-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMultiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
During development, cell polarization is often coordinated to harmonize tissue patterning and morphogenesis. However, how extrinsic signals synchronize cell polarization is not understood. In Caenorhabditis elegans, most mitotic cells are polarized along the anterior-posterior axis and divide asymmetrically. Although this process is regulated by a Wnt-signaling pathway, Wnts functioning in cell polarity have been demonstrated in only a few cells. We analyzed how Wnts control cell polarity, using compound Wnt mutants, including animals with mutations in all five Wnt genes. We found that somatic gonadal precursor cells (SGPs) are properly polarized and oriented in quintuple Wnt mutants, suggesting Wnts are dispensable for the SGPs' polarity, which instead requires signals from the germ cells. Thus, signals from the germ cells organize the C. elegans somatic gonad. In contrast, in compound but not single Wnt mutants, most of the six seam cells, V1–V6 (which are epithelial stem cells), retain their polarization, but their polar orientation becomes random, indicating that it is redundantly regulated by multiple Wnt genes. In contrast, in animals in which the functions of three Wnt receptors (LIN-17, MOM-5, and CAM-1) are disrupted—the stem cells are not polarized and divide symmetrically—suggesting that the Wnt receptors are essential for generating polarity and that they function even in the absence of Wnts. All the seam cells except V5 were polarized properly by a single Wnt gene expressed at the cell's anterior or posterior. The ectopic expression of posteriorly expressed Wnts in an anterior region and vice versa rescued polarity defects in compound Wnt mutants, raising two possibilities: one, Wnts permissively control the orientation of polarity; or two, Wnt functions are instructive, but which orientation they specify is determined by the cells that express them. Our results provide a paradigm for understanding how cell polarity is coordinated by extrinsic signals.
Published in the journal: . PLoS Genet 7(10): e32767. doi:10.1371/journal.pgen.1002308
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002308Summary
During development, cell polarization is often coordinated to harmonize tissue patterning and morphogenesis. However, how extrinsic signals synchronize cell polarization is not understood. In Caenorhabditis elegans, most mitotic cells are polarized along the anterior-posterior axis and divide asymmetrically. Although this process is regulated by a Wnt-signaling pathway, Wnts functioning in cell polarity have been demonstrated in only a few cells. We analyzed how Wnts control cell polarity, using compound Wnt mutants, including animals with mutations in all five Wnt genes. We found that somatic gonadal precursor cells (SGPs) are properly polarized and oriented in quintuple Wnt mutants, suggesting Wnts are dispensable for the SGPs' polarity, which instead requires signals from the germ cells. Thus, signals from the germ cells organize the C. elegans somatic gonad. In contrast, in compound but not single Wnt mutants, most of the six seam cells, V1–V6 (which are epithelial stem cells), retain their polarization, but their polar orientation becomes random, indicating that it is redundantly regulated by multiple Wnt genes. In contrast, in animals in which the functions of three Wnt receptors (LIN-17, MOM-5, and CAM-1) are disrupted—the stem cells are not polarized and divide symmetrically—suggesting that the Wnt receptors are essential for generating polarity and that they function even in the absence of Wnts. All the seam cells except V5 were polarized properly by a single Wnt gene expressed at the cell's anterior or posterior. The ectopic expression of posteriorly expressed Wnts in an anterior region and vice versa rescued polarity defects in compound Wnt mutants, raising two possibilities: one, Wnts permissively control the orientation of polarity; or two, Wnt functions are instructive, but which orientation they specify is determined by the cells that express them. Our results provide a paradigm for understanding how cell polarity is coordinated by extrinsic signals.
Introduction
For tissues and organs to be properly organized, it is often essential that cell polarity be coordinated among cell groups. In the Drosophila wing, for example, cells are polarized in the same proximal-to-distal orientation to produce hairs pointing distally [1]. Similarly, in the mammalian cochlea, stereociliary bundles form at the outer edge of all hair-producing cells [2]. Such coordinated polarizations are often controlled by the Wnt/PCP (planar cell polarity) pathway, which involves the polarized localization of signaling molecules such as Frizzled, Dvl/Dishevelled, and Van Gogh proteins [3]–[5]. One plausible model for cell polarity coordination is that individual cells recognize extrinsic cues that orient their polarity. Although Wnt proteins have been considered candidates for orienting molecules, their functions in regulating cell polarity are not well understood.
In Drosophila, where PCP phenotypes are lacking in some Wnt mutants, including wingless, Wnt proteins are not believed to be required for regulating PCP. Instead, PCP is coordinated by communication between neighboring cells, although the presence of extrinsic cues is still anticipated. In the mammalian cochlea, Wnt7a has been suggested as a cue to instruct cell polarity orientation, based on overexpression and inhibitor studies in organ cultures [6]. However, there is no PCP phenotype in the cochlea of Wnt7a null mice [6], suggesting that other Wnt proteins function redundantly with Wnt7a. In Xenopus and zebrafish, Wnt11 and Wnt5 are required for convergent extension movements during gastrulation, which is also regulated by the PCP pathway [7], [8]. However, these Wnts are thought to function permissively in cell polarization, rather than providing a directional cue. The presence of global extrinsic cues that orchestrate polarity orientations has not been shown in any organism.
In C. elegans, the Wnt/ß-catenin asymmetry pathway controls asymmetry in most somatic cell divisions occurring along the anterior-posterior axis [9]. In this regulation, Wnt pathway components localize asymmetrically. For example, after asymmetric divisions, the ß-catenin homologs WRM-1 and SYS-1 accumulate in the posterior daughter nuclei, while POP-1/TCF localizes more to the anterior than posterior nuclei [10]. Such localization has been observed in most cell divisions, during which cells are accordingly polarized in the anterior-posterior orientation. But how the polarity orientation is determined is not known, except in a few cases. We have shown that Wnts instructively orient the polarity of the EMS blastomere in embryos and of the T cell in larvae [11]. It has also been suggested that MOM-2/Wnt and LIN-44/Wnt expressed in the anchor cell orient the polarity of the P7.p cell, while EGL-20/Wnt expressed near the anus antagonizes these Wnts to orient the P7.p polarity in the opposite orientation [12]. However, it is not known whether or how Wnts globally regulate the polarity of many other cells.
To elucidate the mechanisms of polarity coordination, we focused on a population of epithelial stem cells called seam cells (V cells). At the L1 stage, the six seam cells V1–V6 are positioned on each lateral side of the animals, and repeatedly undergo self-renewing asymmetric cell divisions in each larval stage to produce anterior daughters that fuse with the hypodermal syncytium (hyp7) and posterior daughters that remain as seam cells (Figure 1A) [13]. As with many other cells, the polarity of seam cells is controlled by the Wnt/ß-catenin asymmetry pathway [14], [15], which determines the polarized localization of WRM-1/ß-catenin to the posterior daughter nuclei. Among seam cells, the polarity of the V5 cell reverses fairly frequently in egl-20/Wnt mutants [16]. However, Wnt gene regulation of the polarity of other seam cells has not been reported.
Fig. 1. Analyses of seam cell polarity. 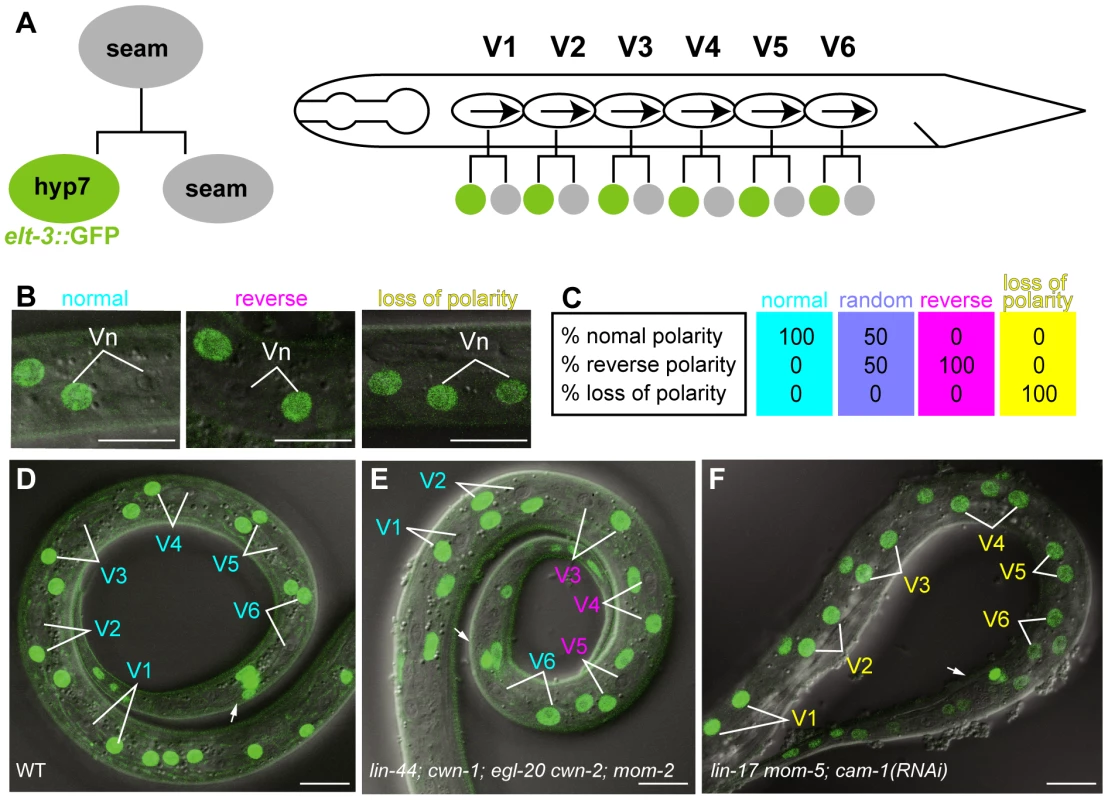
(A) Schematic drawing of seam cell divisions. Left: Anterior daughter of a seam cell fuses with hyp7 and becomes elt-3::GFP-positive. Right: seam cells (V1–V6) on the lateral sides of animals are polarized in the same orientation (represented by arrows) and divide asymmetrically. (B) Examples of the polarity of seam cell (Vn) divisions (normal, reverse, or loss of polarity) as judged by the elt-3::GFP expression in the daughter cells. Merged differential interference contrast (DIC) and fluorescent images are shown. (C) An illustration of sample data for the polarity of seam cell divisions for various genotypes. Each colored box represents the polarity of an individual seam cell. Top, middle, and bottom numbers in the boxes are the percentages of individual seam cells with normal, reverse, and loss of polarity, respectively. An RGB color component was assigned for each polarity phenotype (normal = red; reverse = green; loss of polarity = blue), and the color of each box represents the combination of the calculated intensity of each RGB component per seam cell. Intensity was calculated as follows: 255−(% observed phenotype×2.55), where 255 is the maximum intensity of each RGB component, and 2.55 is a factor for standardizing the phenotype percentage to the RGB scale. The colored boxes shown here represent the resulting four standard colors. Cells with 100% normal division-polarity are cyan (red 0, green 255, blue 255); cells with random division-polarity are lavender (red 128, green 128, blue 255); cells with 100% reverse division-polarity are magenta (red 255, green 0, blue 255); and cells with 100% loss of division-polarity are yellow (red 255, green 255, blue 0). In the similar illustrations in the following Figures, intermediate color compositions indicate relative tendencies towards a certain phenotype in relation to these four standards. (D–F) The expression of elt-3::GFP at the late L1 stage in wild-type C. elegans (D); lin-44(n1792); cwn-1(ok546); egl-20(n585) cwn-2(ok895); mom-2(ne874ts); vpIs1 (E); and lin-17(n3091) mom-5(ne12); cam-1(RNAi); vpIs1 (F). The colors of the cell names indicate the polarity of their divisions (normal in cyan, reverse in magenta, and loss of polarity in yellow), as determined by the elt-3::GFP expression in their daughter cells. Arrows indicate the anus, which is on the ventral side. Scale bars: 10 µm. By analyzing various compound Wnt mutants, including quintuple Wnt mutants, we found that the Wnt genes lin-44, cwn-1, egl-20, and cwn-2 are redundantly required to coordinate the orientation of seam cell polarity at the L1 stage, but three of their receptors are essential for generating the cells' polarity in the first place. The Wnt genes are expressed either anterior or posterior to the seam cells, and each one alone can determine the polarity orientation. Our results provide an important basis for elucidating undiscovered mechanisms in the coordination of cell polarity by Wnt genes.
Results
Multiple Wnts control seam cell polarity
To analyze the polarity of the seam cell divisions, we used elt-3::GFP, which is expressed in hyp7 but not in seam cells [17], [18] (Figure 1A, 1D). About 1 hour after the division of seam cells (V1–V6) at the L1 stage in wild-type animals, the anterior daughters fuse with hyp7; their nuclei immediately begin fluorescing like those of hyp7 cells, because they incorporate GFP from the hyp7 cell. Therefore, we can unambiguously determine the daughter cell fates, from which we can deduce the division polarity type (normal, reverse, or loss of polarity) (Figure 1B). (In Figure 1C and the figures presented below, the proportions of the polarity types of individual seam cells were mathematically converted to RGB colors as described in the figure legend.) The C. elegans genome contains five Wnt genes, lin-44, cwn-1, cwn-2, egl-20, and mom-2. To understand how seam cell polarity is regulated, we first analyzed the phenotypes of animals with mutations in one of the five Wnt genes. Except for egl-20, in which the V5 polarity was reversed [16], the Wnt mutants showed weak phenotypes, if any (Figure 2), raising the possibility that multiple Wnt genes redundantly regulate seam cell polarity.
Fig. 2. Seam cell polarity orientation is redundantly regulated by multiple Wnts. 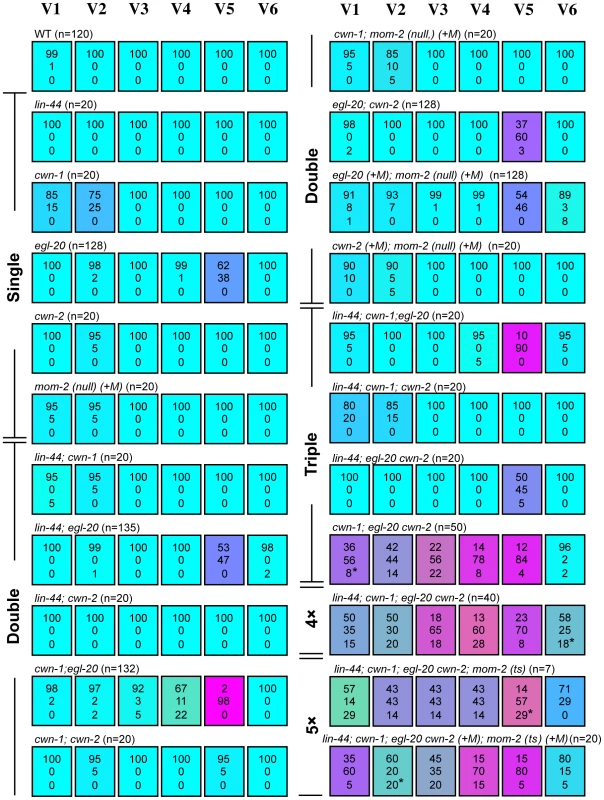
Each colored box represents the polarity of individual seam cell divisions as in Figure 1C. The symbol (+M) indicates maternal contributions. In most cases, “loss of polarity” indicates divisions in which both daughter cells adopted the hyp7 fate, except for some divisions (indicated by asterisks in this and the following Figures; in all cases, one sample per cell) in which both daughters adopted the seam cell fate. Random asymmetry of the V1–V5 divisions in the cwn-1; egl-20 cwn-2 mutants was also observed using scm::GFP (Figure S1). To test this hypothesis, we constructed a strain with mutations in all five Wnt genes (quintuple Wnt mutants). Because a combination of three Wnt null mutations (cwn-1, cwn-2 and mom-2) causes complete embryonic lethality [19], we used the temperature-sensitive (ts) mutation mom-2(ne874), in which endoderm production is strongly affected during embryogenesis at restrictive temperatures [20]. Because quintuple Wnt mutants only occasionally reproduce, even at permissive temperatures, we could analyze only 7 animals born from homozygous quintuple Wnt mutants. In addition, we analyzed quintuple mutants from mothers heterozygous for cwn-2, egl-20 and mom-2, as shown in Figure 2: lin-44; cwn-1; egl-20 cwn-2(+M); mom-2(ts) (+M).
We found that the polarity of all the seam cell divisions was abnormal in the quintuple Wnt mutants (Figure 1E and Figure 2, p<0.01 in V1–V6 by Fisher's exact test), indicating that multiple Wnts are redundantly required for appropriately oriented seam cell polarity. Although the phenotypes varied among the cells, the polarity tended to be either normal or reversed, and symmetric division was less frequent (represented by the absence of yellowish colors in Figure 2). Although we cannot exclude residual mom-2 activity in quintuple mutants with the mom-2(ts) allele, the results suggest that seam cells are mostly polarized even in the absence of Wnt functions.
Most seam cells can be properly polarized by a single Wnt gene
To determine which combinations of Wnt genes are required for the properly oriented polarity of individual seam cells, we analyzed them in double, triple, or quadruple Wnt mutants. The phenotype of quadruple Wnt mutants (lin-44; cwn-1; egl-20 cwn-2) was quite similar to that of quintuple mutants (Figure 2; p>0.1 in V1–V6 for the abnormalities), suggesting that mom-2 has only minor functions, if any, in seam cell polarity. Next, we constructed triple Wnt mutants from these four Wnt mutations. Through these analyses, we found three distinct regulations that depended on cell type, grouped into V1–V4, V5, and V6.
V1–V4
The phenotypes of V1–V4 Wnt triple mutants (cwn-1; egl-20 cwn-2) were similar to those of Wnt quadruple (p>0.1 in V1–V4 for the abnormalities) and quintuple mutants (p>0.1 in V1, V2 and V4; p>0.05 in V3) (Figure 2), suggesting that the polarity in these cells are regulated primarily by these three Wnt genes. In any double combination of these three Wnt mutations, the polarity of the divisions was almost normal, although V4 was weakly affected by cwn-1; egl-20 (Figure 2) (p<0.01). The results indicate that functions of these three Wnts are redundant in all four of these cells.
V6
The most posterior seam cell, V6, was affected in quadruple Wnt mutants (p<0.01), but not in any triple or double combination analyzed (Figure 2). Therefore, the V6 cell polarity is redundantly regulated by the four Wnts.
In summary, V1–V4 and V6 cells are properly polarized by the presence of just one Wnt from among the three Wnts cwn-1, cwn-2 and egl-20 for V1–V4, or among the four Wnts lin-44, cwn-1, cwn-2 and egl-20 for V6.
V5
In contrast to V1–V4 and V6, one Wnt, egl-20, is essential for V5 polarity, as reported previously [16]. In egl-20 mutants, the polarity of the division was reversed in 38% of the V5 cells (Figure 2). This phenotype was strongly enhanced to nearly complete reversal (98%) in cwn-1; egl-20, suggesting that functions of cwn-1 and egl-20 are partially redundant. Although the cwn-2 mutation slightly enhanced polarity reversal in the egl-20 background (p<0.01), it instead suppressed the phenotype in the cwn-1 egl-20 background (p<0.01) (Figure 2), suggesting that cwn-2's functions in the V5 cell polarity are complex. One possibility for the unique regulation of V5 might be its distinct cell lineage compared to the other seam cells. Only the V5 cell produces neurons at the L2 stage. To test this possibility, we analyzed lin-22 mutants, in which not only V5, but also the V1–V4 cells produce neurons [21]. However, even in lin-22 egl-20 double mutants, polarity reversal was observed mostly in the V5 cell (data not shown). Therefore, V5's neuron production is unlikely to be the reason for its unique regulation.
Wnt genes control seam cell polarity through the Wnt/ß-catenin asymmetry pathway
To confirm that Wnt genes regulate the Wnt/ß-catenin asymmetry pathway, we analyzed POP-1/TCF localization in triple Wnt mutants (cwn-1; egl-20 cwn-2), in which the polarity of V1–V5 is disrupted. We found that POP-1 asymmetry was abnormal in V1–V5 cells in the triple Wnt mutants (Figure 3A, 3D, 3E; p<0.01 in V1–V5). As judged by elt-3::GFP expression (Figure 2), polarity reversal is more frequent than loss of polarity (represented by purplish colors in Figure 3A). Therefore, these Wnt genes control seam cell polarity via the Wnt/ß-catenin asymmetry pathway.
Fig. 3. Wnts regulate the Wnt/ß-catenin asymmetry pathway in seam cells. 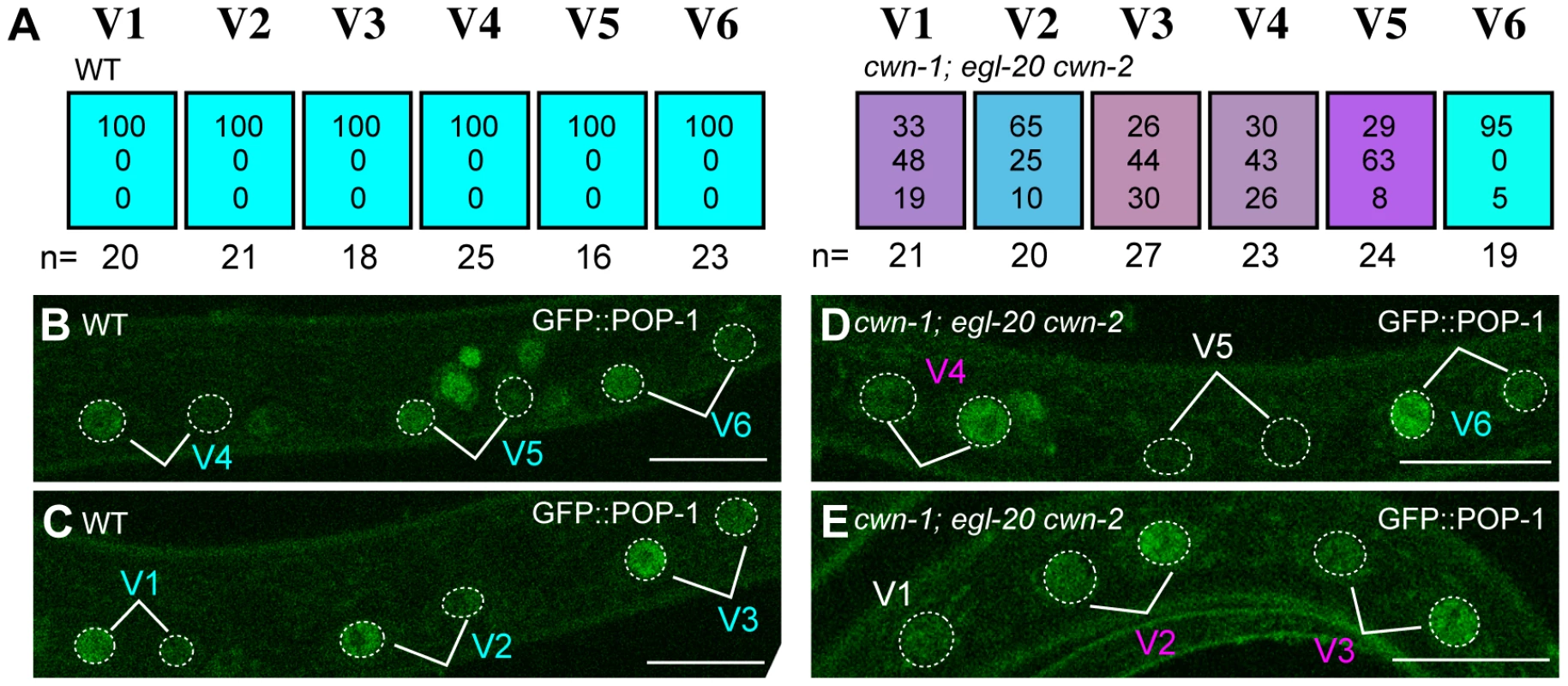
(A) Each colored box represents the polarity of individual seam cell divisions as in Figure 1C, except that polarity was judged by the localization of GFP::POP-1 at the mid-L1 stage. (B–E) Examples of GFP::POP-1 localization in wild-type animals with qIs74 (GFP::POP-1) (B, C) and in cwn-1(ok546); egl-20(n585) cwn-2(ok895); qIs74 (D, E). Cell name colors indicate their polarity, as in Figure 1D-1F. GFP::POP-1 localization can be detected for about 1 hour after each seam cell division. Therefore, we can observe polarity of some but not all seam cells in individual animals. For example the V5 cell polarity in (D) could not be judged due to loss of the expression. A V1 cell is shown in (E) before its division. Scale bars: 10 µm. Since seam cells are polarized in a planar (anterior-posterior) orientation in contact with each other before division, interactions between neighboring cells might coordinate their polarity, as with PCP regulation in the Drosophila wing. However, in triple Wnt mutants (cwn-1; egl-20 cwn-2), we did not observe any significant correlation of polarity reversal between neighboring seam cell pairs (data not shown). In addition, the polarity of the V5 cell division is not affected by laser ablation of the V6 cell [16]. Furthermore, we found that the polarity of the seam cell divisions was normal in mutants of the putative PCP components vang-1/Van Gogh(tm1422) (n = 20) and prkl-1/Prickle(ok3182) (n = 20) (the phenotype of vang-1 was analyzed using scm::GFP, as described in Materials and Methods). Therefore, it is likely that the polarity of each seam cell is independently controlled by Wnt genes.
Three Wnt receptors are required for seam cell polarization
To understand how Wnts control polarity, it is important to identify their receptors. The C. elegans genome contains six Wnt receptors, four Frizzled (MIG-1, LIN-17, CFZ-2, and MOM-5), one Ror (CAM-1) [22], and one Derailed (LIN-18) family members. Among these, it has been reported that cam-1/Ror mutations reverse the polarity of the V1 and V2 cell divisions at a low frequency [23] and that lin-17/Frizzled mutants cause mostly symmetric divisions of a tail seam cell called a T cell [24].
First, we analyzed single mutants of each receptor gene. Similar to cam-1, the mom-5 mutation weakly affected the polarity of the V1 and V2 divisions (p<0.01 in V1 and V2). V1–V2 defects were enhanced in mom-5 cam-1 (RNAi) animals (p<0.01 in V1 and V2 by the comparison with mom-5 mutants or cam-1(RNAi) animals), indicating that MOM-5 and CAM-1 redundantly control V1–V2 polarity (Figure 4). Single mutants for the other receptors showed only minor defects, if any, in the polarity of seam cell divisions, suggesting that their functions are redundant for V3–V6. Since lin-17 and mom-5 show a strong genetic interaction in gonad development [19], we next analyzed lin-17 mom-5 double Frizzled mutants and found that the polarity of all the seam cell divisions was abnormal (p<0.01 in V1–V6) (Figure 4). The mig-1, cfz-2, or lin-18/Derailed mutations slightly modified the phenotype of the lin-17 mom-5 mutants. However, since the mig-1; cfz-2; lin-18 triple mutants showed nearly normal polarity (Figure 4), these receptors are not essential and are likely to function redundantly with other receptors.
Fig. 4. Multiple Wnt receptors redundantly control seam cell polarization. 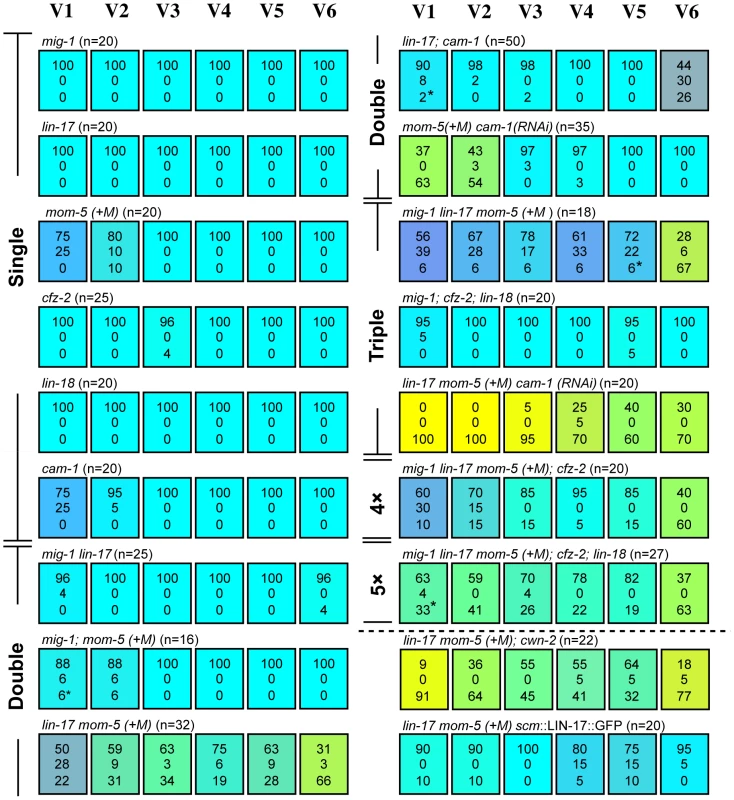
Each colored box represents the polarity of individual seam cell divisions as in Figure 1C; (+M) indicates maternal contributions. Asterisks are as described in Figure 2, legend. Symmetric divisions of the seam cells in lin-17 mom-5; cam-1(RNAi) mutants were also observed using scm::GFP (Figure S1). Next, we constructed lin-17 mom-5; cam-1 triple mutants, and found that this combination was embryonically lethal. Therefore, we inhibited cam-1 by RNAi in lin-17 mom-5, and found that all seam cell divisions were symmetric at high penetrance (Figure 1F) (p<0.01 in V1–V6 and p<0.01 in V1–V4, p<0.05 in V5, p>0.1 in V6 for symmetric division by the comparison with wild type and lin-17 mom-5, respectively; represented by yellowish colors in Figure 4). These results indicate that LIN-17, MOM-5, and CAM-1 are the main receptors that redundantly regulate seam cell polarity, whereas the receptors MIG-1, CFZ-2, and LIN-18 weakly affect polarity in the absence of the main receptors. Most importantly, the phenotype of lin-17 mom-5; cam-1 is clearly distinct from that of quintuple Wnt mutants in which polarity orientation is randomized (p<0.01 in V1–V6 for symmetric division). These results suggest that Wnt receptors can function even in the absence of Wnts to generate polarity, while Wnts are required to orient polarity.
It was previously suggested that CAM-1 functions as a receptor for CWN-2 [25], [26]. If this is the case for seam cell polarity, the cwn-2 mutation should have the same or stronger effects than the cam-1 mutation. However, as described above, the cwn-2 mutation alone did not affect the V1 cell, which was affected in cam-1 mutants. Furthermore, the lin-17 mom-5; cwn-2 mutants had a weaker phenotype than lin-17 mom-5 cam-1(RNAi) (p<0.05 in V2 and V3, p = 0.066 in V4) (Figure 4). Therefore, it is unlikely that CAM-1 is a specific receptor for CWN-2 for seam cell polarity.
It was reported that cam-1p::GFP is expressed in V cells [23]. We found that lin-17p::LIN-17::GFP is also expressed in all V cells (Figure S2). To determine whether the receptors functions in seam cells, we expressed LIN-17 specifically in seam cells using the scm promoter (scm::LIN-17::GFP) [11] and found that the polarity defects in the lin-17 mom-5 animals were significantly rescued in V1–V3 and V6 (p<0.01 in V1, V3, and V6, p<0.05 in V2, p>0.1 in V4 and V5) (Figure 4), suggesting that at least LIN-17 among the Wnt receptors functions in seam cells.
Wnts are expressed either anterior or posterior to the V cells
Wnt genes are expressed in specific regions of the animal, either in the anterior (CWN-2) [25], [26] or posterior (LIN-44, EGL-20, and CWN-1) [16], [27]–[29]. In addition, EGL-20 forms a posterior–to-anterior gradient [30]. We examined CWN-1 and CWN-2 protein localization by full-length translational fusion constructs (cwn-1p::CWN-1::Venus or cwn-2p::CWN-2::Venus). V4 and V5 polarity defects in cwn-1; egl-20 mutants were rescued by cwn-1p::CWN-1::Venus (Figure 5G), and V1–V4 defects in cwn-1; egl-20 cwn-2 mutants were partly rescued by cwn-2p::CWN-2::Venus (Figure 5H), indicating that these fusion proteins are functional. As reported previously for CWN-1 promoter activity [19], cwn-1p::CWN-1::Venus was localized to the cytoplasm and around the cell membrane in posterior muscle cells, both dorsal and ventral (Figure 5A). Although there were variations between animals, the signals clearly tended to be stronger in posterior cells than in the middle of the animal, suggesting that CWN-1 expression may form a gradient.
Fig. 5. Ectopic Wnt expression rescued compound Wnt mutants. 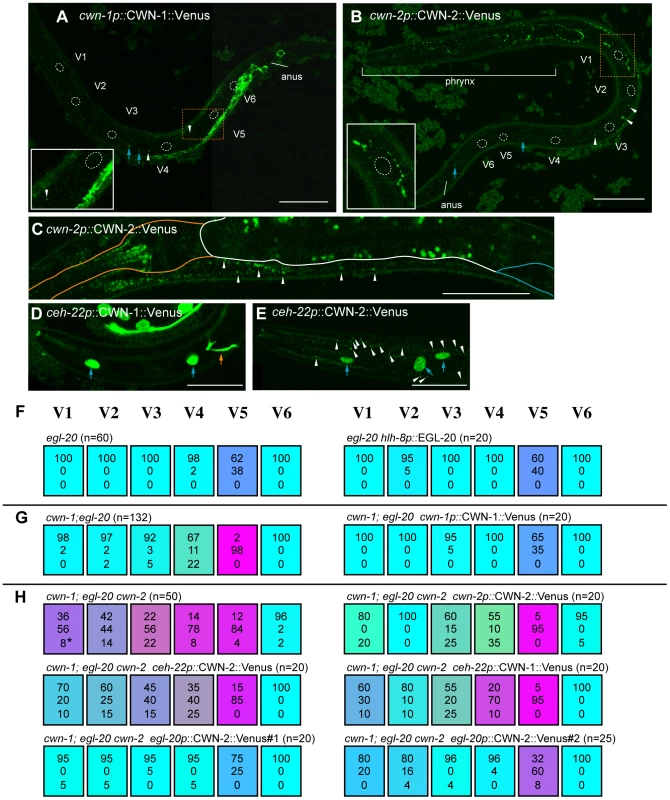
(A–F) The expression of cwn-1p::CWN-1::Venus (A), cwn-2p::CWN-2::Venus (B, C), ceh-22p::CWN-1::Venus (D), and ceh-22p::CWN-2::Venus (E). Anterior is to the left and ventral to the bottom, except for (B), in which anterior is to the upper-left and ventral toward the right. Scale bars: 20 µm. Confocal images in (A, B) and (D, E), were focused on hypodermal cells. The focus is on the pharynx/intestine in (C). (A, B) Dashed lines outline seam cell nuclei. White arrowheads and blue arrows indicate some of the GFP puncta and granule autofluorescence, respectively, which were observed even in the corresponding DIC images. Insets show the area indicated by orange dashed boxes, magnified 2-fold; they illustrate the efficient diffusion of cwn-2p::CWN-2::Venus but not of cwn-1p::CWN-1::Venus. (C) Expression of cwn-2p::CWN-2::Venus in the pharynx and near the intestine. Orange, white, and blue lines outline the pharynx, intestine, and gonad, respectively. Puncta of cwn-2p::CWN-2::Venus near the intestine (white arrowheads) were observed only in the anterior region. Puncta in the intestine are gut granule autofluorescence. (D, E) Diffusion of ceh-22p::CWN-2::Venus (E) but not ceh-22p::CWN-1::Venus (D) was observed in the anterior hypodermis near the pharynx. White arrow heads, blue arrows and the orange arrow indicate GFP puncta, hypodermal cells expressing the elt-3::GFP marker, and neuronal processes expressing the mec-4::GFP marker, respectively. (F–H) Each colored box represents the polarity of individual seam cell divisions, as in Figure 1C. (F) The egl-20 phenotype was not affected by EGL-20 expression in the M cell (hlh-8p::EGL-20). Since the M cell is on the right side of the animals, we scored seam cells only on the right side. (G) cwn-1p::CWN-1::Venus rescued the defect of the cwn-1 mutation in cwn-1; egl-20. (H) The phenotype of cwn-1; egl-20 cwn-2 was rescued by cwn-2p::CWN-2::Venus, by CWN-2 expressed in the pharynx (ceh-22p::CWN-2::Venus) or in the cells near the anus (egl-20p::CWN-2::Venus#1 and #2 with mec-4::GFP and egl-5::GFP as coinjection markers, respectively), or by CWN-1 expressed in the pharynx (ceh-22p::CWN-1::Venus). Consistent with the previous observation that the cwn-2 promoter is strongly active in the pharynx [25], [26], we detected puncta of cwn-2p::CWN-2::Venus mostly around the pharynx. We detected these puncta on the hypodermis, including the seam cells (Figure 5B, white arrowheads), suggesting its diffusion from the pharyngeal region. This is in contrast to cwn-1p::CWN-1::Venus, whose diffusion away from its expressing cells was only occasionally observed (white arrowheads in Figure 5A). Although it was also reported that the cwn-2 promoter is active in the intestine, albeit weaker than in the pharynx [25], [26], we detected cwn-2p::CWN-2::Venus puncta only in the anterior region, along the boundary between the intestine and muscle or hypodermis (Figure 5C, white arrowheads). These observations indicate that CWN-2 is mostly distributed to the anterior side of the animal. To confirm that cwn-2 functions in the pharynx, we used a ceh-22 promoter to express cwn-2 in the pharynx of Wnt triple mutants (cwn-1; egl-20 cwn-2), and found that the phenotype was rescued in V1, V3 and V4 cells (p<0.05 in V1, p<0.1 in V3 and V4, p = 0.14 in V2) (Figure 5H). The weak effects of ceh-22p::CWN-2::Venus compared to cwn-2p: CWN-2::Venus appear to reflect weaker transgene expression. These results suggest that cwn-2 is expressed and functions in the pharynx.
Ectopically expressed Wnts rescued Wnt triple mutants
Our results indicate that each seam cell except V5 can be polarized by a single Wnt gene expressed either anterior or posterior to the cells. For example, V1 is properly polarized merely by cwn-2 expressed nearby and at its anterior, or by egl-20 expressed posterior to and far from V1. To determine whether the position of Wnt expression is important in regulating polarity, we expressed Wnt genes ectopically. If Wnts function permissively, abnormal polarity in Wnt compound mutants should probably be rescued irrespective of the location of Wnt expression. If the Wnts were instructive, we expected that ectopic Wnt expression opposite to its normal location would enhance polarity reversals.
As reported previously, EGL-20 expressed in the pharynx by the myo-2 promoter can rescue V5 polarity defects in egl-20 mutants [16]. However, since the myo-2 promoter is also weakly active in the posterior region [11], the appropriate interpretation of these results was uncertain. We first used the hlh-8 promoter to express egl-20 in the M cell, a mesodermal blast cell positioned between the V4 and V5 cells on the right side, in egl-20 mutants [31]. We found that this had no significant effect on V5 cell polarity (Figure 5F), suggesting that egl-20 does not function (i.e., it is not produced, secreted, or modified) in polarization when it is expressed in the M cell.
We then expressed cwn-1 or cwn-2 ectopically in the anterior (using the ceh-22 promoter) [32], [33] posterior (using the egl-20 promoter) [29] regions in Wnt triple mutants (cwn-1; egl-20 cwn-2). Surprisingly, the posterior expression of CWN-2, which is normally expressed in the pharynx, efficiently rescued the triple mutant phenotype (Figure 5H, p<0.01 in V1–V5). Similarly, the anterior expression of CWN-1, which is normally expressed in the posterior region, appeared to rescue the polarity defects of the V1–V3 divisions (Figure 5H, V1 p = 0.1076, V2 p<0.01, V3 p<0.05). The effect of ceh-22p::CWN-1::Venus was comparable to that of ceh-22p::CWN-2::Venus. These results seem to suggest that the position of Wnt expression is not important and that Wnt functions are not instructive, even though Wnts are required for correct polarity orientation. However, the results can also be explained by assuming that functions of Wnts are determined by the cells that express them (see Discussion).
It is noteworthy that, even though ceh-22p::CWN-1::Venus and ceh-22p::CWN-2::Venus express these Wnts from the same ceh-22 promoter, we detected puncta of CWN-2::Venus but not CWN-1::Venus outside of the pharynx (white arrowheads in Figure 5D, 5E). Together with the efficient diffusion of cwn-2p::CWN-2::Venus but not cwn-1p::CWN-1::Venus described above (Figure 5A and 5B), the results suggest that these Wnts have distinct diffusion properties. Because ceh-22p::CWN-1::Venus rescued V1–V3 polarity, CWN-1 is likely to be diffused, but at such low levels that it was undetectable. The difference may reflect CWN-1's lower diffusion or weaker ability to form puncta as compared to CWN-2.
Wnt-independent regulation of somatic gonad precursor polarity
Similar to seam cells, the Wnts regulating the polarity of Z1 and Z4 cells, which are somatic gonad precursors (SGPs), have not been identified. The SGPs have a mirror-symmetric polarity, which is important for producing the mirror symmetry of the C. elegans gonad [34]. POP-1 asymmetry in the Z1 daughters is reversed compared to other cells, including Z4. POP-1 is higher in the posterior and anterior daughters of Z1 and Z4, respectively (Figure 6A, 6G) [35]. SGP polarity is also regulated by the Wnt/ß-catenin asymmetry pathway [35], although the involvement of Wnt genes has not been demonstrated. We found that the SGP polarity was not affected in quintuple Wnt mutants from mothers heterozygous for cwn-2, egl-20 and mom-2, as judged by the normal POP-1 localization (Figure 6B, 6G) and the presence of distal tip cells (DTCs; data not shown). Although we could not analyze the POP-1 asymmetry in the quintuple Wnt mutants from homozygous mothers, all such animals we examined (n = 85) had two gonad arms as in wild type, indicating that normal numbers of DTCs were produced from SGPs. These results suggest that the polarity of SGPs is regulated by Wnt-independent mechanisms.
Fig. 6. Regulation of SGP polarity. 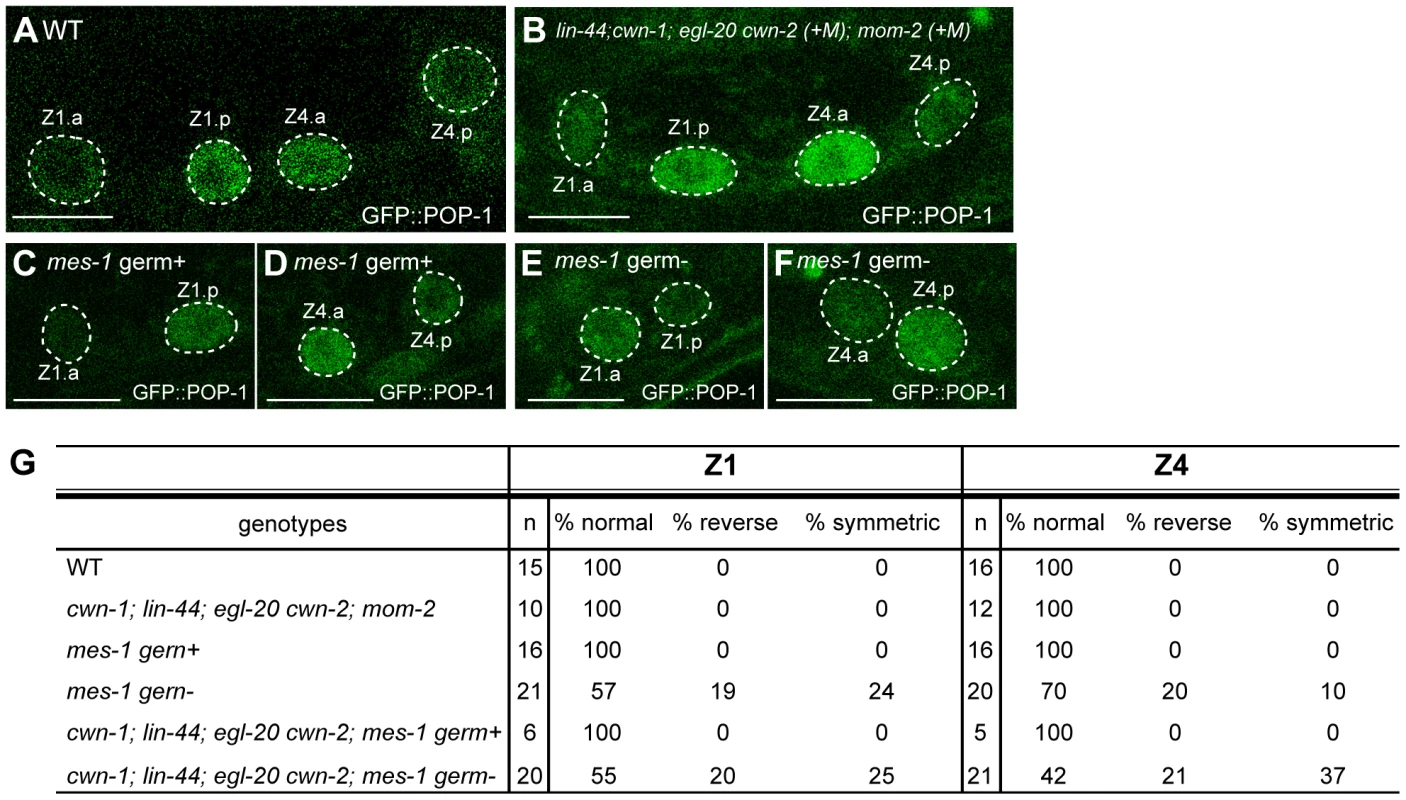
(A–F) GFP::POP-1 localization in SGP daughters in wild-type (A), quintuple Wnt mutants (+M) (B), mes-1 mutants with germ cells (C, D), and mes-1 mutants without germ cells (E, F). Anterior is to the left. Scale bars: 10 µm. (G) The table summarizes the GFP::POP-1 expression data. To explore the polarity-regulating mechanisms in SGPs, we used mes-1 mutants, which frequently lack germ cells [36], to analyze the roles of the germ cells Z2 and Z3, which are positioned between Z1 and Z4. In mes-1 mutants lacking germ cells, the polarity of both Z1 and Z4 was abnormal, although the defect in Z4 was weaker than that in Z1 (Z1 p<0.01, Z4 p<0.05) (Figure 6E, 6F, 6G). Such defects were not observed in mes-1 mutants that had germ cells (Figure 6C, 6D, 6G). These results suggest that non-Wnt signals from germ cells control SGP polarity and hence regulate the proper organization of the somatic gonad.
We also examined the possibility that these germ cell signals function redundantly with Wnts. In quadruple Wnt mutants (lin-44; cwn-1; egl-20 cwn-2) lacking germ cells due to the mes-1 mutation, polarity defects appear to be enhanced in Z4 but not Z1 as compared to mes-1 mutants, although the difference did not reach significance (Figure 6G) (Z1: p = 1.0, Z4: p = 0.11), raising the possibility that Z4 polarity is redundantly controlled by Wnts and signals from germ cells. In contrast, the polarity of the Z1 cell appeared not to be affected by the Wnt mutations, and Z1, in wild type, exhibits a reversed orientation compared with Z4 and the seam cells (i.e., POP-1 is higher in the posterior daughter). Z1 may therefore be regulated by signals from germ cells but may be insensitive to Wnt signals.
Discussion
Redundant regulation by multiple Wnts
We have shown that seam cell polarity is redundantly regulated by multiple Wnt genes. The V1–V4 and V6 cells are affected only by combinations of three and four Wnt mutations, respectively. Such redundancy has been reported in other organisms [37]. For example, double knockout of Wnt1 and Wnt3a in mice causes much stronger CNS developmental abnormalities than the single knockouts [38]. Because all metazoan species have multiple Wnt genes (e.g., 19 in humans), our results suggest that Wnt genes in any organism may have undiscovered functions that can not be identified by the inhibition of one or a few of them.
Distinct regulation of polarity orientation and polarity generation
The defects observed in Wnt mutations in any combination were mostly randomized (normal or reverse) polarity, and less frequently, loss of polarity. Similar observations were reported in a mutant lacking mig-14/Wntless function, which is required for Wnt secretion [39]. Our observations are consistent with a recent report that seam cell numbers are not significantly altered in lin-44; cwn-1; egl-20 cwn-2 animals [15], since the cell numbers were not affected by random orientations of their asymmetry. Even though quintuple Wnt mutants may contain residual mom-2 activity from the ts allele, our results strongly suggest that functions of at least four Wnts (lin-44, cwn-1, cwn-2, and egl-20) determine the polarity orientation of seam cells. In contrast, cell polarization itself appeared to be Wnt-independent, although we cannot eliminate the possibility that cells were not polarized in the complete absence of Wnt functions.
In contrast to the randomized polarity found in compound Wnt mutants, triple receptor mutants (lin-17 mom-5; cam-1) showed a severe loss of polarity. These three receptors are likely to function in the polarity generation that occurs even in the absence of Wnts. Even though the other three receptors (MIG-1, CFZ-2, and LIN-18) appear to be involved in regulating polarity, based on genetic interactions with lin-17 mom-5 mutations, their triple mutants showed nearly normal polarity. Therefore, it is likely that LIN-17, MOM-5, and CAM-1 function in the regulation of polarity orientation as Wnt receptors in addition to having a role in the polarity generation that occurs even in the absence of Wnts, although their activities may be modified by the other three receptors (MIG-1, CFZ-2, and LIN-18). Consistent with this interpretation, strains with mutations in these three receptors showed polarity reversal: V1 and V2 in cam-1 or mom-5 single mutants, and V6 in lin-17 cam-1 double mutants. Our results strongly suggest the presence of distinct mechanisms for polarity orientation, which is Wnt-dependent, and polarity generation, which can occur independently of Wnts.
Do Wnts permissively control polarity orientation?
Our ectopic expression experiments appear to indicate that although Wnt functions are required to correctly orient polarity, those functions are permissive. Assuming Wnts are permissive, how do they control polarity orientation in seam cells? One model is that Wnts act indirectly through other cells that produce real polarity cues in response to Wnts (Figure 7A). In this case, the same Wnt receptors should function in other cells to produce the cues, and in seam cells to generate polarity. For this model, it is strange that, even though Wnts are apparently present near the seam cells, the Wnt receptor activity to polarize seam cells appears not to be affected by Wnts. Together with our finding that LIN-17 functions in seam cells, this model appears unlikely.
Fig. 7. Possible models for polarity orientation by Wnts. 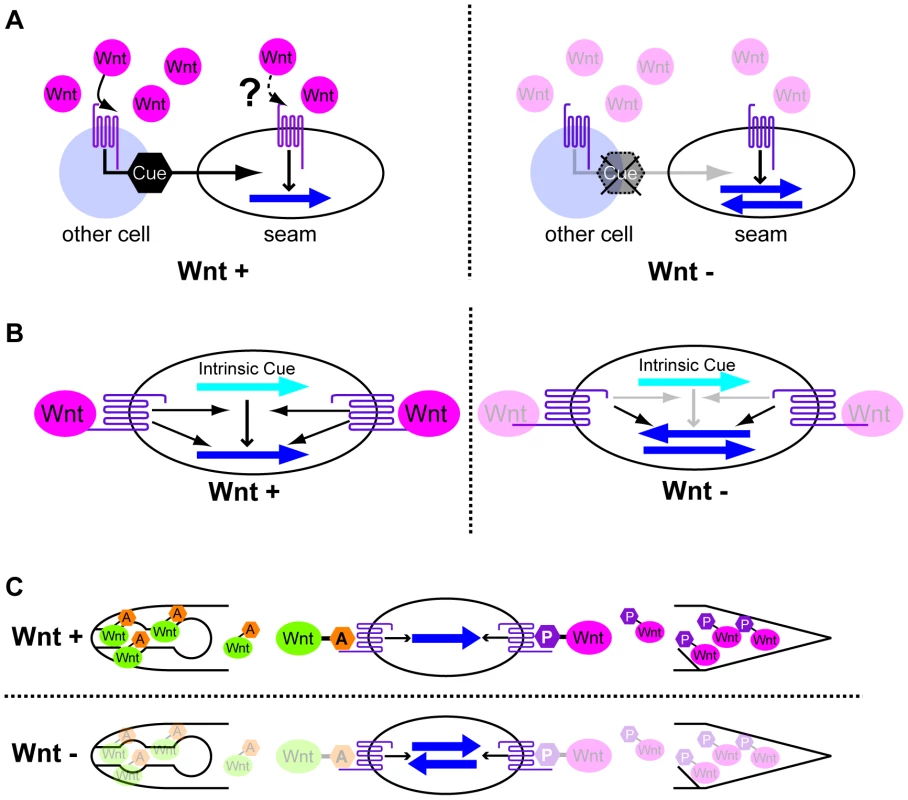
(A) Wnts activate the production of polarity cues (black hexagons) through their receptors in cells other than seam cells. The Wnt receptors also function in polarity generation in seam cells using the cues but not Wnts as directional information. (B) Wnts and their receptors function in the interpretation of intrinsic polarity cues (light blue arrows). The receptors also function in polarity generation even in the absence of Wnts. (C) Polarity is instructively oriented by Wnts that are modified by Wnt-expressing cells. That is, anteriorly and posteriorly expressed Wnts receive “anterior modification” (orange hexagons A) and “posterior modification” (magenta hexagons P), respectively. Wnt receptors recognize the modifications to properly orient the polarity. In all the panels, the dark blue arrows show the polarity orientation that is randomized in the absence of Wnts. Anterior is to the left. A second model is that Wnt receptors function only in seam cells. They have two distinct functions: one to generate polarity via the Wnt/ß-catenin asymmetry pathway, and the other to interpret intrinsic polarity cues (which might be determined by extrinsic cues) through an unknown pathway (orientation pathway) to generate polarity orientation–but only when they are activated by Wnts (Figure 7B). In the absence of Wnts, the receptors still function to polarize cells, but the intrinsic cues cannot be used, resulting in randomly oriented polarity. Although BAR-1/ß-catenin, which functions downstream of LIN-17 in the migration of the Q neuroblast [40], appears to be a good candidate for mediating the orientation pathway, bar-1 single mutants have normal seam cell divisions (H.S. unpublished observation). Whatever the mechanism of the orientation pathway is, the key question regarding this model is how Wnts elicit the function of the receptors to activate the orientation pathway without affecting the receptors' function in the Wnt/ß-catenin asymmetry pathway, which generates polarity even in the absence of Wnts.
Possible Wnt instructive functions in polarity orientation
Because Wnts instruct the polarity of some cells (EMS, T, and P7.p) [11], [12], it is reasonable to imagine that Wnts also instruct seam cells. Assuming that Wnts are instructive, how are the results of ectopic expression explained? One model would be that Wnts' functions depend on the cells that express them. For instance, CWN-2, which is expressed in the pharynx, might receive some specific modification, say, “anterior modification,” whereas CWN-1, which is expressed in the posterior region, might receive a different modification, say, “posterior modification” (Figure 7C). When cells receive CWN-2 with the anterior modification from their anterior side, they recognize the direction of the Wnt source as “anterior” and localize their signaling components accordingly (e.g. POP-1 in the anterior daughter nuclei). When CWN-1 is ectopically expressed in the pharynx, it may receive anterior modification, like CWN-2, and function like CWN-2 to instruct normal seam cell polarity, rather than functioning like CWN-1 with posterior modification.
This model can explain EGL-20's lack of function when expressed in the M cell–assuming that the M cell cannot modify EGL-20. In addition, we have reported that LIN-44 expressed by the egl-5 promoter (egl-5::LIN-44) anterior to a T cell can efficiently reverse T cell polarity in the absence of endogenous LIN-44 expressed at the posterior of the T cell [11]. However, in the presence of endogenous LIN-44 (LIN-44 is expressed in both sides of the T cell), the effect of egl-5::LIN-44 is quite weak despite egl-5's promoter activity being stronger, as judged by egl-5::GFP, than that of the lin-44 promoter, as judged by lin-44::GFP. This observation is also consistent with the model that Wnt functions depend on the cells that express it. Another possibility for cell-specific Wnt functions is that Wnt-expressing cells or their neighbors express specific cofactors of Wnts that bind tightly to Wnts and determine their functions.
Even though there is no direct evidence for the above models, and other explanations may be possible, our results suggest the presence of novel mechanisms that control the orientation of cell polarity. Such mechanisms, as well as the redundancy of Wnt proteins, may also explain Wnt functions that control cell polarity in other organisms.
Materials and Methods
Strains, cloning, culture, and RNAi
N2 Bristol was used as the wild-type strain [41]. The animals were cultured at 22.5°C, except for strains containing mom-2 (ne874ts) [20]. The following alleles were used: lin-44(n1792) (nonsense) [42]; cwn-1(ok546) (deletion) [43]; cwn-2(ok895) (deletion) [43]; egl-20(n585) (missense, but behaves like null) [44]; mom-2(or309) (deletion) [45]; mom-2(ne874ts) (missense); lin-17(n3091) (nonsense) [24]; mig-1(e1787) (nonsense) [28]; mom-5(ne12) (nonsense) [46]; cam-1(gm122) (nonsense) [23]; cfz-2(ok1201) (deletion) [43]; lin-18(e620) (nonsense) [47]; mes-1(bn7) [48]; vang-1(tm1422) (deletion) [49]; prkl-1(ok3182) (deletion); and lin-22(n372) (missense) [50]. Molecular information of cwn-1(ok546), cwn-2(ok895), mom-2(or309) cfz-2(ok1201), vang-1(tm1422) and prkl-1(ok3182) is described in http://www.cbs.umn.edu/CGC/index.html.
The genotypes of compound strains were confirmed either by PCR (cwn-1, cwn-2, cfz-2, vang-1, and prkl-1), sequencing (lin-44, egl-20, mom-2(ne874ts), mig-1, cam-1, and lin-18 ), or by their phenotype (Psa for lin-44, maternal effect lethal for mom-2(or309) and mom-5, bivulva for lin-17, and maternal effect sterile for mes-1). The strains containing mom-5(ne12) mom-2(or309) were maintained as heterozygotes over hT2[qIs48] and nT1[qIs51], respectively, which are marked by GFP expression. Non-fluorescent homozygotes were analyzed for their phenotype. The quintuple Wnt mutants were maintained at 15°C as lin-44; cwn-1; cwn-2 egl-20/nT1[qIs51]; mom-2/nT1. The phenotype was analyzed in non-fluorescent homozygotes or their progeny, which were shifted to 25°C during late embryogenesis. RNAi for cam-1 was performed by feeding RNAi (Ahringer Lab RNAi protocol, http://www.gurdon.cam.ac.uk/~ahringerlab/pages/rnai.html) using the RNAi clone I-6L11.
Analyses of seam cell phenotypes
In most cases, the polarity of seam cell divisions was analyzed using elt-3::GFP (vpIs1) [18] expressed in hyp7, except for cfz-2 single mutants, which were analyzed by scm::GFP (wIs51) [51]; lin-18 single mutants, analyzed by ajm-1::GFP (ncIs13) [52]; vang-1, analyzed by wIs51; and compound strains with lin-18 mutations, also analyzed by wIs51. GFP markers, including GFP::POP-1 (qIs74) [53], cwn-1p::CWN-1::Venus, and cwn-2p::CWN-2::Venus, were analyzed by confocal microscope (Zeiss LSM510). Statistical analysis was performed with the Fisher exact test.
Plasmid construction
cwn-1p::CWN-1::Venus and cwn2p::CWN-2::Venus were constructed from PCR fragments containing their promoter regions (1.8 kb and 6.1 kb, respectively), and the entire coding regions were amplified by PCR from the fosmids WRM0620cE04 and WRM0622bE06, respectively, inserted into a pPD95.75::wVenus derived from pPD95.75 (a gift from A. Fire) and containing the Venus gene optimized for C. elegans codon usage in place of the GFP gene. The plasmids ceh-22p::CWN-1::Venus and ceh-22p::CWN-2::Venus contain a ceh-22 promoter fragment from pCW2.1 [32] and full-length cDNAs (yk236a10 and yk343h8, respectively) inserted into pPD95.75::wVenus. For egl-20p::CWN-2::Venus, a 6.8 kb egl-20 promoter fragment and yk343h8 were inserted into pPD95.75::wVenus. The plasmids were injected as described previously [54], with pBlueScript SK+ DNA and the co-injection markers unc-76-rescuing plasmids for cwn-1p::CWN-1::Venus and cwn2p::CWN-2::Venus injected into unc-76(e911) for expression analyses; ceh-22p::GFP for the cwn-1p::CWN-1::Venus rescue experiment; and mec-4::GFP [55] for ceh-22p::CWN-2::Venus, ceh-22p::CWN-1::Venus, and the cwn-2p::CWN-2::Venus rescue experiment. Either mec-4::GFP or egl-5::GFP [11] was used for egl-20p::CWN-2::Venus. The hlh-8p::EGL-20 plasmid contains a 1.3 kb PCR fragment just upstream of the start codon of the hlh-8 gene from a cosmid C02B8 and an egl-20 cDNA (yk1183a10) subcloned into pPD49.26 (a gift from A. Fire). lin-17p::LIN-17::GFP was constructed by inserting a HindIII-KpnI fragment of pSH6 [24] into pPD95.77 (a gift from A. Fire).
Supporting Information
Zdroje
1. LawrencePAStruhlGCasalJ 2007 Planar cell polarity: one or two pathways? Nature reviews Genetics 8 555 563
2. KellyMChenP 2007 Shaping the mammalian auditory sensory organ by the planar cell polarity pathway. The International journal of developmental biology 51 535 547
3. McNeillH 2010 Planar cell polarity: keeping hairs straight is not so simple. Cold Spring Harbor perspectives in biology 2 a003376
4. SimonsMMlodzikM 2008 Planar cell polarity signaling: from fly development to human disease. Annual review of genetics 42 517 540
5. AxelrodJD 2009 Progress and challenges in understanding planar cell polarity signaling. Seminars in cell & developmental biology 20 964 971
6. DabdoubADonohueMJBrennanAWolfVMontcouquiolM 2003 Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130 2375 2384
7. RohdeLAHeisenbergCP 2007 Zebrafish gastrulation: cell movements, signals, and mechanisms. International review of cytology 261 159 192
8. RoszkoISawadaASolnica-KrezelL 2009 Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Seminars in cell & developmental biology 20 986 997
9. MizumotoKSawaH 2007 Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol 17 465 473
10. MizumotoKSawaH 2007 Cortical beta-catenin and APC regulate asymmetric nuclear beta-catenin localization during asymmetric cell division in C. elegans. Dev Cell 12 287 299
11. GoldsteinBTakeshitaHMizumotoKSawaH 2006 Wnt signals can function as positional cues in establishing cell polarity. Dev Cell 10 391 396
12. GreenJLInoueTSternbergPW 2008 Opposing Wnt pathways orient cell polarity during organogenesis. Cell 134 646 656
13. SulstonJEHorvitzHR 1977 Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Developmental Biology 56 110 156
14. TakeshitaHSawaH 2005 Asymmetric cortical and nuclear localizations of WRM-1/b-catenin during asymmetric cell division in C. elegans. Genes Dev 19 1743 1748
15. GleasonJEEisenmannDM 2010 Wnt signaling controls the stem cell-like asymmetric division of the epithelial seam cells during C. elegans larval development. Developmental biology 348 58 66
16. WhangboJHarrisJKenyonC 2000 Multiple levels of regulation specify the polarity of an asymmetric cell division in C. elegans. Development 127 4587 4598
17. GilleardJSShafiYBarryJDMcGheeJD 1999 ELT-3: A Caenorhabditis elegans GATA factor expressed in the embryonic epidermis during morphogenesis. Developmental biology 208 265 280
18. KohKRothmanJH 2001 ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development 128 2867 2880
19. GleasonJESzyleykoEAEisenmannDM 2006 Multiple redundant Wnt signaling components function in two processes during C. elegans vulval development. Dev Biol 298 442 457
20. NakamuraKKimSIshidateTBeiYPangK 2005 Wnt signaling drives WRM-1/beta-catenin asymmetries in early C. elegans embryos. Genes Dev 19 1749 1754
21. WaringDAWrischnikLKenyonC 1992 Cell signals allow the expression of a pre-existent neural pattern in C. elegans. Development 116 457 466
22. GreenJLKuntzSGSternbergPW 2008 Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol 18 536 544
23. ForresterWCDellMPerensEGarrigaG 1999 A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature 400 881 885
24. SawaHLobelLHorvitzHR 1996 The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev 10 2189 2197
25. SongSZhangBSunHLiXXiangY 2010 A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet 6 e1001056 doi:10.1371/journal.pgen.1001056
26. KennerdellJRFetterRDBargmannCI 2009 Wnt-Ror signaling to SIA and SIB neurons directs anterior axon guidance and nerve ring placement in C. elegans. Development 136 3801 3810
27. HermanMAVassilievaLLHorvitzHRShawJEHermanRK 1995 The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell 83 101 110
28. PanCLHowellJEClarkSGHilliardMCordesS 2006 Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell 10 367 377
29. WhangboJKenyonC 1999 A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell 4 851 858
30. CoudreuseDYRoelGBetistMCDestreeOKorswagenHC 2006 Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312 921 924
31. HarfeBDVaz GomesAKenyonCLiuJKrauseM 1998 Analysis of a Caenorhabditis elegans Twist homolog identifies conserved and divergent aspects of mesodermal patterning. Genes & development 12 2623 2635
32. OkkemaPGHaEHaunCChenWFireA 1997 The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124 3965 3973
33. OkkemaPGFireA 1994 The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120 2175 2186
34. KimbleJHirshD 1979 The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Developmental biology 70 396 417
35. SiegfriedKRKimbleJ 2002 POP-1 controls axis formation during early gonadogenesis in C. elegans. Development 129 443 453
36. StromeSMartinPSchierenbergEPaulsenJ 1995 Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development 121 2961 2972
37. LoganCYNusseR 2004 The Wnt Signaling Pathway in Development and Disease. Annu Rev Cell Dev Biol 20 781 810
38. IkeyaMLeeSMJohnsonJEMcMahonAPTakadaS 1997 Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389 966 970
39. WildwaterMSanderNde VreedeGvan den HeuvelS 2011 Cell Shape and Wnt Signaling Redundantly Control the Division Axis of C. elegans Epithelial Stem Cells. Development In press
40. SilhankovaMKorswagenHC 2007 Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Current opinion in genetics & development 17 320 325
41. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
42. HermanMAHorvitzHR 1994 The Caenorhabditis elegans gene lin-44 controls the polarity of asymmetric cell divisions. Development 120 1035 1047
43. ZinovyevaAYForresterWC 2005 The C. elegans Frizzled CFZ-2 is required for cell migration and interacts with multiple Wnt signaling pathways. Dev Biol 285 447 461
44. MaloofJNWhangboJHarrisJMJongewardGDKenyonC 1999 A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development 126 37 49
45. SugiokaKSawaH 2010 Regulation of asymmetric positioning of nuclei by Wnt and Src signaling and its roles in POP-1/TCF nuclear asymmetry in Caenorhabditis elegans. Genes to cells : devoted to molecular & cellular mechanisms 15 397 407
46. RocheleauCEDownsWDLinRWittmannCBeiY 1997 Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90 707 716
47. InoueTOzHSWilandDGharibSDeshpandeR 2004 C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 118 795 806
48. CapowskiEEMartinPGarvinCStromeS 1991 Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics 129 1061 1072
49. HoffmannMSegbertCHelbigGBossingerO 2010 Intestinal tube formation in Caenorhabditis elegans requires vang-1 and egl-15 signaling. Developmental biology 339 268 279
50. WrischnikLAKenyonCJ 1997 The role of lin-22, a hairy/enhancer of split homolog, in patterning the peripheral nervous system of C. elegans. Development 124 2875 2888
51. ZhongWFengHSantiagoFEKipreosET 2003 CUL-4 ubiquitin ligase maintains genome stability by restraining DNA-replication licensing. Nature 423 885 889
52. LiuZFujiiTNukazukaAKurokawaRSuzukiM 2005 C. elegans PlexinA PLX-1 mediates a cell contact-dependent stop signal in vulval precursor cells. Developmental biology 282 138 151
53. SiegfriedKRKiddAR3rdChesneyMAKimbleJ 2004 The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics 166 171 186
54. MelloCCKramerJMStinchcombDAmbrosV 1991 Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 3959 3970
55. LaiCCHongKKinnellMChalfieMDriscollM 1996 Sequence and transmembrane topology of MEC-4, an ion channel subunit required for mechanotransduction in Caenorhabditis elegans. The Journal of cell biology 133 1071 1081
Štítky
Genetika Reprodukční medicína
Článek Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA MiceČlánek Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse EmbryosČlánek Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 10
-
Všechny články tohoto čísla
- Transcriptional Robustness Complements Nonsense-Mediated Decay in Humans
- Identification, Replication, and Fine-Mapping of Loci Associated with Adult Height in Individuals of African Ancestry
- Genetic Determinants of Serum Testosterone Concentrations in Men
- A One Base Pair Deletion in the Canine Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier
- Three Structure-Selective Endonucleases Are Essential in the Absence of BLM Helicase in
- Identification of Widespread Ultra-Edited Human RNAs
- Multiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
- The Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
- Transcriptome-Wide Binding Sites for Components of the Non-Poly(A) Termination Pathway: Nrd1, Nab3, and Sen1
- Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA Mice
- Genetic Rearrangements Can Modify Chromatin Features at Epialleles
- Novel Function of as a Gap Gene during Spider Segmentation
- A Genome-Wide Screen for Interactions Reveals a New Locus on 4p15 Modifying the Effect of Waist-to-Hip Ratio on Total Cholesterol
- Comparative Genomic Analysis of Human Fungal Pathogens Causing Paracoccidioidomycosis
- Genetic Diversity in Cytokines Associated with Immune Variation and Resistance to Multiple Pathogens in a Natural Rodent Population
- Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
- Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse Embryos
- A Barcode Screen for Epigenetic Regulators Reveals a Role for the NuB4/HAT-B Histone Acetyltransferase Complex in Histone Turnover
- HIF–VEGF Pathways Are Critical for Chronic Otitis Media in and Mouse Mutants
- A Conserved Developmental Patterning Network Produces Quantitatively Different Output in Multiple Species of Drosophila
- Role of Exonic Variation in Chemokine Receptor Genes on AIDS: Association with Pneumocystis Pneumonia
- Whole-Exome Sequencing Identifies Homozygous Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial -AAA Proteases
- Von Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
- A Systems Biology Approach Reveals the Role of a Novel Methyltransferase in Response to Chemical Stress and Lipid Homeostasis
- Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Natural Selection Affects Multiple Aspects of Genetic Variation at Putatively Neutral Sites across the Human Genome
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
- An Adaptive Allelic Series Featuring Complex Gene Rearrangements
- Feed-Forward Microprocessing and Splicing Activities at a MicroRNA–Containing Intron
- Developmental Stability: A Major Role for in
- A Phenomics-Based Strategy Identifies Loci on , , and Associated with Metabolic Syndrome Phenotype Domains
- Association of , , , , and with Systemic Lupus Erythematosus
- Small RNAs Prevent Transcription-Coupled Loss of Histone H3 Lysine 9 Methylation in
- Successive Increases in the Resistance of to Viral Infection through a Transposon Insertion Followed by a Duplication
- Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Insights into Hox Protein Function from a Large Scale Combinatorial Analysis of Protein Domains
- Mutations Cause Seckel and Jawad Syndromes
- Zelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
- Temporal Coordination of Gene Networks by Zelda in the Early Embryo
- Genetic Interaction between MTMR2 and FIG4 Phospholipid Phosphatases Involved in Charcot-Marie-Tooth Neuropathies
- Oxr1 Is Essential for Protection against Oxidative Stress-Induced Neurodegeneration
- Transforming Growth Factor β Receptor Type 1 Is Essential for Female Reproductive Tract Integrity and Function
- Positional Cloning of a Type 2 Diabetes Quantitative Trait Locus; , a Negative Regulator of Insulin Secretion
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Genetic Determinants of Serum Testosterone Concentrations in Men
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



