-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInfection of Induces Antifungal Immune Defenses
Candida albicans yeast cells are found in the intestine of most humans, yet this opportunist can invade host tissues and cause life-threatening infections in susceptible individuals. To better understand the host factors that underlie susceptibility to candidiasis, we developed a new model to study antifungal innate immunity. We demonstrate that the yeast form of C. albicans establishes an intestinal infection in Caenorhabditis elegans, whereas heat-killed yeast are avirulent. Genome-wide, transcription-profiling analysis of C. elegans infected with C. albicans yeast showed that exposure to C. albicans stimulated a rapid host response involving 313 genes (124 upregulated and 189 downregulated, ∼1.6% of the genome) many of which encode antimicrobial, secreted or detoxification proteins. Interestingly, the host genes affected by C. albicans exposure overlapped only to a small extent with the distinct transcriptional responses to the pathogenic bacteria Pseudomonas aeruginosa or Staphylococcus aureus, indicating that there is a high degree of immune specificity toward different bacterial species and C. albicans. Furthermore, genes induced by P. aeruginosa and S. aureus were strongly over-represented among the genes downregulated during C. albicans infection, suggesting that in response to fungal pathogens, nematodes selectively repress the transcription of antibacterial immune effectors. A similar phenomenon is well known in the plant immune response, but has not been described previously in metazoans. Finally, 56% of the genes induced by live C. albicans were also upregulated by heat-killed yeast. These data suggest that a large part of the transcriptional response to C. albicans is mediated through “pattern recognition,” an ancient immune surveillance mechanism able to detect conserved microbial molecules (so-called pathogen-associated molecular patterns or PAMPs). This study provides new information on the evolution and regulation of the innate immune response to divergent pathogens and demonstrates that nematodes selectively mount specific antifungal defenses at the expense of antibacterial responses.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002074
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002074Summary
Candida albicans yeast cells are found in the intestine of most humans, yet this opportunist can invade host tissues and cause life-threatening infections in susceptible individuals. To better understand the host factors that underlie susceptibility to candidiasis, we developed a new model to study antifungal innate immunity. We demonstrate that the yeast form of C. albicans establishes an intestinal infection in Caenorhabditis elegans, whereas heat-killed yeast are avirulent. Genome-wide, transcription-profiling analysis of C. elegans infected with C. albicans yeast showed that exposure to C. albicans stimulated a rapid host response involving 313 genes (124 upregulated and 189 downregulated, ∼1.6% of the genome) many of which encode antimicrobial, secreted or detoxification proteins. Interestingly, the host genes affected by C. albicans exposure overlapped only to a small extent with the distinct transcriptional responses to the pathogenic bacteria Pseudomonas aeruginosa or Staphylococcus aureus, indicating that there is a high degree of immune specificity toward different bacterial species and C. albicans. Furthermore, genes induced by P. aeruginosa and S. aureus were strongly over-represented among the genes downregulated during C. albicans infection, suggesting that in response to fungal pathogens, nematodes selectively repress the transcription of antibacterial immune effectors. A similar phenomenon is well known in the plant immune response, but has not been described previously in metazoans. Finally, 56% of the genes induced by live C. albicans were also upregulated by heat-killed yeast. These data suggest that a large part of the transcriptional response to C. albicans is mediated through “pattern recognition,” an ancient immune surveillance mechanism able to detect conserved microbial molecules (so-called pathogen-associated molecular patterns or PAMPs). This study provides new information on the evolution and regulation of the innate immune response to divergent pathogens and demonstrates that nematodes selectively mount specific antifungal defenses at the expense of antibacterial responses.
Introduction
Candida albicans is a remarkably successful and versatile human pathogen that is found on the skin and mucosal surfaces of virtually all humans. Under most circumstances, C. albicans is a harmless commensal [1]. However, this opportunist can invade host tissues and cause life-threatening infections when the immune system is weakened (e.g. from critical illness) and competing bacterial flora are eliminated (e.g. from broad-spectrum antibiotic use). Accordingly, invasive candidiasis is particularly common in intensive care units where mortality rates reach 45–49% [2]–[4]. Antecedent colonization of mucosal surfaces with C. albicans can also lead to debilitating superficial infections in otherwise normal hosts. Approximately 75% of all women, for example, will have one episode of Candida vaginitis in their lifetime, with half having at least one recurrence [5].
C. albicans can grow vegetatively as yeast or hyphae, and each form contributes to pathogenesis [6]–[8]. C. albicans yeast cells colonize mucosal surfaces and facilitate dissemination of the organism through the blood stream [9]–[11]. Hyphae, by contrast, are important for host invasion and tissue destruction [1], [8], [11], [12]. The factors that influence these diverse growth patterns during infection are poorly understood, but it is clear that innate immune mechanisms in mammalian epithelial cells normally prevent C. albicans from becoming a pathogen [13]–[15]. Recently, genetic analyses of two human families whose members suffered from recurrent or chronic candidiasis on mucosal surfaces identified causative mutations in the innate immune regulators dectin-1 [16] and CARD9 [17]. Dectin-1 is a pattern-recognition receptor important for macrophage phagocytosis of fungi. Interestingly, this protein interacts differently with the C. albicans growth forms. Cell wall components exposed in the bud scar of C. albicans yeast (so-called pathogen-associated molecular patterns or PAMPs) potently stimulate dectin-1, but hyphae are relatively shielded from innate immune detection, which likely contributes to the ability of C. albicans to establish infection [13], [15], [18]. Furthermore, a recent study found that the p38 MAP kinase, a central regulator of mammalian immunity, receives biphasic inputs from C. albicans that are dependent on the morphologic form of the organism and the local fungal burden [14]. These data suggest that the interplay between C. albicans and the mammalian innate immune system dictate the virulence potential of this specialized pathogen, yet relatively little is known about the molecular mechanisms underlying these interactions.
One approach to study evolutionarily conserved aspects of epithelial innate immunity and microbial virulence uses the invertebrate host Caenorhabditis elegans [19], [20]. In nature, nematodes encounter numerous threats from ingested pathogens, which have provided a strong selection pressure to evolve and maintain a sophisticated innate immune system in its intestinal epithelium [21]. Coordination of these defenses involves several highly-conserved elements that have mammalian orthologs [22]–[25]. Furthermore, C. elegans intestinal epithelial cells bear a striking resemblance to human intestinal cells [26] and because the nematode lacks both a circulatory system and cells dedicated to the immune response, the intestinal epithelium constitutes the primary line of defense for the nematode against ingested pathogens. Thus, it is possible to conduct analyses of innate immune mechanisms in a physiologically-relevant, genetically-tractable system.
Much of the characterization of nematode immunity has used nosocomial bacterial pathogens [27]–[30], particularly Pseudomonas aeruginosa [22], [31], [32], but to date, the immune response directed toward a medically-important, fungal pathogen has not been defined. Here, we extend our previously-validated system for the study of hyphal-mediated C. albicans virulence in the nematode [33] to examine C. albicans yeast. Our goal was to use studies of C. elegans-C. albicans interactions to identify novel, conserved features of metazoan innate immunity. We found that the responses to bacterial and fungal pathogens are remarkably distinct. Many of the immune response effectors that are upregulated by either P. aeruginosa or S. aureus are downregulated by infection with C. albicans yeast. We also found that slightly more than half of the immune response genes activated by infection with live C. albicans are also upregulated by heat-killed C. albicans. Our data indicate that the C. elegans immune response to C. albicans most likely involves detection of conserved surface-associated molecular pattern molecules, as well as detection of C. albicans virulence-related factors.
Results
The Yeast Form of C. albicans is Pathogenic to C. elegans
To examine interactions between C. albicans and the innate immune system, we established a novel system using the model host C. elegans. In a previous study, we found that C. albicans hyphae can kill C. elegans in a manner that models key aspects of mammalian pathogenesis [20], [33]. In that assay, yeast cells were ingested by nematodes on solid medium and, after transfer to liquid medium, worms died with true hyphae piercing through their bodies. During these experiments, we noted that when infected worms were maintained on solid media, rather than transferred to liquid media, the C. albicans yeast form caused pathogenic distention of the nematode intestine and premature death of the worms. Thus, we hypothesized that C. albicans yeast, the form commonly found in the mammalian intestine [13], [15], [18], also contain virulence determinants that allow infection of C. elegans. We therefore developed an assay that is conducted exclusively on solid media and allows the direct study of yeast-mediated pathogenesis of the nematode. As shown in Figure 1, the yeast form of the C. albicans laboratory reference strain DAY185 infected and killed C. elegans. Heat-killed C. albicans yeast cells were not pathogenic to the nematode (Figure 1A) and caused less distention of the nematode intestine compared to that seen following exposure to live C. albicans (Figure 1B). We found that the C. albicans clinical isolate SC5314 was also able to establish a lethal infection in nematodes (Figure 2). Furthermore, the C. albicans efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant strain [8], which is attenuated for virulence in mammals, was also unable to efficiently kill C. elegans in this assay (Figure 2). Like its isogenic wild-type parent strain, virulence-attenuated C. albicans yeast enter the nematode intestine during the infection assay (data not shown), suggesting that non-specific occlusion of the intestine with yeast is not the mechanism of C. albicans-mediated worm killing. In addition, we found that C. albicans killed sterile C. elegans fer-15(b26);fem-1(hc17) animals (data not shown) and wild-type worms in the presence of 5-fluoro-2′-deoxyuridine (FUDR), a compound that prevents progeny from hatching (Figure 1A). These results suggest that killing of nematodes by C. albicans yeast in the C. elegans model involves virulence determinants intrinsic to live fungi and not a “matricidal effect” from premature hatching of embryos inside animals, a previously described, non-specific consequence of pathogen stress in wild-type worms [26], [31], [32], [34]. In summary, these data demonstrate that C. albicans yeast are pathogenic to the nematode and establish a second assay, which together with the liquid-media system [33], permit separate in vivo analyses of C. albicans growth states.
Fig. 1. C. albicans yeast can kill C. elegans. 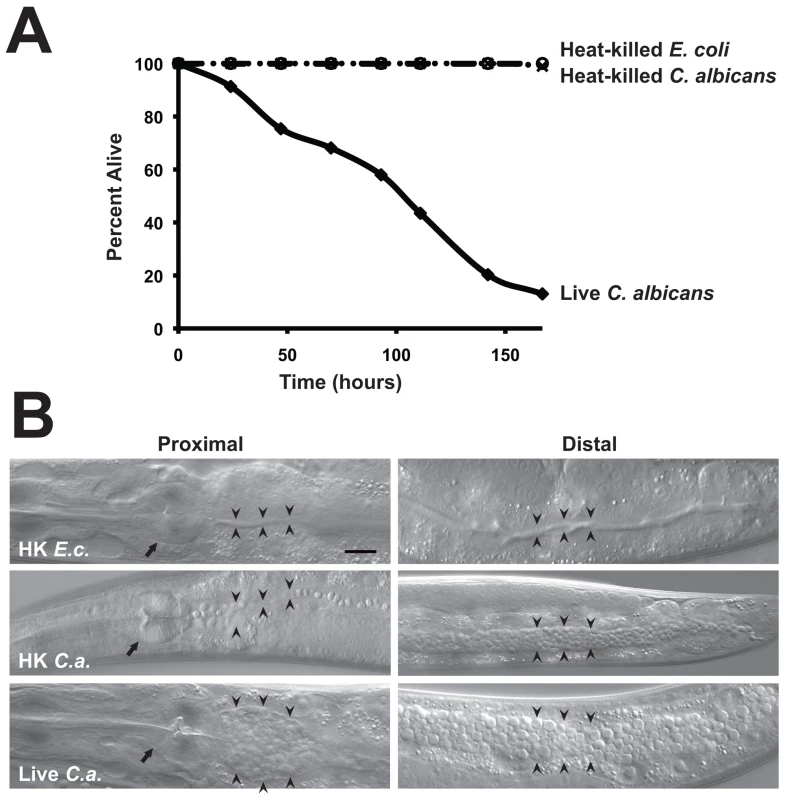
(A) Live C. albicans (closed diamonds) were pathogenic to nematodes on solid media, whereas heat-killed C. albicans (open circles) and E. coli (crosses) were not (P<0.001). The graph presents the average of three plates per strain, each with 30 to 40 animals per plate. Data are representative of two biological replicates. (B) Images of C. elegans animals exposed to heat-killed E. coli (HK E.c.), heat-killed C. albicans (HK C.a.) or live C. albicans (live C.a.) for 16 hours at 25°C are shown. Images of the proximal (left) and distal (right) intestine were obtained using Nomarski optics. Both live and heat-killed C. albicans accumulated within the intestine, but only live C. albicans caused marked distention of the proximal intestine. Arrows point to the pharyngeal grinder and arrowheads outline the lumen of the intestine. The scale bar represents 20 µm. Fig. 2. A C. albicans double mutant strain that is attenuated for pathogenicity in mammals is also unable to efficiently kill C. elegans. 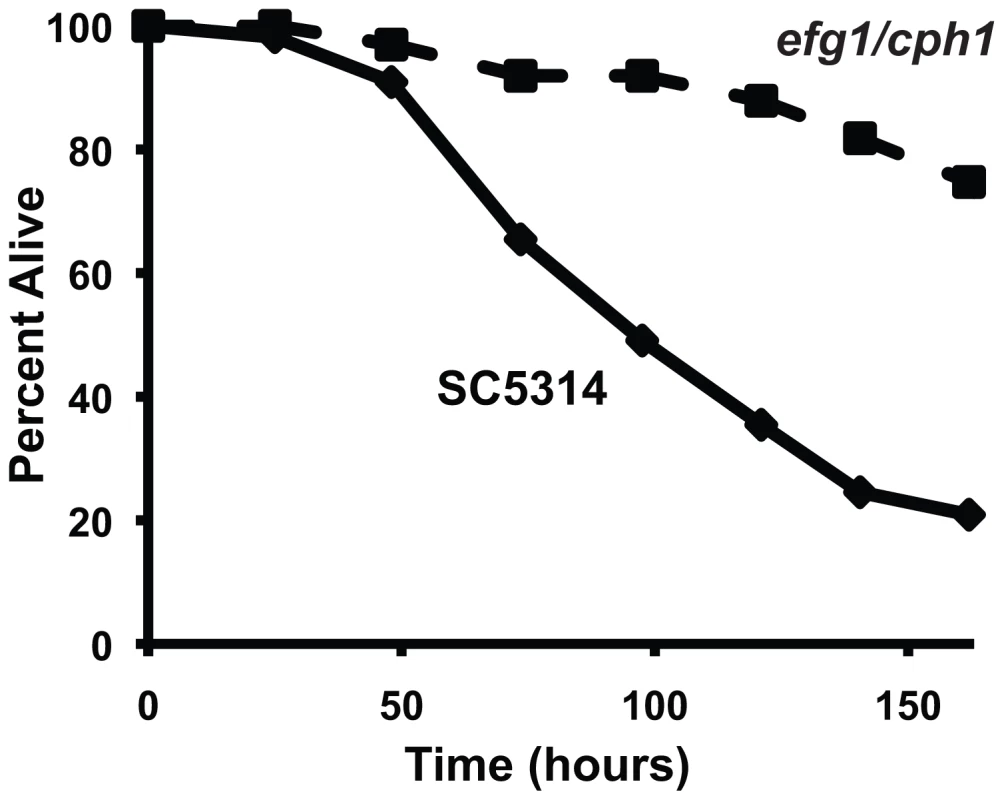
The C. albicans efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant strain (efg1/cph1) exhibited a reduced ability to kill C. elegans compared to its isogenic wild-type parent strain SC5314 (P<0.001). The graph presents the average of three plates per strain, each with 30 to 40 animals per plate. Data are representative of two biological replicates. C. albicans Infection Induces a Rapid Host Response that Involves Antimicrobial, Secreted and Detoxification Genes
Previous studies have shown that C. elegans mounts a rapid and specific immune response toward pathogenic bacteria [32], [35], [36]; however, it is not known how the nematode defends itself against an intestinal fungal pathogen. We therefore used transcriptome profiles of nematodes during an infection with C. albicans yeast to define the antifungal immune response genes in the nematode. We compared gene expression of animals exposed to C. albicans for four hours with control worms fed the non-pathogenic food source, heat-killed E. coli OP50. The short exposure time maximized the yield for transcriptional changes associated with pathogen detection, rather than gene expression changes associated with intestinal damage [36]. It was necessary to use heat-killed E. coli for these experiments because live E. coli were previously shown to be pathogenic to the nematode on C. albicans growth media (brain heart infusion agar) [37]. We found that C. elegans coordinates a rapid and robust transcriptional response to C. albicans that involves approximately 1.6% of the nematode genome (Figure 3). 124 genes were upregulated two-fold or greater in response to C. albicans compared to heat-killed E. coli and 189 genes were downregulated at least two-fold (P<0.01) (Figure 3A and Table S1A). For technical confirmation of the microarray experiment, we selected 11 genes that showed varying degrees of differential regulation and tested their expression by quantitative real-time polymerase chain reaction (qRT-PCR) under each microarray condition (Figure 3B and Table S2). Plotting the fold difference observed in the transcriptome profiles versus the value obtained by qRT-PCR from the three biological replicates used for the microarray analysis yielded an R2 of 0.90 (Figure 3B), which indicates tight correlation between these datasets and is a result that compares favorably with similar analyses of other microarray experiments [38]. We also tested three additional biological replicates and found similar fold changes between the microarray and qRT-PCR analyses in 10 of the 11 genes (Table S2), a correlation rate that is consistent with other microarray analyses of pathogen response genes in the nematode [34]. As a third means to confirm the results of our microarray, we compared the expression of 4 upregulated and 4 downregulated genes in wild-type C. elegans animals infected with a different C. albicans strain than used for the microarray analysis. We exposed animals to the C. albicans clinical isolate SC5314, a strain that is also virulent toward C. elegans (Figure 2), and found similar transcriptional changes between C. albicans SC5314 and DAY185-exposed animals for all 8 genes tested (Table S2). These data suggest that the C. albicans-induced transcriptional changes observed in our microarray analysis are not specific to a particular yeast strain.
Fig. 3. Infection with C. albicans yeast induces a rapid host response. 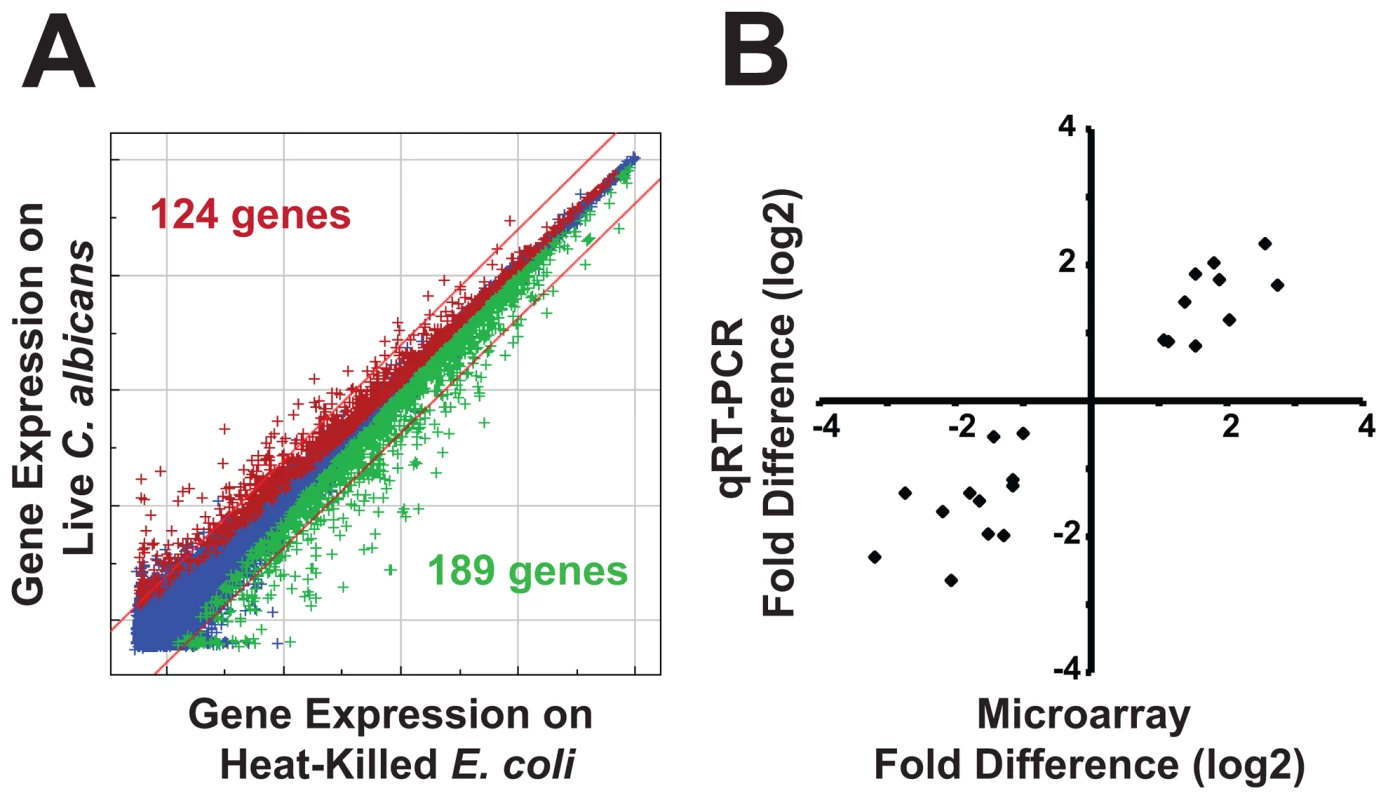
(A) C. elegans genes that were differentially regulated in C. albicans-exposed versus heat-killed E. coli-exposed young adult animals at 4 hours after infection are depicted on a genome-wide intensity plot of 22,548 sequences. Genes colored red were upregulated by C. albicans (P<0.01), those colored green were downregulated (P<0.01) and those colored blue were unchanged. Diagonal lines represent 2-fold change and the numbers of genes differentially regulated greater than 2-fold are indicated (P<0.01)(124 genes were upregulated and 189 genes were downregulated). (B) qRT-PCR was used to confirm the results of the microarray analysis. 11 genes with varying degrees of differential regulation were selected and studied under each condition in which they were differentially regulated in the microarray analysis (see Table S2 for gene identities). Correlation of microarray and qRT-PCR data was determined by plotting the average fold difference observed in the microarray analysis (three biological replicates) versus the average fold difference for the same gene obtained by qRT-PCR (three biological replicates). Linear regression analysis revealed strong correlation between the datasets (R2 of 0.90). Examination of the genes induced by C. albicans in the microarray analysis reveals the footprint of an immune response toward a pathogenic fungus (Table 1). C. albicans infection results in the elaboration of at least seven putative antimicrobial peptides, which are postulated to have antifungal activity in vivo. One of these genes, abf-2, was previously shown to have in vitro activity against the pathogenic fungus Candida krusei [39]. Three genes in this group (fipr-22/23 and two caenacin genes, cnc-4 and cnc-7) are antifungal immune effectors induced by the nematode following exposure to Drechmeria coniospora, an environmental fungal pathogen, which causes a localized infection of the nematode cuticle [40], [41]. fipr-22 and fipr-23 have nearly identical DNA sequences and thus, it is not possible for a probe set to distinguish between these genes. Two chitinase genes (cht-1 and T19H5.1) were also strongly induced by C. albicans. These enzymes are secreted by metazoans and are thought to defend against chitin-containing microorganisms such as C. albicans and other pathogenic fungi [42], [43]. In addition, thn-1, a gene that is postulated to have direct antimicrobial activity and is a homolog of the thaumatin family of plant antifungals [35], [44], was induced 2.5-fold during infection with C. albicans.
Tab. 1. The C. elegans transcriptional response to C. albicans infection involves antimicrobial, detoxification and other pathogen-response genes. 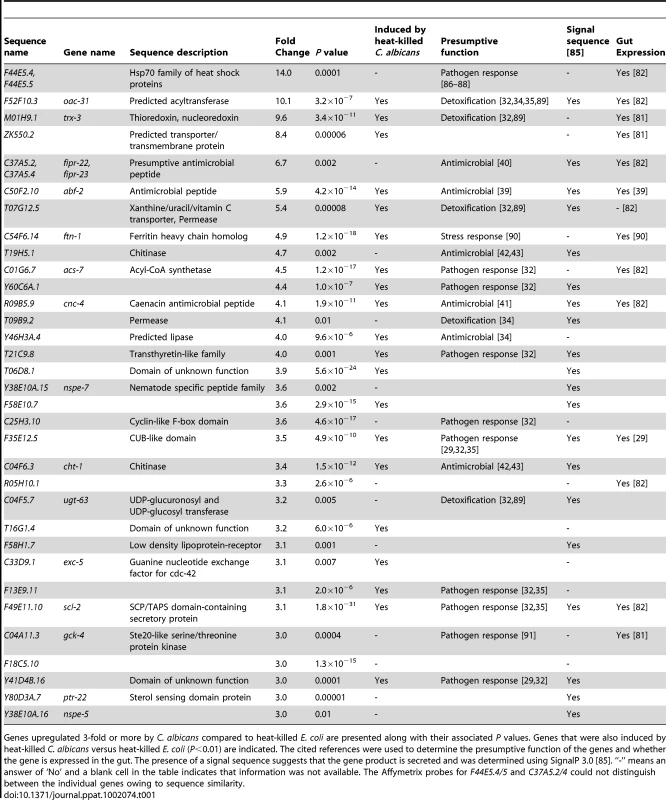
Genes upregulated 3-fold or more by C. albicans compared to heat-killed E. coli are presented along with their associated P values. Genes that were also induced by heat-killed C. albicans versus heat-killed E. coli (P<0.01) are indicated. The cited references were used to determine the presumptive function of the genes and whether the gene is expressed in the gut. The presence of a signal sequence suggests that the gene product is secreted and was determined using SignalP 3.0 [85]. “-” means an answer of ‘No’ and a blank cell in the table indicates that information was not available. The Affymetrix probes for F44E5.4/5 and C37A5.2/4 could not distinguish between the individual genes owing to sequence similarity. Using gene expression analyses, we characterized further the expression pattern of four putative antifungal immune effectors upregulated during C. albicans infection (abf-2, fipr-22/23, cnc-4 and cnc-7). We exposed wild-type nematodes to the C. albicans efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant, a strain that is attenuated for virulence in C. elegans (Figure 2) and mammals [8], and found that the induction of abf-2, fipr-22/23, cnc-4 and cnc-7 was reduced compared to its isogenic parent strain C. albicans SC5314 (P<0.01 for fipr-22/23 and cnc-7, P = 0.06 for abf-2, P<0.025 for cnc-4)(Figure 4). These data suggest that the nematode modulates the expression levels of antifungal immune effectors in response to some aspect of C. albicans virulence, although this yeast may be recognized differently by the nematode innate immune system owing to pleotropic effects of the genetic lesions in this mutant strain. We also found that the induction levels of these four genes appear to be dynamic during infection. Twelve hours after exposure to C. albicans, the expression of abf-2 increases significantly, fipr-22/23 is unchanged and cnc-4 and cnc-7 is reduced (Figure S1).
Fig. 4. The virulence of the infecting C. albicans strain affects the induction of putative antifungal immune effectors. 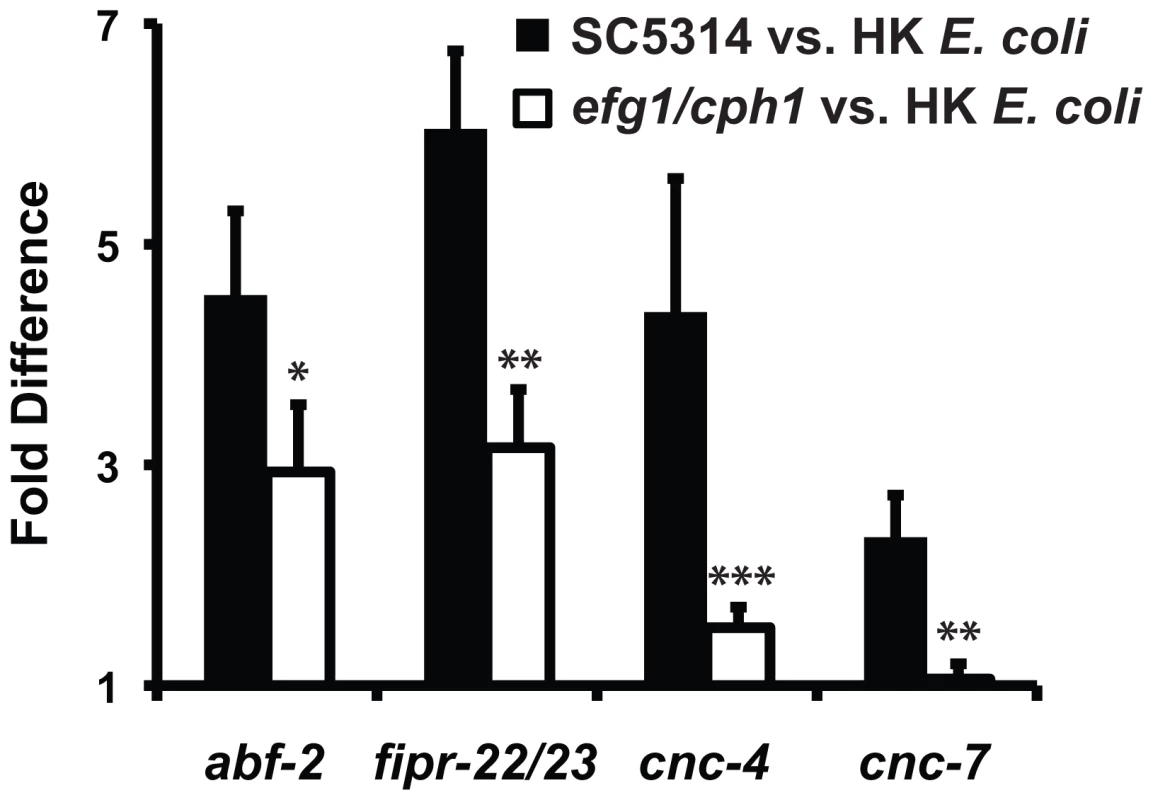
The induction of abf-2, fipr-22/23, cnc-4 and cnc-7 is reduced in wild-type C. elegans animals during infection with the virulence-attenuated C. albicans efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant strain [vs. heat-killed (HK) E. coli] compared to its isogenic wild-type parent strain SC5314 (vs. heat-killed E. coli). Data are presented as the average of three biological replicates, each conducted in duplicate and normalized to a control gene with error bars representing SEM. *P = 0.06, **P<0.01 and ***P<0.025 for the comparison of gene induction on SC5314 versus efg1Δ/efg1Δ cph1Δ/cph1Δ. Among the most highly upregulated C. albicans defense genes (Table 1), we also identified a preponderance of genes encoding secreted proteins, intestinally-expressed proteins and proteins that may function as detoxifying enzymes. Similar types of genes are induced following infection with pathogenic bacteria [32], [34]. As discussed in more detail below, we also found that some of the C. albicans-induced genes were involved in the nematode transcriptional response to bacterial pathogens (Table 1), suggesting that C. albicans and pathogenic bacteria induce a set of common immune response effectors. Although it is possible that the effects of nematode starvation are also reflected in the transcription profiling data as a potential consequence of C. albicans being comparatively non-nutritious relative to heat-killed E. coli, this seems less likely since zero of the eighteen previously-identified, fasting-affected genes [45] were differentially expressed in the dataset. Taken together, these data suggest that the microarray analysis captured the early defense response mounted by C. elegans toward an ingested fungal pathogen.
The Conserved PMK-1/p38 MAP Kinase Mediates Resistance to C. albicans Infection
Genetic, biochemical and molecular analyses have identified a requirement for the PMK-1 mitogen-activated protein (MAP) kinase, orthologous to the mammalian p38 MAPK, in C. elegans immunity [22], [29], [46]–[48]. PMK-1 is a central regulator of nematode defenses [32] that acts cell autonomously both in the intestine to control resistance toward the Gram-negative bacterial pathogens P. aeruginosa [47] and Yersinia pestis [29], and in the hypodermis to defend against the fungus D. coniospora [46]. We found that C. elegans pmk-1(km25) mutants were hypersusceptible to infection with C. albicans yeast (Figure 5A) and that PMK-1 was required for the basal and pathogen-induced expression of three antifungal immune effectors (fipr-22/23, cnc-4 and cnc-7), but not abf-2 (Figure 5B). The full spectrum of nematode sensitivity to C. albicans was not mediated by the genetic control of any of these four effectors because knockdown of each of these genes individually by RNA interference did not result in hypersusceptibility to fungal infection (data not shown). It is likely, however, that there is functional redundancy among immune effectors in C. elegans, as has been suggested previously [29], [32], [44], [49], [50]. That PMK-1 mediates resistance to C. albicans provides another line of evidence that yeast infection of the nematode stimulates host immune defenses. Moreover, the PMK-1-independent genetic regulation of the antifungal effector abf-2 suggests that other pathways are also important in controlling the immune response toward C. albicans.
Fig. 5. The p38 MAP Kinase PMK-1 is Required for the response to C. albicans infection. 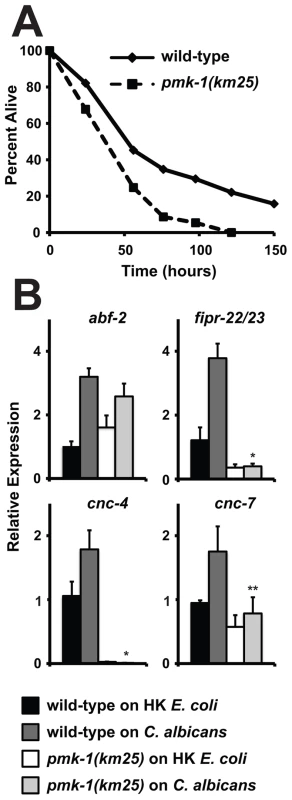
(A) A C. albicans infection assay with wild-type (N2) and pmk-1(km25) animals shows that pmk-1(km25) mutants were more susceptible to C. albicans infection (P<0.01). Each time point represents the average of three plates per strain, each with 30 to 40 animals per plate. Data are representative of two independent experiments. (B) N2 and pmk-1(km25) young adult animals were exposed to the indicated food source and the indicated genes were studied using qRT-PCR (HK equals heat-killed). Expression is relative to N2 on heat-killed E. coli and the data are presented as the average of three biological replicates each normalized to a control gene with error bars representing SEM. *P<0.001 and **P equals 0.05 for the comparison of relative expression of the indicated gene in wild-type animals on C. albicans versus pmk-1(km25) animals on C. albicans. The Host Response to C. albicans Involves Induction of Specific Defenses and Common Immune Genes
To examine the specificity of the antifungal transcriptional response, we compared C. albicans-affected genes with those differentially regulated following infection with the bacterial pathogens P. aeruginosa [32] and Staphylococcus aureus [34] (P<0.01, >2-fold change) (Figure 6). The transcriptional responses induced by fungi, Gram-negative bacteria and Gram-positive bacteria overlapped only to a small extent and the majority of the C. albicans-affected genes were not involved in the response to P. aeruginosa or S. aureus (Figure 6, Table S3A). The C. albicans-specific genes in this comparison included the putative antifungal peptides abf-2, fipr-22/23, cnc-7, thn-1 and the chitinases (cht-1 and T19H5.1). We observed an overlap of 32 induced and 22 repressed genes between the transcriptional responses to P. aeruginosa and C. albicans (1.9 and 1.4 genes expected by chance alone, respectively; P<1.0×10−16 for both comparisons). Likewise, 22 upregulated and 25 downregulated genes were shared in the responses to S. aureus and C. albicans (2.8 and 2.2 genes expected by chance alone, respectively; P<1.0×10−16 for both comparisons). Interestingly, 12 genes were induced and 14 genes were repressed by all three pathogens. Despite the fact that the C. albicans-induced genes were determined using heat-killed E. coli as the control and the genes induced by P. aeruginosa and S. aureus were identified in separate studies that used live E. coli as the control, we detected an overlap of comparable significance between the transcriptional responses to these different organisms. 26% and 18% of C. albicans-induced genes were also upregulated by P. aeruginosa and S. aureus, respectively (Figure 6). Likewise, 17% of genes induced by P. aeruginosa four hours after infection were also upregulated by S. aureus and 11% of S. aureus-upregulated genes were induced by M. nematophilum [34]. Our data suggest that the nematode is able to specifically recognize C. albicans infection and mount a targeted response toward this fungus that involves antifungal defenses and a limited number of common core effectors.
Fig. 6. The transcriptional responses to C. albicans and bacteria comprise specific and overlapping gene sets. 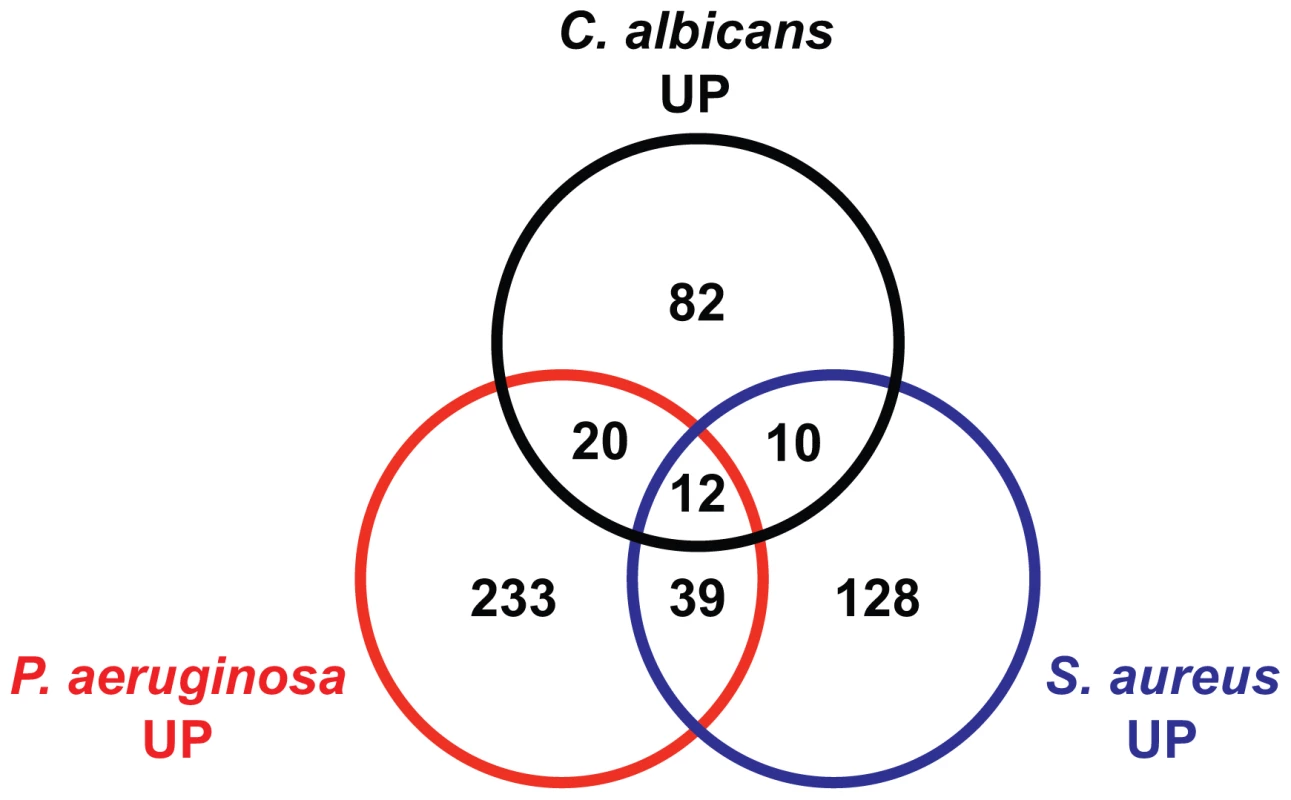
A Venn diagram illustrates the overlap of genes induced 2-fold or greater (P<0.01) by C. albicans (this study), P. aeruginosa [32] and S. aureus [34]. All microarrays were conducted using the Affymetrix platform. Animals were exposed to C. albicans and P. aeruginosa for 4 hours and to S. aureus for 8 hours. See Table S3A for gene identities. Both Heat-Killed and Live C. albicans Yeast Are Immunogenic to the Nematode
Components of the C. albicans cell wall, often referred to as PAMPs, are recognized by mammalian neutrophils, monocytes and macrophages [13], [15], [51]. In this study, we found that heat-killed C. albicans yeast accumulate within the C. elegans intestine (Figure 1B) and therefore postulated that the nematode transcriptional response to nonpathogenic, heat-killed fungi would reflect stimulation of host pathways by immunogenic components of the yeast cell wall. To explore the mechanisms of pathogen detection in the nematode, we fed animals heat-killed C. albicans as an additional condition in the transcriptome profiling experiment. Exposure to heat-killed C. albicans caused a transcriptional response in nematodes involving 287 genes (∼1.4% of the genome, P<0.01) (Table S1B). To determine whether these genes were also involved in defense against live C. albicans infection, we compared the genes differentially regulated by live and heat-killed C. albicans versus the baseline condition of heat-killed E. coli. Interestingly, there was significant overlap (69 genes, 56%) between genes induced by heat-killed C. albicans (vs. heat-killed E. coli) and live C. albicans (vs. heat-killed E. coli)(0.5 genes expected by chance alone, P<1.0×10−16)(Figure 7A, Table S3B). Likewise 106 of 189 genes (56%) repressed by C. albicans were also downregulated by heat-killed C. albicans (0.5 genes expected by chance alone, P<1.0×10−16)(Figure 7B, Table S3B). Interestingly, this overlap includes the majority of the most strongly regulated genes in both directions (Tables 1 and S1A).
Fig. 7. Heat-killed C. albicans yeast cells elicit a transcriptional response in C. elegans that overlaps with the response to live C. albicans. 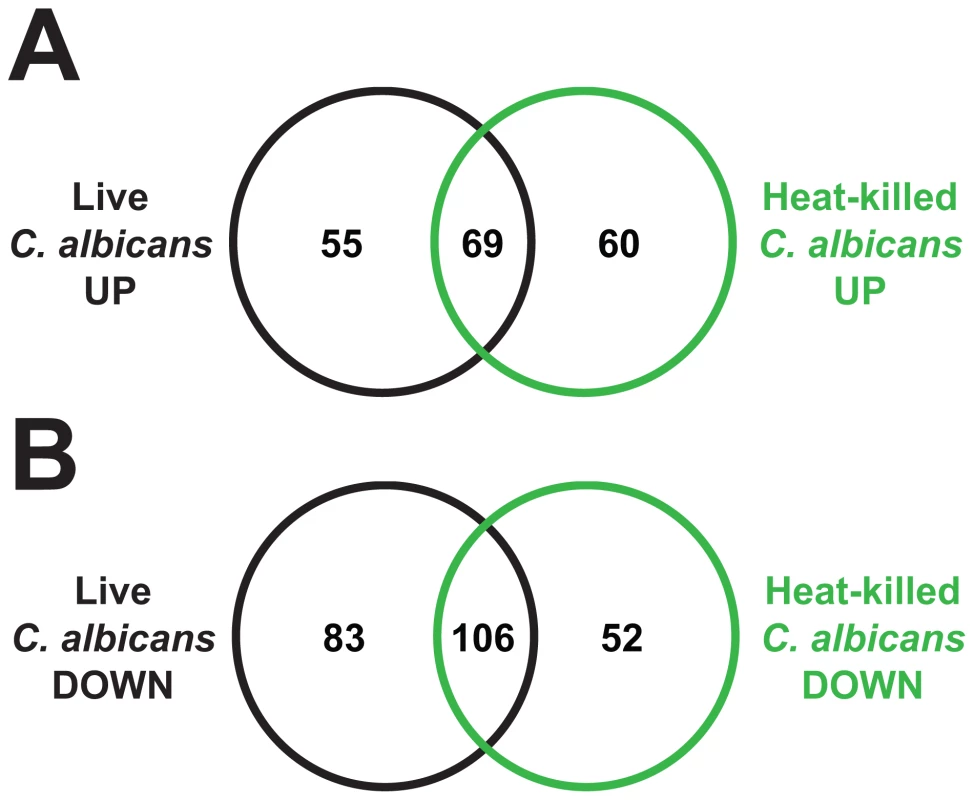
Venn diagrams give the overlap of C. elegans genes upregulated (A) and downregulated (B) at least 2-fold (P<0.01) in response to C. albicans and heat-killed C. albicans, each compared to heat-killed E. coli. See Table S3B for gene identities. These data constitute the first genome-wide analysis of the C. elegans transcriptional response to a heat-killed pathogen and afford several interesting observations. Heat-killed C. albicans yeast cells induce an antifungal transcriptional response in C. elegans despite being non-pathogenic (Figure 1). Genes upregulated by heat-killed C. albicans include several putative antifungal peptides (abf-2, cnc-4, cnc-7, cht-1 and thn-1) and an abundance of secreted or intestinal expressed genes (Table 1), a profile similar to that of live C. albicans. Furthermore, heat-killed C. albicans caused the induction of core immune response genes. The comparison in Figure 6 showed that 42 genes were upregulated by C. albicans and either P. aeruginosa or S. aureus. Thirty-three genes (79%) in this set, including 7 out of 12 genes induced by all three pathogens, were also upregulated by heat-killed C. albicans (Table S3A). Together, these findings suggest that heat-killed C. albicans yeast induce host defenses and imply that a large part of the C. elegans transcriptional response may be mediated by detection of fungal PAMPs through Pattern Recognition Receptors, an evolutionarily-ancient system of pathogen sensing and signaling [52], [53].
Equally interesting, it seems that C. elegans also possesses mechanisms to respond directly to the virulence effects of C. albicans. We identified a smaller group of differentially regulated genes when we compared the transcriptome profiles from nematodes exposed to live C. albicans with those exposed to heat-killed C. albicans. The transcription of 62 genes (22 upregulated and 40 downregulated) changed in this analysis (P<0.01) (Table S1C) presumably in response to the pathogenicity of the fungus. 10 of the 22 genes (45%) upregulated by live C. albicans versus heat-killed C. albicans and 11 of the 40 downregulated genes (28%) were also differentially regulated by live C. albicans versus the baseline condition of heat-killed E. coli (0.12 and 0.36 genes respectively expected by chance alone, P<1.0×10−16 for both comparisons). These data are consistent with our observation that the induction of four putative antifungal effectors was reduced in the virulence-attenuated C. albicans efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant strain compared to its isogenic, wild-type parent strain (Figure 4). Taken together, these data indicate that host recognition of C. albicans infection in the nematode involves at least two mechanisms: recognition of PAMPs and detection of factors associated with fungal virulence.
Immune Specificity towards C. albicans Involves the Targeted Downregulation of Antibacterial Effectors
Closer examination of the genes downregulated by C. albicans revealed an unexpected finding regarding antifungal immune specificity. We noticed that the most over-represented classes among the C. albicans downregulated genes (based on GO annotation) were involved in sugar or carbohydrate binding. Because these gene classes are upregulated in response to P. aeruginosa and S. aureus [32], [34], we postulated that some antibacterial defense effectors are specifically downregulated during infection with C. albicans. We therefore compared the 189 genes that are downregulated by C. albicans with the genes induced during infection with P. aeruginosa and S. aureus, and found a striking overlap (Figure 8A, Table S3C). Twenty-seven of the 189 downregulated C. albicans genes (14%) were induced by P. aeruginosa, which is 25-fold more than expected by chance alone (P<1.0×10−16). Likewise, 22 S. aureus response genes (12%) were downregulated by C. albicans (12-fold more than expected by chance alone, P<1.0×10−16). Thus, it seems that the nematode immune response to C. albicans involves the downregulation of a group of antibacterial defense genes.
Fig. 8. The C. elegans response to C. albicans involves the downregulation of antibacterial effectors. 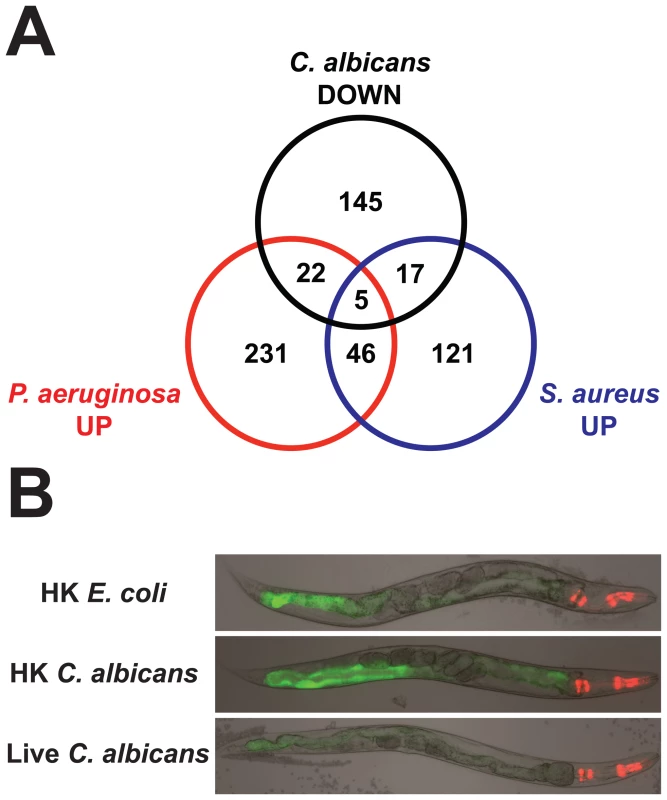
(A) A Venn diagram illustrates that a subset of C. albicans downregulated genes were upregulated after infection of C. elegans by pathogenic bacteria. See Table S3C for gene identities. (B) Transgenic C. elegans animals in which GFP expression was driven by the promoter for the C-type lectin clec-60, a secreted S. aureus immune effector that was downregulated by C. albicans in the microarray analysis, are shown. Worms were exposed to heat-killed (HK) E. coli, heat-killed C. albicans or live C. albicans for 20 hours at 25°C and then imaged. Green is clec-60::GFP. Red is the myo-2::mCherry co-injection marker used to identify transgenic animals. We took two steps to confirm this observation. First, we used qRT-PCR to test the expression of seven genes differentially regulated by C. albicans and previously shown to be part of the P. aeruginosa transcriptional response (irg-3, clec-67, K08D8.5, C17H12.8, F49F1.6, F35E12.5 and F01D5.5) [32]. All seven of these genes were strongly downregulated four hours after C. albicans infection (Table S2). We also assayed the expression of clec-67, K08D8.5, C17H12.8 and F49F1.6 12 hours after infection and found that these genes continue to be transcriptionally repressed at this later time point (Figure S1). Two of these genes, C17H12.8 and F49F1.6, were more strongly repressed at 12 hours compared to 4 hours after infection (P<0.01 and P = 0.07, respectively). As a second approach, we studied transgenic C. elegans animals in which the promoter for the S. aureus immune response gene clec-60 was fused to GFP, allowing a visual readout of gene transcription. clec-60 is a C-type lectin, a gene class important for nematode defense against bacterial pathogens [29], [32], [34], a member of which was shown to have direct antimicrobial activity in a mammalian system [54]. Consistent with the microarray analysis (Table S1A), we found that exposure to live C. albicans dramatically reduced GFP expression in clec-60::GFP transgenic animals compared to the basal expression of this gene on heat-killed E. coli (Figure 8B).
One interpretation of these data is that the downregulation of antibacterial effectors observed in the microarray analysis reflects the absence of bacteria in C. albicans-exposed animals rather than specific transcriptional repression of these genes during infection with pathogenic fungi. We therefore examined the genes that were downregulated in the comparison of live C. albicans versus heat-killed C. albicans, an experiment where bacterial antigens were not present in either condition. Of the 40 genes that were transcriptionally repressed in this comparison, 19 genes were also upregulated by S. aureus [34] or P. aeruginosa [32] (Table S1C)(0.08 genes expected by chance alone, P<1.0×10−6 for this comparison). For reasons that are not clear, only 6 of these 19 genes were also downregulated in the comparison of live C. albicans versus heat-killed E. coli (Table S3C); however, this overlap is significantly more than the 0.08 gene overlap expected by chance alone (P = 0.013). Therefore, we conclude that the nematode downregulates a group of antibacterial defense genes in response to some aspect of C. albicans virulence. It is also interesting that of the 44 antibacterial response genes shown in Figure 8 that were downregulated by C. albicans, 26 (59%) were also repressed by heat-killed C. albicans (Table S3C). Taken together, these data suggest that the nematode responds to components within heat-killed C. albicans, as well as factors associated with fungal virulence, to transcriptionally repress antibacterial immune responses.
One of the antibacterial genes downregulated in the comparison of live C. albicans and heat-killed C. albicans was clec-60. Thus, for additional confirmation of these data, we exposed clec-60::GFP transgenic animals to heat-killed C. albicans. As predicted from the microarray analysis, we found that expression of clec-60::GFP was visually unchanged compared to its basal level on heat-killed E. coli (Figure 8B). Furthermore, our finding that C17H12.8 and F49F1.6 were more strongly downregulated at 12 hours of infection (versus 4 hours)(Figure S1) suggests that the transcriptional repression of these antibacterial immune effectors is an active process associated with progression of fungal infection.
To understand the mechanism underlying the repression of antibacterial immune effectors during C. albicans infection, we assayed gene expression in daf-16(mgDf47) and pmk-1(km25) mutants. Troemel et al. previously showed that the p38 MAP kinase homolog PMK-1 controls the expression of many P. aeruginosa immune response genes [32]. In their analysis, they also observed that the FOXO/forkhead transcription factor DAF-16, a central regulator of nematode longevity, negatively regulates some P. aeruginosa defense genes, including a group of pmk-1-dependent genes. We therefore wondered whether DAF-16 negatively regulates antibacterial defense genes during infection with C. albicans. We determined the overlap of the C. albicans downregulated genes with the group of genes whose basal expression is negatively regulated by DAF-16 (so-called Class II genes from Murphy et al. [55]) and found a 24-gene overlap (more than the 2.6 genes expected by chance alone, P<1.0×10−16). From these analyses, we identified two genes (clec-67 and C17H12.8) whose basal expression was previously reported as being induced by PMK-1 and negatively controlled by DAF-16 [32]. We examined the regulation of these genes during C. albicans infection and found that they were equally downregulated by C. albicans in both wild-type and daf-16(mgDf47) mutants (Figure S2), which suggests that DAF-16 is not responsible for this phenotype. In support of this observation, DAF-16::GFP remained localized to the cytoplasm following exposure to C. albicans and did not translocate into the nucleus, as it does when it is activated to regulate transcription (data not shown). We also wondered whether signaling through the PMK-1 pathway results in the downregulation of antibacterial immune effectors during C. albicans infection. However, the basal expression of clec-67 and C17H12.8 was profoundly affected by PMK-1 (Figure S2), which precluded analysis of differential regulation during C. albicans infection in pmk-1(km25) mutants. In summary, we show that antibacterial response genes are downregulated during C. albicans infection, including a group whose basal expression is repressed by DAF-16 and stimulated by PMK-1. We conclude that an unidentified mechanism, independent of DAF-16, accounts for this phenotype.
Discussion
We show that the yeast form of C. albicans is pathogenic to the nematode and explore the mechanisms of immune activation by pathogenic fungi in vivo. Previous studies of C. elegans infection with bacterial pathogens have led to the characterization of a sophisticated and evolutionarily-conserved innate immune system in the nematode [21]. We found that the C. elegans is also able to specifically recognize and defend itself against C. albicans, the most common fungal pathogen of humans [1]. These data suggest that C. elegans integrates signals from C. albicans yeast and factors associated with its pathogenicity to mount a targeted defense response. We also found that nematode antifungal immunity involves the elaboration of immune effectors and the downregulation of antibacterial response genes.
The C. elegans Immune Response to C. albicans is Mediated by the Detection of PAMPs and Fungal Virulence
Using a C. elegans pathogenesis assay that is conducted on solid agar plates, we show that C. albicans yeast cells kill worms in a manner dependent on live organisms and cause pathogenic distention of the nematode intestine during infection. Furthermore, we found that both heat-killed and virulence-attenuated C. albicans readily enter the nematode intestine, but are less pathogenic than wild-type yeast. While the mechanism of nematode mortality during C. albicans infection is unknown, these data suggest that some aspect of fungal virulence is required for yeast to infect and kill C. elegans.
In response to C. albicans attack, we found that the nematode mounts a pathogen-specific defense response that involves the induction of antifungal effectors and core immune genes. Interestingly, 56% of the genes involved in the transcriptional response to C. albicans infection were also differentially regulated by heat-killed C. albicans. These data suggest that a large part of the transcriptional response to C. albicans is elicited by fungal PAMPs. In mammals, heat-killed fungi also strongly activate host defenses and have been used to study PAMP-mediated immune signaling [13], [56]. In myeloid cells, cell wall components of heat-killed yeast (mannans and β-glucans) activate the pattern recognition receptors TLR2, TLR4, MR and dectin-1 to initiate antifungal immune responses [15]. Indeed, the process of heat killing may actually exaggerate innate immune responses in human cells by exposing fungal PAMPs. For example, β-glucans within the cell wall of C. albicans are normally covered by mannoproteins and thus blocked from detection by dectin-1 [13], [51]. Treatment of yeast cells with heat depletes this protective layer and exposes β-glucans, thereby enhancing dectin-1-mediated proinflammatory cytokine responses [56], [57].
The transcriptome profiling experiments and the expression analyses of nematodes infected with virulence-attenuated C. albicans suggest that factors associated with fungal virulence also elicit a transcriptional response in C. elegans. We do not know, however, whether these factors are derived from the host (e.g. as a consequence of cell damage) or from the pathogen. Recently, Moyes et al. found that human epithelial cells integrate inputs from C. albicans PAMPs via pattern recognition receptors together with “danger signals” perceived by the host during invasive fungal growth [14]. Interestingly, these researchers observed a biphasic activation of the p38 MAP kinase (MAPK) pathway, which was initially dependent on PAMP recognition and later on fungal burden and hyphal formation during invasive growth. We found a requirement for PMK-1, the nematode ortholog of the p38 MAP kinase, in the response to C. albicans infection. We therefore propose that similar mechanisms of pathogen detection involving the PMK-1 pathway exist in C. elegans. As in the human epithelium, the nematode may integrate signals from PAMPs together with inputs associated with fungal virulence to delineate a “pattern of pathogenesis [58]” specific to fungal infection. Further research is needed to determine the PAMPs that are detected by C. elegans, the intestinal pattern recognition receptors that bind them and the mechanisms by which fungal virulence is perceived in the nematode.
Core Immune Effectors Are Activated by Bacterial and Fungal Pathogens
The immune response induced by Gram-negative bacteria, Gram-positive bacteria and fungi involve a small number of overlapping genes, a result that is somewhat surprising given the marked difference between prokaryotic and eukaryotic pathogens. Although others have also reported that the nematode mounts shared responses against different kinds of pathogens [34], [36], [59], our data are the first to define a core set of immune regulators involved in the defense against three prototypical nosocomial pathogens. These findings may ultimately have clinical implications. Our laboratories and others are using C. elegans pathogenesis assays as a means to identify novel antimicrobial therapies with immunomodulatory activity [60]. Thus, identifying compounds that boost these core immune response genes may yield novel therapies that can cure infection by three diverse, nosocomial pathogens and may be a strategy that can be applied in higher order hosts.
Antibacterial Immune Effectors Are Downregulated by C. albicans
Unexpectedly, C. albicans infection of the nematode caused the downregulation of a number of antibacterial response genes including CUB-like genes, C-type lectins and ShK toxins. Moreover, it seems that both heat-killed (non-pathogenic) C. albicans and live (infectious) C. albicans can cause this repression. Interestingly, the basal expression of many of these genes is positively regulated by the p38 MAP kinase homolog PMK-1 and negatively regulated by DAF-16. How might the selective downregulation of these antibacterial response genes be evolutionarily advantageous for the worm? We know that the DAF-2 insulin/insulin-like growth factor receptor signals to the FOXO/forkhead transcription factor DAF-16 to control life span and stress resistance [61]–[63] and that DAF-16 negatively regulates P. aeruginosa immune response genes [32]. Troemel et al. postulated that immune response genes may be energetically expensive to make and thus their downregulation by DAF-16 under normal growth conditions may partially account for the lifespan-enhancing effects of DAF-2/DAF-16 pathway [32]. Irazoqui et al. found that the coordinated regulation of the immune response genes clec-60/61 and clec-70/71 influenced nematode survival. C. elegans animals carrying multiple copies of these gene clusters, which are induced during S. aureus infection, but not by P. aeruginosa or C. albicans, were more resistant to S. aureus, but were paradoxically hypersusceptible to P. aeruginosa [34]. We therefore propose that the transcriptional repression of antibacterial response genes, such as clec-60 and clec-70, during C. albicans infection is an adaptive response. Given the recognized ability of FOXO/forkhead transcription factors to repress immune response genes both in C. elegans and in mammals [64], we hypothesized that DAF-16 activity would be responsible for this phenotype. However, our data suggest that an unidentified mechanism, independent of DAF-16, represses these genes following C. albicans infection.
We are not aware of other examples in metazoans in which activation of specific antimicrobial defenses results in the transcriptional downregulation of another immune response. In contrast, this phenomenon is well described in the immune response of Arabidopsis thaliana, a widely-studied, model laboratory plant [65]. In Arabidopsis, as well as other plants, two low molecular weight immune hormones, salicylic acid and jasmonic acid, are involved in the activation of distinct immune response pathways. Salicylic acid is primarily activated by obligate, biotrophic pathogens that require living plant cells to acquire nutrients. Jasmonic acid, on the other hand, is involved in the response to necrotrophic pathogens that kill host cells and then feed on the carcasses. In most cases, activation of salicylic acid-mediated signaling downregulates jasmonic acid signaling and vice versa. The mutual antagonism of the salicylic acid and jasmonic acid pathways is generally interpreted in terms of evolutionary tradeoffs between biotrophic and necrotrophic defenses [65]. Our data suggest that a similar antagonism may be occurring in C. elegans between bacterial and fungal defenses. That is, when confronted with a virulent fungal pathogen, C. elegans focuses its immune response on the production of specific antifungal effectors at the expense of antibacterial defenses. Our analysis of the genes downregulated by P. aeruginosa or S. aureus did not reveal a statistically significant overlap with the genes induced following exposure to C. albicans. An alternative explanation is that the genes that are downregulated by C. albicans actually encode key immune effectors important for defense against both bacterial and fungal pathogens. Instead of the host downregulating the expression of these genes, the transcriptional repression may reflect an offensive measure by C. albicans to enhance its ability to infect C. elegans.
C. elegans Pathogenesis Assays Enable Analyses of C. albicans Virulence Mechanisms
In this study, we describe a novel C. elegans assay for the study of C. albicans yeast-mediated pathogenesis, which complements our hyphal formation model that we used to identify novel virulence determinants in C. albicans [33]. In our previous study, we screened a C. albicans mutant library containing homozygous mutations in 83 transcription factors [66] for clones attenuated both in their ability to form hyphae in vivo and kill C. elegans [33]. We uncovered several novel mediators of hyphal growth and showed that the efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant [8], which is unable to program filamentation, was also attenuated for virulence in the C. elegans model, as it was in mammalian systems. The efg1Δ/efg1Δ cph1Δ/cph1Δ double mutant contain lesions in transcription factors that are the conserved readouts of the cAMP-mediated cascade (Efg1p) and the MAP-kinase cascade (Cph1p), each with well-described roles in the control of morphogeneis and virulence [8], [67]. In the current study, we show that this mutant was also attenuated for virulence in the C. elegans yeast-mediated pathogenesis assay. These data suggest that the C. albicans cAMP-mediated and MAP-kinase cascades also regulate yeast-specific virulence determinants and support the hypothesis that this morphogenic form is an important contributor to the pathogenic potential of wild-type fungi, as has been suggested by others [11], [68]–[70]. These data also indicate that the C. elegans system can be used in large-scale screens of C. albicans mutant libraries for novel virulence regulators possessed by yeast.
Materials and Methods
Strains and Media
C. elegans were maintained and propagated on E. coli OP50 as described [71]. The C. elegans strains used in this study were: N2 bristol [71], pmk-1(km25) [22], daf-16(mgDf47) [72], fer-15(b26);fem-1(hc17) [55], AU0157 [agEx39(myo-2::cherry,clec-60::GFP)] [28] and TJ356 [zIs356 (pDAF-16::DAF-16-GFP;rol-6)] [73]. The C. albicans strains used in this study were DAY185 (ura3Δ::λimm434/ura3Δ::λimm434 ARG4:URA3::arg4::hisG/arg4::hisG his1::hisG::pHIS/his1::hisG) [74], SC5314 (clinical isolate) [75] and Can34 (ura3Δ::λimm434/ura3Δ::λimm434 cph1Δ::hisG/cph1Δ::hisG efg1Δ::hisG/efg1Δ::hisG-URA3-hisG) [8]. Unless otherwise specified, C. albicans DAY185 was used as the wild-type strain. Yeast strains were grown in liquid yeast extract-peptone-dextrose (YPD,BD) broth or on brain heart infusion agar containing 45 µg of kanamycin/ml at 30°C. Bacteria were grown in Luria Broth (LB, BD).
C. albicans-C. elegans Solid Medium Pathogenesis Assay
The previously described protocol for pathogen infection of C. elegans was modified for these studies [76]. Freshly grown C. albicans of the indicated genotype were picked from a single colony and used to inoculate 1 mL of YPD broth, which was allowed to grow overnight with agitation at 30°C. The following day, 10 µL of yeast were spread into a square lawn in a 4 cm tissue culture plate (BD) containing 4 mL of BHI agar and kanamycin (45 µg/mL). For experiments that compared heat-killed and live C. albicans, cells were subjected to the exact same preparatory conditions. A single colony of yeast was grown in 1 mL BHI at 30°C overnight and then inoculated into 50 mL YPD. After approximately 20 hours of incubation, cells were split into two aliquots, collected by centrifugation and washed twice with sterile PBS (pH 7.4). One aliquot was resuspended in 1 mL PBS, exposed to 75°C for 60 minutes and washed again with sterile PBS. The other aliquot was processed in parallel with the heat-killed sample. Cells were suspended in 25 mL PBS, incubated at room temperature for 60 minutes and washed again with sterile PBS. 10 µL of this sample were added to the killing assay plates. To heat kill E. coli, a similar protocol was followed except that a single colony was inoculated into 50 mL LB and allowed to grow overnight at 37°C. Cells were exposed to 75°C for 30 minutes. In both cases, heat-killed organisms were plated on YPD or LB agar to ensure no viable organisms remained. 50 µL of heat-killed cells were added to the assay plates. The plates were then incubated for approximately 20 hours at 30°C. The next day, a Pasteur pipette molded into the shape of hockey stick was used to gently scrape excess yeast off the top of the thick C. albicans lawn. This step greatly facilitated scoring the animals as live or dead on subsequent days and did not affect the pathogenicity of C. albicans (data not shown). Five-fluoro-2′-deoxyuridine (FUDR; 75–100 µg/mL) was added to the plates 1 to 2 hours before the start of the assay to reduce the growth of progeny and prevent matricidal killing of nematodes by C. albicans. Thirty to forty young adult animals of the indicated genotype were added to each of three assay plates per condition studied. Although it is possible that microorganism inocula varied among individual worms, we doubt that such variation affected the pathogenicity of C. albicans in our assay since we observed similar killing kinetics in replicate experiments. Animals were scored as live or dead on a daily basis by gently touching them with a platinum wire. Worms that crawled onto the wall of the tissue culture plate were eliminated from the analysis. All killing assays were conducted at 25°C. C. elegans survival was examined using the Kaplan-Meier method and differences were determined with the log-rank test (STATA 6; STATA, College Station, TX).
Microarray Analysis of C. albicans Infected Nematodes
N2 animals were synchronized by hypochlorite treatment. Arrested L1s were plated on 10 cm NGM plates seeded with E. coli OP50 and grown at 20°C until they were young adults. Animals were then added to 10 cm plates containing 20 mL of BHI agar (with 45 µg of kanamycin/ml) and live C. albicans, heat-killed C. albicans or heat-killed E. coli. Plates were prepared using the method described above except 50 µL of cells were added to the plates for each condition together with 200 µL of PBS to facilitate even dispersion of the microbes. Three separate biological replicates of nematodes were exposed to these conditions for 4 hours at 25°C. RNA was extracted using TRI Reagent (Molecular Research Center) according to the manufacturer's instructions and purified using an RNeasy column (Qiagen). RNA samples were prepared and hybridized to Affymetrix full-genome GeneChips for C. elegans at the Harvard Medical School Biopolymer Facility following previously described protocols [32] and instructions from Affymetrix. Data were analyzed using Resolver Gene Expression Data Analysis System, version 5.1 (Rosetta Inpharmatics). Three biologic replicates per condition were normalized using the Resolver intensity error model for single color chips [77]. Conditions were compared using Resolver to determine the fold change between conditions for each probe set and to generate a P value using a modified t-test. Probe sets were considered differentially expressed if the fold change was 2-fold or greater (P<0.01). When comparing datasets, the overlap expected by chance alone was determined in 50 groups of randomly selected C. elegans genes using Regulatory Sequence Analysis Tools (http://rsat.ulb.ac.be/rsat/), a technique that has been used for similar analyses [78]. P values were determined using chi-square tests. Analyses for over-representation of GO annotation categories were performed using DAVID Bioinformatics Resources 6.7 from the National Institute of Allergy and Infectious Diseases [79], [80]. Two databases were used to determine the expression patterns for selected genes: Expression Patterns for C. elegans Promoter::GFP Fusions (http://gfpweb.aecom.yu.edu/) [81] and NEXTDB [82].
Quantitative RT-PCR (qRT-PCR) Analyses
Animals were treated and RNA was extracted as described above. RNA was reverse transcribed to cDNA using the Retroscript kit (Ambion). cDNA was analyzed by qRT-PCR using a CFX1000 machine (Bio-Rad) and previously published primers [32], [39], [41]. Primer sequences for fipr-22/23 (GCTGAAGCTCCACACATCC and TATCCCATTCCTCCGTATCC) and cnc-7 (CAGGTTCAATGCAGTATGGCTATGG and GGACGGTACATTCCCATACC) were designed for this study, checked for specificity against the C. elegans genome and tested for efficiency with a dilution series of template. The primer set for fipr-22/23 cannot distinguish between these two genes owing to sequence similarity. All values were normalized against the control gene snb-1, which has been used previously in qRT-PCR studies of C. elegans innate immunity [31], [32], [48], [83]. Analysis of the microarray expression data revealed that the expression of snb-1 did not vary under the conditions tested in our experiment. Fold change was calculated using the Pfaffl method [84] and compared using t-tests.
Microscopy
Nematodes were mounted onto agar pads, paralyzed with 10 mM levamisole (Sigma) and photographed using a Zeiss AXIO Imager Z1 microscope with a Zeiss AxioCam HRm camera and Axiovision 4.6 (Zeiss) software.
Accession Numbers
Accession numbers for the genes and gene products mentioned in this paper are given for Wormbase, a publically available database that can be accessed at http://www.wormbase.org. These accession numbers are pmk-1 (B0218.3), abf-2 (C50F2.10), fipr-22/23 (C37A5.2/4), cnc-4 (F09B5.9), cnc-7 (F53H2.2), cht-1 (C04F6.3), T19H5.1, irg-3 (F53E10.4), clec-67 (F56D6.2), K08D8.5, C17H12.8, F49F1.6, F35E12.5, F01D5.5, clec-60 (ZK666.6) and daf-16 (R13H8.1). The microarray dataset can be downloaded from the National Center for Biotechnology Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo). The accession number for these data is GSE2740.
Supporting Information
Zdroje
1. BermanJSudberyPE 2002 Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 3 918 930
2. LeroyOGangneuxJPMontraversPMiraJPGouinF 2009 Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37 1612 1618
3. GudlaugssonOGillespieSLeeKVande BergJHuJ 2003 Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37 1172 1177
4. LeleuGAegerterPGuidetB 2002 Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J Crit Care 17 168 175
5. AchkarJMFriesBC 2010 Candida infections of the genitourinary tract. Clin Microbiol Rev 23 253 273
6. BraunBRJohnsonAD 1997 Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277 105 109
7. CaoFLaneSRanigaPPLuYZhouZ 2006 The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell 17 295 307
8. LoHJKöhlerJRDiDomenicoBLoebenbergDCacciapuotiA 1997 Nonfilamentous C. albicans mutants are avirulent. Cell 90 939 949
9. GowNABrownAJOddsFC 2002 Fungal morphogenesis and host invasion. Curr Opin Microbiol 5 366 371
10. RosenbachADignardDPierceJVWhitewayMKumamotoCA 2010 Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot Cell 9 1075 1086
11. SavilleSPLazzellALMonteagudoCLopez-RibotJL 2003 Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2 1053 1060
12. KumamotoCAVincesMD 2005 Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol 7 1546 1554
13. GantnerBNSimmonsRMUnderhillDM 2005 Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 24 1277 1286
14. MoyesDLRunglallMMurcianoCShenCNayarD 2010 A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 8 225 235
15. NeteaMGBrownGDKullbergBJGowNA 2008 An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6 67 78
16. FerwerdaBFerwerdaGPlantingaTSWillmentJAvan SprielAB 2009 Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361 1760 1767
17. GlockerEOHennigsANabaviMSchafferAAWoellnerC 2009 A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361 1727 1735
18. JouaultTSarazinAMartinez-EsparzaMFradinCSendidB 2009 Host responses to a versatile commensal: PAMPs and PRRs interplay leading to tolerance or infection by Candida albicans. Cell Microbiol 11 1007 1015
19. KurzCLEwbankJJ 2003 Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet 4 380 390
20. Pukkila-WorleyRMylonakisE 2010 From the outside in and the inside out: antifungal immune responses in Caenorhabditis elegans. Virulence 1 111 112
21. IrazoquiJEUrbachJMAusubelFM 2010 Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10 47 58
22. KimDHFeinbaumRAlloingGEmersonFEGarsinDA 2002 A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 623 626
23. ZieglerKKurzCLCypowyjSCouillaultCPophillatM 2009 Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe 5 341 352
24. RenMFengHFuYLandMRubinCS 2009 Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity 30 521 532
25. CouillaultCPujolNReboulJSabatierLGuichouJF 2004 TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol 5 488 494
26. TroemelERFélixMWhitemanNBarrièreAAusubelFM 2008 Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PloS Biol 6 e309
27. AballayADrenkardEHilbunLRAusubelFM 2003 Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol 13 47 52
28. IrazoquiJENgAXavierRJAusubelFM 2008 Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci USA 105 17469 17474
29. BolzDDTenorJLAballayA 2010 A conserved PMK-1/p38 MAPK is required in Caenorhabditis elegans tissue-specific immune response to Yersinia pestis infection. J Biol Chem 285 10832 10840
30. AnyanfulAEasleyKABenianGMKalmanD 2009 Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5 450 462
31. PowellJRKimDHAusubelFM 2009 The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci U S A 106 2782 2787
32. TroemelERChuSWReinkeVLeeSSAusubelFM 2006 p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2 e183
33. Pukkila-WorleyRPelegAYTampakakisEMylonakisE 2009 Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell 8 1750 1758
34. IrazoquiJETroemelERFeinbaumRLLuhachackLGCezairliyanBO 2010 Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog 6 e1000982
35. O'RourkeDBabanDDemidovaMMottRHodgkinJ 2006 Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res 16 1005 1016
36. WongDBazopoulouDPujolNTavernarakisNEwbankJJ 2007 Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol 8 R194
37. GarsinDASifriCDMylonakisEQinXSinghKV 2001 A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci USA 98 10892 10897
38. MoreyJSRyanJCVan DolahFM 2006 Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online 8 175 193
39. KatoYAizawaTHoshinoHKawanoKNittaK 2002 abf-1 and abf-2, ASABF-type antimicrobial peptide genes in Caenorhabditis elegans. Biochem J 361 221 230
40. PujolNZugastiOWongDCouillaultCKurzCL 2008 Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog 4 e1000105
41. ZugastiOEwbankJJ 2009 Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 10 249 256
42. EliasJAHomerRJHamidQLeeCG 2005 Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol 116 497 500
43. FunkhouserJDAronsonNNJr 2007 Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 7 96
44. ShapiraMHamlinBJRongJChenKRonenM 2006 A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci USA 103 14086 14091
45. Van GilstMRHadjivassiliouHYamamotoKR 2005 A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A 102 13496 13501
46. PujolNCypowyjSZieglerKMilletAAstrainA 2008 Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol 18 481 489
47. ShiversRPKooistraTChuSWPaganoDJKimDH 2009 Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe 6 321 330
48. ShiversRPPaganoDJKooistraTRichardsonCEReddyKC 2010 Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet 6 e1000892
49. KerrySTeKippeMGaddisNCAballayA 2006 GATA transcription factor required for immunity to bacterial and fungal pathogens. PLoS ONE 1 e77
50. MalloGVKurzCLCouillaultCPujolNGranjeaudS 2002 Inducible antibacterial defense system in C. elegans. Curr Biol 12 1209 1214
51. NeteaMGGowNAMunroCABatesSCollinsC 2006 Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116 1642 1650
52. JanewayCAJrMedzhitovR 2002 Innate immune recognition. Annu Rev Immunol 20 197 216
53. JanewayCAJr 1989 Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 Pt 1 1 13
54. CashHLWhithamCVBehrendtCLHooperLV 2006 Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313 1126 1130
55. MurphyCTMcCarrollSABargmannCIFraserAKamathRS 2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
56. GowNANeteaMGMunroCAFerwerdaGBatesS 2007 Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis 196 1565 1571
57. WheelerRTFinkGR 2006 A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2 e35
58. VanceREIsbergRRPortnoyDA 2009 Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6 10 21
59. AlperSMcBrideSJLackfordBFreedmanJHSchwartzDA 2007 Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol Cell Biol 27 5544 5553
60. Pukkila-WorleyRHolsonEWagnerFMylonakisE 2009 Antifungal drug discovery through the study of invertebrate model hosts. Curr Med Chem 16 1588 1595
61. KenyonC 2005 The plasticity of aging: insights from long-lived mutants. Cell 120 449 460
62. KenyonCChangJGenschERudnerATabtiangR 1993 A C. elegans mutant that lives twice as long as wild type. Nature 366 461 464
63. KimuraKDTissenbaumHALiuYRuvkunG 1997 daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277 942 946
64. CofferPJBurgeringBM 2004 Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol 4 889 899
65. SpoelSHDongX 2008 Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3 348 351
66. NobileCJMitchellAP 2005 Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol 15 1150 1155
67. KohAYKohlerJRCoggshallKTVan RooijenNPierGB 2008 Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 4 e35
68. KobayashiSDCutlerJE 1998 Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol 6 92 94
69. FuchsBBEbyJNobileCJEl KhouryJBMitchellAP 2010 Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect 12 488 496
70. HubeBNaglikJ 2001 Candida albicans proteinases: resolving the mystery of a gene family. Microbiology 147 1997 2005
71. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
72. OggSParadisSGottliebSPattersonGILeeL 1997 The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389 994 999
73. HendersonSTJohnsonTE 2001 daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol 11 1975 1980
74. DavisDWilsonRBMitchellAP 2000 RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol 20 971 978
75. GillumAMTsayEYKirschDR 1984 Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198 179 182
76. PowellJRAusubelFM 2007 Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. EwbankJJVivierE Innate Immunity, Methods in Molecular Biology Totowa Humana Press. Vol. 415 403 427
77. WengLDaiHZhanYHeYStepaniantsSB 2006 Rosetta error model for gene expression analysis. Bioinformatics 22 1111 1121
78. KirienkoNVMcEnerneyJDFayDS 2008 Coordinated regulation of intestinal functions in C. elegans by LIN-35/Rb and SLR-2. PLoS Genet 4 e1000059
79. DennisGJrShermanBTHosackDAYangJGaoW 2003 DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4 P3
80. Huang daWShermanBTLempickiRA 2009 Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 44 57
81. Hunt-NewburyRViveirosRJohnsenRMahAAnastasD 2007 High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol 5 e237
82. TadasuSKoharaY 2005 NEXTDB. Available: http://nematode.lab.nig.ac.jp/. Accessed September 2010
83. RichardsonCEKooistraTKimDH 2010 An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463 1092 1095
84. PfafflMW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29 e45
85. BendtsenJDNielsenHvon HeijneGBrunakS 2004 Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340 783 795
86. SinghVAballayA 2006 Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci USA 103 13092 13097
87. PrahladVCorneliusTMorimotoRI 2008 Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320 811 814
88. Mohri-ShiomiAGarsinDA 2008 Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J Biol Chem 283 194 201
89. McElweeJJSchusterEBlancEThomasJHGemsD 2004 Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem 279 44533 44543
90. RomneySJThackerCLeiboldEA 2008 An iron enhancer element in the FTN-1 gene directs iron-dependent expression in Caenorhabditis elegans intestine. J Biol Chem 283 716 725
91. AlperSLawsRLackfordBBoydWADunlapP 2008 Identification of innate immunity genes and pathways using a comparative genomics approach. Proc Natl Acad Sci U S A 105 7016 7021
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



