-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
Malaria-protective CD8+ T cells specific for the circumsporozoite (CS) protein are primed by dendritic cells (DCs) after sporozoite injection by infected mosquitoes. The primed cells then eliminate parasite liver stages after recognizing the CS epitopes presented by hepatocytes. To define the in vivo processing of CS by DCs and hepatocytes, we generated parasites carrying a mutant CS protein containing the H-2Kb epitope SIINFEKL, and evaluated the T cell response using transgenic and mutant mice. We determined that in both DCs and hepatocytes CS epitopes must reach the cytosol and use the TAP transporters to access the ER. Furthermore, we used endosomal mutant (3d) and cytochrome c treated mice to address the role of cross-presentation in the priming and effector phases of the T cell response. We determined that in DCs, CS is cross-presented via endosomes while, conversely, in hepatocytes protein must be secreted directly into the cytosol. This suggests that the main targets of protective CD8+ T cells are parasite proteins exported to the hepatocyte cytosol. Surprisingly, however, secretion of the CS protein into hepatocytes was not dependent upon parasite-export (Pexel/VTS) motifs in this protein. Together, these results indicate that the presentation of epitopes to CD8+ T cells follows distinct pathways in DCs when the immune response is induced and in hepatocytes during the effector phase.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1001318
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001318Summary
Malaria-protective CD8+ T cells specific for the circumsporozoite (CS) protein are primed by dendritic cells (DCs) after sporozoite injection by infected mosquitoes. The primed cells then eliminate parasite liver stages after recognizing the CS epitopes presented by hepatocytes. To define the in vivo processing of CS by DCs and hepatocytes, we generated parasites carrying a mutant CS protein containing the H-2Kb epitope SIINFEKL, and evaluated the T cell response using transgenic and mutant mice. We determined that in both DCs and hepatocytes CS epitopes must reach the cytosol and use the TAP transporters to access the ER. Furthermore, we used endosomal mutant (3d) and cytochrome c treated mice to address the role of cross-presentation in the priming and effector phases of the T cell response. We determined that in DCs, CS is cross-presented via endosomes while, conversely, in hepatocytes protein must be secreted directly into the cytosol. This suggests that the main targets of protective CD8+ T cells are parasite proteins exported to the hepatocyte cytosol. Surprisingly, however, secretion of the CS protein into hepatocytes was not dependent upon parasite-export (Pexel/VTS) motifs in this protein. Together, these results indicate that the presentation of epitopes to CD8+ T cells follows distinct pathways in DCs when the immune response is induced and in hepatocytes during the effector phase.
Introduction
Immunization with irradiated Plasmodium sporozoites to induce sterile protection against live parasite challenge is a powerful model for malaria vaccination [1]. Protective immunity is mediated in part by CD8+ T cells specific for the circumsporozoite (CS) protein of Plasmodium [2], [3]. Plasmodium specific CD8+ T cells have been shown to be primed by dendritic cells (DCs) [4], [5], [6], [7]. In particular, we have found that after sporozoite inoculation into the dermis by infected mosquitoes, antigen is presented by DCs in the skin-draining lymph node to initiate the CD8+ T cell response [4]. Primed CD8+ T cells then exit the priming site and migrate to the liver where they can eliminate infection after recognizing antigen presented by hepatocytes [4]. Thus CD8+ T cell mediated immunity requires antigen presentation by two different cell types – DCs and hepatocytes. Determining how DCs and hepatocytes process and present Plasmodium antigens is essential for the rational identification of vaccine candidates. Since immunization with irradiated sporozoites represents the gold standard for malaria vaccination it is important to know which sporozoite antigens are presented by DCs. Perhaps more vital still, is to understand which molecules are presented by hepatocytes, as only those molecules presented to effector cells can be the targets of protective immunity.
Microbial and tumor epitopes presented by MHC class I usually derive from proteins in the cytosol that are proteolytically cleaved into small peptides by the proteasome. These peptides are translocated from the cytosol into the ER by the TAP transporter for loading onto class I MHC molecules, which then traffic towards the cell surface (reviewed in [8]). Many parasites, however, reside within a parasitophorous vacuole (PV) and their proteins are not necessarily secreted into the host cytosol. The processing and presentation of intracellular parasite antigens is therefore complex and still poorly understood. Toxoplasma gondii antigens have been reported to reach the cytosol for class I processing via fusion of the PV and the host ER; from the host ER antigens may be retrotranslocated into the host cytosol for processing [9]. Leishmania major antigens may bypass the host cytosol altogether as antigen presentation appears to be TAP independent. Instead it is believed that L. major-derived peptides are directly loaded onto MHC Class I in the phagolysosome [10].
The in vivo processing of Plasmodium sporozoite or liver stage antigens has not been studied. Unlike Toxoplasma or Leishmania, Plasmodium does not infect professional APCs and it is not known how DCs acquire sporozoite antigen. Likewise, the presentation of antigens by hepatocytes to effector cells is also poorly understood. In-vitro evidence suggests that hepatocytes are capable of presenting Plasmodium antigen and that this may be proteasome dependent [11], requiring the export of parasite antigen to the hepatocyte cytosol by unknown mechanisms. It has been proposed that Pexel/VTS motifs, known to be important for the export of proteins out of the PV in Plasmodium blood stages [12], [13], could also be involved in the transport of liver stage antigens to the hepatocyte cytosol for processing and presentation by class I MHC [14].
In this study we aimed to identify key cellular and molecular features of the antigen processing pathways employed by DCs and hepatocytes. We aimed to determine if Plasmodium CS processing requires the use of the cytoplasmic TAP dependent pathway to transport the processed epitope from the cytosol to the ER and allow binding of the peptide to class I MHC. In addition, we wanted to investigate whether the CS antigen is phagocytosed by presenting cells or if it is directly deposited or secreted into the cytosol of DCs or hepatocytes. To address these questions we generated P. berghei parasites that express a mutant CS protein containing the model SIINFEKL H-2Kb restricted epitope. Using this parasite in conjunction with knockout and mutant mice we have been able to generate the clearest picture to date of the processing of the CS protein from both sporozoite and liver stages.
Results
Generation of P. berghei CS5M parasites expressing SIINFEKL in the CS protein
A major obstacle to determining how Plasmodium antigens are presented to T cells is the lack of defined H-2b restricted epitopes which severely limits in vivo studies, as many transgenic mice, which are critical to study basic aspects of immunology, are generated on a C57Bl/6 (H-2b) background. To overcome this, we generated P. berghei CS5M parasites in which the endogenous CS gene was replaced with a modified CS gene carrying 5 mutations that changed the natural H-2Kd restricted epitope SYIPSAEKI to SIINFEKL, an H-2Kb restricted epitope (Figure 1A and B). P. berghei CS5M parasites were apparently normal as they infected mosquitoes and mice similarly to parental P. berghei ANKA (Figure S1). Most importantly P. berghei CS5M parasites stimulated a robust SIINFEKL specific response in C57Bl/6 mice upon immunization (Figure 1C), and activated SIINFEKL-specific CD8+ T cells from previously generated TCR transgenic mice [15] were able to eliminate the liver stages of P. berghei CS5M (Figure 1D).
Fig. 1. Generation of P. berghei CS5M parasites. 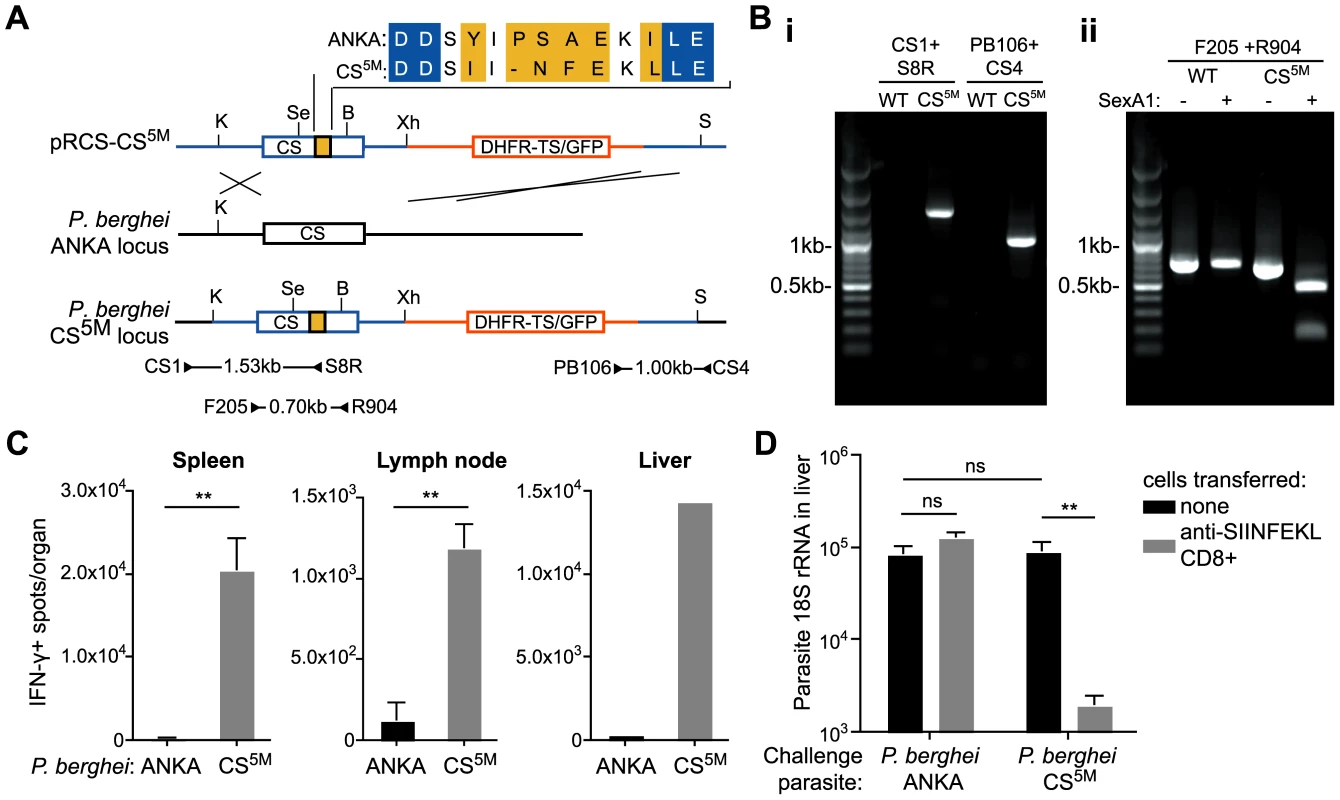
A. Scheme of the strategy used for gene targeting of the replacement CS5M molecule. Location of primers used for PCR verification of recombination is given below (primer sequences given in Table S1). Restriction sites are K – KpnI; Se – SexAI; Bs – BsmF1; X – XhoI; S – SacI. B. Verification of clones – i. genomic DNA from cloned parasites was amplified with the primers CS1 and S8R (giving a 1526 bp product) to verify recombination at the 5′ end, and the primers CS4 and PB106 (giving a 1001 bp product) to verify recombination at the 3′ end, genomic DNA from P. berghei ANKA was used as a control. ii. To verify that the parasite population was clonal, genomic DNA was amplified within the CS sequence with the primers F205 and R904 to give a 699 bp product. The PCR product was then digested with SexA1, which cuts in the P. berghei CS5M product, but not the P. berghei ANKA product, to yield fragments of 510 and 186 bp. C. C57Bl/6 mice were immunized i.d. in the right ear with 5×104 irradiated P. berghei ANKA or P. berghei CS5M parasites. 10 days later the SIINFEKL-specific immune response in the spleen, draining lymph nodes and liver (pooled) was determined by ELISPOT (mean ± SEM; n = 3, data from one of 3 similar experiments; ** = P<0.01). D. C57Bl/6 mice received 2×106 SIINFEKL-specific effector CD8+ T cells 3 hours prior to challenge with 5×103 P. berghei ANKA or P. berghei CS5M sporozoites (grey bars); control mice did not receive effector cells (black bars). 40 hours later livers were taken and parasite rRNA concentration determined by real-time PCR (mean ± SEM; n = 4, data from one of 2 similar experiments, ns = not significant). It is important to emphasize that our approach differs significantly from the more common strategy of inserting an entire foreign gene into a parasite and then tracking the immune responses to the foreign molecule. In the P. berghei CS5M parasite SIINFEKL is inserted in place of a well-defined natural epitope, leaving intact the neighboring residues to ensure correct proteasomal processing, thus the model epitope is presented exactly as the natural CS epitope. This makes the P. berghei CS5M parasite an excellent system in which to study antigen processing and presentation. Moreover, we anticipate that P. berghei CS5M will be a powerful tool for use in future studies of antigen specific immune responses to malaria sporozoites.
The presentation of sporozoite antigen by DCs is TAP dependent
We initiated our studies on the presentation of Plasmodium antigen by investigating whether DCs present irradiated sporozoite antigen via the canonical TAP dependent pathway. Wild type and TAP-1 deficient mice [16] were immunized intra-dermally in the ear with sporozoites and 2 days later CD11c+ DCs were isolated from the draining lymph nodes. To assess antigen presentation the DCs were co-cultured with CFSE-labeled SIINFEKL specific transgenic cells. Antigen presentation was quantified by measuring the expansion of the transgenic cell population 3 days after immunization. While DCs isolated from wild type animals induced extensive proliferation of the SIINFEKL specific cells, DCs from immunized TAP-1 deficient animals were unable to induce proliferation (Figure 2A). The failure of TAP-1 deficient DCs to induce proliferation could only be due to a processing defect as TAP-1 deficient DCs pulsed with exogenous SIINFEKL peptide were fully capable of inducing antigen specific T cell proliferation (Figure S2).
Fig. 2. Antigen presentation by DCs is TAP dependent. 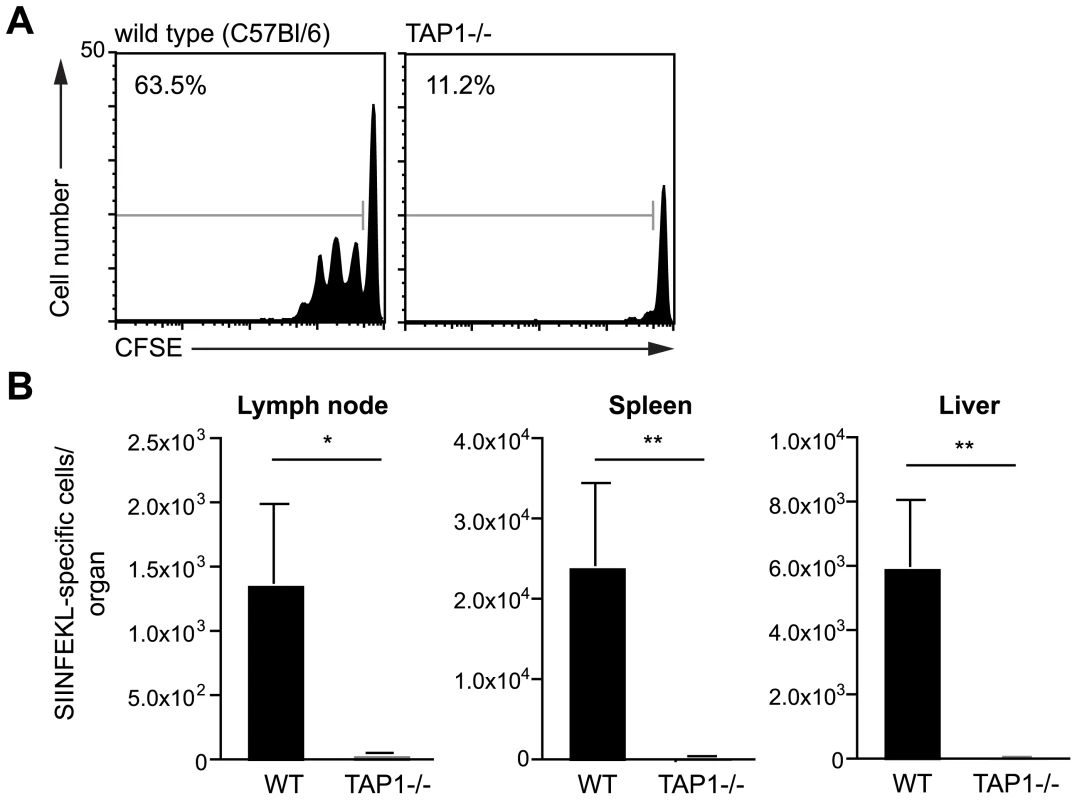
A. CFSE profiles of SIINFEKL specific transgenic cells after incubation with dLN DCs isolated from C57Bl/6 (wild type) or TAP-1 deficient animals 2 days after immunization with 5×104 P. berghei CS5M sporozoites/ear. Data are based on pooled DCs from 6–8 mice per group; values at top left are the percent of cells that have divided. B. TAP1-/- and C57Bl/6 (wild type) mice received 2×103 naïve SIINFEKL-specific CD8+ T cells prior to being fed on by 10–20 P. berghei CS5M infected mosquitoes. 10 days later the mice were sacrificed and the expansion of SIINFEKL-specific cells in the spleen and liver determined. Data are pooled from 2 similar experiments (mean ± SEM; n = 6). To determine if TAP-1 is required in vivo after immunization via the natural route of infection, wild-type and TAP-1 deficient animals that had received SIINFEKL specific TCR transgenic CD8+ T cells were immunized by the bites of irradiated mosquitoes infected with P. berghei CS5M parasites. We observed a robust antigen specific CD8+ T cell response after immunization of wild type mice; however, immunized TAP-1 deficient animals failed to mount a significant CD8+ T cell response in either the draining LN, spleen or liver (Figure 2B). Together these data indicate that the presentation of the CS protein by DCs is strictly TAP dependent.
Sporozoite antigen presentation by DCs occurs via an endosome-to-cytosol pathway
Given that the priming of sporozoite specific T cells is TAP dependent, the CS protein must reach the cytosol of the DC for antigen processing. Since Plasmodium parasites have not been observed to productively infect DCs [17], [18] it is not obvious how sporozoite antigen accesses the DC cytosol. One possibility is that CS antigen from sporozoites is cross-presented via an endosome-to-cytosol pathway in which sporozoite antigen is phagocytosed and then retrotranslocated into the cytosol [19]. Alternatively, CS may be deposited in DCs during the process of cell traversal - a process in which sporozoites pass through the cytosol of cells, without forming a vacuole around themselves [20], [21], [22].
To distinguish between these possibilities we evaluated the induction of CD8+ T cell responses in animals which have a single-point mutation in the molecule Unc93B1 (3d mice). This mutation causes several impairments to endosome function including defects in signaling via the endosomal TLRs and in cross presentation [23]. We reasoned that if there were defects in T cell priming in these animals it would strongly indicate a role for endosomes in antigen processing by DCs. We found that DCs isolated from immunized 3d mice were less capable of priming SIINFEKL specific T cells in vitro compared to wild type controls (Figure 3A). This defect appears to be in the processing of antigen, as exogenous peptide is efficiently presented by DCs from 3d mice (Figure S2). Nonetheless, ex vivo antigen presentation assays provide only a snapshot of sporozoite antigen presentation at a single time point whereas we have recently shown that prolonged antigen presentation is required for full T cell priming [24]. Thus we assessed T cell priming in vivo after immunization by mosquito bites. We found that the difference observed in ex vivo experiments was amplified in vivo as 3d mice had severely decreased SIINFEKL specific responses in the spleen and liver compared to wild type mice (Figure 3B).
Fig. 3. Antigen presentation by DCs occurs via the endosome-to-cytosol pathway. 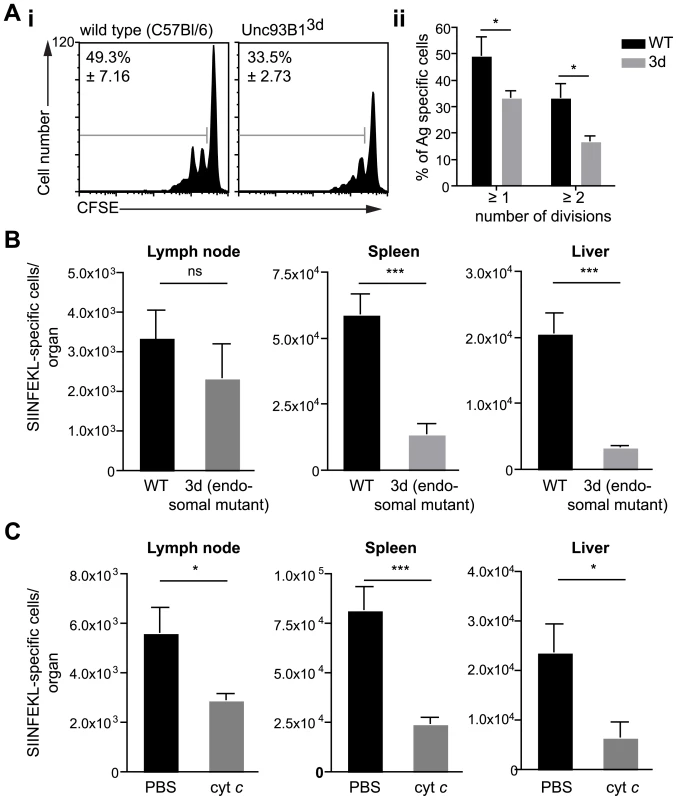
A. i. DCs were purified from the ear draining LN of C57Bl/6 (wild type) or Unc93B13d animals 2 days after immunization with 5×104 P. berghei CS5M sporozoites/ear and incubated with CFSE labeled SIINFEKL specific trangenic cells. i Representative CFSE profiles of the transgenic cells 3 days after immunization values at top left are the percent of cells that have divided (mean ± SEM). ii. Mean number of cells that had divided at least one or twice in 3 independent experiments after incubation with DCs from wild type (black bars) or 3d mice (gray bars) (mean ± SEM; * = P<0.05; for each group in each experiment pooled DCs from 6–8 immunized animals were used). B. 3d (endosomal mutant) and C57Bl/6 mice received 2×103 naïve SIINFEKL-specific cells prior to being fed on by 10–20 P. berghei CS5M infected mosquitoes. 10 days later the mice were sacrificed and the expansion of SIINFEKL-specific cells in the spleen and liver determined. Data are pooled from 2 similar experiments (mean ± SEM; n = 6; *** = P<0.001). C. C57Bl/6 mice received 2×103 naïve SIINFEKL-specific cells prior to being fed on by 10–20 P. berghei CS5M infected mosquitoes. Treated mice received 15 mg of horse cyt c (Sigma) for 3 days starting on the day before immunization, control mice received vehicle alone (PBS). 10 days later the mice were sacrificed and the expansion of SIINFEKL-specific cells in the spleen and liver determined. Data are pooled from 2 similar experiments (mean ± SEM; n = 6). The role of endosomes in the presentation of sporozoite antigen by DCs was further confirmed in experiments in which cross-presenting DCs subsets were depleted in vivo by treatment with cytochrome c (cyt c; Figure S3) [25], [26], [27]. Upon taking up cyt c cross-presenting DCs retrotranslocate it into the cytosol where it can induce apoptosis. In contrast non cross-presenting cell subsets are unaffected as they break down any cyt c that has been taken up in lysosomes. In agreement with the data from 3d mice we found significant reductions in the priming of SIINFEKL specific T cells in cyt c treated animals after immunization via mosquito bites (Figure 3C). Together these data demonstrate that the majority of sporozoite antigen is probably processed via the endosome-to-cytosol pathway.
Opsonization of parasites inhibits their presentation by DCs
Given that the presentation of sporozoite antigen by DCs occurs via the endosome, we hypothesized that opsonization of parasites might enhance the priming of CD8+ T cells [28], [29]. Accordingly we incubated parasites with the anti-CS mAb 3D11 [30] prior to immunization. Unexpectedly, we found that opsonized parasites induced much reduced proliferation of CD8+ T cells compared to sporozoites treated with irrelevant antibody [31] (Figure 4). This intriguing result indicates that opsonization inhibits rather than potentiates the delivery of sporozoite derived CS protein to the DC class I processing pathway. This surprising result is not completely unprecedented – opsonized T. gondii parasites appear to be taken up by DCs via complement and Fc receptors and directed away from the cross presenting pathway and towards break down by lysosomes [9]. To determine if this occurs after opsonization of Plasmodium sporozoites we also treated sporozoites with F(ab′)2 fragments of the 3D11 mAb which cannot be recognized by Fc receptors and do not efficiently fix complement. However 3D11 F(ab′)2 fragments were as efficient as intact antibody at inhibiting T cell priming. Thus it may be that opsonization (and F(ab′)2 treatment) affect T cell priming by immobilizing parasites [32] and thus interfering with a number of processes which may be important for T cell priming. These include parasite migration to the skin draining lymph nodes, invasion of cells in the skin and the shedding of antigen from the sporozoite surface [4], [17], [33].
Fig. 4. Antigen presentation by DCs is inhibited by opsonization. 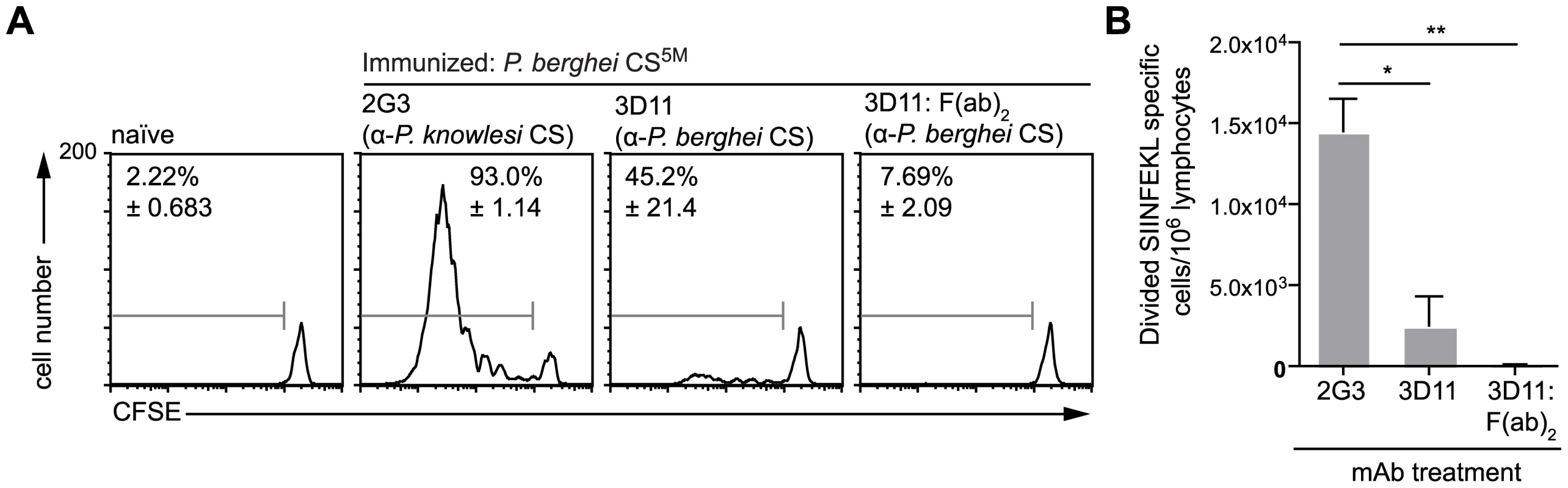
Mice received 5×105 CFSE labeled SIINFEKL-specific cells one day prior to immunization i.d. with 5×104 P. berghei CS5M parasites in the right ear that had either been treated for 20 minutes with 100 µg/ml of either anti-P. knowlesi CS (2G3), anti-P. berghei CS (3D11) or F(ab′)2 fragments prepared from the 3D11 antibody. Three days later the mice were sacrificed and ear draining lymph nodes taken. A. Representative CFSE profiles of the SIINFEKL-specific population, values are the mean % of cells that had proliferated ± SEM in one of 4 similar experiments. B. The size of the expansion of the transferred SIINFEKL-specific cells (mean ± SEM n = 3; representative of 4 similar experiments). Hepatocytes present Plasmodium antigens that are directly secreted into the cytosol
Because effector cells must kill infected hepatocytes, it is also required that hepatocytes present processed antigen to CD8+ T cells. Therefore, in addition to DCs, we were also interested in determining how hepatocytes process antigen for presentation to effector cells. To determine if antigen is processed by hepatocytes via the same endosome-to-cytosol pathway employed by DCs, activated SIINFEKL specific CD8+ T cells were transferred to TAP-1 deficient, 3d and cyt c treated mice that were subsequently infected with P. berghei CS5M parasites. The read-out for epitope presentation is T-cell mediated inhibition of liver stage development i.e. if the epitope is presented, activated CD8+ T cells will recognize it and will eliminate liver stage parasites. We also tried to visualize antigen presentation by immuno-fluorescence with the mAb 25-D1.16 which recognizes Kb-SIINFEKL complexes [34]; however, in common with other researchers we found that this technique was not sensitive enough to detect epitopes on the surface of parasite infected cells [35].
Using our in vivo functional assay we found that effector CD8+ T cells had no inhibitory effect on parasite development in the livers of TAP-1 deficient animals while they were fully capable of eliminating parasites in wild type mice (Figure 5A), clearly indicating that in hepatocytes, as in DCs, CS must reach the cytosol for antigen processing. However, in sharp contrast to DCs, we found that hepatocytes do not process antigen via endosomes since effector CD8+ T cells were capable of efficiently eliminating parasites from the livers of 3d or cyt c treated mice (Figure 5B and C). Thus hepatocytes unlike DCs do not appear to process antigen by an endosome to cytosol pathway, rather, hepatocytes present antigen that has been deposited or secreted by the parasite directly into the cytosol.
Fig. 5. Antigen is directly presented to effector cells by hepatocytes. 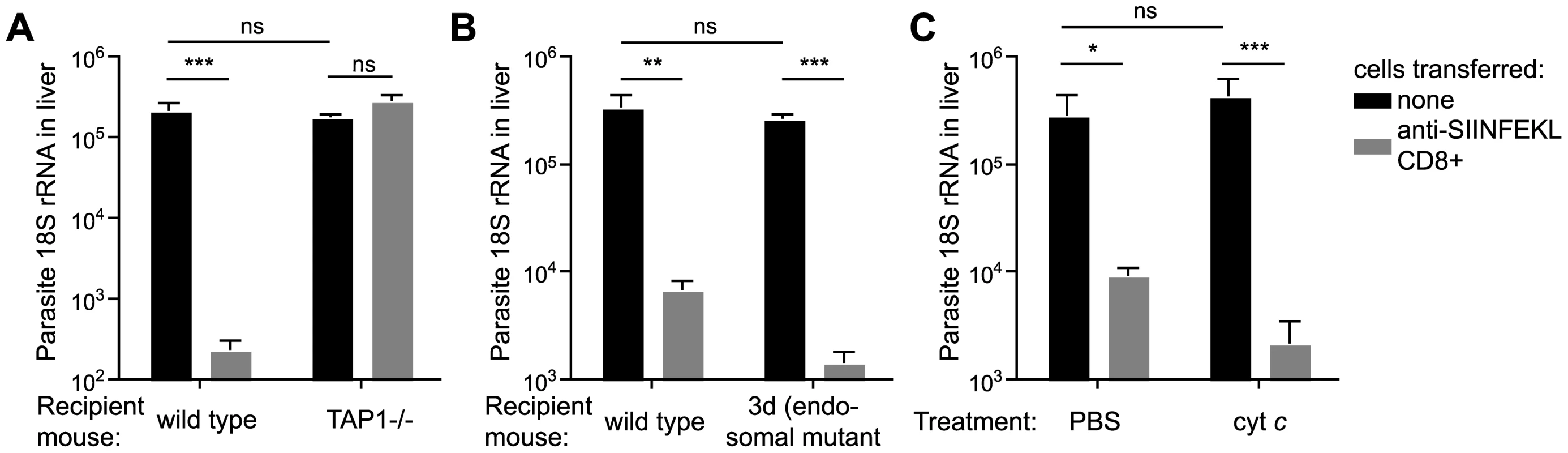
A. C57Bl/6 or TAP1-/- mice received 2×106 SIINFEKL-specific effector CD8+ T cells 3 hours prior to challenge with 5×103 P. berghei CS5M sporozoites (grey bars); control mice did not receive effector cells (black bars). 40 hours later livers were taken and parasite rRNA concentration determined by real-time PCR (mean ± SEM; n = 4, data from one of 2 similar experiments). B. C57Bl/6 or 3d mice received 2×106 SIINFEKL specific effector CD8+ T cells 3 hours prior to challenge with 5×103 P. berghei CS5M sporozoites (grey bars); control mice did not receive effector cells (black bars). 40 hours later livers were taken and parasite rRNA concentration determined by real-time PCR (mean ± SEM; n = 5, data from one of 2 similar experiments). C. Cyt c or PBS treated C57Bl/6 mice received 2×106 SIINFEKL specific effector CD8+ T cells 3 hours prior to challenge with 5×103 P. berghei CS5M sporozoites (grey bars); control mice did not receive effector cells (black bars). Treated mice received 15 mg cyt c for 3 days starting the day before challenge. 40 hours after challenge livers were taken and parasite rRNA concentration determined by real-time PCR (mean ± SEM; n = 4). Presentation of CS by infected hepatocytes and DCs does not require functional Pexel/VTS motifs
Our findings that antigen presentation in hepatocytes requires CS to enter the host cytosol but is independent of the endosomal pathway, raise the question as to how CS traffics to the hepatocyte cytosol. A previous report in which the 2 Pexel/VTS motifs in the N terminal domain of CS were mutated, suggested that CS export to the cytosol was eliminated in the absence of functional Pexel/VTS motifs [14]. To determine whether Pexel/VTS motifs are critical for the entry of CS into the class I processing pathway of infected hepatocytes we generated P. berghei CS5M parasites that carried mutations in key residues of both Pexel/VTS motifs as well as the SIINFEKL epitope (P. berghei CS5MΔP1–2; Figure S4). We mutated the Pexel/VTS sequences to the sequence that was previously suggested to abolish CS export into the cytoplasm of infected hepatocytes [14]. In fact we were able to observe punctate staining of CS in the cytosol of both P. berghei CS5M and P. berghei CS5MΔP1–2 infected Hepa1-6 cells (Figure 6A and B), and more importantly, we found that the P. berghei CS5MΔP1–2 parasites were killed as efficiently as P. berghei CS5M by effector CD8+ T cells (Figure 6C). This indicates that Pexel/VTS motifs are not required for the entry of CS into the cytosol of hepatocytes for antigen presentation to effector CD8+ T cells. However, in agreement with the previous study we did observe that parasites with mutated Pexel/VTS motifs in the CS protein have a ∼10-fold decrease in infectivity (Figure 6C). Finally we found that DCs efficiently present the epitope from the CS protein of parasites lacking the Pexel/VTS motifs (Figure 6D). This was not entirely unexpected as our previous findings suggested that DCs likely acquire the CS antigen by phagocytosis which is unlikely to be affected by host cell targeting sequences.
Fig. 6. Pexel/VTS motifs are not required for the presentation of CD8+ epitopes in the CS protein. 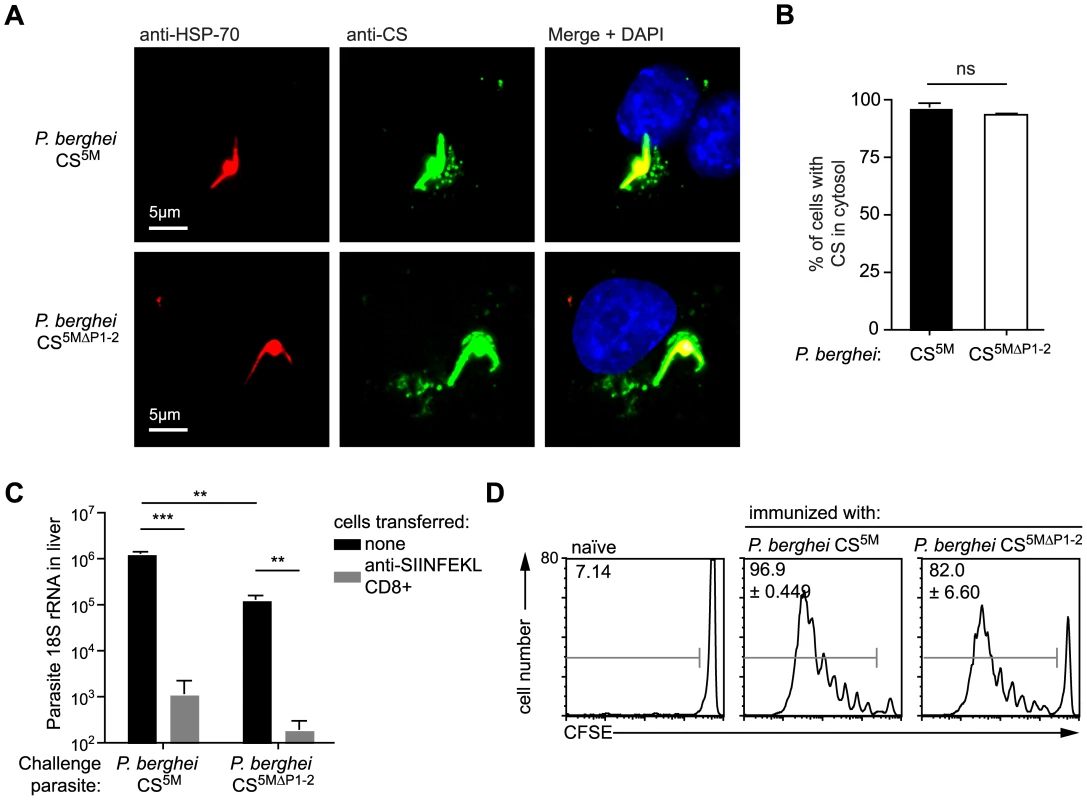
A. Fluorescence microscopy of Hepa1–6 cells 6 hours after infection with P. berghei CS5M and P. berghei CS5MΔP1–2. Parasites were visualized by staining with anti-Plasmodium HSP70 (red) and the localization of the CS protein determined by staining with the 3D11 mAb (green). B. % of parasites with CS visible in the host cell based on microscopy performed as in A, (mean ± SEM; data are based on 3 independent experiments per parasite strain with 50 parasites imaged per experiment). C. C57Bl/6 mice received 2×106 SIINFEKL specific effector CD8+ T cells 3 hours prior to challenge with 5×103 P. berghei CS5M or P. berghei CS5MΔP1–2 sporozoites (grey bars); control mice did not receive effector cells (black bars). 40 hours later livers were taken and parasite rRNA concentration determined by real-time PCR (mean ± SEM; n = 4, data from one of 2 similar experiments). D. C57Bl/6 mice received 5×105 CFSE labeled naïve SIINFEKL-specific cells one day prior to immunization i.d. with 5×104 P. berghei CS5M or P. berghei CS5MΔP1–2 parasites in the right ear. Three days later the mice were sacrificed and ear draining lymph nodes taken. Antigen presentation in vivo was inferred by determining the % of SIINFEKL-specific cells that had proliferated (mean ± SEM; n = 3, data representative of 2 similar experiments). Discussion
In this study we demonstrate that the process of antigen presentation required for the priming of sporozoite specific T cells and for the elimination of liver stage parasites are distinct. The difference in antigen presentation between DCs and hepatocytes has important consequences for malaria vaccine development based on irradiated sporozoites. If other Plasmodium antigens are processed similarly to CS, it is likely that DCs, which acquire antigens by phagocytosis, could stimulate T cell responses to a broad range of secreted and non-secreted antigens. In contrast hepatocytes can only present antigens that are secreted into the cytosol of infected or traversed cells; these antigens are, however, the potential targets of protective immunity as they induce effector cells to eliminate liver stage parasites. Thus, irradiated sporozoites may induce a range of irrelevant as well as protective immune responses. Moreover it is possible that irradiated sporozoites will fail to induce protective responses to various liver stage antigens presented by hepatocytes, that are not expressed by sporozoites. This appears to be the case for the liver stage antigen Hep17: irradiated sporozoites do not induce detectable Hep17 specific CD8+ T cells; however, vaccine-induced T cells specific for this antigen are protective against Plasmodium liver stages [36].
We observed that both T cell priming and parasite elimination by T cells were strictly TAP dependent. Thus in both DCs and hepatocytes antigen must reach the cytosol for presentation. In DCs this appears to occur via an endosome-to-cytosol pathway as determined by two independent in vivo methodologies: the use of 3d mice and treatment of mice with cyt c. However, unlike the defect in TAP1 deficient mice, the reduction in T cell priming in both 3d and cyt c treated mice was not complete. This may indicate that a small amount of antigen is directly deposited in the cytosol of DCs by traversing sporozoites. Alternatively cross-presentation may not be fully ablated in these models. 3d mice carry a single point mutation in one molecule (Unc93B1) which may retain some residual functionality [23], while the depletion of cross-presenting DCs by cyt c may not be absolute, particularly in the lymph nodes. The function of Unc93B1 in antigen presentation is not clear, though it may be involved in translocating elements of the cross-presentation machinery to the endosome similar to the way it mediates the movement of TLRs to endosomes [37]. An intriguing recent study showed that 3d mice were highly susceptible to T. gondii infection [38]. The authors suggest that this was not due to an impairment of CD8+ T cell control of parasites as the activation of CD8+ T cells appeared normal in 3d mice – however they were only able to look at bulk T cell populations and not antigen specific cells.
Further research will be required to determine what receptors DCs use to take up sporozoites and which pattern recognition molecules interact with sporozoites to facilitate cross presentation. One unexpected finding was that opsonization of sporozoites did not enhance the presentation of the CS antigen by DCs. One hypothesis is that opsonization may immobilize parasites [32] and thus interfere with a variety of processes that may be important for T cell priming including antigen shedding, and migration to the draining lymph nodes for presentation [4], [17], [33]. Alternatively opsonization may prevent parasites from infecting cells in the skin where they could continue to provide antigen to the immune system [18], [39]. The inability of DCs to present antigen from immobilized parasites may explain why irradiated parasites are capable of inducing a protective CD8+ T cell response, but heat killed parasites are not [3], [40]. These data also have important implications for vaccine design since they imply that there would be difficulties in priming or boosting sporozoite specific CD8+ T cell responses in individuals with high anti-CS antibody titers. Thus it may be hard to induce effective CD8+ T cell responses in individuals who have already been naturally exposed to parasites or immunized with vaccines such as RTS,S that are designed to induce strong anti-sporozoite antibody responses [41].
Using the 3d and cyt c treated mice we showed that in contrast to T cell priming, parasite elimination was unaffected in mice with reduced capacity to cross-present antigen. This is in agreement with the findings of a previous in vitro study [11] which found no evidence for endosomes having a role in antigen presentation by infected cells. The previous study also showed that proteasome and Golgi inhibitors blocked antigen presentation, which is compatible with our finding that antigen presentation occurs via the classical TAP-dependent pathway [11]. Together these data suggest that cell killing occurs only after direct antigen presentation by the infected hepatocyte itself.
A key direction for future research will be to identify how antigens enter the host cell for presentation. We were unable to find a role for Pexel/VTS motifs in targeting the CS protein to the host cell cytosol as suggested by a previous study [14]. Our data are based on fluorescence microscopy 6 hours post-infection when the highest amounts of CS can be observed in the cytosol [42], [43] and, more importantly, our functional assay to measure the elimination of parasites by T cells. The fact that Pexel/VTS motifs are not required for the entry of CS to the class I processing pathway suggests that liver stage proteins may be exported to the hepatocyte by other mechanisms. In particular, it suggests that the CS protein may contain another motif that facilitates its export out of the PV into the infected host cell. Alternatively, liver-stage antigens might also be exported to the class I processing pathway if the Plasmodium PV can fuse with the hepatocyte ER as appears to occur in Toxoplasma infected DCs [9].
Together our data provide the most complete description to date of the processing of sporozoite and liver stage antigen. Using the P. berghei CS5M parasite we have demonstrated that DCs cross-present sporozoite antigen via an endosome-to-cytosol pathway. Of most importance, we show that CS must be delivered to the hepatocyte cytosol for presentation to effector cells. If this is true for other antigens, it is likely that antigens secreted into the hepatocytes of either infected or traversed cells constitute the major targets of anti-liver stage CD8+ T cell mediated immunity. Secretion to the hepatocyte is likely a complex process given our finding that Pexel/VTS motifs are not required for the entry of CS to the class I processing pathway; however, unraveling this process will be key to the identification of vaccine candidates.
Materials and Methods
Ethics statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University (Protocol Number MO09H41) following the National Institutes of Health guidelines for animal housing and care.
Mice
5–8 week old female C57Bl/6 were purchased from NCI (Frederick, MD). TAP-1 deficient animals were purchased from Jackson (Bar Harbor, ME). Unc93B13d mice were obtained from the Mutant Mouse Resource Center (University of California, Davis, CA). OT-1 mice (carrying a transgene specific for the SIINFEKL epitope) were kindly provided by David Sacks (Laboratory of Parasitic Disease, National Institute of Allergy and Infectious Disease, Bethesda, MD).
Parasites and transfections
P. berghei CS5M parasites were generated by transfection of P. berghei ANKA with the linearized pR-CS5M plasmid as previously described [44]. pR-CS5M was derived from the plasmid pR-CSwt [45] as follows. A Kpn1-Xho1 fragment including the entire CS gene was excised from pR-CSwt into a pBluescript SK - (Stratagene) backbone to generate the plasmid pIC-CSwt. A SexA1 site was introduced by mutation of G to A at position 714 in the CS gene (silent in Gln238) and a BsmF1 site was introduced by a mutation of T to C at position 810 (silent in Asp270) using the QuikChange XL site directed mutagenesis kit (Stratagene). The SexA1-BsmF1 fragment was excised and replaced with a ∼100 bp insert including the SIINFEKL epitope in place of the SYIPSAEKI sequence (formed from the oligos S8ins F and S8insR; see Table S1) to generate the plasmid pIC-CS5M. The Kpn1-Xho1 fragment from pIC-CS5M was excised and ligated into the backbone of pR-CSwt to generate the pR-CS5M plasmid. pR-CS5M was linearized with the enzymes Kpn1 and Sac1.
P. berghei CS5MΔP1–2 parasites were generated similarly to P. berghei CS5M (Figure S1). The plasmid pR-CS5MΔP1–2 was generated as follows. Arg32 and Leu34 in the CS gene on the pIC-CS5M plasmid were mutated to Alanines by using the QuikChange site directed mutagenesis kit with the primers PEXEL1 F and PEXEL1 R (see Table S1), which include a Bsm1 site. Arg66 and Leu68 were mutated similarly with the primers PEXEL2 F and PEXEL2 R that include an ApaB1 site. The resulting plasmid was designated pIC-CS5MΔP1–2. The Kpn1-Xho1 fragment of the pIC-CS5MΔP1–2 plasmid was ligated into the pR-CSwt backbone to generate the pR-CS5MΔP1–2 plasmid used for transfection.
Quantification of T cell priming by DCs ex vivo
Lymph node and spleen myeloid DCs were prepared essentially as described [46]. Briefly, spleens or lymph nodes from immunized mice or naive mice were taken, chopped finely and digested with 1 mg/ml collagenase. The single cell suspension of spleen cells was then separated over a Nycodenz gradient (density, 1.075 g/ml) and the DC-rich low-density fraction was taken. To further enrich the DC population, negative selection was performed on the collected fraction using magnetic bead separation with anti-CD3, anti-GR1, anti TER119, anti-B220 and anti-Thy1.2 antibodies. Final purity of CD11c+ DC was about 70%. To assess Ag presentation ex vivo, splenic myeloid DCs (1×105) were mixed with 5×104 purified naive CFSE-labeled CD8+-transgenic cells in a single V-bottom well of a 96-well plate. 60–65 h later, the cells were harvested, and CFSE dilution in the transgenic cell population was used as a measure of Ag presentation.
Quantification of T cell priming by DCs in vivo
Where possible SIINFEKL-specific T cell priming was measured after immunization by the bites of 10–20 irradiated mosquitoes. Prior to biting, a low number (2×103) of CD45.1+ OT-1 cells were transferred to mice and the expansion of the CD45.1+ CD8+ (SIINFEKL-specific) population were measured by flow cytometry 10 days later to allow time for the responses to reach detectable levels. In some experiments it was necessary to perform immunizations with needle injected sporozoites (e.g. where the sporozoites were treated with antibodies prior to immunization). In these experiments 5×105 congenic CD45.1+ OT-1 cells were adoptively into mice, which were immunized the following day. The cells would be labeled with 0.6 µM CFSE using the Vybrant Cell Tracker kit according to the manufacturer's instructions (Invitrogen Life Technologies), and antigen presentation was inferred from proliferation of CD45.1+ CD8+ cells in the draining lymph nodes after 3 days. Use of a high number of transgenic cells is acceptable in these experiments as we are using the cells as a readout of antigen presentation not measuring particular T cell phenotypes. ELISPOTs to measure peptide-specific IFN-γ secreting cells were performed as described [47] and used to detect endogenous SIINFEKL responses.
Preparation of F(ab′)2 fragments
F(ab′)2 fragments from the 3D11 mAb (class: mouse IgG1) were prepared by incubation with immobilized Ficin in the presence of 4 mM cysteine according to the manufacturer's instructions (Pierce). F(ab′)2 fragments were isolated from intact antibody and Fc fragments by passing twice over a Protein A column. Purity of F(ab′)2 fragments was verified by SDS-PAGE under non-reducing conditions.
Generation of SIINFEKL-specific effector T cells
SIINFEKL-specific effector cells were purified from mice that had received 5×105 naïve CD45.1+ OT-1 cells and then been immunized with 2×106 pfu VV-OVA [48]. 8–10 days later spleens were taken from the immunized mice and the lymphocytes were purified by spinning over lympholyte M (Cedarlane Laboratories). A total of 2×106 effector/SIINFEKL specific CD8+ T cells were transferred to each recipient mouse.
Quantification of parasite RNA
Quantification of liver stage parasites was performed as previously described [49]. Briefly, 40 hours after challenge, livers were excised and parasite load was determined by quantitative PCR for P. berghei 18S rRNA using SYBR Green (Applied Biosystems).
Cell isolation and preparation of samples for flow cytometry
Single cell suspensions of lymphocytes were obtained by grinding spleen cells or lymph node cells between the ground ends of two microscope slides and filtering twice through 100 µm nylon mesh. Liver lymphocytes were isolated from perfused livers by grinding, filtration through a 70 µm mesh and separation over a 35% percol gradient as described [50].
Fluorescence microscopy
Hepa1-6 cells were grown on coverslips in a 48 well plate and allowed to reach ∼80% confluence prior to infection with ∼3×104 parasites. 6 hours later the slides were washed and fixed for 15 minutes with 4% formaldehyde prior to permeablilization with 100% methanol for 10 minutes. The cells were then blocked with 3% BSA for 45 minutes. The parasite cytosol was labeled with anti-Plasmodium HSP70 mAbs [51] followed by secondary staining with Alexa594 anti-mouse IgG (Molecular Probes). The cells were then stained with anti-P. berghei CS mAb (3D11) directly conjugated to FITC. Slides were mounted with ProLong antifade with DAPI (Molecular Probes). Images were acquired on a Nikon Eclipse 90i microscope with a Hamamatsu Orca-ER camera attachment using Volocity software (Perkin Elmer). Images were analyzed and assembled using ImageJ software (open source from NIH).
Data analysis
Statistical analysis was performed using Prism 4 software (GraphPad Software), unless otherwise stated, means were compared by two-tailed Student's t tests. Analysis of all flow cytometry data was performed using FlowJo software (TreeStar).
Supporting Information
Zdroje
1. NussenzweigRS
VanderbergJ
MostH
OrtonC
1967 Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 216 160 162
2. RomeroP
MaryanskiJL
CorradinG
NussenzweigRS
NussenzweigV
1989 Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341 323 326
3. SchofieldL
VillaquiranJ
FerreiraA
SchellekensH
NussenzweigR
1987 Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330 664 666
4. ChakravartyS
CockburnIA
KukS
OverstreetMG
SacciJB
2007 CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med 13 1035 1041
5. JobeO
DonofrioG
SunG
LiepinshD
SchwenkR
2009 Immunization with radiation-attenuated Plasmodium berghei sporozoites induces liver cCD8alpha+DC that activate CD8+T cells against liver-stage malaria. PLoS One 4 e5075
6. JungS
UnutmazD
WongP
SanoG
De los SantosK
2002 In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17 211 220
7. PlebanskiM
HannanCM
BehboudiS
FlanaganKL
ApostolopoulosV
2005 Direct processing and presentation of antigen from malaria sporozoites by professional antigen-presenting cells in the induction of CD8 T-cell responses. Immunol Cell Biol 83 307 312
8. RockKL
ShenL
2005 Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev 207 166 183
9. GoldszmidRS
CoppensI
LevA
CasparP
MellmanI
2009 Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med 206 399 410
10. BertholetS
GoldszmidR
MorrotA
DebrabantA
AfrinF
2006 Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol 177 3525 3533
11. BongfenSE
TorglerR
RomeroJF
ReniaL
CorradinG
2007 Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol 178 7054 7063
12. HillerNL
BhattacharjeeS
van OoijC
LioliosK
HarrisonT
2004 A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306 1934 1937
13. MartiM
GoodRT
RugM
KnuepferE
CowmanAF
2004 Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306 1930 1933
14. SinghAP
BuscagliaCA
WangQ
LevayA
NussenzweigDR
2007 Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell 131 492 504
15. HogquistKA
JamesonSC
HeathWR
HowardJL
BevanMJ
1994 T cell receptor antagonist peptides induce positive selection. Cell 76 17 27
16. Van KaerL
Ashton-RickardtPG
PloeghHL
TonegawaS
1992 TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell 71 1205 1214
17. AminoR
ThibergeS
MartinB
CelliS
ShorteS
2006 Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 12 220 224
18. GueirardP
TavaresJ
ThibergeS
BernexF
IshinoT
2010 Development of the malaria parasite in the skin of the mammalian host. Proc Natl Acad Sci U S A 107 18640 18645
19. den HaanJM
LeharSM
BevanMJ
2000 CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192 1685 1696
20. AminoR
GiovanniniD
ThibergeS
GueirardP
BoissonB
2008 Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3 88 96
21. CoppiA
TewariR
BishopJR
BennettBL
LawrenceR
2007 Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2 316 327
22. MotaMM
PradelG
VanderbergJP
HafallaJC
FrevertU
2001 Migration of Plasmodium sporozoites through cells before infection. Science 291 141 144
23. TabetaK
HoebeK
JanssenEM
DuX
GeorgelP
2006 The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol 7 156 164
24. CockburnIA
ChenYC
OverstreetMG
LeesJR
van RooijenN
2010 Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog 6 e1000877
25. FarrandKJ
DickgreberN
StoitznerP
RoncheseF
PetersenTR
2009 Langerin+ CD8alpha+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J Immunol 183 7732 7742
26. LinML
ZhanY
ProiettoAI
PratoS
WuL
2008 Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A 105 3029 3034
27. QiuCH
MiyakeY
KaiseH
KitamuraH
OharaO
2009 Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol 182 4127 4136
28. KalergisAM
RavetchJV
2002 Inducing tumor immunity through the selective engagement of activating Fc gamma receptors on dendritic cells. J Exp Med 195 1653 1659
29. RafiqK
BergtoldA
ClynesR
2002 Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest 110 71 79
30. YoshidaN
NussenzweigRS
PotocnjakP
NussenzweigV
AikawaM
1980 Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207 71 73
31. CochraneAH
SantoroF
NussenzweigV
GwadzRW
NussenzweigRS
1982 Monoclonal antibodies identify the protective antigens of sporozoites of Plasmodium knowlesi. Proc Natl Acad Sci U S A 79 5651 5655
32. StewartMJ
NawrotRJ
SchulmanS
VanderbergJP
1986 Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect Immun 51 859 864
33. YamauchiLM
CoppiA
SnounouG
SinnisP
2007 Plasmodium sporozoites trickle out of the injection site. Cell Microbiol 9 1215 1222
34. PorgadorA
YewdellJW
DengY
BenninkJR
GermainRN
1997 Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6 715 726
35. BeattieL
PeltanA
MaroofA
KirbyA
BrownN
2010 Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog 6 e1000805
36. DoolanDL
HedstromRC
RogersWO
CharoenvitY
RogersM
1996 Identification and characterization of the protective hepatocyte erythrocyte protein 17 kDa gene of Plasmodium yoelii, homolog of Plasmodium falciparum exported protein 1. J Biol Chem 271 17861 17868
37. KimYM
BrinkmannMM
PaquetME
PloeghHL
2008 UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452 234 238
38. MeloMB
KasperkovitzP
CernyA
Konen-WaismanS
Kurt-JonesEA
2010 UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog 6 e1001071
39. HollingdaleMR
ZavalaF
NussenzweigRS
NussenzweigV
1982 Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol 128 1929 1930
40. HafallaJC
RaiU
MorrotA
Bernal-RubioD
ZavalaF
2006 Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur J Immunol 36 1179 1186
41. MoorthyVS
BallouWR
2009 Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J 8 312
42. HugelFU
PradelG
FrevertU
1996 Release of malaria circumsporozoite protein into the host cell cytoplasm and interaction with ribosomes. Mol Biochem Parasitol 81 151 170
43. KhanZM
NgC
VanderbergJP
1992 Early hepatic stages of Plasmodium berghei: release of circumsporozoite protein and host cellular inflammatory response. Infect Immun 60 264 270
44. MenardR
JanseC
1997 Gene targeting in malaria parasites. Methods 13 148 157
45. WangQ
FujiokaH
NussenzweigV
2005 Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog 1 e9
46. CockburnIA
ChakravartyS
OverstreetMG
Garcia-SastreA
ZavalaF
2008 Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J Immunol 180 64 71
47. MiyahiraY
MurataK
RodriguezD
RodriguezJR
EstebanM
1995 Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods 181 45 54
48. HickmanHD
TakedaK
SkonCN
MurrayFR
HensleySE
2008 Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat Immunol 9 155 165
49. Bruna-RomeroO
HafallaJC
Gonzalez-AseguinolazaG
SanoG
TsujiM
2001 Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31 1499 1502
50. SanoG
HafallaJC
MorrotA
AbeR
LafailleJJ
2001 Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med 194 173 180
51. TsujiM
MatteiD
NussenzweigRS
EichingerD
ZavalaF
1994 Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res 80 16 21
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral EncephalomyelitisČlánek Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



