-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
Model organisms have played an important role in the elucidation of multiple genes and cellular processes that regulate aging. In this study we utilized the budding yeast, Saccharomyces cerevisiae, in a large-scale screen for genes that function in the regulation of chronological lifespan, which is defined by the number of days that non-dividing cells remain viable. A pooled collection of viable haploid gene deletion mutants, each tagged with unique identifying DNA “bar-code” sequences was chronologically aged in liquid culture. Viable mutants in the aging population were selected at several time points and then detected using a microarray DNA hybridization technique that quantifies abundance of the barcode tags. Multiple short - and long-lived mutants were identified using this approach. Among the confirmed short-lived mutants were those defective for autophagy, indicating a key requirement for the recycling of cellular organelles in longevity. Defects in autophagy also prevented lifespan extension induced by limitation of amino acids in the growth media. Among the confirmed long-lived mutants were those defective in the highly conserved de novo purine biosynthesis pathway (the ADE genes), which ultimately produces IMP and AMP. Blocking this pathway extended lifespan to the same degree as calorie (glucose) restriction. A recently discovered cell-extrinsic mechanism of chronological aging involving acetic acid secretion and toxicity was suppressed in a long-lived ade4Δ mutant and exacerbated by a short-lived atg16Δ autophagy mutant. The identification of multiple novel effectors of yeast chronological lifespan will greatly aid in the elucidation of mechanisms that cells and organisms utilize in slowing down the aging process.
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000921
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000921Summary
Model organisms have played an important role in the elucidation of multiple genes and cellular processes that regulate aging. In this study we utilized the budding yeast, Saccharomyces cerevisiae, in a large-scale screen for genes that function in the regulation of chronological lifespan, which is defined by the number of days that non-dividing cells remain viable. A pooled collection of viable haploid gene deletion mutants, each tagged with unique identifying DNA “bar-code” sequences was chronologically aged in liquid culture. Viable mutants in the aging population were selected at several time points and then detected using a microarray DNA hybridization technique that quantifies abundance of the barcode tags. Multiple short - and long-lived mutants were identified using this approach. Among the confirmed short-lived mutants were those defective for autophagy, indicating a key requirement for the recycling of cellular organelles in longevity. Defects in autophagy also prevented lifespan extension induced by limitation of amino acids in the growth media. Among the confirmed long-lived mutants were those defective in the highly conserved de novo purine biosynthesis pathway (the ADE genes), which ultimately produces IMP and AMP. Blocking this pathway extended lifespan to the same degree as calorie (glucose) restriction. A recently discovered cell-extrinsic mechanism of chronological aging involving acetic acid secretion and toxicity was suppressed in a long-lived ade4Δ mutant and exacerbated by a short-lived atg16Δ autophagy mutant. The identification of multiple novel effectors of yeast chronological lifespan will greatly aid in the elucidation of mechanisms that cells and organisms utilize in slowing down the aging process.
Introduction
Model eukaryotic organisms such as Drosophila and C. elegans have played important roles in the identification of genes and the molecular characterization of cellular and biochemical pathways that affect the aging process [1]. For example, large-scale systematic RNAi knockdown screens for lifespan extension with C. elegans have implicated multiple genes that regulate metabolism, signal transduction, protein turnover, and gene expression [2], [3]. The budding yeast, Saccharomyces cerevisiae, has also been particularly useful, especially in characterizing the NAD+-dependent protein deacetylase, Sir2, as a replicative lifespan (RLS) factor [4]. RLS is defined by the number of mitotic cell divisions that a mother cell undergoes prior to senescencing [5].
Yeast lifespan can also be measured chronologically, where the time that non-dividing cells remain viable is monitored [6]. This chronological lifespan (CLS) is typically measured in cells that have entered stationary phase (G0). Both types of yeast aging share multiple effectors of lifespan related to nutrient signaling. Deletion of SCH9 extends both RLS and CLS [6], [7]. Sch9 is related to the serine/threonine kinase (Akt), that in higher eukaryotes functions in insulin-like growth factor (IGF) signaling pathways that have been linked to lifespan regulation [6]. Mutations in the Target of Rapamycin (TOR) signaling pathway also extend both types of lifespan in yeast [8]–[10], as well as in C. elegans [11]. The overlap between CLS and RLS extends to the effects of calorie restriction (CR), a dietary regimen shown to extend the mean and maximum lifespan of rodents [12]. In the yeast system, CR consists of reducing the glucose concentration in the growth medium from the non-restricted (NR) level of 2% (w/v) to the CR level of 0.5% or lower [13], [14]. CR extends both RLS and CLS [13]–[16], consistent with the general theme of conserved nutrient signaling pathways playing major roles in longevity. CR, sch9Δ, and tor1Δ conditions all cause a shift in glucose metabolism from fermentation toward respiration in both lifespan systems [10], [16], [17], revealing a strong link with mitochondrial function. Despite the numerous similarities in nutrient-mediated responses between RLS and CLS, there are also significant differences. One of the most striking is that while SIR2 promotes RLS and is reported to be required for lifespan extension by CR [14], deletion of SIR2 mildly extends CLS and is not required for CR-mediated lifespan extension in this system [15], [16]. Instead, Sir2-mediated deacetylation of the gluconeogenesis enzyme Pck1 limits the large extension of CLS caused by extreme CR conditions [18].
Due to its simplicity, CLS has been amenable to genome-wide functional aging screens. A previous screen for long-lived mutants used the yeast knockout (YKO) collection of individual diploid deletion mutants to individually test each mutant for CLS while incubating in 96-well plates. Several deletion mutants downstream of the TOR signaling pathway were identified, thus implicating TOR signaling in lifespan control [8]. In our study we have utilized the YKO collection to identify additional genetic factors that influence CLS through a different approach. A microarray-based genetic screen was performed on the collection, with the goal of determining which deletion mutants shorten or extend lifespan under NR or CR growth conditions. We report the identification of several classes of short-lived mutants, including those that affect mitochondrial function and the autophagy pathway. We also identify and characterize long-lived mutants in the highly conserved de novo purine biosynthesis pathway that generates IMP, AMP, and GMP. Deletion of genes in this pathway extended lifespan equally to the effect of CR, and CR did not further extend the lifespan of the mutants, suggesting that there are overlapping mechanisms between these two conditions that promote longevity. We show that the de novo purine biosynthesis mutants alter the surrounding growth medium in a way that extends the lifespan of WT cells, pointing to a cell-extrinsic component of CLS regulation.
Results
A microarray-based screen for yeast genes involved in chronological life span
We took advantage of the YKO collection of gene deletion mutants [19], in which each individual gene is replaced by the selection marker (kanMX4) and flanked by specific UPTAG and DNTAG sequences (Figure 1A). Viable mutants from the haploid collection were pooled together and grown in synthetic complete (SC) medium that contained either 2% glucose (non-restricted/NR) or 0.5% glucose (calorie restricted/CR). On days 1, 9, 21, and 33, aliquots were removed and spread onto YPD plates to recover mutants that remained viable (Figure 1B). The TAG sequences present in the recovered cells were PCR amplified using universal primers labeled with Cy3 for day 1, or Cy5 for days 9, 21, and 33 (Figure 1B). Following microarray co-hybridizations, the relative abundance of each mutant was determined by the ratio of Cy5 signal (days 9, 21, or 33) to the Cy3 signal (day 1). (see Table S1 for ratios).
Fig. 1. Microarray-based screen for chronological longevity factors. 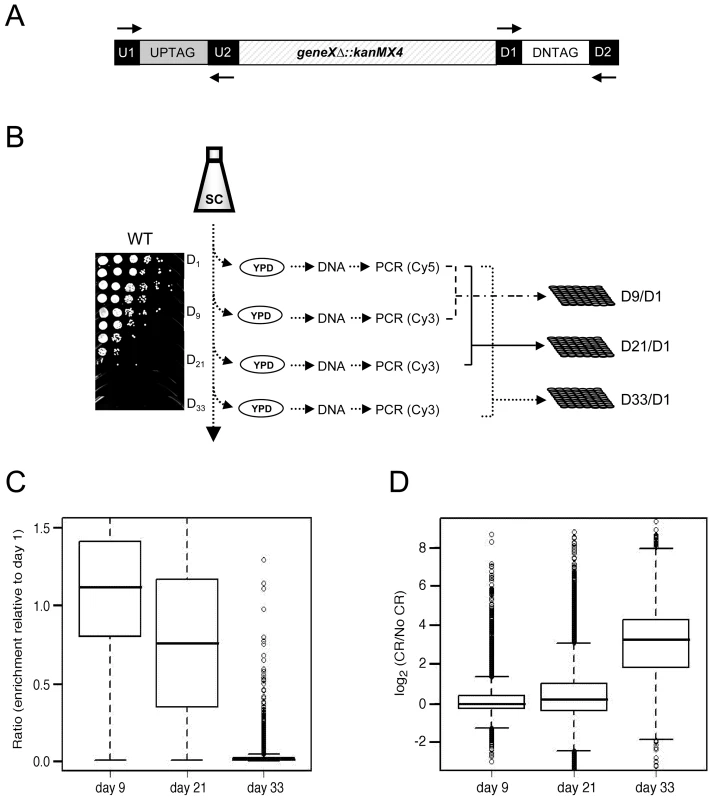
(A) Schematic representation of the UP and DOWN tags flanking KanMX. Universal primer sequences (U1/U2 and D1/D2) flank the UP and DOWN tags. (B) Experimental flow of the screen. Aliquots were removed from SC cultures of the pooled YKO population at days 1, 9, 21, and 33, and spread onto YPD plates to allow growth of survivors. A typical BY4741 CLS time course in 2% glucose (NR) is shown. The TAGs were PCR amplified from genomic DNA and fluorescently labeled with either Cy5 (day 1) or Cy3 (days 9, 21, and 33). The day 1 TAGs were co-hybridized with day 9, 21, or 33 TAGs onto the microarray to generate the abundance ratio for each mutant at that particular day (D9/D1, D21/D1 and D33/D1). (C) Box plot of the mutant abundance ratios within the aging population at days 9, 21, and 33 from the NR medium. (D) Box plot showing the general increase in mutant viability within the aging population for CR medium compared to NR medium. Under - or over-representation of a particular mutant's DNA in the aging population was predicted to be indicative of its CLS relative to the other mutants. As expected, the abundance ratios of the TAG signals for most mutants decreased over time in the NR culture (Figure 1C), indicating that most mutants in the population lost viability (aged). By day 33, when the WT strain was completely dead (Figure 1B, spot assay), there were a limited number of viable mutants in the population that could potentially be extremely long-lived (Figure 1C, data shown for the NR population). The viability of most mutants at day 33 was greater in the CR growth condition than in the NR condition (Figure 1D), suggesting that most mutants respond to CR by extending their CLS.
Specific classes of short-lived and CR–unresponsive mutants
To conservatively choose a subset of mutants for retesting the predicted short CLS phenotype, we set two separate threshold criteria. First, the abundance ratios at day 9 for both TAGs had to be ranked in the bottom 200. Second, the abundance ratio at day 21 had to be less than 0.3 for both TAGs, which represented the bottom quartile for this time point (Figure 1C). The day 33 abundance ratios were not considered because most mutants were dead by then (Figure 1C). The result was 117 candidate mutants predicted to be short-lived (Table S2). Out of this list of 117 mutants, we individually retested 16 of them for CLS, and found 13 (81.3%) to actually be short-lived (Table S2). Interestingly, 42 of the 117 candidate genes were related to mitochondrial function in some way (Table S2), most likely because respiration defects prevent cells from properly transitioning through the diauxic shift, thus reducing stationary phase viability [20]. Another major sub-class from the 117 candidates included 10 of the “ATG” genes involved in autophagy. As shown in Figure 2A, the autophagy mutants that we directly tested generally caused a short CLS in 2% glucose as predicted by the screen. The CLS of these mutants was fully extended by the CR condition (Figure 2A), which was somewhat surprising because earlier work in C. elegans showed that autophagy was required for dietary restriction (DR)-mediated extension of lifespan [21], [22]. All mutants that were tested for various reasons in this study and found to have a short CLS in 2% glucose, including the atg mutants, are listed in Table S3.
Fig. 2. Deletion mutants that shorten CLS. 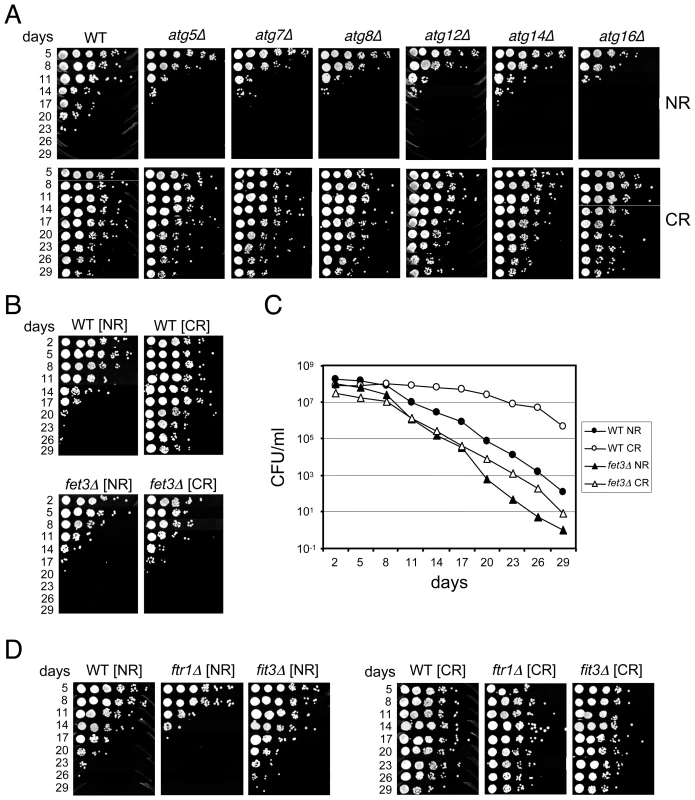
(A) Various deletions of autophagy genes isolated from the screen as short-lived were retested individually for CLS in NR and CR media. (B) Semi-quantitative CLS assay comparing a fet3Δ mutant to WT in NR and CR media. (C) Quantitative CLS assay for the same fet3Δ and WT strains in NR and CR media. Colony forming units (CFU) are plotted over time. (D) CLS assay showing CR-mediated extension of lifespan in ftr1Δ and fit3Δ mutants. We were also interested in identifying mutants whose lifespan was not extended by CR. Such mutants were predicted to have similar abundance ratios in the NR and CR conditions across the time course. Many mutants initially appeared to fit this category, which required them to have average NR and CR log rations within 10% of each other (see Materials and Methods). However, only 2 of 41 mutants retested (4.9%) were actually confirmed as being CR-unresponsive. These two affected genes were NFU1 and FET3, both of which encode proteins involved in iron homeostasis. The CLSs of these two mutants were slightly shorter than WT when grown under NR conditions, and, as predicted from the screen, were not extended by CR (Figure 2B and 2C, and data not shown). NFU1 encodes a mitochondrial matrix protein thought to be involved in iron-sulfur complex biogenesis [23], an important part of the electron transport cascade within the mitochondrial membrane. Its close link with respiration could explain why the nfu1Δ mutant had a shorter lifespan in the CR condition than in the NR condition (data not shown).
FET3 encodes a multicopper oxidase, that along with the iron permease (Ftr1), comprises a high affinity iron uptake system [24], initially suggesting that high affinity transport of iron is required in CR-induced CLS determination. However, even though an ftr1Δ mutant exhibited a slight shortening of CLS in the NR condition similar to the fet3Δ mutant, CR still induced full CLS extension (Figure 2D). Another protein, Fit3, is one of three secreted mannoproteins that functions in the retention of siderophore-iron in the cell wall, which can be released and then imported by the Fet3/Ftr1 transport system [25]. Deletion of FIT3 had no affect on CLS, and like the ftr1Δ mutant, its CLS was extended by CR (Figure 2D). Taken together, these results suggest that Fet3 may have a function independent of Ftr1-mediated iron transport at the plasma membrane that is important for CLS during CR growth conditions.
Identification of long-lived mutants
To identify long-lived mutants, we again defined conservative thresholds in which the day 33/day1 signal ratio had to be in the top 500 for both the UP-and DN-tags. The day 21/day1 ratio also had to be greater than 1.0 for both TAGs, resulting in a list of 40 mutants (Table 1). Twelve out of the 39 mutants retested (30.7%) had a long CLS (several shown in Figure 3A). Isolation of the de novo NAD+ biosynthesis gene, BNA2, was consistent with the long CLS of a strain lacking BNA1 [16]. YPL056C, YLR104W, and YGL085C, were previously uncharacterized and have now been named based on their Long Chronological Lifespan phenotype as LCL1, LCL2, and LCL3, respectively. The lcl1Δ mutant was previously shown to be resistant to the antifungal drug fluconazole [26], and the lcl2Δ mutant has deficient levels of mannosylphosphate in the cell wall [27], suggesting that both of these genes may function in cell wall integrity. DCW1 encodes a putative mannosidase involved in cell well biosynthesis [28], again pointing to the importance of cell wall structure and function in longevity. LCL3 encodes a protein with homology to Staphylococcus aureus nuclease [29]. Three of the long-lived mutants were involved in either de novo purine biosynthesis (ADE3 and ADE4) or purine import (FCY2) [30]–[32]. Ade4 catalyzes the first step of the pathway, while Ade3 functions in one-carbon metabolism, which donates tetrahydrofolate-linked carbon units for synthesis of the purine ring (see Figure 3B). Fcy2 is a purine/cytosine permease that mediates transport of purine bases (adenine, guanine, hypoxanthine), and a specific pyrimidine base (cytosine) across the plasma membrane into the cell. Additional mutants were analyzed for CLS outside of the selection criteria. Those mutants that exhibited an extended lifespan under NR conditions are listed in Table S4, while those with a normal lifespan under NR conditions are listed in Table S5.
Fig. 3. Lifespan-extending mutants include those that block de novo and salvage biosynthesis of purines. 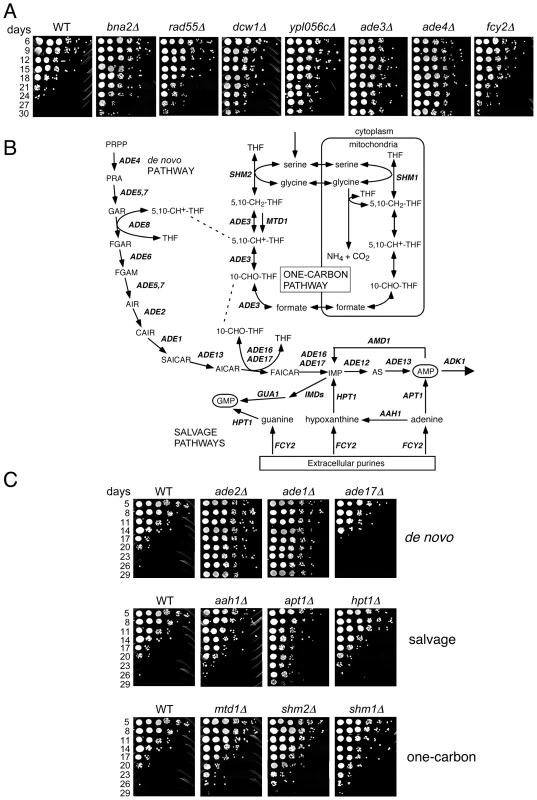
(A) Examples of CLS assays for various long-lived mutants isolated from the screen, including genes related to purine metabolism (ade3Δ, ade4Δ, and fcy2Δ). (B) Schematic diagram of the de novo purine biosynthesis pathway and its connections to one-carbon metabolism and purine import/salvage pathways. Partially adapted from [71]. (C) Examples of additional deletion mutants from the de novo purine biosynthesis, purine salvage, and one-carbon metabolism pathways that were not originally isolated from the screen. Tab. 1. Long-lived mutant candidates chosen from screen. 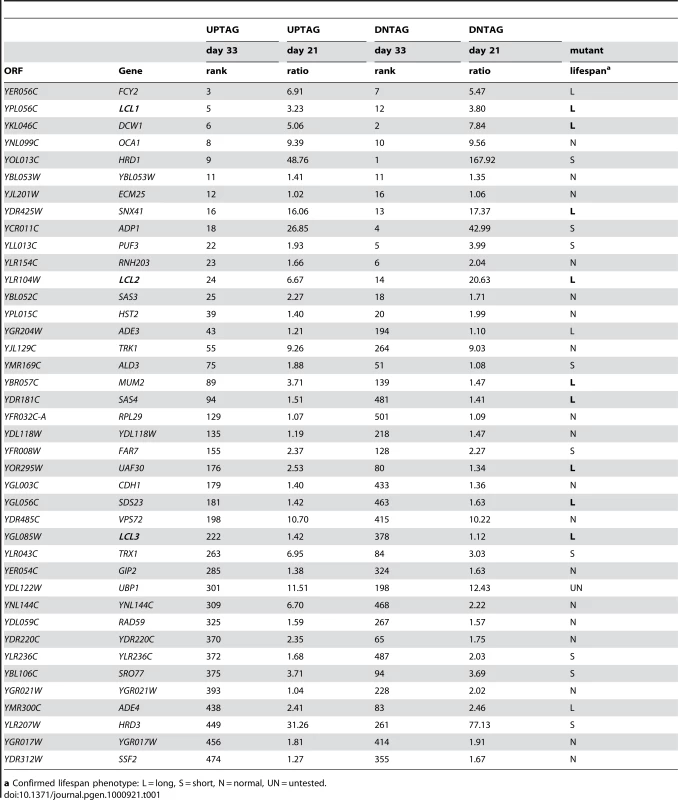
a Confirmed lifespan phenotype: L = long, S = short, N = normal, UN = untested. Characterization of the ADE pathway in CLS regulation
The effects of the de novo purine biosynthesis pathway on aging have not been well studied. In Drosophila melanogaster, mutations in the pathway cause pleiotropic effects due to general purine deficiency, one of them being a short lifespan [33]. In yeast, the pathway was not previously implicated in lifespan regulation. The de novo purine biosynthesis pathway is highly conserved and consists of ten consecutive reactions catalyzed by the ADE gene products that convert 5-phosphoribosyl 1-pyrophosphate (PRPP) to inosine monophosphate (IMP), which is then used for AMP and GMP synthesis (Figure 3B). There are also purine salvage pathways that either import extracellular purines via Fcy2 or utilize endogenous purines to synthesize IMP, GMP or AMP through only a few enzymatic steps (Figure 3C; for review see [34]). Deleting other genes in the de novo synthesis pathway such as ADE1, ADE2, ADE5,7, ADE6, or ADE12 significantly extended CLS (Figure 3C and data not shown). ADE13 is essential and ade8Δ was not available in our KO collection, so they were not tested. The lone exception encountered was an ade17Δ mutant, which had a lifespan modestly, but reproducibly, shorter than WT (Figure 3C). Ade17, as well as Ade16, catalyzes the conversion of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) into 5′-phosphoribosyl-5-formaminoimidazole-4-carboxamide (FAICAR). The major enzyme in this step is Ade17, being responsible for ∼90% of AICAR transformylase activity [35]. Mutants in the purine salvage pathways (AAH1, APT1, or HPT1) or the one-carbon metabolism pathway (MTD1, SHM2, or SHM1) also extended CLS, but to a lesser extent than mutants in the de novo pathway (Figure 3C). The effects of these two pathways on CLS may, therefore, be mediated by a secondary effect on regulation of the de novo pathway.
The de novo synthesis of purine nucleotides is regulated at the genetic and enzymatic levels. Enzymatically, the first step of the pathway catalyzed by Ade4 is feedback-inhibited by the end products ADP and ATP [36]. Genetically, excess adenine has a repressing effect on ADE regulon genes, while depletion of adenine results in transcriptional up-regulation due to the activity of transcription factors Bas1 and Bas2/Pho2 [37], [38]. Regulation of all the de novo pathway genes, with the exception of ADE16, is achieved via the Bas1/Pho2 complex [39]. It is proposed that the AICAR or SAICAR intermediates promote Bas1-Pho2 dimerization, resulting in the up-regulation of ADE-gene transcription [36], [38], [40]. Since we observed CLS extension in ade mutants lacking an enzyme upstream of the AICAR intermediate and CLS shortening for the ade17Δ mutant that likely accumulates AICAR [40], we generated an ade4Δ ade17Δ double mutant and tested CLS. As shown in Figure 4A, the ade4Δ mutation was epistatic to the ade17Δ mutation for lifespan in the double mutant, initially consistent with a hypothesis that accumulation of AICAR shortens CLS of the ade17Δ mutant. However, completely blocking the AICAR to FAICAR step of the de novo pathway with an ade16Δ ade17Δ double mutant, surprisingly resulted in CLS extension (Figure 4A).
Fig. 4. Epistasis analysis of the de novo purine biosynthesis pathway in CLS. 
(A) The lifespan extending ade4Δ mutation was combined with lifespan shortening ade17Δ and atg16Δ mutations through genetic crosses, and the double mutants tested for CLS when grown in SC 2% glucose (NR) medium. An ade16Δ ade17Δ double mutant that completely blocks the AICAR to FAICAR step of the de novo pathway was also tested. (B) CLS assays with WT, ade4Δ, fcy2Δ, tor1Δ, and gln3Δ strains grown in standard SC medium that contains (30 mg/L adenine), or SC medium supplemented with 4 times more adenine (4x Ade; 120 mg/L). Since excess adenine represses the de novo purine synthesis pathway, we next tested whether excess adenine would extend CLS. The SC medium contained either our standard limiting concentration of adenine (30 mg/L) or a 4-fold excess (120 mg/L), which represses the de novo pathway. Surprisingly, excess adenine did not extend the CLS of a WT strain, but instead suppressed the long CLS phenotype of ade2Δ, ade3Δ, or ade4Δ mutants (Figure 4B and data not shown). This effect was specific to the long-lived ade mutants, because excess adenine did not shorten the CLS of two long-lived mutants with inhibited TOR signaling, tor1Δ and gln3Δ (Figure 4B). The fcy2Δ mutation blocks adenine transport, so the addition of excess adenine did not affect CLS.
The long CLS of the ade mutants was reminiscent of the CR effect, suggesting there could be some degree of overlap between the two. To test this idea, CLS of the WT and ade4Δ mutant was measured using the semi-quantitative spot growth assay (Figure 5A), and a quantitative colony forming unit assay that can detect more subtle changes in CLS (Figure 5B). Both assays showed there was no additive effect on CLS when combining the genetic factor (ade4Δ) and the environmental factor (CR), at least for the duration of the experiment (36 days). This was consistent with some overlap in function or involved pathways. To further test this possibility, we also examined the effect of deleting ADE4 on the CLS of an autophagy mutant (atg16Δ). While the CR growth condition fully extended CLS of the atg16Δ mutant (Figure 2A), deleting ADE4 from the atg16Δ mutant only resulted in a partial extension of CLS (Figure 4A). Therefore, one of the differences between CR and the ade4Δ mutant in CLS extension is a differential requirement for autophagy.
Fig. 5. Growth media effects on CLS. 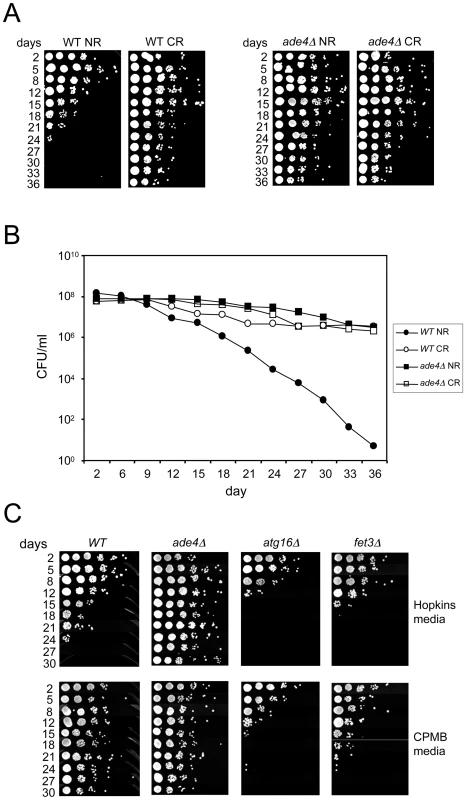
(A) Semi-quantitative CLS assay showing that deleting ADE4 extends CLS to the same degree as CR extends lifespan of a WT strain. The ade4Δ mutation and CR are not additive for CLS extension. (B) Quantitative CLS assay showing similarities between lifespan extension caused by the ade4Δ mutation and the CR growth condition. CFU = colony forming units. (C) SC medium with generally lower concentrations of amino acids (CPMB media) extends CLS when compared to the richer SC medium used in the genetic screen (Hopkins media). Deleting ATG16 (blocking autophagy) or FET3 (iron metabolism) prevented the extension of CLS induced by CPMB medium. Media effects on CLS
An earlier large-scale screen for long-lived yeast mutants did not uncover the de novo purine biosynthesis pathway genes [8]. We noticed that one of the differences between our study and the earlier study was the media composition used for the CLS assays. In general, the SC medium used in our study (Hopkins mix) is relatively rich in most amino acids compared to the SC medium used in the earlier study, which is described in Current Protocols in Molecular Biology [41], and abbreviated here as “CPMB” mix (Table S6). We compared the effects of each SC mix on the CLS of WT, ade4Δ, atg16Δ, and fet3Δ strains. As shown in Figure 5C, CLS of the WT strain was significantly longer in the CPMB media than in the Hopkins medium, even though glucose was 2% in both. As a result, the WT and ade4Δ lifespans were indistinguishable in the CPMB medium. Interestingly, the CPMB media did not extend the short CLS of the atg16Δ mutant (Figure 5C), even though reducing the glucose concentration in Hopkins medium fully extended its CLS (Figure 2A). Similar results were observed with several other autophagy mutants (data not shown), consistent with autophagy being required for mediating the effects of amino acid restriction on CLS. In contrast, the short CLS of the fet3Δ mutant, which was not extended by glucose CR (Figure 2B), was also not extended by the CPMB media (Figure 5C), making Fet3 important for mediating the effects of both glucose restriction and amino acid restriction on CLS.
Cell-extrinsic effects on CLS
Considering the large effects of media composition on CLS, we next investigated whether any of the mutants isolated from the screen could influence longevity via cell-extrinsic factors that are secreted or released into the growth media. For example, secreted purine compounds such as adenine and hypoxanthine have previously been implicated in the regulation of meiosis within a sporulating yeast culture [42]. Additionally, we noticed during this study that expired medium from NR cultures would reverse the long CLS of CR-grown cells, and expired medium from CR cultures would extend CLS of NR-grown cells (D.L. Smith Jr., unpublished data). A similar finding was recently published by the Kaeberlein lab, who reported that acetic acid secreted into the medium during NR growth conditions correlated with the short lifespan, and that CR conditions prevented acetic acid secretion [43]. Reduced exposure to acetic acid in the CR cultures was specifically shown to extend CLS, therefore providing a possible mechanism for how CR extends CLS. Interestingly, other conditions that extend CLS such as high media osmolarity or deletion of SCH9 have been proposed to make the cells more resistant to the acetic acid accumulation, rather than blocking organic acid production and secretion [43]. Taken together, these observations raised the question of whether any mutants isolated from our screen could affect CLS through a similar cell extrinsic mechanism.
To test for cell extrinsic effects we grew WT, ade4Δ, and atg16Δ strains in SC 2% glucose (NR) medium for 5 days into stationary phase. The cells were then pelleted and the expired medium was filtered and swapped in various combinations (Figure 6A). For example, the WT cells received expired medium from the ade4Δ or atg16Δ cells, and vice versa. The media-swapped cultures were then followed through a standard CLS assay (Figure 6B). Interestingly, the CLS of WT and atg16Δ cells was extended when incubated in expired medium from the long-lived ade4Δ cells. In the reciprocal swap, medium from the WT cells largely suppressed the long CLS of the ade4Δ mutant, but had no effect on the atg16Δ mutant. Expired medium from the short-lived atg16Δ mutant did not shorten CLS of the WT strain, but shortened CLS of the ade4Δ mutant (Figure 6B). The expired atg16Δ medium also tended to induce an adaptive regrowth effect, as shown in Figure 6B for the ade4Δ mutant, where nutrients released by dying cells in the stationary phase culture allow some of the remaining viable cells to regrow and populate the culture [44]. The ade4Δ and atg16Δ mutants therefore do alter the growth media in a way that can impact CLS.
Fig. 6. Cell-extrinsic effects of the atg16Δ and ade4Δ mutants on CLS. 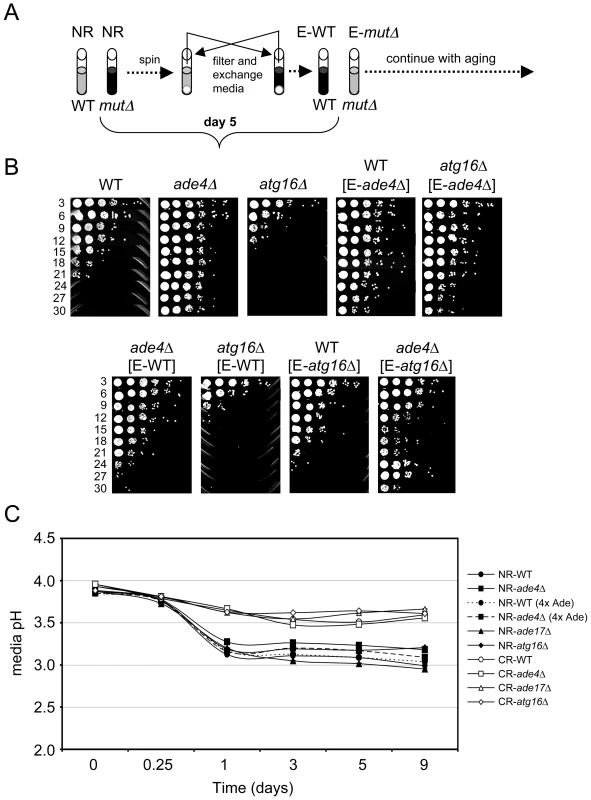
(A) Schematic diagram of a reciprocal media swap experiment. WT and mutant cell cultures were grown to day 5 in standard SC media containing 2% glucose (NR), at which point the cells were pelleted. The media was removed, filtered, and then exchanged such that the cell pellets received expired media derived from the mutant (E-mutΔ) or WT (E-WT) strains. The cultures were then allowed to age and the standard CLS assay continued. (B) CLS assay of the media swap experiment. WT, ade4Δ and atg16Δ strains without the swapped media are included as controls. (C) pH measurements of the SC growth media over time in NR and CR conditions. WT, ade4Δ, ade17Δ, and atg16Δ mutants were tested. A four-fold excess of adenine was added to the WT and ade4Δ strains under the NR condition where indicated. The secretion of organic acids (including acetic acid) and CO2 into the growth medium during fermentation results in a reduction of pH. The toxicity of acetic acid on yeast cells requires a low pH [43]. Therefore, we next tested whether CLS of these mutants correlated with changes in media pH. WT, ade4Δ, ade17Δ, and atg16Δ strains were grown in SC medium containing 2% glucose (NR) or 0.5% glucose (CR), and the pH of the media was measured over time. As expected, the pH of NR medium for WT cells decreased from ∼3.9 to ∼3.15 during the first 24 hr of growth and then leveled off. For WT cells in CR medium, the pH still decreased, but only to ∼3.5 by day 5. Media from the short-lived atg16Δ and ade17Δ mutants had pH profiles across the time course that were similar to the long-lived ade4Δ mutant regardless of the starting glucose concentration, indicating that CLS did not correlate with overall pH of the media. However, the lack of a correlation between pH and CLS did not rule out the possibility that acetic acid could still be involved in the extrinsic CLS regulation, especially since the pH remained relatively low (<4.0) in each conditions. Furthermore, an acidic environment is not sufficient to chronologically age yeast cells in the absence of acetic acid [43]. If acetic acid was involved in the extrinsic CLS effects, then raising the medium pH close to neutral should suppress the relatively short CLS of the WT and atg16Δ strains. Indeed, raising the medium pH to 6.0 either at the time of inoculation (D0) or after two days of growth (D2) (Figure 7A), resulted in a dramatic extension of CLS for the WT and atg16Δ strains that was at least as strong as the ade4Δ mutant effect or the CR growth condition (Figure 7B).
Fig. 7. Effects of elevated pH on CLS. 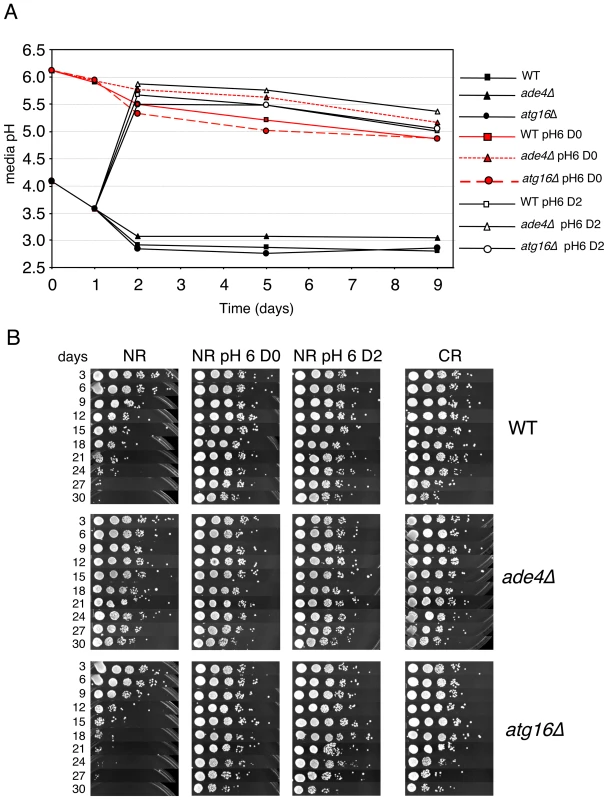
(A) Standard SC cultures (2% glucose) that were either not pH adjusted (started at the default pH of ∼4.0) or pre-adjusted to 6.0 were inoculated with the indicated strains. At day 2 (48 hours), the pH of a subset (open symbols) of the originally untreated cultures was adjusted to a pH of 6.0. For each culture, the pH was measured at the start of incubation (day 0), and then days 1, 2, 5, and 9. (B) CLS assays showing the effect of raising the SC medium pH to 6.0 at either at inoculation (D0), or after 2 days growth (D2). To determine whether the ade4Δ and atg16Δ mutants had any effect on acetic acid accumulation in the growth medium, the acetic acid concentration was measured from log phase, day 2, or day 5 cultures. As shown in Figure 8A, acetic acid accumulated to ∼3 mM in the WT culture on day 5. For the atg16Δ mutant, acetic acid accumulated earlier (day 2) and at a higher concentration by day 5 (∼11 mM), which was consistent with the short CLS of this mutant. In contrast, the long-lived ade4Δ mutant did not accumulate acetic acid at all compared to WT, which was very similar to the effect of CR on blocking acetic acid accumulation (Figure 8A). Therefore, the amount of acetic acid secreted into the medium for these two mutants was inversely correlated with their respective CLSs. Since the short CLS phenotype of the atg16Δ mutant was rescued by raising the pH to 6.0 (Figure 7), we were curious whether the higher pH was accompanied by a decrease in acetic acid concentration. The pH was again adjusted to 6.0 at the time of inoculation for WT and atg16Δ strains, and then acetic acid concentration measured at day 2 and day 5 (Figure 8B). Unexpectedly, the acetic acid concentration was elevated in the WT strain and reduced in the atg16Δ strain at both time points when the pH was adjusted to 6.0 at the time of inoculation (Figure 8B). Such variations in acetic acid accumulation apparently have no effect on CLS because the pH is too high to support the toxicity.
Fig. 8. CLS mutants affect acetic acid secretion and resistance. 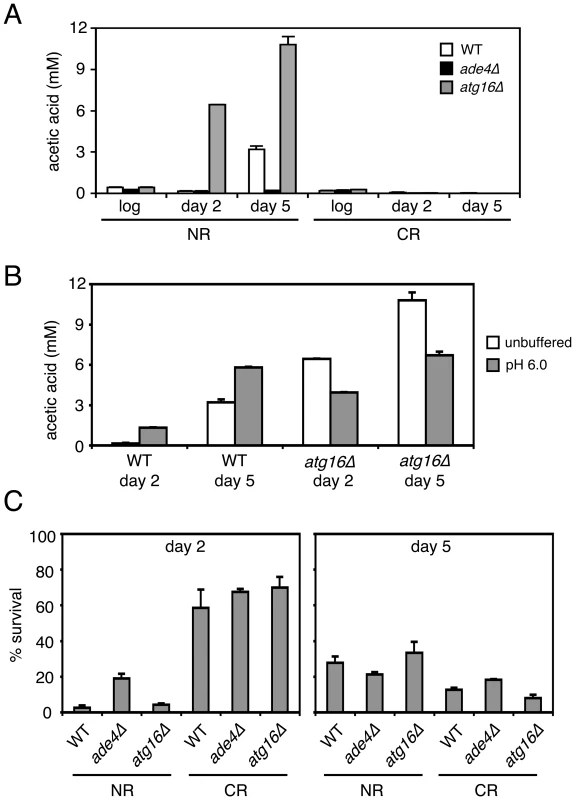
(A) Acetic acid concentrations in the filtered media from cultures of WT, ade4Δ, and atg16Δ strains that were growing in 2% glucose (NR) or 0.5% glucose (CR) conditions. Measurements were taken from log phase cultures, or cultures grown for 2 and 5 days. (B) Acetic acid measurements from WT and atg16Δ strains grown in unbuffered SC, or SC with the pH adjusted to 6 at the time of inoculation. (C) Resistance of the three strains to a 200 minute exposure of 300 mM acetic acid was measured for cells that were grown for 2 or 5 days in SC media (NR or CR levels of glucose). Percent survival for the treated cells is normalized to the untreated cells, which would be 100%. Since a long-lived sch9Δ mutant was previously shown to make yeast cells more resistant to acetic acid [43], we tested whether the ade4Δ and atg16Δ mutations affected cell survival when cultures grown for 2 or 5 days were challenged with 300 mM acetic acid for 200 minutes (Figure 8B). In the day 2 cultures, the ade4Δ mutant was significantly more resistant to acetic acid than the WT strain, again consistent with the long CLS of this mutant. However, resistance of the atg16Δ mutant was indistinguishable from WT. The CR condition made all three strains highly resistant to the acetic acid treatment. In the day 5 NR cultures (the time of the media swaps in Figure 6), there were no significant differences in the acetic acid resistance between the three strains, and surprisingly, the CR growth condition no longer made the cells more resistant. Resistance to acetic acid could potentially play a role in CLS extension for the ade4Δ mutant, which would be consistent with its ability to survive in the pooled mutant culture used for the screen, where many mutants would secrete acetic acid. In contrast, the short CLS of the atg16Δ mutant may not be due to acetic acid hypersensitivity. These results suggest that secreted acetic acid can commonly impact CLS through a cell extrinsic mechanism that is dependent on media pH.
Discussion
A microarray-based screen for short - and long-lived mutants from the YKO collection led to the identification of several pathways that regulate CLS, including autophagy and the de novo purine biosynthesis pathway. An earlier screen for chronologically long-lived deletion mutants revealed that reduced TOR signaling extends CLS [8]. The strongest TOR-related mutant from that screen was a gln3 deletion. In our screen, the gln3Δ mutant just missed the conservative selection criteria because its day 33 abundance ratios fell outside the top 500 (781 for DNTAG and 738 for UPTAG). However, its day 21 ratios were much higher than 1.0, consistent with the long CLS that was observed when tested directly (Figure 4). A direct test of gln3Δ and tor1Δ mutants also confirmed that TOR signaling controlled CLS in haploid yeast and growth media used in our study. A total of 117 potential short-lived mutants were isolated from the screen, with 13 of the 16 individually retested mutants confirmed to have a short CLS. Similarly, a total of 40 potential long-lived mutants were isolated, with 12 of the 39 retested mutants confirmed to have an extended CLS. From all the mutants tested individually for various reasons as part of this study, 69 short-lived and 57 long-lived mutants were found to affect CLS and are listed in Table S3 and Table S4, respectively.
Autophagy is required for chronological longevity in yeast
Autophagy is a multi-step process in which a portion of the cytoplasm is sequestered into a de novo-formed double membrane vesicle called the autophagosome. These vesicles fuse with a lysosome (the vacuole in yeast) and release the inner single-membrane vesicle called the autophagic body. Any sequestered organelle or other cellular matter in the autophagic body is degraded and recycled into amino acids, fatty acids, sugars, etc. [45]. This process is especially important during times of stress when cellular components can become damaged and aggregate, or when nutrients are depleted. Chronological aging of yeast cells is characterized by the ability to survive during extended incubation in starvation phase, making the ability to recycle resources critical. The identification of multiple deletion mutants in the autophagy pathway that shorten CLS therefore makes sense, not only because of the need to regenerate cellular components, but to potentially eliminate damaged proteins that arise as the cells age. Our results are consistent with results in Drosophila where mutation of the ATG7 gene shortens lifespan [46], and a more recent study in yeast showing that atg1Δ and atg7Δ mutants have a short CLS in synthetic growth medium [47]. The atg7Δ mutant was one of the autophagy mutants also isolated from our screen. Surprisingly, most autophagy gene deletion mutants have a normal RLS in rich YPD medium [48]. Similarly, the atg16Δ mutant had a normal RLS when we tested it in SC medium (Figure S1), making it a CLS-specific longevity factor.
Disruption of autophagy in C. elegans prevents the extension of lifespan caused by a daf-2 mutation or dietary restriction [21], [22], [49]. Deleting ATG15, but not the other autophagy genes, blocks CR-mediated RLS extension in yeast [48]. ATG15 was not isolated from our screen, and hence not tested for CLS, but every other autophagy mutant we tested responded to CR with CLS extension (Figure 2A). Interestingly, we found that deleting ATG16, ATG2, or ATG6 (VPS30) prevented CLS extension induced by the CPMB variety of SC media used in the Powers et al. screen, which has a normal 2% glucose level but generally has lower concentrations of amino acids compared to the Hopkins mix (Figure 5C). This result is consistent with the strong stimulation of autophagy triggered by nitrogen limitation or amino acid depletion [50], [51]. Indeed, maintenance of amino acid homeostasis via the general amino acid control system is important for proper CLS [47]. Furthermore, the long CLS of a tor1Δ mutant requires the autophagy gene ATG16 (data not shown). Similarly, autophagy was recently shown to be required for the extension of CLS induced by low concentrations of rapamycin [52], an inhibitor of the TOR signaling pathway. Future studies on the links between autophagy, amino acid depletion, and lifespan extension are clearly warranted.
Iron metabolism and caloric restriction
Two proteins (Fet3 and Nfu1) involved in iron homeostasis/metabolism were isolated as mutants whose CLS was not extended by the CR growth condition. Iron accumulates to high levels in the vacuole of yeast cells where it can be accessed during times of need, such as low iron growth conditions. Another key time of iron release from the vacuole is during the diauxic shift when the balance of iron is shifted to the mitochondria, where it is needed for mitochondrial biogenesis. The iron is incorporated into iron/sulfur complexes within multiple mitochondrial proteins, including aconitase and components of the electron transport chain. A defect in iron homeostasis could affect mitochondrial processes. One of the phenotypes observed during chronological aging is an accumulation of intracellular iron. Much of this iron is likely tied up in lipofuscin, an insoluble aggregate of proteins and lipid that is high in iron and accumulates in aging cells. Interestingly, CR reduces this accumulation of lipofuscin and iron [53]. The reduction in iron could contribute to the corresponding reduction in reactive oxygen species because a major source of reactive oxygen species is generated via iron through the Fenton reaction. It is not clear why a fet3Δ mutant would block the CR effect, but perhaps the iron oxidase activity of Fet3 has an additional function in iron homeostasis beyond its role in high affinity transport. Interestingly, a recent report showed that FET3 is one of several iron related genes that are up-regulated in response to increasing strength of CR [54]. FET3 was also required for the extension of CLS induced by the low amino acid CPMB medium (Figure 5C), pointing to iron and possibly mitochrondrial function being important for both glucose and amino acid restriction effects on CLS.
The de novo purine biosynthesis pathway and longevity
The de novo purine biosynthesis pathway is familiar to yeast researchers because the AIR intermediate that accumulates in ade2 mutants takes on a red pigmentation when it is oxidized and concentrated in the vacuole of respiring cells. Multiple genetic assays have taken advantage of this visual phenotype [55], [56]. Limiting the amount of adenine in the growth medium promotes development of the red color by increasing flux through the pathway. The 30 mg/L of adenine in Hopkins mix SC is limiting in this context. Excess adenine suppresses the red color by reducing flux through the pathway, thus reducing AIR formation. Excess (4X) adenine also suppresses the long CLS of the ade2Δ, ade3Δ, and ade4Δ mutants, but had no effect on CLS of the WT strain. One possible mechanism for a block in this pathway to regulate CLS is that reduced production of AMP and/or IMP leads to lifespan extension. Consistent with this idea, deletion of the adenylate kinase 1 gene ADK1, which leads to a large increase in cellular AMP concentration [57], also shortens CLS (Table S3). AMP is an allosteric effector of multiple enzymes in metabolism, including phosphofructokinase (PFK), a key regulatory step in the glycolytic pathway who's activity is enhanced by AMP binding. CR has been shown to reduce PFK activity in mouse liver [58]. Lower AMP levels could mimic CR by reducing glycolytic flux. This model also fits the extended CLS of the fcy2Δ mutant, which would also reduce AMP production by blocking the import of extracellular adenine. The compensatory increase in AMP production by the de novo purine synthesis pathway would partially suppress the effect, resulting in the more modest increase in lifespan for this mutant compared to the ade4Δ mutant. Since the de novo purine biosynthesis pathway and Fcy2-mediated transport of guanine also regulate GMP production (and subsequently GTP/GDP levels, reduced GMP levels could also contribute to the lifespan extension via effects on the Ras/cAMP/PKA pathway, as inhibition of Ras2 results in extension of CLS [59]. Consistent with this possibility, we have found that deletion of BCY1, which constitutively activates PKA, shortens CLS (Table S3).
A second possible mechanism for the de novo purine biosynthesis pathway to regulate CLS is through the control of AICAR concentration. Severe accumulation of AICAR induced by ADE4 over-expression in an ade16 ade17 double mutant causes synthetic lethality [40]. The less severe accumulation predicted for an ade17Δ mutant is not lethal, but instead leads to a short CLS (Figure 3C). However, any putative negative effect of AICAR accumulation from a defect in this step of the pathway is overcome, in terms of CLS, by a double deletion of ADE16 and ADE17. This double mutant behaves like any other deletion mutant in the de novo pathway (long-lived), suggesting that effects on IMP/AMP production or other unknown mechanisms are dominant to the AICAR effect. If AICAR does have a negative effect on CLS, then it is modest and opposite of that observed in higher eukaryotes. In metazoans, AICAR acts as an agonist of AMP-activated protein kinase (AMPK) [60], an enzyme that functions in mediating some aspects of longevity in C. elegans [61], [62]. The yeast paralog of AMPK, Snf1, is not activated by AMP or AICAR [63]. Furthermore, the snf1Δ mutant was found to have a short CLS (Table S2), a phenotype that is likely due to the roles of Snf1 in promoting respiration and autophagy [64], [65]. Given the complex nature of purine biosynthesis regulation and its links to the regulation of other metabolic pathways, including amino acid biosynthesis, other mechanisms leading to lifespan extension are certainly possible. For example, secreted adenine-related compounds could contribute to the cell-extrinsic effects of the ade mutants on CLS. In fact, the temporal secretion of various purines into the media and their subsequent uptake and utilization is a key signal that synchronizes the sporulation process between cells in a dense culture [42].
Acetic acid and the regulation of CLS
Acetic acid accumulates to low millimolar concentrations in stationary phase yeast cultures that are grown in SC medium with 2% glucose (NR). Exposure to this acetic acid, coupled with the acidic environment of the expired medium contributes to chronological aging [43]. CR growth conditions block the acetic acid accumulation, and long-lived mutants such as sch9Δ and ras2Δ tend to be resistant to acetic acid toxicity, suggesting that resistance to acetic acid may be a general property of chronologically long-lived yeast cells [43]. We found that the long-lived ade4Δ mutant blocked acetic acid accumulation in the growth medium as effectively as CR, while the short-lived atg16Δ mutant accumulated significantly higher concentrations of acetic acid than did the WT strain (Figure 8A). In addition to greatly reducing acetic acid levels (Figure 8A), we found that CR makes all three strains more resistant to acetic acid when the exposure occurs after 2 days growth, but is no longer effective with 5-day cultures (Figure 8B). While the ade4Δ mutant was moderately resistant to acetic acid at day 2 when compared to WT, by day 5 there was very little difference in sensitivity between the two mutants and WT. This is an important point, because the expired media swaps between the WT, ade4Δ, and atg16Δ strains were performed with 5-day old cultures. Perhaps chronologically aged yeast cells are simply programmed to be more resistant to acetic acid as a defense against this by-product of fermentation. These were short-term acetic acid exposures (200 minutes), so it is possible that prolonged exposure of the day 5 cultures, or lack of exposure for the ade4Δ expired media, could still affect CLS. This would also correlate well with the extension of CLS induced by raising the pH to 6.0 (Figure 7B), which would neutralize the toxicity of acetic acid. The ade4Δ mutation therefore both suppresses acetic acid accumulation and promotes acetic acid resistance, a phenotypic combination also induced by the CR growth condition.
It remains unclear why a defect in autophagy (atg16Δ) results in hyper-accumulation of acetic acid, while a block in de novo purine biosynthesis prevents acetic acid accumulation. An important function of autophagy is the turnover of organelles, including mitochondria. In mice deficient for Atg7, mitochondrial dysfunction has been observed that is accompanied by elevated reactive oxygen species [66]. Perhaps a defect in mitochondrial function would promote fermentation during NR conditions by preventing the yeast cells from fully transitioning from fermentation to respiration at the typical diauxic shift, and thus favoring acetic acid production. This would also account for the large number of mitochondria-related mutants that were isolated from the screen as being short-lived. Given the similarities of the ade4Δ CLS phenotype to CR, it is possible that the ade4Δ mutant could also enhance a shift from fermentation toward respiration, which could reduce acetic acid production. For the various mutants isolated from the screen, it will therefore be interesting to further compare the relative CLS contributions of their actual cellular defects with their acetic acid secretion and toxicity profiles. Specific combinations of intracellular and extracellular effects are likely going to be CLS determinants.
Efficacy of large-scale screens for chronological aging factors
The microarray-based genetic screen performed in this study was successful in identifying several novel longevity genes, but its quantitative ability to predict long-lived mutants based on the abundance ratios from the arrays was modest. Similar difficulties were previously observed using the YKO collection in a different type of longevity screen, in which each mutant was individually grown in a 96-well plate, and ability to re-grow was tested over time. In that screen, only 5 of 90 predicted long-lived mutants (5.6%) were confirmed when retested [8], [67]. In our case, 12 of the 39 candidate mutants (30.8%) were confirmed as long-lived when retested (Table 1). Not surprisingly then, only 4 of the 12 confirmed long-lived mutants isolated from our screen (LCL1, DCW1, LCL2, and MUM2) were ranked in the top 1000 long-lived candidates from the earlier Powers et al. CLS screen. These results are likely indicative of inherent variability in large-scale screens for long CLS, as well as subtle differences in the growth conditions. Large-scale screening for short-lived mutants is much more efficient, which is reflected in the fact that 68 of the 117 short-lived candidates from our screen (58.1%) are also in the bottom 1000 short-lived candidates from the Powers et al. screen (Table S2). Having multiple screening approaches is advantageous, as mutants not detected by one method may be detected by another.
There are several possible reasons for the variability associated the microarray-based longevity screen, especially for long-lived mutants. One possibility is the adaptive regrowth phenomenon, in which a subpopulation of cells in an aging stationary phase culture adapts to utilize the nutrients released by dead cells to re-grow and populate the culture [44]. If a mutant underwent gasping during aging of the pooled collection, then it would register an artificially high abundance ratio, and fail to be long-lived when individually retested. Another possibility that would be unique to the mixed population approach is the introduction of competition between the strains, where mutants with improved overall fitness could have an advantage that is lost when they are retested individually. In a related scenario, certain mutants in the mixed population are likely highly resistant or overly sensitive to changes in medium composition (such as acetic accumulation) that occurred as the cultures were aging. Certain mutants could directly influence the medium composition, thus altering the lifespan of the highly sensitive mutants in the process. A good example is the ade4Δ mutant, whose expired SC medium extended the lifespan of the WT and atg16Δ strains (Figure 6), possibly through the reduction of acetic acid accumulation (Figure 8A). In applying the microarray/barcode approach to other aging or age-related problems, it is likely that the amount of variability would be more limited with the addition of duplicate or triplicate screens. However, even with the inherent variability, this microarray screen successfully identified several novel longevity regulators that will be the subject of future studies.
Materials and Methods
Yeast strains and media
Yeast strains used in this study were isogenic to the haploid strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), and were obtained from the yeast gene knockout collection [19]. The ade16Δ ade17Δ mutant strain (Y1093) was kindly provided by Bertrand Daignon-Fornier [68]. Most in vivo assays were performed in synthetic complete (SC) medium following the recipe provided in the Cold Spring Harbor Yeast Genetics Course Manual [69], and sold by QBioGene as “Hopkins mix”. The alternative SC medium is derived from Current Protocols in Molecular Biology [41], which we refer to as “CPMB” mix. Chemical compositions of the various SC media types are listed in Table S6. Glucose was added to the SC media to a final concentration of either 0.5% (CR-Calorie Restricted) or 2% (NR-Non Restricted). Where indicated, the Hopkins mix SC medium was buffered to pH 6.0 with a citrate phosphate buffer (6.42 mM Na2HPO4 and 1.79 mM citric acid, final concentration), as previously described [43]. For buffering the medium at day 2, a 10× concentrate of the citrate phosphate buffer was added to SC. For pH measurements of expired media, small aliquots were removed from the cultures and then discarded to prevent contamination of the long-term culture.
Genetic screen for longevity and TAG–microarray analysis
To begin the screen, 1 ml (15 OD600 units) of the pooled haploid knockout collection was inoculated into 200 ml SC medium containing either 2% glucose (NR) or 0.5% glucose (CR). The next day (day 0), aliquots of 100 µl were transferred into 10 ml of fresh SC-NR and SC-CR media, respectively. Twenty such cultures were inoculated for each glucose concentration and allowed to age at 30°C in the roller drum to provide aeration [16]. Starting with day 1 (D1), 100 µl of each culture was plated onto YPD plates every 3 days to allow viable cells in the population to re-grow. These YPD plates were incubated at 30°C for 2 days and the cell lawns harvested by scraping and pooled together, then washed with ice cold water and stored at −80°C. Once the time course was completed (day 33), genomic DNA was isolated from the cell pellets [41].
The UP - and DNTAGs were labeled with Cy5 (day 1) or Cy3 (days 9, 21, and 33) by PCR amplification of genomic DNA using primer pairs U1/U2 and D1/D2, respectively, as previously described [70]. The Cy5-labeled UP - and DNTAGs from day 1 were then co-hybridized with the Cy3-labeled UP - and DNTAGSs on custom-designed “Hopkins TAG-arrays” from Agilent Technologies (AMADID 011443) as previously described [70]. Fluorescence signal intensities were measured by scanning the arrays with a Genepix 4000B instrument coupled with GenePix Pro software. The signal intensity ratios were then calculated for days 9, 21, and 33 compared to day 1 as the control using Microsoft Excel. The signal ratios for all essential genes on the array were averaged and considered the background. Any non-essential genes with up - or down-tag ratios lower than this background average were eliminated from the analysis, thus ensuring that only genes with signals from both tags were included (2715 genes, which included most of those in the DNTAG list in Table S1). Box plots of the ratios in Figure 1 were assembled from the 3478 genes in the UPTAG list (Table S1) using R Software. Mutants with similar average NR and CR log ratios were identified by applying two criteria to their values at every time point: (1) ratios were within 10% of each other and (2) the null hypothesis that were the same according to a t-test. In the case of (1), we calculated the fractional difference between the average NR and CR log ratios (i.e., difference between these values divided by their average). The absolute value of the fractional difference was required to be less than 0.1. We then applied a t-test to the NR and CR log ratios and required their p-value to be less than 0.05 (i.e., their means are not significantly different).
Chronological life span assays
Quantitative (colony forming unit) and semi-quantitative (10-fold serial dilution spot-test) chronological life span (CLS) assays were performed as previously described [16]. For the media swap experiments, the 10 ml cultures were grown for 5 days. The cultures were then pelleted in a swinging bucket rotor (2500 RPM) at room temperature in an Eppendorf 5810R tabletop centrifuge. The supernatants were removed and passed through a 0.2 micron syringe filter prior to the swap.
Acetic acid measurements and treatments
For the measurement of acetic acid concentration in growth media, cells were grown in the appropriate SC medium (10 ml in culture tubes) to the indicated time points. Log phase cells (OD600 of 0.8) and cells grown to day 2 and day 5 were pelleted by centrifugation, and the clarified media was passed through a 0.2 micron syringe filter. The filtrate was used for measuring the acetic acid concentration using an Acetic Acid Kit (R-Biopharm AG, Darmstadt, Germany), following the manufacturer's directions. Three biological replicas were assayed for each condition to provide mean millimolar concentrations and standard deviations. To determine sensitivity/resistance of the mutant strains to exogenously added acetic acid, cultures were challenged for 200 minutes with 300 mM acetic acid either at day 2 or day 5 of the CLS assay. Cells were diluted in water and then spread onto YPD plates to allow viable cells to grow into colonies, which were then counted. The percent survival was calculated by dividing the colony forming units (CFU) of the treated samples by the untreated samples. Three biological replicates were tested for each condition.
Supporting Information
Zdroje
1. PartridgeL
2008 Some highlights of research on aging with invertebrates, 2008. Aging Cell 7 605 608
2. HamiltonB
DongY
ShindoM
LiuW
OdellI
2005 A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19 1544 1555
3. HansenM
HsuAL
DillinA
KenyonC
2005 New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1 e17 doi:10.1371/journal.pgen.0010017
4. KaeberleinM
McVeyM
GuarenteL
1999 The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13 2570 2580
5. MortimerRK
JohnstonJR
1959 Life span of individual yeast cells. Nature 183 1751 1752
6. FabrizioP
PozzaF
PletcherSD
GendronCM
LongoVD
2001 Regulation of longevity and stress resistance by Sch9 in yeast. Science 292 288 290
7. FabrizioP
PletcherSD
MinoisN
VaupelJW
LongoVD
2004 Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett 557 136 142
8. PowersRW3rd
KaeberleinM
CaldwellSD
KennedyBK
FieldsS
2006 Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 20 174 184
9. KaeberleinM
PowersRW3rd
SteffenKK
WestmanEA
HuD
2005 Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310 1193 1196
10. BonawitzND
Chatenay-LapointeM
PanY
ShadelGS
2007 Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 5 265 277
11. VellaiT
Takacs-VellaiK
ZhangY
KovacsAL
OroszL
2003 Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426 620
12. McCayCM
CrowellMF
1934 Prolonging the life span. Sci Mon 39 405 414
13. JiangJC
JarugaE
RepnevskayaMV
JazwinskiSM
2000 An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J 14 2135 2137
14. LinS-J
DefossezP-A
GuarenteL
2000 Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289 2126 2128
15. FabrizioP
GattazzoC
BattistellaL
WeiM
ChengC
2005 Sir2 blocks extreme life-span extension. Cell 123 655 667
16. SmithDLJr
McClureJM
MatecicM
SmithJS
2007 Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell 6 649 662
17. LinSJ
KaeberleinM
AndalisAA
SturtzLA
DefossezPA
2002 Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418 344 348
18. LinYY
LuJY
ZhangJ
WalterW
DangW
2009 Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 136 1073 1084
19. WinzelerEA
ShoemakerDD
AstromoffA
LiangH
AndersonK
1999 Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901 906
20. GrayJV
PetskoGA
JohnstonGC
RingeD
SingerRA
2004 “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 68 187 206
21. HansenM
ChandraA
MiticLL
OnkenB
DriscollM
2008 A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4 e24 doi:10.1371/journal.pgen.0040024
22. JiaK
LevineB
2007 Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy 3 597 599
23. SchilkeB
VoisineC
BeinertH
CraigE
1999 Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 96 10206 10211
24. KosmanDJ
2003 Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47 1185 1197
25. PhilpottCC
ProtchenkoO
KimYW
BoretskyY
Shakoury-ElizehM
2002 The response to iron deprivation in Saccharomyces cerevisiae: expression of siderophore-based systems of iron uptake. Biochem Soc Trans 30 698 702
26. AndersonJB
SirjusinghC
ParsonsAB
BooneC
WickensC
2003 Mode of selection and experimental evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 163 1287 1298
27. CondeR
PabloG
CuevaR
LarribaG
2003 Screening for new yeast mutants affected in mannosylphosphorylation of cell wall mannoproteins. Yeast 20 1189 1211
28. KitagakiH
WuH
ShimoiH
ItoK
2002 Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphosphatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol Microbiol 46 1011 1022
29. PontingCP
1997 P100, a transcriptional coactivator, is a human homologue of staphylococcal nuclease. Protein Sci 6 459 463
30. FerreiraT
BrethesD
PinsonB
NapiasC
ChevallierJ
1997 Functional analysis of mutated purine-cytosine permease from Saccharomyces cerevisiae. A possible role of the hydrophilic segment 371-377 in the active carrier conformation. J Biol Chem 272 9697 9702
31. GuetsovaML
LecoqK
Daignan-FornierB
1997 The isolation and characterization of Saccharomyces cerevisiae mutants that constitutively express purine biosynthetic genes. Genetics 147 383 397
32. KurtzJE
ExingerF
ErbsP
JundR
1999 New insights into the pyrimidine salvage pathway of Saccharomyces cerevisiae: requirement of six genes for cytidine metabolism. Curr Genet 36 130 136
33. MalmancheN
ClarkDV
2004 Drosophila melanogaster Prat, a purine de novo synthesis gene, has a pleiotropic maternal-effect phenotype. Genetics 168 2011 2023
34. RolfesRJ
2006 Regulation of purine nucleotide biosynthesis: in yeast and beyond. Biochem Soc Trans 34 786 790
35. TibbettsAS
ApplingDR
1997 Saccharomyces cerevisiae expresses two genes encoding isozymes of 5-aminoimidazole-4-carboxamide ribonucleotide transformylase. Arch Biochem Biophys 340 195 200
36. ReboraK
DesmoucellesC
BorneF
PinsonB
Daignan-FornierB
2001 Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. Mol Cell Biol 21 7901 7912
37. Daignan-FornierB
FinkGR
1992 Coregulation of purine and histidine biosynthesis by the transcriptional activators BAS1 and BAS2. Proc Natl Acad Sci U S A 89 6746 6750
38. ZhangF
KirouacM
ZhuN
HinnebuschAG
RolfesRJ
1997 Evidence that complex formation by Bas1p and Bas2p (Pho2p) unmasks the activation function of Bas1p in an adenine-repressible step of ADE gene transcription. Mol Cell Biol 17 3272 3283
39. DenisV
BoucherieH
MonribotC
Daignan-FornierB
1998 Role of the myb-like protein bas1p in Saccharomyces cerevisiae: a proteome analysis. Mol Microbiol 30 557 566
40. ReboraK
LalooB
Daignan-FornierB
2005 Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics 170 61 70
41. AusubelFM
BrentR
KingstonRE
MooreDD
SeidmanJG
2000 Current Protocols in Molecular Biology. New York John Wiley & Sons, Inc
42. JakubowskiH
GoldmanE
1988 Evidence for cooperation between cells during sporulation of the yeast Saccharomyces cerevisiae. Mol Cell Biol 8 5166 5178
43. BurtnerCR
MurakamiCJ
KennedyBK
KaeberleinM
2009 A molecular mechanism for chronological aging in yeast. Cell Cycle 8 1256 1270
44. FabrizioP
BattistellaL
VardavasR
GattazzoC
LiouLL
2004 Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol 166 1055 1067
45. YorimitsuT
KlionskyDJ
2005 Autophagy: molecular machinery for self-eating. Cell Death Differ 12 Suppl. 2 1542 1552
46. JuhaszG
ErdiB
SassM
NeufeldTP
2007 Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev 21 3061 3066
47. AlversA
FishwickL
WoodM
HuD
ChungH
2009 Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 8 353 369
48. TangF
WatkinsJW
BermudezM
GrayR
GabanA
2008 A life-span extending form of autophagy employs the vacuole-vacuole fusion machinery. Autophagy 4 874 886
49. MelendezA
TalloczyZ
SeamanM
EskelinenEL
HallDH
2003 Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 301 1387 1391
50. DrogeW
2004 Autophagy and aging. Mech Aging Dev 125 161 168
51. TakeshigeK
BabaM
TsuboiS
NodaT
OhsumiY
1992 Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119 301 311
52. AlversA
WoodM
HuD
KaywellA
WDJr
2009 Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy 5 847 849
53. Reverter-BranchatG
CaiscolE
TamaritJ
RosJ
2004 Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae. J Biol Chem 279 31983 31989
54. LeeYL
LeeCK
2008 Transcriptional response according to strength of calorie restriction in Saccharomyces cerevisiae. Mol Cells 26 299 307
55. HieterP
MannC
SnyderM
DavisR
1985 Mitotic stability of yeast chromosomes: A colony color assay that measures nondisjunction and chromosome loss. Cell 40 381 392
56. KoshlandD
KentJ
HartwellL
1985 Genetic analysis of the mitotic transmission of minichromosomes. Cell 40 393 403
57. GauthierS
CoulpierF
JourdrenL
MerleM
BeckS
2008 Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol Microbiol 68 1583 1594
58. HagopianK
RamseyJJ
WeindruchR
2003 Influence of age and caloric restriction on liver glycolytic enzyme activities and metabolite concentrations in mice. Exp Gerontol 38 253 266
59. FabrizioP
LongoVD
2003 The chronological life span of Saccharomyces cerevisiae. Aging Cell 2 73 81
60. SullivanJE
BrocklehurstKJ
MarleyAE
CareyF
CarlingD
1994 Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353 33 36
61. NarbonneP
RoyR
2009 Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457 210 214
62. SchulzT
ZarseK
VoigtA
UrbanN
BirringerM
2007 Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6 280 293
63. WilsonWA
HawleySA
HardieDG
1996 Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol 6 1426 1434
64. WangZ
WilsonWA
FujinoMA
RoachPJ
2001 Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol 21 5742 5752
65. WrightRM
PoytonRO
1990 Release of two Saccharomyces cerevisiae cytochrome genes, COX6 and CYC1, from glucose repression requires the SNF1 and SSN6 gene products. Mol Cell Biol 10 1297 1300
66. WuJ
QuijanoC
ChenE
LiuH
CaoL
2009 Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by disruption of autophagy. Aging 1 425 437
67. MurakamiCJ
BurtnerCR
KennedyBK
KaeberleinM
2008 A method for high-throughput quantitative analysis of yeast chronological life span. J Gerontol A Biol Sci Med Sci 63 113 121
68. PinsonB
VaurS
SagotI
CoulpierF
LemoineS
2009 Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev 23 1399 1407
69. BurkeD
DawsonD
StearnsT
2000 Methods in Yeast Genetics. Cold Spring Harbor Cold Spring Harbor Laboratory Press. 205
70. PanX
YuanDS
OoiSL
WangX
Sookhai-MahadeoS
2007 dSLAM analysis of genome-wide genetic interactions in Saccharomyces cerevisiae. Methods 41 206 221
71. GellingCL
PiperMD
HongSP
KornfeldGD
DawesIW
2004 Identification of a novel one-carbon metabolism regulon in Saccharomyces cerevisiae. J Biol Chem 279 7072 7081
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



