-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
article has not abstract
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003851
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003851Summary
article has not abstract
Viral Strategies to Overcome Host Barriers to Infection
As obligate intracellular parasites, viruses must gain entry into target cells and utilize the host cellular machinery for production of viral progeny. After entering the cell and localizing to an intracellular niche, the virus sheds its capsid, replicates its genome, transcribes its RNA, translates its protein components, and assembles the components to form new progeny virions that can infect new cells. At each stage of the virus life cycle, the host has evolved mechanisms to restrict successful infection, and pathogenic viruses have evolved countermechanisms to overcome each restriction. This article will focus on the mechanisms by which the lentivirus human immunodeficiency virus type 1 (HIV-1) overcomes these barriers to establish and propagate infection in human cells.
Lentiviruses Encode Accessory Proteins that Counteract Host Antiviral Responses
While all retroviruses encode proteins required for entry, reverse transcription, integration into host DNA, protein processing, capsid formation, and genome packaging (Env, Pol, and Gag); more complex retroviruses of the lentivirus family, including HIV-1, encode several additional genes. Two of these genes encode proteins that regulate transcription and mRNA nuclear export (Tat and Rev respectively). The remaining genes (nef, vif, vpu, vpr, and/or vpx) encode “accessory proteins” that are not always required for viral infection in in vitro cell culture systems. Instead, these proteins enable pathogenesis in vivo by allowing lentiviruses to evade antiviral responses. Accessory proteins presumably evolved their functions under the selective pressures of continual replication in primate hosts, with each factor serving at least one specific role to enhance viral fitness. Decades of HIV-1 research have led to several key breakthroughs in our understanding of the specific activities and functions of accessory proteins. Interestingly, each accessory protein functions as an adaptor between two or more known host cellular proteins. In this way, the viral pathogen succeeds in dramatically enhancing its capacity to alter the host environment while minimizing its genome size.
HIV-1 Nef Adapts Clathrin Adaptors to Evade Cytotoxic T Lymphocytes (CTLs) and Promote Viral Spread
To establish a successful infection, intracellular pathogens must evade CTLs, which recognize foreign antigens presented in association with host major histocompatibility complex class I (MHC-I). One way that HIV-1 achieves this goal is through the activity of the accessory protein negative effector factor (Nef). Nef enhances the survival of infected cells in the presence of CTLs by mislocalizing and degrading MHC-I [1], [2]. To accomplish this, Nef stabilizes an interaction between MHC-I and the clathrin adaptor protein-1 (AP-1), which regulates clathrin-dependent trafficking of proteins between the trans-Golgi network and endosomes. When stabilized in this complex by Nef, AP-1 directs MHC-I to the endolysosomal pathway where it is degraded at an accelerated rate [3]. Biochemical and structural analysis have revealed that a critical tyrosine residue in the MHC-I cytoplasmic tail mediates the interaction with the tyrosine-binding pocket in the µ1 subunit of AP-1 [4]–[6] (Figure 1A). While this tyrosine can weakly bind AP-1 in some cell types [7], a complex containing MHC-I and AP-1 is normally not detected in T lymphocytes. This is primarily because the MHC-I cytoplasmic tail tyrosine does not conform to a canonical AP-1 tyrosine signal in which there is a downstream hydrophobic amino acid (Yxxφ). Nef stabilizes the weak interaction between MHC-I and AP-1 by providing additional contacts with AP-1 and with the MHC-I cytoplasmic tail. Specifically, an acidic cluster in Nef forms an electrostatic interaction with positively charged residues of AP-1 µ1 [6]. In addition, polyproline (PxxP) repeats in Nef lock the MHC-I cytoplasmic tail onto µ1 (Figure 1) [6].
Fig. 1. Diagrammatic representation of HIV-1 Nef serving as an adaptor of clathrin adaptor proteins in cellular trafficking pathways. 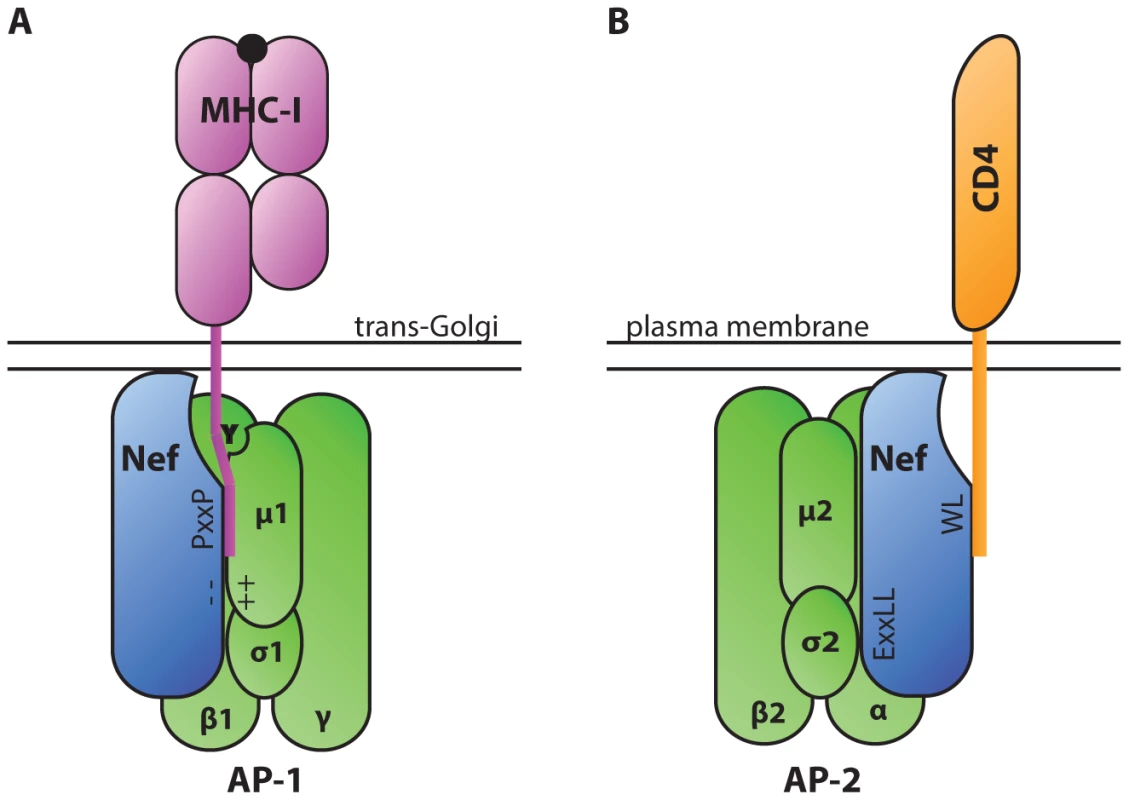
A. The AP-1 µ1 subunit interacts with a tyrosine residue in the cytoplasmic tail of MHC-I via a tyrosine-binding pocket. This interaction is stabilized by electrostatic interactions between a poly-glutamic acid motif of Nef (− −) and a positively charged patch in AP-1 µ1 (++). A polyproline repeat of Nef (PxxP) further stabilizes the complex by forming a wall of the groove that contains the MHC-I tail. These interactions lead to down-modulation of MHC-I from the cell surface. B. AP complexes interact with Nef via a dileucine motif (ExxLL) in the Nef C-terminal loop. AP complexes bind dileucine motifs at an interface between the AP complex σ and heavy chain subunits (α, β, or γ in AP-2, AP-3, and AP-1 respectively) [30]. Nef utilizes the dileucine trafficking signal to down-modulate a number of host proteins, including CD4. Interestingly, Nef also interacts directly with clathrin adaptor proteins AP-1, AP-2, and AP-3 through a canonical dileucine trafficking signal in Nef's C-terminal loop domain (reviewed in [8]). By simultaneously binding to host protein cytoplasmic tails and clathrin adaptor proteins, Nef facilitates the down-modulation of a number of host proteins from the cell surface, including CD4 (Figure 1B) [9]. Down-modulation of CD4, the main cellular receptor for HIV entry, enables HIV-1 to avoid Env-CD4–mediated retention of virions at the cell surface and promotes efficient virus release and dissemination [10], [11].
HIV-1 Vif, Vpu, and Vpr Adapt Cellular Ubiquitin Ligase Adaptors to Counteract Host Antiviral Responses
Ubiquitination is a post-translational protein modification that regulates protein degradation and trafficking. Cellular E3 ubiquitin ligases facilitate the transfer of ubiquitin from E2 ubiquitin-conjugating enzymes to lysine, serine, or threonine residues on specific target proteins. E3 ligases often comprise multi-protein complexes that include a scaffold, an adaptor, and a target protein substrate. By serving as substrate adaptors that simultaneously interact with ubiquitin ligase adaptors and cellular target proteins, three HIV accessory proteins (Vif, Vpu, and Vpr) induce ubiquitination of host targets. This leads to proteasomal degradation and/or mislocalization of targeted host proteins. For example, viral infectivity factor (Vif), an accessory protein encoded by primate lentiviruses, including HIV-1, counteracts the antiviral activities of apolipoprotein B mRNA editing complex 3 (APOBEC3, or A3) proteins, especially APOBEC3G (A3G) [12]. A3 deaminases, which attack single-stranded DNA converting cytidine to uridine, have broad antiviral functions (reviewed in [13]). In the absence of Vif, A3G-mediated cytidine deamination results in uridination of the first strand of DNA synthesized by the viral reverse transcriptase. Guanosine-to-adenosine hypermutation results as uridine residues are paired with adenosine upon second strand synthesis. There is also evidence that A3G has a separate inhibitory effect on the processivity of reverse transcription (reviewed in [13]). In HIV-1-infected T cells, A3G activity can induce a DNA damage response that stimulates up-regulation of natural killer (NK) cell-activating ligands on the surface of the infected cells and activates NK cell lysis of infected cells [14]. To evade A3-mediated responses, the HIV-1 Vif protein simultaneously binds A3G and the ubiquitin ligase adaptor EloBC, causing polyubiquitination by the Rbx2/Cullin5 E3 ubiquitin ligase complex (Figure 2A) [15]. An additional cellular protein, core binding factor β (CBF-β), stabilizes the formation of this complex [16], [17]. By driving the ubiquitin-dependent degradation of A3 family members, Vif enables viral escape from A3-mediated antiviral restriction. The critical importance of A3G as a cellular factor that restricts lentiviruses is evidenced by coevolution of Vif and A3G sequences [18].
Fig. 2. Diagrammatic representations of HIV-1 Vif, Vpu, and Vpr functioning as adaptors of substrate adaptors in cellular ubiquitination pathways. 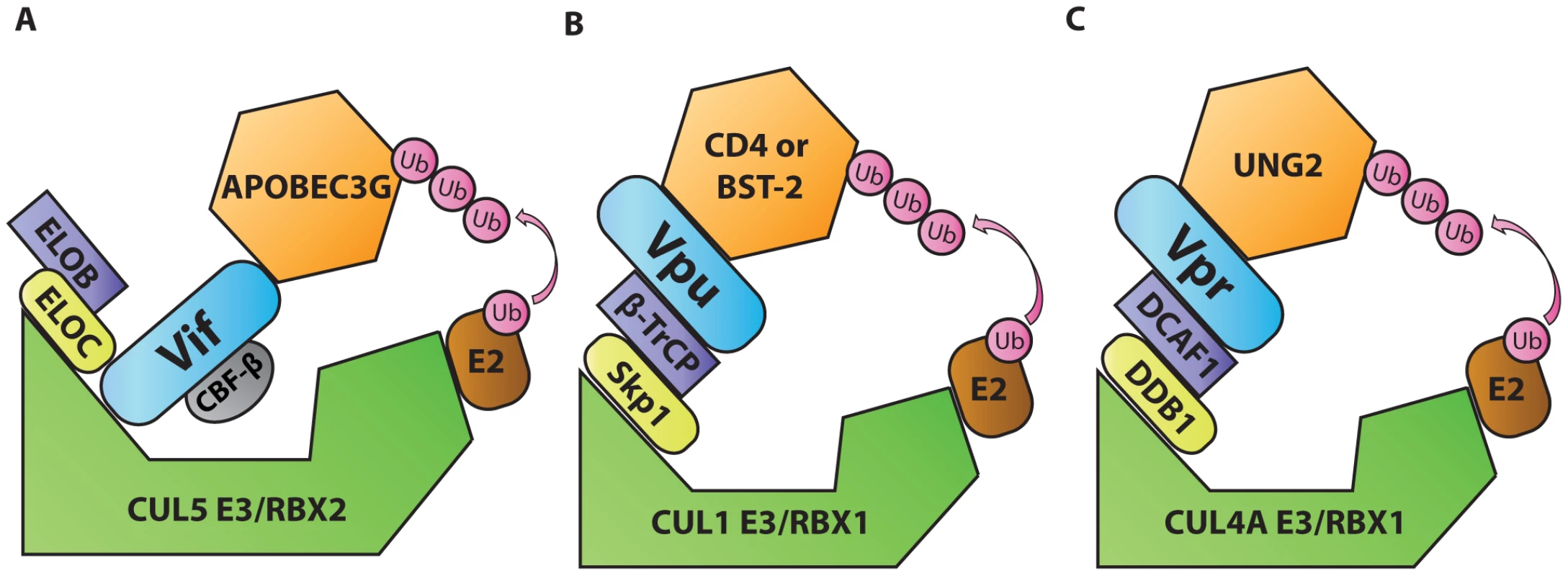
A. Vif, in complex with and stabilized by cellular CBF-β, binds to the EloBC/Rbx2/Cullin5 E3 ubiquitin ligase complex and to APOBEC3G to induce its polyubiquitination and degradation. B. Vpu interacts with the Skp1/Cullin/F-Box (SCF) ubiquitin ligase complex via β-TrCP and with target proteins BST-2 or CD4 to induce their ubiquitination and mislocalization. C. Vpr interacts with the DCAF1/DDB1/Rbx1/Cullin4A E3 ubiquitin ligase complex and with UNG2 to induce its polyubiquitination and degradation. Another example of this tactic is displayed by HIV-1 viral protein U (Vpu). This accessory protein promotes virus release by counteracting the antiviral activities of the interferon-induced restriction factor bone marrow stromal antigen 2 (BST-2/tetherin/CD317/HM1.24) [19], [20]. Vpu also down-modulates the HIV-1 receptor CD4 (reviewed in [21]). In the absence of Vpu, CD4 and BST-2 inhibit the release of infectious viral particles. CD4 binds virions through interactions with Env glycoproteins, and BST-2 tethers virions by virtue of its unusual structure. The general consensus of a number of studies is that BST-2 is attached to membranes via its transmembrane domain at its C-terminus and via its N-terminal glycophosphatidylinositol anchor. By simultaneously binding to viral and cellular membranes, BST-2 tethers virions, preventing their release. To evade BST-2, Vpu acts as an adaptor that promotes an interaction between a ubiquitin ligase substrate adaptor [beta transducing repeat-containing protein (β-TrCP)] and target proteins. In this way, Vpu promotes ubiquitination of the target protein by Skp1/Cullin1/F-box (SCF) ubiquitin ligase complex (Figure 2B) ([22] and reviewed in [21]). Recent studies have expanded the role of BST-2 to include viral sensing and signal transduction to activate NF-κB–dependent pro-inflammatory signals (reviewed in [23]). Like A3, the significance of BST-2–mediated restriction is illustrated by co-evolution of lentiviral genomes with species-specific variations in BST-2. In this regard lentiviruses have demonstrated remarkable flexibility. While HIV-1 utilizes Vpu to target BST-2, most primate lentiviruses use Nef for this purpose, and still others can use Env (reviewed in [21]). In this way, BST-2 variation appears to serve as a barrier to cross-species infection.
Viral protein R (Vpr) is a pleiotropic lentiviral accessory protein that has been shown to activate the DNA damage response, up-regulate NK activating ligands, cause cell-cycle arrest, and promote infection of macrophages (reviewed in [24]). Like Vif and Vpu, Vpr adapts a substrate adaptor of a cellular ubiquitin ligase complex [damaged DNA binding protein 1-cullin 4-associated factor 1 (DCAF1)], promoting ubiquitination by a ubiquitin ligase complex (Rbx1/Cullin4A E3, Figure 2C, reviewed in [24]). One protein targeted by Vpr is the cellular uracil DNA glycosylase 2 (UNG2) [25]. However, the precise role of UNG2 remains controversial as it has both positive and negative effects on HIV-1 replication (reviewed in [26]). Because the interaction with UNG2 does not appear to explain all of Vpr's activities, it is likely that Vpr targets additional cellular proteins that have not yet been identified. A structurally related accessory protein, viral protein X (Vpx), which is encoded by HIV-2 and some viruses of the simian immunodeficiency virus (SIV) family, also interacts with the DCAF1 to promote the degradation of a cellular nucleotide triphosphate phosphohydrolase [SAM domain and HD domain-containing protein 1 (SAMHD1)] [27]. In the absence of Vpx, SAMHD1 inhibits reverse transcription by depleting the intracellular pool of deoxynucleoside triphosphates [28]. Vpx-mediated polyubiquitination of SAMHD1 induces its proteasomal degradation and allows viral replication in myeloid cells [27]. Like the other pairs of viral accessory protein and cellular targets described thus far, Vpx has co-evolved with SAMHD1 from different primate species, acquiring the capacity to utilize different SAMHD1 molecular interfaces to promote its degradation [29].
Accessory Proteins as Tools for Identifying Important Antiviral Defense Mechanisms
The continual battle between host and virus provides constant selective pressure that shapes the viral genome. Research focused on the specific interactions that have evolved between lentiviral accessory proteins and their cellular targets has led to the identification and characterization of several antiviral factors (A3G, BST-2, and SAMHD1) and has informed our understanding of MHC-I trafficking pathways. Importantly, these host factors have broad and significant antiviral effects that can restrict a diverse array of viruses in addition to lentiviruses. The study of interactions between viral and host proteins is likely to continue to yield new information about important host defenses that may facilitate the development of improved treatments for a variety of human diseases.
Zdroje
1. CollinsK, ChenB, KalamsS, WalkerB, BaltimoreD (1998) HIV-1 Nef protein protects infected primary human cells from killing by cytotoxic T lymphocytes. Nature 391 : 397–401.
2. SchwartzO, MarechalV, Le GallS, LemonnierF, HeardJM (1996) Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2 : 338–342.
3. RoethJF, WilliamsM, KasperMR, FilzenTM, CollinsKL (2004) HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol 167 : 903–913.
4. Le GallS, ErdtmannL, BenichouS, Berlloz-TorrentC, LiuL, et al. (1998) Nef interacts with mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8 : 483–495.
5. WonderlichER, WilliamsM, CollinsKL (2008) The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J Biol Chem 283 : 3011–3022.
6. JiaX, SinghR, HomannS, YangH, GuatelliJ, et al. (2012) Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol 19 : 701–706.
7. KulpaDA, Del CidN, PetersonKA, CollinsKL (2013) Adaptor protein 1 promotes cross-presentation through the same tyrosine signal in major histocompatibility complex class I as that targeted by HIV-1. J Virol 87 : 8085–8098.
8. WonderlichER, LeonardJA, CollinsKL (2011) HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv Virus Res 80 : 103–127.
9. LeonardJA, FilzenT, CarterCC, SchaeferM, CollinsKL (2011) HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J Virol 85 : 6867–6881.
10. LamaJ, MangasarianA, TronoD (1999) Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef - and Vpu-inhibitable manner. Curr Biol 9 : 622–631.
11. RossTM, OranAE, CullenBR (1999) Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol 9 : 613–621.
12. SheehyAM, GaddisNC, ChoiJD, MalimMH (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418 : 646–650.
13. MalimMH (2009) APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci 364 : 675–687.
14. NormanJM, MashibaM, McNamaraLA, Onafuwa-NugaA, Chiari-FortE, et al. (2011) The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nature immunology 12 : 975–983.
15. YuX, YuY, LiuB, LuoK, KongW, et al. (2003) Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302 : 1056–1060.
16. ZhangW, DuJ, EvansSL, YuY, YuXF (2012) T-cell differentiation factor CBF-beta regulates HIV-1 Vif-mediated evasion of host restriction. Nature 481 : 376–379.
17. JagerS, KimDY, HultquistJF, ShindoK, LaRueRS, et al. (2012) Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection. Nature 481 : 371–375.
18. ComptonAA, HirschVM, EmermanM (2012) The host restriction factor APOBEC3G and retroviral Vif protein coevolve due to ongoing genetic conflict. Cell Host Microbe 11 : 91–98.
19. NeilSJ, ZangT, BieniaszPD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451 : 425–430.
20. Van DammeN, GoffD, KatsuraC, JorgensonRL, MitchellR, et al. (2008) The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3 : 245–252.
21. HarrisRS, HultquistJF, EvansDT (2012) The restriction factors of human immunodeficiency virus. J Biol Chem 287 : 40875–40883.
22. MargottinF, BourSP, DurandH, SeligL, BenichouS, et al. (1998) A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell 1 : 565–574.
23. HotterD, SauterD, KirchhoffF (2013) Emerging Role of the Host Restriction Factor Tetherin in Viral Immune Sensing. J Mol Biol 425 : 4956–4964.
24. RomaniB, CohenEA (2012) Lentivirus Vpr and Vpx accessory proteins usurp the cullin4-DDB1 (DCAF1) E3 ubiquitin ligase. Curr Opin Virol 2 : 755–763.
25. SchrofelbauerB, YuQ, ZeitlinSG, LandauNR (2005) Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol 79 : 10978–10987.
26. MashibaM, CollinsKL (2013) Molecular mechanisms of HIV immune evasion of the innate immune response in myeloid cells. Viruses 5 : 1–14.
27. LaguetteN, SobhianB, CasartelliN, RingeardM, Chable-BessiaC, et al. (2011) SAMHD1 is the dendritic - and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474 : 654–657.
28. LahouassaH, DaddachaW, HofmannH, AyindeD, LogueEC, et al. (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13 : 223–228.
29. FregosoOI, AhnJ, WangC, MehrensJ, SkowronskiJ, et al. (2013) Evolutionary toggling of Vpx/Vpr specificity results in divergent recognition of the restriction factor SAMHD1. PLoS Pathog 9: e1003496 doi:10.1371/journal.ppat.1003496
30. KellyBT, McCoyAJ, SpateK, MillerSE, EvansPR, et al. (2008) A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456 : 976–979.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



