-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEffectors and Effector Delivery in
article has not abstract
Published in the journal: . PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003826
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003826Summary
article has not abstract
Rice blast caused by Magnaporthe oryzae is one of the most destructive fungal diseases of rice and a model for studying fungal-plant interactions. The fungus penetrates plant cells with appressoria and develops the narrow primary invasive hyphae (IH) and, subsequently, the bulbous secondary IH. As a hemibiotrophic pathogen, biotrophic IH are enclosed in the extra-invasive-hyphal membrane (EIHM) produced by the plant cells [1]. Like many other fungal pathogens [2], M. oryzae secretes effector proteins to manipulate plant immunity and physiology to promote infection.
AVR Effectors
Avr proteins, a special class of effectors encoded by the avirulence (AVR) genes, could be recognized by corresponding R proteins and lead to the race-specific recognition [3]. In the rice–M. oryzae interaction, over 40 AVR genes have been identified. Among the AVR genes that have been cloned, all but ACE1 encode secreted proteins expressed in IH. ACE1 is specifically expressed in appressoria, and it encodes an intracellular hybrid PKS-NRPS protein [4]. Avr-Piz-t and Avr-Pita are the other two Avr proteins with known biochemical functions. Avr-Piz-t functions to suppress pathogen-associated molecular pattern (PAMP)-triggered immunity by inhibiting the ubiquitin ligase activity of the rice RING E3 ubiquitin ligase APIP6 [5]. AVR-Pita encodes a putative neutral zinc metalloprotease [6] and it belongs to a gene family with at least two additional members [7].
PWL1, PWL2, PWL3, and PWL4 are members of another AVR gene family [8]. Pwl effectors are small, glycine-rich proteins that are present in rice pathogens and function as avirulence proteins in infection of weeping lovegrass and finger millet. In contrast, AVR1-CO39 was cloned from a weeping lovegrass isolate. Its specific expression in IH triggers hypersensitive response (HR) and resistance in cultivars carrying the Pi-CO39 R gene [9]. Avr-Pia, Avr-Pik/km/kp, and Avr-Pii were identified in the same re-sequencing study of strain Ina168 [10]. Avr-Pia directly interacts with Rga5-A, which also interacts with Avr-CO39 [11].
Non-AVR Effectors
The best characterized non-Avr effector in M. oryzae is Slp1, a secreted LysM protein that is accumulated at the interface between IH and EIHM [12]. Slp1 is dispensable for appressorium penetration but required for invasive growth in planta. It competes with the chitin elicitor binding protein CEBiP for binding to chitin oligosaccharides. Thus, Slp1 functions to suppress chitin-induced plant immune responses, including generation of reactive oxygen species and expression of defense-related genes [12].
Using an expression profiling approach, a number of low molecular weight secreted proteins specifically expressed or highly induced in biotrophic invasive hyphae were identified, including 58 candidate effectors that were up-regulated over 10-fold during plant infection. Four of them, BAS1–BAS4, were confirmed to be fungal biotrophy-associated secreted (BAS) proteins [13]. MC69 was identified by systematic disruption of in planta expressed secreted protein genes [14]. The 54-aa Mc69 protein was essential for IH development, although it was not translocated into rice cytoplasm. In a separate study, five secreted protein genes named MoCDIP1–5 were found to induce plant cell death in a transient expression assay with rice protoplasts. Four of them also induced cell death in Nicotiana benthamiana [15].
Localization of Fungal Effectors in Plant Cells
M. oryzae effectors can be divided into two distinct types based on their localization in plant cells (Figure 1) [16]. Cytoplasmic effectors, including Avr-Pita, Pwl1, Pwl2, Bas2, and Avr-Piz-T, are preferentially accumulated in the biotrophic interfacial complex (BIC) before being delivered into plant cells. The BIC is a distinct plant-derived, membrane-rich structure developed at the tip of primary IH by M. oryzae. In each newly invaded rice cell, effectors are first secreted into BICs before delivery. The BICs are persistent and left behind when the primary IH differentiates into the secondary IH. In addition, the fungus continues to secrete effectors into BICs even after IH have grown extensively as pseudohyphae and invaded neighboring plant cells [17].
Fig. 1. Localization of M. oryzae effectors during plant infection. 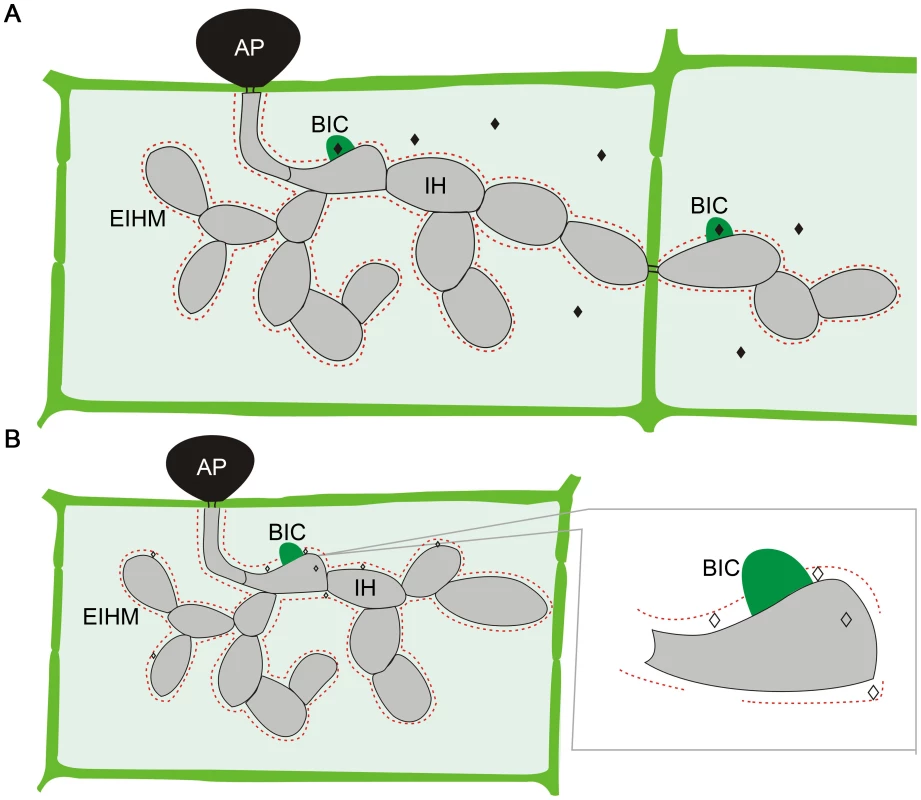
A. Cytoplasmic effectors (♦) are secreted into the biotrophic interfacial complex (BIC) before being translocated into plant cells. B. Apoplastic effectors (◊) are secreted into the space between the fungal cell wall and extra-invasive-hyphal membrane (EIHM). Like IH, effector proteins may move from cell to cell via plasmodesmata. AP, appressorium. On the other hand, apoplastic effectors such as Bas4, Avr1-CO39, and Slp1 are not associated with the BIC. After secretion, they are dispersed in the extracellular space between the fungal cell wall and EIHM. In the rice cells colonized by transformants expressing the Bas4-GFP fusion protein, GFP signals appeared to outline the IH [13], consistent with its extracellular localization. To date, it is not clear how the apoplastic effectors are recognized by surface receptors or translocated into plant cells to interact with their intracellular targets. Furthermore, no specific protein motifs or sequences have been identified in the cytoplasmic or apoplastic effectors that are responsible for their localization in plant cells after being secreted. Therefore, it is impossible to predict whether an effector is apoplastic or cytoplasmic solely based on its amino acid sequence.
Effector Secretion Systems
Consistent with two types of effectors, two distinct effector secretion systems have been identified in M. oryzae (Figure 2) [16]. Cytoplasmic effectors are delivered into plant cells via the BIC, which is independent of the Golgi-dependent secretory system. Instead, secretion into the BIC is associated with a novel form of secretion involving the exocyst complex and t-SNAREs. Targeted deletions of the exocyst components SEC5 and EXO70 resulted in impaired secretion of cytoplasmic effectors and pathogenicity defects but had no effect on the secretion of apoplastic effectors [16]. Mutants deleted of the t-SNARE component SSO1 were defective in BIC development and pathogenesis [16].
Fig. 2. Two effector secretion mechanisms identified in M. oryzae. 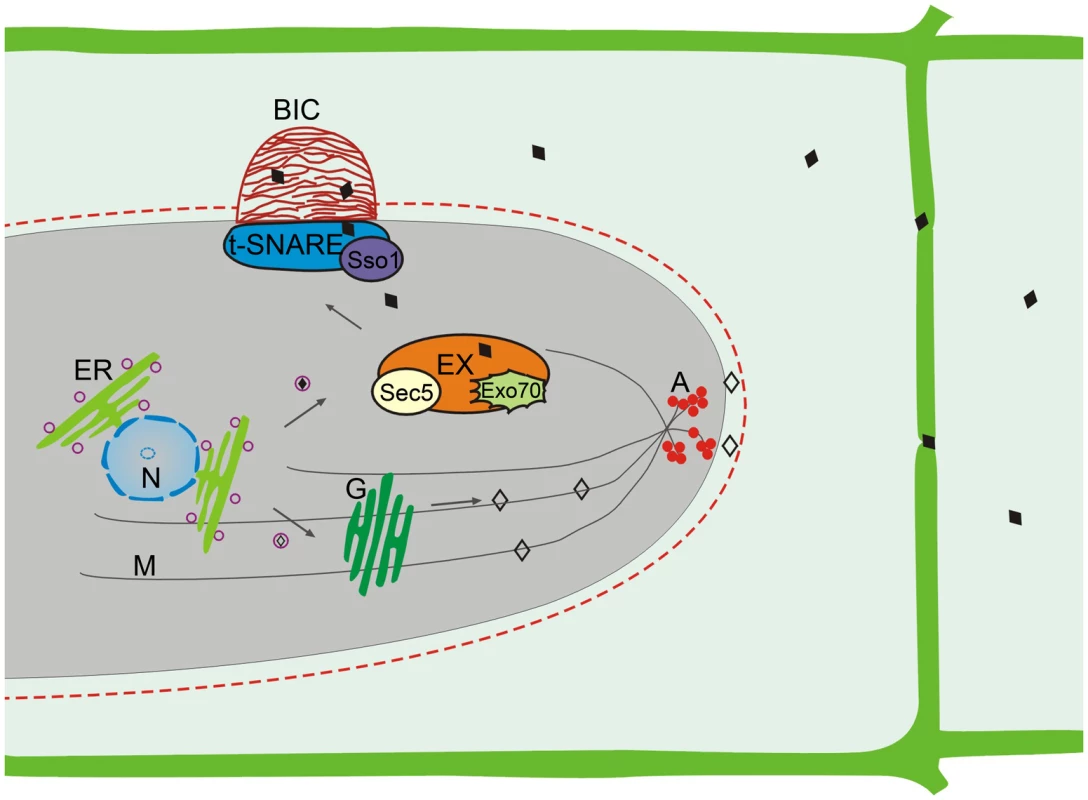
The apoplastic effectors (◊) utilize the conventional ER-Golgi secretion pathway for secretion. Disruption of the cytoskeleton by benzimidazoleand LatA treatments interferes with the secretion of apoplastic effectors. In contract, the secretion of cytoplasmic effectors (♦) into the BIC is independent of the Golgi bodies and cytoskeleton but involves the exocyst complex and t-SNAREs. A, cortical actin; ER, endoplasmic reticulum; EX, exocyst complex; G, Golgi bodies; M, microtubules; N, Nucleus. The apoplastic effectors are secreted from IH by the conserved ER to Golgi secretory pathway that is independent of the BIC. Treatment with Brefeldin A that interferes with Golgi-dependent secretion inhibited the secretion of apoplastic effectors such as Bas4 and Slp1 but had no effect on the localization of cytoplasmic effectors Pwl2, Bas1, and Bas107 to the BIC [16]. Furthermore, actin and microtubules essential for vesicle trafficking are required only for the secretion of apoplastic effectors but not for cytoplasmic effectors. Many genes involved in the ER to Golgi secretory pathway and post-translational modifications also are likely important for the secretion of apoplastic effectors in M. oryzae. For example, the LHS1 ER chaperone gene is required for the translocation and secretion of apoplastic effectors such as Slp1 [18].
Translocation of Effectors into Plant Cells
Several cytoplasmic effectors, including Pwl2, Bas1, and Avr-Piz-t, are translocated into the cytoplasm of rice cells [5], [13]. Whereas some apoplastic effectors may be recognized by host plant surface receptors, the others that have intracellular targets, such as Avr1-CO39, also enter plant cells. In rice leaf sheath cells penetrated by fungal transformants expressing the Pwl2 and Avr-Piz-t proteins tagged with NLS and mRFP sequences, red fluorescence was observed in the nucleus of plant cells [17]. However, the underlying molecular mechanism responsible for effector translocation into plant cells is not clear. In experiments with purified recombinant proteins, the native Avr1-CO39 protein was translocated into rice protoplasts, indicating that it can enter plant cells independent of fungal factors [19]. In Oomycete pathogens such as Phytophthora species, the RXLR sequence is important for the translocation of effector proteins into plant cells [20]. Nevertheless, there is no evidence for the presence of functional RXLR sequences in M. oryzae effector proteins.
Movement of Effectors in Plant Cells
In the rice leaf sheath cells penetrated by M. oryzae, the cytoplasmic effectors Pwl2 and Bas1, but not apoplastic effector Bas4, were moved to neighboring cells ahead of the invasive hyphae [17]. Red fluorescence was observed in the nucleus of rice leaf sheath cells penetrated by a transformant expressing the PWL2-mCherry-NLS fusion construct and a number of surrounding cells without IH. Fungal effectors entering un-colonized plant cells may be able to suppress host defense responses or elicit susceptibility. In fact, it takes a shorter time (2 h) for IH to move through the subsequently invaded cells than in the first colonized cells (12 h) [17].
Interestingly, movement of cytoplasmic effectors from vein-associated cells into neighboring cells was rare and of low efficiency in comparison with effector movement among regular epidermal cells [17], suggesting that effector trafficking depends on rice cell types. In addition, unlike the 39.3 kD Pwl2-mCherry fusion, the 68.3 kD Pwl2-tdTomato protein was defective in the movement from the penetrated cells to surrounding cells [17]. Therefore, cell-to-cell effector translocation also depends on the protein size. These results indicate that Magnaporthe effectors may be moved symplastically through plasmodesmata [17]. The movement of effector proteins and invasion of neighboring cells by IH may be co-regulated because IH of M. oryzae preferentially contacted and crossed the plant cell wall at the pit fields [1].
Although more and more fungal effectors are being discovered, our understanding of effector delivery and cell-to-cell movement in planta is relatively limited in comparison with that of bacterial effectors. Studies in M. oryzae showed that some effectors may be secreted via an unconventional protein secretion system to the BIC [16]. The BIC-like structures may also exist in other plant pathogenic fungi, particularly other hemibiotrophic pathogens. To date, no common plant entry sequence has been identified in fungal effectors, indicating that fungi may utilize a variety of mechanisms for effector translocation. M. oryzae and other fungal pathogens may have conserved mechanisms to recognize plasmodesmata for the movement of effector proteins and spreading of IH.
Zdroje
1. KankanalaP, CzymmekK, ValentB (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19 : 706–724.
2. DjameiA, KahmannR (2012) Ustilago maydis: Dissecting the molecular interface between pathogen and plant. PLoS Pathog 8: e1002955 doi:10.1371/journal.ppat.1002955
3. de WitP, MehrabiR, van den BurgHA, StergiopoulosI (2009) Fungal effector proteins: past, present and future. Mol Plant Pathol 10 : 735–747.
4. BohnertHU, FudalI, DiohW, TharreauD, NotteghemJL, et al. (2004) A putative polyketide synthase peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell 16 : 2499–2513.
5. ParkC, ChenS, ShirsekarG, ZhouB, KhangC, et al. (2012) The Magnaporthe oryzae effector Avrpiz-t targets the ring E3 ubiquitin ligase apip6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 24 : 4748–4762.
6. JiaY, McAdamsSA, BryanGT, HersheyHP, ValentB (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19 : 4004–4014.
7. KhangCH, ParkS-Y, LeeY-H, ValentB, KangS (2008) Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol Plant Microbe Interact 21 : 658–670.
8. KangSC, SweigardJA, ValentB (1995) The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant Microbe Interact 8 : 939–948.
9. PeyyalaR, FarmanML (2006) Magnaporthe oryzae isolates causing gray leaf spot of perennial ryegrass possess a functional copy of the AVR1-CO39 avirulence gene. Mol Plant Pathol 7 : 157–165.
10. YoshidaK, SaitohH, FujisawaS, KanzakiH, MatsumuraH, et al. (2009) Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 21 : 1573–1591.
11. CesariS, ThilliezG, RibotC, ChalvonV, MichelC, et al. (2013) The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors Avr-Pia and Avr1-CO39 by direct binding. Plant cell 25 : 1463–1481.
12. MentlakTA, KombrinkA, ShinyaT, RyderLS, OtomoI, et al. (2012) Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell 24 : 322–335.
13. MosqueraG, GiraldoMC, KhangCH, CoughlanS, ValentB (2009) Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. Plant Cell 21 : 1273–1290.
14. SaitohH, FujisawaS, MitsuokaC, ItoA, HirabuchiA, et al. (2012) Large-scale gene disruption in Magnaporthe oryzae identifies mc69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog 8: e1002711 doi:10.1371/journal.ppat.1002711
15. ChenS, SongkumarnP, VenuRC, GowdaM, BellizziM, et al. (2013) Identification and characterization of in planta-expressed secreted effector proteins from Magnaporthe oryzae that induce cell death in rice. Mol Plant Microbe Interact 26 : 191–202.
16. GiraldoMC, DagdasYF, GuptaYK, MentlakTA, YiM, et al. (2013) Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat Commun 4 : 1996.
17. KhangCH, BerruyerR, GiraldoMC, KankanalaP, ParkS-Y, et al. (2010) Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. The Plant Cell Online 22 : 1388–1403.
18. MentlakTA, TalbotNJ, KrojT (2011) Effector translocation and delivery by the rice blast fungus Magnaporthe oryzae. Effectors in Plant-Microbe Interactions 219–241.
19. RibotC, CesariS, AbidiI, ChalvonV, BournaudC, et al. (2013) The Magnaporthe oryzae effector AVR1CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J 74 : 1–12.
20. JiangRH, TripathyS, GoversF, TylerBM (2008) RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc Natl Acad Sci USA 105 : 4874–4879.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



