-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAllele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
Plant resistance (R) proteins provide a robust surveillance system to defend against potential pathogens. Despite their importance in plant innate immunity, relatively few of the ∼170 R proteins in Arabidopsis have well-characterized resistance specificity. In order to identify the R protein responsible for recognition of the Pseudomonas syringae type III secreted effector (T3SE) HopZ1a, we assembled an Arabidopsis R gene T–DNA Insertion Collection (ARTIC) from publicly available Arabidopsis thaliana insertion lines and screened it for plants lacking HopZ1a-induced immunity. This reverse genetic screen revealed that the Arabidopsis R protein HOPZ-ACTIVATED RESISTANCE 1 (ZAR1; At3g50950) is required for recognition of HopZ1a in Arabidopsis. ZAR1 belongs to the coiled-coil (CC) class of nucleotide binding site and leucine-rich repeat (NBS–LRR) containing R proteins; however, the ZAR1 CC domain phylogenetically clusters in a clade distinct from other related Arabidopsis R proteins. ZAR1–mediated immunity is independent of several genes required by other R protein signaling pathways, including NDR1 and RAR1, suggesting that ZAR1 possesses distinct signaling requirements. The closely-related T3SE protein, HopZ1b, is still recognized by zar1 Arabidopsis plants indicating that Arabidopsis has evolved at least two independent R proteins to recognize the HopZ T3SE family. Also, in Arabidopsis zar1 plants HopZ1a promotes P. syringae growth indicative of an ancestral virulence function for this T3SE prior to the evolution of recognition by the host resistance protein ZAR1. Our results demonstrate that the Arabidopsis resistance protein ZAR1 confers allele-specific recognition and virulence attenuation of the Pseudomonas syringae T3SE protein HopZ1a.
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000894
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000894Summary
Plant resistance (R) proteins provide a robust surveillance system to defend against potential pathogens. Despite their importance in plant innate immunity, relatively few of the ∼170 R proteins in Arabidopsis have well-characterized resistance specificity. In order to identify the R protein responsible for recognition of the Pseudomonas syringae type III secreted effector (T3SE) HopZ1a, we assembled an Arabidopsis R gene T–DNA Insertion Collection (ARTIC) from publicly available Arabidopsis thaliana insertion lines and screened it for plants lacking HopZ1a-induced immunity. This reverse genetic screen revealed that the Arabidopsis R protein HOPZ-ACTIVATED RESISTANCE 1 (ZAR1; At3g50950) is required for recognition of HopZ1a in Arabidopsis. ZAR1 belongs to the coiled-coil (CC) class of nucleotide binding site and leucine-rich repeat (NBS–LRR) containing R proteins; however, the ZAR1 CC domain phylogenetically clusters in a clade distinct from other related Arabidopsis R proteins. ZAR1–mediated immunity is independent of several genes required by other R protein signaling pathways, including NDR1 and RAR1, suggesting that ZAR1 possesses distinct signaling requirements. The closely-related T3SE protein, HopZ1b, is still recognized by zar1 Arabidopsis plants indicating that Arabidopsis has evolved at least two independent R proteins to recognize the HopZ T3SE family. Also, in Arabidopsis zar1 plants HopZ1a promotes P. syringae growth indicative of an ancestral virulence function for this T3SE prior to the evolution of recognition by the host resistance protein ZAR1. Our results demonstrate that the Arabidopsis resistance protein ZAR1 confers allele-specific recognition and virulence attenuation of the Pseudomonas syringae T3SE protein HopZ1a.
Introduction
The retaliatory arms race between host and pathogen has molded the evolution of host immune responses and bacterial virulence strategies. The primary virulence mechanism of Gram-negative bacteria such as Pseudomonas syringae is the type III secretion system (T3SS) that allows for the translocation of type III secreted effector (T3SE) proteins directly into plant cells [1]. T3SEs may promote bacterial proliferation by manipulating host physiology or by suppressing host defenses [2]–[6]. However T3SEs can also betray the bacteria to the plant host by activating effector triggered immunity (ETI) [7]. ETI is a branch of plant immunity in which Resistance (R) proteins recognize specific effector proteins resulting in an effective immune response which is often accompanied by a rapid, localized cell death termed the hypersensitive response (HR) [8],[9]. Resistance proteins have been demonstrated to recognize T3SE proteins in two ways. In one case, the Ralstonia solanacearum T3SE PopP2 interacts directly with its cognate R protein RRS1-R [10]. As well, the Xanthomonas campestris T3SE AvrBs3 binds directly to the promoter of its cognate R gene Bs3, as Bs3 has evolved to mimic virulence targets of AvrBs3 [11],[12]. In most cases however, the resistance protein indirectly recognizes the T3SE by interacting with a host target of the T3SE [9]. In the indirect mode of recognition, R proteins monitor a specific host T3SE target and ETI is initiated when this target is modified by the T3SE [9],[13]. Evolutionary pressure by pathogens has caused the expansion of several R protein families and the diversification of the signaling components which they employ [14].
R proteins are typically defined as having a nucleotide-binding-site (NBS) and leucine-rich-repeat (LRR) domain [15],[16]. In addition to the NBS-LRR domains, the N-terminal region is usually a coiled-coil (CC) domain or a TIR domain, named according to its homology to the Drosophila Toll and mammalian interleukin-1 receptors. Genetic studies of several Arabidopsis R genes have revealed important components of ETI signaling pathways [17]–[19]. ETI induced by CC-NBS-LRR class R proteins like RPS2, RPM1 and RPS5 requires NDR1, a membrane-localized glycosylphatidylinositol (GPI)-anchored protein [20]–[22]. TIR-NBS-LRR R proteins act through EDS1 and its interacting partner PAD4 and include R proteins recognizing effectors from P. syringae (RPS4) and the oomycete Hyaloperonospora arabidopsidis (RPP2, RPP4, RPP5, RPP21) [23]–[25]. R protein stability and accumulation can be mediated by SGT1 and its interacting partner RAR1, which was initially identified by its role in resistance to powdery mildews in barley [26]–[32]. In addition, salicylic acid (SA) and reactive oxygen species have been differentially implicated in the development of ETI and/or its corresponding HR [33],[34]. From the study of several Arabidopsis R proteins it is apparent that multiple ETI signaling pathways exist and more are likely to be uncovered as the ∼170 putative Arabidopsis R proteins are characterized further.
The HopZ family of P. syringae T3SE proteins is part of the larger YopJ superfamily with homologues in Yersinia pestis and Xanthomonas species [2],[35]. Evolutionary analyses demonstrated that the P. syringae pv. syringae (Psy) effector HopZ1aPsyA2 (formerly HopPsyH, hereafter HopZ1a) is most similar to the ancestral allele of the P. syringae HopZ family [35]. YopJ, the founding member of the HopZ/YopJ superfamily, has recently been shown to possess acetyltransferase activity [36]–[38]. YopJ acetylates serine and threonine residues of MAP kinase family members, which blocks the phosphorylation site needed for downstream immune signaling [37],[38]. Similar to YopJ, HopZ1a contains a canonical catalytic triad shared by proteases and acetyltransferases and requires the cysteine residue of this triad for enzymatic activity in a fluorescence-based protease assay [35]. HopZ1a induces defense responses characteristic of ETI in diverse plant hosts, including Arabidopsis, rice, sesame and soybean [35],[39]. The catalytic triad of HopZ1a is required for its recognition in Arabidopsis, indicating that it is recognized via its enzymatic activity [39]. Recognition of HopZ1a-induced immunity is induced independently of the characterized Arabidopsis R proteins RPM1, RPS2, RPS5 and RPS4 [39].
In this study, we demonstrate that the CC-NBS-LRR R gene, HOPZ-ACTIVATED RESISTANCE 1 (ZAR1), is required for recognition of the P. syringae T3SE HopZ1a. We constructed an Arabidopsis R gene T-DNA insertion collection (ARTIC), which was used in a reverse genetic screen to identify ZAR1. T-DNA insertions in the ZAR1 locus result in the loss of HopZ1a recognition, as seen by macroscopic HR assays, trypan blue staining, ion leakage and bacterial growth in planta. Using plants mutated in known signaling components SGT1a, SGT1b, NDR1, RAR1, EDS1, PAD4, RBOHD/F, EDS16 or EDM2, we demonstrate that HopZ1a-induced immunity employs an uncharacterized ETI signaling pathway. Phylogenetic analyses using the ZAR1 CC domain showed that the closest homologues to ZAR1 are from divergent plant species, including Ricinus communis (castor bean), Populus trichocarpa (poplar), Vitis vinifera (grape) and Solanum melongen (eggplant), rather than Arabidopsis. Interestingly, in Arabidopsis plants genetically lacking ZAR1, HopZ1a acts as a virulence factor by promoting bacterial growth, supporting an ancestral virulence function prior to the evolution of ZAR1-mediated immunity. The closely-related HopZ1a family member, HopZ1b, is still recognized in the zar1 knockout demonstrating that Arabidopsis R proteins have diversified to recognize the HopZ family of T3SEs.
Results
HopZ1a-induced immunity is independent of known Arabidopsis resistance signaling genes
We previously demonstrated that HopZ1a induces a resistance response and an associated hypersensitive response in Arabidopsis that is characteristic of effector triggered immunity (ETI) using macroscopic HR assays, trypan blue staining, conductivity assays and bacterial growth assays [35],[39]. Expression of the HopZ1a catalytic mutant (HopZ1aC216A, hereafter HopZ1aC/A) no longer induced ETI [39]. We further showed that this resistance response is independent of known R genes RPM1, RPS2, RPS5, RPS4, RPS6 and the RPM1-interacting protein RIN4 indicating that HopZ1a-induced immunity may involve a novel signaling pathway [39; Lewis et al., unpublished]. To further examine this possibility we investigated HopZ1a-induced immunity in a larger collection of R gene-signaling mutant plants (Table 1).
Tab. 1. <i>Arabidopsis</i> Resistance signaling genes addressed in this study. 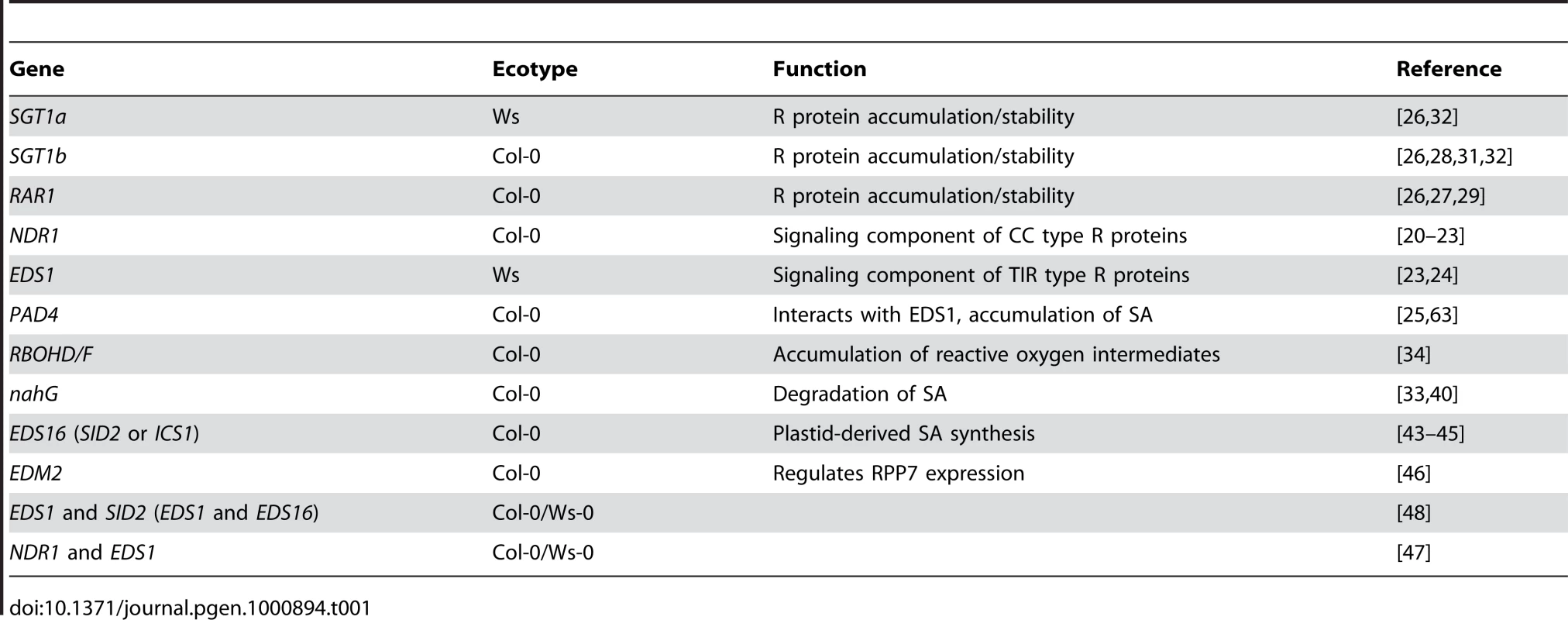
We examined the ability of HopZ1a to induce an ETI-associated hypersensitive response (HR) in Arabidopsis lines with characterized mutations in various defense signaling and response pathways. We tested sgt1a, sgt1b, ndr1rar1, eds1 or pad4 plants by pressure infiltrating each with P. syringae pv. tomato DC3000 (PtoDC3000) carrying a plasmid encoding hopZ1a controlled by its native promoter (Figure 1A). All of these plants displayed a macroscopic HopZ1a-induced HR indicating that these genes do not contribute to HopZ1a-recognition. In contrast, our control infiltration of PtoDC3000 carrying the T3SE AvrRpt2 under the nptII promoter did not induce an HR in ndr1rar1 plants as expected [23],[29].
Fig. 1. HopZ1a recognition is independent of known signaling components of R gene- mediated immunity. 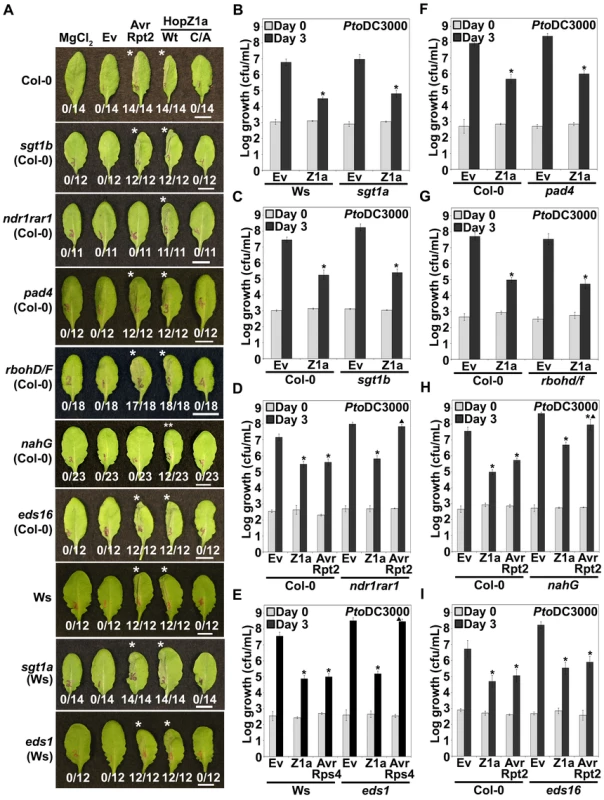
(A) Half-leaves of Arabidopsis Col-0, Ws-0 or mutant plants were infiltrated with 10 mM MgCl2 or with PtoDC3000 expressing the empty vector (Ev), AvrRpt2, or HopZ1a or HopZ1aC216A (C/A) with a C-terminal HA tag under its endogenous promoter. C216 of HopZ1a is part of the predicted catalytic triad and the mutant protein is expressed at a similar level to HopZ1a [39]. The bacteria were syringe infiltrated into the leaves at 5×107 cfu/mL. Photos were taken 22 hours post-infiltration. The number of leaves showing an HR is indicated below the appropriate construct. HRs are marked with an asterisk. Patchy HRs are marked with a double asterisk. Scale bar is 1 cm. (B–I) PtoDC3000 expressing the indicated construct was syringe infiltrated at 1×105 cfu/mL into Arabidopsis Col-0 or mutant leaves and bacterial counts were determined one hour post-infection (Day 0) and 3 days post-infection (Day 3). Two-tailed homoschedastic t-tests were performed to test for significant differences. Within a plant genotype, treatments were compared to empty vector and significant differences are indicated by an asterisk (* P<0.01). To compare between plant genotypes, growth of PtoDC3000 carrying HopZ1a, AvrRpt2 or AvrRps4 was normalized to the average growth of PtoDC3000(Ev). Significant differences in growth of a P. syringae strain between a mutant genotype and wild type Col-0 or Ws are indicated by a triangle (▴ P<0.01). Error bars indicate the standard deviation from the mean of 10 samples. Growth assays were performed at least 3 times. Arabidopsis genotypes are: (B) sgt1a (C) sgt1b (D) ndr1rar1 (E) eds1 (F) pad4 (G) rbohd/f (H) nahG (I) eds16. Other genes involved in the defense response against pathogens include RBOHD and RBOHF, which contribute to reactive oxygen species production [34]. HopZ1a-mediated HR was retained in rbohd/f plants. The plant hormone salicylic acid (SA), which plays a number of critical roles in the defense response, is degraded in nahG transgenic lines via a bacterial salicylate hydroxylase [40]. The HR induced by HopZ1a was partially compromised in nahG plants, with a patchy HR observed in 52% of leaves, whereas the HR induced by AvrRpt2 was completely abrogated [40]. The nahG transgene is known to have pleiotropic effects on plant development, and the breakdown products of salicylic acid suppress resistance responses in Arabidopsis [41],[42]. We therefore also examined HopZ1a-induced defense responses in eds16 plants (also called sid2 or ics1) impaired in the isochorismate synthase responsible for the synthesis of SA during plant immunity [43]–[45]. In contrast to nahG plants, eds16 plants still displayed both HopZ1a - and AvrRpt2-mediated HRs. The gene EDM2 contributes to RPP7-mediated resistance against H. arabidopsidis by maintaining transcript levels of RPP7 [46]. RPP7 resistance does not depend on salicylic acid [46]. In the edm2-2 plants, we still observed a HopZ1a-mediated HR (Figure S1).
Some ETI responses have been demonstrated to cooperatively require disease resistance signaling components [47],[48]. We therefore examined several double mutants for the production of the HopZ1a HR. EDS1 and SID2 (or EDS16) are both necessary for resistance mediated by RPS2 against P. syringae, RPP8 against H. arabidopsidis, and HRT against Turnip Crinkle Virus [48]. eds1sid2 plants still displayed a HopZ1a-induced HR (Figure S1). We also examined the ndr1eds1 mutant, which displays slight impairment of RPP7 - and RPP8-mediated immunity to H. arabidopsidis [47]. We still observed a HopZ1a-induced HR in the ndr1eds1 mutant (Figure S1). Thus, HopZ1a-induced HR is not dependent on R gene-mediated signaling genes SGT1a, SGT1b, NDR1, RAR1, EDS1, PAD4, RBOHD/F, EDS16 or EDM2, and does not require the cooperative action of EDS1 and SID2, or EDS1 and NDR1.
To further quantify the extent of HopZ1a-mediated immunity in Arabidopsis, we compared the in planta growth of the virulent strain PtoDC3000 carrying an empty vector (Ev) to the same strain carrying hopZ1a under the control of its native promoter over the course of three days. HopZ1a caused a 2.0–3.0 log reduction in growth in sgt1a, sgt1b, ndr1rar1, eds1 or pad4 plants comparable to that observed in Ws (for sgt1a and eds1) or Col-0 (for sgt1b, ndr1rar1 and pad4) wild type backgrounds indicating that HopZ1a-mediated resistance is retained in these mutant plants (Figure 1B–1F). As expected, AvrRpt2 and AvrRps4 resistance was abrogated in ndr1rar1 and eds1 mutant plants, respectively [23],[29] (Figure 1D and 1E). Similarly, in rbohD/F plants PtoDC3000(hopZ1a) exhibited typical low levels of bacterial growth (4.5–5.0 logs), comparable to the HopZ1a resistance observed in Col-0 wild type plants (Figure 1G). These experiments provide further support that HopZ1a-mediated immunity does not act through SGT1a, SGT1b, NDR1, RAR1, EDS1, PAD4, or RBOHD/F.
Consistent with the partial loss of HR observed in nahG plants, resistance to both HopZ1a and AvrRpt2 was impaired in the nahG transgenic line (Figure 1H). PtoDC3000(hopZ1a) and PtoDC3000(avrRpt2) exhibited ∼1.5 log and 2.0–2.5 log more growth in nahG than in Col-0 plants, respectively. In contrast to nahG plants, PtoDC3000(hopZ1a) induced a typical defense response in eds16, with a 2.0–2.5 log reduction in bacterial growth, similar to wild type Col-0 plants (Figure 1I). PtoDC3000(avrRpt2) also exhibited a typical defense in eds16, as has been previously observed [43]. Our results show that the HopZ1a-induced HR is not dependent on the plastid-source of SA and that partial impairment of resistance in the nahG background may be due to the pleiotropic effects of nahG on plant development or immunity (see Discussion). In summary, HopZ1a-induced HR and immunity are not dependent on the R gene-mediated signaling genes SGT1a, SGT1b, NDR1, RAR1, EDS1, PAD4, RBOHD/F, EDS16 or EDM2.
The type III effector HopZ1a is recognized by the Arabidopsis ZAR1 resistance protein
We used a reverse genetics approach to identify the R gene responsible for HopZ1a recognition. We generated an Arabidopsis R gene T-DNA insertion collection (ARTIC) comprising publicly available T-DNA insertion lines (or if necessary transposon insertion lines) for all of the canonical R genes identified from the Arabidopsis thaliana Col-0 genome [16],[49]. In order to maximize the chance of obtaining a knock-out line for an individual R gene, preference was given to T-DNA or transposon insertion lines with a high confidence insertion in the locus of the gene of interest (and no other known loci) and preferably an insertion in an exon near the beginning of the gene. If there was no T-DNA or transposon insertion in an exon, lines were chosen in the following order of preference: 5′UTR>3′UTR> within 1000 nt upstream of the start codon (1000-promoter)>intron. T-DNA or transposon insertions were available for 166/170 R genes. Lines were chosen primarily from the Salk [50] and Sail [51] T-DNA insertion collections, with a few representatives from the WiscDsLox [52], and GT [53] transposon insertion collections. ARTIC includes homozygous individuals from 118 Salk lines, 13 Sail lines and 1 WiscDsLox line, as well as heterozygous individuals from 17 Salk and 7 Sail lines (Table S1).
To identify the R gene responsible for HopZ1a recognition, we infiltrated T-DNA insertion lines from ARTIC with PtoDC3000(hopZ1a) and screened for a loss of the HopZ1a-induced HR. One line, SALK_013297 (hereafter referred to as zar1-1), did not develop a HopZ1a-induced HR but was still competent in initiating an AvrRpt2-mediated HR (Figure 2A). We confirmed by sequencing that the T-DNA insertion in Arabidopsis zar1-1 plants was found in the gene At3g50950, which we refer to as HopZ-Activated Resistance or ZAR1. To confirm that ZAR1 was responsible for recognition of HopZ1a, we obtained additional T-DNA insertion lines in At3g50950 and examined them for HopZ1a-induced immunity. We identified four additional alleles of zar1 (Figure 2B) and genotyped them to identify homozygous lines (data not shown). All of the additional zar1 T-DNA insertion lines lacked a macroscopic HR in response to PtoDC3000(hopZ1a) but not PtoDC3000(avrRpt2) confirming the requirement of ZAR1 for HopZ1a-mediated immunity (Figure 2C). To show that the HopZ1a protein is delivered into zar1 plant cells, we performed HR assays in Col-0 and zar1 using a HopZ1a chimeric fusion to the C-terminus of AvrRpt2 (amino acids 80–255) [54], which is recognized by the RPS2 resistance protein in Arabidopsis Col-0 [55],[56]. The HopZ1a-AvrRpt2Δ1-79 fusion still causes a strong HR in Col-0 and zar1-1 demonstrating that lack of recognition of HopZ1a in zar1 plants is not due to lack of HopZ1a translocation (Figure S2). We also tested zar1 plants for recognition of the endogenous HopZ1a allele carried by P. syringae pv. syringae strain A2 (PsyA2) [35]. In Col-0 plants, PsyA2 causes a macroscopic HR (Figure S3) as previously described [35]. In zar1-1 plants, we no longer observed a macroscopic HR, demonstrating that zar1 is responsible for recognition of the HopZ1a native strain, PsyA2 (Figure S3).
Fig. 2. ZAR1 recognizes HopZ1a in Arabidopsis. 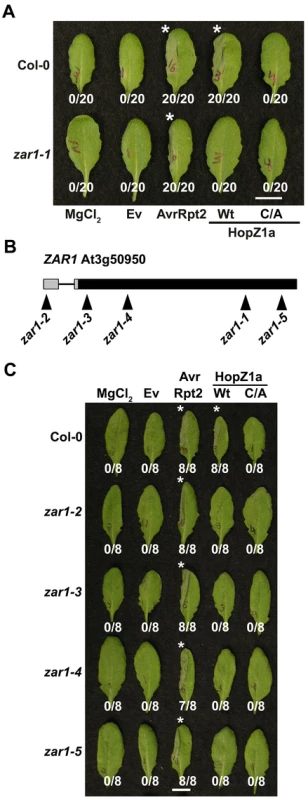
(A) Half-leaves of Arabidopsis Col-0 or zar1-1 plants were infiltrated with 10 mM MgCl2 or with PtoDC3000 expressing the empty vector (Ev), AvrRpt2, or HopZ1a or HopZ1aC216A (C/A) with a C-terminal HA tag under its endogenous promoter. C216 of HopZ1a is part of the predicted catalytic triad and the mutant protein is expressed at a similar level to HopZ1a [39]. The bacteria were syringe infiltrated into the leaves at 5×107 cfu/mL. Photos were taken 22 hours post-infiltration. The number of leaves showing an HR is indicated below the appropriate construct. HRs are marked with an asterisk. Scale bar is 1 cm. (B) At3g50950 is ZAR1. The promoter is shown by grey boxes and the exon by a large black box. There is an intron in the promoter, shown by a black line. The position of the T-DNA insertion lines is shown below the locus. (C) Half-leaves of Arabidopsis Col-0, zar1-2, zar1-3, zar1-4, or zar1-5 plants were infiltrated with 10 mM MgCl2 or with PtoDC3000 expressing the empty vector (Ev), AvrRpt2, or HopZ1a or HopZ1aC216A (C/A) with a C-terminal HA tag under its endogenous promoter. We further verified that HopZ1a-induced immunity and HR were abrogated in zar1-1 plants via a series of qualitative and quantitative avirulence assays. Trypan blue stain is only retained in dead and/or dying cells, and therefore is a qualitative measure of the HR-associated cell death. Heavy trypan blue staining indicative of an HR was observed in zar1-1 leaves infiltrated with PtoDC3000(avrRpt2) and Col-0 leaves infiltrated with PtoDC3000(hopZ1a) or PtoDC3000(avrRpt2) at 12 hours post-infection (Figure 3A). However, zar1-1 leaves infiltrated with PtoDC3000(hopZ1a) did not result in any significant staining with trypan blue indicating the lack of an HR. As a quantitative measure of the HR, we monitored HR-associated electrolyte leakage as measured by changes in media conductivity (Figure 3B). PtoDC3000(hopZ1a) or PtoDC3000(avrRpt2) infiltrated Col-0 leaves increased conductivity by twice as much as PtoDC3000(Ev) at 16 hours post-infection, indicative of an HR, and both were significantly different from PtoDC3000(Ev) in Col-0 (Figure 3B). In the zar1-1 mutant, increased conductivity was observed from leaves infiltrated with PtoDC3000(avrRpt2) but not PtoDC3000(hopZ1a) (Figure 3B). The conductivity measured from PtoDC3000(hopZ1a) infiltrated zar1-1 was significantly different from PtoDC3000(hopZ1a) in Col-0 and was not significantly different from that of Col-0 or zar1-1 leaves infiltrated with PtoDC3000(Ev), demonstrating that HopZ1a associated electrolyte leakage is abrogated in zar1 plants.
Fig. 3. zar1-1 Arabidopsis plants do not display immunity against HopZ1a. 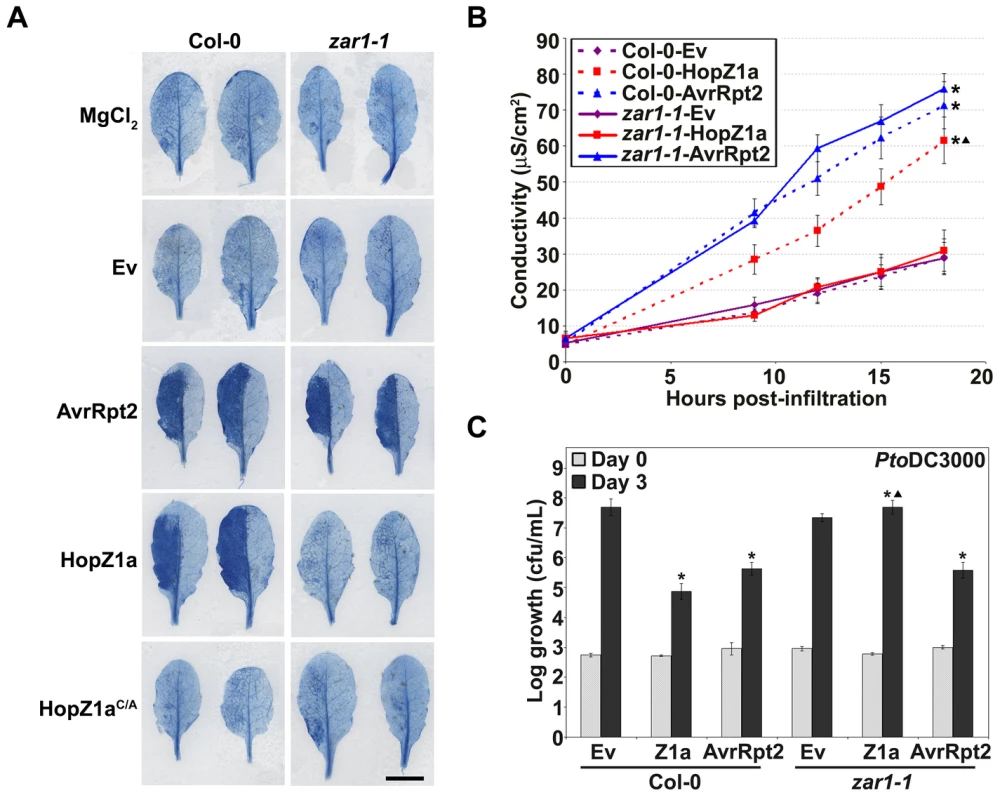
(A) Trypan blue staining of PtoDC3000-infiltrated Arabidopsis Col-0 or zar1-1 leaves. The bacteria were syringe infiltrated into the leaves at 5×107 cfu/mL. Scale bar is 1 cm. C/A indicates the C216A mutation of HopZ1a in the predicted catalytic triad. The mutant protein is expressed at a similar level to HopZ1a [39]. (B) Electrolyte leakage of Arabidopsis Col-0 or zar1-1 leaf discs after infiltration with PtoDC3000 expressing the indicated constructs. The bacteria were syringe infiltrated into the leaves at 2×107 cfu/mL. Error bars indicate the standard deviation from the mean of 6 samples. C/A indicates the C216A mutation. Two-tailed homoschedastic t-tests were performed to test for significant differences. Within a plant genotype, treatments were compared to empty vector and significant differences are indicated by an asterisk (* P<0.01). To compare between plant genotypes, ion leakage from PtoDC3000 carrying HopZ1a or AvrRpt2 was normalized to the average ion leakage of PtoDC3000(Ev) in the same genotype. Significant growth differences between zar1-1 and wild-type Col-0 are indicated by a triangle (▴ P<0.01). (C) PtoDC3000 expressing the indicated construct was syringe infiltrated at 1×105 cfu/mL into Arabidopsis Col-0 or zar1-1 leaves and bacterial counts were determined one hour post-infection (Day 0) and 3 days post-infection (Day 3). Two-tailed homoschedastic t-tests were performed to test for significant differences. Within a plant genotype, treatments were compared to empty vector and significant differences are indicated by an asterisk (* P<0.01). To compare between plant genotypes, growth of PtoDC3000 carrying HopZ1a or AvrRpt2 was normalized to the average growth of PtoDC3000(Ev). Significant growth differences between zar1-1 and wild-type Col-0 are indicated by a triangle (▴ P<0.01). Error bars indicate the standard deviation from the mean of 10 samples. Growth assays were performed at least 3 times. We monitored HopZ1a-mediated immunity through bacterial growth assays in Col-0 and zar1-1 plants with PtoDC3000 carrying Ev, HopZ1a or AvrRpt2. Bacterial growth was strongly restricted in Col-0 infiltrated with PtoDC3000(hopZ1a) or PtoDC3000(avrRpt2) relative to PtoDC3000(Ev) (Figure 3C), while HopZ1a-induced immunity was lost in zar1-1 plants. Importantly, PtoDC3000(hopZ1a) grew slightly, but significantly, better than PtoDC3000(Ev) in zar1-1 plants indicative of a virulence function for HopZ1a in Arabidopsis plants lacking ZAR1. Loss of immunity in zar1-1 plants was specific to HopZ1a as AvrRpt2 still caused a strong restriction of bacterial growth in zar1-1 plants similar to that observed in Col-0 plants.
Taken together, our data demonstrates that the ZAR1 R protein specifically recognizes HopZ1a in Arabidopsis since it is required for the macroscopic HR, rapid ion leakage, and restricted bacterial proliferation induced by HopZ1a. Further, ZAR1 is necessary for recognition of HopZ1a from its native P. syringae strain, PsyA2.
HopZ1a has a virulence function in zar1 Arabidopsis plants
The observation that PtoDC3000(hopZ1a) displayed slightly enhanced growth in zar1-1 relative to PtoDC3000(Ev) prompted us to further investigate whether HopZ1a displays a virulence function in Arabidopsis plants lacking ZAR1 (Figure 3C). We used the non-host strain P. syringae pv. cilantro 0788-9 (hereafter Pci0788-9) as it does not carry an endogenous HopZ allele and is closely-related to P. syringae pv. maculicola ES4326 which carries a HopZ1c allele [35]. Further, we previously demonstrated that the related HopZ2 effector displays an enhanced virulence function in Arabidopsis ecotype Col-0 when delivered by Pci0788-9 [39]. We infiltrated Pci0788-9 carrying HopZ1a, HopZ1aC/A, or empty vector into zar1-1 and Col-0 plants and determined the level of bacterial proliferation after three days of growth. Pci0788-9(hopZ1a) exhibits a significant 0.5–0.75 log increase in growth compared to Pci0788-9(Ev) in zar1-1 (Figure 4). Since the catalytic cysteine residue of HopZ1a was previously shown to be necessary for R gene-mediated recognition (Figure 2A) and enzymatic activity [35],[39], we investigated whether enzymatic activity of HopZ1a is also necessary for virulence, and showed that the catalytic mutant Pci0788-9(hopZ1aC/A) grows to the same level as the vector control Pci0788-9(Ev) (Figure 4). We also confirmed that ZAR1-mediated resistance in Col-0 was not observed with the weakly virulent Pci0788-9, by showing that Pci0788-9(Ev), Pci0788-9(hopZ1a) and Pci0788-9(hopZ1aC/A) grew to equivalent low titers after three days [37]. Thus, HopZ1a promotes bacterial proliferation in the absence of ZAR1 recognition.
Fig. 4. HopZ1a has a virulence function in zar1-1 Arabidopsis plants. 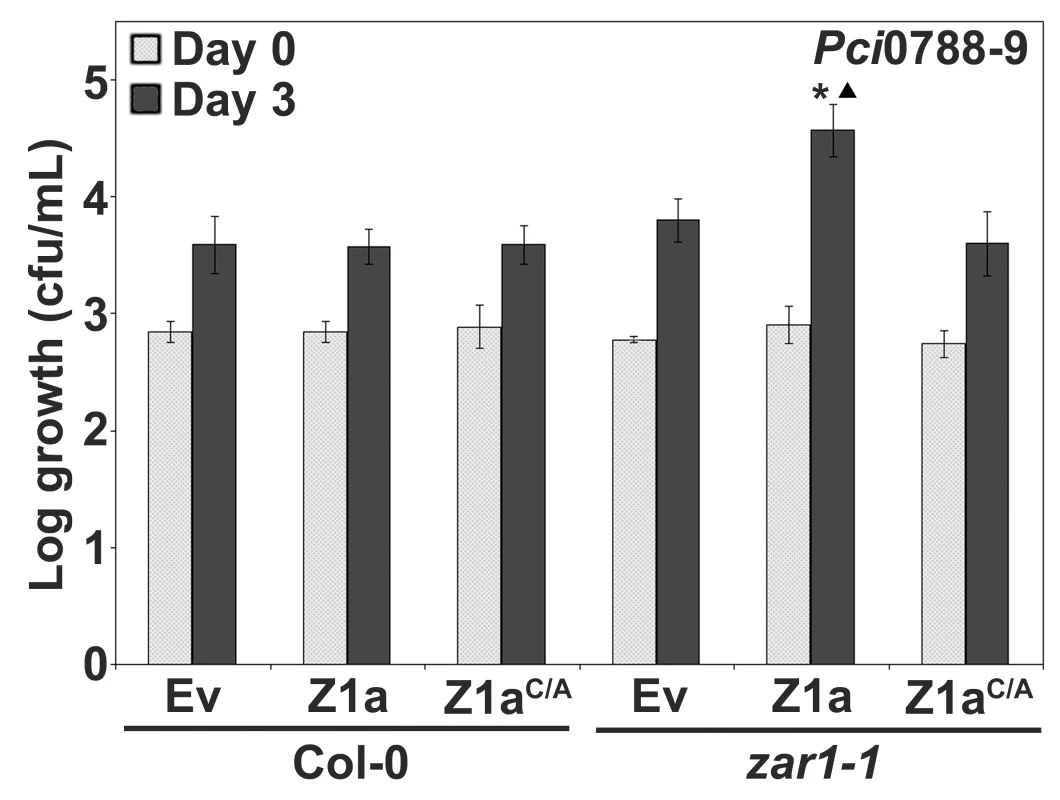
Pci0788-9 expressing the indicated construct was syringe infiltrated at 1×105 cfu/mL into Arabidopsis Col-0 or zar1-1 leaves and bacterial counts were determined one hour post-infection (Day 0) and 3 days post-infection (Day 3). C/A indicates the C216A mutation of HopZ1a in the predicted catalytic triad and the mutant protein is expressed at a similar level to HopZ1a [39]. Two-tailed homoschedastic t-tests were performed to test for significant differences. Within a plant genotype, treatments were compared to empty vector and significant differences are indicated by an asterisk (* P<0.01). To compare between plant genotypes, growth of Pci0788-9 carrying HopZ1a, or HopZ1aC216A (HopZ1aC/A) was normalized to the average growth of Pci0788-9(Ev). Significant differences between zar1-1 and Col-0 are indicated by a triangle (▴ P<0.01). Error bars indicate the standard deviation from the mean of 10 samples. Growth assays were performed at least 3 times. The ZAR1 coiled-coil domain is widespread, yet evolutionarily distinct from other R proteins
ZAR1 is a CC-NBS-LRR type R protein that has an evolutionary history unique from other R genes in the Col-0 genome. Previous phylogenetic analysis using the NBS domain of Arabidopsis R proteins indicated that the most similar resistance proteins to ZAR1 are homologues of RPP13, a downy mildew resistance protein originally identified in the Niederzenz (Nd-1) ecotype of Arabidopsis [16],[57]. While ZAR1 and RPP13 are both clustered into the CNL-C subgroup of NBS-containing proteins their divergence is quite ancient, and in fact ZAR1 shares the same common ancestor with RPP13 as it does with RPP8. Despite this, Meyers et al. [16] classified RPP8 and related sequences as a different subgroup (CNL-D) since they have two introns, while ZAR1, RPP13, and RPP13-related sequences have no introns.
Since the R proteins RPM1, PRF and RPS5 interact through their N-terminal coiled-coil (CC) domain with the T3SE-targeted host protein which they monitor [58]–[60], we reanalyzed the phylogenetic relationships among plant R proteins using only the CC domains, which may provide a basis for identifying R genes that could monitor similar protein families (Figure 5). Bootstrapped neighbor-joining, maximum likelihood, and maximum parsimony analyses provided highly congruent results that identified closely-related ZAR1 homologs in four species, Ricinus communis (castor bean), Populus trichocarpa (poplar), Vitis vinifera (grape), and Solanum melongen (eggplant) (Figure 5, Figure S4). The other Arabidopsis R proteins are highly divergent in their CC domains from ZAR1, and are found in a large distinct and well-supported clade that includes the highly diverse Arabidopsis RPP13 and RPP8 protein families as well as homologues from several other species (Figure 5). While the lack of a reliable root for the phylogenetic analysis complicates the interpretation of the tree, it is clear that the ZAR1 clade is significantly distinct (as shown by bootstrap analysis) from the rest of the CC domain tree.
Fig. 5. Evolutionary relationships of 95 ZAR1 coiled-coil domain homologs. 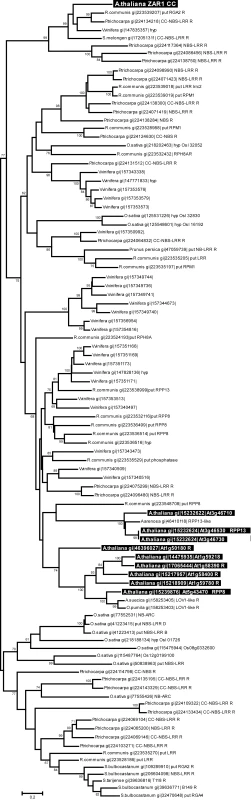
The evolutionary relationships of the homologous amino acid sequences were inferred using Neighbor-Joining, with the robustness of the tree assessed via bootstrapping (500 replicates, with bootstrap values greater than 60% shown above the appropriate nodes). The tree is drawn to scale, with branch lengths scaled to evolutionary distances (scale shown at the bottom of the tree). All Arabidopsis ZAR1 coiled-coil domain homologs are shown in reverse type, while the ZAR1 sequence is found at the top of the tree. The data were parsed to remove redundant sequences as described in the Materials and Methods. “put” indicates a putative R protein while “hyp” is hypothetical. The major structure of this tree (e.g. clustering of ZAR1 and other Arabidopsis homologs) is identical to that observed in trees produced by maximum likelihood and maximum parsimony analysis (data not shown). ZAR1 does not recognize the very closely related HopZ1b allele
We previously demonstrated that HopZ1b induces an HR in ∼24% of Arabidopsis Col-0 leaves when delivered by PtoDC3000 [39]. To further demonstrate that HopZ1b causes an HR, we generated dexamethasone-inducible transgenic HopZ1b plants and tested these for production of the HR upon HopZ1b expression. Two independent HopZ1b transgenic lines induced a strong whole-plant HR within 24–48 hours of dexamethasone-application (Figure 6A). We also tested a HopZ1bC212A transgenic line for production of the HR. HopZ1bC212A did not induce an HR, indicating that the enzymatic activity is necessary for HopZ1b recognition (Figure 6A). The HopZ1b and HopZ1bC212A proteins were all detectable only after application of dexamethasone (Figure 6B).
Fig. 6. ZAR1 does not recognize HopZ1b. 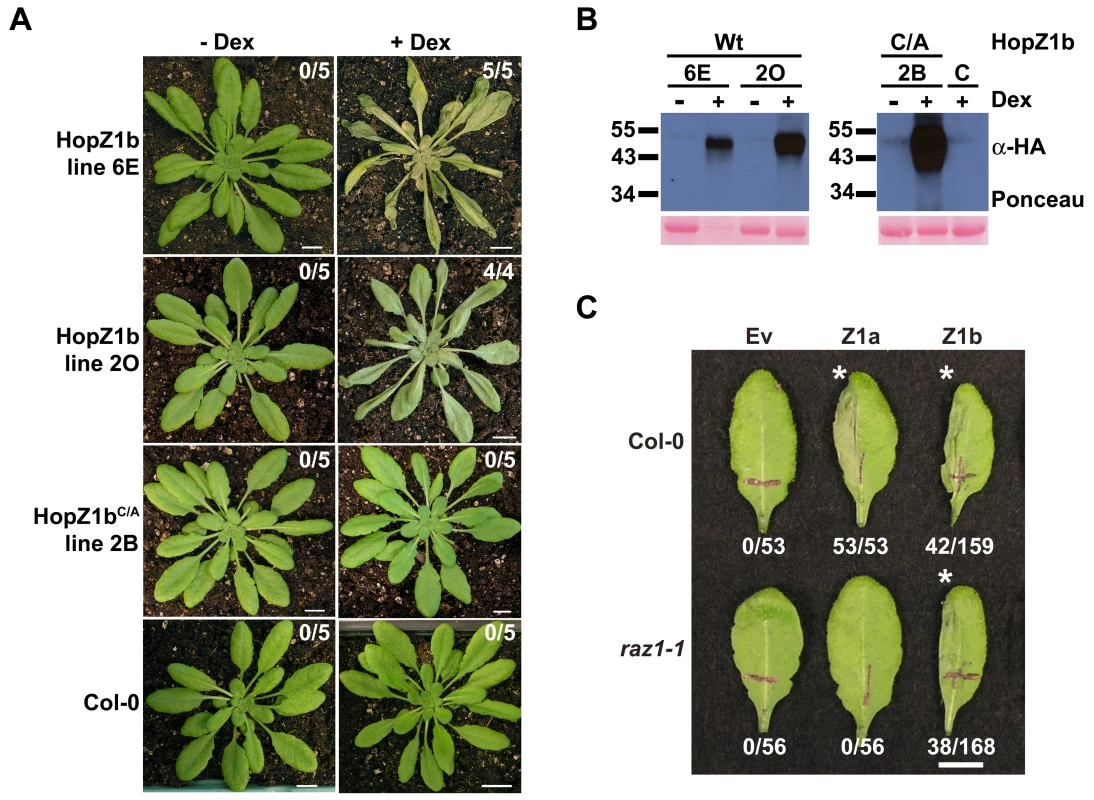
(A) Transgenic homozygous HopZ1b or HopZ1bC/A plants were sprayed with 30 µM dexamethasone or water. C/A indicates the C212A mutation of HopZ1b in the predicted catalytic triad. Photos were taken 24–72 hours post-spraying. The number of plants showing a macroscopic HR is indicated in each box. Scale bar is 1 cm. (B) Immunoblot analysis of HopZ1b or HopZ1bC/A protein expressed in transgenic lines after treatment with 30 µM dexamethasone or water. C/A indicates the C212A mutation of HopZ1b in the predicted catalytic triad. The Ponceau Red stained blot serves as the loading control. The predicted size of HopZ1b-HA is 42.4 kDa. (C) Half-leaves of Arabidopsis Col-0 or zar1-1 plants were infiltrated with 10 mM MgCl2 or with PtoDC3000 expressing the empty vector (Ev), HopZ1a or HopZ1b with a C-terminal HA tag under its endogenous promoter. The bacteria were syringe infiltrated into the leaves at 5×107 cfu/mL. Photos were taken 24 hours post-infiltration. The number of leaves showing an HR is indicated below the appropriate construct. HRs are marked with an asterisk. Scale bar is 1 cm. Given that HopZ1a and HopZ1b are 75% identical at the nucleotide level and 72% identical at the amino acid level, we investigated whether ZAR1 also recognized HopZ1b. We infiltrated Col-0 or zar1-1 with PtoDC3000 carrying Ev, HopZ1a or HopZ1b and monitored for the development of an HR. As expected, 100% of leaves infiltrated with PtoDC3000(hopZ1a) developed an HR in Col-0 and no leaves developed an HR in zar1-1; however, when infiltrated with PtoDC3000(hopZ1b), only 26% of Col-0 leaves and 23% of zar1-1 leaves developed an HR (Figure 6C). HopZ1b therefore causes a macroscopic HR in Arabidopsis Col-0, which like HopZ1a is dependent on its enzymatic activity. However, HopZ1b recognition is not mediated by ZAR1 and must be conferred by a distinct R gene.
Discussion
Resistance proteins are an integral and essential component of the plant immune system. They provide a flexible and readily adaptable means for plants to recognize pathogens that are able to suppress or bypass basal immune responses. In Arabidopsis thaliana alone, there are ∼170 R genes; however, resistance specificities have been determined for relatively few (Table S1). The Arabidopsis R gene T-DNA Insertion Collection (ARTIC) provides a resource to rapidly query the Arabidopsis resistance genome for particular R gene functions. In support of this, we used ARTIC in a reverse genetic screen to identify the CC-NB-LRR resistance protein ZAR1, required for recognition of the P. syringae T3SE HopZ1a.
R genes are frequently present in diverse clusters within a genome [16], which may allow them to evolve new specificities against pathogens through recombination, gene conversion, or by other mutational mechanisms [14] in response to the selection pressure imposed during the infection process [61]. It is also common to find very high diversity in R genes due to pathogen-driven selective diversification. However ZAR1 is not part of a genomic cluster of similar R genes, and unlike the closely-related RPP13 and RPP8 families, no highly similar homologs of the CC domain are found in the Arabidopsis genome (Figure 5, Figure S4). The data available to date indicates that within the ZAR1 CC domain clade, the only homolog to have undergone extensive diversification is found in P. trichocarpa. None of the other species in the ZAR1 CC domain clade carry more than a single homolog, which is again unusual for this family of proteins. This raises the very intriguing possibility that extensive genetic diversity was not selected for in the ancestral ZAR1 CC domain. High genetic diversity, both with respect to gene family expansion as well as maintenance of allelic diversity, is very commonly observed in genes associated with pathogen recognition and immune response. Given the relative paucity of diversity within the ZAR1 CC domain clade, it is possible that this protein or domain was only relatively recently recruited by the plant immune system, perhaps as a means to track HopZ family diversification. This is not to say that the ZAR1 protein has a recent origin, only that it may have originally served an alternative function not directly associated with ETI. What makes this speculation particularly intriguing is that it is at odds with the observation of Ma et al. [35] who showed that HopZ1a is most similar to the ancestral allele of the P syringae HopZ family. It will therefore be interesting to determine if ZAR1 homologs from the other species within the ZAR1 CC domain clade also recognize HopZ1a in these diverse hosts, or if recognition is due to other R proteins.
The majority of R proteins characterized to date require NDR1, EDS1, or PAD4 for proper defense induction. ZAR1 is a notable exception to this rule, along with its relatives which recognize isolates of H. arabidopsidis, RPP13 from the Niederzenz (Nd) ecotype [57], RPP8 from ecotype Landsberg erecta, and the RPP7 R gene from ecotype Col-0 [23],[47]. For example, the Emco5 isolate of H. arabidopsidis induces typical levels of resistance when tested in Arabidopsis ndr1, pad4 or eds1 mutants transformed with the RPP13 Nd allele [57], and in ndr1 or eds1 mutants transformed with the RPP8 Ler allele [47].
Does the lack of NDR1, EDS1, and PAD4 dependence in ZAR1, RPP8, or RPP13 indicate that they signal through the same pathway? Further analysis of these R proteins has demonstrated functional redundancy which may help to answer this question. For example, while RPP8 - or RPP7 - mediated immunity against H. arabidopsidis is not impaired in single ndr1 or eds1 mutant backgrounds, resistance decreases in the ndr1eds1 double mutant [47]. Similarly, RPP8-, HRT-, and RPS2-mediated immunity require both EDS1 and SA, as resistance is lost in eds1nahG or eds1sid2 mutants (sid2 is also known as eds16) [48]. Importantly, ZAR1-mediated immunity differs from RPP8, RPP7, HRT, or RPS2 in that immunity is not impaired in eds1sid2 or ndr1eds1 double mutants (Figure S1). Additionally, unlike ZAR1, HRT requires PAD4 and EDS1 [62], RPS2 depends on NDR1 [23] and RPP7 requires EDM2 [46]. Several R proteins against H. arabidopsidis (RPP2A/B, RPP4, RPP5, RPP7, RPP8) are known to act through SGT1 and/or RAR1 [29],[31]. In contrast, we did not observe any impairment in ZAR1-mediated plant immunity in sgt1a, sgt1b or rar1 mutants (Figure 1). These differences in genetic requirements for ZAR1-mediated immunity suggest that its signaling network is quite different from the characterized networks of other R proteins. Interestingly, the only R protein that also acts independently of the known defense signaling pathways is the closely-related RPP13. At this point we do not know if ZAR1 and RPP13 signal through a common pathway.
We also observed a partial impairment of HopZ1a-induced resistance and a complete loss of AvrRpt2-induced resistance in the nahG background (Figure 1A and 1H). However, nahG has been reported to affect non-host resistance in Arabidopsis to P. syringae pv. phaseolicola, due to the accumulation of catechol [42]. As well, the nahG transgene impairs ethylene signaling, early induction of jasmonate signaling and camalexin production [41]. We therefore tested additional mutants in the SA signaling pathway to clarify these results. The eds16 mutant, which lacks plastid-derived SA [45], did not impair HopZ1a - or AvrRpt2-mediated resistance responses (Figure 1A and 1I). The pad4 mutant, which is impaired in SA signaling [63] and has reduced camalexin and ethylene levels [41], exhibits normal HopZ1a-induced resistance (Figure 1A and 1F). We therefore conclude that SA is not involved in HopZ1a-mediated resistance, and that the impairment in the nahG background is likely due to the accumulation of catechol or the pleiotropic effects of the nahG transgene.
The closely-related HopZ1b allele is only recognized in ∼24% of Arabidopsis ecotype Col-0 leaves in contrast to 100% recognition of HopZ1a (Figure 6C). HopZ1b causes a strong HR when overexpressed in transgenic plants and the HR is dependent on the catalytic cysteine (Figure 6A and 6B). Our data strongly support that HopZ1b is recognized by a distinct R gene. Thus, recognition specificity for the two HopZ1 alleles may have evolved independently. Our phylogenetic analysis provides strong R gene candidates to assay for recognition of HopZ1a in diverse hosts, as well as HopZ1b recognition in Arabidopsis.
HopZ1a demonstrates a virulence function in the zar1 Col-0 background that is dependent on its catalytic function (Figure 4). This virulence function is the putative ancestral state, prior to the development of resistance by the plant. In support of this, recognition of HopZ1a is dependent on its predicted catalytic residues, indicating that HopZ1a is indirectly recognized by ZAR1 via its enzymatic activity. It remains to be determined whether HopZ1a virulence and avirulence activities converge on common or distinct host targets. We previously showed that HopZ2 also has a virulence function in Arabidopsis [39], although it is not clear if HopZ1a and HopZ2 target the same host protein to promote bacterial fitness. HopZ1a and HopZ2 have quite different evolutionary histories; HopZ1a, HopZ1b and HopZ1c evolved by pathoadaptation in response to the host immune system, while HopZ2 was acquired by horizontal gene transfer and is most similar to homologues in Xanthomonas spp., including AvrRxv [35],[64],[65]. Comparing the host targets of HopZ1a and HopZ2 will allow us to evaluate the extent of diversification of HopZ virulence strategies in Arabidopsis.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana plants were grown with 9 h of light (∼130 microeinsteins m−2 s−1) and 15 h of darkness at 22°C in Promix soil supplemented with 20∶20∶20 fertilizer. Unless otherwise indicated, assays were performed in the Col-0 background. T-DNA insertion lines were identified using SIGnAL (Salk Institute Genomic Analysis Laboratory) and obtained from the ABRC (Arabidopsis Biological Resource Center). All generated homozygous lines have been deposited at the ABRC.
For the ZAR1 alleles, zar1-1 is SALK_013297, zar1-2 is SALK_091754, zar1-3 is SALK_033548, zar1-4 is SALK_046916 and zar1-5 is SALK_009040. The following mutants were utilized: sgt1a (in Ws) [32], sgt1b (in Col-0) [28], ndr1-1 rar1-21 (in Col-0) [20],[27], eds1-1 (in Ws) [66], pad4-1 (in Col-0) [67], rbohD/F (in Col-0) [34], eds16 (in Col-0) [43],[44], edm2-2 (in Col-0) [46], eds1-1sid2-1 (Col-0/Ws-0 cross) [48], ndr1-1eds1-2 (Col-0/Ws-0 cross) [47], and the transgenic line nahG (in Col-0) [40].
Genotyping of T–DNA insertion lines
Primers were designed using the iSct feature in the SIGnAL database. Primer sequences are available upon request. PCR-based genotyping was employed to determine the homozygosity or heterozygosity of the individuals. Genomic DNA was extracted from a leaf of 5–6 week old Arabidopsis plants and PCR products were sequenced using Big Dye Terminator 3.1 on an ABI 3730 genetic analyzer.
P. syringae infection assays
The HopZ1a allele was amplified from the Pseudomonas syringae pv. syringae A2, expressed under its native promoter and contained an in-frame hemagglutinin (HA) tag at the C-terminus [39]. Pseudomonas syringae pv. tomato DC3000 (PtoDC3000) or Pseudomonas syringae pv. cilantro 0877-9 (Pci0788-9) carried empty vector (pUCP20) [39], pDSK519-PnptII:AvrRpt2 [68], pUCP20-PhopZ1a::hopZ1a-HA, pUCP20-PhopZ1a::hopZ1aC216A-HA or pUCP20-PhopZ1b::hopZ1b-HA [39] or pV316-1a (carries AvrRps4) [69]. P. syringae pv. syringae A2 contains the endogenous HopZ1a allele [35],[39]. HR, ion leakage and in planta growth assays were performed as has been described [39]. For infiltrations, P. syringae was resuspended to an OD600 = 0.1 (∼5×107 cfu/mL) for HR assays and trypan blue staining, or diluted to 2×107 cfu/mL for ion leakage assays, or diluted to 1×105 cfu/mL for growth curves. Diluted inocula were hand-infiltrated using a needleless syringe as has been described [70]. The HR was scored at 16–20 hours. Leaves for trypan blue staining were harvested at 17–18 hours [39]. For ion leakage assays, 4 disks (1.5 cm2) were harvested, soaked in dH20 for 45 minutes and transferred to 6 mL of dH20. Readings were taken with an Orion 3 Star conductivity meter (Thermo Electron Corporation, Beverly, MA). For growth assays, 4 disks (1 cm2) were harvested, ground in 10 mM MgCl2, and plated on KB with rifampicin and cyclohexamide on day 0 and day 3 for colony counts.
Two-tailed homoschedastic t-tests were performed within genotypes to detect statistical significance. To compare between genotypes, log growth or conductivity was normalized to the average growth or conductivity of PtoDC3000(Ev) or Pci0788-9(Ev) in the appropriate genotype and two-tailed homoschedastic t-tests were performed.
Cloning
The HopZ1a-AvrRpt2 fusion was constructed using a crossover PCR approach, as previously described [39],[71]. For the promoter-full length HopZ1a-HA-AvrRpt2Δ1-79 fusions, the 5′ portion of the fusion was amplified by PCR using a 5′ primer to the HopZ1a promoter and a 3′ primer to the HA tag, plus a portion of the 5′ end of the AvrRpt2 truncation (Δ1-79) [54]. The 3′ portion of the fusion was amplified by PCR using a 5′ primer to the AvrRpt2 truncation plus a portion of 3′ end of the HA tag, and a 3′ primer to AvrRpt2. These two PCR products were then mixed to use as template for the subsequent PCR reaction. The full-length promoter-HopZ1a-HA-AvrRpt2Δ1-79 cassette was amplified using the same 5′ promoter primer and 3′ AvrRpt2 primer and blunt-end cloned into pUCP20 into the SmaI site [39]. The promoter-ATG-AvrRpt2Δ1-79 fusion, driven by the HopZ1a promoter but lacking the signal and translocation sequence, was previously described [39].
To clone into the pBD vector, HopZ1b or HopZ1bC212A with an in-frame HA tag was amplified by PCR using primers to add a unique XhoI site to the 5′ end of the gene and a unique SpeI site to the 3′ end of the HA tag [39]. The pBD vector (a gift from Dr. Jeff Dangl, University of North Carolina, Chapel Hill, NC, USA) was modified from pTA7002 to add an HA tag in the multi-cloning site as has been described [58],[72].
Phylogenetic analysis
ZAR1 homologs were identified from the NCBI nr database via BLASTP analysis using the Arabidopsis ZAR1 protein sequence as the query and default parameters. All similar, full-length sequences with an Expect-value below 10−5 were downloaded. Full length protein sequences were aligned via MAFFT [73] using the E-INS-i algorithm. Coiled-coil domains were then manually examined and extracted from the sequence using GeneDoc, and the alignment was repeated using the MAFFT G-INS-I algorithm. Following alignment, redundant sequences were removed from the dataset via a custom PERL script (written by DSG). Redundant sequences were defined as those sequences from the same species that have more than 95% amino acid identity. The exception to this was A. thaliana, where all Col-0 homologs were retained for the analysis. Neighbor-joining and maximum parsimony phylogenetic analyses were performed with MEGA4 [74] with bootstrapping (1000 pseudo-replicates) and the JTT substitution model. All positions containing alignment gaps were eliminated on a pairwise basis, with a total of 217 positions used in the final dataset. The tree was rooted at the midpoint. Maximum likelihood analysis was performed using the PALM (Phylogenetic Reconstruction by Automated Likelihood Model Selector) [75] server, which performs automated evolution model selection via ProTest [76], and maximum likelihood analysis via PhyML [77]. The best model was determined by AIC to be JTT+G+F.
Transgenic lines
Col-0 plants were transformed with pBD::hopZ1b-HA or pBD::hopZ1b(C212A)-HA using the floral dip method [78]. Transgenic plants were selected by Basta resistance and confirmed by PCR and sequencing to have the correct transgene. Homozygosity of T3 lines was determined by their segregation ratios on plates containing half-strength Murashige and Skoog (MS) media and 6 mg/L bialophos. For the Westerns, leaves were detached from the plants and floated on 30µM dexamethasone or water for 48 hours, and frozen in liquid nitrogen. The leaf tissue was ground in a buffer containing 20mM Tris (pH 8.0), 100mM NaCl, 1mM DTT and 1% Triton X-100. The crude extract was cleared by centrifugation at 5000g for 10 minutes at 4°C. After adding SDS-PAGE loading dye and boiling for 5 minutes, 7.5 µL of protein was separated on 12% SDS-PAGE gels, blotted onto nitrocellulose membranes and detected using HA antibodies (Roche) by chemiluminescence (Amersham Biosciences). Photographs were taken 24–72 hours after spraying 30µM dexamethasone (Sigma) or water onto the plants.
Supporting Information
Zdroje
1. GalanJE
Wolf-WatzH
2006 Protein delivery into eukaryotic cells by type III secretion machines. Nature 444 567 573
2. MudgettMB
2005 New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu Rev Plant Biol 56 509 531
3. GrantSR
FisherEJ
ChangJH
MoleBM
DanglJL
2006 Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu Rev Microbiol 60 425 449
4. SpethEB
LeeYN
HeSY
2007 Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr Opin Plant Biol 10 580 586
5. ZhouJM
ChaiJ
2008 Plant pathogenic bacterial type III effectors subdue host responses. Curr Opin Microbiol 11 179 185
6. LewisJD
DesveauxD
GuttmanDS
2009 The targeting of plant cellular systems by injected type III effector proteins. Semin Cell Dev Biol 20 1055 1063
7. JonesJDG
DanglJL
2006 The plant immune system. Nature 444 323 329
8. HeathMC
2000 Hypersensitive response-related death. Plant Mol Biol 44 321 334
9. DanglJL
JonesJDG
2001 Plant pathogens and integrated defence responses to infection. Nature 411 826 833
10. DeslandesL
OlivierJ
PeetersN
FengDX
KhounlothamM
2003 Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci U S A 100 8024 8029
11. KayS
HahnS
MaroisE
HauseG
BonasU
2007 A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318 648 651
12. RomerP
HahnS
JordanT
StraussT
BonasU
2007 Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318 645 648
13. van der BiezenEA
JonesJDG
1998 Plant disease resistance proteins and the gene-for-gene concept. Trends Biochem Sci 23 454 456
14. FriedmanAR
BakerBJ
2007 The evolution of resistance genes in multi-protein plant resistance systems. Curr Opin Genet Dev 17 493 499
15. van der BiezenEA
JonesJDG
1998 The NB-ARC domain: a novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr Biol 8 R226 R227
16. MeyersBC
KozikA
GriegoA
KuangHH
MichelmoreRW
2003 Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15 809 834
17. BelkhadirY
SubramaniamR
DanglJL
2004 Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol 7 391 399
18. WiermerM
FeysBJ
ParkerJE
2005 Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8 383 389
19. PanstrugaR
DoddsPN
2009 Terrific protein traffic: the mystery of effector protein delivery by filamentous plant pathogens. Science 324 748 750
20. CenturyKS
HolubEB
StaskawiczBJ
1995 NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci U S A 92 6597 6601
21. CenturyKS
ShapiroAD
RepettiPP
DahlbeckD
HolubE
1997 NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278 1963 1965
22. CoppingerP
RepettiPP
DayB
DahlbeckD
MehlertA
2004 Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J 40 225 237
23. AartsN
MetzM
HolubE
StaskawiczBJ
DanielsMJ
1998 Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci U S A 95 10306 10311
24. FalkA
FeysBJ
FrostLN
JonesJDG
DanielsMJ
1999 EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci U S A 96 3292 3297
25. FeysBJ
MoisanLJ
NewmanMA
ParkerJE
2001 Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20 5400 5411
26. AustinMJ
MuskettP
KahnK
FeysBJ
JonesJDG
2002 Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295 2077 2080
27. MuskettPR
KahnK
AustinMJ
MoisanLJ
SadanandomA
2002 Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defenses against multiple pathogens. Plant Cell 14 979 992
28. TorM
GordonP
CuzickA
EulgemT
SinapidouE
2002 Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell 14 993 1003
29. TorneroP
MerrittP
SadanandomA
ShirasuK
InnesRW
2002 RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14 1005 1015
30. ShirasuK
Schulze-LefertP
2003 Complex formation, promiscuity and multi-functionality: protein interactions in disease-resistance pathways. Trends Plant Sci 8 252 258
31. HoltBF
BelkhadirY
DanglJL
2005 Antagonistic control of disease resistance protein stability in the plant immune system. Science 309 929 932
32. AzevedoC
BetsuyakuS
PeartJ
TakahashiA
NoelL
2006 Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J 25 2007 2016
33. RateDN
CuencaJV
BowmanGR
GuttmanDS
GreenbergJT
1999 The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11 1695 1708
34. TorresMA
DanglJL
JonesJDG
2002 Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99 517 522
35. MaWB
DongFFT
StavrinidesJ
GuttmanDS
2006 Type III effector diversification via both pathoadaptation and horizontal transfer in response to a coevolutionary arms race. PLoS Genet 2 e209 doi:10.1371/journal.pgen.0020209
36. OrthK
2002 Function of the Yersinia effector YopJ. Curr Opin Microbiol 5 38 43
37. MittalR
Peak-ChewSY
McMahonHT
2006 Acetylation of MEK2 and I kappa B Kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc Natl Acad Sci U S A 103 18574 18579
38. MukherjeeS
KeitanyG
LiY
WangY
BallHL
2006 Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312 1211 1214
39. LewisJD
AbadaW
MaWB
GuttmanDS
DesveauxD
2008 The HopZ family of Pseudomonas syringae type III effectors require myristoylation for virulence and avirulence functions in Arabidopsis thaliana. J Bacteriol 190 2880 2891
40. DelaneyTP
UknesS
VernooijB
FriedrichL
WeymannK
1994 A central role of salicylic acid in plant disease resistance. Science 266 1247 1250
41. HeckS
GrauT
BuchalaA
MetrauxJP
NawrathC
2003 Genetic evidence that expression of nahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J 36 342 352
42. van WeesSCM
GlazebrookJ
2003 Loss of non-host resistance of Arabidopsis nahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J 33 733 742
43. NawrathC
MetrauxJP
1999 Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11 1393 1404
44. DewdneyJ
ReuberTL
WildermuthMC
DevotoA
CuiJP
2000 Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J 24 205 218
45. WildermuthMC
DewdneyJ
WuG
AusubelFM
2001 Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562 565
46. EulgemT
TsuchiyaT
WangXJ
BeasleyB
CuzickA
2007 EDM2 is required for RPP7-dependent disease resistance in Arabidopsis and affects RPP7 transcript levels. Plant J 49 829 839
47. McDowellJM
CuzickA
CanC
BeynonJ
DanglJL
2000 Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J 22 523 529
48. VenugopalSC
JeongRD
MandalMK
ZhuSF
Chandra-ShekaraAC
2009 Enhanced Disease Susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet 5 e1000545 doi:10.1371/journal.pgen.1000545
49. TanXP
MeyersBC
KozikA
Al WestM
MorganteM
2007 Global expression analysis of nucleotide binding site-leucine rich repeat-encoding and related genes in Arabidopsis. BMC Plant Biol 7 56
50. AlonsoJM
StepanovaAN
LeisseTJ
KimCJ
ChenHM
2003 Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653 657
51. SessionsA
BurkeE
PrestingG
AuxG
McElverJ
2002 A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985 2994
52. WoodyST
Austin-PhillipsS
AmasinoRM
KrysanPJ
2007 The WiscDsLox T-DNA collection: an Arabidopsis community resource generated by using an improved high-throughput T-DNA sequencing pipeline. J Plant Res 120 157 165
53. SundaresanV
SpringerP
VolpeT
HawardS
JonesJDG
1995 Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9 1797 1810
54. GuttmanDS
GreenbergJT
2001 Functional analysis of the type III effectors AvrRpt2 and AvrRpm1 of Pseudomonas syringae with the use of a single-copy genomic integration system. Mol Plant-Microbe Interact 14 145 155
55. BentAF
KunkelBN
DahlbeckD
BrownKL
SchmidtR
1994 RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265 1856 1860
56. MindrinosM
KatagiriF
YuGL
AusubelFM
1994 The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78 1089 1099
57. Bittner-EddyPD
BeynonJL
2001 The Arabidopsis downy mildew resistance gene, RPP13-Nd, functions independently of NDR1 and EDS1 and does not require the accumulation of salicylic acid. Mol Plant-Microbe Interact 14 416 421
58. MackeyD
HoltBF
WiigA
DanglJL
2002 RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108 743 754
59. MucynTS
ClementeA
AndriotisVME
BalmuthAL
OldroydGED
2006 The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell 18 2792 2806
60. AdeJ
DeYoungBJ
GolsteinC
InnesRW
2007 Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc Natl Acad Sci U S A 104 2531 2536
61. RoseLE
Bittner-EddyPD
LangleyCH
HolubEB
MichelmoreRW
2004 The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166 1517 1527
62. Chandra-ShekaraAC
NavarreD
KachrooA
KangHG
KlessigD
2004 Signaling requirements and role of salicylic acid in HRT - and rrt-mediated resistance to Turnip Crinkle Virus in Arabidopsis. Plant J 40 647 659
63. JirageD
TootleTL
ReuberTL
FrostLN
FeysBJ
1999 Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci U S A 96 13583 13588
64. CiesiolkaLD
HwinT
GearldsJD
MinsavageGV
SaenzR
1999 Regulation of expression of avirulence gene avrRxv and identification of a family of host interaction factors by sequence analysis of AvrBsT. Mol Plant-Microbe Interact 12 35 44
65. BonshtienA
LevA
GiblyA
DebbieP
AvniA
2005 Molecular properties of the Xanthomonas AvrRxv effector and global transcriptional changes determined by its expression in resistant tomato plants. Mol Plant-Microbe Interact 18 300 310
66. ParkerJE
HolubEB
FrostLN
FalkA
GunnND
1996 Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 2033 2046
67. GlazebrookJ
RogersEE
AusubelFM
1996 Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143 973 982
68. MudgettMB
StaskawiczBJ
1999 Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol Microbiol 32 927 941
69. HinschM
StaskawiczB
1996 Identification of a new Arabidopsis disease resistance locus, RPS4, and cloning of the corresponding avirulence gene, avrRps4, from Pseudomonas syringae pv. pisi. Mol Plant-Microbe Interact 9 55 61
70. KatagiriF
ThilmonyR
HeSY
2002 The Arabidopsis thaliana-Pseudomonas syringae interaction. In The Arabidopsis Book doi/10.1199/tab. 0039, C.R. Somerville and E.M. Meyerowitz, eds. American Society of Plant Biologists: Rockville
71. LinkAJ
PhillipsD
ChurchGM
1997 Methods for generating precise eeletions and Iinsertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol 179 6228 6237
72. AoyamaT
ChuaNH
1997 A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11 605 612
73. KatohK
KumaK
TohH
MiyataT
2005 MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33 511 518
74. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
75. ChenSH
SuSY
LoCZ
ChenKH
HuangTJ
2009 PALM: a paralleled and integrated framework for phylogenetic inference with automatic likelihood model selectors. PLoS ONE 4 e8116 doi:10.1371/journal.pone.0008116
76. AbascalF
ZardoyaR
PosadaD
2005 ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21 2104 2105
77. GuindonS
DelsucF
DufayardJ.-F
GascuelO
2009 Estimating maximum likelihood phylogenies with PhyML.
PosadaD
Methods in Molecular Biology Totowa, NJ Humana Press 113 137
78. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



