-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCrosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
Intestinal homeostasis is ensured by a subtle balance between bacteria and host immunity. Gut epithelial barriers, such as the mucus layer in mammals and the peritrophic matrix in invertebrates, have a protective function for the host, as they are impermeable to invading intestinal microbes. Here we found that, in the fly Drosophila melanogaster, transglutaminase (TG), a molecular glue involved in protein-protein covalent bond formation, is essential for peritrophic matrix formation by converting the peritrophic protein drosocrystallin into a stable fiber-like structure and inhibition of pathogenic bacteria. Knockdown of the TG gene led to increased permeability of the peritrophic matrix and greatly increased the susceptibility to a toxic bacterial protease. TG contributes to form a stable fiber-like barrier on the peritrophic matrix and increase tolerance to pathogenic microorganisms.
Published in the journal: . PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005244
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005244Summary
Intestinal homeostasis is ensured by a subtle balance between bacteria and host immunity. Gut epithelial barriers, such as the mucus layer in mammals and the peritrophic matrix in invertebrates, have a protective function for the host, as they are impermeable to invading intestinal microbes. Here we found that, in the fly Drosophila melanogaster, transglutaminase (TG), a molecular glue involved in protein-protein covalent bond formation, is essential for peritrophic matrix formation by converting the peritrophic protein drosocrystallin into a stable fiber-like structure and inhibition of pathogenic bacteria. Knockdown of the TG gene led to increased permeability of the peritrophic matrix and greatly increased the susceptibility to a toxic bacterial protease. TG contributes to form a stable fiber-like barrier on the peritrophic matrix and increase tolerance to pathogenic microorganisms.
Introduction
Gut epithelia are the first line of defense against invading microorganisms. Drosophila has several gut defense systems, including the production of antimicrobial peptides [1–3] and reactive oxygen species [4–6], peritrophic matrix formation [7], and stem cell activation for cell renewal [8,9]. Transglutaminase (TG) is involved in the regulation of antimicrobial peptide production and peritrophic matrix formation [10]. TG catalyzes the isopeptide bond formation between lysine and glutamine residues, and has diverse physiologic roles in vertebrates and invertebrates [11,12]. Drosophila TG is involved in cuticular formation [13] and hemolymph coagulation, which traps invading pathogens [14,15]. The concept of hemolymph coagulation in invertebrates as a part of the early innate immune system has been extended to vertebrates [14,15]. Recently, we reported that systemic and gut-specific TG-knockdown flies have a shorter lifespan than control flies, concomitant with severe apoptosis of cells in the gut epithelium [10]. Moreover, we found that TG crosslinks N-terminal Relish in the immune deficiency pathway to suppress antimicrobial peptide expression, thereby enabling immune tolerance against gut microbes. Further, RNA interference (RNAi) of the TG gene causes peritrophic matrix defects and penetration of dextran beads from the gut lumen (endoperitrophic space) into the ectoperitrophic space [10].
The peritrophic matrix in insects is a non-cellular sieve-like structure that lines the midgut epithelium, and comprises chitin fibrils and chitin-binding proteins [16]. This matrix has a role analogous to that of the mucosal layer of the vertebrate intestine, and is thought to support digestion and provide protection against abrasive food particles and enteric pathogens [17]. The drosocrystallin gene, which encodes a 52-kDa glycoprotein with Ca2+-binding ability, was originally identified in Drosophila eyes, but its function was not clear [18,19]. Drosocrystallin was recently reported to have an important role in protecting against entomopathogenic bacteria such as Pseudomonas entomophila [7]. Drosocrystallin expression is induced upon oral infection by bacteria, and the peritrophic matrix of drosocrystallin-knockout flies is more permeable than that of wild-type flies, demonstrating the essential role of drosocrystallin in peritrophic matrix formation [7]. Drosocrystallin is a secreted glycoprotein containing a chitin-binding R&R motif [20,21]. Cuticular chitin-binding proteins in horseshoe crabs are substrates for TG [22], suggesting that drosocrystallin could be a potential TG substrate. Here we demonstrate that TG enhances the structural strength of the peritrophic matrix by crosslinking drosocrystallin fibers, and that the crosslinked drosocrystallin fibers, but not non-crosslinked drosocrystallin fibers, are essential for protection against exotoxins secreted by gut-invading pathogenic bacteria.
Results and Discussion
Drosocrystallin is crosslinked by TG in vitro and in vivo
Wild-type drosocrystallin and a lysine-to-arginine substituted mutant (KR) were prepared in Escherichia coli. To examine the functional activity of these recombinants, we evaluated chitin-binding activity. Both recombinants clearly bound to chitin (Fig 1A, left and right panels). The recombinants were then incubated with TG in the presence of monodansylcadaverine (MDC) or biotin pentylamine, an amino-substrate of TG. MDC was incorporated into these recombinants in a time-dependent manner (Fig 1B, left panel). MDC was incorporated into the KR mutant at the same rate as in the wild-type recombinant (Fig 1B, right panel), because the amino-substrate is incorporated in glutamine residues. The biotin pentylamine-incorporated recombinants were also detected using streptavidin, but incorporation was inhibited in the presence of a TG inhibitor, EDTA (Fig 1C). In wild-type drosocrystallin, a protein band was observed on the stacking gel, but not in the KR mutant in the absence of MDC and EDTA (Fig 2A), indicating that drosocrystallin is covalently crosslinked by TG to form homopolymers. To clarify the crosslinking profile of drosocrystallin in vivo, we generated systemic TG-knockdown flies (Da>TG IR). Western blotting using anti-drosocrystallin antibody revealed a protein band on the stacking gel in the gut of control flies (Da>+), but not TG-knockdown flies (Fig 2B), indicating that drosocrystallin was covalently crosslinked by TG in vivo. In sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the non-glycosylated wild-type recombinant expressed in E. coli and intact drosocrystallin in the gut had apparent molecular masses of 52 kDa and 75 kDa, respectively (Fig 2A and 2B).
Fig. 1. TG-dependent incorporation of monodansylcadaverine (MDC) or biotin pentylamine into drosocrystallin recombinants. 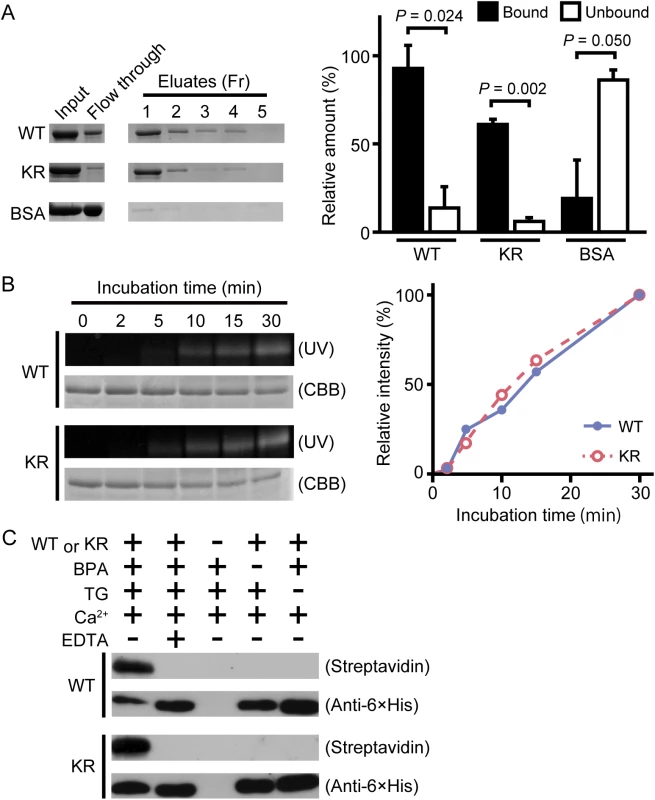
(A) Wild-type drosocrystallin (WT), the KR mutant (KR), or bovine serum albumin (BSA) was incubated with chitin, and each fraction (Fr) was analyzed by SDS-PAGE in 10% slab gels (left panel). The intensity of each fraction relative to that of the input was summed as the bound fraction. Bars indicate the mean and standard deviations of experiments performed in triplicate (right panel). BSA was used as a negative control. Open bars, unbound fraction; closed bars, bound fraction. (B) Wild-type drosocrystallin (WT) or the KR mutant (KR) was incubated with MDC in the presence of TG, and analyzed by SDS-PAGE in 10% slab gels. The proteins were stained with Coomassie brilliant blue (CBB), and the MDC-incorporated protein was detected by the emission intensity of the dansyl group. Data are representative of three independent experiments (left panel). The relative emission intensity of each fraction compared to that of CBB-stained protein was calculated using ImageJ software (right panel). (C) Wild-type drosocrystallin (WT) or the KR mutant (KR) was incubated with or without biotin pentylamine (BPA) in the presence of TG, and subjected to SDS-PAGE in 10% slab gels. Incorporation of BPA was detected with horseradish peroxidase-conjugated streptavidin. Loaded recombinant proteins were detected by Western blotting with a horseradish peroxidase-conjugated anti-6× His tag antibody. Data are representative of at least three independent experiments. Fig. 2. TG-dependent polymerization of drosocrystallin in vitro and in vivo. 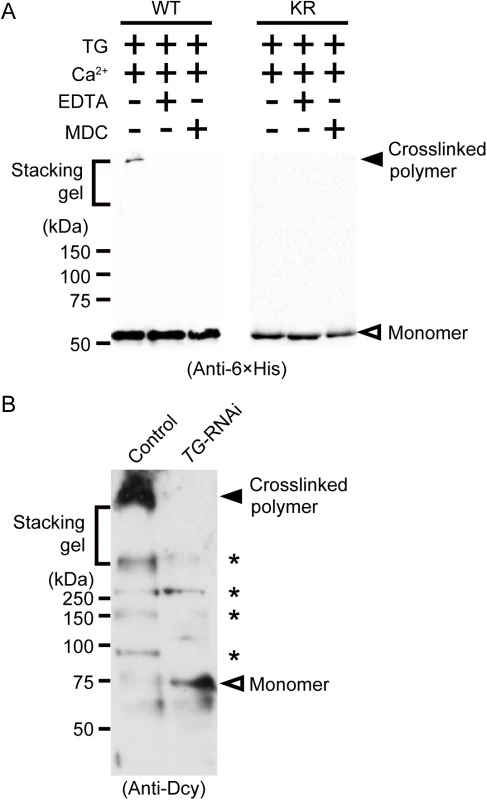
(A) Wild-type drosocrystallin (WT) or the KR mutant (KR) was incubated with TG, and subjected to SDS-PAGE in 10% TGX FastCast gels (Bio-Rad Laboratories). These recombinants were detected by Western blotting with a horseradish peroxidase-conjugated anti-6 × His tag antibody. Monodansylcadaverine (MDC) was used as an inhibitor of protein-protein crosslinking. Data are representative of at least three independent experiments. (B) Gut extracts from systemic TG-RNAi flies (Da>TG IR) and their counterparts (Da>+) were subjected to SDS-PAGE in 12% slab gels. Native drosocrystallin in the extracts was detected by Western blotting with an anti-drosocrystallin antibody (Anti-Dcy). Asterisks indicate unknown cross-reacted proteins. Data are representative of three independent experiments. Crosslinked drosocrystallin, but not non-crosslinked drosocrystallin, is resistant to proteases secreted by pathogenic bacteria
Drosocrystallin is important for host defense against bacterial protease AprA secreted by P. entomophila [7]. Protease AprA is a metalloprotease important for local infection [2,23,24]. Wild-type drosocrystallin was degraded by adding the culture supernatant from P. entomophila in the absence of TG, but recombinant drosocrystallin crosslinked by TG was not degraded (Fig 3A). In Drosophila, TG-mediated crosslinking of Fondue and hexamerin is important for trapping invading microbes in the hemocoel [14,25]. In the case of Trichoplusia ni, the chitin-binding proteins (CBP1 and CBP2) and the insect intestinal mucin bind to chitin fibers on the peritrophic matrix, which protect the insect from food-derived digestive proteases [26–28]. These previous findings together with our results indicate that crosslinked drosocrystallin on the peritrophic matrix could act as a protective physical barrier against P. entomophila exotoxins.
Fig. 3. Polymerized drosocrystallin protects against AprA. 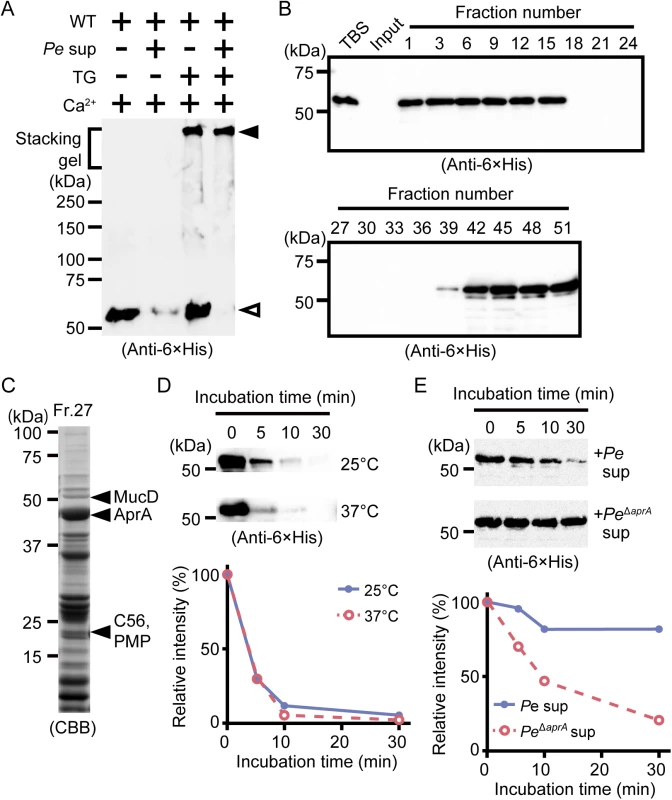
(A) Wild-type drosocrystallin (WT) was incubated at 37°C for 30 min with or without TG, and then the culture supernatant from P. entomophila (Pe) was added, and the mixture was subjected to SDS-PAGE in 10% TGX FastCast gels. Open arrowhead, the monomeric recombinant; closed arrowhead, the crosslinked recombinant. Data are representative of at least three independent experiments. (B) Wild-type drosocrystallin was subjected to SDS-PAGE in 10% slab gels and detected by Western blotting after incubating with each fraction obtained by gel filtration of the culture supernatant from P. entomophila. (C) Fraction No. 27 from the gel filtration was subjected to SDS-PAGE in 15% slab gels, and proteases in this fraction were identified by liquid chromatography tandem mass spectroscopy analysis. (D) Wild-type drosocrystallin was incubated with purified AprA at 25°C or 37°C and analyzed by SDS-PAGE in 10% slab gels, and detected by Western blotting using anti-6 × His tag antibody (upper panel). Western blotting data are representative of four independent experiments. The relative intensity of each band compared to that of the untreated protein (0 min) was calculated using ImageJ software (lower panel). (E) Wild-type drosocrystallin was incubated with the culture supernatant from P. entomophila (Pe) or the AprA-knockout strain (PeΔaprA), analyzed by SDS-PAGE in 10% slab gels, and detected by Western blotting using anti-6 × His tag antibody. Western blotting data are representative of three independent experiments (upper panel). The relative intensity of each band compared to that of the untreated protein (0 min) was calculated using ImageJ software (lower panel). To identify the pathogenic protease(s) involved in the degradation of non-crosslinked drosocrystallin, the culture supernatant from P. entomophila was fractionated by gel filtration, and wild-type drosocrystallin was incubated with each fraction. The non-crosslinked recombinant was not detected in the fractions (Nos. 18–36) by Western blotting, possibly due to proteolytic digestion (Fig 3B). Metalloprotease AprA [2,23,24] and three proteases with unknown function, including MucD, C56, and PMP, were identified from one fraction (No. 27) by mass spectrometry, and AprA was the most abundant protease in this fraction (Fig 3C). Therefore, we purified AprA from a fraction (No. 26), as described in the Materials and Methods. Wild-type drosocrystallin was completely degraded by the purified AprA (Fig 3D). To confirm the involvement of AprA in the digestion of drosocrystallin, culture supernatant without AprA was prepared using AprA-knockout P. entomophila. The resulting supernatant did not significantly degrade wild-type drosocrystallin (Fig 3E, upper and lower panels), clearly indicating that AprA is a key protease for the degradation of non-crosslinked drosocrystallin.
Drosocrystallin fibers stabilized by TG-catalyzed crosslinking are resistant to bacterial toxins
TG-catalyzed fibers or a mesh formation of several proteins, such as proxin and stablin in horseshoe crabs, is important for wound healing and bacterial entrapment [29,30]. To determine whether drosocrystallin forms fibers or a mesh by protein-protein crosslinking of TG activity, wild-type drosocrystallin was incubated on cover glass under several conditions and observed by immunofluorescence microscopy (Fig 4A). Interestingly, fiber-like structures were observed in the absence of TG, but the fibers were not detected in the presence of EDTA, indicating that Ca2+ induces non-covalent self-association of drosocrystallin (Fig 4B). This finding is consistent with a previous finding that drosocrystallin exhibits Ca2+-binding ability [19], and suggests that Ca2+ is required not only for TG activation, but also for non-covalent fiber formation of drosocrystallin. To clarify the importance of covalent crosslinking of drosocrystallin mediated by TG, the effect of culture supernatant including protease AprA from P. entomophila on the stability of drosocrystallin fibers was observed with or without active TG. In the absence of active TG, the fiber structure of wild-type drosocrystallin gradually collapsed in proportion to the amount of the culture supernatant from P. entomophila and the fluorescence intensity of the fibers decreased further (Fig 4C). On the other hand, in the presence of active TG, the fiber structure and fluorescence intensity of drosocrystallin were not affected by culture supernatant of P. entomophila. These findings suggest that TG-mediated covalent crosslinking of drosocrystallin is required for the protection against proteolytic digestion. P. entomophila secretes another exotoxin, monalysin, that acts as a pore-forming toxin against cell membranes, causing host cell death [24]. To examine whether the crosslinked fibers of drosocrystallin protect against penetration of monalysin into the peritrophic matrix, crosslinked or non-crosslinked fibers of wild-type drosocrystallin were mixed with wild-type monalysin in the presence or absence of the culture supernatant, and both recombinants were observed by immunofluorescence microscopy. Wild-type monalysin colocalized with the crosslinked fibers of wild-type drosocrystallin (Fig 4D, left panels of Pe sup +). In contrast, the non-crosslinked fibers in the absence of TG were degraded by proteases in the culture supernatant and wild-type monalysin did not colocalize with wild-type drosocrystallin (Fig 4D, right panels of Pe sup +). In the absence of the culture supernatant, however, wild-type monalysin colocalized with both the TG-dependent crosslinked fibers and the Ca2+-induced non-covalent associated fibers of wild-type drosocrystallin (Fig 4D, left and right panels of Pe sup −). These findings indicate that the non-covalent fiber formation of drosocrystallin leads to co-localization of monalysin and that the protease-resistant drosocrystallin fibers crosslinked by TG, but not non-crosslinked drosocrystallin fibers, trap monalysin released from P. entomophila in the presence of proteases such as metalloprotease AprA.
Fig. 4. Covalently crosslinked drosocrystallin fibers are resistant to proteolytic digestion and trap a pore-forming exotoxin, monalysin. 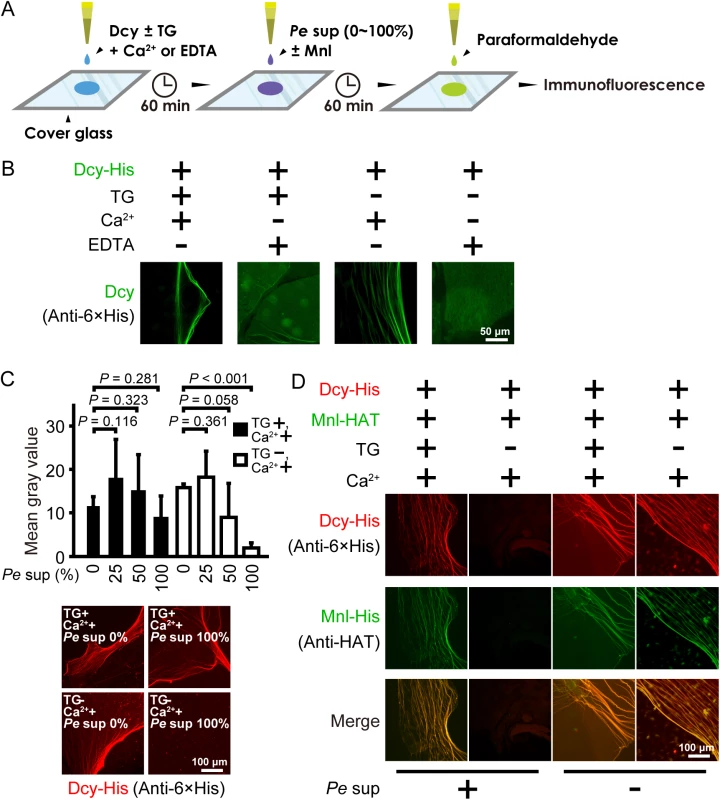
(A) A schematic of the assay for the fiber formation of drosocrystallin. Briefly, wild-type drosocrystallin (Dcy) was incubated on cover glass in the presence or absence of TG and Ca2+ or EDTA for 1 h. The culture supernatant from P. entomophila (Pe sup) containing protease AprA was added on the cover glass with or without monalysin (Mnl). After incubation, proteins on the cover glass were fixed with paraformaldehyde and detected by immunofluorescence. (B) Wild-type drosocrystallin was incubated on coverslip glass and observed by immunofluorescence microscopy. Wild-type drosocrystallin was detected using an anti-His tag antibody and CF488-conjugated secondary antibody (green). Representative data from at least five experiments are shown. (C) Wild-type drosocrystallin was incubated with or without TG on coverslip glass in the presence of Ca2+, and then 2-fold serial dilutions of the culture supernatant from P. entomophila (Pe sup) were added. The concentration of the culture supernatant is indicated. Bars indicate the mean and standard deviations of the mean gray values from four independent experiments (upper panel). Lower panels show representative data of drosocrystallin fibers formed in the presence or absence of TG and Pe sup. Wild-type drosocrystallin was detected by anti-His tag antibody and CF568-conjugated secondary antibody (red). (D) Wild-type drosocrystallin was incubated with or without TG on coverslip glass in the presence of Ca2+, and then wild-type monalysin was added in the presence (Pe sup +) or absence (Pe sup −) of the culture supernatant from P. entomophila. Wild-type drosocrystallin was detected by anti-His tag antibody and CF568-conjugated secondary antibody (red), and wild-type monalysin was detected by anti-histidine affinity tag (HAT) antibody and CF488-conjugated secondary antibody (green). One representative experiment from at least five independent experiments is shown. TG is important for protection against protease AprA
The survival rate of flies ingesting P. entomophila was analyzed. No significant differences were observed between gut-specific TG-knockdown flies and control flies after ingesting a non-lethal pathogen, Erwinia carotovora carotovora 15 (Ecc15), but gut-specific TG-knockdown flies had a significantly shorter lifespan than control flies after ingesting P. entomophila, and the lifespan returned to the control level after ingesting AprA-knockout P. entomophila (Fig 5A). In a previous study, we found that TG-induced dampening of the immune-eliciting signals in the gut and TG-RNAi (NP1>TG IR)-induced shortened lifespan occurred at least 7 days after eclosion [10]. Here, we confirmed that TG-RNAi itself did not affect the survival rate in the time span of ~5 days after eclosion (Fig 5A, TG-RNAi + sucrose). These data indicate that TG is involved in host defense in the fly gut after infection with P. entomophila to preserve the proteolytic digestion of drosocrystallin. Protease AprA is a member of the metzincin superfamily and was originally identified in P. aeruginosa [31,32]. Flies injected with or ingesting P. entomophila have a shorter lifespan than untreated flies, because AprA facilitates bacterial survival by degrading antimicrobial peptides produced by host immunity [2] and activates monalysin by cleaving the N-terminal pro-peptide [24]. In the present study, we demonstrate for the first time that AprA is directly involved in degrading the peritrophic matrix protein drosocrystallin to shorten the lifespan of Drosophila. On the other hand, monalysin causes apoptosis of gut epithelial cells [7,24]. To determine the cause of the shortened lifespan of TG-RNAi flies after ingestion of wild-type P. entomophila, we evaluated gut epithelial cell death. In this experiment, 3 to 5-day old adult flies were used because of the negligible effect of commensal community on the survival rate of TG-RNAi flies (Fig 5A). After ingesting P. entomophila, a significant number of dead cells was detected in the gut, and the ratio of cell death was increased in gut-specific TG-knockdown flies (Fig 5B). These findings demonstrate that TG is essential for protection against pathogenic bacterial infection in the gut (Fig 5C).
Fig. 5. TG-dependent protection against P. entomophila infection in the gut. 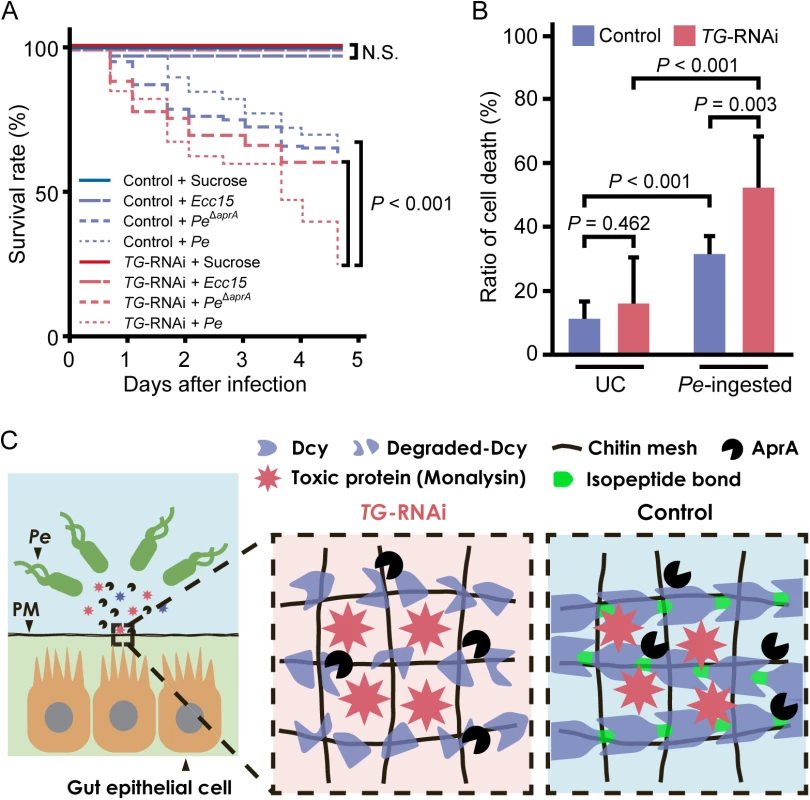
(A) Survival analysis of gut-specific TG-RNAi flies (NP1>TG IR) and their counterparts (NP1>+) upon oral infection with P. entomophila (Pe) or Ecc15. Statistical analysis was performed using a log-rank test. At least 50 flies were used. N.S., not significant. (B) Cell-death was quantified by propidium iodide staining. Results represent the percentage of dead cells (propidium iodide-positive nuclei) in the midguts of flies infected for 4 h with P. entomophila (Pe). Results represent the mean of 10 independent experiments. Statistical analysis was performed by one-way analysis of variance followed by Bonferroni correction for multiple comparisons to evaluate the pairwise difference. UC, unchallenged. (C) A schematic model of the TG-mediated peritrophic matrix formation. TG crosslinks drosocrystallin (Dcy) on the peritrophic matrix (PM). Crosslinked drosocrystallin is not digested by AprA, and the crosslinked drosocrystallin strengthens the peritrophic matrix to function as a physical barrier against exotoxins of pathogenic microbes. Conclusion
Drosocrystallin non-covalently self-associated to form fiber-like structures in the presence of Ca2+. Non-crosslinked fibers were not stable and were degraded more quickly by AprA than the crosslinked fibers. TG stabilized the drosocrystallin fibers through intermolecular crosslinking. Such a crosslinking reaction could "mask" potential proteolytic cleavage sites of AprA. Peritrophic matrix proteins, such as insect intestinal mucin and chitin-binding proteins containing multiple chitin-binding domains, are proposed to form a bridge-like structure on chitin fibers on the peritrophic matrix in T. ni [26,28]. There is no genetic evidence for involvement of these peritrophic matrix proteins with multiple chitin-binding domains in peritrophic matrix formation in Drosophila, but the TG-catalyzed crosslinked fibers could promote the formation of a rigid peritrophic matrix structure to protect against exotoxins. Importantly, flies die within 5 h after injection of purified AprA into the hemocoel [2]. Ingestion of a high concentration of AprA has little effect on fly survival, and thus AprA itself is not critical for the virulence of naturally infecting P. entomophila [2]. These findings suggest that the peritrophic matrix inhibits the penetration of AprA secreted by P. entomophila from the gut lumen into the ectoperitrophic space. In addition, fluorescein isothiocyanate-labeled dextran-feeding assays performed in our previous study indicated increased permeability of the peritrophic matrix in systemic TG-RNAi flies [2]. In the present paper, we did not examine the survival of gut-specific TG-knockdown flies after ingesting monalysin and/or AprA, but the TG-mediated crosslinking of drosocrystallin in the peritrophic matrix clearly reduced AprA-mediated peritrophic matrix damage and blocked the movement of monalysin and other virulent factor(s) from the endoperitrophic space into the ectoperitrophic space. The drosocrystallin fibers crosslinked by TG, but not non-crosslinked drosocrystallin fibers, appear to form an important physical barrier against exotoxins of invading pathogenic microbes in the Drosophila gut.
Materials and Methods
Fly stocks
Flies were maintained on standard yeast medium at 25°C. Da-GAL4 and w1118 flies were obtained from the Bloomington Stock Center (Bloomington, IL). NP1-GAL4 flies were obtained from the Drosophila Genetics Resource Center (Kyoto, Japan). UAS-TG IR flies were obtained from Dr. Ryu Ueda at the National Institute of Genetics (Mishima, Japan). Strain w1118 was used as a control strain.
Bacterial stocks
P. entomophila L48 [23], P. entomophilaΔaprA [2], and Ecc 15 were grown in Luria-Bertani (LB) medium for all experiments. Bacteria were grown at 29°C and allowed to reach the stationary phase. Cells were then concentrated at OD600 = 200 except when indicated.
Infection and survival assays
For oral infection, female flies were starved for 2 h at 29°C. Ecc 15 or P. entomophila (OD600 = 200) was added to a filter disk (Whatman) that completely covered the surface of the standard fly medium, and the flies were placed on the medium. Flies were maintained at 29°C, and mortality was monitored at different time-points.
Expression of recombinant drosocrystallin
To construct expression vectors, cDNA fragments were amplified by polymerase chain reaction (PCR). An amplimer encoding the drosocrystallin-coding sequence without a putative signal sequence (1–60) was inserted into expression vector pET-22b (Novagen) between the NdeI and EcoRI sites. The construct was verified by DNA sequencing. The construct, which contained C-terminal His-tags, was expressed in the E. coli strain BL21 (DE3) (Novagen). Bacteria were cultured in LB medium, and expression was induced by the addition of isopropyl-β-D-thiogalactoside at a final concentration of 1 mM at 15°C for 24 h. Bacterial pellets were harvested by centrifugation and sonicated in 10 ml of 20 mM Tris-HCl, pH 8.0, 200 mM NaCl containing 1% Nonidet P-40 and 1 mM phenylmethylsulfonylfluoride. After sonication, the supernatants were recovered by centrifugation and purified according to the manufacturer’s protocol using Ni-NTA agarose (Qiagen). To produce protein insensitive to TG, all lysine residues of drosocrystallin were substituted with arginine by PCR-based site-directed mutagenesis. Each amino acid substitution was generated by PCR using specific 5’-phosphorylated primers. The lysine-substituted mutation was verified by DNA sequencing and expressed in BL21 (DE3)/pLysS by the same method as used for the wild type. K35R-sense primer, GGTCCTCCAACCTTCAGCAG; K35R-antisense primer, TGGCTAGCTGGTTAAGATCG; K92, 96, 103R-sense primer, GGCGGCAGGAGGAGAGGCGCGATGGCGACCTGGTCAGGGGT; K92, 96, 103R-antisense primer, TGTCATCGCCAGTCAGCGAG; K135R-sense primer, GGCAGCGTCTGGATGAGCAG; K135R-antisense primer, TAGACACAATGGCATTGAAG; K470R-sense primer, GGAACTGGCCGAATTCGAGCTCCGTCGACAGGCTT; K470R-antisense primer, TAGAGCGACGTTCGGCACTG.
Expression of recombinant TG
The whole sequence encoding the TG gene was cloned into expression vector pET-22b. The construct was verified by DNA sequencing. The construct, which contained no tags, was expressed in the E. coli strain BL21 (DE3). Bacteria were cultured in LB medium, and expression was induced by the addition of isopropyl-β-D-thiogalactoside at a final concentration of 30 μM at 15°C for 24 h. Bacterial pellets were harvested by centrifugation and sonicated in 50 mM Tris-HCl, pH 8.8, 50 mM NaCl, 10 mM dithiothreitol (DTT), 2 mM EDTA, 10% glycerol, and 1% 3-[(3-cholamidepropyl)dimethylammonio]-1-propanesulphonate. After sonication, the supernatants were recovered by centrifugation. Then, buffer was exchanged with 50 mM Tris-HCl, pH 8.0, 10 mM DTT, 1 mM tris(2-carboxyethyl)phosphine, and 0.5 mM EDTA using a Sephadex G-25 Superfine column, and stored at -80°C before use.
Expression of wild-type monalysin
The whole sequence encoding the monalysin gene was generated by PCR with C-terminal HAT encoding primers and cloned into expression vector pET-15b. The construct was verified by DNA sequencing. The construct was expressed in the E. coli strain Rosseta-gami B. Bacteria were cultured in LB medium, and expression was induced by the addition of isopropyl-β-D-thiogalactoside at a final concentration of 0.1 mM at 18°C for 24 h. Bacterial pellets were harvested by centrifugation and sonication buffer (50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1 mM phenylmethylsulfonylfluoride, 0.5 mM lysozyme) was added and frozen. After thawing and sonicating the pellets, the supernatant was recovered by centrifugation. The supernatant was purified using HisTrap crude FF column (1 mL, GE Healthcare). After purification, the buffer was exchanged with PBS using Sephadex G-25 Superfine column.
Preparation of a polyclonal antibody against drosocrystallin
To prepare the polyclonal antibody, recombinant drosocrystallin without a putative signal sequence (61–472) was expressed in E. coli strain BL21 (DE3) (Novagen). An inclusion body containing the recombinant protein was isolated and subjected to SDS-PAGE under reducing conditions, and negatively stained. The protein band corresponding to the recombinant protein was excised from the gel band recovered by electroelution for immunization of rabbits (MBL International). The polyclonal antibody was purified sequentially from the anti-serum using Protein A Sepharose CL-4B (GE Healthcare) and antigen-conjugated Affi-Gel 15 (Bio-Rad Laboratories).
Chitin-binding assay
The recombinant proteins were mixed with chitin in 50 mM Tris-HCl, pH 7.5, and 150 mM NaCl, and incubated at 4°C for 30 min. Supernatants were separated by centrifugation and precipitates were washed with the same buffer. Proteins bound to chitin were eluted with 10% acetic acid. Eluted fractions (100 μL each) were evaporated using a speed-vac (Labconco). Input, bound, and unbound fractions were subjected to SDS-PAGE and detected by Coomassie brilliant blue staining. The relative intensity of each fraction compared to the input protein was calculated by ImageJ software.
Incorporation of biotin pentylamine into wild-type drosocrystallin
Recombinant proteins were incubated with TG in 50 mM Tris-HCl, pH 8.5, containing 10 mM CaCl2, 10 mM DTT, and 500 μM biotin pentylamine at 37°C for 1 h. Following the reaction, the aliquots were subjected to SDS-PAGE and electroblotted on a polyvinylidene difluoride membrane. After blocking with 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 5% dry milk, the membrane was incubated at room temperature for 1 h with the horseradish peroxidase-conjugated streptavidin diluted 1 : 1,000 with blocking buffer, followed by development with Chemi-Lumi One-Super reagent (Nacalai Tesque).
Quantification of MDC incorporation
Recombinant proteins were incubated with TG in 50 mM Tris-HCl, pH 8.5, 10 mM CaCl2, 10 mM DTT, and 5 mM MDC at 37°C for the durations indicated in Fig 1B. Following the reaction, aliquots were subjected to SDS-PAGE and visualized by ultraviolet irradiation. Band intensity was calculated using ImageJ software.
SDS-PAGE and western blotting
SDS-PAGE was performed in slab gels according to the method of Laemmli. Precision Plus protein standards (Bio-Rad Laboratories) were used to determine the apparent molecular masses. Protein bands were visualized by Coomassie brilliant blue staining. Samples were subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane. After blocking with 5% dry milk, the membrane was incubated at room temperature for 1 h with the anti-drosocrystallin antibody and then with the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG; Bio-Rad Laboratories), followed by development with Chemi-Lumi One, Chemi-Lumi One-super (Nacalai), or WesternBright Sirius (Advansta). For detection of the His-tag, horseradish peroxidase-conjugated anti-6 × His tag antibody (MBL International) was used. Chemifluorescence was detected using an Omega Lum G fluorescence imager (Aplegen) or X-ray film.
Detection of crosslinked drosocrystallin
Wild-type drosocrystallin or the KR mutant was incubated with TG in 50 mM Tris-HCl, pH 8.5, 10 mM CaCl2, and 10 mM DTT at 37°C for 1 h. Following the reaction, samples were subjected to SDS-PAGE and detected by Western blotting using anti - 6 × His tag antibody. Guts from wild-type and TG-knockdown flies (Da>TG IR) were homogenized in 50 mM Tris-acetate, pH 7.5, 1% Nonidet P-40, and protein inhibitor cocktail (Nacalai Tesque), and centrifuged at 15,000 rpm at 4°C for 15 min to collect the supernatant. The supernatant was precipitated by 10% trichloroacetic acid, subjected to SDS-PAGE, and detected by Western blotting using anti-drosocrystallin antibody.
Live imaging
Quantification of dead cells was performed as follows: 4 h after ingestion of P. entomophila, the guts were dissected and stained with Hoechst 33342 (1 : 1,000, Dojindo Molecular Technologies) and propidium iodide (1 : 2,000, Life Technologies). Pictures were obtained with a fluorescence microscope. From these pictures, 100 Hoechst 33342-stained nuclei, representing all nuclei, were randomly defined and the number of propidium iodide-positive nuclei, representing dead cells, was determined. Three parcels per gut were analyzed. Results represent the mean of 10 independent experiments. In this experiment, 3 to 5-day-old adult flies were used.
Preparation of AprA
Culture supernatant from the wild-type P. entomophila was fractionated using ÄKTA start with a HiPrep 16/60 Sephacryl S-100 HR column (GE Healthcare). Each fraction was incubated with the wild-type recombinant. The sample from fraction No. 26 was dialyzed with 20 mM Tris-HCl, pH 7.5, and applied to a DEAE Sepharose CL-6B column (1×2 cm). The flow-through fraction was applied to a CM Sepharose CL-6B column (1×2 cm). After washing with 20 mM Tris-HCl, pH 7.5, the protein was eluted with a linear NaCl gradient (100–500 mM) in the same buffer.
Observation of drosocrystallin fibers
One microgram of wild-type drosocrystallin in 50 mM Tris-HCl, pH 8.5, and 10 mM DTT with 10 mM CaCl2 or 50 mM EDTA was placed on a coverslip and incubated at 37°C for 1 h with or without TG. Next, the culture supernatant from P. entomophila and/or HAT-tagged monalysin was added to the coverslip and incubated at 37°C for 1 h. After incubation, the proteins were fixed with 4% paraformaldehyde for 20 min, washed with 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and blocked with 2% bovine serum albumin in the same buffer. The proteins were then incubated for 1 h with anti-His tag monoclonal antibody (MBL International) for wild-type drosocrystallin and the anti-HAT polyclonal antibodies (GenScript) for monalysin. For detection, CF488 or CF568-conjugated goat anti-mouse secondary antibody (Biotium) and CF568-conjugated goat anti-rabbit secondary antibody (Biotium) were used. The proteins were imaged with a ZOE fluorescence microscope (Bio-Rad) for detection of the structure of wild-type drosocrystallin or MZ10 F (Leica) for calculating the mean gray value. The mean gray value of the signal for wild-type drosocrystallin was calculated using ImageJ software. The sum of gray values in the protein-coated area was divided by the number of pixels. The mean gray value of uncoated-area was subtracted from the value of the protein-coated area.
Zdroje
1. Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLOS Pathog 2007;3: e173. 18039029
2. Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLOS Pathog 2006;2: e56. 16789834
3. Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol 2007;25 : 697–743. 17201680
4. Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol 2009;10 : 949–957. doi: 10.1038/ni.1765 19668222
5. Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, et al. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell 2009;16 : 386–397. doi: 10.1016/j.devcel.2008.12.015 19289084
6. Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, et al. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 2013 May 9;153(4):797–811. doi: 10.1016/j.cell.2013.04.009 23663779
7. Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci USA 2011;108 : 15966–15971. doi: 10.1073/pnas.1105994108 21896728
8. Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 2009;5 : 200–211. doi: 10.1016/j.chom.2009.01.003 19218090
9. Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep 2013;3 : 1725–1738. doi: 10.1016/j.celrep.2013.04.001 23643535
10. Shibata T, Sekihara S, Fujikawa T, Miyaji R, Maki K, Ishihara T, et al. Transglutaminase-catalyzed protein-protein cross-linking suppresses the activity of the NF-kappaB-like transcription factor relish. Sci Signal 2013;6: ra61. doi: 10.1126/scisignal.2003970 23882120
11. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003;4 : 140–156. 12563291
12. Makarova KS, Aravind L, Koonin EV. A superfamily of archaeal, bacterial, and eukaryotic proteins homologous to animal transglutaminases. Protein Sci 1999;8 : 1714–1719. 10452618
13. Shibata T, Ariki S, Shinzawa N, Miyaji R, Suyama H, Sako M, et al. Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila. PLOS One 2010;5: e13477. doi: 10.1371/journal.pone.0013477 20976106
14. Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, et al. Pathogen entrapment by transglutaminase—a conserved early innate immune mechanism. PLOS Pathog 2010;6: e1000763. doi: 10.1371/journal.ppat.1000763 20169185
15. Loof TG, Morgelin M, Johansson L, Oehmcke S, Olin AI, Dickneite G, et al. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood 2011;118 : 2589–2598. doi: 10.1182/blood-2011-02-337568 21613262
16. Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol 1997;42 : 525–550. 15012322
17. Terra WR. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol 2001;47 : 47–61. 11376452
18. Janssens H, Gehring WJ. Isolation and characterization of drosocrystallin, a lens crystallin gene of Drosophila melanogaster. Dev Biol 1999;207 : 204–214. 10049575
19. Komori N, Usukura J, Matsumoto H. Drosocrystallin, a major 52 kDa glycoprotein of the Drosophila melanogaster corneal lens. Purification, biochemical characterization, and subcellular localization. J Cell Sci 1992;102 : 191–201. 1400628
20. Rebers JE, Riddiford LM. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J Mol Biol 1988;203 : 411–423. 2462055
21. Karouzou MV, Spyropoulos Y, Iconomidou VA, Cornman RS, Hamodrakas SJ, Willis JH. Drosophila cuticular proteins with the R&R Consensus: annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem Mol Biol 2007;37 : 754–760. 17628275
22. Iijima M, Hashimoto T, Matsuda Y, Nagai T, Yamano Y, Ichi T, et al. Comprehensive sequence analysis of horseshoe crab cuticular proteins and their involvement in transglutaminase-dependent cross-linking. FEBS J 2005;272 : 4774–4786. 16156796
23. Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci USA 2005;102 : 11414–11419. 16061818
24. Opota O, Vallet-Gely I, Vincentelli R, Kellenberger C, Iacovache I, Gonzalez MR, et al. Monalysin, a novel β-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality. PLOS Pathog 2011;7: e1002259. doi: 10.1371/journal.ppat.1002259 21980286
25. Lindgren M, Riazi R, Lesch C, Wilhelmsson C, Theopold U, Dushay MS. Fondue and transglutaminase in the Drosophila larval clot. J Insect Physiol 2008;54 : 586–592. doi: 10.1016/j.jinsphys.2007.12.008 18222466
26. Wang P, Li G, Granados RR. Identification of two new peritrophic membrane proteins from larval Trichoplusia ni: structural characteristics and their functions in the protease rich insect gut. Insect Biochem Mol Biol 2004;34 : 215–227. 14871618
27. Fang S, Wang L, Guo W, Zhang X, Peng D, Luo C, et al. Bacillus thuringiensis bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl Environ Microbiol 2009;75 : 5237–5243. doi: 10.1128/AEM.00532-09 19542344
28. Wang P, Granados RR. Molecular cloning and sequencing of a novel invertebrate intestinal mucin cDNA. J Biol Chem 1997;272 : 16663–16669. 9195982
29. Matsuda Y, Osaki T, Hashii T, Koshiba T, Kawabata S. A cysteine-rich protein from an arthropod stabilizes clotting mesh and immobilizes bacteria at injury sites. J Biol Chem 2007;282 : 33545–33552. 17855345
30. Osaki T, Okino N, Tokunaga F, Iwanaga S, Kawabata S. Proline-rich cell surface antigens of horseshoe crab hemocytes are substrates for protein cross-linking with a clotting protein coagulin. J Biol Chem 2002;277 : 40084–40090. 12189150
31. Guzzo J, Murgier M, Filloux A, Lazdunski A. Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J Bacteriol 1990;172 : 942–948. 2153662
32. Duong F, Lazdunski A, Cami B, Murgier M. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene 1992;121 : 47–54. 1427098
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



