-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVon Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
Renal tumor heterogeneity studies have utilized the von Hippel-Lindau VHL gene to classify disease into molecularly defined subtypes to examine associations with etiologic risk factors and prognosis. The aim of this study was to provide a comprehensive analysis of VHL inactivation in clear cell renal tumors (ccRCC) and to evaluate relationships between VHL inactivation subgroups with renal cancer risk factors and VHL germline single nucleotide polymorphisms (SNPs). VHL genetic and epigenetic inactivation was examined among 507 sporadic RCC/470 ccRCC cases using endonuclease scanning and using bisulfite treatment and Sanger sequencing across 11 CpG sites within the VHL promoter. Case-only multivariate analyses were conducted to identify associations between alteration subtypes and risk factors. VHL inactivation, either through sequence alterations or promoter methylation in tumor DNA, was observed among 86.6% of ccRCC cases. Germline VHL SNPs and a haplotype were associated with promoter hypermethylation in tumor tissue (OR = 6.10; 95% CI: 2.28–16.35, p = 3.76E-4, p-global = 8E-5). Risk of having genetic VHL inactivation was inversely associated with smoking due to a higher proportion of wild-type ccRCC tumors [former: OR = 0.70 (0.20–1.31) and current: OR = 0.56 (0.32–0.99); P-trend = 0.04]. Alteration prevalence did not differ by histopathologic characteristics or occupational exposure to trichloroethylene. ccRCC cases with particular VHL germline polymorphisms were more likely to have VHL inactivation through promoter hypermethylation than through sequence alterations in tumor DNA, suggesting that the presence of these SNPs may represent an example of facilitated epigenetic variation (an inherited propensity towards epigenetic variation) in renal tissue. A proportion of tumors from current smokers lacked VHL alterations and may represent a biologically distinct clinical entity from inactivated cases.
Published in the journal: . PLoS Genet 7(10): e32767. doi:10.1371/journal.pgen.1002312
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002312Summary
Renal tumor heterogeneity studies have utilized the von Hippel-Lindau VHL gene to classify disease into molecularly defined subtypes to examine associations with etiologic risk factors and prognosis. The aim of this study was to provide a comprehensive analysis of VHL inactivation in clear cell renal tumors (ccRCC) and to evaluate relationships between VHL inactivation subgroups with renal cancer risk factors and VHL germline single nucleotide polymorphisms (SNPs). VHL genetic and epigenetic inactivation was examined among 507 sporadic RCC/470 ccRCC cases using endonuclease scanning and using bisulfite treatment and Sanger sequencing across 11 CpG sites within the VHL promoter. Case-only multivariate analyses were conducted to identify associations between alteration subtypes and risk factors. VHL inactivation, either through sequence alterations or promoter methylation in tumor DNA, was observed among 86.6% of ccRCC cases. Germline VHL SNPs and a haplotype were associated with promoter hypermethylation in tumor tissue (OR = 6.10; 95% CI: 2.28–16.35, p = 3.76E-4, p-global = 8E-5). Risk of having genetic VHL inactivation was inversely associated with smoking due to a higher proportion of wild-type ccRCC tumors [former: OR = 0.70 (0.20–1.31) and current: OR = 0.56 (0.32–0.99); P-trend = 0.04]. Alteration prevalence did not differ by histopathologic characteristics or occupational exposure to trichloroethylene. ccRCC cases with particular VHL germline polymorphisms were more likely to have VHL inactivation through promoter hypermethylation than through sequence alterations in tumor DNA, suggesting that the presence of these SNPs may represent an example of facilitated epigenetic variation (an inherited propensity towards epigenetic variation) in renal tissue. A proportion of tumors from current smokers lacked VHL alterations and may represent a biologically distinct clinical entity from inactivated cases.
Introduction
Von Hippel-Lindau (VHL) alteration leading to protein inactivation is considered a frequent, early event in renal carcinogenesis that can be used as a biomarker of tumor heterogeneity, to strengthen etiologic relationships with risk factors, and study mechanistic pathways of disease [1]–[4]. The most common established risk factors that are associated with approximately 50% of renal cell cancer (RCC) cases include obesity, hypertension, and tobacco smoking. Less-established risk factors include occupational exposure to pesticides and the organic solvent trichloroethylene (TCE). Dietary intake of vegetables and fruits has been inversely associated with renal cancer, whereas intake of red meat and milk products have been associated with increased RCC risk, although not consistently [5]. Common genetic variation has also been shown to modify RCC risk [5].
Germline sequence alterations of the VHL gene were first identified and have been observed in almost all families with VHL disease, a hereditary cancer syndrome in which affected individuals are at risk for renal cysts and clear cell RCC (ccRCC) [1]. In sporadic ccRCC, alterations in the VHL gene have been reported in up to 91% of case tumors [6]. The VHL gene plays a role in tissue-specific responses to oxygen concentration and delivery. Under normal oxygen conditions, the VHL protein forms a complex with elongin B, elongin C, and cullin 2 which targets hydroxylated hypoxia inducible factor-alpha (HIFα) for ubiquitin-mediated degradation [7], [8]. Under hypoxic conditions, the VHL complex cannot bind HIFα for degradation because it is in the non-hydroxylated form. Therefore, HIFα accumulates, resulting in transcription of additional genes that facilitate oxygen delivery, cellular adaptation to oxygen deprivation, and angiogenesis. Similarly, alteration of the VHL gene prevents formation of the protein complex required for HIF degradation, resulting in an excess of HIF and a similar gene expression pattern as that observed under hypoxic conditions.
Recently, we applied sensitive and high-throughput mutation detection methods in a pilot study of 205 well-characterized sporadic ccRCC cases to comprehensively evaluate tumor DNA for VHL sequence alterations and promoter methylation, and to identify associations between the prevalence, type, and location of VHL alterations with etiologic and clinical factors [6]. In the current study, we expand upon our previous analysis [6], and report findings from the entire case series of 507 sporadic RCC cases, including 470 ccRCC patients. The combined dataset has increased statistical power to identify associations between risk factors and heterogeneous subgroups of cases defined by the type of VHL alterations. Using questionnaire data on patient characteristics, nutritional intake, and occupational exposures known or suspected to modify risk, we attempted to replicate findings from previously published studies in which evidence of tumor heterogeneity had been reported [9]–[13]. A previously reported VHL polymorphism, rs779805 and epigenetic inactivation of the VHL gene were examined among these cases using a tag SNPs method that is routinely used in association studies. The VHL gene locus was examined using highly correlated SNPs to comprehensively evaluate common genetic variation across this gene region and risk of developing a specific type of VHL alteration [14].
Results
Patient and Tumor Characteristics
In Table S1, sporadic RCC cases from the Central and Eastern European Case-Control study (CEERCC) that were included in this analysis are compared to cases not included in this analysis by their personal/clinical characteristics, and risk factors that have been previously associated with the prevalence of RCC or VHL alterations in tumor tissue. Cases not included in this study were those from whom we were unable to collect frozen tumor biopsy material for analyses. Collection of frozen tissue was most successful among cases from the Czech Republic than the other study regions. This analysis also included more cases with high body mass index (BMI), with a lower level of education, and included more M0 compared to M1 cases compared to those not included in this analysis. The distribution of other factors was similar across groups.
VHL Alteration Prevalence in RCC and ccRCC Tumor DNA
The prevalence of having any VHL alteration (i.e. inactivating, silent, and intronic mutations, and promoter hypermethylation) was 83.0% among all RCC cases, 88.3% among the subset of ccRCC cases, and 16.2% among non-ccRCC cases (Table 1). The higher percentage observed in ccRCC compared to all RCC cases was due to a greater proportion of cases with VHL inactivating alterations and a lower proportion of wild type ccRCC cases [i.e. cases without inactivating VHL gene alterations], that were primarily observed among non-ccRCC cases. The overall prevalence of VHL promoter hypermethylation was similar in RCC, ccRCC, and non-ccRCC cases. Among all ccRCC cases, the highest alteration prevalence was located at codon 117 (N = 13). These included 5 deletions, 5 missense, 2 insertions, and 1 nonsense sequence alteration at Codon 65 had the second highest sequence alteration prevalence (N = 11), and included 6 missense, 4 nonsense, and one deletion (data not shown). Thirty putative splice junction sequence alterations, (intronic variants located within 3 bases of an exon) were observed. VHL promoter epigenetic and genetic inactivation was mutually exclusive in all tumors analyzed. Because VHL genetic alterations were rarely observed among non-ccRCC cases, all additional analyses were restricted to confirmed ccRCC cases.
Tab. 1. VHL gene alterations among histologically confirmed RCC, clear cell RCC, and non-clear cell RCC cases. 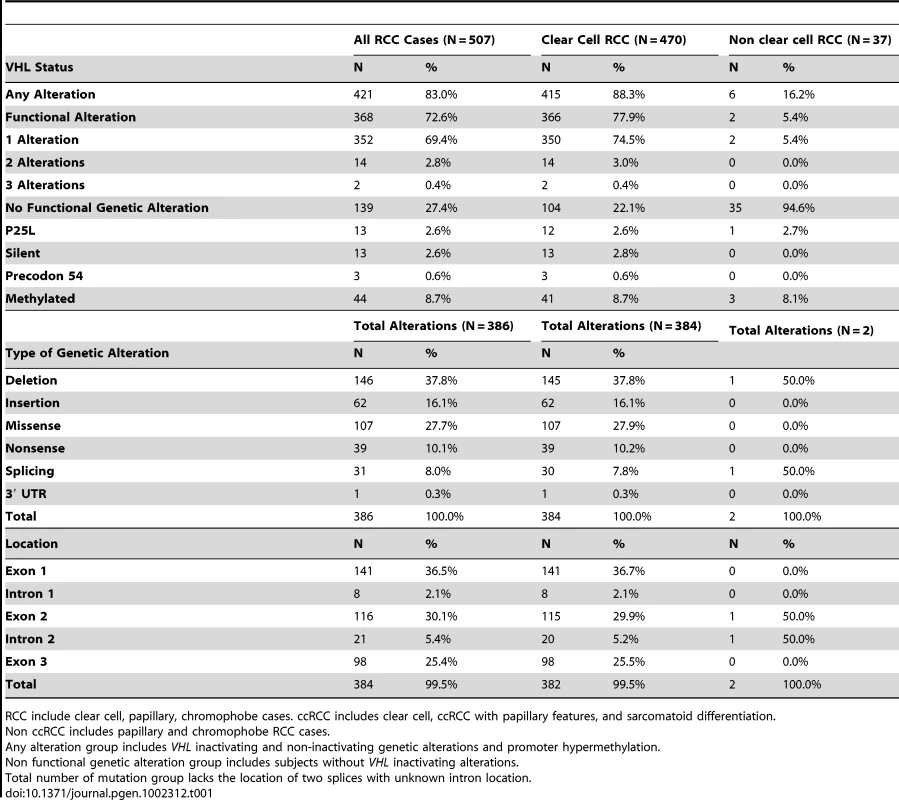
RCC include clear cell, papillary, chromophobe cases. ccRCC includes clear cell, ccRCC with papillary features, and sarcomatoid differentiation. Univariate Analysis of VHL Alteration Subtypes by Tumor and Patient Characteristics in ccRCC
From the univariate analysis (Table S2), risk factors with a p-value<0.20 were selected as adjustment variables in multivariate analyses. In addition to sex, age, and country, variables remained in models if their inclusion changed risk estimates by at least 10%. High blood pressure (p = 0.16), family history of cancer (p = 0.12), and fruit intake frequency (p = 0.09), were inversely associated with VHL promoter hypermethylation and were selected for initial inclusion in multivariate models. In addition to smoking (p = 0.05), family history of cancer (p = 0.10) and fruit intake (p = 0.14) were initially included the multivariate analysis of genetic inactivation prevalence.
In M1 compared to M0 cases, a lower prevalence of genetic inactivation was observed in exon 1 [from 43.4% to 11.8%, p = 0.02] and a higher prevalence was observed in exon 3 [from 25.4% to 52.9%, p = 0.08]. Inactivating VHL alterations were found in 9/12 (75%) patients with a self-reported family history of kidney cancer; 8 case tumors had inactivating alterations, and 1 had a hypermethylated VHL promoter. Specifically, two cases had deletions located at codons 137 and 180, two had insertions located at codons 184 and 203, and four had missense mutations, two A/G transitions at codons 120 and 147, one T/A transversion at codon 158, and one C/A transversion at amino acid 65 which resulted in a stop (ATG) codon (data not shown). Of the three VHL wild type cases, one had an alteration within intron 2 (-11) that was not considered as an inactivating alteration in this study.
Multivariate Analyses
In Table 2, Odds Ratios (OR) and 95% confidence intervals (CI) estimated from multivariate models of risk factors and VHL alteration prevalence among ccRCC case tumor DNAs are presented. Only tobacco smoking and fruit intake, were significantly associated with VHL genetic inactivation in tumor tissue, and/or were required for adjustment. Tobacco smoking was inversely associated with VHL genetic inactivation in ccRCC tumors. The OR associated with having a ccRCC tumor with a genetic VHL inactivating alteration decreased in a dose-dependent manner among former [OR = 0.70(0.20–1.31)] and current smokers [OR = 0.56(0.32–0.99)] compared to never smokers (p-trend = 0.04). The association in the adjusted analysis was very similar to that observed in the univariate analysis as was the association with fruit intake that was not statistically significant (Table S2). The association with fruit intake and genetic alterations in tumor DNA did not differ with respect to smoking status. The lower prevalence of genetically inactivated cases among current smokers was due to a higher prevalence of wild type cases (∼8%) (Table 2). Low fruit intake frequency was associated with having a VHL promoter hypermethylated tumor (p = 0.02). Compared to cases with high fruit intake frequency, the association with promoter hypermethylation increased in a dose-dependent manner among cases with medium [OR = 1.37(0.62–3.03)] and low fruit intake [OR = 3.03(1.16–8.33); p-trend = 0.02]. After stratification by smoking status (ever/never), the association between fruit intake and promoter hypermethylation was observed among smokers when the high intake group was used as a referent [medium: OR = 1.72(0.59–3.13), and low: OR = 4.35(1.09–16.67); p-trend = 0.03]. A significant trend was not observed among never smokers (p-trend = 0.59), however, the formal test for heterogeneity between models was not statistically significant (p-interaction = 0.28). In contrast, tumor histopathologic factors that are generally associated with disease progression (i.e. stage, nuclear grade, node positivity, metastases) were not associated with alteration prevalence. Likewise, occupational exposure to the solvent TCE was not associated with VHL alteration prevalence in tumor DNA, when compared to unexposed cases.
Tab. 2. Multivariate analysis of VHL inactivating alterations and ccRCC risk factors. 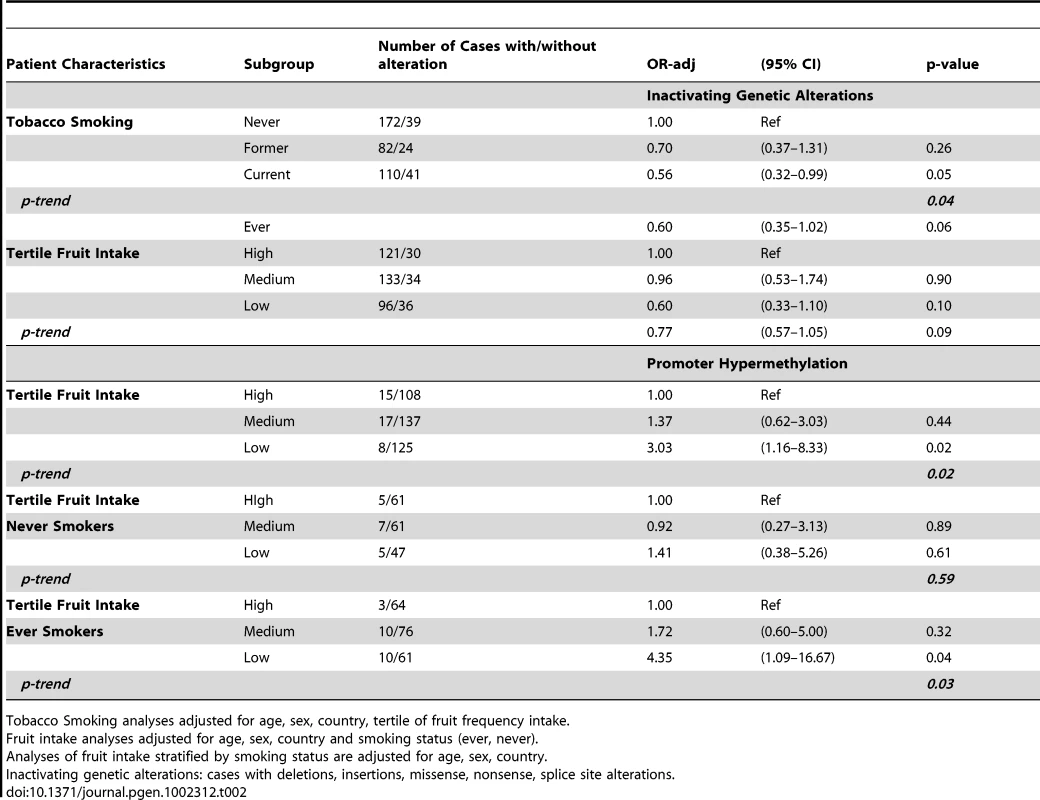
Tobacco Smoking analyses adjusted for age, sex, country, tertile of fruit frequency intake. In Table 3, the association between chromosome 3p loss and specifically the 3p clone CTB110j24, which harbors the VHL gene locus, were analyzed with respect to VHL gene inactivation through either epigenetic or sequence alterations. The proportion of both chromosome 3p loss, and loss of clone CTB110j24 were significantly higher among cases with VHL gene inactivation compared to cases without VHL inactivation (92.4%/93.9% vs 70.2%/61.7%; p<0.00001). Visual examination of each array CGH profile among cases without and with VHL inactivation but for whom chromosome 3p loss was observed, did not differ with respect to loss of clone CTB110j24, and did not support the hypothesis that biallelic loss of the VHL locus had occurred among cases without VHL inactivation (i.e. wild type cases). In adjusted multivariate analysis, the only factor that was significantly associated with chromosome 3p loss was having a VHL gene alteration, and the association was highly significant (OR = 1250; 95% CI:476–3125, p<0.00001). Additional analysis of array CGH data did not indicate that loss of chromosome 3p or clone CTB110j24 differed significantly in tumor DNA from never (92.1%), former (91.3%), or current smoking cases (92.3%), nor did we observe a significant trend with tobacco exposure (data not shown).
Tab. 3. Cases with/without VHL genetic or epigenetic inactivation and chromosome 3p and VHL locus loss (clone CTB-110j24) using array CGH. 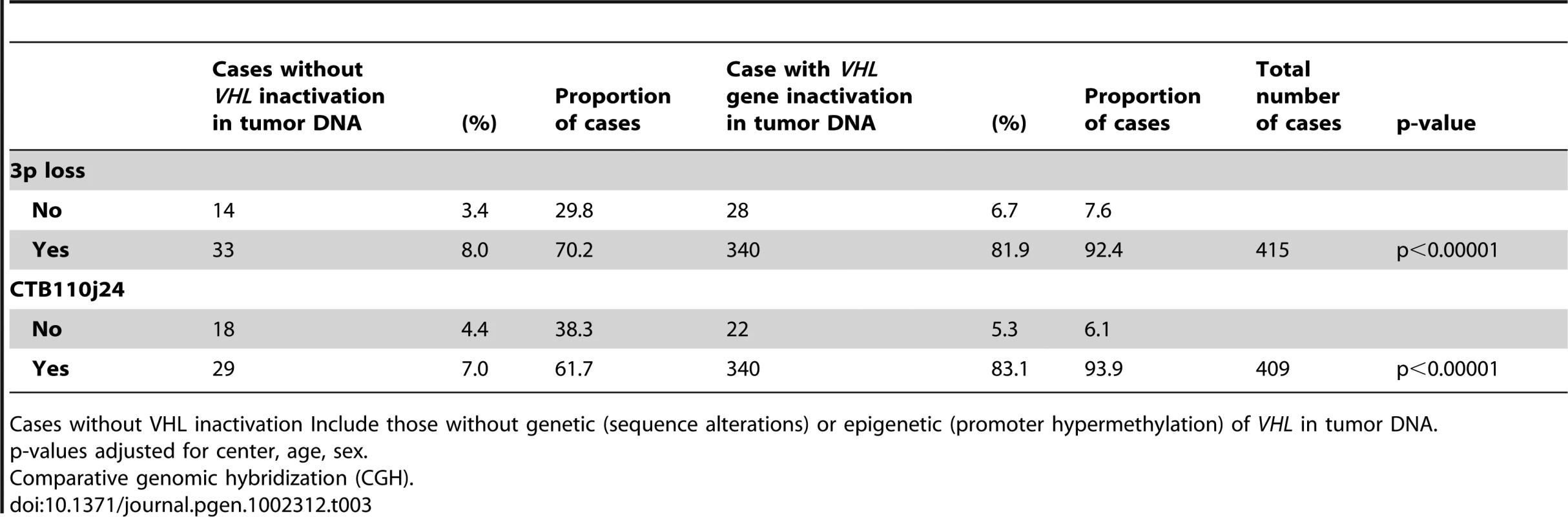
Cases without VHL inactivation Include those without genetic (sequence alterations) or epigenetic (promoter hypermethylation) of VHL in tumor DNA. Germline VHL Polymorphisms and VHL Promoter Hypermethylation in Tumor Tissue DNA
In Table 4, associations between germline VHL tag SNPs and VHL gene promoter hypermethylation in tumor tissue were evaluated in an attempt to replicate a previously reported association between the presence of VHL SNP rs779805 (Ex1+19G>A) in germline DNA, and the prevalence of promoter hypermethylation in tumor DNA [15]. A tag-SNP method was used that relies upon linkage disequilibrium (LD) of highly correlated SNPs to identify gene regions that could be associated with disease susceptibility, or heterogeneous subgroups of cases [14]. A significant association was observed between cases with VHL promoter hypermethylation with SNP rs779805, and six additional tag SNPs across the VHL gene region. Some associations between SNPs in germline and promoter hypermethylation in tumor DNA were stronger when analyses were restricted to ccRCC cases. Significant trends were observed in ccRCC cases that had germline minor alleles at VHL SNPs rs265318 (−2872 A>G), rs779805 (Ex1 +19 G>A), rs779812 (IVS1 −1184 G>A), rs1678607 (IVS2 +108 T>G), rs1642742 (Ex3 +473 G>A), rs1642739 (3835 bp 3′ST), and rs457414 (IRAK2 −3754 A>C). Examination of this region using Haploview revealed that some tag SNPs were highly correlated (Figure 1). To identify regional associations between germline variants with promoter hypermethylation in tumor DNA, a sliding window analysis was conducted with a fixed window size ranging from 2 to 9 consecutive SNPs. The most significant global association across all inherited haplotype variants and tumor-specific VHL promoter hypermethylation was observed when a 9-SNP window spanning across the entire VHL gene region was evaluated. This window spanned from tag SNP rs6442154 (c3orf10, Ex3 −90, T>C) through rs457414 (IRAK2 −3754 A>C) (p-global = 8E-5). When specific VHL haplotypes were evaluated individually and compared to the common 9-SNP referent haplotype T-A-A-A-G-G-A-C-A, two germline haplotypes were significantly associated with tumor-specific promoter hypermethylation, specifically T-A-C-G-A-T-G-A-C (OR = 6.10; 95% CI:2.28–16.35, p = 3.76E-4) and C-G-A-G-G-G-G-C-A (OR = 4.65; 95% CI:1.75–12.32; p = 2.21E-3, respectively). A similar association was observed with a 4-SNP haplotype, as rs779805 was able capture common variation across a highly correlated 6-SNP block, spanning from rs779805 through rs457414. Haplotype analyses spanning a 3-SNP sliding window, conducted to capture smaller regional variations, suggested that two regions may be driving the association observed, one centered within the VHL promoter at rs265318 (p = 0.005) and the other located 3′ of the VHL stop codon centered at rs457414 (p = 0.0004) (data not shown). No associations were observed between these inherited polymorphisms and ccRCC cases with genetically inactivated VHL or wild type tumors.
Fig. 1. Risk of VHL promoter hypermethylation associated with inherited VHL gene polymorphisms. 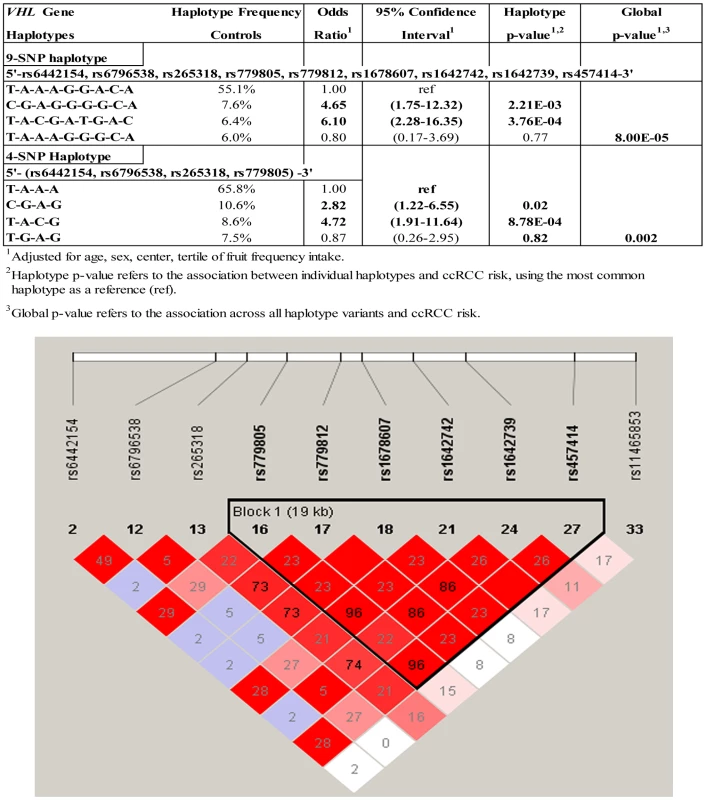
A haploview plot showing the correlation (r2) between the VHL SNPs genotyped. The number in each square represents the value of pair wise r2 between any two markers. Tab. 4. Tagging SNP analysis of common VHL polymorphisms in genomic DNA and promoter hypermethylation in somatic tissue from ccRCC cases. 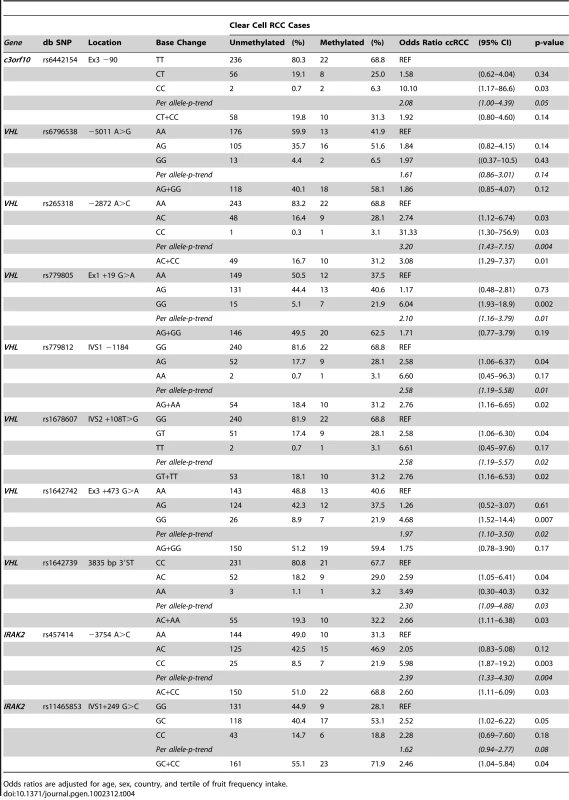
Odds ratios are adjusted for age, sex, country, and tertile of fruit frequency intake. Discussion
The prevalence of VHL inactivation in ccRCC tumor DNA from this large well-characterized case-series of sporadic RCC is one of the highest reported in the literature, and is consistent with recent publications including our previous report [6], [9], [13], [15], [16]. As in our previous study, VHL inactivation in ccRCC tumors occurred either through genetic or epigenetic mechanisms. Notably, VHL alteration prevalence was associated uniquely with etiologic risk modifiers such as tobacco use, fruit intake, and VHL tag SNPs that were examined to capture common germline genetic variation across this region. We observed a slightly higher prevalence of tumors without genetic or epigenetic inactivation of VHL gene among current and former smokers, compared to never smokers. The addition of array CGH analysis of this same case-series of chromosome 3p loss and specifically the clone that harbors the VHL locus, clone CTB110j24, enabled us to evaluate the VHL gene region for evidence of biallelic loss among cases in which we did not detect sequence alterations or evidence of epigenetic inactivation. Multivariate analysis of chromosome 3p loss revealed that the only patient/tumor characteristic or RCC risk factor associated with 3p or clone CTB110j24 loss was inactivation of the VHL gene, and the association was highly significant. In addition, statistically significant associations between VHL promoter hypermethylation and low fruit intake frequency were observed, particularly in tumor DNA from smokers. Interestingly, associations were observed with several tag SNPs spanning the VHL gene in germline DNA and tumor-specific VHL promoter hypermethylation. Of the ten tag SNPs selected to capture common genetic variation across the VHL gene region and evaluate associations with heterogeneous case subgroups, seven were significantly associated with VHL promoter hypermethylation in tumors. Analysis of haplotypes revealed the strongest association was observed using a 9-SNP sliding window that spanned across the entire VHL gene region. Lastly, we did not observe an increased prevalence of inactivating alterations, multiple mutations, nor a specific “hot spot” among TCE exposed cases, compared to unexposed cases.
The association between inherited VHL polymorphisms and promoter hypermethylation in sporadic ccRCC case tumor DNA observed in the current study was similar to a previous report of 97 sporadic RCC cases [15] in which germline SNP rs779805 (Ex1 +19 A>G) was significantly associated with tumor-specific VHL promoter hypermethylation. In the current study, along with rs779805, nine additional SNPs were selected to capture (tag) common variation across the VHL gene region to evaluate associations with heterogeneous subgroups of cases. The global p-values indicated that germline variation across the VHL gene region was significantly associated with risk of having a VHL hypermethylated tumor. When compared to the most common SNP or haplotype as a common referent, subsequent analyses revealed that several individual SNPs and two high risk haplotypes were associated with tumor-specific VHL promoter hypermethylation. These findings are similar to several reports of other cancer types, in which associations between constitutional (germline) mutations were associated with gene specific hypermethylation and silencing in tumors. In one report, a MGMT germline polymorphism (rs16906252 C>T) located within the transcriptional enhancer element of the MGMT promoter was strongly associated with susceptibility to CpG island methylation and gene silencing in colorectal cancer (OR = 18.0; 95% CI:6.2–52.1, p<.0001) [17], [18]. Epigenetic silencing and transcriptional suppression of the death associated protein kinase 1 gene DAPK1, an underlying factor in familial B cell chronic lymphocytic leukemia, was found to be attributable to a germline SNP [c.1–6531 A>G] located upstream of the DAPK1 promoter. Presence of this SNP resulted in higher binding affinity for the HOXB7 protein [19]. Similarly, hypermethylation of the MLH1 and MLH2 genes, also referred to as “epimutations” in inherited cases, have been associated with germline variants in some cases [20], [21]. In contrast, in Beckwith-Wiedemann syndrome, an IGF2 gene polymorphism has been associated with loss of imprinting of the maternal allele-specific methylation of the KCNQ1 gene [22]. The association observed in the current study between particular inherited VHL haplotypes and promoter hypermethylation in renal tumors may be an example of facilitated epigenetic variation, or an increased inherited propensity towards epigenetic variation with respect to a particular genotype [23]. Because we did not analyze VHL promoter hypermethylation in germline DNA (or in normal somatic tissues), it is unknown whether this particular finding is an example of an epimutation, however additional studies are warranted. This observation could be important for future translational research, as identification of individuals with high risk haplotypes could benefit from increased surveillance. Also of note, haplotype analyses spanning a 3-SNP sliding window across the VHL gene suggested that two regions may be driving the association observed, one centered within the VHL promoter and the other located 3′ of the VHL stop codon. The first region of interest, which spanned the promoter region of the VHL gene, raises the possibility that SNP variants in the promoter region could directly influence gene expression [24]. The second region associated with promoter hypermethylation in tumor DNA was centered 3′ to the VHL stop codon. It is possible that this region could be in linkage disequilibrium with a cis-acting structural or copy number alteration that might permit a mechanistic explanation to the associations observed [25].
VHL inactivation in tumor tissue was not associated with any of the clinical parameters examined that are normally considered to reflect disease progression such as stage, grade, and the presence of metastases or positive lymph nodes. The lack of an association between VHL alteration prevalence and indicators of tumor progression was similar to some previous reports [26]–[31]. However, this finding was in contrast to our previous report of 205 sporadic cases [6], and a recent study (analyzing 177 patient tumors) that reported an association between VHL promoter hypermethylation and tumor grade [13]. The mutational spectrum observed in our study was similar to others reporting that the majority of genetic alterations in RCC were located in exon 1 (codons 54–114) (an excellent summary of recent studies is provided in ref 13). The prevalence of promoter hypermethylation observed among cases in our study was within the range observed in other studies (∼5–15%), and was lower than that observed in a recent large study (a prevalence of 31% compared to ∼9% in our study) [13]. Unlike Young et al., [13] we did not observe a difference in the prevalence of alterations between tumors from male and female cases. These dissimilarities could be partially explained by differences in the laboratory methods used to detect promoter hypermethylation and/or the case selection criteria used in each study. Our case series came from a hospital-based case-control study that attempted to include all cases diagnosed at hospitals serving a specific geographic region per study center, and therefore may be more representative of the general study population of sporadic cases than those acquired through studies that uniquely include cases from tertiary care centers.
We observed a higher prevalence (∼8%) of VHL wild type cases among current smokers compared to never smokers in this study. The higher prevalence of VHL wild type cases among smokers had been previously observed in our study of 205 cases but was not statistically significant (p = 0.07). A Dutch study also observed more VHL wild type tumors among smokers [16]. A Swedish study did not observe more VHL wild type tumors among smokers overall, however, they observed that smoking modified the associations observed between VHL alteration prevalence and citrus fruit and vegetable intake [9]. Unlike the Swedish study, we observed an association between VHL promoter hypermethylation and low fruit intake frequency among smokers, rather than genetic alteration prevalence. One reason for these differences may be the use of formalin fixed tissues in both studies, compared to the use of frozen tissue in the current study.
The 8% difference in the prevalence of VHL wild type tumors among current smokers compared to never smokers could be clinically meaningful as this subgroup is considered biologically distinct from those with an inactivated VHL protein. Molecular studies have shown that VHL wild type and VHL inactivated tumors demonstrate differential signaling pathways [32]–[35] and methylation profiles [36]. Clinical studies have reported mixed results with respect to disease progression and survival. VHL wild type tumors have shown reduced treatment response rates [37] and lower median progression free survival [35]. In contrast, other studies have reported that cases without VHL alterations had better cancer-free and overall survival [34], [35], [37]–[41], with the exception of Stage IV disease, as found in one study [39]. However, two studies reported no differences [13], [27]. Clearly, elucidation of the association between heterogeneous tumor subtypes with respect to progression and survival warrants further large studies of well-characterized cases to enable pooled analyses across studies. Follow-up of cases in the current study is ongoing.
Occupational exposure to TCE was associated with an increased risk of RCC in this case-control study [42], however we were unable to replicate previous findings from a German study of exposed workers in which a higher VHL mutation prevalence and a hotspot located at nt454 (codon 81) was reported [11]. In the current study, only one unexposed case had a VHL mutation located at this location and the VHL mutation prevalence was similar in TCE exposed and unexposed cases. Although the German study result may have been a false-positive finding due to small sample size, it was unlikely that mutations were caused by artifacts introduced by formalin fixation, as each tumor DNA sample was analyzed in duplicate [11]. Another possibility was that the German workers may have been exposed to higher TCE levels than those in the current study. A second study of RCC tumor heterogeneity recently conducted among TCE-exposed workers in France, also did not replicate the high mutation prevalence and hotspot observed in tumors from the German exposed workers [43]. However, the French study reported an unusually low VHL alteration prevalence overall, which may have been due to the sensitivity of the laboratory methods applied and the use of DNA extracted from formalin fixed rather than frozen tumor material, as frozen tissue generally results in higher yields of good quality DNA. Both differences could cause misclassification of cases by their alteration status which would have biased results toward the null.
Strengths of the current study include a large sample size, a high participation rate, histological confirmation of all cases and also the tumor tissue used for DNA extraction. The study also included a high number of subjects from most case centers. We applied accurate and sensitive, mutation detection methods in a large study of well-characterized RCC and ccRCC cases to provide a clear picture of VHL inactivation through sequence alteration and promoter hypermethylation in a study conducted in a region with the highest incidence of RCC world-wide [44]. CGH analysis of chromosome 3p and specifically the clone harboring the VHL locus enabled us to evaluate the VHL region for biallelic loss. The multivariate analysis provided additional evidence to support that the only patient characteristic or risk factor associated with 3p or VHL locus loss was inactivation of the VHL gene through either genetic or epigenetic mechanisms, and the association was highly statistically significant. To our knowledge, this study is one of the largest conducted to date. However, additional large studies, data pooling, and meta-analyses will be required to clarify many of the associations observed across study populations. Lastly, we did not observe an increased risk associated with smoking in this case-control study as would have been expected; however by conducting case-only analyses, biases caused by control selection were eliminated.
Some weaknesses of this study included misclassification of fruit, vegetable, and alcohol intake frequency due to retrospective recall using a limited 23-item food frequency questionnaire. There may also have been misclassification of BMI and self-reported hypertension among cases, as this information was collected post - rather than prior to diagnosis. These qualities are strengths of the ongoing Dutch cohort study. Lastly, the prevalence of VHL gene epigenetic inactivation in tumor tissue was only about 9%. Therefore, this analysis relied upon few cases that were both heterozygous/homozygous for the VHL germline variants which also had promoter hypermethylation in tumor tissue. This resulted in unstable point estimates, and is reflected by some of the wide confidence intervals observed.
In summary, this comprehensive analysis of 470 well-characterized ccRCC patient tumor DNA samples observed VHL inactivation through genetic or epigenetic mechanisms in 86.6% of cases. The remaining 13.4% of cases in which we did not observe evidence of VHL inactivation (VHL wild type cases) may represent a biologically distinct subgroup, one that was observed more frequently in tumor DNA from smokers than never-smokers. Moreover, common germline VHL SNPs and haplotypes were associated with promoter hypermethylation in RCC tumor tissue and may demonstrate an example of facilitated epigenetic variation with respect to inherited high risk genotypes [23]. These findings show for the first time in a well-defined ccRCC case series that somatic VHL gene alterations in tumors were uniquely associated with exposures (i.e. tobacco smoking, diet) and inherited VHL polymorphisms in germline DNA, rather than factors associated with disease progression. Additional work is required to elucidate the consequences in these VHL molecularly defined subtypes in terms of etiology, biological mechanisms, treatment, and survival.
Methods
Ethics Statement
The study protocol was approved by relevant ethics committees and institutional review boards of all participating centers, the International Agency for Research on Cancer (IARC), and the U.S. National Cancer Institute (NCI) at the U.S. National Institutes of Health. All study subjects and their physicians provided written informed consent.
Study Population
Cases were from a hospital-based case-control study of sporadic RCC that was conducted between 1999 and 2003 in seven centers in four countries of Central and Eastern Europe (Moscow, Russia; Bucharest, Romania; Lodz, Poland; and Prague, Olomouc, Ceske-Budejovice, and Brno, Czech Republic) as previously described [45], [46]. All newly diagnosed and histologically confirmed cases of sporadic kidney cancer (ICD-O2 code C.64) were identified at participating hospitals in each study region between 1999 and 2003. Histological slides of renal tumor tissue from all cases were reviewed by an international renal cancer pathology expert at the U.S. National Cancer Institute for standardized confirmation and disease classification (MM). Only confirmed cases of RCC and ccRCC were included in the analyses. There were 1097 cases included in the final case-control study: 524 of the 1097 cases (48%) originally diagnosed with RCC from hospital reports that provided frozen tumor biopsies for genetic analysis. Of the 524 cases, 507 (97%) cases were confirmed with RCC by review of their biopsy material provided, and 470 of the 507 (93%) were confirmed with the ccRCC subtype.
Questionnaires were administered in person by trained interviewers at each center. Subjects were asked about their lifestyle habits, in particular tobacco consumption, anthropometric measures one year before diagnosis, personal and familial medical history, and dietary habits. Lifetime occupational information for jobs of at least 12 months duration was also collected during interviews through the use of general occupational and job-specific questionnaires. Job-specific questionnaires covered (1) possible organic and chlorinated solvent exposures, (2) hours per week of solvent exposure, (3) source of solvent exposure, and (4) a description of solvent use as previously described [42]. All coding in the re-assessment was performed while blinded with respect to the previous assessment (with respect to chlorinated solvents and TCE exposures) as well as disease status.
Frequencies of fruit, vegetable, and alcohol intake were examined as they have been inversely associated with the prevalence of VHL alterations and particular alteration subtypes [9]. The dietary questionnaire was comprised of 23 food items which the study investigators selected by consensus during the planning stage of the study, which had been validated as previously described [47]. The questionnaire was repeated for two different time periods: 1) the year prior to interview, and 2) prior to political and market changes in 1989 (1991 in Russia). A lifetime weighted average intake for the two time periods was calculated as previously described [47].
Tumor DNA Samples
Frozen tumor biopsies were collected from a subset of cases enrolled in the case-control study in which detailed information on tumor pathology, patient characteristics, and occupational risk factors for RCC, had been collected (Table S1). Tumor DNA extraction was performed following an additional pathology review of each tissue sample (FW) followed by macrodissection to remove non-tumor tissue, as previously described [6]. Sample areas estimated to contain at least 70% tumor cells were removed for DNA extraction. For each sample, 5 mm3 of tissue was sectioned and digested with 0.4 µg Proteinase K per µl of digestion buffer (500 mM KCl, 100 mM Tris-HCl, 15 mM MgCl2, 0.5% Tween 20) at 50°C overnight. A standard protocol1 was used to extract DNA from the digested samples.
Analysis of VHL Sequence Alterations in Tumor DNA
PCR of VHL coding sequences, endonuclease scanning, and sequencing were performed as previously described [6]. PCR primers for this study for exons 1–3 were the following: VHL Exon1 - Forward:5′-CTACGGAGGTCGACTCGGGAG, VHL Exon 1 - Reverse:5′-GGGCTTCAGACCGTGCTATCG (amplicon length:495 bp); Exon 2 - Forward:5′-CCGTGCCCAGCCACCGGTGTG, Exon 2 - Reverse:5′-GGATAACGTGCCTGACATCAG (amplicon length:288 bp), Exon 3 - Forward:5′-CGTTCCTTGTACTGAGACCCTAG, Exon 3 - Reverse:5′-GAACCAGTCCTGTATCTAGATCAAG (amplicon length:317 bp).
PCR
Amplification was carried out in 50 µL reactions using 10 to 15 ng tumor DNA and HotMaster Taq DNA Polymerase (Eppendorf). Thermal cycling was accomplished using a MJ Research (Bio-Rad) DNA Engine and a touchdown PCR program with an annealing temperature of 58C. PCR products were heteroduplexed using standard conditions. PCR products were analyzed on 2% agarose gels and electrophoresis.
Endonuclease Scanning
Heteroduplexed PCR samples were analyzed using SURVEYOR Nuclease (Transgenomic, Inc.) and standard non-denaturing HPLC conditions appropriate for DNA fragment sizing as previously described [6]. A 100-bp DNA ladder (New England Biolabs) was run as a size marker. Positive and negative controls were included with each plate of PCR products to monitor endonuclease cleavage efficiency.
Sequencing
Excess PCR primers were removed using the AMPure PCR Purification system (Agencourt Bioscience Corp.). Reaction chemistry using BigDye version 3.1 (Applied Biosystems) and cycle sequencing on an MJ Research thermal cycler were adapted from the manufacturer's recommendations. Sequencing products were purified using CleanSEQ reagents (Agencourt Bioscience). Sequence chromatograms were analyzed using Sequencher (GeneCodes, Ann Arbor, MI).
VHL Promoter Methylation
Standard methods were used for bisulfite modification of 100 to 500 ng of tumor DNA (Zymo Research Laboratories). Primers were designed to amplify both methylated and unmethylated alleles across 11 CpG dinucleotides of the VHL promoter. Primers for the methylation analysis were the following: wild-type (WT) amplicon: VHL WT-Forward-CTACGGAGGTCGACTCGGGAG, WT-Reverse-GCGATTGCAGAAGATGACCTG (amplicon length:335 bp); VHL nested primers: WT Forward-CGGGTGGTCTGGATCG, WT-Reverse AGTTCACCGAGCGCAGCA (nested amplicon length:226 bp). Post-bisulfite modification primers that annealed to both methylated (M) and unmethylated (U) alleles were as follows: VHL M/U Forward-5′-TTAYGGAGGTYGATTYGGGAG, and VHL M/U Reverse - ACRATTACAAAAAATAACCTA, (amplicon length:335 bp), nested primers VHL M/U Forward-YGGGTGGTTTGGATYG, VHL M/U Reverse AATTCACCRAACRCAACA, nested (amplicon length: 226 bp). PCR was performed using an MJ Research PTC200 thermal cycler and a touchdown PCR program with an annealing temperature of 50°C. Nested PCR included 1 µL of a 1∶10 dilution of first-round product using cycling conditions as described above. PCR products were visualized in 2.0% agarose and bi-directionally sequenced. Cytosine positions in CpGs were inspected for thymine or cytosine signals in chromatograms, and scoring was conducted as follows: T only, not methylated; both cytosine and thymine, partially methylated; C only, fully methylated. Tumor samples with at least four of 11 methylated CpGs (>36%) were considered as hypermethylated. All analyses were run in duplicate, blinded to VHL mutation status, and with positive (CpGenome Universal Methylated DNA, Chemicon/Millipore) and negative (K562 Human Genomic DNA, Promega) controls.
Array CGH
Each DNA sample from the same case-series was analyzed using Scanning and OncoBAC arrays. Scanning arrays were comprised of 2464 BACs selected at approximately megabase intervals along the genome as described previously [48], [49]. OncoBAC arrays were comprised of 960 Pa, PAC or BAC clones. About three-quarters of the clones on the OncoBAC arrays contained genes and STSs implicated in cancer development or progression. All cones were printed in quadruplicate. DNA samples for array CGH were labeled as described previously [48], [49]. Briefly, 500 ng each of cancer and normal female genomic DNA sample was labeled by random priming with CY3 - and CY5-dUTP, respectively; denatured; and hybridized with unlabeled Cot-1 DNA to CGH arrays. After hybridization, the slides were washed and imaged using a 16-bit CCD camera through CY3, CY5, and DAPI filters [50]. Array CGH data image analyses were performed as described previously [51], [52].
Tag SNP Analysis of Inherited VHL Polymorphisms
Genomic DNA was extracted from blood samples as described previously [42]. Single nucleotide polymorphisms (SNPs) were selected from the HapMap Project, using expected minor allele frequencies reported among Caucasians, to capture 80–90% of common genetic variation across the locus encompassing the VHL gene, covering an average of 15 kb upstream and downstream of the gene. This method relies upon linkage disequilibrium (LD) of highly correlated SNPs in order to identify gene regions that are associated with disease susceptibility, or in this case, specific heterogeneous subgroups of cases defined by their VHL alteration status [14]. SNPs selected (from 5′ to 3′ included the following: rs6442154 (c3orf10, Ex3 −90 T>C), rs6796538 (VHL, −5011 A>G), rs265318 (VHL, −2872 A>C), rs779805 (VHL, Ex1 +19 A>G), rs779812 (VHL, IVS1 −1184 G>A), rs1678607 (VHL, IVS2 +108 G>T), rs1642742 (VHL, Ex3 +473 A>G), rs1642739 (VHL 3835 bp 3′STP C>A), rs457414 (IRAK2, −3754 A>C), rs11465853 (IRAK2, IVS1 +249 G>C). Assays were validated at the NCI Core Genotyping Facility (CGF) in 280 control samples from the human diversity panel that included 76 African/African Americans, 66 Caucasians, 49 Native Americans/Hispanics, and 89 Pacific Rim Asians. Subsequently, minor allele frequencies (MAFs) were determined from the CGF panel among Caucasians. All selected SNPs had a minor allele frequency of at least 10%. Methods for assays can be found at: http://snp500cancer.nci.nih.gov. DNA samples were blinded and randomized on PCR plates to avoid any potential bias and duplicate genotyping was performed for a randomly selected 5% of the total series for quality control. Concordance rates were >99% for all SNPs. All SNPs analyzed were within the expected distributions of Hardy-Weinberg equilibrium (p<0.05).
Statistical Analyses
Tumor and patient characteristics including clinical stage, Fuhrman nuclear grade (I–IV), node stage (N0, N1, N2–3), body mass index (BMI) (<25, 25–35, >35), and smoking status were considered as categorical variables. Smoking status (never, former, or current smoker) was defined as status 2 years prior to the interview. Specifically, participants who were smoking in the 24 months prior interview were classified as current smokers. Other variables such as metastasis (M0, M1) self-reported hypertension (no/yes), family history of cancer or kidney cancer (no/yes), sex, and age at diagnosis (<50, ≥50 years) were analyzed as dichotomous variables. Additional exposures and risk factors were selected based upon previous published reports of renal tumor heterogeneity including our own [6]. These additional factors included fruit, vegetable, and alcohol intake frequency, and VHL tagging SNPs [9]–[13]. Because occupational trichloroethylene (TCE) exposure was positively associated with RCC risk in this population [42] and because TCE exposure had previously been associated with an increased prevalence of VHL mutation among occupationally exposed workers [11], [12], we also evaluated associations between occupational exposure to organic and chlorinated solvents and trichloroethylene with VHL alteration prevalence. For nutritional variables, intake frequencies of related foods were summed to form food group intake categories based on tertile cutoff points that were defined by consumption frequency among controls. Categories of consumption for food-specific items were grouped as low (never to <once per month), medium (≥once per month but <once per week), and high (≥once per week to daily). Categories of alcohol consumption as grams per week of ethanol among weekly drinkers were none, low (<36.5), medium (36.5–137.5), and high ≥137.5) as described previously [47].
The prevalences of VHL genetic (sequence alterations) and epigenetic alterations (promoter hypermethylation) observed in tumor DNA samples were considered as dichotomous variables per case (no/yes). VHL gene nonsynonymous sequence alterations were considered as inactivating alterations if they were located within exons 1–3 and would lead to an altered amino acid sequence or a truncated VHL protein. Splice site mutations and promoter hypermethylation were also considered as inactivating alterations. DNA sequence alterations that were synonymous or SNPs were not considered as inactivating alterations. Similarly, sequence alterations that were located 5′ of codon 54, which were very rare, were not considered as inactivating alterations unless they were nonsynonymous and also would cause an alteration in the coding sequence of both pVHL19 and pVHL30 translation products. These included alterations such as insertions, deletions, and nonsense mutations. As in our previous study, the P25L variant was only considered an inactivating alteration if the case also possessed a second VHL alteration that would be considered inactivating using the above criteria. The prevalence of cases with a particular type of alteration was calculated by dividing the number of cases with that type of alteration by the total number of cases analyzed in the group. Chi-square tests were applied to contingency table (2×2) analyses to test for differences between the proportion of cases with or without a particular alteration subtype within each group. Trend tests were used to analyze associations between categorical variables and cases with particular types of alterations. Ordered logistic regression was used for multivariate analyses to evaluate associations between categorical variables and case subgroups, initially adjusting for variables that were associated with the same alteration subtype in our univariate analyses (p<0.20; Table S2). With the exception of sex, age, and country, other variables remained in multivariate models if their inclusion modified risk estimates by at least 10%.
For genotyping analyses, each SNP was assessed in three categories, (G0 = homozygote for the major allele, G1 = heterozygote, G2 = homozygote for the minor allele) using the most common allele as a referent. LD between SNPs was measured using Lewontin's D' statistic. Correlation (r2) between tagged regions was evaluated in Haploview. To evaluate associations between SNPs and VHL promoter hypermethylation prevalence, logistic regression models were used to calculate odds ratios (OR) and 95% confidence intervals (CI), adjusting for sex, age, country, and fruit intake. Risks were estimated using both additive and dominant models. Risk per allele and trends were calculated using unconditional logistic regression. Analyses were conducted using STATA 10.0 (Stata Corporation, College Station, TX) and all statistical tests were two-sided.
Haplotype analysis was used to explore the combined contribution of consecutive VHL tagging SNPs. A sliding window analysis was first conducted in MatLab to identify regions of interest (The MathWorks, Inc., Natick, MA). For the 10 SNPs examined, sliding windows of 2–9 SNPs were evaluated, accounting for multiple testing and correlations between SNPs. The significance of haplotype-based associations was assessed using the score test [53]. Haplotypes of interest were analyzed using an R package Haplostats (Version 1.3.1) in (version 2.4.1) adjusting for sex, age, and country. The most common haplotype was used as the reference group and rare haplotypes with frequencies of <5% were combined into one group. Generalized linear models accounting for phase ambiguity were used to estimate haplotype-specific ORs per copy [54], [55].
For array CGH analyses of these same cases, those with loss of chromosome arm 3p or clone CTB110j24 to those without, the presence of a loss was considered as a dependent variable in stepwise logistic regression models to first evaluate associations with clinical risk factors including: T stage (T1, T2, T3–4), Fuhrman nuclear grade (1–2, 3–4), node positivity (N0, N1, N2–3), and presence of metastases (M0, M1, Mx). In addition, co-variates and etiologic risk factors (described above) that were previously associated with RCC risk were initially analyzed using univariate analyses, including the presence of a VHL inactivating alteration in tumor tissue (no/yes-any). The criterion for initial inclusion of a variable into multivariate models was a p-value<0.20. Frequency matching variables inherent to the study design (i.e. country, sex and age) were included into all of the models regardless of associations with outcome. Variables selected were then fitted in logistic models adjusted for sex, country, and age, to obtain odds ratios (OR) and 95% confidence intervals (95% CI) as estimates of risk for both types of alterations. All analyses were conducted using SAS 9.1.3 software (SAS Institute Inc.) and STATA 10.0 and statistical tests were two-sided.
Supporting Information
Zdroje
1. LinehanWMWaltherMMZbarB 2003 The genetic basis of cancer of the kidney. J Urol 170 2163 2172
2. Garcia-ClosasMVermeulenRShermanMMooreLSmithM 2006 Application of biomarkers in cancer epidemiology. SchotenfeldDFraumeniJ Cancer Epidemiology and Prevention. 3rd ed New York: Oxford University Press 70 88
3. LatifFToryKGnarraJYaoMDuhFM 1993 Identification of the von Hippel-Lindau disease tumor-suppressor gene. Science 260 1317 1320
4. SignorettiSBratslavskyGWaldmanFMReuterVEHaagaJ 2008 Tissue-based research in kidney cancer: Current challenges and future directions. Clin Cancer Res 14 3699 3705
5. ChowWHDevesaSS 2008 Contemporary epidemiology of renal cell cancer. Cancer J 14 288 301
6. NickersonMLJaegerEShiYDurocherJAMahurkarS 2008 Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 14 4726 4734
7. MaxwellPHWeisenerMSChangGWCliffordSCVauxEC 1999 The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399 271 75
8. KaelinWGJr 2002 Molecular basis of the VHL heteditary cancer syndropme. Nat Rev Cancer 2 673 82
9. HemminkiKJiangYWMaXYangKEgevadL 2002 Molecular epidemiology of VHL gene mutations in renal cell carcinoma patients: relation to dietary and other factors. Carcinogenesis 23 809 815
10. van DijkBACSchoutenLJOosterwijkEHulsbergen-van de KaaCAKiemeneyL 2006 Cigarette smoking, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Br J Cancer 95 374 377
11. BrauchHWeirichGHornauerMAStorkelSWohlT 1999 Trichloroethylene exposure and specific somatic mutations in patients with renal cell carcinoma. J Natl Cancer Inst 91 854 861
12. BruningTWeirichGHornauerMAHoflerHBrauchH 1997 Renal cell carcinomas in trichloroethene (TRI) exposed persons are associated with somatic mutations in the von Hippel-Lindau (VHL) tumour suppressor gene. Arch Toxicol 71 332 5
13. YoungACCravenRACohenDTaylorCBoothC 2009 Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin Cancer Res 15 7582 7592
14. StramDO 2004 Tag SNP selection for association studies. Gen Epidemiol 27 365 74
15. BanksRETirukondaPTaylorCHornigoldNAstutiD 2006 Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res 66 2000 2011
16. Van DijkBASchoutenLJHulsbergen-van de KaaCAvan HouwelingenKPSchalkenJA 2002 Risk factors, genetic predisposition and mutations in the VHL gene in sporadic renal cell carcinoma: The Netherlands Cohort Study. IARC Sci Publ 156 509 10
17. OginoSHazraATranahGJKirknerGJKawasakiT 2007 MGMT germline polymorphism is associated with somatic MGMT promoter methylation and gene silencing in colorectal cancer. Carcinogenesis 28 1985 1990
18. HawkinsNJLeeJHFWongJJLKwokCTWardRL 2009 MGMT methylation is associated primarily with the germline C>T SNP (rs16906252) in colorectal cancer and normal colonic mucosa. Mod Pathol 22 1588 1599
19. RavalATannerSMByrdJCAngermanEBPerkoJD 2007 Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell 129 879 890
20. ChenHPTaylorNPSotamaaKMMutchDGPowellMA 2007 Evidence for heritable predisposition to epigenetic silencing of MLH1. Int J Cancer 120 1684 1688
21. HitchinsMPWardRL 2009 Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet 46 793 802
22. MurrellAHeesonSCooperWNDouglasEApostolidouS 2004 An association between variants in the IGF2 gene and Beckwith-Wiedemann syndrome: interaction between genotype and epigenotype. Hum Mol Genet 13 247 255
23. RichardsEJ 2006 Inherited epigenetic variation-revisiting soft inheritance. Nat Rev Genet 7 395 4000
24. OyerJAChuABrarSTurkerMS 2009 Aberrant epigenetic silencing is triggered by a transient reduction in gene expression. PLoS ONE 4 e4832 doi:10.1371/journal.pone.0004832
25. LiuWHeLRamirezJKrishnaswamySKantetiR 2011 Functional EGFR germline polymorphisms may confer risk for EGFR somatic mutations in non-small cel lung cancer, with a predominant effect on exon 19 microdeletions. Cancer Res 71 2423 7
26. KondoKYaoMYoshidaMKishidaTShuinT 2002 Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: Relationship to clinicopathological parameters. Genes Chromosomes Cancer 34 58 68
27. SmitsKMSchoutenLJvan DijkBACde KaaCWoutersKAD 2008 Genetic and epigenetic alterations in the von Hippel-Lindau gene: The influence on renal cancer prognosis. Clin Cancer Res 14 782 787
28. SuzukiHUedaTKomiyaAOkanoTIsakaS 1997 Mutational state of von Hippel-Lindau and adenomatous polyposis coli genes in renal tumors. Oncology 54 252 257
29. HamanoKEsumiMIgarashiHChinoKMochidaJ 2002 Biallelic inactivation of the von Hippel-Lindau tumor suppressor gene in sporadic renal cell carcinoma. J Urol 167 713 717
30. SchramlPStruckmannKHatzFSonnetSKullyC 2002 VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol 196 186 93
31. van HouwelingenKPvan DijkBACHulsbergen-van de KaaCASchoutenLJGorissenHJM 2005 Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: results from the Netherlands cohort study. BMC Cancer 5 57
32. GordanJDLalPDondetiVRLetreroRParekhKN 2008 HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14 435 46
33. AtkinsDJGingertCJustenhovenCSchmahlGEBonatoMS 2005 Concomitant deregulation of HIF1 alpha and cell cycle proteins in VHL-mutated renal cell carcinomas. Virchows Arch 447 634 642
34. PetrellaBLBrinckerhoffCE 2009 PTEN suppression of YY1 induces HIF-2 activity in von hippel lindau null renal cell carcinoma. Cancer Biol Ther 8 1389 1401
35. PatardJJFergelotPKarakiewiczPIKlatteTTrinhQD 2008 Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer 123 395 400
36. McRonaldFEMorrisMRGentleDWinchesterLBabanD 2009 CpG methylation profiling in VHL related and VHL unrelated renal cell carcinoma. Mol Cancer 8 31
37. GolshayanARBrickAJChoueiriTK 2008 Predicting outcome to VEGF-targeted therapy in metastatic clear-cell renal cell carcinoma: data from recent studies. Future Oncol 4 85 92
38. PatardJJRioux-LeclercqNMassonDZerroukiSJouanF 2009 Absence of VHL gene alteration and high VEGF expression are associated with tumour aggressiveness and poor survival of renal-cell carcinoma. Br J Cancer 101 1417 1424
39. YaoMYoshidaMKishidaTNakaigawaNBabaM 2002 VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. Journal Natl Cancer Inst 94 1569 1575
40. KimJHJungCWChoYHLeeJLeeSHKim 2005 Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol Rep 13 859 864
41. RiniBIJaegerEWeinbergVSeinNChewK 2006 Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and Von Hippel-Lindau gene status. BJU Int 98 756 762
42. MooreLEBoffettaPKaramiSBrennanPStwartPS 2010 Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res 70 16 6527 36
43. CharbotelBGadSCaiolaDBeroudCFevotteJ 2007 Trichloroethylene exposure and somatic mutations of the VHL gene in patients with Renal Cell Carcinoma. J Occup Med Toxicol 2 13
44. CuradoMPEdwardsBShinHRFerlayJHeanueM 2008 Cancer Incidence in Five Continents, Volume IX IARC Scientific Publications
45. BrennanPvan der HelOMooreLEZaridzeDMatveevV 2008 Tobacco smoking, body mass index, hypertension, and kidney cancer risk in central and eastern Europe. Br J Cancer 99 1912 1915
46. HungRJMooreLBoffettaPFengBJToroJR 2007 Family history and the risk of kidney cancer: A multicenter case-control study in Central Europe. Cancer Epidemiol Biomarkers Prev 16 1287 1290
47. HsuCCChowWHBoffettaPMooreLZaridzeD 2007 Dietary risk factors for kidney cancer in Eastern and Central Europe. Am J Epidemiol 166 62 70
48. HodgsonGHagerGHVolikSWernickMMooreD 2001 Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet 29 4 459 64
49. WilhelmMVeltmanJAOlshenABJainANMooreDH 2002 Array-based comparative genomic hybridizaiont for the differential diagnosis of renal cell cancer. Cancer Res 62 4 957 60
50. PinkelDSegravesRSudarDClarkSPooleI 1998 High resloultion analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat Genet 20 2 207 211
51. JainANTokuyasuTASnijdersAMSegravesRAlbsertsonDG 2002 Fully automatic quantification of microarray impage data. Genome Res 12 325 332
52. FridlyandJSnijdersAMYlstraBLiHOlshenA 2006 Breast tumor copy number aberration phenotypes and genomic instability. BMC Cancer 18 : 6 96
53. SchaidDJRowlandCMTinesDEJacobsonRMPolandGA 2002 Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70 425 434
54. LakeSLLyonHTantisiraKSilvermanEKWeissST 2003 Estimation and tests of haplotype-environment interaction when linkage phase is ambiguous. Hum Hered 55 56 65
55. SchaidDJ 2004 Evaluating associations of haplotypes with traits. Genet Epidemiol 27 348 364
Štítky
Genetika Reprodukční medicína
Článek Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA MiceČlánek Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse EmbryosČlánek Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 10
-
Všechny články tohoto čísla
- Transcriptional Robustness Complements Nonsense-Mediated Decay in Humans
- Identification, Replication, and Fine-Mapping of Loci Associated with Adult Height in Individuals of African Ancestry
- Genetic Determinants of Serum Testosterone Concentrations in Men
- A One Base Pair Deletion in the Canine Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier
- Three Structure-Selective Endonucleases Are Essential in the Absence of BLM Helicase in
- Identification of Widespread Ultra-Edited Human RNAs
- Multiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
- The Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
- Transcriptome-Wide Binding Sites for Components of the Non-Poly(A) Termination Pathway: Nrd1, Nab3, and Sen1
- Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA Mice
- Genetic Rearrangements Can Modify Chromatin Features at Epialleles
- Novel Function of as a Gap Gene during Spider Segmentation
- A Genome-Wide Screen for Interactions Reveals a New Locus on 4p15 Modifying the Effect of Waist-to-Hip Ratio on Total Cholesterol
- Comparative Genomic Analysis of Human Fungal Pathogens Causing Paracoccidioidomycosis
- Genetic Diversity in Cytokines Associated with Immune Variation and Resistance to Multiple Pathogens in a Natural Rodent Population
- Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
- Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse Embryos
- A Barcode Screen for Epigenetic Regulators Reveals a Role for the NuB4/HAT-B Histone Acetyltransferase Complex in Histone Turnover
- HIF–VEGF Pathways Are Critical for Chronic Otitis Media in and Mouse Mutants
- A Conserved Developmental Patterning Network Produces Quantitatively Different Output in Multiple Species of Drosophila
- Role of Exonic Variation in Chemokine Receptor Genes on AIDS: Association with Pneumocystis Pneumonia
- Whole-Exome Sequencing Identifies Homozygous Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial -AAA Proteases
- Von Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
- A Systems Biology Approach Reveals the Role of a Novel Methyltransferase in Response to Chemical Stress and Lipid Homeostasis
- Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Natural Selection Affects Multiple Aspects of Genetic Variation at Putatively Neutral Sites across the Human Genome
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
- An Adaptive Allelic Series Featuring Complex Gene Rearrangements
- Feed-Forward Microprocessing and Splicing Activities at a MicroRNA–Containing Intron
- Developmental Stability: A Major Role for in
- A Phenomics-Based Strategy Identifies Loci on , , and Associated with Metabolic Syndrome Phenotype Domains
- Association of , , , , and with Systemic Lupus Erythematosus
- Small RNAs Prevent Transcription-Coupled Loss of Histone H3 Lysine 9 Methylation in
- Successive Increases in the Resistance of to Viral Infection through a Transposon Insertion Followed by a Duplication
- Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Insights into Hox Protein Function from a Large Scale Combinatorial Analysis of Protein Domains
- Mutations Cause Seckel and Jawad Syndromes
- Zelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
- Temporal Coordination of Gene Networks by Zelda in the Early Embryo
- Genetic Interaction between MTMR2 and FIG4 Phospholipid Phosphatases Involved in Charcot-Marie-Tooth Neuropathies
- Oxr1 Is Essential for Protection against Oxidative Stress-Induced Neurodegeneration
- Transforming Growth Factor β Receptor Type 1 Is Essential for Female Reproductive Tract Integrity and Function
- Positional Cloning of a Type 2 Diabetes Quantitative Trait Locus; , a Negative Regulator of Insulin Secretion
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Genetic Determinants of Serum Testosterone Concentrations in Men
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



