-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUnsupervised primaquine for the treatment of malaria relapses in southern Papua: A hospital-based cohort study
In a pragmatic hospital-based cohort study, Ric Price and colleagues compared risk of re-presentation with vivax malaria in patients prescribed dihydroartemisinin-piperaquine combined with primaquine versus patients who could not be given primaquine due to stock outages.
Published in the journal: . PLoS Med 14(8): e32767. doi:10.1371/journal.pmed.1002379
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002379Summary
In a pragmatic hospital-based cohort study, Ric Price and colleagues compared risk of re-presentation with vivax malaria in patients prescribed dihydroartemisinin-piperaquine combined with primaquine versus patients who could not be given primaquine due to stock outages.
Introduction
Primaquine is the only licensed drug known to be active against Plasmodium vivax hypnozoites. It is critically important for preventing relapses and thus reducing the global burden of vivax malaria [1]. The World Health Organization (WHO) recommends a total dose of 3.5–7 mg/kg primaquine divided over 14 days for the treatment of vivax malaria in those who are not deficient in the glucose-6-phosphate dehydrogenase (G6PD) enzyme [2]. Outside of clinical trials, adherence to this long regimen is commonly believed to be poor [3, 4]. Prospective studies of treatment supervision have yielded inconsistent results [5–8] and are challenging to interpret since study involvement itself tends to have an effect on treatment compliance [9]. Despite widespread use since the 1960s, there have not been any rigorous studies of the effectiveness of prescribing primaquine in routine practice in vivax-endemic regions.
Many National Malaria Control Programs have been reluctant to advocate primaquine for the radical cure of P. vivax infections because of the risk of inducing severe hemolysis in G6PD-deficient patients and the difficulty of promoting practical pretreatment testing to reduce exposure to those individuals at greatest risk [10]. However, the risk of drug toxicity needs to be balanced against the cumulative morbidity caused by recurrent P. vivax relapses arising from the dormant liver stages of the parasite [11–13]. Recent guidelines from WHO advocate that, where possible, all patients should be tested for G6PD deficiency prior to the administration of primaquine; however, few malaria-endemic countries have incorporated this into clinical practice.
We aimed to determine the effectiveness of primaquine in combination with dihydroartemisinin-piperaquine (DHP), prescribed according to national policy without prior testing for G6PD deficiency, for preventing recurrent P. vivax malaria. Three stock outages of primaquine at a hospital in Papua, Indonesia provided an opportunity for a pragmatic comparison of the risk of re-presentation to hospital with vivax malaria in patients treated with DHP plus primaquine compared to those treated with DHP alone.
Methods
Ethical approval
Ethical approval for this study was obtained from the Health Research Ethics Committees of the University of Gadjah Mada (KE/FK/544/EC), Indonesia and Menzies School of Health Research, Darwin, Australia (HREC 10.1397).
Study design
This was a retrospective cohort study which used routinely-collected hospital surveillance data to determine the comparative rate of re-presentation to hospital with vivax malaria in patients treated with DHP with or without primaquine. The analysis followed an a priori statistical plan that is available in S1 Text. The RECORD checklist is provided in S2 Text.
Study site, hospital characteristics, and treatment protocols
The geography, climate, and demographics of Mimika District and its capital, Timika, have been described previously [14, 15]. Briefly, this region lies in south-central Papua, Indonesia. Timika has an expanding population—currently approximately 200,000 people—of whom half are native highland or lowland Papuans, and half are migrants from elsewhere in Indonesia [15].
Malaria transmission is perennial with minimal seasonal variation. In 2013, the point prevalence of parasitemia was estimated to be 13.9%, 46% due to P. vivax and 2% due to mixed P. falciparum/P. vivax infections [16]. Local P. vivax strains have a typical equatorial relapse interval of 3–4 weeks [17, 18] and are highly resistant to chloroquine [19–21].
Rumah Sakit Mitra Masyarakat (RSMM) is the largest health care facility in Timika and one of two public hospitals in the district. The other hospital opened in 2008, and since then, RSMM has received an estimated 80% of malaria patients attending either of the two hospitals. RSMM has an active outpatient department, a 24-hour emergency department, four wards with 110 beds for inpatient care, and a “high care” facility.
DHP replaced quinine and chloroquine as the first-line treatment of uncomplicated malaria due to any Plasmodium species at RSMM in March 2006. An unsupervised, 14-day course of primaquine was also recommended for the treatment of patients with P. vivax mono - or mixed infections with a change in policy from low-dose (3.5 mg/kg total dose) to high-dose (7 mg/kg) primaquine in March 2006, coinciding with the policy change for blood schizontocidal treatment. In the outpatient clinic, primaquine is commenced at the same time as schizontocidal treatment. For inpatients, primaquine is administered once clinical recovery has begun and patients are able to take oral medication.
Based on a local prevalence survey, 2.6% of the population have at least intermediate G6PD deficiency (16). Testing for G6PD deficiency prior to prescribing primaquine is not done routinely. Patients with very dark urine, potentially indicative of severe hemolysis, are advised to stop taking primaquine and return to the hospital for clinical review.
Laboratory and data collection procedures
All patient presentations to RSMM between 2004 and 2013 were recorded in a Q-Pro database by hospital administrators. Details entered for each presentation included individual hospital identification number, demographic details, dates of presentation and discharge, and clinical diagnoses assigned by the attending physician. Details of all prescriptions at the hospital pharmacy were also recorded in a separate database.
Strict hospital protocols required that all patients presenting to the outpatient department with fever or other symptoms potentially compatible with malaria and all inpatients, regardless of clinical picture, had a blood smear taken for Giemsa staining and examination under a light microscope. In most cases, parasitological diagnosis was made from a thick film. In a minority of cases, thin films were also read. Rapid diagnostic tests based on the Histidine Rich Protein-2 (HRP2) antigen were used occasionally to confirm P. falciparum infection.
Data preparation
Clinical data for each patient presentation were merged with antimalarial prescription data using individual hospital identification numbers and dates. Data were concatenated such that multiple presentations with malaria within 14 days of the initial presentation were grouped as a single “episode,” the rationale being that recurrent parasitemia due to reinfection or relapse is highly unlikely within this period [18].
Total primaquine doses were estimated in milligrams per kilogram from the numbers of tablets provided by the pharmacy and the predicted weight of each patient, which was derived from the mean weight of age-, sex-, and ethnicity-matched patients in a local cross-sectional survey [15]. Prescribed doses were then categorized as: (a) no primaquine (matching antimalarial prescription data but no primaquine prescribed), (b) single-dose primaquine (<1.5 mg/kg), (c) low-dose primaquine (≥1.5 mg/kg and <5 mg/kg), (d) high-dose primaquine (≥5 mg/kg), or (e) unknown dose (matched primaquine prescription record but unable to determine dose in mg/kg—mostly due to nonsensical dose fractions).
Statistical analysis
Recurrent malaria following P. vivax infection can be due to: (i) recrudescence of a partially treated infection (which usually occurs early and has been accounted for in this analysis by concatenating clinical episodes into 14-day events), (ii) reinfection in patients continuing to reside in a malaria-endemic area, or (iii) relapse from reactivation of the dormant hypnozoite stage. In Papua and other equatorial regions, relapses start to occur 21 days after the initial infection and can potentially continue for over a year. An individual will therefore be at excess risk of vivax malaria over and above the baseline risk of reinfection whilst the relapses continue to occur.
In order to determine the optimal duration of patient follow-up that would capture the excess rate of re-presentation due to relapse whilst minimizing the influence of background reinfection rates, graphs of the first and second derivatives of the overall failure curve were generated (Fig 1). These provided graphical representation of the noncumulative risk of re-presentation by time and the rate of change in risk of re-presentation by time, respectively. The point of inflexion of the first derivative and the point at which the second derivative settled at 0 represent the time at which the baseline risk of presentation with vivax malaria is reached; this occurred at about 1 year after the initial episode of P. vivax malaria (Fig 1). Follow-up data were therefore censored at 365 days.
Fig. 1. Noncumulative risk of re-presentation to hospital with vivax malaria following initial <i>P</i>. <i>vivax</i> infection (main figure) and the rate of change of the risk of re-presentation (inset). 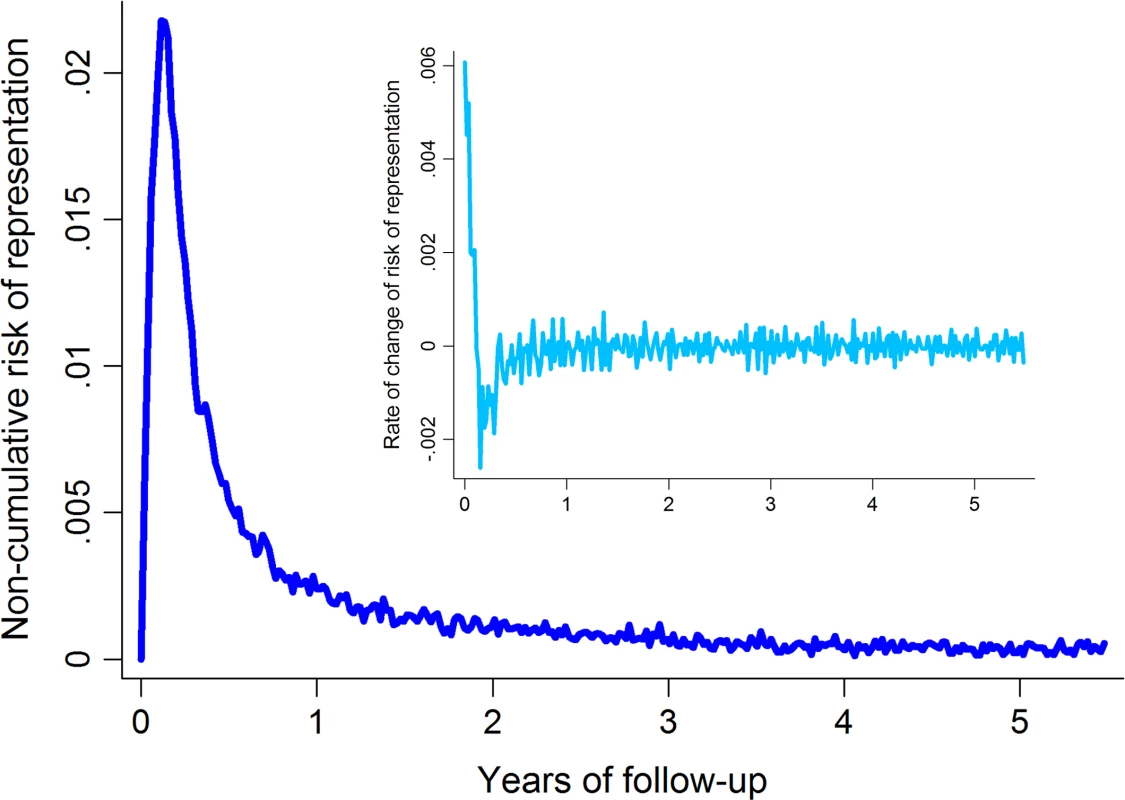
To ensure comparability of patients in this study, only episodes of P. vivax malaria that were treated with DHP as the blood schizontocidal agent were included. As hospital treatment policy precluded treatment of pregnant women and infants aged less than 1 year with primaquine, these individuals were excluded from analyses. The primary comparison was between patients treated without primaquine and those treated with either a low or high total dose of primaquine—in other words, with a potentially curative regimen. All prespecified subgroup analyses were limited to patients receiving either no primaquine or high-dose primaquine (hospital protocol) and included: [1] individual age categories (1 to <5 years, 5 to <15 years, and ≥15 years), [2] patients initially treated as outpatients, [3] patients treated during, or 12 months before and after, the largest primaquine stock outage from July 2007 to February 2008, and [4] patients prescribed either no primaquine or a total primaquine dose greater than 7 mg/kg. Since a longer period of follow-up will result in a greater proportion of recurrent episodes of vivax malaria attributable to reinfection, subanalyses were also conducted in which follow-up was restricted to 3 months; during this period, relapsing infections will have constituted the majority of recurrences. All analyses were performed in STATA version 12.1 (College Station, Texas). Kaplan-Meier failure curves for the risk of re-presentation to hospital with P. vivax infection were plotted, stratified by the baseline characteristics sex, pregnancy status, ethnicity (non-Papuan, Highland Papuan, Lowland Papuan), age group (as above), year of initial presentation, presence or absence of Plasmodium coinfection, episode number (first through fifth), initial admission status (outpatient or inpatient), and primaquine dose category.
Cox proportional hazards regression was used for analyses of primaquine effectiveness. The proportional hazards assumption was assessed by visually comparing the log(cumulative hazard) by time of follow-up curves for each covariable category and subsequently by fitting and comparing models with and without time of follow-up interaction terms. All multivariable models were stratified by year to control for changes in the background malaria endemicity over the 8-year study period.
Approximately 40% of patients in the database had multiple episodes of vivax malaria. Up to 5 episodes per individual were included in the Cox models, and the variance–covariance matrices were corrected for intraindividual correlation using robust standard errors. Since number of episodes was predictive of a further presentation, this was also included as a covariable in the final models. Kaplan-Meier estimates were based on the risk of recurrence after the first episode only.
Results
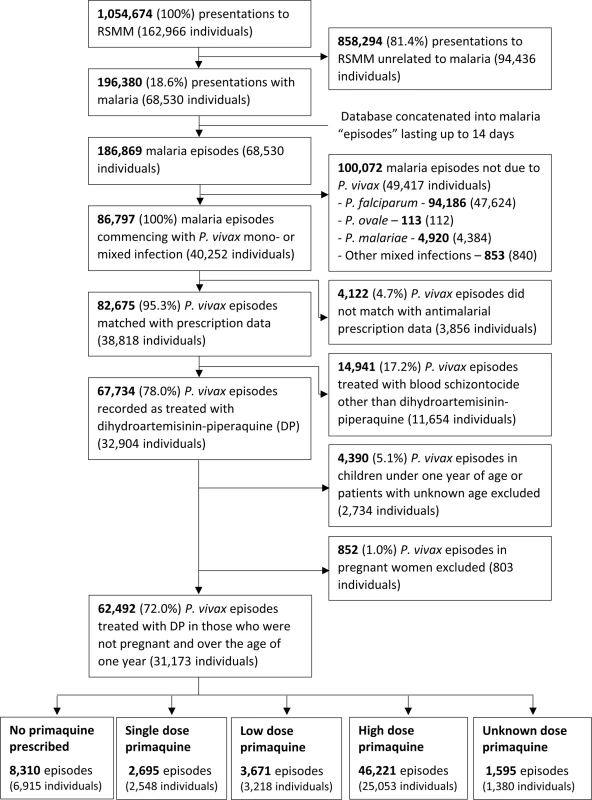
Between April 2004 and December 2013, there were 1,054,674 presentations to Mitra Masyarakat Hospital made by 162,966 individuals. The exclusion of presentations without malaria and concatenation of the dataset left 186,869 malaria episodes, of which 86,797 (46.4%) were due to P. vivax infection, and 82,675 (95.3%) could be matched with pharmacy records (Fig 2).
The total monthly prescriptions of oral antimalarials for patients with P. vivax infection are shown in Fig 3. There were 3 complete interruptions in the supply of primaquine, the largest from July 2007 to February 2008. After excluding pregnant women, children younger than 1 year of age and patients treated with non-DHP treatments, 62,492 (72.0%) clinical cases in 31,173 individuals, contributed to the primaquine effectiveness analysis (see Fig 2 and Table 1). Overall, 59.9%, 18.5%, and 21.7% of patients had 1, 2, or ≥3 (up to 24) episodes of vivax malaria during the study period, respectively.
Fig. 3. The absolute number of blood schizontocide and primaquine prescriptions at Mitra Masyarakat Hospital by year. 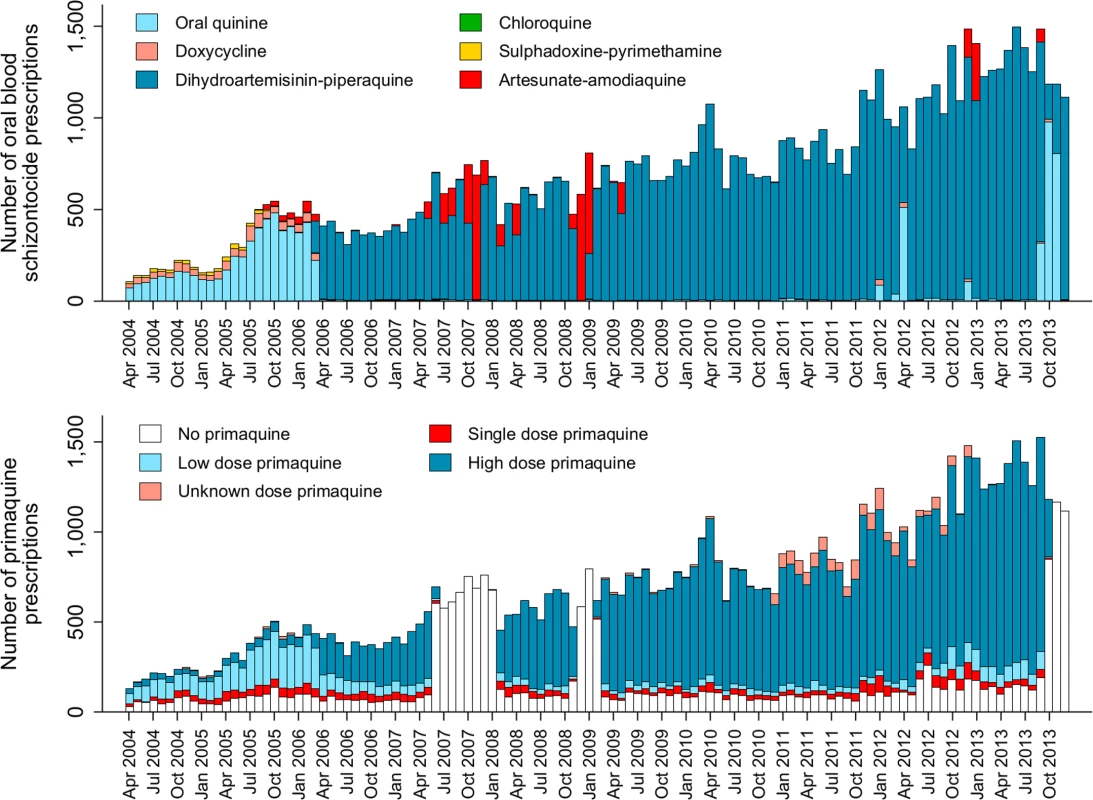
Tab. 1. Distribution of demographic and clinical characteristics for patients included and excluded in the analysis. 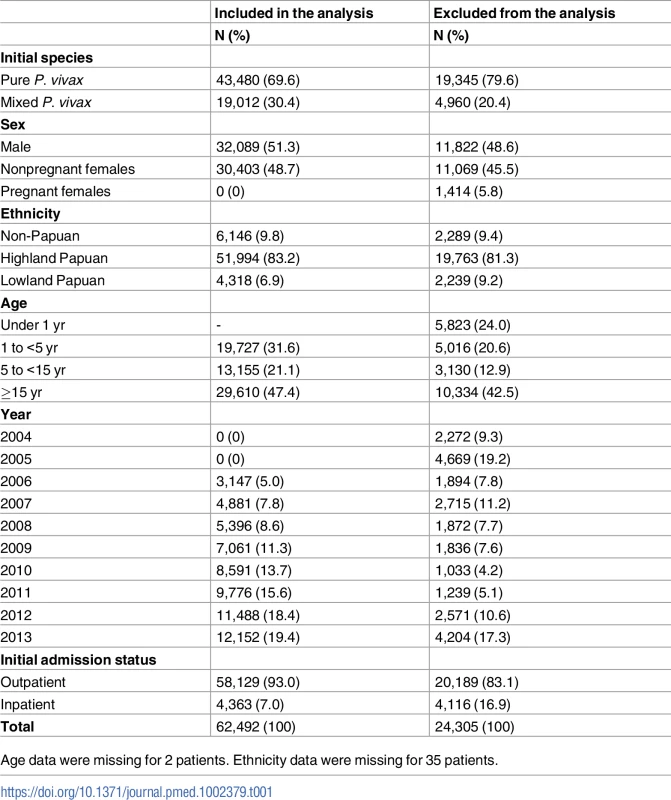
Age data were missing for 2 patients. Ethnicity data were missing for 35 patients. A total of 86.7% (54,182/62,492) of P. vivax episodes were treated with primaquine, and the dose prescribed could be determined in 52,587 (97.1%) of these cases (Fig 4). Single-dose primaquine was prescribed in 2,695 (5.1%) of these vivax malaria episodes, a 14-day low-dose primaquine regimen in 3,672 (7.0%) episodes, and a 14-day high-dose primaquine regimen in 46,221 (87.9%) episodes. In total, 9.1% (335/3,672) of patients who were prescribed low-dose primaquine were predicted to have received less than 3.5 mg/kg of primaquine, and 39.8% (18,373/46,221) of patients prescribed a high dose regimen were administered a total dose less than 7 mg/kg. Patients receiving an unknown dose of primaquine were more likely to be less than 5 years old (72.4%, 1,154/1,595) compared to those with a known primaquine dose (30.5%, 18,573/60,897); Table 2. Patients receiving doses in the single-dose category were more likely to be adults than those receiving higher doses of primaquine (60.2% [1,622/2,695] versus 47.3% [21,883/46,221]). Other baseline characteristics are presented in Table 2.
Fig. 4. Histogram of the derived mg/kg total dose of primaquine administered. 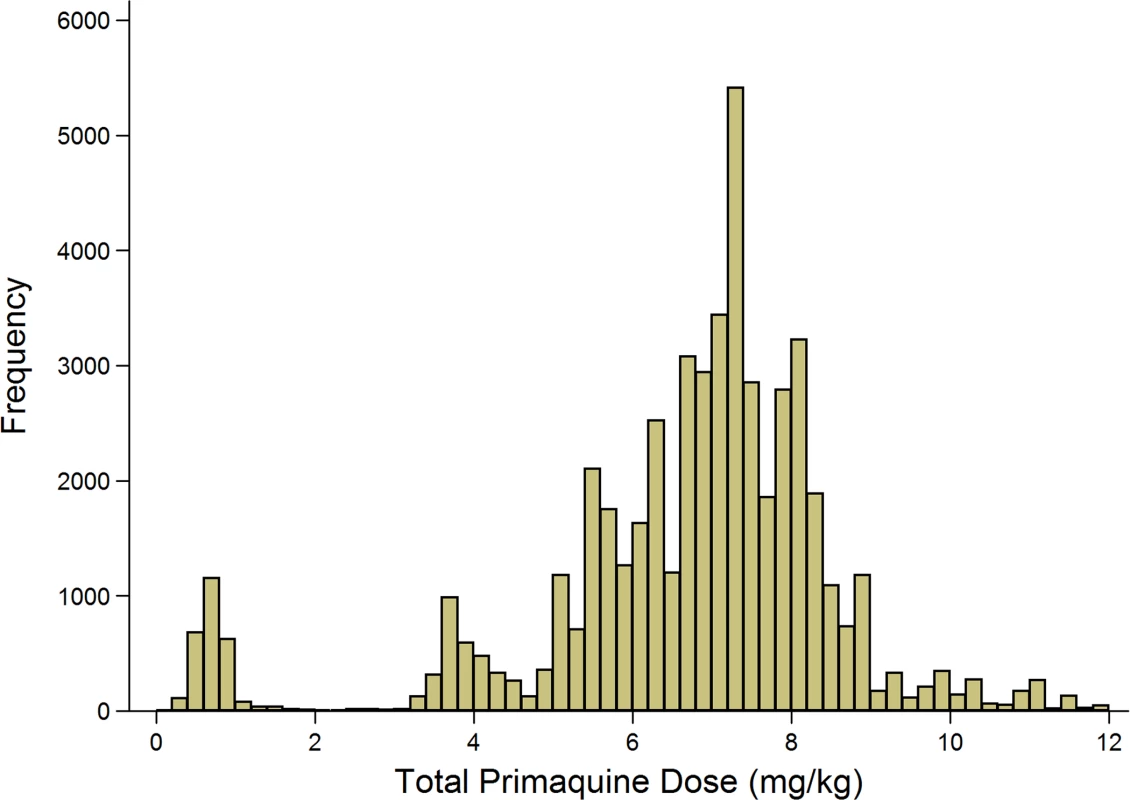
Tab. 2. The distribution of risk factors for re-presentation with P. vivax malaria by primaquine dose category. 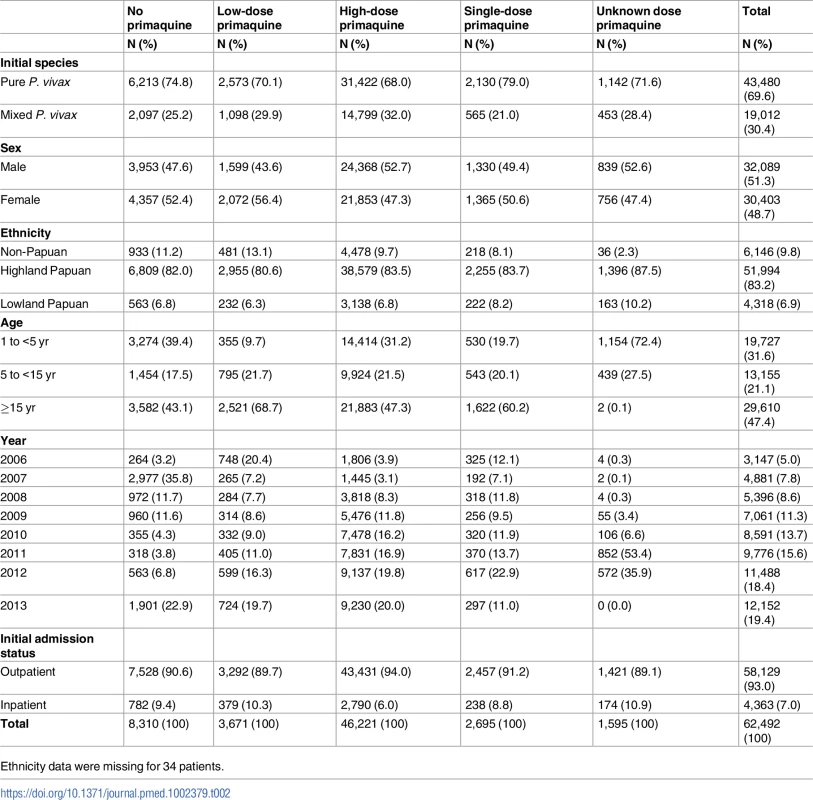
Ethnicity data were missing for 34 patients. Baseline risk factors for re-presentation with P. vivax infection
The cumulative risk of re-presentation with clinical P. vivax infection within 1 year was 33.8% (95% Confidence Interval [95% CI] 33.1%–34.5%) after initial P. vivax monoinfection, and 29.2% (95% CI 28.1%–30.4%) after mixed-species infection (Fig 5 panel A). The rate of re-presentation reduced markedly with advancing age with children 1 to <5 years of age having an Adjusted Hazard Ratio (AHR) of 2.23 (95% CI 2.15–2.31) compared to patients 15 years of age or older (Table 3). The risk of re-presentation with vivax malaria was also lower in patients requiring admission to a hospital for treatment (23.9%, 95% CI 22.2%–25.8%) compared to those treated as outpatients (33.5% [95% CI 32.9%–34.2%]); AHR = 0.81 (95% CI: 0.76–0.86), p = <0.001. The greater the number of previous P. vivax infections an individual had had, the more likely they were to re-present with another P. vivax recurrence (Table 3; Fig 5 panel H).
Fig. 5. The cumulative risk of re-presentation with P. vivax malaria by baseline risk factor. 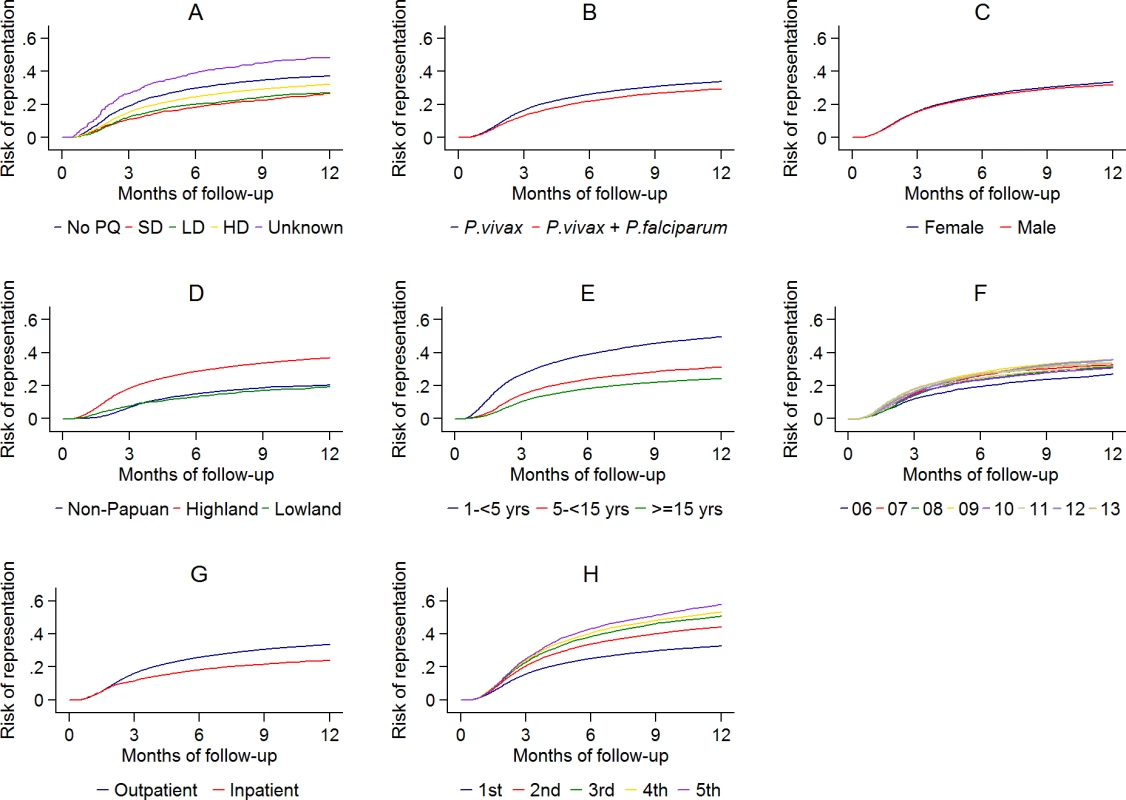
A: primaquine dose category; B: P. vivax mono- or mixed infection; C: gender; D: ethnicity; E: age group; F: year; G: initial treatment as an outpatient or inpatient; H: vivax malaria episode number. (PQ, Primaquine; SD, Single Dose; LD, Low Dose; HD, High Dose). Tab. 3. Baseline risk factors for re-presenting with P. vivax malaria after initial P. vivax malaria. 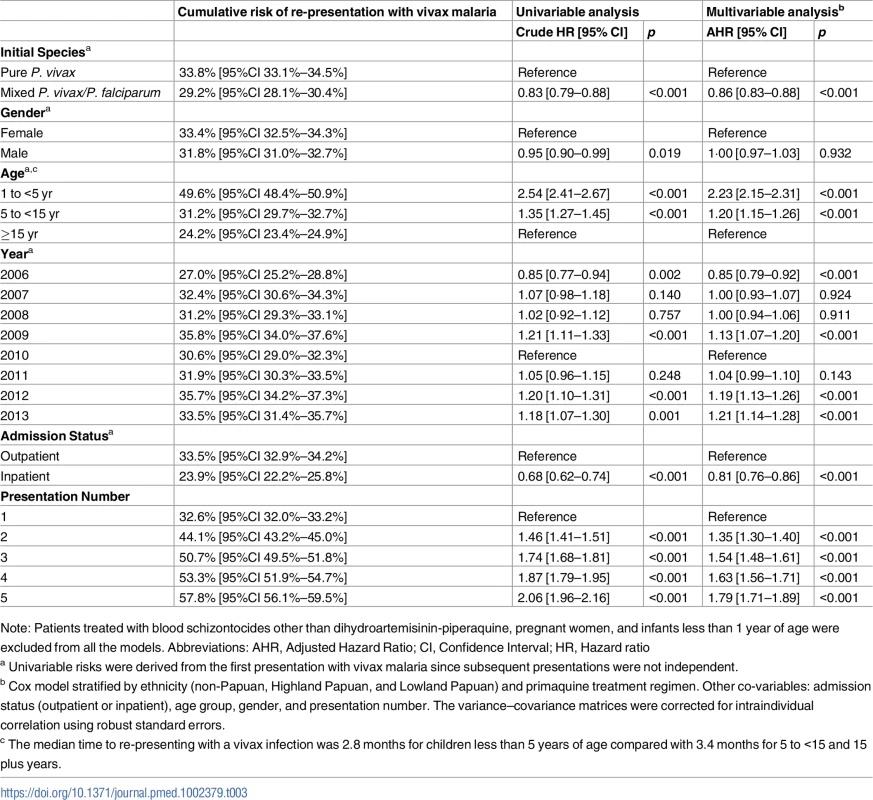
Note: Patients treated with blood schizontocides other than dihydroartemisinin-piperaquine, pregnant women, and infants less than 1 year of age were excluded from all the models. Abbreviations: AHR, Adjusted Hazard Ratio; CI, Confidence Interval; HR, Hazard ratio Primaquine effectiveness
The unadjusted cumulative risk of re-presentation with P. vivax malaria was 37.2% (95% CI 35.6%–38.8%) after no primaquine, 26.7% (24.1%–29.5%) after single-dose primaquine, 26.9% (24.8%–29.0%) after low-dose primaquine, 32.1% (31.4%–32.9%) after high-dose primaquine, and 48.3% (44.0%–52.9%) after unknown dose primaquine. Overall patients treated with any radically curative primaquine regimen (low dose or high dose) had a risk of re-presentation of 31.6% (95% CI 30.9%–32.3%) with an AHR for re-presentation with vivax malaria of 0.90 (95% CI 0.86%–0.95, p < 0.001) compared to patients who did not receive any primaquine (Table 4). Restricting the model to patients receiving either high-dose primaquine or no primaquine in the period during or 12 months before and after the largest primaquine stock outage did not alter the estimated protective effect of primaquine (AHR 0.91, 95% CI 0.85–0.97, p = 0.003). High-dose primaquine was associated with a 15% reduction in the rate of re-presentation compared with no primaquine in children less than 5 years of age (AHR 0.85, 95% CI 0.79–0.90, p < 0.001), whereas no significant effect was seen in 5 to <15 year olds (AHR 0.98, 95% CI 0.86–1.12, p = 0.76) and those ≥15 years of age (AHR 0.99, 95% CI 0.91–1.08, p = 0.88). To reduce potential confounding due to reinfection, the overall effectiveness and subgroup analyses were restricted to 3 months of follow-up; the results were similar (Table 5).
Tab. 4. Results of Cox proportional hazards models for the effect of primaquine on the risk of re-presentation to hospital with P. vivax malaria within 12 months of being treated for P. vivax malaria. 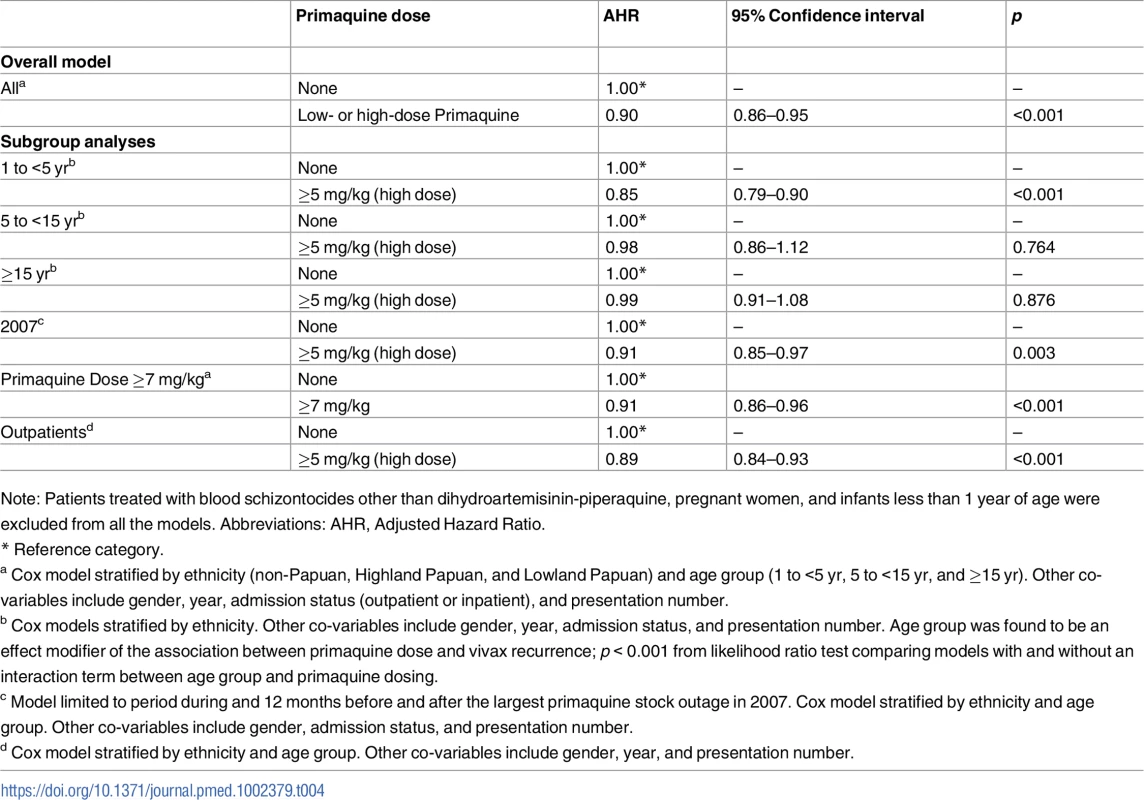
Note: Patients treated with blood schizontocides other than dihydroartemisinin-piperaquine, pregnant women, and infants less than 1 year of age were excluded from all the models. Abbreviations: AHR, Adjusted Hazard Ratio. Tab. 5. Results of Cox proportional hazards models for the effect of primaquine on the risk of re-presentation to hospital with P. vivax malaria within 3 months of being treated for P. vivax malaria. 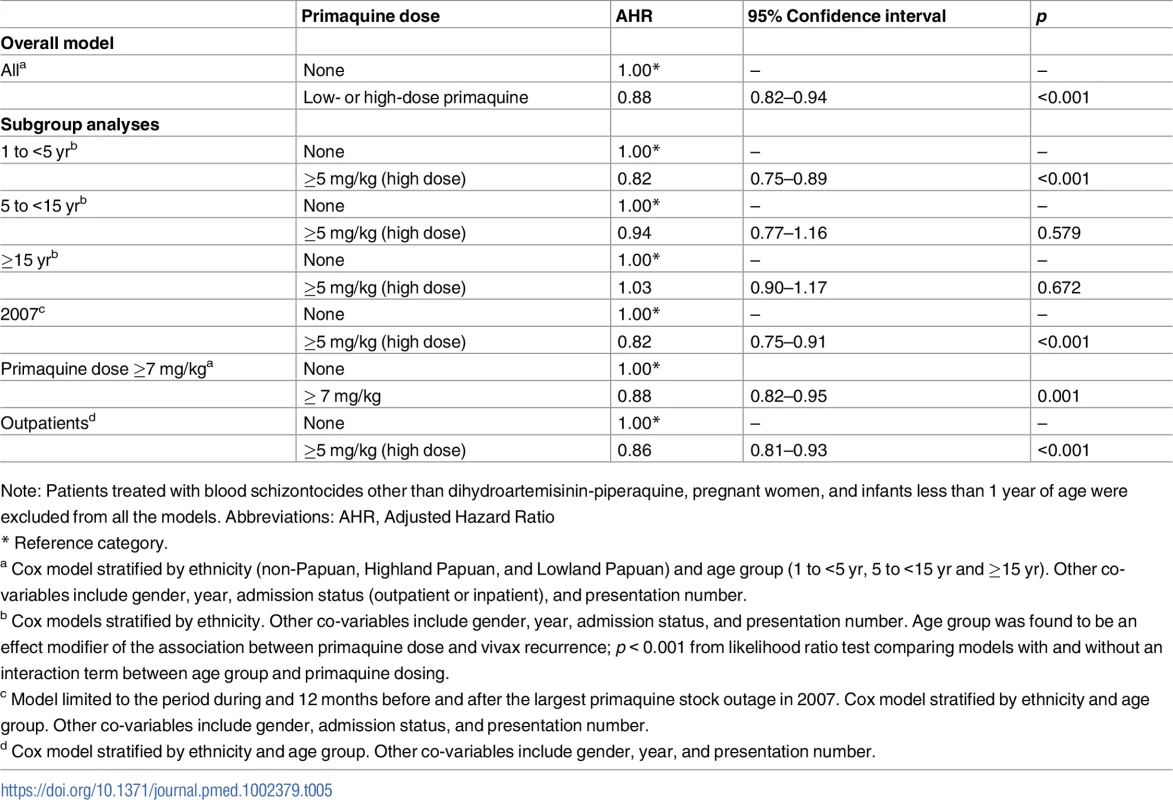
Note: Patients treated with blood schizontocides other than dihydroartemisinin-piperaquine, pregnant women, and infants less than 1 year of age were excluded from all the models. Abbreviations: AHR, Adjusted Hazard Ratio Discussion
We present a large-scale comparative assessment of the effectiveness of unsupervised primaquine for preventing recurrent P. vivax malaria in a nontrial setting. Despite good staff adherence to the WHO-endorsed dosing regimen, prescription of primaquine at RSMM was associated with, at best, a modest reduction of approximately 10% in the rate of re-presentation to hospital with vivax malaria within 1 year.
Recurrent P. vivax malaria has important implications both for individuals and populations in endemic regions [22], resulting in cumulative anemia due to repeated episodes of hemolysis, impaired nutrition, poor cognitive performance, and school absenteeism [12, 23]. Furthermore, at a community level, recurrent infections are an important source of transmission and the main barrier to elimination of this parasite [24–26]. Safe and effective radical cure of P. vivax is therefore an essential component of optimal treatment guidelines. Our analysis suggests that the strategy of long, unsupervised treatment courses of primaquine is compromised significantly in this resource-poor setting.
Our pragmatic study has a number of important strengths. Due to very large numbers, our estimates of the risk of re-presentation are unlikely to be attributable to chance. Hospital policy dictates that all patients presenting with fever are offered laboratory tests for the diagnosis of malaria. Microscopy services at RSMM have performed well in quality assurance procedures [15]; thus, parasitological diagnoses are comparatively accurate when considered in the context of field laboratory services. Crucially, patients included in our analyses received their medications according to normal hospital procedures and were free from the powerful biases and clinical influences associated with many study designs [9]. Although this is an important strength, it is also a notable limitation. In the absence of randomized and contemporaneous allocation, our data are susceptible to the effects of residual bias due to unknown confounders. Restriction of the effectiveness model to the period during and 12 before and after the largest primaquine stock outage was intended to reduce confounders associated with the clinical decision to give or withhold primaquine. Importantly, this model did not substantially change the estimate of the effectiveness of primaquine, suggesting minimal residual confounding in the overall model.
The potential explanations for the modest observed effectiveness of primaquine can be divided broadly into those pertaining to the host, the parasite, the drug, or the study design. Poor adherence to an unsupervised 14-day treatment regimen is likely to be a significant host factor. It is well recognized that the duration of antimalarial treatment is a critical determinant of adherence [9]. The 14-day primaquine regimen currently recommended in the WHO antimalarial treatment guidelines is more than 4 times longer than most schizontocidal regimens. Since the symptoms of malaria usually subside within 2 or 3 days of initiation of treatment, the incentive to take all doses of primaquine is low. Recent randomized controlled trials conducted in Thailand and Ethiopia demonstrated that directly observed primaquine therapy was associated with a 3-to-4-fold lower rate of vivax recurrence when compared to unsupervised therapy [7, 27]. In our study, primaquine effectiveness was highest in young children, and this may reflect parental encouragement and supervision of tablet administration. Another host factor could be a reduced ability to metabolise primaquine to its active metabolite. Recent evidence has implicated polymorphisms in the cytochrome (CYP)450 2D6 gene in the treatment failure of patients following primaquine therapy [28]. However, whilst these genetic variants have been documented across most of the globe, they usually occur in about 10% of the population [29], and thus would be unlikely alone to account for high rates of treatment failure in our analysis.
Tolerance or resistance of the parasite to primaquine could have played a role. Although strains of P. vivax from Southeast Asia and the Pacific, and, in particular, the Chesson strain of the Island of New Guinea, are thought to be relatively unresponsive to primaquine and other 8-aminoquinoline compounds [30, 31], a recent study of Indonesian soldiers with P. vivax acquired in Papua Province demonstrated that a supervised regimen of DHP plus a high-dose primaquine regimen had excellent efficacy [32]. Significant primaquine resistance or tolerance in this region therefore seems unlikely.
Poor quality and/or counterfeit antimalarials have been recognized as a substantial threat to malaria-control programs, generally, and a catalyst for the propagation of multidrug-resistant parasites [33–35]. Although poor-quality primaquine could have contributed to the poor effectiveness observed in this study, previous studies in Papua of supervised primaquine regimens, with a drug from the same manufacturer showed excellent prophylactic efficacy, so this is unlikely to have been a major contributor to our findings [32, 36, 37]. Further studies are underway to confirm the quality of the primaquine tablets currently available in Timika; however; the possibility of significant batch to batch variation over the 8 years of the study cannot be ruled out. Alternatively, the observed poor effectiveness could have arisen from low-primaquine concentrations due to patients being underdosed. There was a wide range in the total mg/kg primaquine dose prescribed, with almost 40% of those prescribed the high-dose regimen receiving a dose below the 7 mg/kg target dose. However, in a subgroup analysis in which patients receiving a total dose of primaquine greater than 7 mg/kg were compared with those prescribed no primaquine, the adjusted hazards ratio was unchanged (Table 4), suggesting that underdosing alone did not account for primaquine’s poor effectiveness (Table 4).
Our observational study design and passive follow-up will have resulted in attrition bias. Previous clinical trials in this location have shown that recurrent P. vivax parasitemia can be detected in up to 40% of patients by day 42 after schizontocidal treatment alone, with a high proportion of these recurrences being asymptomatic [17, 38]. In our hospital-based surveillance, only a third of patients re-presented to hospital with P. vivax malaria within a year, potentially reflecting less severe or asymptomatic recurrent infections, seeking treatment at alternative facilities, or migration out of the area. Since our study was not designed to identify asymptomatic relapses, which are a major source of transmission potential [26], we cannot rule out the possibility that unsupervised primaquine may have had a significant public health benefit through the reduction of the asymptomatic parasite reservoir [39].
In summary, our study highlights, at best, a modest benefit of unsupervised high-dose primaquine for preventing clinical relapses of vivax malaria in southern Papua. The elimination of P. vivax will require practicable, safe, and effective radical cure of both blood and liver stage infections. Building stronger health systems, educating patients on the importance of treatment adherence, and supervision of therapy will be critical for improving the outcomes of primaquine radical cure for P. vivax [1, 40]. However, ultimately, a hypnozoitocidal treatment regimen is needed, which is more amenable to high prescriber and patient adherence than the current prolonged, and potentially toxic, course of primaquine. Radical cure has been achieved with a single dose of tafenoquine, and this drug is now in phase III clinical trials [41]. Until tafenoquine or an alternative agent is licensed and widely available, public health measures aimed at increasing the adherence to primaquine regimens, either through supervision or more practical dosing regimens, need to be implemented as a matter of urgency.
Supporting Information
Zdroje
1. Thriemer K, Ley B, Bobogare A, Dysoley L, Alam MS, Pasaribu AP, et al. Challenges for achieving safe and effective radical cure of Plasmodium vivax: a round table discussion of the APMEN Vivax Working Group. Malar J. 2017;16(1):141. doi: 10.1186/s12936-017-1784-1 28381261
2. World Health Organization. Guidelines for the treatment of malaria - 3rd edition. Geneva: World Health Organization; 2015.
3. Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39 : 1336–45. doi: 10.1086/424663 15494911
4. John GK, Douglas NM, von Seidlein L, Nosten F, Baird KJ, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11(1):280.
5. Maneeboonyang W, Lawpoolsri S, Puangsa-Art S, Yimsamran S, Thanyavanich N, Wuthisen P, et al. Directly observed therapy with primaquine to reduce the recurrence rate of Plasmodium vivax infection along the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2011;42(1):9–18. 21323159
6. Grietens KP, Soto V, Erhart A, Ribera JM, Toomer E, Tenorio A, et al. Adherence to 7-day primaquine treatment for the radical cure of P. vivax in the Peruvian Amazon. Am J Trop Med Hyg. 2010;82(6):1017–23. doi: 10.4269/ajtmh.2010.09-0521 20519594
7. Takeuchi R, Lawpoolsri S, Imwong M, Kobayashi J, Kaewkungwal J, Pukrittayakamee S, et al. Directly-observed therapy (DOT) for the radical 14-day primaquine treatment of Plasmodium vivax malaria on the Thai-Myanmar border. Malar J. 2010;9(1):308.
8. Leslie T, Rab MA, Ahmadzai H, Durrani N, Fayaz M, Kolaczinski J, et al. Compliance with 14-day primaquine therapy for radical cure of vivax malaria: a randomized placebo-controlled trial comparing unsupervised with supervised treatment. Trans R Soc Trop Med Hyg. 2004;98 : 168–73. 15024927
9. Bruxvoort K, Goodman C, Kachur SP, Schellenberg D. How patients take malaria treatment: a systematic review of the literature on adherence to antimalarial drugs. PLoS ONE. 2014;9(1):e84555. doi: 10.1371/journal.pone.0084555 24465418
10. von Seidlein L, Auburn S, Espino F, Shanks D, Cheng Q, McCarthy J, et al. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12 : 112. doi: 10.1186/1475-2875-12-112 23537118
11. Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: Clinical Spectrum, Risk Factors and Pathogenesis. Adv Parasitol. 2012;80 : 151–201. doi: 10.1016/B978-0-12-397900-1.00003-7 23199488
12. Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, et al. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11(1):135.
13. Douglas NM, Lampah DA, Kenangalem E, Simpson JA, Poespoprodjo JR, Sugiarto P, et al. Major burden of severe anemia from non-falciparum malaria species in southern papua: a hospital-based surveillance study. PLoS Med. 2013;10(12):e1001575. doi: 10.1371/journal.pmed.1001575 24358031
14. Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5(6):e128. doi: 10.1371/journal.pmed.0050128 18563962
15. Karyana M, Burdarm L, Yeung S, Kenangalem E, Wariker N, Rilia, et al. Epidemiology of multidrug resistant P. vivax and P. falciparum infection in Southern Papua, Indonesia. Malar J. 2008;7 : 148. doi: 10.1186/1475-2875-7-148 18673572
16. Pava Z, Burdam FH, Handayuni I, Trianty L, Utami RA, Tirta YK, et al. Submicroscopic and Asymptomatic Plasmodium Parasitaemia Associated with Significant Risk of Anaemia in Papua, Indonesia. PLoS ONE. 2016;11(10):e0165340. doi: 10.1371/journal.pone.0165340 27788243
17. Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung M, Laihad F, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369 : 757–65. doi: 10.1016/S0140-6736(07)60160-3 17336652
18. Battle KE, Karhunen MS, Bhatt S, Gething PW, Howes RE, Golding N, et al. Geographical variation in Plasmodium vivax relapse. Malar J. 2014;13 : 144. doi: 10.1186/1475-2875-13-144 24731298
19. Ratcliff A, Siswantoro H, Kenangalem E, Wuwung M, Brockman A, Edstein MD, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in Southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101 : 351–9. doi: 10.1016/j.trstmh.2006.06.008 17028048
20. Tjitra E, Baker J, Suprianto S, Cheng Q, Anstey NM. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob Agents Chemother. 2002;46 : 3947–53. doi: 10.1128/AAC.46.12.3947-3953.2002 12435700
21. Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52(3):1040–5. doi: 10.1128/AAC.01334-07 18180357
22. Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22 : 430–5. doi: 10.1097/QCO.0b013e32832f14c1 19571748
23. Hutchinson RA, Lindsay SW. Malaria and deaths in the English marshes. Lancet. 2006;367(9526):1947–51. doi: 10.1016/S0140-6736(06)68850-8 16765764
24. Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, et al. Gametocyte Dynamics and the Role of Drugs in Reducing the Transmission Potential of Plasmodium vivax. The Journal of infectious diseases. 2013.
25. Noviyanti R, Coutrier F, Utami RA, Trimarsanto H, Tirta YK, Trianty L, et al. Contrasting Transmission Dynamics of Co-endemic Plasmodium vivax and P. falciparum: Implications for Malaria Control and Elimination. PLoS Negl Trop Dis. 2015;9(5):e0003739. doi: 10.1371/journal.pntd.0003739 25951184
26. Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891 26505753
27. Abreha T, Hwang J, Thriemer K, Tadesse Y, Girma S, Melaku Z, et al. Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: A randomized controlled trial. PLoS Med. 2017;14(5):e1002299. doi: 10.1371/journal.pmed.1002299 28510573
28. Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. The New England journal of medicine. 2013;369(14):1381–2. doi: 10.1056/NEJMc1301936 24088113
29. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–43. doi: 10.1517/14622416.3.2.229 11972444
30. Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55(3):243. 8842108
31. Cooper WC, Myatt AV, Hernandez T, Jeffery GM, Coatney GR. Studies in human malaria. XXXI. Comparison of primaquine, isopentaquine, SN-3883, and pamaquine as curative agents against Chesson strain vivax malaria. Am J Trop Med Hyg. 1953;2(6):949–57. 13104803
32. Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA, et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother. 2013;57(3):1128–35. doi: 10.1128/AAC.01879-12 23254437
33. Newton PN, Green MD, Fernandez FM. Impact of poor-quality medicines in the 'developing' world. Trends in pharmacological sciences. 2010;31(3):99–101. doi: 10.1016/j.tips.2009.11.005 20117849
34. Petralanda I. Quality of antimalarial drugs and resistance to Plasmodium vivax in Amazonian region. Lancet. 1995;345(8962):1433.
35. Kron MA. Substandard primaquine phosphate for US Peace Corps personnel. Lancet. 1996;348(9039):1453–4.
36. Baird JK, Fryauff DJ, Basri H, Bangs MJ, Subianto B, Wiady I, et al. Primaquine for prophylaxis against malaria among nonimmune transmigrants in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1995;52(6):479–84. 7611550
37. Fryauff DJ, Baird JK, Basri H, Sumawinata I, Purnomo, Richie TL, et al. Randomised placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet. 1995;346(8984):1190–3. 7475658
38. Hasugian AR, Purba HLE, Kenangalem E, Wuwung RM, Ebsworth EP, Maristela R, et al. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin Infect Dis. 2007;44 : 1067–74. doi: 10.1086/512677 17366451
39. White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3.
40. Tanner M, Greenwood B, Whitty CJ, Ansah EK, Price RN, Dondorp AM, et al. Malaria eradication and elimination: views on how to translate a vision into reality. BMC Med. 2015;13 : 167. doi: 10.1186/s12916-015-0384-6 26208740
41. Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383(9922):1049–58. doi: 10.1016/S0140-6736(13)62568-4 24360369
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Harmonization of community health worker programs for HIV: A four-country qualitative study in Southern Africa
- Gestational diabetes mellitus and interpregnancy weight change: A population-based cohort study
- Association of pre-pregnancy body mass index with offspring metabolic profile: Analyses of 3 European prospective birth cohorts
- Pregnant and breastfeeding women: A priority population for HIV viral load monitoring
- Malaria, malnutrition, and birthweight: A meta-analysis using individual participant data
- Childhood adiposity and risk of type 1 diabetes: A Mendelian randomization study
- Unsupervised primaquine for the treatment of malaria relapses in southern Papua: A hospital-based cohort study
- Effectiveness of a brief behavioural intervention on psychological distress among women with a history of gender-based violence in urban Kenya: A randomised clinical trial
- Impact of fortified versus unfortified lipid-based supplements on morbidity and nutritional status: A randomised double-blind placebo-controlled trial in ill Gambian children
- Identification of factors associated with stillbirth in the Indian state of Bihar using verbal autopsy: A population-based study
- Counting stillbirths and achieving accountability: A global health priority
- Evaluating the impact of Affordable Care Act repeal on America's opioid epidemic
- Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized controlled trials
- Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: An observational, multicenter study
- Assessing the impact of healthcare research: A systematic review of methodological frameworks
- Lay worker-administered behavioral treatments for psychological distress in resource-limited settings: Time to move from evidence to practice?
- Antimicrobial resistance: The complex challenge of measurement to inform policy and the public
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Childhood adiposity and risk of type 1 diabetes: A Mendelian randomization study
- Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized controlled trials
- Lay worker-administered behavioral treatments for psychological distress in resource-limited settings: Time to move from evidence to practice?
- Evaluating the impact of Affordable Care Act repeal on America's opioid epidemic
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání