-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Next Generation Becomes the Now Generation
article has not abstract
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000906
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000906Summary
article has not abstract
In recent years, several so-called next-generation DNA sequencing platforms have begun to challenge the well-established Sanger sequencing method. In two important ways—cost and speed—these next-gen technologies provide improvements over Sanger sequencing. Several technical drawbacks (short read length, lack of paired end reads, and quality problems, particularly with homonucleotide stretches [1]), however, render assembly difficult and limit the use of post-Sanger sequencing. These obstacles limited the effective use of next-generation sequencing to the sequencing of prokaryotes [2], the resequencing of individuals [3], and transcriptomics studies, recently termed RNA-Seq [4] and effectively precluded de novo eukaryotic sequencing. Realizing the shortcomings of next-generation technology, manufacturers have continued to improve the read length and have recently implemented paired end methods. Capitalizing on these improvements, the publication by Nowrousian et al. describes the team's success in completely bypassing Sanger sequencing to produce a de novo assembly (to draft quality) of a complete genome, that of the filamentous fungus Sordaria macrospora [5], using Solexa sequencing-by-synthesis and 454 pyrosequencing.
The technical merits of this publication make it an excellent starting point for future genome sequencing using post-Sanger platforms. The assembly phase has been a particular sticking point for de novo genome sequencing in eukaryotes, as the complexity of the genomes makes it difficult to correctly place short reads. By sequencing to high depth (nearly 100 times the length of the genome), the authors were able to pull the assembly together in large pieces (contigs) and obtain a reasonable N50 = 117 kb (defined as the smallest length of the longest contigs that cover 50% of the genome). The authors also experimented with different levels of coverage and different combinations of reads to produce assemblies of various qualities. They determined that the depth to which S. macrospora was sequenced may not be necessary, and that closing gaps with 454 reads resulted in a large improvement. Interestingly, this is similar to the blend of long - and short-insert libraries that were used for the whole genome shotgun version of the human genome project [6]. By leveraging the short inexpensive Solexa reads for the bulk of the genome, the longer 454 reads can add valuable contig order and orienting information and vastly improve quality while dramatically reducing the associated cost. Nowrousian et al. [5] have provided the assembly statistics for various depths and platforms, paving the way for future studies using high throughput sequencing.
The researchers also showed that post-Sanger sequencing technologies can be used to reliably assemble difficult areas of the genome. One region of the genome, that which controls nonself recognition, could have been a particularly troublesome stumbling block. Anastomosis is a process by which hyphae, the thread-like projections of filamentous fungi, fuse and bring genetically distinct nuclei into contact. Fungi from the same species with different het (heterokaryon incompatibilty) loci will fuse, but the resulting heterokaryotic cells are subject to either severely restricted growth or cell death. This process has benefits that the authors describe briefly. Although incompatibility has never been observed in S. macrospora, the investigators report that the genome contains apparent heterokaryon incompatibility genes, with the twist that the region is inverted and contains duplications of key genes near the ends of the inversion. Such a duplication might be difficult to resolve with short Solexa data and even the longer 454 reads. However, the authors used polymerase chain reaction (PCR) to amplify across the boundaries of the inverted and duplicated region, and end-sequenced the PCR products to confirm the genome structure predicted by the genome assembler Velvet [7]. Given this demonstrated success in resolving a difficult region containing duplicate genes, researchers and physicians can consider the previously unfeasible next-gen sequencing technologies when deciding whether to sequence an entire genome.
The quality of sequence produced, and ability to compare the Sanger and post-Sanger sequence scores, were additional sticking points to relying completely on the lower cost next-gen technologies. On this front, Nowrousian's team gave us a glimpse of the error rate and how it compares to that of Sanger sequencing by choosing several possible frame shifts in predicted coding regions for resequencing. The outcome of this investigation, although based on a small (21 kb total) sample, shows that the next-gen technologies can achieve error rates similar to those of Sanger sequencing. This leaves no obvious reason to use any Sanger sequencing for future whole genome sequencing projects.
Beer, Wine, and Advancements in Science and Technology
The selection of organism to sequence in this venture was critical, and a wise choice was made. Fungi, as the authors mention, are not only important to broad areas from ecology and agriculture to medicine and biotechnology, but are also important test platforms due to several characteristics of the genomes inherent to the fungal kingdom. Such traits were important in selecting the yeast Saccharomyces cerevisiae as the first sequenced eukaryote, a fungus only distantly related to the filamentous S. macrospora. Similar attributes are of value here, chiefly low-repeat content (critical for clean assemblies) and manageable size (S. macrospora genome of approximately 40 Mb). The low-repeat content in the genome of S. macrospora is possibly due to the effect of repeat-induced point mutation or RIP [8], which has been well documented in the closely related Neurosopora crassa [9]. The authors suggest that RIP might have been active at some point in its evolutionary history, but that S. macrospora may no longer have an active RIP process. Still, by some mechanism S. macrospora is able to keep repeat elements low in copy number. In addition, haploid genomes are much more easily assembled because of a lack of allelic heterozygosity. It remains to be seen how amenable large, diploid genomes will be to assembly using similar technologies.
For one other key reason, S. macrospora was an excellent candidate for this next-gen sequencing effort. The close relation to N. crassa offers both a good companion for comparative genomics as well as a verification of assembly quality, as large sections of the genomes were known to be similar enough to align extensively [10]. This relationship was also used to pull the assembled fragments together and produce a very clean high-quality assembly with few scaffolds (152 in total).
Terabyte Is the New Gigabyte
Now that any academic department or perhaps even lab around the world can sequence a draft quality genome inexpensively, the amount of sequence data will predictably explode. While the number of genomes sequenced to date is more than one thousand (Figures 1 and 2) [11]—if we count both eukaryotic and prokaryotic projects—this advancement opens the door to an exponential expansion in the number of available genomes. Can we handle it? The National Center for Biotechnology Information (NCBI) currently deals well with several strains of the same species, but are we ready for individuals of the same strain? While technical hurdles to individual sequencing (the need for multiple copies of the same genome to fragment) remain for single-celled organisms, for fungi, and other eukaryotes with small genomes, this is a likely next level of study. Clearly the expected flood of data and the potential for finding answers to biological questions on this new level make it imperative to develop robust tools for referencing and storing sequence information on an individual by individual basis, and perhaps doing away with the current system of using a single reference genome.
Fig. 1. Number of genomes entered into GenBank by year as of September 2009. 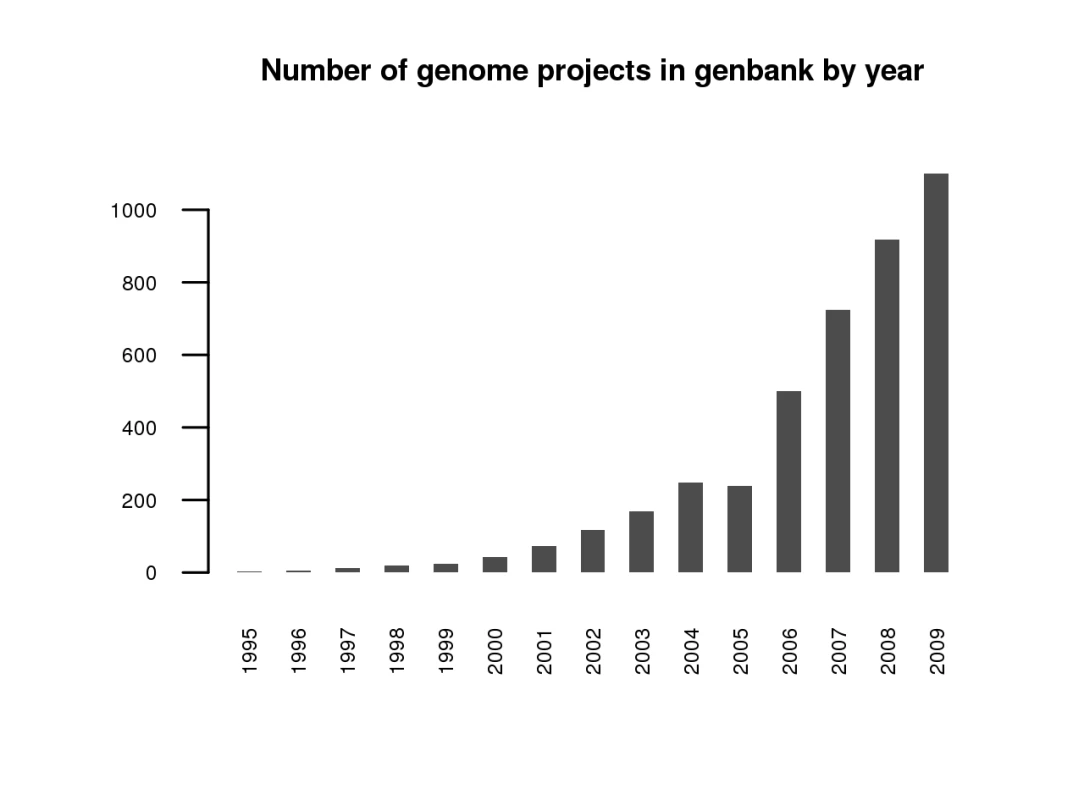
Adapted from http://www.genomesonline.org/ [11]. Fig. 2. Number of projects per phylogenetic group as of September 2009. 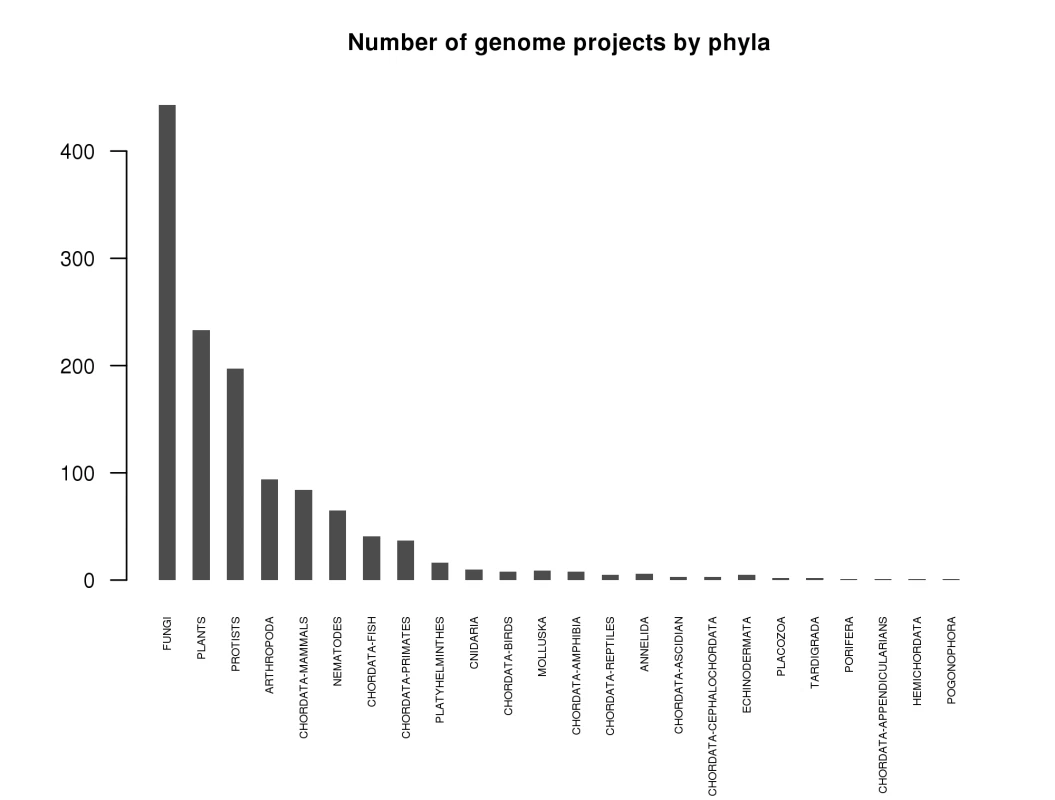
Adapted from http://www.genomesonline.org/ [11]. At least for the fungal research community, the quality, cost, and speed of next-gen sequencing technologies are now such that we can sequence at will and add to the rapidly growing list of available fungal genomes, as shown in Figure 2. This may be the case for mammalian genomes as well, as suggested in a recent publication (the giant panda [12]). Still, we have not yet attained the “1,000-dollar genome” widely thought to be necessary for broad medical use in diagnosis and selection of treatments [13].
What is the new next-gen sequencing? One answer to this question might come from Pacific Biosciences Corporation. In a recent publication [14], it appears they are able to detect the addition of a nucleotide to a growing strand of DNA by the polymerase enzyme. This “real-time” sequencing technology may be the next point in the race for fast and inexpensive whole-genome sequencing. Additional companies such as Complete Genomics and Ion Torrent Systems are unveiling new instruments and techniques and it is likely the speed with which data are produced will continue to increase while the costs will decrease. Until then, we will have plenty of data to sift through while we wait.
Zdroje
1. ShendureJ
JiH
2008 Next-generation DNA sequencing. Nat Biotech 26 1135 1145
2. SrivatsanA
HanY
PengJ
TehranchiAK
GibbsR
2008 High-Precision, Whole-Genome Sequencing of Laboratory Strains Facilitates Genetic Studies. PLoS Genet 4 e1000139 doi:10.1371/journal.pgen.1000139
3. WheelerDA
SrinivasanM
EgholmM
ShenY
ChenL
2008 The complete genome of an individual by massively parallel DNA sequencing. Nature 452 872 876
4. WilhelmBT
MargueratS
WattS
SchubertF
WoodV
2008 Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature 453 1239 1243
5. NowrousianM
StajichJE
ChuM
EnghI
EspagneE
2010 De novo Assembly of a 40 Mb eukaryotic genome from short sequence reads: Sordaria macrospora, a model organism for fungal morphogenesis. PLoS Genet 6 e1000891 doi:10.1371/journal.pgen.1000891
6. VenterJC
AdamsMD
MyersEW
LiPW
MuralRJ
2001 The Sequence of the Human Genome. Science 291 1304 1351
7. ZerbinoDR
BirneyE
2008 Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18 821 829
8. GalaganJ
SelkerE
2004 RIP: the evolutionary cost of genome defense. Trends Genet 20 417 423
9. GalaganJ
CalvoS
BorkovichK
SelkerE
ReadN
2003 The genome sequence of the filamentous fungus Neurospora crassa. Nature 422 859 868
10. NowrousianM
WürtzC
PöggelerS
KückU
2004 Comparative sequence analysis of Sordaria macrospora and Neurospora crassa as a means to improve genome annotation. Fungal Genet Biol 41 285 292
11. LioliosK
MavromatisK
TavernarakisN
KyrpidesNC
2008 The Genomes On Line Database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucl Acids Res 36 475 479
12. LiR
FanW
TianG
ZhuH
HeL
2010 The sequence and de novo assembly of the giant panda genome. Nature 463 311 317
13. MardisER
2006 Anticipating the 1,000 dollar genome. Genome Biol 7 112
14. EidJ
FehrA
GrayJ
LuongK
LyleJ
2009 Real-Time DNA Sequencing from Single Polymerase Molecules. Science 323 133 138
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



