-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLongistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Ixodid ticks are notorious blood-sucking ectoparasites and are completely dependent on blood-meals from hosts. In addition to the direct severe effects on health and productivity, ixodid ticks transmit various deadly diseases to humans and animals. Unlike rapidly feeding vessel-feeder hematophagous insects, the hard ticks feed on hosts for a long time (5−10 days or more), making a large blood pool beneath the skin. Tick's salivary glands produce a vast array of bio-molecules that modulate their complex and persistent feeding processes. However, the specific molecule that functions in the development and maintenance of a blood pool is yet to be identified. Recently, we have reported on longistatin, a 17.8-kDa protein with two functional EF-hand Ca++-binding domains, from the salivary glands of the disease vector, Haemaphysalis longicornis, that has been shown to be linked to blood-feeding processes. Here, we show that longistatin plays vital roles in the formation of a blood pool and in the acquisition of blood-meals. Data clearly revealed that post-transcriptional silencing of the longistatin-specific gene disrupted ticks' unique ability to create a blood pool, and they consequently failed to feed and replete on blood-meals from hosts. Longistatin completely hydrolyzed α, β and γ chains of fibrinogen and delayed fibrin clot formation. Longistatin was able to bind with fibrin meshwork, and activated fibrin clot-bound plasminogen into its active form plasmin, as comparable to that of tissue-type plasminogen activator (t-PA), and induced lysis of fibrin clot and platelet-rich thrombi. Plasminogen activation potentiality of longistatin was increased up to 4 times by soluble fibrin. Taken together, our results suggest that longistatin may exert potent functions both as a plasminogen activator and as an anticoagulant in the complex scenario of blood pool formation; the latter is critical to the feeding success and survival of ixodid ticks.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1001312
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001312Summary
Ixodid ticks are notorious blood-sucking ectoparasites and are completely dependent on blood-meals from hosts. In addition to the direct severe effects on health and productivity, ixodid ticks transmit various deadly diseases to humans and animals. Unlike rapidly feeding vessel-feeder hematophagous insects, the hard ticks feed on hosts for a long time (5−10 days or more), making a large blood pool beneath the skin. Tick's salivary glands produce a vast array of bio-molecules that modulate their complex and persistent feeding processes. However, the specific molecule that functions in the development and maintenance of a blood pool is yet to be identified. Recently, we have reported on longistatin, a 17.8-kDa protein with two functional EF-hand Ca++-binding domains, from the salivary glands of the disease vector, Haemaphysalis longicornis, that has been shown to be linked to blood-feeding processes. Here, we show that longistatin plays vital roles in the formation of a blood pool and in the acquisition of blood-meals. Data clearly revealed that post-transcriptional silencing of the longistatin-specific gene disrupted ticks' unique ability to create a blood pool, and they consequently failed to feed and replete on blood-meals from hosts. Longistatin completely hydrolyzed α, β and γ chains of fibrinogen and delayed fibrin clot formation. Longistatin was able to bind with fibrin meshwork, and activated fibrin clot-bound plasminogen into its active form plasmin, as comparable to that of tissue-type plasminogen activator (t-PA), and induced lysis of fibrin clot and platelet-rich thrombi. Plasminogen activation potentiality of longistatin was increased up to 4 times by soluble fibrin. Taken together, our results suggest that longistatin may exert potent functions both as a plasminogen activator and as an anticoagulant in the complex scenario of blood pool formation; the latter is critical to the feeding success and survival of ixodid ticks.
Introduction
Blood coagulation is a very complex but well-synchronized biochemical process by which blood forms a clot and the damaged blood vessel is sealed by a platelet-rich fibrin plug leading to hemostasis. Any damage to the vascular beds due to laceration of tissues exposes tissue factor from the endothelium to the circulating blood, which initiates coagulation cascades. Once coagulation is initiated, the process leads to the generation of thrombin, which converts fibrinogen to fibrin, the building block of a hemostatic plug [1]–[5]. In contrast, fibrinolysis is an enzymatic process wherein a fibrin clot is dissolved. Normally, in the body, both coagulation and fibrinolytic processes are precisely regulated by the measured participation of zymogens, activators, inhibitors, cofactors and receptors. Plasmin is the major fibrinolytic enzyme and is derived from the limited proteolytic cleavage of plasminogen, the circulating plasma zymogen, by its physiological activators such as tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) [6]–[9]. Homeostasis in blood fluidity is not only vital for humans but also for hematophagous animals, which have to counteract their hosts' hemostatic mechanisms and/or facilitate the fibrinolytic process to keep the blood in a fluid state during acquisition and digestion of blood-meals. Therefore, it is believed that blood sucking-animals require an extensive spectrum of anticoagulant and/or fibrinolytic mechanisms to maximize their feeding as part of their diverse survival strategies. Thus, hematophagous animals are thought to possess anticoagulant and/or fibrinolytic proteins that have been acquired during their evolution [1], [10], [11].
The ixodid ticks (Arthropoda: Ixodidae), popularly known as hard ticks, are notorious ectoparasites and live entirely on nutrients derived from host's blood protein, hemoglobin. Blood-feeding is an essential biologic phenomenon for the survival of hard ticks [12]–[14]. These ectoparasites firmly attach to hosts using their deeply penetrating mouthparts on hosts' skin and cause dermatitis and severe anemia. In addition to the direct severe adverse effects on health and productivity, ticks serve as a unique vector of various deadly diseases, such as lyme disease, tick-borne encephalitis, Rocky Mountain spotted fever, babesiosis, theileriosis and anaplasmosis during hematophagy. Ticks are only second to mosquitoes as vectors of diseases of humans and animals [15]–[20]. Unlike rapidly feeding hematophagous insects that suck blood directly from the blood vessels within a couple of seconds, the hard ticks feed on blood-meals for a long time (5−10 days or more), making a large blood pool beneath the host's skin. Although size of the blood pool produced by different species of ticks varies but it is an essential feature in the feeding mechanism of ticks [13], [21]. Blood pools contain copious unclotted blood and exudates, especially in the rapid phase of feeding [1], [21]. It is of current interest to look at the molecular scenario inside the blood pool that maintains a large volume of unclotted blood underneath the skin. Prior studies suggest that ixodid ticks, the smart pharmacologists, produce a vast array of pharmacologically active bio-molecules that are injected into the feeding lesions during persistent blood-feeding processes, and play crucial modulatory roles in their feeding success, especially to keep the blood in a fluid state in the blood pool and within the gut as well [22]–[26]. However, the precise molecular mechanism(s) that prevents blood coagulation and initiates fibrinolysis in the blood pool to facilitate successful acquisition of blood-meals is still unclear. Previously, we have shown that longistatin, an ixodid tick salivary gland-derived bioactive molecule, is functionally linked to the blood-feeding processes. The molecule was shown to be secreted into the blood pool created by ticks while they attach on a robust mammalian host and suck blood to engorge [27].
Here, we demonstrate that longistatin-specific gene-knockdown ixodid ticks such as Haemaphysalis longicornis completely lost their unique ability to create a blood pool and consequently were unable to feed and engorge on blood-meals as verified by RNA interference (RNAi) tool. Furthermore, we describe that longistatin binds with fibrin and specifically catalyzes the activation of plasminogen into plasmin and hydrolyzes fibrinogen in vitro; the latter is known to be a major component of cross-linked fibrin polymer. To the best of our knowledge, longistatin is the first plasminogen activator, isolated and characterized from hematophagous arthropods.
Results
Injection of dslongistatin inhibits the transcription and translation of endogenous longistatin
Total RNA isolated from salivary glands of ticks of different feeding phases of both control and treated groups was analyzed by reverse transcription-PCR (RT-PCR) and quantitative RT-PCR (qRT-PCR) to demonstrate the effect of RNAi on the expression of longistatin-specific mRNA. The RT-PCR data revealed that injection of dslongistatin completely abolished the detectable mRNA expression corresponding to the longistatin-specific gene in ticks (Figure 1A). Our qRT-PCR data also supported this finding. However, in the RNAi-treated group, longistatin-specific transcript, although very low compared with that of control, was detected in ticks at 24, 48 and 72 h of feeding only by qRT-PCR (Figure 1B). This variation in the detection of longistatin-specific mRNA by RT-PCR and qRT-PCR may be due to the sensitivity of the techniques. Furthermore, the presence of a detectable level of mRNA in the RNAi-treated group of ticks might be due to individual variations in the tick population. Here, we used pools of salivary-gland extracts, isolated from three randomly selected ticks in each feeding phase; thus, it is quite reasonable that the effects of RNAi were not exactly uniform in each and every microinjected tick. Longistatin-specific gene expression was detected in its normal pattern [27] in ticks injected with dsRNA complementary to the gene encoding maltose-binding protein in Escherichia coli (dsmalE) (Figures 1A and B), indicating that injection of dslongistatin caused disruption of longistatin-specific mRNA transcription. For in situ detection of the effect of RNAi on the translation of endogenous longistatin, salivary glands were collected from partially fed (96 h) ticks of both treated and control groups and were subjected to immunofluorescence staining using mouse anti-longistatin sera. Longistatin-specific reactions were almost absent in dslongistatin-injected ticks whereas binding of mouse anti-longistatin antibody was detected in the salivary glands of dsmalE-injected ticks (Figure 1C), suggesting that injection of dslongistatin efficiently silenced longistatin-specific mRNA expression and subsequent translation of longistatin. To further validate our results regarding in vivo longistatin gene silencing, we conducted Western blot analysis using salivary gland extracts collected from both treated and control ticks. Longistatin-specific bands were detected only in the salivary gland extracts of dsmalE-injected ticks and longistatin was upregulated with the feeding process of ticks (Figure 1D). These findings further reinforced the evidence of longistatin-specific gene silencing by dslongistatin injection in ticks.
Fig. 1. Post-transcriptional silencing of longistatin-specific gene in adult ticks by injecting dsRNA. 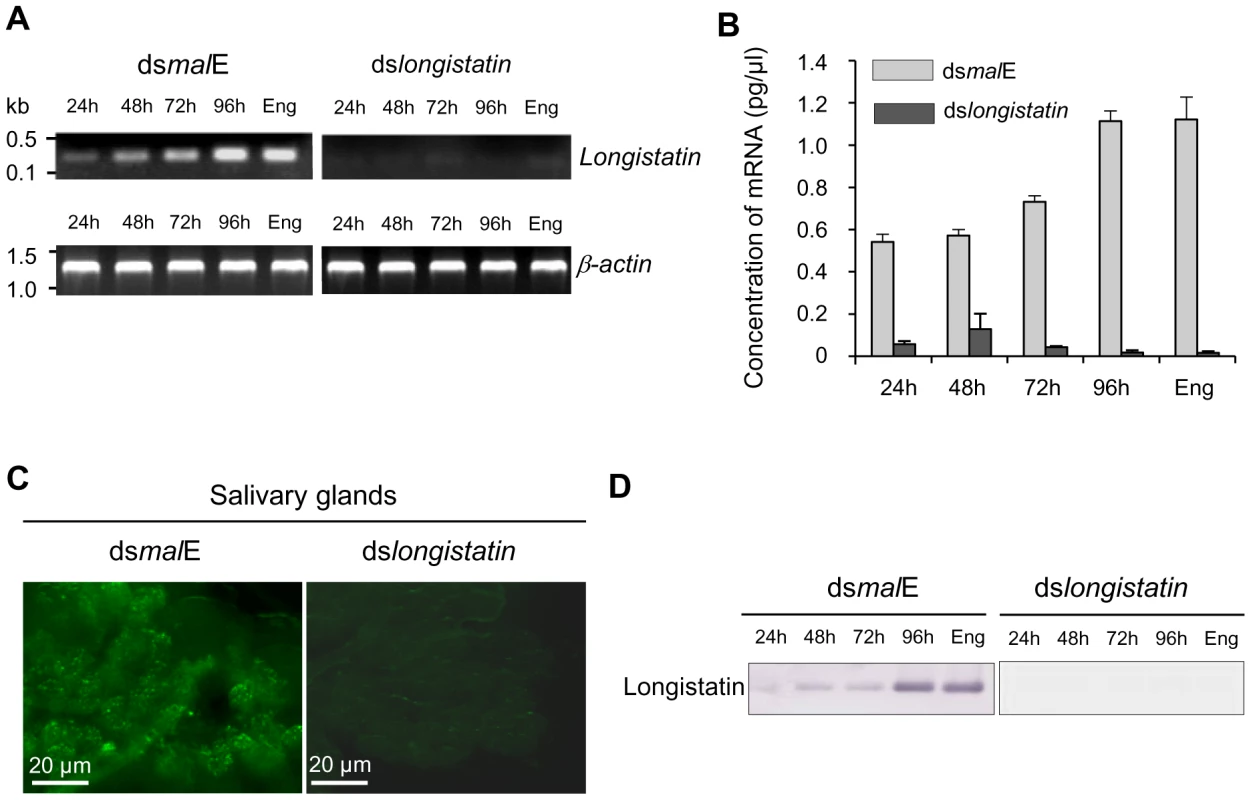
(A) Semiquantitative RT-PCR analysis. One microgram of longistatin dsRNA was injected into the hemolymph of ticks of the RNAi-treated group. Ticks of the control group were treated with 1 µg of malE dsRNA. Actin was used as an internal control. Eng, engorged. (B) Quantitative RT-PCR using total RNA and primers specific for longistatin as in A. Eng, engorged. (C) In situ detection of longistatin expression in ticks' salivary glands. Salivary glands from the ticks of control and RNAi-treated groups. Endogenous longistatin was reacted with mouse anti-longistatin sera (1∶100). (D) Effect of gene silencing on longistatin post-translation by Western blot analysis. Salivary gland extracts were electrophoresed and transferred onto nitrocellulose membrane. Endogenous longistatin was probed with mouse anti-longistatin (1: 100). Eng, engorged. RNAi-treated ticks failed to develop a blood pool and were unable to feed blood-meals from hosts
All ticks microinjected with dsRNA were active and healthy during the incubation period. After placement on rabbits' ears, all ticks of both treated and control groups actively attached. However, in the dslongistatin-injected group, 3 (2.5%) ticks were found dead at 72 h of attachment. All ticks in the dsmalE-injected group reached to repletion and detached by day 6 post-attachment. Notably, dslongistatin injection was shown to hamper the feeding of ticks. These ticks were poorly fed and most of them failed to engorge (Figure 2A). Only two ticks (1.66%) dropped off the host following engorgement in the RNAi group. The mean body weight of the ticks collected after 6 days of feeding was significantly (P<0.01) lower in the RNAi-treated group (53.53±50.38 mg) than that of the control group (253.43±57.91 mg) (Figure 2B). Visible phenotypic changes were also obvious among the ticks of the treated and control groups. Ticks of the treated group, despite 6 days of feeding, were very small with a dull cuticle and devoid of normal cuticular wrinkling. In contrast, ticks of the control group, which dropped off the host following full engorgement, were large and glossy in appearance with dorsal cuticular wrinkling (Figure 2B).
Fig. 2. Effects of post-transcriptional silencing of longistatin gene on blood pool formation and blood feeding. 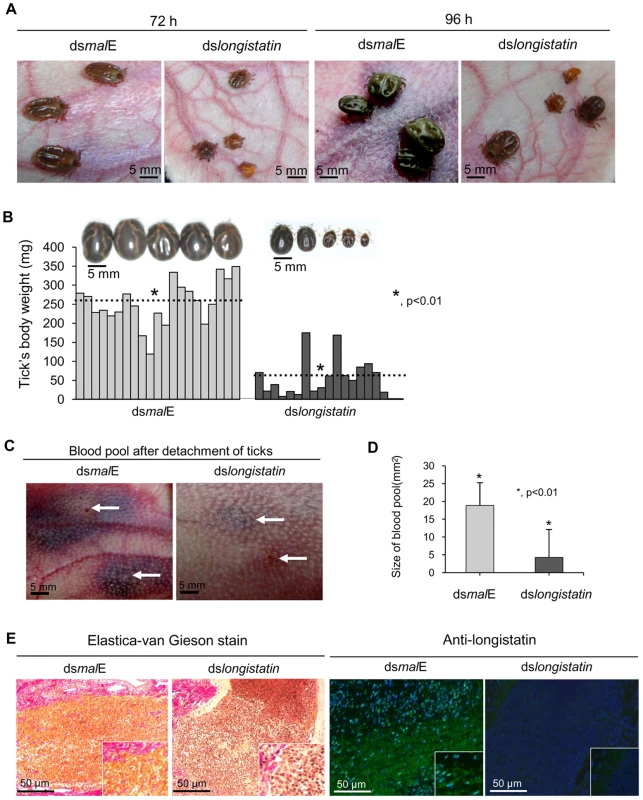
(A) Impact of longistatin-specific mRNA silencing on blood-meal feeding from hosts. Adult ticks were injected with longistatin dsRNA or malE dsRNA (1 µg/tick) and were allowed to feed on a tick-naïve rabbit. RNAi-treated ticks failed to replete. (B) Postengorgement weight was significantly (p<0.01) lower in the RNAi-treated group than that of the control group. Dotted lines indicate mean±SD of body weight of ticks. (C) Effects of post-transcriptional silencing of longistatin gene on blood pool formation. RNAi was performed and ticks were fed in the same manner as in A. RNAi-treated ticks failed to establish a prominent blood pool. Arrows indicate site of tick attachment. (D) Blood pools were significantly (p<0.01) smaller in the RNAi-treated group. (E) Histopathological changes were studied using EVGS. Longistatin was detected in the feeding lesions of ticks on the host's tissues using mouse anti-longistatin sera (1∶100). A marked difference between blood pools induced by the ticks of RNAi-treated and control groups was observed. Large blood pools were developed at the site of attachment of each tick of the control group. Grossly, blood pools were large enough with an estimated mean size of 19.53±7.85 mm2, containing a considerable amount of exudates and blood. The affected area was cyanotic or dark bluish in color. Blood vessels in the vicinity of the blood pool were congested and distended. The surrounding area of the blood pool was markedly hyperemic. On the contrary, most of the ticks of the RNAi-treated group failed to establish a feeding lesion. Importantly, significantly (p<0.01) smaller blood pools (4.25±6.38 mm2) were detected in few individual ticks. Also, no prominent gross pathological changes were detected at the site of attachment of RNAi-treated ticks (Figures 2C and D). To study the histological features, we stained the thin tissue sections of the blood pool areas with Elastica-van Gieson stain (EVGS) where hemorrhagic areas exhibited a bright golden yellow color, indicating that blood pools, developed at the site of attachment of the dsmalE-injected ticks, were flooded with RBC. Notably, hemorrhagic changes were not detected at the biting areas of ticks of the RNAi-treated group (Figure 2E), suggesting that the longistatin gene plays vital roles in the formation and maintenance of a blood pool as preceded by marked hemorrhage into tick-feeding lesions. In addition, an attempt was made to detect endogenous longistatin in the feeding lesions produced by the ticks of both groups using mouse anti-longistatin sera. Longistatin-specific bright green fluorescence was detected at the well-developed blood pool areas produced by the dsmalE-injected ticks but no such reaction was detected at the feeding lesions induced by dslongistatin-injected ticks (Figure 2E), which further suggests a possible role of longistatin in the blood pool formation.
Longistatin degrades fibrinogen and delays fibrin clot formation
To judge the anticoagulant function, longistatin was reacted with several coagulation factors (viz., thrombin, factors VIIa and Xa) using different approaches but none of them was affected by longistatin. Interestingly, longistatin was found to delay fibrin clot formation, degrade fibrinogen and efficiently activate plasminogen to its active form, plasmin. To evaluate the anticoagulation potential of longistatin, fibrinogen (7.5 mM) was pre-incubated in the absence or presence of longistatin (0.1, 0.2, 0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM) and then thrombin (0.10 NIH unit/µl) was added as described in Materials and Methods. Longistatin significantly (p<0.01) interrupted the normal fibrin clot formation in a concentration-dependent manner. In the absence of longistatin, fibrin clot was formed within 15 min, but in the presence of longistatin (1.6 µM) or plasmin (1.6 µM), no visible clot was developed within this time (Figure 3A). Longistatin was shown to delay the formation of a visible fibrin clot. Fibrin clotting time was significantly extended (up to 90 min) at a concentration of 1.6 µM longistatin (Figure 3B), indicating potent anticoagulant activity of longistatin. To explore the fibrinogenolytic potential, fibrinogen was incubated in the absence or presence of longistatin (0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM) and was subjected to SDS–PAGE analysis. Longistatin potently degraded the α, β and γ chains of fibrinogen in a concentration-dependent manner. Longistatin completely hydrolyzed all three chains of fibrinogen at 1.6 µM concentration, as it was done by 1.6 µM plasmin (Figure 3C), implying that longistatin is an efficient fibrinogenolytic protease of ixodid ticks.
Fig. 3. Anti-coagulation and fibrinogenolytic activity of longistatin. 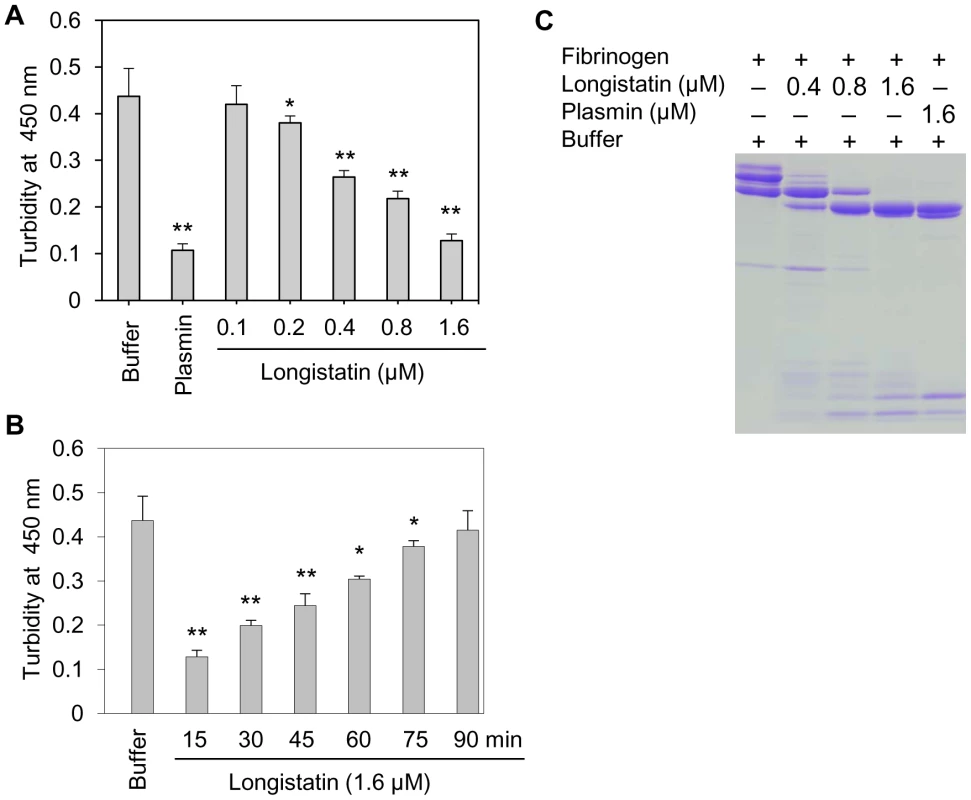
(A) Effects of longistatin on the formation of fibrin clot. Fibrinogen (7.5 mM) was pre-incubated in a buffer in the absence or presence of longistatin (0.1, 0.2, 0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM) and then thrombin was added (0.10 NIH unit/µl) as described in Materials and Methods. Clot formation was detected visually and also by determining changes in turbidity at OD450 using a spectrophotometer after 15 min. (B) Longistatin (1.6 µM) delayed fibrin clot formation up to 90 min. Fibrinogen was incubated in the absence or presence of longistatin (1.6 µM) following the same procedures as mentioned in A and then thrombin was added. OD450 was measured at 15 min intervals. (C) Fibrinogenolytic effect of longistatin. Fibrinogen (7.5 mM) was incubated in the absence or presence of longistatin (0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM). Samples were collected at the indicated time period and were subjected to 12.5% SDS–PAGE analysis under reducing conditions. A gradual degradation of the α, β and γ chains of fibrinogen was detectable with the concomitant deposition of degraded products. Asterisks (*) indicate that the difference compared with the negative control group (buffer only) is significant as determined by Student's t-test with unequal variance (*p<0.05, **p<0.01). Longistatin activates plasminogen in the presence of soluble fibrin
Initially, we determined the plasminogen activation potential of longistatin by measuring the amidolytic activity of activated plasminogen on the plasmin-specific fluorogenic substrate. Data revealed that the initial rate of plasminogen activation was proportional to the concentration of longistatin. Hydrolysis of plasmin-specific substrate sharply increased with the increase of concentration of longistatin. However, longistatin alone, even at a higher concentration (640 nM), was not able to induce hydrolysis of plasmin-specific fluorogenic synthetic substrate (Figure 4A), indicating that longistatin activated plasminogen into its active form, plasmin, which hydrolyzed the synthetic substrate releasing MCA. Most interestingly, plasminogen activation potential of longistatin was significantly increased in the presence of soluble fibrin and induced robust amidolytic activity. Addition of fibrin cyanogen bromide (CNBr) fragments (4 µg) to the assay mixture caused an increase of plasminogen activation rate up to 4 times higher than that in the absence of fibrin CNBr fragments (Figure 4B). CNBr fragments of fibrin, the soluble fibrin, is usually regarded as a t-PA stimulator and increases the rate of plasminogen activation about 5 times [28], [29]. Plasminogen was sparingly affected by longistatin in the absence of soluble fibrin (Figure 4B), indicating the extraordinary specificity of longistatin towards fibrin-bound plasminogen rather than free circulating plasminogen.
Fig. 4. Plasminogen activation by longistatin. 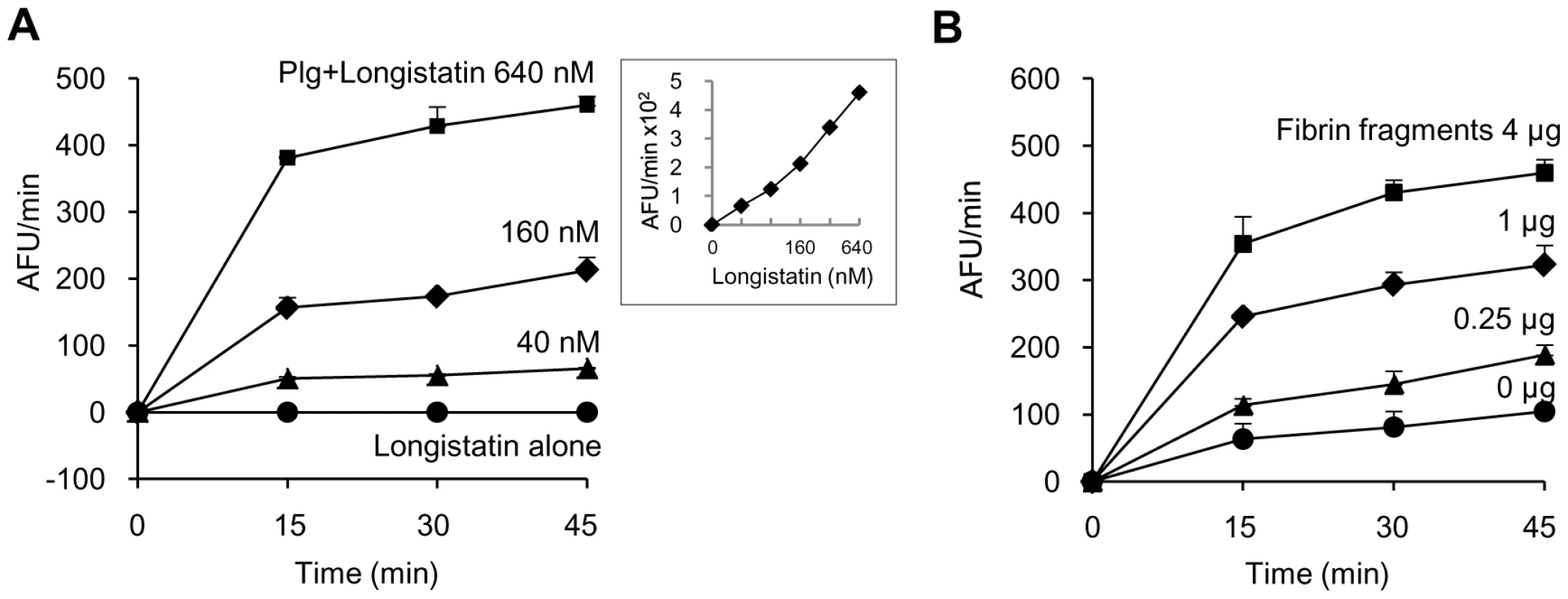
(A) Longistatin (40, 80, 160, 320 and 640 nM) was incubated without or with plasminogen (0.24 units) adding fibrin CNBr fragments (4 µg) in a total volume of 200 µl of buffer (50 mM Tris–HCl, pH 7.5; 100 mM NaCl and 5 mM CaCl2) at 25°C for 2 h. Then, plasmin-specific fluorogenic substrate (100 µM, final concentration) was added and substrate hydrolysis was monitored by measuring excitation and emission wavelengths of 360 nm and 460 nm, respectively, at 15 min intervals. Inset, initial rate of plasminogen activation at different concentrations of longistatin. (B) Effects of fibrin CNBr fragments on the activation of plasminogen by longistatin. Plasminogen (0.24 units) was incubated with longistatin (640 nM) in the absence or presence of fibrin CNBr fragments (0.25, 1 and 4 µg) as described in Materials and Methods. All assays were performed in triplicate. Longistatin is able to lyse fibrin clot by activating plasminogen
Although plasmin has enzymatic activity towards a broad spectrum of substrates, but the fibrin clot is considered as its main native substrate [30]. To further evaluate the plasminogen activation efficiency of longistatin, fibrin polymer was incubated with plasminogen–longistatin/-t-PA mixture or with buffer only at 25°C for 24 h. Data revealed that, in the presence of either longistatin or t-PA, plasminogen was able to induce lysis of fibrin clot. Visual observation and spectrometric analysis at 450 nm (OD450) revealed that the fibrinolytic potential of plasminogen, in the presence of longistatin, was directly proportional to the concentration of the recombinant protein used. These results firmly suggest that longistatin activates plasminogen to an active plasmin in a concentration-dependent manner, which in turn lyses the visible fibrin clot. Longistatin caused complete lysis of fibrin clot in the nanomolar range (Figures 5A and B). SDS–PAGE analysis showed that longistatin efficiently cleaved plasminogen into its heavy and light chains. These results are comparable to those of purified, active and commercially available t-PA (Figure 5C). Here, we also observed that longistatin activated a sufficient amount of plasminogen only in the presence of fibrin clot and induced profound fibrinolysis (data not shown).
Fig. 5. Longistatin induced fibrinolysis by activating plasminogen. 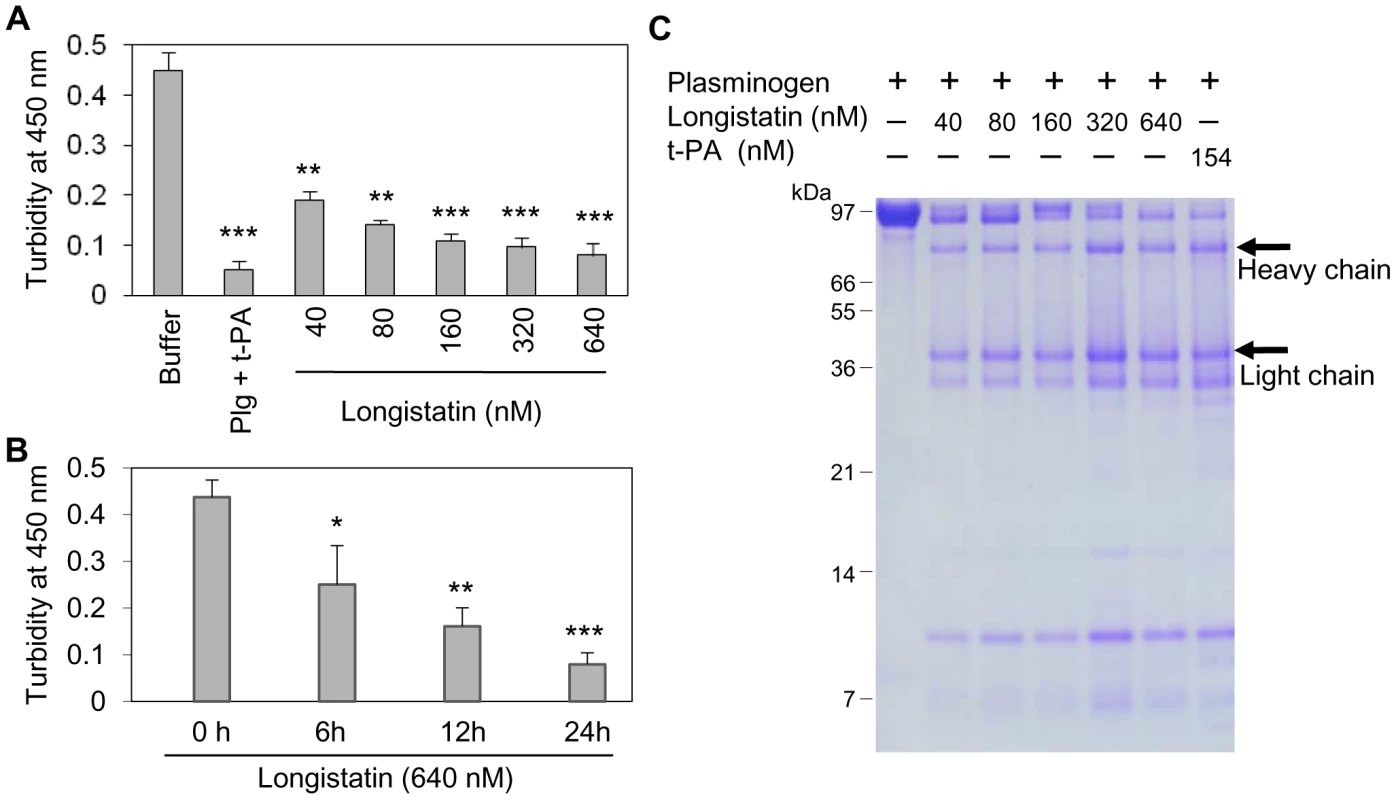
(A) Fibrin clot was formed by incubating fibrinogen (7.5 mM) and thrombin (0.10 NIH unit/µl) and was incubated in the presence of plasminogen-t-PA/-longistatin (40, 80, 160, 320 and 640 nM) mixture or buffer only at 25°C for 24 h. Clot lysis was measured at OD450. Plasminogen induced complete lysis of fibrin clot in the presence of 640 nM longistatin or 154 nM t-PA. (B) Time-dependent activation of plasminogen by longistatin with concomitant lysis of fibrin clot. (C) Cleavage of plasminogen into the heavy and light chains. Digested product of fibrin clot was electrophoresed by 12.5% SDS–PAGE. Asterisks (*) indicate that the difference compared with the negative control group (buffer only) is significant as determined by Student's t-test with unequal variance (*p<0.05, **p<0.01, ***p<0.001). Longistatin binds with fibrin
To explore the fibrin-binding capability of longistatin, we conducted fibrin-binding assays. Longistatin-specific green fluorescence was detected in the fibrin meshwork when purified recombinant longistatin was incorporated with fibrin and reacted with anti-longistatin sera. However, longistatin-specific fluorescent reaction was completely absent in the absence of longistatin in the reaction mixture or when longistatin-impregnated fibrin meshwork was treated with pre-immune sera (1∶100), indicating that longistatin is able to bind with fibrin clot. To compare the fibrin binding of longistatin, we used t-PA as a positive control because t-PA is known to bind with fibrin [31]. Here, t-PA-specific fluorescence was only detected when t-PA added fibrin was treated with anti-t-PA mouse monoclonal antibody (1∶100), but no such reaction was visible in the presence of pre-immune sera (normal serum) at the same concentration. To evaluate the specificity of the fibrin-binding assays, we used u-PA as a negative control because u-PA does not bind with fibrin [32]. u-PA-specific reaction was not detected in the fibrin meshwork even in the presence of a relatively high concentration (1∶20) of anti-u-PA antibody (Figure 6A). By using SDS−PAGE analysis, we also observed that the concentration of residual longistatin was markedly reduced after fibrin clot formation (Figure 6B), which reinforced the assertion that longistatin was bound with the fibrin clot. Furthermore, we determined the concentration of longistatin/t-PA in the supernatant obtained from the fibrin-binding assay mixer and data revealed that the percentage of binding of longistatin/t-PA with fibrin meshwork was directly proportional to the amount of fibrin. Longistatin binding was 97.93%±1.68% when 60 mM fibrinogen was used to produce fibrin clot and, under the same conditions, binding of t-PA was 90.6%±1.2%. Specificity of the binding was evidenced by the absence of u-PA binding (Figure 6C). To evaluate the fibrin-binding potentials of longistatin, we determined the fibrin-binding parameters of longistatin and compared them with those of t-PA. Longistatin was shown to bind with fibrin with the estimated Kd, Bmax and molar binding ratio (MBR) of 145.5±3.3 nmol/L, 3.1±0.6 µmol/L and 42.3±7.4, whereas those of t-PA were 159.2±7.4 nmol/L, 1.4±0.4 µmol/L and 19.3±4.7, respectively (Table 1), suggesting that longistatin potently binds with fibrin. Fibrin binding is an essential feature for the plasminogen activators that specifically activate fibrin clot-bound plasminogen. For example, t-PA is a very weak activator of plasminogen in the absence of fibrin. The affinity between t-PA and plasminogen is significantly increased in the presence of fibrin of blood clot [6], [33]. Fibrin binding was also assumed as critical in the activation process of plasminogen by longistatin.
Fig. 6. Binding of longistatin with fibrin clot. 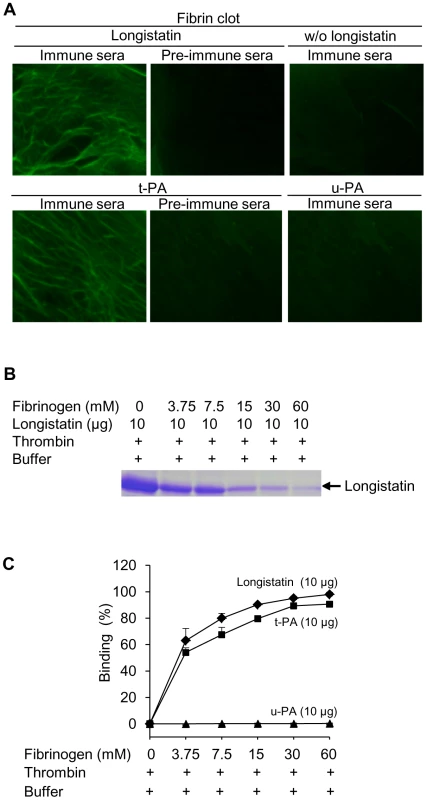
(A) Detection of longistatin bound on fibrin meshwork. Fibrinogen at different concentrations (3.75, 7.5, 15, 30 and 60 mM; final concentration) was mixed in the absence or presence of longistatin (10 µg) or an equal amount of t-PA or u-PA in a buffer (50 mM Tris–HCl, pH 7.5; 100 mM NaCl and 5 mM CaCl2) and thrombin (0.10 NIH unit/µl) was added immediately and was incubated at 25°C for 1 h. The clot was treated with anti-longistatin (1∶100), anti-t-PA (1∶100), anti-u-PA (1∶20) or pre-immune sera (1∶100). Bound antibodies were detected using green fluorescent-labeled secondary antibody (Alexa Flour 488 goat anti-mouse IgG). (B) Supernatant was analyzed by 12.5% SDS–PAGE under reducing conditions. (C) The target protein was extracted from the supernatant and its concentration was determined using micro-BCA reagent as described in Materials and Methods. The results are expressed as percentage of longistatin/t-PA/u-PA bound to the fibrin clot. Data represent mean ± SD, n = 3. Tab. 1. Comparison of fibrin-binding parameters of longistatin with those of t-PA. 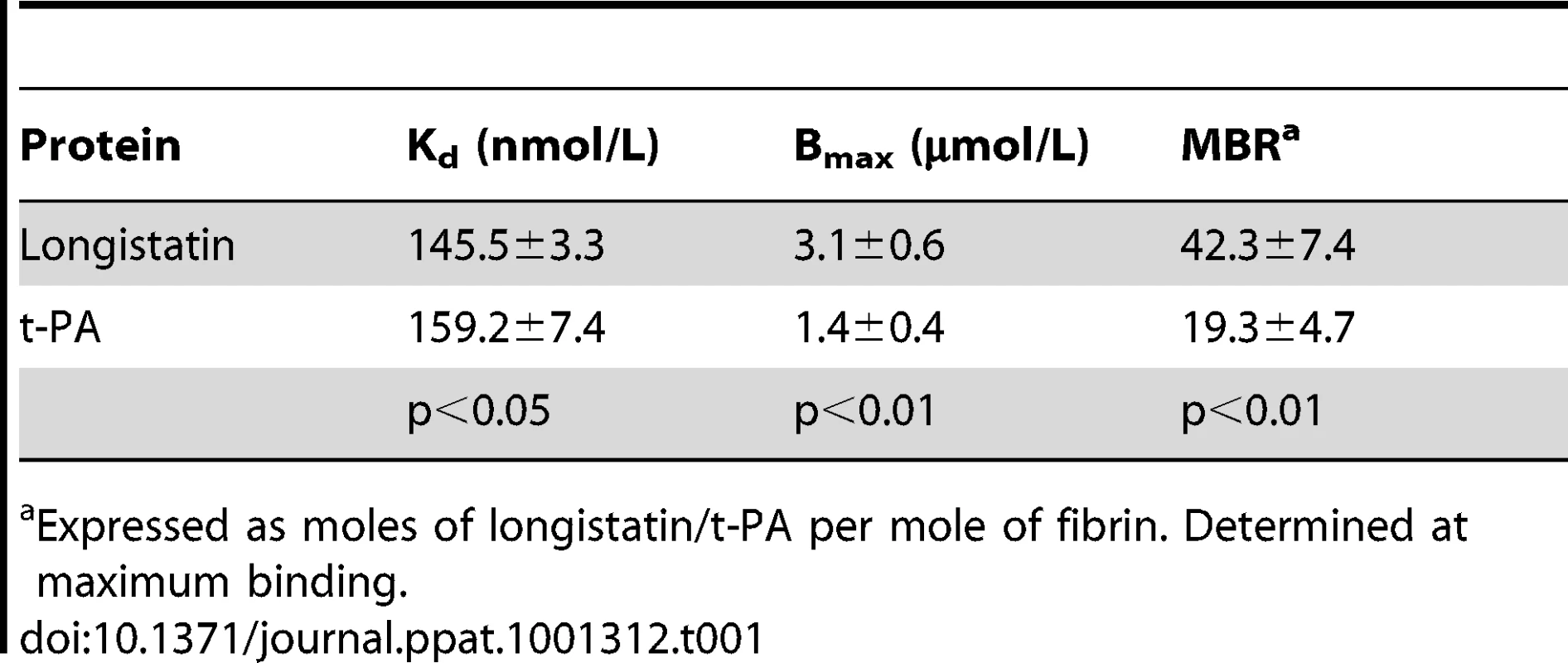
Expressed as moles of longistatin/t-PA per mole of fibrin. Determined at maximum binding. Longistatin activates plasminogen present in plasma milieu and induces thrombolysis
To demonstrate the thrombolytic capability of longistatin, we treated freshly prepared platelet-rich thrombi kept in fresh plasma. Longistatin was able to cause lysis of platelet-rich thrombi in the presence of fresh plasma in a concentration-dependent manner and efficiently lysed thrombi at 640 nM concentration (Figure 7A). Longistatin induced more than 50% lysis of thrombi within 2 h at 640 nM concentration (data not shown). In the same experimental setup, longistatin (640 nM) and t-PA (154 nm) induced 93.62%±2.33% and 98.78%±2.11% lysis of thrombi, respectively, by 12 h (Figure 7B). Moreover, like t-PA, longistatin efficiently digested fibrin clot produced from purified, commercially available fibrinogen and thrombin in the presence of plasma (data not shown). Taken together, our results suggest that longistatin is capable of causing thrombolysis and subsequent recanalization of occluded thrombosed vascular tree by activating the physiological level of plasminogen into plasmin.
Fig. 7. Lysis of platelet-rich thrombi by longistatin in dog plasma. 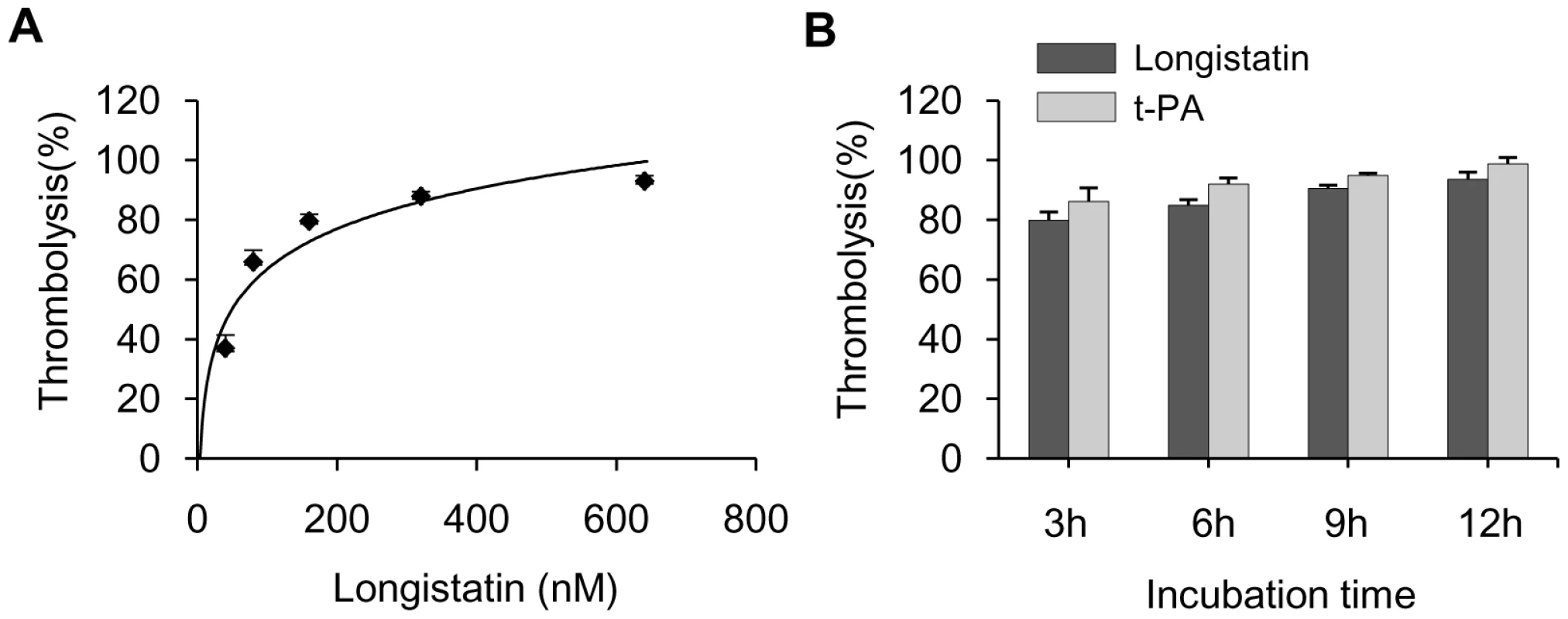
(A) Longistatin hydrolyzes platelet-rich thrombi ex vivo in dog plasma. Platelet-rich clot was produced by incubating 0.2 ml of dog blood and the thrombi were treated with longistatin at various concentrations in 0.5 ml of dog plasma at 37°C for 12 h and weighed at the indicated period. (B) Comparison of thrombolytic activity of longistatin with that of t-PA. Thrombi were treated with longistatin (640 nM)/t-PA (154 nM) under the same ex vivo experimental conditions. Functional implications of longistatin in blood coagulation and fibrinolysis cascades
We hypothesized that ixodid ticks synthesize longistatin and possibly other functionally related bioactive molecules to efficiently counteract host's hemostatic ability and/or to activate its own fibrinolytic machinery to create blood pools for the acquisition of blood-meals and engorgement. Our in vivo and in vitro data strongly support this hypothesis and that longistatin exerts its multifunctional roles both in coagulation cascades and in fibrinolytic pathways. In this study, we proposed a longistatin-induced anti-coagulation and fibrinolytic mechanism that works persistently against host's hemostatic pathways until ticks ensure full blood-meals (Figure 8).
Fig. 8. A schematic diagram showing roles of longistatin in blood coagulation and fibrinolysis events. 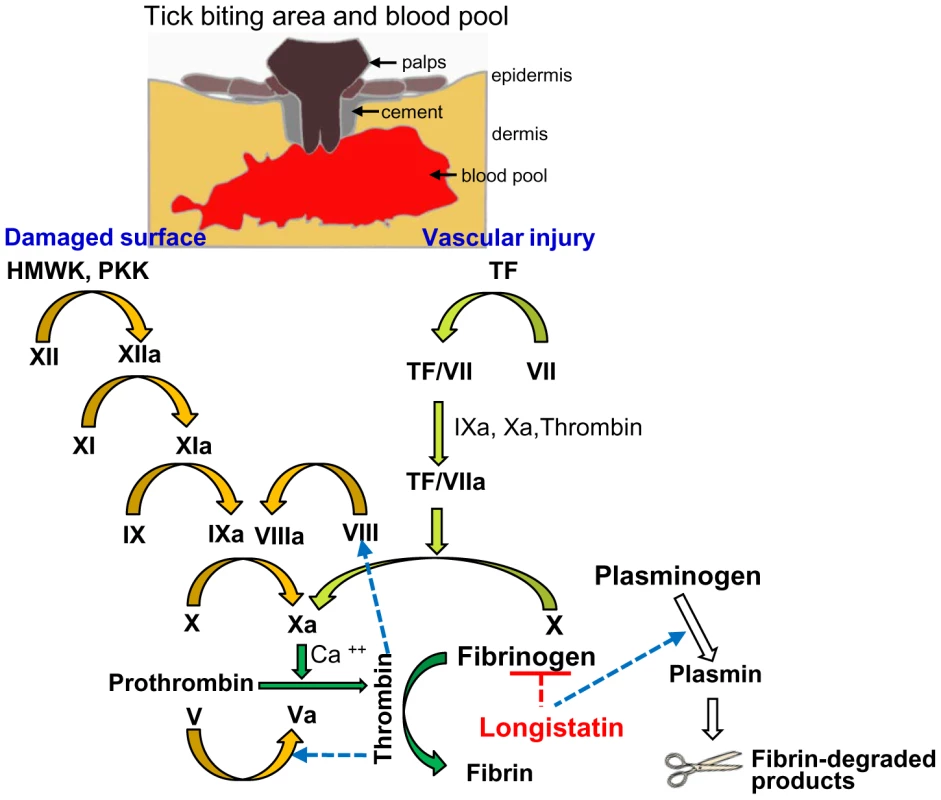
In the initial phase, the tick bites and lacerates tissues at the site of attachment and damages vascular beds, which results in hemorrhage leading to the development of a blood pool. Longistatin is synthesized in and secreted from the salivary glands and injected into the blood pool during feeding process [27]. Longistatin degrades fibrinogen and activates plasminogen to its active form, plasmin. HMWK, high-molecular-weight kininogen; PKK, prekallikrein; TF, tissue factor. Yellow arrows, contact activation (intrinsic) pathway; olive-green arrows, tissue factor (extrinsic) pathway; green arrows, common pathway of coagulation cascade and white arrows, fibrinolytic pathway. Figure adapted from ref. [1], [6], [32]. Discussion
Previous literatures on the feeding behavior and physiology of hematophagous ixodid ticks suggest that acquisition of blood-meals from mammalian hosts is modulated by a vast array of pharmacologically active bio-molecules secreted from the salivary glands of these amazing tiny arthropods. Ticks' saliva is thought to efficiently manipulate the hosts' strong defense mechanisms, such as hemostasis, inflammatory reactions and immune responses that are induced against the tick during hematophagy [1], [26], [34]–[37]. In the present study, we provide evidence that longistatin, a salivary gland protein with two functional EF-hand Ca++-binding domains isolated from the ixodid tick, H. longicornis, binds with fibrin and specifically activates plasminogen to its active form, plasmin. We also demonstrate that longistatin degrades fibrinogen and is able to delay fibrin clot formation; thus, longistatin appears to be essential to keep the blood in a fluid state in the blood pool, which enables ticks to feed and replete on blood-meals from hosts.
Our in vivo gene silencing study revealed that longistatin-specific gene-knockdown ticks were unable to produce a blood pool and consequently failed to replete. These findings prompted us to unveil the underlying complex mechanism(s) by which longistatin exerts its modulatory roles in blood pool formation and maintenance, which is an essential pre-requisite to ticks for successful feeding and engorgement. Anticoagulation of host's blood is a crucial step for the development and maintenance of a blood pool. To verify this, we have conducted several anticoagulation and fibrinolysis assays. Interestingly, we have shown that longistatin significantly extends the time of fibrin clot formation up to 90 min when fibrinogen is treated with longistatin compared with untreated fibrinogen (control), which takes only 15 min to complete fibrin clot formation. Data on fibrinogenolytic assays also support this observation that longistatin is capable of degrading the α, β and γ chains of fibrinogen in a concentration-dependent manner and completely hydrolyzes these three bands. Therefore, it may be assumed that the delay in the fibrin clot formation during in vitro anticoagulation assays in the presence of longistatin is due to the gradual degradation of coagulable proteins in the reaction mixture. Fibrinogenolytic activity has also been reported in tick metalloprotease isolated from Ixodes scapularis [38] and in several proteases identified from snake venoms [39] and spider toxins [40]. Fibrinogen is the key component for the formation of cross-linked fibrin polymer. In general, after laceration of blood vessels, platelets are exposed to the subendothelial collagen and become activated and aggregated around the injuries. Then, fibrin strands, derived from cleavage of fibrinogen, intertwine about the aggregated platelets giving rigidity and stability of the initial and preliminary platelet plugs. Finally, activated factor XIII forms covalent bonds that crosslink the fibrin polymers and thus the injured blood vessels are sealed by an extra strengthened, stable fibrin clot leading to hemostasis [1], [41], [42]. Therefore, it may be predicted that fibrinogenase activity of longistatin hampers hemostasis and may facilitate hemorrhage into the blood pools from which ticks persistently feed blood-meals.
We have shown that longistatin specifically activated plasminogen in the presence of fibrin clot and cleaved it into plasmin, heavy chain (α) and light chain (β). Although plasminogen is unable to cleave fibrin clot but it has a strong affinity for fibrin. Plasminogen has a secondary structure known as a kringle domain, which anchors plasminogen specifically to the carboxy-terminal arginine and lysine residues of the fibrin; thus, plasminogen becomes more concentrated on the surface of the clot, whenever and wherever it develops. As soon as plasminogen is converted into plasmin by its activators, it functions like a serine protease. Fibrin acts as a cofactor in the enzymatic activation reactions when plasminogen is activated by the fibrin-selective agents, like t-PA and its derivatives or staphylokinase and its derivatives. Through a highly orchestrated biochemical process, plasmin initially creates nicks on the fibrin and further digestion leads to the complete dissolution of fibrin meshwork into soluble fibrin degradation products [6], [32], [43]. Our results clearly demonstrate that longistatin contributes to this well-coordinated fibrinolytic pathway by activating plasminogen into plasmin. Plasminogen activators have been purified from the venomous snake, Trimeresurus stejnegeri [44] and from the saliva of common vampire bat, Desmodus rotundus [10], [45]. In vampire bats, plasminogen activator is thought to be a key enzyme that plays a crucial role in the maintenance of the flow of blood during the feeding process [46] and snake-venom plasminogen activator is associated with the pathogenesis of envenomation rendering the blood incoagulable [44]. Maintenance of hosts' blood fluidity at the site of biting is also very critical for the development of blood pool and subsequent blood feeding, and eventually for the survival of ixodid ticks. From the available evidence, here we assume that fibrin-specific, plasminogen-activation-dependent thrombolytic potentiality of longistatin plays significant functional roles in the pathobiology of vector ticks through the formation of feeding lesion and by the maintenance of its homeostasis throughout the entire period of blood feeding.
In conclusion, longistatin, a salivary-gland protein of ixodid ticks with multitarget potential, shows high specificity for fibrin clot-bound plasminogen, and degrades fibrinogen. These findings indicate that longistatin plays significant roles in the formation and maintenance of blood pools leading to the successful acquisition of blood-meals and may be critical for the survival of ixodid ticks. Furthermore, our data suggest that longistatin may serve as a novel therapeutic target against ticks and tick-borne diseases, including human diseases such as thrombosis or other occlusive cardiovascular accidents.
Materials and Methods
Ticks and animals
We propagated parthenogenetic Okayama strains of H. longicornis at the Laboratory of Parasitic Diseases, National Institute of Animal Health (NIAH), Tsukuba, Japan, by feeding on the ear of tick-naïve, specific-pathogen-free Japanese White rabbits according to methods described previously [27]. Ticks were collected after detachment following full engorgement or at the indicated period of feeding. After collection, ticks were weighed and phenotypic differences between the ticks of RNAi and control groups were recorded. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Animal Health. The protocol was approved by the Committee of the Ethics of Animal Experiments of the NIAH (Permit Number: 09-017, 09-018, 10-008, 10-010). All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize the animals' suffering.
Reagents
Green fluorescent-labeled secondary antibody (Alexa Flour 488 goat anti-mouse IgG (H+L)) was purchased from Invitrogen and alkaline phosphatase-conjugated goat anti-mouse IgG (H+L) was from ZYMED. Nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (BCIP/NBT) and T7 RNA polymerase were from Promega. Purified human plasmin, bovine thrombin and fibrinogen were obtained from Sigma. Purified human plasminogen, t-PA and anti-t-PA (Ab-1) mouse monoclonal antibody (GMA-043) were purchased from Calbiochem. Human u-PA was from Cosmo Bio Co. LTD and polyclonal antibody to u-PA was from Acris Antibodies GmbH. Fibrin CNBr fragment was obtained from Technoclone and Boc-Glu-Lys-Lys-MCA from Peptide Institute. Total RNA extraction kit and DNA gel extraction kit were purchased from QIAGEN.
RNA interference
We performed RNAi using dsRNA following a protocol described previously [47]. The sequence coding longistatin was cloned into pBluescript II SK+ vector in XhoI and EcoRI restriction sites following a protocol described previously [27]. The dsRNA complementary to the E. coli malE gene that encodes maltose-binding protein was used as a negative control. cDNA corresponding to malE mRNA was synthesized and was cloned into pBluescript II SK+ plasmid using the primers 5′-CCGCTCGAGCGGTTATGAAAATAAAAACAGGTGCA-3′ and 5′-GAATTCGCTTGTCCTGGAACGCTTTGTC-3′. The inserted sequences of longistatin and malE were amplified by PCR using primers T7 (5′-GTAATACGACTCACTATAGGGC-3′) and CMO422 (5′-GCGTAATACGACTCACTATAGGGAACAAAAGCTGGAGCT-3′) to attach to T7 promoter recognition sites at either end. The PCR products were purified using gel extraction kit (QIAGEN). dsRNA complementary to the respective DNA inserts was synthesized by in vitro transcription using T7 RNA polymerase (Promega). One microgram of longistatin dsRNA (dslongistatin, treated group) or malE dsRNA (dsmalE, control group) was injected into each tick through the 4th coxa. Ticks were observed in a humified incubator for 24 h at 25°C prior to attaching them on the host for feeding. A total of 120 ticks, each of 60 in RNAi and control groups, were attached on the ear of tick-naïve rabbits. Ticks were collected at 24, 48, 72 and 96 h of feeding or after repletion. All ticks collected after they had dropped off the host following full engorgement were weighed individually using a digital balance (Sartorius).
Semiquantitative RT-PCR and qRT-PCR
Salivary glands from adult ticks of both control and RNAi groups at different feeding periods (24, 48, 72, 96 h and engorged) were collected as described previously [27]. Shortly after collection, salivary glands were submerged in RNAlater, an RNA Stabilization Reagent (QIAGEN). Total RNA was isolated using an RNeasy Mini Kit (QIAGEN) according to the manufacturer's protocol and 500 ng of total RNA was used for reverse transcription before PCR. Single-stranded cDNA was prepared using Takara RNA PCR Kit (AMV) Ver.3.0 (Takara) following the manufacturer's instructions. A series of PCRs were carried out using 500 ng of cDNA from each sample and longistatin-specific oligonucleotides (5′GCTATCTCGGCTCCTGTGTC 3′ and 5′ATCTTCGCCAGGTCCTTCTT 3′) or oligonucleotides specific for a control cDNA encoding β-actin in a final volume of 20 µl. The PCR product was subjected to electrophoresis in 1% agarose gel. The qRT-PCR was performed in a LightCycler 1.5 instrument (Roche Instrument Centre AG) using LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics) following a procedure described previously [48]. The reaction mixture of 20 µl contained 4 mM MgCl2, 0.5 µM of each primer (forward and reverse as described above) and 2 µl (250 ng/µl) of the single-stranded DNA template. The data obtained were analyzed using LightCycler Software Version 3.5.
Recombinant longistatin and polyclonal antibody production
Recombinant longistatin was produced, purified, dialyzed and its concentration was determined as previously described [27]. Purified longistatin was kept at –20°C until further use. Polyclonal antibody was produced in BALB/c mice following a procedure described elsewhere [27].
Immunofluorescence and histopathology
We collected salivary glands from partially fed (96 h) adult ticks of both control and RNAi groups as described above. Salivary glands were placed on slides and were fixed with 4% paraformaldehyde. Salivary glands were then permeabilized with 0.1% tritonX-100 and were treated with mouse anti-longistatin sera (1∶100). Bound antibodies were detected using green fluorescent-labeled secondary antibody (Alexa Flour 488 goat anti-mouse IgG (H+L), Invitrogen). Slides were mounted with VECTASHIELD mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories) and examined under a fluorescent microscope (Leica). In addition, thin sections were prepared from tissues collected from the feeding lesions developed on a rabbit's ear at the site of attachment of the ticks. Tissue sections were then subjected to immunofluorescent staining using mouse anti-longistatin sera as described previously[27]. Rabbit's tissues were also stained with EVGS as described previously [49].
Immunoblot analysis
Equal numbers of adult ticks from both RNAi and control groups collected at different feeding intervals (24, 48, 72, 96 h and engorged) were dissected separately in PBS and salivary glands were isolated. Antigens were prepared as previously described [50]. Equal amounts of protein (4 µg) were separated by 12.5% SDS−PAGE under reducing conditions and were transferred onto nitrocellulose membrane. The membrane was treated with mouse anti-longistatin sera (1∶100) overnight at 4°C. Bound antibodies were probed with alkaline phosphatase-conjugated goat anti-mouse IgG (H+L) (ZYMED). The membranes were developed with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (BCIP/NBT, Promega).
Anticoagulation and fibrinogenolytic assays
Fibrinogen (7.5 mM) was pre-incubated in a total volume of 397 µl of buffer containing 25 mM Hepes (pH 7.2) and 25 mM NaCl in the absence or presence of longistatin (0.1, 0.2, 0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM) at 25°C for 3 h. Fibrin clot formation was initiated by the addition of 3 µl of thrombin (0.10 NIH unit/µl). Fibrin clot formation was detected visually and also by determining changes in turbidity at OD450 using a spectrophotometer (Beckman Coulter) at 15 min intervals. To detect the fibrinogenolytic activity of longistatin, fibrinogen (7.5 mM) was incubated in a total volume of 100 µl of buffer (25 mM Hepes, pH 7.2, and 25 mM NaCl) in the absence or presence of longistatin (0.4, 0.8 and 1.6 µM) or plasmin (1.6 µM) at 25°C for 48 h. Aliquots were collected and separated by 12.5% SDS–PAGE.
Plasminogen activation assays
Longistatin at various concentrations (40, 80, 160, 320 and 640 nM) was incubated in a 96-well cell culture plate without or with plasminogen (0.24 units) in the absence or presence of fibrin CNBr fragments (0.25, 1 and 4 µg) in a total volume of 200 µl of buffer (50 mM Tris–HCl, pH 7.5; 100 mM NaCl and 5 mM CaCl2) at 25°C for 2 h. Then, 2 µl (100 µM, final concentration) of plasmin-specific fluorogenic substrate (Boc-Glu-Lys-Lys-MCA) was added. Substrate hydrolysis was monitored by measuring excitation and emission wavelengths of 360 nm and 460 nm, respectively, at 15 min intervals using a Spectra Fluor fluorometer (TECAN, Männedorf, Switzerland). All assays were performed in triplicate and activity of activated plasminogen was expressed in an arbitrary fluorescent unit per min (AFU/min).
Fibrinolytic assays
Fibrin clot was incubated in the presence or absence of longistatin. An initial fibrin clot was produced by incubating 10 µl of fibrinogen (7.5 mM) and 5 µl of thrombin (0.10 NIH unit/µl) in a total volume of 400 µl of buffer (50 mM Tris–HCl, pH 7.5; 100 mM NaCl and 5 mM CaCl2) at 25°C for 1 h. An equal volume of plasminogen (2.4 units), t-PA (154 nM) or longistatin at various concentrations (40, 80, 160, 320 and 640 nM) in a total volume of 100 µl of buffer, as mentioned above, was added to the fibrin clot and incubated at 25°C for 24 h. Lysis of fibrin clot was detected visually and also by measuring changes in turbidity at OD450 using a spectrophotometer (Beckman Coulter) at different time intervals (0, 6, 12 and 24 h). Furthermore, to verify the plasminogen activation potential of longistatin, 20 µl of digested products of clots corresponding to each concentration were collected and subjected to 12.5% SDS–PAGE analysis under reducing conditions.
Fibrin clot binding assays
Fibrin binding assays were conducted with the modifications of procedures described previously [31]. Briefly, purified fibrinogen (3.75, 7.5, 15, 30 and 60 mM; final concentration) was mixed in the absence or presence of longistatin (10 µg) or an equal amount of t-PA or u-PA in a total volume of 200 µl of buffer (50 mM Tris–HCl, pH 7.5; 100 mM NaCl and 5 mM CaCl2). Fibrin clot formation was initiated by adding 3 µl of thrombin (0.10 NIH unit/µl) immediately and was incubated at 25°C for 1 h. The clot was centrifuged at 10,000 g for 10 min and supernatant was collected. The remaining clot was extensively washed in PBS and treated with anti-longistatin (1∶100), anti-t-PA (1∶100), anti-u-PA (1∶20) or pre-immune sera (1∶100) overnight at 4°C. Bound antibodies were detected using green fluorescent-labeled secondary antibody. Supernatant was analyzed by 12.5% SDS−PAGE under reducing conditions. The target protein band was excised and protein was extracted from the gel using negative zinc staining kit following the manufacturer's instructions (Bio-Rad). Double volume of cold (−20°C) acetone was mixed thoroughly with the extracted protein, incubated at −20°C for 1 h and centrifuged at 10,000 g for 30 min. The pellet was air-dried and dissolved with distilled water. The concentration of the extracted protein was determined using micro-BCA reagents (Pierce). To determine the binding parameters, longistatin (2−10 µg) or an equal amount of t-PA was incorporated into the fibrin clot. Residual longistatin/t-PA was extracted from the supernatant and the concentration of the relevant protein was determined following the same methods as mentioned above. Kd, Bmax and MBR were calculated according to the procedures as previously described [51].
Ex vivo thrombolysis assays
Platelet-rich thrombi were produced by incubating 0.2 ml of fresh dog blood in a 96-well flat-bottom cell culture plate for 15 min. Thrombi were removed and washed gently with normal saline and weighed using a digital balance (Sartorius). The thrombi were then treated with t-PA (154 nM) or longistatin at various concentrations (40, 80, 160, 320 and 640 nM) in a total volume of 0.5 ml of fresh dog plasma at 37°C for 12 h and weighed at 3 h intervals. Furthermore, fibrin clot was produced by incubating purified, commercially available fibrinogen (7.5 mM) and thrombin (0.10 NIH unit/µl) as mentioned above and was treated with t-PA (154 nM) or longistatin (640 nM) in fresh dog plasma following the same experimental procedures and conditions.
Accession numbers of the proteins and the genes used
Plasmin from human plasma (NP_000292), plasminogen from human plasma (AAN85555), t-PA from human plasma (NP_000921), u-PA from human plasma (NP_002649.1), thrombin (as prothrombin) from bovine plasma (NP_776302), fibrinogen from bovine plasma (α chain, AAI02565; β chain, NP_001136389 and γ chain, AAI02630), longistatin from H. longicornis (AB519820) and malE from E. coli (ZP_02999303).
Statistical analysis
Data were presented as mean ± standard error, where appropriate. Statistical significance was determined using Student's t test with unequal variance.
Zdroje
1. StarkKR
JamesAA
1996
Anticoagulant in vector arthropods.
Parasitol Today
12
430
437
2. DavieEW
RatnoffOD
1964
Waterfall sequence for intrinsic blood clotting.
Science
145
1310
1312
3. RiddelJPJr
AouizeratBE
MiaskowskiC
LillicrapDP
2007
Theories of blood coagulation.
J Pediatr Oncol Nurs
24
123
131
4. ByzovaTV
PlowEF
1997
Networking in the hemostatic system, integrin αIIbβ3 binds prothrobin and influences its activation.
J Biol Chem
272
27183
27188
5. NeymanM
GewirtzJ
PonczM
2008
Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots.
Blood
112
1101
1108
6. Cesarman-MausG
HajjarKA
2005
Molecular mechanisms of fibrinolysis.
Br J Haematol
129
307
321
7. CollenD
LijnenHR
2004
Tissue-type plasminogen activator: a historical perspective and personal account.
J Thromb Haemost
2
541
546
8. ImuraY
StassenJM
KurokawaT
IwasaS
LijnenHR
1992
Thrombolytic and pharmacokinetic properties of an immunoconjugate of single-chain urokinase-type plasminogen activator (u-PA) and a bispecific monoclonal antibody against fibrin and against u-PA in baboons.
Blood
79
2322
2329
9. BooyseFM
OsikowiczG
FederS
ScheinbuksJ
1984
Isolation and characterization of a urokinase-type plasminogen activator (Mr = 54,000) from cultured human endothelial cells indistinguishable from urinary urokinase.
J Biol Chem
259
7198
7205
10. GardellSJ
DuongLT
DiehlRE
YorkJD
HareTR
1989
Isolation, characterization, and cDNA cloning of a vampire bat salivary plasminogen activator.
J Biol Chem
264
17947
17952
11. YoshidaS
SudoT
NiimiM
TaoL
SunB
2008
Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito.
Blood
111
2007
2014
12. HajdusekO
SojkaD
KopacekP
BuresovaV
FrantaZ
2009
Knockdown of proteins involved in iron metabolism limits tick reproduction and development.
Proc Natl Acad Sci U S A
106
1033
1038
13. SonenshineDE
1991
Biology of Ticks.
Vol 1, New York , (New York)
Oxford University Press
14. AkovS
1982
Blood digestion in ticks.
ObenchainFD
GalunR
Physiology of ticks
Oxford
Pergamon Press
197
212
15. MurrayTS
ShapiroED
2010
Lyme disease.
Clin Lab Med
30
311
328
16. KlompenH
2005
Ticks, the ixodida.
MarquardtWC
Biology of Disease Vectors
London
Elsevier Academic Press
45
55
17. TelfordSR3rd
GoethertHK
2004
Emerging tick-borne infections: rediscovered and better characterized, or truly ‘new’?
Parasitology
129
S301
327
18. CableRG
LeibyDA
2003
Risk and prevention of transfusion-transmitted babesiosis and other tick-borne diseases.
Curr Opin Hematol
10
405
411
19. HoT
HtweKK
YamasakiN
ZhangGQ
OgawaM
1995
Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan.
Micribiol Immunol
39
663
671
20. FujisakiK
KawajuS
KamioT
1994
The Taxonomy of the bovine Theileria spp.
Parasitol Today
10
31
33
21. KempDH
StoneBF
BinningtonKC
1982
Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions, and the host response.
ObenchainFD
GalunR
Physiology of Ticks
New York
Pergamon Press Inc
119
168
22. WaxmanL
SmithDE
ArcuriKE
VlasukGP
1990
Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa.
Science
248
593
596
23. NarasimhanS
MontgomeryRR
DePonteK
TschudiC
MarcantonioN
2004
Disruption of Ixodes scapularis anticoagulation by using RNA interference.
Proc Natl Acad Sci U S A
101
1141
1146
24. HoviusJW
LeviM
FikrigE
2008
Salivating for knowledge: potential pharmacological agents in tick saliva.
PLoS Med
5
e43
25. KohCY
KazimirovaM
TrimnellA
TakacP
LabudaM
2007
Variengin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick.
J Biol Chem
282
29101
29113
26. Maritz-OlivierC
StutzerC
JongejanF
NeitzAW
GasparAR
2007
Tick anti-hemostatics: targets for future vaccines and therapeutics.
Trends Parasitol
23
397
407
27. Anisuzzaman
IslamMK
MiyoshiT
AlimMA
HattaT
2010
Longistatin, a novel EF-hand protein from the ixodid tick Haemaphysalis longicornis, is required for acquisition of host blood-meals.
Int J Parasitol
40
721
729
28. ZamarronC
LijnenHR
CollenD
1984
Kinetics of the activation of plasminogen by natural and recombinant tissue-type plasminogen activator.
J Biol Chem
259
2080
2083
29. HoylaertsM
RijkenDC
LijnenHR
CollenD
1982
Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin.
J Biol Chem
257
2912
2919
30. WimanB
2000
The fibrinolytic enzyme system. Basic principles and links to venous and arterial thrombosis.
Hematol Oncol Clin North Am
14
325
338
31. LiXK
LijnenHR
NellesL
Van HoefB
StassenJM
1992
Biochemical and biologic properties of rt-PA del (K296-G302), a recombinant human tissue-type plasminogen activator deletion mutant resistant to plasminogen activator inhibitor-1.
Blood
79
417
429
32. MurrayV
NorrvingB
SandercockPA
TeréntA
WardlawJM
2010
The molecular basis of thrombolysis and its clinical application in stroke.
J Intern Med
267
191
208
33. TateKM
HigginsDL
HolmesWE
WinklerME
HeynekerHL
1987
Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis.
Biochemistry
26
338
343
34. MonteiroRQ
RezaieAR
RibeiroJM
FrancischettiIM
2005
Ixolaris: a factor Xa heparin-binding exosite inhibitor.
J Biochem
387
871
877
35. IwanagaS
OkadaM
IsawaH
MoritaA
YudaM
2003
Identification and characterization of novel salivary thrombin inhibitors from ixodidae tick, Haemaphysalis longicornis.
Eur J Biochem
270
1926
1934
36. KarczewskiJ
EndrisR
ConnollyTM
1994
Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata.
J Biol Chem
269
6702
6708
37. van de LochtA
StubbsMT
BodeW
FriedrichT
BollschweilerC
1996
The ornithodorin-thrombin crystal structure, a key to the TAP enigma?
EMBO J
15
6011
6017
38. FrancischettiIM
MatherTN
RibeiroJM
2003
Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis.
Biochem Biophys Res Commun
305
869
875
39. MatsuiT
SakuraiY
FujimuraY
HayashiI
Oh-IshiS
1998
Purification and amino acid sequence of halystase from snake venom of Agkistrodon halys blomhoffii, a serine protease that cleaves specifically fibrinogen and kininogen.
Eur J Biochem
252
569
575
40. da SilveiraRB
dos Santos FilhoJF
MangiliOC
VeigaSS
GremskiW
2002
Identification of proteases in the extract of venom glands from brown spiders.
Toxicon
40
815
822
41. FurieB
FurieBC
2005
Thrombus formation in vivo.
J Clin Invest
115
3355
3362
42. MacFarlaneRG
1964
An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier.
Nature
202
498
499
43. NesheimM
2003
Thrombin and fibrinolysis.
Chest
124
33
39
44. ZhangY
WisnerA
XiongY
BonC
1995
A novel plasminogen activator from snake venom. Purification, characterization, and molecular cloning.
J Biol Chem
270
10246
10255
45. LiberatoreGT
SamsonA
BladinC
SchleuningWD
MedcalfRL
2003
Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration.
Stroke
34
537
543
46. Tellgren-RothA
DittmarK
MasseySE
KemiC
Tellgren-RothC
2009
Keeping the blood flowing-plasminogen activator genes and feeding behavior in vampire bats.
Naturwissenschaften
96
39
47
47. IslamMK
TsujiN
MiyoshiT
AlimMA
HuangX
2009
The Kunitz-like modulatory protein, Haemangin, is vital for hard tick blood feeding success.
PLoS Pathog
5
e1000497
48. TsujiN
MiyoshiT
BattsetsegB
MatsuoT
XuanX
2008
A cysteine protease is critical for Babesia spp. transmission in Haemaphysalis ticks.
PLoS Pathog
4
e1000062
49. ChinoD
FujitaY
IshiiK
NakayamaK
2003
Effects of DX-9065a, an inhibitor of factor Xa, on ellagic acid-induced plantar skin thrombosis assessed in tetrodotoxin - and N(omega)-nitro-L-arginine-treated rats.
J Pharmacol Sci
91
319
329
50. AlimMA
TsujiN
MiyoshiT
IslamMK
HuangX
2007
Characterization of asparaginyl endopeptidase, legumain induced by blood feeding in the ixodid tick Haemaphysalis longicornis.
Insect Biochem Mol Biol
37
911
922
51. SiddiqiF
OdrljinTM
FayPJ
CoxC
FrancisCW
1998
Binding of tissue-plasminogen activator to fibrin: effect of ultrasound.
Blood
91
2019
2025
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral Encephalomyelitis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



