-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInfluenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest
Like all viruses, Influenza A virus (IAV) is absolutely dependent on host-cell protein synthesis machinery. This dependence makes the virus vulnerable to the innate ability of cells to inhibit protein synthesis in response to various types of stress. This inhibition, termed translation arrest, helps cells survive adverse conditions by re-dedicating their energy to stress responses. When cells arrest translation, they form stress granules: depots of untranslated mRNAs and associated proteins. Translation arrest and formation of stress granules can be induced pharmacologically, and in this work we sought to determine whether stress granule induction would be effective in blocking IAV replication. Here we demonstrate that treatment of cells with inducers of stress granules at early times after infection resulted in blockade of viral protein synthesis and stopped viral replication. At later times post-infection, by contrast, IAV proteins prevented pharmacological induction of stress granules. We identified three viral proteins – more than in any virus to date – that work in concert to prevent stress granule formation. Taken together, our studies reveal a multipronged approach for viral suppression of translation arrest, and identify a window of opportunity early in infection when pharmacological induction of stress granules has a strong antiviral effect.
Published in the journal: . PLoS Pathog 10(7): e32767. doi:10.1371/journal.ppat.1004217
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004217Summary
Like all viruses, Influenza A virus (IAV) is absolutely dependent on host-cell protein synthesis machinery. This dependence makes the virus vulnerable to the innate ability of cells to inhibit protein synthesis in response to various types of stress. This inhibition, termed translation arrest, helps cells survive adverse conditions by re-dedicating their energy to stress responses. When cells arrest translation, they form stress granules: depots of untranslated mRNAs and associated proteins. Translation arrest and formation of stress granules can be induced pharmacologically, and in this work we sought to determine whether stress granule induction would be effective in blocking IAV replication. Here we demonstrate that treatment of cells with inducers of stress granules at early times after infection resulted in blockade of viral protein synthesis and stopped viral replication. At later times post-infection, by contrast, IAV proteins prevented pharmacological induction of stress granules. We identified three viral proteins – more than in any virus to date – that work in concert to prevent stress granule formation. Taken together, our studies reveal a multipronged approach for viral suppression of translation arrest, and identify a window of opportunity early in infection when pharmacological induction of stress granules has a strong antiviral effect.
Introduction
Transcription of Influenza A virus (IAV) genes is performed by a viral polymerase that generates 5′-capped and polyadenylated (poly[A]) messenger RNAs (mRNAs) structurally similar to host mRNAs [1]. Despite this similarity, IAV transcripts gain preferential access to cellular translation machinery through a host shutoff mechanism executed by the viral non-structural protein 1 (NS1) [2], [3] and the recently discovered viral PA-X protein [4].
The reliance on cap-dependent translation initiation makes viral mRNAs susceptible to host-cell stress-induced translation inhibition mechanisms. This inhibition results from phosphorylation of eukaryotic translation initiation factor-2α (eIF2α) by any of four kinases activated by distinct types of stress [5]. Heme-regulated translation inhibitor (HRI) kinase is activated in response to oxidative stress, GCN2 senses nutrient deprivation and ultraviolet damage, double-stranded RNA (dsRNA)-dependent protein kinase R (PKR) is activated in response to viral infections, and the PKR-like endoplasmic reticulum kinase (PERK) signals in response to endoplasmic reticulum stress. Inhibition of translation initiation leads to runoff of elongating ribosomes from mRNA and the accumulation of stalled translation preinitiation complexes. Translationally inactive messenger ribonucleoproteins (mRNPs) recruit RNA-binding proteins with self-aggregating properties, including the T-cell intracellular antigen 1 (TIA-1), TIA-1-related protein (TIAR), and ras GTPase-activating protein-binding protein 1 (G3BP1), which nucleate the formation of large cytoplasmic mRNP foci known as stress granules (SGs; [6]). Many viruses have evolved specific mechanisms that modulate SG responses (reviewed in [7]).
Previously we demonstrated that SGs do not form at any point during IAV infection [8]. Importantly, complete inhibition of SG formation is dependent on NS1. In cells infected with NS1-mutant viruses, SG formation is triggered by PKR activation. However, more than 50% of cells infected with NS1-mutant viruses remained SG-free and allowed IAV replication cycle progression, suggesting the existence of additional NS1-independent mechanisms of SG suppression. In this work, by analyzing SG formation in IAV-infected cells in response to a variety of stresses, we report a robust mechanism of SG inhibition that becomes engaged at later times post-infection and acts despite strong eIF2α phosphorylation. Maximal SG inhibition coincided with a striking depletion of cytoplasmic poly(A) mRNA and the nuclear re-localization of poly(A)-binding protein 1 (PABP1) at later stages of viral replication, effects reminiscent of host shutoff mechanisms observed in other viral systems [9]–[11]. Screening of known IAV ORFs derived from A/PuertoRico/8/34(H1N1) strain (PR8) revealed the identities of two additional viral SG inhibitors, nucleoprotein (NP) and polymerase-acidic protein-X (PA-X), which both act independently of eIF2α phosphorylation. Furthermore, we provide evidence that in the early stages of infection, before the accumulation of sufficient quantities of SG-inhibiting proteins, viral replication is vulnerable to translation-inhibiting drugs that induce SGs and block viral replication.
Results
Influenza A virus inhibits SG formation by an NS1-independent mechanism
Previously we have observed SG formation triggered by infection with recombinant influenza viruses with amino acid substitutions in NS1 that compromise its ability to counteract PKR [8]. For two different NS1 mutant viruses, SG formation peaked at 18 hpi; however, a majority of infected cells remained SG-free throughout a 24 h time course, suggesting that either these cells are not competent to form SGs, or that IAV has additional mechanisms that block PKR activity and/or SG formation. To test whether these infected cells are competent to form SGs, we treated cells infected with either WT or NS1 mutant viruses with sodium arsenite, a potent inducer of SGs that activates the oxidative stress-responsive eIF2α kinase HRI [12]. By 18 hpi, sodium arsenite had induced the formation of large, well-defined SGs in the cytoplasm of all uninfected cells, while most of the cells infected with either WT or NS1 dsRNA binding mutant viruses failed to form SGs (Fig. 1A). We reinforced these observations through use of an additional NS1-mutant virus whose NS1 protein retains the functional dsRNA-binding domain but completely lacks the C-terminal effector domain (PR8 NS1 N80; Fig. 1B upper panel), and a mutant virus completely lacking functional NS1 (PR8 NS1 N15). The latter virus triggered formation of PKR-mediated SGs, but was still able to partially inhibit arsenite-induced SG formation at 18 hpi (Fig. 1B lower panel, and Fig. 1D).
Fig. 1. Influenza A virus inhibits sodium arsenite-induced SG formation at later times post-infection by an NS1-independent mechanism. 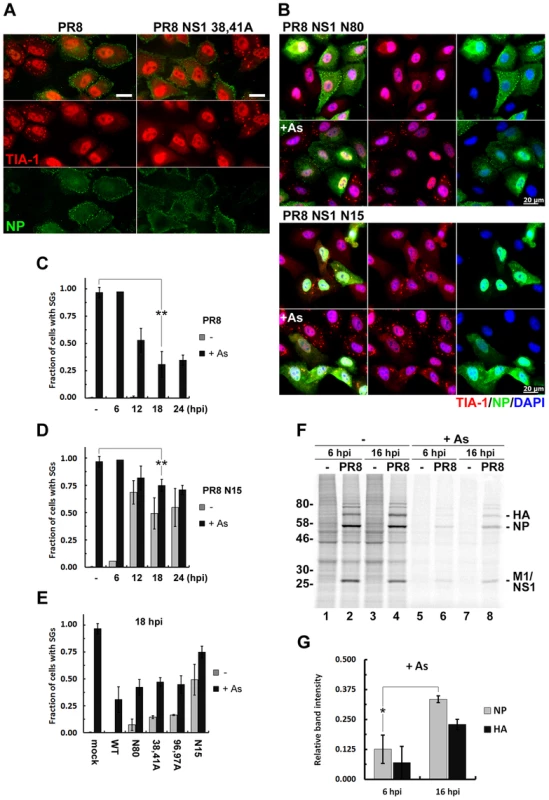
(A and B). SG formation was analysed using immunofluorescent staining of A549 cells infected with the wild type (PR8) and the indicated NS1-mutant influenza A viruses treated with sodium arsenite (As) at 18 hours post-infection (hpi). Virus-infected cells were identified by antibody staining for NP (green) and SGs were visualized using staining for TIA-1 (red). In panel (B) nuclei were stained with DAPI (blue). Scale bars represent 20 µm. (C) Virus and arsenite-induced SG formation was quantified in A549 cells infected with WT PR8 virus at 6, 12, 18 and 24 hours post-infection (hpi). (D) Same quantification as in (C) was done in cells infected with PR8 NS1 N15 virus. (E) SG formation at 18 hpi was compared in mock-infected cells and cells infected with recombinant PR8 viruses with the indicated NS1 mutations. (F) Autoradiogram of the whole cell lysates of mock and PR8 virus-infected A549 cells. At 6 or 16 hpi cells were either left untreated (-) or treated with sodium arsenite (+As) for 1 hour and then immediately pulse-labelled with [35S]methionine-[35S]cysteine for 30 min. (G) Viral NP and HA translation rates post-arsenite treatment are compared between 6 and 16 hpi. Band intensities after arsenite treatment from (F) were normalized to untreated sample band intensities. We observed striking inhibition of arsenite-induced SGs in WT PR8-infected cells from 12 hpi onwards, peaking at 18 hpi (Fig. 1C). For this reason, we evaluated a panel of NS1-mutant viruses at this time point (Fig. 1E). These NS1 mutant viruses, while triggering SG formation to varying degrees in infected cells, all inhibited arsenite-induced SGs compared to mock-infected cells. Furthermore, this effect was not cell-line specific, as we observed the same magnitude of SG inhibition by PR8 in U2OS osteosarcoma cells (Fig. S1A–M). Importantly, SG inhibition did not result from blockade of arsenite-induced eIF-2α phosphorylation (Fig. S1N), and the levels of SG constituent proteins TIA-1, G3BP, PABP1 and eIF4A did not change throughout a 24 h infectious cycle (Fig. S1O).
What is the significance of SG inhibition at later times post-infection? Using pulse-labeling experiments, we queried the rate of viral protein synthesis and sensitivity to sodium arsenite treatment. We observed a high rate of viral protein synthesis, which was maintained at later times post-infection (Fig. 1F lanes 1–4). Sodium arsenite treatment inhibited the production of viral proteins at 6 hpi and 16 hpi; however, the remaining translation rate was significantly higher at 16 hpi (Fig. 1F, G), coincident with SG inhibition. Collectively, our data suggest that at later times post-infection the virus establishes an environment in which accumulation of viral proteins becomes less sensitive to stress-induced translation arrest.
SG inhibition is independent of vRNP export and coincides with depletion of cytoplasmic poly(A) RNA
At later times post-infection, IAV vRNPs are exported from the nucleus to the cytoplasm and transit to the cell surface for assembly and egress. We observe inhibition of SG formation late in infection, coincident with the cytoplasmic export of vRNPs. To test whether cytoplasmic accumulation of vRNPs is necessary for SG inhibition, we treated mock - and IAV-infected cells with leptomycin B (LMB), an inhibitor of CRM1-dependent nuclear export. Consistent with previous reports [13], LMB treatment prevented cytoplasmic accumulation of vRNPs (Fig. 2A). Importantly, in our system LMB treatment did not affect SG inhibition in virus-infected cells, and did not alter SG formation in mock-infected cells. Thus, cytoplasmic localization of vRNPs is dispensable for SG inhibition.
Fig. 2. vRNP export-independent SG inhibition by influenza virus coincides with depletion of cytoplasmic poly(A) RNA and nuclear relocalization of PABP1. 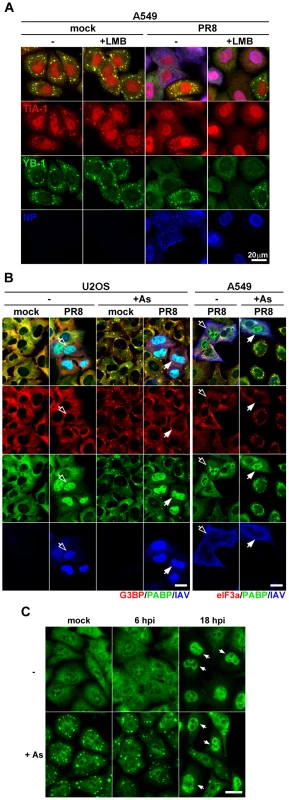
(A and B) Immunofluorescent analysis of mock and PR8 virus-infected cells at 18 hpi, treated with sodium arsenite where indicated. (A) At 6 hpi some A549 cells received 10 ng/ml leptomycin B (+LMB) to block nuclear export of viral ribonucleoproteins up to the time of arsenite treatment and subsequent fixation at 18 hpi. SG formation was analysed by staining for TIA-1 (red) and YB-1 (green) markers and viral ribonucleoproteins were detected with antibody to NP (blue). (B) SG formation and sub-cellular distribution of cellular G3BP, eIF3A (red), PABP1 (PABP, green), and influenza virus proteins (IAV, blue) were analysed in U2OS and A549 cells. Note that while in U2OS cells most of the IAV antigens remain nuclear at 18 hpi, virus material is exported from the nucleus by this time point in A549 cells (open arrows). In both cell lines, however, virus causes redistribution of PABP1 to the nucleus and the SG formation is blocked (filled arrows). (C) Sub-cellular distribution of poly(A) RNA in mock and PR8 virus-infected A549 cells was analysed at indicated times post-infection by fluorescence in situ hybridization. At 18 hpi, many PR8-infected cells have decreased cytoplasmic and increased nuclear poly(A) signal. Upon arsenite treatment (+As) these cells fail to form cytoplasmic poly(A) RNA-containing stress granules (arrows). Scale bars = 20 µm. Because SGs are comprised of stalled poly(A) mRNAs and certain translation initiation factors, we determined their intracellular location at late times post-infection. We saw a remarkable relocalization of PABP1 to the nucleus of infected cells (Fig. 2B). PABP1 is nucleocytoplasmic shuttling protein that binds newly polyadenylated mRNAs in the nucleus [14]. Thus, the nuclear accumulation of PABP1 strongly suggested that the normal processing and transport of nascent mRNAs is impaired in infected cells, and/or cytoplasmic mRNA pools are severely depleted. Using fluorescence in situ hybridization (FISH), we analyzed the nucleocytoplasmic localization of poly(A) mRNA at early and late times post-infection. Subcellular distribution of poly(A) RNA was comparable in mock - and IAV-infected cells at early times post-infection (Fig. 2C and S2). By contrast, at later stages post-infection, we observed striking loss of poly(A) RNA signal from the cytoplasm, and a noticeable increase in the nuclear poly(A) signal (Fig. S2). Importantly, upon arsenite treatment of mock - and IAV-infected cells at early times post-infection, bright cytoplasmic poly(A) foci were observed, consistent with the accretion of mRNAs into SGs. By contrast, no cytoplasmic foci were observed in cells that displayed nuclear accumulation of poly(A) RNA. Taken together, these data suggest that IAV SG inhibition coincides with bulk depletion of cytoplasmic poly(A) mRNA and the nuclear accumulation of PABP1.
Influenza A virus inhibits SG formation downstream of eIF-2α kinase activation
In eukaryotes, eIF2α integrates signals from four stress-activated kinases, and we have established that IAV inhibits SG formation in response to either HRI - or PKR-mediated eIF2α phosphorylation. To determine whether the virus acts downstream of eIF2α phosphorylation, we assessed SG formation triggered by thapsigargin and UV light, which activate the two remaining eIF2α kinases, PERK and GCN2, respectively. As a control, we also tested pateamine A (PatA), which has been shown to induce SGs by translation inhibition but without eIF2α phosphorylation [15] (Fig. 3A–C). In mock-infected cells, these treatments induced varying degrees of SG formation. Nevertheless, consistent with our sodium arsenite data, IAV inhibited SG formation in response to all three treatments without affecting eIF2α phosphorylation (Fig. 3A–C). Most notably, IAV inhibited SG formation in response to PatA treatment, which did not induce eIF2α phosphorylation. Together, these findings establish that IAV can block SG formation in a manner independent of eIF2α phosphorylation.
Fig. 3. Influenza A virus blocks SG formation independent of inducing stimuli and downstream of eIF2α phosphorylation. 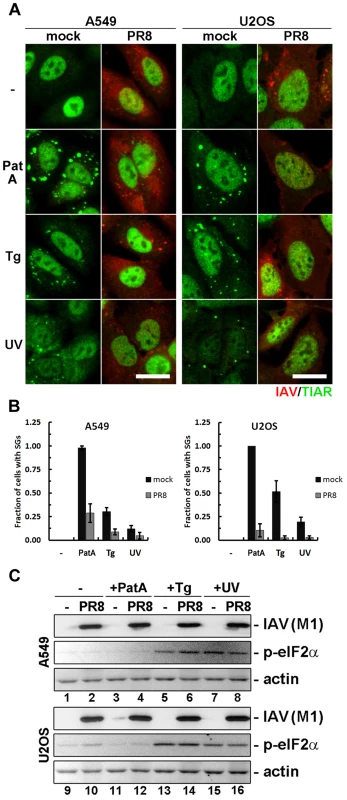
SG formation and eIF2α phosphorylation was analysed in mock and PR8 virus infected A549 and U2OS cells at 18 hpi by immunofluorescent staining and western blot. Cells were either left untreated (-), treated for 1 hour with pateamine A (PatA), treated for 1 hour with thapsigargin (Tg), or exposed to UV light and incubated for 2 hours (UV) prior to analysis. (A) Immunofluorescent staining with antibodies to influenza A virus (IAV, red) and TIAR (red). Scale bars = 20 µm. (B) SG formation was quantified from the experiment presented in (A). (C) Western blot analysis of the whole cell lysates of A549 and U2OS cells treated as in (A). Influenza A virus PA and NP ORFs inhibit SG formation
To identify additional IAV gene products that could function in this NS1-independent mechanism of SG inhibition, we analyzed arsenite-induced SG formation in cells ectopically expressing myc epitope-tagged constructs for each of the known IAV ORFs (Fig. 4A). Among 11 ORFs analyzed, only PA and NP significantly inhibited arsenite-induced SG formation. PA-myc was predominantly localized to the cytoplasm, consistent with requirement of the PB1 viral subunit for its nuclear import [16]. In cells in which PA failed to suppress SG formation, PA-myc maintained a diffuse cytoplasmic localization and was not recruited to SGs (Fig. 4A). By contrast, NP was found predominantly in the nucleus of transfected cells, with only a fraction of the total signal coming from the cytoplasm. NP-mediated inhibition of SG formation was dependent on NP expression level (Fig. S3), and many cells with low levels of NP formed SGs upon arsenite treatment. In cells in which NP failed to inhibit SG formation, NP was recruited to SGs, consistent with previous reports [17]. Finally, NS1 and the viral protein M1 were similarly recruited to arsenite-induced SGs, but did not interfere with their formation (Fig. 4B). The distinct subcellular distribution and SG-recruitment characteristics of PA and NP suggested distinct mechanisms of SG suppression. Importantly, in cells expressing high levels of PA, SG inhibition coincided with striking relocalization of PABP1 to the nucleus (Fig. 4B), similar to what we observed in IAV-infected cells at late times post-infection (Fig. 2B). Importantly, in both PA-transfected and IAV-infected cells nuclear PABP accumulation invariably coincided with complete inhibition of SG formation in all our experiments. To confirm that both PA and NP interfere with SG formation regardless of eIF2α phosphorylation, we tested PatA-induced SG formation in cells expressing these IAV ORFs (Fig. 4C,D). Both proteins were able to block SG formation in response to PatA. NS1, as expected for this PKR-specific SG inhibitor, did not interfere with arsenite - and PatA-induced SG formation (Fig. 4A–D).
Fig. 4. Ectopic expression of influenza A virus PA and NP ORFs inhibits SG formation induced by arsenite and pateamine A. 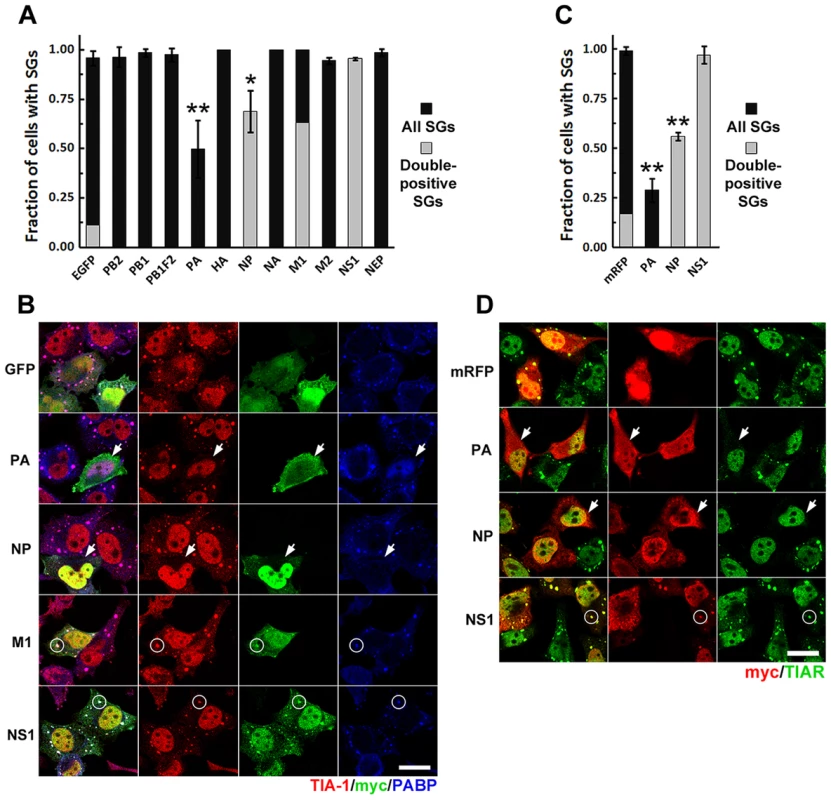
HeLa-TetOff cells were transfected with the expression vectors for the indicated c-terminally myc-tagged IAV ORFs or the EGFP or mRFP expression vectors and treated with sodium arsenite or PatA 24 hours post-transfection. 50 min post-treatment SG formation was analyzed by immunofluorescent staining for TIA-1 or TIAR and SG co-localization of viral ORF-encoded proteins by staining for myc epitope. (A). Arsenite-induced SG formation (dark grey bars) and SG recruitment (double-positive SGs, light grey bar overlays) was quantified from 3 independent experiments. (B). Representative immunofluorescence images demonstrating arsenite-induced SG inhibition by PA-myc and NP-myc (arrows), SG co-localization of M1-myc and NS1-myc proteins (circles), and nuclear PABP1 relocalization in PA-myc expressing cells. (C). PatA-induced SG formation (dark grey bars) and SG recruitment (double-positive SGs, light grey bar overlays) in cells expressing the indicated IAV ORFs and the control mRFP protein was quantified from 2 independent experiments. (D). Representative immunofluorescence images demonstrating SG inhibition by PA-myc and NP-myc (arrows) in response to PatA treatment, and SG co-localization of NS1-myc protein (circles). NP oligomerization is required for efficient SG inhibition
NP associates with IAV genomic and anti-genomic segments and is essential for vRNP synthesis, genome trafficking and virus assembly [18]. Extensive mutational analysis and atomic-resolution structural studies have identified important structural features of NP, including an amino-terminal nuclear-localization signal (NLS) and a carboxy-terminal oligomerization motif [19]–[21], both critical for NP interaction with nascent vRNPs in the nucleus (Fig. 5C). Mutation of arginine 8 to an alanine residue (R8A) prevented nuclear localization of NP, but did not impair its ability to suppress arsenite-induced SG formation, suggesting that NP nuclear accumulation is not required for SG inhibition (Fig. 5A, B). NP oligomerization was experimentally disrupted by substitution of key resides in the tail loop (F412A, R416A) required for the formation of intermolecular salt bridges [19], [20]. Disruption of NP oligomerization completely restored arsenite-induced SG formation in transfected cells (Fig. 5A, B). During infection, NP oligomerization is essential for polymerase activity and for the stability and transport of vRNPs, but our experiments clearly demonstrate that NP-mediated inhibition of SG formation is independent of virion RNAs or polymerase subunits. NP has not been shown to interact with host RNAs, but a number of high-affinity interactions of IAV ribonucleoproteins with host factors have been identified [22], many of which could plausibly be dependent on the ability of NP to oligomerize.
Fig. 5. NP oligomerisation is required for SG inhibition. 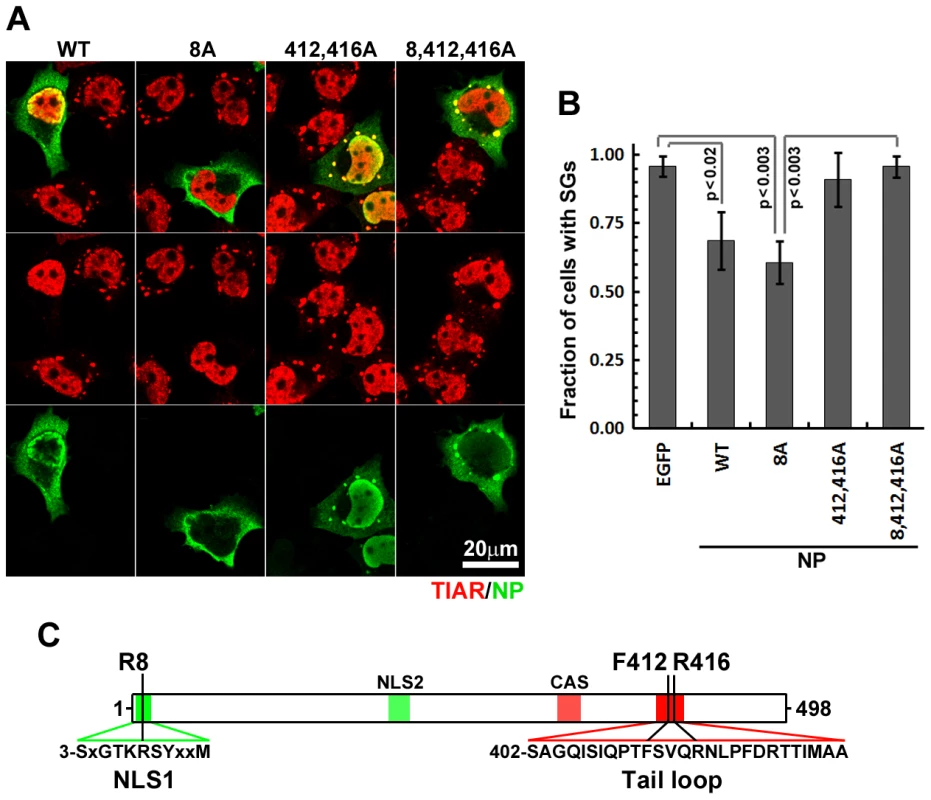
(A and B). HeLa-TetOff cells were transfected with the expression vectors for the indicated wild-type and mutant NP proteins and treated with sodium arsenite 24 hours post-transfection. 50 min post-treatment sub-cellular distribution of NP constructs (green) and SG formation (TIAR, red) was analyzed by immunofluorescent staining (A) and quantified (B). (C). Schematic representation of NP protein showing selected primary sequence features (color shading) and positions of amino acids mutated in this study. Unlike N-terminal nuclear localization signal (NLS1), downstream NLS (NLS2) is believed to be non-functional in the folded protein. The cytoplasmic accumulation sequence (CAS) harbours residues that participate in oligomerisation. PA-X mediated depletion of cytoplasmic mRNA coincides with SG inhibition
Influenza virus infection results in the rapid decline of host protein synthesis, a process referred to as shutoff. The viral PA protein has been implicated in this host shutoff, but the mechanism remained obscure until the recent discovery of an additional protein, PA-X, produced from the PA gene by ribosomal frameshifting [4] (Fig. 6F). PA-X was shown to inhibit cellular gene expression and modulate viral virulence and the global host response [4], [23]. The PA-myc plasmid shown above to inhibit arsenite-induced SG formation and cause nuclear accumulation of PABP1 is predicted to also encode PA-X (Fig. 4). To determine whether the full-length PA protein, or the alternative PA-X protein product, is responsible for SG inhibition and PABP1 relocalization, we transiently transfected HeLa Tet-Off cells with a carboxy-terminal epitope-tagged PA expression vector, or the same construct with optimized codons for F191 and R192 that would greatly reduce the probability of frameshifting and PA-X production (PA(fs)-myc), or a frameshift mutant constructed to express PA-X exclusively (PA-X, Fig. 6F). Results with these plasmids strongly indicate that the PA-X protein produced as a result of ribosomal frameshifting is responsible for the SG inhibition and PABP1 relocalization observed in PA-myc transfected cells. In cells transfected with the PA-X construct, we observed the strongest nuclear accumulation of PABP1, which was completely concordant with SG inhibition. By contrast, in PA(fs)-myc transfected cells, we could not detect any relocalization of PABP1, and arsenite-induced SG formation was unperturbed. As expected, the PA-myc construct displayed an intermediate phenotype, consistent with a lower expected level of PA-X production (Fig. 6B). To directly confirm that PA-X ORF is expressed in cells showing nuclear PABP relocalisation, we created an additional expression construct with a C-terminally tagged PA-X ORF. Due to high toxicity of PA-X, only cells expressing low levels of this fusion protein remained viable at 24 h post-transfection. Nevertheless, these levels were sufficient to cause nuclear relocalisation of PABP1 and complete blockade of SG formation in response to arsenite treatment (Fig. S4). In the study by Jagger et al. (2012), PA-X expression was linked to host gene expression shutoff, which required an endonuclease function of the N-terminal domain shared by the full-length PA and PA-X proteins (Fig. 6F). It was proposed that this endonuclease activity of PA-X is responsible for depletion of host cytoplasmic mRNA pools. To determine whether PA-X endonuclease activity is responsible for depletion of cytoplasmic PABP1 (Fig. 2B) and poly(A) mRNA (Fig. 2C) late in IAV infection, we disrupted the endonuclease activity of PA and PA-X by a single amino acid substitution at D108 (PA(108A)-myc), and analyzed SG formation and subcellular distribution of poly(A) RNA using FISH (Fig. 6A, D). Similar to PA(fs)-myc transfected cells, cells transfected with PA(108A)-myc displayed normal cytoplasmic levels of poly(A) RNA and formed SGs in response to sodium arsenite.
Fig. 6. Viral PA-X protein participates in SG inhibition by depleting host cytoplasmic mRNAs. 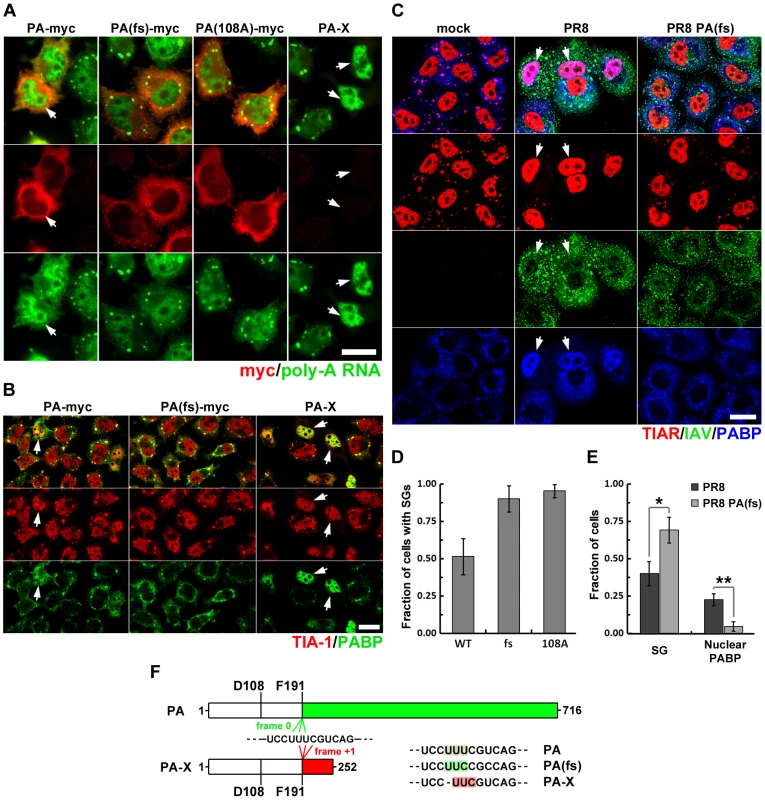
(A, B, D) HeLa-TetOff cells were transfected with the indicated expression vectors and treated with sodium arsenite 24 hours post-transfection. (A) Sub-cellular distribution of poly(A) RNA was analyzed by fluorescence in situ hybridization (green). Expression of the full-length wild type and mutant PA constructs was detected by subsequent immunofluorescent staining for myc epitope (red). (B) SG formation (TIA-1, red) and sub-cellular distribution of PABP1 (PABP, green) was analyzed by immunofluorescent staining. (D) SG formation was quantified from experiments described in (A). (C and E) Immunofluorescent analysis of sodium arsenite-treated mock, wild-type (PR8), and recombinant PA-X deficient (PR8 PA(fs)) virus-infected A549 cells reveals that PA-X expression is required for nuclear PABP1 relocalization and efficient SG inhibition (18 hpi). (C) Representative images showing strong SG inhibition and nuclear PABP1 relocalization in the wild-type, but not the PR8 PA(fs) mutant virus-infected cells treated with sodium arsenite at 18 hpi. (E). Quantification of SG formation and nuclear PABP1 localization from (C). (F). Schematic representation of PA and PA-X proteins synthesized from the same segment 3 transcript and sharing first 191 amino-acids encompassing the endonuclease domain. Continued ORF translation (frame 0, green) produces full-length viral polymerase subunit PA, while +1 frameshifting at F191 codon (frame +1, red) produces smaller PA-X protein. Position of D108 residue that is required for endonuclease activity of PA and PA-X is indicated on the diagram. To the right, frame shift site sequences mutagenized in this study are presented. Note that single nucleotide deletion in PA-X construct completely prevents full-length PA protein expression, as evidenced by the lack of myc-tag detection in the right column of panel (A). To examine the contribution of PA-X to the SG-inhibition phenotype at late times post-infection, we created a recombinant IAV with a codon-optimized PA ORF, termed PR8 PA(fs), expected to have an attenuated host-shutoff phenotype [4]. At 18 hpi, the PR8 PA(fs) infected cells showed dramatically decreased nuclear PABP1 relocalization and attenuated SG inhibition in response to sodium arsenite (Fig. 6C, E). Notably, a fraction of cells infected with PR8 PA(fs) remained refractory to arsenite-induced SG formation, perhaps due to inhibition of SG formation by NP protein.
Pateamine A induces SG formation and blocks influenza A virus replication
We have established that at later stages of its replication cycle IAV efficiently blocks SG formation downstream of eIF2α phosphorylation, while early in infection IAV is particularly sensitive to treatments that induce translation arrest and SG formation (Fig. 1C, F). This early part of the infectious cycle may therefore provide a window of opportunity for pharmacological induction of viral translation arrest and SG formation. Among the treatments that we have tested, we observed strongest induction of SG formation in response to sodium arsenite (Fig. 1) and PatA (Fig. 3). To test the effects of these drugs on viral replication, we pulse-treated IAV-infected cells at 4 hpi, with subsequent removal of drug after 1 h. This 1 h treatment with PatA was sufficient to completely block IAV replication, as evidenced by lack of accumulation of viral proteins at later times post-infection (Fig. 7A). Interestingly, sodium arsenite had minimal effects on IAV protein accumulation. Despite the high toxicity of sodium arsenite, upon its removal cells are reported to rapidly dephosphorylate eIF2α, dissolve SGs, and resume robust translation [24]. By contrast, PatA binds and inhibits the function of eIF4A helicase irreversibly [25]. These properties could explain the differential effect of these SG-inducing agents on viral replication. To test this, we compared translation rates in mock - and PR8-infected cells immediately post-treatment with sodium arsenite or PatA, and at regular intervals post-drug withdrawal. As shown in Fig. 7B, D, soon after removal of sodium arsenite, production of IAV proteins was largely restored. By contrast, translation rates remained low long after PatA was removed (Fig. 7C, D). This sustained inhibition is in agreement with lack of viral protein accumulation up to 14 hours after removal of PatA-containing medium (Fig. 7A), and is marked by the accumulation and persistence of SGs, both in mock - and PR8-infected cells (Fig. 7F). Importantly, persistence of SGs prevented the accumulation of progeny vRNPs in the cytoplasm (Fig. 7F), viral genomic RNA accumulation (Fig. 7G), and infectious virus production (Fig. 7E). By contrast, PatA treatment did not prevent viral mRNA accumulation, and even increased its abundance at later times post-infection (Fig. 7H). This finding is consistent with the model in which viral replication is dependent on de novo protein synthesis [26], and earlier observations that treatment with translation inhibitors prevents viral polymerase from initiating genome replication while still allowing viral mRNA synthesis [27].
Fig. 7. Pateamine A treatment causes sustained arrest of viral protein and genomic RNA synthesis. 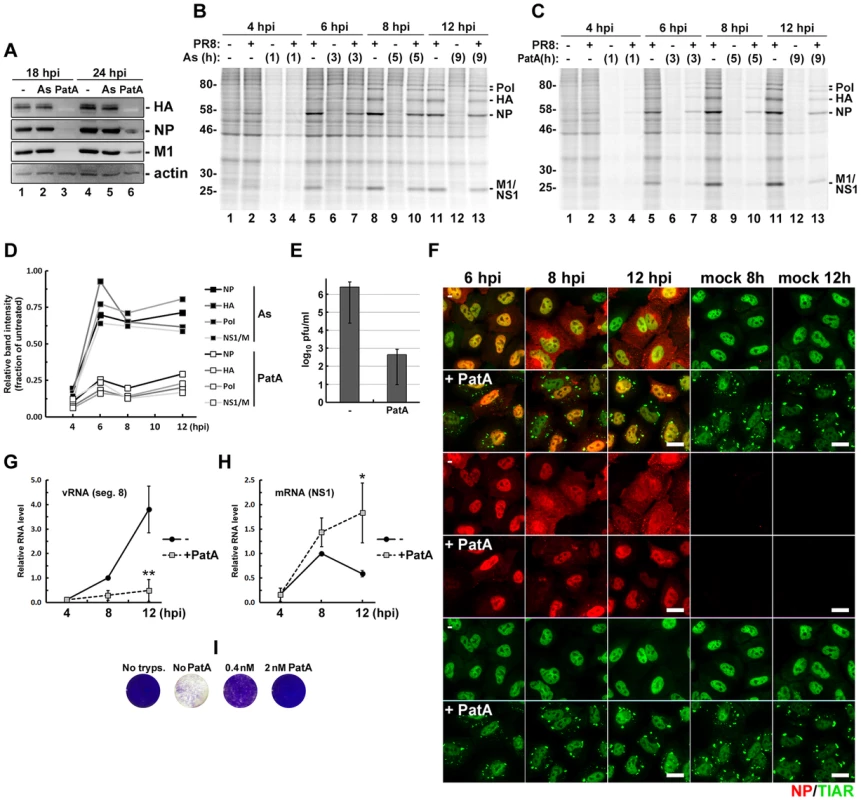
(A). Effects of 1-hour arsenite and pateamine A treatments on IAV protein accumulation. A549 cells were infected with PR8 virus and treated with either arsenite or pateamine A at 4 hpi. After 1 hour, drug-containing media was removed, cells were washed with PBS and received fresh media. Whole cell lysates were collected at 18 and 24 hpi and analysed by western blot. (B and C) Autoradiograms of the lysates of mock and PR8 virus-infected cells. At 3 hpi cells were either left untreated (-) or treated with sodium arsenite (B) or pateamine A (C) for 1 hour and then pulse-labelled with [35S]methionine-[35S]cysteine immediately after treatment and at the indicated 2-hour intervals. (D) Recovery of viral protein translation post-arsenite and post-pateamine A treatments was quantified from autoradiograms in (B) and (C). Band intensities from post-treatment lanes were normalized to untreated sample band intensities for each time point. (E to H). SG formation, viral RNA accumulation, and infectious virus production were analysed in mock and PR8 virus-infected A549 cells treated with 10 nM pateamine A at 4 hpi. (E) Virus titers were analysed in culture media collected at 24 hpi by plaque assays. (F) Cells were fixed and immunofluorescently stained for influenza NP (red) and TIAR (green) at indicated times post-infection (hpi). (G) Accumulation of viral genomic RNA segment 8 was quantified by real-time quantitative RT-PCR. (H) Accumulation of viral NS1 mRNA was quantified by real-time quantitative RT-PCR. (I) Pateamine A protects cells from influenza virus-induced cell death. Vero cells were inoculated with PR8 virus at MOI 0.1. After 1 h, inoculums were replaced with infection medium containing 2.5 µg/ml trypsin and the indicated concentrations of pateamine A, or control media lacking trypsin (No tryps.). 2 days post-infection cells were fixed with 4% paraformaldehyde and stained with crystal violet (purple). Given the potency of PatA for IAV inhibition, we tested the effects of low-dose PatA treatment on multi-round IAV infection and observed an inhibitory effect at 2 and 0.4 nM (Fig. 7I). It is notable that we observed no adverse effects of PatA on cell viability at these low doses, consistent with published observations in other model systems [28].
Discussion
Despite being generated by a viral RNA-dependent RNA polymerase complex, IAV mRNAs bear structural similarity to the products of host RNA polymerase II, with 5′ caps and 3′ poly(A) modifications that ensure efficient translation by host machinery [1]. These features render IAV mRNAs susceptible to negative regulation by eIF2α kinases that have evolved to sense a variety of environmental stresses, including viral infection [5]. Triggering of eIF2α phosphorylation during IAV infection would therefore be expected to arrest translation of a large fraction of viral mRNAs as well as host mRNAs, and entrap them in SGs. Remarkably, we observed that SGs do not form at any point during the IAV replication cycle and efficient translation of viral gene products is maintained in the later stages of infection, when a variety of eIF2α-kinase activating signals might be anticipated. Here we have identified three IAV proteins capable of inhibiting SG formation: NS1, NP and PA-X (Fig. 8). NS1 binds to viral dsRNA and prevents PKR activation and consequent eIF2α phosphorylation. High levels of oligomeric NP block SG formation in an eIF2α-independent manner, through a yet to be identified mechanism. By contrast, inhibition of SG formation by PA-X, the alternative product of PA mRNA that is made as a result of translational frameshifting, is dependent on its endoribonuclease activity, and coincides with depletion of a large fraction of cytoplasmic poly(A) RNA. During PA mRNA translation, less than 2% of the ribosomes frame-shift at the Phe-191, downstream of the endoribonuclease domain that is shared between PA and PA-X proteins [4]. This low rate of frame-shift ensures slow accumulation of PA-X, the potent shutoff factor that acts at later times post-infection. Indeed, mutations that inhibit frame-shifting and PA-X production by IAV disrupt the viral inhibition of host gene expression [4]. Our finding that PA-X inhibits SG formation led us to explore PA-X host shutoff function, and our findings support the recently proposed model; we find that PA-X causes striking depletion of poly(A) RNA from the cytoplasm of cells transfected with a PA-X expression vector, or in IAV-infected cells late in infection, but not in cells infected with a mutant virus with defects in PA-X protein production. Notably, in addition to the depletion of cytoplasmic poly(A) RNA and nuclear relocalization of PABP1, we also observe a remarkable accumulation of poly(A) RNA in the nuclei of cells expressing PA-X and in virus-infected cells at later times post-infection. While this phenomenon has not been demonstrated previously for IAV infections, a similar accumulation of poly(A) RNA and PABP1 has been reported in other viral infections, and in each case the described perturbations of PABP1 has been linked to host shutoff [9]–[11]. For example, host shutoff in cells infected by Kaposi's sarcoma-associated herpesvirus (KSHV) is enforced by the shut-off exonuclease protein SOX, which accumulates during infection and degrades host cytoplasmic mRNAs [29]. SOX expression leads to strong nuclear accumulation of PABP1 and hyper-adenylated mRNAs. It was demonstrated that relocalization of PABP1 to the nucleus results in stimulation of poly(A) polymerase and synthesis of extended poly(A) tails [30]. Given the general inhibition of host mRNA synthesis by IAV, it is plausible that an increase in nuclear poly(A) RNA signal is due to hyperadenylation rather than a blockade of nascent mRNA export, similar to the hyperadenylation observed in cells ectopically expressing KSHV SOX protein or a mutant PABP1 that is restricted to the nucleus [31]. PABP1 localizes to SGs, but is not required for SG formation [6], [32]. Therefore, it remains to be determined whether PA-X directly affects nucleocytoplasmic shuttling of PABP1, or whether PABP1 nuclear accumulation simply results from bulk depletion of cytoplasmic poly(A) RNA. In either case, we have demonstrated here that the endonuclease activity of PA-X is important for SG inhibition, forging the first links between host shutoff and SG dynamics.
Fig. 8. A model for concerted action of NS1, NP, and PA-X proteins in the inhibition of translation arrest and SG formation in influenza A virus-infected cells. 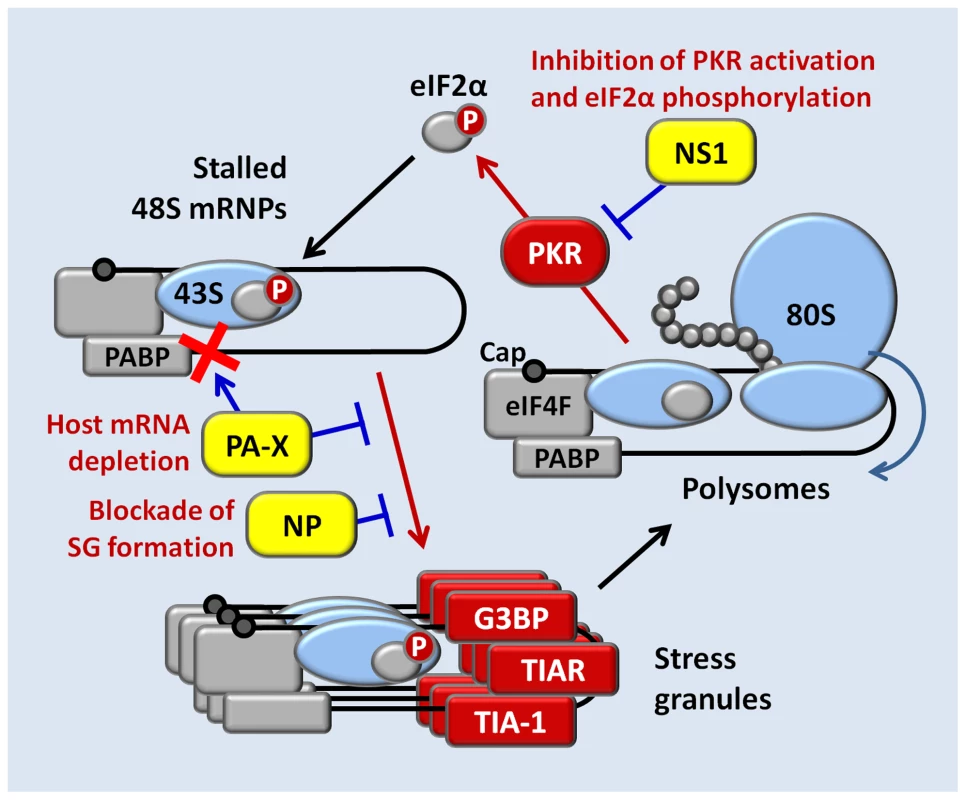
Host shutoff is an important feature of influenza virus infection. In human-adapted strains of IAV, host shutoff is mediated at least in part by the NS1 proteins that are able to bind and inactivate the cellular cleavage and polyadenylation specificity factor 30 (CPSF30). This inactivation prevents polyadenylation and nuclear export of host pre-mRNAs [2]. The host shutoff mechanisms employed by IAV strains with NS1 proteins that lack CPSF30-binding activity remain poorly characterized, but the recent discovery of the PA-X protein significantly advances our understanding of host shutoff by these viruses. Curiously, our study reveals that PA-X expression causes nuclear accumulation of poly(A) RNA, which would require polyadenylation of at least some RNA species in the nucleus. At present, we cannot predict the cumulative effects on sub-cellular poly(A) RNA distribution of CPSF30 inactivation by NS1 and nuclear PABP1 relocalization by PA-X, because the NS1 of PR8 strain employed in this study does not bind CPSF30. Interestingly, the PA segment of WSN/33 strain of IAV, which is competent for CPSF inactivation, was less effective at inhibiting host protein synthesis than the PA segment from A/California/04/2009 strain [23]. This difference suggests that the host-shut-off function of PA-X, similar to that of NS1, may vary significantly between strains of IAV.
NP is a highly conserved RNA-binding protein that plays essential roles in IAV transcription, genome replication, and genome packaging into nascent virions. NP binds to RNA with high affinity (Kd approx. 20 nM), but there is little evidence for sequence specificity, and no links have yet been made to the control of mRNA translation [33], [34]. Our data demonstrate that ectopic expression of NP alone, in the absence of viral RNA or other viral proteins, is sufficient to inhibit arsenite-induced SG formation. Structural studies have shown that NP forms oligomers in physiological ionic conditions [19]. The formation of these oligomers depends on intermolecular salt bridges, and we demonstrate that experimental disruption of these salt bridges by substitution of key residues in the tail loop (F412A, R416A) disabled the SG-inhibition function of NP. We are unable to assess the impact of these mutations on NP function during viral infection because they prevent IAV transcription and genome replication [35]. Proteomic studies have identified a number of host proteins bound by viral ribonucleoproteins [22], several of which play roles in mRNA processing, stability and translation. In addition, a number of proteins recruited to SGs and involved in their formation have been shown to bind NP [36], [37], [38]. NP interacts with nuclear factor 90 (NF90) that participates in virus-induced PKR activation and SG formation [38]. Another host antiviral factor that is recruited to SGs and interacts with both NP and NS1 is the RNA-associated protein 55 (RAP55) [36]. When unable to block SG formation (e.g. at early times post-infection) NS1 and NP can be sequestered in SGs by RAP55 where their normal functions are likely to be inhibited. Thus, it is possible that IAV NS1 and NP can be both the antagonists and the targets of host antiviral SG responses. Recently, RNA-dependent interaction of IAV NP with Fragile X mental retardation protein (FMRP) was demonstrated and FMRP was shown to stimulate viral ribonucleoprotein assembly [37]. Unlike the antiviral factors NF90 or RAP55, FMRP contributes to efficient IAV replication, and may be sequestered through recruitment to SGs. This would further impair viral replication and contribute to the antiviral effect of SGs. In our ongoing studies, we plan to employ WT and mutant NP constructs to screen for interactions with these and other host factors, and determine their relative impact on SG dynamics.
We have established that early in infection, before significant accumulation of NP and PA-X proteins, IAV is particularly sensitive to treatments that induce translation arrest and SG formation. This may provide a window of opportunity for pharmacological induction of viral translation arrest and SG formation. Among the treatments tested, we observed strongest induction of SG formation in response to arsenite and to PatA. Until now, PatA and related structural analogs have been evaluated for their antitumor properties, and found to represent promising and selective anti-tumor agents [39], [40]. Like tumor cells, IAV-infected cells require high levels of translation to support their metabolic needs. Moreover, at the concentrations used in our studies, PatA inhibits the translation of only a fraction of cellular RNAs, specifically ones important for tumor cell growth and metastasis [39]. Ongoing in vitro studies will address the mechanism of action of pateamine A and other translation inhibitor drugs in IAV restriction.
In summary, we report that translation of IAV proteins continues uninterrupted until late in infection, long after the viral polymerase complex has been re-tasked to viral genome replication. We report the remarkable discovery of three functionally distinct IAV-encoded inhibitors of SG formation, which together strongly influence the translational landscape of infected cells. The existence of three distinct mechanisms of IAV-mediated SG inhibition reveals the magnitude of the threat (to the virus) of stress-induced translation arrest during viral replication, and hints that other viruses, large and small, may also encode multiple SG-regulatory proteins. Investigation of the mechanisms of action of these viral SG inhibitors may reveal important clues about how these viruses enforce host shutoff and ensure preferential production of viral gene products.
Materials and Methods
Reagents and cells
Unless specifically indicated, all chemical reagents were purchased from Sigma. Pateamine A was a kind gift from Dr. Jerry Pelletier (McGill University, Montreal, QC, Canada). HeLa-TetOff cells were purchased from Clontech. A549, U2OS, Vero, and HeLa-TetOff cells were maintained in Dulbecco's modified Eagle's medium (DMEM; HiClone) supplemented with 10% fetal bovine serum (FBS, Life Technologies) and 100 U/ml penicillin+100 µg/ml streptomycin+20 µM L-glutamine (Pen/Strep/Gln; Wisent) at 37°C in 5% CO2 atmosphere.
Viruses, infections and treatments
The A/PuertoRico/8/34/(H1N1) virus (PR8) and the recombinant mutant viruses PR8 NS1 38A,41A and PR8 NS1 96,97A are described in [8]. To generate NS1 deletion mutant viruses PR8 NS1 N80 and PR8 NS1 N15, the plasmid encoding segment 8 of PR8 [41] was subjected to Phusion PCR mutagenesis (Finnzymes/Thermo Scientific) to introduce termination codons at amino acid positions 81 and 16, respectively, in the NS1 open reading frame. Resulting constructs were combined with 7 wild-type PR8 plasmids for rescue of the mutant viruses as described in [41], producing PR8 mutants expressing either just the dsRNA binding domain of NS1 (PR8 NS1 N80) or completely lacking functional NS1 and expressing only 15 N-terminal amino acids shared between NS1 and NEP upstream of the unaltered transcript splice site (PR8 NS1 N15). Recombinant virus PR8 PA(fs) was generated using the general approach described above. In the segment 3 rescue plasmid, sequence TTTCGT encoding Phe-191 and Arg-192 of the PA ORF was substituted with the optimized codon sequence TTCCGC to generate the recombinant virus with strongly attenuated +1 ribosomal frame shifting during PA mRNA translation. Mutagenesis primer sequences are available upon request. Virus stocks used for experiments were produced and titrated by plaque assays as described [8]. Genomic RNA segments 3 and 8 of each virus stock were verified by sequencing.
For virus infection experiments, unless specified otherwise, infections were done at multiplicity of infection (MOI) of 0.5. After inoculation, cells received infection medium containing 0.5% bovine serum albumin (BSA) in DMEM and incubated at 37°C in 5% CO2 atmosphere. When indicated, mock and virus-infected cells were treated with 0.75 mM sodium arsenite, 10 nM pateamine A (except when other concentrations are given), or 1 µM Thapsigargin. For UV treatment, cells were washed briefly with PBS, exposed to 10,000 µJ/cm2 of 254 nm light in HL-2000 Hybrilinker chamber (UVP) and promptly returned to 37°C incubator.
Plasmids and primers
Rescue plasmids encoding 8 segments of PR8 virus (pHW191-PB2 to pHW198-NS) were kindly provided by Dr. Richard Webby (St. Jude Children's Research Hospital, Memphis, TN, USA). pCR3.1-Myc and pCR3.1-NS1-myc expression vectors are described in [8]. Remaining expression vectors for c-terminally tagged PR8 ORF library (with the exception of M2) were constructed by inserting PCR-amplified coding sequences from the rescue vectors between KpnI and Mlu sites of pCR3.1-Myc vector (flanking restriction sites were introduced by PCR). M2 expression vector was generated by removal of the intervening intron sequence from pHW197-M plasmid using Phusion PCR mutagenesis protocol. Untagged NP expression vector was generated by inserting the PCR-amplified coding sequences from the pHW195-NP plasmid into pCR3.1 vector (Invitrogen/Life Technologies) between EcoRI and XhoI sites. Subsequent substitutions in the NP ORF were done by PCR mutagenesis to produce pCR3.1-NP(8A), pCR3.1-NP(412,416A) and pCR3.1-NP(8,412,416A) vectors. pCR3.1-PA(fs)-myc, pCR3.1-PA(108A)-myc and pCR3.1-PA-X were generated by PCR mutagenesis of the pCR3.1-PA-myc vector from the PR8 ORF library. Cloning primer sequences are available upon request.
Transient transfection
HeLa - TetOff cells were seeded at a density of 100,000 cells/well of a 12-well culture plate and transfected the next day with 0.5 µg total DNA/well using FuGene HD reagent (Promega) according to manufacturer's protocol. At 24 h post-transfection, cells were treated as described and analysed by immunofluorescent staining.
Immunostaining
Cells grown on glass coverslips were fixed and immunostained according to the protocol in [6] using mouse monoclonal antibodies to IAV nucleoprotein (AAH5; Abcam), G3BP (clone 23, BD Transduction Labs), and PABP1 (sc-32318, Santa Cruz Biotechnology); goat polyclonal antibody to TIA-1 (sc-1751, Santa Cruz Biotechnology), influenza virus (ab20841, Abcam), rabbit antibody to TIAR (clone D32D3, Cell Signaling), YB-1 (ab12148, Abcam) and myc-tag (9B11; Cell Signaling) at manufacturer-recommended dilutions. AlexaFluor-conjugated secondary antibodies (Molecular Probes) were used at 1∶1000 dilution. Images were captured using Zeiss Axioplan II microscope or Zeiss LSM 510 laser scanning microscope. Quantification of stress granules was done in at least 3 random fields of view with greater than 200 cells analysed on each slide. Cells were considered stress granule-positive if two or more stress granule marker foci were present in the cytoplasm. For western blot analysis, whole cell lysates were resolved on denaturing 10% polyacrylamide gels and analyzed using primary antibodies described above and the antibodies to phospho-Ser-51 - eIF2α (rabbit, D9G8, Cell Signaling), eIF4A (goat, sc-14211, Santa Cruz Biotechnology), and actin (rabbit, 4968; Cell Signaling).
Isotopic labelling
As described in [8].
Fluorescence in situ hybridisation
Cells grown on glass coverslips were washed briefly with PBS, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, and permeabilized using 0.1% Triton X-100 in PBS for 10 min. Coverslips were equilibrated in wash buffer (2× SSC, 20% formamide, 0.02% BSA) for 15 min and then incubated overnight with 100 µl of hybridization mix (wash buffer with 10% dextran sulphate, 0.4 mg/ml S. cerevisiae tRNA, and 100 nM Fluorescein-conjugated oligo-dT-40 probe) at 42°C. Then, coverslips were washed in wash buffer for 15 min at 42°C, for 15 min at room temperature, and finally with PBS for 15 min prior to either mounting on slides or blocking for subsequent immunostaining.
Real time quantitative PCR
Total RNA was isolated using the RNeasy mini-prep kit (Qiagen) according to the manufacturer's instructions, and the cDNA synthesis was performed using the Super Script III Reverse Transcriptase Kit (Life Technologies). Quantitative PCR analysis was performed using MX6000P unit (Agilent Technologies) and GoTaq PCR master mix (Promega). For analysis of NS1 mRNA reverse transcription was performed using oligo-dT(20) primer. For reverse transcription of segment 8 vRNA the following primer was used: 5′-CAA ACA CTG TGT CAA GCT TTC AG. Same primer set was used for the mRNA and vRNA-derived cDNA amplification: NS1-Left 5′-CTG TGT CAA GCT TTC AGG TAG A and NS1-Right 5′ - GGT ACA GAG GCC ATG GTC AT. Relative initial template quantities were determined using the standard curve method. For each biological replicate, reactions were done in duplicate and mean cycle threshold (Ct) values were used.
Statistical analyses
All error bars represent standard deviations calculated from values obtained in at least 3 independent biological replicates. Analysis of variance (ANOVA) single-factor algorithm was applied to the select datasets using Microsoft Excel data analysis module (Microsoft) to calculate p values. Where indicated, asterisks denote p values lower than 0.05 (*) or 0.005 (**).
Supporting Information
Zdroje
1. PlotchSJ, BouloyM, UlmanenI, KrugRM (1981) A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23 : 847–858.
2. NemeroffME, BarabinoSM, LiY, KellerW, KrugRM (1998) Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol Cell 1 : 991–1000.
3. YángüezE, NietoA (2011) So similar, yet so different: selective translation of capped and polyadenylated viral mRNAs in the influenza virus infected cell. Virus Res 156 : 1–12.
4. JaggerBW, WiseHM, KashJC, WaltersKK-A, WillsNM, et al. (2012) An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science (80-) 165 : 199–204.
5. YamasakiS, AndersonP (2008) Reprogramming mRNA translation during stress. Curr Opin Cell Biol 20 : 222–226.
6. KedershaN, AndersonP (2007) Mammalian stress granules and processing bodies. Methods Enzymol 431 : 61–81.
7. WhiteJP, LloydRE (2012) Regulation of stress granules in virus systems. Trends Microbiol 20 : 175–183.
8. KhaperskyyDA, HatchetteTF, McCormickC (2012) Influenza A virus inhibits cytoplasmic stress granule formation. FASEB J 26 : 1629–1639.
9. CovarrubiasS, RichnerJM, ClydeK, LeeYJ, GlaunsingerBa (2009) Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J Virol 83 : 9554–9566.
10. HarbM, BeckerMM, VitourD, BaronCH, VendeP, et al. (2008) Nuclear localization of cytoplasmic poly(A)-binding protein upon rotavirus infection involves the interaction of NSP3 with eIF4G and RoXaN. J Virol 82 : 11283–11293.
11. BlakqoriG, van KnippenbergI, ElliottRM (2009) Bunyamwera orthobunyavirus S-segment untranslated regions mediate poly(A) tail-independent translation. J Virol 83 : 3637–3646.
12. McEwenE, KedershaN, SongB, ScheunerD, GilksN, et al. (2005) Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem 280 : 16925–16933.
13. WatanabeK, TakizawaN, KatohM, HoshidaK, KobayashiN, et al. (2001) Inhibition of nuclear export of ribonucleoprotein complexes of influenza virus by leptomycin B. Virus Res 77 : 31–42.
14. AfoninaE, StauberR, PavlakisGN (1998) The human poly(A)-binding protein 1 shuttles between the nucleus and the cytoplasm. J Biol Chem 273 : 13015–13021.
15. MazrouiR, SukariehR, BordeleauM, KaufmanRJ, NorthcoteP, et al. (2006) Inhibition of Ribosome Recruitment Induces Stress Granule Formation Independently of Eukaryotic Initiation Factor 2 alpha Phosphorylation. Mol Biol Cell 17 : 4212–4219.
16. DengT, EngelhardtOG, ThomasB, AkoulitchevAV, BrownleeGG, et al. (2006) Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex. J Virol 80 : 11911–11919.
17. OnomotoK, JogiM, YooJ-S, NaritaR, MorimotoS, et al. (2012) Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS One 7: e43031.
18. PortelaA, DigardP (2002) The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol 83 : 723–734.
19. EltonD, MedcalfE, BishopK, DigardP (1999) Oligomerization of the influenza virus nucleoprotein: identification of positive and negative sequence elements. Virology 260 : 190–200.
20. YeQ, KrugRM, TaoYJ (2006) The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 444 : 1078–1082.
21. WangP, PaleseP, O'NeillRE (1997) The NPI-1/NPI-3 (karyopherin alpha) binding site on the influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal. J Virol 71 : 1850–1856.
22. MayerD, MolawiK, Martínez-sobridoL, GhanemA, BaginskyS, et al. (2008) Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res 6 : 672–682.
23. DesmetEa, BusseyKa, StoneR, TakimotoT (2013) Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis. J Virol 87 : 3108–3118.
24. KedershaN, ChenS, GilksN, LiW, MillerIJ, et al. (2002) Evidence That Ternary Complex (eIF2-GTP-tRNAiMet) – Deficient Preinitiation Complexes Are Core Constituents of Mammalian Stress Granules. Mol Biol Cell 13 : 195–210.
25. BordeleauM-E, MatthewsJ, WojnarJM, LindqvistL, NovacO, et al. (2005) Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc Natl Acad Sci U S A 102 : 10460–10465.
26. JorbaN, ColomaR, OrtínJ (2009) Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLoS Pathog 5: e1000462.
27. HatadaE, HasegawaM, MukaigawaJ, ShimizuK, FukudaR (1989) Control of influenza virus gene expression: quantitative analysis of each viral RNA species in infected cells. J Biochem 105 : 537–546.
28. Di MarcoS, CammasA, LianXJ, KovacsEN, MaJF, et al. (2012) The translation inhibitor pateamine A prevents cachexia-induced muscle wasting in mice. Nat Commun 3 : 896.
29. GlaunsingerB, ChavezL, GanemD (2005) The exonuclease and host shutoff functions of the SOX protein of Kaposi's sarcoma-associated herpesvirus are genetically separable. J Virol 79 : 7396–7401.
30. LeeYJ, GlaunsingerBA (2009) Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol 7: e1000107.
31. KumarGR, ShumL, GlaunsingerBA (2011) Importin alpha-mediated nuclear import of cytoplasmic poly(A) binding protein occurs as a direct consequence of cytoplasmic mRNA depletion. Mol Cell Biol 31 : 3113–3125.
32. OhnT, KedershaN, HickmanT, TisdaleS, AndersonP (2008) A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol 10 : 1224–1231.
33. BaudinF, BachC, CusackS, RuigrokRW (1994) Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent. EMBO J 13 : 3158–3165.
34. YamanakaK, IshihamaA, NagataK (1990) Reconstitution of influenza virus RNA-nucleoprotein complexes structurally resembling native viral ribonucleoprotein cores. J Biol Chem 265 : 11151–11155.
35. LiZ, WatanabeT, HattaM, WatanabeS, NanboA, et al. (2009) Mutational analysis of conserved amino acids in the influenza A virus nucleoprotein. J Virol 83 : 4153–4162.
36. MokB, SongW, WangP, TaiH, ChenY, et al. (2012) The NS1 protein of influenza A virus interacts with cellular processing bodies and stress granules through RNA-associated protein 55 (RAP55) during virus infection. J Virol 86 : 12695–12707.
37. ZhouZ, CaoM, GuoY, ZhaoL, WangJ, et al. (2014) Fragile X mental retardation protein stimulates ribonucleoprotein assembly of influenza A virus. Nature Communications 5 : 3259.
38. WenX, HuangX, MokB, ChenY, ZhengM, et al. (2014) NF90 exerts antiviral activity through regulation of PKR phosphorylation and stress granules in infected cells. J Immunol 192 : 3753–3764.
39. LindqvistL, PelletierJ (2009) Inhibitors of translation initiation as cancer therapeutics. Future Med Chem 1 : 1709–1722.
40. KuznetsovG, XuQ, Rudolph-OwenL, TendykeK, LiuJ, et al. (2009) Potent in vitro and in vivo anticancer activities of des-methyl, des-amino pateamine A, a synthetic analogue of marine natural product pateamine A. Mol Cancer Ther 8 : 1250–1260.
41. HoffmannE, NeumannG, KawaokaY, HobomG, WebsterRG (2000) A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97 : 6108–6113.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDSČlánek The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV InfectionČlánek Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
- Defensins and Viral Infection: Dispelling Common Misconceptions
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- The Wide World of Ribosomally Encoded Bacterial Peptides
- Microbial Egress: A Hitchhiker's Guide to Freedom
- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1
- Tetherin Can Restrict Cell-Free and Cell-Cell Transmission of HIV from Primary Macrophages to T Cells
- The Frustrated Host Response to Is Bypassed by MyD88-Dependent Translation of Pro-inflammatory Cytokines
- Larger Mammalian Body Size Leads to Lower Retroviral Activity
- The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection
- Lytic Gene Expression Is Frequent in HSV-1 Latent Infection and Correlates with the Engagement of a Cell-Intrinsic Transcriptional Response
- Phase Variation of Poly-N-Acetylglucosamine Expression in
- A Screen of Mutants Reveals Important Roles for Dot/Icm Effectors and Host Autophagy in Vacuole Biogenesis
- Structure of the Trehalose-6-phosphate Phosphatase from Reveals Key Design Principles for Anthelmintic Drugs
- The Impact of Juvenile Coxsackievirus Infection on Cardiac Progenitor Cells and Postnatal Heart Development
- Vertical Transmission Selects for Reduced Virulence in a Plant Virus and for Increased Resistance in the Host
- Characterization of the Largest Effector Gene Cluster of
- Novel Drosophila Viruses Encode Host-Specific Suppressors of RNAi
- Pto Kinase Binds Two Domains of AvrPtoB and Its Proximity to the Effector E3 Ligase Determines if It Evades Degradation and Activates Plant Immunity
- Genetic Analysis of Tropism Using a Naturally Attenuated Cutaneous Strain
- Plasmacytoid Dendritic Cells Suppress HIV-1 Replication but Contribute to HIV-1 Induced Immunopathogenesis in Humanized Mice
- A Novel Mouse Model of Gastroenteritis Reveals Key Pro-inflammatory and Tissue Protective Roles for Toll-like Receptor Signaling during Infection
- Pathogenicity of Is Expressed by Regulating Metabolic Thresholds of the Host Macrophage
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Independent Bottlenecks Characterize Colonization of Systemic Compartments and Gut Lymphoid Tissue by
- Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
- G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated mRNAs and Are Targeted by a Dengue Virus Non-coding RNA
- Cytolethal Distending Toxins Require Components of the ER-Associated Degradation Pathway for Host Cell Entry
- The Machinery at Endoplasmic Reticulum-Plasma Membrane Contact Sites Contributes to Spatial Regulation of Multiple Effector Proteins
- Arabidopsis LIP5, a Positive Regulator of Multivesicular Body Biogenesis, Is a Critical Target of Pathogen-Responsive MAPK Cascade in Plant Basal Defense
- Plant Surface Cues Prime for Biotrophic Development
- Real-Time Imaging Reveals the Dynamics of Leukocyte Behaviour during Experimental Cerebral Malaria Pathogenesis
- The CD27L and CTP1L Endolysins Targeting Contain a Built-in Trigger and Release Factor
- cGMP and NHR Signaling Co-regulate Expression of Insulin-Like Peptides and Developmental Activation of Infective Larvae in
- Systemic Hematogenous Maintenance of Memory Inflation by MCMV Infection
- Strain-Specific Variation of the Decorin-Binding Adhesin DbpA Influences the Tissue Tropism of the Lyme Disease Spirochete
- Distinct Lipid A Moieties Contribute to Pathogen-Induced Site-Specific Vascular Inflammation
- Serovar Typhi Conceals the Invasion-Associated Type Three Secretion System from the Innate Immune System by Gene Regulation
- LANA Binds to Multiple Active Viral and Cellular Promoters and Associates with the H3K4Methyltransferase hSET1 Complex
- A Molecularly Cloned, Live-Attenuated Japanese Encephalitis Vaccine SA-14-2 Virus: A Conserved Single Amino Acid in the Hairpin of the Viral E Glycoprotein Determines Neurovirulence in Mice
- Illuminating Fungal Infections with Bioluminescence
- Comparative Genomics of Plant Fungal Pathogens: The - Paradigm
- Motility and Chemotaxis Mediate the Preferential Colonization of Gastric Injury Sites by
- Widespread Sequence Variations in VAMP1 across Vertebrates Suggest a Potential Selective Pressure from Botulinum Neurotoxins
- An Immunity-Triggering Effector from the Barley Smut Fungus Resides in an Ustilaginaceae-Specific Cluster Bearing Signs of Transposable Element-Assisted Evolution
- Establishment of Murine Gammaherpesvirus Latency in B Cells Is Not a Stochastic Event
- Oncogenic Herpesvirus KSHV Hijacks BMP-Smad1-Id Signaling to Promote Tumorigenesis
- Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection
- Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection
- Chitin-Degrading Protein CBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees
- Influenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest
- Nsp9 and Nsp10 Contribute to the Fatal Virulence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Emerging in China
- Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis
- Syk Signaling in Dendritic Cells Orchestrates Innate Resistance to Systemic Fungal Infection
- A Repetitive DNA Element Regulates Expression of the Sialic Acid Binding Adhesin by a Rheostat-like Mechanism
- T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection
- Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health
- Influence of ND10 Components on Epigenetic Determinants of Early KSHV Latency Establishment
- Antibody to gp41 MPER Alters Functional Properties of HIV-1 Env without Complete Neutralization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



