously described [12]. With the small amount of embryos in each sample, the ultra-centrifugation was carried out with a SW41 rotor, and the volumes of buffers, detergents, and CsCl2 solutions were adjusted accordingly as detailed in the previous protocol.
ChIP and sequencing
The chromatin obtained was fragmented to sizes ranging from 100 to 300 bp using a Bioruptor (Diagenode, Inc.) for a total of processing time of 140 min (15 s on, 45 s off), with power setting at “H”. We used 3.7 µg chromatin from cycle 8, 6.6 µg from cycle 13 and 6 µg from cycle 14 in the chromatin immunoprecipitation reaction, using the affinity purified anti-ZLD antibody, following the procedure described previously [12]. The sequencing libraries were prepared from the ChIP and Input DNA samples, and subjected to ultra-high throughput sequencing on a Solexa Genome Analyzer IIx as previously described [23], except that the DNA fragments ranged from 200–350 bp in size.
Mapping sequencing reads to the genome
Sequenced reads were mapped to the April 2006 assembly of the D. melanogaster genome, (UCSC version dm3, BDGP Release 5) using Bowtie [13] using the command-line options ‘-n 2 -l 36 -m 2’, thereby keeping for further analyses only tags that mapped uniquely to the genome with at most two mismatches. Each read was extended to 150 bp based on its orientation, and the total number of reads per timepoint was normalized to 10,000,000.
Peak calling
We developed a model-based multi-peak algorithm—Grizzly Peak—to accurately identify significant ZLD bound loci across the genome. Grizzly Peak is an iterative model-based peak fitting method, which we modified from Capaldi et al. [41]. In brief, Grizzly Peak estimates the expected shape of a binding event in the ChIP-seq data. The algorithm then iteratively scans the genome and identifies enriched regions with high protein occupancy. These regions are expanded and analyzed, aiming at finding a minimal set β of peaks (each with a genomic position and an occupancy level) optimizing the fit to the measured data. To allow for overlapping peaks, we devised a simple heuristic for considering actions such as adding or removing peaks. Each step is then assigned a score, and steps are taken if a significant improvement in the score is achieved. Once a genomic region has been analyzed and fitted, the optimized set of peaks is recorded, and this genomic region is discarded from future fitting. This process is repeated until no significantly bound loci remain. The Grizzly Peak algorithm is available at http://eisenlab.org/software/grizzly.
Motif analysis
Identified peaks were expanded to 300 bp around each binding event (peak center), and were analyzed for enriched motifs. We used three de novo motif discovery tools. First we used MEME (version 4.5.0) [14], searching in a zero or one binding site per peak (“zoops”) mode, and allowing for up to 10 motifs, while testing both strands. In addition, we used another motif analysis algorithm using Expectation-Maximization (EM), and assuming at least one binding site per peak [15]. We accompanied our analysis by Weeder (version 1.4.2) [16], an exhaustive enumeration algorithm that tests the enrichment of each motif among the input sequences.
Genomic annotations
Each called peak was assigned a genomic functional annotation based on FlyBase gene annotations (UCSC, release dm3), including the position of exons and transcript start and end points. According to the position of the peak center position, we categorized each peak into one of six genomic categories: (1) Promoter peaks – from 500 bp upstream to 150 bp downstream to an annotated start site; (2) Coding sequence (CDS) peaks – overlapping any exon; (3) 5′-UTR peaks – overlapping a transcript, but not CDS or promoter; (4) 3′-UTR peaks; (5) Intron peaks; and (6) Intergenic peaks – downstream of genes or more than 500 bp upstream. Each peak was then assigned to the nearest gene.
Randomization via genomic shuffling of peak positions
To estimate the random expected distribution of ZLD peaks relative to genome annotations, we devised a simple strategy to assign every peak to a new randomized position that maintained the number of peaks, their sizes, their distribution over chromosomes and their relative distances from each other. First we randomly reordered the peaks in each chromosome, practically mixing between strong and weak peaks. Second, we randomly shuffled the linker distances between every pair of adjacent peaks. Finally, we repositioning each peak at a new randomized position and repeated the analyses at hand.
Zygotic expression—data, class, and estimate onset time
We used single-embryo zygotic expression data from Lott et al. [17], including gene classification to (1) zygotic; (2) zygotic/maternal; and (3) maternal only. These were done according to the zygotic expression patterns of each gene and its genotypic signature. Onset times for zygotic genes were determined as the first time for each gene with zygotic mRNA abundance above 5 RPKM (reads per kilobase per million reads mapped, limited to autosomal chromosomes), after interpolating the eight measured timepoints using a shape-preserving piecewise cubic interpolation (MATLAB R2010a, interp1 function, “pchip” model).
Analysis of expression in embryos depleted for maternal ZLD
Raw gene expression data from Liang et al. [10] for wildtype embryos and embryos depleted for maternal ZLD were downloaded from GEO (accession GSE11231) and reanalyzed. Up - and down-regulation were estimated by comparing expression levels in wildtype to ZLD-depleted embryos. Probes marked as “absent” in both strains (“noise” vs. “noise”) were discarded from further analyses of these data.
Overlap with developmental transcription factors
ChIP-chip data for 21 transcription factors during early developmental stages (cycle 14) were obtained from MacArthur et al. [18], at http://bdtnp.lbl.gov/Fly-Net. We applied Grizzly Peak to identify the exact binding position for each factor within the 1% FDR (symmetrical) enriched regions. We then analyzed the co-occurrence of ZLD peaks vs. the top 300 peaks of each factor. As a control, we repeated this analyses after randomly repositioning all the peaks per TF using the random shuffling approach described above. We then compared the coverage of these randomized positions with ZLD, and calculated the percent of recognition elements that are bound in the presence or absence of ZLD. We focused on eight well-studied factors with simple recognition motifs (BCD: TAATCC; CAD: TTTATTG; GT: TTACGTAA; HKB: GGGCGTG; TLL: TTGACTTT; D: CCATTGT; H: CACGCGCC; and PRD: GTCACGC). We identified all genomic occurrences of these motifs, and calculated the fraction of bound motifs (using a 1% FDR threshold from MacArthur et al. [18]). These fractions were then compared to the number of bound motifs given overlapping ZLD binding (ZLD occupancy above 100 RPKM in late cycle 14). Finally, we repeated this analysis with a randomly shuffled set of genomic positions (instead of the real occurrences of recognition motif for each TF) to test the different basal correlations of each factor and ZLD. Merged data with ZLD binding and data from previously published transcription factors are provided in Table S5.
Data availability
Raw and mapped sequencing reads are available from the National Center for Biotechnology Information's GEO database (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE30757. A browser with ZLD binding and other related data discussed in the manuscript can be accessed at http://eisenlab.org/data/ZLD.
Supporting Information
Zdroje
1. NewportJKirschnerM 1982 A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell 30 687 696
2. TadrosWLipshitzHD 2009 The maternal-to-zygotic transition: a play in two acts. Development 136 3033 3042
3. FoeVEAlbertsBM 1983 Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 61 31 70
4. AndersonKVLengyelJA 1979 Rates of synthesis of major classes of RNA in Drosophila embryos. Dev Biol 70 217 231
5. McKnightSLMillerOLJr 1976 Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell 8 305 319
6. PritchardDKSchubigerG 1996 Activation of transcription in Drosophila embryos is a gradual process mediated by the nucleocytoplasmic ratio. Genes Dev 10 1131 1142
7. ten BoschJRBenavidesJAClineTW 2006 The TAGteam DNA motif controls the timing of Drosophila pre-blastoderm transcription. Development 133 1967 1977
8. De RenzisSElementoOTavazoieSWieschausEF 2007 Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLoS Biol 5 e117 doi:10.1371/journal.pbio.0050117
9. HarrisonMMBotchanMRClineTW 2010 Grainyhead and Zelda compete for binding to the promoters of the earliest-expressed Drosophila genes. Dev Biol 345 248 255
10. LiangHLNienCYLiuHYMetzsteinMMKirovN 2008 The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 456 400 403
11. StaudtNFellertSChungHRJackleHVorbruggenG 2006 Mutations of the Drosophila zinc finger-encoding gene vielfaltig impair mitotic cell divisions and cause improper chromosome segregation. Mol Biol Cell 17 2356 2365
12. LiXYMacArthurSBourgonRNixDPollardDA 2008 Transcription factors bind thousands of active and inactive regions in the Drosophila blastoderm. PLoS Biol 6 e27 doi:10.1371/journal.pbio.0060027
13. LangmeadBTrapnellCPopMSalzbergSL 2009 Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10 R25
14. BaileyTLElkanC 1994 Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2 28 36
15. BarashYElidanGFriedmanNKaplanT 2003 Modeling dependencies in protein-DNA binding sites. Proceedings of the seventh annual international conference on Research in computational molecular biology Berlin, Germany ACM 28 37
16. PavesiGMereghettiPMauriGPesoleG 2004 Weeder Web: discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Res 32 W199 203
17. LottSEVillaltaJESchrothGPLuoSTonkinLA 2011 Noncanonical Compensation of the Zygotic X Transcription in Early Drosophila melanogaster Development Revealed through Single-Embryo RNA-Seq. PLoS Biol 9 e1000590 doi:10.1371/journal.pbio.100059
18. MacArthurSLiXYLiJBrownJBChuHC 2009 Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome Biol 10 R80
19. KaplanTLiX-ySaboPJThomasSStamatoyannopoulosJA 2011 Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Drosophila Development. PLoS Genet 7 e1001290 doi:10.1371/journal.pgen.1001290
20. ThomasSLiXYSaboPJSandstromRBThurmanRE 2011 Dynamic reprogramming of chromatin accessibility during Drosophila embryo development. Genome Biol 12 R43
21. TweedieSAshburnerMFallsKLeylandPMcQuiltonP 2009 FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37 D555 559
22. LiXYThomasSSaboPJEisenMBStamatoyannopoulosJA 2011 The role of chromatin accessibility in directing the widespread, overlapping patterns of Drosophila transcription factor binding. Genome Biol 12 R34
23. BradleyRKLiXYTrapnellCDavidsonSPachterL 2010 Binding site turnover produces pervasive quantitative changes in transcription factor binding between closely related Drosophila species. PLoS Biol 8 e1000343 doi:10.1371/journal.pbio.1000343
24. YangJTanCDarkenRSWilsonPAKleinPS 2002 Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development 129 5743 5752
25. BlytheSAChaSWTadjuidjeEHeasmanJKleinPS 2010 beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell 19 220 231
26. GualdiRBossardPZhengMHamadaYColemanJR 1996 Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev 10 1670 1682
27. CirilloLALinFRCuestaIFriedmanDJarnikM 2002 Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 9 279 289
28. Gaspar-MaiaAAlajemAMeshorerERamalho-SantosM 2011 Open chromatin in pluripotency and reprogramming. Nature reviews Mol Cell Biol 12 36 47
29. BultmanSJGebuhrTCPanHSvobodaPSchultzRM 2006 Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev 20 1744 1754
30. VastenhouwNLZhangYWoodsIGImamFRegevA 2010 Chromatin signature of embryonic pluripotency is established during genome activation. Nature 464 922 926
31. BernsteinBEMikkelsenTSXieXKamalMHuebertDJ 2006 A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125 315 326
32. MikkelsenTSKuMJaffeDBIssacBLiebermanE 2007 Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448 553 560
33. Rada-IglesiasABajpaiRSwigutTBrugmannSAFlynnRA 2011 A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470 279 283
34. SchuettengruberBGanapathiMLeblancBPortosoMJaschekR 2009 Functional anatomy of polycomb and trithorax chromatin landscapes in Drosophila embryos. PLoS Biol 7 e1000013 doi:10.1371/journal.pbio.1000013
35. AkkersRCvan HeeringenSJJacobiUGJanssen-MegensEMFrancoijsKJ 2009 A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev Cell 17 425 434
36. TsurumiAXiaFLiJLarsonKLafranceR 2011 STAT Is an Essential Activator of the Zygotic Genome in the Early Drosophila Embryo. PLoS Genet 7 e1002086 doi:10.1371/journal.pgen.1002086
37. PeterlinBMPriceDH 2006 Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23 297 305
38. RemusDBeallELBotchanMR 2004 DNA topology, not DNA sequence, is a critical determinant for Drosophila ORC-DNA binding. The EMBO journal 23 897 907
39. RoySErnstJKharchenkoPVKheradpourPNegreN 2010 Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330 1787 1797
40. MacAlpineHKGordanRPowellSKHarteminkAJMacAlpineDM 2010 Drosophila ORC localizes to open chromatin and marks sites of cohesin complex loading. Genome Res 20 201 211
41. CapaldiAPKaplanTLiuYHabibNRegevA 2008 Structure and function of a transcriptional network activated by the MAPK Hog1. Nat Genet 40 1300 1306
Štítky
Genetika Reprodukční medicína
Článek Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA MiceČlánek Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse EmbryosČlánek Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 10
-
Všechny články tohoto čísla
- Transcriptional Robustness Complements Nonsense-Mediated Decay in Humans
- Identification, Replication, and Fine-Mapping of Loci Associated with Adult Height in Individuals of African Ancestry
- Genetic Determinants of Serum Testosterone Concentrations in Men
- A One Base Pair Deletion in the Canine Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier
- Three Structure-Selective Endonucleases Are Essential in the Absence of BLM Helicase in
- Identification of Widespread Ultra-Edited Human RNAs
- Multiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
- The Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
- Transcriptome-Wide Binding Sites for Components of the Non-Poly(A) Termination Pathway: Nrd1, Nab3, and Sen1
- Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA Mice
- Genetic Rearrangements Can Modify Chromatin Features at Epialleles
- Novel Function of as a Gap Gene during Spider Segmentation
- A Genome-Wide Screen for Interactions Reveals a New Locus on 4p15 Modifying the Effect of Waist-to-Hip Ratio on Total Cholesterol
- Comparative Genomic Analysis of Human Fungal Pathogens Causing Paracoccidioidomycosis
- Genetic Diversity in Cytokines Associated with Immune Variation and Resistance to Multiple Pathogens in a Natural Rodent Population
- Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
- Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse Embryos
- A Barcode Screen for Epigenetic Regulators Reveals a Role for the NuB4/HAT-B Histone Acetyltransferase Complex in Histone Turnover
- HIF–VEGF Pathways Are Critical for Chronic Otitis Media in and Mouse Mutants
- A Conserved Developmental Patterning Network Produces Quantitatively Different Output in Multiple Species of Drosophila
- Role of Exonic Variation in Chemokine Receptor Genes on AIDS: Association with Pneumocystis Pneumonia
- Whole-Exome Sequencing Identifies Homozygous Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial -AAA Proteases
- Von Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
- A Systems Biology Approach Reveals the Role of a Novel Methyltransferase in Response to Chemical Stress and Lipid Homeostasis
- Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Natural Selection Affects Multiple Aspects of Genetic Variation at Putatively Neutral Sites across the Human Genome
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
- An Adaptive Allelic Series Featuring Complex Gene Rearrangements
- Feed-Forward Microprocessing and Splicing Activities at a MicroRNA–Containing Intron
- Developmental Stability: A Major Role for in
- A Phenomics-Based Strategy Identifies Loci on , , and Associated with Metabolic Syndrome Phenotype Domains
- Association of , , , , and with Systemic Lupus Erythematosus
- Small RNAs Prevent Transcription-Coupled Loss of Histone H3 Lysine 9 Methylation in
- Successive Increases in the Resistance of to Viral Infection through a Transposon Insertion Followed by a Duplication
- Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Insights into Hox Protein Function from a Large Scale Combinatorial Analysis of Protein Domains
- Mutations Cause Seckel and Jawad Syndromes
- Zelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
- Temporal Coordination of Gene Networks by Zelda in the Early Embryo
- Genetic Interaction between MTMR2 and FIG4 Phospholipid Phosphatases Involved in Charcot-Marie-Tooth Neuropathies
- Oxr1 Is Essential for Protection against Oxidative Stress-Induced Neurodegeneration
- Transforming Growth Factor β Receptor Type 1 Is Essential for Female Reproductive Tract Integrity and Function
- Positional Cloning of a Type 2 Diabetes Quantitative Trait Locus; , a Negative Regulator of Insulin Secretion
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Genetic Determinants of Serum Testosterone Concentrations in Men
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



