-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
Cyclic dimeric GMP (c-di-GMP) is a bacterial second messenger that modulates many biological processes. Although its role in bacterial pathogenesis during mammalian infection has been documented, the role of c-di-GMP in a pathogen's life cycle within a vector host is less understood. The enzootic cycle of the Lyme disease pathogen Borrelia burgdorferi involves both a mammalian host and an Ixodes tick vector. The B. burgdorferi genome encodes a single copy of the diguanylate cyclase gene (rrp1), which is responsible for c-di-GMP synthesis. To determine the role of c-di-GMP in the life cycle of B. burgdorferi, an Rrp1-deficient B. burgdorferi strain was generated. The rrp1 mutant remains infectious in the mammalian host but cannot survive in the tick vector. Microarray analyses revealed that expression of a four-gene operon involved in glycerol transport and metabolism, bb0240-bb0243, was significantly downregulated by abrogation of Rrp1. In vitro, the rrp1 mutant is impaired in growth in the media containing glycerol as the carbon source (BSK-glycerol). To determine the contribution of the glycerol metabolic pathway to the rrp1 mutant phenotype, a glp mutant, in which the entire bb0240-bb0243 operon is not expressed, was generated. Similar to the rrp1 mutant, the glp mutant has a growth defect in BSK-glycerol medium. In vivo, the glp mutant is also infectious in mice but has reduced survival in ticks. Constitutive expression of the bb0240-bb0243 operon in the rrp1 mutant fully rescues the growth defect in BSK-glycerol medium and partially restores survival of the rrp1 mutant in ticks. Thus, c-di-GMP appears to govern a catabolic switch in B. burgdorferi and plays a vital role in the tick part of the spirochetal enzootic cycle. This work provides the first evidence that c-di-GMP is essential for a pathogen's survival in its vector host.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002133
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002133Summary
Cyclic dimeric GMP (c-di-GMP) is a bacterial second messenger that modulates many biological processes. Although its role in bacterial pathogenesis during mammalian infection has been documented, the role of c-di-GMP in a pathogen's life cycle within a vector host is less understood. The enzootic cycle of the Lyme disease pathogen Borrelia burgdorferi involves both a mammalian host and an Ixodes tick vector. The B. burgdorferi genome encodes a single copy of the diguanylate cyclase gene (rrp1), which is responsible for c-di-GMP synthesis. To determine the role of c-di-GMP in the life cycle of B. burgdorferi, an Rrp1-deficient B. burgdorferi strain was generated. The rrp1 mutant remains infectious in the mammalian host but cannot survive in the tick vector. Microarray analyses revealed that expression of a four-gene operon involved in glycerol transport and metabolism, bb0240-bb0243, was significantly downregulated by abrogation of Rrp1. In vitro, the rrp1 mutant is impaired in growth in the media containing glycerol as the carbon source (BSK-glycerol). To determine the contribution of the glycerol metabolic pathway to the rrp1 mutant phenotype, a glp mutant, in which the entire bb0240-bb0243 operon is not expressed, was generated. Similar to the rrp1 mutant, the glp mutant has a growth defect in BSK-glycerol medium. In vivo, the glp mutant is also infectious in mice but has reduced survival in ticks. Constitutive expression of the bb0240-bb0243 operon in the rrp1 mutant fully rescues the growth defect in BSK-glycerol medium and partially restores survival of the rrp1 mutant in ticks. Thus, c-di-GMP appears to govern a catabolic switch in B. burgdorferi and plays a vital role in the tick part of the spirochetal enzootic cycle. This work provides the first evidence that c-di-GMP is essential for a pathogen's survival in its vector host.
Introduction
Bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP), discovered by Benziman and colleagues in the mid-80s [1], is now widely recognized as a ubiquitous second messenger that modulates many aspects of biological processes in bacteria (for reviews, see [2], [3], [4]). C-di-GMP is synthesized by diguanylate cyclases (DGCs), a group of GGDEF domain-containing proteins, and is broken down by phosphodiesterases (PDEs) that contain a conserved EAL or HD-GYP domain [5], [6], [7], [8], [9], [10], [11]. GGDEF, EAL and HD-GYP domains are among the most abundant domains encoded in bacterial genomes [5], [12]. Numerous studies on c-di-GMP signaling pathways in the Proteobacteria revealed that c-di-GMP controls the transition between planktonic and biofilm lifestyles by stimulating the biosynthesis of adhesins and exopolysaccharide matrix substances in biofilms while inhibiting various forms of motility [13], [14], [15], [16], [17], [18], [19]. Several classes of c-di-GMP receptor/effector proteins have been identified [20]. Despite tremendous progress, the role of c-di-GMP in bacterial pathogenesis and the mechanisms of action of c-di-GMP remain poorly understood [4], [21], [22]. Further, very little is known about the function of c-di-GMP beyond Proteobacteria.
Borrelia burgdorferi is a spirochete that causes Lyme disease, the most prevalent vector-borne infection in the United States [23]. As an obligate pathogen, B. burgdorferi has a reduced genome that contains a limited number of genes that are known to be involved in signal transduction and gene regulation [24], [25]. For instance, the genome only has two sets of two-component signal transduction systems: Hk1-Rrp1 (BB0420-BB0419) and Hk2-Rrp2 (BB0764-BB0763), in addition to the chemotaxis CheA-CheY system. On the other hand, the enzootic life cycle of B. burgdorferi is complex. It involves two markedly different hosts, an arthropod vector and a small mammal. This unique lifestyle requires B. burgdorferi to utilize its limited signaling capabilities for adapting to dramatic changes in host environments during its natural cycle. In this regard, the Hk2-Rrp2 two-component signaling pathway has been shown to modulate differential expression of numerous surface lipoprotein genes and plays an essential role for spirochetal transmission and mammalian infection [26], [27], [28], [29], [30].
Little is known about the function of the second two-component system present in B. burgdorferi, Hk1-Rrp1. The response regulator Rrp1 contains an N-terminal response regulator receiver domain and a C-terminal GGDEF domain [8]. Ryjenkov et al. demonstrated that recombinant Rrp1 has DGC activity that strictly depends on the phosphorylation status of Rrp1 [8]. The complete enzootic cycle of B. burgdorferi and the pathogenesis of the disease can be largely reproduced in the laboratory [31]. Rrp1 is the only GGDEF-domain protein in B. burgdorferi, making this organism attractive for uncovering the role of c-di-GMP-mediated signaling in bacterial pathogenesis [5], [8].
Two recent studies have shed light on the potential role that c-di-GMP plays in the life cycle of B. burgdorferi. Rogers et al. showed that rrp1 is significantly upregulated upon tick feeding [32]. They also generated an rrp1 mutant in the non-infectious clone B31 5A13. The mutant showed altered expression of more than 140 genes (8% of the genome) whose functions covered almost all functional categories, including cell envelope biosynthesis, transport, metabolism, chemotaxis, and flagellar biosynthesis [32]. The rrp1 mutant also showed reduced growth at room temperature and increased serum sensitivity [32]. Another study focused on BB0363, the only EAL-domain protein encoded in the B. burgdorferi genome [33]. Recombinant BB0363 was shown to have c-di-GMP phosphodiesterase activity. The bb0363 mutant, which likely has high intracellular levels of c-di-GMP, was found to be defective in motility in vitro [33]. In vivo, the bb0363 mutant was able to survive in ticks but failed to establish infection in mice, suggesting that high levels of c-di-GMP are detrimental for spirochetes to replicate in a mammalian host. However, whether c-di-GMP is required for any stage of the infectious cycle of B. burgdorferi remains undetermined. In this study, we generated an rrp1 mutant in the infectious clone of B. burgdorferi, B31 5A4NP1. We show that Rrp1 is dispensable for mammalian infection but is essential for spirochetal survival in the tick vector. We further show that the Rrp1 requirement is, in part, due to its control over the expression of glycerol transport and metabolism in B. burgdorferi.
Results
Generation of the rrp1 mutant and the repaired strain
To determine the role of c-di-GMP in B. burgdorferi pathogenesis, we constructed an rrp1 mutant in the infectious B. burgdorferi strain 5A4NP1 (See Table 1 for a list of strains used in this study). This was accomplished by replacing the wild-type chromosomal rrp1 copy with a disrupted gene via homologous recombination (Fig. 1A). A similar approach was used to repair the wild-type rrp1 gene by replacing the mutated copy with a wild-type rrp1 (Fig. 1A). The genotypes of the rrp1 mutant and the repaired strain (rrp1com) were confirmed by PCR (Fig. 1B) and by immunoblot analyses (Fig 1C).
Fig. 1. Construction of the rrp1 mutant and the repaired strain. 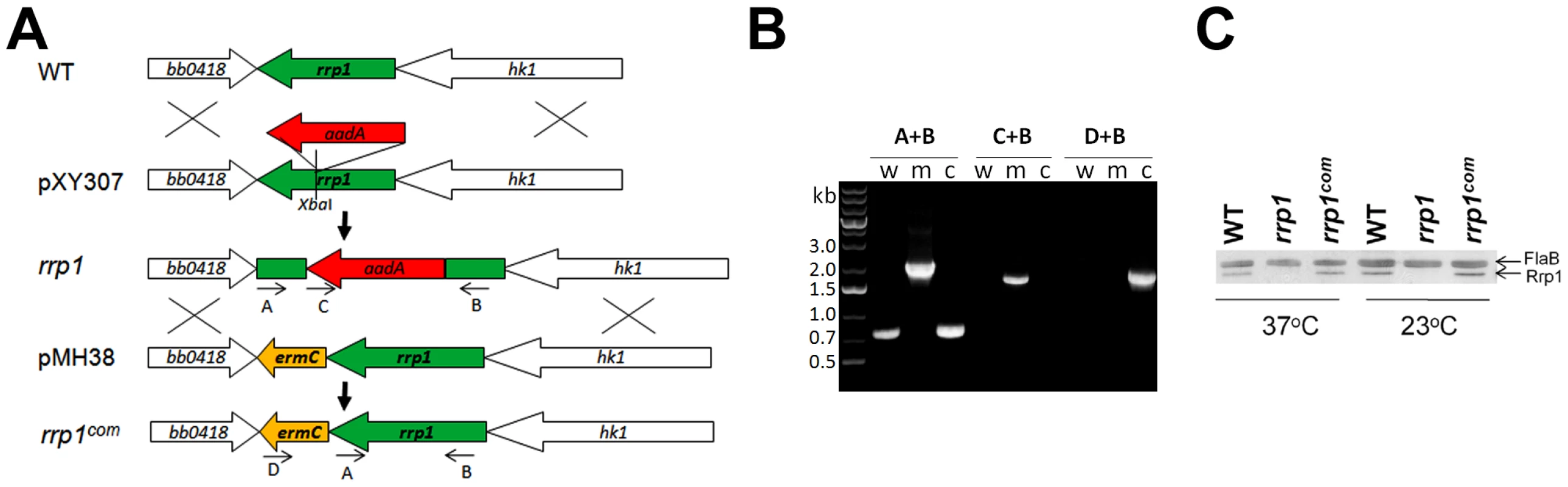
(A) Strategy for construction of the rrp1 mutant and the repaired strain (rrp1com). Arrows indicate the approximate positions of the oligonucleotide primers used for PCR analysis. (B) PCR analysis of strains. The specific primer pairs used in PCR are indicated above lanes. Lane W, wild-type (5A4NP1); lane M, the rrp1 mutant; lane C, rrp1com. (C) Western blot analysis of whole-cell lysates of WT, rrp1, and rrp1com spirochetes probed with α-Rrp1 and α-FlaB monoclonal antibodies. Tab. 1. <i>B. burgdorferi</i> strains used in this study. 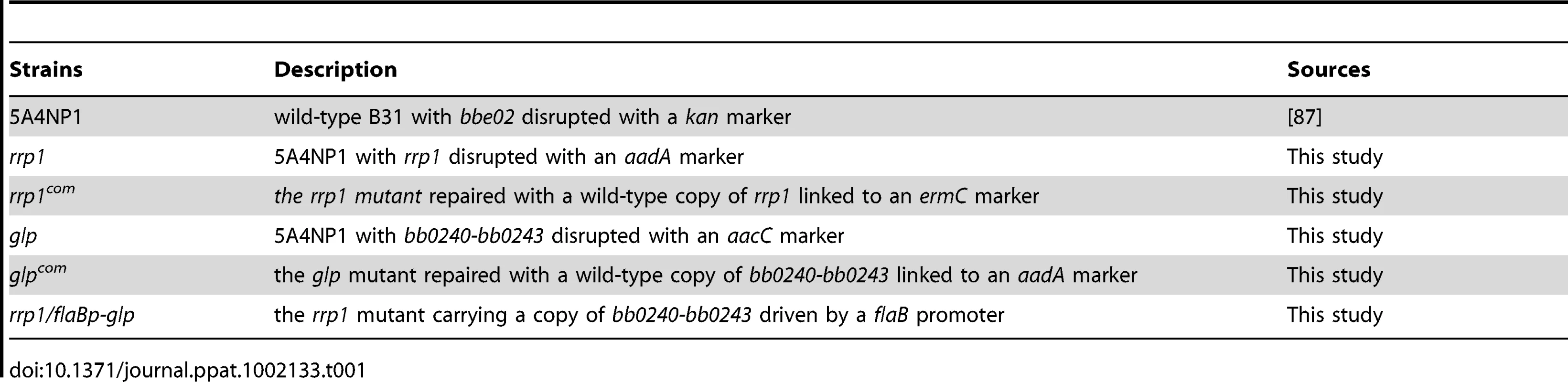
Rrp1 is not required for spirochetal infection in mammals
To determine the role of c-di-GMP in mammalian infection, we needle-inoculated groups of mice with various B. burgdorferi strains (105 spirochetes/mouse). Two-weeks post inoculation, ear punch biopsies were cultured in BSKII medium for the presence of spirochetes. Similar to wild-type spirochetes, the rrp1 mutant was readily detected in either immunocompetent (C3H/HeN) or immunocompromised (C3H-SCID) mouse strains (Table 2). No major difference in ID50 values between wild-type and the rrp1 mutant (Table 3). Further analysis of histopathology revealed that the rrp1 mutant elicited Lyme arthritis similar to that induced by wild-type B. burgdorferi. (Supplemental Fig. S1). This result indicates that abrogation of c-di-GMP synthesis does not affect the ability of B. burgdorferi to infect mice. We conclude that c-di-GMP is dispensable for mammalian infection. This is in contrast with an avirulent phenotype observed in B. burgdorferi lacking the c-di-GMP phosphodiesterase BB0363 [33].
Tab. 2. Mouse infectivity of the <i>rrp1</i> mutant. 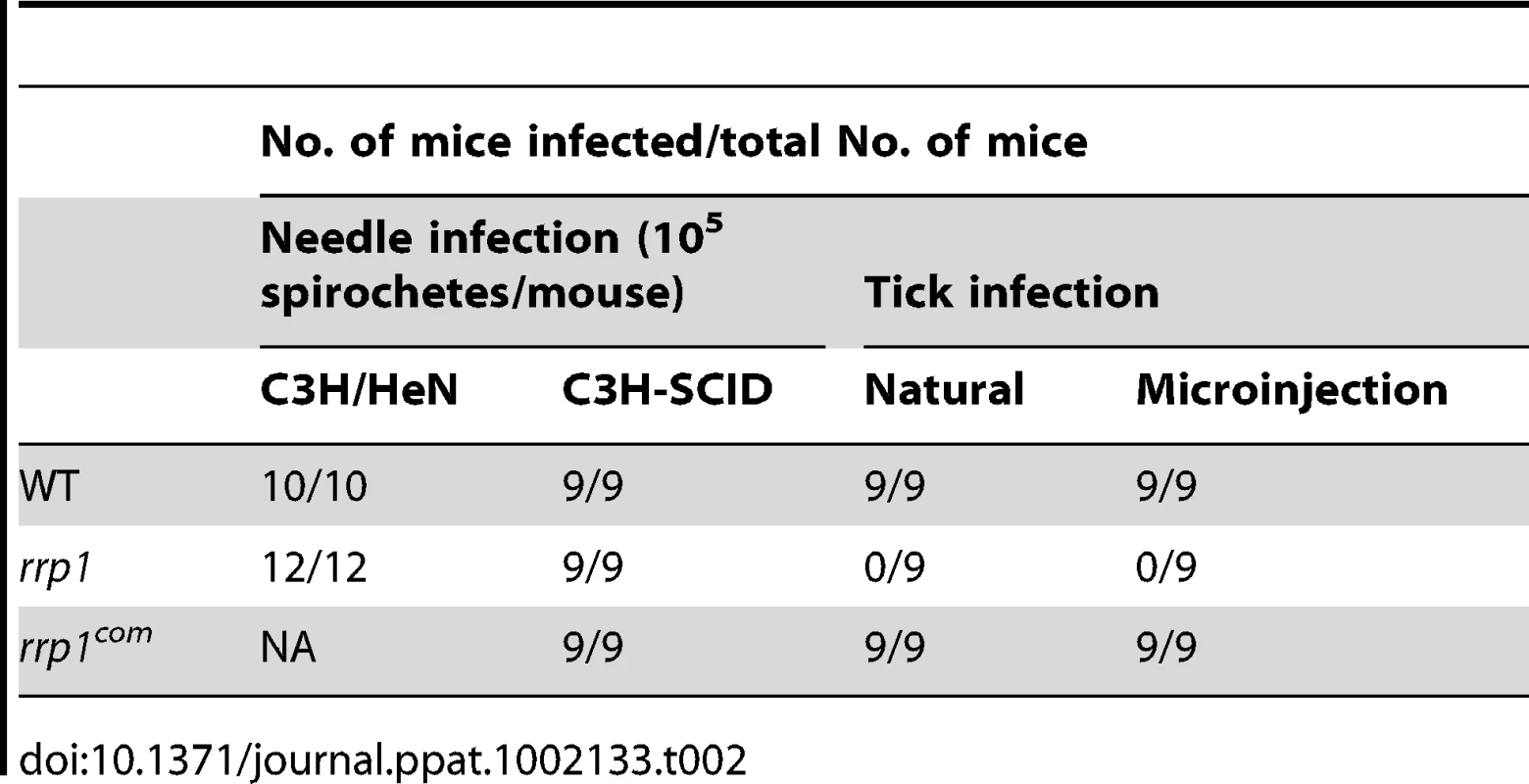
Tab. 3. ID<sub>50</sub> values of various <i>B. burgdorferi</i> strains. 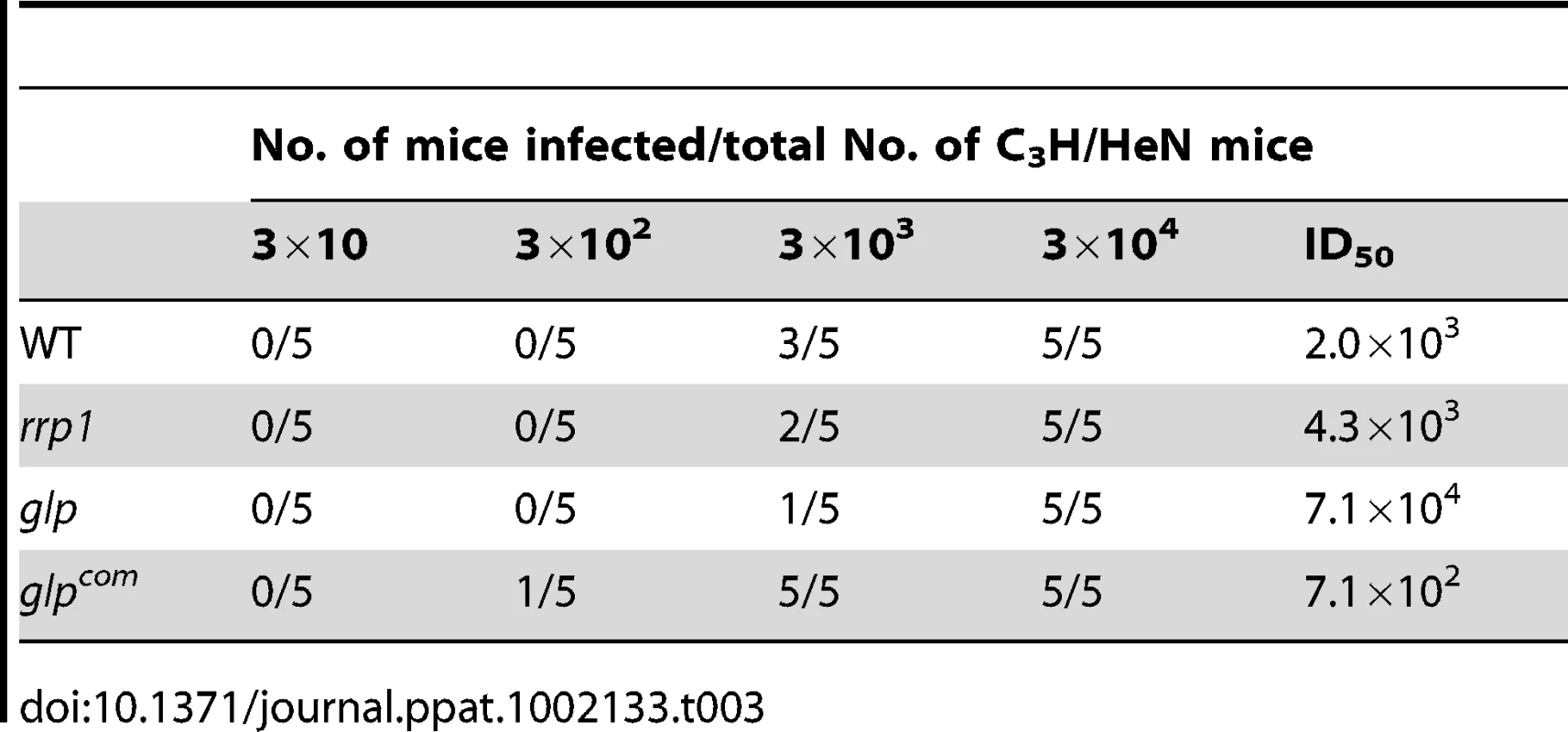
Rrp1 is essential for spirochetal survival in ticks
To examine the rrp1 mutant's phenotype in the tick cycle, groups of pathogen-free Ixodes scapularis larvae were fed on C3H/SCID mice that were needle-infected with the wild-type, rrp1mut or rrp1com strains two weeks after infection. Engorged larvae were collected after repletion and tick contents were subjected to immunofluorescence assay (IFA). In contrast to the wild-type and rrp1com strains that were readily detectable in fed larvae, virtually no rrp1 mutant spirochetes were observed (Fig. 2A). Further quantitative PCR analysis revealed that there were significantly lower numbers of the rrp1 mutant than that of wild-type or rrp1com strains in ticks (Fig. 2B).
Fig. 2. The rrp1 mutant failed to survive in ticks upon acquisition. 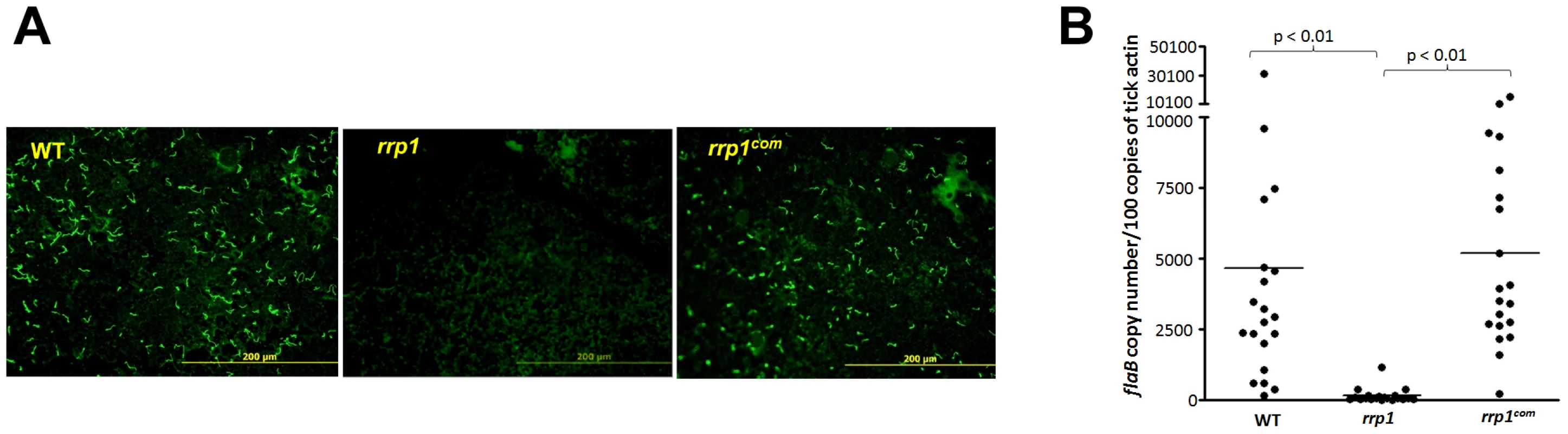
(A) IFA analysis of spirochetes from fed larvae. I. scapularis larvae were fed on needle infected C3H/SCID mice harboring wild-type (5A4NP1), rrp1, or rrp1com spirochetes. Engorged larvae were collected after repletion and subjected to IFA analysis using fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody. Twelve ticks were examined in each group and a representative image for each group of ticks is shown. (B) qPCR analyses of spirochete burden in fed larvae. Quantitative PCR for the B. burgdorferi flaB gene was performed with DNA extracted from fed larvae. Each dot represents one data point from one three larval tick (a total of 20 samples with 60 ticks examined for each group from two independent experiments). The horizontal bar represents the mean value of each group. The inability to detect the rrp1 mutant in tick midguts after feeding could be due either to a defect in tick midgut survival or a defect in migration from the mouse to the tick. To test these two possibilities, we used microinjection to directly place spirochetes into midguts of nymphal ticks [34], [35]. These artificially infected ticks then fed on naïve mice. Detached ticks were collected and subjected to IFA analysis. As shown in Fig. 3, the wild-type and rrp1com strains were readily detectable in ticks, whereas the rrp1 mutant remained undetected.
Fig. 3. The rrp1 mutant could not survive in artificially infected ticks upon feeding. 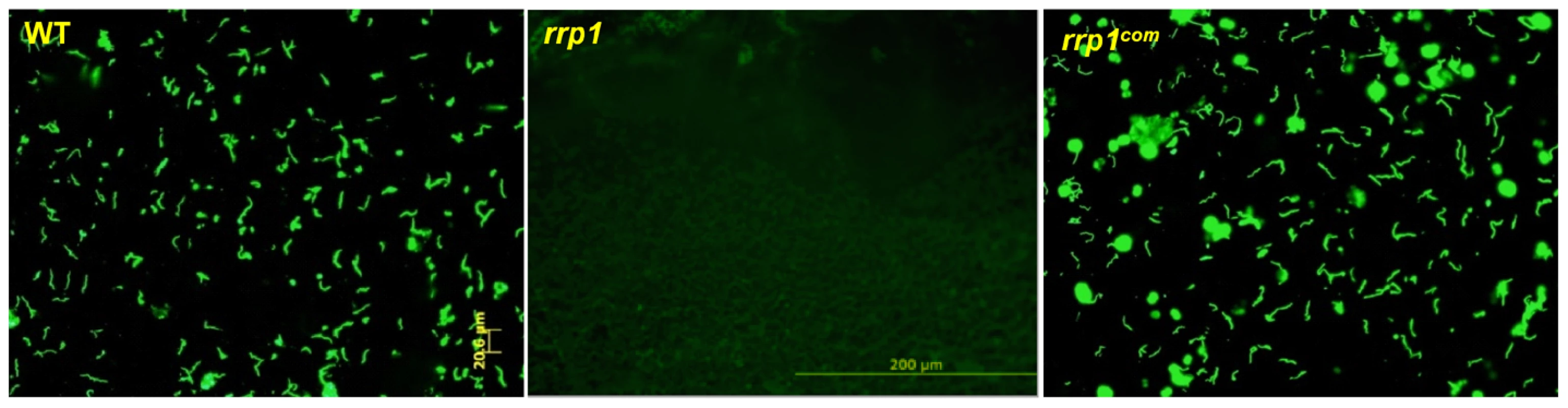
Unfed I. scapularis nymphs were microinjected with wild-type (5A4NP1), rrp1, or rrp1com spirochetes and then fed on naïve mice. The engorged nymphs were collected after repletion and subjected to IFA analysis using fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody. Ten ticks were examined in each group and a representative image for each group of ticks is shown in this figure. To confirm that the rrp1 mutant is defective in the ability to survive in ticks, engorged larvae that were fed on infected mice from the experiments described above were allowed to molt to nymphs in an environmental chamber. Unfed nymphs were then fed on naïve mice. Ticks that were infected with either the wild-type or rrp1com strains could readily infect naïve mice, whereas ticks infected with the rrp1 mutant could not (Table 2). Similarly, ticks that were artificially infected with the rrp1 mutant were also unable to infect C3H/SCID mice (Table 2). These results support the notion that the rrp1 mutant is unable to survive in the tick vector.
Transcriptome analyses of the rrp1 mutant
To investigate the molecular mechanisms underlying the requirement of c-di-GMP for spirochete survival in ticks, we sought to identify genes whose expression was affected by the deletion of rrp1. To do so, we performed two independent microarray analyses: one comparing transcriptional profiles of the wild-type and rrp1 mutant and the other comparing transcriptional profiles of the rrp1 mutant and the rrp1com strain. The comparison of the transcriptomes of the wild-type and rrp1 mutant revealed 120 genes whose expressions were up - or down-regulated by Rrp1 (cut-off >3-fold) (Text S1). Among these, 39 genes whose dependence on Rrp1 could be confirmed by the comparison of the transcriptomes of the rrp1 and rrp1com strains (cut-off >3-fold) (Table 4). We considered these genes to be the most reliable candidates for Rrp1-dependent regulation.
Tab. 4. Rrp1-regulated genes in B. burgdorferi. 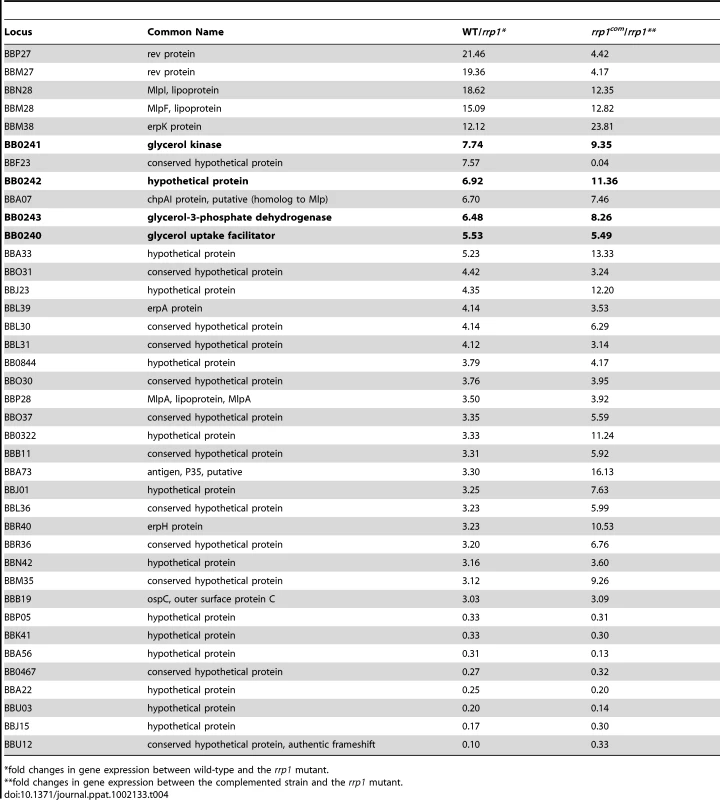
*fold changes in gene expression between wild-type and the rrp1 mutant. Rrp1 controls expression of glycerol transport and metabolism
Genes regulated by Rrp1 are distributed throughout the genome and extra-chromosomal segments of B. burgdorferi (Table 4, Locus numbers start with BB and a letter are extra-chromosomal genes [24]). Among these genes, an intriguing target of Rrp1 regulation was an apparent glp operon encoding glycerol transport/metabolism genes, bb0240-bb0243 [24], [25], [36], [37]. The first gene of the operon, bb0240, encodes a putative glycerol uptake facilitator (GlpF), followed by a putative glycerol kinase gene (bb0241, glpK), a small putative hypothetical gene (bb0242), and a putative glycerol-3-phosphate dehydrogenase gene (bb0243, glpA/glpD). Glycerol can be utilized in energy production as a biosynthetic precursor to membrane lipids or lipoproteins [24], [25], [36], [37].
qRT-PCR analysis confirmed that induction of bb0240-bb0243 was indeed under the control of Rrp1 (Fig. 4A). We hypothesized that if bb0240-bb0243 is involved in glycerol transport and metabolism, the rrp1 mutant may have a defect in the utilization of glycerol as a carbon source. To test this hypothesis, the wild-type, rrp1 and rrp1com strains were cultivated in either standard BSKII medium or in a modified BSKII medium where glucose was replaced with glycerol (BSK-glycerol, which was prepared from glucose free CMRL) [37]. The rrp1 mutant was not impaired in growth in the standard BSKII medium at either 35°C (Fig. 4B) or 23°C (Fig. 4D). However, when grown in the BSK-glycerol medium, the rrp1 mutant failed to reach the cell density of the wild-type or rrp1com (Fig. 4C and 4E). Thus, glycerol transport and metabolism appeared to be particularly important at later time points in the growth curve. BSK medium is a complex medium containing many undefined components including rabbit serum and BSA as well as other potential carbon source such as pyruvate. Presence or absence of pyruvate did not significantly affect the growth of either wild-type or the rrp1 mutant in BSK-II or BSK-glycerol medium (data not shown). BSK-glycerol medium also contains 0.1 g/L of glucose, determined by D-Glucose Kit (Roche Applied Science, Indianapolis, IN), which may contribute to the initial growth of the rrp1 mutant in BSK-glycerol medium (the standard BSK-II medium contains 6 g/L of glucose). Nevertheless, these experiments verified the involvement of Rrp1 in glycerol transport/metabolism.
Fig. 4. Rrp1 controls expression of the glycerol gene operon (bb0240-0243). 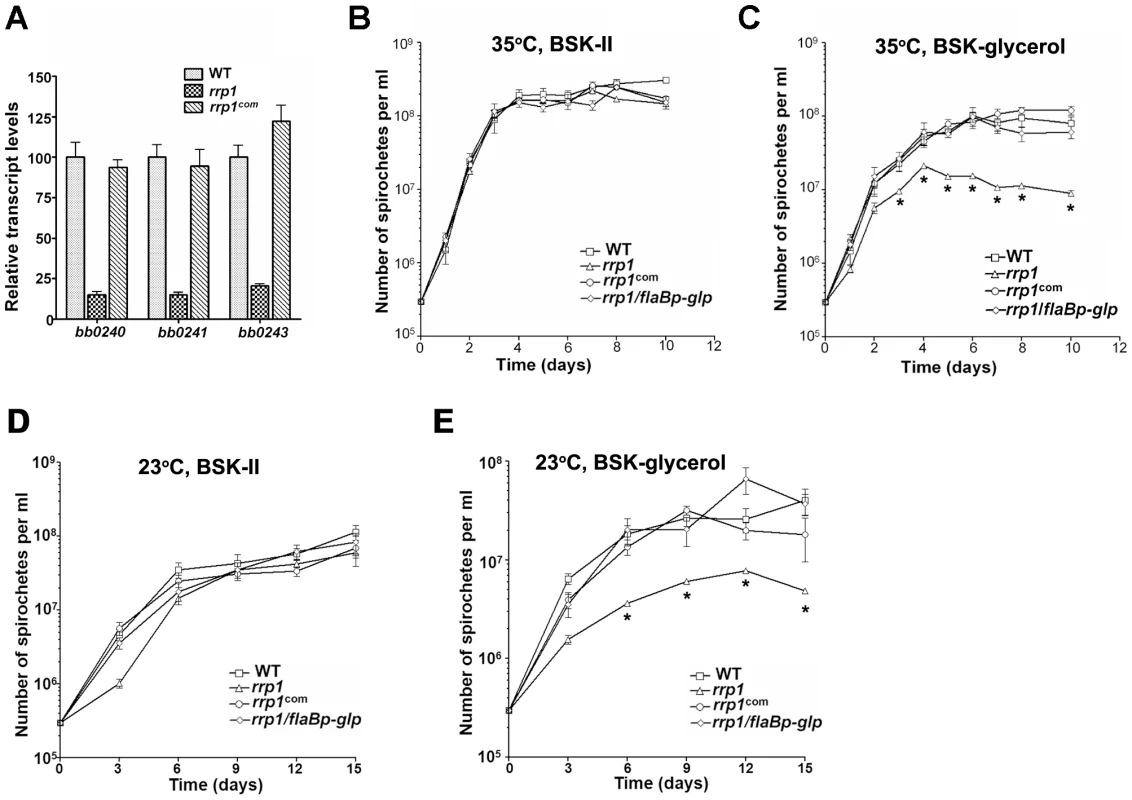
(A) Relative transcript levels of the glycerol operon glp (bb0240-bb0243) in wild-type, rrp1, and rrp1com by real-time RT-PCR. RNA was isolated from late logarithmic phase cultures grown at 35°C in standard BSK-II medium. Values represent the average copy number for each gene (± standard deviation) normalized per 1000 copies of flaB. (B–E) growth curves of wild-type, rrp1, rrp1com or rrp1/flaBp-glp at 23°C (D, E) or 35°C (B, C) in standard BSK-II medium (B, D) or BSK-glycerol medium (C, E). The initial cell density was 3×105 cells/ml for each strain. Spirochetes were enumerated under dark-field microscopy. Data presented here is from one representative experiment with three independent cultures. Each data point was the average of data from three independent cultures. *, P<0.05. Glycerol enhances expression of rrp1
We further tested the possibility that expression of rrp1 is also influenced by glycerol. RNA was extracted from wild-type B. burgdorferi grown in either standard BSKII or BSKII-glycerol medium. The extracted RNAs were subjected to qRT-PCR analysis. Growth in the BSKII-glycerol medium did not significantly alter expression of Rrp2-dependent genes such as rpoS and ospC. However, the transcript level of rrp1 as well as the glycerol metabolic genes bb0240-bb0243 were dramatically upregulated when grown in BSKII-glycerol medium (Fig. 5A). However, Rrp1 protein level is much less influenced by this growth condition (1.7 fold) (Fig. 5B). Nevertheless, this observation suggests that glycerol may potentially enhance rrp1 expression.
Fig. 5. Glycerol induces expression of rrp1 and bb0240-bb0243. 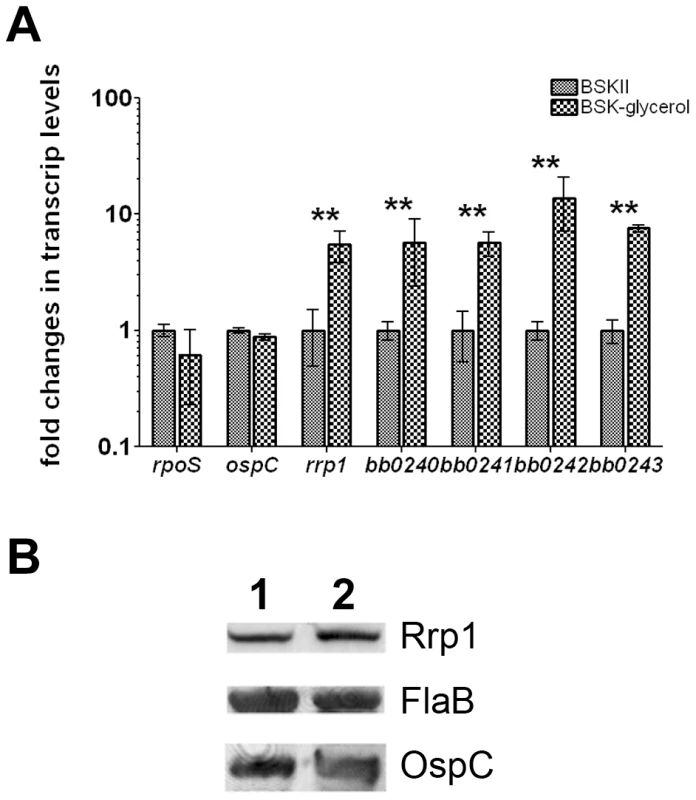
Wild-type B. burgdorferi strain B31 5A4NP1 was grown at 35°C in either standard BSKII or BSK-glycerol medium. Cells were harvested at late logarithmic phase. (A) qRT-PCR. RNAs were extracted and subjected to real-time RT-PCR analyses for rrp1, bb0240, bb0241, bb0242, bb0243, rpoS, ospC, and flaB. Levels of expression of each gene were normalized with the level of flaB expression in each sample. Relative fold change of gene expression between the two growth conditions were reported (with levels of expression of each gene in standard BSKII media as values of 1). **, p<0.01. (B) immunoblot against Rrp1, FlaB, or OspC. Lane 1, spirochetes cultivated in BSK-II medium. Lane 2, spirochetes cultivated in BSK-glycerol medium. Upon normalization against FlaB, Rrp1 level is 1.7 fold higher in BSK-glycerol medium than that in standard medium. Glycerol transport and/or metabolism is required for maximal spirochete burden in ticks
Because Rrp1 was required for full induction of the glycerol operon and for maximal growth in the BSKII-glycerol medium, we hypothesized that defective glycerol metabolism by the rrp1 mutant could contribute to the mutant's inability to survive in ticks. If so, a mutant defective in glycerol metabolism would be expected to have a phenotype similar to that of the rrp1 mutant. To test this hypothesis, we constructed a glp mutant by deleting a portion of the first gene bb0240 and its upstream promoter of the bb0240-bb0243 operon (Fig. 6A). qRT-PCR analysis confirmed that the glp mutant lacks bb0240 bb0241, bb0242, and bb0243 mRNA (Fig. 6B). Expression of bb0240-bb0243 was restored when the mutated bb0240 gene and the promoter region was replaced by the wild-type copy of bb0240 at the native location (designated as glpcom. Fig. 6A and 6B).
Fig. 6. Construction of the glp mutant and the repaired strain. 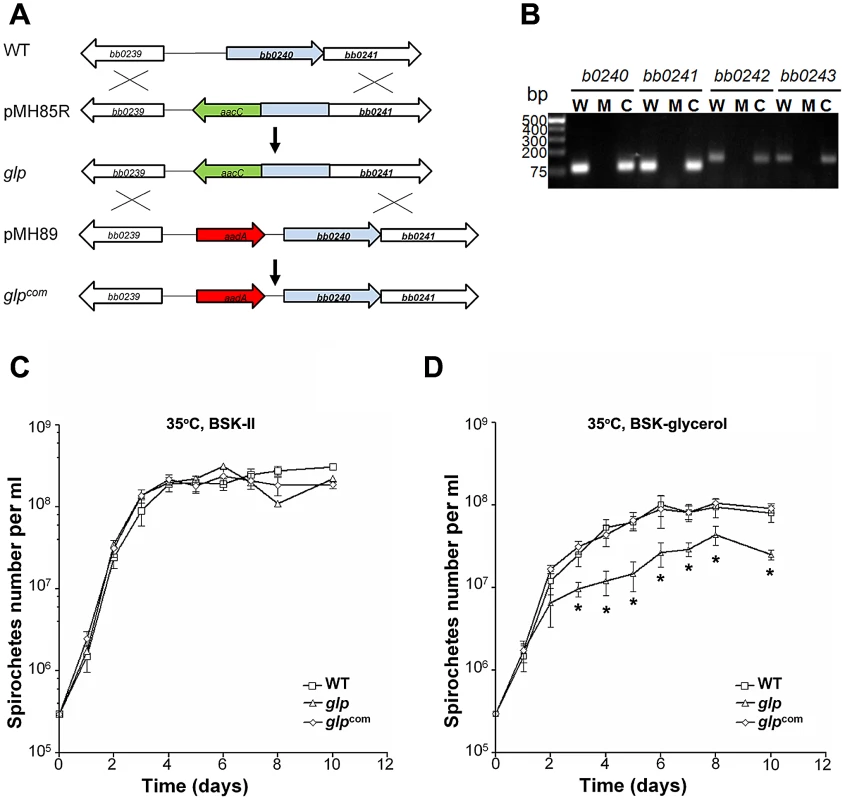
(A) Strategy for construction of the glp mutant and the repaired strain (glpcom). (B) RT-PCR analysis of expression of bb0240-bb0243. RNA was isolated from late logarithmic phase cultures grown at 35°C in the BSK-II medium (pH 7.5). W: wild-type strain 5A4NP1; M: the glp mutant; C: the repaired strain (glpcom). (C) and (D) Growth curve of wild-type, glp and glpcom at 35°C in standard BSK-H medium (C) or BSK-glycerol medium (D). The initial cell density was 3×105 cells/ml for each strain. Spirochetes were enumerated under dark-field microscopy. Data presented here is from one representative experiment with three independent cultures. Each data point was the average of data from three independent cultures. *, p< 0.05. We first examined the growth phenotype of the glp mutant in vitro. The mutant had no detectable growth defect when grown in standard BSKII medium (Fig. 6C). However, similar to the rrp1 mutant, the glp mutant could not reach the same cell density as the parent wild-type strain when grown in the BSK-glycerol medium (Fig. 6D). This defect resulted from abrogation of bb0240-0243 expression, as the growth defect was readily restored upon restoration of bb0240-bb0243 expression in glpcom (Fig. 6A & 6D). This result is consistent with the prediction that the growth defect of the rrp1 mutant in the BSK-glycerol medium is due to the loss of expression of bb0240-bb0243.
We then examined the phenotype of the glp mutant in vivo. The wild-type, glp mutant or glpcom spirochetes (105 spirochetes/mouse), were intradermally inoculated into C3H/HeN mice. Two weeks after inoculation, ear punch biopsies from all mice were culture-positive for spirochetes, suggesting that BB0240-BB0243 are not required for mammalian infection (Table 5). Further determination of the ID50 values showed that the glp mutant has a slight infectivity deficit relative to wild-type B. burgdorferi, with 1-log-unit increase in the ID50 (Table 3). To examine the role of bb0240-0243 in the tick-mouse cycle, pathogen-free unfed larvae were placed on infected mice. Fed larvae were collected and allowed to molt to nymphs. Unfed nymphs then fed on groups of naïve C3H/HeN mice. Ticks at various stages were collected for IFA and/or qRT-PCR analyses. We observed that although detectable in ticks, the glp mutant had reduced spirochetal loads compared to the wild-type or glpcom strains (Fig. 7A & 7B, only results from nymphs were shown). These data suggest that, similar to Rrp1, the glycerol transport/metabolic pathway is required for the optimal colonization of B. burgdorferi in ticks and that the loss of bb0240-bb0243 expression in the rrp1 mutant contributes to its poor survival in ticks.
Fig. 7. Glycerol transport/metabolism is important during tick residence. 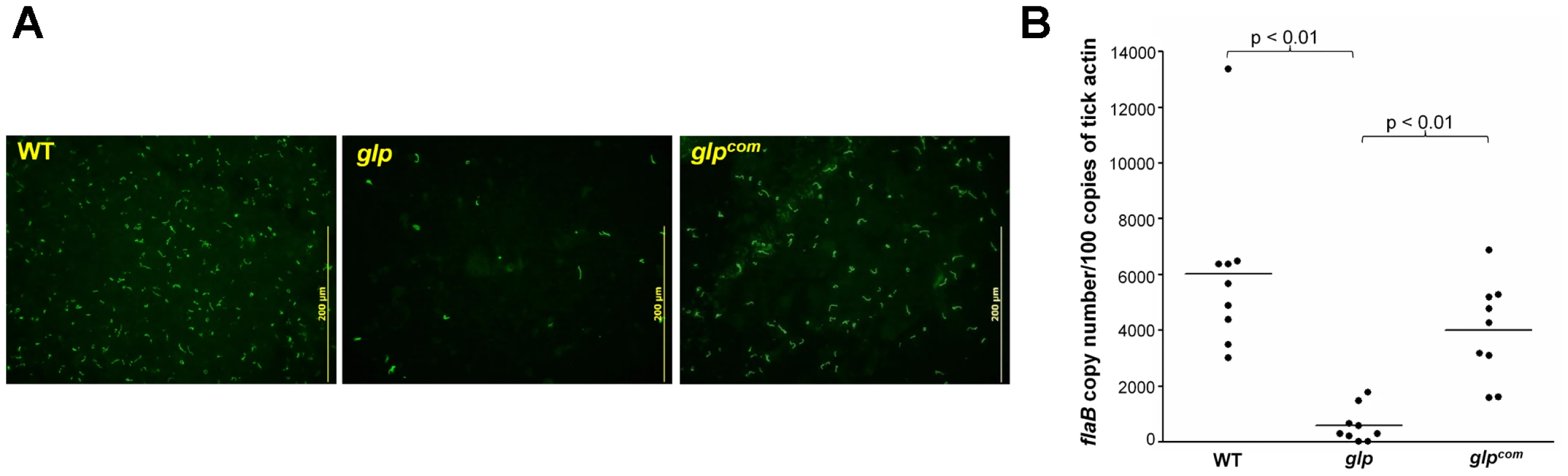
Naïve larvae fed on needle inoculated C3H/HeN mice infected with wild-type, glp, or glpcom. Fed larvae were allowed to molt to nymphs. Infected unfed nymphs were then fed on naïve mice to produce fed nymphs. Fed nymphs were then subjected to IFA analysis (A) and qPCR analysis (B). For qPCR, copies of the B. burgdorferi flaB genes were chosen to represent spirochete numbers and the values were reported relative to 100 copies of the tick actin gene. Each data point is from one tick. Tab. 5. Mouse infectivity of the <i>glp</i> mutant. 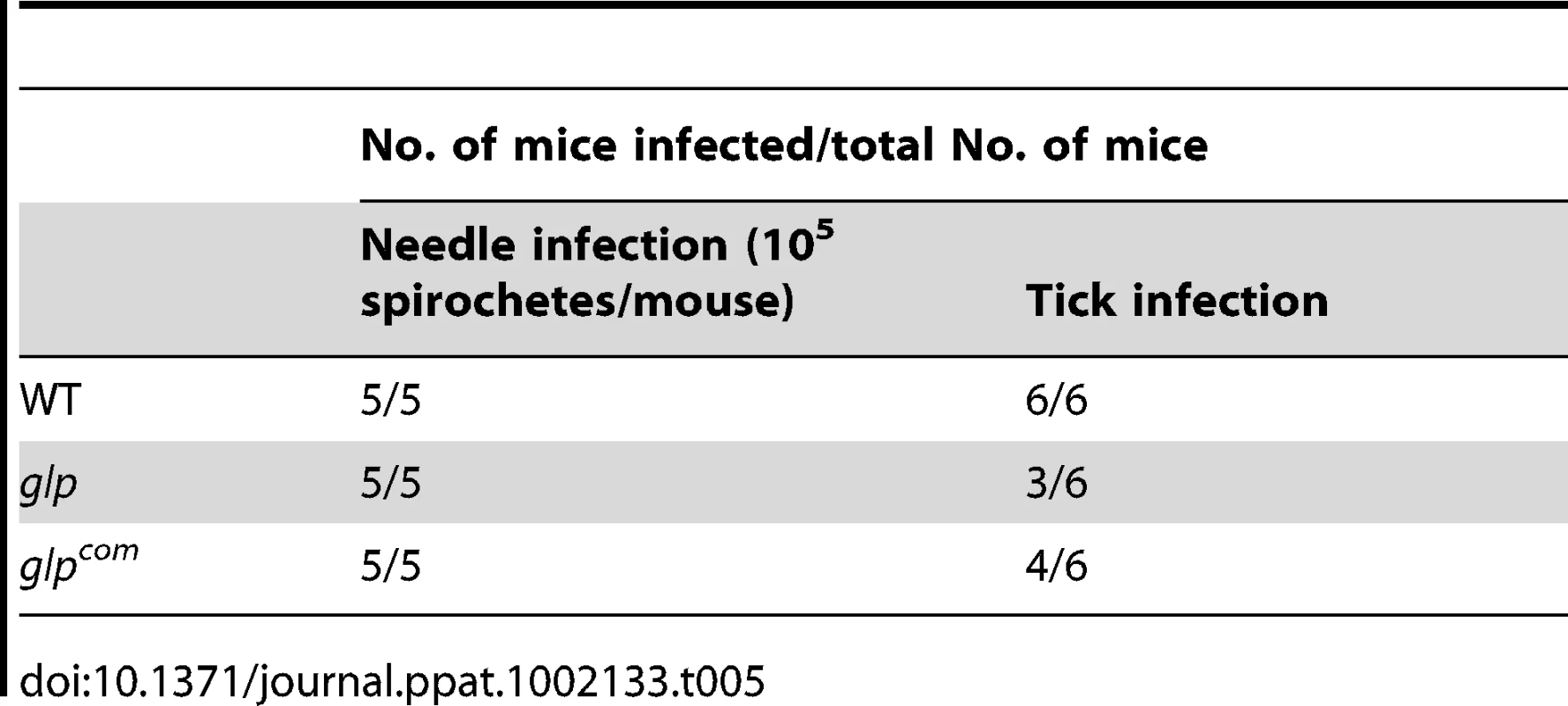
Mice two weeks post tick feeding were also examined for the presence of spirochetes in various tissue samples (skin, heart, and joint). Unlike the rrp1 mutant that failed to infect mice via tick bites, the glp mutant was capable of completing the tick-mouse cycle and subsequently infecting naïve mice upon tick feeding (Table 5), despite its reduced survival in ticks. Note that both the glp mutant and glpcom strains showed partially reduced infectivity via tick bites, indicating that this reduction of infectivity is not due to the loss of bb0240-bb0243 (Table 5). These results indicate that loss of bb0240-bb0243 expression of the rrp1 mutant could not fully account for the inability of the rrp1 mutant to complete its enzootic cycle and that Rrp1 controls additional factor(s) involved in the spirochetal life cycle in ticks.
Constitutive expression of glycerol metabolic pathway partially rescues the rrp1 mutant's phenotype in ticks
To further investigate the role of glycerol transport and metabolism during tick infection, we constitutively expressed the bb0240-bb0243 operon in the rrp1 mutant using an independent flaB promoter (Fig. 8A). The resulting strain, designated as rrp1mut/flaBp-glp, expressed bb0240-bb0243 in an Rrp1-independent fashion (Fig. 8B) and fully rescued the growth defect of the rrp1 mutant in the BSK-glycerol medium (Fig. 4B & 4C). This observation provides additional genetic evidence that the growth defect of the rrp1 mutant is due to impaired glycerol transport/metabolism.
Fig. 8. Constitutive expression of glycerol metabolism genes in the rrp1 mutant partially restored spirochetal survival in ticks. 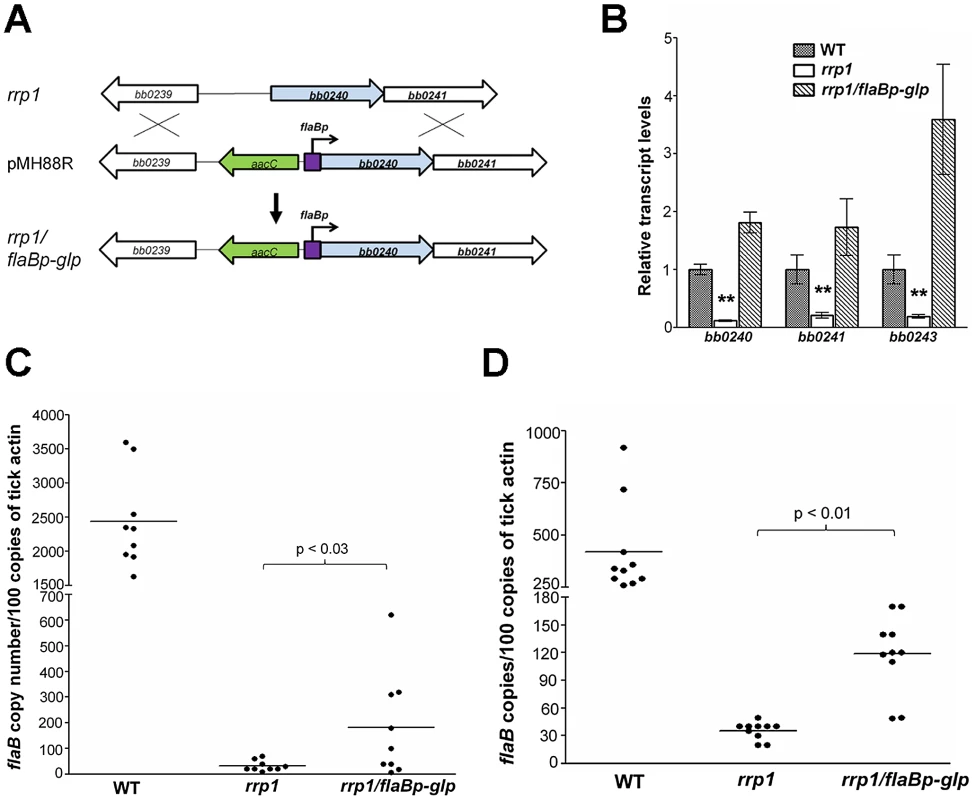
(A) Strategy for construction of an rrp1 mutant with constitutive expression of bb0240-bb0243, by replacing the promoter region of bb0240 with the flaB promoter. (B) Real-time RT-PCR analysis of expression of b0240, bb0241, and bb0243. RNA was isolated from late logarithmic phase cultures of wild-type (WT), the rrp1 mutant (rrp1), or rrp1/flaBp-glp that had been grown at 35°C in the BSK-II medium (pH 7.5). Levels of gene expression were normalized with the level of flaB gene expression. Fold changes of gene expression relative to wild-type strain (set as 1) were reported. (C) and (D), qPCR analysis of spirochetal burden in fed larvae one day (C) or fourteen days (D) after repletion. Copies of the B. burgdorferi flaB genes were chosen to represent spirochete numbers and are normalized with 100 copies of the tick actin gene in each sample. Each data point is from three fed larval ticks. **, p<0.01. To compare the phenotypes in ticks, the wild-type, rrp1 mutant, or rrp1/flaBp-glp strains were needle-infected into naïve mice. Unfed larvae were allowed to feed on these infected mice. qPCR analyses on fed larvae showed that the rrp1mut/flaBp-glp spirochetes had a 4 - to 5-fold increase in spirochetal load compared to the load of the rrp1 mutant (Fig. 8C & 8D). This increase suggests that restoration of expression of glycerol transport/metabolism can improve survival of the rrp1 mutant in ticks. However, the spirochete load of rrp1/flaBp-glp was still drastically lower than that of wild-type spirochetes (Fig. 8C & 8D). To determine if the rrp1/flaBp-glp spirochetes are able to migrate to mice, fed larvae were collected and allowed to molt to nymphs. Infected nymphs were then used to infect naïve C3H/SCID mice. The result showed that, similar to the rrp1 mutant, the rrp1mut/flaBp-glp strain was incapable of completing the tick-mouse cycle to infect naïve mice (Table 6). These data further support the conclusions that while glycerol transport/metabolism is important during tick residence, additional Rrp1-dependent factor(s) are involved in the tick-mouse cycle of B. burgdorferi.
Tab. 6. Mouse infectivity of the <i>rrp1</i> mutant with constitutive expression of <i>bb0240-bb0243.</i> 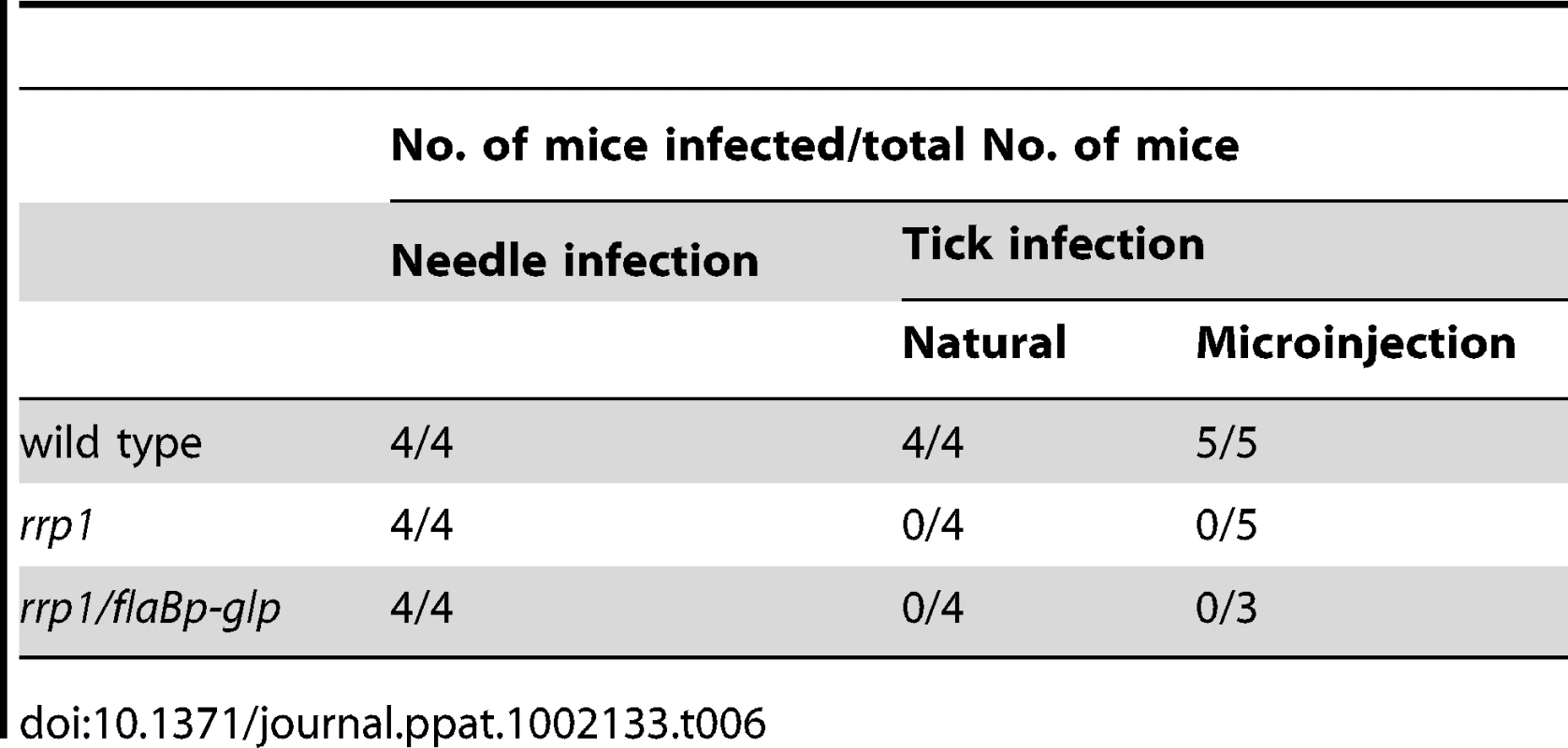
Discussion
During the transmission process between mammals and ticks, B. burgdorferi dramatically alters the expression of many genes that are essential for spirochete survival in either host (for reviews, see [31], [38]). In the past few years, we and others have shown that one of the B. burgdorferi two-component signaling systems, Hk2-Rrp2, functions as a key signaling pathway that governs expression of genes necessary for mammalian host infection [26], [27], [29], [30], [39]. In this study, we provide genetic evidence that the other two-component system, Hk1-Rrp1, is dispensable for mammalian infection, yet plays a vital role in the tick, in part, by controlling expression of the glycerol transport/metabolic pathway of B. burgdorferi.
Rrp1 is a diguanylate cyclase responsible for synthesis of the second messenger c-di-GMP [8], [32]. The importance of c-di-GMP to bacterial pathogenesis has been well documented [4], [21], [22]. In many cases, the impact of c-di-GMP on pathogenesis is due to its effect on biofilm formation or motility [40], [41], [42], [43]. An interesting example that is related to this study involves another vector-borne pathogen, Yersinia pestis. Similar to the phenotype of the rrp1 mutant in ticks that we have described herein, disruption of hmsT, a gene encoding diguanylate cyclase in Y. pestis, reduces the transmission of plague bacteria from fleas to mammals [44], [45], [46], [47]. However, the mechanisms of influencing transmission by c-di-GMP in these two pathogens seem to be different. Inactivation of hmsT results in a defect in biofilm formation but not in replication of Y. pestis in fleas, which is important for the spread of Y. pestis from fleas to mammals. Currently there is no evidence that B. burgdorferi forms biofilms. The B. burgdorferi genome encodes a luxS gene responsible for the autoinducer AI2 synthesis, which is necessary for biofilm formation in some bacteria [48], [49], [50]. However, inactivation of luxS does not affect the life cycle of B. burgdorferi in either ticks or mice [51], [52]. Therefore, the mechanism of action of c-di-GMP in the enzootic cycle of B. burgdorferi is different from that of Y. pestis.
In addition to affecting biofilm formation and motility, c-di-GMP modulates many other activities that may not be related to multicellular behavior such as cell division, phage resistance, heavy metal resistance, etc. [2], [3], [4]. With regards to bacterial pathogenesis, c-di-GMP has been reported to affect the processes of adhesion, invasion, and toxin production by modulating the production or activities of virulence factors [21], [22], [53], [54], [55]. However, modulation of bacterial infection by the control of glycerol metabolism is observed here for the first time. In this study, we provide the following lines of evidence supporting the notion that c-di-GMP controls glycerol transport and metabolism in B. burgdorferi, which in turn is important for its survival in ticks. 1) Expression of bb0240-bb0243 is significantly downregulated by abrogation of Rrp1 (Table 4 & Fig. 4A). 2) Both the rrp1 mutant and the glp mutant show growth defects in BSK-glycerol medium (Fig. 4C & Fig. 6D). 3) The glp mutant has reduced survival in ticks (Fig. 7). 4) Restoration of bb0240-bb0243 expression in the rrp1 mutant rescues the growth defect in vitro and enhances the survival of the rrp1 mutant in ticks.
What roles does glycerol transport/metabolism play in B. burgdorferi physiology? As an obligate pathogen, B. burgdorferi has a reduced genome and lacks many metabolic pathways such as the TCA cycle and those for synthesis of amino acids, nucleotides, and fatty acids [24], [25], [36]. B. burgdorferi does encode proteins for the utilization of several sugars in addition to glucose [24], [25], [36], [37]. Notably, a complete pathway for transport and utilization of glycerol (BB0240-BB0243) is preserved. Bioinformatics analysis suggests that upon uptake of glycerol (by glycerol uptake facilitator BB0240, GlpF), glycerol is converted to glycerol-3-P by glycerol kinase (BB0241, GlpK) [36], [37]. Glycerol-3-P can either feed into lipid/lipoprotein biosynthesis or enter the ATP-generating stage of glycolysis via conversion to glyceraldehyde 3-phosphate by glycerol-3-P dehydrogenase (BB0243, GlpA/GlpD) and triosephosphate isomerase (BB0561) [36], [37]. In other words, the glycerol and glucose pathways interconnect, and glycerol can be an important carbon and energy source at times when glucose becomes limited. This notion is supported by a previous study [37] as well as the in vitro growth data from this study (Fig. 4B & 4C). Based on the observation that the glycerol pathway-defective glp mutant replicates normally in mice but has reduced growth in ticks, we postulate that B. burgdorferi utilizes different carbon/energy sources within each host environment. During mammalian infection when glucose is readily available (0.1–0.2% in mouse blood) [56], B. burgdorferi utilizes glucose as the main carbon and energy source. Thus, inactivation of the glp operon does not dramatically affect spirochete replication in mammals. When spirochetes enter the tick vector, initially the glp mutant may be able to replicate with the presence of glucose from blood. Then, glucose may become limiting, while glycerol, on the other hand, may be available in ticks. This notion is consistent with the fact that the glp mutant remains capable of surviving in ticks but with reduced spirochetal numbers (Fig. 7). It is noteworthy that many insects including ticks produce glycerol as an anti-freezing molecule [57]. Therefore, activation of the glycerol transport and metabolism via Rrp1 could ensure optimal growth of B. burgdorferi in the tick vector. Further, growth on glycerol appears to provide a positive feedback on rrp1 gene expression (Fig. 5).
How does c-di-GMP control the expression of bb0240-bb0243? One of the characterized mechanisms employed by c-di-GMP to influence gene regulation is through a unique riboswitch RNA structure. It was shown that c-di-GMP can directly bind to a riboswitch located in the 5′ UTR region of target genes and can influence gene transcription and/or translation [58]. We did not find c-di-GMP-specific riboswitches upstream of bb0240. C-di-GMP can also modulate gene expression by affecting expression or activity of transcription factors [59], [60], [61], [62], [63]. Some of these transcription factors bind c-di-GMP directly [64], [65]. Interestingly, c-di-GMP controls DNA binding of a subgroup of CRP (cAMP receptor protein) transcription factors that activate genes involved in utilization of alternative carbon and energy sources (other than glucose). For example, Clp, a CRP homolog from Xanthomonas campestris binds c-di-GMP and regulates virulence gene expression [65], [66], [67]. In Vibrio cholerae, it was shown that cAMP-CRP controls expression of a DGC that, in turn, governs the production of c-di-GMP and biofilm formation [68]. Bioinformatic analysis did not identify any CRP homologue encoded in the B. burgdorferi genome. Recently, it was reported that another transcriptional regulator in B. burgdorferi, BosR, also affects glp expression [69], [70]. Thus, it is possible that c-di-GMP may influence glp via BosR. Nevertheless, elucidating the mechanism of how Rrp1 controls expression of the glycerol pathway in B. burgdorferi will shed light on the interplay between c-di-GMP and carbon utilization networks.
Work on Rrp1 from this study and previous studies [8], [32] strongly supports the notion that c-di-GMP is essential for spirochetal adaptation in the tick vector but is not required for mammalian infection. In fact, c-di-GMP is not only dispensable, shutting down the synthesis of c-di-GMP is necessary for B. burgdorferi to successfully establish infection in the mammalian host. This was recently demonstrated by Sultan et al., when they showed that the B. burgdorferi mutant missing c-di-GMP phosphodiesterase (BB0363) failed to infect mice [33]. This is consistent with an emerging theme that uncontrolled production of c-di-GMP is detrimental to the acute phase of bacterial infection [3], [21], [22]. Thus, a tight regulation of the synthesis of c-di-GMP is important for Borrelia adaptation in both the tick vector and the mammalian host.
What are the downstream effectors of c-di-GMP in B. burgdorferi? The bb0363 mutant showed a defect in motility, suggesting that flagellar proteins or gene transcription of B. burgdorferi may be direct targets of c-di-GMP, as shown in other bacteria [15], [17], [68], [71]. The rrp1 mutant did not have an apparent defect in motility, suggesting that c-di-GMP controls other bacterial factor(s) that are important to spirochetal survival in ticks. Note that although c-di-GMP may regulate transcription of flagellar genes [71], our microarray analysis indicates that flaB expression is not affected by rrp1 deletion and thus using the flaB as the reference gene in this study remains valid. In addition, expression of previously identified genes important for spirochetal survival in ticks, including ospA/B, bptA, dps, bb0365 and lp6.6 [34], [72], [73], [74], [75], [76], were not affected by Rrp1 (Table 4). Although glycerol transport/metabolism is important to the optimal growth of B. burgdorferi in ticks, independent expression of the glycerol transport/metabolism genes in the rrp1 mutant does not fully rescue spirochete survival in ticks, and the rrp1/flaB-glp spirochetes remain incapable of completing its entire enzootic cycle (Table 6). Thus, c-di-GMP likely controls yet-to-be-identified factor(s) that contribute to B. burgdorferi proliferation in ticks. In this regard, relatively few c-di-GMP targets have been identified in other bacteria to date. The best characterized c-di-GMP targets are PilZ domain-containing proteins, such as cellulose synthase subunit BcsA in Gluconacetobacter xylinus and motility regulatory protein YcgR in Escherichia coli [77], [78]. The B. burgdorferi genome encodes one PliZ protein, PlzA (BB0733) [79]. Interestingly, Freedman et al. showed that plzA expression is upregulated during tick feeding, suggesting a potential role of PlzA in the tick vector [79]. Whether PlzA plays a role in the enzootic cycle of B. burgdorferi remains to be determined.
Microarray analyses from this study and previous studies by Roger et al [32] suggest that expressions of several membrane-associated proteins including Rev, Mlps, and Erps are influenced by Rrp1. Whether these proteins/lipoproteins contribute to B. burgdorferi survival in ticks needs to be further determined. In addition, there are some significant differences between these two microarray results. Roger et al. showed that Rrp1 influences expression of more than 140 genes, most of which are chromosome-encoded core genes [32]. Our study reveals only few chromosome-encoded genes whose expression was affected by rrp1 deletion and such effect could be further restored in rrp1com. One difference between the two studies is the strain used. In this study, an infectious strain B31 5A4NP1 that contains all endogenous plasmids was used, whereas Rogers et al., a non-infectious strain B31 5A13 that lost lp25 was used [32]. In addition, differences in media used for cultivation of B. burgdorferi might also contribute to differences of the results (we used BSK-II whereas Roger et al. used commercially purchased BSK-H complete medium [32]). Another factor that may contribute to this discrepancy is that many genes revealed by WT/rrp1 microarray analysis could not be confirmed by rrp1com/rrp1 analysis. In fact, there are only 39 genes whose dependence on Rrp1 could be confirmed by rrp1com/rrp1 microarray analysis. We do not fully understand what might contribute to this phenomenon, but it may reflect the complexity of B. burgdorferi plasmid contents and gene regulation. Nevertheless, since the expression of rrp1 as well as the in vitro growth defect and the tick survival defect of the rrp1 mutant were fully restored in rrp1com, the difference between the microarray results of WT/rrp1 and rrp1com/rrp1 is not due to Rrp1 and does not affect the overall conclusion of the work presented in the manuscript. The difference of microarray results observed herein also raises caution on microarray analysis of B. burgdorferi gene expression and reinforces the importance of performing complementation experiments for identification of genes that are truly affected by inactivation of the target gene.
What signal activates Rrp1 during tick feeding? As a two-component response regulator, the diguanylate cyclase activity of Rrp1 is dependent on phosphorylation [8]. The predicted cognate histidine kinase for Rrp1 is Hk1. Bioinformatics analysis suggests that Hk1 contains a periplasm-located sensor domain homologous to the family 3 periplasmic substrate-binding proteins (SBP_3) [80]. Proteins in this family often bind to amino acids or opine molecules [80], suggesting that B. burgdorferi may sense such a molecule and activates the c-di-GMP signaling pathway to achieve successful adaptation of the harsh environments of feeding ticks.
In summary, the findings on Hk1-Rrp1 and Hk2-Rrp2 two-component systems suggest a seemingly simple signal transduction model in B. burgdorferi. Through evolution, B. burgdorferi reduced its genome and only kept these two sets of two-component systems for the adaptation to each of the two hosts encountered in its entire enzootic life cycle. When spirochetes migrate from ticks to the mammalian host, the Hk2-Rrp2 pathway is activated during tick feeding, leading to the production of OspC, DbpA/B, BBK32 BBA64 and many factors that are important for B. burgdorferi to establish infection in the mammalian host [81], [82], [83], [84], [85], [86]. Prior and/or after spirochetes enter the tick gut from mammals, the Hk1-Rrp1 pathway becomes activated, leading to activation of the glycerol pathway and other yet-to-be identified factors to ensure that spirochetes can successfully adapt and replicate in the tick vector.
Materials and Methods
Ethics statement
All animal experimentation was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol of using ticks and mice was approved by the Committee on the Ethics of Animal Experiments and the Institutional Animal Care and Use Committee of Indiana University (Permit Number: 2976). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Bacterial strains and culture conditions
Low–passage, virulent B. burgdorferi strain 5A4NP1 (Table 1) (a gift from Drs. H. Kawabata and S. Norris, University of Texas Health Science Center at Houston) was derived from wild-type strain B31 by inserting a kanamycin-resistance marker into the restriction modification gene bbe02 on plasmid lp25 [87]. Borreliae were cultivated in Barbour-Stoenner-Kelly (BSK-II) medium [88] supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at 35°C with 5% CO2. BSK-glycerol medium was prepared as previously reported by von Lackum and Stevenson, by replacing glucose with an equal amount of glycerol (0.6%) and regular CMRL 1066 with glucose-free CMRL [37]. Relevant antibiotics were added to the cultures with the following final concentrations: 200 µg/ml for kanamycin, 100 µg/ml for streptomycin, 50 µg/ml for gentamicin, and 50 ng/ml for erythromycin. The constructed suicide vectors were maintained in E. coli strain TOP10.
Construction of the rrp1 mutant and the repaired (cis-complementation) strain
The rrp1 (bb0419) mutant was created by allelic exchange in 5A4NP1 by transforming a suicide vector pXY307 (Fig. 1A). To construct pXY307, a 2663bp sequence from B. burgdorferi chromosome DNA between the coordinates 428794 and 431456 was PCR cloned into pGEM-T (Promega, Madison, WI) using primers pri-Rrp1-40 and pri-Rrp1-41 (Text S1). Then an antibiotic marker flaBp-aadA was inserted into the XbaI site within the rrp1 gene. The protocol used for transformation was described previously [34], [89]. Numerous streptomycin - and kanamycin-resistant transformants were obtained and the loss of Rrp1 was confirmed by immunoblotting analyses. Endogenous plasmid profiles were determined as previously described [90], [91]. One of the rrp1 mutant clones that had plasmid profiles identical to the parental strain (5A4NP1) was chosen for further study.
For cis-complementation of the rrp1 mutant, a suicide vector, pMH38, was constructed (Fig. 1A). pMH38 contains an ermC antibiotic marker flanked by 1) a PCR fragment of rrp1 and part of the hk1 (bb0420) region (using primers priRrp1-F2-PstI-3 and priRrp1-F2-XhoI-5, Text S1) and 2) a PCR fragment of the bb0418 region (with primers priRrp1-F1-SpeI-5 and priRrp1-F1-BamHI-3). pMH38 DNA was transformed into the rrp1 mutant. Erythromycin - and kanamycin-resistant transformants were subjected to immunoblot analyses to confirm the restoration of rrp1 expression. A successfully complimented clone (rrp1com) with endogenous plasmid profiles identical to the parental strain was then chosen for further study.
Inactivation and cis-complementation of bb0240-bb0243 (the glp operon)
To construct a suicide vector for inactivation of bb0240-bb0243, regions of DNA corresponding to 1.9 kb upstream of bb0240 and 1.9 kb downstream of bb0240 (including part of bb0240) were PCR amplified from B31-A3 genomic DNA. The resulting DNA fragments were then cloned upstream and downstream of a gentamicin-resistant marker (aacC) within the pCR-XL-TOPO cloning vector, resulting in suicide vector pMH85R (Fig. 6A). The construct was confirmed by sequencing. The plasmid DNA was transformed into B. burgdorferi B31 strain 5A4NP1, resulting in a mutant with a disrupted bb0240 and its promoter region by an aacC marker. Since bb0240-bb0243 constitute an operon, the loss of bb0240, bb0241, bb0242, and bb0243 expression was confirmed by RT-PCR analysis. One of the bb0240-bb0243 mutant clones (designated as the glp mutant) that had all the endogenous plasmids (identical to the parental strain 5A4NP1) was chosen for further study. However, this clone subsequently lost lp28-4 during storage, which may contribute to the reduced infectivity in mice with tick infestation (Table 5).
For cis-complementation of the glp mutant, the fragment containing the aacC marker and the disrupted bb0240 in pMH85R was replaced with an aadA marker linked to a wild-type copy of bb0240, to generate the suicide vector pMH89 (Fig. 6A). pMH89 DNA was then transformed into the glp mutant. Restoration of bb0240-bb0243 expression in the streptomycin/kanamycin-resistant transformants were confirmed by RT-PCR analysis. A positive clone (designated as glpcom) with a plasmid profile identical to the parental strain was selected for further study.
Construction of the rrp1 mutant harboring a bb0240-bb0243 operon (the glp operon) driven by the flaB promoter
A flaB promoter and bb0240 fusion fragment was constructed using a two-step PCR method. First, a flaB promoter fragment was PCR amplified from B31 genomic DNA with primers 240P7Aat2 and 240P8 (Text S1). Second, a promoter-less bb0240 fragment was PCR amplified with primers 240P3B and 240P4. These two overlapping fragments were mixed together and subjected to RCR reaction with 5 cycles. The mixture was then served as template for PCR amplification of the fused flaBp-bb0240 fragment with primers 240P7Aat2 and 240P4. The flaBp-bb0240 fragment was cloned into a cloning vector, pSCB-kan/amp, to generate plasmid pMH86. A 1.6 kbp fragment upstream of bb0240 (starting from 205 bp upstream of the bb0240 ORF) was PCR amplified with primers 240P9Sal1 and 240P10Aat2. This fragment was then cloned into pMH86 upstream of the flaB promoter to generate plasmid pMH87. Lastly, a gentamicin-resistant marker, aacC, was inserted into pMH8 upstream of the flaB promoter to generate the suicide vector pMH88R (Fig. 8A). pMH88R DNA was transformed into the rrp1 mutant, and gentamicin/streptomycin/kanamycin-resistant clones were selected and subjected to PCR analysis to confirm the replacement of the native bb0240 with flaB-bb0240 in the rrp1 mutant. Constitutive expression of bb0240-bb0243 in these clones was also determined by quantitative RT-PCR analysis (Fig. 8B). Plasmid profiles were then performed, and a clone having a plasmid profile identical to the parental strain was selected for further study. This strain is designated as rrp1/flpB-glp.
Mouse infection via needle inoculation
Four-week-old C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with 1×105 spirochetes. Ear punch biopsies were collected 14 days after inoculation, and mice were sacrificed by CO2 asphyxiation at 21 days post-inoculation. To culture B. burgdorferi, ear punch tissue samples were transferred to 2 ml of the BSK-II medium (Sigma-Aldrich, St. Louis, MO) containing an antibiotic mixture of fosfomycin (2 mg/ml), rifampin (5 mg/ml), and amphotericin B (250 µg/ml) (Sigma-Aldrich). All cultures were maintained at 34°C and examined for the presence of spirochetes every 5 to 7 days by dark-field microscopy beginning 5 days after inoculation. A single growth-positive culture was used as the criterion for infection of each mouse.
Tick-mouse cycle of B. burgdorferi
The colony of Ixodes scapularis originated from females obtained from Bridgeport, Connecticut, and was maintained in the Tick-Borne Disease Activity Laboratory at the Centers for Disease Control and Prevention, Ft. Collins, Colorado. The tick-mouse experiments were conducted in the Vector-Borne Diseases Laboratory at Indiana University School of Medicine, Indianapolis, IN. Unfed, larvae were fed on groups of mice (C3H/HeN, three mice/group, 100–150 larvae/mouse) that were needle-infected with either 5A4NP1 or various mutant spirochetes. Ticks were allowed to feed to repletion (3–5 days) and then collected within 24 hrs. A portion of fed larvae were subjected to IFA or qPCR analysis (see below). The remaining fed larvae were maintained in the tick incubator and allowed to molt to the nymphal stage (about 5 weeks). One month after molting, unfed nymphs were then allowed to feed on naïve mice (10 ticks per mouse). Fully engorged nymphal ticks were collected within 24 hrs of repletion and subjected to IFA or qPCR analyses. Two weeks after tick feeding, mouse tissues were collected and tested for infection by cultivation for positive growth of spirochetes in BSK-H medium, as described above.
To generating artificially infected ticks with B. burgdorferi, a previously described microinjection method was used [26], [34], [35]. Briefly, 0.1 µl of B. burgdorferi culture with a concentration of 108 spirochetes per ml was injected into the rectal aperture of unfed nymphal ticks by using a femtojet microinjector system (Eppendorf AG). After microinjection, ticks were placed on naïve C3H/HeN mice (10 ticks per mouse), allowed to feed to repletion (4–5 days), and then collected for IFA or qPCR analysis.
SDS-PAGE and immunoblot analysis
Spirochetes were harvested by centrifugation at 7,000×g and washed three times with PBS (pH 7.4) at 4°C. Pellets were resuspended in SDS buffer containing 50 mM Tris-HCl (pH 8.0), 0.3% sodium dodecyl sulfate (SDS) and 10 mM dithiothreitol (DTT). Total protein lysates (5×107 cells per lane) were separated by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (GE-Healthcare, Milwaukee, WI). Protein bands were detected using a 1∶20 dilution of monoclonal antibody against Rrp1 or FlaB, and a 1∶1000 anti-mouse IgG-peroxidase-conjugate secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), followed by development with 4-chloro-1-naphthol as the substrate. Monoclonal antibody against FlaB, 8H3-33, has been described previously [92], [93]. Anti-Rrp1 monoclonal antibody was generated by immunizing BALB/c mice with the full-length fusion protein according to previously published protocols [92].
Quantitative RT-PCR
RNA samples were extracted from B. burgdorferi cultures using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. Three independent culture samples were used for each strain. Digestion of contaminating genomic DNA in the RNA samples was performed using RNase-free DNase I (Promega), and removal of DNA was confirmed by PCR amplification for the B. burgdorferi flaB gene. The cDNA was synthesized using the SuperScript III reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). To quantify the transcript levels of interested genes, an absolute quantitation method was used by creating a standard curve in qPCR assay by following the manufacturer's protocol (Strategene, La Jolla, CA). Briefly, a cloning vector containing the flaB gene serves as standard template. A series of ten-fold dilution (100 to 107 copies/µl) of the standard template was prepared and qPCR was performed to generate a standard curve by plotting the initial template quantity against the Ct values for the standards. The quantity of the targeted genes and flaB in cDNA samples were calculated by comparing their Ct values of the Standard Curve plot. Both standards and samples were performed in triplicate on an ABI 7000 Sequence Detection System using GREEN PCR Master Mix (ABI, Pleasanton, CA). Levels of target gene transcript were reported as per 1000 copies of flaB transcripts.
Indirect immunofluorescence assay (IFA)
IFA was performed as described previously [26]. Briefly, the entire contents of a fed tick were smeared and fixed on a silylated microscope slide (CEL Associates, Pearland, TX). The slides were incubated with BacTrace fluorescein isothiocyanate-conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories Gaithersburg, MD) at 37°C. Samples were observed using an Olympus BX50 fluorescence microscope. Ten ticks from each group were examined by IFA.
Enumeration of spirochetes in ticks by qPCR
DNA was isolated from engorged larvae (pools of 3 larvae per sample), and replete nymphs (one nymph per sample) using the DNeasy Blood & Tissue Kit B (QIAGEN, CA) according to the manufacturer's instructions. Spirochete burdens within infected ticks were assessed by qPCR with primer pairs of qflaB-F/R for the B. burgdorferi flaB gene and qTactin-F/R for the tick actin gene (Text S1). Calculations of relative DNA copy number (represented by flaB) were normalized with the copy numbers of the tick actin gene.
Microarray analysis
Wild-type, the rrp1 mutant and rrp1com strains were cultivated in BSK-II at 35°C and harvested at the mid-logarithmic growth. RNA was extracted from three biological replicates using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Digestion of contaminating genomic DNA in the RNA samples was performed using RNase-free DNase I (GenHunter Technology, Nashville, TN), and removal of DNA was confirmed by PCR amplification using primers specific for the B. burgdorferi flaB gene. RNA quality was determined using the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). 70-mer oligonucleotides arrays of B. burgdorferi were prepared as previously reported [26], [29], [94]. cDNA synthesis, sample labeling, hybridization, and data analysis were also described previously [26]. A cutoff value of a 3-fold change was used for selecting candidate genes. Statistical analyses were performed using the one and two-sample significance test (p<0.05) in the Acuity program. The array data has been deposited at http://www.ncbi.nlm.nih.gov/geo/ (accession number GSE26968).
Statistical analysis
To determine the statistical significance of differences observed in qRT-PCR, qPCR, and growth curves, values were compared using an unpaired t test. The P values are indicated in each figure.
Supporting Information
Zdroje
1. RossPWeinhouseHAloniYMichaeliDWeinberger-OhanaP 1987 Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325 279 281
2. HenggeR 2009 Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7 263 273
3. JenalUMaloneJ 2006 Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet 40 385 407
4. WolfeAJVisickKL 2010 The second messenger cyclic di-GMP. Washington D.C. ASM Press
5. GalperinMYNikolskayaANKooninEV 2001 Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett 203 11 21
6. BobrovAGKirillinaOPerryRD 2005 The phosphodiesterase activity of the HmsP EAL domain is required for negative regulation of biofilm formation in Yersinia pestis. FEMS Microbiol Lett 247 123 130
7. ChristenMChristenBFolcherMSchauerteAJenalU 2005 Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J Biol Chem 280 30829 30837
8. RyjenkovDATarutinaMMoskvinOVGomelskyM 2005 Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol 187 1792 1798
9. SchmidtAJRyjenkovDAGomelskyM 2005 The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187 4774 4781
10. RyanRPFouhyYLuceyJFCrossmanLCSpiroS 2006 Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci U S A 103 6712 6717
11. PaulRWeiserSAmiotNCChanCSchirmerT 2004 Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev 18 715 727
12. GalperinMY 2005 A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol 5 35
13. RomlingUGomelskyMGalperinMY 2005 C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol 57 629 639
14. NewellPDMondsRDO'TooleGA 2009 LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc Natl Acad Sci U S A 106 3461 3466
15. BoehmAKaiserMLiHSpanglerCKasperCA 2010 Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141 107 116
16. FangXGomelskyM 2010 A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol Microbiol 76 1295 1305
17. PaulKNietoVCarlquistWCBlairDFHarsheyRM 2010 The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell 38 128 139
18. SimmRMorrMKaderANimtzMRomlingU 2004 GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53 1123 1134
19. TischlerADCamilliA 2004 Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 53 857 869
20. GomelskyM 2011 cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol 79 562 565
21. TamayoRPrattJTCamilliA 2007 Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol 61 131 148
22. CotterPAStibitzS 2007 c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol 10 17 23
23. SteereACCoburnJGlicksteinL 2004 The emergence of Lyme disease. J Clin Invest 113 1093 1101
24. FraserCMCasjensSHuangWMSuttonGGClaytonR 1997 Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390 580 586
25. DasRHegyiHGersteinM 2000 Genome analyses of spirochetes: a study of the protein structures, functions and metabolic pathways in Treponema pallidum and Borrelia burgdorferi. J Mol Microbiol Biotechnol 2 387 392
26. BoardmanBKHeMOuyangZXuHPangX 2008 Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun 76 3844 3853
27. CaimanoMJIyerREggersCHGonzalezCMortonEA 2007 Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol 65 1193 1217
28. YangXFAlaniSMNorgardMV 2003 The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci U S A 100 11001 11006
29. OuyangZBlevinsJSNorgardMV 2008 Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154 2641 2658
30. FisherMAGrimmDHenionAKEliasAFStewartPE 2005 Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A 102 5162 5167
31. RosaPATillyKStewartPE 2005 The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 3 129 143
32. RogersEATerekhovaDZhangHHovisKMSchwartzI 2009 Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol 71 1551 1573
33. SultanSZPitzerJEMillerMRMotalebMA 2010 Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol Microbiol 77 128 142
34. YangXFPalUAlaniSMFikrigENorgardMV 2004 Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 199 641 648
35. PalUYangXChenMBockenstedtLKAndersonJF 2004 OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest 113 220 230
36. GherardiniFBoylanJLawenceKSkareJ 2010 Metabolism and Physiology of Borrelia. SamulesDSRadolfJD Borrelia: Molecular Biology, Host Interaction and Pathogenesis Norfolk, UK Caister Academic Press 103 138
37. von LackumKStevensonB 2005 Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol Lett 243 173 179
38. SinghSKGirschickHJ 2004 Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect Dis 4 575 583
39. CaimanoMJEggersCHGonzalezCARadolfJD 2005 Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol 187 7845 7852
40. HickmanJWTifreaDFHarwoodCS 2005 A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A 102 14422 14427
41. LiuXBeyhanSLimBLiningtonRGYildizFH 2010 Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. J Bacteriol 192 4541 4552
42. KazmierczakBILebronMBMurrayTS 2006 Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Mol Microbiol 60 1026 1043
43. KuchmaSLBrothersKMMerrittJHLiberatiNTAusubelFM 2007 BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189 8165 8178
44. HinnebuschBJEricksonDL 2008 Yersinia pestis biofilm in the flea vector and its role in the transmission of plague. Curr Top Microbiol Immunol 322 229 248
45. HinnebuschBJPerryRDSchwanTG 1996 Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273 367 370
46. SimmRFetherstonJDKaderARomlingUPerryRD 2005 Phenotypic convergence mediated by GGDEF-domain-containing proteins. J Bacteriol 187 6816 6823
47. BobrovAGKirillinaORyjenkovDAWatersCMPricePA 2011 Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol Microbiol 79 533 551
48. StevensonBBabbK 2002 LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun 70 4099 4105
49. BabbKvon LackumKWattierRLRileySPStevensonB 2005 Synthesis of autoinducer 2 by the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol 187 3079 3087
50. SchauderSShokatKSuretteMGBasslerBL 2001 The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol 41 463 476
51. HubnerARevelATNolenDMHagmanKENorgardMV 2003 Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect Immun 71 2892 2896
52. BlevinsJSRevelATCaimanoMJYangXFRichardsonJA 2004 The luxS gene is not required for Borrelia burgdorferi tick colonization, transmission to a mammalian host, or induction of disease. Infect Immun 72 4864 4867
53. KumagaiYMatsuoJHayakawaYRikihisaY 2010 Cyclic di-GMP signaling regulates invasion by Ehrlichia chaffeensis of human monocytes. J Bacteriol 192 4122 4133
54. TamayoRSchildSPrattJTCamilliA 2008 Role of cyclic di-GMP during El Tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic Di-GMP phosphodiesterase CdpA. Infect Immun 76 1617 1627
55. LamprokostopoulouAMonteiroCRhenMRomlingU 2010 Cyclic di-GMP signalling controls virulence properties of Salmonella enterica serovar Typhimurium at the mucosal lining. Environ Microbiol 12 40 53
56. WongFSJanewayCA 1999 Insulin-dependent diabetes mellitus and its animal models. Curr Opin Immunol 11 643 647
57. LeeREChenCPDenlingerDL 1987 A Rapid Cold-Hardening Process in Insects. Science 238 1415 1417
58. SudarsanNLeeERWeinbergZMoyRHKimJN 2008 Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321 411 413
59. HickmanJWHarwoodCS 2008 Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69 376 389
60. WeberHPesaventoCPosslingATischendorfGHenggeR 2006 Cyclic-di-GMP-mediated signalling within the sigma network of Escherichia coli. Mol Microbiol 62 1014 1034
61. PesaventoCBeckerGSommerfeldtNPosslingATschowriN 2008 Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev 22 2434 2446
62. LimBBeyhanSYildizFH 2007 Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J Bacteriol 189 717 729
63. WatersCMLuWRabinowitzJDBasslerBL 2008 Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol 190 2527 2536
64. KrastevaPVFongJCShikumaNJBeyhanSNavarroMV 2010 Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327 866 868
65. LeducJLRobertsGP 2009 Cyclic di-GMP allosterically inhibits the CRP-like protein (Clp) of Xanthomonas axonopodis pv. citri. J Bacteriol 191 7121 7122
66. GomelskyM 2009 Cyclic-di-GMP-binding CRP-like protein: a spectacular new role for a veteran signal transduction actor. J Bacteriol 191 6785 6787
67. ChinKHLeeYCTuZLChenCHTsengYH 2010 The cAMP receptor-like protein CLP is a novel c-di-GMP receptor linking cell-cell signaling to virulence gene expression in Xanthomonas campestris. J Mol Biol 396 646 662
68. FongJCYildizFH 2008 Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol 190 6646 6659
69. OuyangZKumarMKariuTHaqSGoldbergM 2009 BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol 74 1331 1343
70. HydeJASeshuJSkareJT 2006 Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology 152 2599 2609
71. WolfeAJVisickKL 2008 Get the message out: cyclic-di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol 190 463 475
72. LiXPalURamamoorthiNLiuXDesrosiersDC 2007 The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol 63 694 710
73. NeelakantaGLiXPalULiuXBeckDS 2007 Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog 3 e33 doi:10.1371/journal.ppat.0030033
74. PalUDaiJLiXNeelakantaGLuoP 2008 A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J Infect Dis 197 148 155
75. PromnaresKKumarMShroderDYAndersonJFPalU 2009 Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol Microbiol 74 112 125
76. RevelATBlevinsJSAlmazanCNeilLKocanKM 2005 bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci U S A 102 6972 6977
77. RyjenkovDASimmRRomlingUGomelskyM 2006 The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem 281 30310 30314
78. AmikamDGalperinMY 2006 PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22 3 6
79. FreedmanJCRogersEAKostickJLZhangHIyerR 2010 Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol Med Microbiol 58 285 294
80. TamRSaierMH 1993 Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev 57 320 346
81. BlevinsJHagmanKENorgardMV 2008 Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol 8 82
82. GilmoreRDHowisonRRDietrichGPattonTGCliftonDR 2010 The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc Natl Acad Sci U S A 107 7515 7520
83. GrimmDTillyKByramRStewartPEKrumJG 2004 Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101 3142 3147
84. SeshuJEsteve-GassentMDLabandeira-ReyMKimJHTrzeciakowskiJP 2006 Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol 59 1591 1601
85. WeeningEHParveenNTrzeciakowskiJPLeongJMHookM 2008 Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun 76 5694 5705
86. MaruskovaMEsteve-GassentMDSextonVLSeshuJ 2008 Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect Immun 76 391 402
87. KawabataHNorrisSJWatanabeH 2004 BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun 72 7147 7154
88. BarbourAG 1984 Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57 521 525
89. SamuelsDS 1995 Electrotransformation of the spirochete Borrelia burgdorferi. NickoloffJA Methods in molecular biology Totowa, NJ Humana Press 253 259
90. PurserJENorrisSJ 2000 Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97 13865 13870
91. XuHCaimanoMJLinTHeMRadolfJD 2010 Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog 6 e1001104 doi:1001110.1001371/journal.ppat.1001104
92. AkinsDRBourellKWCaimanoMJNorgardMVRadolfJD 1998 A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest 101 2240 2250
93. XuHHeMHeJJYangXF 2010 Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect Immun 78 2910 2918
94. TerekhovaDIyerRWormserGPSchwartzI 2006 Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J Bacteriol 188 6124 6134
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



