-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaComposite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTL
Many agriculturally, evolutionarily, and medically important characters vary in a quantitative fashion. Unfortunately, the genes and sequence variants accounting for this variation remain largely unknown due to a variety of biological and technical challenges. Drosophila melanogaster contains high levels of sequence variation and low linkage disequilibrium, allowing us to dissect the effects of many causative variants within a single locus. Here, we take advantage of these features to identify and characterize the sequence polymorphisms that comprise major effect QTL alleles segregating at the bric-a-brac locus. We show that natural bric-a-brac alleles with large effects on cuticular pigmentation reflect a cumulative impact of polymorphisms that affect three functional regions: a promoter, a tissue-specific enhancer, and a Polycomb response element. Analysis of allele-specific expression at the bric-a-brac locus confirms that these polymorphisms modulate transcription at the cis-regulatory level. Our results establish that a single QTL can act through a confluence of multiple molecular mechanisms and that sequence variation in regions flanking experimentally validated functional elements can have significant quantitative effects on transcriptional activity and phenotype. These findings have important design and conceptual implications for basic and medical genomics.
Published in the journal: . PLoS Genet 7(1): e32767. doi:10.1371/journal.pgen.1001275
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001275Summary
Many agriculturally, evolutionarily, and medically important characters vary in a quantitative fashion. Unfortunately, the genes and sequence variants accounting for this variation remain largely unknown due to a variety of biological and technical challenges. Drosophila melanogaster contains high levels of sequence variation and low linkage disequilibrium, allowing us to dissect the effects of many causative variants within a single locus. Here, we take advantage of these features to identify and characterize the sequence polymorphisms that comprise major effect QTL alleles segregating at the bric-a-brac locus. We show that natural bric-a-brac alleles with large effects on cuticular pigmentation reflect a cumulative impact of polymorphisms that affect three functional regions: a promoter, a tissue-specific enhancer, and a Polycomb response element. Analysis of allele-specific expression at the bric-a-brac locus confirms that these polymorphisms modulate transcription at the cis-regulatory level. Our results establish that a single QTL can act through a confluence of multiple molecular mechanisms and that sequence variation in regions flanking experimentally validated functional elements can have significant quantitative effects on transcriptional activity and phenotype. These findings have important design and conceptual implications for basic and medical genomics.
Introduction
Quantitative trait loci (QTLs) have traditionally been identified by linkage analysis in experimental crosses [1]. In a complementary approach, DNA polymorphisms causing trait variation can be inferred from statistical associations between sequence and phenotype in large panels of natural genotypes [2]. These methods have been advanced with creative applications [3], [4], but in most cases QTL regions have only been narrowed to a single gene. Understanding the identity, distribution, and functional nature of nucleotide polymorphisms that make up QTL alleles remains a critical challenge in molecular and evolutionary genetics. In this report, we take a QTL study to its logical conclusion by describing the fine structure of functional variation that underlies large-effect QTL alleles segregating in a natural population.
To meet this aim, we sequenced the 148 kb bric a brac (bab) genomic region from 94 inbred lines of D. melanogaster sampled from a single location. The bab locus is composed of two anciently duplicated paralogs, bab1 and bab2, which are involved in patterning the adult abdomen, legs, and ovaries [5]. In particular, Bab expression in the pupal abdominal epidermis is responsible for the sex-specific pigmentation of adult cuticle, and evolutionary changes in bab regulation have played a central role in the macroevolutionary diversification of Drosophila color patterns [6]–[8]. Moreover, segregation of bab alleles is responsible for ∼60% of the genetic variation in female abdominal pigmentation within a natural population of D. melanogaster [9] (Figure 1). Linkage disequilibrium (LD) at the bab locus extends for less than 1 kb, [10] providing an opportunity to dissect the causative nucleotide variation that contributes to the cumulative effect of QTL alleles. Previous analysis revealed evidence of directional selection in two regions within the bab locus, including the previously identified cis-regulatory element (CRE) that controls sex-specific abdominal pigmentation [8], [10]. This confluence of population - and developmental-genetic evidence sets the stage for connecting specific DNA polymorphisms to variation in gene expression and, ultimately, in phenotypic traits. We have sequenced the bab locus from 96 D. melanogaster lines to identify the genetic variation responsible for pigmentation differences.
Fig. 1. Three representative females (lines W3-123, W3-112, and W3-135) showing the range of pigmentation variation. 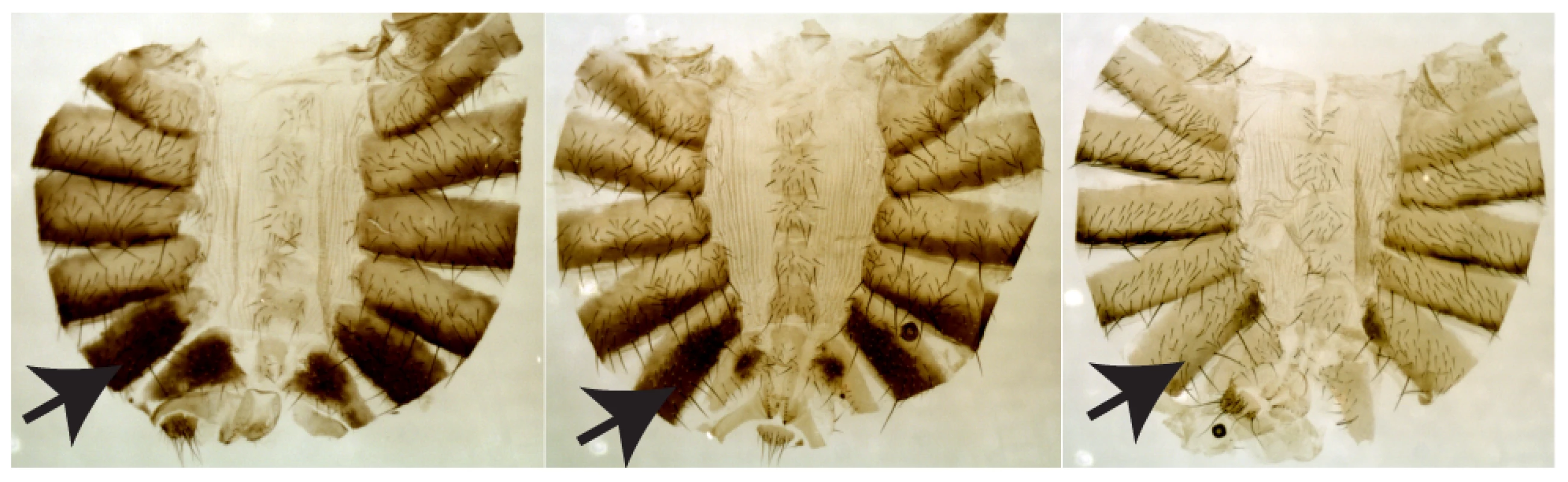
The arrow indicates A6, the segment analyzed. Results/Discussion
Causative polymorphisms cluster in three functional regions
We identified 6766 sequence variants, including 5566 single nucleotide polymorphisms (SNPs) and 1211 insertion/deletion (indel) polymorphisms, in the bab region. After removing polymorphisms with frequencies below 5%, which are difficult to associate with phenotypic variation, 3821 polymorphism (SNPs and indels) remained. We tested each polymorphism individually for an association with female pigmentation, and identified 266 polymorphisms that showed significant association at the false discovery rate (FDR) <0.4 (p<.033) (Spearman rank correlation, qvalue correction) [11] (Figure 2A). All associated polymorphisms are located in non-coding DNA.
Fig. 2. Association mapping of female A6 pigmentation at the bab locus. 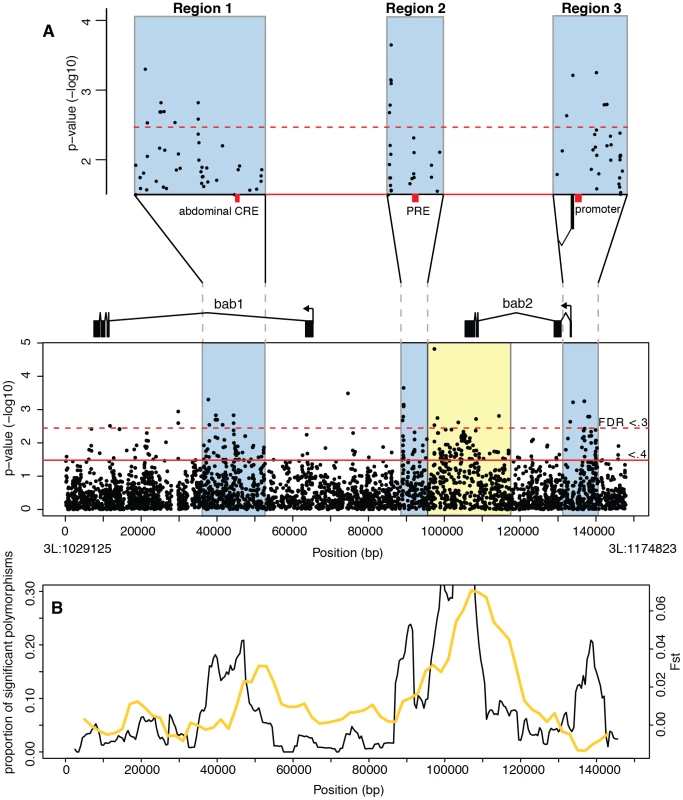
(A) P-values from a Spearman rank correlation for each polymorphism identified in the bab locus. The dotted and solid red lines indicate FDR values of 0.3 and 0.4 respectively. 3 functional regions containing most associated polymorphisms (shaded blue rectangles) are shown in greater detail above. The sex-specific abdominal CRE (in region 1), Polycomb response element (region 2), and the bab2 promoter (region 3) are indicated by red bars. The yellow region contains an excess of linked polymorphisms, but is attributed to cryptic structure in our samples. (B) Black line shows the proportion of all polymorphisms in 5 kb sliding windows that are significantly associated with variation in pigmentation (FDR <.4), plotted against the midpoint of the window. Overlayed in yellow is Fst between samples collected in separate years (see text for details). To determine whether the significant polymorphisms were randomly distributed across the locus or clustered into functional regions, we looked at the proportion of associated polymorphisms in 5 kb sliding windows (Figure 2B) (see Materials and Methods for details). We found that most associated polymorphisms are located in three clusters: a 15 kb region in the first intron of bab1, an 8 kb region between the bab1 and bab2 coding regions, and a 9.5 kb region near the transcription start site of bab2.
These clusters of significantly associated points could be the result of a high number of causative polymorphisms or alternatively a single causative polymorphism with high levels of LD resulting in a cluster of associated polymorphisms. We examined the pairwise levels of LD for significant polymorphisms within each region (see Figure S1). Based on the LD pattern, we conservatively estimate that there are at least 28 associated polymorphisms, or groups of polymorphisms, assorting independently in region 1, 8 polymorphisms in region 2 and 14 polymorphisms in region 3. Thus, there are multiple polymorphisms within each region that are likely to be causative variation.
The first region resides within the large first intron of bab1 and contains the cis-regulatory element that drives expression in the epidermis of the last three abdominal segments in females [8] (red bar in the blowup of region 1 in Figure 2A). This enhancer, which is the only identified sex-specific enhancer in the bab locus, is thought to control the sex-specific expression of both bab1 and bab2 [8]. This enhancer contains functionally characterized binding sites for the Abdominal-B (Abd-B) and doublesex (dsx) transcription factors [8]. None of the significantly associated polymorphisms affect these binding sites, and indeed there are no common polymorphisms in the 624 bp core enhancer. However, the variation surrounding this location is strongly associated with pigmentation variation (Figure 2A, 2B). Macroevolutionary changes in bab expression are largely due to sequence changes within the core enhancer but outside of the Abd-B and Dsx binding sites, indicating that CREs present large targets for functionally relevant mutations [8]. Our results reveal an even larger mutational target by demonstrating that sequence variation surrounding the core CRE can result in significant phenotypic variation without disrupting the primary function of the enhancer. This region also shows an excess of high-frequency derived alleles [10], suggesting that genetic variation in the region flanking the sex-specific abdominal CRE has been shaped by directional selection.
The second identified region is located in the intergenic region between bab1 and bab2 and contains a predicted and experimentally verified Polycomb response element (PRE) [12]–[14] (red bar in the blowup of region 2, Figure 2A). PREs regulate transcriptional activity over multiple cell generations by affecting chromatin state. As in the CRE region, the distribution of significantly associated polymorphisms is considerably wider than the functionally characterized PRE, with most effects located outside of the core region. These results suggest that PREs, as well as tissue-specific enhancers, present a large target for functionally and evolutionarily important mutations.
The third region showing a high concentration of significantly associated polymorphisms spans the promoter and transcription start site of bab2 (red bar in the blowup of region 3, Figure 2A). While promoters have been traditionally thought of as passive players in transcription, recent research suggests that promoters are often active in transcriptional regulation [15]. Several studies have shown that polymerases remain stalled on developmentally important genes, poised to begin transcription [16], [17]. This pattern is observed on the bab2 promoter during embryonic development and in S2 cells [18], while the pupal stages are yet to be investigated. Our results suggest that not only are promoter regions involved in transcriptional regulation, but that sequence variation in these regions can have a significant impact on gene expression and phenotype. It is intriguing that no similar concentration of associated SNPs is observed around the bab1 promoter (Figure 2A, 2B). We believe that the explanation for this discrepancy may lie in the differences between the transcriptional activity of bab1 and bab2 at the pupal stage (see below).
In addition to these three regions, there appears to be an excess of associated polymorphisms near the downstream exons of bab2. However, this observation can be attributed to cryptic population structure. The lines that comprise our sample were collected in two different years. The region of highly significant Fst between the two collections [10] coincides with the region of apparent association near the 3′ exons of bab2 (Figure 2B). When the samples from different years are examined separately, no association is observed in this region (Figure 3A), suggesting that it contains no causative polymorphisms.
Fig. 3. Association mapping of adult pigmentation and transcriptional variation in the subset of lines used to measure bab expression. 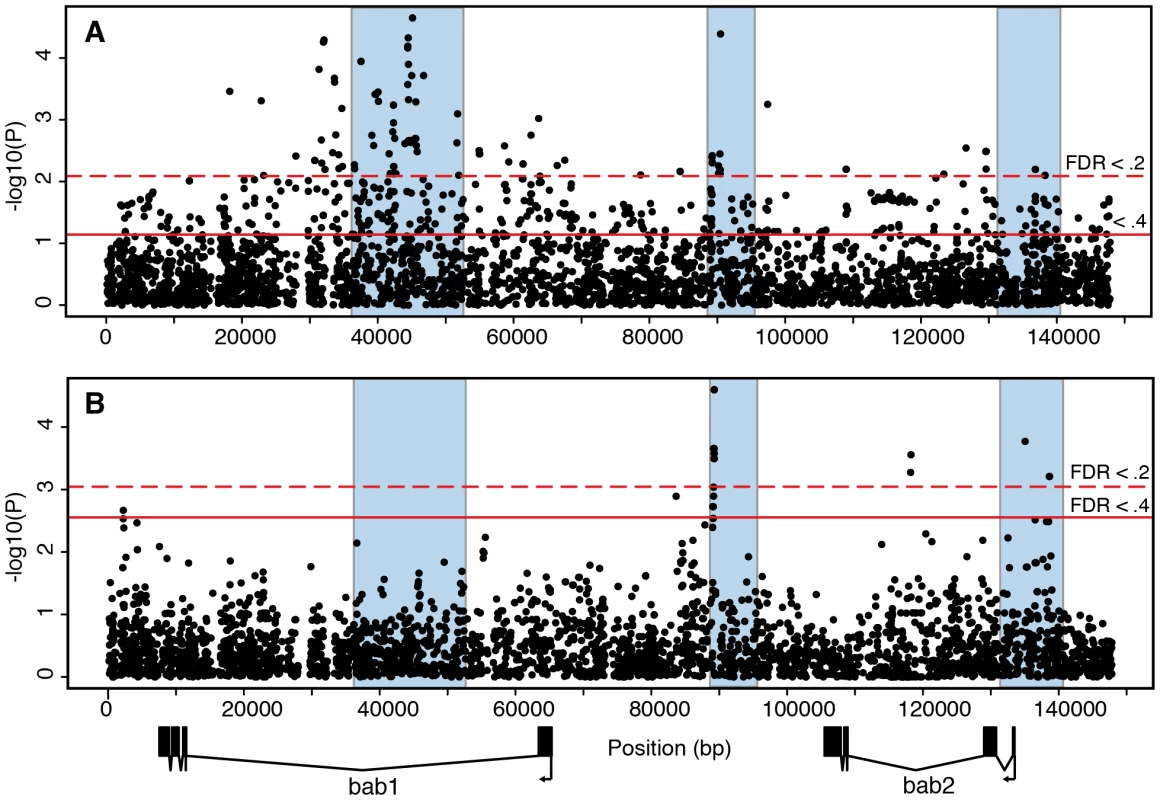
(A) Association between polymorphisms and pigmentation. (B) Association between polymorphisms and bab2 transcript levels. The p-values are from a Spearman rank correlation test for all polymorphisms. Regions shaded in blue are the same as in Figure 2. The existence of females with completely pigmented sixths abdominal segment distinguishes D. melanogaster from its close relatives D. simulans, D. mauritiana, and D. yakuba. To test whether derived SNP alleles are associated with darker phenotypes, we polarized DNA polymorphisms in the bab region using the genome of D. simulans. The 107 SNPs associated with phenotypic variation at FDR <.2 (Figure 3A) show a marginal bias towards derived alleles having a darker phenotype (53 of 76, 2-tailed Fisher's exact test P = 0.034; 31 polymorphisms not polarized).
Causative polymorphisms have small individual effect sizes
The effect size of each of these associated polymorphisms is small, but within each region, these polymorphisms form haplotypes that have a large effect on pigmentation. To examine these effects, we assigned each individual a haplotype score, which indicates the relative number of dark and light alleles they contain at the significant polymorphisms (see Material and Methods for details). A negative haplotype score indicates more light alleles than dark alleles, a positive score more dark than light and a score of zero indicates an equal number of dark and light alleles. There is a strong correlation between the haplotype score and pigmentation for all three regions (Figure 4) (p<.001).
Fig. 4. Pigmentation and the haplotype of associated polymorphisms. 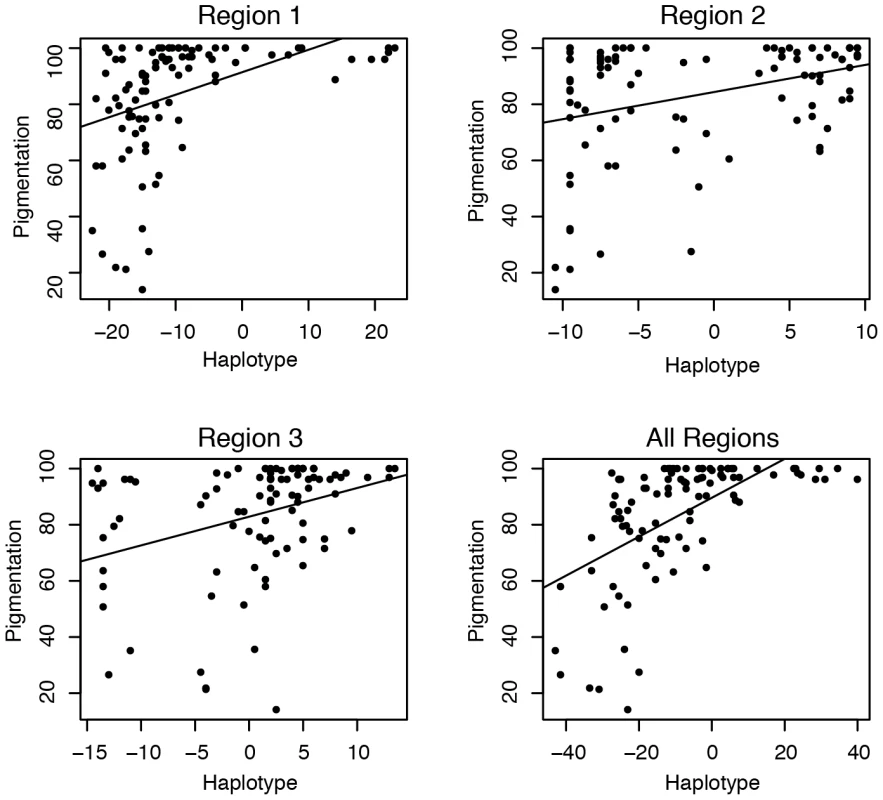
The haplotype represents the relative number of dark and light alleles at the associated polymorphisms within the region. Individuals with negative scores have more light alleles than dark alleles and positive scores have more dark alleles than light alleles (see Materials and Methods for details). The line represents the best fit of the pigmentation as a function of haplotype score. Each of the three regions is displayed individually and all three together. Within both regions 1 and 2 each polymorphism increases pigmentation by about 1% percent of the total variation (see Table 1). Some of these associated polymorphisms are likely not causative due to LD and false positives, but the effect size of each polymorphism is still small.
Tab. 1. Estimation of polymorphism and region effect size. 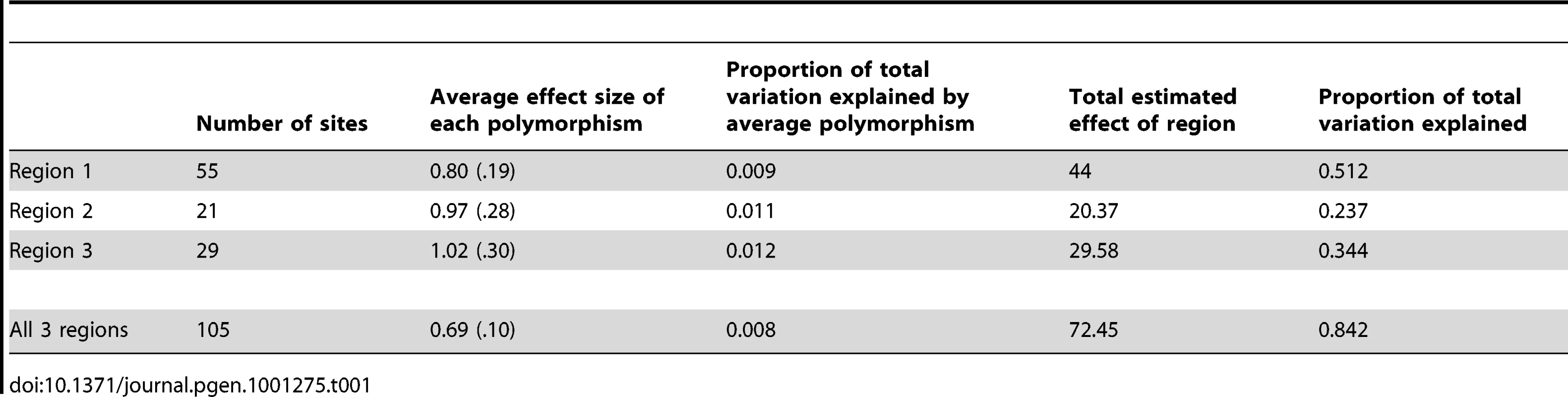
Despite each polymorphism only having a small effect on phenotype, together the polymorphisms result in haplotypes with large potential effects. Each region accounts for between 20% and 50% of the pigmentation variation. When we perform this analysis using haplotypes from all 3 regions, a similar pattern emerges; there is a small effect of each individual polymorphism, but a large overall effect (Table 1) (Figure 4). Together these three regions explain 84% of the pigmentation variation among the studied genotypes. The sum of regional effects appears to be greater than the total effect of polymorphisms in all three regions because our sample had a number of lines with completely pigmented A6 segments. Since pigmentation score cannot be higher than 100, such lines are saturated with dark alleles. In those haplotypes, each individual dark allele appears to have a smaller effect size.
Polymorphisms act in cis to influence transcript abundance
The location of associated polymorphisms in three non-coding regions suggests that QTL effects are due to differences in transcriptional regulation rather than protein function. Variation in gene expression has become a recent frontier in molecular and evolutionary genetics [19]. Local (cis-acting) regulatory variation may consist of expression quantitative trait nucleotides (eQTNs) in sequences responsible for controlling transcription. In yeast, for instance, transcriptional variation is frequently associated with eQTNs located close to promoter regions [20]. Other possible mechanisms include variation in splicing efficiency [21] and conformation [22]. A natural allele can incorporate multiple eQTNs with a complex interplay of these mechanisms [23], and cis-acting variants can also interact with distant (trans) regulatory factors in complex ways [24].
We examined cis-acting transcriptional variation in bab1 and bab2 directly using allele specific expression (ASE) [25], [26]. We selected 51 of our lines that were all collected in a single year. Analysis of association between sequence variation and pigmentation in this sub-panel reveals the concentration of significant polymorphisms in the same three non-coding regions (Figure 3A). We crossed each of these lines to two tester strains that contained low frequency SNPs in the coding regions of bab1 and bab2, allowing us to differentiate the two alleles. The use of two different tester strains allowed us to identify consistent cis effects and minimize complex interactions between cis and trans factors. Heterozygous female abdomens were collected at 48–56 hours after pupariation, when the bab genes are expressed in the abdominal epidermis[6]. In these heterozygotes, we measured the relative transcript levels of the bab1 and bab2 alleles from the line of interest and the tester strain, and corrected for differences in the tester strains (see Materials and Methods for details). In this experimental design, any differences in expression between the two parental alleles are due to cis-acting polymorphisms, and all 51 lines can be compared directly since they are measured relative to the same testers.
We used these transcription measurements to identify polymorphisms affecting transcription at the bab locus. We found a number of SNPs that are associated with variation in bab2 transcript abundance (Figure 3B). Of the three previously identified regions affecting pigmentation, the regions flanking the PRE and the bab2 transcription start site contain polymorphisms with the strongest effects on bab2 transcript levels (p<.001; FDR <.2). This overlap suggests that at least some of the variation in sex-specific pigmentation is the result of differential regulation of bab2 in different QTL alleles. We did not detect any effect of the region straddling the sexually dimorphic abdominal CRE (region 1 in Figure 2A) on transcript abundance. This may be due to the fact that, in contrast to the PRE and the promoter, this CRE is active only in the epidermis of the last three abdominal segments, whereas the RNA samples used in the analysis of transcriptional variation comprised multiple other tissues where the bab genes are expressed, including the anterior abdominal segments, the ovaries, the oenocytes, muscles, and the genitalia. Alternatively, sequence variation in this CRE may influence transcription during a time period that was not sampled in our experiments.
Although both bab1 and bab2 are expressed in the abdominal epidermis and contribute to the patterning of pigmentation [6], [8], no sequence variants at the bab locus affect bab1 transcript levels (FDR >.5) (data not shown). Chromatin immunoprecipitation data show extensive binding of RNA polymerase II (polII) to the region surrounding the transcription start site of bab2, but not bab1, at the pupal stage [27]. In contrast, both promoter regions show similar polII binding during embryonic stages. Thus, it appears that sequence variation at the bab locus affects adult pigmentation primarily by modulating the transcription of bab2. It is possible, of course, that bab1 also contributes to phenotypic variation but at a different developmental stage that falls outside of the sampled period. Since pigmentation pattern is determined in a specific cell type at precise times during development [5], [6], [28], a more detailed understanding of how sequence variation affects transcription and translates into phenotypic differences will require quantitative analysis of gene expression with greater spatial and temporal resolution.
Previous research has identified two direct transcriptional regulators of bab, Abd-B and dsx [8]. Although the CREs responsible for bab expression in the abdominal epidermis are well defined [8], it is conceivable that additional regions modulate bab transcription in a redundant manner [29], [30]. A search of the entire bab locus reveals 1412 potential Abd-B core binding sites (TTTAY) and 73 potential Dsx binding sites (ACWAWGT), which is higher than expected based on sequence composition (485 and 47 sites, respectively). However, none of the associated polymorphisms were found in potential Dsx binding sites, and only a few in potential Abd-B binding sites, in any of our analyses. This observation confirms that most variation in transcript levels and adult phenotype is due to sequence variation outside of known transcription factor binding sites.
Understanding the causative variation at the bab locus
The bab locus was identified as a large effect QTL responsible for most of the natural variation in female abdominal pigmentation [9]. By investigating the function of bab at two different levels, we were able to elucidate the fine structure of QTL alleles and characterize DNA sequence variation that affects transcription and phenotypic differences. Three major lessons emerge from this analysis. First, large-effect QTL alleles can reflect the cumulative effects of many nucleotide polymorphisms. This finding is in contrast to several cases where large QTLs are due to single coding or regulatory mutations with major phenotypic effects [31]–[33]. On the other hand, detailed analysis of a single gene responsible for 100% of a phenotypic difference between two Drosophila species revealed contributions from several independent regulatory regions [34]. Within Drosophila melanogaster, multiple polymorphisms in a single cis regulatory element of the ebony gene contribute to pigmentation differences between populations [35]. In addition many studies have shown the contribution of multiple polymorphisms to variation in protein function (e.g. [36]–[39]). These results suggest that accumulation of multiple mutations at the same locus may be a relatively common mechanism of micro - and macroevolutionary change.
Second, the causative polymorphisms are not distributed randomly throughout the locus, but are located in multiple, distinct functional regions. In the case of bab, the vast majority of polymorphisms associated with transcriptional and phenotypic variation are concentrated around three regions: the core promoter, tissue-specific enhancer, and Polycomb response element – the elements expected to contribute the most to transcriptional regulation. Thus, functional annotation of the human and model system genomes, such as that generated by the ENCODE and modENCODE projects [27], [40], may go some way towards predicting the loci of evolutionary variation. Conversely, quantitative-genetic analyses of natural variation may help identify and annotate functional genomic regions.
The third major lesson is that the functional elements present larger targets than previously thought for phenotypically and evolutionarily important mutations. The regions detected in our analysis are much larger than the core elements defined previously by functional assays. Over 90% of significant polymorphisms are found around, but not precisely inside, the core CRE, PRE, and promoter. This pattern indicates that DNA sequence variation in regions surrounding the core elements has major phenotypic consequences. These sequence variants may act by modulating the regulatory activity of the functional elements, perhaps through subtle changes in chromatin structure or transcription factor recruitment, without disrupting their primary functions. As a result, important evolutionary changes may occur through mutations in DNA sequences that lack readily identifiable molecular functions. The rapidly growing body of genome-wide association studies will put this proposition to the test. However, the final lesson from the Drosophila bab locus is that fine-scale dissection of functional variation is only possible in genes with little linkage disequilibrium, underscoring the importance of model systems in framing the analysis of human genetic variation.
Materials and Methods
Lines selection and sequencing
96 inbred lines of D. melanogaster were chosen for sequencing the bab locus. 86 lines were chosen at random from the W1 and W3 inbred line collections maintained in the Nuzhdin lab. Progenitors of these lines were originally collected from Wolfskill Orchard in Winters, CA, and inbred by full sib mating for a minimum of 20 generations. 10 additional lines with low pigmentation scores were selected, including 8 lines from Winters and 2 from North Carolina. Sequencing was performed as described [10].
Pigmentation phenotyping and association mapping
3–5 day old females were scored for pigmentation as described [9]. All statistical tests were preformed using the R Statistical package (http://www.r-project.org/). Spearman's correlation test and associated p-values were calculated using “cor.test” command in the base Stats package for each polymorphism individually. False Discovery rates were calculated using the q-value package.
Identification of enriched regions and estimating effects size
To identify regions with an excess of associated points, we scanned the bab locus in 5 kb sliding windows, shifting the window in 500 bp increments. Regions where more than 0.12 of polymorphisms were significant were tentatively identified as enriched regions. We manually excluded the region that contained the cryptic population structure and then manually manipulated each remaining region to only include the significant points (i.e. we narrowed the region if the high score was the result of all of the polymorphisms being clustered on one side of the window).
To understand the effect size for each region, we created a region haplotype score for each individual. This haplotype score is the difference between the number of dark alleles and light alleles for significant polymorphisms divided by 2. ((Number of dark alleles – number of light alleles)/2). Thus an increase in the haplotype score by one indicates a change of a single light allele to a dark allele. A score of zero would indicate an equal number of dark and light alleles for that region. This formula was necessary because not all individuals had a genotype assigned for all polymorphism.
We then fit a linear model with the formula: pigmentation score = haplotype. The coefficient of the haplotype term is the average effect of each allele on the pigmentation score. To calculate the average effect size of the entire region we multiplied the average effect of each allele by the number of associated polymorphisms in that region.
Quantification of bab transcripts
We measured allele-specific expression of both bab1 and bab2 in 51 lines out of the 96 used for pigmentation mapping. Two lines that carried rare SNPs in the coding regions of bab1 and bab2 were chosen as the tester strains. Each of these tester strains was crossed to the 51 experimental lines in several replicates, creating F1 genotypes that were heterozygous for the tester and experimental alleles.
We collected 48–56 hour whole pupal abdomens from the female F1s. Individuals from replicate crosses were combined into pools of 10, and we attempted to obtain at least 2 pools from each cross. From each of these samples, a 218 bp fragment of bab1 and a 142 bp fragment of bab2, spanning the SNPs of interest, were amplified. We used Illumina high throughput sequencing of barcoded samples to measure the relative amounts of each allele as described [26]. To normalize the samples from the two tester lines, we took the residuals from a fixed cofactor ANOVA. We then mapped the expression variation across the bab region using the same procedures as for the adult phenotype.
Binding site predictions were made using custom R and Perl scripts for the sequences ACWAWGT (Dsx) and TTTAY (Abd-B).
Supporting Information
Zdroje
1. MackayTF
2001
Quantitative trait loci in Drosophila.
Nat Rev Genet
2
11
20
2. ReichDE
LanderES
2001
On the allelic spectrum of human disease.
Trends Genet
17
502
510
3. GenisselA
PastinenT
DowellA
MackayTF
LongAD
2004
No evidence for an association between common nonsynonymous polymorphisms in delta and bristle number variation in natural and laboratory populations of Drosophila melanogaster.
Genetics
166
291
306
4. De LucaM
RoshinaNV
Geiger-ThornsberryGL
LymanRF
PasyukovaEG
2003
Dopa decarboxylase (Ddc) affects variation in Drosophila longevity.
Nat Genet
34
429
433
5. CoudercJL
GodtD
ZollmanS
ChenJ
LiM
2002
The bric a brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila.
Development
129
2419
2433
6. KoppA
DuncanI
CarrollSB
2000
Genetic control and evolution of sexually dimorphic characters in Drosophila.
Nature
408
553
559
7. GompelN
CarrollSB
2003
Genetic mechanisms and constraints governing the evolution of correlated traits in drosophilid flies.
Nature
424
931
935
8. WilliamsTM
SelegueJE
WernerT
GompelN
KoppA
2008
The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila.
Cell
134
610
623
9. KoppA
GrazeRM
XuSZ
CarrollSB
NuzhdinSV
2003
Quantitative trait loci responsible for variation in sexually dimorphic traits in Drosophila melanogaster.
Genetics
163
771
787
10. BickelRD
SchackwitzWS
PennacchioLA
NuzhdinSV
KoppA
2009
Contrasting patterns of sequence evolution at the functionally redundant bric a brac paralogs in Drosophila melanogaster.
J Mol Evol
69
194
202
11. StoreyJD
TibshiraniR
2003
Statistical significance for genomewide studies.
Proc Natl Acad Sci U S A
100
9440
9445
12. RingroseL
RehmsmeierM
DuraJM
ParoR
2003
Genome-wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster.
Dev Cell
5
759
771
13. SchwartzYB
KahnTG
NixDA
LiXY
BourgonR
2006
Genome-wide analysis of Polycomb targets in Drosophila melanogaster.
Nat Genet
38
700
705
14. KwongC
AdryanB
BellI
MeadowsL
RussellS
2008
Stability and dynamics of polycomb target sites in Drosophila development.
PLoS Genet
4
e1000178
doi:10.1371/journal.pgen.1000178
15. GoodrichJA
TjianR
2010
Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation.
Nat Rev Genet
11
549
558
16. WuJQ
SnyderM
2008
RNA polymerase II stalling: loading at the start prepares genes for a sprint.
Genome Biol
9
220
17. ZeitlingerJ
StarkA
KellisM
HongJW
NechaevS
2007
RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo.
Nat Genet
39
1512
1516
18. MuseGW
GilchristDA
NechaevS
ShahR
ParkerJS
2007
RNA polymerase is poised for activation across the genome.
Nat Genet
39
1507
1511
19. SkellyDA
RonaldJ
AkeyJM
2009
Inherited variation in gene expression.
Annu Rev Genomics Hum Genet
10
313
332
20. RonaldJ
BremRB
WhittleJ
KruglyakL
2005
Local regulatory variation in Saccharomyces cerevisiae.
PLoS Genet
1
e25
doi:10.1371/journal.pgen.0010025
21. Telonis-ScottM
KoppA
WayneML
NuzhdinSV
McIntyreLM
2009
Sex-specific splicing in Drosophila: widespread occurrence, tissue specificity and evolutionary conservation.
Genetics
181
421
434
22. DuanS
HuangRS
ZhangW
BleibelWK
RoeCA
2008
Genetic architecture of transcript-level variation in humans.
Am J Hum Genet
82
1101
1113
23. StamLF
LaurieCC
1996
Molecular dissection of a major gene effect on a quantitative trait: the level of alcohol dehydrogenase expression in Drosophila melanogaster.
Genetics
144
1559
1564
24. WangHY
FuY
McPeekMS
LuX
NuzhdinS
2008
Complex genetic interactions underlying expression differences between Drosophila races: analysis of chromosome substitutions.
Proc Natl Acad Sci U S A
105
6362
6367
25. WittkoppPJ
HaerumBK
ClarkAG
2004
Evolutionary changes in cis and trans gene regulation.
Nature
430
85
88
26. MainBJ
BickelRD
McIntyreLM
GrazeRM
CalabresePP
2009
Allele-specific expression assays using Solexa.
BMC Genomics
10
422
27. CelnikerSE
DillonLA
GersteinMB
GunsalusKC
HenikoffS
2009
Unlocking the secrets of the genome.
Nature
459
927
930
28. WittkoppPJ
CarrollSB
KoppA
2003
Evolution in black and white: genetic control of pigment patterns in Drosophila.
Trends Genet
19
495
504
29. FrankelN
DavisGK
VargasD
WangS
PayreF
2010
Phenotypic robustness conferred by apparently redundant transcriptional enhancers.
Nature
30. HongJW
HendrixDA
LevineMS
2008
Shadow enhancers as a source of evolutionary novelty.
Science
321
1314
31. HoekstraHE
HirschmannRJ
BundeyRA
InselPA
CrosslandJP
2006
A single amino acid mutation contributes to adaptive beach mouse color pattern.
Science
313
101
104
32. ProtasME
HerseyC
KochanekD
ZhouY
WilkensH
2006
Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism.
Nat Genet
38
107
111
33. ChanYF
MarksME
JonesFC
VillarrealGJr
ShapiroMD
2010
Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer.
Science
327
302
305
34. McGregorAP
OrgogozoV
DelonI
ZanetJ
SrinivasanDG
2007
Morphological evolution through multiple cis-regulatory mutations at a single gene.
Nature
448
587
590
35. RebeizM
PoolJE
KassnerVA
AquadroCF
CarrollSB
2009
Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population.
Science
326
1663
1667
36. BabbittCC
SilvermanJS
HaygoodR
ReiningaJM
RockmanMV
2010
Multiple Functional Variants in cis Modulate PDYN Expression.
Mol Biol Evol
27
465
479
37. HoranM
MillarDS
HedderichJ
LewisG
NewswayV
2003
Human growth hormone 1 (GH1) gene expression: complex haplotype-dependent influence of polymorphic variation in the proximal promoter and locus control region.
Hum Mutat
21
408
423
38. SunC
SouthardC
WitonskyDB
OlopadeOI
Di RienzoA
2010
Allelic imbalance (AI) identifies novel tissue-specific cis-regulatory variation for human UGT2B15.
Hum Mutat
31
99
107
39. TishkoffSA
ReedFA
RanciaroA
VoightBF
BabbittCC
2007
Convergent adaptation of human lactase persistence in Africa and Europe.
Nature Genetics
39
31
40
40. BirneyE
StamatoyannopoulosJA
DuttaA
GuigoR
GingerasTR
2007
Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project.
Nature
447
799
816
Štítky
Genetika Reprodukční medicína
Článek Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in ProkaryotesČlánek Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple TraitsČlánek Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene ExpressionČlánek H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone DeacetylasesČlánek A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 1
-
Všechny články tohoto čísla
- A Meta-Analysis of Genome-Wide Association Scans Identifies IL18RAP, PTPN2, TAGAP, and PUS10 As Shared Risk Loci for Crohn's Disease and Celiac Disease
- Composite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTL
- Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in Prokaryotes
- Genome-Wide Association Study SNPs in the Human Genome Diversity Project Populations: Does Selection Affect Unlinked SNPs with Shared Trait Associations?
- Friedreich's Ataxia (GAA)•(TTC) Repeats Strongly Stimulate Mitotic Crossovers in
- Zebrafish Mutation Leads to mRNA Splicing Defect and Pituitary Lineage Expansion
- Histone H4 Lysine 12 Acetylation Regulates Telomeric Heterochromatin Plasticity in
- Bub1-Mediated Adaptation of the Spindle Checkpoint
- Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple Traits
- Signaling Role of Fructose Mediated by FINS1/FBP in
- RNF12 Activates and Is Essential for X Chromosome Inactivation
- Comparative Study between Transcriptionally- and Translationally-Acting Adenine Riboswitches Reveals Key Differences in Riboswitch Regulatory Mechanisms
- Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene Expression
- Application of a New Method for GWAS in a Related Case/Control Sample with Known Pedigree Structure: Identification of New Loci for Nephrolithiasis
- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
- Transcription Initiation Patterns Indicate Divergent Strategies for Gene Regulation at the Chromatin Level
- The Transposon-Like Correia Elements Encode Numerous Strong Promoters and Provide a Potential New Mechanism for Phase Variation in the Meningococcus
- Proteins Encoded in Genomic Regions Associated with Immune-Mediated Disease Physically Interact and Suggest Underlying Biology
- A Novel RNA-Recognition-Motif Protein Is Required for Premeiotic G/S-Phase Transition in Rice ( L.)
- The Mucin-Like Protein OSM-8 Negatively Regulates Osmosensitive Physiology Via the Transmembrane Protein PTR-23
- Genome Sequencing and Comparative Transcriptomics of the Model Entomopathogenic Fungi and
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Quaking Regulates Expression through Its 3′ UTR in Oligodendrocyte Precursor Cells
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



