-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaComparative Genomics of Plant Fungal Pathogens: The - Paradigm
article has not abstract
Published in the journal: . PLoS Pathog 10(7): e32767. doi:10.1371/journal.ppat.1004218
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004218Summary
article has not abstract
The closely related smut fungi Ustilago maydis, U. hordei, and Sporisorium reilianum f. sp. zeae are facultatively biotrophic basidiomycetes that occur ubiquitously. Teliospores germinate to produce sporidia of different mating type that grow saprophytically and multiply mitotically by budding [1]. For mass proliferation and sexual genetic exchange, successful colonization of economically important crop plants like maize, barley, and oats is a prerequisite. Mating of compatible haploid yeast cells leads to the formation of dikaryotic filaments that are infection competent. These filaments enter their hosts by penetration of the leaf surface [2]. Once inside the plant, filaments multiply in the affected tissue and induce spore formation in tumors near the penetration site (U. maydis) [3] or spread through the entire plant and form spores in inflorescences (S. reilianum and U. hordei) [4], [5]. Although presence of the fungus is clearly detected [6], defense reactions of native host plants are very limited, allowing fungal spread initially without major plant tissue damage. In fact, a living host plant is required to provide nutrients for massive fungal proliferation and successful spore formation.
What Did We Know before Genome Sequencing?
Smut fungi have intrigued scientists for more than a century for many different reasons, among which are their host specificity, mating behavior, and ability to cause plant disease [7]. Before the molecular era, smut fungi were typically classified by identification of the plant on which symptoms were found, since smuts have a limited host range and many form spores only on a single plant species [8].
Mating behavior was one of the first things studied in smut fungi. U. hordei is bipolar [9], which means that germination of the diploid spore leads to haploid yeast-like meiosis products with two different mating types. In contrast, U. maydis and S. reilianum are tetrapolar, and spore germination gives rise to four different haploid yeast cell types, of which only two combinations are mating competent [10]–[12]. These early observations already led to the proposal of the presence of two independently segregating mating type loci, a and b, that each exist in several alleles. U. maydis possesses two a and more than 20 b alleles and was thought to represent the typical tetrapolar smut fungus. Only much later was it discovered that S. reilianum had three a alleles with two pheromone genes each [13], and even later that the occurrence of three a alleles might have been the earlier state during smut fungal evolution (see below) [14].
The start of the molecular era made it possible to identify pathogenicity genes. The first ones identified were the mating type genes and genes involved in signal transduction of the pheromone stimulus [15]. This research unraveled the complex signaling pathways that take place when two mating competent cells meet and form an infectious dikaryotic filament and supports the notion that mating is a prerequisite for plant infection. However, identification of bona fide virulence genes involved in the plant-fungus communication was not successful until the start of the genome sequencing era.
What Did We Learn from Sequencing Smut Genomes?
Because of its virulence in maize and its molecular accessibility, U. maydis was the first smut fungus to be sequenced [3]. After sequencing by two private companies, it was also sequenced by the public sector using classical Sanger sequencing. Genome sequences of S. reilianum f. sp. zeae and U. hordei were assembled from Roche/454 sequencing reads, and S. reilianum was one of the first eukaryotes to be de novo sequenced using this technology [4]. In contrast to the S. reilianum genome, which could be well assembled from the sequencing reads, assembly of the U. hordei genome was only possible after data integration of a whole genome shotgun and a 10 kb paired-end, as well as an end-sequenced bacterial artificial chromosome (BAC) clone library [5].
S. reilianum and U. maydis have small genomes of about 20 Mb, encoding around 6,700 genes distributed on 23 chromosomes. U. hordei has the same number of chromosomes, but its genome is larger (26.1 Mb) and encodes more genes (7,113) [5]. This increase in genome size is not explained by the higher number of genes (since these have a smaller average size) but by a high amount of repetitive DNA [5]. The high content of repetitive DNA resulted in almost 5 Mb of small nonassembled contigs [5] and the sequence assembly problems mentioned above.
All three fungi contain a smaller amount of genes encoding cell-wall-degrading enzymes compared to necrotrophic pathogens [3]–[5], [16]. Having only a few cell-wall-degrading enzymes turned out to be a hallmark of biotrophic fungi that depend on living host tissue and thus need to avoid major damage to the plant [16]. In the U. maydis genome sequence, 12 gene clusters were found that encode small secreted proteins without homologs in any database and lacking enzyme-associated functional domains. This finding, combined with the knowledge that the clustered genes were highly up-regulated during plant infection, prompted a cluster-deletion study. Deletion of five of the 12 clusters resulted in an altered virulence phenotype of U. maydis, confirming involvement of cluster-encoded proteins in virulence [3].
What Did We Learn from Comparing Smut Genomes?
U. maydis and S. reilianum f. sp. zeae are both able to form spores on the same host plant, maize, but cause different symptoms (Figure 1A, left panels). Likely, fungal proteins involved in determining symptom specificity during fungal growth in planta are proteins in need of constant change to escape recognition by the plant. Therefore, these proteins are expected to show weak conservation of encoded amino-acid sequences and might thus be recognized by genome comparison. Comparison of the U. maydis and S. reilianum genomes revealed a very high similarity: about 95% of all genes occur in both organisms, and most of them are located at syntenic positions [4]. This allowed a gene-by-gene comparative analysis, which revealed 43 genomic regions containing at least three consecutive genes with predicted protein sequence identities well below average. Some of these divergence regions corresponded to the gene clusters encoding secreted proteins [4] previously identified in the genome of U. maydis [3]. Notably, all U. maydis clusters whose virulence effect was proven were reidentified as divergence regions, while three of the U. maydis clusters whose deletion did not affect virulence were not. Six newly found divergence regions were deleted in U. maydis, and four of them affected virulence [4]. These results confirmed that virulence factors can be efficiently identified using a comparison approach of related pathogens.
Fig. 1. Overview of mig1-related genes in U. hordei, U. maydis, and S. reilianum. 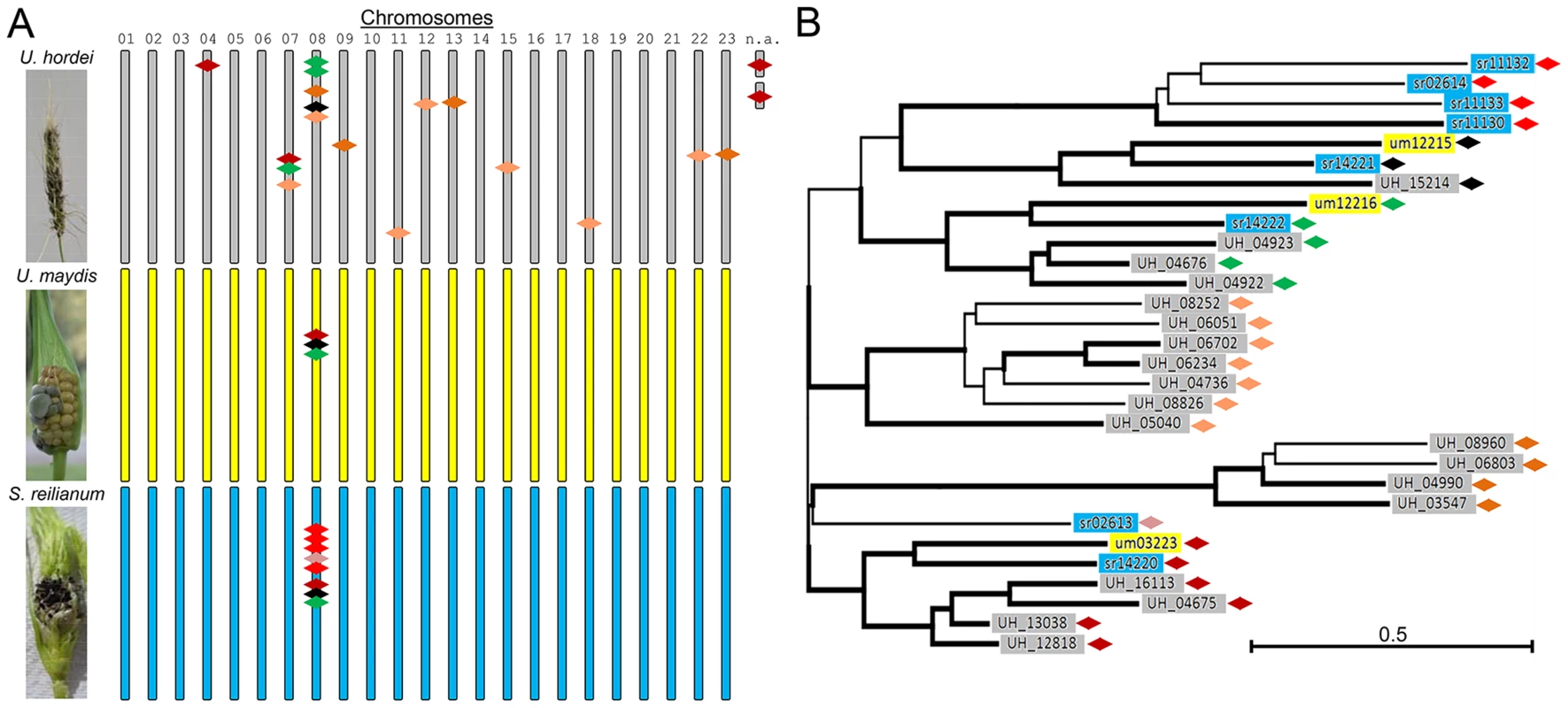
The family of mig1-related secreted effectors in U. maydis are on chromosome 8 and form a cluster of secreted proteins, whose deletion leads to hypervirulence [4]. In S. reilianum, the gene family is increased, but the genes are still clustered on chromosome 8 and have been identified as a divergence region [4]. In U. hordei, many more copies of mig1-related genes are present that likely have been shuffled all over the genome by TE activity [5]. (A) Typical symptoms of smut infection on barley (U. hordei) and maize (U. maydis and S. reilianum) (left panels) and schematic location of mig1-related genes (colored diamonds) on the 23 chromosomes of U. hordei (grey), U. maydis (yellow), and S. reilianum (blue) (right panels). (B) Tree of Mig1 proteins showing similarities of individual Mig1 proteins of the different organisms. Bold subtrees showed bootstrap support (≥50%) after 10,000 iterations. UH Mig2 was used as outgroup (not shown). Key: 01–23, chromosome number; n.a., location not assigned. Abbreviations in gene names: UH, U. hordei; um, U. maydis; sr, S. reilianum f. sp. zeae. Green: um12216 and related genes, black: um12215 and related genes, red and derivatives: um03223 and related genes—shades of red depict relatedness among the genes. The largest divergence region between U. maydis and S. reilianum (cluster 19A) is located on chromosome 19 and has been shown to contain symptom specificity determinants. Deletion of the 44-kb cluster 19A region in U. maydis led to loss of typical U. maydis-specific symptoms. Deletion mutants were unable to induce anthocyanin formation and did not induce tumors on leaves. Dissection of the cluster led to identification of one factor (Tin2) as responsible for induction of anthocyanin and nine factors (Tin1-1, Tin1-2, Tin1-3, Tin1-4, Tin1-5, Tin2, Tin3, Tin4, and Tin5) together responsible for tumor induction of U. maydis on leaves [17]. Dissection of the complete 59 kb cluster 19A region in S. reilianum resulted in identification of a factor (Sad1) that enables S. reilianum to suppress apical dominance in infected maize plants (H. Ghareeb, F. Drechsler, C. Löfke, T. Teichmann, J. Schirawski, unpublished). Affected plants develop more female inflorescences due to outgrowth of subapical ears [18], which increases the number of sites for fungal spore formation. Interestingly, Sad1 also increases inflorescence branching when expressed heterologously in transgenic Arabidopsis thaliana plants, indicating that it functions via a mechanism conserved between maize and A. thaliana (H. Ghareeb, F. Drechsler, C. Löfke, T. Teichmann, J. Schirawski, unpublished).
What Did We Learn about the Evolution of Smuts?
The major chromosomal differences between S. reilianum, U. maydis, and U. hordei can be explained by two independent chromosome rearrangements that happened during speciation and separated the most recent common ancestor with a genome organization as in S. reilianum from the U. maydis and the U. hordei lineages. In the U. hordei lineage, the chromosome rearrangement had a profound effect on fungal biology because it placed the before independently segregating a and b mating type loci on the same chromosome, introducing a physical linkage and forcing a bipolar mating behavior on U. hordei [5]. Accumulation of repetitive elements in the intervening regions between the a and b part of the mating locus of U. hordei may have led to suppression of recombination [5], [19], which represents a step towards evolution of a sex chromosome.
The most common ancestor of the three fungi was likely tetrapolar and had several b and three different a alleles. The number of a and b alleles that were retained in U. hordei diminished to two combinations through chromosomal linkage. In the tetrapolar U. maydis lineage, one a allele got lost (remnants of a second pheromone gene can still be found in the a1 locus of U. maydis), while the number of b alleles increased by mutation and intra-allelic recombination events [20]. In support of this scenario, a recent investigation of mating factor distribution in smut fungi revealed a high prevalence of the third a allele in other smut fungi distributed along the smut fungal tree [14]. In addition to the one major chromosomal rearrangement event, transposable elements (TEs) and repetitive sequences have spread in the U. hordei lineage. This led to shuffling within the U. hordei genome that shows small regions of conserved gene order placed at rearranged chromosomal locations [5]. TE activity also seems to have contributed to distribution of duplicated effector genes. For example, 19 genes related to the U. maydis avirulence effector mig1 (maize-induced gene 1) exist in U. hordei, and they are distributed over at least 11 chromosomes, while in U. maydis three and in S. reilianum eight mig1-related genes lie clustered solely on chromosome 8 (Figure 1).
U. hordei is capable of RNA silencing and possesses enzymes necessary for RNA interference (RNAi). These enzymes are also present in S. reilianum and therefore were likely also present in their common ancestor. However, the genes for RNAi-associated enzymes are lacking in U. maydis. They seem to have been cleanly excised from the genome by the efficient recombination system present in U. maydis [4]. One explanation for the necessity to rid the genome of RNAi components may be the occurrence of killer viruses in U. maydis. These provide a growth advantage to U. maydis cells due to virally encoded killer proteins toxic to other yeasts [21]. Since the viruses carry genomic RNA, RNAi would likely silence their beneficial effects on spread of U. maydis.
Will More Sequencing Be Necessary?
Absolutely. As outlined above, a lot can be learned from sequencing and comparing smut fungal genomes. In addition, sequencing of smut fungal transcriptomes at different stages of plant colonization will tell us when or in which tissues virulence effectors are expressed, which will help in the identification of effector targets in the host plant. With each new genome and transcriptome sequence at hand, the prediction of which factors are responsible for the colonization of particular niches (e.g., host plant or host tissue) will become more precise. At the moment it is possible to compare the effector proteins of U. maydis, S. reilianum f. sp. zeae, and U. hordei [5] and generate lists with effectors conserved among all three species (which would be expected to have a general role in virulence) and those that are conserved only in U. maydis and S. reilianum (and would thus be involved in colonization of maize rather than barley). The problem with the current lists is that they are too long to experimentally verify involvement of the target effectors in adaptation to a particular host and that they likely still include many “false positives”, which decreases the chance of experimental validation. Therefore, we need more genome and transcriptome sequences of closely related fungi colonizing different ecological niches. For example, much progress in virulence effector prediction can be expected from sequencing and comparison of the smut fungi S. scitamineum, a pathogen on sugarcane, U. bromivora, a pathogen on Brachypodium, or Thecaphora thlaspeos, a smut fungus able to infect Brassicaceae. However, the more closely related the compared fungi are, the more likely it is that genomic differences reflect host adaptation. Therefore, sequencing of the sorghum pathogen S. reilianum f. sp. reilianum and comparison to its maize-pathogenic relative S. reilianum f. sp. zeae is most promising to identify genes involved in host adaptation.
Zdroje
1. HaliskyPM (1965) Physiologic specialization and genetics of the smut fungi III. Bot Rev 31 : 114–150.
2. Christensen JJ (1963) Corn smut caused by Ustilago maydis. Monograph 2. St. Paul (Minnesota): American Phytopathological Society. pp. 1–40.
3. KämperJ, KahmannR, BölkerM, MaLJ, BrefortT, et al. (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 : 97–101.
4. SchirawskiJ, MannhauptG, MünchK, BrefortT, SchipperK, et al. (2010) Pathogenicity determinants in smut fungi revealed by genome comparison. Science 330 : 1546–1548.
5. LaurieJD, AliS, LinningR, MannhauptG, WongP, et al. (2012) Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species-specific presence of transposable elements. Plant Cell 24 : 1733–1745.
6. DoehlemannG, WahlR, HorstRJ, VollLM, UsadelB, et al. (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J 56 : 181–195.
7. HoltonCS, HoffmanJA (1968) Variation in the smut fungi. Annual Rev Phytopathol 6 : 213–242.
8. Begerow D, Göker M, Lutz M, Stoll M (2004) On the evolution of smut fungi on their hosts. In: Agerer R, Blanz P, Piepenbring M, editors. Frontiers in Basidiomycete Mycology. Eching, Germany: IHW-Verlag. pp. 81–98.
9. BakkerenG, KronstadJW (1994) Linkage of mating-type loci distinguishes bipolar from tetrapolar mating in basidiomycetous smut fungi. PNAS 91 : 7085–7089.
10. PuhallaJE (1968) Compatibility reactions on solid medium and interstrain inhibition in Ustilago maydis. Genetics 60 : 461–474.
11. SnetselaarKM, MimsCW (1992) Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia 84 : 193–203.
12. HannaWF (1929) Studies in the physiology and cytology of Ustilago zeae and Sorosporium reilianum. Phytopathol 19 : 415–442.
13. SchirawskiJ, HeinzeB, WagenknechtM, KahmannR (2005) Mating type loci of Sporisorium reilianum: Novel pattern with three a and multiple b specificities. Euk Cell 4 : 1317–1327.
14. KellnerR, VollmeisterE, FeldbrüggeM, BegerowD (2011) Interspecific sex in grass smuts and the genetic diversity of their pheromone-receptor system. PLoS Genet 7: e1002436 doi:10.1371/journal.pgen.1002436
15. FeldbrüggeM, KämperJ, SteinbergG, KahmannR (2004) Regulation of mating and pathogenic development in Ustilago maydis. Curr Opin Microbiol 7 : 666–672.
16. ZhaoZ, LiuH, WangC, XuJR (2013) Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics 14 : 274 doi:10.1186/1471-2164-14-274
17. BrefortT, TanakaS, NeidigN, DoehlemannG, VinconV, et al. (2014) Characterization of the largest effector gene cluster of Ustilago maydis. PLoS Path 10: e1003866.
18. GhareebH, BeckerA, IvenT, FeussnerI, SchirawskiJ (2011) Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiol 156 : 2037–2052.
19. BakkerenG, JiangG, WarrenRL, ButterfieldY, ShinH, et al. (2006) Mating factor linkage and genome evolution in basidiomycetous pathogens of cereals. Fungal Genet Biol 43 : 655–666.
20. KämperJ, ReichmannM, RomeisT, BölkerM, KahmannR (1995) Multiallelic recognition: Nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81 : 73–83.
21. DrinnenbergIA, FinkGR, BartelDP (2011) Compatibility with killer explains the rise of RNAi-deficient fungi. Science 333 : 1592.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDSČlánek The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV InfectionČlánek Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
- Defensins and Viral Infection: Dispelling Common Misconceptions
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- The Wide World of Ribosomally Encoded Bacterial Peptides
- Microbial Egress: A Hitchhiker's Guide to Freedom
- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1
- Tetherin Can Restrict Cell-Free and Cell-Cell Transmission of HIV from Primary Macrophages to T Cells
- The Frustrated Host Response to Is Bypassed by MyD88-Dependent Translation of Pro-inflammatory Cytokines
- Larger Mammalian Body Size Leads to Lower Retroviral Activity
- The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection
- Lytic Gene Expression Is Frequent in HSV-1 Latent Infection and Correlates with the Engagement of a Cell-Intrinsic Transcriptional Response
- Phase Variation of Poly-N-Acetylglucosamine Expression in
- A Screen of Mutants Reveals Important Roles for Dot/Icm Effectors and Host Autophagy in Vacuole Biogenesis
- Structure of the Trehalose-6-phosphate Phosphatase from Reveals Key Design Principles for Anthelmintic Drugs
- The Impact of Juvenile Coxsackievirus Infection on Cardiac Progenitor Cells and Postnatal Heart Development
- Vertical Transmission Selects for Reduced Virulence in a Plant Virus and for Increased Resistance in the Host
- Characterization of the Largest Effector Gene Cluster of
- Novel Drosophila Viruses Encode Host-Specific Suppressors of RNAi
- Pto Kinase Binds Two Domains of AvrPtoB and Its Proximity to the Effector E3 Ligase Determines if It Evades Degradation and Activates Plant Immunity
- Genetic Analysis of Tropism Using a Naturally Attenuated Cutaneous Strain
- Plasmacytoid Dendritic Cells Suppress HIV-1 Replication but Contribute to HIV-1 Induced Immunopathogenesis in Humanized Mice
- A Novel Mouse Model of Gastroenteritis Reveals Key Pro-inflammatory and Tissue Protective Roles for Toll-like Receptor Signaling during Infection
- Pathogenicity of Is Expressed by Regulating Metabolic Thresholds of the Host Macrophage
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Independent Bottlenecks Characterize Colonization of Systemic Compartments and Gut Lymphoid Tissue by
- Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
- G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated mRNAs and Are Targeted by a Dengue Virus Non-coding RNA
- Cytolethal Distending Toxins Require Components of the ER-Associated Degradation Pathway for Host Cell Entry
- The Machinery at Endoplasmic Reticulum-Plasma Membrane Contact Sites Contributes to Spatial Regulation of Multiple Effector Proteins
- Arabidopsis LIP5, a Positive Regulator of Multivesicular Body Biogenesis, Is a Critical Target of Pathogen-Responsive MAPK Cascade in Plant Basal Defense
- Plant Surface Cues Prime for Biotrophic Development
- Real-Time Imaging Reveals the Dynamics of Leukocyte Behaviour during Experimental Cerebral Malaria Pathogenesis
- The CD27L and CTP1L Endolysins Targeting Contain a Built-in Trigger and Release Factor
- cGMP and NHR Signaling Co-regulate Expression of Insulin-Like Peptides and Developmental Activation of Infective Larvae in
- Systemic Hematogenous Maintenance of Memory Inflation by MCMV Infection
- Strain-Specific Variation of the Decorin-Binding Adhesin DbpA Influences the Tissue Tropism of the Lyme Disease Spirochete
- Distinct Lipid A Moieties Contribute to Pathogen-Induced Site-Specific Vascular Inflammation
- Serovar Typhi Conceals the Invasion-Associated Type Three Secretion System from the Innate Immune System by Gene Regulation
- LANA Binds to Multiple Active Viral and Cellular Promoters and Associates with the H3K4Methyltransferase hSET1 Complex
- A Molecularly Cloned, Live-Attenuated Japanese Encephalitis Vaccine SA-14-2 Virus: A Conserved Single Amino Acid in the Hairpin of the Viral E Glycoprotein Determines Neurovirulence in Mice
- Illuminating Fungal Infections with Bioluminescence
- Comparative Genomics of Plant Fungal Pathogens: The - Paradigm
- Motility and Chemotaxis Mediate the Preferential Colonization of Gastric Injury Sites by
- Widespread Sequence Variations in VAMP1 across Vertebrates Suggest a Potential Selective Pressure from Botulinum Neurotoxins
- An Immunity-Triggering Effector from the Barley Smut Fungus Resides in an Ustilaginaceae-Specific Cluster Bearing Signs of Transposable Element-Assisted Evolution
- Establishment of Murine Gammaherpesvirus Latency in B Cells Is Not a Stochastic Event
- Oncogenic Herpesvirus KSHV Hijacks BMP-Smad1-Id Signaling to Promote Tumorigenesis
- Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection
- Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection
- Chitin-Degrading Protein CBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees
- Influenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest
- Nsp9 and Nsp10 Contribute to the Fatal Virulence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Emerging in China
- Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis
- Syk Signaling in Dendritic Cells Orchestrates Innate Resistance to Systemic Fungal Infection
- A Repetitive DNA Element Regulates Expression of the Sialic Acid Binding Adhesin by a Rheostat-like Mechanism
- T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection
- Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health
- Influence of ND10 Components on Epigenetic Determinants of Early KSHV Latency Establishment
- Antibody to gp41 MPER Alters Functional Properties of HIV-1 Env without Complete Neutralization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



