-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRe-emerging and newly recognized sexually transmitted infections: Can prior experiences shed light on future identification and control?
How do we spot the next sexually transmitted infection? Kyle Bernstein and colleagues look for lessons from past discovery.
Published in the journal: . PLoS Med 14(12): e32767. doi:10.1371/journal.pmed.1002474
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1002474Summary
How do we spot the next sexually transmitted infection? Kyle Bernstein and colleagues look for lessons from past discovery.
Summary points
Determining sexual contact as a mode of pathogen transmission and quantifying the risk of sexual transmission pose epidemiologic challenges.
Prior experiences with nontraditional sexually transmitted infections present valuable epidemiologic lessons, including comparisons of disease rates by sex, molecular analyses among sexually linked clusters, and methods to control for other potential modes of transmission.
Applying lessons learned from prior infections might be critical for rapid and effective detection, prevention, and control of other reemerging and newly recognized sexually transmitted infections.
The spectrum of pathogens that have a sexually transmitted component is broad. Globally, there are more than 30 recognized sexually transmitted infections (STIs), including those transmitted primarily by sexual contact and those that are sexually transmissible but whose primary mode of transmission is by food, vector, or droplet [1]. This latter group of nontraditional STIs poses unique methodologic and epidemiologic challenges for public health practitioners and researchers, who need to anticipate, identify, and contain the next new STI outbreak. In this paper, we explore these challenges using examples of nontraditional STIs, including 2 (shigellosis and Neisseria meningitidis) that have recently reemerged as sexually transmissible and 2 (Zika and Ebola) that are newly recognized as being sexually transmissible (Table 1).
Tab. 1. Modes of emergence and key characteristics of selected sexually transmitted infections. 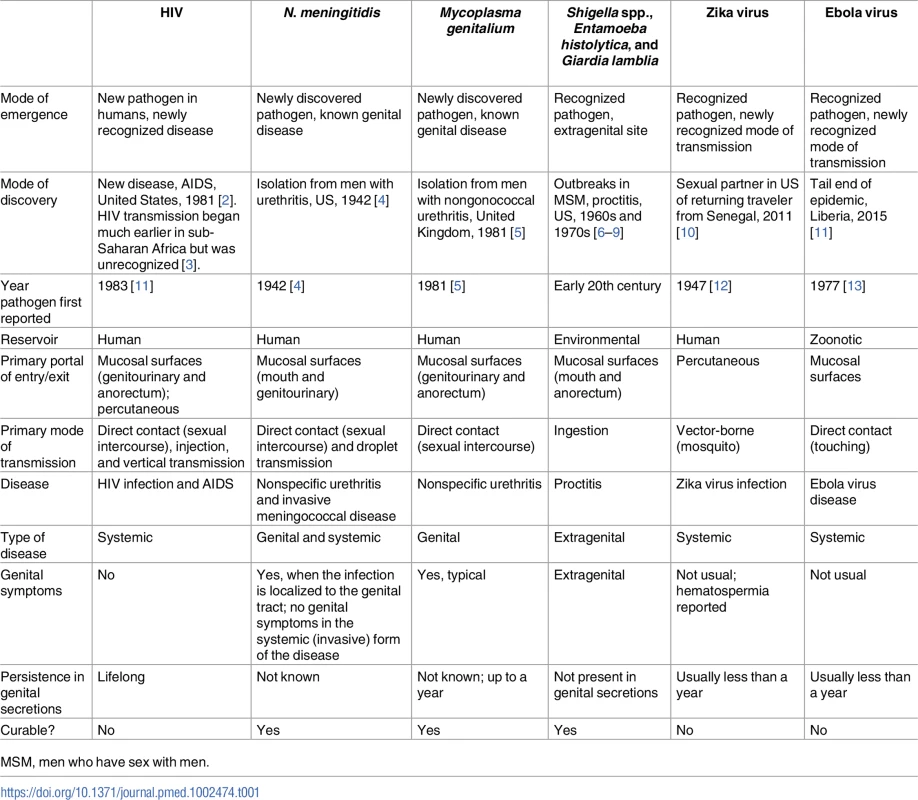
MSM, men who have sex with men. Shigella: Raised male-to-female ratios in routine surveillance data
Shigellosis is a diarrheal illness caused by several species of the bacterium Shigella. Shigella is transmitted by direct or indirect contact with human feces, often via contaminated food, water, or fomites [13]. Prior to the 1970s, Shigella incidence was highest among children <5 years of age, their caretakers, and travelers to less developed countries. Recognition of Shigella as a potential STI began in the 1970s with outbreaks among men who have sex with men (MSM) in the US [8, 9, 14, 15]. Sexual transmission of Shigella likely occurs during oral-anal sex (e.g., anilingus or rimming) or digital-anal sex (e.g., fisting) [16, 17]. During the 1970s and 1980s, Shigella flexneri rates increased in the US among adult males, even as overall rates and rates among children declined [18]. Routine case reports for Shigella do not include information about sexual practices, but the widening disparity between adult male and female case rates strongly suggested male-male sexual transmission. Increases in Shigella among men in the US and England between 2004 and 2015—despite declining or steady rates among women and children—support the reemergence of Shigella as an STI among MSM [19, 20]. Shigella strains among MSM have demonstrated increasing multidrug resistance over the past 5 years [21–28], and recent genomic analyses suggest international spread of an antimicrobial-resistant S. flexneri serotype among MSM [23].
N. meningitidis: The role of dyad and cluster analyses
N. meningitidis, the bacterium that causes invasive meningococcal disease (IMD), spreads primarily by droplet transmission and infection of respiratory mucosa. Approximately 5%–10% of healthy adults are nasopharyngeal carriers. N. meningitidis has been isolated from men with urethritis [4]. A 1972 study described the transmission of N. meningitidis from a male chimpanzee’s nasopharynx to his own urethra via oral-genital autoinoculation [29]. The authors concluded that N. meningitidis in the human urogenital tract might be the result of oral-genital sexual contact. Dyad and cluster analyses showing related strains of meningococci among partners epidemiologically linked by female-to-male oral sex strengthen the argument for sexual transmission leading to meningococcal urethritis [30, 31]. Recent molecular analyses suggest that N. meningitidis has genetically adapted to the urogenital tract [32]. Recent IMD outbreaks among MSM in Europe, Canada, and the US have also raised questions about the role that sexual networks play in N. meningitidis transmission [33–38]. Droplet transmission within MSM sexual networks could explain consistently higher N. meningitidis nasopharyngeal carriage rates relative to heterosexual men [39, 40]. It remains challenging, however, to determine whether the primary mode of transmission in IMD outbreaks among MSM is oral-genital contact, open-mouth kissing, or droplet transmission via “close contact,” including the sharing of living and sleeping spaces.
Ebola virus: Viral persistence in semen and genetic epidemiology
The largest outbreak of Ebola virus disease started in December 2013 in West Africa and led to >28,000 confirmed cases and 11,310 deaths [41]. Almost all infections resulted from exposure to acutely symptomatic infected persons or recently deceased Ebola patients. Concern about possible sexual transmission of Ebola grew as the outbreak continued [42]. Anecdotal reports of new Ebola infections occurring among persons not in close proximity to a symptomatic or recently deceased person were followed by a report of a Liberian woman with Ebola, whose only possible source of infection was her husband, a convalescing Ebola survivor [43]. The husband had a positive PCR test for Ebola RNA in his semen 199 days after symptom onset, and the homology between genetic sequences of the Ebola RNA from the man and woman suggested that the only possible source of her infection was through sexual transmission [43, 44]. Ebola virus persistence in the semen of male survivors was documented in previous sporadic outbreaks [45, 46]. However, in the most recent West African outbreak, more robust systematic assessments found male survivors with Ebola virus RNA detected by PCR up to 565 days after symptom onset [47–49]. There is little evidence supporting viral persistence in other body fluids [42]. Female-to-male sexual transmission of Ebola is likely inefficient, but data are limited. Although the risk of transmission from semen exposure is considered small, the sheer number of male Ebola survivors raised concern about potential flare-ups and new clusters as the West African outbreak waned [50, 51]. Little is known about the public health impact of Ebola persistence among high-risk groups such as sex workers and MSM.
Zika virus: Infections in sexual partners of travelers returning from endemic areas
A large outbreak of Zika virus in Latin America and the Caribbean in 2015–2016 drew international attention because of its reported association with microcephaly. By 2017, 84 countries and territories had evidence of Zika transmission [52]. While the predominant mode of Zika transmission is through the bite of an infected Aedes spp. mosquito, sexual transmission was documented when a scientist returned to the US from Senegal in 2008 and transmitted Zika to his female sex partner who had not travelled [10]. Case reports from 13 countries have since described probable sexual transmission of Zika—via oral, anal, and vaginal sex—to partners of travelers returning from endemic areas. Suspected male-to-female sexual transmission was reported in 27 couples, while only 1 case of female-to-male and 1 case of male-to-male sexual transmission have been documented [53]. Most sexual transmission events occurred in symptomatic couples, but the timing of suspected transmission relative to symptom onset ranges greatly.
Persistent detection of Zika in genital fluids by reverse transcriptase (RT)-PCR and culture provides additional evidence for the biological plausibility of sexual transmission. In Puerto Rico, Zika was detected in the semen of 56% of convalescing men, with a median of 34 days between symptom onset and undetectable virus levels [54]. The maximum reported durations of RNA detection in genital fluids are as follows: 188 days in semen by RT-PCR [55], 69 days in semen by culture [56], 3 days in vaginal fluid in RT-PCR, and 11 days in cervical mucus by RT-PCR [57]. Investigation of Zika sexual transmission is complicated by several factors, including difficulty distinguishing vector and sexual transmission in endemic settings and difficulty obtaining viral cultures to confirm that viral persistence represents infectiousness. It is unclear whether controlling sexual transmission of Zika will contribute substantially to overall control of Zika epidemics; mathematical models estimate the population attributable risk of sexual transmission to be from 3% to 23% [58, 59]. Sustained sexual transmission of Zika is unlikely in a general population, but clusters may occur within high-risk sexual networks [60].
Lessons learned to inform future efforts
Lessons learned from the STIs discussed above can help the public health community identify newly emerging STIs and estimate the potential for an epidemic by sexual transmission. Sexual transmissibility of Zika and Ebola viruses was identified when infections were detected in persons who could only have been infected through sexual intercourse. In the case of meningococcal urethritis, detection of the pathogen in an unexpected anatomic niche and molecular linkage between index patients and their sexual partners established sexual transmissibility. Sexual transmission of Shigella was identified by attention to epidemiological changes in the affected populations. M. genitalium, another predominantly sexually transmitted pathogen, was discovered in 1981 by scientists searching for causes of nonspecific urethritis (Table 1) [56]. This discussion harkens back to the global HIV/AIDS epidemic. HIV crossed a species barrier and spread undetected as a new pathogen and STI among humans for years, before its identification as AIDS in MSM with unexplained immune suppression in San Francisco in 1981 [2,3].
Now is the optimal time to establish methods to assess whether an infectious agent is sexually transmissible and prepare for a new STI with pandemic potential. New or reemerging pathogens that are sexually transmissible will continue to arise. Since HIV was discovered, new molecular tools, such as phylogenetics and the omics, have become available to complement etiological epidemiological investigations of causal associations. For example, genotypic data were critical in linking sexual partners in the context of Ebola [44] and Shigella [23]. Standardized definitions and approaches to the investigation of sexual transmission of infectious agents and criteria for considering a pathogen to be sexually transmissible would greatly aid this effort. For example, is transmission by close, intimate contact (i.e., skin-to-skin) considered sexual transmission? Bradford Hill’s classic viewpoints about causation could be adapted; for example, molecular concordance of strains between sexual partners might be a requirement for demonstrating specificity of association. The establishment of an objective criteria for determining the necessary components to consider a pathogen sexually transmitted could complement the existing framework developed by Hill. If sexual transmission is established, quantitative information about parameters such as incubation period, serial interval, transmission probability per coital act, and reproductive number will be needed for mathematical modelling studies of transmission dynamics, the proportion of cases attributable to sexual transmission, the potential for epidemic spread, and the effects of control measures. These quantities are particularly difficult to estimate when the agent has an alternative, predominant mode of transmission in endemic areas, e.g., mosquito-borne Zika. Developing criteria and methodologic approaches to sexual transmission could prove invaluable if they can be applied to key pathogens with epidemic potential, including those that the World Health Organization has published in its blueprint of action to prevent epidemics [61]. A proactive initiative to understand the potential for sexual transmission of such pathogens will help us to stay ahead of the curve.
Zdroje
1. World Health Organization. WHO Sexually Transmitted Infections (STIs) Fact Sheet. 2017 [3/8/17]. Available from: http://who.int/mediacentre/factsheets/fs110/en/.
2. Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morbidity and mortality weekly report. 1981;30(21):250–2. 6265753
3. Rambaut A, Posada D, Crandall KA, Holmes EC. The causes and consequences of HIV evolution. Nat Rev Genet. 2004;5(1):52–61. doi: 10.1038/nrg1246 14708016
4. Carpenter CM, Charles R. Isolation of Meningococcus from the Genitourinary Tract of Seven Patients. Am J Public Health Nations Health. 1942;32(6):640–3. 18015632
5. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288–91. 6112607
6. Most H. Manhattan: "a tropic isle"? Am J Trop Med Hyg. 1968;17(3):333–54. 5658876
7. Schmerin MJ, Gelston A, Jones TC. Amebiasis. An increasing problem among homosexuals in New York City. Jama. 1977;238(13):1386–7. 197276
8. Mildvan D, Gelb AM, William D. Venereal transmission of enteric pathogens in male homosexuals. Two case reports. Jama. 1977;238(13):1387–9. 197277
9. William DC, Felman YM, Marr JS, Shookhoff HB. Sexually transmitted enteric pathogens in male homosexual population. N Y State J Med. 1977;77(13):2050–2. 270619
10. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerging infectious diseases. 2011;17(5):880–2. doi: 10.3201/eid1705.101939 21529401
11. Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). 1983. Rev Invest Clin. 2004;56(2):126–9. 15378805
12. World Health Organization. Emergencies: The history of Zika virus. 2017 [04/20/17]. Available from: http://www.who.int/emergencies/zika-virus/timeline/en/.
13. Bowen A. Shigellosis 2016. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/shigellosis
14. Dritz SK, Back AF. Letter: Shigella enteritis venereally transmitted. The New England journal of medicine. 1974;291(22):1194.
15. Bader M, Pedersen AH, Williams R, Spearman J, Anderson H. Venereal transmission of shigellosis in Seattle-King county. Sexually transmitted diseases. 1977;4(3):89–91. 337539
16. Gilbart VL, Simms I, Jenkins C, Furegato M, Gobin M, Oliver I, et al. Sex, drugs and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sexually transmitted infections. 2015;91(8):598–602. doi: 10.1136/sextrans-2015-052014 25921020
17. Aragon TJ, Vugia DJ, Shallow S, Samuel MC, Reingold A, Angulo FJ, et al. Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;44(3):327–34.
18. Tauxe RV, McDonald RC, Hargrett-Bean N, Blake PA. The persistence of Shigella flexneri in the United States: increasing role of adult males. American journal of public health. 1988;78(11):1432–5. 3052115
19. Simms I, Field N, Jenkins C, Childs T, Gilbart VL, Dallman TJ, et al. Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men—Shigella flexneri and S. sonnei in England, 2004 to end of February 2015. Euro Surveill. 2015;20(15).
20. Centers for Disease Control and Prevention. FoodNet Fast [2/27/17]. Available from: https://wwwn.cdc.gov/foodnetfast/.
21. Gaudreau C, Ratnayake R, Pilon PA, Gagnon S, Roger M, Levesque S. Ciprofloxacin-resistant Shigella sonnei among men who have sex with men, Canada, 2010. Emerging infectious diseases. 2011;17(9):1747–50. doi: 10.3201/eid1709.102034 21888811
22. Gaudreau C, Barkati S, Leduc JM, Pilon PA, Favreau J, Bekal S. Shigella spp. with reduced azithromycin susceptibility, Quebec, Canada, 2012–2013. Emerging infectious diseases. 2014;20(5):854–6. doi: 10.3201/eid2005.130966 24750584
23. Baker KS, Dallman TJ, Ashton PM, Day M, Hughes G, Crook PD, et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis. 2015;15(8):913–21. doi: 10.1016/S1473-3099(15)00002-X 25936611
24. Bowen A, Hurd J, Hoover C, Khachadourian Y, Traphagen E, Harvey E, et al. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin—United States, May 2014-February 2015. MMWR Morbidity and mortality weekly report. 2015;64(12):318–20. 25837241
25. Chiou CS, Izumiya H, Kawamura M, Liao YS, Su YS, Wu HH, et al. The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin Microbiol Infect. 2016;22(4):383 e11–6.
26. Heiman KE, Karlsson M, Grass J, Howie B, Kirkcaldy RD, Mahon B, et al. Notes from the field: Shigella with decreased susceptibility to azithromycin among men who have sex with men—United States, 2002–2013. MMWR Morbidity and mortality weekly report. 2014;63(6):132–3. 24522098
27. Lane CR, Sutton B, Valcanis M, Kirk M, Walker C, Lalor K, et al. Travel Destinations and Sexual Behavior as Indicators of Antibiotic Resistant Shigella Strains—Victoria, Australia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62(6):722–9.
28. Mook P, McCormick J, Bains M, Cowley LA, Chattaway MA, Jenkins C, et al. ESBL-Producing and Macrolide-Resistant Shigella sonnei Infections among Men Who Have Sex with Men, England, 2015. Emerging infectious diseases. 2016;22(11):1948–52. doi: 10.3201/eid2211.160653 27767929
29. Brown WJ, Kraus SJ, Arko RJ. Chimpanzee urethral meningococci. Br J Vener Dis. 1973;49(1):88. 4632813
30. Talbot MD, Collins BN. Presumed sexual transmission of meningococci. J Infect. 1981;3(3):273–6. 6821095
31. Wilson AP, Wolff J, Atia W. Acute urethritis due to Neisseria meningitidis group A acquired by orogenital contact: case report. Genitourin Med. 1989;65(2):122–3. 2502493
32. Taha MK, Claus H, Lappann M, Veyrier FJ, Otto A, Becher D, et al. Evolutionary Events Associated with an Outbreak of Meningococcal Disease in Men Who Have Sex with Men. PLoS ONE. 2016;11(5):e0154047. doi: 10.1371/journal.pone.0154047 27167067
33. Marcus U, Vogel U, Schubert A, Claus H, Baetzing-Feigenbaum J, Hellenbrand W, et al. A cluster of invasive meningococcal disease in young men who have sex with men in Berlin, October 2012 to May 2013. Euro Surveill. 2013;18(28).
34. Euopean Center for Disease Control. Invasive meningococcal disease among men who have sex with men. Rapid risk assessment. 2013.
35. Aubert L, Taha M, Boo N, Le Strat Y, Deghmane AE, Sanna A, et al. Serogroup C invasive meningococcal disease among men who have sex with men and in gay-oriented social venues in the Paris region: July 2013 to December 2014. Euro Surveill. 2015;20(3).
36. Kratz MM, Weiss D, Ridpath A, Zucker JR, Geevarughese A, Rakeman J, et al. Community-Based Outbreak of Neisseria meningitidis Serogroup C Infection in Men who Have Sex with Men, New York City, New York, USA, 2010–2013. Emerging infectious diseases. 2015;21(8):1379–86. doi: 10.3201/eid2108.141837 26197087
37. Kamiya H, MacNeil J, Blain A, Patel M, Martin S, Weiss D, et al. Meningococcal disease among men who have sex with men—United States, January 2012-June 2015. MMWR Morbidity and mortality weekly report. 2015;64(44):1256–7. doi: 10.15585/mmwr.mm6444a6 26562570
38. Nanduri S, Foo C, Ngo V, Jarashow C, Civen R, Schwartz B, et al. Outbreak of Serogroup C Meningococcal Disease Primarily Affecting Men Who Have Sex with Men—Southern California, 2016. MMWR Morbidity and mortality weekly report. 2016;65(35):939–40. doi: 10.15585/mmwr.mm6535e1 27606798
39. Janda WM, Bohnoff M, Morello JA, Lerner SA. Prevalence and site-pathogen studies of Neisseria meningitidis and N gonorrhoeae in homosexual men. Jama. 1980;244(18):2060–4. 6776296
40. Salit IE, Frasch CE. Seroepidemiologic aspects of Neisseria meningitidis in homosexual men. Can Med Assoc J. 1982;126(1):38–41. 6800626
41. World Health Organization. Ebola outbreak 2014–2015 2017 [cited 3/15/17]. Available from: http://www.who.int/csr/disease/ebola/en/.
42. Thorson A, Formenty P, Lofthouse C, Broutet N. Systematic review of the literature on viral persistence and sexual transmission from recovered Ebola survivors: evidence and recommendations. BMJ Open. 2016;6(1):e008859. doi: 10.1136/bmjopen-2015-008859 26743699
43. Christie A, Davies-Wayne GJ, Cordier-Lassalle T, Blackley DJ, Laney AS, Williams DE, et al. Possible sexual transmission of Ebola virus—Liberia, 2015. MMWR Morbidity and mortality weekly report. 2015;64(17):479–81. 25950255
44. Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T, et al. Molecular Evidence of Sexual Transmission of Ebola Virus. The New England journal of medicine. 2015;373(25):2448–54. doi: 10.1056/NEJMoa1509773 26465384
45. Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum JJ, Bressler D, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. The Journal of infectious diseases. 1999;179 Suppl 1:S28–35.
46. Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. The Journal of infectious diseases. 2007;196 Suppl 2:S142–7.
47. Soka MJ, Choi MJ, Baller A, White S, Rogers E, Purpura LJ, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health. 2016;4(10):e736–43. doi: 10.1016/S2214-109X(16)30175-9 27596037
48. Deen GF, Knust B, Broutet N, Sesay FR, Formenty P, Ross C, et al. Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors—Preliminary Report. The New England journal of medicine. 2015.
49. Sissoko D, Duraffour S, Kerber R, Kolie JS, Beavogui AH, Camara AM, et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health. 2017;5(1):e80–e8. doi: 10.1016/S2214-109X(16)30243-1 27955791
50. Wolrd Health Organization. Clinical care for survivors of Ebola virus disease: Interim guidence. 2016 April 11, 2016.
51. Vetter P, Kaiser L, Schibler M, Ciglenecki I, Bausch DG. Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect Dis. 2016;16(6):e82–91. doi: 10.1016/S1473-3099(16)00077-3 27020309
52. World Health Organization. Situation Report: Zika Virus. 2017 March 9, 2017.
53. Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23(5):296–305. doi: 10.1016/j.cmi.2016.12.027 28062314
54. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika Virus in Body Fluids—Preliminary Report. The New England journal of medicine. 2017.
55. Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21(32).
56. Arsuaga M, Bujalance SG, Diaz-Menendez M, Vazquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16(10):1107.
57. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16(9):1000–1.
58. Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, et al. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci Rep. 2016;6 : 28070. doi: 10.1038/srep28070 27312324
59. Towers S, Brauer F, Castillo-Chavez C, Falconar AK, Mubayi A, Romero-Vivas CM. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics. 2016;17 : 50–5. doi: 10.1016/j.epidem.2016.10.003 27846442
60. Althaus CL, Low N. How Relevant Is Sexual Transmission of Zika Virus? PLoS Med. 2016;13(10):e1002157. doi: 10.1371/journal.pmed.1002157 27780196
61. World Health Organization. An R&D Blueprint for Action to Prevent Epidemics. 2016.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO)
- Re-emerging and newly recognized sexually transmitted infections: Can prior experiences shed light on future identification and control?
- Antiretroviral therapy and population mortality: Leveraging routine national data to advance policy
- Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: A multicentre prospective cohort study
- Research on HIV cure: Mapping the ethics landscape
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- Effects of women’s groups practising participatory learning and action on preventive and care-seeking behaviours to reduce neonatal mortality: A meta-analysis of cluster-randomised trials
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
- Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: A modeling study
- Sexually transmitted infections—Research priorities for new challenges
- Healthcare provider perspectives on managing sexually transmitted infections in HIV care settings in Kenya: A qualitative thematic analysis
- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Dual-strain genital herpes simplex virus type 2 (HSV-2) infection in the US, Peru, and 8 countries in sub-Saharan Africa: A nested cross-sectional viral genotyping study
- Association between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries
- Bridging the quality chasm in maternal, newborn, and child healthcare in low- and middle-income countries
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



