-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
All skeletal muscle progenitor cells in the body derive from the dermomyotome, the dorsal epithelial domain of developing somites. These multipotent stem cells express Pax3, and this expression is maintained in the myogenic lineage where Pax3 plays an important role. Identification of Pax3 targets is therefore important for understanding the mechanisms that underlie the onset of myogenesis. In a microarray screen of Pax3-GFP sorted cells, with analysis on Pax3 gain and loss of function genetic backgrounds, we identify Dmrt2, expressed in the dermomyotome, as a Pax3 target. In vitro gel shift analysis and chromatin immunoprecipitation with in vivo extracts show that Pax3 binds to a conserved 286 bp sequence, situated at −18 kb from Dmrt2. This sequence directs reporter transgene expression to the somite, and this is severely affected when the Pax3 site is mutated in the context of the locus. In Dmrt2 mutant embryos, somite maturation is perturbed and the skeletal muscle of the myotome is abnormal. We now report that the onset of myogenesis is also affected. This depends on activation, in the epaxial dermomyotome, of the myogenic determination gene, Myf5, through its early epaxial enhancer. This sequence contains sites that bind Dmrt2, which belongs to the DM class of DNA–binding proteins. Mutation of these sites compromises activity of the enhancer in transgenic embryos where the reporter transgene is under the control of the Myf5 epaxial enhancer. Transactivation of this site by Dmrt2 is demonstrated in vitro, and conditional overexpression of Dmrt2 in Pax3 expressing cells in the somite confirms the role of this factor in the activation of Myf5. These results reveal a novel genetic network, comprising a Pax3/Dmrt2/Myf5 regulatory cascade that operates in stem cells of the epaxial dermomyotome to initiate skeletal muscle formation.
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000897
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000897Summary
All skeletal muscle progenitor cells in the body derive from the dermomyotome, the dorsal epithelial domain of developing somites. These multipotent stem cells express Pax3, and this expression is maintained in the myogenic lineage where Pax3 plays an important role. Identification of Pax3 targets is therefore important for understanding the mechanisms that underlie the onset of myogenesis. In a microarray screen of Pax3-GFP sorted cells, with analysis on Pax3 gain and loss of function genetic backgrounds, we identify Dmrt2, expressed in the dermomyotome, as a Pax3 target. In vitro gel shift analysis and chromatin immunoprecipitation with in vivo extracts show that Pax3 binds to a conserved 286 bp sequence, situated at −18 kb from Dmrt2. This sequence directs reporter transgene expression to the somite, and this is severely affected when the Pax3 site is mutated in the context of the locus. In Dmrt2 mutant embryos, somite maturation is perturbed and the skeletal muscle of the myotome is abnormal. We now report that the onset of myogenesis is also affected. This depends on activation, in the epaxial dermomyotome, of the myogenic determination gene, Myf5, through its early epaxial enhancer. This sequence contains sites that bind Dmrt2, which belongs to the DM class of DNA–binding proteins. Mutation of these sites compromises activity of the enhancer in transgenic embryos where the reporter transgene is under the control of the Myf5 epaxial enhancer. Transactivation of this site by Dmrt2 is demonstrated in vitro, and conditional overexpression of Dmrt2 in Pax3 expressing cells in the somite confirms the role of this factor in the activation of Myf5. These results reveal a novel genetic network, comprising a Pax3/Dmrt2/Myf5 regulatory cascade that operates in stem cells of the epaxial dermomyotome to initiate skeletal muscle formation.
Introduction
The Pax family of transcriptional regulators play key roles in the onset of organogenesis and cell lineage specification during development [1]. Pax3 and Pax7 regulate skeletal muscle formation. Skeletal muscle progenitors in the trunk and limbs of vertebrates derive from somites, from the dorsal compartment known as the dermomyotome. In the mouse embryo, Pax3 is expressed throughout this epithelial structure, whereas Pax7 expression is restricted to the central domain. Myogenesis is initiated by the delamination of Pax3 positive cells from the edges of the dermomyotome; at certain axial levels, cells from the hypaxial domain migrate from the somite, before activating the myogenic determination genes Myf5 and MyoD, whereas other cells, most notably in the epaxial domain, have already activated Myf5 and immediately differentiate, on delamination, to form the first skeletal muscle of the epaxial myotome. Transcription of Myf5 at this site depends on an early epaxial enhancer, which lies at −5.5 kb from the gene [2],[3]. This sequence is regulated by Shh [4]–[6] and by canonical Wnt signalling [6] from the adjacent axial structures, acting through Gli and TCF binding sites. Subsequently, as the somite matures, the central dermomyotome loses its epithelial structure and cells that are positive for Pax3 and Pax7 enter the underlying muscle masses. These provide an essential population of muscle stem cells for all subsequent muscle growth. In Pax3−/−; Pax7−/− double mutants, these cells fail to activate myogenic determination genes and many of them die [7]. In the absence of Pax3 alone, cell death is also observed at the extremities of the dermomyotome, where it is most evident in the hypaxial domain [1].
Pax3 target genes that are important for the onset of myogenesis are beginning to be identified. A classic example is provided by the gene encoding c-Met, a receptor required for delamination and migration of muscle progenitor cells [8]. More recently, a Myf5 regulatory sequence required for activation of this myogenic determination gene in the limb and hypaxial somite has been identified as a direct Pax3 target [9]. The second myogenic determination gene, MyoD, has been shown to be a target of Pax3/7 in a myogenic cell line and in postnatal muscle progenitor cells [10] and indeed other Pax7 [11],[12] targets have been identified in this cellular context. Activation of myogenic determination genes leads to entry into the myogenic programme, which is accompanied by down-regulation of Pax3/7, to which microRNAs contribute [13]. However, maintenance of a muscle stem cell population is essential in the growing organism and this is achieved through signalling systems that maintain a balance between self-renewal and differentiation [14]. In the context of FGF signalling, Pax3 directly regulates Fgfr4 and acts genetically upstream of other components of the pathway also, such as Sprouty1, to control self-renewal versus differentiation of Pax3 positive muscle progenitors [15].
In order to identify further Pax3 target genes acting at earlier stages within the dermomyotome, we have performed a microarray screen that has led to the identification of Dmrt2. This gene family was first characterized as doublesex in Drosophila [16]–[18] and as mab-3 in C.elegans [19], where these genes play important roles in sex determination [20],[21]. Vertebrate homologues have since been identified, some of which also play a role in sexual development [22]. However Dmrt genes are also expressed outside the gonads and have been implicated in other developmental processes. Dmrt2/Terra is expressed during somitogenesis in vertebrates [23], where transcripts are present in the presomitic mesoderm (PSM) and then confined to the dermomyotome of somites. In the chick embryo, Terra is expressed symmetrically in the PSM, but has a transient asymmetrical expression around the node, implicating it in left-right axis determination and normal development of bilateral somites through interaction with the segmentation clock, as indicated by morpholino experiments in zebrafish [24]. Mouse embryos lacking Dmrt2 show somite patterning defects, culminating in malformed ribs and sternum leading to postnatal death due to respiratory problems [25]. Such malformations are often associated with abnormal dermomyotome and myotome development [1] and indeed Dmrt2−/− embryos have morphological defects in these somite compartments, with perturbation of the expression of myogenic markers reported at E10.5 and E11.5. In Dmrt2 and Pax3 compound mutants perturbations in myogenesis were also observed [26], as evidenced by muscle differentiation markers at E10.5. However cell death in the absence of Pax3 [1] complicates the interpretation.
The molecular function of Dmrt2 has not been investigated. Dmrt proteins are characterised by the DM domain, an intertwined Zn finger-like motif that interacts with the minor groove of DNA [27]. The conserved DM domains of mammalian Dmrt factors bind to similar DNA consensus sequences [28]. Drosophila Dsxm acts as a transcriptional activator whereas DsxF has repressor function [29]. When human DMRT1 is fused to the VP16 activation domain, transactivation through the consensus DMRT1 binding sequence is observed, however DMRT1 alone did not show much activity [28]. Regulation of the Dmrt2 gene, present in a cluster with Dmrt1, 3 in mice, has not been examined, although the human DMRT2 gene has been shown to encode alternatively spliced transcripts [30].
In this report, we identify Dmrt2 as a target of Pax3, acting directly through a regulatory sequence that directs expression in the somite. We show that Dmrt2, in turn, acts on the epaxial enhancer of Myf5. Perturbation of this regulatory network, functioning in the dermomyotome, has consequences for the onset of myogenesis.
Results
In a screen designed to detect Pax3 targets in the dermomyotome of interlimb somites at E9.5, this region of the somite was dissected from Pax3GFP/+ heterozygote and Pax3PAX3-FKHR-IRESnlacZ/GFP gain of function embryos [31]. After dissociation, GFP positive cells were separated by flow cytometry. Microarray comparisons of the two classes of samples led to identification of a series of sequences that are up - or down-regulated in the presence of PAX3-FKHR, a constitutively active form of the Pax3 transcription factor [31]. Among these sequences, Dmrt2 was up-regulated 1.87 fold (data not shown).
Dmrt2 is transcribed in mouse somites (Figure 1), as previously reported [25]. Expression is detected as a band in presomitic mesoderm (S0) and in the first somites at E8.5, where transcripts accumulate in the epaxial domain, as shown for immature caudal somites at E9.5 (C″). Sections of a Pax3IRESnlacZ/+ embryo at this stage (Figure 1F) show that Dmrt2 expression is confined to the Pax3 positive dermomyotome of the somite, in the epaxial domain of the most immature caudal somites (Figure 1G), and then throughout this epithelium in more mature somites (Figure 1H). Already, at this stage, the most mature anterior somites (Figure 1I) are beginning to lose Dmrt2 expression at the epaxial and hypaxial extremities of the dermomyotome. This is detected also by whole mount in situ hybridization at E9.5 (Figure 1C and 1C′) and at E10.5 (Figure 1D), when labelled cells are also detectable in the mesodermal core of the 1st and 2nd branchial arches and in the forelimb bud, to which myogenic progenitor cells have begun to migrate from the somites [1]. By E11.5 (Figure 1E), Dmrt2 transcripts are only detectable in caudal somites.
Fig. 1. The expression of Dmrt2, visualised by whole mount in situ hybridization. 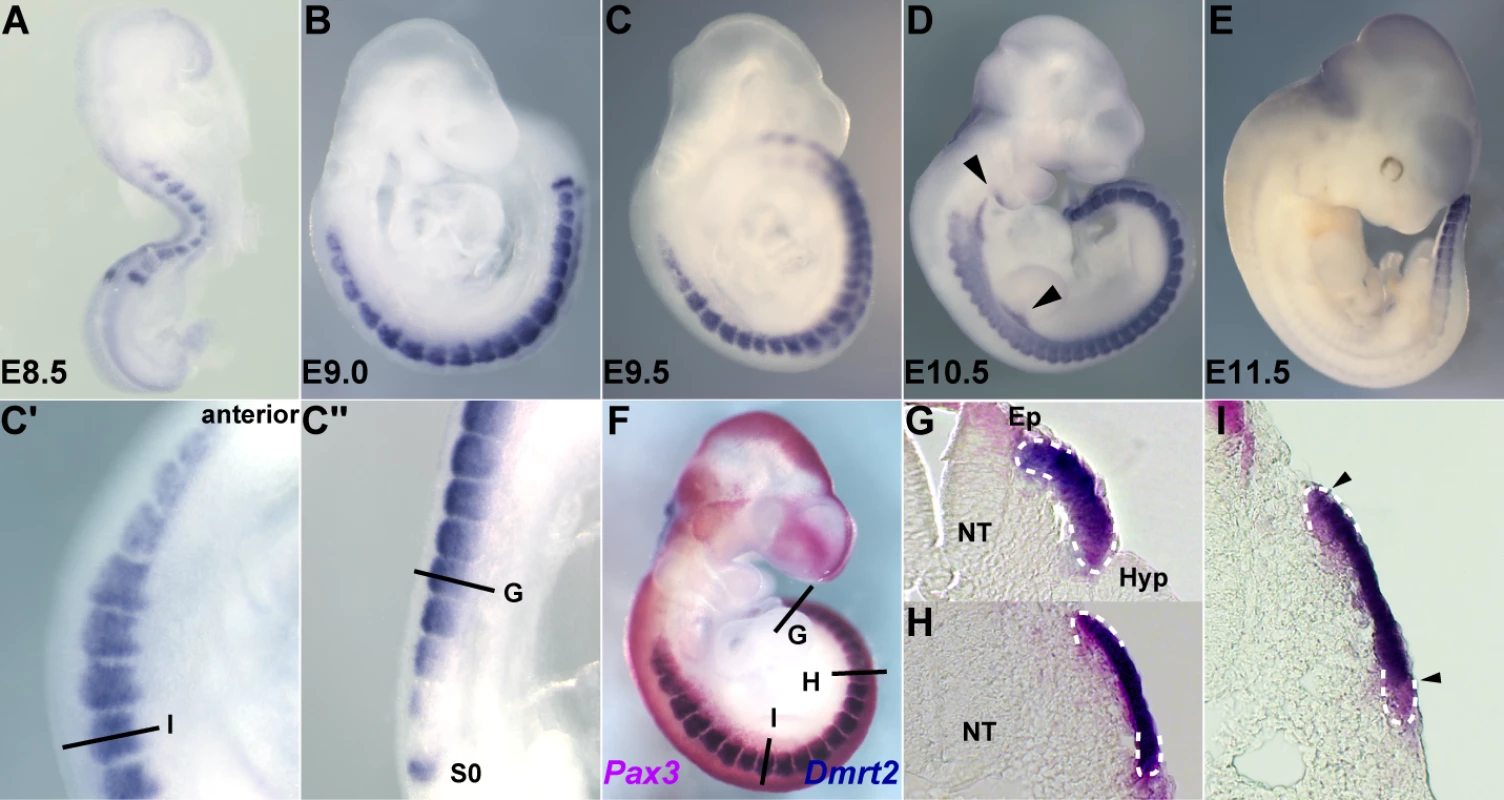
Embryos were hybridized with a Dmrt2 RNA probe. (A) an E8.5 embryo, (B) an E9.0 embryo, (C, F) E9.5 embryos, (D) an E10.5 embryo, (E) an E11.5 embryo. C′ and C″ are magnified views of C. Dmrt2 transcripts are present in all somites until E11.5 when they are only detected caudally. (F) A Pax3IRES-nlacZ/+ embryo at E9.5 hybridized with a Dmrt2 probe (blue) and treated with Red-gal to reveal β-galactosidase (β-gal) expression from the Pax3IRES-nlacZ allele (red). Dmrt2 expression was also detected in proximal forelimb buds and branchial arches (arrowheads in D). (G–I) Transverse sections of the embryos shown in F. Equivalent axial levels are also indicated in C′ and C″. White dotted lines outline the dermomyotome. Dmrt2 expression is observed in the epaxial domain of immature somites (C″, G), and then throughout the Pax3 positive dermomyotome (C′, H). In more mature anterior somites, the expression of Dmrt2 in the epaxial and hypaxial edges of the dermomyotome has decreased (I). S0, presomitic mesoderm prior to the first somite; NT, neural tube; Ep, epaxial somite; Hyp, hypaxial somite. The expression of Dmrt2 is similar in Pax3GFP/+ heterozygote embryos (Figure 2A and 2D–2F) to that in wild type embryos (Figure 1). However Dmrt2 expression levels are higher in the dermomyotome of Pax3PAX3-FKHR-IRESnlacZ/GFP gain of function embryos (Figure 2B and 2G–2I), whereas they are notably lower in Pax3Pax3-En-IRESnlacZ/+ embryos, where the presence of a fusion protein in which the DNA binding domain of Pax3 is fused to the repression domain of Engrailed, results in a partial loss of function phenotype [9], attenuating the cell death seen in the absence of Pax3. Diminution of Dmrt2 expression is also seen in Pax3 mutant embryos at E9.25 (Figure S1), prior to extensive cell death. Maintenance of Dmrt2 expression in the central dermomyotome may reflect the expression of Pax7 in this domain. These results confirm that Dmrt2 lies genetically downstream of Pax3.
Fig. 2. The expression of Dmrt2 is controlled by Pax3. 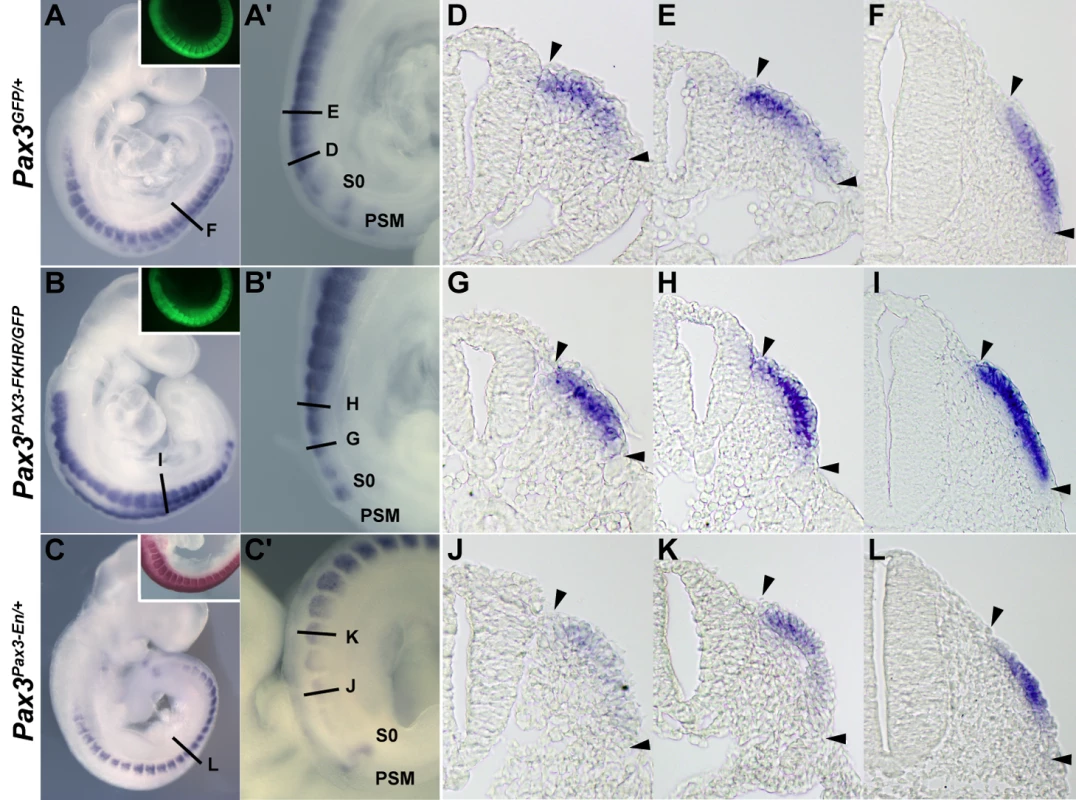
(A–C) Whole mount in situ hybridization with a Dmrt2 probe of Pax3GFP/+ (A), Pax3PAX3-FKHR-IRESnlacZ/GFP (Pax3PAX3-FKHR/GFP, B) and Pax3Pax3-En-IRESnlacZ/+ (Pax3Pax3-En/+, C) embryos at E9.5. Pax3 expression is shown as GFP fluorescence (inserts in A, B), or as Salmon-gal staining (insert in C) of trunk somites. A′–C′ show enlargements of the posterior somite regions used for sections. (D–L) Sections at equivalent axial levels showing Dmrt2 transcripts in the dermomyotome of control Pax3GFP/+ (D–F), gain of function Pax3PAX3-FKHR/GFP (G–I) or partial loss of function Pax3Pax3-En/+ (J–L) embryos. Dmrt2 transcripts are higher in gain of function embryos, where they are now detectable throughout the dermomyotome, whereas when Pax3 activity is altered they are barely detectable in the epaxial domain of immature somites (J) and detectable in a restricted region of more mature somites (L). Arrowheads indicate the full extent of the dermomyotome. PSM, presomitic mesoderm; S0, PSM prior to the first somite. In order to see whether Dmrt2 is a direct Pax3 target, we examined the Dmrt2 locus on mouse chromosome 19, which includes other Dmrt genes (1, 3), for non-coding sequences that are conserved between species (Figure 3A). A conserved 286 bp region at about −18 kb from the Dmrt2 gene attracted our attention. This region has five putative Pax3 binding sites (Figure 3B). Electrophoretic mobility gel shift assays with oligonucleotides encompassing these sites and in vitro synthesized Pax3 protein showed that site2 binds Pax3, a result confirmed by a super-shift experiment with a Pax3 antibody (Figure 3C). Chromatin immunoprecipitation of embryo extracts at E9.5 demonstrated that Pax3 binds specifically to the 286 bp sequence at −18 kb from the Dmrt2 gene in vivo. The function of the 286 bp sequence was tested in transgenic embryos. It directs transgene expression in the dermomyotome, where the endogenous gene is expressed, with additional ectopic expression in the ventral somite (Figure 3E). Mutation of Pax3 site2 in the 286 bp sequence interferes with the expression of the transgene in the dermomyotome (Figure 3F). Other conserved regions, notably at +20 kb and +37 kb did not direct reporter gene expression in transgenic embryos. A BAC transgenic analysis had shown that 150 kb of 5′ and 50 kb of 3′ genomic sequence flanking the Dmrt2 gene directed somitic expression in transgenic embryos. When the Pax3 binding site in the 286 bp sequence was mutated in the context of this BAC, somitic expression was severely affected (Figure S2). This result therefore indicates that the Pax3 site within this sequence plays an important role in the regulation of the Dmrt2 gene.
Fig. 3. A conserved 286 bp element 5′ of the Dmrt2 gene directs Pax3-dependent expression in the somite. 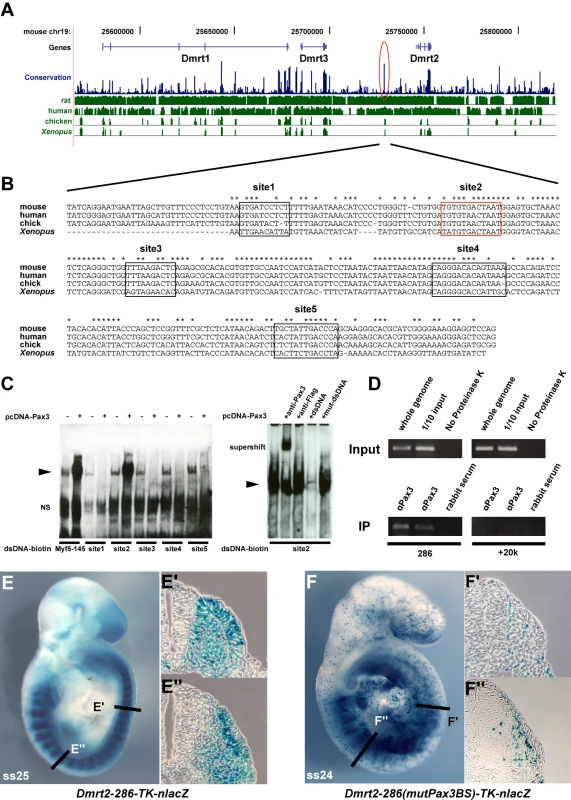
(A) A schematic representation of the mouse Dmrt genomic locus showing overall sequence conservation (blue bars) between human, rat, chicken and Xenopus (green bars). (B) The nucleotide sequence of the conserved 286 bp Dmrt2 element (red circle in A) in mouse (the position on ch19; 25728951 to 25729236, about 18 kb 5′ of Dmrt2) and comparison with a homologous region of human, chick and Xenopus genomes, with asterisks indicating conserved nucleotides between all these species. Five putative Pax3 binding sites are framed in black and red. (C) Electrophoretic mobility shift assays (EMSA) performed with oligonucleotides conjugated with biotin, containing putative Pax3 binding sites (1–5) as indicated in (B), incubated with extracts of HEK293 cells, with (+) or without (−) a Pax3 expression vector, or oligonucleotides without biotin (+dsDNA), including mutated oligonucleotides (+mut-dsDNA). The Pax3 site in the 145 bp regulatory element at −57.5 kb from the Myf5 gene provides a positive control (Myf5-145; [9]). Significant binding is seen on site2 when Pax3 protein is present. For supershift assays, monoclonal Pax3 antibody (DSHB) was used (+anti-Pax3); +anti-Flag provides a negative control. Arrowheads show the band due to binding of the Pax3 protein. NS; non-specific. (D) Chromatin immunoprecipitation (ChIP) analysis of Pax3 binding to the 286 bp sequence in vivo was performed with chromatin prepared from somites of E9.5 embryos (without head, neural tube, and internal tissues). Upper panels show the evaluation of sheared genomic DNA (1/10 input) with two sets of Dmrt2 primers, for the potential regulatory sequence (286) and for an upstream control sequence (+20 k) without Pax3 binding sites (results not shown), in left and right panels respectively. As an additional positive or negative control, whole genome sheared genomic DNA, with (whole genome) or without proteinase K (No Proteinase K) treatment is shown (In the absence of proteinase K cross-linked chromatin blocks the PCR reaction). Lower panels show ChIP (IP) with two different Pax3 antibodies (αPax3, Bajard et al. (2006), Lagha et al. (2008)) and control rabbit serum. Left lower bands indicate Pax3 binding to the 286 bp sequence, not seen with the control +20 kb sequence (right panels). (E) A transient transgenic embryo (E9.5) with the 286 bp Dmrt2 fragment, fused to the TK promoter and nlacZ reporter shows dermomyotome expression. (F) The transgene, with mutated Pax3 binding site2, shows loss of β-galactosidase activity in the dermomyotome (F′), but some ectopic expression in the myotome and ventral somite (F″), seen in more mature somites in the non-mutated transgenic embryo (E″). Observations on transgenic embryos are summarised in Table 1. Dmrt2 has been implicated in somite patterning, with morphological abnormalities reported in mature somites from E10.5 [25]. These include abnormal expression of myotomal markers. Since Dmrt2 is first expressed in the epaxial dermomyotome where activation of the myogenic determination gene, Myf5, initiates the onset of myogenesis [1], we examined Myf5 expression in Dmrt2 mutant embryos. Myf5 activation in the epaxial domain is retarded in the absence of Dmrt2. This is detectable on whole mount in situ hybridization (Figure 4A and 4B). On sections of somites at equivalent axial levels, Myf5 expression is reduced. The delay in the colonisation of the myotome reflects the reduction in Myf5 transcription in the epaxial domain. (Figure 4D and 4F). This perturbation in Myf5 activation has consequences for myogenesis, as evidenced by the delay in expression of the gene for the myogenic differentiation factor, myogenin (Figure 4G and 4H), in the absence of Dmrt2. Pax3 transcription, on the other hand, is not reduced at the onset of somitogenesis (Figure 4I and 4J).
Fig. 4. Dmrt2 is required for the maturation of the epaxial somite, where Myf5 is first expressed. 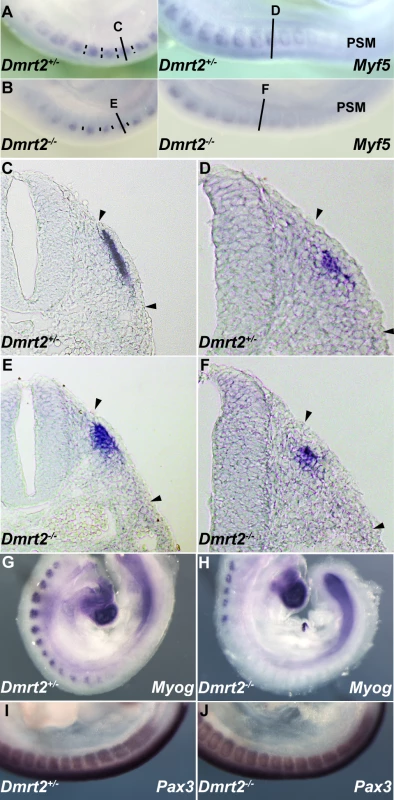
Whole mount in situ hybridization of Dmrt2+/− (A) and Dmrt2−/− (B) embryos at E9.5, with a Myf5 probe. (C–F) Sections of embryos shown in (A, B) at different axial levels - left hand panels show more mature somites; immature somites where the epaxial myotome is beginning to form are shown in right hand panels. The onset of Myf5 expression is perturbed in the absence of Dmrt2. (G, H) Whole mount in situ hybridization of Dmrt2+/− (G) and Dmrt2−/− (H) embryos with a myogenin (Myog) probe, showing a striking delay of myogenin expression in the absence of Dmrt2. (I, J) Whole mount in situ hybridization of Dmrt2+/− (1) and Dmrt2−/− (J) embryos showing Pax3 transcripts. The initiation of Myf5 expression in the somite depends on an early epaxial enhancer [2],[3]. This element, used here in its extended form (Myf5-EpExt; [6]), is regulated by Gli and TCF binding sites, targets of Hedgehog and canonical Wnt signalling. The enhancer contains four sites that are close to the consensus for Dmrt2 binding ([28], Figure 5A). Of these, sites 1, 3 and 4 bind Dmrt2, as shown by electrophoretic mobility shift assays (Figure 5B–5D). This is specific since it is abolished by competition with the Myf5 sequence, but not by this sequence with mutations in the Dmrt2 binding sites (Figure 5C). No good antibodies are available for Dmrt2 detection, so we used a human influenza hemagglutinin (HA) tagged version of the protein, and anti-HA antibodies to show that the complex is disrupted (Figure 5D). The Myf5-EpExt enhancer, with a TK or Myf5BA promoter region drives nlacZ transgene expression in somites. When the Dmrt2 sites are mutated in the Myf5-EpExt enhancer, this expression is very reduced in newly forming somites and is perturbed or absent in more mature somites (Figure 5E and 5F). Transactivation of the Myf5-EpExt enhancer by Dmrt2 was shown by co-transfection experiments in NIH3T3 cells, with this enhancer driving a luciferase reporter and increasing amounts of a Dmrt2 expression vector; mutation of the Dmrt2 sites (1,3,4) in the Myf5-EpExt sequence showed that activity depends on Dmrt2 binding (Figure 6A). Strong transactivation by Dmrt2 was also seen in HEK293 cells (results not shown). In order to look at Dmrt2 activation in vivo, a transgene was constructed in which expression of a sequence encoding Dmrt2, under the transcriptional control of a strong universal CAG promoter, depends on Cre recombinase removal of an intervening CAT sequence. When crossed with a PGK-Cre line [32], Dmrt2 expression is seen throughout the embryo (Figure 6C, the left hand embryo), as also evidenced by expression of the Tomato red reporter (Figure 6C′). When crossed with a Pax3Cre/+ line [33], the Dmrt2 transgene is transcribed at sites of Pax3 expression, including the somites and dorsal neural tube (Figure 6D), where Tomato red coloration is also detected (Figure 6D′). Expression of the endogenous Myf5 gene was monitored in these transgenic embryos at E9.5 (ss 23). When the Dmrt2 transgene is activated by Pax3-Cre, Myf5 expression is more extensive in the newly formed somites (Figure 6E) than in the control embryo (Figure 6H). Myogenin transcripts are also more widely expressed (Figure 6F and 6I) and sections of an immature somite show premature presence of assembled laminin, which marks the myotome basement membrane (Figure 6G, compared to Figure 6J). Together, these data indicate that myogenesis is accelerated in these embryos, leading to an earlier entry and organisation of cells in the myotomal space. The effect on Myf5 expression is also seen when the Dmrt2 transgene is expressed in Myf5nlacZ/+ mice (Figure 6K, compared to Figure 6L) and sections show that Myf5 is now ectopically expressed in the central/hypaxial dermomyotome in the presence of Pax3-Cre (Figure 6K′ and 6K″ compared to Figure 6L′ and 6L″). A similar result was seen with PGK-Cre (Figure S3). This therefore demonstrates that Dmrt2 activates the Myf5 gene in vivo in the somite, leading to ectopic expression. Acceleration of the onset of myogenesis and myotome formation are probably the consequence of over-expression of Dmrt2 and over-activation of the epaxial enhancer of Myf5.
Fig. 5. Dmrt2 binding sites in the Myf5 epaxial enhancer are essential for normal activity in vivo. 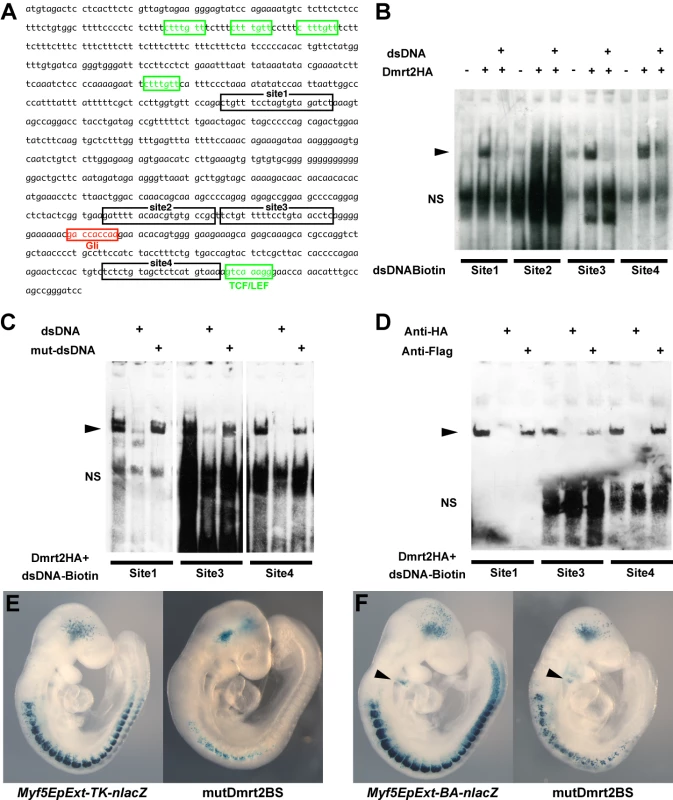
(A) The sequence of the Myf5 epaxial enhancer (EpExt), which drives early Myf5 expression in the epaxial domain of newly formed somites. TCF/LEF binding sites are boxed in green, the Gli1 binding site in red, and four putative Dmrt2 binding sites in black. (B–D) EMSA performed with oligonucleotides conjugated with biotin, containing Dmrt2 binding sites (1–4) as indicated in A, incubated with extracts of HEK293 cells, with (+) or without (−) a Dmrt2HA expression vector, or oligonucleotides without biotin (dsDNA) (including mutated oligonucleotides; mut-dsDNA). In the supershift assay (D), binding was disrupted by adding anti-HA (Monoclonal; Roche) to HA-tagged Dmrt2, whereas control anti-Flag antibody had no effect. The arrowheads show the binding between the oligos and the tagged protein. NS; non-specific. (E, F) Transient transgenic analysis to examine the role of the Dmrt2 binding sites in the Myf5 EpExt enhancer. The transgenes, containing mutated Dmrt2 binding sites1, 3, 4, showed reduced β-galactosidase activity in developing somites with either the TK (E) or Myf5-BA (branchial arch) promoter region (F). The BA element that directs transgene expression to the branchial arches, provides a positive control (arrowheads in F). Observations on transgenic embryos are summarised in Table 1. Fig. 6. Overexpression of Dmrt2 accelerates myogenesis. 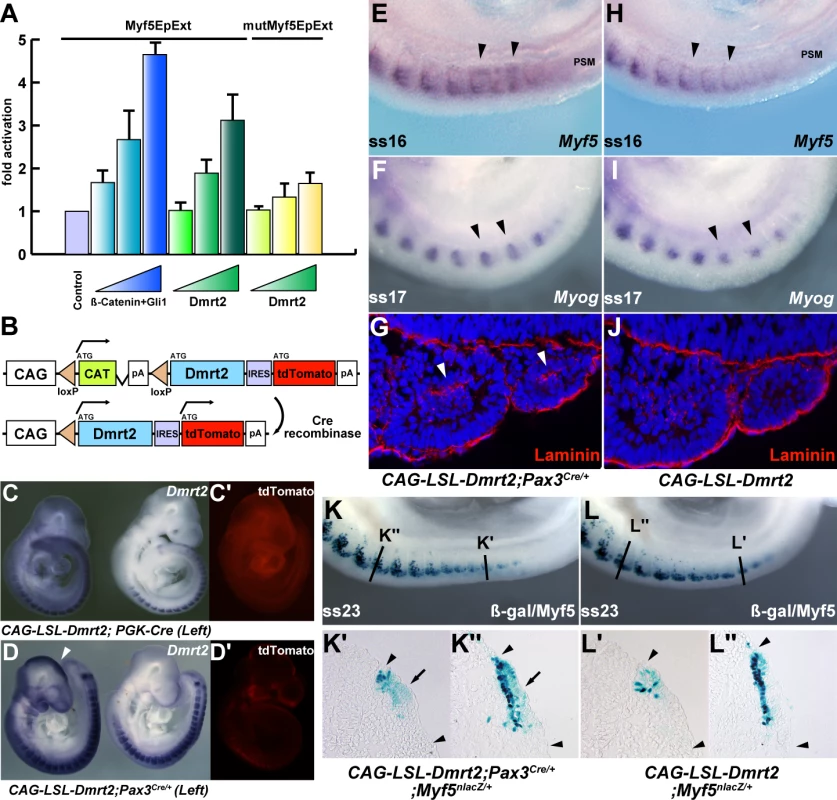
(A) Luciferase assay in NIH3T3 cells with the Myf5EpExt fragment with (yellow bars) or without (green bars) mutated Dmrt2 binding sites 1, 3, 4 (mutMyf5EpExt) as used in Figure 5, and increasing concentrations of a Dmrt2-expression vector. As a positive control, β-catenin and Gli1 expression vectors were used (blue bars). Progressive increase in Dmrt2 expression, leads to increased activation of the Myf5EpExt which is lost when Dmrt2 binding sites 1, 3, 4 are mutated. (B) The transgene for Dmrt2 conditional over-expression, in which Cre recombination removes the CAT sequence leading to Dmrt2 and reporter Tomato red expression under the strong CAG promoter. (C, C′, D, D′) Transgenic embryos, containing the CAG-floxedstop-Dmrt2-IRES-tdTomato (CAG-LSL-Dmrt2) transgene, crossed with PGK-Cre mice (left in C, C′), and Pax3Cre/+ mice (left in D, D′). Ectopic Dmrt2 expression is seen in PGK-Cre or Pax3Cre/+ expressing embryos reflecting Cre expression (arrowheads in C, D). (E–I) Whole mount in situ hybridization of transgenic control or Pax3-Cre activated embryos at E9.5. Myf5 (E.H) and myogenin (F, I) expression are up-regulated in developing somites of embryos overexpressing the Dmrt2 transgene in Pax3 positive cells (arrowheads in E, F) compared to controls (H, I). (G, J) Sections showing somites of control or activated Dmrt2 transgenic embryos at the same axial level, treated by immunohistochemistry with a laminin antibody. Laminin is detected in the premature myotome, where it is not normally present at this developmental stage. (arrowheads in G). (K, L) Whole mount X-gal staining of Myf5nlacZ/+ embryos crossed onto the Dmrt2 expressing transgenic line, with (K) or without (L) activation by Pax3-Cre, show perturbed location of β-galactosidase activity. This is shown on sections at the axial levels indicated in K and L, in which Pax3 activation of the Dmrt2 transgene results in more extensive dermomyotome (arrows in K′, K″) and early myotome (K′) expression (not seen in the control (L′, L″). Arrowheads indicate somite extent. Discussion
We establish a genetic network that is initiated in the dermomyotome by Pax3 regulation of Dmrt2 and which, through Dmrt2 regulation of Myf5, orchestrates the onset of myogenesis in the myotome. This direct transcriptional cascade and further genetic targets discussed in this paper are presented schematically in Figure 7.
Fig. 7. A schematic representation of the regulatory network presented in this paper. 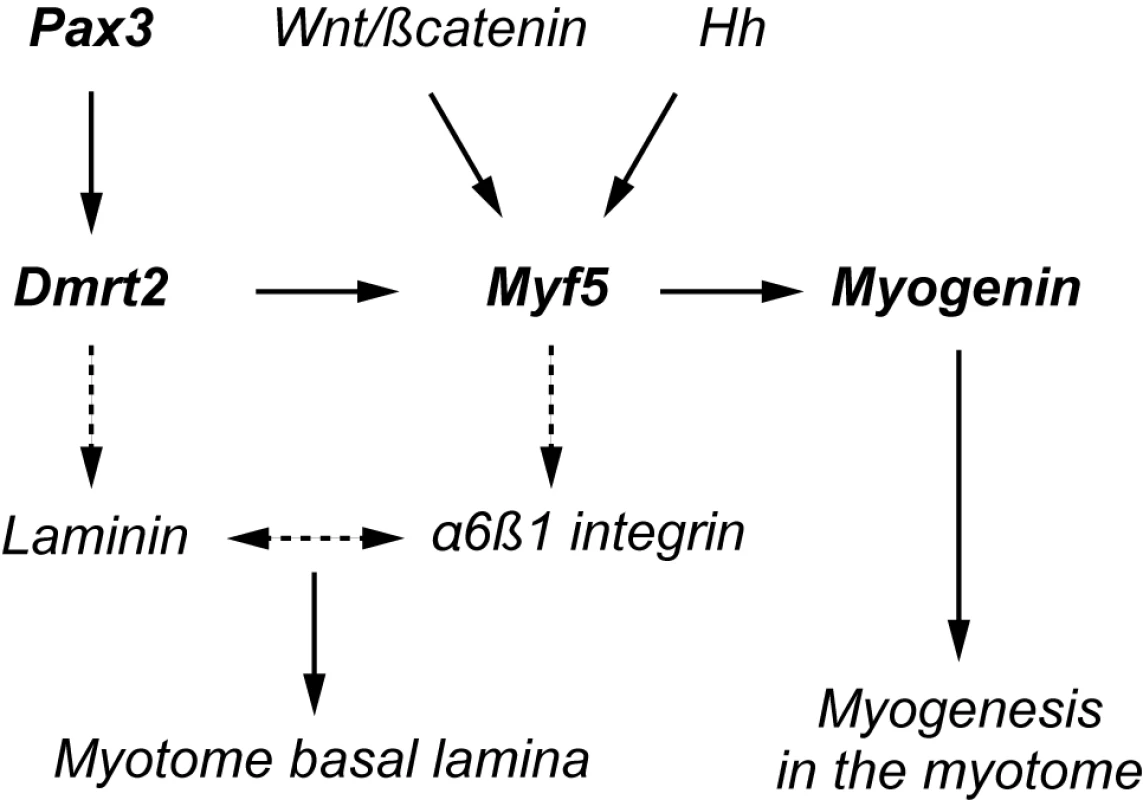
Direct targets are shown in heavy type. Indirect targets are indicated by a discontinuous arrow. The myogenin promoter depends on a critical Ebox [47],[48] targeted by Myf5. α6β1 integrin expression depends on Myf5, in cells entering the myotome [39]. This receptor interacts with Laminin1 as a ligand, leading to formation of the basement membrane of the myotome. Laminin expression is modulated by Dmrt2 (see also [25]). Our demonstration that Dmrt2 is a Pax3 target was initially based on a genetic analysis that showed up-regulation of this gene in the presence of PAX3-FKHR. This transcriptional activator of Pax3 target genes may introduce a bias due to the FKHR domain, however down-regulation of Dmrt2 in the presence of the Pax3-Engrailed fusion protein and in the absence of Pax3 reinforces the interpretation. This is confirmed by the identification of a Pax3 dependent regulatory sequence, located at 18 kb 5′ of the Dmrt2 gene, which directs transgene expression to the somite. Mutation of the Pax3 site shown to bind this factor in vitro and in vivo, abolishes most of the activity in the dermomyotome. Since Pax7 can replace Pax3 in the trunk [34], it is probable that this site also binds Pax7. Indeed in Pax3 mutants, Dmrt2 continues to be expressed in the remaining dermomyotome [26], where Pax7 prevents cell death in the absence of Pax3 [1]. Expression of the transgene is not confined to the dermomyotome, suggesting that the 286 bp element also responds to factors in the ventral somite, where transcription of the endogenous gene is repressed by other regulatory sequences. Dmrt2 is present in a locus that includes Dmrt1 and Dmrt3. The latter lies about 30 kb 5′ of the 286 bp sequence. However, Dmrt3 is not transcribed in the somites and there is no indication that this regulatory element directs any aspect of Dmrt3 expression, which is characteristically seen at sites in the head [35],[36] for example, where no labelling with the Dmrt2-286-TKnlacZ transgene is observed. Other regulatory regions, namely 2.6 kb immediately 5′ of Dmrt1 which directs transgene expression to the testis [37] or a highly conserved sequence between Dmrt1 and Dmrt3, associated with XY sex reversal in humans [38], which directs Dmrt3-like transgene expression, also show regulatory properties characteristic of the adjacent downstream gene only.
It is well established that the DM domains of Dmrt2 factors bind to a similar consensus sequence [28], however direct target genes have not been identified. We now show that the Myf5 gene is targeted by Dmrt2 through the expected DM consensus sequence. The transcriptional activity of mammalian Dmrt factors is not well defined, in contrast to Drosophila Dsx which acts as a transcriptional activator or repressor, when produced from a male or female allele, respectively [29]. We demonstrate that Dmrt2 functions as a transcriptional activator on a DM consensus binding site in the Myf5 early epaxial element. This was observed in assays with the NIH3T3 fibroblast cell line, and with HEK293 cells in which transcriptional activity of Dmrt1 was not detectable under conditions in which a Dmrt1-VP16 fusion protein transactivated a luciferase reporter through DM sites [28]. Our transgenic experiments which lead to Dmrt2 over-expression in vivo also point to the action of this factor as an activator of Myf5 expression.
Dmrt2 mutant phenotypes in which somite disorganisation and effects on myogenesis had been documented previously, focussed on E10.5 and E11.5 embryos [25],[26]. We observed that Dmrt2 transcription begins to be down-regulated from E10.5 and transcripts are no longer detectable in most somites by E11.5. The muscle recovery noted in Dmrt2 mutants at later stages [25] is probably due to the invasion of the underlying muscle by Pax3/7 positive cells, from the central dermomyotome, which takes place from E10.5 [7]. Indeed in Dmrt2 mutants at E10.5, the absence of apically polarised N-cadherin, in the dermomyotome ([25], Figure 4), may indicate de-epithelialization and premature disaggregation of the central domain which is the source of these muscle progenitor cells. We have concentrated our analysis on the onset of myogenesis when Dmrt2 is strongly expressed in the dermomyotome. In the absence of Dmrt2, the onset of Myf5 expression is affected. This depends on the epaxial enhancer [2],[3], which we show is regulated through Dmrt2 binding sites. This enhancer is activated in the epaxial somite by signals from the adjacent axial structures [6]. The requirement for Dmrt2 will restrict this activation to the dermomyotome. Dmrt2 alone does not direct ectopic expression of Myf5 outside the somite, indicating that other factors also play a role in this restriction. Myf5 plays a key role in the initiation of myogenesis, manifested by the formation of the early epaxial myotome. The perduration of β-galactosidase, either from a Myf5nlacZ allele or from a Myf5-EpExt regulated transgene, marks these first myogenic cells. When Myf5 expression is perturbed by the lack of Dmrt2, the formation of the myotome is affected. In contrast, over-expression of Dmrt2 promotes Myf5 expression and myotome formation. Activation of the myogenic differentiation programme is evidenced by expression of the myogenic differentiation gene, myogenin, which is delayed in somites in the Dmrt2 mutant and increased in the early myotome when Dmrt2 is over-expressed. An important aspect of myotome formation is the laying down of the basal lamina that confines this somite compartment. In the absence of Dmrt2, the expression of myogenic markers, such as myogenin, in the myotome is more diffuse ventrally and this correlates with a lack of laminin [25]. In contrast, when Dmrt2 is over-expressed, we observed premature expression of laminin, associated with an acceleration of myotome formation. Laminin is the ligand of α6β1 integrin, required for the formation of the myotome basal lamina. Interestingly, α6β1 integrin is not expressed on myogenic cells delaminating from the dermomyotome in Myf5 mutant mice, in which cells fail to locate correctly and the early myotome does not form [39]. Since this early expression of Myf5 depends on the epaxial enhancer, Dmrt2 lies genetically upstream of both this integrin receptor and its laminin ligand (Figure 7).
The Pax3-Dmrt2-Myf5 regulatory cascade, identified by genetic and molecular approaches, has consequences for the onset of skeletal muscle formation, both in terms of the activation of the myogenic regulatory programme and of the organisation of myogenic cells and their progenitors within the somite. In Pax3 mutants, epaxial myogenesis occurs, however perturbations in epaxial derivatives, such as deep back muscles, are observed [40]. Other Myf5 regulatory sequences control myotomal expression [2]. Pax3 also directly activates another Myf5 regulatory element at later developmental stages [9]. This key factor therefore, directly or indirectly influences different spatiotemporal aspects of myogenesis. Expression of Dmrt2 is not limited to myogenic progenitors in the epaxial domain, but is present throughout the developing dermomyotome, where it may be implicated in maintaining this epithelial structure [25]. We have recently identified Foxc2 as a gene that is negatively regulated by Pax3 in the dermomyotome and that promotes non-myogenic cell fates [41]. Identification of these targets begins to reveal how Pax3 controls cell behaviour in the somite before the determination of myogenic cells. Integration of Pax3 targets into a regulatory network, comprising elements such as those discussed in this paper (Figure 7), is essential for understanding how such a key regulatory factor orchestrates the progression from stem cell towards differentiated tissue. Defining such regulatory networks that control developmental processes is a major challenge, taken up by the emerging field of systems biology.
Materials and Methods
Mice
Generation and genotying of Pax3IRES-nlacZ/+, Pax3GFP/+ [7], Pax3PAX3FKHR-IRESnlacZ/+ [31], Pax3Pax3-En-IRESnlacZ/+ [9], Pax3Cre/+ [33], PGK-Cre [32], Myf5nlacZ/+ [42], Dmrt2+/−, and Dmrt2−/− [25] mice was carried out as previously described.
Screening
Pax3GFP/+ mice were crossed with PGK-Cre transgenic mice to obtain Pax3GFP/+; PGK-Cre females, and these females were crossed with Pax3PAX3FKHR-IRESnlacZ/+ males to obtain embryos with one Pax3GFP allele and one floxed Pax3PAX3FKHR-IRESnlacZ allele [31]. Somites were dissected from the interlimb region of E9.5 embryos. The GFP positive cells were collected by FACs from Pax3GFP/+ and Pax3Pax3FKHR–IRESnlacZ/GFP mutant embryos. About 3.0×105 cells were collected for RNA preparation, cDNA synthesis and subsequent analysis using Affymetrix microarrays. Detailed description of the microarray analysis will be published elsewhere (Lagha and Sato, in preparation).
Plasmid construction and generation of transgenic mice
The mouse Dmrt2 cDNA (contains the complete coding region; NM145831) was isolated by RT-PCR from cDNA of RNA prepared from C57BL/6 mouse embryos at E9.5. The Dmrt2 cDNAs with or without a 3′ sequence coding HA tag were subcloned into pBS and pcDNA3 vectors for in situ hybridization, electrophoresis mobility shift, and overexpression assays.
To generate transgenes with the conserved 286 bp sequence located at −18 kb from the Dmrt2 gene (Dmrt2-286), or the extended Myf5 epaxial enhancer (Myf5-EpExt, [6]), these sequences were obtained by PCR from total DNA of E9.5, C57BL/6 embryos using KOD DNA polymerases (Novagen) and ligated into pBS plasmids to lie 5′ of TK-nlacZ or Myf5BA-nlacZ transgenic sequences [43]. The primers used were as follows, Dmrt2-286-Fwd; 5′-GCAGCGGCCGCTATCAGGAATGAATTAGCTTGTTTCCCTCC-3′, Dmrt2-286-Rev; 5′ - GCCACTAGTCTGGACCTCCTTTCCCCGATGCGTGCCCT-3′, Myf5-EpExt-Fwd; 5′-GCAGCGGCCGCATGTAGACTCCTCACTTCTCGTTAGTAGA-3′, Myf5-EpExt-Rev; 5′ - GCCACTAGTGGATCCCGGCTGGCAAATGTTTGGTTCCCT-3′.
Transgenes with mutated Pax3 or Dmrt2 binding sites were created with the QuikChange Multi Site-Directed Mutagenesis kit (Stratagene). The Myf5EpExt-TK fragment was digested with NheI and NcoI, and ligated into the pGL4 vector (Promega) in front of the luciferase reporter for transactivation assays.
For transgenic expression of Dmrt2, the HindIII and BamHI fragment digested from the pBS-CAG-floxedCAT vector [44], the HindIII and XbaI fragment digested from pBS-Dmrt2, and the XbaI and BamHI fragment digested from the pCMVTnT-IRES2-tdTomato-pA vector (pCMVTnT; Promega, pIRES2; Clontech, tdTomato; a gift from Dr. Tsien) were all ligated together to make the pBS-CAG-floxedCAT-Dmrt2-IRES2-tdTomato vector (pBS-CAG-LSL-Dmrt2). pBS-Dmrt2-286-TK-nlacZ, pBS-Myf5EpExt-TK-nlacZ, pBS-Myf5EpExt-BA-nlacZ and pBS-CAG-LSL-Dmrt2 vectors were linearized to produce transgenic mice according to standard techniques. The primers used for genotyping of CAG-LSL-Dmrt2 mice were as follows, F502; CTCCGGAGGCAGCAGGCCACAG, R620; ATGCTTTTGGCCAGCAAACTCG; R794; CGCGATGTCCCAAATGGACCTAA (wildtype; 293 bp, transgene; 119 bp).
For BAC transgenic analysis, a BAC containing Dmrt2 genomic DNA (−150 kb/+50 kb: clone RP24-290E12 purchased from BACPAC resources center, CHORI) was used for targeting with an nlacZ reporter into the ATG site of Dmrt2. The Dmrt2 BAC with a mutated Pax3 binding site in the 286 bp sequence was created using the fragment from the transgene with the mutated Pax3 binding site in Dmrt2-286. All BAC recombineering were performed with SW105 and SW106 strains [45].
Whole mount staining of embryos and immunohistochemistry
Whole mount in situ hybridization analyses and X-gal staining were performed as previously described [15]. For double detection of β-galactosidase (β-gal) from a Pax3 nlacZ allele and Dmrt2 transcripts, the former was visualized by Red-gal (Sigma), and the latter using a digoxigenin (DIG)-labeled probe visualized with BM purple (Roche). For immunohistochemistry of laminin, the antibody against laminin (Chemicon; AL-4 clone) was used as described [39].
Electrophoretic mobility shift assays (EMSA)
EMSA was performed using the LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific). Protein extracts containing Pax3 or Dmrt2 protein were prepared from HEK293 cells transfected with pcDNA3-Pax3 [46] or pcDNA3-Dmrt2HA. Cells were then lysed on ice in extraction buffer (15 mM Tris pH 7.4; containing protease inhibitor cocktail (Roche)) supplemented with 0.5% NP40. Lysates were centrifuged at 2000×g for 5 min at 4°C and the supernatant was stored in 25% glycerol at −80°C until ready for use. The 5′-biotin-conjugated oligos and unlabeled probes used were as follows, Pax3BS1; 5′-CTTGTTTCCCTCCTGTAAGTGATCC-3′, Pax3BS2; 5′-CTGTGGTGTGTGACTAATGGAGTGC-3′, Pax3BS3; 5′-CTCTCAGGGCTGGTTTAAGACTCA-3′, Pax3BS4; 5′-CATAGCAGGGACACAGTAAAGCCAC-3′, mutPax3BS2; 5′ - CTGTGGTGACGTCTAAATGGAGTGC-3′, Dmrt2BS1; 5′-CTGTTTCCTAGTGTAGATCT-3′, Dmrt2BS2; 5′ - GATTTTACAACGTGTGCCGC-3′, Dmrt2BS3; 5′-TCTGTTTTTCCTGTAACCTC-3′, Dmrt2BS4; 5′-TCTCTGTAGCTCTCATGTAAA-3′, mutDmrt2BS1; 5′-CTGTTTCCTTGTTTTGATCT-3′, mutDmrt2BS3; 5′-TCTGTTTTTCCTTTTATCTC-3′, mutDmrt2BS4; 5′-TCTCTTTTGCTCTCATTTTAA-3′. Sense and anti-sense strands were synthesized and 40 fmol biotin-labeled double-stranded DNA was mixed with 1 µg protein from a HEK293 cell extract. Gel mobility shift assays and hybridization were performed according to the instructions of Invitrogen, using 6% DNA retardation gels (Invitrogen).
Chromatin Immunoprecipitation (ChIP)
For ChIP experiments, somites were collected from E9.5 embryos with heads, neural tubes and internal organs removed. These samples were mechanically digested through a syringe and dissociated cells were fixed with 1% formaldehyde at room temperature for 10 min. The ChIP procedure was performed according to an enzymatic digestion protocol (ChIP-IT Express; ActiveMotif) with Pax3 antibody raised from Rabbit (ActiveMotif and Geneka; Bajard et al., 2006 and Lagha et al., 2008) or normal Rabbit serum (Sigma) as a control. Input DNA and immunoprecipitated DNA were analyzed by PCR. The sequences of primers were designed as follows. The Dmrt2-18 kb conserved region (286 bp) Fwd; 5′-GCTTGTTTCCCTCCTGTAAGT-3′, Rev; 5′ - GTGTAGGATCTGTGGCTTTAC-3′ and the Dmrt2+20 kb control conserved region (+20 kb) Fwd; 5′-GGTTCTCATAATTTACATGCT-3′, Rev; 5′ - TCCAACATCTGATTGTACTTA-3′.
Luciferase assay
For luciferase assays, pGL4-Myf5EpExt vectors were transfected into NIH3T3 cells together with a pRL-TK plasmid (Promega; for normalizing) and test plasmids (Gli1 and β-catenin expression vectors were kindly provided by Dr. S. Brunelli). Transfected cells were cultured for 24 hours, and subjected to luciferase assays using the Dual-Luciferase Reporter Assay System (Promega).
Tab. 1. Transient transgenic embryos and lines (where indicated) (at E9.5, unless otherwise indicated). 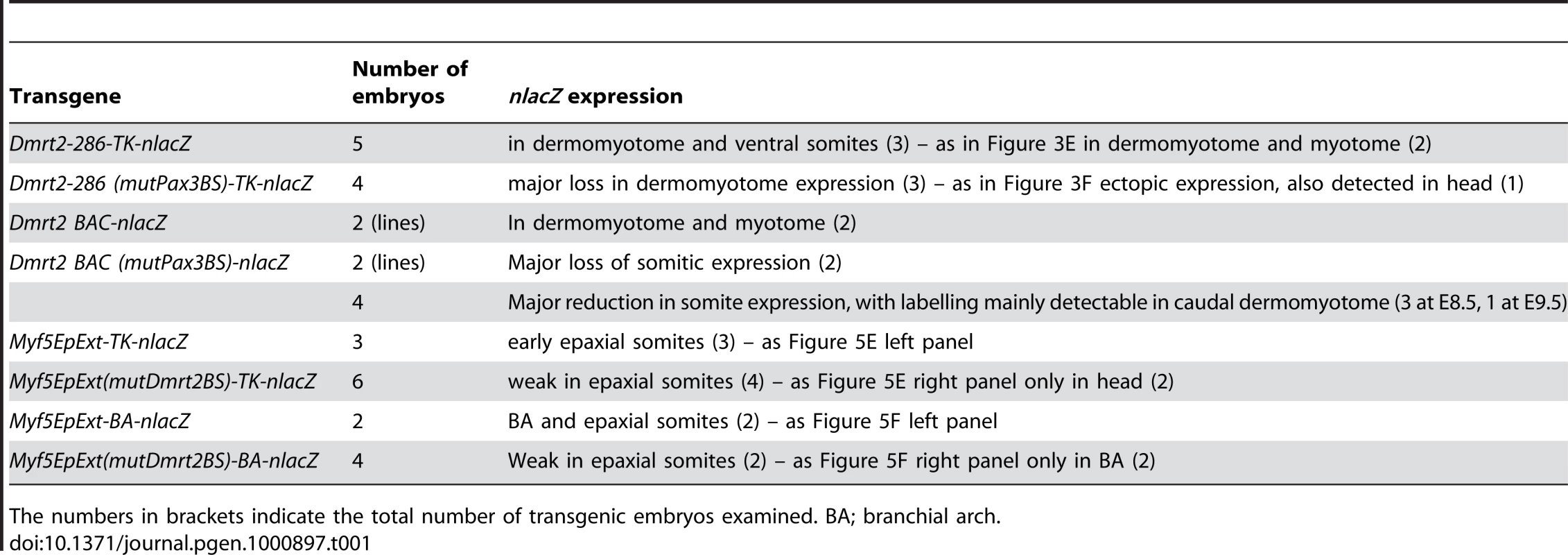
The numbers in brackets indicate the total number of transgenic embryos examined. BA; branchial arch. Supporting Information
Zdroje
1. BuckinghamM
RelaixF
2007 The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol 23 645 673
2. SummerbellD
AshbyPR
CoutelleO
CoxD
YeeS
2000 The expression of Myf5 in the developing mouse embryo is controlled by discrete and dispersed enhancers specific for particular populations of skeletal muscle precursors. Development 127 3745 3757
3. TeboulL
HadchouelJ
DaubasP
SummerbellD
BuckinghamM
2002 The early epaxial enhancer is essential for the initial expression of the skeletal muscle determination gene Myf5 but not for subsequent, multiple phases of somitic myogenesis. Development 129 4571 4580
4. GustafssonMK
PanH
PinneyDF
LiuY
LewandowskiA
2002 Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev 16 114 126
5. TeboulL
SummerbellD
RigbyPW
2003 The initial somitic phase of Myf5 expression requires neither Shh signaling nor Gli regulation. Genes Dev 17 2870 2874
6. BorelloU
BerarducciB
MurphyP
BajardL
BuffaV
2006 The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development 133 3723 3732
7. RelaixF
RocancourtD
MansouriA
BuckinghamM
2005 A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435 948 953
8. EpsteinJA
ShapiroDN
ChengJ
LamPY
MaasRL
1996 Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci U S A 93 4213 4218
9. BajardL
RelaixF
LaghaM
RocancourtD
DaubasP
2006 A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev 20 2450 2464
10. HuP
GelesKG
PaikJH
DePinhoRA
TjianR
2008 Codependent activators direct myoblast-specific MyoD transcription. Dev Cell 15 534 546
11. McKinnellIW
IshibashiJ
Le GrandF
PunchVG
AddicksGC
2008 Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol 10 77 84
12. KumarD
ShadrachJL
WagersAJ
LassarAB
2009 Id3 is a direct transcriptional target of Pax7 in quiescent satellite cells. Mol Biol Cell 20 3170 3177
13. CristGC
MontarrasD
PallafacchinaG
RocancourtD
CumanoA
2009 Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci USA 106 13383 13387
14. BuckinghamM
VincentS
2009 Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev 19 444 453
15. LaghaM
KormishJD
RocancourtD
ManceauM
EpsteinJA
2008 Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev 22 1828 1837
16. HildrethPE
1965 Doublesex, recessive gene that transforms both males and females of Drosophila into intersexes. Genetics 51 659 678
17. BakerBS
RidgeKA
1980 Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94 383 423
18. BakerBS
WolfnerMF
1988 A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev 2 477 489
19. ShenMM
HodgkinJ
1988 mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell 54 1019 1031
20. RaymondCS
ShamuCE
ShenMM
SeifertKJ
HirschB
1998 Evidence for evolutionary conservation of sex-determining genes. Nature 391 691 695
21. YiW
ZarkowerD
1999 Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development 126 873 881
22. HongCS
ParkBY
Saint-JeannetJP
2007 The function of Dmrt genes in vertebrate development: it is not just about sex. Dev Biol 310 1 9
23. MengA
MooreB
TangH
YuanB
LinS
1999 A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development 126 1259 1268
24. SaudeL
LourencoR
GoncalvesA
PalmeirimI
2005 terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat Cell Biol 7 918 920
25. SeoKW
WangY
KokuboH
KettlewellJR
ZarkowerDA
2006 Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Dev Biol 290 200 210
26. SeoKW
2007 Dmrt2 and Pax3 double-knockout mice show severe defects in embryonic myogenesis. Comp Med 57 460 468
27. ZhuL
WilkenJ
PhillipsNB
NarendraU
ChanG
2000 Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev 14 1750 1764
28. MurphyMW
ZarkowerD
BardwellVJ
2007 Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol Biol 8 58
29. SacconeG
PaneA
PolitoLC
2002 Sex determination in flies, fruitflies and butterflies. Genetica 116 15 23
30. OttolenghiC
VeitiaR
BarbieriM
FellousM
McElreaveyK
2000 The human doublesex-related gene, DMRT2, is homologous to a gene involved in somitogenesis and encodes a potential bicistronic transcript. Genomics 64 179 186
31. RelaixF
PolimeniM
RocancourtD
PonzettoC
SchaferBW
2003 The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev 17 2950 2965
32. LallemandY
LuriaV
Haffner-KrauszR
LonaiP
1998 Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res 7 105 112
33. EnglekaKA
GitlerAD
ZhangM
ZhouDD
HighFA
2005 Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280 396 406
34. RelaixF
RocancourtD
MansouriA
BuckinghamM
2004 Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev 18 1088 1105
35. SmithCA
HurleyTM
McClivePJ
SinclairAH
2002 Restricted expression of DMRT3 in chicken and mouse embryos. Mech Dev 119 S73 76
36. KimS
KettlewellJR
AndersonRC
BardwellVJ
ZarkowerD
2003 Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene Expr Patterns 3 77 82
37. BoyerA
DornanS
DaneauI
LussierJ
SilversidesDW
2002 Conservation of the function of DMRT1 regulatory sequences in mammalian sex differentiation. Genesis 34 236 243
38. AhituvN
ZhuY
ViselA
HoltA
AfzalV
2007 Deletion of ultraconserved elements yields viable mice. PLoS Biol 5 e234 doi:10.1371/journal.pbio.0050234
39. BajancaF
LuzM
RaymondK
MartinsGG
SonnenbergA
2006 Integrin alpha6beta1-laminin interactions regulate early myotome formation in the mouse embryo. Development 133 1635 1644
40. TajbakhshS
RocancourtD
CossuG
BuckinghamM
1997 Redefining the genetic hierarchies controling skeletal myogenesis: Pax3 and Myf-5 act upstream of MyoD. Cell 89 127 138
41. LaghaM
BrunelliS
MessinaG
CumanoA
KumeT
2009 Pax3:Foxc2 reciprocal repression in the somite modulates musclar versus vascular cell fate choice in multipotent progenitors. Dev Cell 17 892 899
42. TajbakhshS
BoberE
BabinetC
PourninS
ArnoldH
1996 Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev Dyn 206 291 300
43. HadchouelJ
TajbakhshS
PrimigM
ChangTH
DaubasP
2000 Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development 127 4455 4467
44. SakaiK
MiyazakiJ
1997 A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun 237 318 324
45. WarmingS
CostantinoN
CourtDL
JenkinsNA
CopelandNG
2005 Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33 e36
46. RelaixF
MontarrasD
ZaffranS
Gayraud-MorelB
RocancourtD
2006 Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172 91 102
47. ChengTC
WallaceMC
MerlieJP
OlsonEN
1993 Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science 261 215 218
48. YeeSP
RigbyPW
1993 The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev 7 1277 1289
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



