-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRnf12—A Jack of All Trades in X Inactivation?
article has not abstract
Published in the journal: . PLoS Genet 7(1): e32767. doi:10.1371/journal.pgen.1002002
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002002Summary
article has not abstract
Placental mammals compensate the dosage imbalance of X-linked genes between males (XY) and females (XX) by silencing one randomly chosen X chromosome in females. This process is initiated during early embryonic development and can be recapitulated during differentiation of murine embryonic stem cells (mESCs). X chromosome inactivation (XCI) is initiated by up-regulation of a non-coding RNA on the future inactive X chromosome, named Xist, which lies within a large complex locus, called the X inactivation center (Xic). Subsequently, Xist RNA induces silencing of the entire chromosome in cis [1]. Although central to the XCI process, the molecular mechanisms underlying Xist's regulation still remain to be deciphered. In particular, it is unclear (1) how the up-regulation of Xist is triggered at the onset of differentiation, (2) why this is restricted to female cells, and (3) why one allele and not the other is affected? Although each aspect could in principle be controlled by distinct factors and sequence elements, one protein has recently been proposed to regulate Xist at all three levels: the E3 ubiquitin ligase Rnf12/Rlim [2]. The X-linked Rnf12 gene acts as a dose-dependent activator of Xist, which is expressed at elevated levels in female relative to male cells and is up-regulated during differentiation. Two recent studies shed further light on the precise role of Rnf12 in XCI [3], [4].
Developmental Regulation of Xist
Multiple stem cell–specific factors have been proposed to repress Xist in undifferentiated cells, and trigger its up-regulation when down-regulated during differentiation (Figure 1A and 1B, dark blue). The ability of some of these factors, e.g., Nanog, Oct3/4, and Sox2, to bind within the first intron of Xist led to the hypothesis that they could directly inhibit Xist expression [5]. However, the present study by Gribnau and colleagues reveals that deletion of this intronic site in mESCs is insufficient to activate Xist [3], thereby demonstrating the existence of additional mechanisms repressing Xist prior to differentiation. These could include repression by Tsix, Xist's repressive antisense transcript. Indeed, Tsix is also thought to be regulated by stem cell factors (Figure 1A) [6], [7]. However, abrogating Tsix transcription does not lead to Xist derepression prior to differentiation [8]. As neither the sole deletion of Tsix, nor of Xist intron 1, result in Xist up-regulation, they might be targeted by two independent redundant pathways. Alternatively, stem cell factors might affect Xist by controlling another Xist regulator, such as Rnf12 itself. Gribnau and colleagues now provide evidence that Rnf12 can trans-activate the Xist promoter independently of Tsix [3]. As Rnf12 is itself up-regulated during early mESC differentiation (Figure 1B, red line), stem cell factors might control the correct developmental expression of Xist by repressing its activator Rnf12 (Figure 1A). Their strong binding within the Rnf12 promoter would suggest a direct repression, but this remains to be explored [9].
Fig. 1. The X chromosome inactivation network. 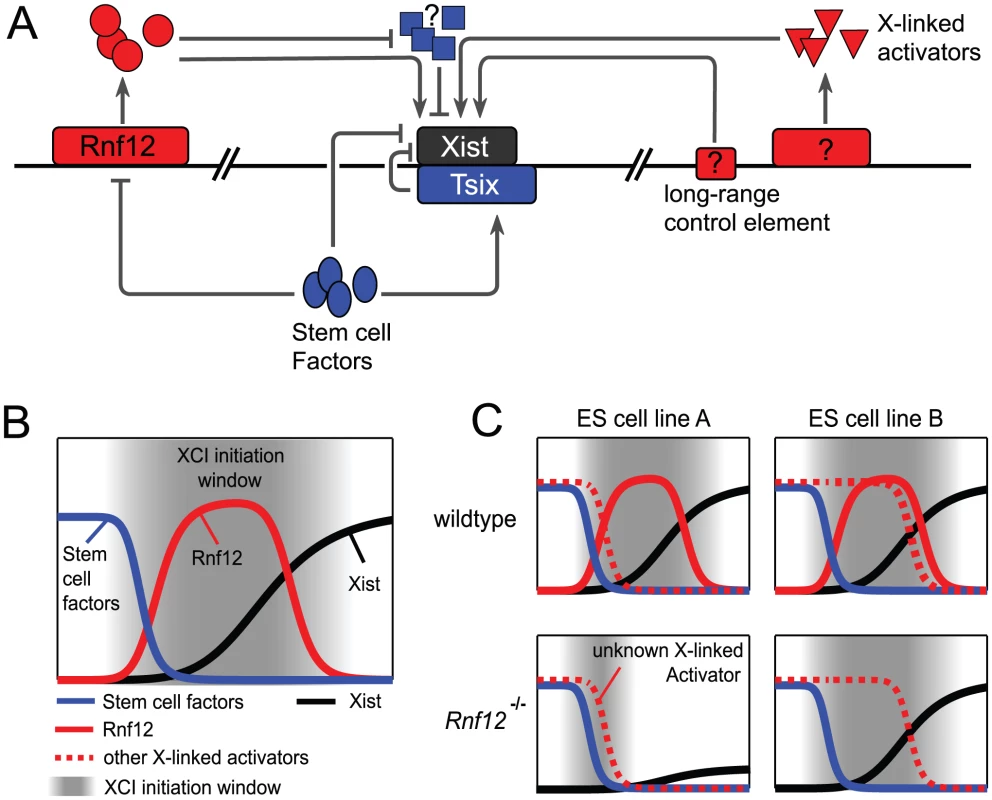
(A) Xist expression is controlled by counteracting activators (red) and repressors (blue). Stem cell factors (blue ovals) might repress Xist directly or indirectly via activating the repressive transcript Tsix or repressing the activator Rnf12. Rnf12 is the only known activator, and may function by targeting the Xist promoter directly and/or by inducing degradation of an unknown Xist repressor (blue squares). The existence of additional X-linked activators (red triangles) and long-range control elements such as Xpr, Xce, Xite, and others (red box) has been suggested [10]. (B) The time window when XCI can be initiated (grey) could be controlled by the down-regulation of Xist repressors such as stem cell factors (blue) and up-regulation of Xist activators like Rnf12 (red). (C) Different cell lines might require Rnf12 (ESC line B) or not (ESC line A), depending on the expression kinetics of other X-linked activators (dotted red line). Female-Specific Expression of Xist
The fact that XCI is only initiated in XX, but not XY cells, suggests that Xist up-regulation is controlled by an X-linked activator, which could be Rnf12. If a double dose of Rnf12 was the sole mechanism to ensure female-specific expression, then a cell heterozygous for an Rnf12 deletion should fail to initiate XCI. However, heterozygous deletion of Rnf12 delays, but does not prevent, random XCI in mESCs [2], [4], which points to the existence of additional X-linked activators of Xist (Figure 1). Nevertheless, in mice as well as in differentiating mESCs, XCI is skewed towards the mutated Rnf12 allele, suggesting either preferential up-regulation of Xist on the mutated allele, or a selective disadvantage of XX cells that have chosen to silence the wild-type allele, resulting in functional Rnf12 deficiency [2], [4]. Importantly, Rnf12−/Y mice are fully viable and fertile, implying that the counter-selection mentioned above may be due to an initial inability to induce XCI. The capacity of complete null Rnf12−/− ES cells to initiate random XCI was also investigated in the two studies, though the conclusions diverged. Bach and colleagues report similarly delayed kinetics as for Rnf12+/− cells [4], whereas the Gribnau lab observes almost complete abrogation of XCI [3]. What could be the reasons for this discrepancy? First, Gribnau and co-workers generated the deletion in vitro in mESCs, while Bach and colleagues derived their ES cells from Rnf12−/− embryos. In the latter case, ES cells could have adapted to or have been selected for compensation of the Rnf12 deletion. Second, ES cells can be subject to genetic or epigenetic differences, and in particular, female ES cells often lose one X chromosome and thus can survive differentiation without XCI. However, the ES cells in both studies were reported to have XX status in most cells. Alternatively, the two ES cell lines studied might carry polymorphisms in Xist cis-acting control elements, or differ in the levels or expression kinetics of trans-acting Xist regulators. For example, if an unknown X-linked activator (Figure 1C, dotted line) were to be down-regulated more quickly in one cell line (Figure 1C, left) than in the other (Figure 1C, right), XCI might occur only in the latter case. This raises the exciting possibility that comparison of the two lines might enable identification of these unknown activators.
Monoallelic Expression of Xist
Does Rnf12 participate in the mechanism that ensures that only one out of two X chromosomes up-regulates Xist? Rnf12 has been suggested to act as a negative feedback regulator to ensure monoallelic Xist up-regulation: its silencing on the inactive X might prevent Xist up-regulation on the second X chromosome [2]. While this hypothesis still remains to be investigated, monoallelic Xist expression is much better understood in the other, imprinted form of XCI in the mouse. In female pre-implantation embryos, the paternal X is inactivated initially, while the maternal X is prevented from XCI by a repressive maternal imprint on Xist [1]. This situation is maintained in extra-embryonic tissues, but reversed in cells giving rise to the embryo, where it is followed by random XCI. The maternally transmitted deletion of Rnf12, which results in a loss of the maternal Rnf12 pool in the zygote, leads to female-restricted embryonic lethality [4]. The Rnf12 maternal pool thus seems to be essential for triggering paternal XCI during early development. It should be noted, however, that imprinted XCI differs from random XCI in that it initially occurs independently of the number of X chromosomes in the cell and is controlled by a smaller genomic region. Consequently, additional cis-regulators must be involved in random XCI [10], as well as additional trans-activators [2].
The characterization of Rnf12 in XCI is clearly an important step towards a better understanding of Xist regulation. However, important pieces of the puzzle are still missing. How does Rnf12 activate Xist? As a ubiquitin ligase, does it induce degradation of a repressor of Xist? Is the Xist promoter the direct target of Rnf12, or are other Xic sequences also involved, such as Xce, Xpr, or others [10]? What are the missing X-linked activators that compensate for a heterozygous Rnf12 mutation in females? And more generally, at the very heart of X chromosome inactivation, why is only one and not both Xist alleles up-regulated during the random form of XCI? This could be due to stochastic activation of Xist, followed by cis-silencing of dose-dependent activators such as Rnf12. But even if this was the case, it still remains to be understood why Xist is up-regulated with such a low probability that it is initially triggered in a mono-allelic fashion. Whatever the answers, the recent work on Rnf12 has provided us with exciting new insights into the regulatory network acting on Xist.
Zdroje
1. ChowJHeardE 2009 X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol 21 359 366
2. JonkersIBarakatTSAchameEMMonkhorstKKenterA 2009 RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell 139 999 1011
3. BarakatTSGunhanlarNPardoCGAchameEMGhazviniM 2011 RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet 7 e1002001 doi:10.1371/journal.pgen.1002001
4. ShinJBossenzMChungYMaHByronM 2010 Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature 467 977 981
5. NavarroPChambersIKarwacki-NeisiusVChureauCMoreyC 2008 Molecular coupling of Xist regulation and pluripotency. Science 321 1693 1695
6. DonohoeMESilvaSSPinterSFXuNLeeJT 2009 The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 460 128 132
7. NavarroPOldfieldALegoupiJFestucciaNDuboisA 2010 Molecular coupling of Tsix regulation and pluripotency. Nature 468 457 460
8. LeeJTLuN 1999 Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99 47 57
9. MarsonALevineSSColeMFFramptonGMBrambrinkT 2008 Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134 521 533
10. NoraEPHeardE 2009 X chromosome inactivation: when dosage counts. Cell 139 865 867
Štítky
Genetika Reprodukční medicína
Článek Composite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTLČlánek Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in ProkaryotesČlánek Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple TraitsČlánek Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene ExpressionČlánek H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone DeacetylasesČlánek A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 1
-
Všechny články tohoto čísla
- A Meta-Analysis of Genome-Wide Association Scans Identifies IL18RAP, PTPN2, TAGAP, and PUS10 As Shared Risk Loci for Crohn's Disease and Celiac Disease
- Composite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTL
- Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in Prokaryotes
- Genome-Wide Association Study SNPs in the Human Genome Diversity Project Populations: Does Selection Affect Unlinked SNPs with Shared Trait Associations?
- Friedreich's Ataxia (GAA)•(TTC) Repeats Strongly Stimulate Mitotic Crossovers in
- Zebrafish Mutation Leads to mRNA Splicing Defect and Pituitary Lineage Expansion
- Histone H4 Lysine 12 Acetylation Regulates Telomeric Heterochromatin Plasticity in
- Bub1-Mediated Adaptation of the Spindle Checkpoint
- Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple Traits
- Signaling Role of Fructose Mediated by FINS1/FBP in
- RNF12 Activates and Is Essential for X Chromosome Inactivation
- Comparative Study between Transcriptionally- and Translationally-Acting Adenine Riboswitches Reveals Key Differences in Riboswitch Regulatory Mechanisms
- Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene Expression
- Application of a New Method for GWAS in a Related Case/Control Sample with Known Pedigree Structure: Identification of New Loci for Nephrolithiasis
- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
- Transcription Initiation Patterns Indicate Divergent Strategies for Gene Regulation at the Chromatin Level
- The Transposon-Like Correia Elements Encode Numerous Strong Promoters and Provide a Potential New Mechanism for Phase Variation in the Meningococcus
- Proteins Encoded in Genomic Regions Associated with Immune-Mediated Disease Physically Interact and Suggest Underlying Biology
- A Novel RNA-Recognition-Motif Protein Is Required for Premeiotic G/S-Phase Transition in Rice ( L.)
- The Mucin-Like Protein OSM-8 Negatively Regulates Osmosensitive Physiology Via the Transmembrane Protein PTR-23
- Genome Sequencing and Comparative Transcriptomics of the Model Entomopathogenic Fungi and
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Quaking Regulates Expression through Its 3′ UTR in Oligodendrocyte Precursor Cells
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



